Abstract
We report outcomes from 52 patients with chronic osteomyelitis from implant infection treated with a single stage protocol including debridement augmented with application of CERAMENT™/G biocomposite after resection of Cierny-Mader (C-M) stage III and IV chronic osteomyelitis. Mean age was 53 years with a mean follow up of 17 months. Infection was eradicated in 48 (92.3%) patients. There were four (7.7%) recurrences. Eighteen patients (35%) had a flap. Staphylococci (51%) and Enterococci (15%) were the commonest microorganisms. Local antibiotic augmentation (CERAMENT™/G biocomposite) with dead space management is effective in the treatment of implant related chronic osteomyelitis.
Level of evidence
Prognostic Level IV.
Keywords: Implant related osteomyelitis, Augmented debridement, Absorbable, Gentamycin loaded calcium sulphate/hydroxyapatite biocomposite
1. Introduction
Chronic osteomyelitis in orthopaedic surgery is a limb threatening pathology.1 Different factors can contribute to chronicity. The presence of implants (i.e. plates, screws, intramedullary nails) and devascularised or dead bone contributes to chronicity. The pathogens with their ability of producing a protective biofilm and reduction of their metabolism compromise the host's potential to eliminate infection. The host's physiological status, smoking, obesity or systemic diseases (diabetes mellitus, peripheral vascular disease) are also related to inadequate response to infection.1
Surgical treatment with repeated debridement, fixation in stages and soft tissue coverage has been the classical treatment over the past decades.2 Antibiotic-impregnated acrylic beads and antibiotic-loaded polymethylmethacrylate (PMMA) cement may be used for dead space sterilization and management3 but need removal and increase the number of operative stages. Furthermore, failure to remove can lead to secondary infection due to retained foreign material.2 Calcium sulphate (CAS) carriers loaded with antibiotics are often for bone defects after excision of infected bone but bone formation does not always happen, and pathological fractures have been reported in up to 10% of patients in some studies.3,4
Recently, CAS crystals and hydroxyapatite (HA) particles have been combined in a liquid, injectable product called CERAMENT™ Fig. 1.5 This promising product has also been loaded with antibiotics (175 mg gentamycin in 10 mls CAS/HA: Cerament G; 66 mg vancomycin in 10 mls CAS/HA: CERAMENT V; Bonesupport, Lund, Sweden). The rational is that after injection of the biocomposite into a bone void both active resorption and passive dissolution begin and what left is a HA scaffold for new bone formation. Literature regarding CERAMENT™ as a void filler is restricted.6 Even fewer reports exist for the use of CERAMENT™ in the treatment of osteomyelitis in animal models or humans.7 In a series of 100 patients with chronic osteomyelitis, McNally et al. report 96% eradication of infection with a single-stage protocol including debridement, dead space management with CERAMENT G, culture-specific systemic antibiotics, primary skin closure and stabilization if needed.7
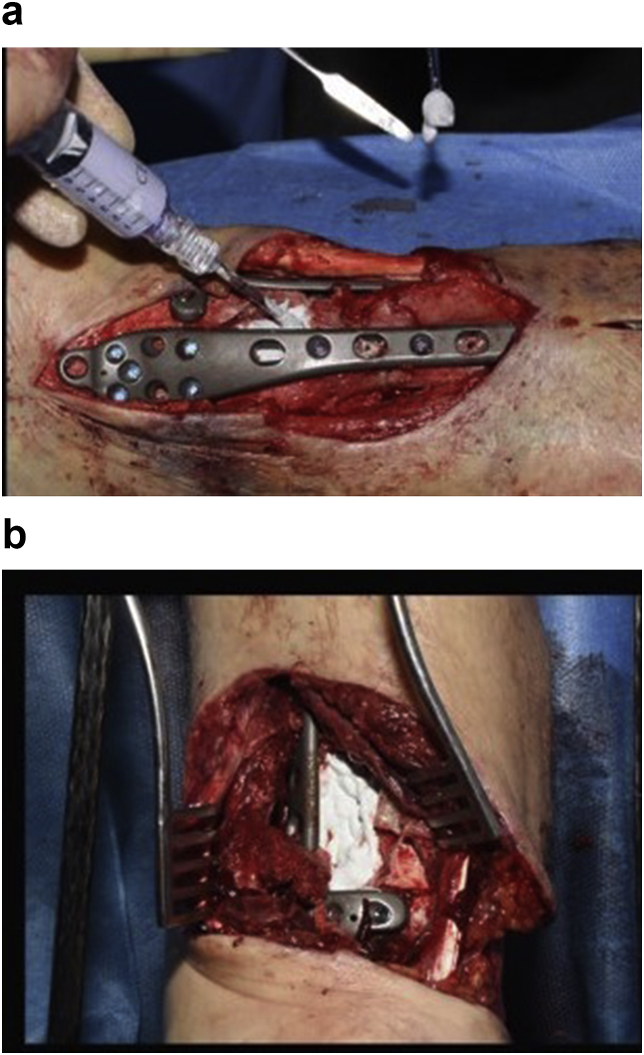
Fig. 1.
a–b: Application of injectable Cerament G in a cavitary defect of distal tibia after excision of dead bone in a patient with chronic osteomyelitis.
We present a series of 52 patients all with implant related chronic osteomyelitis treated in a single stage surgical protocol, with removal of the metalwork, debridement augmented by CERAMENT G, culture-based antibiotics, appropriate soft tissue cover and stabilization. This is a single center, single surgeon study.
2. Methods
This is a review of prospectively collected data from 52 patients with chronic osteomyelitis from implant infection. Informed consent was taken from all patients included in the study. The retrospective observational study was approved by the local Ethical Committee as part of the Clinical Effectiveness and Audit Department (number 6654) and was performed in accordance with the ethical standards of the 1964 Declaration of Helsinki as revised in 2000.
Chronic osteomyelitis in the presence of metalwork was defined as symptomatology of at least six months with associated radiological, microbiology, hematological and clinical findings with at least one of the following: discharging sinus, exposed metalwork, abscess or pus.8 Infected non-unions were also included in the study. A dedicated ortho-plastic approach along with input from infectious diseases was undertaken in all cases. The Cierny-Mader (C-M) staging system was used to describe the extent of bony involvement Table 1 and the host's physiological status Table 2.9 Patients with C-M Type III and IV chronic osteomyelitis were included into the study. Exclusion criteria were diabetic foot infections, chronic osteomyelitis without previous metalwork in the area and known allergy to CS/HA or aminoglycosides.
Table 1.
Cierny-Mader anatomical type.
| I | Medullary (endosteal) |
| II | Superficial (outer cortex) |
| III | Localised (cortex and endosteal) |
| IV | Diffuse (involvement of bone segment) |
Table 2.
Cierny-Mader host's physiological status.
| Class A | No comorbidities |
| Class BL | Local compromise of affected limb |
| Class BS | Systemic compromise |
| Class BLS | Local and systemic compromise |
| Class C | Treatment worse than disease |
Data including demographics, anatomical site, clinical, radiological and laboratory features, previous treatments/type of infected metalwork, need/type of stabilisation performed, soft tissue coverage, antibiotic treatment, duration of therapy and follow up were collected prospectively.
2.1. Augmented debridement -single stage protocol
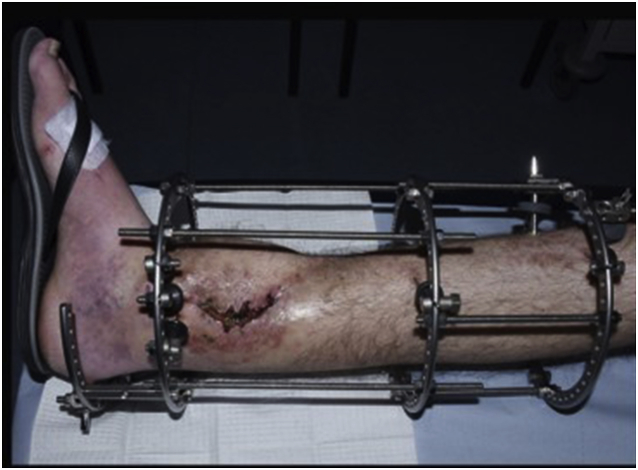
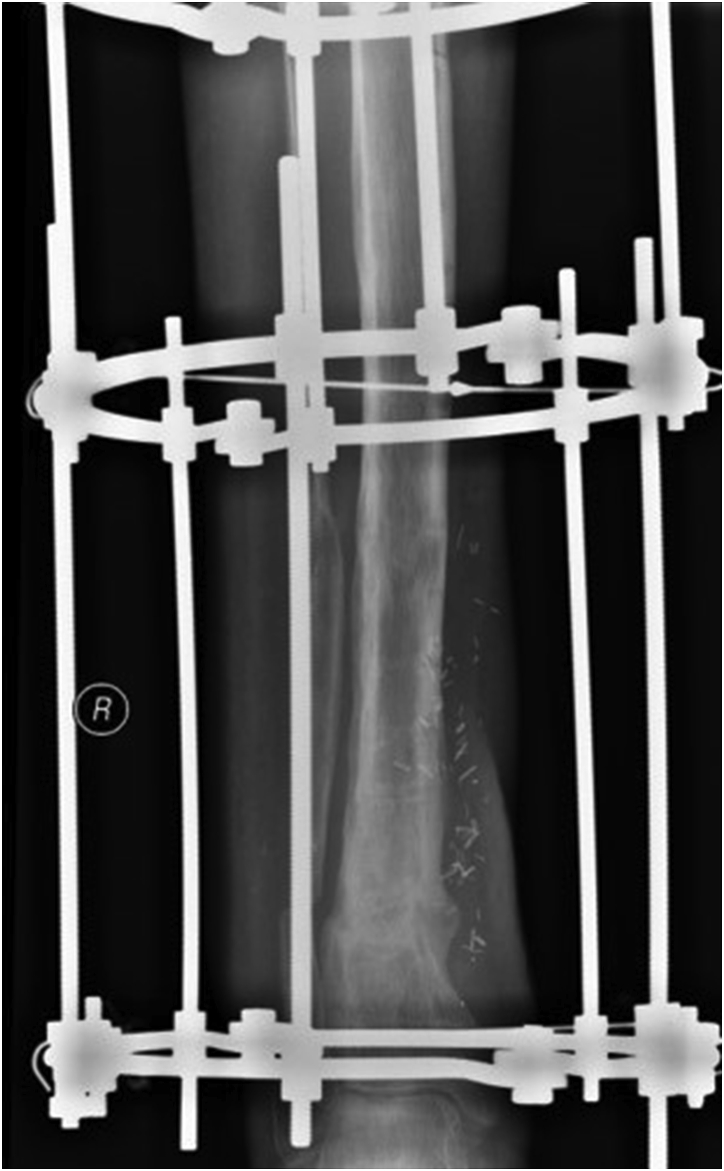
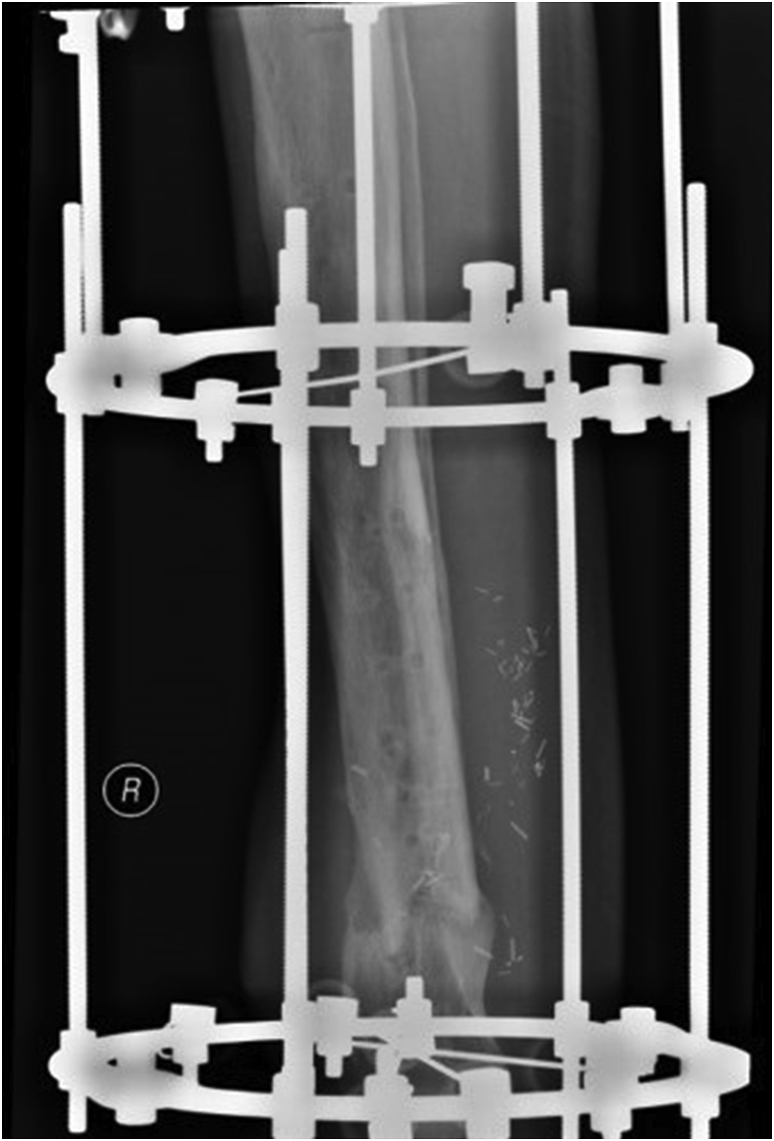
All patients underwent a single stage operative protocol. All infected/devitalised skin, sinus tracts and soft tissues and bone was excised. When necessary, a plastic surgeon performed or advised on extension of the skin incision in order not to jeopardise performance of successive flaps or skin viability. Infected metalwork was removed until healthy and bleeding bone. Multiple deep tissue samples were collected, sent for microbiology and intravenous antibiotics were started according advice from an infectious disease specialist. The remaining cavity, after excision of infected bone, was washed then irrigated with dilute (50/50) hydrogen peroxide solution and dried by gauze packing. The dry cavity was now filled with CERAMENT G. If instability was present (i.e. CM stage IV osteomyelitis) external fixation (usually an Ilizarov frame) was added to the construct Fig. 2a–e. Provision was always taken to facilitate access to the area when a free/local flap, split thickness skin graft (STSG) or primary closure was required from the plastic surgery team. Post operatively, antibiotic treatment continued for a period up to 12 weeks. After removal of the circular frame a fracture boot was used for a period of 4 weeks and full weight bearing was allowed.
Fig. 2.
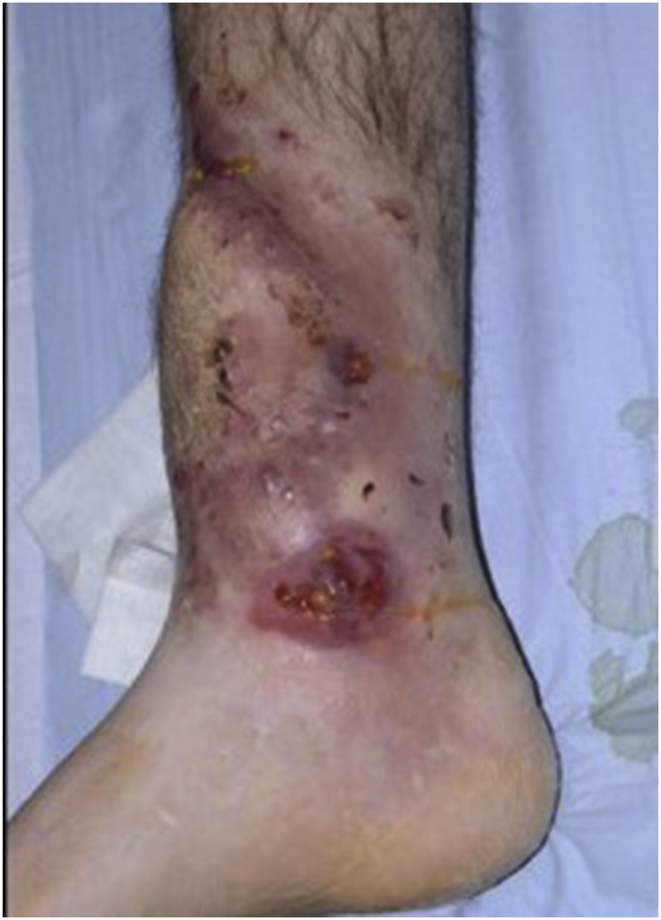
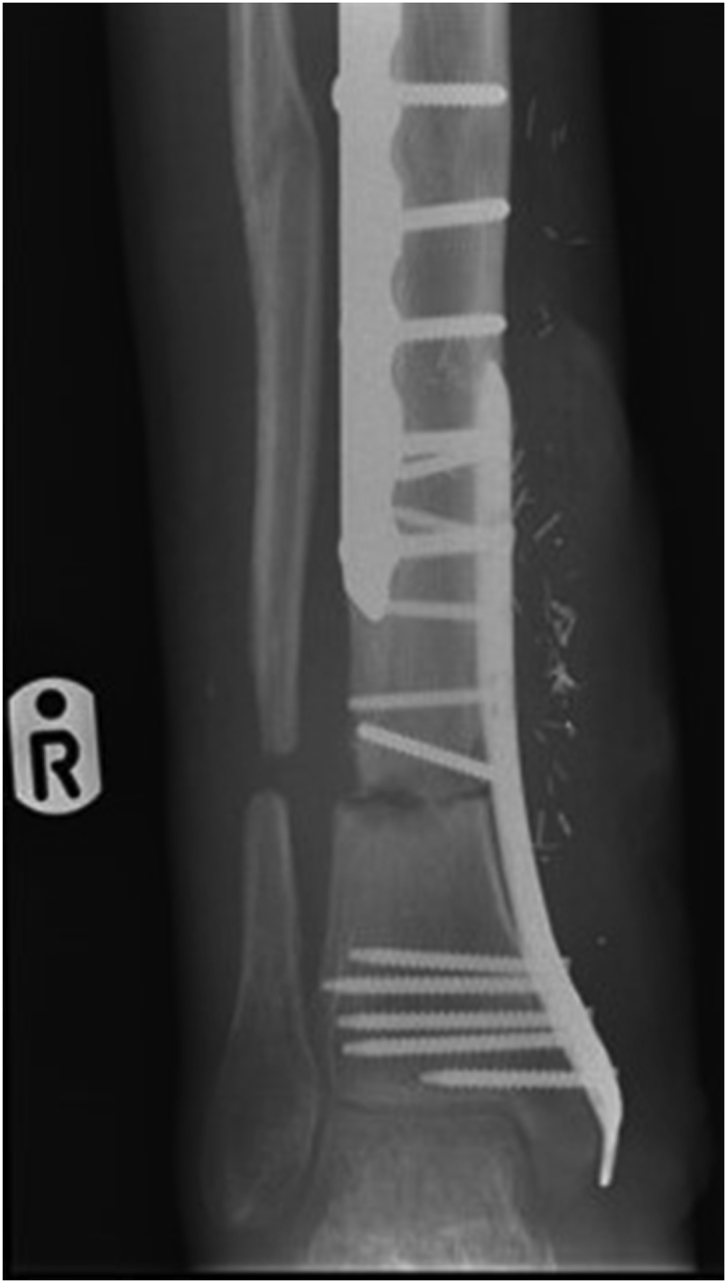
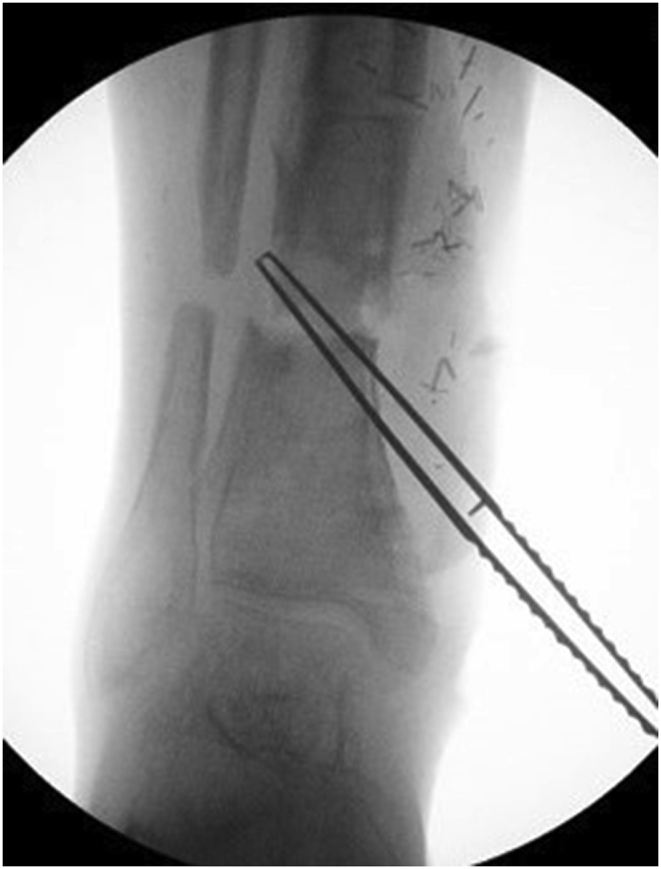
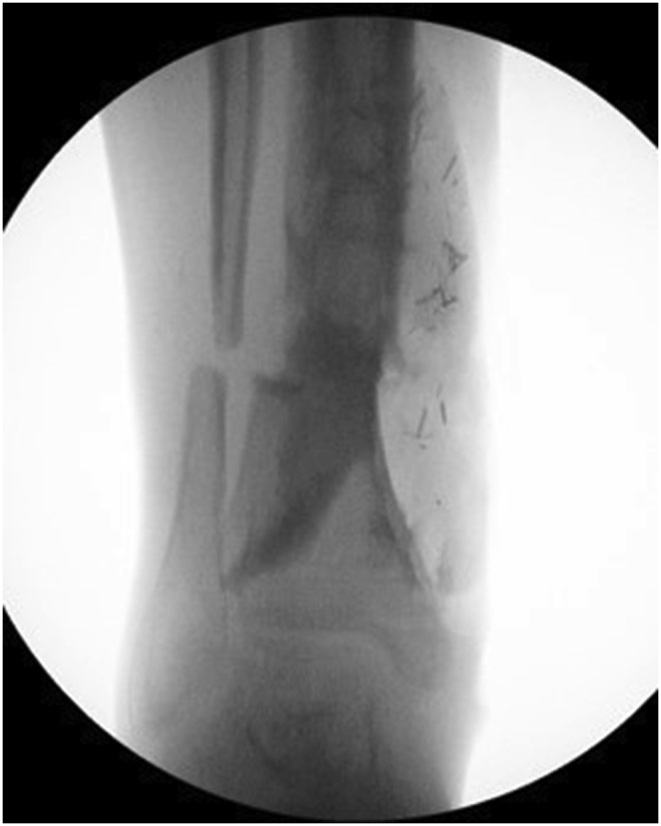
a–e: (a) Implant related chronic osteomyelitis of the tibia with sinuses. (b) Tibial shaft non-union in the same patient treated with plate/screws for open fracture; (c) During surgery, infected skin, soft tissues and infected/dead bone were excised; (d) The medullary canal was opened both sides and the remaining cavity was adequately prepared and filled with CERAMENT G; (e) Instability was present (CM stage IV osteomyelitis) and an Ilizarov frame was added to the construct.2f–g: At 8 months post-surgery the tibial non-union has gone on to complete healing and the biocomposite has been replaced by bone and started remodelling.
The primary outcome measure was eradication of infection at a minimum of 12 months follow up after surgery. Recurrence of infection of the soft tissues or bone, recurrent sinus formation, further surgery for infection or patient requiring antibiotic treatment for persisting symptoms was defined as a failure. Any deaths, wound healing problems, pathological fractures and need for re-operation were considered as secondary outcomes.
For collection and analysis of data the Microsoft Excel (Redmond, Washington, USA) and SPSSv19 software was used for windows (IBM SPSS Statistics 21NY, United States, Package for the Social Sciences). Consent was taken from all patients included in the study. The study was approved by the local Ethical Committee as part of the Clinical Effectiveness and Audit Department and was performed in accordance with the ethical standards of the 1964 Declaration of Helsinki as revised in 2000.
3. Results
Using the C-M classification 34 patients (65%) were defined as Type III and 18 (35%) as Type IV. A total of 37 (71%) patients were Class B hosts. In 11 cases (21%), there was an infected non-union and one patient had septic arthritis. Mean age was 53 years (range 22–81). Patients were followed for a mean of 17 months (range 12–48).
The tibia was the most commonly involved bone (47 patients/90%) followed by the femur (2 patients/4%), the foot (2 patients/4%) and in 1 (2%) patient there was an infected total ankle replacement (TAR). The infected metalwork was a plate in 41 patients (78%), an intramedullary nail in 5 (9.6%) cases, an Ilizarov frame or external fixator pin in 4 (7.7%) patients, one (2%) TAR and one (2%) patient with bone anchors. Additional stabilisation was needed in 35 (67%) cases and a circular frame was most commonly used (27 patients) with 7 patients having a plate/screws or intramedullary nail (IM nail) construct and one bone anchors. In 9 patients there was an infected non-union together with the implant related chronic osteomyelitis. Eighteen (35%) patients had a flap performed by the plastic surgeon (4 local and 14 a free flaps) and in the rest 65% primary closure was achieved Table 3. The antibiotic treatment (parental and oral) was used for a mean of 8 weeks (range 6–12).
Table 3.
Location of osteomyelitis by Cierny and Mader (C-M) stage, soft-tissue cover, and fixation method used.
| Bone | Total | C-M stagea III A | III B | IV A | IV B | Non-Union | Soft tissue cover Direct closure | Local or free flap | Fixation None | External Fixation | Internal Fixation |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Tibia | 47 | 7 | 23 | 7 | 10 | 7 | 31 | 16 | 17 | 25 Ilizarov | 3 Plate 2 IMN |
| Femur | 2 | 1 | 1 | 2 | 2 | 0 | 1 Ilizarov | 1 IMNb | |||
| Foot | 2 | 2 | 0 | 2 | 1 Plate | 1 Screws | |||||
| Ankle |
1 |
1 |
1 |
0 |
1 Ilizarov |
||||||
| Total | 52 | 8 | 26 | 7 | 11 | 9 |
A, Host with no comorbidities; B, Host with local and or systemic compromise.
Intramedullary nail.
3.1. Microbiology
Staphylococci (26 cultures or 51%) and Enterococci (8 cultures or 15%) were the most common microorganisms. Pseudomonas aeruginosa was more common in polymicrobial infection usually with Staphylococcus aureus (Table 4). The microorganisms grown in 13 of the patients (26%) were found to be resistant to gentamycin using the EUCAST criteria.10
Table 4.
Aetiology of infection and microbiology.
| Aetiology of infection | |
|---|---|
| Organisms | Number |
| Polymicrobial – 27% | 9 (5) |
| Methicillin Sensitive Staph aureus | 19 (1) |
| Enterococci | 4 (4) |
| Methicillin Resistant Staph aureus | 4 (2) |
| Corynebacterium | 5 |
| E. Coli | 4 |
| Pseudomonas spp | 3 (1) |
| Proteus spp | 3 |
| Corynebacteria spp | 3 |
| Morganella morganii | 2 (1) |
| Bacteriodes species | 2 |
| Klebsiella spp | 2 |
| Serratia spp | 1 (1) |
| Achromobacter xylosoxidans | (1) |
| Fusobacterium necrophorum | (1) |
| Cryptococcus | (1) |
| Stenotrophomonas | 1 |
| Achromobacter xylosoxidans | (1) |
3.2. Outcomes
The infection was controlled in 92.3% of the patients (48 cases) with the single stage protocol described.
There were 4 recurrences [Table 5]. Three were recurrence of osteomyelitis and one soft tissue/flap infection. One of the cases of recurrent osteomyelitis (Class A host) was treated successfully with reoperation repeating the same protocol used for the first treatment. We attribute this failure, to our learning curve in the early part of the development of the technique. The second case of recurrent osteomyelitis was a Class BLS (physiological status with local and systemic compromise from bowel cancer, smoking) host with a type III chronic osteomyelitis after treatment of his open ankle fracture with open reduction and internal fixation (ORIF). The microbiology report included Methicillin Resistant Staph aureus (MRSA) resistant also to Gentamycin and Klebsiella spp. The patient received suppressive antibiotic therapy as per patient's choice. We believe that both the host type and the microbiology (high virulence of Staph aureus resistant to Methicillin and Gentamycin, polymicrobial infection) contributed to failure. The third patient with recurrence of bone infection was again a Class BLS host (physiological status with local and systemic compromise from previous injury, diabetes, smoking, chronic obstructive pulmonary disease/COPD) with an infected intramedullary tibial nail after an open tibial shaft fracture. The microbiology showed a polymicrobial flora with Staph aureus (Methicillin sensitive). Once again, the high virulence of Staph aureus in a patient with compromised physiological status contributed to failure of initial treatment in our view. The patient had re-operation, but osteomyelitis persisted, and a below knee amputation was subsequently performed. The fourth failure involved a Class BLS host (local and systemic compromise from previous trauma, smoking, atrial fibrillation) with an infected intramedullary tibial nail after an open tibial shaft fracture. The microbiology showed a polymicrobial flora including Staph aureus (Methicillin sensitive and Gentamycin resistant). This case was successfully treated with soft tissue debridement. To summarise, the non-ideal physiological status, all with grade III chronic osteomyelitis and needing initially treatment with complex techniques (including a circular frame and plastic input with flaps) are common characteristics of the above cases. One out of the 9 (11.1%) infected non-unions did not unite but was infection-free at the last follow-up.
Table 5.
Cases with recurrent infection.
| Patient | Site | Cierny-Mader Staging | Aetiology/Infected Metalwork | Initial Treatment | Initial Microbiology | Revision Treatment |
|---|---|---|---|---|---|---|
| 1 | Tibia | III A | Open fracture with IM Nail | Excision, 25 cc Cerament G, free flap and Ilizarov | E. Coli | Successful revision: excision 20 cc Cerament G |
| 2 | Tibia | III B | Open fracture with ORIF | Excision, 30 cc Cerament G, free flap and Ilizarov | Resistant MRSA, Klebsiella spp | Suppressive antibiotic therapy |
| 3 | Tibia | III B | IM Nail for open fracture | Excision, 20 cc Cerament G, local flap and Ilizarov | MSSA, Pseudomonas spp | Revision excision 15 cc Cerament G, failure and BKA |
| 4 | Tibia | III B | IM Nail for open fracture | Excision, 20 cc Cerament G, free flap and Ilizarov | Resistant MSSA, Proteus spp, | Soft tissue debridement |
IM Nail: Intramedullary Nail; ORIF: Open Reduction and Internal Fixation.
No patient died during the period of our study. In 4 patients we noticed persisting wound oozing with a white fluid like liquefied CAS/HA biocomposite. All healed completely with regular change of dressings and none evolved into persisting sinus or recurrence of infection.
4. Discussion
Chronic osteomyelitis is difficult to treat and eradicate and particularly when combined with the presence of infected metalwork can be a devastating complication in orthopaedic surgery. The ability of the bacteria to establish the infection is related to the formation biofilms that allow them to adhere to each other and to any orthopaedic hardware. The presence of a biofilm in proximity of the metalwork complicates effective treatment as antibiotics usually are unable to penetrate.
Staphylococcus aureus (S. aureus) is one of the primary pathogens responsible for implant associated osteomyelitis.11 The natural history of formation of the biofilm begins when the bacteria meet the implant's surface and attach via one or more cell wall-associated adhesins, collectively known as MSCRAMMs (microbial surface components recognizing adhesive matrix molecules). The MRCRAMMs can recognize mammalian structures likely to be present at a wound site facilitating adhesion. The surface-bound bacteria then enter a robust proliferation phase and secrete including carbohydrate polymers, proteins and DNA, all forming the biogel matrix. The current theory is that as the local nutrient supply depletes, two phenotypes emerge: residual bacteria that remain in the biofilm, and bacteria activating the master regulator gene AGR (accessory gene regulator), setting in motion a series of events resulting in the S. aureus populating distant sites.11
Once established, osteomyelitis is rarely eradicated without any surgical intervention. The principle is that all infected bone must be excised and the surrounding soft tissue, frequently with part of the skin, extensively debrided. Insufficient bone resection will lead to recurrence of the osteomyelitis. But clinically there is a need to preserve as much bone as possible in order not to jeopardize stability of the residual bone and to maintain function of the limb. Furthermore, soft tissue coverage can be a crucial determinant of success.
Dead space sterilization and management is important to decrease the chance of recurrence of infection or the need for further procedures for bone grafting.
In 1970 Buchholz and Engelbrecht proposed the delivery of antibiotics to the infected site using antibiotic impregnated PMMA beads offering high local antibiotic concentrations with low systemic levels and risk of toxicity.12 Staged surgery with repeated excision of infected dead tissues has since been developed as treatment for chronic osteomyelitis. Further operations may be needed to remove the PMMA beads and deal with the loss of bone. With the evolution of plastic surgery techniques, local or more complex free fascio-cutaneous flaps have been used more commonly for loss of skin and soft tissues.1
Unfortunately, surgery in repeated stages increases costs for health systems and patient morbidity and seems to have variable success, usually less than 90% cure rate and often as low as 55% in various published series.13,14 With the introduction of biodegradable antibiotic impregnated bone substitutes, the integration of advanced techniques for plastic cover and systemic culture based antibiotic therapy increases the potential for a more patient focused approach to the treatment with a single-stage surgery.
Recent articles of patients with chronic osteomyelitis (but not necessary with implant associated) and using various methods of dead space management (PMMA rods, CAS) have reproduced recurrence rates of 9–45% at similar follow up periods.3,13,14 Literature regarding use of Cerament G in osteomyelitis is limited and McNally et al. report a series of 100 cases with chronic osteomyelitis treated using a similar single-stage protocol.7 This series included patients with haematogenous infection (19 cases) and patients without any metalwork. In our study implant related chronic osteomyelitis was effectively treated with a single surgery in 92.3% of the patients. Considering the presence of metalwork at the site of infection and the type of host patient frequently with local or even systemic comorbidities (72% of patients were hosts type B according to CM classification) the low recurrence rate at 12 months of follow up is promising. Also, only one out of the 9 (11.1%) infected non-unions did not unite but was infection-free at the last follow-up. The rest 8 went on to union with no recurrence of infection Fig. 2f–g
Our low recurrence rate and very low amputation risk (only one amputation or 2%) in a cohort of patients with infected metalwork, including 71% Class B hosts may be due to the combination of different factors. With the Cerament G delivery system we could deliver locally, often into avascular or not well perfused areas, high doses of antibiotic targeting any residual biofilm or bacteria. Local application of antibiotic reduces problems of compliance and with no systemic toxicity. According to the manufacturer Cerament G provides antibiotic elution above the Minimum Inhibitory Concentration (MIC) for gentamycin sensitive micro-organisms for at least 28 days and at least 100 times above the MIC for at least 7 days. Rapid dissolution of the biocomposite removes concerns of a prolonged period of low-grade local antibiotic release and the risk of gentamycin resistance.7
Bacteria resistance to gentamycin on laboratory testing were successfully treated in this series. Like McNally et al. we believe that lab culture reported gentamycin resistance refers to concentrations which can be given systemically without toxicity and the effectiveness of antibiotic at levels up to 1000 the MIC is not known.7 In our series there was no case of systemic toxicity. Four patients had persisting wound oozing with a white fluid like liquefied CAS/HA biocomposite but all healed completely with regular change of dressings and none evolved in persisting sinus or recurrence of infection.
Another advantage of the Cerament G is related to management of the dead space after the surgical debridement. Its use is simple, reproducible and in all patients the CAS/HA biocomposite was dissolved and replaced by bone Fig. 2a–g. After application to a void, tissue fluids fill microporous channels in the material, and both passive dissolution and active resorption of the CAS begins, leaving behind a HA scaffold for osteoblast colonization and formation of new bone.
According to Nilsson et al. the presence of HA, which does not dissolve passively, increases compressive strength of Cerament G like that of cancellous bone.15 We did not have any fractures after application of Cerament G in our study and we had bone remodelling in all patients. In our opinion this confers an additional advantage to other CAS carriers with higher reported fracture rates.
Our study is limited from its retrospective nature and the lack of a control group. We would highlight though that adequate debridement, removal of the metalwork, multiple bacteriological sampling, osseous stabilisation, soft tissue coverage and the collaborative multidisciplinary team (MDT) approach including plastic surgeons and infectious disease specialists is an important element of success with these challenging cases. We accept that the results reported by this study are not due only to the Cerament G application. However, our results of high cure rate and low recurrence is superior in comparison with other studies referring to PMMA spacers and CAS carriers with healing rates of 55–80%.3,13,14 Our results would therefore suggest that this new biodegradable CAS/HA composite can offer significant added advantages in the management of this cohort of patients.
5. Conclusion
In conclusion, we believe that chronic implant related osteomyelitis needs to be managed in specialised units. Our results with a single-stage protocol, using Cerament G as a bioabsorbable dead space filler and local antibiotic carrier show low recurrence rates with fewer operations. This improves patient experience and reduces healthcare costs. Additional, long term studies are required, and will help to improve our understanding of the use of local antibiotic delivery systems for bone and joint infection.
Patient consent
Informed consent was taken from all patients included in the study.
Conflict of interest statement
The authors report no conflict of interest concerning the materials or methods used in this study or the findings specified in this paper.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Ethical approval
The retrospective observational study was approved by the local Ethical Committee as part of the Clinical Effectiveness and Audit Department (number 6654) and was performed in accordance with the ethical standards of the 1964 Declaration of Helsinki as revised in 2000.
List of abbreviations
- Multidisciplinary team
MDT
- Cierny-Mader
C-M stage III and IV chronic osteomyelitis
- Antibiotic-impregnated acrylic beads and antibiotic-loaded polymethylmethacrylate
PMMA cement
- Calcium sulphate
CAS
- Hydroxyapatite
HA
- Split thickness skin graft
STSG
- Total ankle replacement
TAR
- Intramedullary
IM nail
- Microbial surface components recognizing adhesive matrix molecules
(MSCRAMMs)
- Accessory gene regulator
AGR
- Minimum Inhibitory Concentration
MIC
References
- 1.Gogia J.S., Meehan J.P., Di Cesare P.E., Jamali A.A. Local antibiotic therapy in osteomyelitis. Semin Plast Surg. 2009;23(2):100–107. doi: 10.1055/s-0029-1214162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Neut D., van de Belt H., van Horn J.R., van der Mei H.C., Busscher H.J. Residual gentamicin-release from antibiotic-loaded polymethylmethacrylate beads after 5years of implantation. Biomaterials. 2003;24:1829–1831. doi: 10.1016/s0142-9612(02)00614-2. [DOI] [PubMed] [Google Scholar]
- 3.Chang W., Colangeli M., Colangeli S. Adult osteomyelitis: debridement versus debridement plus Osteoset T pellets. Acta Orthop Belg. 2007;73:238–243. [PubMed] [Google Scholar]
- 4.Ferguson J.Y., Dudareva M., Riley N.D., Stubbs D., Atkins B.L., McNally M.A. The use of a biodegradable antibiotic-loaded calcium sulphate carrier containing tobramycin for the treatment of chronic osteomyelitis: a series of 195 cases. Bone Joint Lett J. 2014;96-B:829–836. doi: 10.1302/0301-620X.96B6.32756. [DOI] [PubMed] [Google Scholar]
- 5.Nilsson M., Zheng M.H., Tagil M. The composite of hydroxyapatite and calcium sulphte: a review of preclinical evaluation and clinical applications. Expert Rev Med Devices. 2013;10:675–684. doi: 10.1586/17434440.2013.827529. [DOI] [PubMed] [Google Scholar]
- 6.Lundusi R., Gasbarra E., D'Arienzo M., Piccioli A., Tarantino U. Augmentation of tibial plateau fractures with an injectable bone substitute: CERAMENT™. Three-year follow-up from a prospective study. BMC Muscoskelet Disord. 2015;16:115. doi: 10.1186/s12891-015-0574-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.McNally M.A., Ferguson J.Y., Lau A.C. Single-stage treatment of chronic osteomyelitis with a new absorbable, gentamicin-loaded, calcium sulphate/hydroxyapatite biocomposite: a prospective series of 100 cases. Bone Joint Lett J. 2016;98-B(9):1289–1296. doi: 10.1302/0301-620X.98B9.38057. [DOI] [PubMed] [Google Scholar]
- 8.Cierny G., III, DiPasquale D. Treatment of chronic infection. J Am Acad Orthop Surg. 2006;14:S105–S110. doi: 10.5435/00124635-200600001-00025. [DOI] [PubMed] [Google Scholar]
- 9.Cierny, Mader J.T., Penninck J.J. A clinical staging system for adult osteomyelitis. Clin Orthop Relat Res. 2003;414:7–24. doi: 10.1097/01.blo.0000088564.81746.62. [DOI] [PubMed] [Google Scholar]
- 10.No authors listed Clinical breakpoints. 7 September 2018. http://www.eucast.org/clinical_breakpoints/
- 11.Foster T.J., Geoghegan J.A., Ganesh V.K. Adhesion, invasion and evasion: the many functions of the surface proteins of Staphylococcus aureus. Nat Rev Microbiol. 2014;12:49–62. doi: 10.1038/nrmicro3161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Buchholz H.W., Engelbrecht H. Uber die depotwirkung einiger antibiotica bei vermischung mit dem kunstharz Palacos. Chirurg. 1970;41(11):511–515. [PubMed] [Google Scholar]
- 13.McNally M., Sendi P. Implant-Associated osteomyelitis of long bone. In: Zimmerli W., editor. Bone and Joint Infections: From Microbiology to Diagnostics and Treatment. John Wiley & Son; Chichester, West Sussex: 2014. pp. 303–323. [Google Scholar]
- 14.Cho S.H., Song H.R., Koo K.H., Jeong S.T., Park Y.J. Antibiotic-impregnated cement beads in the treatment of chronic osteomyelitis. Bull Hosp Jt Dis. 1997;56(3):140–144. [PubMed] [Google Scholar]
- 15.Nilsson M., Wang J.-S., Wielanek L., Tanner K.E., Lidgren L. Biodegradation and biocompatibility of a calcium sulphate-hydroxyapatite bone substitute. J Bone Joint Surg [Br] 2004;86-B:120125. [PubMed] [Google Scholar]