ABSTRACT
Mural cells (MCs) are essential for blood vessel stability and function; however, the mechanisms that regulate MC development remain incompletely understood, in particular those involved in MC specification. Here, we investigated the first steps of MC formation in zebrafish using transgenic reporters. Using pdgfrb and abcc9 reporters, we show that the onset of expression of abcc9, a pericyte marker in adult mice and zebrafish, occurs almost coincidentally with an increment in pdgfrb expression in peri-arterial mesenchymal cells, suggesting that these transcriptional changes mark the specification of MC lineage cells from naïve pdgfrblow mesenchymal cells. The emergence of peri-arterial pdgfrbhigh MCs required Notch signaling. We found that pdgfrb-positive cells express notch2 in addition to notch3, and although depletion of notch2 or notch3 failed to block MC emergence, embryos depleted of both notch2 and notch3 lost mesoderm- as well as neural crest-derived pdgfrbhigh MCs. Using reporters that read out Notch signaling and Notch2 receptor cleavage, we show that Notch activation in the mesenchyme precedes specification into pdgfrbhigh MCs. Taken together, these results show that Notch signaling is necessary for peri-arterial MC specification.
KEY WORDS: Mural cells, Pericytes, Vascular smooth muscle cells, Zebrafish, Notch
Summary: Live imaging of mural cell development in zebrafish uncovers the fact that specification into mural cells is positively regulated by Notch signaling.
INTRODUCTION
All blood vessels are built from two principal cell types: endothelial cells (ECs) and mural cells (MCs). ECs line the vascular lumen whereas MCs localize on the abluminal side of ECs, where they regulate vessel stability and function. MCs are further categorized into at least two major cell types, vascular smooth muscle cells (VSMCs) and pericytes (PCs). VSMCs encircle the EC and cover most (in arterioles) or all (in arteries with multiple-layered VSMC coats) of the vascular abluminal surface. PCs instead extend longitudinally along capillaries, one PC in contact with multiple ECs and covering only part of the abluminal surface.
The population of MCs expands by migration and proliferation during vascular growth (reviewed by Armulik et al., 2011). Before this expansion, however, MC precursors have to emerge from mesodermal or neural crest-derived mesenchyme, depending on the position in the vascular tree (Ando et al., 2016; Majesky, 2007; Wasteson et al., 2008; Winkler et al., 2011). In mammals, VSMCs in the gut and liver have a proposed mesothelial origin, whereas most VSMCs and PCs in the head and thymus are thought to derive from the neural crest. Similarly, VSMCs that cover the ascending aorta and arch, as well as the pulmonary trunk, are thought to originate from neural crest cells, whereas those in the descending aorta have a presumed somite origin (Armulik et al., 2011; Sinha and Santoro, 2018). This lineage commitment appears to be evolutionarily conserved, as zebrafish vascular development follows a similar pattern; MC progenitors in trunk and hindbrain vessels originate from mesoderm, whereas those in the forebrain and eye originate from the neural crest (Ando et al., 2016).
A number of signaling mechanisms have been shown to be important for MC development (Armulik et al., 2011; Sinha and Santoro, 2018). Following their specification, MCs proliferate and migrate to cover the expanding vessel network in a process that is dependent on platelet-derived growth factor (PDGF) receptor-β, which is expressed by the MCs, and its ligand PDGF-BB, which is secreted from growing ECs (Ando et al., 2016; Armulik et al., 2011; Hellstrom et al., 1999; Lindahl et al., 1997). Also TGFβ signaling has been implicated in MC differentiation, both via the TGFβ and activin branches of the pathway. For example, SMAD2 is important for aortic arch VSMC differentiation, whereas loss of ALK1 (ACVRL1) function (in the activin branch) results in reduced MC coverage (Chen et al., 2013; Garg et al., 2014). TGFβ signaling has also been implicated in MC differentiation in mouse skin vasculature (Yamazaki et al., 2017) and in cell culture (Tang et al., 2010). Sphingosine-1-phosphate (S1P), heparin-binding epidermal growth factor/ErbB and stromal cell-derived factor 1/C-X-C chemokine receptor type 4-mediated signaling have further been suggested to induce VSMC migration towards the vascular wall (Allende and Proia, 2002; Allende et al., 2003; Iivanainen et al., 2003; Liu et al., 2000; Song et al., 2009; Stratman et al., 2010).
Another important signaling mechanism is Notch signaling, which exerts important functions both in EC and MC development (Siebel and Lendahl, 2017). Mice specifically targeted for the Notch ligand Jagged1 in EC show loss of MC coverage (High et al., 2008), presumably by failure to activate Notch receptors on the MC side. Among the Notch receptors (Notch1, 2, 3, 4 in mammals), Notch3 is selectively expressed in MC, and loss of Notch3 function in the mouse leads to progressive loss of arterial VSMC (Henshall et al., 2014; Liu et al., 2010; Ruchoux et al., 2003; Wang et al., 2012; Volz et al., 2015). Furthermore, mutations in the human NOTCH3 gene cause cerebral autosomal dominant arteriopathy with subcortical infarct and leukoencephalopathy (CADASIL), a genetic stroke syndrome that is associated with morphological changes in the wall of small arteries and arterioles (Joutel et al., 1996). Experiments using notch3 loss- and gain-of-function zebrafish mutants have revealed that Notch3 signaling positively regulates MC proliferation also in this species (Coupland et al., 2018; Wang et al., 2014).
Although we have a relatively good understanding of some of the later maturation steps in MC development, we are still largely ignorant about signaling mechanisms that control early stages of the process, such as the commitment of undifferentiated mesenchymal cells to the MC lineage. Available data suggest that mesenchymal progenitors adjacent to ECs – in particular arterial ECs (aECs) – are induced to become MC progenitors, which subsequently expand during vessel growth and eventually differentiate into quiescent VSMC and PC of mature vessels (Ando et al., 2016; Armulik et al., 2011). In the absence of aEC, MCs fail to emerge, which indicates that ECs may play an instructive role in MC development (Ando et al., 2016). Further insights into early MC specification have been hampered by the lack of tools to visualize the process in vivo. However, with the recent development of transgenic reporter lines, we can now study the process of MC specification in greater detail. pdgfrb reporter transgenic (Tg) zebrafish lines allow visualization of MC location, abundance and recruitment (Ando et al., 2016; Vanlandewijck et al., 2018). Studies of this line show that all vessels in the zebrafish brain are covered by pdgfrb-positive MCs. Other MC reporter fish lines provide complementary information: tagln-positive MCs (presumably VSMCs) are restricted to arteries and/or arterioles, and abcc9-positive MCs (PCs) are restricted to capillaries and veins in the juvenile and adult brain (Vanlandewijck et al., 2018). The distribution of these markers across the different types of MCs in zebrafish matches the expression pattern of gene orthologs in the mouse brain vasculature, suggesting that MC heterogeneity and subtype distribution is evolutionarily conserved (Vanlandewijck et al., 2018). In addition, zebrafish and mammals seemingly share the same fundamental regulatory mechanisms of MC development, as pdgfrbsa16389 mutant zebrafish vessels lack MC coverage owing to defective MC migration and proliferation (Ando et al., 2016), analogous to the consequences of perturbed PDGFRβ signaling for MC development in the mouse (Hellstrom et al., 1999). Therefore, it is likely that general insights into the mechanisms that govern MC development can be inferred from studies in zebrafish.
In this study, we took advantage of MC reporter zebrafish in which the timing and location of MC emergence could be observed at high resolution, in order to gain new insights into the molecular mechanisms underlying MC specification. We reveal that Notch signaling in MC precursors is indispensable for their specification into MC lineage cells.
RESULTS
Characterization of discrete steps in zebrafish mural cell development
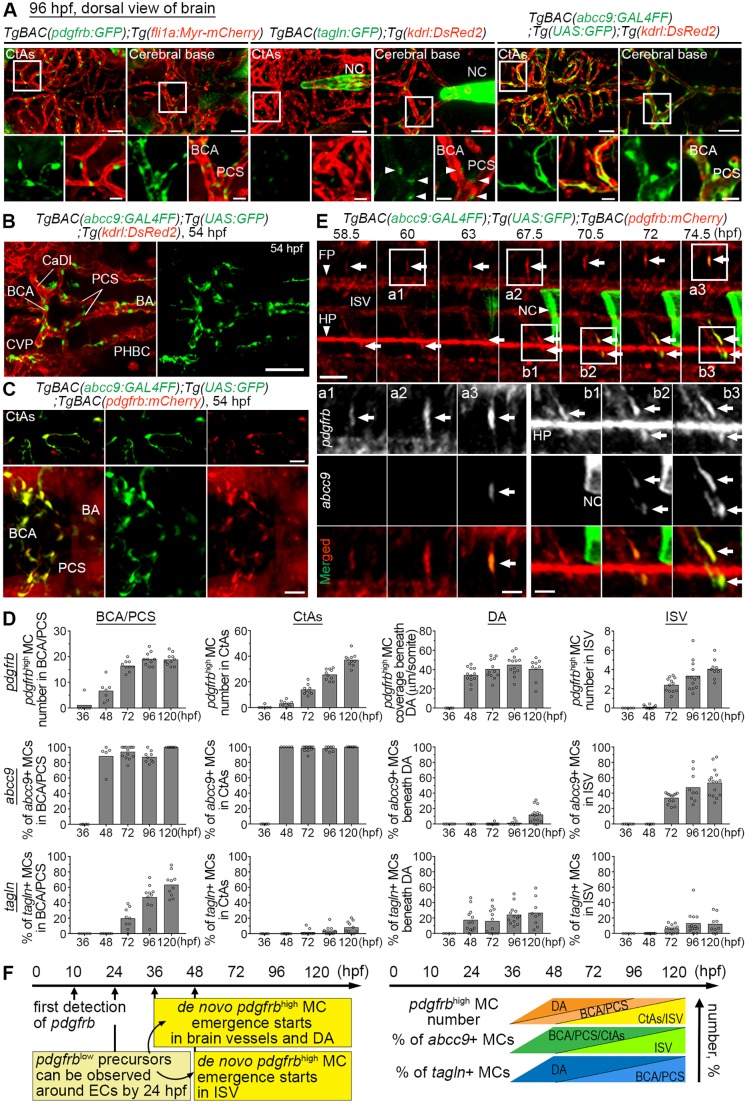
The progression from a founding cell population of MCs to the different subtypes of VSMCs and PCs that are observed in the adult quiescent vasculature is likely to proceed through a series of distinct intermediate steps. In order to be able to delineate these steps, new insights into the molecular mechanisms that control MC specification and differentiation would be desirable. Progress has, however, been hampered by the lack of good markers. To overcome this hurdle, we set out to establish and characterize zebrafish reporter lines that reflect different steps in MC differentiation. We first corroborated previous findings (Ando et al., 2016) that a pdgfrb reporter line, TgBAC(pdgfrb:GFP), displays weakly pdgfrb-positive (pdgfrblow) cells widely distributed in the animal, and that cells adjacent to arterial ECs show increased reporter expression and start to cover brain and trunk arterial vessels before 4 days post-fertilization (dpf) (Fig. 1A and data not shown). At this age, VSMC markers, such as tagln, were detected in only a subset of the pdgfrb-positive cells and were hardly detectable at all in central arteries (CtAs) before 4 dpf (Fig. 1A,D). This is in agreement with previous data (Ando et al., 2016; Chen et al., 2017; Seiler et al., 2010; Whitesell et al., 2014) and indicates that additional markers would be helpful to map the early development of MCs, in particular with regard to the time point(s) of MC specification. We next assessed the distribution of reporter gene activity driven by the regulatory elements of abcc9, a relatively specific marker for PCs in the mouse brain, using a recently developed TgBAC(abcc9:GAL4FF) zebrafish line that specifically labels PCs in the juvenile/adult zebrafish brain (Vanlandewijck et al., 2018). By observing Tg(abcc9:GAL4FF);Tg(UAS:GFP) larvae at 4 dpf, we found that brain and trunk vessels were already covered by abcc9-positive cells that were also positive for pdgfrb:mCherry expression (Fig. 1A,D; Fig. S1A-D). Earlier, at 54 h post-fertilization (hpf) and soon after the emergence of pdgfrbhigh cells around brain vessels (Fig. 1D) (Ando et al., 2016), abcc9 reporter expression was already detected in pdgfrbhigh cells around vessels (Fig. 1B-D). The TgBAC(abcc9:GAL4FF) reporter labeled almost all pdgfrbhigh MCs in the brain from their first emergence (Fig. 1D; Fig. S1). However, approximately half of pdgfrbhigh MCs in intersegmental vessels (ISVs) and most MCs beneath the dorsal aorta (DA) were abcc9 reporter negative at 5 dpf (Fig. 1D; Fig. S1). Time-lapse imaging of TgBAC(abcc9:GAL4FF);Tg(UAS:GFP);TgBAC(pdgfrb:mCherry) embryos showed that abcc9 expression occurred in pdgfrbhigh cells soon after the transition from pdgfrblow to pdgfrbhigh in the pdgfrb:mCherry reporter (Fig. 1E). In line with previous suggestions (Ando et al., 2016), these results indicate that the increase in pdgfrb expression reflects the commitment of naïve pdgfrblow mesenchymal cells into pdgfrbhigh MC, and that this specification takes place primarily around arteries during 36-72 hpf (after 36 hpf in brain vessels and beneath the DA and after 48 hpf in ISV) (Fig. 1D,F). Subsequently, pdgfrbhigh MCs around larger vessels start to express tagln or acta2 and seemingly acquire VSMC properties, which is obvious in brain vessels (Fig. 1D).
Fig. 1.
MC marker expression during early developmental stages. (A) Confocal stack images of the brain vasculature of 96 hpf TgBAC(pdgfrb:GFP);Tg(fli1a:Myr-mCherry) (left), TgBAC(tagln:GFP);Tg(kdrl:DsRed2) (middle) or TgBAC(abcc9:GAL4FF);Tg(UAS:GFP);Tg(kdrl:DsRed2) (right) larvae. CtAs or the vessels in the cerebral base such as the BCA, PCS and BA are shown in the top rows, and the boxed regions are enlarged in the bottom rows. Note that pdgfrb- and abcc9 reporter-positive MCs were readily observed, whereas tagln reporter expression was faint in BCA and PCS (arrowheads) and not detected in CtAs. (B,C) Confocal stack images of the brain vasculature of 54 hpf TgBAC(abcc9:GAL4FF);Tg(UAS:GFP);Tg(kdrl:DsRed2) (B) or TgBAC(abcc9:GAL4FF);Tg(UAS:GFP);Tg(pdgfrb:mCherry) embryos (C). abcc9-positive cells were observed only around vessels (B) and these abcc9-positive cells were positive for pdgfrb:mCherry expression (C). Note that pdgfrb:mCherry expression was broadly detected in the cerebral base but only pdgfrbhigh cells around these vessels expressed the abcc9 reporter. (D) Changes of pdgfrbhigh MC number (top row), percentage of abcc9 reporter-positive (middle row) or percentage of tagln reporter-positive (bottom row) pdgfrbhigh MCs at indicated time points and vessels. The number of pdgfrbhigh MCs in ISV is shown per somite in one side of the trunk. (E) Time-lapse imaging of the trunk region of TgBAC(abcc9:GAL4FF);Tg(UAS:GFP);TgBAC(pdgfrb:mCherry) embryo at indicated time points. Boxed regions are enlarged to the bottom. Increase of pdgfrb:mCherry signal could be observed after 67.5 hpf (arrows). Soon after the increase of pdgfrb:mCherry signal, GFP expression was observed only in these pdgfrbhigh cells (arrows). (F) Schematic time course of pdgfrb-positive cell emergence (left) (Ando et al., 2016) and the changes of pdgfrbhigh-, abcc9-positive or tagln-positive MCs in brain vessels (BCA/PCS, CtAs), DA or ISV, based on D (right). Peri-arterial pdgfrblow precursors can be detected by 24 hpf. Subsequently, the specification from those pdgfrblow precursors into pdgfrbhigh MCs takes place, and the expression of other MC markers is induced in pdgfrbhigh MCs at the cerebral base and beneath the DA after 36 hpf, as well as in the ISV after 48 hpf. BA, basilar artery; CaDl, caudal division of the internal carotid artery; CVP, choroidal vascular plexus; FP, floor plate; HP, hypochord; NC, notochord; PHBC, primordial hindbrain channel. Scale bars: 50 µm in A,E; 100 µm in B; 30 µm in C; 20 µm in enlarged images in A,E.
Notch but not TGFβ signaling is essential for the early emergence of mural cells
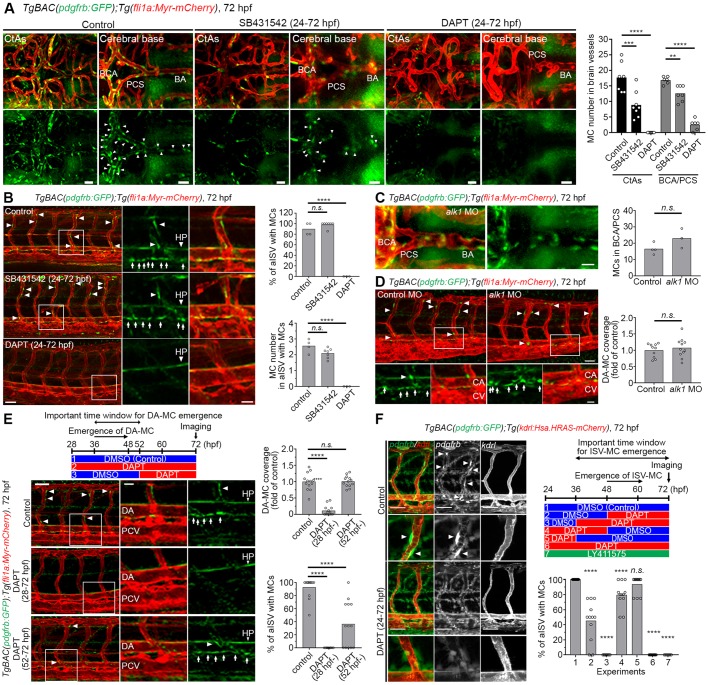
The molecular mechanisms that control specification of MC are only partially understood, and both TGFβ and Notch signaling have been proposed as regulators of MC differentiation (Tang et al., 2010). Based on the delineation of reporter activity above, we focused on the time window 36-72 hpf, a period preceding and encompassing the emergence of pdgfrbhigh MCs, as this is likely a crucial window in MC specification. We first explored the role of TGFβ signaling by treating embryos with the TGFβ1 receptor inhibitor SB431542. Even though the number of pdgfrbhigh MCs in the brain was partially reduced, the emergence of MCs in trunk vessels was not affected by SB431542 treatment (Fig. 2A,B). As the SB431542 inhibitor does not inhibit TGFβ signaling through the Alk1 receptor, and because loss of ALK1 function has been shown to cause a reduction in the MC coverage (Chen et al., 2013; Garg et al., 2014), we investigated the effect of alk1 depletion on MC emergence. Recruitment of MCs from the brain base to the CtAs was inhibited by alk1 morpholino (MO), but the emergence of MCs in the brain base and beneath the DA was not affected (Fig. 2C,D; Fig. S2A,B), which suggests a role for alk1 in recruitment of MC, but not in the early inductive phase.
Fig. 2.
The emergence of pdgfrbhigh MCs was inhibited by DAPT treatment during the specification period. (A,B) Confocal stack images of pdgfrbhigh MCs at 72 hpf in brain vessels (A) or trunk vessels (B) after treatment with vehicle-DMSO (control), 50 µM SB431542 or 100 μM DAPT from 24 hpf through 72 hpf. E3 medium-containing inhibitors were replaced with fresh ones every 12 h. (C,D) Brain vessels (C) and trunk vessels (D) of 72 hpf TgBAC(pdgfrb:GFP);Tg(fli1a:Myr-mCherry) larvae injected with 6 ng alk1 MO. Note that alk1 MO induced abnormal vasculature in the cerebral base or caudal vein but did not inhibit the emergence of pdgfrbhigh MCs. (E) Trunk vessels of TgBAC(pdgfrb:GFP);Tg(fli1a:Myr-mCherry) larvae treated with vehicle-DMSO or 100 µM DAPT at the time point indicated in the top schematic. Boxed regions are enlarged to the right. (F) Confocal stack images of TgBAC(pdgfrb:GFP);Tg(kdrl:Hsa.HRAS-mCherry) larvae treated with either 1% DMSO, 25 μM DAPT or 5 μM LY411575 at the time point indicated in the schematic on the right. Arrows and arrowheads indicate pdgfrbhigh MCs beneath DA and in BSA/PCS/BA/ISVs, respectively. Quantitative results are shown on the right of each representative image. Bars and circles in the graph indicate averages and each value of observed larvae, respectively. ****P<0.0001, ***P<0.001 and **P<0.01, significant difference between control and indicated group. BA, basilar artery; CA, caudal artery; CV, caudal vein; HP, hypochord; n.s., not significant; PCS, posterior communicating segment. Scale bars: 30 µm in A,C; 50 µm in B,D,E; 20 µm in enlarged images in B,D,E; 15 µm in F.
To next assess the role of Notch signaling, we treated embryos with the γ-secretase inhibitor DAPT, which abrogates Notch receptor cleavage and almost completely blocked the emergence of pdgfrbhigh MCs around vessels in the brain base (Fig. 2A). A similar result was obtained on pdgfrbhigh MC emergence beneath the DA or around ISVs (Fig. 2B). To corroborate these data, we also used an alternative γ-secretase inhibitor, LY411575, which also completely blocked MC emergence in ISVs (Fig. 2F; Fig. S2D). To better define the crucial time point for when Notch signaling is required, we treated embryos with DAPT before, during or after the emergence of pdgfrbhigh MCs. When inhibiting γ-secretase during the period of normal emergence, pdgfrbhigh MCs completely failed to appear beneath the DA and ISVs (Fig. 2E,F; Fig. S2C-E). In contrast, when γ-secretase was inhibited at an earlier or later time window, the emergence of pdgfrbhigh MCs beneath the DA and ISVs was comparable with controls (Fig. 2E,F; Fig. S2C,E). These data suggest that the loss of pdgfrbhigh MCs in DAPT-treated embryos might not be due to defects in the precursors or to apoptosis of MCs, the latter being observed in Notch3 knockout mouse retina and brain (Henshall et al., 2014). Taken together, these results indicate that Notch, but not TGFβ, signaling is essential for the specification of pdgfrblow mesenchymal cells into pdgfrbhigh MCs.
The emergence of pdgfrbhigh mural cells is redundantly controlled by Notch2 and Notch3
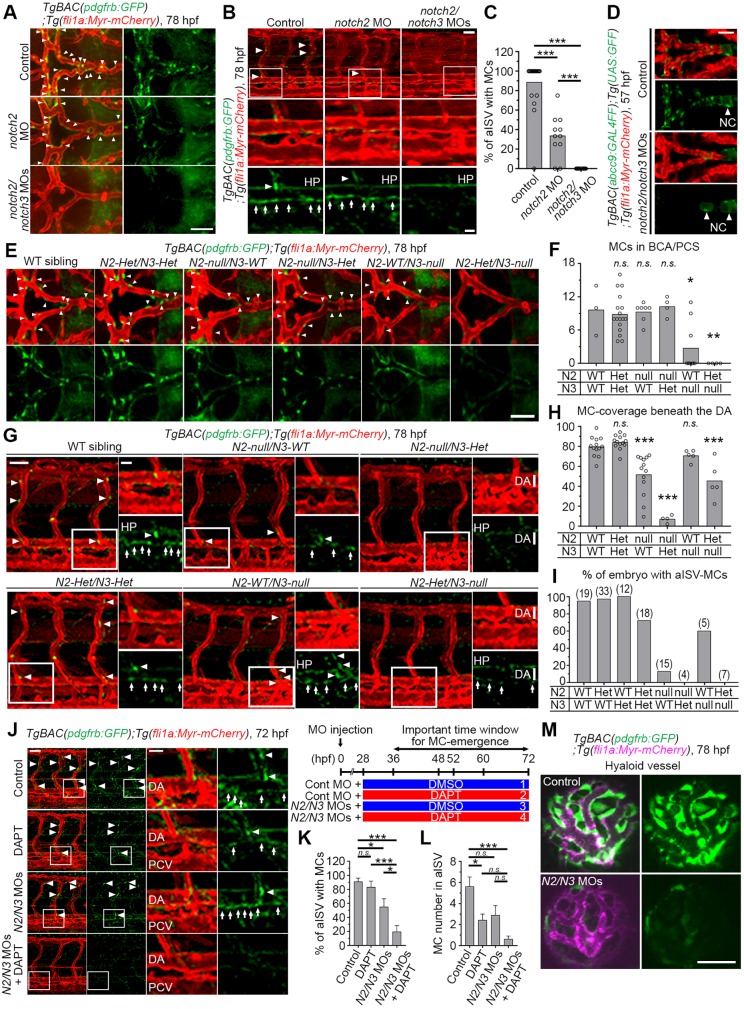
There are several Notch receptor paralogs and their individual functions during vascular differentiation are not fully understood. Hence, we wished to establish which of the paralogs were the most important for the early emergence of MCs. Notch2 and Notch3 are of particular interest, as Notch3 has been shown to have a role in zebrafish MC development (Wang et al., 2014) and NOTCH2 is expressed at a higher level than NOTCH3 in human aortic VSMCs (Tang et al., 2010). Furthermore, it has been reported that both Notch2 and Notch3 regulate VSMC-development in mouse dorsal aorta (Wang et al., 2012). In agreement with previous observations, the number of MC in CtAs decreased when notch3 was depleted by MO (Fig. S3A) (Wang et al., 2014). However, the distribution of pdgfrbhigh MCs at the cerebral base was not affected in notch3 morphants (Fig. S3A), which suggests that the reduction in coverage with pdgfrbhigh MCs in CtAs may be due to an impaired recruitment of already established pdgfrbhigh MCs from the cerebral base to the CtAs. In trunk vessels, some pdgfrbhigh MCs were observed even after high dose notch3 MO treatment (Fig. S3B). To learn whether notch2 could act redundantly with notch3, we first established that both notch3 and notch2 were expressed in zebrafish pdgfrb-positive cells (Fig. S3C,D). Depletion of notch2 alone by MO suppressed pdgfrbhigh MC emergence in the brain base (Fig. 3A; Fig. S3E), whereas these cells were completely lost in larvae that were injected with both notch2 and notch3 MOs, even when the individual doses of each MO were low (Fig. 3A,K). A similar scenario reemerged in the region beneath the DA and arterial ISVs (aISV), in which the emergence of pdgfrbhigh MCs was also completely blocked when both notch2 and notch3 were depleted (Fig. 3B,C). We checked the notch2 or notch3 mRNA levels in the notch3 or notch2 morphants, respectively, and found no compensatory upregulation of the other transcript (Fig. S3F,G). As expected, abcc9- or tagln-positive MCs were also almost completely lost in notch2 and notch3 morphants (Fig. 3D; Fig. S4A-D). To corroborate the involvement of notch2 and notch3 in MC specification, we next investigated notch2 and notch3 mutant larvae. Consistent with the results using MOs, pdgfrbhigh MC emergence was more severely blocked in larvae carrying mutations in both notch2 and notch3 than in larvae with a mutation in either notch2 or notch3 alone (Fig. 3E-I). Interestingly, we found that pdgfrblow cells around the basal communicating artery (BCA)/posterior communicating segment (PCS) were higher in notch2-het/notch3-null mutants than in controls (Fig. S4E), implying that pdgfrblow cells are recruited but failed to undergo specification into pdgfrbhigh MCs in the mutants.
Fig. 3.
Both Notch2 and Notch3 are essential for pdgfrbhigh MC emergence. (A,B) Confocal stack images of the brain (A) or trunk vessels (B) of 78 hpf TgBAC(pdgfrb:GFP);Tg(fli1a:Myr-mCherry) larvae injected with 10 ng control MO, 10 ng notch2 MO or 5 ng each of notch2 and notch3 MOs. (C) The percentage of aISV with pdgfrbhigh MCs, as observed in B (n≥11). (D) Confocal stack images of the brain vessels of 57 hpf TgBAC(abcc9:GAL4FF);Tg(UAS:GFP);Tg(kdrl:DsRed2) embryos injected with 10 ng control MO or 5 ng each of notch2 and notch3 MOs. (E,G) Confocal images of brain (E) and trunk (G) vasculature of the wild-type (WT) siblings or mutants carrying both heterozygous notch2 and notch3 mutations (N2-Het/N3-Het), homozygous notch2 mutation (N2-null/N3-WT), both homozygous notch2 and heterozygous notch3 mutations (N2-null/N3-Het), homozygous notch3 mutation (N2-WT/N3-null) or both heterozygous notch2 and homozygous notch3 mutations (N2-Het/N3-null) with the TgBAC(pdgfrb:GFP);Tg(fli1a:Myr-mCherry) background at 78 hpf. (F,H,I) The number of pdgfrbhigh MCs in BCA and PCS in notch2 and notch3 mutants, as shown in panel E (F), the percentage of the DA with MC coverage (MC-covered/total DA length in observed areas were measured using Imaris software) (H) or the percentage of embryos with pdgfrbhigh MCs in aISV (I) in notch2 and notch3 mutants, as shown in G. The total number of observed larvae are shown on the top of each bar in I. (J) Confocal stack image of the trunk vessels of 72 hpf TgBAC(pdgfrb:GFP);Tg(fli1a:Myr-mCherry) larvae injected with control MO or low dose (0.75 ng each) of notch2 and notch3 MO combined with subsequent treatment with DMSO or low dose (5 µM) of DAPT from 36 to 72 hpf as shown in the scheme to the top right of J. (K,L) The percentage of aISV with pdgfrbhigh MCs (K) and pdgfrbhigh MCs number in aISV on one side of the observed trunk (L), as observed in J. Data are mean±s.e.m. (n≥4). (M) Confocal stack image of the hyaloid vessels of 78 hpf TgBAC(pdgfrb:GFP);Tg(fli1a:Myr-mCherry) larvae injected with 10 ng control MO (top) or 5 ng each of notch2 and notch3 MO (bottom). Boxed regions are enlarged to the bottom or right. Arrows and arrowheads indicate pdgfrbhigh MCs beneath the DA and in ISV/BCA/PCS, respectively. *P<0.05, **P<0.01, ***P<0.001, significant difference between two groups. n.s., not significant. In F and H, significant difference between WT and indicated mutant group. HP, hypochord; NC, notochord; PCV, posterior cardinal vein. Scale bars: 50 µm; 20 µm in enlarged images.
These analyses also revealed vessel-specific differences in the single mutants. In brain vessels, notch3-null mutants showed a stronger reduction of pdgfrbhigh MCs than notch2-null mutants (Fig. 3E,F) and conversely, the MC emergence beneath the DA was more severely affected in notch2 mutants (Fig. 3G,H). In addition, the emergence of MCs in aISVs was almost completely blocked in notch2-null mutants, whereas aISVs covered by pdgfrbhigh MCs were found in more than 60% of notch3-null mutants (Fig. 3I; Fig. S4F). To gain insight into the time point at which Notch2 and Notch3 function is most crucial, we injected moderate amounts of notch2 and notch3 MOs into one-cell stage embryos and subsequently treated the embryos with a low dose of DAPT during the MC specification period (Fig. 3J-L). DAPT treatment at 5 µM, or notch2 and notch3 MOs at 0.75 ng each, partially blocked the emergence of pdgfrbhigh MCs beneath the DA and at aISVs (Fig. 3J-L). In line with this result, when DAPT treatment was combined with MOs at the same levels pdgfrbhigh MCs were almost completely lost (Fig. 3J-L).
We were next interested in learning whether Notch2 and Notch3 are important also for specification into MCs from other cellular origins. To explore this, we analyzed MCs that cover the hyaloid vessels, which are derived from neural crest, in contrast to the mesoderm-derived MCs in the cerebral base or trunk vessels (Ando et al., 2016). When depleting both notch2 and notch3, pdgfrbhigh MC emergence around the hyaloid vessels was completely abrogated (Fig. 3M). These data suggest that Notch2 and Notch3 play a crucial role in the specification of pdgfrbhigh MCs in zebrafish embryos regardless of their mesodermal or neural crest origin. Taken together, the results indicate that both Notch2 and Notch3 jointly regulate MC specification but exhibit region-specific differences in MC specification, with Notch2 playing a more prominent role for MC specification in trunk vessels and Notch3 in brain vessels.
Notch signaling in the endothelium is dispensable for MC specification
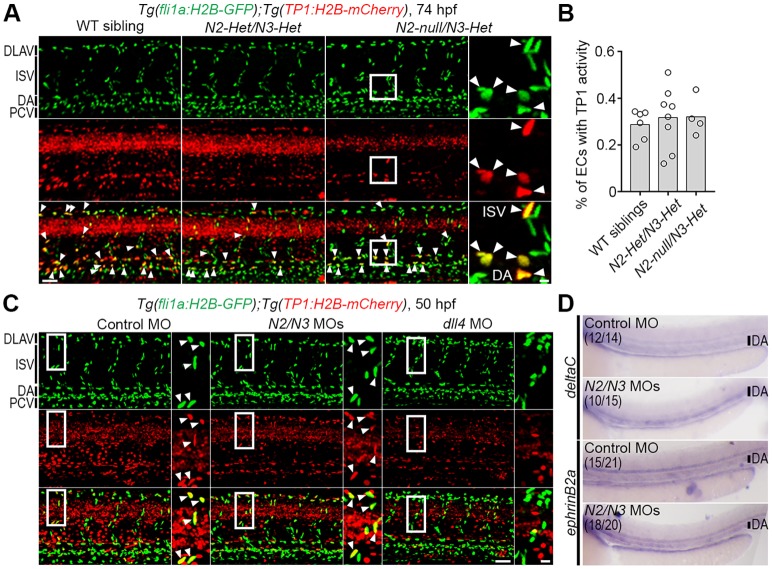
The experiments described above provide evidence for a crucial role of Notch signaling in the early steps of MC specification, but do not establish the cell type in which Notch signaling is important. Although a cell-autonomous effect in the emerging MCs is plausible, there are also indications that Notch signaling within ECs may influence MC development. Notably, MCs emerge around aECs, which are essential for the induction of pdgfrbhigh MCs (Ando et al., 2016). Moreover, we found that notch2 is expressed in aECs in addition to the pdgfrb-positive cell population (Fig. S3C). To test the possibility that the Notch signal emanated from the EC rather than the MC lineage, we used a dual reporter system, in which a reporter reading out the immediate downstream Notch signaling (based on multimerized CSL/RBPJ binding sites) was combined with a reporter for ECs. The Notch downstream reporter system was based on Tg(TP1:H2B-mCherry), in which the nuclei of Notch-activated cells are labeled by mCherry tagged to Histone H2B (H2B-mCherry). To visualize ECs, we used Tg(fli1a:H2B-GFP), in which all EC nuclei are labeled by GFP-tagged Histone H2B (H2B-GFP). In notch2-null/notch3-Het mutants, which display a complete loss of MC emergence (Fig. 3), H2B-mCherry signals were still observed in ISV- or DA-ECs, and the percentage of ECs that were positive for H2B-mCherry was comparable between mutants and control siblings (Fig. 4A,B), which indicates preserved activation of Notch signaling in ECs. Similarly, H2B-mCherry signals in ISVs and DA-ECs were detected in notch2 and notch3 morphants (Fig. 4C). In contrast, knockdown of dll4 dramatically reduced the H2B-mCherry signal in ECs (Fig. 4C), which is consistent with the known role of dll4 in ECs. To corroborate the lack of any apparent abnormalities in aEC upon notch2 and notch3 knockdown, we next investigated the expression of aEC markers (deltaC, ephrinB2a, dll4) in notch2 and notch3 morphants by in situ hybridization (Fig. 4D) or qPCR analysis (Fig. S5A). We found similar expression levels of these transcripts in control siblings and notch2 and notch3 morphants (Fig. 4D; Fig. S5A). Collectively, these results suggest that immediate downstream Notch signaling is not affected in ECs in notch2 and notch3 mutants, but that Dll4 serves a crucial role in Notch signaling in ECs, as expected. As Notch1b-Dll4 is the most likely ligand-receptor pair operating in ECs (Fig. 4C), we knocked both these genes down with MOs and assessed the consequences for MC specification. We found that neither dll4 nor notch1b MO affected the emergence of pdgfrbhigh MCs in trunk and brain vessels (Fig. S5B,C), in spite of the fact that Rbpj-mediated transcriptional activity in ECs was dramatically reduced, and that these morphants showed abnormal vasculature (Fig. 4C; Fig. S5C). In summary, these data indicate that disruption of Notch signaling in the EC, which generates an aberrant vasculature, does not impact on the early phases of MC specification.
Fig. 4.
Depletion of both notch2 and notch3 hardly affected Notch-Rbpj-mediated transcriptional activity in ECs. (A) Confocal stack images of the trunk vasculature of the wild-type (WT) sibling or notch2/notch3 mutants with the Tg(fli1a:H2B-GFP);Tg(TP1:H2B-mCherry) background at 74 hpf. (B) The percentage of ECs in observed trunk region with TP1:H2B-mCherry expression, as observed in A. Total number of EC nuclei marked by fli1a:H2B-GFP and the number of EC nuclei positive for both fli1a:H2B-GFP and TP1:H2B-mCherry were counted using Imaris software. Bars and circles indicate averages and each value of observed larvae, respectively. (C) Confocal images of trunk vasculature of the 50 hpf Tg(fli1a:H2B-GFP);Tg(TP1:H2B-mCherry) embryos injected with 10 ng control MO (left), 5 ng each of notch2 and notch3 MOs (middle, N2/N3 MOs) or 10 ng of dll4 MO (right). (D) The expression of deltaC or ephrin-B2a mRNA, as detected by whole-mount in situ hybridization, in 48 hpf embryos injected with 10 ng control MO or 5 ng each of notch2 and notch3 MOs. Numbers indicate embryos with the similar expression as shown in each image/total number of embryos. Two independent experiments were performed for each marker with at least seven embryos per experiment. In A and C, arrowheads indicate ECs with TP1:H2B-mCherry expression; boxed regions are enlarged to the right. Scale bars: 50 µm; 10 µm in enlarged images. DLAV, dorsal longitudinal anastomotic vessels; PCV, posterior cardinal vein.
Activation of Notch precedes the switch from pdgfrblow to pdgfrbhigh mural cells
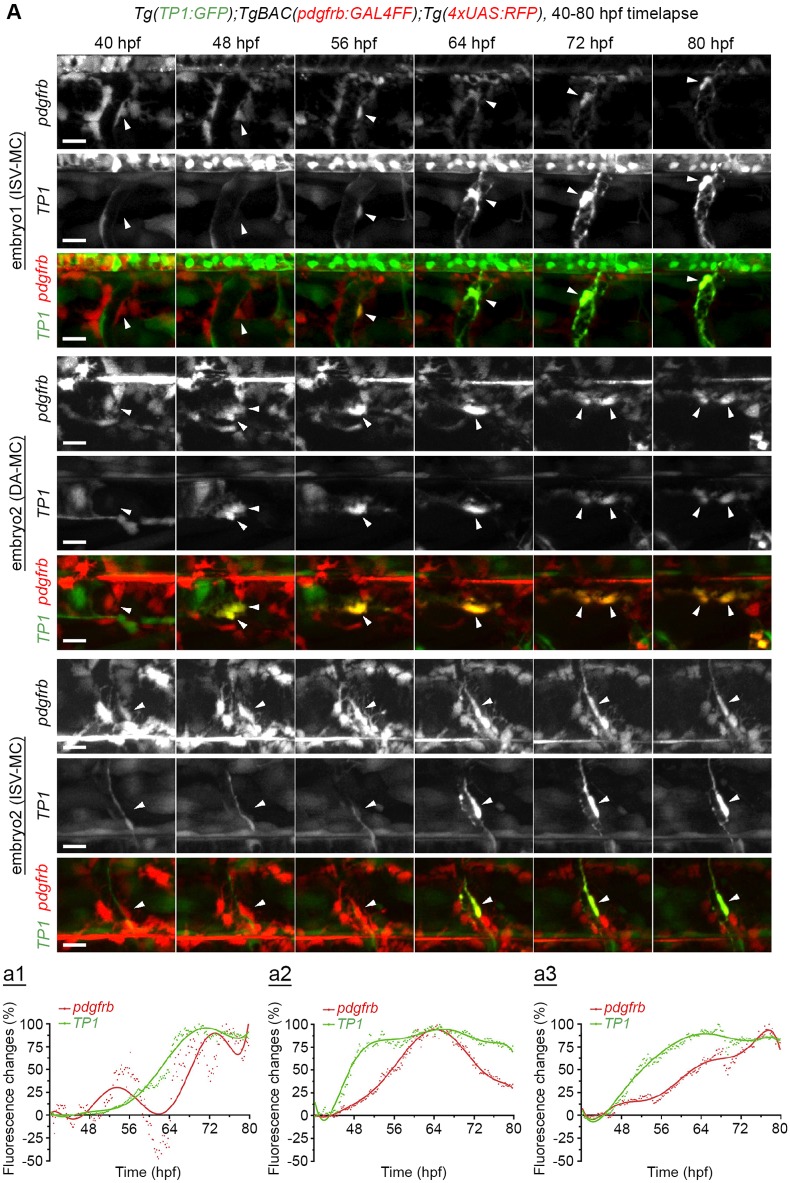
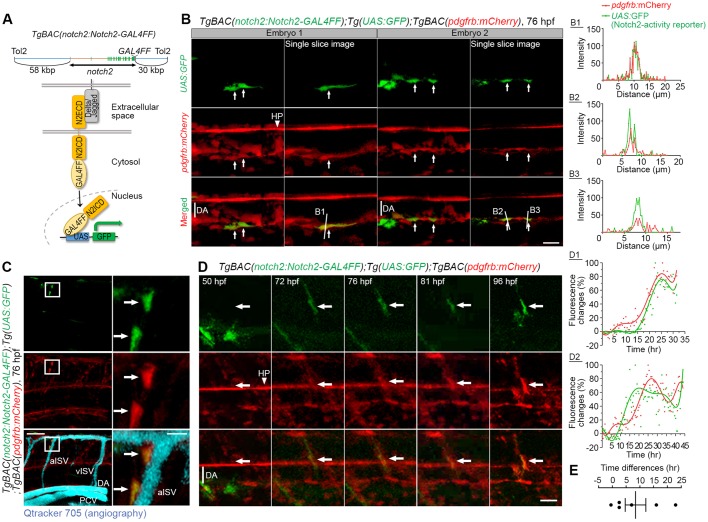
We next explored when Notch signaling becomes activated in mesenchymal cells during the specification into MCs. To measure Notch downstream signaling, we used a similar Notch-activity reporter as described above but instead labeling cell cytoplasm with GFP expression upon Notch activation, Tg(TP1:GFP), combined with reporter cassettes, TgBAC(pdgfrb:GAL4FF):Tg(4xUAS:RFP), which read out RFP expression in the pdgfrb-expressing cells. TP1:GFP signals were detected in pdgfrbhigh MCs in ISVs and beneath the DA during the differentiation period (Fig. 5A). Quantitative analysis of reporter expression over time indicates that Notch activation occurs before or simultaneously with the increase of pdgfrb reporter (Fig. 5A). Moreover, Movie 1 shows that pdgfrb-positive cells change their behavior after the onset of TP1:GFP expression, i.e. they associate tightly with ISV, extend processes, migrate and proliferate along the vessel, whereas pdgfrb-positive cells without Notch activation did not behave in this way, but instead gradually decreased their pdgfrb reporter expression. To gain further insights into Notch activation in the MC lineage, we established a new Tg line, TgBAC(notch2:Notch2-GAL4FF), in which a GAL4 moiety was directly linked to the C terminus of Notch2 and the Notch2-GAL4FF fusion protein is expressed under the control of the notch2 promoter. This reporter directly monitors liberation of the Notch2 intracellular domain (N2ICD) by nuclear localization of the N2ICD-GAL4FF fragment and activation of a UAS promoter (Fig. 6A). To test the validity of the novel reporter, we first showed that the GFP signals from TgBAC(notch2:Notch2-GAL4FF);Tg(UAS:GFP) were detected in skeletal myocytes, cells in the eye, face or heart before 3 dpf in control siblings, but not in notch2 morphants (Fig. S6A), which indicates that these signals are indeed dependent on Notch2 expression. To confirm that this GFP expression reflects Notch2 activation, we treated TgBAC(notch2:Notch2-GAL4FF);Tg(UAS:GFP) embryos with DAPT and found that GFP expression in the eye and heart decreased (Fig. S6B). Interestingly, the GFP signal increased in rbpj morphants (Fig. S6A), which indicates that endogenous Rbpj may act as a sink for N2ICD-GAL4FF and inhibit the access of N2ICD-GAL4FF to the UAS promoter. Using the TgBAC(notch2:Notch2-GAL4FF);Tg(UAS:GFP) reporter system, we next set out to define more precisely when Notch2 activation occurred. Consistent with the TP1 reporter line, pdgfrbhigh MCs were positive for GFP (Fig. 6B-D; Fig. S6C), which indicates that Notch2 is active in pdgfrbhigh MCs. Importantly, time-lapse imaging revealed that Notch2 activation preceded the increase in pdgfrb expression in perivascular mesenchymal cells (Fig. 6D,E), i.e. Notch2 was activated in pdgfrblow cells that subsequently become pdgfrbhigh MCs on ISV. Taken together, the results show that activation of the Notch2 receptor precedes the transition from pdgfrblow to pdgfrbhigh MCs. The TP1 reporter was still expressed at 5 dpf in pdgfrbhigh MCs (Fig. S6D), which suggests that the Notch activity persists after the MC specification.
Fig. 5.
Time-lapse imaging of TP1 reporter expression in MCs. (A) Time-lapse imaging of the trunk region (ISV or DA) of Tg(TP1:GFP);TgBAC(pdgfrb:GAL4FF);Tg(4xUAS:RFP) every 12 min (see also Movie 1). Increase of RFP signal was observed in the cells positive for GFP expression (Notch activation) (arrows). Representative images at indicated time points and fluorescence changes (a1-a3) showing % of the peak value of pdgfrb or TP1 reporter fluorescence intensity after the subtraction of each fluorescent intensity of the first flame. Quantifications a1 (dorsal side) and a2 (ventral side) correspond to one of the ISV-MCs in embryo 1 (see Movie 1), and a3 corresponds to an ISV-MC in embryo 3 (images not shown). Red dots, pdgfrb reporter; green dots, TP1 reporter. Polynomial fitting curves for the pdgfrb (red line) and TP1 (green line) were assigned using the polyfit function in MATLAB with the degree of 7. Scale bars: 15 μm.
Fig. 6.
Notch activation in pdgfrbhigh MCs. (A) Schematic structure of the BAC clone (CH211-39M8) which was used to generate TgBAC(notch2:Notch2-GAL4FF) for visualizing Notch2-activated cells. A cDNA encoding GAL4FF was inserted at the C terminus of the notch2 gene. Notch2 intracellular region (N2ICD) fused with GAL4FF is proteolytically released and translocated to the nucleus upon Notch2 activation, therefore, Notch2 activated cells can be monitored by the expression of proteins such as GFP driven by the UAS/GAL4FF system. (B) Confocal images of the DA in TgBAC(notch2:Notch2-GAL4FF);Tg(UAS:GFP);TgBAC(pdgfrb:mCherry) larvae at 76 hpf. Left, stack images. Right, single slice images from stack images on the left. Arrows indicate pdgfrbhigh MCs beneath the DA with Notch2 activation. The fluorescence intensity profile of pdgfrb:mCherry (red line) and UAS:GFP (green line) along the line in the single slice images (B1-B3) are shown on the right, indicating the colocalization of mCherry and GFP expression. The x-axes show distance from the beginning of the line. (C) Confocal stack images of the trunk vessels of TgBAC(notch2:Notch2-GAL4FF);Tg(UAS:GFP);TgBAC(pdgfrb:mCherry) larvae at 76 hpf. Vascular structure was visualized by injection of Qtracker 705 vascular labels (aqua) into the circulation. Boxed regions depicting pdgfrbhigh MCs in aISV are enlarged to the right. Arrows indicate the Notch2-activated pdgfrbhigh MCs. Notch2-activity-positive MCs in ISVs/DA at 74 hpf were seen in 31.6% (12/38) of embryos shown. (D) Time-lapse imaging of the trunk region (ISV) of TgBAC(notch2:Notch2-GAL4FF);Tg(UAS:GFP);TgBAC(pdgfrb:mCherry) embryo at the indicated time points. Note that Notch2 activation precedes the increase of pdgfrb expression. GFP signal was absent at 50 hpf, but induced in pdgfrblow cells after 72 hpf. Subsequently, pdgfrb:mCherry expression was increased in Notch2-activated pdgfrblow cells (arrows). Representative fluorescence changes (% of the peak value of pdgfrb- or Notch2-activity reporter fluorescence) are shown on the right (D1, ISV-MC; D2, DA-MC). Red dots, pdgfrb reporter. Green dots, Notch2-activity reporter. Polynomial fitting curves for the pdgfrb (red line) and Notch2-activity reporter (green line) were assigned using the polyfit function in MATLAB with the degree of 7. The x-axes show the relative time. (E) Comparison of the time showing the highest value of Notch2 activity and the pdgfrb reporter in fitting curve. The data are mean±s.e.m. (n=6). Note that the time showing the highest GFP fluorescence preceded that of mCherry fluorescence by more than 8 h on average, indicating that Notch2 is activated before the onset of pdgfrb increase. HP, hypochord; N2ECD, Notch2 extracellular region; PCV, posterior cardinal vein. Scale bars: 20 µm in B,D: 50 µm in C: 10 µm in enlarged images in C.
Finally, having established that Notch2 and Notch3 are necessary for MC specification, we asked the converse question, i.e. whether expression of activated forms of these two receptors would accelerate the specification into pdgfrbhigh MCs. To test this, we injected a plasmid encoding GFP-N2ICD or GFP-N3ICD, or GFP-N3ICD mRNA. In spite of GFP-N2ICD being functional (Fig. S7A,B), we found that neither GFP-N2ICD nor GFP-N3ICD enhanced pdgfrb, abcc9 or tagln reporter expression (Fig. S7C-G), which indicates that ectopic or super-physiological levels of Notch signaling do not augment MC specification.
DISCUSSION
Defects in the MC coverage have been associated with several vascular diseases (French et al., 2014; Garg et al., 2014; Joutel et al., 1996), as well as with developmental abnormalities and disease-like vascular conditions (Armulik et al., 2010, 2011; Bell et al., 2010; Daneman et al., 2010; Enge et al., 2002; Lindahl et al., 1997; Lindblom et al., 2003). Malfunctioning MC is also of central importance to the pathogenesis of common cardiovascular diseases such as atherosclerosis, aneurysms and various forms of stroke. Significant attention has therefore been given to the area of MC development. Although it is well established in the mouse that PDGF-B/PDGFRβ paracrine signaling is pivotal in the recruitment of MC to expanding vascular networks (Enge et al., 2002; Hellstrom et al., 1999; Hellström et al., 2001; Lindahl et al., 1997; Lindblom et al., 2003), the molecular mechanisms that underlie specification of MC around blood vessels during early development have remained enigmatic (Hellstrom et al., 1999; Sinha and Santoro, 2018). One reason for this knowledge void has been the lack of models in which the MC differentiation can be monitored and analyzed in vivo with sufficiently high spatial and temporal resolution. In this study, we utilized a set of recently developed MC-visualizing Tg-zebrafish lines (pdgfrb, abcc9 and tagln reporter lines) to dissect the process of MC differentiation and to assess the molecular mechanisms that underlie MC specification. Of these markers, pdgfb is a well-established marker for all types of vascular MCs, whereas abcc9 and tagln are specific for PCs and VSMCs, respectively. In the present study, we found that abcc9 signals were detected in pdgfrbhigh cells soon after their appearance. Considering the short time gap between pdgfrbhigh and abcc9:GAL4FF-driven GFP, we conclude that abcc9 expression is likely coincidental with the increment of pdgfrb, suggesting that the pdgfrbhigh phenotype indeed marks the time when peri-endothelial mesenchymal cells acquire MC properties. In contrast to pdgfrb, which is broadly expressed in the fish body, abcc9 expression is generally restricted to MCs. We noticed that abcc9 labels almost all pdgfrbhigh MCs in the brain, but most MCs beneath the DA were abcc9 negative. Considering that abcc9 is selectively expressed by PCs and not by the VSMC that cover arteries and arterioles in adult mouse and zebrafish brains (Vanlandewijck et al., 2018), it is possible that MCs that develop beneath the DA might have an arterial VSMC phenotype with suppressed abcc9 expression from the start, i.e. they develop into arterial VSMC without first passing through a PC-like MC phenotype. Indeed, the MCs beneath the DA show tagln (Fig. 1D) and acta2 expression early on (Chen et al., 2017; Whitesell et al., 2014). Even though pdgfrb-negative but tagln-positive cells can be observed around the DA or some ISVs (Ando et al., 2016), it is important to note that acta2 (Chan and Mably, 2011; Whitesell et al., 2014) and tagln (Fig. 1) are not the first markers of brain MCs. Moreover, tagln-positive pdgfrbhigh MCs constitute less than 20% of the MC in trunk vessels before 72 hpf (Fig. 1). Hence, lack of acta2- or tagln-positive cells does not necessarily imply impaired MC emergence, but may instead indicate defective MC maturation into arterial VSMC. Indeed, blood flow is dispensable for the emergence of pdgfrbhigh (Ando et al., 2016) whereas it is indispensable for acta2 expression beneath the DA (Chen et al., 2017), which suggests that blood flow may promote MC maturation into VSMC.
Taking advantage of the MC reporter lines, we asked whether Notch and TGFβ signaling, which are both known to play crucial roles in MC survival and proliferation, were important for MC specification. TGFβ signaling turned out to be largely dispensable for this step, as neither pharmacological inhibition of TGFβ signaling by SB431542 nor depletion of alk1 had any major effect on the emergence of MCs. The reduction of MC number observed on brain vessels and ISVs following treatment with SB431542 was small and might represent indirect effects. In contrast, functional Notch signaling was absolutely required for the emergence of MCs both of mesodermal and neural crest origin, as judged both by pharmacological inhibition, MO knockdown and the use of notch2 and notch3 mutant zebrafish. To more precisely map the activation of the Notch2 receptor, we generated a new reporter, TgBAC(notch2:Notch2-GAL4FF), which gets activated only upon receptor cleavage and therefore represents a direct readout of Notch receptor activation. Activation of this reporter preceded the switch from pdgfrblow to pdgfrbhigh, supporting the notion that Notch regulates this crucial step. This conclusion is also supported by the TP1:GFP reporter expression, that reads out immediate downstream Notch signaling activation. We also assessed where the Notch signaling was required to affect the emergence of the MC lineage. Our data suggest that Notch activation is needed in the MC progenitors, and not in the surrounding EC, because knockdown of notch2 and notch3 did not affect Notch signaling levels in the EC, and the knockdown of dll4 or notch1b, which strongly reduces Notch signaling in the EC and causes an aberrant vasculature, did not affect the MC specification step. This low contribution from Notch signaling within EC in the MC specification step may contrast with later steps in the EC lineage, in which Notch signaling in ECs promote the α-SMA-positive MC coverage in zebrafish (Chen et al., 2017).
Our data also reveal that Notch2 and Notch3 act in part redundantly in the control of MC lineage initiation. We noted some spatial differences however; notch2 depletion alone tended to affect MC emergence in trunk vessels, whereas notch3 depletion alone had a stronger effect in the brain. This might be because of the differences in gene expression level. Notch3 expression in mouse brain MCs is more than ten times higher than Notch2 expression (Fig. S8). It is also possible that this initial difference may contribute to heterogeneity of MCs in different organs.
The partially redundant role of Notch2 and Notch3 signaling in the emergence of the MC lineage, as revealed in the present study in zebrafish, may in fact be evolutionarily conserved. Indeed, a similar redundant relationship is also indicated in mice. It has been reported that mice with both Notch2 hypomorphic and Notch3 null mutations show defects in the VSMC coverage of vessels (Wang et al., 2012), which appear to be more severe than those observed in Notch3-deficiency alone (Henshall et al., 2014). The more severe phenotype in the Notch2 hypomorphic and Notch3 null mutation mice may originate from a problem in MC specification that is not seen in the Notch3 null mice, which exhibit an almost normal vasculature at birth (Jin et al., 2008).
Our data reveal a link between Notch activation and an increase in pdgfrb expression, but somewhat surprisingly, exogenous N2ICD or N3ICD expression failed to upregulate pdgfrb expression. This may be linked to an absence of putative rbpj binding sites predicted by the Match (Kel et al., 2003) or Patch program (Matys et al., 2003) in the CH1073-606I16 BAC clone that was used to establish the pdgfrb reporter lines. Regulation of pdgfrb under physiological Notch levels may therefore be more indirect, although Notch regulates pdgfrb expression also in mammals (Jin et al., 2008; Kofler et al., 2015), and activation in those situations may occur directly via a CSL-binding site in the pdgfrb promoter. The interplay between PDGFRβ and Notch may also be important in vascular differentiation, as this synergistic signaling can induce transdifferentiation from skeletal muscle to MC lineage (Cappellari et al., 2013). However, it is important to note that we cannot entirely rule out the possibility that MC specification might be regulated via an Rbpj-independent (non-canonical) mode of Notch signaling. This possibility would be consistent with our finding that the ratio of TP1- or Notch2-GAL4FF reporter-positive MCs was low during MC emergence. Interestingly, MCs that are positive for TP1 activity increased after 4-5 dpf (data not shown). Because pdgfrbhigh MCs start to express acta2 or tagln after 3 dpf, it is possible that high Notch-Rbpj activation in MCs may promote the further differentiation of these cells into aortic VSMCs. Further work to more precisely distinguish between these different scenarios will require the generation of novel tools.
MATERIALS AND METHODS
Zebrafish husbandry
Zebrafish (Danio rerio) were maintained as previously described (Fukuhara et al., 2014). Embryos and larvae were staged by hpf at 28-28.5°C (Kimmel et al., 1995). The experiments using zebrafish were approved by the Uppsala animal ethical board (5.2.18-8927/16) and performed according to Swedish national legislation.
Plasmids
Construction of Tol2-based plasmids used to establish Tg zebrafish lines, N2ICD and N3ICD plasmids are described in the supplementary Materials and Methods.
Tg and mutant fish lines
Transgenic and mutant zebrafish lines were established or provided as described below. Tg(UAS:GFP) and Tg(UAS:RFP) zebrafish lines were provided by K. Kawakami (National Institute of Genetics, Japan) (Asakawa et al., 2008). Tg(fli1:GFP)y1 zebrafish line was provided by Nathan Lawson (University of Massachusetts Medical School, MA, USA) (Lawson and Weinstein, 2002). notch2el515 mutant was described previously (Barske et al., 2016). notch3fh332 mutant (Alunni et al., 2013), Tg(tp1-MmHbb:eGFP)um14 (Parsons et al., 2009), abbreviated Tg(TP1:GFP) and Tg(-7kdrl;DsRed2)pd27 [abbreviated Tg(kdrl:DsRed2)] (Kikuchi et al., 2011) were obtained from the Zebrafish International Resource Center. Tg(Tp1bglob:H2BmCherry)S939 [abbreviated Tg(TP1:H2B-mCherry)] was described previously (Ninov et al., 2012). TgBAC(pdgfrb:GFP)ncv22, TgBAC(pdgfrb:mCherry)ncv23, TgBAC(abcc9:GAL4FF)ncv34, TgBAC(tagln:GFP)ncv25, Tg(fli1a:Myr-mCherry)ncv1, and Tg(kdrl:Hsa.HRAS-mCherry)s896 zebrafish lines were described previously (Ando et al., 2016; Chi et al., 2008; Fukuhara et al., 2014; Hogan et al., 2009; Vanlandewijck et al., 2018). TgBAC(notch2:Notch2-GAL4FF)cbz1 and Tg(fli1a:H2B-GFP)ncv69 were established as described in the supplementary Materials and Methods. Throughout the text, all Tg lines and mutant used in this study are described without their line numbers, e.g. TgBAC(pdgfrb:GFP)ncv22 is abbreviated to TgBAC(pdgfrb:GFP).
Microinjection of oligonucleotide and mRNA
For gene knockdown, one-cell stage embryos were injected with MO. For protein overexpression, one-cell stage embryos were injected with plasmid or mRNA as described in the supplementary Materials and Methods.
Image acquisition and processing
Embryos and larvae were anesthetized and mounted in 1% low-melting agarose on a 35-mm-diameter glass-base dish (Asahi Techno Glass or Thermo Fisher Scientific Nunc), as previously described (Fukuhara et al., 2014). Confocal images were obtained using a FluoView FV1200 confocal upright microscope (Olympus) equipped with a water-immersion 20× (XLUMPlanFL, 1.0 NA) lens or with a Leica TCS SP8 confocal microscope (Leica Microsystems) equipped with a water-immersion 25× (HCX IRAPOL, 0.95 NA) or a dry 10× (HC PLAPO CS, 0.40 NA) lens. The 473 nm (for GFP), 559 nm (for mCherry) and 633 nm (for Qdot 655) laser lines in the FluoView FV1200 confocal microscope and the 488 nm (for GFP), 563 nm (for DsRed2), 587 nm (for mCherry) and 670 nm (for Qtracker 705) in the Leica TCS SP8 confocal microscope were employed, respectively. Obtained confocal images were processed using Olympus Fluoview (FV10-ASW), Leica Application Suite 3.2.1.9702 or an IMARIS 8 software (Bitplane). All Leica confocal images are represented as maximum intensity (Leica Application Suite 3.2.1.9702). Bright-field images were taken with a fluorescence stereozoom microscope (SZX12, Olympus).
Whole-mount in situ hybridization
Embryos at 48 hpf were hybridized with digoxigenin-labeled antisense RNA probes, as described in the supplementary Materials and Methods.
FACS and qPCR analyses
Zebrafish embryos were incubated in Protease solution (1% trypsin, 1 mM EDTA, pH 8.0 in PBS) at 37°C for 10 min and dissociated by gentle pipetting. The dissociated cells were sorted using a FACS Aria III or Melody cell sorter (BD Biosciences). Then, total RNA from sorted cells was purified using a NucleoSpin RNA XS kit (Macherey-Nagel) following the manufacturer's instructions, and subjected to cDNA synthesis using iScript cDNA Synthesis Kit (Bio-Rad). Using these cDNA libraries as a template, qPCR analysis was performed using the primers in Table S1.
Statistical analysis
Data are expressed as mean±s.e.m. Statistical significance was determined by a Student's t-test for paired samples, one-way analysis of variance with Tukey’s test for multiple comparisons, or Dunnett's Multiple Comparison Test. Data were considered statistically significant if P<0.05.
Supplementary Material
Acknowledgements
We thank S. Schulte-Merker for providing plasmids for BAC recombineering, K. Kawakami for the Tol2 system as well as the Tg(UAS:GFP) and Tg(UAS:RFP) lines, and N. Lowson for the Tg(fli1:GFP) line. We are grateful to Y. Ando, L. Ebarasi, M. Sone, Y. Wakayama, H. Fukui, T. Babazono, W. Koeda, K. Hiratomi and E. Liébanas for technical assistance.
Footnotes
Competing interests
The authors declare no competing or financial interests.
Author contributions
Conceptualization: K.A., B.M.H., S.F., N.M., C.B.; Methodology: K.A., A.C., L.B., J.G.C., D.Y.R.S.; Validation: B.M.H.; Formal analysis: K.A.; Investigation: K.A., W.W., D.P., K.K.; Resources: K.A., A.C., L.B., J.G.C., D.Y.R.S.; Data curation: K.A., W.W., D.P., K.K.; Writing - original draft: K.A., U.L., C.B.; Writing - review & editing: K.A., W.W., D.P., A.C., J.G.C., D.Y.R.S., U.L., K.K., B.M.H., S.F., N.M., C.B.; Visualization: K.A., W.W., D.P.; Supervision: A.L., K.K., B.M.H., S.F., N.M., C.B.; Project administration: S.F., N.M., C.B.; Funding acquisition: K.A., D.Y.R.S., K.K., S.F., N.M., C.B.
Funding
This work was supported by Vetenskapsrådet (2015-00550 to C.B. and 2016-01437 to K.K.), the European Research Council (AdG294556 to C.B.), the Fondation Leducq (14CVD02 to C.B.), the Cancerfonden (150735 to C.B.), the Knut och Alice Wallenbergs Stiftelse (2015.0030 to C.B. and 2017.0144 to K.K.), the Louis-Jeantet Foundation (2018 Louis-Jeantet Prize to C.B.), the Ragnar Söderbergs stiftelse (M13/17 to K.K.) and the Max-Planck-Gesellschaft (to D.Y.R.S.). It was also supported by the Ministry of Education, Culture, Sports, Science, and Technology, Japan, through Grants-in-Aid for Scientific Research on Innovative Areas ‘Fluorescence Live Imaging’ (22113009 to S.F.), and by the Japan Society for the Promotion of Science through Grants-in-Aid for Young Scientists (Start-up) (26893336 to K.A.), for Scientific Research (B) (25293050 to S.F.), for Scientific Research (A) (16806432 to N.M.), for Exploratory Research (26670107 and 17K19689 to S.F.) and for Overseas Research Fellowships (to K.A.). The work also received grants from the Ministry of Health, Labour, and Welfare of Japan (to N.M.); the Core Research for Evolutional Science and Technology (CREST) program of the Japan Agency for Medical Research and Development (AMED) (JP18gm0610010 to N.M.); the PRIME program of AMED (JP17gm5810010 to S.F.); the Takeda Science Foundation (to S.F., N.M.); the Naito Foundation (to S.F.); the Mochida Memorial Foundation for Medical and Pharmaceutical Research (to S.F.); and the Daiichi Sankyo Foundation of Life Science (to S.F.).
Supplementary information
Supplementary information available online at http://dev.biologists.org/lookup/doi/10.1242/dev.165589.supplemental
References
- Allende M. L. and Proia R. L. (2002). Sphingosine-1-phosphate receptors and the development of the vascular system. Biochim. Biophys. Acta Mol. Cell Biol. Lipids 1582, 222-227. 10.1016/S1388-1981(02)00175-0 [DOI] [PubMed] [Google Scholar]
- Allende M. L., Yamashita T. and Proia R. L. (2003). G-protein-coupled receptor S1P1 acts within endothelial cells to regulate vascular maturation. Blood 102, 3665-3667. 10.1182/blood-2003-02-0460 [DOI] [PubMed] [Google Scholar]
- Alunni A., Krecsmarik M., Bosco A., Galant S., Pan L., Moens C. B. and Bally-Cuif L. (2013). Notch3 signaling gates cell cycle entry and limits neural stem cell amplification in the adult pallium. Development 140, 3335-3347. 10.1242/dev.095018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ando K., Fukuhara S., Izumi N., Nakajima H., Fukui H., Kelsh R. N. and Mochizuki N. (2016). Clarification of mural cell coverage of vascular endothelial cells by live imaging of zebrafish. Development 143, 1328-1339. 10.1242/dev.132654 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Armulik A., Genové G., Mäe M., Nisancioglu M. H., Wallgard E., Niaudet C., He L., Norlin J., Lindblom P., Strittmatter K., Johansson B.R. and Betsholtz C. (2010). Pericytes regulate the blood-brain barrier. Nature 468, 557-561. 10.1038/nature09522 [DOI] [PubMed] [Google Scholar]
- Armulik A., Genové G. and Betsholtz C. (2011). Pericytes: developmental, physiological, and pathological perspectives, problems, and promises. Dev. Cell 21, 193-215. 10.1016/j.devcel.2011.07.001 [DOI] [PubMed] [Google Scholar]
- Asakawa K., Suster M. L., Mizusawa K., Nagayoshi S., Kotani T., Urasaki A., Kishimoto Y., Hibi M. and Kawakami K. (2008). Genetic dissection of neural circuits by Tol2 transposon-mediated Gal4 gene and enhancer trapping in zebrafish. Proc. Natl Acad. Sci. USA 105, 1255-1260. 10.1073/pnas.0704963105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barske L., Askary A., Zuniga E., Balczerski B., Bump P., Nichols J. T. and Crump J. G. (2016). Competition between jagged-notch and endothelin1 signaling selectively restricts cartilage formation in the zebrafish upper face. PLoS Genet. 12, e1005967 10.1371/journal.pgen.1005967 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bell R. D., Winkler E. A., Sagare A. P., Singh I., LaRue B., Deane R. and Zlokovic B. V. (2010). Pericytes control key neurovascular functions and neuronal phenotype in the adult brain and during brain aging. Neuron 68, 409-427. 10.1016/j.neuron.2010.09.043 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cappellari O., Benedetti S., Innocenzi A., Tedesco F. S., Moreno-Fortuny A., Ugarte G., Lampugnani M. G., Messina G. and Cossu G. (2013). Dll4 and PDGF-BB convert committed skeletal myoblasts to pericytes without erasing their myogenic memory. Dev. Cell 24, 586-599. 10.1016/j.devcel.2013.01.022 [DOI] [PubMed] [Google Scholar]
- Chan J. and Mably J. D. (2011). Dissection of cardiovascular development and disease pathways in zebrafish. Prog. Mol. Biol. Transl. Sci. 100, 111-153. 10.1016/B978-0-12-384878-9.00004-2 [DOI] [PubMed] [Google Scholar]
- Chen W., Guo Y., Walker E. J., Shen F., Jun K., Oh S. P., Degos V., Lawton M. T., Tihan T., Davalos D. et al. (2013). Reduced mural cell coverage and impaired vessel integrity after angiogenic stimulation in the Alk1-deficient brain. Arterioscler. Thromb. Vasc. Biol. 33, 305-310. 10.1161/ATVBAHA.112.300485 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen X., Gays D., Milia C. and Santoro M. M. (2017). Cilia control vascular mural cell recruitment in vertebrates. Cell Rep. 18, 1033-1047. 10.1016/j.celrep.2016.12.044 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chi N. C., Shaw R. M., De Val S., Kang G., Jan L. Y., Black B. L. and Stainier D. Y. R. (2008). Foxn4 directly regulates tbx2b expression and atrioventricular canal formation. Genes Dev. 22, 734-739. 10.1101/gad.1629408 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coupland K., Lendahl U. and Karlström H. (2018). Role of NOTCH3 mutations in the cerebral small vessel disease cerebral autosomal dominant arteriopathy with subcortical infarcts and leukoencephalopathy. Stroke 49, 2793-2800. 10.1161/STROKEAHA.118.021560 [DOI] [PubMed] [Google Scholar]
- Daneman R., Zhou L., Kebede A. A. and Barres B. A. (2010). Pericytes are required for blood–brain barrier integrity during embryogenesis. Nature 468, 562 10.1038/nature09513 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Enge M., Bjarnegård M., Gerhardt H., Gustafsson E., Kalén M., Asker N., Hammes H. P., Shani M., Fässler R. and Betsholtz C. (2002). Endothelium-specific platelet-derived growth factor-B ablation mimics diabetic retinopathy. EMBO J. 21, 4307-4316. 10.1093/emboj/cdf418 [DOI] [PMC free article] [PubMed] [Google Scholar]
- French C. R., Seshadri S., Destefano A. L., Fornage M., Arnold C. R., Gage P. J., Skarie J. M., Dobyns W. B., Millen K. J., Liu T. et al. (2014). Mutation of FOXC1 and PITX2 induces cerebral small-vessel disease. J. Clin. Invest. 124, 4877-4881. 10.1172/JCI75109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukuhara S., Zhang J., Yuge S., Ando K., Wakayama Y., Sakaue-Sawano A., Miyawaki A. and Mochizuki N. (2014). Visualizing the cell-cycle progression of endothelial cells in zebrafish. Dev. Biol. 393, 10-23. 10.1016/j.ydbio.2014.06.015 [DOI] [PubMed] [Google Scholar]
- Garg N., Khunger M., Gupta A. and Kumar N. (2014). Optimal management of hereditary hemorrhagic telangiectasia. J. Blood Med. 5, 191-206. 10.2147/JBM.S45295 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hellstrom M., Lindahl P., Abramsson A. and Betsholtz C. (1999). Role of PDGF-B and PDGFR-beta in recruitment of vascular smooth muscle cells and pericytes during embryonic blood vessel formation in the mouse. Development 126, 3047-3055. [DOI] [PubMed] [Google Scholar]
- Hellström M., Gerhardt H., Kalén M., Li X., Eriksson U., Wolburg H. and Betsholtz C. (2001). Lack of pericytes leads to endothelial hyperplasia and abnormal vascular morphogenesis. J. Cell Biol. 153, 543-554. 10.1083/jcb.153.3.543 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henshall T. L., Keller A., He L., Johansson B. R., Wallgard E., Raschperger E., Mäe M. A., Jin S., Betsholtz C. and Lendahl U. (2014). Notch3 is necessary for blood vessel integrity in the central nervous system. Arterioscler. Thromb. Vasc. Biol. 35, 409-420. 10.1161/ATVBAHA.114.304849 [DOI] [PubMed] [Google Scholar]
- High F. A., Lu M. M., Pear W. S., Loomes K. M., Kaestner K. H. and Epstein J. A. (2008). Endothelial expression of the Notch ligand Jagged1 is required for vascular smooth muscle development. Proc. Natl Acad. Sci. USA 105, 1955-1959. 10.1073/pnas.0709663105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hogan B. M., Bos F. L., Bussmann J., Witte M., Chi N. C., Duckers H. J. and Schulte-Merker S. (2009). Ccbe1 is required for embryonic lymphangiogenesis and venous sprouting. Nat. Genet. 41, 396 10.1038/ng.321 [DOI] [PubMed] [Google Scholar]
- Iivanainen E., Nelimarkka L., Elenius V., Heikkinen S.-M., Junttila T. T., Sihombing L., Sundvall M., Määttä J. A., Laine V. J. O., Ylä-Herttuala S. et al. (2003). Angiopoietin-regulated recruitment of vascular smooth muscle cells by endothelial-derived heparin binding EGF-like growth factor. FASEB J. 17, 1609-1621. 10.1096/fj.02-0939com [DOI] [PubMed] [Google Scholar]
- Jin S., Hansson E. M., Tikka S., Lanner F., Sahlgren C., Farnebo F., Baumann M., Kalimo H. and Lendahl U. (2008). Notch signaling regulates platelet-derived growth factor receptor-beta expression in vascular smooth muscle cells. Circ. Res. 102, 1483-1491. 10.1161/CIRCRESAHA.107.167965 [DOI] [PubMed] [Google Scholar]
- Joutel A., Corpechot C., Ducros A., Vahedi K., Chabriat H., Mouton P., Alamowitch S., Domenga V., Cécillion M., Maréchal E. et al. (1996). Notch3 mutations in CADASIL, a hereditary adult-onset condition causing stroke and dementia. Nature 383, 707 10.1038/383707a0 [DOI] [PubMed] [Google Scholar]
- Kel A. E., Gossling E., Reuter I., Cheremushkin E., Kel-Margoulis O. V. and Wingender E. (2003). MATCHTM: a tool for searching transcription factor binding sites in DNA sequences. Nucleic Acids Res. 31, 3576-3579. 10.1093/nar/gkg585 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kikuchi K., Holdway J. E., Major R. J., Blum N., Dahn R. D., Begemann G. and Poss K. D. (2011). Retinoic acid production by endocardium and epicardium is an injury response essential for zebrafish heart regeneration. Dev. Cell 20, 397-404. 10.1016/j.devcel.2011.01.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kimmel C. B., Ballard W. W., Kimmel S. R., Ullmann B. and Schilling T. F. (1995). Stages of embryonic development of the zebrafish. Dev. Dyn. 203, 253-310. 10.1002/aja.1002030302 [DOI] [PubMed] [Google Scholar]
- Kofler N. M., Cuervo H., Uh M. K., Murtomäki A. and Kitajewski J. (2015). Combined deficiency of Notch1 and Notch3 causes pericyte dysfunction, models CADASIL, and results in arteriovenous malformations. Scientific Reports 5, 16449 10.1038/srep16449 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawson N. D. and Weinstein B. M. (2002). In vivo imaging of embryonic vascular development using transgenic zebrafish. Dev. Biol. 248, 307-318. 10.1006/dbio.2002.0711 [DOI] [PubMed] [Google Scholar]
- Lindahl P., Johansson B. R., Levéen P. and Betsholtz C. (1997). Pericyte loss and microaneurysm formation in PDGF-B-deficient mice. Science 277, 242-245. 10.1126/science.277.5323.242 [DOI] [PubMed] [Google Scholar]
- Lindblom P., Gerhardt H., Liebner S., Abramsson A., Enge M., Hellström M., Bäckström G., Fredriksson S., Landegren U., Nyström H. C. et al. (2003). Endothelial PDGF-B retention is required for proper investment of pericytes in the microvessel wall. Genes Dev. 17, 1835-1840. 10.1101/gad.266803 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y., Wada R., Yamashita T., Mi Y., Deng C.-X., Hobson J. P., Rosenfeldt H. M., Nava V. E., Chae S.-S., Lee M.-J. et al. (2000). Edg-1, the G protein–coupled receptor for sphingosine-1-phosphate, is essential for vascular maturation. J. Clin. Invest. 106, 951-961. 10.1172/JCI10905 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu H., Zhang W., Kennard S., Caldwell R. B. and Lilly B. (2010). Notch3 is critical for proper angiogenesis and mural cell investment. Circ. Res. 107, 860-870. 10.1161/CIRCRESAHA.110.218271 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Majesky M. W. (2007). Developmental basis of vascular smooth muscle diversity. Arterioscler. Thromb. Vasc. Biol. 27, 1248-1258. 10.1161/ATVBAHA.107.141069 [DOI] [PubMed] [Google Scholar]
- Matys V., Fricke E., Geffers R., Gößling E., Haubrock M., Hehl R., Hornischer K., Karas D., Kel A. E. and Kel-Margoulis O. V. (2003). TRANSFAC®: transcriptional regulation, from patterns to profiles. Nucleic Acids Res. 31, 374-378. 10.1093/nar/gkg108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ninov N., Borius M. and Stainier D. Y. R. (2012). Different levels of Notch signaling regulate quiescence, renewal and differentiation in pancreatic endocrine progenitors. Development 139, 1557-1567. 10.1242/dev.076000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parsons M. J., Pisharath H., Yusuff S., Moore J. C., Siekmann A. F., Lawson N. and Leach S. D. (2009). Notch-responsive cells initiate the secondary transition in larval zebrafish pancreas. Mech. Dev. 126, 898-912. 10.1016/j.mod.2009.07.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruchoux M. M., Domenga V., Brulin P., Maciazek J., Limol S., Tournier-Lasserve E. and Joutel A. (2003). Transgenic mice expressing mutant Notch3 develop vascular alterations characteristic of cerebral autosomal dominant arteriopathy with subcortical infarcts and leukoencephalopathy. Am. J. Pathol. 162, 329-342. 10.1016/S0002-9440(10)63824-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seiler C., Abrams J. and Pack M. (2010). Characterization of zebrafish intestinal smooth muscle development using a novel sm22α-b promoter. Dev. Dyn. 239, 2806-2812. 10.1002/dvdy.22420 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siebel C. and Lendahl U. (2017). Notch Signaling in Development, Tissue Homeostasis, and Disease. Physiol. Rev. 97, 1235-1294. 10.1152/physrev.00005.2017 [DOI] [PubMed] [Google Scholar]
- Sinha S. and Santoro M. M. (2018). New models to study vascular mural cell embryonic origin: implications in vascular diseases. Cardiovasc. Res. 114, 481-491. 10.1093/cvr/cvy005 [DOI] [PubMed] [Google Scholar]
- Song N., Huang Y., Shi H., Yuan S., Ding Y., Song X., Fu Y. and Luo Y. (2009). Overexpression of platelet-derived growth factor-BB increases tumor pericyte content via stromal-derived factor-1α/CXCR4 axis. Cancer Res. 69, 6057-6064. 10.1158/0008-5472.CAN-08-2007 [DOI] [PubMed] [Google Scholar]
- Stratman A. N., Schwindt A. E., Malotte K. M. and Davis G. E. (2010). Endothelial-derived PDGF-BB and HB-EGF coordinately regulate pericyte recruitment during vasculogenic tube assembly and stabilization. Blood 116, 4720-4730. 10.1182/blood-2010-05-286872 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang Y., Urs S., Boucher J., Bernaiche T., Venkatesh D., Spicer D. B., Vary C. P. H. and Liaw L. (2010). Notch and transforming growth factor-β (TGFβ) signaling pathways cooperatively regulate vascular smooth muscle cell differentiation. J. Biol. Chem. 285, 17556-17563. 10.1074/jbc.M109.076414 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vanlandewijck M., He L., Mäe M. A., Andrae J., Ando K., Del Gaudio F., Nahar K., Lebouvier T., Laviña B., Gouveia L. et al. (2018). A molecular atlas of cell types and zonation in the brain vasculature. Nature 554, 475 10.1038/nature25739 [DOI] [PubMed] [Google Scholar]
- Volz K. S., Jacobs A. H., Chen H. I., Poduri A., McKay A. S., Riordan D. P., Kofler N., Kitajewski J., Weissman I. and Red-Horse K. (2015). Pericytes are progenitors for coronary artery smooth muscle. eLife 4, e10036 10.7554/eLife.10036 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Q., Zhao N., Kennard S. and Lilly B. (2012). Notch2 and Notch3 function together to regulate vascular smooth muscle development. PLoS ONE 7, e37365 10.1371/journal.pone.0037365 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y., Pan L., Moens C. B. and Appel B. (2014). Notch3 establishes brain vascular integrity by regulating pericyte number. Development 141, 307-317. 10.1242/dev.096107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wasteson P., Johansson B. R., Jukkola T., Breuer S., Akyurek L. M., Partanen J. and Lindahl P. (2008). Developmental origin of smooth muscle cells in the descending aorta in mice. Development 135, 1823-1832. 10.1242/dev.020958 [DOI] [PubMed] [Google Scholar]
- Whitesell T. R., Kennedy R. M., Carter A. D., Rollins E.-L., Georgijevic S., Santoro M. M. and Childs S. J. (2014). An α-smooth muscle actin (acta2/αsma) zebrafish transgenic line marking vascular mural cells and visceral smooth muscle cells. PLoS ONE 9, e90590 10.1371/journal.pone.0090590 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winkler E. A., Bell R. D. and Zlokovic B. V. (2011). Central nervous system pericytes in health and disease. Nat. Neurosci. 14, 1398-1405. 10.1038/nn.2946 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamazaki T., Nalbandian A., Uchida Y., Li W., Arnold T. D., Kubota Y., Yamamoto S., Ema M. and Mukouyama Y.-S. (2017). Tissue myeloid progenitors differentiate into pericytes through TGF-β signaling in developing skin vasculature. Cell Rep. 18, 2991-3004. 10.1016/j.celrep.2017.02.069 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.