Abstract
Dementia among elderly is a serious problem worldwide. This study was conducted to estimate the prevalence and associated risk factors of dementia and mild cognitive impairment (MCI) in nursing homes (NHs) and common communities (CCs) among elderly in China.
A cross-sectional survey was conducted in 4 communities across 12 cities in Southern China from May to November of 2014. Qualified psychiatrists and trained nurses carried out relevant diagnosis, assessments, interviews, and information collection. Screening test of mini-mental state examination was conducted among participants firstly, then confirmed diagnosis was carried out among the ones with positive results. Student t test, χ test, univariate, and multivariate logistic regression analysis were conducted to analyze data.
A total of 2015 participants aged 65 or older were included in the final analysis; 908 came from NHs while 1107 came from CCs. The crude prevalence rates of dementia and MCI were 22.0% and 15.8%, respectively among all the participants. Dementia prevalence was 42.4% among those living in NHs, which was significantly higher than that of 5.3% in CCs (P < .0001). There were more moderate and severe dementia in NHs compared with CCs (P < .0001). It showed that older age, illiterate compared with high level of education (adjusted odds ratio, AOR = 3.32, 95% CI: 1.53–7.21), heavy drinking (AOR = 1.51 (1.00–2.24), having a medical history of diabetes (AOR = 1.41, 95% CI: 1.02–2.33), and stroke (AOR = 1.21, 95% CI: 1.01–1.23) were associated with dementia in NHs, and middle socioeconomic status might be a protective factor for dementia (AOR = 0.33, 95% CI: 0.21–0.51).
The problem of senile dementia in NHs is much more serious than our estimation, and there are not enough trained nursing staffs in NHs. More population-based strategies in NHs, including conducting cognitive screening accompanied with routine physical examination among elderly population, carrying out related primary prevention policies and public health services, and paying attention to some modifiable associated risk factors such as heavy smoking and drinking are needed.
Keywords: cognitive function status, elderly, prevalence
1. Introduction
Dementia is a syndrome with acquired cognitive impairment at its core, which leads to a significant decline in patients’ ability to live, learn, work, and communicate. It is a major cause of death and disability among the elderly.[1] Dementia prevalence is estimated to be 4.3%–6.4% at the age of 60 years or older, and 22.1%–30.1% at the age of 85 years or more in western countries.[2,3] China is facing substantial challenge of ageing, and the increasing numbers might have some degree of dementia. There are an estimated 7.4 million elderly in China with dementia, and this number will grow to 18 million by 2030.[4,5] A recent meta-analysis which included 76,980 subjects 60 years or more in China from 2000 to 2015 showed the prevalence for the following conditions: 5.15% for dementia, 3.56% for Alzheimer's disease (AD), and 1.11% for vascular dementia (VaD). The prevalence of senile dementia was found to be increasing in the past 15 years.[6]
Senile dementia often starts insidiously and aggravates memory disorder slowly. In the early stage, it is often ignored by their families, patients themselves, and even doctors, which is considered as a normal aging phenomenon. Patients often delay early diagnosis and treatment. With the progression of dementia, the cognitive function of patients continues to decline, which has a significant impact on life, leading to the loss of self-care ability. Some of the patients show obvious mental behavior abnormalities, such as anxiety, delusion, irritability, aggression, etc, which increases the mental and economic burden of caregivers. Dementia is not curable, but intervention in early stage is more effective.[7] A number of studies have shown that early intervention for dementia patients could control the cognitive function better, reduce the functional and behavioral disorders, and prolong the time of patients’ self-care.[2]
Cognition is the process by which the brain receives and processes information from the outside in order to recognize the world actively. Cognitive functions involve memory, attention, language, execution, reasoning, computation, and orientation. Cognitive impairment refers to impairment of one or more of these areas, which can affect a patient's social functioning and quality of life to varying degrees, and even lead to death in severe cases.[8] The most common cognitive impairment is mild cognitive impairment (MCI) and dementia. MCI is a transitional state between normal cognitive function and dementia. Research shows that about 10%–20% of patients with MCI convert to AD every year, and the rate is 10 times higher than that of healthy elderly people.[9] More than half of MCI patients will progress to dementia within 5 years, while only a small number of MCI patients can maintain stable cognitive function or even return to normal.[10] Therefore, timely intervention of MCI is very important to delay the occurrence and development of dementia. Dementia is a group of diseases with cognitive impairment as the core, accompanied by mental behavior symptoms, which lead to a decline in the ability of daily life. AD is the most common type of dementia, accounting for about 60% of all dementia types.[11]
Zhejiang province is located in eastern China, and it has an ageing population (60 years or older) of 7.5 million in 2013 which accounted for 13.4% of the total population.[11,12] Nowadays, more and more old people choose to spend their old age in nursing homes (NHs) in order to reduce the burden on their children, which leads to a high prevalence of dementia in NHs and MCI. There is an urgent need for a study to get the prevalence of MCI and dementia, including the associated risk factors. At present, there is lack of such data in eastern China.
2. Methods
2.1. Sampling
This study was a cross-sectional survey administrated in 4 communities across 12 cities in Zhejiang province. A multistage stratified random cluster sampling was used. According to the formula for calculating the sample size of stratified cluster random sampling, 5% was taken as the estimated value of the dementia prevalence, the tolerance error was taken as 1.0%, and the required minimum sample size was calculated as 1825. In consideration of sampling error, information loss, and other factors, a total of 2063 cases needed to be investigated, and 2015 cases of the research were finally included in this study.
Firstly, 12 administrative cities were divided into 4 groups based on economic levels. By the comparison, selection, and calculation, the variables of gender ratio, proportion of aged population (65 years or more), GDP, birth rate, mortality and natural growth rate, the proportion of employees in the tertiary industry, proportion of urban population, total population, the population with education level of high school or more, and the number of employees were used to measure the economic levels of 12 cities in Zhejiang province. We used the method of square sum of deviations with a better classification effect to conduct the clustering analysis. The distance between samples was estimated using the square Euclidean distance, and 12 cities were divided into 4 groups, that the first group included Hangzhou, Ningbo, and Wenzhou, the second group included Jiaxing, Huzhou, and Shaoxing, the third group included Jinhua, Yiwu, and Taizhou, and the fourth group included Zhoushan, Quzhou, and Lishui. Secondly, one city was randomly selected from the 4 types of groups respectively, then Hangzhou, Shaoxing, Yiwu, and Lishui was selected. Thirdly, one district/county was randomly chosen from each city. Finally, one common community (CC) and a NH were randomly chosen from the selected district/county. Referring to the sixth population census in Zhejiang province and the local situation of each district/county, we used the age (65–75, 75–85, 85 or more), gender (male, female), and education levels (illiterate, primary school, middle school, high school) stratification ratio of the elderly with 65 years old or more in Zhejiang province to recruit participants in selected communities, which make the sample be more representative.
Inclusion criteria of participants included subjects living in the selected communities/nursing homes, aged 65 or more, permanent residents who lived for more than half a year,[13] native Chinese speaker, and signed informed consents. Exclusion criteria included of hypothyroidism, depression, coma, aphasia, and end-of-life status. About 11 participants were excluded from the study at the recruiting phase because of depression, 7 came from CCs, and 4 came from NHs.
From May 1 to November 31 of 2014, 2063 subjects were recruited, including 1139 from CCs and 924 from NHs. About 31 cases were excluded from CCs because of low compliance and 1 because of death. Fourteen cases were excluded from NHs because of low compliance and 2 because of death. A total of 2015 subjects, 1107 from CCs and 908 from NHs, were included in the final data set (Fig. 1).
Figure 1.
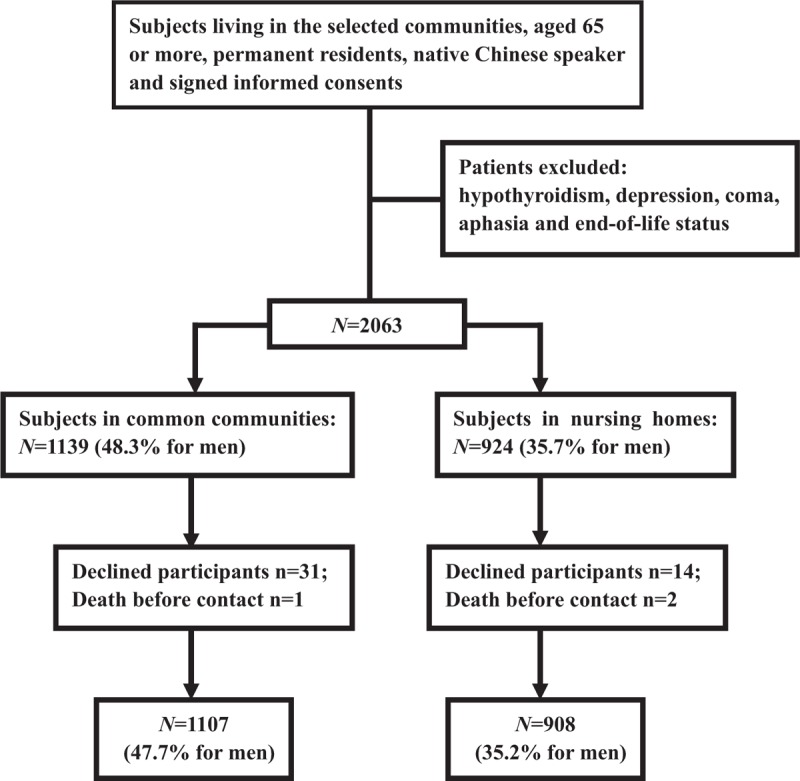
Schematic diagram of study participant selection.
2.2. Ethical review
The protocol of this study was approved by the Medical Ethics Committee of Zhejiang Hospital. The participants were informed about the objectives and methods of the study. They were informed that their participation was totally voluntary and that they could withdraw from the study at any time without citing any reason. Written and signed or thumb printed informed consent was obtained from those who agreed to participate, or from their guardians.
2.3. Measures
The survey was performed by qualified psychiatrists and trained nurses, and conducted in the residence of participants, community health service center, or NHs. Data collection of this field survey included a questionnaire interview and basic physical examination, which were conducted by trained nurses. The demographic information was obtained from subjects and caregivers or relatives, including age, areas of residence, education, and health behaviors. We collected relevant examination data, including blood pressure (BP), height, weight, and so on.
Screening test of mini-mental state examination (MMSE) was conducted among participants, which were conducted by qualified psychiatrists. MMSE score that was <20 among the illiterate (<1 year education) or <23 among participants with primary school education level (1–6 years) or <27 among participants with middle school or higher education level (>7 years) were considered as positive results, which have the tendency of dementia.[8]
The confirmed diagnosis of dementia was conducted among the subjects with positive result of MMSE test. The confirmed diagnosis procedure of dementia includes the history and personal information inquiry, and the work of combination with relevant scales results and magnetic resonance imaging (MRI) results, then the primary diagnosis was made. The MRI was implemented on all patients with dementia, and the remaining tests including cerebrospinal fluid (CSF) test would depend on the discretion of the physicians. Auxiliary examinations such as CSF test were often used to classify different types of dementia better. Dementia mainly included of AD, VaD, dementia with Lewy bodies (DLB), and Parkinson's disease dementia (PDD). Detailed diagnostic criteria are as follows.
The diagnostic criteria for AD used the recommendations from the National Institute on Aging-Alzheimer's Association (NIA-AA) workgroups on diagnostic guidelines for AD in 2011. Diagnosis of AD dementia[14]: (1) The neuropsychological scale indicated a significant decline in episodic memory. (2) MRI including coronal position confirmed the atrophy of medial temporal lobe and/or hippocampus. (3) Cerebrospinal fluid Aβ42 decreased, total Tau/phosphorylation Tau increased, and Position Emission Computed Tomography showed a lower glucose metabolism rate in bilateral temporal parietal lobe and of Aβ deposition. All of these helped to diagnose AD dementia and differentiate other types of dementia. Diagnosis of VaD[15]: (1) There were more than 1 vascular risk factor. (2) There were more than one cognitive domain impairment. (3) Vascular events were associated with cognitive impairment. (4) Cognitive function impairment showed rapid deterioration or volatility. The diagnosis of dementia with DLB used consensus guidelines for the clinical and pathologic diagnosis of DLB.[16] The diagnosis of PDD used European Federation of Neurological Societies-European Neurological Societies Guidelines on the diagnosis and management of disorders associated with dementia.[17]
According to the severity, dementia is classified as mild dementia, moderate dementia, and severe dementia. Mild dementia is manifested as memory loss, prominent forgetfulness of recent events, and decreased judgment ability. Patients are unable to analyze, think, and judge events, and feel difficult to deal with complex problems. Patients with moderate dementia show severe impairment of near and far memory, disorientation of time and place, severe impairment in processing problems and identifying similarities and differences in things, and also show various neurological symptoms.[8] Severe dementia patients have been completely dependent on caregivers, with severe memory loss, unable to take care of themselves in daily life. The severity of dementia was generally distinguished by MMSE score, 17–24 score was for mild dementia, 10–16 score was for moderate dementia, and 0–9 score was for severe dementia.[8]
2.4. Quality control
Participants were required to wear light clothes, no shoes, and no headgear while being measured for height and weight. Body mass index (BMI) was defined as weight in kilograms divided by height in square meters.[18] BP was measured twice on the right upper arm after 5 minutes of rest in a seated position with a standardized electronic blood pressure monitor (Omron HEM-7430). If the difference between the 2 systolic blood pressure readings was greater than 10 mm Hg, a third measurement was obtained and the average of the last 2 readings was used.[18]
MMSE scale is the most commonly used rapid screening tool for cognitive dysfunction in the world. This scale has been translated and revised into many languages and has been widely used in clinical practice. The original author's study confirmed that the English MMSE scale had good validity and reliability.[19] Domestic studies also showed that the Chinese MMSE have good reliability among evaluators (0.95–1) and good content validity with good item discrimination validity.[8]
2.5. Statistical analysis
Epidata 3.0 was used for data entry and validation and SAS 9.2 for data management and analysis. Socio-demographic characteristics, physical measurements, dementia, and MCI status of participants were summarized using frequencies (percentages) or means and standard deviations, and they were compared by Student t test and χ2 test, respectively. Adjusted prevalence standardized by age and gender were calculated by a direct method with a standard population (the sixth population census in Zhejiang). Logistic regression models including crude and adjusted models were used to assess associated risk factors associated with dementia and MCI. Crude associations were first assessed using univariate models, multivariate models were used for the adjusted associations adjusted by age, gender, education levels, family income, status of marriage. Significance level was set at P < .05 for all hypothesis tests.
3. Results
3.1. Socio-demographic characteristics of participants
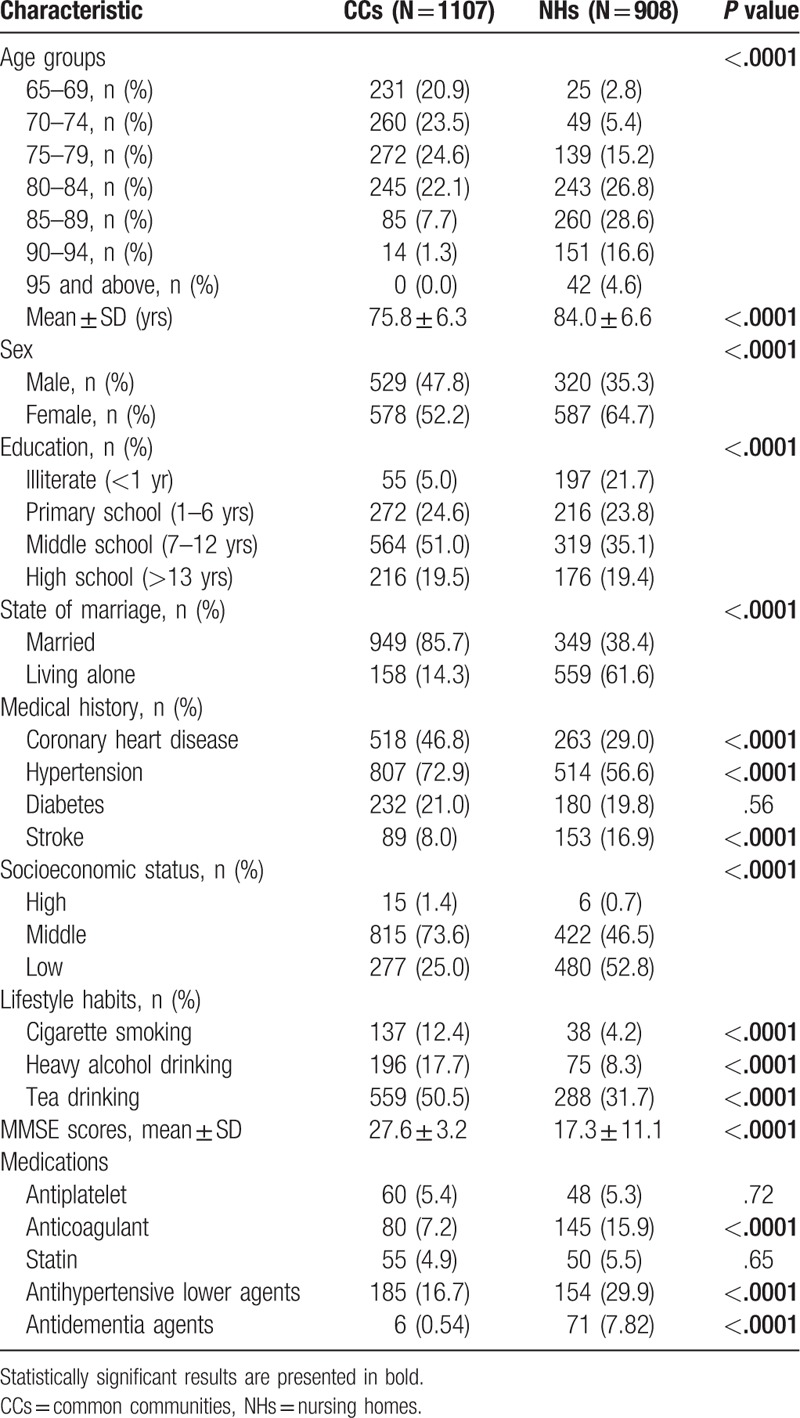
A total of 2063 subjects were included in this study, 45 subjects were excluded because of low compliance, and 3 were because of death. A total of 2015 participants with a mean age of 79.5 years from 4 communities of Zhejiang province were included in the final data set. Among the 2015 subjects, 1107 came from CCs, while 908 came from NHs. Participants in NHs were older than that in CCs. There were more males, married subjects in CCs compared with that in NHs. And subjects in CCs had higher levels of education and family income levels compared with that in NHs. General characteristics including several common medications of the participants were presented in Table 1.
Table 1.
Baseline characteristics of subjects in CCs and NHs.
3.2. Prevalence of dementia and MCI
Among the total subjects, 444 (22.0%) were diagnosed with some form of dementia, the prevalence of dementia standardized by age and sex with a standard population (the sixth population census in Zhejiang) was 13.0%. Screening test and diagnosis were conducted among 1107 and 908 elderly patients in CCs and NHs. There were 926/327 cases with normal cognitive function, 122/196 with MCI, and 59/385 with dementia in CCs/NHs. There were 41/200 cases of possible and probable AD, 1/17 case of VaD, 7/16 cases of AD with vascular dementia, 2/0 case of DLB, 2/0 case of PDD, 2/0 cases of drug-induced dementia, 1/0 case of schizophrenia-induced dementia, 0/7 cases of dementia with other causes, and 3/145 cases of unexplained dementia in CCs/NHs.
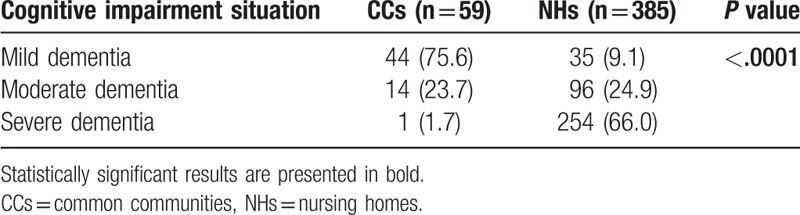
The crude prevalence rates of dementia and MCI were 22.0% and 15.8%, respectively, among all the participants, and with significantly higher dementia and MCI rates in NHs compared to CCs. Subjects living alone had higher rates of dementia compared with married ones in both NHs and CCs (Table 2). And the prevalence of dementia was higher among the participants with medical history of diabetes mellitus and stroke in CCs. There were more moderate and severe dementia in NHs compared with CCs (P < .0001) (Table 3).
Table 2.
Prevalence of dementia and MCI among elderly in CCs and NHs.
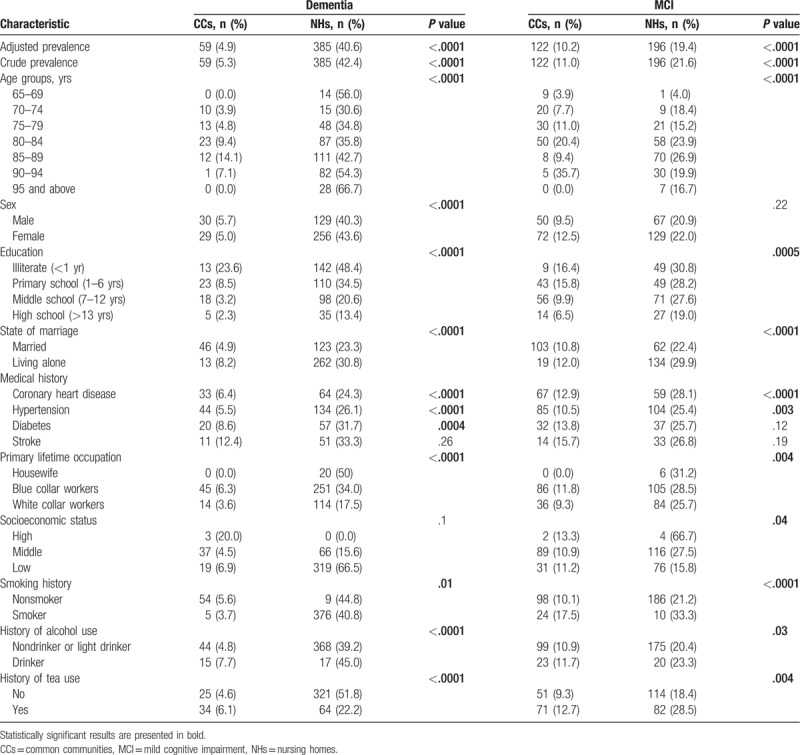
Table 3.
Severity of cognitive impairment in CCs and NHs (n (%)).
3.3. Associated risk factors of dementia and MCI in NHs
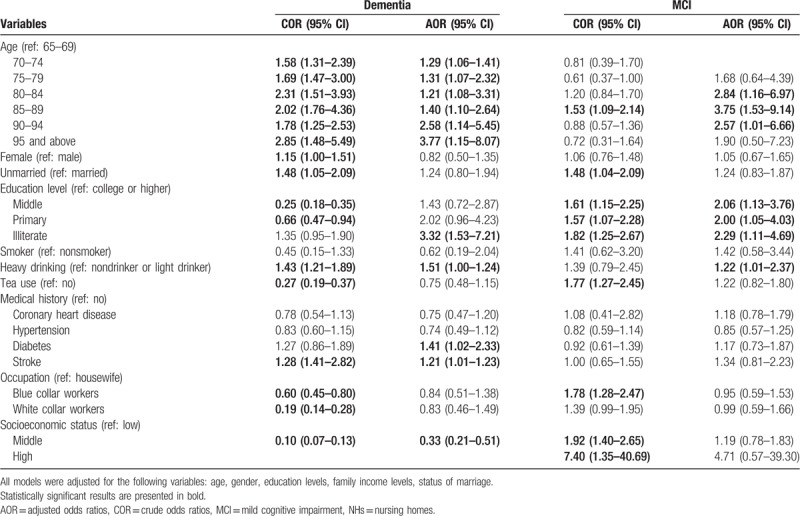
Logistic regression models including crude and adjusted models were used to assess associated risk factors associated with dementia and MCI in NHs. Crude associations were first assessed using univariate models, then multivariate models were used for the adjusted associations, which were adjusted by age, gender, education levels, family income levels, status of marriage. It showed that older age, illiterate compared with high level of education (adjusted odds ratio, AOR = 3.32, 95% CI: 1.53–7.21), heavy drinking (AOR = 1.51 (1.00–2.24), having a medical history of diabetes (AOR = 1.41, 95% CI: 1.02–2.33), and stroke (AOR = 1.21, 95% CI: 1.01–1.23) were associated with dementia in NHs, while middle socioeconomic status might be a protective factor for dementia (AOR = 0.33, 95% CI: 0.21–0.51). Several variables were associated with MCI in adjusted models in NHs. These included older age, lower education levels of middle (AOR = 2.06, 95% CI: 1.13–3.76), primary (AOR = 2.00, 95% CI: 1.05–4.03), illiterate (AOR = 2.29, 95% CI: 1.11–4.69), and heavy drinking (AOR = 1.22, 95% CI: 1.01–2.37) (Table 4).
Table 4.
Logistic regression analysis of risk factors of dementia and MCI among elderly in NHs.
4. Discussion
The population aged 60 years or older are expected to increase from 605 million in 2000 to 1.2 billion by 2025 and 2 billion by 2050 in the world.[20] Dementia is an age-related disease, the prevalence of dementia rises sharply with age. Most people with dementia are 65 and older, which places a heavy burden on families and society.
Our study showed that the prevalence of dementia among elderly aged 65 or more was 4.9% in CCs, which was consistent with the result conducted among Chinese people aged 60 and older (5.14%).[6] The prevalence of dementia and MCI among elderly in NHs was significantly higher than that of CCs. The dementia rate in NHs was very high (40.6%), but lower than that in NHs of other countries and regions: 48.2% in United States,[21] 50.7% in Italy,[22] 47.6% to 51.8% in Germany,[23,24] and 59.1% in Hong Kong.[25] The extremely high prevalence of dementia and MCI in NHs was probably a kind of indication bias or reverse causality. Elderly people moved into NHs, because they were demented and could get appropriate care in NHs.
The dementia prevalence among elderly aged 65 years or more reported by NHs’ directors was only 23.4% (about 90 dementia patients), which was far lower than the survey's results (40.6%). The problem of senile dementia in NHs was much more serious than our estimation, and we should pay more attention. Patients with dementia needed professional long-term care, and NHs were short of nursing staffs who received professional training. Most dementia patients in NHs transitioned to moderate or severe dementia quickly who suffered from mild dementia at the beginning and could not get prompt intervention, their quality of lives was greatly reduced. Dementia care should be one of the core businesses of NHs activities as dementia is becoming a serious problem in China due to the ageing population.
Although dementia had become a serious problem, the rate of use of anti-dementia agents such as memantine was very low. The medication use rate among the senile dementia patients was 10.2% (6/59) in CCs, and the rate was 18.4% (71/385) in NHs. There were higher rates of common medications uses among elderly patients in NHs compared with CCs, including the uses of anticoagulant, anti-hypertensive lower agents, and that might because the medications reminding of nursing staff in NHs.
In our study, we found a rise tendency in the prevalence of dementia with age, which had similar results with other studies.[26] Our data showed that the prevalence of dementia was higher in female than male in NHs, but there were no significant differences, whereas some other studies found that there was a higher dementia prevalence in female than male.[27]
Our result showed that lower education levels were associated with high risk for dementia and MCI compared with higher levels of education, which was confirmed by other studies.[25,28] Shadlen et al did a study in the United States and found that low education level (≤10 years) had twice the risk of dementia as high education level (>10 years). Another Chinese study revealed that high educated people had significantly lower AD prevalence after adjustment for age, but no significant educational effects were observed for VaD.[28]
The result in our study also revealed that heavy drinking increased the risk of dementia and MCI in NHs, which was similar with that in other study. Sabia et al believed that the risk of dementia was increased in people who abstained from alcohol in midlife or consumed >14 units/week.[29] The negative impact of heavy drinking on the risk of dementia had been suggested to involve nutritional deficiency, the direct neurotoxic effects of ethanol, and the indirect negative impacts through increased risk of diabetes, hypertension, and stroke. Heavy alcohol intake was a modifiable lifestyle, which should attract our attention. At the same time, having medical histories of diabetes and stroke were associated risk factors of dementia, middle socioeconomic status was a protective factor of dementia compared with too low socioeconomic status in NHs.
The prevalence of VaD in NHs was 1.87%, the VaD rate among the elderly participants having a medical history of stroke, with a habit of smoking and heavy drinking was found to have a sharp rise (P < .0001). Current smokers had an increased risk of VaD, that was proven by other studies.[30] That might because that smoking was a proven risk factor for stroke,[31] and stroke tended to lead VaD. High alcohol consumption could be neurotoxic and increased the risk of VaD, while low and moderate alcohol consumption had an opposite effect. This trend was verified in other studies.[30]
Compared with developed countries, the problem of dementia in China would face greater challenges due to the rapid growth of a large elderly population and the lack of mental health services. We assumed that the problem of low rate of active consultation might be overcome by large-scale cognitive function screening among the elderly population over 60 years or older, and the early diagnosis rate of dementia might be improved, and we should provide more support and help for the families with dementia patients, that might extend the time and quality of home-based care. If goals and requirements for the construction of long-term care system for senile dementia in NHs were put forward, the great pressure caused by the rapid growth of senile dementia on nursing care might be alleviated, and the service ability of NHs’ care could be improved.
At present, there is no precedent to include cognitive screening in routine physical examination among elderly in NHs or CCs of China. If the elderly population in highly concentrated places such as NHs could be screened for cognitive function by the community health care centers’ personnel, and then patients with suspected abnormalities are referred to professional medical institutions for more detailed diagnosis by specialists, we would find the dementia patients earlier and in time. In other words, it is of great significance to adopt the method of “hierarchical diagnosis” to detect cognitive impairment patients at an early stage, and to intervene, control, and delay the development process of dementia in a timely manner, which has already been implemented in the UK.[5] Our study provided the evidence for the early screening program for the large population in China.
This study had several strengths and limitations. The main strength of this study was the rejection rate to the study was very low (<3%), because the survey was carried out among the participants together with the free physical examination, which was conducted by the government. Moreover, it provided a picture and comparison on the epidemiology prevalence of dementia and MCI among elderly between NHs and CCs. To our knowledge, the study was the first to examine the difference in the prevalence and associated factors of dementia and MCI between NHs and CCs in Zhejiang province.
Despite these strengths, there were several limitations. The main limitation of the study was its cross-sectional design and the lack of information about risk factors that developed over time, which were related to the occurrence and progression of cognitive decline, and could also represent targets for prevention.[32] Because of lacking the relatively strict admission and exclusion criteria of longitudinal data, we failed to establish cause-and-effect relationship between the observed associations which might be very useful to develop preventive policies. There is an urgent need of future research addressing this problem. Otherwise, some confounding variables such as dietary habits were not included, we should consider them in the future study. Finally, we excluded participants with depression at the recruiting phase, although the number was small that might cause some selection bias.
5. Conclusion
In conclusion, dementia prevalence among elderly who were 65 years old or more in NHs were significantly higher than that in CCs, and there were not enough trained nursing staffs in NHs. Most dementia patients in NHs could not get the corresponding care in time, which should attract our attention. More population-based strategies in NHs, including conducting cognitive function screening accompanied with routine physical examination among elderly population, carrying out related primary prevention policies and public health services, and paying attention to some modifiable associated risk factors such as heavy smoking and drinking are needed.
Author contributions
Conceptualization: Jing Yan.
Data curation: Shanhu Xu.
Funding acquisition: Li Yang, Jing Yan.
Investigation: Shanhu Xu, Ying Xu, Caixia Liu.
Methodology: Xiaoqing Jin, Yu Jin.
Project administration: Yu Jin.
Resources: Xiaoqing Jin, Ying Xu, Caixia Liu.
Software: Li Yang.
Supervision: Xiaoqing Jin, Jing Yan, Yu Jin.
Validation: Caixia Liu.
Visualization: Li Yang.
Writing – original draft: Li Yang.
Footnotes
Abbreviations: AD = Alzheimer's disease, AOR = adjusted odds ratio, CCs = common communities, COR = crude odds ratios, DLB = dementia with Lewy bodies, MCI = mild cognitive impairment, NHs = nursing homes, NIA-AA = National Institute on Aging-Alzheimer's Association, PDD = Parkinson's disease dementia, VaD = vascular dementia.
How to cite this article: Yang L, Jin X, Yan J, Jin Y, Xu S, Xu Y, Liu C, Yu W, Zheng P. Comparison of prevalence and associated risk factors of cognitive function status among elderly between nursing homes and common communities of China: A STROBE-compliant observational study. Medicine. 2019;98:49(e18248).
This work was supported by Project supported by the National Science Foundation for Young Scientists of China (81803314), General project of Medical Science and Technology in Zhejiang province (2019KY001), General project of Medical Science and Technology in Zhejiang province (2018KY193), State Commission of Science Technology and Ministry of Science and Technology of China (2014DFT30100) and Key project of the Fourth-Round Three-Year Plan of Action in Public Health in Shanghai (15GWZK1001).
The authors have no conflicts of interest to disclose.
References
- [1].Lin YT, Wu PH, Kuo MC, et al. Comparison of dementia risk between end stage renal disease patients with hemodialysis and peritoneal dialysis-a population based study. Sci Rep 2015;5:8224–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Noguchi-Shinohara M, Yuki S, Dohmoto C, et al. Differences in the prevalence of dementia and mild cognitive impairment and cognitive functions between early and delayed responders in a community-based study of the elderly. J Alzheimers Dis 2013;37:691–8. [DOI] [PubMed] [Google Scholar]
- [3].Ferri CP, Prince M, Brayne C, et al. Global prevalence of dementia: a Delphi consensus study. Lancet 2005;366:2112–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Yang L, Jin X, Yan J, et al. Prevalence of dementia, cognitive status and associated risk factors among elderly of Zhejiang province, China in 2014. Age Ageing 2016;45:708–12. [DOI] [PubMed] [Google Scholar]
- [5].Yang L, Yan J, Jin X, et al. Screening for dementia in older adults: comparison of Mini-Mental State Examination, Mini-Cog, Clock Drawing Test and AD8. PLoS One 2016;11:e0168949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Liu BY, Wang JL, Xiao YZ, et al. [Prevalence of senile dementia in people aged ≥60 years in China: a meta-analysis]. Zhonghua Liu Xing Bing Xue Za Zhi 2016;37:1541–5. [DOI] [PubMed] [Google Scholar]
- [7].Carmona P, Molina M, Toledano A, et al. Blood-based biomarkers of Alzheimer's disease: diagnostic algorithms and new technologies. Curr Alzheimer Res 2016;13:450–64. [DOI] [PubMed] [Google Scholar]
- [8].Consensus writing group of experts on diagnosis and treatment of cognitive impairment in the elderly, Geriatric neurology group of geriatric medicine branch of Chinese medical association. Expert advice on the diagnosis and treatment process of cognitive impairment in the elderly in China. Chin J Geriatr 2014;33:817–25. [Google Scholar]
- [9].Petersen RC. Clinical practice. Mild cognitive impairment. N Engl J Med 2011;364:2227–34. [DOI] [PubMed] [Google Scholar]
- [10].Gauthier S, Reisberg B, Zaudig M, et al. Mild cognitive impairment. Lancet 2006;367:1262–70. [DOI] [PubMed] [Google Scholar]
- [11].Yang L, Xu X, Yan J, et al. Analysis on associated factors of uncontrolled hypertension among elderly hypertensive patients in Southern China: a community-based, cross-sectional survey. BMC Public Health 2014;14:903–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Yang L, Yan J, Tang X, et al. Prevalence, awareness, treatment, control and risk factors associated with hypertension among adults in southern China, 2013. PLoS One 2016;11:e0146181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Moore DC, Keegan TJ, Dunleavy L, et al. Factors associated with length of stay in care homes: a systematic review of international literature. Syst Rev 2019;8:56–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Sperling RA, Aisen PS, Beckett LA, et al. Toward defining the preclinical stages of Alzheimer's disease: recommendations from the National Institute on Aging-Alzheimer's Association workgroups on diagnostic guidelines for Alzheimer's disease. Alzheimers Dement 2011;7:280–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Sachdev P, Kalaria R, O’Brien J, et al. Diagnostic criteria for vascular cognitive disorders: a VASCOG statement. Alzheimer Dis Assoc Disord 2014;28:206–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].McKeith IG. Consensus guidelines for the clinical and pathologic diagnosis of dementia with Lewy bodies (DLB): report of the Consortium on DLB International Workshop. J Alzheimers Dis 2006;9:417–23. [DOI] [PubMed] [Google Scholar]
- [17].Sorbi S, Hort J, Erkinjuntti T, et al. EFNS-ENS Guidelines on the diagnosis and management of disorders associated with dementia. Eur J Neurol 2012;19:1159–79. [DOI] [PubMed] [Google Scholar]
- [18].Lu J, Lu Y, Yang H, et al. Characteristics of high cardiovascular risk in 1.7 million Chinese Adults. Ann Intern Med 2019;170:298–308. [DOI] [PubMed] [Google Scholar]
- [19].Anthony JC, LeResche L, Niaz U, et al. Limits of the ‘Mini-Mental State’ as a screening test for dementia and delirium among hospital patients. Psychol Med 1982;12:397–408. [DOI] [PubMed] [Google Scholar]
- [20].World Health Organization. Teaching Geriatrics in Medical Education II. 2010;Geneva:Department of Ageing and Life Course and IFMSA. World Health Organization, Available at: http://www.who.int/ageing/projects/TeGeMe II. Accessed November 12, 2018. [Google Scholar]
- [21].Magaziner J, German P, Zimmerman SI. The prevalence of dementia in a statewide sample of new nursing home admissions aged 65 and older: diagnosis by expert panel. Epidemiology of Dementia in Nursing Homes Research Group. Gerontologist 2000;40:663–72. [DOI] [PubMed] [Google Scholar]
- [22].Cherubini A, Ruggiero C, Dell’Aquila G, et al. Underrecognition and undertreatment of dementia in Italian nursing homes. J Am Med Dir Assoc 2012;13: 759.e7-13. [DOI] [PubMed] [Google Scholar]
- [23].Jakob A, Busse A, Riedel-Heller SG, et al. [Prevalence and incidence of dementia among nursing home residents and residents in homes for the aged in comparison to private homes]. Z Gerontol Geriatr 2002;35:474–81. [DOI] [PubMed] [Google Scholar]
- [24].Hoffmann F, Kaduszkiewicz H, Glaeske G, et al. Prevalence of dementia in nursing home and community-dwelling older adults in Germany. Aging Clin Exp Res 2014;26:555–9. [DOI] [PubMed] [Google Scholar]
- [25].Cheng ST, Lam LC, Chow PK. Under-recognition of dementia in long-term care homes in Hong Kong. Aging Ment Health 2012;16:516–20. [DOI] [PubMed] [Google Scholar]
- [26].Zhang ZX, Zahner GE, Román GC, et al. Socio-demographic variation of dementia subtypes in China: methodology and results of a prevalence study in Beijing, Chengdu, Shanghai, and Xian. Neuroepidemiology 2006;27:177–87. [DOI] [PubMed] [Google Scholar]
- [27].Kalaria RN, Maestre GE, Arizaga R, et al. Alzheimer's disease and vascular dementia in developing countries: prevalence, management, and risk factors. Lancet Neurol 2008;7:812–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Shadlen MF, Siscovick D, Fitzpatrick AL, et al. Education, cognitive test scores, and black-white differences in dementia risk. J Am Geriatr Soc 2006;54:898–905. [DOI] [PubMed] [Google Scholar]
- [29].Sabia S, Fayosse A, Dumurgier J, et al. Alcohol consumption and risk of dementia: 23 year follow-up of Whitehall II cohort study. BMJ 2018;362:k2927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Rusanen M, Kivipelto M, Quesenberry CP, Jr, et al. Heavy smoking in midlife and long-term risk of Alzheimer disease and vascular dementia. Arch Intern Med 2011;171:333–9. [DOI] [PubMed] [Google Scholar]
- [31].Srinivasan V, Braidy N, Chan EK, et al. Genetic and environmental factors in vascular dementia: an update of blood brain barrier dysfunction. Clin Exp Pharmacol Physiol 2016;43:515–21. [DOI] [PubMed] [Google Scholar]
- [32].Lattanzi S, Brigo F, Vernieri F, et al. Visit-to-visit variability in blood pressure and Alzheimer's disease. J Clin Hypertens 2018;20:918–24. [DOI] [PMC free article] [PubMed] [Google Scholar]


