Abstract
The aim of this study was to investigate possible associations of nutrient intake on glaucoma in subjects of Japanese descent living in Los Angeles, CA.
In this cross-sectional study, 581 Japanese American participants in Los Angeles underwent an interview, fundus photography, comprehensive physical, and blood examinations, along with determining the body mass index status and any confounding factors. CDSketch was used to measure the cup-disc ratio and rim width of each fundus in the retinal photographs. A multivariate logistic regression test with adjustment for confounding factors was used to assess the association between glaucoma and nutrient intake.
A total of 61 of 581 participants were diagnosed with glaucoma in this study. Multivariate logistic regression analysis showed that a high intake of iron (odds ratio [OR]: 1.303, P = .004), low intake of vitamin A (OR: 0.365, P = .019), and vegetable fat (OR: 0.957, P = .004) were associated with an increased risk of glaucoma.
Current findings showed that high iron intake and low vitamin A and vegetable fat intake appeared to be associated with an increased risk of glaucoma in subjects of Japanese descent living in the Los Angeles populations.
Keywords: glaucoma, iron, nutrient intake, vegetable fat, vitamin A
1. Introduction
Glaucoma is a progressive optic neuropathy characterized by degeneration of the retinal ganglion cells (RGCs); it is the second leading cause of visual loss in the world.[1] This progressive neurodegenerative disease leads to glaucomatous visual field loss. It is estimated that >60.5 million people globally were affected by primary open angle glaucoma (POAG) in 2010, with up to 111.8 million estimated to be affected by 2040 owing to population aging. Individuals of African and Asian descent have the highest prevalence of POAG, whereas Europeans have the lowest.[1,2]
Although the pathogenesis of glaucoma has yet to be fully established, intraocular pressure (IOP) is the most important and treatable risk factor. Furthermore, glaucoma can develop even in the presence of low or normal IOP and can progress despite having low ocular pressure. This suggests that independent factors associated with IOP might also influence glaucoma progression. Other proposed mechanisms that have been reported include impaired blood flow, oxidative stress, and ocular rigidity.[3–6]
Recent studies have shown that vitamins A, B1, B2, and B3 may have potential effects on the incidence of glaucoma.[7,8] Owaifeer et al[9] performed a review of diet in glaucoma and found that alcohol, tea, and diets rich in fruits and vegetables can decrease the risk of glaucoma. Moreover, individual diet may also have an effect on IOP and the incidence and progression of glaucoma. However, the Nurse's Health Study and the Health Professionals Follow-up Study,[10] 2 large prospective cohorts involving >100,000 participants, investigated the influence of nutrients with antioxidative effects on POAG and reported finding no significant associations.
Since 1970, we have conducted an epidemiological study called the Hawaii–Los Angeles–Hiroshima Study. Participants in this study, which include Japanese migrants who emigrated from Japan to the United States and their children, have agreed to undergo regular medical surveys and examinations.[11–13] In 2015, we performed dietary assessments using a computer-assisted menu planning system and took fundus photographs of Japanese American participants living in Los Angeles, CA.[14] Computer-assisted menu planning systems help nutritionists create best-fit menus for individuals by evaluating information on intakes of nutritional energy, vitamin, cholesterol, and fat. The aim of the present cross-sectional study was to investigate the possible association of nutrition with glaucoma among Japanese American participants.
2. Materials and methods
2.1. Study design population
The present cross-sectional study included 590 Japanese Americans who underwent medical examinations conducted in August 2015 in Los Angeles. After all participants received an explanation of the study procedures and provided their written informed consent, they underwent examination by a team of well-trained dietitians, along with an ophthalmologist, internist, optometrist, and nurses. We excluded participants with unreadable retinal photographs and missing data of body mass index (BMI), dietary intake, and smoking. After exclusion, 581 participants were included in the statistical analysis. This study was carried out in accordance with the Declaration of Helsinki and was approved by the Ethics Committee of Hiroshima University (Approval number: E-139).
2.2. Dietary assessment
Dietary information at baseline was collected using the food-frequency method, which has been described in detail elsewhere.[15] The frequency and amount of intake per meal and the cooking method for each food group were ascertained using food models, along with personal interviews carried out by a dietitian. The mean daily intake of each food group was calculated as (mean amount of intake per meal) × (frequency of intake per day); the nutritional intake from each food group was calculated as (nutritional value per gram of each food) × (mean daily intake of each food group).[15] The nutritional value of each food group was determined according to the US Department of Agriculture Nutritive Value of American Foods in Common Units.[16] Simple carbohydrate intake was defined as the sum of intakes of sugar and fructose.
2.3. Evaluating optic disc and nerve fibre layer defects
Bilateral fundus photographs were captured using a 45 degree nonmydriatic retinal camera (NIDEK AFC-300; NIDEK Co., Ltd., Gamagori, Japan). As the fundus examinations were carried out by 3 different examiners (YK, KH, MY), each investigator independently evaluated the color photographs using CDSketch Version 1.4 2009–2012 (Kowa Company, Ltd., Nagoya, Japan). This software was used to screen for glaucomatous optic disc appearances. The rim border was determined based on shadows, gradations of color, texture, and the course of blood vessels. After the examiner manually drew a line on the disc and cup of the optic disc photo, the software calculated the vertical cup/disc ratio and rim width at the superior or inferior portion. When at least 1 examiner noted any findings that suggested the presence of glaucomatous changes, consensus was obtained in discussions, to make a final judgment. In general, we used right-eye optic disc photographs unless the image could not be evaluated, in which case the left-eye photo was used. All fundus photographs obtained from participants were included in the study analysis. The criteria for glaucoma diagnosis were based on previously reported diagnostic definitions for category 2 glaucoma and glaucoma suspects.[17]
2.4. Assessment of covariates
All participants underwent an interview and physical examination and provided blood samples after fasting overnight. Venous blood was collected for the purpose of examining cholesterol, triglycerides, C-reactive protein, and blood glucose. Height and weight were measured using a digital scale and a stadiometer. BMI was then calculated as weight divided by height squared (kg/m2). BMI categories were standardized according to the National Institute of Health classification of BMI for adults: underweight (BMI <18.5), normal weight (18.5–24.9), overweight (25–29.9), and obesity (>30).[18] All participants without diabetes underwent a 75-g oral glucose tolerance test (OGTT). Diabetes status was obtained by either self-report of a previous diagnosis, receiving treatment with diabetic medications or insulin, and OGTT results. Diabetes was defined based on American Diabetes Association guidelines, which require a fasting serum glucose level of ≥126 mg/dL or a 2-hour serum glucose level ≥200 mg/dL after OGTT.[19] Mean arterial blood pressure (MABP) was calculated as one-third of the systolic blood pressure plus two-thirds of the diastolic blood pressure. The classification of MABP was as follows: optimal MABP <93.33, normal MABP 93.33–99.00, high normal MABP: 99.01–105.67, grade 1 hypertension 105.68 –119.00, grade II hypertension 119.01–132.33, and grade III hypertension >132.33.[20] Based on data collected during the interview, participants were also categorized as nonsmoker or former and current smoker.
2.5. Statistical analysis
Statistical analyses were performed using IBM SPSS software version 21 (IBM Corp., Armonk, NY). As the total number of participants with glaucoma was not very large, we combined participants with glaucoma and suspected glaucoma before the statistical analysis. Continuous variables are presented as mean ± standard deviation. Demographic factors between participants with and those without glaucoma were compared using the Kruskal–Wallis test and χ2 analysis. Relationships between the categorical variables were analyzed using a χ2 test. Logarithmic transformation was considered for continuous variables to improve the normality of distribution. Logistic regression analysis was also performed so as to develop models for predicting glaucoma using the following variables: age, sex, BMI, diabetes, MABP, animal protein, animal fat, vegetable protein, vegetable fat, carbohydrates, fructose, fibre, salt, calcium, iron, potassium, vitamins (A, B1, B2, C), polyvalent fatty acids, saturated fatty acids, and cholesterol. Factors found to be nonsignificant (P > .2) in the univariate analysis were then excluded from the stepwise multiple logistic regression analysis used to identify independent factors associated with glaucoma. Statistically significant variables determined in the multivariate regression analysis by backward elimination were subsequently included in the new model. A P value <.05 was considered statistically significant.
3. Results
Among the 590 included Japanese American participants in Los Angeles, 6 who lacked an optic disc photo and three with no data were excluded from the analysis. After exclusion, a total 581 participants were included in the study (Fig. 1).
Figure 1.
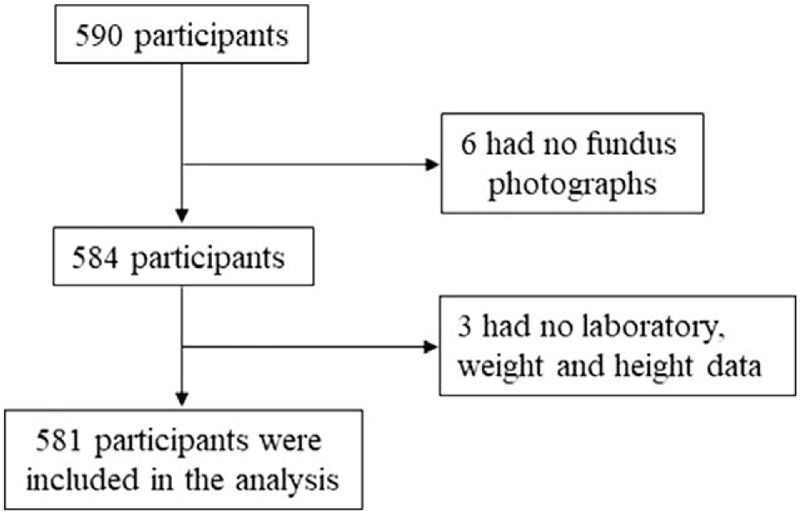
Flow diagram displaying selection of study subjects.
Table 1 presents the demographics for participants. A total of 61 participants were diagnosed with glaucoma. No significant differences were observed for age (P = .84), sex (P = .20), BMI (P = .50), smoking (P = .36), diabetes (P = .57), or hypertension (P = .22) between both groups.
Table 1.
Baseline participant characteristics.
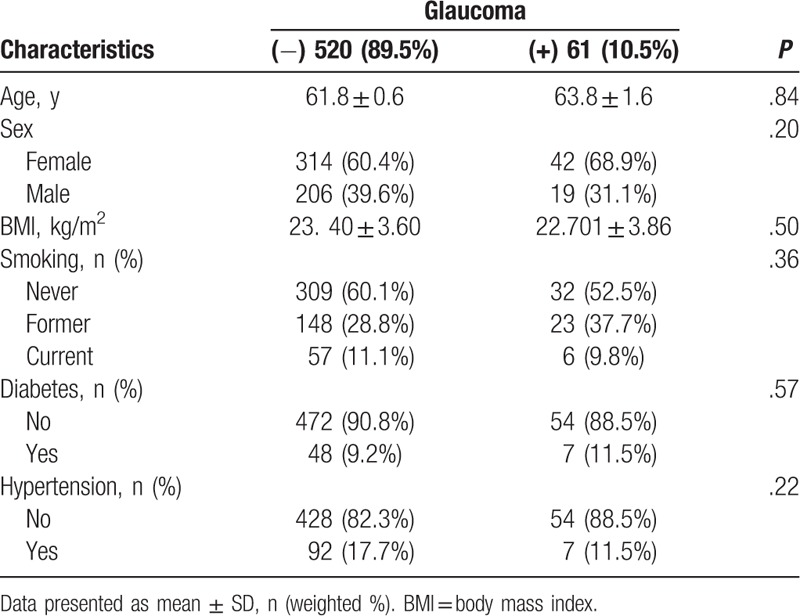
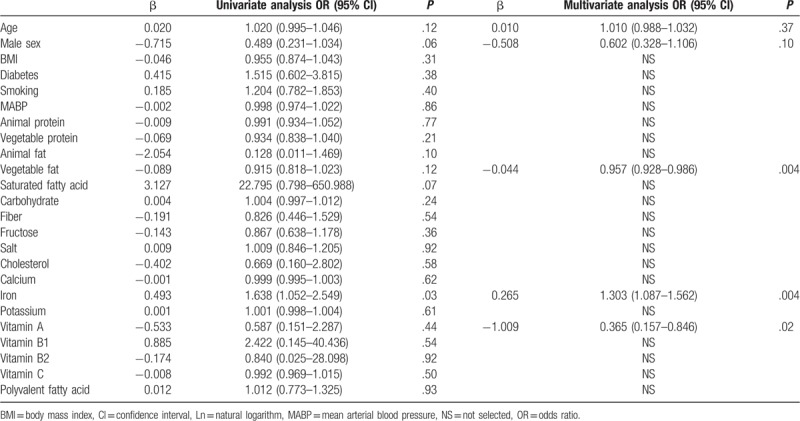
Table 2 shows descriptive data of the mean daily dietary intake of nutrients. Subjects with glaucoma were significantly lower intake of vegetable fat compared with normal subjects (P = .03). However, intakes of other nutrient were similar between both groups. Multivariate logistic regression analysis showed that the distribution of potential risk factors for glaucoma included the intake level of vegetable fat, iron, and vitamin A (Table 3). These data showed that a low intake of vegetable fat was associated with a higher risk for glaucoma (odds ratio [OR]: 0.957, P = .004). A high intake of iron also exhibited an association with a higher risk of glaucoma (OR: 1.303, P = .004). A low intake of vitamin A additionally showed a higher risk of glaucoma (OR: 0. 365, P = .019).
Table 2.
Nutrient intakes of normal subjects and glaucoma subjects.
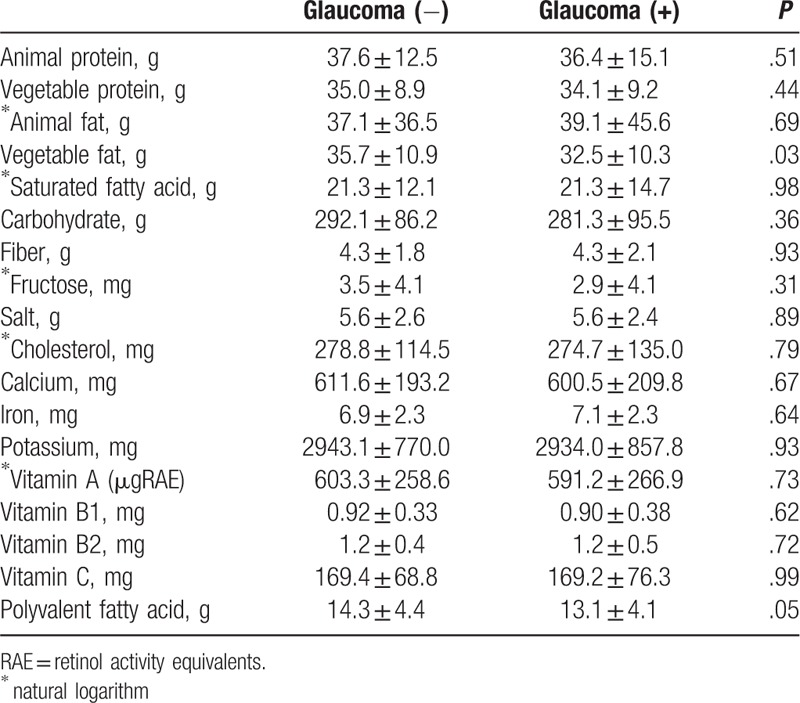
Table 3.
Univariate and multivariate logistic regression model for prediction of glaucoma.
4. Discussion
Our results demonstrated that low intakes of vitamin A and vegetable fat were significantly associated with the risk of glaucoma, whereas a high intake of iron was significantly associated with glaucoma.
Glaucoma is a slowly developing eye disease that may not always be detected during the early stages, with approximately half of all cases of open-angle glaucoma (OAG) initially going undetected.[21] Oxidative stress has been reported as an etiological factor in the pathophysiology of RGC death in glaucoma, along with involvement of elevated IOP, retinal ischemia, and nutritional status.[5,6] Neurodegeneration owing to oxidative stress may play a role in the deterioration that occurs in OAG. This deterioration has been suggested to damage the trabecular meshwork, thereby resulting in increased IOP.[5,22,23] Furthermore, it has also been shown that in glaucoma, oxidative stress occurs mainly in the mitochondria of RGCs and their axons.[4]
Bussel et al[24] published a review of dietary factors in glaucoma and reported that high intakes of calcium and iron were associated with a higher risk of developing glaucoma. In their study, Farkas et al[25] showed that calcium and iron as oxidants appeared to reduce the function of the trabecular meshwork and RGCs in glaucoma. Wang et al[26] conducted a cross-sectional study and found that high consumption of iron increased the risk of glaucoma. Our study also showed that high consumption of iron had a significant association with glaucoma.
A few common antioxidants are known to have a beneficial effect, such as vitamin A and carotenoids (present in fruits and vegetables); vitamins B, C, and E; and polyphenolic flavonoids (mostly present in green tea and coffee). Tanito et al[27] reported that a low systemic antioxidant capacity was associated with more severe visual field progression in patients with OAG. The Rotterdam Study demonstrated a protective effect of vitamin B1 in OAG; patients with OAG had lower thiamine levels than controls.[8] Although a high intake of retinol equivalents and vitamin B1 has been found to be associated with decreased risk of OAG, a high intake of magnesium (Mg) shows increased risk of OAG.[8] In our current study, we found that a low intake of vitamin A was associated with glaucoma, which is consistent with the results of previous studies.
Another presumed mechanism associated with development of disease is impaired blood flow. It has been reported that vascular dysregulation increases the risk of normotension glaucoma, central serous choroidopathy, retinal artery and vein occlusion, and anterior ischemic neuropathy without atherosclerosis.[4] Mg, which is involved in many metabolic processes in normal cells, also acts as a natural physiologic calcium channel blocker, thereby improving ocular blood flow, reducing oxidative stress, and providing neuroprotection. Furthermore, it has been reported that Mg has a potential beneficial role in the treatment of glaucoma.[28]
A few previous studies reported finding a relationship between an increased BMI and elevated IOP.[29–31] However, this is in contrast to the Barbados Eye Study, which reported that low BMI was related to POAG.[32] In our current study, we did not find any association between BMI and glaucoma.
A study by Pavljasevic et al[33] found that higher cholesterol levels were related to POAG. A feasible explanation for this is that excess intraorbital fat along with increased blood viscosity in obesity, which is usually related to a lipid abnormality, may disturb aqueous outflow capacity, thereby causing increased IOP. Another study speculated that prolonged disturbance of plasma lipids could potentially cause degenerative changes in the retinal and choroidal vessels, which might then result in premature ischemic retinoneural angiopathy. This can then create hypo perfusions that cause microcirculation disorders related to the RGCs, which could then induce permanent visual disturbance.[34] In a case-control study by Davari et al,[35] the investigators found that serum levels of cholesterol and triglyceride were significantly higher in patients with POAG than in controls. Brachium et al[36] reported that higher cholesterol intake might increase the risk of oxidative stress-related ocular diseases such as cataracts and age-related macular degeneration but this did not increase the risk for glaucoma. A meta-analysis by Hwang et al[37] demonstrated that hyperlipidemia did not increase the risk of glaucoma. A further study examining the use of statins (drugs used to lower cholesterol) in patients with glaucoma showed that statin use was associated with a significant reduction in the risk of OAG in patients with hyperlipidemia.[38] Several mechanisms have been proposed to describe these findings. Statins have been shown to increase the cellular response of endothelial nitric oxide synthase.[39] This results in vasodilation followed by an increase in the retinal and choroidal blood flow, which helps to maintain the health of the optic nerve. Another suggested mechanism is that statins affect molecular intermediaries in the aqueous outflow pathway, which includes Rho kinase and myosin II ATPase activity. As a result, this increases aqueous outflow facility via the trabecular meshwork, thereby leading to a reduction in IOP.[40] In our study, we found that a low intake of vegetable fat might be associated with glaucoma. However, at the current time we have not found any other published studies that confirm our present findings.
In 2017, Williams et al[41] reported that oral administration of vitamin B3 and/or gene therapy could protect the RGCs and axon from degeneration in an aged mouse model. The authors of that previous study used DBA/2J (D2) mice, as these animals have been shown to develop a form of chronic glaucoma with iris abnormalities, high IOP, and RGC loss.[42] The decrease in nicotinamide adenine dinucleotide (NAD+) that subsequently occurred then induced mitochondrial dysfunction and disturbed the redox metabolism. Furthermore, those authors reported that the decline in NAD+, which has a potential role in protecting mitochondrial metabolism and protecting against oxidative stress, could be corrected by giving the animals a high dose of the oral biosynthetic precursor of NAD+, nicotinamide/vitamin B3 (NAM). The findings of that previous study, showing that vitamin B3/NAM could protect against both elevated IOP and neuronal susceptibility, suggest that vitamin B3/NAM has a potential role in glaucoma treatment. However, further studies in humans will need to be undertaken to confirm these findings. In our study, however, we found no significant relationship between vitamin B and glaucoma.
Possible limitations of our current study include the limited number of participants who were diagnosed with glaucoma, and apart from fundus photography, no other glaucoma examinations were carried out. Second, measurements taken by the investigators in this study were based on color photographs and information self-reported by patients. Thus, there could have been variation in the measurements taken by different investigators and participants may not have correctly reported their actual dietary intakes. Third, because this was a cross-sectional study, it was not possible to determine a causal relationship between dietary nutrition and glaucoma. Another possible limitation is that we did not measure IOP. The participants in this study have a Japanese genetic disposition; however, they live in an American environment with corresponding dietary and exercise habits. Because the Hawaii–Los Angeles–Hiroshima Study was carried out so as to investigate the effects of a Western lifestyle on the prevalence of metabolic diseases, only fundus photographs were available with which to investigate the prevalence of glaucoma. The Japanese ethnic population is more likely than other populations to have normal-tension glaucoma.[43] Therefore, it is important to clarify the IOP of the cohort and whether this differs from the population with glaucoma in this study.
In conclusion, our current findings showed that a high intake of iron and low intakes of vitamin A and vegetable fat were associated with the risk of glaucoma in participants of Japanese descent currently living in Los Angeles. A future prospective study to measure nutrients in serum, using a larger and more general population, will need to be undertaken to determine whether there is a causal link between nutrition and glaucoma.
Acknowledgment
The authors thank Analisa Avila, ELS, of Edanz Group (www.edanzediting.com/ac) for editing a draft of this manuscript.
Author contributions
Conceptualization: Kazuyuki Hirooka, Yoshiaki Kiuchi
Data curation: Masayasu Yoneda, Haruya Ohno, Kazuhiro Kobuke
Formal analysis: Reo Kawano
Funding acquisition: Masayasu Yoneda
Investigation: Muhammad Yoserizal, Kazuyuki Hirooka, Yoshiaki Kiuchi
Methodology: Kazuyuki Hirooka, Yoshiaki Kiuchi
Project administration: Masayasu Yoneda, Yoshiaki Kiuchi
Supervision: Kazuyuki Hirooka
Validation: Kazuyuki Hirooka, Masayasu Yoneda, Yoshiaki Kiuchi
Visualization: Kazuyuki Hirooka
Writing – original draft: Muhammad Yoserizal
Writing – review & editing: Kazuyuki Hirooka, Masayasu Yoneda, Haruya Ohno, Kazuhiro Kobuke, Reo Kawano, Yoshiaki Kiuchi
Footnotes
Abbreviations: BMI = body mass index, IOP = intraocular pressure, MABP = mean arterial blood pressure, MG = magnesium, NAD+ = nicotinamide adenine dinucleotide, NAM = nicotinamide/vitamin B3, OAG = open angle glaucoma, OGTT = oral glucose tolerance test, OR = odds ratio, POAG = primary open angle glaucoma, RGC = retinal ganglion cell.
How to cite this article: Yoserizal M, Hirooka K, Yoneda M, Ohno H, Kobuke K, Kawano R, Kiuchi Y. Associations of nutrient intakes with glaucoma among Japanese Americans. Medicine. 2019;98:49(e18314).
References
- [1].Tham YC, Li X, Wong TY, et al. Global prevalence of glaucoma and projections of glaucoma burden through 2040: systemic review and meta-analysis. Ophthalmology 2014;121:2081–90. [DOI] [PubMed] [Google Scholar]
- [2].Pascolini D, Mariotti SP. Global estimates of visual impairment: 2010. BrJ Ophthalmol 2012;96:614–8. [DOI] [PubMed] [Google Scholar]
- [3].Abu-Amero K, Kondkar AA, Chalam KV. An updated review on the genetics of primary open angle glaucoma. Int J Mol Sci 2015;16:28886–911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Flammer J, Konieczka K, Flammer AJ. The primary vascular dysregulation syndrome: implications for eye diseases. EPMA J 2013;4:14–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Kumar DM, Agarwal N. Oxidative stress in glaucoma: a burden of evidence. J Glaucoma 2007;16:334–43. [DOI] [PubMed] [Google Scholar]
- [6].Weinreb RN, Aung T, Medeiros FA. The pathophysiology and treatment of glaucoma: a review. JAMA 2014;311:1901–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Jung KI, Kim YC, Park CK. Dietary niacin and open-angle glaucoma: The Korean National Health and Nutrition Examination Survey. Nutrients 2018;10:ii:E387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Ramdas WD, Wolfs RC, Kiefte-de Jong JC, et al. Nutrient intake and risk of open-angle glaucoma: the Rotterdam Study. Eur J Epidemiol 2012;27:385–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Al Owaifeer AM, Al Taisan AA. The role of diet in glaucoma: a review of the current evidence. Ophthalmol Ther 2018;7:19–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Kang JH, Pasquale LR, Willett W, et al. Antioxidant intake and primary open-angle glaucoma: a prospective study. Am J Epidemiol 2003;158:337–46. [DOI] [PubMed] [Google Scholar]
- [11].Kawate R, Yamakido M, Nishimoto Y, et al. Diabetes mellitus and its vascular complications in Japanese migrants on the Island of Hawaii. Diabetes Care 1979;2:161–70. [DOI] [PubMed] [Google Scholar]
- [12].Nakanishi S, Okubo M, Yoneda M, et al. A comparison between Japanese-Americans living in Hawaii and Los Angeles and native Japanese: the impact of lifestyle westernization on diabetes mellitus. Biomed Pharmacother 2004;58:571–7. [DOI] [PubMed] [Google Scholar]
- [13].Yoneda M, Yamane K, Jitusiki K, et al. Prevalence of metabolic syndrome compared between native Japanese and Japanese-Americans. Diabetes Res Clin Pract 2008;79:518–22. [DOI] [PubMed] [Google Scholar]
- [14].Kubota M, Yoneda M, Maeda N, et al. Westernization of lifestyle affects quantitative and qualitative changes in adiponectin. Cardiovascular Diabetol 2017;16:83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Sugihiro T, Yoneda M, Ohno H, et al. Associations of nutrient intake with obesity and diabetes mellitus in the longitudinal medical surveys of Japanese Americans. J Diabetes Investig 2019;10:1229–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].US Department of Agriculture, The Agricultural Research Service.. Nutritive Value of American Foods in Common Units, Agriculture Handbook No. 456. Washington, DC: U.S:Dept of Agriculture; 1975. [Google Scholar]
- [17].Iwase A, Suzuki Y, Araie M, et al. The prevalence of primary open-angle glaucoma in Japanese: the Tajimi Study. Ophthalmology 2004;111:1641–8. [DOI] [PubMed] [Google Scholar]
- [18].Dwyer JT, Melanson KT, Sriprachy-anunt U, Cross P, Wilson, M. Dietary Treatment of Obesity. (MDText.com, Inc, 2000). [PubMed] [Google Scholar]
- [19].American Diabetes Association. Classification and Diagnosis of Diabetes: standards of medical care in diabetes-2018. Diabetes Care 2018;41: suppl 1: S13–27. [DOI] [PubMed] [Google Scholar]
- [20].Kundu RN, Biswas S, Das M. Mean arterial pressure classification: a better tool for statistical interpretation of blood pressure related risk covariates. Cardiol Angiol 2017;6:1–7. [Google Scholar]
- [21].Hollows FC, Graham PA. Intra-ocular pressure, glaucoma, and glaucoma suspects in a defined population. Br J Ophthalmol 1966;50:570–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Cheung W, Guo L, Cordeiro MF. Neuroprotection in glaucoma: drug-based approaches. Optom Vis Sci 2008;85:406–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Izzotti A, Bagnis A, Saccà SC. The role of oxidative stress in glaucoma. Mutat Res 2006;612:105–14. [DOI] [PubMed] [Google Scholar]
- [24].Bussel II, Aref AA. Dietary factors and the risk of glaucoma: a review. Ther Adv Chronic Dis 2014;5:188–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Farkas RH, Chowers I, Hackam AS, et al. Increased expression of iron-regulating genes in monkey and human glaucoma. Invest Ophthalmol Vis Sci 2004;45:1410–7. [DOI] [PubMed] [Google Scholar]
- [26].Wang SY, Singh K, Lin SC. The association between glaucoma prevalence and supplementation with the oxidants calcium and iron. Invest Ophthalmol Vis Sci 2012;53:725–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Tanito M, Kaidzu S, Takai Y, et al. Association between systemic oxidative stress and visual field damage in open-angle glaucoma. Sci Rep 2016;6:25792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Ekici F, Korkmaz S, Karaca EE, et al. The role of magnesium in the pathogenesis and treatment of glaucoma. Inter Sch Rese Notices 2014;2014:745439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Mori K, Ando F, Nomura H, et al. Relationship between intraocular pressure and obesity in Japan. Int J Epidemiol 2000;29:661–6. [DOI] [PubMed] [Google Scholar]
- [30].Klein BE, Klein R, Linton KL. Intraocular pressure in an American community. The Beaver Dam Eye Study. Invest Ophthalmol Vis Sci 1992;33:2224–8. [PubMed] [Google Scholar]
- [31].Yoshida M, Ishikawa M, Kokaze A, et al. Association of life-style with intraocular pressure in middle-aged and older Japanese residents. Jpn J Ophthalmol 2003;47:191–8. [DOI] [PubMed] [Google Scholar]
- [32].Leske MC, Connell AM, Wu SY, et al. Risk factors for open-angle glaucoma. The Barbados Eye Study. Arch Ophthalmol 1995;113:918–24. [DOI] [PubMed] [Google Scholar]
- [33].Pavljasevic S, Asceric M. Primary open-angle glaucoma and serum lipids. Bosn J Basic Med Sci 2009;9:85–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Modrzejewska M, Grzesiak W, Zaborski D, et al. The role of lipid dysregulation and vascular risk factors in glaucomatous retrobulbar circulation. Bosn J Basic Med Sci 2015;15:50–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Davari MH, Kazemi T, Rezai A. A survey of the relationship between serum cholesterol and triglyceride to glaucoma: a case control study. J Basic Appl Sci 2014;10:39–43. [Google Scholar]
- [36].Braakhuis A, Raman R, Vaghefi E. The association between dietary intake of antioxidants and ocular disease. Diseases 2017;5: [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Hwang IC, Lee YJ, Bae JH. A meta-analysis of glaucoma risk in hyperlipidemic individuals: a critical problem in design. Invest Ophthalmol Vis Sci 2016;57:6339–40. [DOI] [PubMed] [Google Scholar]
- [38].Stein JD, Newman-Casey PA, Talwar N, et al. The relationship between statin use and open-angle glaucoma. Ophthalmology 2012;119:2074–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Nagaoka T, Takahashi A, Sato E, et al. Effect of systemic administration of simvastatin on retinal circulation. Arch Ophthalmol 2006;124:665–70. [DOI] [PubMed] [Google Scholar]
- [40].Song J, Deng P-F, Stinnett SS, et al. Effects of cholesterol-lowering statins on the aqueous humor outflow pathway. Invest Ophthalmol Vis Sci 2005;46:2424–32. [DOI] [PubMed] [Google Scholar]
- [41].Williams PA, Harder JM, Foxworth NE, et al. Vitamin B(3) modulates mitochondrial vulnerability and prevents glaucoma in aged mice. Science 2017;355:756–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].John SW, Smith RS, Savinova OV, et al. Essential iris atrophy, pigment dispersion, and glaucoma in DBA/2J mice. Invest Ophthalmol Vis Sci 1998;39:951–62. [PubMed] [Google Scholar]
- [43].Kim KE, Park KH. Update on the prevalence, etiology, diagnosis, and monitoring of normal-tension glaucoma. Asia Pac J Ophthalmol 2016;5:23–31. [DOI] [PubMed] [Google Scholar]


