Abstract
Chikungunya fever (CHIKF) is an arbovirus characterized by acute fever, myalgia and polyarthralgia. Lymphedema in the lower limbs (LL) was observed in several patients during an outbreak of CHIKF in the state of Pernambuco (Brazil) in 2016. No reports on lymphatic vessels disease due to CHIKF have been described. The aim of the study was to follow lymphatic abnormalities in the LL of 16 patients with CHIKF, using lymphoscintigraphy.
An observational, prospective study with patients in the acute phase of CHIKF (confirmed serological diagnosis) with LL edema submitted to clinical evaluation and lymphoscintigraphy at baseline and after 90 days.
Sixteen patients (81% females) participated in this study. All patients presented with lower limb lymphedema, being 15 (94%) bilateral. Of the 31 limbs affected by lymphedema, 24 (77%) presented abnormalities in lymphatic drainage by lymphoscintigraphy. The delay to visualize pelvic lymph nodes was the most frequent lymphoscintigraphic abnormality, observed in 16 (51,6%) LL. Nine (56%) patients were clinically reevaluated after 90 days, and all 18 LL remained with lymphedema. A second lymphoscintigraphy showed persistent abnormalities in 13 (72%) of the 18 LL.
CHIKF can lead to lymphedema, and lymphedema may persist or progress after 3 months of the acute phases of the disease.
Keywords: Chikungunya fever, lower limb lymphedema, lymphatic abnormalities, lymphatic vessels, lymphoscintigraphy
Author summary
-
1.
Chikungunya fever (CHIKF) is an acute disease accompanied by arthralgia, intense asthenia, myalgia, headache and skin rash. The diagnosis of CHIKF is typically clinical, since the association between acute fever and arthralgia is highly predictive in areas where the disease is endemic or epidemics occur. A definitive diagnosis can be made by detecting the virus by polymerase chain reaction (PCR) or through serological test by detecting immunoglobulin M (IgM) and immunoglobulin G (IgG).
-
2.
The most common vascular manifestations are peripheral arterial disorders and the Raynaud phenomenon but lymphedema in the lower limbs (LL) was observed in several patients during an outbreak of CHIKF in the state of Pernambuco (Northeastern Brazil) in 2016. For the first time, lymphoscintigraphic abnormalities were demonstrated in patients with CHIKF and LL edema. CHIKF can lead to lymphedema, and lymphedema may persist or progress after three months of the acute or subacute phases of the disease.
1. Introduction
Chikungunya virus is an alphavirus transmitted by the Aedes Aegypti and Aedes Albopictus infected mosquitoes widely spread across Brazil.[1–7] This virus has shown an amazing ability to spread and infect a large proportion of the population, as it has been observed in recurrent Chikungunya fever outbreaks. This arbovirus has an epidemic character with high attack rates ranging from 38% to 63%.[1] In 2016, 146,914 cases of CHIKF were confirmed in Brazil, with a higher incidence rate in the Northeast. The state of Pernambuco, located on the east coast of Brazil, was responsible for 25,692 cases of CHIKF[8] and for the highest number of deaths in 2016.[1,8]
CHIKF is typically an acute disease accompanied by arthralgia, intense asthenia, myalgia, headache and skin rash. The disease has an abrupt start after a 3-day incubation period and can evolve into 3 phases: acute - after the incubation period until the 10th day; subacute - if the joint pain persists after the acute phase, lasting up to 3 months; and chronic – the persistence of symptoms after 3 months and lasting up to 3 years.[1,2,4,5,9]
The diagnosis of CHIKF is typically clinical, since the association between acute fever and arthralgia is highly predictive in areas where the disease is endemic or epidemics have occurred,[9] such as in Pernambuco. The main laboratory finding is leukopenia with lymphopenia.[1,9] The definitive diagnosis can be made by detecting the virus by polymerase chain reaction (PCR) in the first week of illness. Serological diagnosis is made through detecting immunoglobulin M (IgM) from the 2nd to the 3rd day of illness, remaining present for 1 up to 3 months, and immunoglobulin G (IgG) starting from the 5th to the 10th day of illness, and remaining active for years.[1,2,9]
Atypical manifestations involving different systems, including the arterial vascular system, have been described in the literature involving CHIKF.[1,10,11] The most common vascular manifestations are peripheral arterial disorders and the Raynaud phenomenon.
Lymphedema in the lower limbs (LL) was observed in several patients during an outbreak of CHIKF in the state of Pernambuco (Northeastern Brazil) in 2016. As far as lymphatic manifestations are concerned, based on our knowledge there is only one study in the literature describing isolated cases of lymphedema in patients with CHIKF, however without lymphoscintigraphic confirmation.[12]
Thus, the aim of the present study was to evaluate lymphatic changes in the LL of patients with CHIKF and LL edema through lymphoscintigraphy.
2. Methods
The study population consisted of patients attended at the Ambulatory Clinic of Angiology and Vascular Surgery - Vascular Surgery Clinic of the Hospital das Clínicas of the Federal University of Pernambuco (HC - UFPE), sent for evaluation of lower limb edema with clinical epidemiological and serological diagnosis of CHIKF. Sixteen patients with positive serological diagnosis (detection of IgM antibodies for CHIKF) were included. All research stages were developed from March 2016 to November 2016, and a prospective observational study model was employed. In the same period, at the Hospital das Clínicas of the Federal University of Pernambuco (HC – UFPE), 248 cases of CHIKF were notified.[8]
The selected patients were subjected, LL doppler ultrasonography to exclude deep vein thrombosis, and 2 lymphoscintigraphies; one during the study admission period and another 90 days after.
Children, pregnant women, patients with a body mass index above 40, patients with LL ulcers of any etiology or advanced chronic venous disease of the LL (C3–C6 according to the Clinical-Etiology-Anatomy-Pathophysiology Classification of the Chronic Venous Disorders – CEAP classification), patients with renal, hepatic and/or cardiac failure, patients with a previous history of LL edema of any etiology, deep venous thrombosis, LL lymphangitis, orthopedic, vascular and/or oncologic LL surgery, patients under radiotherapy and patients with clinically evident lymphedema prior to CHIKF were all excluded from the study.
LL lymphoscintigraphy was performed at the HC – UFPE Nuclear Medicine Service following subcutaneous administration in the first inter-toe space of each foot of about 37 MBq (1 mCi) with a volume of 0.1 mL of dextran-70 solution marked with 99mTc. The 99mTc radionuclide was obtained from a molybdenum-technetium generator, manufactured by IPEN-CNEN-São Paulo.
Each patient walked for 10 minutes and then radiopharmaceutical bolus injection was performed with a 13x3 29G 1/2" needle after previous aspiration with the patient in the decubitus position. On-site massage was performed and patients performed flexion and ankle extension during the examination.
Dynamic images of the anterior pelvic region were acquired for 30 minutes (1 frame/minute) immediately after radiopharmaceutical injection, and whole-body images in the anterior and posterior projections (capture rate 7.5 cm/minute) about 1 hour after. Whole body images were also acquired 4 hours after the injection in case radiopharmaceutical ascension was not observed up to the height of the thoracic duct in the 1-hour images. The images were acquired by an all-purpose STARCAM 3200 gamma camera (General Electric, CA, USA) of one detector, with a collimator for low energy, 140 kiloelectronvolt (keV) photopic and 20% window. The following lymphoscintigraphic changes were analyzed for interpreting the images: asymmetry and early or delayed in radiotracer arrival in topography of inguinal and pelvic lymph nodes in the 30-minute dynamic images, poor visualization of the lymphatic vessels (medial superficial trunk of the lower limb), dermal reflux of the radiopharmaceutical, presence of collateral circulation, dilatation and tortuosity of lymphatic vessels and visualization of deep lymph nodes.
The obtained data was organized into spreadsheets (Microsoft Office Excel 2003). Descriptive statistics were used for analyzing the social, clinical and epidemiological profile and the results obtained from the lymphoscintigraphies, and the data were presented as n (absolute number) and % (relative number).
All patients were informed as to the nature of the research and the benefits of the diagnostic investigation, and those who agreed to participate in the study signed the Free and Informed Consent Terms. The study project was approved by the Ethics and Research Committee on Human Beings of the Federal University of Pernambuco (Opinion number: 1.710.092).
3. Results
In the periodo of the study a 248 cases of CHIKF was notified at the Hospital das Clínicas of the Federal University of Pernambuco (HC – UFPE), from them 31 were refer to the clinic as suspect Lymphedema and finally 16 was diagnosis by Lymphoscintigraphy of LL. (prevalence of confined LL: 6.4%).
Those 16 patients with soft and pitting LL edema and positive IgM serology for CHIKF in the acute or subacute phases of CHIKF selected, all from Pernambuco, have an average age of 59 years, and 13 (81%) patients were female (Fig. 1).
Figure 1.
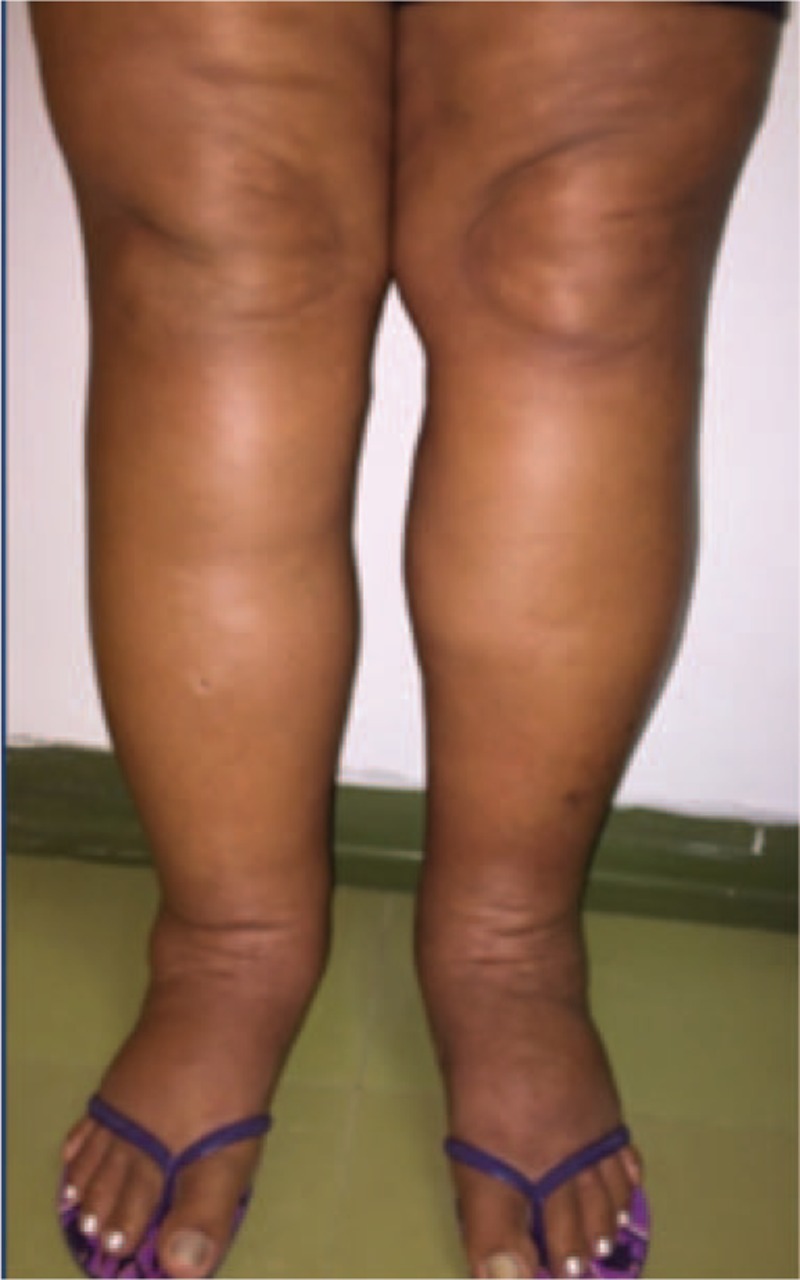
Patient from this study with LL lymphedema (cylindrical shape) and an edema in the dorsum of the foot included in the study.
Concerning LL affected by lymphedema, 15 (93.7%) patients presented bilateral edema and 1 (6.2%) unilateral edema.
Among the 31 LL of the 16 patients evaluated in the first lymphoscintigraphy, the presence of lymphopathy was observed in 24 (77.4%) LL. The delay in pelvic lymph nodes visualization was the most frequent alteration present in 16 (51.6%) LL, accelerated visualization of the pelvic lymph nodes in 5 (16%) LL, presence of dermal reflux in 2 (6.4%) LL, visualization of deep lymph nodes (popliteal, femoral and/or tibial) in 1 (3.2%) LL (Table 1, Figs. 2 and 3).
Table 1.
Lymphoscintigraphic results of the initial lymphoscintigraphy (Lympho. 1).
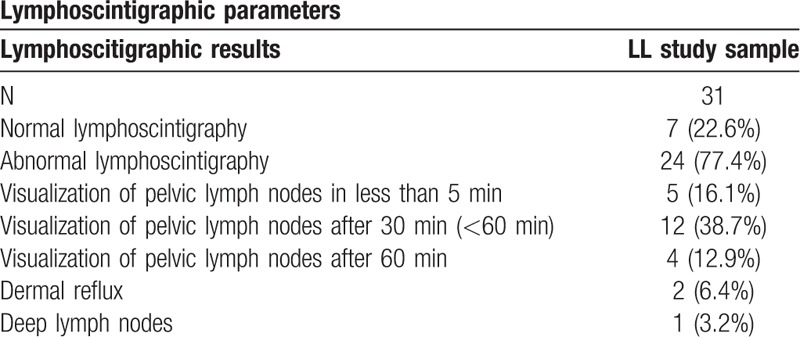
Figure 2.
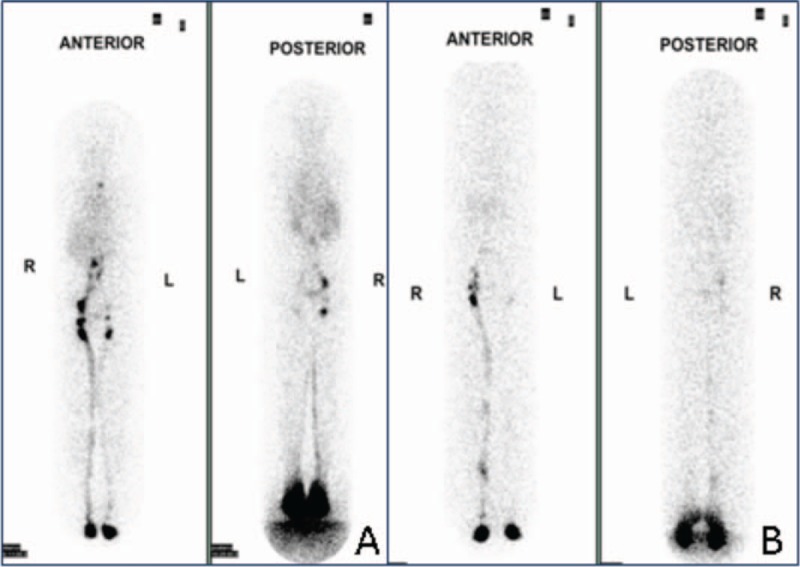
A: Whole body images from lymphoscintigraphy demonstrating poorly defined lymphatic vessels on the left and pelvic lymph nodes visualized just on 1-hour imaging post- 99m Tc- Dextran-70 injection. B: Whole body images from lymphoscintigraphy demonstrating poorly defined lymphatic vessels and poorly visualized pelvic lymph nodes on the left.
Figure 3.
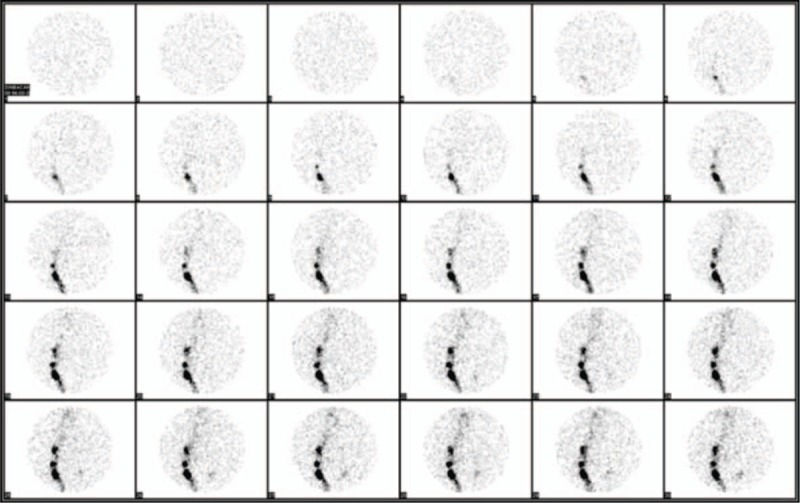
Lymphoscintigraphy images of the anterior pelvis acquired at 1-minute intervals during 30 minutes with absence of pelvic lymph node visualization on the left after 30 minutes of radiotracer injection.
After the first evaluation, compression stockings (20–30 mmHg) were prescribed for all patients and the patients were advised to raise the legs when possible.
Out of the 16 study patients, 9 (56.2%) returned for a clinical reevaluation 90 days later and all of these patients continued to present bilateral LL edema.
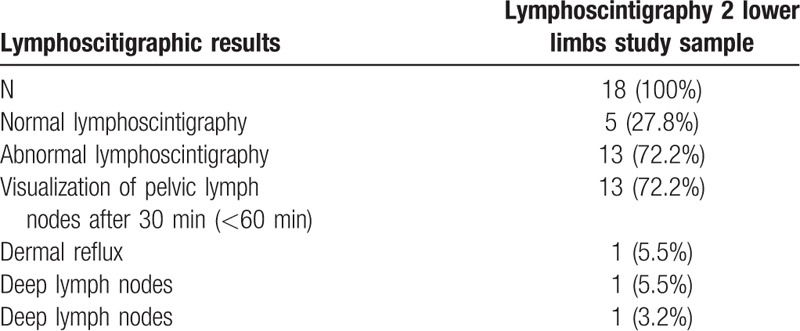
All those 9 patients underwent a second lymphoscintigraphy. The delay in the visualization time of the pelvic lymph nodes was the most frequent abnormality, present in 13 (72.2%) LL, followed by the presence of dermal reflux in one (5.5%) LL and visualization of deep lymph nodes (popliteal, femoral and/or tibial) in one (5.5%) LL. Among the 18 LL underwent a second lymphoscintigraphy, 13(72,2%) presented lymphoscintigraphic abnormalities (Fig. 4 and Table 2).
Figure 4.
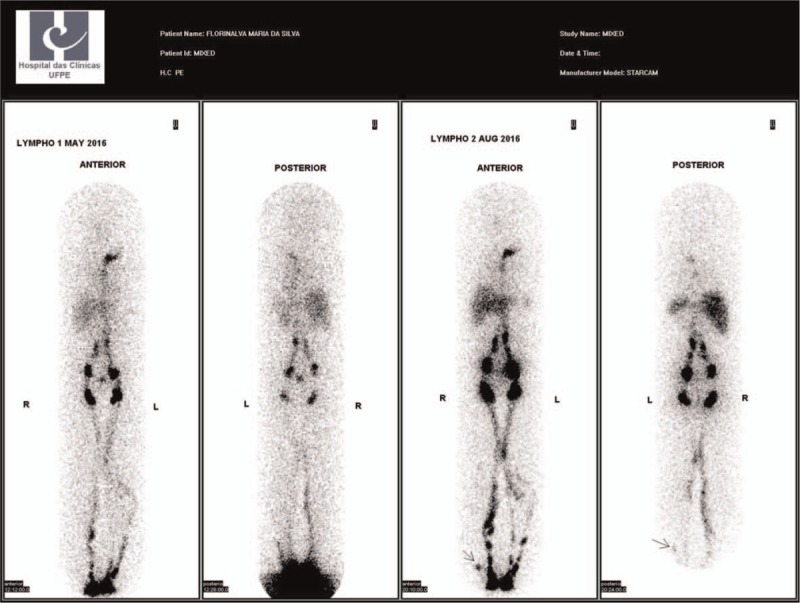
– LL lymphoscintigraphy showing increase of the collateral lymphatic vessels and increase in the number of deep lymph nodes in the second exam.
Table 2.
Lymphoscintigraphic results of the second lymphoscintigraphy 90 days after the first exam (Lymphoscintigraphy 2).
4. Discussion
This was the first study to investigate lymphatic damage in patients with CHIKF and LL edema. Our results showed that CHIKF can cause acute lymphedema, and that lymphatic damage can progress or persist for more than 3 months causing chronic lymphedema.
The pathophysiology of lymphedema is complex[11–14] and the mechanism of lymphatic edema formation in CHIKF remains unknown. Recent studies in animal and human models suggest that inflammation is the key factor, if not the most important in the progression of lymphedema, particularly regarding interstitial changes and lymph transport.[12,15] Inflammatory joint involvement characterized by periarticular edema, tenosynovitis and arthritis is typical of CHIKF, and characterizes the main manifestation in the acute and chronic phases,[1,9] and may be associated with the development of lymphedema in these patients.
Another possibility is that CHIKF induces a mixed type II, II-III or III cryoglobulinemia. In 2009, Oliver et al evaluated the presence and persistence of cryoglobulinemia in 51 patients with CHIKF. About 20% of the patients presented vascular manifestations, and 94% of these presented cryoglobulinemia.[16] The presence of cryoglobulins may be related to vascular manifestations, but this statement still lacks studies for confirmation.
The innate and adaptive immune response to acute chikungunya virus infection has received considerable attention. Nevertheless, the pathophysiology of the manifestations of various CHIKF afflictions remains poorly characterized. Research should be performed to understand the pathophysiology of the atypical and chronic manifestations of this disease,[10] particularly of the manifestations in the lymphatic vessels. This problem will probably require new studies in order to discover biomarkers related to the severity and chronicity of the disease and genomic analysis involving these patients.[9]
This study considered normal lymphoscintigraphic findings: gradual and symmetrical rise of the radiotracer in the anterior and medial LL regions through a maximum of 5 lymphatic vessels in the calf, and up to 2 lymphatic vessels in the thigh; bilateral visualization of 2 and up to 10 pelvic lymph nodes between 10 and 15 minutes of injection; and absence of radiotracer accumulation in the subcutaneous cellular tissue in the late images of 1 and/or 4 hours. Similar criteria have been adopted by other authors.[17–21]
The delay in radiotracer ascent was the main lymphoscintigraphic alteration found in majority of the assessed LL. This lymphocytic pathological pattern is described in patients with lymphedema secondary to infectious lymphangitis of the LL,[20,22] and was the main alteration found by Soo et al in 2007 when investigating the presence of lymphopathy in patients with infectious lymphangitis.[22]
About 60% of the patients in the present study returned three months later for reassessment, and the persistence of lymphedema was observed in the majority of them. The delay in visualization time of the pelvic lymph nodes was also the most frequent alteration in the second lymphoscintigraphy. Moreover, some patients reassessed presented worsening in the lymphoscintigraphic pattern. These findings seem to indicate progression of the injury in the lymphatic vessels, thus demonstrating the possibility of chronic lymphatic vascular manifestations caused by CHIKF for the first time.
Unlike the present study, most studies in the literature describe lymphoscintigraphic changes in the LL of patients with chronic and/or secondary lymphedema. Advanced lymphedema phases are more likely to exhibit prominent lymphatic vessels or lymphangiectasia, the presence of collaterals, more pronounced delay in radiotracer transport, and dermal reflux in delayed images.[23] Otherwise, the present study evaluated lymphatic abnormalities in patients with the duration of edema for less than 6 months. Studies on acute lymphedema are scarce, and there is insufficient data in the literature describing expected lymphoscintigraphic changes.
Another alteration evidenced in this study was the accelerated radiotracer ascension time, with visualization of the pelvic lymph nodes varying from 1 to 9 minutes after the injection of 99mTc-Dextran 70 in about 16% of the assessed LL. Lymph flow may be normal, increased or decreased. Increased flow is characterized by pelvic lymph node visualization up to 10 minutes after radiopharmaceutical injection, and has already been associated with the presence of venous insufficiency and lymphedema.[24,25] It is possible that this accelerated pelvic lymph node visualization time may be related to lymphatic hyperflow as an attempt to compensate or increase lymphangiogenesis, and pre-existing lymphatic vessel expansion (lymphatic hyperplasia) in acute inflammatory processes.[26] More studies involving the Chikungunya virus are necessary to confirm its action over the lymphatic vessels.
5. Study limitations
Although it is considered the gold standard, lymphoscintigraphy lacks a single protocol and all the technical aspects of the exam are variable.[27–30] For this study, we adopted the protocol developed by our institution, which has already been used in the elaboration of other studies.[31] The lack of current knowledge on the incidence of lymphoscintigraphic abnormalities in asymptomatic individuals and the absence of a control group of patients with CHIKF and no LL edema (denied for ethical reasons) also limits our findings. However, all patients had a clinical diagnosis of lymphedema and a second lymphoscintigraphy was performed after 90 days, thus proving the persistence or progression of lymphoscintigraphic changes in more than half of them, and serving as a control for the first examination.
6. Conclusion
This study shows the lymphoscintigraphic abnormalities in patients with CHIKF are recurrent. CHIKF can lead to lymphedema, and lymphedema may persist or progress after 3 months of the acute or subacute phases of the disease.
Author contributions
Conceptualization: Esdras Marques Lins.
Formal analysis: Flavia Cristina Morone Pinto.
Investigation: Catarina Coelho Almeida, Simone Cristina Soares Brandão.
Methodology: Catarina Coelho Almeida, Simone Cristina Soares Brandão, Flavia Cristina Morone Pinto.
Project administration: José Lamartine de Andrade Aguiar, José Luiz de Lima Filho.
Resources: José Lamartine de Andrade Aguiar.
Supervision: Esdras Marques Lins.
Validation: Flavia Cristina Morone Pinto, Fernanda Appolonio Rocha.
Visualization: José Luiz de Lima Filho.
Writing – original draft: Catarina Coelho Almeida.
Writing – review & editing: Esdras Marques Lins, Simone Cristina Soares Brandão, Fernanda Appolonio Rocha.
Esdras Marques Lins orcid: 0000-0001-6603-6944.
ESDRAS MARQUES LINS orcid: 0000-0001-6603-6944.
Footnotes
Abbreviations: Ceap Classification = clinical-etiology-anatomy- pathophysiology classification of the chronic venous disorders, Chikf = Chikungunya fever, IgG = immunoglobulin G, IgM = immunoglobulin M, LL = lower limbs, PCR = polymerase chain reaction.
How to cite this article: Almeida CC, Lins EM, Soares Brandão SC, Pinto FCM, de Andrade Aguiar JL, de Lima Filho JL, Rocha FA. Secondary lower limbs lymphedema in patients with Chikungunya fever. Medicine. 2019;98:49(e18274).
The authors report no conflicts of interest.
References
- [1]. Brasil. Ministério da Saúde. Secretaria de Vigilância em Saúde. Secretaria de Atenção Básica Chikungunya: Manejo Clínico /Ministério da Saúde, Secretaria de Vigilância em Saúde, Secretaria de Atenção Básica. – Brasília: Ministério da Saúde, 2017. [Google Scholar]
- [2].Mohan A, Kiran DH, Manohar IC, et al. Epidemiology, clinical manifestations, and diagnosis of chikungunya fever: lessons learned from the re-emerging epidemic. Indian J Dermatol 2010;55:54–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Borgherini G, Poubeau P, Staikowsky F, et al. Outbreak of chikungunya on reunion island: early clinical and laboratory features in 157 adult patients. Clin Infect Dis 2007;44:1401–7. [DOI] [PubMed] [Google Scholar]
- [4]. Brasil. Ministério da Saúde. Secretaria de Vigilância em Saúde. Departamento de Vigilância das Doenças Transmissíveis. Plano de Contingência Nacional para a Febre Chikungunya /Ministério da Saúde, Secretaria de Vigilância em Saúde, Departamento de Vigilância das Doenças Transmissíveis. – Brasília: Ministério da Saúde, 2014. [Google Scholar]
- [5].Taubitz W, Cramer JP, Kapaun A, et al. Chikungunya fever in travelers: clinical presentation and course. Clin Infect Dis 2007;45:e1–4. [DOI] [PubMed] [Google Scholar]
- [6].Furuya-Kanamori L, Liang S, Milinovich G, et al. Co-distribution and co-infection of chikungunya and Dengue viruses. BMC Infect Dis 2016;16:84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Rosso F, Pacheco R, Rodríguez S, et al. Co-infection by Chikungunya virus (CHIK-V) and dengue virus (DEN-V) during a recent outbreak in Cali, Colombia: Report of a fatal case. Rev Chilena Infectol 2016;33:464–7. doi: 10.4067/S0716-10182016000400013. [DOI] [PubMed] [Google Scholar]
- [8]. Secretaria de Saúde do Estado de Pernambuco. Secretaria Executiva de Vigilância em Saúde. Diretoria Geral de Controle de Doenças e Agravos. Gerência de Vigilância às arboviroses. Informe Epidemiológico No 47/SE 49. 2016. [Google Scholar]
- [9].Weaver SC, Lecuit M. Chikungunya virus and the global spread of a mosquito – borne disease. N Eng J Med 2015;372:1231–9. [DOI] [PubMed] [Google Scholar]
- [10].Staples JE, Breiman RF, Powers AM. Chikungunya fever: an epidemiological review of a re – emerging infectious disease. Clin Infect Dis 2009;49:942–8. [DOI] [PubMed] [Google Scholar]
- [11].Torres JR, Leopoldo Códova G, Castro JS, et al. Chikungunya fever: atypical and lethal cases in the western hemisphere a Venezuelan experience. IDCases 2014;2:6–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Inamadar AC, Palit A, Sampagavi VV, et al. Cutaneous manifestations of chikungunya fever: observations made during a recent outbreak in South India. Int J Dermatol 2008;47:154–9. [DOI] [PubMed] [Google Scholar]
- [13].Simon F, Parola P, Grandadam M, et al. Chikungunya infection an emerging rheumatism among travelers returned from Indian Ocean island. Report of 47 cases. Medicine (Baltimore) 2007;86:123–37. [DOI] [PubMed] [Google Scholar]
- [14].Dabrowski J, Merkert R, Kuśmierek J. Optimized lymphoscintigraphy and diagnostics of lymphatic o edema of the lower extremities. Nucl Med Rev 2008;11:26–9. [PubMed] [Google Scholar]
- [15].Dixon B, Weiler M. Bridging the divide between pathogenesis and detection in lymphedema. Semin Cell Dev Biol 2015;38:75–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Oliver M, Grandadam M, Marimoutou C, et al. Persisting mixed cryoglobulinemia in chikungunya infection. PLoS Negl Trop Dis 2009;3:e374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Godoy JRP, Silva VZM, Souza HA. Lymphedema: A Literature Review. Univ Ciênc Saúde 2004;2:267–80. [Google Scholar]
- [18].Moshiri M, Katz D, Marvin B, et al. Using lymphoscintigraphy to evaluate suspected lymphedema of the extremities. Am J Roentgenol 2002;178:405–12. [DOI] [PubMed] [Google Scholar]
- [19].Lam MC, Luk WH, Tse KH. Lymphoscintigraphy in the evaluation of lower extremity lymphedema: local experience. Hong Kong Med J 2014;20:121–5. [DOI] [PubMed] [Google Scholar]
- [20].Gloviczki P, Calcagno D, Schi Noninvasive evaluation of the swollen extremity: experiences with 190 lymphoscintigraphic examinations. J Vasc Surg 1989;9:683–90. [DOI] [PubMed] [Google Scholar]
- [21].Dalia RM, Martins GRP, Barbosa R, et al. Qualitative and quantitative lymphoscintigraphy in the evaluation of lower limbs lymphedema. Braz Arch Biol Tech 2005;48:159–62. [Google Scholar]
- [22].Soo JK, Bicanic TA, Heenan S, et al. Lymphatic abnormalities demonstrated by lymphoscintigraphy after lower limb cellulitis. Br J Dermatol 2008;158:1350–3. [DOI] [PubMed] [Google Scholar]
- [23].Barral CM, Stehling AP, Silva ACM, et al. Lymphoscintigraphy of the lower limbs: a retrospective study of 154 cases from March 2009 to June 2010. Rev Med Minas Gerais 2013;23:185–95. [Google Scholar]
- [24].Pecking P, Alberíni JL, Wartski M, et al. Relationship between lymphoscintigraphy and clinical findings in lower limb lymphedema (LO): toward a comprehensive staging. Lymphology 2008;41:1–0. [PubMed] [Google Scholar]
- [25].Collins PS, Villavicencio JL, Abreu SH, et al. Abnormalities of lymphatic drainage in lower extremities: a lymphoscintigraphic study. J Vasc Surg 1989;9:145–52. [DOI] [PubMed] [Google Scholar]
- [26].Adamczyk LA, Gordon K, Kholová I, et al. Lymph vessels: the forgotten second circulation in health disease. Virchows Arch 2016;469:3–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Varrichi G, Granata F, Loffredo S, et al. Angiogenesis and lymphangiogenesis in inflammatory skin disorders. J Am Acad Dermatol 2015;73:144–53. [DOI] [PubMed] [Google Scholar]
- [28].Yuan Z, Chen L, Luo Q, et al. The role of radionuclide lymphoscintigraphy in extremity lymphedema. Ann Nucl Med 2006;20:341–4. [DOI] [PubMed] [Google Scholar]
- [29].Cambria RA, Gloviczki P, Naessens JM, et al. Noninvasive evaluation of the lymphatic system with lymphoscintigraphy: a prospective, semiquantitative analysis in 386 extremities. J Vasc Surg 1993;18:733–82. [DOI] [PubMed] [Google Scholar]
- [30].Sadeghi R, Kazemzadeh G, Keshtgar M. Diagnostic application of lymphoscintigraphy in the management of lymphedema. Hell J Nucl Med 2010;13:6–10. [PubMed] [Google Scholar]
- [31].Marques SRB, Lins EM, Marchetti F, et al. Lymphoscintigraphic visualization of the thoracic duct confluence. J Vasc Bras 2005;4:349–52. [Google Scholar]


