Abstract
INTRODUCTION:
Gastric emptying scintigraphy (GES) or wireless motility capsules (WMCs) can evaluate upper gastrointestinal symptoms in suspected gastroparesis; WMC tests can also investigate lower gut symptoms. We aimed to determine whether these tests impact treatment plans and needs for additional diagnostic evaluation.
METHODS:
In a prospective, multicenter study, 150 patients with gastroparesis symptoms simultaneously underwent GES and WMC testing. Based on these results, investigators devised management plans to recommend changes in medications, diet, and surgical therapies and order additional diagnostic tests.
RESULTS:
Treatment changes were recommended more often based on the WMC vs GES results (68% vs 48%) (P < 0.0001). Ordering of additional test(s) was eliminated more often with WMC vs GES (71% vs 31%) (P < 0.0001). Prokinetics (P = 0.0007) and laxatives (P < 0.0001) were recommended more often based on the WMC vs GES results. Recommendations for prokinetics and gastroparesis diets were higher and neuromodulators lower in subjects with delayed emptying on both tests (all P ≤ 0.0006). Laxatives and additional motility tests were ordered more frequently for delayed compared with normal WMC colonic transit (P ≤ 0.02). Multiple motility tests were ordered more often on the basis of GES vs WMC findings (P ≤ 0.004). Antidumping diets and transit slowing medications were more commonly recommended for rapid WMC gastric emptying (P ≤ 0.03).
DISCUSSION:
WMC transit results promote medication changes and eliminate additional diagnostic testing more often than GES because of greater detection of delayed gastric emptying and profiling the entire gastrointestinal tract in patients with gastroparesis symptoms.
TRANSLATIONAL IMPACT:
Gastric scintigraphy and WMCs have differential impact on management decisions in suspected gastroparesis.
INTRODUCTION
Gastric emptying testing is performed to diagnose gastroparesis (1). Gastric emptying scintigraphy (GES) measures retention of 99mTc-labeled meals (2,3). Wireless motility capsules (WMCs) also measure gastric emptying by detecting pH increases during capsule passage into the duodenum (4,5). Breath tests also quantify gastric emptying by measuring 13CO2 production after 13C-labeled meals (6). Two prospective concurrent GES and WMC studies and other investigations performing each test separately determined these tests identify similar but not identical subgroups with emptying delays (4,7,8).
Patients with suspected gastroparesis may report symptoms referable to other transit impairments in the stomach and extragastric regions (9–12). Rapid gastric emptying can present with symptoms indistinguishable from gastroparesis (13–15). Furthermore, delays in small intestinal and colon transit with WMCs have been described in 15.5% and 33.5% of suspected gastroparesis, respectively (13). Scintigraphy is usually limited to the stomach because of multiple visits required to measure small bowel and colon transit (16).
Whether transit findings influence management of suspected gastroparesis is uncertain. In an older series, GES had little impact on clinical decisions, although most patients had postvagotomy complications (17). WMC findings promoted medication and nutritional changes in 60% and 14%, and additional tests were eliminated in one retrospective series (18). New prokinetic, antiemetic, antidepressant, and laxative therapies were described in 50% in another report (18,19). In the only prospective study published to date, GES findings influenced management decisions in 60% of patients including recommendations to change the diet in those with delayed gastric emptying and to eliminate selected medications in individuals with normal gastric emptying (20). These investigations did not determine whether GES and WMCs have differential impact on decisions in suspected gastroparesis.
We performed a prospective, multicenter concurrent GES and WMC study in patients with suspected gastroparesis. Site investigators completed management plans, recommending treatment changes and additional testing based on transit findings. We aimed to characterize whether: (i) GES and WMCs had differential impact on clinical decisions, (ii) specific treatments and testing recommendations were influenced by gastric emptying, and (iii) additional management recommendations were made based on extragastric delays or rapid transit.
METHODS
Subject population
One hundred sixty-seven patients (18–80 years) with ≥2 symptoms suggesting gastroparesis (nausea/vomiting, fullness/early satiety, bloating/distension, and upper abdominal discomfort/pain) for ≥12 weeks were referred for gastroparesis care to 10 centers from 2013 to 2016 (ClinicalTrials.gov NCT02022826). Esophagogastroduodenoscopy or radiography excluded organic disorders. Exclusion criteria included dysphagia, previous gastrointestinal surgery (except appendectomy, cholecystectomy, and hysterectomy), abdominopelvic surgery within 3 months, inflammatory bowel disease, chronic nonsteroidal anti-inflammatory drug use, diverticulitis, intestinal strictures, HbA1c > 10%, implanted cardiac devices, and body mass index > 40 kg/m2. Institutional review board approval was obtained at each center. Subjects provided written informed consent.
GES and WMC methodology
Subjects underwent concurrent GES and WMCs; transit was determined for subjects completing management plans (see Supplemental Methods, Supplementary Digital Content 1, http://links.lww.com/CTG/A100) (5,21–23). Transit abnormalities on GES and WMC testing were determined using previously reported values. Delayed gastric emptying on GES testing was defined as >10% meal retention at 4 hours (3). Rapid gastric emptying on GES testing was defined as <38% meal retention at 1 hour (2,3). Delayed gastric emptying on WMC testing was defined as >5-hour gastric emptying time (GET) (21). Rapid gastric emptying on WMC testing was defined as GET <1:45 hours (21). Delayed transit times in the small bowel (SBTT) and colon (CTT) on WMC testing were defined as >6 and >58:45 hours, respectively (21). Rapid SBTT on WMC testing was defined as <2:15 hours (21).
Management decision protocol
Analyses were conducted according to a priori planned study endpoints (MA-501 Clinical Trial Protocol, 3/15/2015, pages 25–27, see Supplementary Digital Content 2, http://links.lww.com/CTG/A101). Five to 28 days after testing, site investigators completed 3 management plans on standardized forms describing treatment recommendations, including changes to medications and diet (gastroparesis diets, liquid or other diets, and enteral or parenteral nutrition) and referrals for surgery (feeding tube and gastric stimulator or resection). Management forms also documented recommendations for additional diagnostic testing. Investigators entered names of medications and additional tests on management forms. The first plan was based on one test (GES or WMCs) in alternating order with blinding to the other test; the second plan was based on the other test but unblinded to the first on separate days; the third plan was based on combining GES and WMC results. Management forms were reviewed separately by 2 investigators (W.L.H. and A.A.L.), confirming their accuracy. These investigators initially disagreed on management decisions for 6/150 subjects (4%). A final arbiter (B.K.) uninvolved in initial reviews resolved these differences.
Data comparisons
Primary endpoints
Coprimary endpoints were defined as changes in treatment and diagnostic testing based on transit tests. Additional forms were completed that recorded whether medication (prokinetics, antiemetics, and neuromodulators) or diet changes or surgical referrals were recommended. Medication changes were defined as starting a new drug category. Starting a prokinetic de novo or switching from a prokinetic to antiemetic was considered a change, whereas switching from one prokinetic to another (e.g., metoclopramide to domperidone) was not a change, unless the original and new drugs targeted different gut regions. Adding a neuromodulator with potential mechanistic benefits in patients with gastroparesis symptoms (e.g., tricyclic agent, mirtazapine, olanzapine, or neuropathic pain modulator for nausea and/or abdominal pain; buspirone to enhance fundic accommodation) who already were prescribed neuromodulators in different classes for other indications (e.g., serotonin or serotonin-norepinephrine reuptake inhibitors for depression; benzodiazepines for anxiety) was considered a medication change. A separate item on the form asked whether transit findings prompted investigators to recommend eliminating additional tests. Investigators determined whether management changes were based on GES, WMCs, or both tests together.
Specific decisions
Specific changes in medication categories, diets, referrals for surgery, and additional testing were determined from the lead author (W.L.H.) review of site management forms. Greater impact of one test (GES or WMCs) vs the other on management decisions was defined when that test led to more treatment or fewer additional test recommendations. Specific medication categories included prokinetics, antiemetics, neuromodulators, laxatives, and agents to slow transit (retardants) (see Supplemental Methods, Supplementary Digital Content 1, http://links.lww.com/CTG/A100). Gastroparesis (low fat, fiber, or residue and/or liquid and/or frequent, small meals) and antidumping (separate liquids from solids and/or avoid simple sugars) diets were recorded. Additional testing categories included endoscopy/imaging, motility, and other tests (see Supplemental Methods, Supplementary Digital Content 1, http://links.lww.com/CTG/A100). Motility test ordering was further stratified into recommending (i) any motility tests and (ii) multiple motility tests (≥2 tests for any subject). Additional testing was recommended by site investigators based on the resources available at each study center. As is commonly observed in clinical practice, methods of additional ordered tests likely exhibited differences between sites. However, these testing methods were not queried as part of this study. We excluded recommendations for additional GES made on the basis of WMC findings or WMCs made on the basis of GES findings.
Relating decisions to transit
Management recommendations were related to transit (see Supplemental Methods, Supplementary Digital Content 1, http://links.lww.com/CTG/A100). Site investigators made decisions based on individual practice patterns. The first analyses compared new treatment and testing recommendations for delayed vs normal GES or WMC gastric emptying. Second, comparisons related management to delayed vs normal WMC extragastric and generalized transit. Third, comparisons examined decisions for rapid vs normal GES or WMC gastric emptying. Additional comparisons ascertained whether specific medications were preferentially advocated for particular transit profiles, including comparing specific prokinetics and neuromodulators for delayed vs normal gastric emptying and specific laxatives with delayed vs normal CTT.
Statistical analysis
Descriptive statistics and confidence intervals associated with binary variables were computed (24). Comparisons of treatment changes and additional test ordering between tests were performed using an exact calculation McNemar test for binary endpoints. Fisher exact testing analyzed binary endpoints on independent subgroup comparisons of GES or WMCs. Assessing subgroup differences on GES and WMCs with overlapping samples used permutation testing based on portion differences as appropriate, with P values obtained from permutation test distributions based on 10,000 Monte Carlo simulations. Analyses used SAS version 9.4 software (Cary, NC).
RESULTS
Clinical features
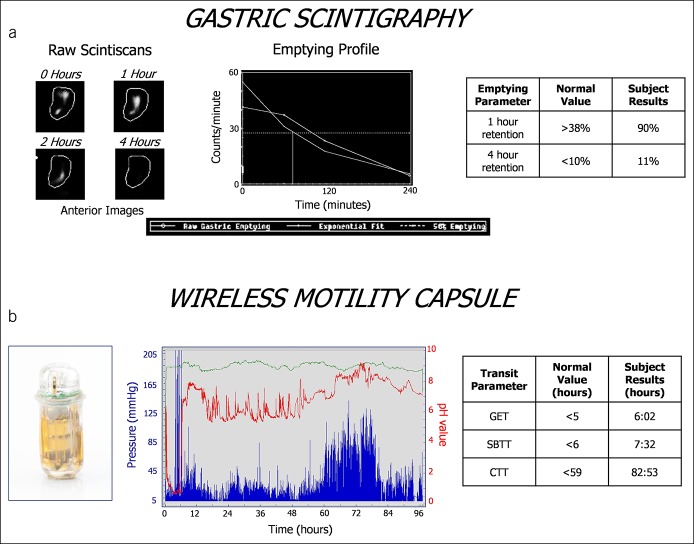
Management plans were completed for 150/167 subjects. Most subjects reported diverse symptoms of gastroparesis; many also reported lower abdominal pain and bowel disturbances (see Table 1A, Supplementary Digital Content 3, http://links.lww.com/CTG/A102). Figure 1A shows scintiscans displaying increased 4-hour retention (delayed gastric emptying). Figure 1B shows that subject's WMC tracing displaying prolonged GET, SBTT, and CTT (generalized delays). Subject subsets exhibited transit delays in the stomach, small bowel, and/or colon, some of which were isolated to single regions, whereas others were generalized to ≥2 regions (see Table 1B, Supplementary Digital Content 3, http://links.lww.com/CTG/A102). Smaller numbers showed rapid gastric or small bowel transit. Gastric emptying delays were more common with WMC than GES testing (P < 0.001), whereas rapid gastric emptying was detected more often by GES than WMCs (P < 0.001).
Figure 1.
GES and WMC findings are shown for a subject with suspected gastroparesis. Scintigraphy images and emptying profiles of the radiolabeled meal are shown in (a). This individual exhibited mildly delayed gastric emptying at 4 hours. The WMC and tracing with the pH tracing in red, pressure tracing in blue, and temperature tracing in green are shown in (b). This subject exhibited generalized transit delays in GET, SBTT, and CTT. CTT, colon transit time; GES, gastric emptying scintigraphy; GET, gastric emptying time; SBTT, small bowel transit time; WMC, wireless motility capsule.
Primary endpoints
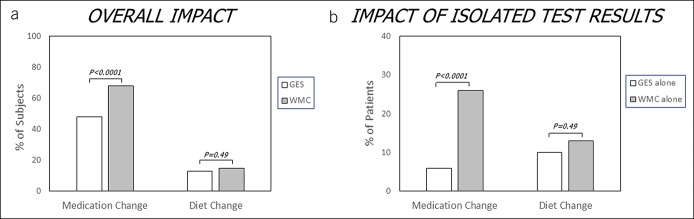
Medication and diet changes were recommended for 74% and 25% of subjects based on combined GES and WMC results. Medication changes were more often recommended based on WMCs vs GES (68% vs 48%, P < 0.0001) (Figure 2A) and more often when only WMC was abnormal (and GES was normal) than when only GES was abnormal (and WMC was normal) (26% vs 6%, P < 0.0001) (Figure 2B), showing greater impact of WMCs than GES on treatment decisions.
Figure 2.
Differential effects of GES and WMC findings on treatment recommendations in suspected gastroparesis are shown. WMC testing led to greater changes in medication therapies vs GES (a). Of the 74% of subjects with recommended medication changes, more were informed by WMC results alone compared with GES results alone (b). More than 40% of medication changes were recommended based on both abnormal GES and WMC findings. There were no differences in diet changes made in response to WMC vs GES testing. GES, gastric emptying scintigraphy; WMC, wireless motility capsule.
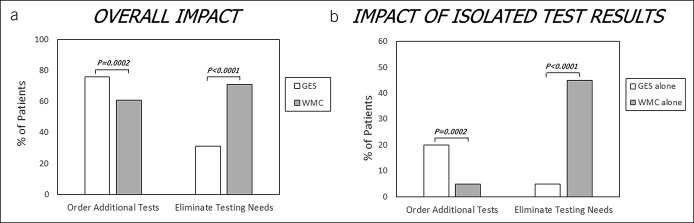
Additional tests were recommended for 81% of subjects based on combined GES and WMC results. Fewer tests were ordered based on WMCs (P = 0.0002), and more were eliminated based on WMCs (P < 0.0001) vs GES (Figure 3A). Fewer additional tests were ordered (P = 0.0002) and more eliminated (P < 0.0001) when only WMC was abnormal (and GES was normal) than when only GES was abnormal (and WMC was normal) (Figure 3B), showing greater impact of WMCs than GES on additional test recommendations.
Figure 3.
Differential effects of GES and WMC findings on recommendations for additional diagnostic testing in suspected gastroparesis are shown. WMC testing promoted less additional test ordering and higher rates of eliminating additional testing (a). Of subjects who were recommended to undergo additional testing, fewer were referred based on WMC alone vs GES alone (b). Of those with elimination of additional testing, more tests were eliminated by WMC alone vs GES alone. More than 50% of additional diagnostic test ordering was recommended based on both abnormal GES and WMC findings. GES, gastric emptying scintigraphy; WMC, wireless motility capsule.
Specific decisions
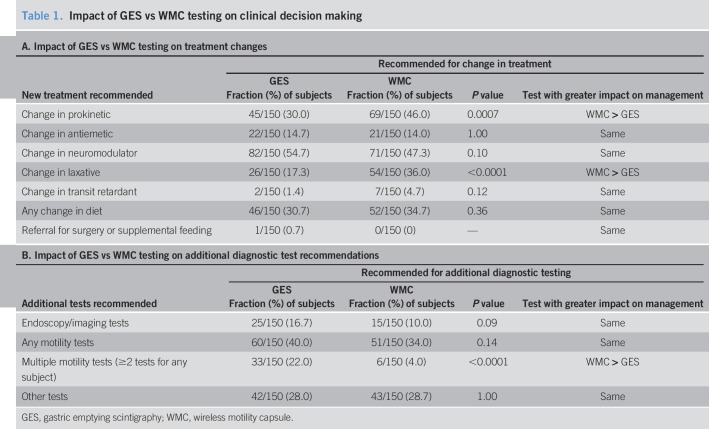
Prokinetics (P = 0.0007) and laxatives (P < 0.0001) but not other therapies were recommended more often based on WMC vs GES results (Table 1A). Recommendations for endoscopy/imaging tests trended higher (P = 0.09) based on the GES vs WMC results (Table 1B). Recommendations for any additional motility testing were similar based on GES vs WMCs. Multiple motility tests were recommended more often based on GES than WMCs (P < 0.0001), showing greater impact of WMCs than GES on specific treatment and testing decisions.
Table 1.
Impact of GES vs WMC testing on clinical decision making
Relating transit to decisions
Related to delayed gastric emptying
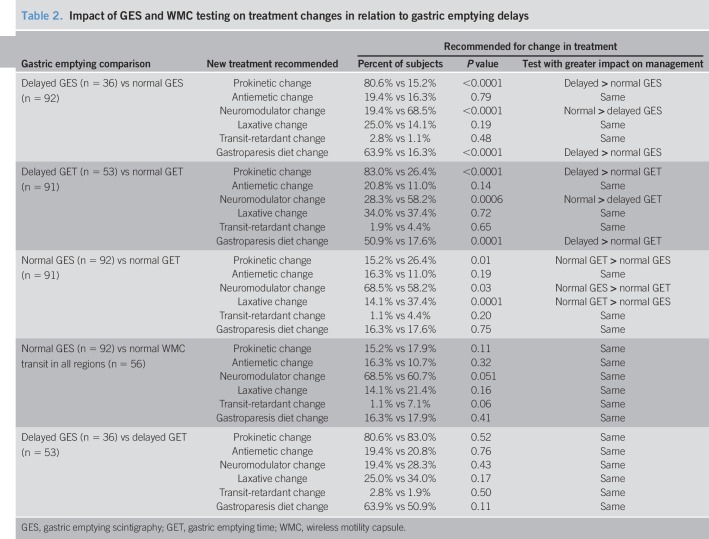
Prokinetics and gastroparesis diets were recommended more often and neuromodulators less for delayed vs normal gastric emptying by GES or WMCs (P ≤ 0.0006) (Table 2). Prokinetics (P = 0.01) and laxatives (P = 0.0001) were recommended more often and neuromodulators less (P = 0.03) for normal WMC gastric emptying vs normal GES. Differences disappeared when normal WMC transit in all regions was compared with normal GES. Treatment recommendations were similar for delayed GES vs WMC gastric emptying. This shows that finding delayed vs normal gastric emptying has a different impact on treatment decisions made after both WMC and GES testing.
Table 2.
Impact of GES and WMC testing on treatment changes in relation to gastric emptying delays
Specific recommended prokinetics included metoclopramide, domperidone, macrolides, and pyridostigmine. Common neuromodulators were mirtazapine, tricyclics, and gabapentin. Recommendations for specific prokinetics and neuromodulators were similar for both tests regardless of emptying delays (see Table 2A, Supplementary Digital Content 3, http://links.lww.com/CTG/A102).
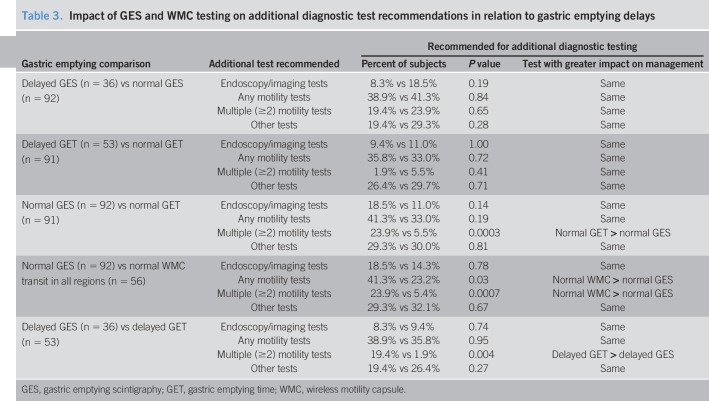
Multiple motility tests were recommended more often for normal GES vs normal WMC gastric emptying (P = 0.0003) and normal WMC transit in all regions (P = 0.0007) and for delayed gastric emptying by GES vs WMCs (P = 0.004) (Table 3). Recommendations for any motility test (P = 0.03) and multiple motility tests (P = 0.006) were greater for normal GES vs normal WMC transit in all regions, showing greater impact of WMCs vs GES on test ordering in different gastric emptying subsets.
Table 3.
Impact of GES and WMC testing on additional diagnostic test recommendations in relation to gastric emptying delays
Specific motility tests included radiopaque markers and anorectal and antroduodenal manometry (see Table 2B, Supplementary Digital Content 3, http://links.lww.com/CTG/A102). Radiopaque markers were recommended more often for GES vs WMCs when gastric emptying was delayed (P = 0.02) or normal (P = 0.001).
Related to delayed extragastric transit
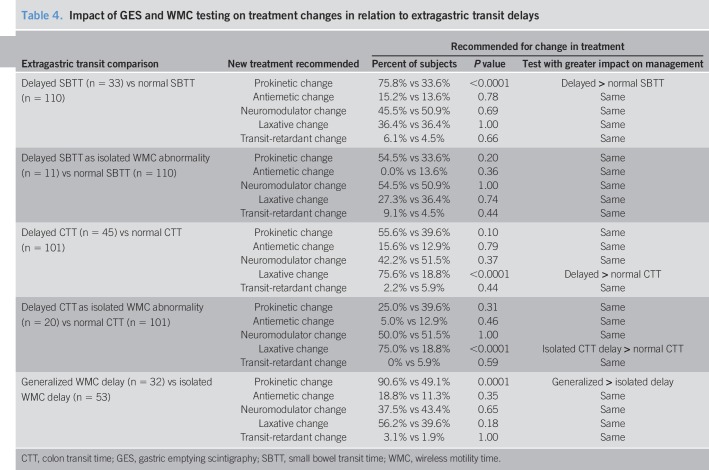
Prokinetics were recommended more often for delayed vs normal SBTT (P < 0.0001) (Table 4). This difference disappeared when SBTT delays were isolated. Laxatives were recommended more often for delayed vs normal CTT (P < 0.0001). This difference persisted when CTT delays were isolated abnormalities (P < 0.0001). Specific laxatives (polyethylene glycol 3350, linaclotide, and lubiprostone) were similarly advocated for delayed vs normal CTT (see Table 3A, Supplementary Digital Content 3, http://links.lww.com/CTG/A102). Prokinetics capable of stimulating colon transit (pyridostigmine and prucalopride) were recommended more often for delayed (7/45, 15.6%) than normal CTT (2/101, 2.0%) (P = 0.004). Prokinetics were recommended more often (P = 0.0001) with generalized vs isolated delays (Table 4). This shows differential impact of different WMC extragastric findings on treatment decisions.
Table 4.
Impact of GES and WMC testing on treatment changes in relation to extragastric transit delays
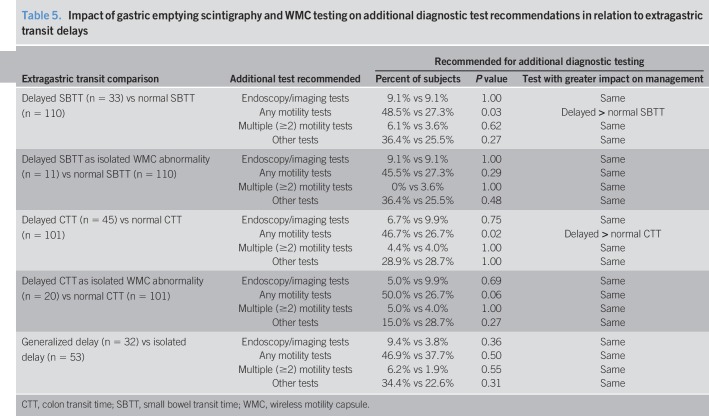
Additional motility tests were recommended more often with delayed vs normal SBTT (P = 0.03) (Table 5). This difference disappeared when SBTT delays were isolated. Additional motility tests were recommended more often with delayed vs normal CTT (P = 0.02). This difference still trended higher when CTT delays were isolated (P = 0.06). Specific motility test recommendations were similar for delayed and normal CTT (see Table 3B, Supplementary Digital Content 3, http://links.lww.com/CTG/A102). Testing recommendations were similar for generalized and isolated delays. This shows differential impact of different WMC extragastric findings on test ordering.
Table 5.
Impact of gastric emptying scintigraphy and WMC testing on additional diagnostic test recommendations in relation to extragastric transit delays
Related to rapid transit
Transit-retardant medications were recommended more often for rapid vs normal GET (P = 0.03) (see Table 4A, Supplementary Digital Content 3, http://links.lww.com/CTG/A102). Antidumping diets were recommended more often for rapid GET (P = 0.01) and trended higher for rapid GES (P = 0.09) vs normal emptying. Prokinetic medications were recommended more often when SBTT was rapid than when it was normal (P = 0.04). Additional testing recommendations did not relate to rapid transit (see Table 4B, Supplementary Digital Content 3, http://links.lww.com/CTG/A102). This shows the impact of rapid GES and WMC transit on management decisions.
DISCUSSION
Although gastric emptying testing is used for patients with suspected gastroparesis, there is little evidence to suggest these tests influence management choices. This investigation prospectively compared how GES and WMC testing informs recommendations for treatments and additional diagnostic evaluations in these patients. Its unique features include its large size, diverse patient cohort, standardized transit methods, and structured characterization of management recommendations. This comprehensive, multicenter study did not have the drawbacks of previous retrospective publications. There was also no bias from practice patterns of any single provider. Thus, these decisions more closely reflected the diverse management approaches of clinicians in varied medical settings.
Previous studies evaluating impact of transit tests on decisions in suspected gastroparesis had deficiencies. Three limited retrospective series used medical record review, which may not consistently capture management choices (13,16,17). One prospective study described the influence of GES on medication and diet changes made by 30 physicians but offered few details on specific medications and did not discuss additional diagnostic testing or compare GES with WMCs (20).
Our coprimary endpoints uncovered differences in how clinicians use GES and WMCs to make decisions. Medication changes were recommended more frequently, and ordering of diagnostic tests was less frequent with WMCs vs GES. Specific choices included greater recommendations for prokinetics and laxatives for WMCs and increased ordering of multiple motility tests with GES. Because WMC results led to recommending more treatments and fewer additional tests than GES, we concluded that WMCs have greater impact on decision making in managing suspected gastroparesis.
Subgroup analyses were designed to test whether these coprimary endpoint findings resulted from greater sensitivity of detecting delayed gastric emptying, extragastric delays, or rapid transit. Delayed gastric emptying by either test increased prokinetic recommendations, indicating a pathophysiological management approach. Prokinetics were recommended more often after WMC testing because of the greater sensitivity of WMCs over GES to detect delayed gastric emptying. Higher recommendations of prokinetics for normal WMC gastric emptying vs normal GES disappeared when small bowel and colon transit delays were excluded, indicating that prokinetics were likely prescribed for extragastric transit impairments. Although treatment of functional dyspepsia (including postprandial distress) has been extensively studied, the literature on managing suspected gastroparesis patients with normal gastric emptying is limited. This study provides the first report affirming preferential neuromodulator use in this large patient subset with normal emptying. Tricyclics are reportedly ineffective in gastroparesis but are beneficial for functional dyspepsia with normal gastric emptying; thus, our observations corroborate previous publications (25–27). Neuromodulators were recommended more often for normal GES than normal WMCs unless all gut regions had normal transit, suggesting that the WMC extragastric results influenced these decisions.
In addition to experiencing symptoms of gastroparesis, some subjects reported lower abdominal pain and bowel disturbances potentially originating in the distal gut. Similar high degrees of lower abdominal symptoms have been observed in published large gastroparesis cohorts (9,10,28). Our findings illustrate advantages of WMC extragastric measurements, which may be relevant to these symptoms. Small bowel delays promoted more prokinetic recommendations, but sample sizes were small and differences disappeared after excluding other regional delays. Delayed colon transit correlated with recommending increased laxative use. Some patients with CTT delays were prescribed colonic prokinetic agents, confirming that WMCs can direct specific decisions in treating constipation. Further investigations will determine whether interventions targeting extragastric findings will translate into better outcomes in suspected gastroparesis, although one pediatric study reported that laxatives can reduce gastroparesis symptoms (29). Prokinetics were recommended more often for generalized vs isolated delays, because of increased gastric emptying delays with generalized (28/32, 87.5%) vs isolated (22/53, 41.5%) transit impairments (P < 0.0001).
Agents that slow transit were advocated more often for rapid WMC gastric emptying. Prokinetics were ordered more for rapid SBTT. Although this might seem counterintuitive, 2 of 3 patients with rapid SBTT exhibited delayed gastric emptying by GES or WMCs providing a pathophysiologic rationale for use of this drug class. Rapid CTT was not evaluated because it is not an established measure, as defecation can occur any time after the onset of colonic high-amplitude propagating contractions (30,31).
Diet recommendations were influenced by transit results. Gastroparesis diets were preferentially advocated for delayed gastric emptying, supporting a controlled diabetic gastroparesis trial reporting benefits of small particle diets (32). Antidumping diets were advocated more for rapid gastric emptying, again reflecting a physiologic basis for treatment (33). Although rapid emptying can be found in functional dyspepsia, diabetes, and cyclic vomiting, the pathophysiologic relevance of rapid transit remains unproved (15,16,34).
Recommendations for additional testing differed based on transit. Multiple motility tests were more often advised based on GES vs WMCs regardless of delays probably because GES provides no extragastric information. More additional motility tests were recommended for delayed vs normal small bowel and colon transit; differences trended higher when colon delays were isolated. WMC testing did not eliminate decisions to perform anorectal manometry to exclude dyssynergia as a cause of constipation (35). Anorectal manometry was similarly advocated for 39 patients after obtaining GES results vs 42 patients after obtaining WMC results. However, anorectal manometry was not ordered significantly more often in those with delayed WMC CTT (17/45, 37.8%) than with normal CTT (25/101, 24.8%) (P = 0.12), indicating that the WMC colon transit interpretation was not the sole determining factor in ordering this test. By contrast, radiopaque marker studies were ordered more often after GES reflecting the inability of gastric scintigraphy to measure colon transit. Curiously, small numbers of radiopaque marker tests were ordered on the basis of WMC findings, although WMC CTT results were available. Older studies suggested that marker retention profiles may be different in slow transit constipation than with outlet obstruction, although this was not subsequently confirmed (36). It is possible that some site investigators wished to measure regional colon transit delays in some of their study patients.
This investigation had limitations. Each center had different resources and practice standards, which influenced local management options. We considered this a strength of this study, as it reflected real-life practice patterns across diverse settings. Site investigators were afforded leeway in making decisions, which did not consider medication affordability, insurance coverage, or acceptance of invasive testing. It was beyond the scope of this investigation to determine whether differences in the impact of WMC vs GES findings on management decisions translated into different symptom and quality of life outcomes. Furthermore, it was not possible to quantify longitudinal outcomes relating to management decisions that were made based on findings of either WMCs or GES as individual tests; rather, any outcome would be influenced by clinical decisions that were made by site investigators based on knowledge of both the WMC and GES results taken together. We plan to correlate transit findings with symptom profiles at baseline and follow-up in future reports. Nevertheless, our detailed findings provide unique insights into how clinicians approach management decisions in patients with pretest possibilities of having gastroparesis.
In conclusion, gastric emptying and gut transit testing influenced management decisions in suspected gastroparesis. WMC findings led to greater medication changes and fewer recommendations for additional motility testing compared with GES. Recommendations for prokinetics, neuromodulators, and gastroparesis diets were influenced by gastric emptying delays, whereas laxative recommendations and ordering of additional coloanal motility tests were influenced by extragastric delays.
CONFLICTS OF INTEREST
Guarantor of the article: William L. Hasler, MD.
Specific author contributions: W.L.H.: Study design, experimental conduct, data collection, data analysis, manuscript preparation, and approval of the final manuscript version. S.S.C.R: Experimental conduct, data collection, manuscript preparation, and approval of the final manuscript version. R.W.M.: Experimental conduct, data collection, and approval of the final manuscript version. R.A.K.: Experimental conduct, data collection, and approval of the final manuscript version. L.A.N.: Experimental conduct, data collection, and approval of the final manuscript version. M.I.S.: Experimental conduct, data collection, and approval of the final manuscript version. A.A.L.: Experimental conduct, data collection, manuscript preparation, and approval of the final manuscript version. B.M.: Experimental conduct, data collection, and approval of the final manuscript version. J.M.W.: Experimental conduct, data collection, and approval of the final manuscript version. H.P.P.: Experimental conduct, data collection, and approval of the final manuscript version. I.S.: Experimental conduct, data collection, and approval of the final manuscript version. G.E.W.: Study design and statistical analysis of data. B.K.: Study design, experimental conduct, data collection, data analysis, manuscript preparation, and approval of the final manuscript version.
Financial support: The parent study supporting the data and analyses included in this article were funded by Medtronic (grant MA501). Matilde Lourd, a Medtronic employee, provided some initial data analyses for the primary endpoints of this study before its presentation at the 2017 World Congress of Gastroenterology meeting; statistical analyses of these data were verified by Gregory E. Wilding, and all additional statistical analyses relating to specific management decisions and decisions related to gut transit abnormalities were conducted by Gregory E. Wilding, a consultant for Medtronic as described below. All initial data collection was performed by site investigators. Data were transferred to a central database supervised by Medtronic. Medtronic did not participate in study design, data interpretation, writing support, or other preparation assistance for this article.
Potential competing interests: W.L.H.: Received grant funding from Medtronic for conduct of research as site principal investigator at University of Michigan, Ann Arbor, MI. S.S.C.R.: Received grant funding from Medtronic for conduct of research as site principal investigator at Medical College of Georgia, Augusta, GA. R.W.M.: Received grant funding from Medtronic for conduct of research as site co-principal investigator at Texas Tech University, El Paso, TX. R.A.K.: Received grant funding from Medtronic for conduct of research as site principal investigator at ClinSearch, Chattanooga, TN. L.A.N.: Received grant funding from Medtronic for conduct of research as site principal investigator at Stanford University, Palo Alto, CA. M.I.S.: Received grant funding from Medtronic for conduct of research as site principal investigator at Florida Digestive Health Associates, Largo, FL. A.A.L.: Received grant funding from Medtronic for conduct of research as site principal investigator at the University of Vermont, Burlington, VT (he has since moved to the University of Michigan, Ann Arbor, MI). B.M.: Received grant funding from Medtronic for conduct of research as site principal investigator at the University of Miami, Miami, FL (she has since moved to Carolinas HealthCare System, Charlotte, NC). J.M.W.: Received grant funding from Medtronic for conduct of research as site principal investigator at Indiana University, Indianapolis, IN. H.P.P.: Received grant funding from Medtronic for conduct of research as site principal investigator at Temple University, Philadelphia, PA. I.S.: Received grant funding from Medtronic for conduct of research as site co-principal investigator at Texas Tech University, El Paso, TX. G.E.W.: Received compensation as a consultant by Medtronic for statistical analyses of study data. B.K.: Received grant funding from Medtronic for conduct of research as site principal investigator at Massachusetts General Hospital, Boston, MA. None of the investigators is an employee of Medtronic, none owns stocks or shares in Medtronic, and none owns patents with Medtronic.
Clinical trial registry: Analyses included in this article were conducted according to a priori planned secondary study endpoints of a registered clinical trial (ClinicalTrials.gov NCT02022826).
Study Highlights.
WHAT IS KNOWN
✓ Gastric emptying is measured by GES or WMCs; the latter also assesses extragastric transit.
✓ Patients with suspected gastroparesis report diffuse symptoms suggesting possible generalized dysmotility.
✓ Impacts of GES and WMCs on management decisions are poorly defined.
WHAT IS NEW HERE
✓ More treatment changes were recommended, and ordering of additional motility tests was eliminated more based on WMCs—testing which detected more gastric and extragastric abnormalities.
✓ These tests provided information that differentially influenced clinical decisions in suspected gastroparesis.
TRANSLATIONAL IMPACT
✓ These detailed findings provide novel insight about how motility specialists use gut transit data to make decisions on treating and ordering other testing in patients with suspected gastroparesis.
Supplementary Material
Footnotes
SUPPLEMENTARY MATERIAL accompanies this paper at http://links.lww.com/CTG/A100, http://links.lww.com/CTG/A101, http://links.lww.com/CTG/A102
REFERENCES
- 1.Hasler WL. Gastroparesis: Pathogenesis, diagnosis and management. Nat Rev Gastroenterol Hepatol 2011;8:438–53. [DOI] [PubMed] [Google Scholar]
- 2.Abell TL, Camilleri M, Donohoe K, et al. Consensus recommendations for gastric emptying scintigraphy: A joint report of the American Neurogastroenterology and Motility Society and the Society of Nuclear Medicine. Am J Gastroenterol 2008;103:753–63. [DOI] [PubMed] [Google Scholar]
- 3.Tougas G, Eaker EY, Abell TL, et al. Assessment of gastric emptying using a low fat meal: Establishment of international control values. Am J Gastroenterol 2000;95:1456–62. [DOI] [PubMed] [Google Scholar]
- 4.Kuo B, McCallum RW, Koch KL, et al. Comparison of gastric emptying of a nondigestible capsule to a radiolabelled meal in healthy and gastroparetic subjects. Aliment Pharmacol Ther 2008;27:186–96. [DOI] [PubMed] [Google Scholar]
- 5.Hasler WL. The use of SmartPill for gastric monitoring. Expert Rev Gastroenterol Hepatol 2014;8:587–600. [DOI] [PubMed] [Google Scholar]
- 6.Szarka LA, Camilleri M, Vella A, et al. A stable isotope breath test with a standard meal for abnormal gastric emptying of solids in the clinic and in research. Clin Gastroenterol Hepatol 2008;6:635–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lee AA, Rao S, Nguyen LA, et al. Validation of diagnostic and performance characteristics of the wireless motility capsule in patients with suspected gastroparesis. Clin Gastroenterol Hepatol 2019;17:1770–9.e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Stein E, Berger Z, Hutfless S, et al. Wireless Motility Capsule Versus Other Diagnostic Technologies for Evaluating Gastroparesis and Constipation: A Comparative Effectiveness Review. Agency for Healthcare Research and Quality; 2013. [PubMed] [Google Scholar]
- 9.Parkman HP, Yates K, Hasler WL, et al. Clinical features of idiopathic gastroparesis vary with sex, body mass, symptom onset, delay in gastric emptying, and gastroparesis severity. Gastroenterology 2011;140:101–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Parkman HP, Yates K, Hasler WL, et al. Similarities and differences between diabetic and idiopathic gastroparesis. Clin Gastroenterol Hepatol 2011;9:1056–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Stanghellini V, Chan FK, Hasler WL, et al. Gastroduodenal disorders. Gastroenterology 2016;150:1380–92. [DOI] [PubMed] [Google Scholar]
- 12.Arora Z, Parungao JM, Lopez R, et al. Clinical utility of wireless motility capsule in patients with suspected multiregional gastrointestinal dysmotility. Dig Dis Sci 2015;60:1350–7. [DOI] [PubMed] [Google Scholar]
- 13.Hasler WL, May KP, Wilson LA, et al. Relating gastric scintigraphy and symptoms to motility capsule transit and pressure findings in suspected gastroparesis. Neurogastroenterol Motil 2018;30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Delgado-Aros S, Camilleri M, Cremonini F, et al. Contributions of gastric volumes and gastric emptying to meal size and postmeal symptoms in functional dyspepsia. Gastroenterology 2004;127:1685–94. [DOI] [PubMed] [Google Scholar]
- 15.Bharucha AE, Camilleri M, Forstrom LA, et al. Relationship between clinical features and gastric emptying disturbances in diabetes mellitus. Clin Endocrinol (Oxf) 2009;70:415–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rao SS, Camilleri M, Hasler WL, et al. Evaluation of gastrointestinal transit in clinical practice: Position paper of the American and European Neurogastroenterology and Motility Societies. Neurogastroenterol Motil 2011;23:8–23. [DOI] [PubMed] [Google Scholar]
- 17.Galil MA, Critchley M, Mackie CR. Isotope gastric emptying tests in clinical practice: Expectation, outcome, and utility. Gut 1993;34:916–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kuo B, Maneerattanaporn M, Lee AA, et al. Generalized transit delay on wireless motility capsule testing in patients with clinical suspicion of gastroparesis, small intestinal dysmotility, or slow transit constipation. Dig Dis Sci 2011;56:2928–38. [DOI] [PubMed] [Google Scholar]
- 19.Rao SS, Mysore K, Attaluri A, et al. Diagnostic utility of wireless motility capsule in gastrointestinal dysmotility. J Clin Gastroenterol 2011;45:684–90. [DOI] [PubMed] [Google Scholar]
- 20.Tseng AS, Crowell MD, DiBaise JK. Clinical utility of gastric emptying scintigraphy: Patient and physician perspectives. Neurogastroenterol Motil 2018;30:e13279. [DOI] [PubMed] [Google Scholar]
- 21.Wang YT, Mohammed SD, Farmer AD, et al. Regional gastrointestinal transit and pH studied in 215 healthy volunteers using the wireless motility capsule: Influence of age, gender, study country and testing protocol. Aliment Pharmacol Ther 2015;42:761–72. [DOI] [PubMed] [Google Scholar]
- 22.Rao SS, Kuo B, McCallum RW, et al. Investigation of colonic and whole-gut transit with wireless manometry capsule and radiopaque markers in constipation. Clin Gastroenterol Hepatol 2009;7:537–44. [DOI] [PubMed] [Google Scholar]
- 23.Camilleri M, Thorne NK, Ringel Y, et al. Wireless pH-motility capsule for colonic transit: Prospective comparison with radiopaque markers in chronic constipation. Neurogastroenterol Motil 2010;22:874–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Clopper CJ, Pearson ES. The use of confidence or fiducial limits illustrated in the case of binomial. Biometrika 1934;26:404–13. [Google Scholar]
- 25.Parkman HP, Van Natta ML, Abell TL, et al. Effect of nortriptyline on symptoms of idiopathic gastroparesis: The NORIG randomized clinical trial. JAMA 2013;310:2640–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Talley NJ, Locke GR, Saito YA, et al. Effect of amitriptyline and escitalopram on functional dyspepsia: A multicenter, randomized controlled study. Gastroenterology 2015;149:340–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hasler WL, Koch KL. Amitriptyline for functional dyspepsia: Importance of symptom profile and making a case for gastric emptying testing. Gastroenterology 2015;149:270–2. [DOI] [PubMed] [Google Scholar]
- 28.Koch KL, Hasler WL, Yates KP, et al. Baseline features and differences in 48 week clinical outcomes in patients with gastroparesis and type 1 vs type 2 diabetes. Neurogastroenterol Motil 2016;28:1001–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Boccia G, Buonavolontà R, Coccorullo P, et al. Dyspeptic symptoms in children: The result of a constipation-induced cologastric brake? Clin Gastroenterol Hepatol 2008;6:556–60. [DOI] [PubMed] [Google Scholar]
- 30.Bharucha AE. High amplitude propagated contractions. Neurogastroenterol Motil 2012;24:977–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Rao SS, Sadeghi P, Beaty J, et al. Ambulatory 24-hour colonic manometry in slow-transit constipation. Am J Gastroenterol 2004;99:2405–16. [DOI] [PubMed] [Google Scholar]
- 32.Olausson EA, Störsrud S, Grundin H, et al. A small particle size diet reduces upper gastrointestinal symptoms in patients with diabetic gastroparesis: A randomized controlled trial. Am J Gastroenterol 2014;109:375–85. [DOI] [PubMed] [Google Scholar]
- 33.van Beek AP, Emous M, Laville M, et al. Dumping syndrome after esophageal, gastric or bariatric surgery: Pathophysiology, diagnosis, and management. Obes Rev 2017;18:68–85. [DOI] [PubMed] [Google Scholar]
- 34.Hejazi RA, Lavenbarg TH, McCallum RW. Spectrum of gastric emptying patterns in adult patients with cyclic vomiting syndrome. Neurogastroenterol Motil 2010;22:1298–302. [DOI] [PubMed] [Google Scholar]
- 35.Carrington EV, Scott SM, Bharucha A, et al. Expert consensus document: Advances in the evaluation of anorectal function. Nat Rev Gastroenterol Hepatol 2018;15:309–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Chaussade S, Khyari A, Roche H, et al. Determination of total and segmental colonic transit time in constipated patients. Results in 91 patients with a new simplified method. Dig Dis Sci 1989;34:1168–72. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.