Abstract
The objective of this study is to investigate the role of paraspinal muscles in the progression of different types of spondylolisthesis by examining the correlation between cross-sectional area (CSA) of lumbar paraspinal muscle and slip percentage (SP) in degenerative spondylolisthesis and isthmic spondylolisthesis.
A multicenter retrospective analysis was carried out including 219 subjects diagnosed with lumbar spondylolisthesis. Using T2-weighted axial magnetic resonance imgaging, CSAs of the psoas major (PM), multifidus (MU), and erector spinae were measured and divided by L5 vertebral body (VB) CSA. SP was measured using sagittal T2-weighted images. Correlations between muscle CSA ratio and SP were calculated in each group. Regression analysis was performed to predict the influence of each muscle CSA/VB CSA ratio on SP.
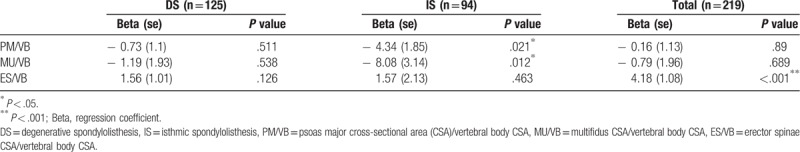
No significant correlation was found in the degenerative spondylolisthesis group between any of the muscle CSA ratios and SP. Both PM/VB ratio (r = −0.24, P = .021) and MU/VB ratio (r = −0.26, P = .012) were negatively correlated with SP in the isthmic spondylolisthesis group. MU had more influence on SP than PM in the isthmic spondylolisthesis group (regression coefficient MU/VB: −8.08, PM/VB: −4.34).
Both PM and MU muscle CSA ratios were negatively correlated with SP in the isthmic group. MU had more influence on SP than PM. No muscles had any correlations with SP in the degenerative group. This discrepancy between the two groups suggests that exercise programs or interventions regarding the segmental stability of isthmic spondylolisthesis and degenerative spondylolisthesis should be distinguished in clinical practice.
Clinical Research Information Service of Korea Centers for Disease control and Prevention, KCT0002588. Registered on 12 December 2017, https://cris.nih.go.kr/cris/search/search_result_st01.jsp?seq=10702
Keywords: cross-sectional area, degenerative spondylolisthesis, isthmic spondylolisthesis, lumbar paraspinal muscle, slip percentage
1. Introduction
According to the North American Spine Society's (NASS) Evidence-Based Clinical Guideline for spondylolisthesis, the best working definition of degenerative spondylolisthesis (DS) is “an acquired anterior displacement of one vertebra over the subjacent vertebra without an associated disruption or defect in the vertebral ring,” while isthmic spondylolisthesis (IS) is defined as “an anterior translation of one lumbar vertebra relative to the next caudal segment as a result of an abnormality in the pars interarticularis”.[1,2] The reported incidence of DS varies among studies from 4.1% in cadaveric material, 13.6% in a population-based cohort study, and up to 28.6% in a clinical cohort study.[3–5] The incidence of IS in the general adult population ranges from 3.7% to 8%.[6–9]
NASS recommends lateral radiographs to detect DS and standing plain radiographs with/without oblique views or dynamic radiographs to detect IS. If symptoms indicate myelopathy or radiculopathy, magnetic resonance imaging (MRI) should be considered to confirm the diagnosis of both DS and IS.[1,2] This emphasis on MRI evaluations in symptomatic cases provides the grounds for soft tissue evaluation in lumbar spondylolisthesis, especially lumbar paraspinal muscles (PSMs), which are essential for vertebrae stabilization and movement.[10] Several studies on the role of lumbar PSMs and spondylolisthesis have been performed by measuring the cross-sectional areas (CSA) of PSMs, but the conclusions are inconsistent.[11–15] To further investigate the correlation between muscles and lumbar spondylolisthesis, we previously performed an observational study on the correlation between muscle CSA and slip percentage (SP) in 120 subjects diagnosed with lumbar spondylolisthesis.[16] The results showed correlation of multifidus (MU) atrophy and erector spinae (ES) hypertrophy with SP, and subgroup analysis only showed significant psoas major (PM) and ES CSA differences between the two subgroups (DS and IS). However, the correlation between muscle CSA and SP was not analyzed in the respective subgroup.
This study is a retrospective analysis of 219 subjects diagnosed with DS and IS that assesses lumbar PSM CSAs using MRI. The aim of this study is to compare the relationship between SP and lumbar PSM CSAs in the two most common types of spondylolisthesis, degenerative and isthmic.
2. Materials and methods
2.1. Study participants and design
A multicenter, retrospective, observational study using MRI was conducted in 2 medical centers in Seoul, Korea from January 1st 2010 to July 31st 2017. Inclusion criteria were
-
1)
age 19 to 80 years;
-
2)
available MRI images;
-
3)
diagnosed with degenerative or isthmic spondylolisthesis by a radiologist. If a patient had more than one available MRI, the most recent one was chosen.
Exclusion criteria were
-
1)
diagnosed with conditions other than spondylolisthesis such as malignant tumors, infection, and inflammatory spondylitis;
-
2)
poor MRI image quality;
-
3)
previous lumbar surgery;
-
4)
diagnosed with congenital, traumatic, metabolic, and iatrogenic spondylolisthesis.
A total of 219 subjects were screened by the inclusion and exclusion criteria. Subjects were then classified into two groups, degenerative and isthmic, according to the radiologist. The DS group had 125 subjects and the IS group had 94 subjects (Fig. 1). Personal information regarding subjects was coded using patient initials. All parties were well informed of confidentiality with regards to the Helsinki declaration. This study was approved by the Institutional Review Board (approval number KOMCIRB-170817-HR-028, KHNMCOH 2017–09–006) of each hospital, and the protocol was registered with the Clinical Research Information Service (approval number KCT0002588). This study adheres to CONSORT guidelines for reporting clinical trials and the CONSORT checklist for this study is attached in Additional file 1.
Figure 1.
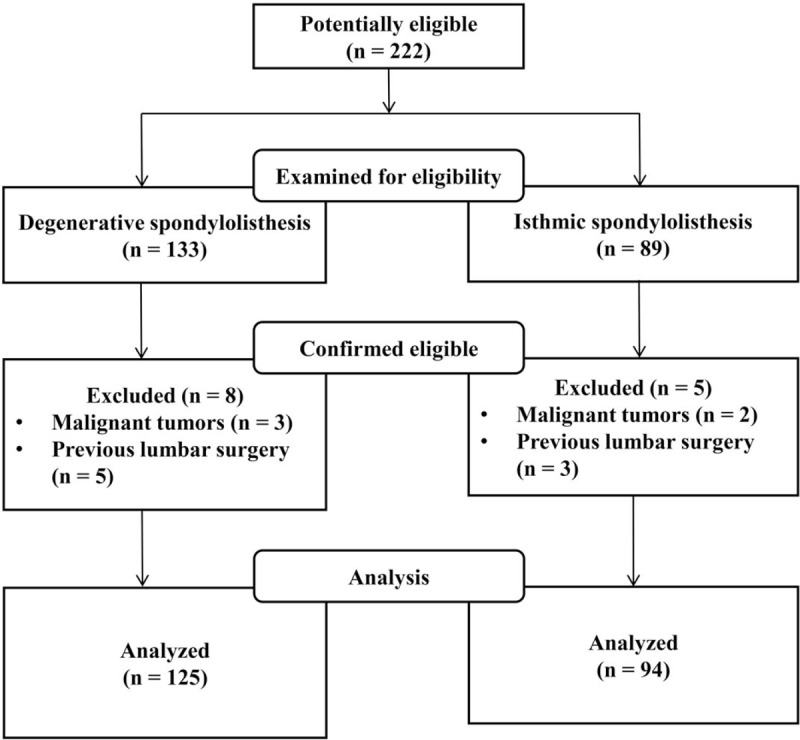
Study flow chart.
2.2. Data extraction and management
The data were collected through paper-based documents written by the clinical research coordinator and outcome assessors. Two independent assessors participated in this study at each medical center. They were blinded to the radiologist's conclusion and group allocation. Assessors were educated with the same standards of measurement prior to initiation.
2.3. Measurement of slip percentage
The slip percentage of the translated vertebra was measured using the sagittal section of T2-weighted MRI images. Slip percentage is the ratio of the overhanging part of the superior vertebral body to the diameter of the inferior vertebral body (Fig. 2). If there were more than 2 different levels involved, the segment with the largest degree of slip was chosen. The mean SP of the 2 assessors was used for analysis.
Figure 2.

Sagittal T2-weighted image obtained for measurement of slip percentage. Slip percentage was obtained by dividing (A) the distance of superior vertebral body translation into (B) the diameter of inferior vertebral body.
2.3.1. Measurement of lumbar paraspinal muscle cross-sectional area
CSAs of PM, MU, and ES were measured by assessing axial sections of conventional T2-weighted MRI images. The section from the upper endplate of the L5 vertebra was used. CSAs were measured bilaterally using the Picture Archiving and Communication Systems (PACS, INFITT PACS, INFITT Healthcare Company, Korea) to create a free line region of interest for each muscle (Fig. 3). Given the strong interrelation between muscle volume and skeletal CSA,[17,18] muscle areas were speculated to be biomechanically associated with vertebral body (VB) CSA. Muscle CSA/VB CSA ratios were therefore used to eliminate biases rising from differences in physical builds. The sum of left and right muscle CSA/VB CSA was calculated. The mean value of the two assessors was used for analysis.
Figure 3.
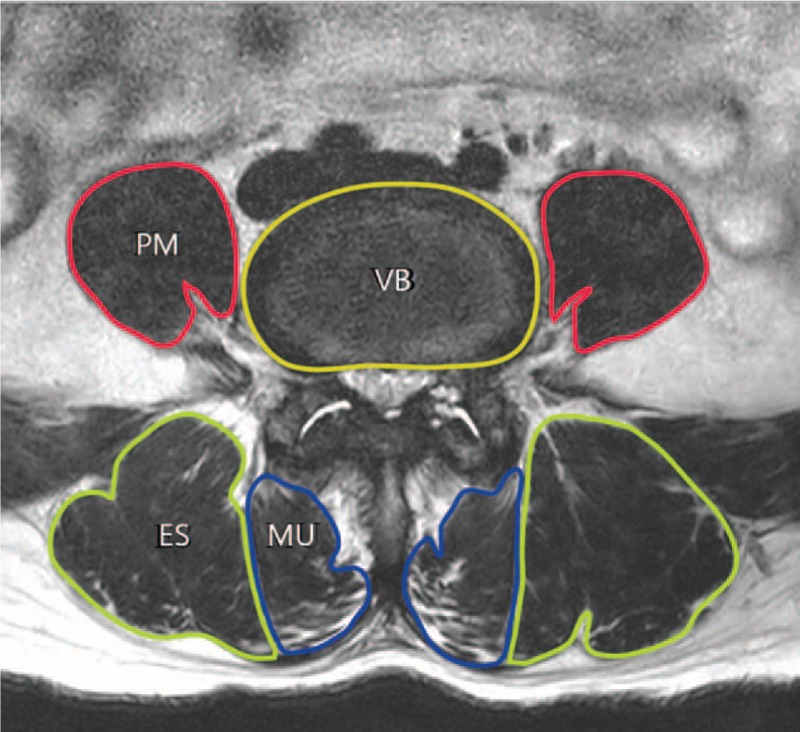
Axial T2-weighted image of paraspinal muscles obtained at upper endplate of L5. Cross-sectional areas of superior endplate of L5 vertebra, psoas major (PM), multifidus (MU), and erector spinae (ES) were measured.
2.4. Statistical analysis
Data were entered into an Excel spreadsheet (Microsoft Corp., Redmond, Washington, USA) and analyzed using Statistical Package for Social Science (SPSS) for Windows (version 18.0; IBM Corp., Armonk, NY). The continuous variables were measured as the mean and standard deviation. The student t-test was used to examine differences between age, SP, PM CSA/VB CSA (PM/VB), MU CSA/VB CSA (MU/VB), and ES CSA/VB CSA (ES/VB) in the DS and IS group. Chi-square test was used to calculate intergroup gender differences. Correlation of SP with muscle area ratios was analyzed using Pearson correlation test. A simple linear regression analysis was performed to predict the influence of each muscle CSA/VB CSA ratio on SP. Inter-assessor agreement was measured using the intraclass correlation coefficient (ICC) and standardized ratings of agreement.[19] All statistical analysis was advised and reviewed by a statistician.
3. Results
3.1. Demographics
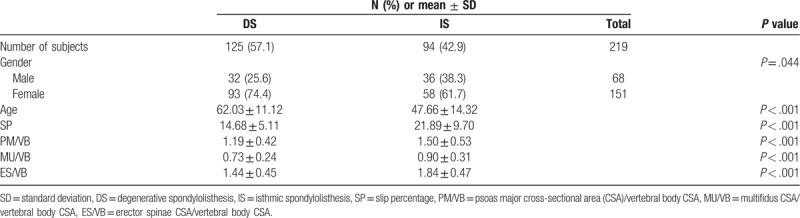
The degenerative group included 32 males and 93 females with a mean age of 62.03 ± 11.12 years. The isthmic group consisted of 36 males and 58 females with a mean age of 47.66 ± 14.32 years. SP, PM/VB, MU/VB, and ES/VB were all significantly lower in the DS group (Table 1).
Table 1.
Demographics of degenerative and isthmic group.
3.2. Correlation analysis between PSM CSA/VB CSA, age, and SP
Pearson correlation analysis was used to determine the correlation between muscle CSA/VB ratio and SP. In the degenerative group, no muscle CSA/VB CSA values showed significant correlation with SP. The only statistically significant factor that had any correlation with SP in the DS group was age (P < .05). The isthmic group demonstrated negative correlation between PM/VB and MU/VB with SP (P < .05) (Table 2 and Fig. 4).
Table 2.
Correlation between cross-sectional area ratio of the lumbar paraspinal muscles, age, and slip percentage.
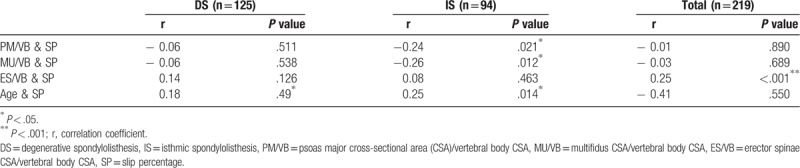
Figure 4.
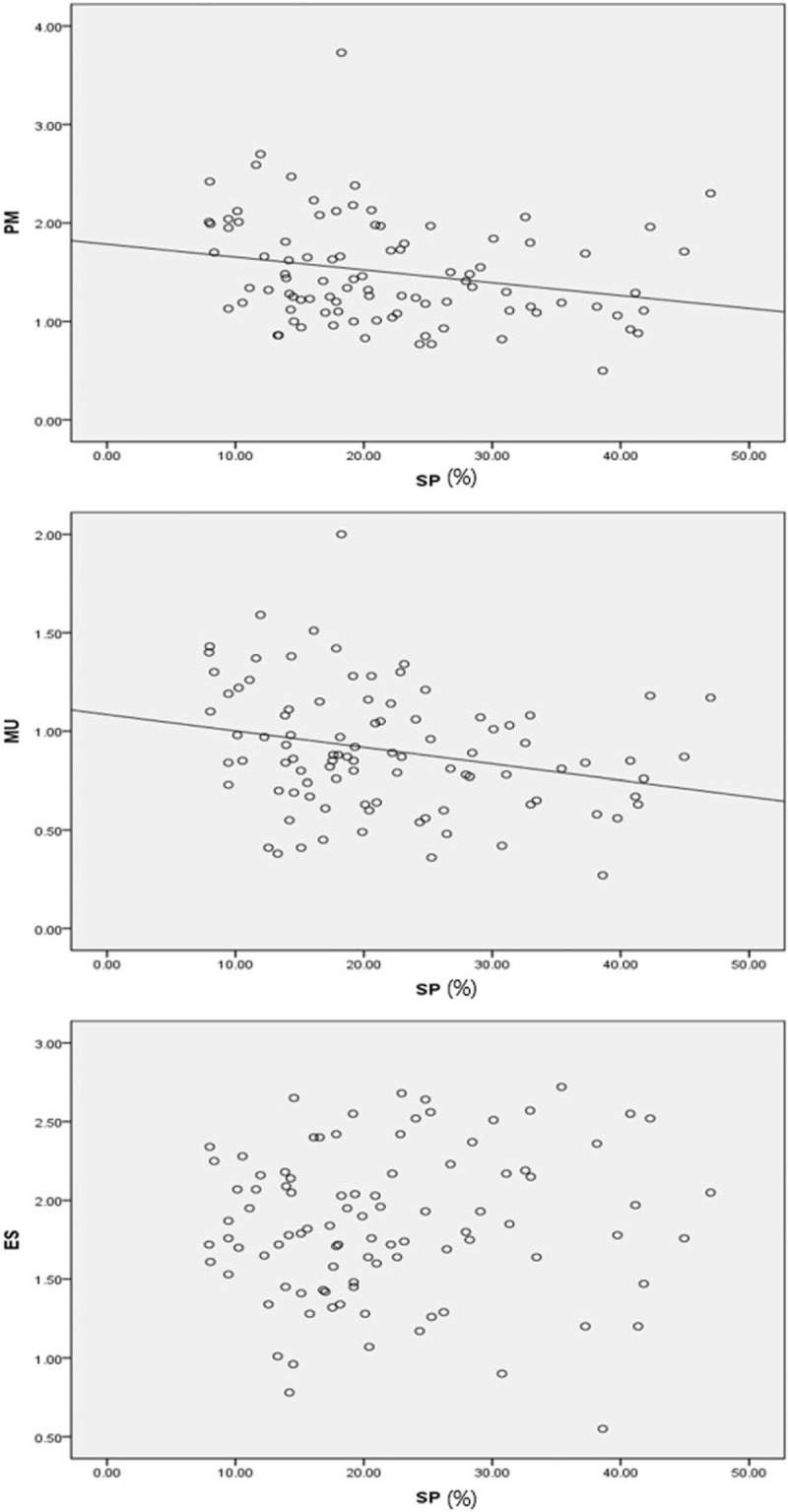
Scatterplots demonstrating degree of correlation between slip percentage and lumbar paraspinal muscle cross-sectional area ratio. ES = erector spinae, MU = multifidus, PM = psoas major, SP = slip percentage.
3.3. Regression analysis of PSM CSA/VB CSA on SP
A simple linear regression analysis was performed to predict the influence of each muscle CSA/VB CSA ratio on SP. In the isthmic group, MU/VB and PM/VB predicted lower SP (Table 3). MU/VB had more influence on SP than PM/VB (regression coefficient PM/VB: −4.34, MU/VB: −8.08). There were no significant findings in the degenerative group.
Table 3.
Linear regression analysis of lumbar paraspinal muscle cross-sectional area ratio on slip percentage.
3.4. Inter-assessor agreement analysis
Agreement between the 2 assessors for measurement of the muscle and vertebral body CSAs was nearly perfect at both hospitals. The measurement of MU CSA was substantial at hospital 1 and moderate at hospital 2 (Table 4).
Table 4.
Inter-assessor agreement analysis.

4. Discussion
DS is the leading cause of low back pain in adults over 50 years of age.[20] IS is more frequently seen in young adults and athletes.[21] Paraspinal muscles play an important role in stabilization of the lumbar vertebrae.[10] However, contradictory results have been shown in studies on DS. A case-control study reported PSM hypertrophy compared to controls,[11] while an observational study showed that MU CSA was not influenced by disc degeneration or the degree of slip.[12] Another case-control study reported that disc height and MU CSA ratio were lower, but the ES CSA ratio was higher in the DS group.[13] Reports on IS are still controversial as well. A case-control computed tomography analysis showed that PSM areas were significantly larger in the IS group.[14] Another study of surgically managed IS subjects found that the mean CSA value of ES was significantly higher, whereas that of MU was significantly lower compared to that of normative controls.[15] An observational study including both DS and IS subjects showed a negative correlation of SP with MU CSA value and a positive correlation of SP with ES CSA value, yet the two groups were not distinguished.[16] To analyze the difference between DS and IS regarding correlation of muscle CSA and SP, we initiated a retrospective, multi-centered review of medical records using MRI images. This novel approach may help clinicians differentiate between the two types of spondylolisthesis for patient treatment and management.
Two conflicting outcomes were drawn from our study. No significant correlation was found in the DS group between any of the muscle CSA ratios and SP. Conversely, PM/VB and MU/VB both had negative correlation with SP in the IS group, and MU had more influence on SP than PM (regression coefficient MU/VB: −8.08, PM/VB: −4.34). There is a significantly higher mean age in the degenerative group than the isthmic group. This is consistent with the normal epidemiology of DS in that DS mostly occurs in middle-aged women over 50.[22] A study on the relationship between age and lumbar PSM CSAs reported significantly decreased CSAs with age.[23] It is possible to infer that individual PSMs do not have a significant impact on slip progression in the DS group because older age leads to overall atrophy of PSMs. It is well known that degenerative changes of the lumbar vertebrae are proportional to age. Therefore, it stands to reason that SP in the DS group increases with age. The overall age-related atrophy of PSMs and progression of degenerative changes explain the positive correlation between age and SP rather than the correlation between muscle CSA and SP in the DS group.
Independent analysis of the DS group shows that our findings are parallel with the result of a previous case-control study in that PSM CSA ratio did not have any significant correlations with SP within DS group.[13] However, the same study showed MU atrophy and ES hypertrophy when compared to normal controls. Positive correlation between muscle CSAs and SP indicates the role of PSM in progression of vertebral slip, while the discrepancy in muscle CSA compared to normal controls suggests the role of PSM in the initiation or aftermath of listhesis. We can comprehensively conclude that muscle atrophy is an initiating factor of DS or a consequence of existing DS, but its role in the progression of DS is questionable.
The IS group did show correlations of MU and PM with SP. Deep multifidus fibers control intervertebral movement and intersegmental motion.[24] Therefore, MU atrophy can cause lumbar segmental instability and predispose to unfavorable prognosis.[25] A study on surgically managed subjects with IS[15] reported that MU CSA values negatively correlate with bone marrow signal changes of the lumbar pedicle, which represent the degree of stress-related biochemical bony changes of pars or pedicle fractures.[26] Resolutions in marrow signal changes and improved pain levels have been related to healing and reduced biomechanical stress in pars interarticularis fractures.[27] It is inferred that MU strength provides biochemical stability and prevents further development of pars fractures. Although bone marrow signal changes were not considered in our research, a negative correlation was shown between SP and the MU CSA ratio. Higher SP indicates progression of IS, which is also a sign of segmental instability. MU atrophy and its correlation with progression of IS was conclusively shown in both studies.
Deep fibers of the MU have a higher percentage of Type 1 muscle fibers than other muscles of the lumbar spine.[28] These slow-twitch, fatigue-resistant fibers are more susceptible to the adverse effects of immobilization and pain than Type 2 fast-twitch fibers.[29] MU atrophy observed in subjects with IS in our study may be due to this discrepancy. Although medical records other than MRI images were not evaluated and patient symptoms could not be entirely accounted for, all subjects were either inpatients or outpatients in a hospital. This suggests that almost all of them had painful conditions. MU atrophy may also be due to posterior primary ramus denervation.[30] The posterior primary ramus of the lumbar nerve root splits into a lateral branch, which innervates the iliocostalis and skin, an intermediate branch, which innervates the longissimus, and a medical branch, which innervates the MU and facet joint.[31] The posterior primary ramus travels underneath the mammilo-accessory ligament, where it can be entrapped and stretched.[32,33] Segmental hypermobility or instability, which is frequently seen in spondylolisthesis, the posterior primary ramus may be stretched.[30] This can lead to denervation of the MU, eventually predisposing the muscle to atrophy.
To our knowledge, this is the first study demonstrating that PM atrophy is involved in progression of IS. PM CSA ratio negatively correlates with SP, but it is less influential than the MU CSA ratio. PM in the lumbar region acts as a vertical stabilizer rather than an extensor or a flexor.[34,35] Ipsilateral PM atrophy has been reported in unilateral back pain and sciatica with single-level disc herniation.[36,37] PM atrophy can be due to inhibition of a symptomatic vertebral level along a long loop reflex to protect compromised tissue.[38,39] This explanation adequately accounts for PM atrophy due to pain. However, it lacks a structural interpretation of PM atrophy, which is the focus of our research. Aggravation of coronal instability in degenerative scoliosis can theoretically cause stretching and thinning of the paraspinal musculature on the convex side with subsequent shortening and thickening of muscles on the concave side.[40] Similar changes are expected with increased sagittal instability due to spondylolisthesis because forward slip of a vertebra in spondylolisthesis leads to increased lumbar lordosis.[41] Since the PMs are located anterior to the spinal column, which is the more convex area in hyper-lordotic spines, CSA thinning can be predicted. This sufficiently explains structural changes and their relationship to PM.
Our research provides a basis for exercise and physiotherapy. Recently, specific exercise programs targeting the MU and deep abdominal muscles in the first stages of rehabilitation have been proposed.[42] Exercise therapy has been reported to decrease clinical symptoms and increase MU CSA in patients with LBP.[43,44] Exercises that incorporate PM coactivate MU and/or transversus abdominis.[36] Most research has focused on stretching the iliopsoas muscle with the assumption that stretching improves lumbar mobility.[45] However, an observational study on degenerative disc disease showed decreased pain after 8 weeks of PM strengthening exercises.[43] While the long-term relationship between lumbar PSM exercises and progression of spondylolisthesis has not been established, previous studies showed that selective MU and PM strengthening exercises can alleviate symptoms.[43,44] Based on the correlations shown in our research, strengthening of the MU and PM can also beneficial in preventing progression of IS as well as alleviating symptoms. According to our data, preventing progression of DS (SP increase) cannot be achieved by specific muscle strengthening exercises. Isolated MU strengthening exercises can be helpful in symptom control, as past research has shown muscle atrophy of the MU compared to normative controls.
The discrepancy between DS and IS conclusively suggests different approaches in the clinical management of spondylolisthesis. Simple X-rays should be considered for the differential diagnosis of IS and DS. Treatment of symptoms (pain, functionality) should be grossly identical because previous research has shown MU atrophy and ES hypertrophy compared to normal control groups in both cases. However, prophylactic exercise programs or interventions intended to prevent progression of IS and DS should be distinguished. One limitation of this study is that our data do not contain asymptomatic control groups. Therefore, the effect of symptoms such as pain was ignored. Also, due to the retrospective design of this study, we cannot be sure of the causal relationship of muscle CSA ratio and SP.
5. Conclusion
In this study, MU and PM CSA ratio were both negatively correlated with the SP of listhetic vertebrae in the isthmic group. Using simple linear regression models, MU was found to have more influence on SP than PM. None of the muscles were correlated with SP in the degenerative group. This discrepancy between two groups suggests different clinical approaches.
Acknowledgments
The authors would like to appreciate the contributions of all the participants.
Author contributions
Conceptualization: Jae-Hyun Park, Koh-Woon Kim, Jae-Heung Cho.
Data curation: Yousuk Youn, Won-Seok Chung, Mi-Yeon Song.
Formal analysis: Jae-Hyun Park, Won-Seok Chung, Jae-Heung Cho.
Investigation: Jae-Hyun Park, Koh-Woon Kim, Jae-Heung Cho.
Methodology: Koh-Woon Kim, Yousuk Youn, Mi-Yeon Song, Jae-Heung Cho.
Writing – original draft: Jae-Hyun Park, Jae-Heung Cho.
Writing – review & editing: Koh-Woon Kim, Hyungsuk Kim.
Koh-Woon Kim orcid: 0000-0002-0353-6041.
Footnotes
Abbreviations: CSA = cross-sectional area, DS = degenerative spondylolisthesis, ES = erector spinae, IS = isthmic spondylolisthesis, MRI = magnetic resonance imaging, MU = Multifidus, PM = psoas major, PSM = paraspinal muscle, SD = standard deviation, SP = slip percentage, VB = vertebral body.
How to cite this article: Park JH, Kim KW, Youn Y, Kim H, Chung WS, Song MY, Cho JH. Association of MRI-defined lumbar paraspinal muscle mass and slip percentage in degenerative and isthmic spondylolisthesis: a multicenter, retrospective, observational study. Medicine. 2019;98:49(e18157).
J-HP and K-WK contributed equally to this work.
This study was reviewed and approved by the Institutional Review Board of the Kyung Hee University Oriental Medical Center [KOMCIRB-170817-HR-028] and the Korean Medicine Hospital of Kyung Hee University at Gangdong [KHNMCOH 2017–09–006]. Any important protocol modifications were made known to relevant parties and/or individuals (e.g., investigators and IRB). All participants signed a written informed consent.
The authors have no conflicts of interest to disclose.
References
- [1].Matz PG, Meagher RJ, Lamer T, et al. Guideline summary review: An evidence-based clinical guideline for the diagnosis and treatment of degenerative lumbar spondylolisthesis. Spine J 2016;16:439–48. [DOI] [PubMed] [Google Scholar]
- [2].Kreiner DS, Baisden J, Mazanec DJ, et al. Guideline summary review: an evidence-based clinical guideline for the diagnosis and treatment of adult isthmic spondylolisthesis. Spine J 2016;16:1478–85. [DOI] [PubMed] [Google Scholar]
- [3].Farfan HF. The pathological anatomy of degenerative spondylolisthesis A cadaver study. Spine (Phila Pa 1976) 1980;5:412–8. [DOI] [PubMed] [Google Scholar]
- [4].Kauppila LI, Eustace S, Kiel DP, et al. Degenerative displacement of lumbar vertebrae. A 25-year follow-up study in Framingham. Spine (Phila Pa 1976) 1998;23:1868–73. discussion 1873-1864. [DOI] [PubMed] [Google Scholar]
- [5].Morgan FP, King T. Primary instability of lumbar vertebrae as a common cause of low back pain. J Bone Joint Surg Br 1957;39-B:6–22. [DOI] [PubMed] [Google Scholar]
- [6].Fredrickson BE, Baker D, McHolick WJ, et al. The natural history of spondylolysis and spondylolisthesis. J Bone Joint Surg Am 1984;66:699–707. [PubMed] [Google Scholar]
- [7].Beutler WJ, Fredrickson BE, Murtland A, et al. The natural history of spondylolysis and spondylolisthesis: 45-year follow-up evaluation. Spine (Phila Pa 1976) 2003;28:1027–35. discussion 1035. [DOI] [PubMed] [Google Scholar]
- [8].Kalichman L, Kim DH, Li L, et al. Spondylolysis and spondylolisthesis: prevalence and association with low back pain in the adult community-based population. Spine (Phila Pa 1976) 2009;34:199–205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Sakai T, Sairyo K, Takao S, et al. Incidence of lumbar spondylolysis in the general population in Japan based on multidetector computed tomography scans from two thousand subjects. Spine (Phila Pa 1976) 2009;34:2346–50. [DOI] [PubMed] [Google Scholar]
- [10].Nizard RS, Wybier M, Laredo JD. Radiologic assessment of lumbar intervertebral instability and degenerative spondylolisthesis. Radiol Clin North Am 2001;39:55–71. v-vi. [DOI] [PubMed] [Google Scholar]
- [11].Kalpakcioglu B, Altinbilek T, Senel K. Determination of spondylolisthesis in low back pain by clinical evaluation. J Back Musculoskelet Rehabil 2009;22:27–32. [DOI] [PubMed] [Google Scholar]
- [12].Nava-Bringas TI, Ramirez-Mora I, Coronado-Zarco R, et al. Association of strength, muscle balance, and atrophy with pain and function in patients with degenerative spondylolisthesis. J Back Musculoskelet Rehabil 2014;27:371–6. [DOI] [PubMed] [Google Scholar]
- [13].Wang G, Karki SB, Xu S, et al. Quantitative MRI and X-ray analysis of disc degeneration and paraspinal muscle changes in degenerative spondylolisthesis. J Back Musculoskelet Rehabil 2015;28:277–85. [DOI] [PubMed] [Google Scholar]
- [14].Ergun T, Sahin MS, Lakadamyah H. Evaluation of the relationship between L5-S1 spondylolysis and isthmic spondylolisthesis and lumbosacral-pelvic morphology by imaging via 2- and 3-dimensional reformatted computed tomography. J Comput Assist Tomo 2011;35:9–15. [DOI] [PubMed] [Google Scholar]
- [15].Thakar S, Sivaraju L, Aryan S, et al. Lumbar paraspinal muscle morphometry and its correlations with demographic and radiological factors in adult isthmic spondylolisthesis: a retrospective review of 120 surgically managed cases. J Neurosurg Spine 2016;24:679–85. [DOI] [PubMed] [Google Scholar]
- [16].Park HS, Kim JI, Kim KW, et al. The correlation between cross-sectional area of lumbar paraspinal muscles and spondylolisthesis: a retrospective study. J Korean Medicine Rehabilitation 2016;26:95–102. [Google Scholar]
- [17].Sun X, Lei SF, Deng FY, et al. Genetic and environmental correlations between bone geometric parameters and body compositions. Calcified Tissue Int 2006;79:43–9. [DOI] [PubMed] [Google Scholar]
- [18].Thakar S, Mohan D, Furtado SV, et al. Paraspinal muscle morphometry in cervical spondylotic myelopathy and its implications in clinicoradiological outcomes following central corpectomy: clinical article. J Neurosurg Spine 2014;21:223–30. [DOI] [PubMed] [Google Scholar]
- [19].Landis JR, Koch GG. The measurement of observer agreement for categorical data. Biometrics 1977;33:159–74. [PubMed] [Google Scholar]
- [20].Rosenberg NJ. Degenerative spondylolisthesis. Predisposing factors. J Bone Joint Surg Am 1975;57:467–74. [PubMed] [Google Scholar]
- [21].Micheli LJ, Wood R. Back pain in young athletes. Significant differences from adults in causes and patterns. Arch Pediatr Adolesc Med 1995;149:15–8. [DOI] [PubMed] [Google Scholar]
- [22].Sengupta DK, Herkowitz HN. Degenerative spondylolisthesis: review of current trends and controversies. Spine (Phila Pa 1976) 2005;30:S71–81. [DOI] [PubMed] [Google Scholar]
- [23].Takayama K, Kita T, Nakamura H, et al. New predictive index for lumbar paraspinal muscle degeneration associated with aging. Spine (Phila Pa 1976) 2016;41:E84–90. [DOI] [PubMed] [Google Scholar]
- [24].Moseley GL, Hodges PW, Gandevia SC. Deep and superficial fibers of the lumbar multifidus muscle are differentially active during voluntary arm movements. Spine (Phila Pa 1976) 2002;27:E29–36. [DOI] [PubMed] [Google Scholar]
- [25].Danneels LA, Vanderstraeten GG, Cambier DC, et al. CT imaging of trunk muscles in chronic low back pain patients and healthy control subjects. Eur Spine J 2000;9:266–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Ulmer JL, Elster AD, Mathews VP, et al. Lumbar spondylolysis - reactive marrow changes seen in adjacent pedicles on Mr-Images. Am J Roentgenol 1995;164:429–33. [DOI] [PubMed] [Google Scholar]
- [27].Borg B, Modic MT, Obuchowski N, et al. Pedicle marrow signal hyperintensity on short tau inversion recovery- and t2-weighted images: prevalence and relationship to clinical symptoms. AJNR Am J Neuroradiol 2011;32:1624–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Porterfield JA, DeRosa C. Mechanical Low Back Pain: Perspectives in Functional Anatomy. Philadelphia:W.B. Saunders; 1991. [Google Scholar]
- [29].Appell HJ. Muscular atrophy following immobilisation. A review. Sports Med 1990;10:42–58. [DOI] [PubMed] [Google Scholar]
- [30].Haig AJ, London Z, Sandella DE. Symmetry of paraspinal muscle denervation in clinical lumbar spinal stenosis: support for a hypothesis of posterior primary ramus stretching? Muscle Nerve 2013;48:198–203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Bogduk N, Wilson AS, Tynan W. The human lumbar dorsal rami. J Anat 1982;134:383–97. [PMC free article] [PubMed] [Google Scholar]
- [32].Fisher MA, Kaur D, Houchins J. Electrodiagnostic examination, back pain and entrapment of posterior rami. Electromyogr Clin Neurophysiol 1985;25:183–9. [PubMed] [Google Scholar]
- [33].Wu PB, Russel K HS. The dorsal ramus myotome: anatomical description and clinical implications in electrodiagnosis. Muscle Nerve 1991;14:887–8. [Google Scholar]
- [34].Hansen L, de Zee M, Rasmussen J, et al. Anatomy and biomechanics of the back muscles in the lumbar spine with reference to biomechanical modeling. Spine (Phila Pa 1976) 2006;31:1888–99. [DOI] [PubMed] [Google Scholar]
- [35].Santaguida PL, McGill SM. The psoas major muscle: a three-dimensional geometric study. J Biomech 1995;28:339–45. [DOI] [PubMed] [Google Scholar]
- [36].Barker KL, Shamley DR, Jackson D. Changes in the cross-sectional area of multifidus and psoas in patients with unilateral back pain: the relationship to pain and disability. Spine (Phila Pa 1976) 2004;29:E515–519. [DOI] [PubMed] [Google Scholar]
- [37].Dangaria TR, Naesh O. Changes in cross-sectional area of psoas major muscle in unilateral sciatica caused by disc herniation. Spine (Phila Pa 1976) 1998;23:928–31. [DOI] [PubMed] [Google Scholar]
- [38].Hides JA, Stokes MJ, Saide M, et al. Evidence of lumbar multifidus muscle wasting ipsilateral to symptoms in patients with acute/subacute low back pain. Spine (Phila Pa 1976) 1994;19:165–72. [DOI] [PubMed] [Google Scholar]
- [39].Cooper RG, St Clair Forbes W, Jayson MI. Radiographic demonstration of paraspinal muscle wasting in patients with chronic low back pain. Br J Rheumatol 1992;31:389–94. [DOI] [PubMed] [Google Scholar]
- [40].Syed HR, Yaeger K, Sandhu FA. Resolution of the more anteriorly positioned psoas muscle following correction of spinal sagittal alignment from spondylolisthesis: case report. J Neurosurg Spine 2017;26:441–7. [DOI] [PubMed] [Google Scholar]
- [41].Frymoyer JW, Pope MH, Clements JH, et al. Risk factors in low-back pain. An epidemiological survey. J Bone Joint Surg Am 1983;65:213–8. [DOI] [PubMed] [Google Scholar]
- [42].O'Sullivan PB, Phyty GD, Twomey LT, et al. Evaluation of specific stabilizing exercise in the treatment of chronic low back pain with radiologic diagnosis of spondylolysis or spondylolisthesis. Spine (Phila Pa 1976) 1997;22:2959–67. [DOI] [PubMed] [Google Scholar]
- [43].Kim S, Kim H, Chung J. Effects of spinal stabilization exercise on the cross-sectional areas of the lumbar multifidus and psoas major muscles, pain intensity, and lumbar muscle strength of patients with degenerative disc disease. J Phys Ther Sci 2014;26:579–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Danneels LA, Vanderstraeten GG, Cambier DC, et al. Effects of three different training modalities on the cross sectional area of the lumbar multifidus muscle in patients with chronic low back pain. Br J Sports Med 2001;35:186–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Jorgensson A. The iliopsoas muscle and the lumbar spine. Aust J Physiother 1993;39:125–32. [DOI] [PubMed] [Google Scholar]


