Abstract
The introduction of chemoimmunotherapy and more recently the implementation of novel agents into first-line and relapse treatment have substantially improved treatment outcomes in patients with chronic lymphocytic leukaemia (CLL). With longer progression-free survival and more frequently observed deep remissions there is an emerging need for sensitive methods quantitating residual disease after therapy. Over the last decade, assessment of minimal residual disease (MRD) has increasingly been implemented in CLL trials. The predictive value of MRD status on survival outcomes has repeatedly been proven in the context of chemoimmunotherapy and cellular therapies. Recent data suggests a similar correlation for Bcl-2 inhibitor-based therapy. While the relevance of MRD assessment as a surrogate endpoint in clinical trials is largely undisputed, its role in routine clinical practice has not yet been well defined.
This review outlines current methods of MRD detection in CLL and summarizes MRD data from relevant trials. The significance of MRD testing in clinical studies and in routine patient care is assessed and new MRD-guided treatment strategies are discussed.
Introduction
Treatment outcomes in chronic lymphocytic leukaemia (CLL) have improved considerably over the last decades with an increasing number of patients experiencing long lasting progression-free survival (PFS) after first-line therapy.1 This undeniably positive development does, however, pose a significant challenge to clinical researchers when designing trials that compare new, potentially superior therapies against a highly effective standard treatment. A primary endpoint solely based on PFS or overall survival (OS) could excessively prolong the course of the clinical trial and delay approval of new treatments regimens.
Minimal residual disease (MRD) in CLL is defined as the number of leukemic cells that can be detected in peripheral blood (PB) or bone marrow (BM) following treatment. Undetectable MRD (uMRD) is currently defined as the presence of less than 1 CLL cell in 10,000 leukocytes (<10−4).2
When the first deep responses were achieved by combining fludarabine with cyclophosphamide (FC), detection of minimal residual disease slowly gained momentum in CLL.3 Over the last decades, the significance of MRD in CLL has steadily grown. Finally, several large randomized controlled trials (RCT) showed that MRD status after induction treatment is an independent predictor of survival and progression-free survival.4–13 Based on these data, in 2016 the European Medicines Agency (EMA) in contrast to the FDA allowed the use of uMRD as an intermediate endpoint in RCTs that were used for drug approval.14
While this regulatory decision has underscored the significance of MRD assessment in the context of clinical trials, it remains unclear whether routine MRD testing should also be implemented in clinical practice.15–17 In this review, we discuss current methods for MRD testing, MRD data from relevant trials, new MRD-guided treatment strategies and the role of MRD assessment in patient care outside of clinical trials.
Methods of MRD testing
The majority of CLL patients are not cured after frontline treatment but have some degree of residual disease.1,18 Over the last decades, residual disease status after treatment has been quantified by physical examination, blood counts, imaging studies and MRD status. MRD detection is performed using peripheral blood (PB) or bone marrow aspirate (BM). A compartment effect has been demonstrated with some substances being more effective in PB than in the BM. Hence, differences in the MRD levels of PB and BM can be observed, which are of relevant prediction for the duration of response.8
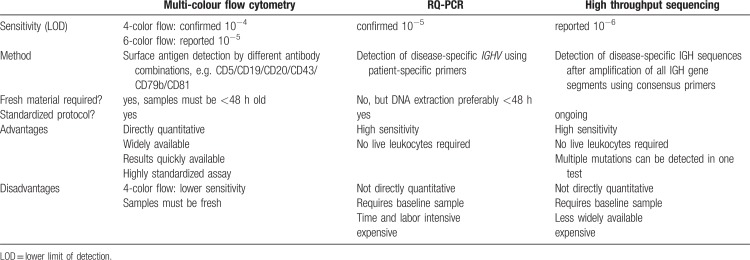
The established MRD detection level of <10−4 in PB can currently be achieved by 3 different methods: multi-colour flow cytometry, real-time quantitative polymerase chain reaction (RQ-PCR) and high-throughput sequencing (HTS), each with their specific advantages (Table 1).
Table 1.
Differences Between Multi-colour Flow Cytometry, RQ-PCR-, and HTS-Based Detection Methods
The European Research Initiative on CLL (ERIC) has established a harmonized multicolour flow cytometry (FCM) protocol that has been widely implemented and constantly optimized over the last years.19–22 The widespread use of this standardized assay in prospective CLL trials has led to highly comparable MRD results.
The antibody panel according to the ERIC protocol distinguishes B-cells from other leukocytes by using CD19 expression and differentiates normal B-cells from CLL cells based on their CD5, CD20, CD43, CD79b and CD81 expression.20,22 In contrast to PCR-based methods this assay does not need pre-treatment samples and can be adapted by most laboratories using cytometers with 6 or more colours. The panel can be extended beyond the 6 marker core panel by combination with additional markers, if necessary. Using this simple and reliable method, a detection limit of 10−5 can be achieved. Although studies correlating different MRD detection limits with clinical outcomes are sparse, it is conceivable that post-treatment MRD below a level of 10−5 or 10−6 might be associated with even longer survival when compared to standard uMRD as defined by the iwCLL guidelines (<10−4).
A detection limit below 10−5 has also been reported for MRD assessment methods using RQ-PCR with patient-specific primers.23–27 In this method, the disease-specific IGHV is sequenced and allele-specific oligonucleotide (ASO) probes are designed in order to expand the malignant IGHV gene during PCR. As opposed to consensus PCR approaches, the assay using patient-specific primers and RQ-PCR methodology is quantitative. While this method also achieves a detection limit of 10−5 it is less widely used, as it is more time and labour intensive and costly. Moreover, the detection limit for each specific patient primer set has to be determined and IGHV sequencing before treatment start is necessary. Its main advantage compared to flow cytometry is that it can be performed on less material and non-fresh samples including frozen samples. In contrast to RQ-PCR approaches, digital droplet PCR (ddPCR) does not require preparation of a disease-specific reference standard curve. IGH-based MRD assessment methods using ddPCR are currently developed by numerous groups.
Another DNA-based method of MRD detection that does not require patient-specific primers is high-throughput IGH sequencing (IGH-HTS).28 This method uses consensus primers for amplification of all IGH genes followed by HTS for quantification of the disease-specific IGH sequence that has been identified in pre-treatment blood samples.11 With this approach, an MRD detection limit of 10−6 is reported to be achievable.20
Multi-colour flow cytometry remains the most widely applied method for MRD assessment owing to its extensive availability, its degree of standardization, its reliable detection of currently applied MRD limits (<10−4) and relatively low costs.19 In treatment strategies aiming at disease eradication (e.g. allogeneic stem cell transplantation [allo-HCT] or possibly in the future CAR T-cell therapy), there is a rationale for using more sensitive methods for MRD detection like HTS.
If future studies show that lower post-treatment MRD levels of <10−6 translate into improved survival in CLL patients, HTS, alone or in combination with flow cytometry assays, could become the future gold standard of MRD assessment in CLL.
MRD assessment in clinical trials
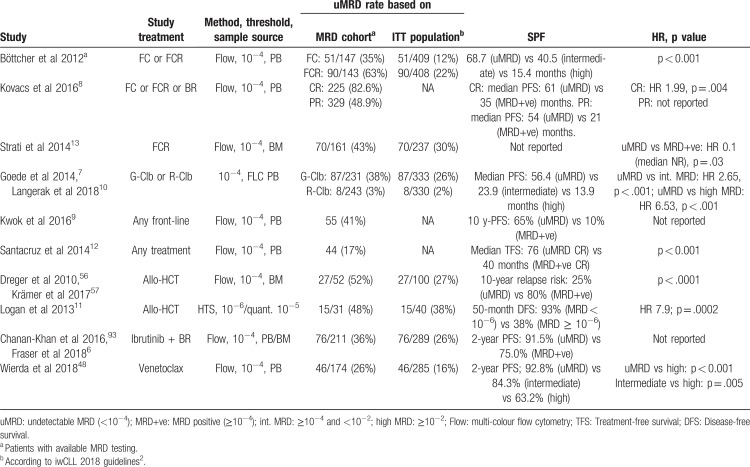
MRD was assessed in a large number of pivotal CLL trials using diverse treatments and different MRD detection methods. In numerous of these trials, MRD correlated with survival (Table 2). Shown uMRD rates of randomized trials below in the text are based on Intention-to-treat (ITT) populations if not otherwise specified.
Table 2.
Key Data Establishing the Association of Posttreatment MRD and Clinical Outcome
MRD with chemotherapy (CT) and chemoimmunotherapy (CIT)
Chemoimmunotherapy with fludarabine, cyclophosphamide and rituximab (FCR) was the first treatment regimen besides allo-HCT showing remarkable rates of uMRD. In the German CLL Study Group (GCLLSG) CLL8 trial, MRD was analyzed in patients receiving FC or FCR treatment, with 35% (ITT-based: 12%) of FC-treated patients achieving uMRD (<10−4) in PB vs 63% (ITT-based: 22%) after FCR chemoimmunotherapy.29 uMRD after end of treatment (EOT) was associated with significantly longer PFS than intermediate (≥10−4 to <10−2) or high MRD (≥10−2).4 Additionally, uMRD seemed to correlate better with PFS than clinical response assessment. Another analysis also showed that patients with uMRD and a partial response (PR) due to residual splenomegaly had similar outcomes as patients with uMRD and a complete response (CR).8
The superiority of FCR in achieving uMRD was also shown compared to bendamustine/rituximab (BR) chemoimmunotherapy in the GCLLSG CLL10 trial. Patients treated with BR achieved uMRD less frequently than those who received FCR (ITT-based: 38% vs 49% in PB; p = 0.041; MRD cohort: 63% vs 74%; p = 0.029).30
A predictive effect of PB uMRD was also shown in patients treated with chlorambucil and obinutuzumab (G-Clb) in the GCLLSG CLL11 trial.10 Patients achieving uMRD after EOT showed significantly prolonged PFS compared to those with positive MRD testing (not reached [NR] vs 19.4 months).7
On the basis of these 3 trials (CLL8, CLL10, CLL11), Dimier et al designed a predictive model, that for the first time quantitated the relationship between the effect of treatment on MRD and the effect of treatment on PFS.5 Consequently it allows prediction of treatment effect on PFS using treatment effect on PB MRD, which will prove useful in the design of MRD-based end points and the calculation of sample sizes in large clinical trials.31 As the prediction model is solely based on CT/CIT trials, it remains unclear whether it can be used to estimate relationships between MRD and PFS in trials using novel agents.
MRD assessment with novel agents
An overview of MRD data from clinical trials using novel agents can be found in Table 3.
Table 3.
Overview of MRD Data from Relevant Studies of Novel Agents
Kinase inhibitors
Ibrutinib
The Bruton Tyrosine Kinase (BTK) inhibitor ibrutinib has shown promising results in previously untreated and relapsed/refractory (r/r) CLL patients and has therefore been widely approved as a monotherapy in both settings.32–35 While CR rates increase gradually during the course of treatment, they remain low and uMRD is only achieved by a minority of patients. Ahn and colleagues have reported a uMRD rate (FCM, <10−4) of 5/49 (10.2%; ITT-based: 5.8%) in PB after 4 years of ibrutinib treatment.36 Additionally, the group compared PFS in patients with low MRD (<10−2) and high MRD (≥10−2) after 3 years of ibrutinib and no statistical difference could be shown.
While uMRD is not regularly achievable in ibrutinib monotherapy, combinations of ibrutinib with CIT or anti-CD20 monoclonal antibodies have shown higher uMRD rates. In the recent HELIOS trial of ibrutinib and BR (BR+I) vs BR only, the reported PB uMRD (FCM) rate at 36 months was 26.3% in the BR+I arm. The 3-year PFS rate for patients with uMRD was significantly higher than those for patients with MRD ≥ 10−4 (88.6% vs 60.1%).6
Other currently investigated front-line combinations include ibrutinib and rituximab (IR) and ibrutinib and obinutuzumab (IG). While uMRD seemed rare in IR, treatment with IG has shown a substantial PB MRD negativity rate of 35% in the recently published phase 3 iLLUMINATE trial.37–39
Combinations of ibrutinib and anti-CD20-antibodies after an optional debulking with bendamustine were also tested in the CLL2-BIG and CLL2-BIO trial, in which the time-limited combination treatment of ibrutinib and obinutuzumab led to PB uMRD in 48% (FCM, ITT-based: 44%) of the patients whereas the combination of ibrutinib and ofatumumab only yielded a PB uMRD rate of 14% (FCM, ITT-based: 14%).40,41
An intensive treatment regime consisting of ibrutinib, fludarabine, cyclophosphamide and obinutuzumab (iFCG) has recently yielded a BM uMRD rate of 90% (FCM, ITT-based: 88%) after only 3 months of treatment in a cohort of IGHV mutated patients.42
Undetectable MRD is rarely achieved in ibrutinib monotherapy, thus the assessment of MRD is not informative in this context. Its assessment is certainly reasonable in more intensive combination treatments using ibrutinib. As uMRD rates gradually improve over the course of ibrutinib treatment it may be sensible to set different time points for MRD assessment in trials using BTK inhibitors than those defined for CIT.
Other kinase inhibitors
No MRD data have yet been reported for the pivotal studies of the PI3K inhibitor idelalisib in combination with rituximab or with BR, as well as for the newer PI3K inhibitor duvelisib.43 The currently ongoing CLL2-BCG trial will show whether deep remissions can be achieved by combining idelalisib with obinutuzumab. The CLLRUmbrella1 and 2 studies will assess whether dual kinase inhibition (PI3K/ BTK in Umbrella 1 or SYK/BTK in Umbrella 2) in combination with obinutuzumab can lead to high rates of uMRD and thus MRD-based treatment termination.44
Trials evaluating the use of other BTK inhibitors like acalabrutinib or zanubrutinib have not reported MRD data so far.45
Venetoclax
The Bcl-2 inhibitor venetoclax has shown very impressive response rates in r/r CLL patients with or without high risk cytogenetics.46,47 A comprehensive MRD analysis of the two large phase II trials M13-982 and M14-032 evaluated venetoclax monotherapy in mostly r/r CLL patients with or without del (17p).48 MRD in PB was <10−4 (FCM) in 26% of the 174 patients with available MRD assessment. Among MRD-positive patients 23% showed intermediate (≥10−4 to <10−2) MRD levels and 51% had high MRD (≥10−2). PFS rates at 24 months were significantly higher for patients who achieved uMRD or intermediate MRD levels compared with patients who never achieved MRD < 10−2 (92.8%, 84.3%, and 63.2% respectively).
Combinations of venetoclax with antibodies have yielded even higher rates of uMRD.49 The MURANO study that investigated the use of rituximab and venetoclax in r/r CLL, showed uMRD in PB in 62% of all patients 2 to 3 months after the end of combination treatment with uMRD translating into considerably longer PFS.49,50
In the CLL2-BAG study, an optional debulking with bendamustine was followed by an induction and MRD-guided maintenance treatment of venetoclax and obinutuzumab (VG) in patients with treatment-naïve or r/r CLL.44 After induction treatment, a rate of PB uMRD of 87% (ITT-based: 83%) was achieved, with the majority of patients completing maintenance treatment at the earliest possible time point due to confirmed uMRD. The same drug combination has been investigated in the large phase III CLL14 trial. An interim safety analysis that comprised 12 patients showed PB uMRD in 10/12 (83%) patients.51 The phase II HOVON139 trial used the same combination with a different treatment scheme: after 2 months of obinutuzumab, 6 months of combined VG and 6 additional months of venetoclax 26 of 28 assessed patients (92.9%) achieved uMRD in PB.52
The oral combination treatment of ibrutinib and venetoclax has also shown promising preliminary results. In the phase II CLARITY trial, 23/40 (58%) patients with r/r CLL have achieved PB uMRD (<10−4) after 12 months of combined therapy, out of which 13 (33%) have even reached MRD levels of <10−5.53 In another phase II trial of ibrutinib and venetoclax in treatment-naïve CLL patients, the reported rate of BM uMRD at 12 months was 17/25 (68%).54 In both trials, the proportion of patients with uMRD increased steadily over the treatment course, promising even higher rates of uMRD with longer follow-up data available.
In summary combination treatments using ibrutinib and/or venetoclax yield high rates of uMRD in PB and BM that appear to increase with longer exposure to the targeted agents. First analyses have associated uMRD with increased PFS in patients treated with venetoclax-containing regimens, but more mature data from large trials are required to confirm the value of MRD status as an independent predictive factor for survival in the context of novel agents.
MRD assessment in allo-HCT and CAR T-cell therapy
Allo-HCT is a procedure with curative potential in CLL, yielding high rates of uMRD. Post-allo-HCT detection of residual disease is closely associated with impaired overall survival. In the prospective CLL3X trial, in which 90 patients have undergone allo-HCT, 10-year-OS was 51%, indicating that a certain proportion of patients can be considered cured after allo-HCT.55 MRD status of these patients at the 12-month landmark post-allo-HCT highly correlated with long-term clinical outcome. Patients who had BM uMRD at this time point showed a significantly lower 10-year relapse incidence than those with detectable MRD (25% vs 80%, p < 0.0001).56
Studies investigating anti-CD19 CAR T-cell therapies have yielded promising, but still limited results in extensively pre-treated patients with CLL.57 In the initial trial by Porter et al, 4 of 14 patients, who were treated with CAR T-cell therapy have achieved a CR with no evidence of MRD in PB and BM assessed by deep sequencing 1 or 3 months after CAR T-cell infusion. In none of the patients with uMRD and a CR relapse has been reported so far.58
With the addition of ibrutinib to CAR T-cell therapy, high clinical response rates as well as a high proportion of patients with uMRD might be achievable. Gauthier et al report BM uMRD by flow cytometry in 12/16 (75%) of all evaluable patients who received additional ibrutinib vs 11/18 (65%) in patients not treated with ibrutinib.59 In another recent study, 19 patients were treated with anti-CD19 CAR T-cells and ibrutinib. Of these, 15 patients had BM uMRD by flow cytometry and 14 by IGH sequencing 3 months after CAR T-cell infusion, at 12 months, 7 of 11 patients with evaluable BM remained without detectable MRD.60
Limitations of MRD
MRD eradication: a goal for every patient?
In light of these data, absence of MRD is a goal for CLL patients, in particular when fixed-duration therapies are used. Given the excellent results obtained by continuous therapy with BTK-inhibitors such as ibrutinib, the question is whether MRD eradication should be a treatment goal in every patient.36,39,61
Due to the median age of 72 years at diagnosis, the majority of CLL patients have comorbid conditions that may lead to ineligibility for intensive treatment regimens aiming at uMRD.1,62–65 In older patients with comorbidities and co-medication combinations of 2 or more antileukemic drugs may lead to higher toxicities and worse tolerability.66 Thus, patients with comorbidities, whose life expectancy is limited due to conditions other than CLL or simply due to advanced age might not always benefit from a maximization of treatment-free intervals but may profit from less intensive treatment strategies aiming at durable disease control rather than residual disease eradication at all costs. Despite low CR and uMRD rates, ibrutinib monotherapy has shown excellent progression-free survival (18-month PFS on ibrutinib in the pivotal RESONATE-2 trial: 89%, 2-year PFS in the ALLIANCE A041202 trial: 87%), durable remissions and a favourable safety profile as well as a considerable increase in patient-reported quality of life.39,67,68 These results demonstrate that achievement of uMRD may not be required to achieve long-term control of CLL.
Undetectable MRD is not equivalent to eradicated MRD
CLL is a disease that virtually always involves multiple compartments including blood, bone marrow, lymph nodes, spleen and liver, as well as cerebrospinal fluid, lung, bones, kidneys or skin.69–71 Several studies have shown that certain agents, in particular anti-CD20-antibodies, preferentially deplete cells in the peripheral blood and have less efficacy in other compartments.72 Consequently, MRD assessment in PB after anti-CD20-antibody-containing therapies might lead to an underestimation of residual disease across all compartments. In contrast, B-cell receptor pathway inhibitors (BCRi) like ibrutinib and idelalisib have been associated with a redistribution of tissue-residing CLL cells into the blood, often leading to a transient lymphocytosis.61 In light of these varyingly concordant results of residual disease assessment in bone marrow and peripheral blood, the EMA has stated that “if MRD is not detectable in PB, it is mandatory to confirm MRD status in the BM” in their approval of MRD status as an intermediate endpoint for licensure.14
However, even in absence of residual disease in the bone marrow, patients may have significant residual disease in unassessed sites. In a recent analysis, we have shown that residual abdominal lymphadenopathy after front-line CIT is associated with inferior PFS and OS regardless of MRD status, suggesting that in spite of uMRD, patients’ lymph nodes act as a reservoir for residual disease and consequently cause relapse.73
With current methods of MRD assessment showing limitations in detecting residual disease in the lymphatic system, the development of more sensitive methods like circulating tumour DNA (ctDNA)-based approaches is underway and promises a more comprehensive monitoring of disease burden across different topographical sites within the body.74
Until these refined methods can be utilized it is sensible to continue using a multimodal approach that includes MRD assessment, physical examinations and imaging studies to estimate residual disease burden.
Predictive value of MRD status in relationship to disease biology
The predictive value of uMRD is influenced by several factors as treatment modalities, the residual disease site and particularly by subclones of the disease, which are not necessarily measured by a quantitative method like MRD assessment.75–79 Long-term follow-up data from the original phase II FCR cohort at MD Anderson Cancer Center (MDACC) showed a 12.8-year PFS rate of 79.8% for patients with mutated IGHV that had uMRD after CIT.80 Interestingly, patients with mutated IGHV, who were MRD positive had superior 12.8-year PFS compared to patients with uMRD and unmutated IGHV (36.9% vs 16.3%) despite the evident residual disease after completion of CIT.
These data are in line with the work of Dimier et al that established the validity of MRD status as a surrogate for PFS.5 Their calculations showed that approximately one third of the variability in the PFS hazard ratio (HR) could be attributed to the observed MRD results, which in turn implies that a considerable part of the variability is caused by factors other than MRD status, for instance disease biology.
These observations demonstrate that re-growth of the malignant clone and subsequent timing of relapse cannot be sufficiently described by the solely quantitative technique of MRD detection but needs to be complemented by biological information on the disease like IGHV, TP53 status or other mutations like ATM, SF3B1, BIRC3, and NOTCH1.
Using MRD to guide treatment decisions
The improved treatment outcomes due to novel drugs allow further refinement and individualization of treatment strategies, possibly with the help of MRD assessment. Interim MRD analysis could be used to identify patients who benefit from treatment de-escalation or cessation to avoid unnecessary treatment-related toxicity. Similarly, assessment of MRD, together with genetic aspects, could identify patient groups in need of longer or more intensive treatment.
In BCR-ABL positive chronic myeloid leukemia (CML), MRD assessment is an integral part of the treatment strategy.81 Patients who achieve durable and deep molecular responses may discontinue treatment with BCR-ABL-directed TKIs with a good chance of entering a treatment-free period. Increase in BCR-ABL transcripts will prompt reinitiation of treatment in these patients. Although CLL does not have a single, targetable genetic aberration, a similar model could possibly be used in CLL in the future.
Currently evaluated MRD-guided treatment strategies
A retrospective analysis of the MDACC evaluated the outcome of FCR-treated patients who achieved uMRD after 3 (of 6 planned) treatment cycles.13 Patients with uMRD who stopped treatment after 3 cycles had similar PFS and OS as patients with uMRD who continued to receive additional FCR courses, suggesting that MRD-guided stopping of FCR treatment after 3 cycles is feasible without affecting long term survival and possibly sparing unnecessary treatment-related toxicities. Based on these observations and various sub-analyses, a risk-adapted treatment strategy for the first-line treatment of CLL is proposed, that includes interim MRD assessment as well as risk categorization on the basis of disease biology.76 Besides an MRD-triggered end of treatment, the scheme envisages yearly follow-up MRD analyses and early treatment interventions in the case of molecular relapse.
While this exact strategy needs to be validated in the context of large prospective trials, further non-randomized MRD-guided approaches have already been tested. One trial evaluated the MRD-guided use of ibrutinib, fludarabine, cyclophosphamide and obinutuzumab (iFCG) in the first-line treatment of patients with a favourable genetic risk profile (IGHV mutated, no del (17p)/TP53 mutation).42 After 3 courses of iFCG, BM MRD was assessed and patients with uMRD and CR (i) received 3 additional cycles of ibrutinib and obinutuzumab (IG) and 6 more cycles of ibrutinib (I). Patients who did not achieve uMRD, received 9 additional cycles of IG after iFCG, uMRD at 1 year prompted stopping of all therapy. Seventeen of 42 (40.4%) patients had uMRD and CR (i) and thus received the reduced intensity treatment while all 28 patients who reached the 12 months time-point had uMRD and stopped therapy per protocol.
In the CLL2-BAG trial, optional bendamustine debulking was followed by obinutuzumab (month 1–8) and venetoclax (month 2–8) induction treatment and a maintenance treatment consisting of the same agents with a flexible duration between 6 and 24 months.44 Undetectable MRD in PB, confirmed by 2 subsequent FCM measurements in patients with (clinical) CR (i) led to treatment termination. At the end of induction treatment, 55/63 (87%) analyzed patients had achieved uMRD in PB and at the latest data cut, 21 patients had regularly finished maintenance treatment thanks to uMRD. The remissions seem durable after EOT, even in patients with genetic high risk features.82
Another individualized approach to MRD-guided treatment decisions is evaluated by Hillmen et al in the context of the phase II CLARITY trial with ibrutinib and venetoclax (IV).53 The group uses an innovative MRD-guided treatment scheme that extrapolates the optimal treatment duration from the time it took to achieve uMRD. Patients are tested for MRD after 6 and 12 months of combined treatment and continue IV for the same duration of time it took them to achieve uMRD. 24% of all patients showed uMRD in BM after 6 months and could thus finish treatment after 12 months of IV; PB uMRD rate at 12 months was 58%. This method of individualized treatment durations depending on time to uMRD is currently tested in the large phase III FLAIR trial comparing FCR, I, IR and IV in previously untreated CLL.83
Another reasonable use of MRD could be the implementation of MRD-guided decision-making in the context of the now approved MURANO treatment scheme. It is not clear if MRD-positive patients should continue on single agent venetoclax while the data clearly show that patients with uMRD can stop after 24 months of venetoclax treatment.
MRD-guided treatment in CLL: too complex for routine practice?
As the example of CML treatment shows, an ideal MRD-guided treatment strategy in CLL would have to be simple, affordable, use widely available methods and improve treatment outcomes in terms of safety and/or efficacy.
The above mentioned examples of currently investigated MRD-guided treatment strategies seem to either improve tolerability by sparing drug exposure or efficacy by prompting extended treatment for those who require it. However, so far it is unclear how patients with detectable MRD after induction should be further treated. It has to be assessed whether these patients should just continue on the same treatment if applicable or if patients should change the substance, particularly when new subclones are detected.84
Considering that in CLL not only MRD levels, but also genetic aberrations in subclones could be considered for future treatment approaches, it will be a challenge to transfer the complexity of these approaches into clinical practice.
Other than in BCR-ABL positive CML, CLL disease activity cannot be comprehensively captured by one method. Patients might for instance show fulminant nodal relapses that are not detectable by flow cytometry and the value of uMRD greatly depends on the therapeutic agents that are used. Furthermore, the appropriate time points for MRD assessment have not yet been established in the context of novel agents and access to MRD detection methods is still limited.
Until a simple and cost-effective MRD-guided treatment approach is implemented in clinical routine, further exploration of MRD-guided treatment strategies in clinical trials will help to determine ideal time points to assess MRD and identify appropriate treatment durations for different patient groups. Once these groups are well defined, it might even be more practicable in routine patient care to use MRD-independent treatment schemes with different yet fixed durations that are selected depending on individual risk factors.
Outside of clinical trials there are only a few scenarios, in which MRD assessment is indicated or could be at least considered.
Highly sensitive assessment of residual disease is particularly vital in treatment settings aiming at cure of CLL like allo-HCT and CAR T-cell therapy. In allo-HCT continued serial assessments are useful to detect low-level molecular relapse that might influence immunosuppression or maintenance therapy.55
MRD testing can also be useful in selected patients receiving highly effective combination treatments of novel agents, as venetoclax plus rituximab outside of clinical trials to evaluate treatment outcome and allow for prognostic estimations.44,50,52,53,82,85
In contrast, in patients receiving indefinite treatments that rarely lead to uMRD (e.g. ibrutinib, idelalisib), MRD assessment might not be routinely indicated as it appears to lack predictive value.36 Conversely, positive MRD results might worry patients and necessitate additional counselling.
According to the recently updated iwCLL guidelines, symptomatic disease remains the main criterion for treatment and MRD status should not play a role in establishing treatment indications outside of clinical trials and the post-allo-HCT setting.2
Whether MRD testing will indeed be utilized as comprehensively as currently foreseen, will ultimately depend on which treatment paradigm will prevail in the near future: time-limited combination therapies of novel agents aiming at uMRD or indefinite treatment with 1 substance aiming at durable disease control rather than eradication. Direct randomized comparisons of these 2 concepts are much-needed to advance this debate. Shanafelt et al recently suggested a survival benefit in patients treated with an indefinite ibrutinib-based regimen (IR) when compared to FCR-treated patients in the randomized E1912 trial, making a compelling case for indefinite treatment approaches even in fit patients.86 Despite this data, indefinite treatment may come with drawbacks that are not captured by survival curves, an increased financial burden, higher cumulative toxicities or development of resistance mutations.76,87–89 In the light of the promising results from the above mentioned, time-limited and MRD-guided CLARITY (IV) and CLL2-BAG (GV) combinations, results from the phase III FLAIR (FCR vs I vs IR vs IV) and GAIA/CLL13 (FCR/BR vs RV vs GV vs GIV) trials are eagerly awaited to fuel this discussion with new data.83,90
Conclusions
The addition of rituximab to chemotherapy and more recently the implementation of novel agents into first-line and relapse treatment have led to substantially prolonged survival in CLL patients, raising the need for new study and treatment endpoints. By allowing the use of MRD status as an intermediate endpoint for licensure, the European Medicines Agency has responded to this need and established the basis for an expedited access to new CLL treatments.
Undetectable MRD and longer PFS also seem to correlate in first analyses of venetoclax-based regimens, while this association has not yet been shown for the first-line approved and widely used monotherapy with ibrutinib as well as for other kinase inhibitors. In any case, the predictive value of MRD status on survival has to be prospectively evaluated for all different treatment regimens, ideally including detailed analyses of different genetic subgroups.
New MRD-guided treatment approaches will probably soon find their way into clinical practice, allowing for a more individualized therapy, possibly leading to less treatment-related toxicities and better outcomes. Until this has become reality, it is necessary to eliminate the remaining weaknesses of current MRD assessment approaches and develop more sensitive methods to also detect residual disease beyond the BM and PB compartment.
The joint effort of ERIC and other CLL groups that has ultimately led to highly standardized methods for MRD detection by flow cytometry can serve as a model in this process to not only standardize the use of MRD assessment but to also make it more affordable and accessible in the routine clinical setting.
Footnotes
Citation: Fürstenau M,De Silva N, Eichhorst B, Hallek M. Minimal Residual Disease Assessment in CLL: Ready for Use in Clinical Routine?. HemaSphere, 2019;00:00–00. http://dx.doi.org/10.1097/HS9.0000000000000287.
All authors participated in researching and analyzing the data, writing and reviewing the manuscript.
Barbara Eichhorst (Research grants from Abbvie, Gilead, Janssen, Roche. Honoraria and advisory boards from the same companies and Celgene and Novartis in addition) and Michael Hallek (Research support for Roche, Gilead, Mundipharma, Janssen, Celgene, Pharmacyclics, Abbvie. Speakers bureau and Advisory Board: Roche Gilead, Mundipharma, Janssen, Celgene, Pharmacyclics, Boehringer).
Moritz Fürstenau and Nisha De Silva declare no conflict of interest.
References
- 1.Hallek M, Shanafelt TD, Eichhorst B. Chronic lymphocytic leukaemia. Lancet (London, England) 2018; 391:1524–1537. [DOI] [PubMed] [Google Scholar]
- 2.Hallek M, Cheson BD, Catovsky D, et al. iwCLL guidelines for diagnosis, indications for treatment, response assessment, and supportive management of CLL. Blood 2018; 131:2745–2760. [DOI] [PubMed] [Google Scholar]
- 3.Eichhorst BF, Busch R, Hopfinger G, et al. Fludarabine plus cyclophosphamide versus fludarabine alone in first-line therapy of younger patients with chronic lymphocytic leukemia. Blood 2006; 107:885–891. [DOI] [PubMed] [Google Scholar]
- 4.Bottcher S, Ritgen M, Fischer K, et al. Minimal residual disease quantification is an independent predictor of progression-free and overall survival in chronic lymphocytic leukemia: a multivariate analysis from the randomized GCLLSG CLL8 trial. J Clin Oncol: Off J Am SocClin Oncol 2012; 30:980–988. [DOI] [PubMed] [Google Scholar]
- 5.Dimier N, Delmar P, Ward C, et al. A model for predicting effect of treatment on progression-free survival using MRD as a surrogate end point in CLL. Blood 2018; 131:955–962. [DOI] [PubMed] [Google Scholar]
- 6.Fraser G, Cramer P, Demirkan F, et al. Updated results from the phase 3 HELIOS study of ibrutinib, bendamustine, and rituximab in relapsed chronic lymphocytic leukemia/small lymphocytic lymphoma. Leukemia 2019; 33:969–980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Goede V, Fischer K, Busch R, et al. Obinutuzumab plus chlorambucil in patients with CLL and coexisting conditions. N Engl J Med 2014; 370:1101–1110. [DOI] [PubMed] [Google Scholar]
- 8.Kovacs G, Robrecht S, Fink AM, et al. Minimal residual disease assessment improves prediction of outcome in patients with chronic lymphocytic leukemia (CLL) who achieve partial response: comprehensive analysis of two phase III studies of the German CLL study group. J Clin Oncol: Off J Am Soc Clin Oncol 2016; 34:3758–3765. [DOI] [PubMed] [Google Scholar]
- 9.Kwok M, Rawstron AC, Varghese A, et al. Minimal residual disease is an independent predictor for 10-year survival in CLL. Blood 2016; 128:2770–2773. [DOI] [PubMed] [Google Scholar]
- 10.Langerak AW, Ritgen M, Goede V, et al. Prognostic value of MRD in CLL patients with comorbidities receiving chlorambucil plus obinutuzumab or rituximab. Blood 2019; 133:494–497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Logan AC, Zhang B, Narasimhan B, et al. Minimal residual disease quantification using consensus primers and high-throughput IGH sequencing predicts post-transplant relapse in chronic lymphocytic leukemia. Leukemia 2013; 27:1659–1665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Santacruz R, Villamor N, Aymerich M, et al. The prognostic impact of minimal residual disease in patients with chronic lymphocytic leukemia requiring first-line therapy. Haematologica 2014; 99:873–880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Strati P, Keating MJ, O’Brien SM, et al. Eradication of bone marrow minimal residual disease may prompt early treatment discontinuation in CLL. Blood 2014; 123:3727–3732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.European Medicines Agency. Appendix 4 to the guideline on the evaluation of anticancer medicinal products in man – condition specific guidance. Ref number: EMA/CHMP/703715/2012 Rev. 2. Published Feb 15, 2016. [Google Scholar]
- 15.Ghia P, Rawstron A. Minimal residual disease analysis in chronic lymphocytic leukemia: a way for achieving more personalized treatments. Leukemia 2018; 32:1307–1316. [DOI] [PubMed] [Google Scholar]
- 16.Owen C, Christofides A, Johnson N, et al. Use of minimal residual disease assessment in the treatment of chronic lymphocytic leukemia. Leukemia Lymphoma 2017; 58:2777–2785. [DOI] [PubMed] [Google Scholar]
- 17.Thompson M, Brander D, Nabhan C, et al. Minimal residual disease in chronic lymphocytic leukemia in the era of novel agents: a review. JAMA Oncol 2018; 4:394–400. [DOI] [PubMed] [Google Scholar]
- 18.Thompson PA, Wierda WG. Eliminating minimal residual disease as a therapeutic end point: working toward cure for patients with CLL. Blood 2016; 127:279–286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rawstron AC, Bottcher S, Letestu R, et al. Improving efficiency and sensitivity: European Research Initiative in CLL (ERIC) update on the international harmonised approach for flow cytometric residual disease monitoring in CLL. Leukemia 2013; 27:142–149. [DOI] [PubMed] [Google Scholar]
- 20.Rawstron AC, Fazi C, Agathangelidis A, et al. A complementary role of multiparameter flow cytometry and high-throughput sequencing for minimal residual disease detection in chronic lymphocytic leukemia: an European Research Initiative on CLL study. Leukemia 2016; 30:929–936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rawstron AC, Kreuzer KA, Soosapilla A, et al. Reproducible diagnosis of chronic lymphocytic leukemia by flow cytometry: an European Research Initiative on CLL (ERIC) & European Society for Clinical Cell Analysis (ESCCA) Harmonisation project. Cytometry Part B Clin Cytometry 2018; 94:121–128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rawstron AC, Villamor N, Ritgen M, et al. International standardized approach for flow cytometric residual disease monitoring in chronic lymphocytic leukaemia. Leukemia 2007; 21:956–964. [DOI] [PubMed] [Google Scholar]
- 23.Bottcher S, Ritgen M, Pott C, et al. Comparative analysis of minimal residual disease detection using four-color flow cytometry, consensus IgH-PCR, and quantitative IgH PCR in CLL after allogeneic and autologous stem cell transplantation. Leukemia 2004; 18:1637–1645. [DOI] [PubMed] [Google Scholar]
- 24.Bottcher S, Stilgenbauer S, Busch R, et al. Standardized MRD flow and ASO IGH RQ-PCR for MRD quantification in CLL patients after rituximab-containing immunochemotherapy: a comparative analysis. Leukemia 2009; 23:2007–2017. [DOI] [PubMed] [Google Scholar]
- 25.Raponi S, Della Starza I, De Propris MS, et al. Minimal residual disease monitoring in chronic lymphocytic leukaemia patients. A comparative analysis of flow cytometry and ASO IgH RQ-PCR. Brit J Haematol 2014; 166:360–368. [DOI] [PubMed] [Google Scholar]
- 26.van der Velden VH, Cazzaniga G, Schrauder A, et al. Analysis of minimal residual disease by Ig/TCR gene rearrangements: guidelines for interpretation of real-time quantitative PCR data. Leukemia 2007; 21:604–611. [DOI] [PubMed] [Google Scholar]
- 27.van der Velden VH, Hochhaus A, Cazzaniga G, et al. Detection of minimal residual disease in hematologic malignancies by real-time quantitative PCR: principles, approaches, and laboratory aspects. Leukemia 2003; 17:1013–1034. [DOI] [PubMed] [Google Scholar]
- 28.Logan AC, Gao H, Wang C, et al. High-throughput VDJ sequencing for quantification of minimal residual disease in chronic lymphocytic leukemia and immune reconstitution assessment. Proc Natl Acad Sci USA 2011; 108:21194–21199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hallek M, Fischer K, Fingerle-Rowson G, et al. Addition of rituximab to fludarabine and cyclophosphamide in patients with chronic lymphocytic leukaemia: a randomised, open-label, phase 3 trial. Lancet (London, England) 2010; 376:1164–1174. [DOI] [PubMed] [Google Scholar]
- 30.Eichhorst B, Fink AM, Bahlo J, et al. First-line chemoimmunotherapy with bendamustine and rituximab versus fludarabine, cyclophosphamide, and rituximab in patients with advanced chronic lymphocytic leukaemia (CLL10): an international, open-label, randomised, phase 3, non-inferiority trial. Lancet Oncol 2016; 17:928–942. [DOI] [PubMed] [Google Scholar]
- 31.Thompson PA. MRD negativity as a surrogate for PFS in CLL? Blood 2018; 131:943–944. [DOI] [PubMed] [Google Scholar]
- 32.Byrd JC, Brown JR, O’Brien S, et al. Ibrutinib versus ofatumumab in previously treated chronic lymphoid leukemia. N Engl J Med V 371 2014; 213–223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Byrd JC, Furman RR, Coutre SE, et al. Targeting BTK with ibrutinib in relapsed chronic lymphocytic leukemia. N Engl J Med 2013; 369:32–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Byrd JC, O’Brien S, James DF. Ibrutinib in relapsed chronic lymphocytic leukemia. N Engl J Med 2013; 369:1278–1279. [DOI] [PubMed] [Google Scholar]
- 35.O’Brien S, Furman RR, Coutre SE, et al. Ibrutinib as initial therapy for elderly patients with chronic lymphocytic leukaemia or small lymphocytic lymphoma: an open-label, multicentre, phase 1b/2 trial. Lancet Oncol 2014; 15:48–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ahn IE, Farooqui MZH, Tian X, et al. Depth and durability of response to ibrutinib in CLL: 5-year follow-up of a phase 2 study. Blood 2018; 131:2357–2366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Jain P, Keating MJ, Wierda WG, et al. Long-term follow-up of treatment with ibrutinib and rituximab in patients with high-risk chronic lymphocytic leukemia. Clin Cancer Res: Off J Am Assoc Cancer Res 2017; 23:2154–2158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Moreno C, Greil R, Demirkan F, et al. Ibrutinib plus obinutuzumab versus chlorambucil plus obinutuzumab in first-line treatment of chronic lymphocytic leukaemia (iLLUMINATE): a multicentre, randomised, open-label, phase 3 trial. Lancet Oncol 2018; 20:43–56. [DOI] [PubMed] [Google Scholar]
- 39.Jones JA, Mato AR, Wierda WG, et al. Venetoclax for chronic lymphocytic leukaemia progressing after ibrutinib: an interim analysis of a multicentre, open-label, phase 2 trial. Lancet Oncol 2018; 19:65–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Cramer P, von Tresckow J, Robrecht S, et al. Bendamustine followed by ofatumumab and ibrutinib in patients with chronic lymphocytic leukemia (CLL): CLL2-BIO trial of the German CLL study group (GCLLSG). Blood 2017; 130 Suppl. 1:S494–S1494. [Google Scholar]
- 41.Cramer P, von Tresckow J, Bahlo J, et al. CLL2-BXX phase II trials: sequential, targeted treatment for eradication of minimal residual disease in chronic lymphocytic leukemia. Future Oncol (London, England) 2018; 14:499–513. [DOI] [PubMed] [Google Scholar]
- 42.Jain N, Thompson PA, Burger JA, et al. Ibrutinib, fludarabine, Cyclophosphamide, and obinutuzumab (iFCG) for firstline treatment of patients with CLL with mutated <em>IGHV</em> and without <em>TP53</em> aberrations. Blood 2018; 132 Suppl. 1:S695–S1695. [Google Scholar]
- 43.Zelenetz AD, Barrientos JC, Brown JR, et al. Idelalisib or placebo in combination with bendamustine and rituximab in patients with relapsed or refractory chronic lymphocytic leukaemia: interim results from a phase 3, randomised, double-blind, placebo-controlled trial. Lancet Oncol 2017; 18:297–311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Cramer P, von Tresckow J, Bahlo J, et al. Bendamustine followed by obinutuzumab and venetoclax in chronic lymphocytic leukaemia (CLL2-BAG): primary endpoint analysis of a multicentre, open-label, phase 2 trial. Lancet Oncol 2018; 19:1215–1228. [DOI] [PubMed] [Google Scholar]
- 45.Byrd JC, Harrington B, O’Brien S, et al. Acalabrutinib (ACP-196) in relapsed chronic lymphocytic leukemia. N Engl J Med 2016; 374:323–332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Roberts AW, Davids MS, Pagel JM, et al. Targeting BCL2 with venetoclax in relapsed chronic lymphocytic leukemia. N Engl J Med 2016; 374:311–322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Stilgenbauer S, Eichhorst B, Schetelig J, et al. Venetoclax for patients with chronic lymphocytic leukemia with 17p deletion: results from the full population of a phase II pivotal trial. J Clin Oncol: Off J Am Soc Clin Oncol 2018; 36:1973–1980. [DOI] [PubMed] [Google Scholar]
- 48.Wierda WG, Roberts AW, Ghia P, et al. Minimal residual disease status with venetoclax monotherapy is associated with progression-free survival in chronic lymphocytic leukemia. Blood 2018; 132 Suppl. 1:S3134–S13134. [Google Scholar]
- 49.Seymour JF, Kipps TJ, Eichhorst B, et al. Venetoclax-rituximab in relapsed or refractory chronic lymphocytic leukemia. N Engl J Med 2018; 378:1107–1120. [DOI] [PubMed] [Google Scholar]
- 50.Kater AP, Seymour JF, Hillmen P, et al. Fixed duration of venetoclax-rituximab in relapsed/refractory chronic lymphocytic leukemia eradicates minimal residual disease and prolongs survival: post-treatment follow-up of the MURANO phase III study. J Clin Oncol: Off J Am Soc Clin Oncol 2019; 37:269–277. [DOI] [PubMed] [Google Scholar]
- 51.Fischer K, Al-Sawaf O, Fink AM, et al. Venetoclax and obinutuzumab in chronic lymphocytic leukemia. Blood 2017; 129:2702–2705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Kater AP, Kersting S, van Norden Y, et al. Obinutuzumab pretreatment abrogates tumor lysis risk while maintaining undetectable MRD for venetoclax + obinutuzumab in CLL. Blood Adv 2018; 2:3566–3571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Hillmen P, Rawstron A, Brock K, et al. Ibrutinib plus venetoclax in relapsed/refractory CLL: results of the bloodwise TAP clarity study. Blood 2018; 132 Suppl. 1:S182–S1182. [Google Scholar]
- 54.Jain N, Keating MJ, Thompson PA, et al. Combined ibrutinib and venetoclax in patients with treatment-naïve high-risk chronic lymphocytic leukemia (CLL). Blood 2018; 132 Suppl. 1:S696–S1696. [Google Scholar]
- 55.Dreger P, Dohner H, Ritgen M, et al. Allogeneic stem cell transplantation provides durable disease control in poor-risk chronic lymphocytic leukemia: long-term clinical and MRD results of the German CLL Study Group CLL3X trial. Blood 2010; 116:2438–2447. [DOI] [PubMed] [Google Scholar]
- 56.Kramer I, Stilgenbauer S, Dietrich S, et al. Allogeneic hematopoietic cell transplantation for high-risk CLL: 10-year follow-up of the GCLLSG CLL3X trial. Blood 2017; 130:1477–1480. [DOI] [PubMed] [Google Scholar]
- 57.Turtle CJ, Hay KA, Hanafi LA, et al. Durable molecular remissions in chronic lymphocytic leukemia treated with CD19-specific chimeric antigen receptor-modified T cells after failure of ibrutinib. J Clin Oncol: Off J Am Soc Clin Oncol 2017; 35:3010–3020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Porter DL, Hwang WT, Frey NV, et al. Chimeric antigen receptor T cells persist and induce sustained remissions in relapsed refractory chronic lymphocytic leukemia. Sci Transl Med 2015; 7:303ra139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Gauthier J, Hirayama AV, Hay KA, et al. Comparison of efficacy and toxicity of CD19-specific chimeric antigen receptor T-cells alone or in combination with ibrutinib for relapsed and/or refractory CLL. Blood 2018; 132 Suppl. 1:S299–S1299. [Google Scholar]
- 60.Gill SI, Vides V, Frey NV, et al. Prospective clinical trial of anti-CD19 CAR T cells in combination with ibrutinib for the treatment of chronic lymphocytic leukemia shows a high response rate. Blood 2018; 132 Suppl. 1:S298–S1298. [Google Scholar]
- 61.Woyach JA, Smucker K, Smith LL, et al. Prolonged lymphocytosis during ibrutinib therapy is associated with distinct molecular characteristics and does not indicate a suboptimal response to therapy. Blood 2014; 123:1810–1817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Satram-Hoang S, Reyes C, Hoang KQ, et al. Treatment practice in the elderly patient with chronic lymphocytic leukemia-analysis of the combined SEER and Medicare database. Ann Hematol 2014; 93:1335–1344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Stauder R, Eichhorst B, Hamaker ME, et al. Management of chronic lymphocytic leukemia (CLL) in the elderly: a position paper from an international Society of Geriatric Oncology (SIOG) Task Force. Ann Oncol: Off J Eur Soc Med Oncol 2017; 28:218–227. [DOI] [PubMed] [Google Scholar]
- 64.Strati P, Parikh SA, Chaffee KG, et al. Relationship between co-morbidities at diagnosis, survival and ultimate cause of death in patients with chronic lymphocytic leukaemia (CLL): a prospective cohort study. Brit jJ Haematol 2017; 178:394–402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Woyach JA. What is the optimal management of older CLL patients? Best Pract Res Clini Haematol 2018; 31:83–89. [DOI] [PubMed] [Google Scholar]
- 66.Goede V, Hallek M. Which drugs in hemato-oncology are unnecessary or inappropriate for use in the elderly? Deutsche med Wochenschr (1946) 2018; 143:244–252. [DOI] [PubMed] [Google Scholar]
- 67.Barrientos JC, O’Brien S, Brown JR, et al. Improvement in parameters of hematologic and immunologic function and patient well-being in the phase III RESONATE study of ibrutinib versus ofatumumab in patients with previously treated chronic lymphocytic leukemia/small lymphocytic lymphoma. Clin Lymphom, Myeloma Leukemia 2018; 18:803–813.e7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Burger JA, Tedeschi A, Barr PM, et al. Ibrutinib as initial therapy for patients with chronic lymphocytic leukemia. N Engl J Med 2015; 373:2425–2437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.McMillan P, Mundy G, Mayer P. Hypercalcaemia and osteolytic bone lesions in chronic lymphocytic leukaemia. Brit Med J 1980; 281:1107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Robak E, Robak T. Skin lesions in chronic lymphocytic leukemia. Leukemia Lymphoma 2007; 48:855–865. [DOI] [PubMed] [Google Scholar]
- 71.Wanquet A, Birsen R, Bonnet C, et al. Management of central nervous system involvement in chronic lymphocytic leukaemia: a retrospective cohort of 30 patients. Brit J Haematol 2017; 176:37–49. [DOI] [PubMed] [Google Scholar]
- 72.Rawstron AC, Howard D, McParland L, et al. Compartment effect on the prognostic significance of MRD detection in CLL: impact of treatment type and duration of follow-up. Blood 2016; 128:3226–13226. [Google Scholar]
- 73.Fürstenau M, Bahlo J, Fink A-M, et al. Residual abdominal lymphadenopathy after intensive frontline chemoimmunotherapy is associated with inferior outcome regardless of MRD status in advanced chronic lymphocytic leukemia (CLL). Blood 2018; 132 Suppl. 1:S4430–S14430. [DOI] [PubMed] [Google Scholar]
- 74.Yeh P, Hunter T, Sinha D, et al. Circulating tumour DNA reflects treatment response and clonal evolution in chronic lymphocytic leukaemia. Nat Commun 2017; 8:14756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Boettcher S, Ritgen M, Fischer K, et al. Minimal residual disease (MRD) re-growth kinetics are an independent predictor for progression free survival (PFS) in chronic lymphocytic leukemia (CLL) and are related to biologically defined CLL-subgroups – results from the CLL8 trial of the German CLL study group (GCLLSG). Blood 2011; 118:1777–11777. [Google Scholar]
- 76.Chen LS, Bose P, Cruz ND, et al. A pilot study of lower doses of ibrutinib in patients with chronic lymphocytic leukemia. Blood 2018; 132:2249–2259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Nadeu F, Delgado J, Royo C, et al. Clinical impact of clonal and subclonal TP53, SF3B1, BIRC3, NOTCH1, and ATM mutations in chronic lymphocytic leukemia. Blood 2016; 127:2122–2130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Stamatopoulos B, Timbs A, Bruce D, et al. Targeted deep sequencing reveals clinically relevant subclonal IgHV rearrangements in chronic lymphocytic leukemia. Leukemia 2017; 31:837–845. [DOI] [PubMed] [Google Scholar]
- 79.Ljungstrom V, Cortese D, Young E, et al. Whole-exome sequencing in relapsing chronic lymphocytic leukemia: clinical impact of recurrent RPS15 mutations. Blood 2016; 127:1007–1016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Thompson PA, Tam CS, O’Brien SM, et al. Fludarabine, cyclophosphamide, and rituximab treatment achieves long-term disease-free survival in IGHV-mutated chronic lymphocytic leukemia. Blood 2016; 127:303–309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Tantravahi SK, Guthula RS, O’Hare T, et al. Minimal residual disease eradication in CML: does it really matter? Currt Hematol Malignancy Rep 2017; 12:495–505. [DOI] [PubMed] [Google Scholar]
- 82.Cramer P, von Tresckow J, Bahlo J, et al. Durable Remissions after discontinuation of combined targeted treatment in patients with chronic lymphocytic leukemia (CLL) harbouring a high-risk genetic lesion (del (17p)/TP53 mutation). Blood 2018; 132 Suppl 1:694–1694.29907599 [Google Scholar]
- 83.Collett L, Howard DR, Munir T, et al. Assessment of ibrutinib plus rituximab in front-line CLL (FLAIR trial): study protocol for a phase III randomised controlled trial. Trials 2017; 18:387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Herling CD, Abedpour N, Weiss J, et al. Clonal dynamics towards the development of venetoclax resistance in chronic lymphocytic leukemia. Nat Commun 2018; 9:727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Seymour JF, Kipps TJ, Eichhorst B, et al. MURANO trial establishes feasibility of time-limited venetoclax-rituximab (VenR) combination therapy in relapsed/refractory (R/R) chronic lymphocytic leukemia (CLL). Blood 2018; 132 Suppl 1:184–1184. [Google Scholar]
- 86.Shanafelt TD, Wang V, Kay NE, et al. A randomized phase III study of ibrutinib (PCI-32765)-based therapy vs standard fludarabine, cyclophosphamide, and rituximab (FCR) chemoimmunotherapy in untreated younger patients with chronic lymphocytic leukemia (CLL): a trial of the ECOG-ACRIN cancer research group (E1912). Blood 2018; 132 Suppl. 1:SLBA-4-LBA-4. [Google Scholar]
- 87.Barnes JI, Divi V, Begaye A, et al. Cost-effectiveness of ibrutinib as first-line therapy for chronic lymphocytic leukemia in older adults without deletion 17p. Blood Adv 2018; 2:1946–1956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Chen Q, Jain N, Ayer T, et al. Economic burden of chronic lymphocytic leukemia in the era of oral targeted therapies in the United States. J Clin Oncol 2017; 35:166–174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Quinquenel A, Fornecker LM, Letestu R, et al. High prevalence of BTK mutations on ibrutinib therapy after 3 years of treatment in a real-life cohort of CLL patients: a study from the French innovative leukemia organization (FILO) group. Blood 2018; 132 Suppl. 1:S584–S1584. [Google Scholar]
- 90.Tresckow v, J, Kater NC, et al. The GAIA (CLL13) trial: an international intergroup phase III study for frontline therapy in chronic lymphocytic leukemia (CLL). J Clin Oncol: Off J Am Soc Clin Oncol 2018; 36.2018 (suppl; abstr TPS7582). [Google Scholar]
- 91.Chanan-Khan A, Cramer P, Demirkan F, et al. Ibrutinib combined with bendamustine and rituximab compared with placebo, bendamustine, and rituximab for previously treated chronic lymphocytic leukaemia or small lymphocytic lymphoma (HELIOS): a randomised, double-blind, phase 3 study. Lancet Oncol 2016; 17:200–211. [DOI] [PubMed] [Google Scholar]
- 92.Davids MS, Kim HT, Brander DM, et al. Initial results of a multicenter, phase II study of ibrutinib plus FCR (iFCR) as frontline therapy for younger CLL patients. Blood 2016; 128:3243–13243. [Google Scholar]
- 93.Rogers KA, Huang Y, Ruppert AS, et al. Phase 2 study of combination obinutuzumab, ibrutinib, and venetoclax in treatment-naive and relapsed/refractory chronic lymphocytic leukemia. Blood 2018; 132 Suppl 1:693–1693. [Google Scholar]
- 94.Siddiqi T, Soumerai JD, Wierda WG, et al. Rapid MRD-negative responses in patients with relapsed/refractory CLL treated with Liso-Cel, a CD19-directed CAR T-cell product: preliminary results from transcend CLL 004, a phase 1/2 study including patients with high-risk disease previously treated with ibrutinib. Blood 2018; 132 Suppl. 1:S300–S1300. [Google Scholar]