Abstract
Background:
Tpeak-Tend interval (TpTe), a measurement of transmural dispersion of repolarization (TDR), has been shown to predict ventricular tachyarrhythmia in cardiac resynchronization therapy with defibrillator (CRT-D) patients. However, the ability of TpTe to predict ventricular tachyarrhythmia and mortality for heart failure patients with a cardioverter-defibrillator (ICD) is not clear. The purpose of this study was to assess the predictive ability of TpTe in heart failure patients with ICD.
Methods and results:
We enrolled 318 heart failure patients treated after ICD. Patients were divided into 3 groups according to their post-implantation TpTe values and were evaluated every 6 months. The primary endpoint was appropriate ICD therapy. The secondary endpoint was all-cause mortality. During long-term follow-up, the TpTe > 110 ms group (n = 111) experienced more VT/VF episodes (45%) and all-cause mortality (25.2%) than the TpTe 90–110 ms group (n = 109) (26.4%, 14.5%) and TpTe < 90 ms group (n = 98) (11.3%, 11.3%) (overall P < .05, respectively). In Cox regression, longer post-implantation TpTe was associated with an increased number of VT/VF episodes [HR: 1.017; 95% CI: 1.008-1.026; P < .001], all-cause mortality [HR: 1.015; 95% CI: 1.004-1.027; P = .010] and the combined endpoint [HR: 1.018; 95%CI: 1.010-1.026; P < .001].
Conclusions:
Post-implantation TpTe was an independent predictor of both ventricular arrhythmias and all-cause mortality in heart failure patients with an implanted ICD.
Keywords: implantable cardioverter-defibrillator, sudden cardiac death, dispersion of repolarization, Tpeak-Tend interval
1. Introduction
Implantable cardioverter-defibrillator (ICD) has been proven to be an effective treatment method to prevent sudden cardiac death caused by malignant rapid ventricular arrhythmias in heart failure patients. In addition, cardiac resynchronization therapy (CRT) with ICD (CRT-D) can improve the cardiac functional class as well as quality of life and reduce both HF hospitalizations and overall mortality in patients with a prolonged QRS complex duration.[1,2] However, the risk of ventricular arrhythmias and sudden death after device implantation has not disappeared and cannot be ignored.
The predictive value of the Tpeak-Tend interval (TpTe) in malignant ventricular arrhythmias has been a popular area of research for several years. A series of studies confirmed the practicality of TpTe as a risk factor in long QT syndrome[3] and Brugada syndrome.[4] Moreover, immediate postimplant TpTe has proven to be useful in appropriate ICD therapy predictions among CRT-D patients.[5] However, fewer studies have examined the predictive ability of TpTe for appropriate ICD therapy and all-cause mortality in ICD patients. Morin et al[6] and Rosenthal et al[7] suggested that instead of TpTe, baseline rate-corrected TpTe (TpTec) predicts both ventricular tachycardia (VT)/ventricular fibrillation (VF) and overall mortality in patients with systolic dysfunction and implanted ICDs. However, TpTec may be limited by cumbersome calculations; thus, its viability and effectiveness have yet to be established. Considering that TpTe may be prolonged by biventricular pacing,[8] the present long-term follow-up study investigated the relationship between immediate postimplantation TpTe and ventricular arrhythmias as well as death in heart failure patients with ICD/CRT-D implantation.
2. Methods
2.1. Subjects
This study consecutively enrolled patients who experienced ICD implantation at Fuwai Hospital from January 2014 to January 2016, and all subjects met the following inclusion criteria:
-
1.
Severe LV systolic dysfunction (LVEF ≤30%) and
-
2.
New York Heart Association (NYHA) class ≥II.
The exclusion criteria were ventricular tachyarrhythmia with a transient, reversible cause (drugs, electrolyte abnormalities, acute myocardial infarction); structurally normal heart or isolated right ventricular heart disease without left ventricular involvement; Long QT syndrome; and age < 18 years. The secondary prevention of sudden cardiac death was defined as patients who had a history of sustained VT or VF or who had survived cardiac arrest.
All of the patients signed informed consent forms. This study complied with the Declaration of Helsinki and was approved by the Research Ethics Board of Fuwai Hospital.
Before device operation, the following baseline demographic data were collected: age, gender, NYHA functional class, LVEF (assessed by the modified biplane Simpson's method[9]), 12-lead electrocardiogram (ECG), medical history and medical therapy. Figure 1 was a flow diagram illustrating the method of this study.
Figure 1.
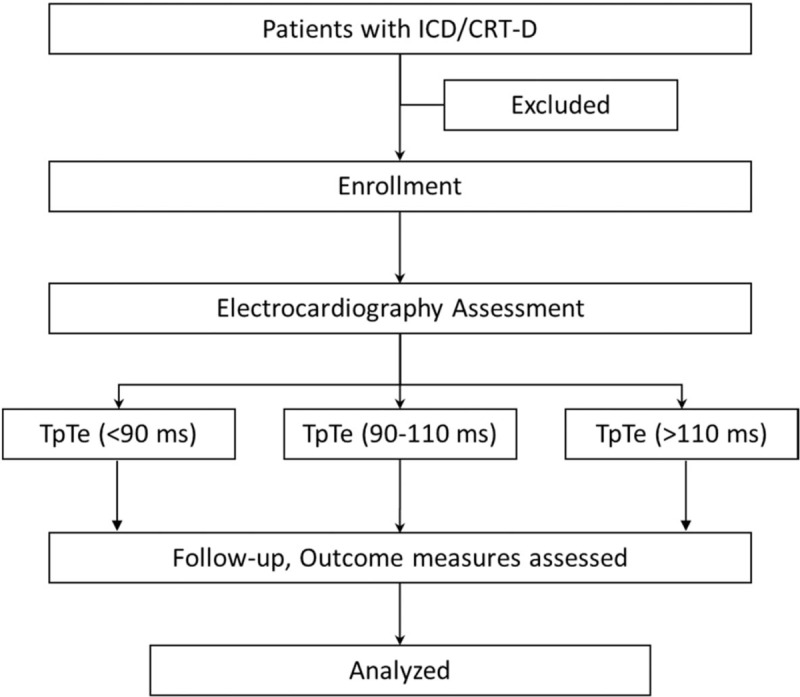
Protocol for selection participants. ICD, cardioverter-defibrillator; CRT-D, cardiac resynchronization therapy with defibrillator; TpTe = peak-Tend interval.
2.2. Device implantation
Device implantation was performed according to the ICD and CRT guidelines. The right ventricular lead was implanted in the apex in all patients. For double chamber ICD, the right atrial lead was implanted in the right atrial appendage. For CRT-D implantation, the left ventricular lead was positioned in the lateral or posterolateral vein, which was the preferable target. If these veins were not accessible, the lead was implanted in the other branch of the coronary sinus, close to the lateral LV wall. Initial device programming was performed immediately after ICD implantation. The VT zone was set to at least 170 beats/min or 10 beats/min below the slowest episode of VT in those who had VT; antitachycardia pacing (ATP) was the first-line therapy in all patients. The VF zone was set to at least 200 beats/min, treated by shock(s).
2.3. Electrocardiography assessment
ECG recording was conducted both before and within 24 hours post implantation using a standard digital recorder with 12 simultaneous leads at a paper speed of 25 mm/s. TpTe was averaged for all 12 leads and was defined as the interval from the peak of a positive T wave or the nadir of a negative T wave to the end of the T wave. The first peak in the bimodal T-wave was selected. All ECG measurements were performed independently by two physicians in a blinded fashion. When measurements were not identical, the mean values were calculated. If the values differed by > 10 ms, the measurements were adjudicated by a third reviewer. According to a large sample study (n = 36,299), the mean TpTe interval to be 85 ± 11ms in normal and recommended adoption of 110ms as the upper limit of normal[10]. Therefore, patients were divided into TpTe (<90 ms) (T1), TpTe (90–110 ms) (T2) and TpTe (>110 ms)(T3) groups according to their post-operation TpTe values.
2.4. Follow-up
Long-term follow-up after device implantation was performed every 6 months via in-person, remote device interrogation or a telephone interview. The primary endpoint was the occurrence of the first appropriate ICD therapy (ATP or shock) for sustained VT or VF. The secondary endpoint was all-cause mortality (due to heart failure, sudden cardiac death, or a non-cardiac cause). The occurrence of ICD shocks or antitachycardia pacing after ICD implantation was confirmed in all patients by device interrogation at the Pacemaker follow-up center. Two electrophysiologists who were blinded to the patient follow-up data reviewed all of the ICD therapy events and identified the appropriate ICD therapy. Inappropriate therapy was excluded from analysis. Data on patients’ survival and the causes of death were surveyed for all patients. HF death was diagnosed when patients died of terminal heart failure, progressive failure of cardiac pump function, or cardiac asthma under maximum inotropic drug support.
2.5. Statistical analyses
Continuous variables are expressed as the mean ± SD, and categorical variables are presented as numbers and/or percentages. Comparison of continuous variables between 3 different TpTe categories was performed with 1-way analysis of variance (ANOVA) and Bonferroni post-hoc testing. Categorical variables were analyzed using the Chi-square test or Fisher exact test. Differences between the baseline and post-implantation values were tested using the Wilcoxon signed rank test for continuous variables. Cumulative event rates were evaluated with the Kaplan-Meier method, and a log rank test was utilized to compare between groups. The annual event rate was calculated as the number of adverse clinical events divided by the average number of follow-up years. Univariate and multivariate Cox proportional hazards models were performed for appropriate ICD therapy, all-cause mortality and the combined endpoint. Variables with P < .1 in univariate analysis were retained in the multivariate model. Cox model findings are shown as hazard ratios (HRs), 95% confidence intervals (CIs), and tests of significance. All statistical analyses were two-tailed, and a P value < .05 was considered significant. All data were analyzed using SPSS statistical software (version 19.0; SPSS Inc., Chicago, IL).
3. Results
3.1. Baseline characteristics
A total of 318 consecutive patients were included, and their baseline characteristics are described in Table 1. The mean age was 57.59 ± 11.36 years; there were 239 men (75.2%), and the mean LVEF was 27.93 ± 6.65%. The main underlying cardiac condition was idiopathic dilated cardiomyopathy (n = 272, 85.5%), while the other underlying cardiomyopathies were ischemic cardiomyopathy (n = 41, 12.9%) and hypertrophic cardiomyopathy (n = 5, 1.6%). Forty-nine patients (15.4%) had a history of atrial fibrillation. Forty-six patients (14.5%) received an implanted defibrillator for secondary prevention of sudden cardiac death. Twenty-one patients had a history of VF, and 25 patients had VT. The average baseline TpTe was 104 ± 23 ms; post-operation, TpTe increased to 106 ± 21 ms (P < .05).
Table 1.
Baseline characteristics of all patients.
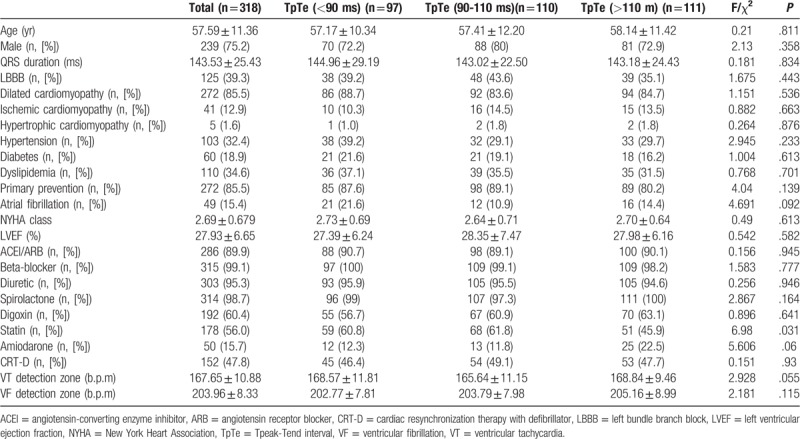
All patients were divided into 3 groups according to TpTe post-operation. There were no significant differences in drug therapy between the 3 groups, with the exception of statins. All groups had a high usage of ACEI/ARB, β-blockers, diuretics and spirolactones.
3.2. Appropriate device therapies
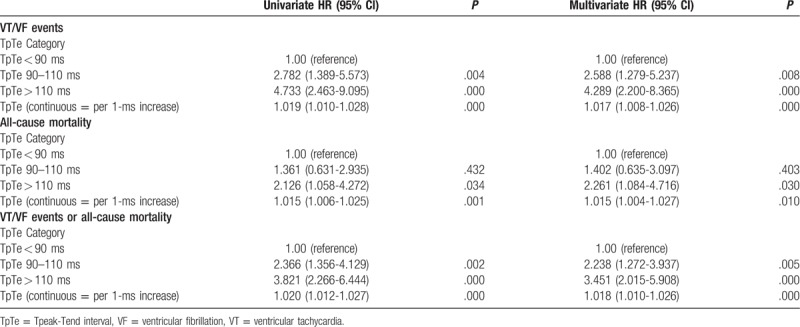
The clinical outcome of patients were described in Table 2. During the follow-up of 25.14 ± 22.83 months, 90 patients (28.3%) experienced their first appropriate device therapy (ATP and/or shocks) for ventricular arrhythmias, with 64% appearing in the first year. The three groups had similar follow-up times (29.24 ± 22.12, 23.16 ± 22.56, and 23.51 ± 23.44, respectively; all P = .104). The annual rate of VT/VF episodes was highest in T3 (23.0%), intermediate in T2 (13.7%), and lowest in T1 (4.7%) (P = .00). Kaplan-Meier event-free survival analysis demonstrated that T1 had a better arrhythmia-free survival than the other two groups ((Log rank P = .002 and P = .000, respectively) (Fig. 2). In Cox regression modeling (Table 3), when treated as a continuous covariate, prolonged TpTe was associated with an increased number of VT/VF episodes (hazard ratio [HR] = 1.019 for every 1 ms increase in TpTe; 95% CI: 1.010-1.028; P = .000) in an unadjusted model but also in a multivariate model (HR = 1.017; 95% CI: 1.008-1.026; P = .000) (adjusted for age, gender, comorbidities, primary prevention, baseline electrocardiograph parameters, medications, and device type). When assessed as a category variable, in univariate analysis, both T2 (HR = 2.782; 95% CI: 1.389-5.573; P = .004) and T3 (HR: 4.733; 95% CI: 2.463-9.095; P = .000) were associated with VT/VF episodes, but this was also the case in a multivariate model (HR: 2.588; 95% CI: 1.279-5.237; P = .008; and HR: 4.289; 95% CI: 2.200-8.365; P = 0).
Table 2.
Clinical outcome of patients.
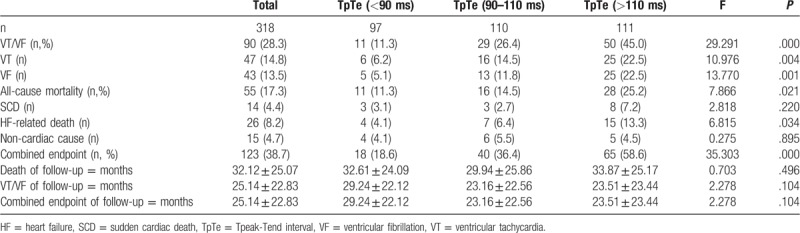
Figure 2.
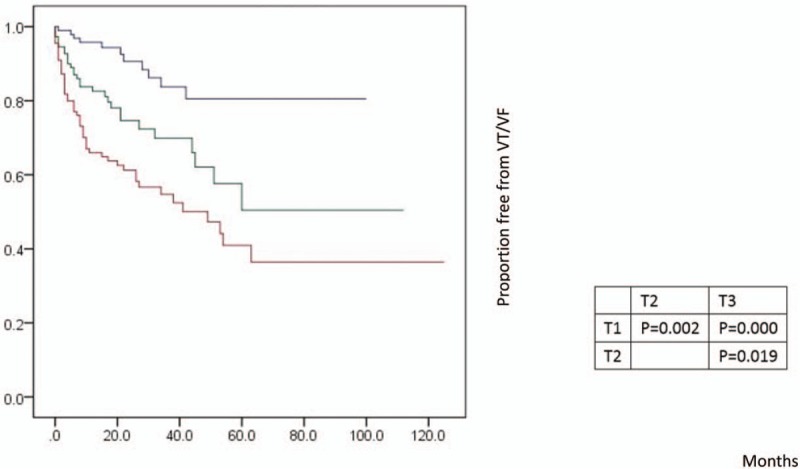
Kaplan-Meier estimates of cumulative incidence of VT/VF episodes in the groups TpTe (<90 ms), TpTe (90-110 ms), and TpTe (>110 ms). TpTe = Tpeak-Tend interval, VT = ventricular tachycardia, VF = ventricular fibrillation.
Table 3.
Cox proportional hazards analyses of VT/VF events = mortality = and combined endpoints according to admission TpTe.
3.3. Mortality
After a mean mortality follow-up of 32.12 ± 25.07 months, 55 patients (17.3%) died: 14 cases were sudden death, 26 were heart failure-related and 15 were due to a non-cardiac cause. The annual all-cause mortality in T3 was approximately 8.9% and declined stepwise to 5.8% and 4.2% in T2 and T1, respectively (overall P = .021). In univariate analysis, for all-cause mortality, T1 had the highest event-free survival compared with those in T3 (Log rank P = .032), with intermediate survival among patients in T2 (Log rank P = .420) (Fig. 3). However, there were no significant differences in the causes of death among the three groups (Fig. 4).
Figure 3.
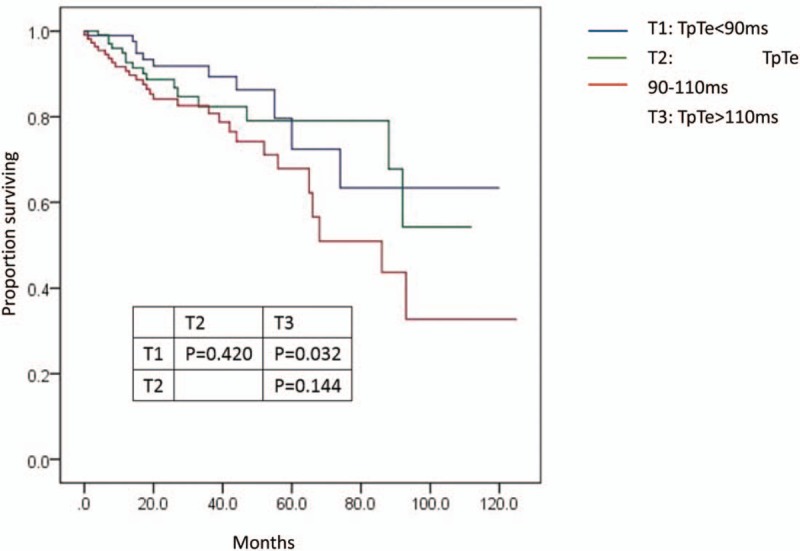
Kaplan-Meier estimates of all-death mortality in the groups of TpTe (<90 ms), TpTe (90-110 ms), and TpTe (>110 ms). TpTe = Tpeak-Tend interval.
Figure 4.
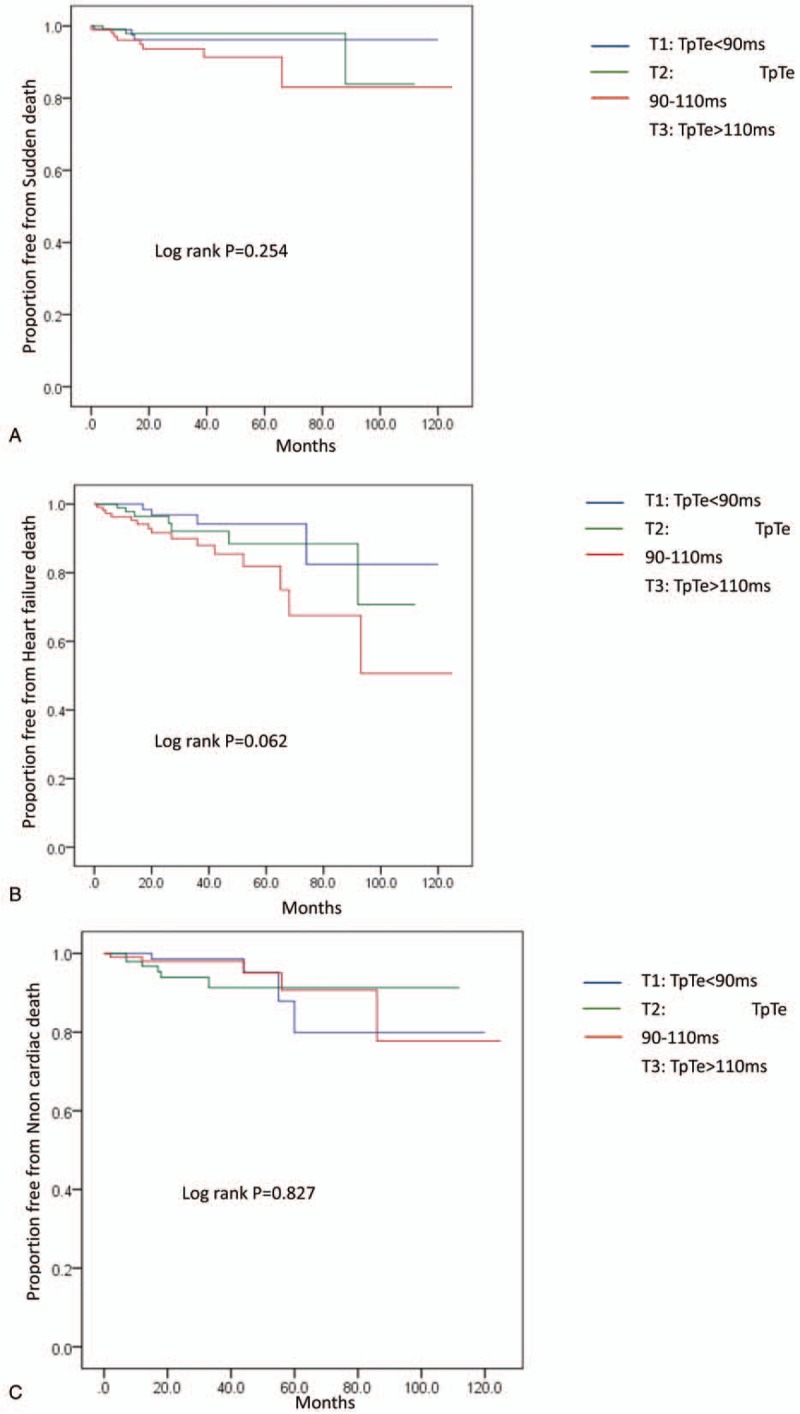
Kaplan-Meier estimates of different causes of death (A. Sudden death, B. Heart failure death, and C. Non-cardiac death) in the groups TpTe (<90 ms), TpTe (90-110 ms), and TpTe (>110 ms). TpTe = Tpeak-Tend interval.
According to Cox regression modeling (Table 3), TpTe post-operation was also associated with all-cause mortality upon both unadjusted (HR: 1.015; 95% CI: 1.006-1.025; P = .001) and adjusted analyses (HR: 1.015; 95% CI: 1.004-1.027; P = .010). TpTe was also able to stratify the risk of death; when examined as categorical variables, T3 showed significantly worse event-free survival compared with T1 not only in univariate Cox models (HR: 2.126; 95% CI: 1.058-4.272; P = .034) but also after adjustment (HR: 2.261; 95% CI: 1.084-4.716; P = .03).
3.4. Combined endpoint
Of the study population, a total of 123 patients (38.7%) experienced the combined endpoint of VT/VF episodes or death during follow-up. The annual combined endpoint events of death or VT/VF episodes was also highest in T3 (29.9%) and was lowest in T1 (7.6%); T2 (18.8%) was intermediate. Kaplan-Meier analysis showed a decreased probability of death or ventricular arrhythmias with shorter TpTe post-operation, and event-free survival was highest among T1 compared with T2 and T3 (Fig. 5).
Figure 5.
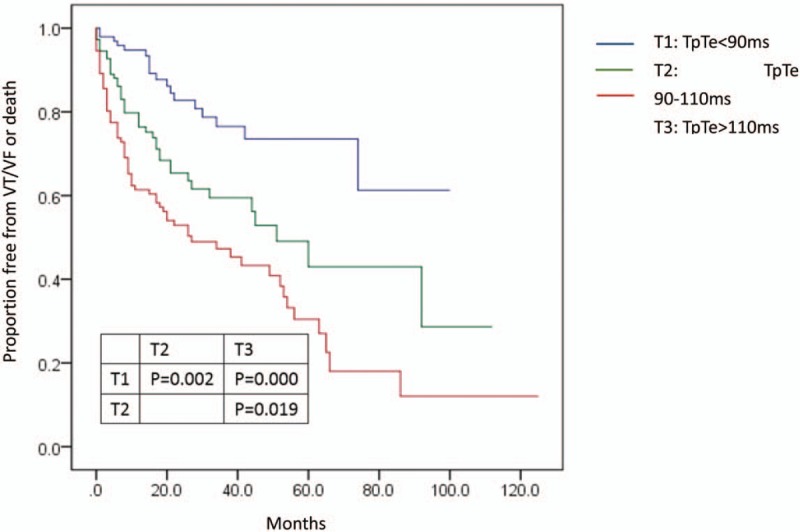
Kaplan-Meier estimates of cumulative incidence of VT/VF episodes or death in the groups TpTe (<90 ms), TpTe (90-110 ms), and TpTe (>110 ms). TpTe = Tpeak-Tend interval, VT = ventricular tachycardia, VF = ventricular fibrillation.
In Cox regression modeling (Table 3), TpTe was also associated with the combined endpoint of death or VT/VF episodes in both unadjusted (HR: 1.020; 95% CI: 1.012-1.027; P = 0) and adjusted analyses (HR: 1.018; 95% CI: 1.010-1.026; P = 0). When assessed as a category variable, after multivariate adjustment, T2 and T3 were associated with a higher risk of death or ventricular arrhythmias (HR = 2.366; 95% CI: 1.356-4.129; P = .002 and HR: 3.821; 95% CI: 2.266-6.444; P = .000, respectively) compared to the T1 category.
4. Discussion
4.1. Major finding
The most important finding of this study was the correlation between TpTe and the outcome of heart failure patients with an ICD implanted for primary or secondary prevention. After multivariate Cox regression model analysis, prolonged TpTe was a strong independent predictor of appropriate ICD therapy, death and the combined endpoint in heart failure patients after ICD implantation.
It is known that TpTe represents the difference between the epimyocardial repolarization time and midmyocardial repolarization time and is traditionally accepted as a measurement of the transmural dispersion of repolarization. The T peak reflects the epimyocardial repolarization endpoint, and the T end corresponds to the midmyocardial repolarization endpoint. Yamaguchi et al[11] showed that the TpTe interval was more accurate than QT dispersion at reflecting repolarization dispersion. Several studies have proposed that TpTe is responsible for mortality and life-threatening arrhythmias in congenital and acquired long-QT syndrome [3], Brugarda Syndrome [4], and patients undergoing primary percutaneous coronary intervention for myocardial infarction.[12] Golcuk et al,[13] found that TpTe is also useful to discriminate malignant RV/VAs and idiopathic RVOT tachycardia. For patients with a cardiovascular implantable electronic device, Lellouche et al[5] found that prolonged post-operation TpTe was associated with an increased incidence of ventricular arrhythmias after CRT-D. Morin et al[6] and Rosenthal et al[7] subsequently reported that the baseline TpTec independently predicts both VT/VF and overall mortality in patients with systolic dysfunction and implanted ICDs.
The results of our study support the important predictive value of TpTe for ventricular arrhythmia and mortality. Furthermore, there are some significant differences between the aforementioned studies and our own: first, the study population was heart failure patients with ICD implantation for either primary or secondary prevention. Second, we investigated TpTe post-operation instead of at baseline because of the potential impact of the eft ventricular epicardial pacing and biventricular pacing on repolarization dispersion in CRT-D patients. Furthermore, this study only evaluated TpTe based on previous studies showing that TpTe and TpTec share predictive value in ventricular arrhythmias.[14] Lastly, we explored the relationship between TpTe and causes of death.
4.2. TpTe and ventricular arrhythmia
This study verified that post implant TpTe was a strong independent predictor of appropriate ICD therapy in heart failure patients with ICD implantation. TpTe > 110 ms, in particular, had a high risk of ventricular arrhythmia.
Although many studies have confirmed TpTe to be an indicator of increased risk of arrhythmia, the details of the underlying mechanism of the TpTe interval are still unclear. Possible reasons include the following:
-
1.
TDR is a major predictor of malignant rapid ventricular arrhythmias, and an abnormal increase in TDR enables arrhythmic substrate for VT, ventricular fibrillation and flutter, and sudden cardiac death.[14] As mentioned earlier, it is generally recognized that TpTe, an index of TDR in ECG, reflects the gap of action potential duration between the endo-epicardium and M (mid-layer) cell.[15] Prolonged TpTe represents increased TDR, which means more vulnerability to reentrant arrhythmias.
-
2.
On the other hand, others argue that TpTe may be more representative of the global dispersion of repolarization than TDR, which has been confirmed by experimental investigation.[16] Therefore, TpTe could still be used in heart failure and cardiomyopathy conditions, which would create a large-scale repolarization dispersion change. The increase in transmural dispersion of repolarization due to augmentation of the global dispersion of repolarization would be expressed in TpTe.[16]
In the present study, the risk of appropriate ICD therapies was not equally distributed over time. The highest risk of VT/VF was noted at the beginning of follow-up, with 56% of appropriate therapies occurring during the first year after ICD implantation. It is of particular interest that ventricular arrhythmias are a constant risk, and even the rate of first appropriate therapy decreases over time. In the study by Boule et al,[17] one patient received a first appropriate therapy nearly 10 years after ICD implantation. Therefore, generator replacement is still necessary for patients who never experienced ventricular tachyarrhythmia after ICD implantation.
It should be noted that a VT detection zone was associated with appropriate ICD therapies according to multivariate Cox regression analyses. Although ICD programming was standardized at the time of operation, doctors may change the detection rates for different conditions (e.g., eliminate distractions by supraventricular tachycardia) to avoid inappropriate therapies. However, a higher threshold for the VT detection rate would result in a lower incidence of appropriate ICD therapies. The tachycardia detection programming strategies still require further research.
4.3. TpTe and mortality
According to previous clinical trials, the benefits of ICD are embodied in the 28% reduction in total mortality, which is mainly due to a 50% reduction in arrhythmic death.[18] In the study by Thijssen et al[19], 2,859 patients with ICD or CRT-D implantation were followed for a median of 3.4 years, and heart failure and non-cardiac death were found to be the most common type of death. Sudden death was low (7%–8%) and was comparable between primary and secondary prevention ICD and CRT-D patients.
In the present study, heart failure was the major cause of death for all patients. The predictive factors of morbidity are a popular topic of study in the field of ICD. Poole et al[20] demonstrated that appropriate ICD shock was associated with a substantially higher risk of death in heart failure patients who received an ICD for primary prevention. In the study by Zhang et al[21], changes in LVEF were inversely associated with all-cause mortality among primary prevention ICD patients. TpTe has been studied as well, but the results vary. In the Porthan et al[22] study, TpTe was not a significant mortality predictor in the general population. However, Panikkath et al[23] suggested that prolongation of TpTe was independently associated with SCD for patients without ICD. Recently, Rosenthal et al[7] found that baseline TpTec could independently predict overall mortality in patients with implanted ICD for primary prevention. The variable results of these studies may be due to the different measurement methods of TpTe as well as the study population. In our study, TpTe post-operation was measured on 12 leads in ECG. The average was calculated, and we found that prolonged TpTe post-operation was associated with all-cause mortality and the combined endpoint of death or VT/VF episodes in both unadjusted and adjusted Cox regression analysis. Patients with TpTe greater than 110 ms had the highest heart failure death rates; however, there was no significant difference between the groups according to Kaplan-Meier event-free survival analysis. In addition, there was no intergroup difference in sudden cardiac death. It should be noted that TpTe is liable to be affected by cardiac morphology, electrocardiogram leads and medication. Therefore, the clinical and practical value of TpTe requires further discussion and study.
5. Limitations
This was a single-center study with a relatively small patient cohort. Therefore, further large-scale studies are needed to confirm the findings and to discover the possible mechanisms behind the association. Moreover, the mechanism of T wave formation is still unclear, and the measurement and calculation methods of TpTe remain in conflict. The reliability of this indicator as a predictor of risk merits further investigation. Another limitation is that ventricular arrhythmias were evaluated by appropriate ICD therapies that had occurred, which were restricted by tachycardia detection programming and might have omitted arrhythmias that fell short of the mark.
6. Conclusion
Heart failure patients with ICD/CRT-D implanted for primary and secondary prevention of SCD have a high risk of appropriate ICD therapy and death over prolonged follow-up. The first appropriate ICD therapy attributable to ventricular arrhythmias tends to occur in the early stage after device implantation. Post implantation TpTe was an independent predictor of both ventricular arrhythmias and all-cause mortality in patients with heart failure and an implanted ICD.
Author contributions
Data curation: Cong Xue, Chi Cai, Zhi-Min Liu, Min Gu, Yun-Zi Zhao.
Formal analysis: Cong Xue, Hong-Xia Niu.
Investigation: Cong Xue, Xiao-Han Fan.
Methodology: Wei Hua, Li-Gang Ding.
Resources: Shu Zhang.
Writing – original draft: Cong Xue.
Writing – review & editing: wei Hua.
Footnotes
Abbreviations: ATP = antitachycardia pacing, CIs = confidence intervals, CRT = cardiac resynchronization therapy, CRT-D = cardiac resynchronization therapy with defibrillator, HRs = hazard ratios, ICD = implantable cardioverter-defibrillator, NYHA = New York Heart Association, TDR = transmural dispersion of repolarization, TpTe = Tpeak-Tend interval, TpTec = baseline rate-corrected TpTe, VF = ventricular fibrillation, VT = ventricular tachycardia.
How to cite this article: Xue C, Hua W, Cai C, Ding LG, Niu HX, Fan XH, Liu ZM, Gu M, Zhao YZ, Zhang S. Predictive value of Tpeak-Tend interval for ventricular arrhythmia and mortality in heart failure patients with an implantable cardioverter-defibrillator: A cohort study. Medicine. 2019;98:49(e18080).
The authors have no conflicts of interest to disclose.
References
- [1].Cleland JG, Daubert JC, Erdmann E, et al. The effect of cardiac resynchronization on morbidity and mortality in heart failure. N Engl J Med 2005;352:1539–49. [DOI] [PubMed] [Google Scholar]
- [2].Momomura S, Tsutsui H, Sugawara Y, et al. Clinical efficacy of cardiac resynchronization therapy with an implantable defibrillator in a Japanese population: results of the MIRACLE-ICD outcome measured in Japanese indication (MOMIJI) study. Circul J 2012;76:1911–9. [DOI] [PubMed] [Google Scholar]
- [3].Topilski I, Rogowski O, Rosso R, et al. The morphology of the QT interval predicts torsade de pointes during acquired bradyarrhythmias. J Am Coll Cardiol 2007;49:320–8. [DOI] [PubMed] [Google Scholar]
- [4].Zumhagen S, Zeidler EM, Stallmeyer B, et al. Tpeak-Tend interval and Tpeak-Tend/QT ratio in patients with Brugada syndrome. Europace 2016. [DOI] [PubMed] [Google Scholar]
- [5].Lellouche N, De Diego C, Akopyan G, et al. Changes and predictive value of dispersion of repolarization parameters for appropriate therapy in patients with biventricular implantable cardioverter-defibrillators. Heart Rhythm 2007;4:1274–83. [DOI] [PubMed] [Google Scholar]
- [6].Morin DP, Saad MN, Shams OF, et al. Relationships between the T-peak to T-end interval, ventricular tachyarrhythmia, and death in left ventricular systolic dysfunction. Europace 2012;14:1172–9. [DOI] [PubMed] [Google Scholar]
- [7].Rosenthal TM, Stahls PF, Abi Samra FM, et al. T-peak to T-end interval for prediction of ventricular tachyarrhythmia and mortality in a primary prevention population with systolic cardiomyopathy. Heart Rhythm 2015;12:1789–97. [DOI] [PubMed] [Google Scholar]
- [8].Medina-Ravell VA, Lankipalli RS, Yan GX, et al. Effect of epicardial or biventricular pacing to prolong QT interval and increase transmural dispersion of repolarization: does resynchronization therapy pose a risk for patients predisposed to long QT or torsade de pointes? Circulation 2003;107:740–6. [DOI] [PubMed] [Google Scholar]
- [9].Lang RM, Bierig M, Devereux RB, et al. Recommendations for chamber quantification: a report from the American Society of Echocardiography's Guidelines and Standards Committee and the Chamber Quantification Writing Group,developed in conjunction with the European Association of Echocardiography, a branch of the European Society of Cardiology. J Am Soc Echocardiogr 2005;18:1440–63. [DOI] [PubMed] [Google Scholar]
- [10].Rautaharju PM, Zhang Z, Gregg RE, et al. Normal standards for computer-ECG programs for prognostically and diagnostically important ECG variables derived from a large ethnically diverse female cohort: the Women's Health Initiate (WHI). J Electrocardiol 2013;46:707–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Yamaguchi M, Shimizu M, Ino H, et al. T wave peak-to-end interval and QT dispersion in acquired long QT syndrome: a new index for arrhythmogenicity. Clinical Sci (London, England: 1979) 2003;105:671–6. [DOI] [PubMed] [Google Scholar]
- [12].Haarmark C, Hansen PR, Vedel-Larsen E, et al. The prognostic value of the Tpeak-Tend interval in patients undergoing primary percutaneous coronary intervention for ST-segment elevation myocardial infarction. J Electrocardiol 2009;42:555–60. [DOI] [PubMed] [Google Scholar]
- [13].Golcuk E, Yalin K, Kaya Bilge A, et al. Usefulness of T(peak) -T(end) interval to distinguish arrhythmogenic right ventricular cardiomyopathy from idiopathic right ventricular outflow tract tachycardia. Pacing Clin Electrophysiol 2014;37:1665–70. [DOI] [PubMed] [Google Scholar]
- [14].Watanabe N, Kobayashi Y, Tanno K, et al. Transmural dispersion of repolarization and ventricular tachyarrhythmias. J Electrocardiol 2004;37:191–200. [DOI] [PubMed] [Google Scholar]
- [15].Yan GX, Antzelevitch C. Cellular basis for the normal T wave and the electrocardiographic manifestations of the long-QT syndrome. Circulation 1998;98:1928–36. [DOI] [PubMed] [Google Scholar]
- [16].Arteyeva NV, Goshka SL, Sedova KA, et al. What does the T(peak)-T(end) interval reflect? An experimental and model study. J Electrocardiol 2013;46: 296.e291–e298. [DOI] [PubMed] [Google Scholar]
- [17].Boule S, Semichon M, Guedon-Moreau L, et al. Long-term outcome of implantable cardioverter-defibrillator implantation in secondary prevention of sudden cardiac death. Arch Cardiovasc Dis 2016. [DOI] [PubMed] [Google Scholar]
- [18].Connolly SJ, Hallstrom AP, Cappato R, et al. Meta-analysis of the implantable cardioverter defibrillator secondary prevention trials AVID, CASH and CIDS studies. Antiarrhythmics vs Implantable Defibrillator study. Cardiac Arrest Study Hamburg. Canad Implant Defibr Study Eur Heart J 2000;21:2071–8. [DOI] [PubMed] [Google Scholar]
- [19].Thijssen J, van Rees JB, Venlet J, et al. The mode of death in implantable cardioverter-defibrillator and cardiac resynchronization therapy with defibrillator patients: results from routine clinical practice. Heart Rhythm 2012;9:1605–12. [DOI] [PubMed] [Google Scholar]
- [20].Poole JE, Johnson GW, Hellkamp AS, et al. Prognostic importance of defibrillator shocks in patients with heart failure. New Engl J Med 2008;359:1009–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Zhang Y, Guallar E, Blasco-Colmenares E, et al. Changes in follow-up left ventricular ejection fraction associated with outcomes in primary prevention implantable cardioverter-defibrillator and cardiac resynchronization therapy device recipients. J Am Coll Cardiol 2015;66:524–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Porthan K, Viitasalo M, Jula A, et al. Predictive value of electrocardiographic QT interval and T-wave morphology parameters for all-cause and cardiovascular mortality in a general population sample. Heart Rhythm 2009;6:1202–8. 1208.e1201. [DOI] [PubMed] [Google Scholar]
- [23].Panikkath R, Reinier K, Uy-Evanado A, et al. Prolonged Tpeak-to-tend interval on the resting ECG is associated with increased risk of sudden cardiac death. Circul Arrhyth Electrophysiol 2011;4:441–7. [DOI] [PMC free article] [PubMed] [Google Scholar]


