Abstract
Only a few high-volume centers have reported the efficacy of laparoscopic liver resection (LLR) for patients with intrahepatic cholangiocarcinoma (ICC). The minimally invasive approach is still controversial for ICC, especially when dealing with large (≥5 cm) or multiple (≥2) ICCs.
Patients with large and multiple ICCs who underwent LLR or open hepatectomy (OH) between January 2012 and June 2017 were included. Furthermore, 1:2 propensity score matching (PSM) was performed between the LLR group and the OH group. Short- and long-term outcomes were compared between the different techniques.
After PSM, LLR resulted in significantly longer operation time (median 225 minutes vs 190 minutes, P = .006) and pringle maneuver time (median 50 minutes vs 32.5 minutes, P = .001). There was no statistically significant difference in postoperative hospital stay between the different approaches (median 6 days vs 7 days, P = .092). The grade III/IV complication rates were comparable between the groups (5.6% vs 11.1%, P = .868). In the PSM subset, there was no significant difference in terms of overall survival (P = .645) or disease-free survival (P = .827) between patients in the LLR group and in the OH group.
The present study showed that patients who underwent LLR for large or multiple ICCs could obtain similar short- and long-term outcomes compared with those who underwent OH, and lymph node dissection (LND) was technically difficult but feasible during LLR.
Keywords: hepatectomy, intrahepatic cholangiocarcinoma, laparoscopic liver resection
1. Introduction
Intrahepatic cholangiocarcinoma (ICC), an epithelial cell malignancy that stems from the intrahepatic biliary tree, is the second most common primary hepatic tumor and a type of fatal malignant disease.[1] Despite the fact that ICCs account for only 5% to 10% of liver malignancies, data from studies suggest a rising incidence worldwide.[2,3] Although only 30% to 40% of ICCs are resectable upon first diagnosis, surgical resection is still the leading curative treatment for ICC.[1,4] The aim of surgery is to completely resect the tumor and achieve a negative surgical margin.
Laparoscopic liver resection (LLR), which has progressed over the last 20 years, has become a feasible choice for various kinds of liver lesions owing to the development of high-tech surgical techniques and equipment.[5,6] However, the LLR approach is not widely adopted by surgeons for ICC, especially for large or multiple tumors. Although large or multiple malignancies are not contraindications for LLR, debates focusing on the risks of positive surgical margins, massive hemorrhage and difficulty with lymphadenectomy in LLR still exist. Few reports[7–10] referring to LLR for ICC are available, and there has only one study[11] with a relatively small sample size that has focused on the feasibility of LLR for patients with large or multiple ICCs. Therefore, whether patients with large or multiple ICCs benefit from the minimally invasive approach requires further scientific research.
The aim of this study was to compare the difference between open hepatectomy (OH) and LLR in patients with large or multiple intrahepatic cholangiocarcinomas in a case-matched analysis using propensity score matching (PSM).
2. Methods
2.1. Participants and assessment
Patient selection is shown in Fig. 1. All patients who underwent hepatectomy for ICC at West China Hospital, Sichuan University from January 2012 to June 2017 were retrospectively reviewed. Patients who met the following inclusion criteria were selected: ≥18 years old; Child-Pugh class A or B hepatic function; and pathology confirmed large (≥5 cm) or multiple (≥2) ICCs. The exclusion criteria were as follows: re-resection; palliative resections; concurrent other malignancy; and requiring vascular or biliary reconstruction. Data from the selected patients were analyzed thoroughly. Selected patients were divided into the LLR group or OH group based on which surgical procedure they underwent. Preoperative assessment included blood biochemical examination, routine blood examination, carbohydrate antigen 19–9 (CA19-9), and 3-phase contrast-enhanced computed tomography (CT) or magnetic resonance imaging (MRI) scans. The diagnosis of ICC was confirmed by pathology. The review board of our institute approved this study. Written informed consent was obtained from all participants.
Figure 1.
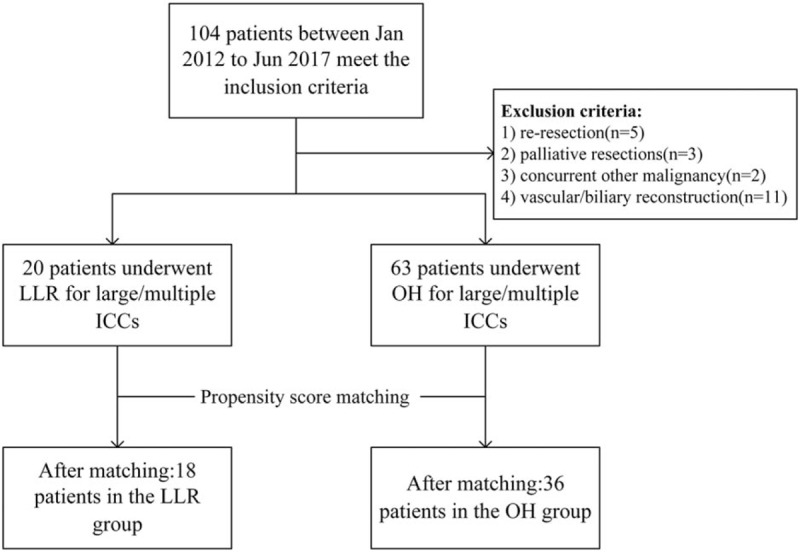
Flow chart for patient selection.
2.2. Surgical techniques
For OH, patients were placed in the supine position after general anesthesia. A reverse L-incision was used for laparotomy. Parenchyma transections were performed with a Cavitron Ultrasonic Surgical Aspirator (CUSA Excel+, Intergra, NJ) or clamp crushing. To control bleeding, intermittent pringle maneuvers and bipolar forceps were routinely applied. Pringle maneuvers were performed with a 15 minutes of inflow occlusion followed by 5 minutes of reperfusion, and they were repeated until the end of the surgery.
The exact surgical procedure for LLR has been described in our earlier reports.[12,13] In short, general anesthesia was performed, and based on the tumor location, the patient was adjusted to a 30° reverse Trendelenburg position. Four trocars were used for the operation and 1 for laparoscopy. Intraoperative ultrasonography was routinely performed to confirm the tumor load and main hepatic structures. After confirming the extent of excision, a tourniquet surrounding the first porta hepatis was prepared for hepatic inflow occlusion. During resection, a laparoscopic Cavitron Ultrasonic Surgical Aspirator, Harmonic scalpel (Ethicon, Somerville, NJ) and Hem-o-Lok clips (Weck, Telefex Medical, NC) were used. The prepared tourniquet was used to perform the laparoscopic intermittent pringle maneuver. A wide lymphadenectomy was avoided, and lymph node dissection (LND) was only performed when an enlarged lymph node around the hepatoduodenal ligament was detected preoperatively or during surgery. The port near the linea alba was opened longitudinally to extract the specimen. Peritoneal drainage was placed on the cutting surface of the liver.
2.3. Postoperative monitoring and follow-up
Routine blood tests and blood biochemical examinations were performed on postoperative days (PODs) 1, 3, and 5. Blood pancreatic enzyme levels were tested if LND involved peripancreatic lymph nodes. Chest radiography was performed when suspicious pulmonary infection existed. An abdominal ultrasound evaluation was performed on POD 5 to confirm that there were no abnormalities. Peritoneal drainage fluid was routinely sent for testing of bilirubin levels, and the drainage tube was pulled out when the drainage fluid was serous and contained no bile. During the first year after discharge, patients were followed up every 3-months. A follow up strategy of every 3 to 6 months was adopted based on the patient's condition from the second year after surgery. At follow-up, all patients received laboratory tests for biochemistry, CA19-9 levels, HBV-DNA levels (for HBsAg-positive patients), and abdominal ultrasounds. Enhanced MRI or CT was performed when recurrence was suspected from the ultrasound or when an evaluated tumor marker was observed during evaluation. In terms of postoperative treatment, patients with R1 resection were transferred to oncology department to receive chemotherapy. Chemotherapy was recommended for the remaining patients. The interval between the date of operation and the date of the last follow-up/death was defined as overall survival (OS). Once recurrence happened, the interval between the operation and recurrence was deemed disease-free survival (DFS).
2.4. Definitions
Resection of >3 segments or resection of the right posterior segments was defined as major resection based on the second consensus meeting for LLR.[14] Postoperative mortality was defined as any death within 30 days postoperation. All complications were graded according to the Clavien–Dindo classification, and grade III/IV complications were considered major complications.[15] Histopathology confirmed vascular invasion was defined as microvascular invasion (MVI). The “50–50 criteria” on POD 5 were used to identify liver failure.[16] At least a threefold bilirubin concentration in the drainage fluid than in the serum on or after POD 3 was defined as biliary leakage.[17] Hemorrhage was defined as a drop in hemoglobin level >3 g/dL postoperatively compared with the postoperative baseline level and/or any postoperative transfusion of packed red blood cells for a falling hemoglobin level.[18] Drainage of >500 mL/d and lasting for over 3 days was defined as ascites. Positive sputum cultures and/or positive pneumonia imaging characteristics associated with fever and hyperleukocytosis were diagnosed as pulmonary infection.[19]
2.5. Statistical analysis
Normally distributed continuous data were statistically analyzed using Student t test and expressed as mean (s.d.). Non-normally distributed continuous data were statistically analyzed using the Mann–Whitney U test and expressed as median (range). The chi-squared or Fisher exact test was used for categorical data, and categorical data were expressed as n (%). Survival outcomes including OS and DFS were analyzed using Kaplan–Meier method and compared using log-rank test. To alleviate the selective bias between the groups, PSM was performed based on the following factors: age, tumor characteristics (tumor size, tumor number, nodule status, tumor differentiation, and microvascular invasion), American Society of Anesthesiologists (ASA) grade, underlying liver cirrhosis, liver function (Child-Pugh grade), and resection extent. Then, 1:2 propensity score matching was performed with the nearest neighbor matching method within 0.2 of the standard deviation of the logit-transformed propensity score. SPSS version 22.0 (IBM SPSS Inc, Chicago, IL) was used to perform all analyses. A P value of <.05 was considered as statistically significant.
3. Results
3.1. Baseline characteristics
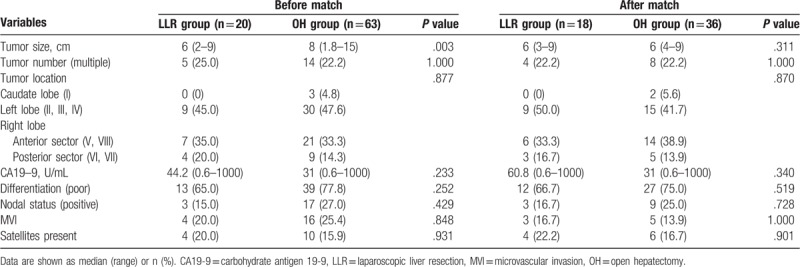
A total of 83 patients with large and multiple ICCs who underwent LLR or OH between January 2012 and June 2017 were included in this study (Fig. 1). Among them, 20 (24.1%) patients who received LLR were categorized into the LLR group, and 63 (75.9%) patients who received OH were categorized into the OH group. In terms of the patient characteristics shown in Table 1, age, sex, body mass index (BMI), ASA grade, biometrics, comorbidities (hypertension, diabetes mellitus), underlying liver cirrhosis, HBsAg positive rate and history of previous abdominal surgery were comparable between the 2 groups. With regard to disease characteristics (Table 2), no differences were noted in terms of tumor number, tumor location, histological grade or nodule status between the LLR group and OH group were noted. However, the maximum tumor size before PSM showed a significant difference (median 6 cm vs 8 cm, P = .003). After 1:2 PSM, 18 patients remained in the LLR group and 36 patients were in the OH group with comparable patient characteristics and disease characteristics.
Table 1.
Patients characteristic before and after propensity score matching.
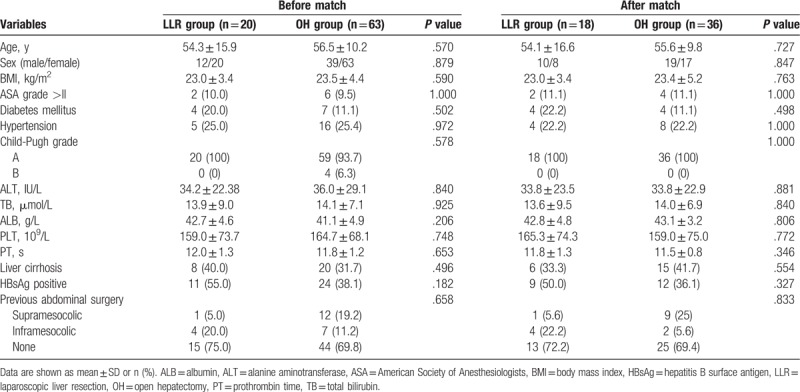
Table 2.
Disease characteristic before and after propensity score matching.
3.2. Intraoperative outcomes
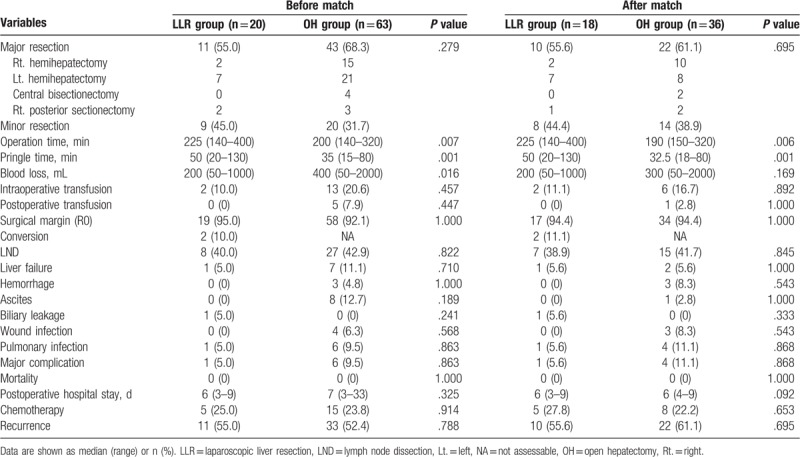
The intraoperative data are reported in Table 3. LLR resulted in a significantly longer operation time (median 225 minutes vs 200 minutes, P = .007; median 225 minutes vs 190 minutes, P = .006, respectively) both before and after PSM. Occlusion time was longer in the LLR group than in the OH group (median 50 minutes vs 35 minutes, P = .001; median 50 minutes vs 32.5 minutes, P = .001, respectively) both before and after matching. Laparoscopic liver resection was completed in 18 patients. Two patients experienced conversions because of severe adhesion (n = 1) and uncontrolled bleeding (n = 1). In terms of the type of resection, the proportion of major liver resection was comparable between different treatment groups (55.0% vs 68.3%, P = .279; 56.6% vs 61.1%, P = .695, respectively) both before and after PSM. Before matching, patients receiving LLR experienced a significantly lower amount of blood loss than patients receiving OH (median 200 mL vs 400 mL, P = .016), however, the difference was not significant (median 200 mL vs 300 mL, P = .169) after PSM. The intraoperative and postoperative blood transfusion rates were comparable between the groups.
Table 3.
Intraoperative data and postoperative outcomes before and after propensity score matching.
3.3. Postoperative morbidity and mortality
Postoperative morbidity and mortality are shown in Table 3. Before PSM, a lower proportion of patients encountered major complication in the LLR cohort than in the OH cohort (5.0% vs 9.5%, P = .863). Although there was no significant difference, the major complication rate was still lower in LLR group than in the OH group after PSM (5.6% vs 11.1%, P = .868). For liver-related specific morbidities, transient liver failure occurred in 1 versus 7 patients before PSM (5.0% vs 11.1%, P = .710) and in 1 versus 2 patients after PSM (5.6% vs 5.6%, P = 1.000). For ascites, no significant difference was noted (0% vs 12.7%, P = .189; 0% vs 2.8%, P = 1.000, respectively) between the groups. The bile leakage rate was comparable between different treatment groups (5.0% vs 0%, P = .241; 5.6% vs 0%, P = .333, respectively) before and after matching. The rates of general complications including wound infection and pulmonary infection were also comparable between the different surgical procedures. The postoperative hospital stay was shorter in the LLR cohort (median 6 days vs 7days, P = .325; median 6 days vs 7 days, P = .092, respectively), although this difference was not statistically significant. Overall, no patient died within 30 days postoperation in either group.
3.4. Survival
The follow-up duration ranged from 1 to 78 months (median 24 months). During follow-up, 11 patients in the LLR group and 33 patients in the OH group experienced recurrence. After PSM, 10 patients and 22 patients in the LLR group and OH group experienced recurrence, respectively. The recurrence rates were comparable between different treatment groups (55% vs 52.4%, P = .788; 55.6% vs 61.1%, P = .695, respectively) before and after matching. In the PSM cohort, no significant difference was observed in OS between the 2 groups (P = .645) with a median OS of 21 months in the LLR group and 17 months in the OH group. The 1- and 3-year OS rates were 66.7% and 45.8% in the LLR group and 72.2% and 38.2% in the OH group, respectively (Fig. 2A). In terms of DFS, there was no statistically significant difference between the groups (P = .827). The median DFS was 17 months in the LLR group and 12 months in the OH group. The 1- and 3-year DFS rates were 53.1% and 37.8% in the LLR group and 48.7% and 34.9% in the OH group, respectively (Fig. 2B).
Figure 2.
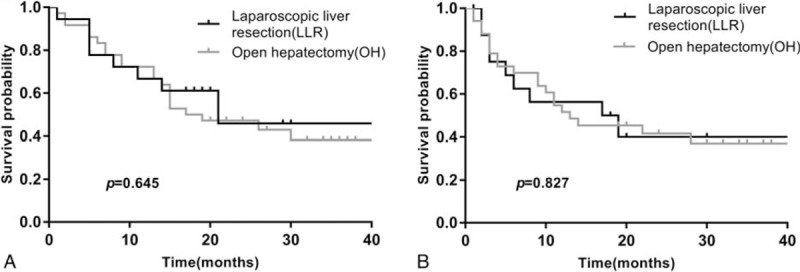
(A) Overall survival for LLR versus OH in the matched cohort; (B) disease-free survival for LLR versus OH in the matched cohort. LLR = laparoscopic liver resection, OH = open hepatectomy.
4. Discussion
Although few studies[20,21] have reported that patients with “very early” ICC might benefit from liver transplantation. ICC is still typically a contraindication for liver transplantation because of the high risk of recurrence and poor long-term outcomes.[1,22] Thus, liver resection remains the first-line treatment option for patients with resectable ICC. Many studies have shown the feasibility of minimally invasive approach for patients with liver malignancy including hepatocellular carcinoma (HCC) and colorectal liver metastases (CLM).[23–25] However, only a few small studies from high-volume centers have discussed the efficacy of LLR in patients with ICC but they mainly focused on small solitary ICC.[7–11]
Nevertheless, the size of the largest tumor and the number of tumors were identified as independent risk factors for DFS after liver resection for ICC.[3] Furthermore, large or multiple tumors make liver resection under laparoscopy more technical challenging. Wei's et al[11] report[11] discussed the safety and feasibility of LLR for large or multiple ICCs, however, the main purpose was compared in patients who underwent laparoscopic liver resection for small solitary ICC and yet shed little light on the difference between LLR and OH. Therefore, minimally invasive approach is still a controversial area for patients with ICC due to concerns about compromising the oncological efficacy, especially when dealing with large or multiple ICCs. The aim of this study was not only to analyze the feasibility and safety of LLR for large or multiple ICCs but also to compare the short- and long-term outcomes in patients who underwent OH.
The second consensus meeting for LLR showed that laparoscopic minor liver resection usually related to small wound, less blood loss, lower transfusion rates, superior complication rates and shorter hospital stays, however, the operative mortality was comparable to that of OH.[14] In terms of major liver resection, a study that included >10,000 major hepatectomy patients registered over 3 years in Japan demonstrated similar conclusions.[26] In the present study, the better visualization of vessels provided by laparoscopy made parenchyma transection more precise in the LLR group, together with high intra-abdominal pressure caused by the pneumoperitoneum resulted in a lower blood loss in the LLR group than in the OH group, although the difference was not statistically significant after PSM. However, highlighted precision procedures and technical difficulty resulted in longer hepatic inflow occlusion times and operation times in the LLR cohort than in the OH cohort. Moreover, even without statistical significance, a shorter postoperative length of stay was observed in the LLR cohort than in the OH cohort, corresponding to previous researches.[9] Early oral intake and off-bed activity facilitate recovery and reduce the stress response following surgery, which may contribute to the shorter postoperative hospital stay in patients receiving LLR than in patients receiving OH.
The overall conversion rate in the present study was 10%, which is comparable to previous report.[6] A total of 2 patients experienced conversion in the current study, 1 was a 63 year-old woman with multiple lesions, and the maximum tumor was located in segment VII. Wedge resection was performed, however, massive hemorrhage occurred during parenchyma transection. Inadequate exposure restrained the hemostatic steps, titanium clips were used to temporarily control the bleeding, and laparotomy was performed. This patient also accounted for the maximum blood loss in the LLR cohort and underwent intraoperative transfusion. Another 56-year-old man had underwent open cholecystectomy in the 1990s. A CT scan showed that the tumor was located in segment V with enlarged hilar lymph nodes. A previous history of abdominal surgery caused severe adhesion and resulted in conversion to laparotomy. Above cases agreed with the results of previous researches which reported that bleeding and adhesion were the most common causes of conversion.[5,27] Moreover, the resections of postero-superior segments (SI, SIVa, SVII, SVIII) was identified as an independent risk factor for conversion in LLR and thus requires further careful assessment before LLR.[27]
Another concern for the use of minimally invasive approach in the treatment of ICCs is the technical difficulty of performing LND by using laparoscopic apparatus. Although the role of routine lymphadenectomy is still controversial, routine lymphadenectomy is recommended by many experts and is highlighted in the 8th edition of the AJCC/UICC ICC staging schema for accurate staging.[28,29] A recent study[30] from the National Institutes of Health based on National Cancer Database reported that the use of LLR for ICC is associated with inadequate nodal evaluation. A total of 2309 patients who underwent LLR or OH for ICC between 2010 and 2015 were retrospectively analyzed, and LND was performed more commonly in patients who underwent OH (61%, n = 1210) than in patients who underwent LLR (39%, n = 120), P < .001. In addition, we reviewed the literature aimed to comparing LLR with OH for ICC. Five studies[7–11] included 90 patients who underwent LLR and 150 patients who underwent OH were found. The pooled result also showed that LND was performed more frequently in OH (73.3%, n = 110) than in LLR (31.1%, n = 28). This finding indicated that the nodal evaluation strategy may need to be more aggressive in LLR in the future. In the present study, LND was only performed when suspected lymph node metastasis (LNM) was detected by preoperative imaging or intraoperative ultrasound or palpable enlarged regional lymph nodes during surgery. After all, a total of 35 patients underwent LND, and the LND rates were comparable between the groups (40.0% vs 42.9%, P = .822; 38.9% vs 41.7%, P = .845, respectively, Table 3) both before and after matching. Additionally LNM was confirmed in 20 of these patients by pathology. Except for the previously mentioned male patients who experienced conversion, the remaining 7 patients in the LLR group completed the LND procedure via pure laparoscopy. Ratti et al[31] reported that the laparoscopic approach for lymphadenectomy is associated with lower lymphadenectomy-related morbidity in patients with biliary cancers, however, no patients encountered lymphadenectomy-related morbidity in either group in the present study. In general, the present study showed that LND is feasible and safe under laparoscopy.
In terms of short-term outcomes, the results of the current study showed that LLR did not increase liver-specific complications, general complications, or major complications. Moreover, although there was no significant difference, ascites together with hemorrhage occurred more frequently in the OH group than in the LLR group after PSM. Consistently, 2 other studies focused on LLR for HCC in patients with cirrhosis have reported that the rate of liver-specific complications was lower after laparoscopy.[24,32] Regarding general complications, a lower wound infection rate was noted in the LLR cohort as well as pulmonary complications which corresponded to the previous literature.[19,33] The major complication rate was minimal and comparable between the groups after PSM (5.6% in the LLR and 11.1% in the OH group, P = .868, Table 3). The major complication rate was consistent with published reports’ rates of 9.5% to 27.5% in LLR for large HCC or CLM,[34,35] which is lower than the reported rate of 16.7% in a previous similar study for large or multiple ICCs.[11] Furthermore, no patients encountered death within 30 days postoperation in either group.
Tumor-free surgical margins affect DFS after resection of ICCs. The main concern was that LLR would not guarantee sufficient tumor-free surgical margins due to the lack of palpation. However, in contrast to relying solely on surgeon's impressions and instincts, the application of ultrasonography during surgery can help locate tumors and guarantee adequate surgical margins during resection. The R0 resection rate in the present study population was 92.8%, which is comparable to the previously reported rates of 92.5% to 95.6% for LLR in large CLM and HCC.[34,35] The present study also showed that the R0 resection rate was comparable between the different techniques (95% vs 92.1%, P = 1.000; 94.4% vs 94.4%, P = 1.000, respectively, Table 3) both before and after matching. Moreover, DFS was comparable between the groups, and there was no statistically significant difference in terms of OS between the LLR and OH groups.
The current study carries several inevitable limitations. First, although the PSM method was used, the retrospective nature might weaken the generalizability and accuracy of the results. Second, the low incidence of ICC resulted in a relatively small sample size, and the number of patients may not be sufficient to provide solid evidence. Third, to illuminate the long-term differences between the different approaches, a longer follow-up period is still needed.
In conclusion, the present study showed that patients who underwent LLR for large or multiple ICCs could obtain similar short- and long-term outcomes compared with those who underwent OH, and LND was more technically difficult but feasible in LLR. Further studies with larger number of patients and a longer follow-up duration should be performed in the future to confirm this result.
Acknowledgments
The authors are grateful for the support of the liver surgery department, West China Hospital of Sichuan University.
Author contributions
Conceptualization: Yunfeng Zhu, Jiulin Song.
Methodology: Yifei Tan.
Software: Xi Xu, Yifei Tan.
Writing – original draft: Yunfeng Zhu, Jiulin Song.
Writing – review & editing: Jiayin Yang.
Footnotes
Abbreviations: ASA = American Society of Anesthesiologists, BMI = body mass index, CA19-9 = carbohydrate antigen 19-9, CLM = colorectal liver metastases, CT = computed tomography, CUSA = Cavitron Ultrasonic Surgical Aspirator, DFS = disease-free survival, HCC = hepatocellular carcinoma, ICC = intrahepatic cholangiocarcinoma, LLR = laparoscopic liver resection, LND = lymph node dissection, LNM = lymph node metastasis, MRI = magnetic resonance imaging, MVI = microvascular invasion, OH = open hepatectomy, OS = overall survival, POD = postoperative day, PSM = propensity score match.
How to cite this article: Zhu Y, Song J, Xu X, Tan Y, Yang J. Safety and feasibility of laparoscopic liver resection for patients with large or multiple intrahepatic cholangiocarcinomas: a propensity score based case-matched analysis from a single institute. Medicine. 2019;98:49(e18307).
YZ and JS contributed equally to this article.
This study was supported by grants from the 1.3.5 project for discipline of excellence, West China Hospital, Sichuan University (ZY20170308).
The authors have no conflicts of interest to disclose.
References
- [1].Razumilava N, Gores GJ. Cholangiocarcinoma. Lancet 2014;383:2168–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Shaib YH, Davila JA, McGlynn K, et al. Rising incidence of intrahepatic cholangiocarcinoma in the United States: a true increase? J Hepatol 2004;40:472–7. [DOI] [PubMed] [Google Scholar]
- [3].Endo I, Gonen M, Yopp AC, et al. Intrahepatic cholangiocarcinoma: rising frequency, improved survival, and determinants of outcome after resection. Ann Surg 2008;248:84–96. [DOI] [PubMed] [Google Scholar]
- [4].Bridgewater J, Galle PR, Khan SA, et al. Guidelines for the diagnosis and management of intrahepatic cholangiocarcinoma. J Hepatol 2014;60:1268–89. [DOI] [PubMed] [Google Scholar]
- [5].Nguyen KT, Gamblin TC, Geller DA. World review of laparoscopic liver resection-2,804 patients. Ann Surg 2009;250:831–41. [DOI] [PubMed] [Google Scholar]
- [6].Ciria R, Cherqui D, Geller DA, et al. Comparative short-term benefits of laparoscopic liver resection: 9000 cases and climbing. Ann Surg 2016;263:761–77. [DOI] [PubMed] [Google Scholar]
- [7].Uy BJ, Han H-S, Yoon Y-S, et al. Laparoscopic liver resection for intrahepatic cholangiocarcinoma. J Laparoendosc Adv Surg Tech A 2015;25:272–7. [DOI] [PubMed] [Google Scholar]
- [8].Ratti F, Cipriani F, Ariotti R, et al. Safety and feasibility of laparoscopic liver resection with associated lymphadenectomy for intrahepatic cholangiocarcinoma: a propensity score-based case-matched analysis from a single institution. Surg Endosc 2016;30:1999–2010. [DOI] [PubMed] [Google Scholar]
- [9].Lee W, Park JH, Kim JY, et al. Comparison of perioperative and oncologic outcomes between open and laparoscopic liver resection for intrahepatic cholangiocarcinoma. Surg Endosc 2016;30:4835–40. [DOI] [PubMed] [Google Scholar]
- [10].Kinoshita M, Kanazawa A, Takemura S, et al. Indications for laparoscopic liver resection of mass-forming intrahepatic cholangiocarcinoma. Asian J Endosc Surg 2019;1–3. 10.1111/ases.12703 [DOI] [PubMed] [Google Scholar]
- [11].Wei F, Lu C, Cai L, et al. Can laparoscopic liver resection provide a favorable option for patients with large or multiple intrahepatic cholangiocarcinomas? Surg Endosc 2017;31:3646–55. [DOI] [PubMed] [Google Scholar]
- [12].Song JL, Yang J, Wu H, et al. Pure laparoscopic right hepatectomy of living donor is feasible and safe: a preliminary comparative study in China. Surg Endosc 2018;32:4614–23. [DOI] [PubMed] [Google Scholar]
- [13].Liu F, Wei Y, Li H, et al. LigaSure versus CUSA for parenchymal transection during laparoscopic hepatectomy in hepatocellular carcinoma patients with cirrhosis: a propensity score-matched analysis. Surg Endosc 2018;32:2454–65. [DOI] [PubMed] [Google Scholar]
- [14].Chana P, Burns EM, Arora S, et al. A systematic review of the impact of dedicated emergency surgical services on patient outcomes. Ann Surg 2016;263:20–7. [DOI] [PubMed] [Google Scholar]
- [15].Clavien PA, Barkun J, de Oliveira ML, et al. The Clavien-Dindo classification of surgical complications: five-year experience. Ann Surg 2009;250:187–96. [DOI] [PubMed] [Google Scholar]
- [16].Balzan S, Belghiti J, Farges O, et al. The “50–50 Criteria” on postoperative day 5. Ann Surg 2005;242:824–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Taguchi Y, Ebata T, Yokoyama Y, et al. The determination of bile leakage in complex hepatectomy based on the guidelines of the International Study Group of Liver Surgery. World J Surg 2014;38:168–76. [DOI] [PubMed] [Google Scholar]
- [18].Rahbari NN, Garden OJ, Padbury R, et al. Post-hepatectomy haemorrhage: a definition and grading by the International Study Group of Liver Surgery (ISGLS). HPB (Oxford) 2011;13:528–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Fuks D, Cauchy F, Fteriche S, et al. Laparoscopy decreases pulmonary complications in patients undergoing major liver resection: a propensity score analysis. Ann Surg 2016;263:353–61. [DOI] [PubMed] [Google Scholar]
- [20].Sapisochin G, Rodriguez de Lope C, Gastaca M, et al. “Very early” intrahepatic cholangiocarcinoma in cirrhotic patients: should liver transplantation be reconsidered in these patients? Am J Transplant 2014;14:660–7. [DOI] [PubMed] [Google Scholar]
- [21].Sapisochin G, Facciuto M, Rubbia-Brandt L, et al. Liver transplantation for “very early” intrahepatic cholangiocarcinoma: International retrospective study supporting a prospective assessment. Hepatology 2016;64:1178–88. [DOI] [PubMed] [Google Scholar]
- [22].Goldaracena N, Gorgen A, Sapisochin G. Current status of liver transplantation for cholangiocarcinoma. Liver Transpl 2018;24:294–303. [DOI] [PubMed] [Google Scholar]
- [23].Soubrane O, Goumard C, Laurent A, et al. Laparoscopic resection of hepatocellular carcinoma: a French survey in 351 patients. HPB (Oxford) 2014;16:357–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Yoon YI, Kim KH, Kang SH, et al. Pure laparoscopic versus open right hepatectomy for hepatocellular carcinoma in patients with cirrhosis: a propensity score matched analysis. Ann Surg 2017;265:856–63. [DOI] [PubMed] [Google Scholar]
- [25].Ratti F, Fiorentini G, Cipriani F, et al. Laparoscopic vs open surgery for colorectal liver metastases. JAMA Surg 2018;153:1028–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Takahara T, Wakabayashi G, Konno H, et al. Comparison of laparoscopic major hepatectomy with propensity score matched open cases from the National Clinical Database in Japan. J Hepatobiliary Pancreat Sci 2016;23:721–34. [DOI] [PubMed] [Google Scholar]
- [27].Troisi RI, Montalti R, Van Limmen JG, et al. Risk factors and management of conversions to an open approach in laparoscopic liver resection: analysis of 265 consecutive cases. HPB (Oxford) 2014;16:75–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Weber SM, Ribero D, O’Reilly EM, et al. Intrahepatic cholangiocarcinoma: expert consensus statement. HPB (Oxford) 2015;17:669–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Bagante F, Spolverato G, Weiss M, et al. Assessment of the lymph node status in patients undergoing liver resection for intrahepatic cholangiocarcinoma: the New Eighth Edition AJCC staging system. J Gastrointest Surg 2018;22:52–9. [DOI] [PubMed] [Google Scholar]
- [30].Martin SP, Drake J, Wach MM, et al. Laparoscopic approach to intrahepatic cholangiocarcinoma is associated with an exacerbation of inadequate nodal staging. Ann Surg Oncol 2019;26:1851–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Ratti F, Fiorentini G, Cipriani F, et al. Perioperative and long-term outcomes of laparoscopic versus open lymphadenectomy for biliary tumors: a propensity-score-based, case-matched analysis. Ann Surg Oncol 2019;26:564–75. [DOI] [PubMed] [Google Scholar]
- [32].Xu HW, Liu F, Li HY, et al. Outcomes following laparoscopic versus open major hepatectomy for hepatocellular carcinoma in patients with cirrhosis: a propensity score-matched analysis. Surg Endosc 2018;32:712–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Franken C, Lau B, Putchakayala K, et al. Comparison of short-term outcomes in laparoscopic vs open hepatectomy. JAMA Surg 2014;149:941–6. [DOI] [PubMed] [Google Scholar]
- [34].Shelat VG, Cipriani F, Basseres T, et al. Pure laparoscopic liver resection for large malignant tumors: does size matter? Ann Surg Oncol 2015;22:1288–93. [DOI] [PubMed] [Google Scholar]
- [35].Nomi T, Fuks D, Louvet C, et al. Outcomes of laparoscopic liver resection for patients with large colorectal liver metastases: a case-matched analysis. World J Surg 2016;40:1702–8. [DOI] [PubMed] [Google Scholar]


