Abstract
Intrahepatic cholangiocarcinoma (ICC) is an aggressive malignancy with poor prognosis and increasing incidence. Due to its asymptomatic manifestation, ICC often progresses to a metastatic stage on diagnosis. The current study attempted to evaluate the prognostic value of site-specific metastases in patients with metastatic ICC.
Surveillance, Epidemiology, and End Results (SEER) database (2010–2015) was queried and metastatic ICC patients were classified according to the metastatic sites. Kaplan–Meier analysis was used for survival comparisons and multivariate analysis was performed to elicit characteristics independently associated with survival.
A total of 1567 patients were identified and included in the analysis. Compared with those with multiple-site metastases, patients with single-site metastases had better prognostic outcomes. Among the single-site metastases, regional lymph nodes metastases had the best prognosis; liver metastases had better prognostic outcomes than bone metastases; no significant difference was found between lung and bone or liver metastasis. Local treatment of primary tumor might benefit patients with isolated lymph nodes metastases and few exceptional cases of patients with liver metastases.
Different metastatic sites have distinct impact on the survival outcomes of patients with advanced ICC and highly selected subset of them might benefit from the local treatment of the primary tumor.
Keywords: intrahepatic cholangiocarcinoma, metastatic site, prognosis, SEER
1. Introduction
Intrahepatic cholangiocarcinoma (ICC) is the second most common primary liver cancer and accounts for 10–15% of all primary liver cancers.[1,2] It is highly malignant and has an extremely poor prognosis[3] and its incidence rate has prominently increased over the past several decades worldwide.[4,5] Currently, radical surgical resection seems to be the only potentially curative therapy for patients with ICC. However, as the symptoms of ICC manifest mainly late and not specific, most patients present with advanced stages at the point of diagnosis. Only 30% to 60% of ICC patients are candidates for such a surgical resection.[6] Even in patients who undergo successful resection, the overall 5-year postoperative survival rate is usually lower than 40%[7] and that for advanced patients who cannot be operated on is even worse, falling short of 5%.[8]
With the progress of disease process, advanced intrahepatic cholangiocarcinomas often preferentially metastasize to liver and extrahepatic organs including lungs, bones, brain, and lymph nodes.[5] Patients with different metastatic sites might present different tumor biologic patterns, face different prognostic prospects, and need distinct therapeutic approaches.[9] Nevertheless, due to rare data of ICC metastases, few detailed studies have explored the profiles of liver and extrahepatic metastasis. Therefore, metastatic patterns of ICC still await further clarification and it remains unsettled whether different metastatic sites would be translated into distinct clinical outcomes. Besides, patients with different metastatic sites might react differently to the treatments. A retrospective cohort study suggests that benefits of overall survival from surgical resection might be obtained in some advanced ICC patients with intrahepatic metastasis, vascular invasion, or regional lymph node metastasis.[10] Another study reports that percutaneous radiofrequency ablation (RFA) may provide successful local tumor control in some patients with unresectable intrahepatic cholangiocarcinomas, which are of intermediate or small diameter.[11] However, these studies are based on a rather small single-center patient cohort. The effects of surgery or local tumor destructions including RFA on the metastatic ICC are still disputable. Therefore, it is meaningful to elucidate the metastatic distribution of ICC in order to reap better treatment outcomes and more survival benefits.
So far, population-based data on the prognostic value of site-specific metastases for metastatic ICC are insufficient. Thus, this study attempted to review the presentation and treatment trends of metastatic ICC patients registered within the Surveillance, Epidemiology, and End Results (SEER) database with a particular focus on the prognostic value of different metastatic sites.
2. Materials and methods
2.1. Cohort population
The data for this study were extracted from the SEER database of the US National Cancer Institute, which contains information from various locations and sources throughout the United States, representing approximately 28% of the US population.[12] The data were retrieved by utilizing the SEER∗Stat software (Version 8.3.5). Patients diagnosed with intrahepatic cholangiocarcinomas from 2010 to 2015 were identified and the detailed population selection procedure was summarized in Figure 1. Briefly, we included cases with a primary site of “intrahepatic bile duct”, with ICD-O-Histology/behavior codes of 8160/3 (cholangiocarcinoma) and with a clinical stage of N1 or M1 (American Joint Committee on Cancer (AJCC) 7th edition). We excluded patients without sufficient survival data. Cases with multiple primary tumors were also excluded.
Figure 1.
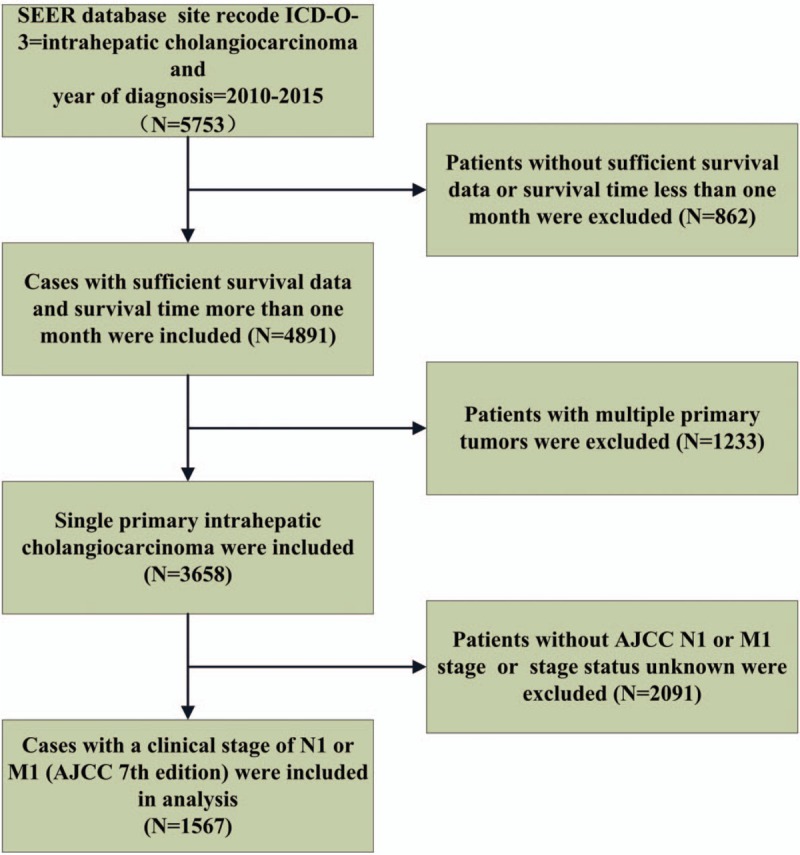
Flowchart of population inclusion of the study. AJCC = American Joint Committee on Cancer.
2.2. Covariates and follow-up information
Covariates of interest extracted for each case included age on diagnosis, gender, race, tumor size and grade, site of extrahepatic metastasis, local treatments of the primary tumor (surgery or local tumor destruction). The follow-up information included survival months and cause-specific death classification. The primary endpoints of the study were overall survival (OS) and cancer-specific survival (CSS). OS time was calculated from the date of diagnosis to the date of death from any cause or the last follow-up. CSS time was from the date of diagnosis to death from ICC or the last follow-up. The study was approved by the review board of Fujian Medical University Union Hospital, Fujian, China. SEER dataset was accessed through the reference number 10372-Nov2017. Extraction of data from the SEER database does not require informed consent.
2.3. Statistical analysis
In this study, metastatic intrahepatic cholangiocarcinoma patients were classified according to the site of metastases (bone, brain, liver, lung, and regional lymph nodes). Chi-square test and Fisher's exact test were utilized to compare the clinicopathological characteristics among different metastatic sites. Kaplan–Meier analysis and log rank testing were used for survival comparisons. Multivariate Cox proportional hazards regression analysis was employed to evaluate the prognostic factors, and hazard ratios (HRs) along with 95% confidence interval (CI) were calculated. A 2-tailed P value <.05 was considered as statistically significant. All of the statistical analyses were performed using SPSS Statistics 19.0 (IBM, NY).
3. Results
3.1. Patients’ characteristics
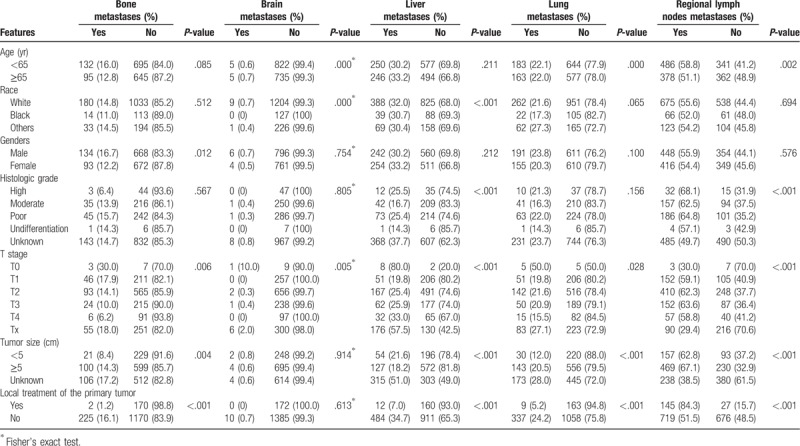
A total of 1567 patients with metastatic intrahepatic cholangiocarcinoma were identified and included in the current study according to the inclusion and exclusion criteria. Table 1 summarizes the distribution of different metastatic sites and baseline characteristics for all enrolled patients. A total of 227 (14.5%) patients were diagnosed with bone metastasis, 10 (0.6%) patients with brain metastasis, 496 (31.7%) with liver metastasis, 346 (22.1%) with lung metastasis and 864 (55.1%) with regional lymph nodes metastases. A total of 927 (59.2%) patients had a single-site of metastases while 640 patients (40.8%) had multi-site metastases, the median follow-up time was 6 months and 5 months. In patients with single-site metastases, 70 (4.3%) cases were diagnosed with single bone metastasis, 193 (12.3%) with liver metastasis, 91 (5.8%) with lung metastasis, and 571 (36.4%) were with regional lymph nodes metastases, the median follow-up time was 4 months, 5 months, 5 months, 7 months, respectively. Only 2 patients were diagnosed with single brain metastasis. Surgery to the primary tumor was performed on 162 (10.3%) patients and other local tumor destructions of the primary tumor were performed on 10 (0.6%) patients. No information on systemic treatment was provided in the SEER database. Statistically significant correlations were obtained between different characteristics and each site of metastases.
Table 1.
Clinical features and metastatic sites.
3.2. Survival analysis
The overall survival and ICC specific survival were compared according to different metastatic sites. For both endpoints, Kaplan–Meier analysis showed that patients with single-site metastases have better survival outcomes than patients with multiple-site metastases (P < .001 for both end points) (Fig. 2). Among patients with single-site metastases, patients with regional lymph nodes metastases had the best prognosis; patients with isolated liver metastasis had better outcomes than patients with bone metastasis (lymph nodes vs liver metastasis: P = .011 for OS and P = .048 for CSS; lymph nodes vs lung metastasis: P =.004 for OS and P = .001 for CSS; lymph nodes vs bone metastasis: P < .001 for both OS and CSS; liver vs lung metastasis: P = .414 for OS and P = .158 for CSS; liver vs bone metastasis: P = .025 for OS and P = .041 for CSS; lung vs bone metastasis: P = .172 for OS and P = .567 for CSS) (Fig. 3). Patients with isolated brain metastasis were excluded from this analysis due to the small sample size (n = 2).
Figure 2.
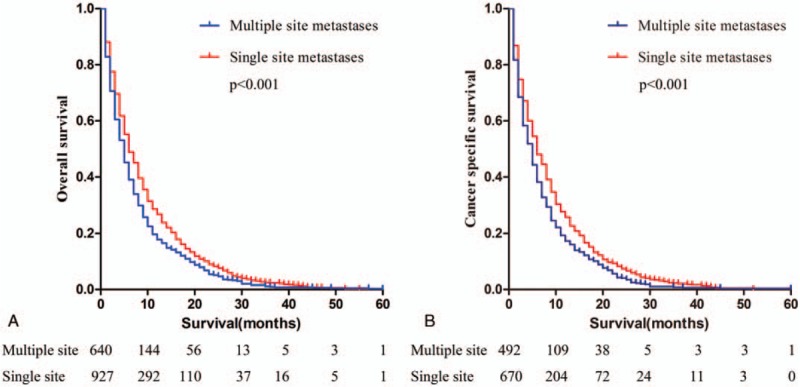
Kaplan–Meier curves of overall survival (a) and cancer-specific survival (b) according to metastasis to single or multiple sites. The difference in survival between patients with single-site metastases and with multiple-site metastases was statistically significant (P < .001 for both end points).
Figure 3.
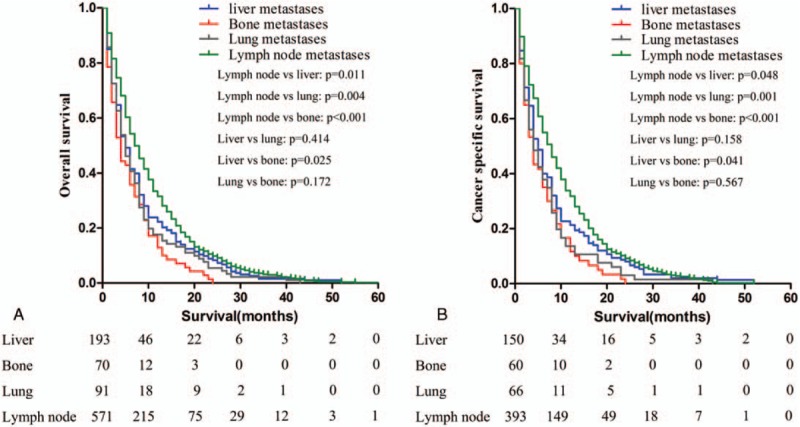
Kaplan–Meier curves of overall survival (a) and cancer-specific survival (b) according to the sites of metastases in patients with single metastatic site. Patients with regional lymph nodes metastases had the best prognosis (P < .05). Patients with isolated liver metastasis had better outcomes than patients with bone metastasis (P < .05).
In addition, OS and CSS were assessed according to whether local treatment of the primary tumor was performed on patients with single liver (the median follow-up time was 22 months for yes and 5 months for no), lung (the median follow-up time was 7.5 months for yes and 5 months for no), or regional nodal metastasis (the median follow-up time was 11.5 months for yes and 6 months for no). Patients with single bone metastasis were not included because there was only 1 case. The results showed that patients with isolated regional nodal metastasis may benefit from local treatment of the primary tumor. Besides that, few exceptional cases of patients with isolated liver metastases might also benefit from local treatment of the primary tumor (Fig. 4).
Figure 4.
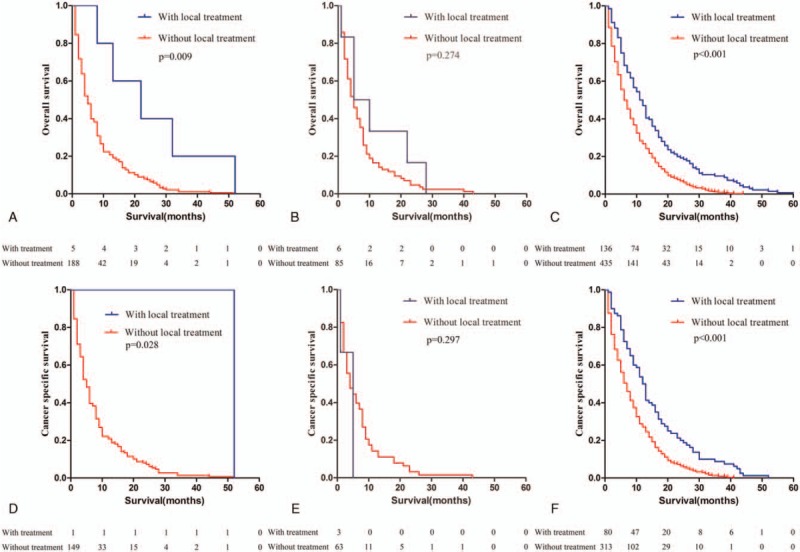
Kaplan–Meier curve of overall survival (a–c) and cancer-specific survival (d–f) according to the performance of local treatment to the primary tumor. (a and d) Few exceptional cases of patients with liver metastases benefited from local treatment of the primary tumor. (b and e) No significant differences were found in the prognosis between patients with single lung metastasis regardless of the reception of local treatment. (c and f) Patients with isolated regional lymph node metastases benefited from local treatment of the primary tumor.
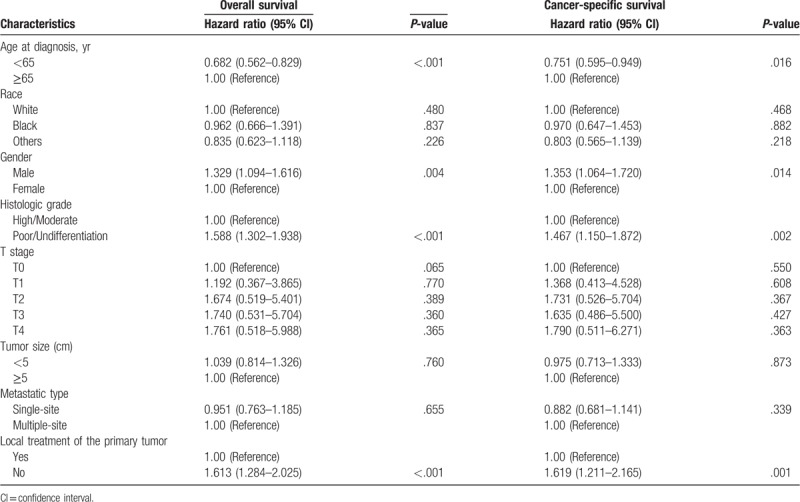
Furthermore, multivariate Cox regression analysis was performed to identify independent factor that might influence the survival. We found that the age (<65 years) and local treatment of the primary tumor were associated with better overall survival and intrahepatic cholangiocarcinoma specific survival. Patients with well/moderately-differentiated carcinomas had better prognosis compared with patients with poorly-differentiated or undifferentiated carcinomas (Table 2).
Table 2.
Multivariate Cox regression analysis of prognostic factors influencing survival outcomes in overall patient cohort.
4. Discussion
ICC represents a subtype of bile duct adenocarcinoma involving the small ducts within the liver.[13] After hepatocellular carcinoma (HCC), ICC is the second most common primary hepatic malignant tumor and its incidence is increasing throughout the world.[14] Compared with HCC, it is even more invasive and has a higher percentage of metastasis.[15] Up to date, radical surgical treatment has been considered to be the only effective therapy for ICC patients. However, the resectability rate of ICC remains low[16] and the prognosis of ICC patients is usually poor, especially for advanced-stage cases with intrahepatic or extrahepatic metastasis. The 5-year relative survival rate is reported to be about 3.1%.[17] Thus, a clear understanding of clinical features and metastatic sites becomes urgent. In this study, we analyzed a large, contemporary series of patients with metastatic ICC from a representative national cohort and discussed the prognostic value of site-specific metastases and local treatment of the primary tumor.
It was reported that patients afflicted with single-site metastases had a better prognosis than patients with multiple-site metastases by 2 studies of metastatic bladder cancer, in which the SEER database was also used.[9,18] Using Kaplan–Meier analysis and log-rank test, we also found a significant difference between patients of ICC with single-site metastases and multiple-site metastases in this study. The former seemed to have a much better prognosis. But in multivariate Cox regression analysis, single-site metastasis was not found to be a significant independent prognostic predictor of OS and CSS. In fact, one of the above mentioned study about metastatic gallbladder cancer has the similar problem. In Dong et al's study, the results of multivariate analysis also did not support multiple metastases emerge as an independent risk factor for poor prognosis. The difference might arise from the different choices of the covariates in the Cox model, and further studies are needed to expound how the number of metastatic sites could affect the prognosis of patients with metastatic ICC.
Moreover, our data revealed prognostic differences of the site of metastases. Patients with isolated regional lymph node involvement were more common and, as expected had better outcomes than patients with any other isolated metastases. Liver was the second most common metastasis site and patients with isolated liver metastasis had better prognosis than patients with bone metastasis, but not than patients with lung metastasis. There were no significant differences in survival between patients with bone metastasis and lung metastasis. Additionally, we also evaluated the efficacy of local therapy to the primary tumor for patients with advanced ICC. Benefits of local treatment of the primary tumor in cases of a metastatic solid tumor have been suggested for metastatic breast cancer and renal cell carcinoma.[19,20] Primary tumor removal can reverse immune suppression caused by bulky tumor even in the presence of extensive metastatic disease.[21] Given that ICC is a primary liver cancer, local liver-directed treatments are central to the management of patients with ICC.[22] Liver-directed treatments include surgical resection and other local tumor destructions, such as thermal ablation therapy.[23] However, data on outcomes after local liver-directed treatments for advanced ICC are limited due to the rarity of the disease.
In the present study, we found that the majority of these patients received surgical resection while a small subset was treated with other local tumor destructions. The use of radiofrequency ablation, cryotherapy and microwave ablation is less used may because of common large size of ICC and increased risk of biliary complications for tumors near the hilum and pedicles.[24] In current study, survival analysis showed that for patients with isolated lymph regional nodal metastasis, those undergoing local treatment of the primary tumor had a better prognosis. Nodal status is important among patients with ICC, as lymph node metastasis is one of the strongest factors associated with poor long-term survival.[25,26] However, published literature regarding the benefit of surgical resection for patients with positive lymph node disease remains controversial. Some authors have suggested that lymph node metastases should be considered as a relative contraindication to extensive surgery, claiming that lymphadenectomy does not enhance the long-term survival.[27–29] But other authors advocate that a selected group of patients with positive lymph node status can benefit from surgery. Suzuki et al[30] have suggested that hepatic resection with lymph node dissection may be curative in patients with a single lymph node metastasis and a solitary ICC tumor. Nakagawa et al[31] reported that curative resection with lymphadenectomy improved the survival of patients with solitary tumor and no more than 2 positive lymph nodes. One retrospective study of the National Cancer Database reported that surgery is an independent prognostic factor for better prognosis of patients who suffered ICC with lymph node metastasis.[32] In our study, local treatment of the primary tumor, together with age <65 and high histological grade, was associated with better overall and cancer-specific survival. It seems that some patients with lymphatic metastasis may benefit from local treatments of the primary tumor. Even so, what we need to pay attention is that performance of lymphadenectomy may influence the survival analysis. Although the 2015 The National Comprehensive Cancer Network (NCCN) guidelines on hepatobiliary malignancies state that lymphadenectomy may be considered in addition to resection, no definitive conclusion has been made regarding the role of routine lymphadenectomy.[25] Only 49% to 78% of patients undergoing resection of ICC have data available on lymph node status.[25]
Furthermore, Kaplan–Meier survival analysis also showed that for patients with isolated liver metastasis might benefit from liver-directed therapies. But the number of patients who received local treatment was very limited. We consider it is the exception but not the rule. However, it still suggested that there are exceptional cases of patients with indolent metastatic disease that might be considered for resection or other local treatment. Methods for the differentiation of them are needed.
Our study provides some valuable information about the prognostic impact of different metastatic sites and the potential role of local treatment in each metastatic site. However, some limitations should also be considered here. The first limitation comes from the SEER dataset itself. The database only covers 28% of the US population. Certain regions, such as the populations in the Northeast and Midwest are not included in this database.[33] Furthermore, the SEER database provides only 5 specific metastatic sites but information about other extrahepatic metastatic sites is unclear. In addition, there is also a lack of details concerning systemic therapies such as chemotherapy, which might influence the survival analysis. Secondly, there may be a patient selection bias, since patients who were offered surgical or ablative treatments are more likely to have better general health and smaller comorbidity. Therefore, prospective randomized trials are needed to provide more trustworthy evidence.
In conclusion, the current study reveals that different metastatic sites have distinct impact on the survival outcomes of patients with advanced ICC. Patients with isolated regional lymphatic metastasis enjoy the best survival outcomes and may benefit from local treatment to the primary tumor. Certain high selected subset of ICC patients with isolated liver metastases might also benefit from liver-directed therapies, but methods for the differentiation of them are needed.
Acknowledgments
We would like to thank Dr Bang-Wei Zeng, MD, from the Department of Hospital Infection-Control, Fujian Medical University Union Hospital, for his help with statistical analysis.
Author contributions
Conceptualization: Yanling Chen.
Data curation: Jingmin Ye.
Formal analysis: Bi Wang.
Project administration: Rui Cheng, Qiang Du.
Software: Jingmin Ye.
Visualization: Bi Wang.
Writing – original draft: Rui Cheng, Qiang Du.
Writing – review & editing: Yanling Chen.
Footnotes
Abbreviations: AJCC = American Joint Committee on Cancer, CI = confidence interval, CSS = cancer-specific survival, HR = hazard ratio, ICC = intrahepatic cholangiocarcinoma, NCCN = National Comprehensive Cancer Network, OS = overall survival, SEER = Surveillance, Epidemiology, and End Results.
How to cite this article: Cheng R, Du Q, Ye J, Wang B, Chen Y. Prognostic value of site-specific metastases for patients with advanced intrahepatic cholangiocarcinoma: A SEER database analysis. Medicine. 2019;98:49(e18191).
RC and QD contributed equally.
This study was supported by Joint Funds for Innovative Science and Technology, Fujian Province [Grant number: 2017Y9022], and Startup Fund for Scientific Research, Fujian Medical University [Grant number: 2017XQ1046], and Middle-aged and Young Teachers’ Education Scientific Research Projects in Fujian Province, China [Grant number: JAT170221].
The authors have no conflicts of interest to disclose.
References
- [1].Torre LA, Bray F, Siegel RL, et al. Global cancer statistics, 2012. CA: Cancer J Clin 2015;65:87–108. [DOI] [PubMed] [Google Scholar]
- [2].Kamarajah SK. Evaluation of the AJCC 8th Edition staging system for pathologically versus clinically staged intrahepatic cholangiocarcinoma (iCCA): a time to revisit a dogma? A Surveillance, Epidemiology, and End Results (SEER) analysis. J Gastrointest Cancer 2019;50:392–9. [DOI] [PubMed] [Google Scholar]
- [3].Meng ZW, Pan W, Hong H J, et al. Modified staging classification for intrahepatic cholangiocarcinoma based on the sixth and seventh editions of the AJCC/UICC TNM staging systems. Medicine 2017;96:e7891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].West J, Wood H, Logan RF, et al. Trends in the incidence of primary liver and biliary tract cancers in England and Wales 1971–2001. Br J Cancer 2006;94:1751–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Razumilava N, Gores GJ. Cholangiocarcinoma. Lancet 2014;383:2168–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Endo I, Gonen M, Yopp AC, et al. Intrahepatic cholangiocarcinoma: rising frequency, improved survival, and determinants of outcome after resection. Ann Surg 2008;248:84–96. [DOI] [PubMed] [Google Scholar]
- [7].Bridgewater J, Galle PR, Khan SA, et al. Guidelines for the diagnosis and management of intrahepatic cholangiocarcinoma. J Hepatol 2014;60:1268–89. [DOI] [PubMed] [Google Scholar]
- [8].Macias RIR, Banales JM, Sangro B, et al. The search for novel diagnostic and prognostic biomarkers in cholangiocarcinoma. Biochim Biophys Acta 2017. [DOI] [PubMed] [Google Scholar]
- [9].Dong F, Shen Y, Gao F, et al. Prognostic value of site-specific metastases and therapeutic roles of surgery for patients with metastatic bladder cancer: a population-based study. Cancer Manag Res 2017;9:611–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Yoh T, Hatano E, Yamanaka K, et al. Is surgical resection justified for advanced intrahepatic cholangiocarcinoma? Liver Cancer 2016;5:280–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Kim JH, Won HJ, Shin YM, et al. Radiofrequency ablation for the treatment of primary intrahepatic cholangiocarcinoma. Am J Roentgenol 2011;196:W205–9. [DOI] [PubMed] [Google Scholar]
- [12].Wang Y, Ma S. Racial differences in mantle cell lymphoma in the United States. BMC Cancer 2014;14:764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Patel T. Cholangiocarcinoma—controversies and challenges: nature reviews. Gastroenterol Hepatol 2011;8:189–200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Lendoire JC, Gil L, Imventarza O. Intrahepatic cholangiocarcinoma surgery: the impact of lymphadenectomy. Chin Clin Oncol 2018;7:53. [DOI] [PubMed] [Google Scholar]
- [15].Wu W, He X, Andayani D, et al. Pattern of distant extrahepatic metastases in primary liver cancer: a SEER based study. J Cancer 2017;8:2312–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Bektas H, Yeyrek C, Kleine M, et al. Surgical treatment for intrahepatic cholangiocarcinoma in Europe: a single center experience. J Hepatobiliary Pancreat Sci 2015;22:131–7. [DOI] [PubMed] [Google Scholar]
- [17].Ryerson AB, Eheman CR, Altekruse SF, et al. Annual Report to the Nation on the Status of Cancer, 1975–2012, featuring the increasing incidence of liver cancer. Cancer 2016;122:1312–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Chen C, Hu L, Chen Y, et al. The prognostic value of histological subtype in patients with metastatic bladder cancer. Oncotarget 2017;8:28408–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Akay CL, Ueno NT, Chisholm GB, et al. Primary tumor resection as a component of multimodality treatment may improve local control and survival in patients with stage IV inflammatory breast cancer. Cancer 2014;120:1319–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Heng DY, Wells JC, Rini BI, et al. Cytoreductive nephrectomy in patients with synchronous metastases from renal cell carcinoma: results from the International Metastatic Renal Cell Carcinoma Database Consortium. Eur Urol 2014;66:704–10. [DOI] [PubMed] [Google Scholar]
- [21].Danna EA, Sinha P, Gilbert M, et al. Surgical removal of primary tumor reverses tumor-induced immunosuppression despite the presence of metastatic disease. Cancer Res 2004;64:2205–11. [DOI] [PubMed] [Google Scholar]
- [22].Amini N, Ejaz A, Spolverato G, et al. Temporal trends in liver-directed therapy of patients with intrahepatic cholangiocarcinoma in the United States: a population-based analysis. J Surg Oncol 2014;110:163–70. [DOI] [PubMed] [Google Scholar]
- [23].Guro H, Kim JW, Choi Y, et al. Multidisciplinary management of intrahepatic cholangiocarcinoma: current approaches. Surg Oncol 2017;26:146–52. [DOI] [PubMed] [Google Scholar]
- [24].Dodson RM, Weiss MJ, Cosgrove D, et al. Intrahepatic cholangiocarcinoma: management options and emerging therapies. J Am Coll Surg 2013;217:736–50. e734. [DOI] [PubMed] [Google Scholar]
- [25].Jutric Z, Johnston WC, Hoen HM, et al. Impact of lymph node status in patients with intrahepatic cholangiocarcinoma treated by major hepatectomy: a review of the National Cancer Database. HPB 2016;18:79–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Bagante F, Spolverato G, Weiss M, et al. Assessment of the lymph node status in patients undergoing liver resection for intrahepatic cholangiocarcinoma: the New Eighth Edition AJCC Staging System. J Gastrointest Surg 2018;22:52–9. [DOI] [PubMed] [Google Scholar]
- [27].Inoue K, Makuuchi M, Takayama T, et al. Long-term survival and prognostic factors in the surgical treatment of mass-forming type cholangiocarcinoma. Surgery 2000;127:498–505. [DOI] [PubMed] [Google Scholar]
- [28].Isa T, Kusano T, Shimoji H, et al. Predictive factors for long-term survival in patients with intrahepatic cholangiocarcinoma. Am J Surg 2001;181:507–11. [DOI] [PubMed] [Google Scholar]
- [29].Kizy S, Altman AM, Marmor S, et al. Surgical resection of lymph node positive intrahepatic cholangiocarcinoma may not improve survival. HPB 2019;21:235–41. [DOI] [PubMed] [Google Scholar]
- [30].Suzuki S, Sakaguchi T, Yokoi Y, et al. Clinicopathological prognostic factors and impact of surgical treatment of mass-forming intrahepatic cholangiocarcinoma. World J Surg 2002;26:687–93. [DOI] [PubMed] [Google Scholar]
- [31].Nakagawa T, Kamiyama T, Kurauchi N, et al. Number of lymph node metastases is a significant prognostic factor in intrahepatic cholangiocarcinoma. World J Surg 2005;29:728–33. [DOI] [PubMed] [Google Scholar]
- [32].Tran Cao HS, Zhang Q, Sada YH, et al. The role of surgery and adjuvant therapy in lymph node-positive cancers of the gallbladder and intrahepatic bile ducts. Cancer 2018;124:74–83. [DOI] [PubMed] [Google Scholar]
- [33].Newsome JR, Venkatramani R, Heczey A, et al. Cholangiocarcinoma among children and adolescents: a review of the literature and surveillance, epidemiology, and end results program database analysis. J Pediatr Gastroenterol Nutr 2018;66:e12–8. [DOI] [PubMed] [Google Scholar]


