Abstract
Background:
MPT64 and PstS1 are the earliest known immune-dominant antigens of Mycobacterium tuberculosis and have been commonly used as candidates in the diagnosis of tuberculosis.
Methods:
We constructed recombinant plasmids pET-32a-Rv0934 and pET-32a-Rv1980c to express both wild and mutant forms of MPT64 and PstS1 and purified them. From November 9 to December 9, 2016, and November 9 to December 10, 2017, 96 patients with tuberculosis, 53 patients without tuberculosis, and 96 healthy volunteers were enrolled in this study. We used the purified proteins as antigens to perform T-spot and enzyme-linked immunosorbent assay (ELISA) for samples obtained from healthy volunteers and tuberculosis patients.
Results:
Regarding T-spot, the area under the curve (AUC) values for MPT64-wild protein (MPT64-H37Rv) and MPT64-mutant protein (MPT64-FJ05395) were 0.723 and 0.750, respectively. The AUC values for PstS1-H37Rv, PstS1-FJ05132, and PstS1-JL06035 were 0.817, 0.796, and 0.745, respectively. With regard to ELISA, the AUC values for MPT64-H37Rv and MPT64-FJ05395 were 0.525 and 0.528, respectively, while those for PstS1-H37Rv, PstS1-FJ05132, PstS1-JL06035 were 0.588, 0.509, and 0.560, respectively. There was no difference between wild and mutant proteins when we used them as antigens to perform T-spot and ELISA assays.
Conclusion:
MPT64 and PstS1 are likely candidate diagnostic antigens for M tuberculosis T-spot test, at least in combination with other proteins. Polymorphisms of MPT64 and PstS1 had little effect on cell-mediated and humoral immunity in the host.
Keywords: cell-mediated immunity, humoral immunity, MPT64, Mycobacterium tuberculosis, polymorphism, PstS1
1. Introduction
In 2017, the World Health Organization (WHO) reported about 1.7 billion people worldwide have been infected with Mycobacterium tuberculosis (M tuberculosis), and the number of new cases is close to 10 million. Rapid diagnosis of tuberculosis is an important way to control and prevention of the disease. MPT64 and PstS1 are 2 important proteins in M tuberculosis and are commonly used as candidates for diagnosis and vaccines. MPT64 (Rv1980c), a 24-kDa protein of M tuberculosis, is an important secreted protein of the pathogen.[1,2] It is hypothesized actively secreted proteins in M tuberculosis are the first to interact with the host immune system, and hence such proteins are important for activating the immune response in individuals infected with M tuberculosis. The mycobacterial PstS1 antigen, that is, Rv0934, belongs to the gene family of ABC transporters and is the phosphate-binding subunit of the inorganic phosphate uptake system from M tuberculosis.[3] It is one of highly immunogenic and immunostimulatory components of the mycobacterial cell membrane.[4] PstS1 is also a glycosylated lipoprotein, which can be found intracellularly and secreted into the extracellular culture supernatant.[5] Additionally, PstS1 has been regarded as an immunodominant antigen, and antibodies against it can distinguish inactive TB from active TB.[6,7]
In our previous study, we found that there was polymorphism existed in MPT64 and PstS1, and the polymorphism may reflect ongoing immune evasion.[8,9] Among 180 clinical isolates of M tuberculosis complex, some M tuberculosis strains harbor a 63 bp deletion in sequence of MPT64 gene, which may cause changes of related functions and allowing immune evasion.[10] Meanwhile, we found that some of the mutations, especially 2 frameshift mutations, occurred in the PstS1 antigen, which may have resulted in the protein function alteration and ongoing immune evasion. There was a base insertion in the FJ05132 and JL06035 strains that resulted in a frameshift mutation and led to an early stop in PstS1.
There are some reports about enzyme-linked immunosorbent assay (ELISA) test containing MPT64 and PstS1,[11–15] while T-spot test containing these 2 proteins is rare. The purpose of this study is to evaluate the diagnostic efficacy of T-spot and ELISA tests using MPT64 and PstS1 in wild and mutant forms and find whether the polymorphism of these 2 proteins affected relative cell-mediated immunity and humoral immunity in host.
2. Materials and methods
2.1. Construction of the recombinant plasmid pET-32a-Rv0934 and pET-32a-Rv1980c
In this study, we chose H37Rv as the wild strain, JL06035 and FJ05132 as 2 PstS1 mutant strains and FJ05395 as MPT64 mutant strain. Fragments of Rv1980c and Rv0934 were amplified from MTB H37Rv DNA, and the primers (from the 5′ to 3′ end) used in polymerase chain reaction (PCR) were described in Table 1.
Table 1.
The primers used in this study for PCR amplification.

The PCR was carried out in a total volume of 25 μL. The PCR mix contained 1 μL DNA, 1 U Ex Taq HS (Takara Bio, Inc., Otsu, Japan), 1 μL forward primer, 1 μL reverse primer, 2.5 μL 10× Ex Taq buffer and 8.5 μL ddH2O. An initial denaturation of 5 minutes at 94°C was followed by 30 cycles of denaturation at 94°C for 30 seconds, annealing at 60°C for 45 seconds and extension at 72°C for 1 minute, and a final extension at 72°C for 5 minutes. Negative controls (no DNA, reagents only) were included each time when the PCR was performed. A DNA purification kit (Tiangen Biotech, Beijing, China) was used to purify the PCR products. After digestion with EcoRI and HindIII, the fragments were cloned into a pET-32a vector and the recombinant plasmids were transformed into Escherichia coli DH5α cells. The recombinant plasmids pET-32a-Rv1980c and pET-32a-Rv0934 (wild and mutant forms) were isolated from the E coli DH5α cells and chemically transformed into E coli BL21 (DE3) cells after the identity of fragments were confirmed by endonuclease restriction digestion and DNA sequencing.
2.2. Expression and purification of MPT64 and PstS1 proteins in wild and mutant forms
The DE3 cells with the recombinant plasmid were cultured in Luria–Bertani medium overnight at 37°C. When OD600 value was in the range from 0.6 to 0.8, isopropyl β-D-1-thiogalactopyranoside was added to the medium to a concentration of 1.0 mmol/L. Then the culture was incubated at 37°C for 3 hours. The cells were collected by centrifugation at 12000 g for 3 minutes. The supernatant and cell pellet were analyzed by using 12% sodium dodecyl sulfate-polyacrylamide gels after the cells were processed by ultrasonication. The sodium dodecyl sulfate-polyacrylamide gelelectrophoresis (SDS-PAGE) was performed by using 1.5-mm thick 10.1 cm × 7.3 cm glass plates. The electrophoresis was performed at 80 V for 30 minutes, and the gels were stained by Coomassie blue. SDS-PAGE indicated that the MPT64 and PstS1 proteins (in wild and mutant forms) were expressed in the form of inclusion bodies. The protein inclusion bodies were washed twice with Tris-HCl buffer containing 1 M NaCl, 2 M urea, and 0.5% Triton X-100 and then dissolved in binding buffer (8 M urea, 0.5 M NaCl, 20 mM Tris-HCl, and 5 mM imidazole). The recombinant Rv1980c or Rv0934 proteins were purified by using nickel column chromatography, and the purified lysate was then loaded onto a 5-mL Ni-NTA column (His Trap HP, GE Life Sciences). The column was washed with wash buffer containing 8 M urea, 0.5 M NaCl, 20 mM Tris-HCl, and 60 mM imidazole, the proteins were eluted with elution buffer (8 M urea, 0.5 mM NaCl, 20 mM Tris-HCl, and 1 M imidazole) and the column was then stripped with stripping buffer containing 8 M urea, 0.5 M NaCl, 20 mM Tris-HCl, and 10 mM Ethylene Diamine Tetraacetic Acid (EDTA). The fractions that contained the Rv0934 or Rv1980c proteins were pooled and dialyzed in phosphate buffer (0.2 mM EDTA, 0.9 mM L-Glutathione, 0.18 mM L-Glutathione (Oxidized)) with different concentrations of urea (6, 4, 2, 1, and 0.5 M, and no urea). The refolded proteins were concentrated to 1 mg/mL after analyzed by a bicinchoninic acid protein assay kit (Thermo). The purified PstS1 or MPT64 proteins (in wild and mutant forms) were analyzed by SDS-PAGE.
2.3. Study subjects
Two groups of people participated in the study. First group included 42 patients with TB from Fujian and 42 healthy donors from Beijing, which were enrolled and subjected to analysis with the ELISA assay using the wild and mutant MPT64 or PstS1 protein. Second group included 54 patients with TB, 53 patients with no TB from Fujian and 54 healthy donors from Beijing, which were enrolled and subjected to analysis with the T-spot assay using the wild and mutant MPT64 or PstS1 proteins. The inclusion criteria for the subjects are as follows:
-
(1)
Active TB patients were those with clinical and radiographical features of TB confirmed by sputum smear and sputum culture.
-
(2)
The healthy donors included those with no clinical tuberculosis symptoms, no history of tuberculosis exposure, and normal X-rays.
-
(3)
Non-TB patients were those with other pulmonary diseases than TB.
The sputum samples of Non-TB patients were collected, smeared, subjected to acid-fast staining and cultured on Löwenstein–Jensen medium. The samples were determined as bacteriologically positive when the sputum smear and/or the bacterial culture was positive, and they were categorized as bacteriologically negative when the result was negative. 5 to 10 mL subcutaneous venous blood of each subject was collected.
This research was approved by the Ethics Committee of the National Institute for Communicable Disease Control and Prevention at the Chinese Center for Disease Control and Prevention. Each subject participated in this study provided written informed consent.
2.4. T-spot test
To evaluate the magnitude of the response in stimulation by Rv1980c or Rv0934 proteins (in wild and mutant forms), a diagnostic kit for MTB-specific T cells (ELISpot) (QuanBio, China) was used. The procedure was performed as previously described.[16]
2.5. ELISA test
Indirect ELISA technique was used in our study. Dilute the MPT64 or PstS1 proteins (in wild and mutant forms) with the coating buffer and the final concentration of the proteins are 20 μg/mL. ELISA plates were coated with the proteins (20 μg/mL) overnight at 4°C. In the next morning, plates were washed 3 times with phosphate-buffered saline tween-20 (PBST), dried and blocked with PBS containing 3% BSA for 2 hours at 37°C. Following the blocking step, the plates were washed 3 times with PBST and then dried. The sera samples were diluted 1:100 with PBS and added to each well (100 μL/well), and horseradish peroxidase-labeled goat anti-human Immunoglobulin G antibody were diluted (1:1000) and added to each well (100 μL/well). The plates were incubated at 37°C for 50 minutes and washed 3 times with PBST. Finally, the Tetramethylbenzidine substrate was then added to the plates and incubated at 37°C for 50 minutes. Optical delnsity values were calculated at 450 nm wavelength.
2.6. Statistical analysis
Medcalc software (version 9) was used to compare the receiver operating characteristic (ROC) curve of wild and mutant proteins. Z tests were used to compare the diagnose ability. P < 0.05 were considered significant between the experimental groups.
3. Results
3.1. Expression and purification of recombinant proteins
A 687-bp fragment (624 bp fragment in mutant protein) of Rv1980c and an 1125-bp fragment (1126 bp fragment in mutant proteins) of Rv0934 were successfully inserted into the pET32a vector (Solarbio, China) respectively and then confirmed by DNA sequencing. As shown in Figure 1, the result of the SDS-PAGE analysis indicated that the wild Rv1980c and Rv0934 were expressed in the form of inclusion bodies and were purified as an approximately 32 kD and 62 kD recombinant proteins respectively (Fig. 1, lane 5 and 2). The mutant proteins were also expressed in the form of inclusion bodies. The mutant MPT64 protein was purified as an approximately 30 kD recombinant proteins (Fig. 1, lane 6), while the mutant PstS1 proteins were purified as 29 kD and 32 kD recombinant proteins (Fig. 1, lane 3 and 4).
Figure 1.
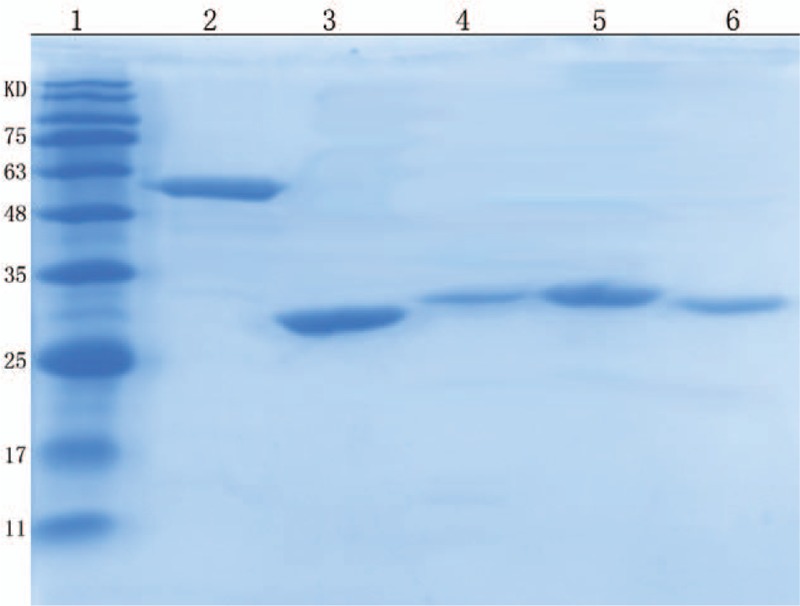
SDS-PAGE of purified recombinant wild and mutant proteins expression. Lanes: 1, Standard protein marker; 2, induced pET-32a-PstS1-H37Rv; 3, induced pET-32a-PstS1-FJ05132; 4, induced pET-32a-PstS1-JL06035; 5, induced pET-32a-MPT64-H37Rv; 6, induced pET-32a-MPT64-FJ05395. SDS-PAGE = sodium dodecyl sulfate-polyacrylamide gel electrophoresis.
3.2. Characteristics of the subjects
From November 9 to December 9, 2016, a total of 84 subjects including 42 patients with pulmonary TB were recruited from the Fuzhou Pulmonary Hospital, Fujian, and 42 healthy donors were recruited from the Chinese Center for Disease Control and Prevention, Beijing, China. From November 9 to December 10, 2017, a total of 161 subjects including 54 patients with pulmonary TB, 53 patients with no TB were recruited from the Fuzhou Pulmonary Hospital, Fujian, and 54 healthy donors were recruited from the Chinese Center for Disease Control and Prevention, Beijing, China. A total of 245 subjects with valid results and diagnostic information were enrolled for the statistical analyses. All the subjects were vaccinated with Bacillus Calmette-Guérin. The patients in the TB group included microbiologically positive subjects.
3.3. Diagnostic performance of the wild and mutant proteins in T-spot
When using MPT64-H37Rv as antigen to perform T-spot, the sensitivity is 66.67% and the specificity is 70%. The sensitivity of MPT64-FJ05395 is 76.19% while the specificity decreased to 66.67%. When using PstS1-H37Rv protein as antigen to perform T-spot, the sensitivity is 87.04% and the specificity is 62.26%. The sensitivity of PstS1-FJ05132 protein is 74.07% while the specificity increased to 75.47%. The sensitivity of PstS1-JL06035 protein declined to 59.26% while the specificity is 77.63%. There was no difference between wild and mutant proteins to perform T-spot assay to detect patients, which means the humoral immunity induced by the proteins (wild and mutant forms) is affected little by the polymorphism of the 2 proteins. (Table 2)
Table 2.
Comparison of wild and mutant MPT64 and PstS1 proteins for T-spot assay.
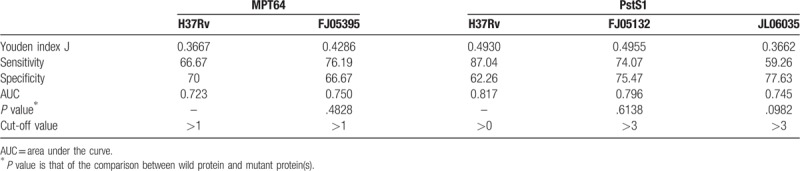
The data obtained from T-spot for each antigen was analyzed through ROC curve (Figs. 2 and 3). The area under curve (AUC) is an indication of the diagnostic sensitivity of the antigen variant. AUC was 0.723 for MPT64-H37Rv protein as compared to 0.750 in the case of MPT64-FJ05395 protein. That was 0.817 for PstS1-H37Rv as compared to 0.796 in the case of PstS1-FJ05132 and 0.745 in PstS1-JL06035.
Figure 2.
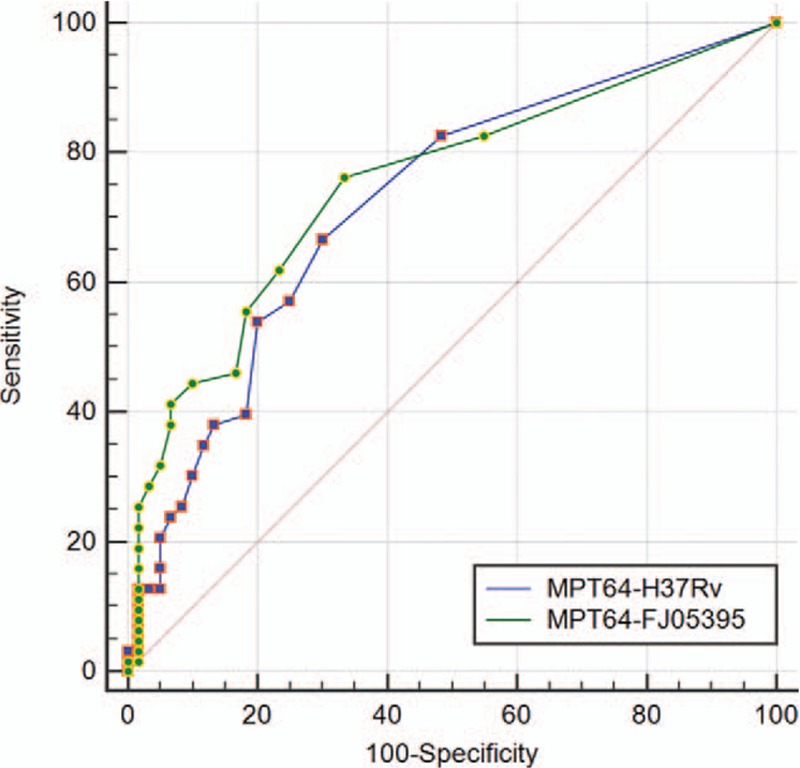
ROC curve comparison of wild and mutant MPT64 proteins for T-spot (wild form: PstS1-H37Rv; mutant form: PstS1-FJ05132 and PstS1-FJ06035). ROC = receiver operating characteristic.
Figure 3.
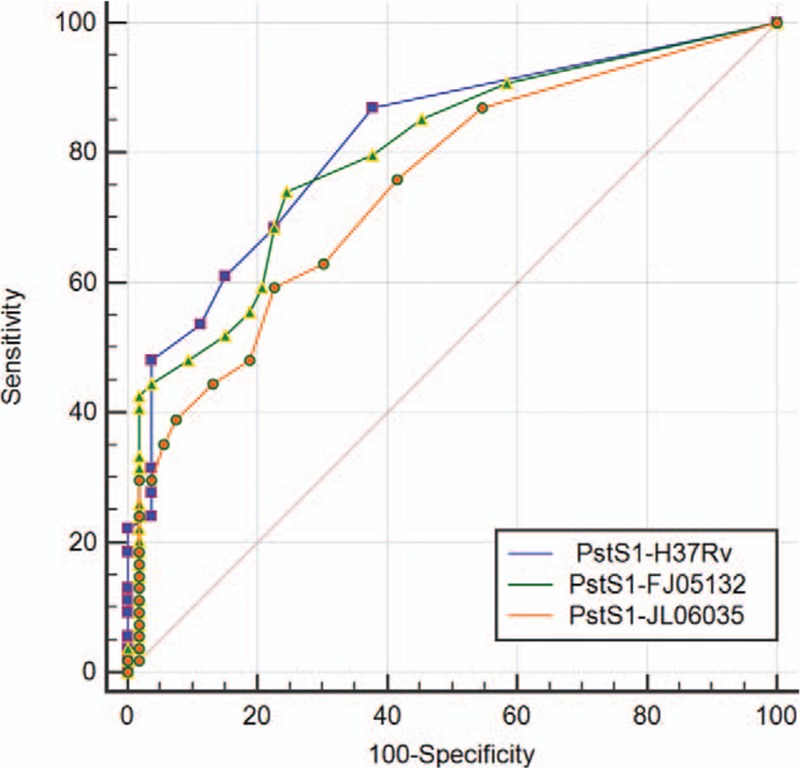
ROC curve comparison of wild and mutant PstS1 proteins for T-spot (wild form: PstS1-H37Rv; mutant form: PstS1-FJ05132 and PstS1-FJ06035). ROC = receiver operating characteristic.
3.4. Diagnostic performance of the wild and mutant proteins in ELISA
When using MPT64-wild protein (MPT64-H37Rv) as antigen to perform ELISA, the sensitivity and specificity were 73.81% and 2.83%, respectively. The sensitivity of mutant protein (MPT64-FJ05395) is 23.81%, and the specificity reached to 100%. The sensitivity is 42.86% and the specificity is 88.10% using PstS1-H37Rv protein as antigen to perform ELISA. The sensitivity of PstS1-FJ05132 protein is 19.05% while the specificity increased to 100%. The sensitivity of PstS1-JL06035 protein is 26.19% while the specificity increased to 95.24%. There was no difference between wild and mutant proteins (MPT64 or PstS1) to perform ELISA assay to detect patients, which means the humoral immunity induced by the proteins (wild and mutant forms) is affected little by the polymorphism of MPT64 and PstS1 proteins. (Table 3)
Table 3.
Comparison of wild and mutant MPT64 and PstS1 proteins for ELISA assay.
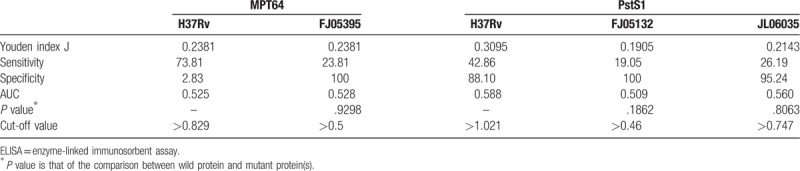
The data obtained from ELISA for each antigen was also analyzed through ROC curve (Figs. 4 and 5). AUC was 0.525 for MPT64-wild protein (MPT64-H37Rv) as compared to 0.528 in the case of MPT64-mutant protein (MPT64-FJ05395). That was 0.588 for PstS1-H37Rv as compared to 0.509 in the case of PstS1- FJ05132 and 0.560 in PstS1- JL06035.
Figure 4.
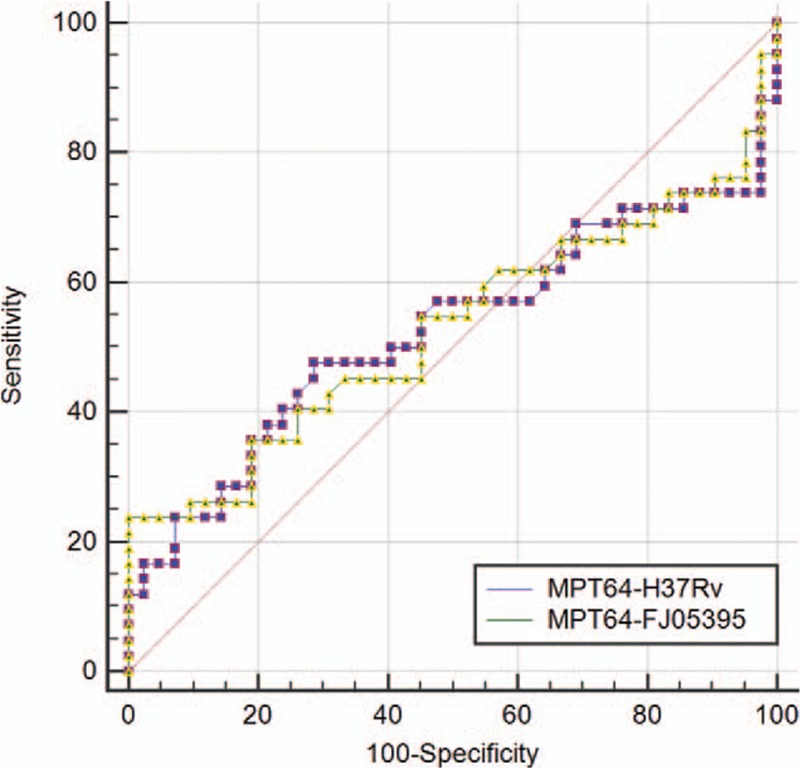
ROC curve comparison of wild and mutant MPT64 proteins for ELISA (wild form: MPT64-H37Rv; mutant form: MPT64-FJ05395). ELISA = enzyme-linked immunosorbent assay; ROC = receiver operating characteristic.
Figure 5.
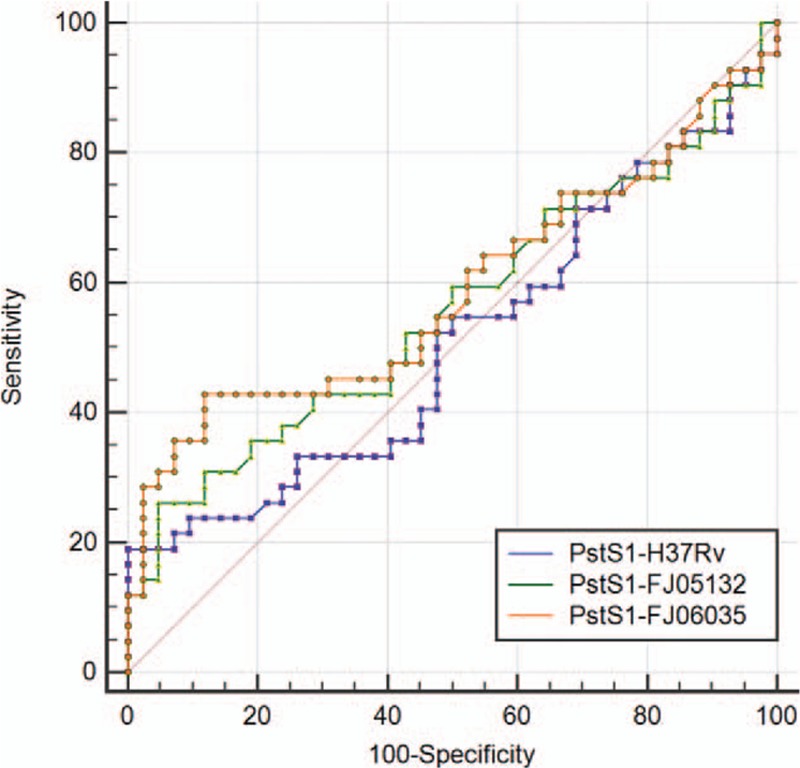
ROC curve comparison of wild and mutant PstS1 proteins for ELISA (wild form: MPT64-H37Rv; mutant form: MPT64-FJ05395). ELISA = enzyme-linked immunosorbent assay; ROC = receiver operating characteristic.
4. Discussion
Cellular immunity plays a leading role in tuberculosis immunity, and immunity to tuberculosis in humans mainly depends on T lymphocytes.[17,18] T-SPOT. TB tests are in vitro blood tests that measure the T-cell release of IFN-γ after stimulation with antigens unique to M tuberculosis.[19] Currently, the T-spots kits widely used in the market contain CFP10 and ESTA6. In a meta-analysis of 16 T-spot studies, the sensitivity and specificity were 84.0% and 65.8%, respectively.[20] In a recent study in China, the AUC value for T-spot containing CFP10 and ESTA6 was 0.906; the AUC value declined to 0.884 and 0.877 when a single protein was used. The sensitivity values for ESTA6, CFP10, and ESTA6-CFP10 were 81.3%, 65.9%, and 80.9% while the corresponding specificity values were 88.9%, 94.1%, and 91.3%.[21] For T-spot in our study, the AUC values for MPT64-H37Rv and MPT64-FJ05395 proteins were 0.723 and 0.750, respectively, while those for PstS1-H37Rv, PstS1-FJ05132, and PstS1-JL06035 were 0.817, 0.796, and 0.745, respectively. The sensitivity and specificity values were 59.26% to 87.04% and 62.26% to 77.63%, respectively. Although the diagnostic efficacy of MPT64 or PstS1 in T-spot in our study was relatively lower than that of the combination (CFP10 and ESTA6), it could be improved by combining 2 or more antigens. This suggests that MPT64 and PstS1 are likely candidate diagnostic antigens for the M tuberculosis T-spot test, at least in combination with other proteins. Further study is required to evaluate the diagnostic efficacy of the combination of these 2 proteins or their combination with other proteins.
In previous studies, the results of serological tests using a single antigen for M tuberculosis were always unsatisfactory. The WHO recommended that serological tests not be used for the diagnosis of M tuberculosis infection.[22] Commercial serological tests provide imprecise and inconsistent results with highly variable values for sensitivity and specificity, and high proportions of false-negative and false-positive results adversely affect patient safety.[13–15] In a recent study, the sensitivity values for the 2 antigens (MPT64 and PstS1) in 200 PTB patients and 152 healthy controls were 36.5% and 67.0%, respectively, while the corresponding specificity values were 86.8% and 74.3%.[23] In our study, The AUC values for wild and mutant MPT64 protein in ELISA were 0.525 and 0.528, respectively, indicating low specificity or low sensitivity. The sensitivity value for wild PstS1 in ELISA was 42.86%; the finding was is in agreement with those of previous studies.[7,11,12] However, the sensitivity values for mutant PstS1 were 19.05% and 26.19%. The specificity values for wild and mutant PstS1 in ELISA were 88.10%, 100%, and 95.24% respectively. Our results show the limitations of antibodies for the diagnosis of M tuberculosis infection.
There was no difference between wild and mutant proteins when we used them as antigens to perform the T-spot assay, which revealed that M tuberculosis is a relatively conservative strain and the polymorphism of some functional genes, such as MPT64 and PstS1, had little effect on cell-mediated immune reactions in humans. Despite the shortcomings in the diagnosis of serological antibodies, we still found that there was no difference between wild and mutant proteins (MPT64 or PstS1) in the ELISA assay. This also suggests that the humoral immunity induced by the proteins (wild and mutant forms) is affected little by polymorphisms of the MPT64 and PstS1 proteins.
The polymorphism of MPT64 had little effect on cell-mediated immunity and humoral immunity in the host, which suggested that strain diversity might not be considered during further development of new vaccines containing MPT64. It showed that the insertion of FJ05132 and JL06035 in the PstS1 protein had little effect on cell-mediated immunity in humans compared to that associated with the wild-type PstS1 protein. This may due to the fact that the insertions in FJ05132 and JL06035 located near the C-terminus of the PstS1 protein, which had little effect on the function of PstS1. It was deduced that the function of the PstS1 protein is determined more by the AA sequence of the N-terminus. It has been reported that PstS1 (285–374) showed higher immunoreactivity in latent tuberculosis infection (LTBI) than in active TB.[24] The base insertions in FJ05132 and JL06035 were at positions 135 and 208, which led to an early stop and sequence deletion of PstS1 (285–374). Further investigations are needed to determine whether these insertions affect the diagnostic ability of PstS1 for LTBI detection. Moreover, we should perform familiar study to compare other proteins than the 2 proteins which have polymorphisms in MTB.
There is a limitation of this study. We collected specimens for ELISA and T-spot tests from different subjects at different time periods. Therefore, the results of ELISA and the T-spot test cannot be compared.
Acknowledgments
The authors thank all participants and acknowledge the contribution of the staffs from the State Key Laboratory for Infectious Disease Prevention and Control, and the nurses, clinicians and laboratory technicians from Fujian provinces for their assistance in this study.
Author contributions
Data curation: Tongyang Xiao.
Funding acquisition: Yi Jiang, Kanglin Wan.
Investigation: Tongyang Xiao.
Project administration: Yi Jiang, Kanglin Wan.
Resources: Guilian Li.
Supervision: Xiuqin Zhao.
Validation: Hui Pang, Lili Zhao.
Writing – review and editing: Yi Jiang.
Footnotes
Abbreviations: AUC = area under curve, LTBI = latent tuberculosis infection, M tuberculosis = Mycobacterium tuberculosis, PBST = phosphate-buffered saline tween-20, ROC = receiver operating characteristic, SDS-PAGE = sodium dodecyl sulfate-polyacrylamide gelelectrophoresis.
How to cite this article: Xiao T, Jiang Y, Li G, Pang H, Zhao L, Zhao X, Wan K. Polymorphism of MPT64 and PstS1 in Mycobacterium tuberculosis is not likely to affect relative immune reaction in human. Medicine. 2019;98:49(e18073).
This work was supported by the Major Project of the Thirteenth Five Year Special for Infectious Diseases of China (2018ZX10101002), Major Project of the Thirteenth Five Year Special for Infectious Diseases of China (2018ZX10731301-002), and the project 81401647 of Natural Science Foundation of China. The funders had no role in the study design, data collection and analysis, manuscript preparation, or decision to publish.
The authors have no conflicts of interest to disclose.
References
- [1].Mustafa AS. HLA-promiscuous Th1-cell reactivity of MPT64 (Rv1980c), a major secreted antigen of Mycobacterium tuberculosis, in healthy subjects. Med Princ Pract 2009;18:385–92. [DOI] [PubMed] [Google Scholar]
- [2].Mustafa AS, Shaban F. Mapping of Th1-cell epitope regions of Mycobacterium tuberculosis protein MPT64 (Rv1980c) using synthetic peptides and T-cell lines from M. tuberculosis-infected healthy humans. Med Princ Pract 2010;19:122–8. [DOI] [PubMed] [Google Scholar]
- [3].Braibant M, Lefevre P, de Wit L, et al. A Mycobacterium tuberculosis gene cluster encoding proteins of a phosphate transporter homologous to the Escherichia coli Pst system. Gene 1996;176:171–6. [DOI] [PubMed] [Google Scholar]
- [4].Vordermeier HM, Coombes AG, Jenkins P, et al. Synthetic delivery system for tuberculosis vaccines: immunological evaluation of the M. tuberculosis 38 kDa protein entrapped in biodegradable PLG microparticles. Vaccine 1995;13:1576–82. [DOI] [PubMed] [Google Scholar]
- [5].Young DB, Garbe TR. Lipoprotein antigens of Mycobacterium tuberculosis. Res Microbiol 1991;142:55–65. [DOI] [PubMed] [Google Scholar]
- [6].Davidow A, Kanaujia GV, Shi L, et al. Antibody profiles characteristic of Mycobacterium tuberculosis infection state. Infect Immun 2005;73:6846–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Espitia C, Cervera I, Gonzalez R, et al. A 38-kD Mycobacterium tuberculosis antigen associated with infection. Its isolation and serologic evaluation. Clin Exp Immunol 1989;77:373–7. [PMC free article] [PubMed] [Google Scholar]
- [8].Jiang Y, Liu H, Wan K. MPT64 polymorphisms of Mycobacterium tuberculosis strains suggest ongoing immune evasion. Tuberculosis (Edinb) 2014;94:712–4. [DOI] [PubMed] [Google Scholar]
- [9].Liu H, Jiang Y, Dou X, et al. pstS1 polymorphisms of Mycobacterium tuberculosis strains may reflect ongoing immune evasion. Tuberculosis (Edinb) 2013;93:475–81. [DOI] [PubMed] [Google Scholar]
- [10].Jiang Y, Liu H, Wang H, et al. Polymorphism of antigen MPT64 in Mycobacterium tuberculosis strains. J Clin Microbiol 2013;51:1558–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Bothamley GH, Rudd R, Festenstein F, et al. Clinical value of the measurement of Mycobacterium tuberculosis specific antibody in pulmonary tuberculosis. Thorax 1992;47:270–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Daniel TM, Debanne SM. The serodiagnosis of tuberculosis and other mycobacterial diseases by enzyme-linked immunosorbent assay. Am Rev Respir Dis 1987;135:1137–51. [DOI] [PubMed] [Google Scholar]
- [13].Steingart KR, Dendukuri N, Henry M, et al. Performance of purified antigens for serodiagnosis of pulmonary tuberculosis: a meta-analysis. Clin Vaccine Immunol 2009;16:260–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Steingart KR, Flores LL, Dendukuri N, et al. Commercial serological tests for the diagnosis of active pulmonary and extrapulmonary tuberculosis: an updated systematic review and meta-analysis. PLoS Med 2011;8:e1001062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Dowdy DW, Steingart KR, Pai M. Serological testing versus other strategies for diagnosis of active tuberculosis in India: a cost-effectiveness analysis. PLoS Med 2011;8:e1001074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Wang X, Chen S, Xu Y, et al. Identification and evaluation of the novel immunodominant antigen Rv2351c from Mycobacterium tuberculosis. Emerg Microbes Infect 2017;6:e48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].North RJ, Jung YJ. Immunity to tuberculosis. Annu Rev Immunol 2004;22:599–623. [DOI] [PubMed] [Google Scholar]
- [18].Comas I, Chakravartti J, Small PM, et al. Human T cell epitopes of Mycobacterium tuberculosis are evolutionarily hyperconserved. Nat Genet 2010;42:498–503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Redelman-Sidi G, Sepkowitz KA. IFN-gamma release assays in the diagnosis of latent tuberculosis infection among immunocompromised adults. Am J Respir Crit Care Med 2013;188:422–31. [DOI] [PubMed] [Google Scholar]
- [20].Lu P, Chen X, Zhu LM, et al. Interferon-gamma release assays for the diagnosis of tuberculosis: a systematic review and meta-analysis. Lung 2016;194:447–58. [DOI] [PubMed] [Google Scholar]
- [21].Wang L, Yu Y, Chen W, et al. Evaluation of the characteristics of the enzyme-linked immunospot assay for diagnosis of active tuberculosis in China. Clin Vaccine Immunol 2015;22:510–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22]. World Health Organization Commercial Serodiagnostic Tests for Diagnosis of Tuberculosis: Policy Statement. Geneva 2011. [PubMed] [Google Scholar]
- [23].Yan ZH, Yi L, Wei PJ, et al. Evaluation of panels of Mycobacterium tuberculosis antigens for serodiagnosis of tuberculosis. Int J Tuberc Lung Dis 2018;22:959–65. [DOI] [PubMed] [Google Scholar]
- [24].de Araujo LS, de Barbara Moreira da Silva Lins N, Leung JA, et al. Close contact interferon-gamma response to the new PstS1 (285-374):CPF10: a preliminary 1-year follow-up study. BMC Res Notes 2017;10:59. [DOI] [PMC free article] [PubMed] [Google Scholar]


