Abstract
Background:
Pre-treatment sleep disturbance has been shown to predict antidepressant treatment outcomes. How changes in sleep disturbance during acute treatment affect longitudinal outcomes, or whether continuation-phase treatment further improves sleep disturbance, is unclear.
Methods:
We assessed sleep disturbance repeatedly in: a) 523 adults with recurrent MDD who consented to 12-14 weeks of acute-phase cognitive therapy (A-CT) and b) 241 A-CT responders at elevated risk for depression relapse/recurrence who were randomized to 8 months of continuation-phase treatment (C-CT vs. fluoxetine vs. matched pill placebo) and followed protocol-treatment-free for 24 months. Trajectories of change in sleep and depression during and after A-CT were evaluated with multilevel models; individual intercepts and slopes were retained and input into Cox regression models to predict remission, recovery, relapse, and recurrence of MDD.
Results:
Sleep disturbance improved over the course of A-CT, but most patients continued to report clinically significant sleep complaints. Response and remission were more likely in patients with less overall sleep disturbance and those with greater reduction in sleep disturbance during A-CT; these patients also achieved post-A-CT remission and recovery sooner. Sleep improvements endured throughout follow-up but were not enhanced by continuation-phase treatment. Sleep disturbance did not predict relapse or recurrence consistently.
Limitations:
Objective sleep disturbance was not assessed. Analyses were not specifically powered to use sleep changes to predict outcomes.
Conclusions:
Improvements in sleep disturbance during A-CT are linked to shorter times to remission and recovery, supporting consideration of monitoring and targeting sleep disturbance in adults with depression.
Keywords: depression, sleep, relapse, recurrence, randomized clinical trial
Introduction
Major depressive disorder (MDD) is a debilitating disorder prevalent in approximately 4.4% of the population worldwide (Baxter et al., 2014). Because depression frequently recurs following acute-phase treatment, research has aimed to identify risk factors for depression relapse and recurrence, most notably incomplete remission. Sleep disturbance is known to precede incident depression (Fernandez-Mendoza et al., 2015), and frequently remains following treatment (Carney et al., 2007; Nierenberg et al., 2010; Taylor et al., 2010).
Objective and subjective measures of sleep disturbance are associated with poor depression-treatment response. Decreased REM latency has been associated with slower, more variable response to depression treatment (Dew et al., 1997), and abnormal electroencephalographic (EEG) profiles have been associated with lower recovery rates following Cognitive Behavioral Therapy (CBT; Thase et al., 1996). Greater objective sleep-onset latency combined with subjectively reported insomnia at baseline also has been associated with increased risk of non-remission (Troxel et al., 2012). Increased subjective sleep disturbance during pharmacotherapy treatment has been linked to a lower likelihood of remission (Jha et al., 2018); however, studies exploring how the rate of improvement of subjective sleep disturbance during treatment affects stability or speed of remission are needed. As individuals with recurrent MDD are already at higher risk of relapse relative to individuals experiencing a first episode (Keller et al., 1983), real-time monitoring of the change rate of modifiable risk factors may be key to optimal outcomes.
The literature is mixed regarding relations of sleep disturbance to risk for relapse and recurrence. Some studies document increased risk for relapse or recurrence associated with greater sleep disturbance (e.g., Cho et al., 2008; Combs et al., 2014; Dombrovski et al., 2007, 2008; Perlis et al., 1997; Thase et al., 1996), but others fail to show a relation between residual insomnia and depression relapse or recurrence (e.g., Iovieno et al., 2011; Nierenberg et al., 2010; Taylor et al., 2010; Yang et al., 2010). These conflicting reports may be due to methodological differences. There is broad heterogeneity in depression treatment types and assessment of sleep disturbance. Sleep disturbance items from the same depression rating scales used to assess outcome (e.g., the Hamilton Rating Scale for Depression; HRSD; Hamilton, 1960) are often used as predictors, which can influence results. Validated sleep measures such as the Pittsburgh Sleep Quality Index (PSQI; Buysse et al., 1989), the Insomnia Severity Index (ISI; Morin et al., 2001), or polysomnography (PSG) are used, albeit less frequently.
Discrepant findings may also be due to the fact that individuals with similar sleep disturbance severity at baseline may differ in their trajectories of sleep change during treatment. Overwhelmingly, studies use single baseline or post-acute-treatment assessments of sleep disturbance to predict response, relapse or recurrence after acute-phase treatment, but because rate of change of sleep disturbance can be actively monitored in treatment, its relation to depression outcome may be particularly useful in treatment planning. In the insomnia treatment literature, Manber et al. (2016) showed that early improvements in depression following treatment with Cognitive Behavioral Therapy for Insomnia (CBT-I) plus escitalopram predicted depression remission in adults with comorbid insomnia and MDD. Additionally, individuals in that cohort classified as “optimal responders” in terms of depression symptoms were found to have the most rapid early improvement in insomnia (Bei et al., 2018). To our knowledge, only a few studies focused solely on depression treatments have taken this approach. Gulec et al. (2011) and Perlis et al. (1997) reported significantly greater sleep disturbance in patients who relapsed/recurred following depression treatment relative to those who did not, with Perlis et al. also reporting steadily increasing sleep disturbance in the weeks leading up to recurrence. Gebara et al. (2018a) found that worse depression at baseline was associated with a poor sleep-improvement trajectory, and Hartwig et al. (2019) found that inpatients with comorbid MDD and GAD with a non-responsive sleep disturbance trajectory exhibited greater disability and lower levels of well-being at both discharge and 6-month follow-up. What remains to be tested is whether changes in sleep disturbance during acute depression treatment are associated with likelihood of depression treatment response or remission, a finding that could inform real-time treatment planning.
Whether continuation-phase treatment for depression further improves sleep is also unclear. Continuation-phase treatment effectively reduces the risk for relapse/recurrence (Frank et al., 1990; Jarrett et al., 2001, 2013; Kocsis et al., 1996; Kupfer et al., 1992; Rapaport et al.,2004). Indeed, the parent randomized controlled trial (RCT) of the present analysis demonstrated that both continuation-phase cognitive therapy (C-CT) and fluoxetine (FLX) were associated with reduced odds of relapse relative to matched pill placebo (PBO) (Jarrett et al., 2013). Whether sleep complaints continued to improve post-acute phase or were linked to longitudinal outcomes in that study was not tested. A handful of studies examined the effects of continuation treatment on sleep disturbance with positive effects; however, they were pharmacotherapy studies with limited generalizability to psychotherapy (Kupfer et al., 1994; Reynolds et al., 1991), or did not extend analyses past the first month of continuation treatment (Reynolds et al., 1997). A greater understanding of the longer-term effects of continuation treatment on sleep disturbance could inform optimization of long-term outcomes.
The current study examined the extent to which changes in sleep disturbance predicted response across 12-14 weeks of acute-phase cognitive therapy (A-CT) in adults with recurrent MDD. We hypothesized that a steeper reduction of subjective sleep disturbance during A-CT and a better sleep-improvement trajectory would be associated with increased likelihood of acute-phase response. Next, in A-CT responders, we examined whether these changes, or presence or absence of sleep disturbance after A-CT, predicted time to post-acute remission, recovery and relapse/recurrence over 32 months of follow-up, including 8 months of continuation-phase treatment with: C-CT, FLX plus clinical management, or PBO plus clinical management, followed by a 24-month protocol-treatment-free follow-up. We hypothesized that greater reductions in sleep disturbance over A-CT and absence of sleep disturbance would both predict shorter time to remission and recovery and longer time to relapse/recurrence. For comparability to previous reports that use mean values of sleep disturbance to predict outcome, we conducted parallel analyses using A-CT responders’ overall levels of sleep disturbance throughout A-CT as predictors. Finally, we examined the impact of continuation-phase treatment on sleep disturbance among higher risk A-CT responders. Prior research did not inform specific hypotheses as to the degree to which the continuation treatments, C-CT or FLX, would affect sleep complaints; thus, this analysis was exploratory.
Methods
Methods are detailed in previous reports (Jarrett et al., 2010, 2013); here we highlight methods relevant to current aims. Data collection took place at the University of Texas Southwestern Medical Center (UT Southwestern; Dallas) and the Western Psychiatric Institute and Clinic of the University of Pittsburgh Medical Center (UPMC). All study procedures were approved by the trial’s Data Safety Monitoring Board, and the UT Southwestern and UPMC institutional review boards. All patients provided verbal and written informed consent for participation.
Sample
Participants responded to community advertisements or were referred by providers between 2000 and 2008. Eligibility criteria included: 1) diagnosis of recurrent MDD via the Structured Clinical Interview for the DSM-IV (APA, 1994) and 2) a 14 or higher score on the 17-item version HRSD in both initial and second diagnostic interviews. Adults were excluded if they (1) experienced uncontrolled medical conditions that may be linked to depression or treated by medications with the capacity to alter mood; (2) were diagnosed with psychosis, bipolar disorder, active substance dependence, obsessive compulsive disorder (as the prominent presenting disorder), or any eating disorder; (3) were unable to complete written questionnaires in English; (4) were actively suicidal; (5) had previous history of non-response to CT or 40mg of FLX; (6) were pregnant or had plans to become pregnant during the first 11 months post baseline, or (7) did not provide informed consent. Of 1359 individuals screened, 523 began A-CT. Each participant who was taking antidepressant medications at evaluation began a medication-washout period supervised by a highly experienced psychopharmacologist. Taper time matched the medication being withdrawn and was conducted to minimize discomfort. Participants were free of a mood-altering medication prior to study entry.
Procedures
Acute phase cognitive therapy.
Participants received 12-14 weeks of A-CT from experienced psychotherapists, consisting of 16 to 20, 50-to-60-minute individual sessions. The A-CT was based on the Beck et al. (1979) manual, which includes cognitive-behavioral models and strategies. Therapists were experienced in using the HRSD-17 to guide selection of symptom targets during sessions, which could have included sleep when it was a predominant complaint. Therapist competence was monitored using the Cognitive Therapy Scale (CTS; Young & Beck, 1980) and weekly group supervision. Sessions occurred twice weekly for 4 weeks followed by weekly sessions for patients who achieved a 40% or greater reduction in HRSD-17 scores. Those who had yet to reach that threshold continued with twice weekly sessions for an additional 4 weeks before switching to weekly therapy.
After 12-14 weeks of A-CT, patients who achieved response (i.e., no DSM-IV MDD and an HRSD-17 score ≤ 12), were stratified into lower and higher risk groups based on their final seven HRSD-17 evaluations. Lower risk participants (n = 50) were those patients who had HRSD-17 scores of < 7 during all final seven assessments (i.e., achieved acute-phase remission). Higher risk patients (n = 241) were those who had at least one HRSD-17 score ≥ 7 during the final seven acute-phase assessments, reflecting unstable or partial remission and consequently a greater likelihood of relapse. As the goal of the parent trial was to test the relative efficacy of C-CT in preventing relapse/recurrence, only higher risk patients were randomized to the continuation phase of the RCT; lower risk patients participated in follow-up assessments.
8-month continuation-phase treatment.
Higher risk participants were randomly assigned to eight months of C-CT, FLX plus clinical management, or PBO plus clinical management. The C-CT consisted of 10 approximately 60-minute sessions every other week for the first 2 months, followed by six monthly sessions. The C-CT was tailored to the individual’s specific target problems, symptoms, and symptom severity. Clinical management plus FLX or PBO (Fawcett et al., 1987) was provided in sessions of equivalent length and frequency as C-CT and conducted by experienced psychiatrists blinded to randomization. Patients began with 10 mg of FLX or PBO pills that appeared identical. Dosages were titrated up to 40 mg as tolerated.
24-month protocol-treatment-free follow-up phase.
All consenting participants (both higher and lower risk) entered a 2-year longitudinal, protocol-treatment-free follow-up phase lasting 32 months post-A-CT. During this time, responders agreed not to take mood-altering medications. All patients were instructed to contact research staff if they experienced symptoms of relapse or recurrence, at which time a blinded evaluation occurred. If the participant met DSM-IV criteria for a major depressive episode, he or she was referred for non-protocol treatment. Participants continued in the longitudinal follow-up period regardless of their status for relapse/recurrence and/or non-protocol treatment.
Measures
Assessment of depression outcomes.
Analyses examined the following depression treatment outcomes, clinically based on consensus conceptual definitions from the MacArthur Foundation Research Network of the Psychobiology of Depression task force (Frank et al., 1991): (1) “response” marks the beginning of clinically significant improvement in depressive symptoms, defined as absence of DSM-IV MDD and an HRSD-17 score of ≤ 12; (2) acute-phase “stable remission,” is consistent evidence of asymptomatic status, defined as the last seven consecutive acute phase HRSD-17 scores < 7; (3) post-acute “remission” is defined as six Psychiatric Status Ratings (PSR) of two or less over the 6 weeks post randomization using the Longitudinal Interview Follow-up Evaluation (LIFE; Keller et al., 1987) a semi-structured interview used to assess psychiatric disorders in longitudinal studies; (4) “recovery” is sustained remission, defined as remission lasting ≥ 8 consecutive months; (5) “relapse” is defined as exacerbation of the presenting episode after a response but before recovery; and (6) “recurrence” is defined as meeting DSM-IV criteria for MDD after recovery. Depression outcomes are consistent with the parent RCT, which used a more liberal definition of response. This had the effect of increasing the rates of relapse and recurrence, examination of which was the goal of the parent RCT.
Assessment of depression symptoms.
Depression-symptom severity was measured by the following instruments administered on approximately the same schedule as sleep measures (i.e., pre-A-CT; A-CT weeks 1, 4, 8, 12; post-acute months 1, 4, 8, 12, 16, 18 20, 24, 28, 32):
Hamilton Rating Scale for Depression (Hamilton, 1960).
Considered the gold-standard clinician-rated scale of depression-symptom severity, the HRSD-17 was used to assess symptom severity and to help operationalize clinical outcomes of remission, recovery, relapse and recurrence.
Inventory of Depressive Symptomatology Self Report (IDS-SR; Rush et al., 2000).
The IDS-SR is a well-validated 30-item self-report measure of depressive symptoms that correlates highly with the HRSD-17 (Rush et al., 2000).
Beck Depression Inventory (Beck, Steer & Brown, 1996).
The BDI is a 21-item self-report instrument assessing severity of affective, cognitive, motivational, and somatic symptoms of depression. Internal consistency and retest reliability of this measure are well-established (Beck et al., 1988).
The HRSD-17, IDS-SR, and BDI scales were re-scored after removing items reflecting insomnia or hypersomnia, standardized (M = 50, SD =10) using their distributions at study intake, and averaged to form a depression severity composite (cf. Vittengl et al., 2013). Alpha internal consistency was .95.
Assessment of sleep disturbance.
We operationalized “subjective sleep disturbance” to include insomnia-like symptoms such as difficulty falling asleep, waking after sleep onset, waking too early, and/or sleeping too little. To capture this construct, we used the following measures:
Pittsburgh Sleep Quality Index Global Scale (PSQI; Buysse et al., 1989).
The PSQI is a widely used self-report measure of sleep disturbance, assessing 19 items related to sleep quality over the previous month. It has good sensitivity and specificity for identification of sleep disorders (Buysse et al., 1989). The global scale summed the seven component scores: sleep quality, latency, duration, efficiency, disturbance, medication use, and daytime sleepiness. Alpha internal consistency was .71.
Sleep-problems scale.
For consistency with the extant literature, we conducted parallel analyses using a scale composed of sleep items from two depression measures. Six items were extracted from the HRSD-17 (items 4-6) and IDS-SR-30 (items 1-3) to form a sleep-problems scale, with two items each for early, middle, and late insomnia. The IDS-SR item 4 (hypersomnia) was excluded because it correlated weakly with the insomnia items, and the BDI item 16 (late insomnia) was excluded to keep the sleep-problems scale content balanced. These six items were standardized as the percent of maximum score (HRSD-17 items were rated 0-2, whereas the IDS-SR items were rated 0-3) and averaged. Alpha internal consistency was .77. Higher scores on the sleep-problems scale indicate greater subjective sleep disturbance.
Sleep disturbance trajectories.
Using the cutoff of PSQI ≥ 5 to define clinically significant sleep disturbance, we formed 4 groups of patients using the pre-A-CT and A-CT week-12 assessments: Decreasing (pre PSQI ≥ 5 and week-12 PSQI < 5), persisting (both pre and week-12 PSQI ≥ 5), absent (both pre and week-12 PSQI < 5), and emerging (pre PSQI < 5 and week-12 PSQI ≥ 5) sleep disturbance. At randomization, we categorized patients in terms of presence or absence of sleep disturbance, again using a cutoff of PSQI > 5.
Statistical Analyses
Acute-phase (N = 523) and post-acute (N = 241) analyses were conducted on the intent-to-treat sample. To address missing data, we generated 10 complete data sets with missing values imputed via the Markov chain Monte Carlo method in PROC MI, computed standard analyses on each dataset, and pooled the results via PROC MIANALYZE in SAS software version 9.3 (SAS Institute, Inc., Cary, NC). All reported statistical tests and parameter estimates used multiply imputed data.
Changes in the PSQI, sleep-problems, and depression-severity scales during A-CT were first analyzed in a series of repeated-measures multilevel models that included the fixed effect of assessment as a discrete variable plus random intercepts and slopes for each patient. Main effects of site (Dallas/Pittsburgh) and site-by-time interactions on sleep-disturbance variables were non-significant (p > .05) and were excluded from final models.
These multilevel models captured the sample’s average level (fixed intercept) and change (fixed slope), as well as individual patients’ deviations from the average level (random intercept) and change (random slope), in repeated assessments of sleep during A-CT. Larger intercepts marked more sleep disturbance, and smaller (i.e., more strongly negative) slopes marked greater reductions in sleep disturbance during A-CT. Individual patients’ sleep-disturbance intercepts and slopes were tested as predictors of treatment outcomes. Models of dichotomous outcomes were logistic, whereas models of continuous depression severity were linear.
Among higher risk responders randomized to continuation treatment, we predicted time to relapse (over the 8-month continuation phase), relapse/recurrence (32 months), post-acute remission (32 months), and recovery (32 months) in a series of Cox regression analyses. Due to limited variability in sleep disturbance during the continuation phase, and to minimize regression to the mean, intercepts and slopes from acute-phase models were used to predict continuation-phase outcomes. Patients were censored at the earliest of: the end of the study, dropping out of the study, or relapse/recurrence. Time was measured in weeks with the LIFE retrospective interview. Post-acute-phase models controlled treatment arm. Non-significant interactions of acute-phase sleep-disturbance intercept and slope with treatment arm were removed from the final models.
Among higher risk responders to A-CT, we tested the main effects of time, continuation treatment (C-CT, FLX, or PBO), and their interactions on post-acute sleep disturbance. The repeated-measures multilevel models focused on months 0-8 after A-CT, during which continuation treatment was provided and sleep disturbance was measured approximately every 4 months. Time and treatment condition were modeled as discrete fixed effects, and the models included random intercepts and slopes.
For sleep-disturbance trajectories, logistic regression models were used to calculate the probability of A-CT response and remission. Time to relapse, recurrence, remission and recovery based on presence or absence of clinically significant sleep disturbance was assessed with Cox regression models.
Results
Sample Characteristics
Participants (N = 523) were predominantly female (67.5%) and white (80.9%) with a mean age of 42.4 (SD = 12.1). Based on HRSD scores at diagnostic evaluation, 38.4%, 58.5%, and 3.1% had mild, moderate, and severe depression, respectively. Demographic and clinical characteristics of the study sample are displayed in Table 1.
Table 1.
Demographic and clinical characteristics of sample at diagnostic evaluation
| Variable | M or % | SD |
|---|---|---|
| Patient Characteristics at Diagnostic Evaluation Prior to the Acute Phase (N = 523) | ||
| Age (years) | 42.37 | 12.11 |
| Gender | ||
| Female | 67.5% | |
| Male | 32.5% | |
| Race/ethnicity | ||
| Asian | 1.9% | |
| Black | 10.3% | |
| Hispanic | 5.2% | |
| Other | 1.7% | |
| White | 80.9% | |
| Education (years) | 15.06 | 2.93 |
| Hamilton Rating Scale for Depression | 21.22 | 4.19 |
| Mild (< 20) | 38.4% | |
| Moderate (20-29) | 58.5% | |
| Severe (≥ 30) | 3.1% | |
| Acute-phase Cognitive Therapy Outcomes (N = 523) | ||
| Non-response | 38.2% | |
| Response | 61.8% | |
| Stable response / remission | 9.4% | |
| Observed Outcomes among Randomized Higher-risk Responders after the Acute Phase (N = 241) | ||
| Remission | 69.7% | |
| Recovery | 46.9% | |
| Relapse / recurrence | 33.2% | |
Do Subjective Sleep Disturbance and Depression Improve After A-CT?
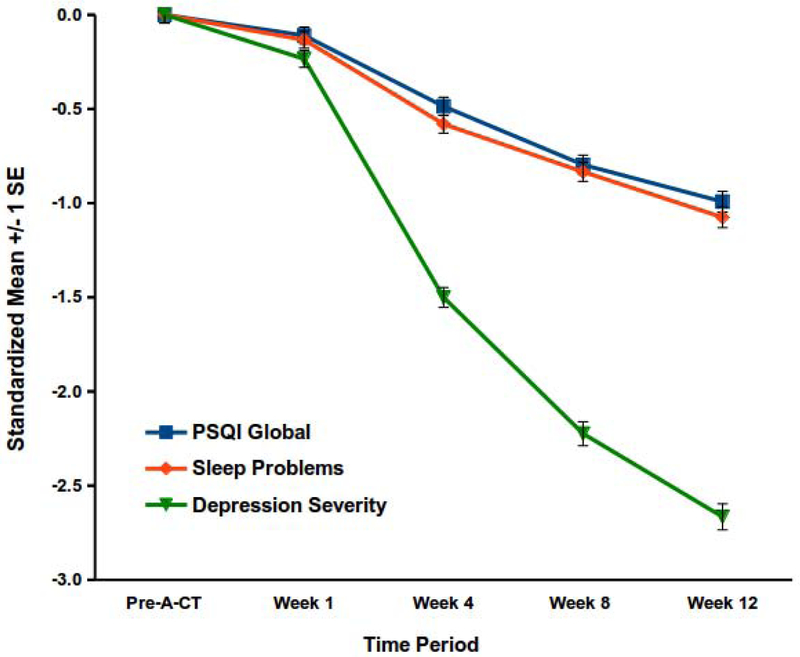
Yes, both depression and sleep-disturbance severity decreased during A-CT (see Figure 1). Mean PSQI global scores dropped from 9.98 to 6.10 during A-CT (F(4,1385) = 132.37, p < .001), although scores ≥ 5 indicate “poor sleep.” Using this threshold, 93.7% (SE = 1.1%) and 64.1% (SE = 2.1%) of patients reported “poor sleep” before and after A-CT, respectively. Table 2 shows percentages of individuals demonstrating poor sleep on the PSQI at each time point. Means on the sleep-problems scale (F(4,1340) = 156.70) and the depression-symptom scale (F(4,804) = 560.39) decreased substantively during A-CT, p < .001. Figure 1 depicts standardized mean changes in the PSQI, sleep-problems scale, and depression severity scores during A-CT estimated in the discrete-time models. Changes depicted in this figure can be interpreted in d effect-size units. Overall decreases in the PSQI, d = 0.99 (SE = 0.05), and sleep-problems, d = 1.08 (SE = 0.05), scales were large but not as large as depression-symptom change, d = 2.67 (SE = 0.07). Data regarding convergent and discriminant validity of the sleep and depression scales and descriptive statistics for these scales during the acute phase is available in supplemental material.
Figure 1.
Changes in sleep and depression during A-CT.
Decreases in the Pittsburgh Sleep Quality Index (PSQI) global score, sleep-problems scale, and the depression-symptom severity index (which excluded sleep items) were statistically significant, ps < .001, during acute-phase cognitive therapy for recurrent depression (A-CT).
Table 2.
Estimated proportions of patients with clinically significant sleep disturbance on the Pittsburgh Sleep Quality Index.
| Time Point | % |
|---|---|
| Acute-phase Cognitive Therapy (N = 523) | |
| Diagnostic Intake | 93.7% |
| Week 1 | 92.2% |
| Week 4 | 81.1% |
| Week 8 | 72.1% |
| Week 12 | 64.1% |
| Acute-phase Responders Randomized to Continuation-Phase Treatment (N = 241) | |
| Month 0 (randomization) | 58.1% |
| Month 4 | 56.5% |
| Month 8 | 51.5% |
| Month 12 | 47.9% |
| Month 16 | 52.8% |
| Month 20 | 51.2% |
| Month 24 | 51.7% |
| Month 28 | 53.4% |
| Month 32 | 52.9% |
Note. Scores ≥ 5 on the Pittsburgh Sleep Quality Index (PSQI) mark clinically significant sleep disturbance.
Does Subjective Sleep Disturbance Predict Response, Remission, and Depression-symptom severity During A-CT?
Yes, patients with less overall subjective sleep disturbance, and those with greater reduction in sleep disturbance, experienced larger decreases in depression severity and were more likely to achieve response and acute-phase remission. Table 3 shows prediction of response, acute-phase remission, and change in depression-symptom severity from sleep-disturbance intercepts and slopes. A one-SD decrease in the change of sleep disturbance (slope) over the course of A-CT was associated with 48% (SE = 7%) and 56% (SE = 7%) lower odds of response per the PSQI and the sleep-problems scales respectively. Similarly, each unit increase in overall levels of sleep disturbance (intercept) was associated with 47% (SE = 6%) and 54% (SE = 6%) lower odds of response per the PSQI and sleep-problems scales. Odds of remission were similar: a one-SD decrease in the change of sleep disturbance over the course of A-CT was associated with 27% (SE = 7%) and 32% (SE = 8%) lower odds of acute-phase remission, and each unit increase in overall levels of sleep disturbance was associated with 32% (SE = 9%) and 43% (SE = 11%) lower odds of response per the PSQI and sleep-problems scales, respectively.
Table 3.
Prediction of response, remission, and depression-symptom change in all patients during acute phase cognitive therapy (N=523).
| Model: Outcome | Predictor | Beta | SE | OR |
|---|---|---|---|---|
| 1: Response | PSQI Global: Acute-phase intercept | −0.760*** | 0.124 | 0.47 |
| PSQI Global: Acute-phase slope | −0.740*** | 0.138 | 0.48 | |
| 2: Response | Sleep problems: Acute-phase intercept | −0.612*** | 0.112 | 0.54 |
| Sleep problems: Acute-phase slope | −0.572*** | 0.130 | 0.56 | |
| 3. Acute-phase remission | PSQI Global: Acute-phase intercept | −1.127*** | 0.254 | 0.32 |
| PSQI Global: Acute-phase slope | −1.307*** | 0.259 | 0.27 | |
| 4. Acute-phase remission | Sleep problems: Acute-phase intercept | −0.841*** | 0.243 | 0.43 |
| Sleep problems: Acute-phase slope | −1.142*** | 0.254 | 0.32 | |
| 5. Depression-symptoms at week 12 | PSQI Global: Acute-phase intercept | 0.305*** | 0.044 | |
| PSQI Global: Acute-phase slope | 0.455*** | 0.042 | ||
| 6. Depression-symptoms at week 12 | Sleep problems: Acute-phase intercept | 0.239*** | 0.044 | |
| Sleep problems: Acute-phase slope | 0.402*** | 0.046 |
Note. N = 523. PSQI = Pittsburgh Sleep Quality Index. Sleep-problems scale derived from depression inventories. Depression severity scale does not include sleep items. Models 1-4 are logistic, and models 5-6 are linear, regression. All predictors and the symptoms outcome are standardized (M = 0, SD = 1). All models control depression severity at intake to the acute phase. OR = odds ratio.
p < .001, two-tailed. Note: “Response” marks the beginning of clinically significant improvement in depression symptoms, defined as absence of DSM-IV MDD and an HRSD-17 score of ≤ 12. “Remission” is consistent evidence of asymptomatic status, defined as the last seven consecutive acute-phase HRSD scores <7.
A similar pattern of results emerged from analyses of sleep-disturbance improvement trajectories. Patients with decreasing versus persisting sleep disturbance had significantly higher probabilities of A-CT response (79.6 vs. 52.1%) and acute-phase remission (18.4 vs. 2.6%), ps < .001 (see Table 4).
Table 4.
Patterns of clinically significant sleep problems during acute-phase cognitive therapy.
| Clinically significant sleep disturbance |
Proportion of A-CT sample |
Probability of A-CT response |
Probability of A-CT remission |
|---|---|---|---|
| Decreasing | 31.4% | 79.6% | 18.4% |
| Persisting | 62.3% | 52.1% | 2.6% |
| Absent | 4.5% | --- | --- |
| Emerging | 1.8% | --- | --- |
Note. N = 523. A-CT = Acute-phase Cognitive Therapy. Probabilities of response and remission were not estimated for absent or emerging sleep disturbance groups due to small sample sizes.
Does Subjective Sleep Disturbance Predict Post-A-CT Relapse at 8 months?
Findings were mixed. Table 5 shows prediction of relapse, relapse/recurrence, post-acute remission, and recovery from sleep-disturbance intercepts (levels during A-CT) and slopes (reductions during A-CT), among the 241 higher risk A-CT responders. Overall levels of sleep disturbance on the PSQI and sleep-problems measure did not predict relapse at 8 months. Changes in subjective sleep disturbance measured with the PSQI, or presence versus absence of sleep disturbance at randomization likewise did not predict relapse (see Table 6). When measured with the sleep-problems scale, however, less improvement in sleep disturbance predicted longer time to relapse (HR = 0.70), an unexpected, counter-intuitive finding. We explored whether floor effects or incomplete control of pre-A-CT sleep problems may have contributed to this non-hypothesized result. Consistent with this possibility, prediction of relapse from sleep-problems slopes became non-significant, p = .36, when baseline sleep problems score was added to the model as a covariate.
Table 5.
Prediction of time to relapse, recurrence, remission, and recovery among A-CT responders across 8 and 32 months (N=241).
| Model: Outcome | Predictor | Beta | SE | HR |
|---|---|---|---|---|
| 1: Relapse (8 months) | PSQI Global: Acute-phase intercept | 0.078 | 0.168 | 1.08 |
| PSQI Global: Acute-phase slope | 0.035 | 0.176 | 1.04 | |
| 2: Relapse (8 months) | Sleep problems: Acute-phase intercept | −0.103 | 0.151 | 0.90 |
| Sleep problems: Acute-phase slope | −0.353* | 0.178 | 0.70 | |
| 3. Relapse/recurrence (32 months) | PSQI Global: Acute-phase intercept | −0.023 | 0.125 | 0.98 |
| PSQI Global: Acute-phase slope | 0.034 | 0.127 | 1.03 | |
| 4. Relapse/recurrence (32 months) | Sleep problems: Acute-phase intercept | −0.072 | 0.108 | 0.93 |
| Sleep problems: Acute-phase slope | −0.148 | 0.121 | 0.86 | |
| 5. Post-acute remission (32 months) | PSQI Global: Acute-phase intercept | −0.139 | 0.093 | 0.87 |
| PSQI Global: Acute-phase slope | −0.276** | 0.099 | 0.76 | |
| 6. Post-acute remission (32 months) | Sleep problems: Acute-phase intercept | −0.015 | 0.074 | 0.98 |
| Sleep problems: Acute-phase slope | −0.184* | 0.092 | 0.83 | |
| 7. Recovery (32 months) | PSQI Global: Acute-phase intercept | −0.267* | 0.117 | 0.77 |
| PSQI Global: Acute-phase slope | −0.345** | 0.113 | 0.71 | |
| 8. Recovery (32 months) | Sleep problems: Acute-phase intercept | |||
| SS: Continuation cognitive therapy | 0.122 | 0.170 | 1.13 | |
| SS: Continuation fluoxetine | −0.399* | 0.159 | 0.67 | |
| SS: Continuation pill placebo | 0.139 | 0.170 | 1.15 | |
| Sleep problems: Acute-phase slope | −0.307** | 0.107 | 0.74 |
Models 1-8 are Cox regression. PSQI = Pittsburgh Sleep Quality Index. SS = simple slope. Sleep-problems scale derived from depression inventories. Depression severity does not include items referencing sleep directly. All predictors and the symptoms outcome are standardized (M = 0, SD = 1). HR = hazard ratio. All models controlled depression-symptom severity at intake to the acute phase, as well as continuation treatment arm (cognitive therapy, fluoxetine, or pill placebo with clinical management). Non-significant (p > .05) interactions of acute phase intercept and slope with treatment arm were removed from the final models. Note: “Post-acute Remission” is defined as six Psychiatric Status Ratings of 2 or less over the six weeks post randomization using the Longitudinal Interview Follow-up Evaluation; “Recovery,” is sustained remission, defined as remission lasting ≥ eight consecutive months; “Relapse,” is defined as exacerbation of the presenting episode after a response but before recovery; and “Recurrence,” defined as meeting DSM-IV criteria for MDD after recovery.
p < .05,
p < .01, two-tailed.
Table 6.
Predictors of time to post-acute outcomes from clinically significant sleep disturbance.
| Model: Outcome | Predictor | Beta | SE | HR |
|---|---|---|---|---|
| 1: Relapse (8 months) | PSQI ≥ 5 | −0.004 | 0.328 | 1.00 |
| 2: Relapse/recurrence (32 months) | PSQI ≥ 5 | −0.085 | 0.234 | 0.92 |
| 3. Post-acute remission (32 months) | PSQI ≥ 5 | −0.366* | 0.175 | 0.69 |
| 4. Recovery (32 months) | PSQI ≥ 5 | −0.530** | 0.199 | 0.59 |
Note. N = 241. Models are Cox regression. PSQI = Pittsburgh Sleep Quality Index. HR = hazard ratio. All models controlled depression-symptom severity at randomization to continuation treatment, as well as continuation-treatment arm (cognitive therapy, fluoxetine, or pill placebo with clinical management). Non-significant (p > .05) interactions of sleep problems with treatment arm were removed from the final models.
p < .05,
p < .01, two-tailed
Does Subjective Sleep Disturbance Throughout A-CT and at Randomization Predict Post-A-CT Relapse/Recurrence at 32 months?
No. Neither sleep-disturbance intercepts nor slopes measured throughout the acute phase, nor presence versus absence of sleep disturbance at randomization predicted depression relapse or recurrence over the full 32 months of follow-up.
Does Subjective Sleep Disturbance Throughout A-CT and at Randomization Predict Post-A-CT Remission and Recovery at 32 months?
Yes. Both the PSQI and sleep-problems scales predicted remission and recovery. Patients with greater reduction in sleep disturbance during A-CT remitted and recovered sooner. In addition, patients with less sleep disturbance overall on the PSQI recovered sooner. For the sleep-problems scale, the intercept interacted significantly with treatment arm, F(2, 2.25*108) = 3.58, p = .03, when predicting recovery. Simple slopes analysis showed that fewer sleep problems predicted recovery in the FLX arm only (see Table 5, Model 8, for simple slopes).
Among the sample of higher risk A-CT responders randomized to continuation treatment (N = 241), 60.2% had clinically significant sleep disturbance (PSQI ≥ 5) at randomization. Controlling depression-symptom severity at randomization, the presence (versus absence) of clinically significant sleep disturbance predicted longer time to remission (HR = 0.69) and recovery (HR = 0.59; see Table 6). At the end of the continuation phase, the estimated proportions of patients reaching remission were 86.7 versus 94.5%, and 25.8 versus 39.7% reaching recovery, for those with versus without clinically significant sleep disturbance at randomization. Similarly, at the end of the follow-up phase (32 months post-randomization), the estimated proportions of patients reaching remission were 97.4 versus 99.5%, and 90.4 versus 98.1% reaching recovery, for those with versus without clinically significant sleep disturbance at randomization.
Does Subjective Sleep Disturbance Continue to Improve During 8 months of Continuation-phase Treatment?
Improvements in sleep endured, but no additional reduction in sleep disturbance was observed. For both scales, the main effects of time, continuation treatment, and their interaction, were non-significant (ps > .16). Parallel analyses contrasting active treatment (C-CT or FLX) with PBO also yielded no significant main effects or interactions (ps > .22).
Does Subjective Sleep Disturbance Continue to Improve 32 Months After A-CT?
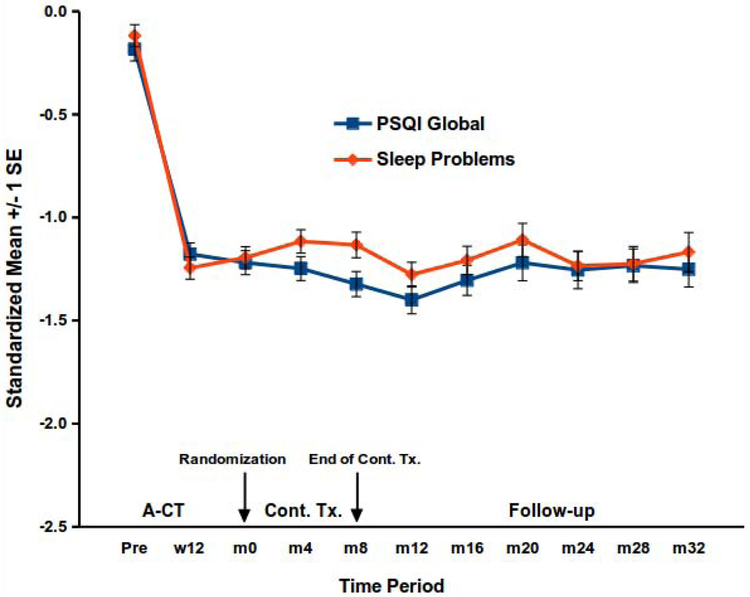
Again, enduring improvement but no additional gains were evident. All main effects of time, continuation treatment, and their interaction were non-significant for both scales (ps > .20). Parallel analyses contrasting active treatment (C-CT or FLX) with PBO also yielded no significant main effects or interactions, (ps > .26). The lack of significant effects suggests that sleep-disturbance means were relatively stable for 32 months after A-CT. Figure 2, which displays estimated scale means, and Table 2 reporting proportions of patients with clinically elevated PSQI scores, also support this conclusion.
Figure 2.
Changes in sleep disturbance among higher risk A-CT responders.
PSQI = Pittsburgh Sleep Quality Index. A-CT = Acute-phase cognitive therapy. Cont. Tx. = continuation treatment. w = week, m = month. Among higher risk responders to A-CT, additional changes in the PSQI global score and sleep-problems scale were not statistically significant through 8 months of continuation treatment (Cont. Tx; continuation cognitive therapy, fluoxetine with clinical management, or pill placebo with clinical management) or through 32 total months of follow-up.
Conclusions
The aim of these analyses was to clarify the extent to which changes in subjective sleep disturbance over the course of A-CT may affect treatment response, remission, episode recovery, and risk for relapse/recurrence during and after continuation-phase treatments. Results offered mixed support for links between changes in sleep and shorter- and longer-term outcomes in depressive symptoms. Findings support that A-CT can improve subjective sleep disturbance compared to baseline; however, the average participant and roughly 64% of the sample still met the threshold for “poor sleep,” which is consistent with reports of high rates of residual sleep disturbance following depression treatment (e.g., Carney et al., 2011; Pigeon et al., 2009). Furthermore, patients with “persisting” sleep disturbance had only a 2.6% probability of acute-phase remission in our sample. Responders to A-CT with greater subjective improvement in sleep are more likely to achieve response and acute-phase remission and have a shorter time to post-acute remission and recovery compared to responders with less improvement in sleep disturbance. However, we found a significant interaction with treatment, such that overall sleep problems during A-CT predicted slower recovery only in the FLX arm. This may be because FLX has negative effects on sleep architecture, including increased stage-1 sleep and reduced REM sleep (Armitage et al., 1997; Rush et al., 1998). Individuals already experiencing sleep problems who begin FLX treatment may experience continued sleep disturbance that could prolong depression symptoms. This finding should be evaluated in future studies with exploration of directionality and potential mechanisms.
For the most part, sleep changes during A-CT did not predict relapse or recurrence during or after continuation phase treatments. We did, however, observe a contrary finding. Less reduction in subjective sleep disturbance (measured only by the sleep-problems scale) predicted a longer time to relapse over the 8-month continuation phase. This finding may reflect a Type I error because it did not replicate across measures and time points and could have been linked to baseline sleep-disturbance severity (e.g., floor effects) that was not fully captured by the sleep-problems intercept. Indeed, when baseline sleep-problem scores were added to the model, the effect was no longer significant. Future studies that stratify patients at baseline according to their initial levels of sleep disturbance, including hypersomnolence, and then examine trajectories over time would shed light on this finding.
None of the continuation-phase treatments further improved sleep; however, the importance of achieving quicker recovery and maintaining gains in sleep improvement should not be underestimated. Sleep disturbance is independently associated with negative health outcomes including greater risk of cardiovascular and metabolic disorders (Grandner et al., 2012), cognitive impairment (Blackwell et al., 2006), and suicide (Pigeon et al., 2012). Given that these outcomes are likewise associated with MDD (Angst et al., 1999; Musselman et al., 1998; Rock et al., 2014; Vancampfort et al., 2015), treatment of sleep disturbance in the context of MDD has the potential to reduce overall burden of illness, a hypothesis that should be tested in future research. Our finding that sleep improvements predicted quicker depression remission and recovery supports careful targeting of sleep disturbance in acute-phase treatment, pending replication. Notably, the parent RCT was designed prior to research that established the efficacy of CBT-I (Trauer et al., 2015). Nevertheless, our findings are in line with burgeoning research showing that insomnia treatment is associated with improvements in depressive symptoms (Ballesio et al., 2018; Cunningham & Shapiro, 2018; Gebara et al., 2018b). The efficacy of CBT-I as an adjunct to continuation-phase treatment for residual sleep disturbance has not been tested as a relapse-prevention strategy and is an important area for further research.
The present study has several strengths. First, building on the foundation of Manber et al. (2016) who explored how changes in insomnia severity following CBT-I affected depression symptoms during acute-phase depression treatment, our analyses incorporate post-continuation outcomes, demonstrating longer term effects of improved sleep on depression recovery. Second, we both used a widely validated measure of sleep disturbance (PSQI) and derived a psychometrically sound scale of sleep disturbance from clinician-rated and self-reported measures of depression symptoms, demonstrating that results are consistent across measures and lessening the likelihood that mixed findings in the literature are predominantly due to heterogeneity in measurement. Analyses were performed on a large two-site sample with 32 months of post-randomization follow-up.
Notwithstanding these strengths, the study did not include objective measures of sleep disturbance and did not assess for sleep disorders such as obstructive sleep apnea or restless leg syndrome. Findings are limited by design characteristics (e.g., a focus on adults with recurrent depression, highly experienced CT therapists providing a relatively large “dose” of therapy). Our findings are generalizable only to those with similar sample characteristics.
As sample size was generated based on power calculations for the original RCT, current analyses may be under powered to examine relations between sleep disturbance and relapse/recurrence. Additionally, we did not systematically assess the degree to which sleep disturbance was addressed during A-CT. Finally, our derived sleep-problems scale was constructed in part from items from the HRSD-17, the remaining items of which were used to assess treatment outcome.
Our findings highlight the importance of addressing sleep disturbance in both acute- and continuation-phase treatment to enhance depression outcomes. Monitoring and treating changes in self-reported sleep disturbance during acute-phase treatment could prove key to hastening recovery. Given mounting evidence that insomnia treatment yields antidepressant effects, future research should explore individual factors that may predict which patients are more likely to need adjunctive insomnia treatment to achieve faster and more stable remission.
Supplementary Material
Highlights.
Greater reductions in sleep disturbance predicted increased likelihood of remission
Patients with greater reductions in sleep disturbance recovered sooner
Sleep disturbance did not predict relapse/recurrence of major depression consistently
Continuation treatment did not further improve sleep disturbance
Improvements in sleep achieved during the acute phase were maintained
Acknowledgements
We appreciate the careful review by members of the trial’s Data Safety and Monitoring Board. We are indebted to our research teams and our colleagues at The University of Texas Southwestern Medical Center, the University of Pittsburgh (where Dr. Thase was located during patient accrual), and the University of Pennsylvania (Dr. Thase’s current affiliation). We appreciate the participation of colleagues, previously named, and study participants without whom such research could not have been completed.
Role of Funding Source
This report was supported by the National Institute of Mental Health (R.B.J., grant numbers K24 MH001571, R01 MH58397, R01 MH69619; and M.E.T., grant numbers R01 MH58356 and R01 MH69618). Dr. Boland’s time was supported by Clinical Science Research and Development, Department of Veterans Affairs, grant number IK2 CX001501. The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIMH, National Institutes of Health, or the U.S. Government.
Footnotes
COI
Dr. Thase has no conflicts of interest pertaining to this paper, although he does report the following relationships with companies that develop treatment for depression or provide education pertaining to those treatments: Dr. Thase has consulted with and/or served on advisory boards for Alkermes, Allergan (includes Forest Laboratories), AstraZeneca, Cerecor, Johnson & Johnson (includes Janssen), Lundbeck, MedAvante, Merck, Moksha8, Otsuka, Pfizer Pharmaceuticals, Shire, Sunovion, and Takeda; he has received grant support from Alkermes, Allergan (includes Forest Laboratories), Assurerx, Johnson & Johnson, Takeda, the Agency for Healthcare Research and Quality, Patient Centered Outcomes Research Institute and the NIMH. He has equity holdings for MedAvante, Inc. and has received royalties from American Psychiatric Publishing, Inc. (APPI), Guilford Publications, Herald House, and W.W. Norton & Company, Inc. Dr. Thase’s spouse is an employee of Peloton Advantage, which does business with several pharmaceutical companies. Dr. Jarrett is a paid reviewer for the National Institute of Mental Health (NIMH). Dr. Jarrett’s medical center collects the payments from the cognitive therapy she provides to patients. Drs. Vittengl and Jarrett are paid reviewers for UpToDate. Drs. Boland and Clark have no financial interest or conflicts of interest related to the treatments studied.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Armitage R, Yonkers K, Cole D, Rush AJ 1997. A multicenter, double-blind comparison of the effects of nefazodone and fluoxetine on sleep architecture and quality of sleep in depressed outpatients. J. Clin. Psychopharmacol 17, 161–168. Doi: 10.1097/00004714-199706000-00004 [DOI] [PubMed] [Google Scholar]
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition. Washington, DC: American Psychiatric Association; 1994. [Google Scholar]
- Angst J, Angst F, Stassen HH 1999. Suicide risk in patients with major depressive disorder. J. Clin. Psychiatry 60(Suppl2):57–62. [PubMed] [Google Scholar]
- Ballesio A, Aquino MRJV, Feige B, Johann AF, Kyle SD, Spiegelhalder K, Lombardo C, Rucker G, Riemann D and Baglioni C 2018. The effectiveness of behavioural and cognitive behavioural therapies for insomnia on depressive and fatigue symptoms: a systematic review and network meta-analysis. Sleep Med. Rev 37:114–129. 10.1016/j.smrv.2017.01.006 [DOI] [PubMed] [Google Scholar]
- Baxter AJ, Scott KM, Ferrari AJ, Norman RE, Vos T, Whiteford HA 2014. Challenging the myth of an “epidemic” of common mental disorders: trends in the global prevalence of anxiety and depression between 1990 and 2010. Depress. Anxiety 31, 506–516. Doi: 10.1002/da.22230 [DOI] [PubMed] [Google Scholar]
- Beck AT, Steer RA, Brown GK 1996. Beck Depression Inventory-II. The Psychological Corporation, San Antonio. [Google Scholar]
- Beck AT, Steer RA, Carbin MG 1988. Psychometric properties of the Beck Depression Inventory: Twenty-five years of evaluation. Clin. Psychol. Rev 8, 77–100. Doi: 10.1016/0272-7358(88)90050-5 [DOI] [Google Scholar]
- Beck AT (Ed.). 1979. Cognitive Therapy of Depression. Guilford Press, New York. [Google Scholar]
- Bei B, Asarnow LD, Krystal A, Edinger J>D, Buysse DJ, Manber R 2018. Treating insomnia in depression: Insomnia related factors predict long-term depression trajectories. J. Consult. Clin. Psychol 86(3): 282–293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blackwell T, Yaffe K, Ancoli-Israel S, Schneider JL, Cauley JA, Hillier TA, Fink HA, Stone KL 2006. Poor sleep is associated with impaired cognitive function in older women: the study of osteoporotic fractures. J. Gerontol. A Biol. Sci. Med. Sci 61(4):405–410. 10.1093/gerona/61.4.405 [DOI] [PubMed] [Google Scholar]
- Buysse DJ, Reynolds CF III, Monk TH, Berman SR, Kupfer DJ 1989. The Pittsburgh Sleep Quality Index: a new instrument for psychiatric practice and research. Psychiatry Res. 28, 193–213. Doi: 10.1016/0165-1781(89)90047-4 [DOI] [PubMed] [Google Scholar]
- Carney CE, Harris AL, Friedman J, Segal ZV 2011. Residual sleep beliefs and sleep disturbance following cognitive behavioral therapy for major depression. Depress. Anxiety 28, 464–470. Doi: 10.1002/da.20811. [DOI] [PubMed] [Google Scholar]
- Carney CE, Segal ZV, Edinger JD, Krystal AD 2007. A comparison of rates of residual insomnia symptoms following pharmacotherapy or cognitive-behavioral therapy for major depressive disorder. J. Clin. Psychiatry 68, 254–260. Doi: 10.4088/JCP.v68n0211 [DOI] [PubMed] [Google Scholar]
- Combs K, Smith PJ, Sherwood A, Hoffman B, Carney RM, Freedland K, Craighead EW, Blumenthal JA 2014. Impact of sleep complaints and depression outcomes among participants in the standard medical intervention and long-term exercise study of exercise and pharmacotherapy for depression. J. Nerv. Ment. Dis 202, 167–171. Doi: 10.1097/NMD.0000000000000085. [DOI] [PubMed] [Google Scholar]
- Cho HJ, Lavretsky H, Olmstead R, Levin MJ, Oxman MN, Irwin MR 2008. Sleep disturbance and depression recurrence in community-dwelling older adults: A prospective study. Am. J. Psychiatry 165, 1543–1550. Doi: 10.1176/appi.ajp.2008.07121882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cunningham JE, Shapiro CM 2018. Cognitive Behavioural Therapy for Insomnia (CBT-I) to treat depression: A systematic review. J. Psychosom. Res 106:1–12. 10.1016/j.jpsychores.2017.12.012 [DOI] [PubMed] [Google Scholar]
- Dew MA, Reynolds CF, Houck PR, Hall M, Buysse DJ, Frank E, Kupfer DJ 1997. Temporal profiles of the course of depression during treatment: Predictors of pathways toward recovery in the elderly. Arch. Gen. Psychiatry 54, 1016–1024. Doi: 10.1001/archpsyc.1997.01830230050007 [DOI] [PubMed] [Google Scholar]
- Dombrovski AY, Cyranowski JM, Mulsant BH, Houck PR, Buysse DJ, Andreescu C, Thase ME, Mallinger AG, Frank E 2008. Which symptoms predict recurrence of depression in women treated with maintenance interpersonal psychotherapy? Depress. Anxiety. 25, 1060–1066. Doi: 10.1002/da.20467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dombrovski AY, Mulsant BH, Houck PR, Mazumdar S, Lenze EJ, Andreescu C, Cyranowski JM, Reynolds CM III 2007. Residual symptoms and recurrence during maintenance treatment of late-life depression. J. Affect. Disord 103, 77–82. 10.1016/j.jad.2007.01.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fawcett J, Epstein P, Fiester S, Elkin I, Autry J 1987. Clinical management imipramine/placebo administration manual. Psychopharmacol. Bull 23(2):309–24. [PubMed] [Google Scholar]
- Fernandez-Mendoza J, Shea S, Vgontzas AN, Calhoun SL, Liao D, & Bixler EO 2015. Insomnia and incident depression: role of objective sleep duration and natural history. J. Sleep Res 24(4): 390–398. 10.1111/jsr.12285 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frank E, Prien RF, Jarrett RB, Keller MB, Kupfer DJ, Lavori PW, Rush AJ, Weissman MM 1991. Conceptualization and rationale for consensus definitions of terms in major depressive disorder: remission, recovery, relapse, and recurrence. Arch. Gen. Psychiatry 48(9): 851–855. Doi: 10.1001/archpsyc.1991.01810330075011 [DOI] [PubMed] [Google Scholar]
- Frank E, Kupfer DJ, Perel JM, Cornes C, Jarrett DB, Mallinger AG, Thase ME, McEachran AB, Grochocinski VJ 1990. Three-year outcomes for maintenance therapies in recurrent depression. Arch. Gen. Psychiatry 47, 1093–1099. Doi: 10.1001/archpsyc.1990.01810240013002 [DOI] [PubMed] [Google Scholar]
- Gebara MA, Kasckow J, Smagula SF, DiNapoli EA, Karp JF, Lenze EJ, Mulsant BH, Reynolds CF III. 2018a. The role of late life depressive symptoms on the trajectories of insomnia symptoms during antidepressant treatment. J. Psychiatr. Res 96: 162–166. Doi: 10.1016/j.jpsychires.2017.10.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gebara MA, Siripong N,. DiNapoli EA, Maree RD, Germain A, Reynolds CF, Kasckow JW, Weiss PM, Karp JF 2018b. Effect of insomnia treatments on depression: A systematic review and meta-analysis. Depress. Anxiety 35: 717–731. 10.1002/da.22776 [DOI] [PubMed] [Google Scholar]
- Grandner MA, Jackson NJ, Pak VM, Gehrman PR 2012. Sleep disturbance is associated with cardiovascular and metabolic disorders. J. Sleep Res 21(4):427–33. 10.1111/j.1365-2869.2011.00990.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gulec M, Selvi Y, Boysan M, Aydin A, Besiroglu L, Agargun MY 2011. Ongoing or re-emerging subjective insomnia symptoms after full/partial remission or recovery of major depressive disorder mainly with the selective serotonin reuptake inhibitors and risk of relapse or recurrence: a 52-week follow-up study. J. Affect Disord 134(1-3):257–265. 10.1016/j.jad.2011.05.056 [DOI] [PubMed] [Google Scholar]
- Hartwig EM, Rufino KA, Palmer CA, Shepard C, Alfano CA, Schanzer B, Mathew SJ, Patriquin MA 2019. Trajectories of self-reported sleep disturbance across inpatient treatment predict clinical outcome in comorbid major depressive disorder and generalized anxiety disorder. J. Affect. Disord 251:248–255. [DOI] [PubMed] [Google Scholar]
- Hamilton M 1960. A rating scale for depression. J. Neurol. Neurosurg. Psychiatry. Res 23, 56 Doi: 10.1136/jnnp.23.1.56 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iovieno N, van Nieuwenhuizen A, Clain A, Baer L, Nierenberg AA 2011. Residual symptoms after remission of MDD with fluoxetine and risk of relapse. Depress. Anxiety 28, 137–144. Doi: 10.1002/da.20768 [DOI] [PubMed] [Google Scholar]
- Jarrett RB, Kraft D, Doyle J, Foster BM, Eaves GG, Silver PC 2001. Preventing recurrent depression using cognitive therapy with and without a continuation phase: A randomized clinical trial. Arch. Gen. Psychiatry 58, 381–388. Doi: 10.1001/archpsyc.58.4.381 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jarrett RB, Minhajuddin A, Gershenfeld H, Friedman ES, Thase ME 2013. Preventing depressive relapse and recurrence in higher-risk cognitive therapy responders. A randomized trial of continuation phase cognitive therapy, fluoxetine, or matched pill placebo. JAMA. Psychiatry 11, 1152–1160. Doi: 10.1001/jamapsychiatry.2013.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jarrett RB, Thase ME 2010. Comparative efficacy and durability of continuation phase cognitive therapy for preventing recurrent depression: Design of a double-blinded, fluoxetine-and pill placebo controlled, randomized trial with 2-year follow up. Contemp. Clin. Trials 4, 335–377. Doi: 10.1016/j.cct.2010.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jha MK, Wakhlu S, Dronamraju N, Minhajuddin A, Greer TL, Trivedi MH 2018. Validating pre-treatment body mass index as moderator of antidepressant treatment outcomes: Findings from CO-MED trial. J. Affect. Disord 234, 34–37. Doi: 10.1016/j.jad.2018.02.089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keller MB, Lavori PW, Friedman B, Nielsen E, Endicott J, McDonald-Scott P, Andreasen NC 1987. The Longitudinal Interval Follow-up Evaluation: a comprehensive method for assessing outcome in prospective longitudinal studies. Arch. Gen. Psychiatry 44, 540–548. Doi: 10.1001/archpsyc.1987.01800180050009 [DOI] [PubMed] [Google Scholar]
- Keller MB, Lavori PW, Lewis CE, Klerman GL 1983. Predictors of relapse in major depressive disorder. JAMA. 250(24): 3299–3304. Doi: 10.1001/jama.1983.03340240025024 [DOI] [PubMed] [Google Scholar]
- Kocsis JH, Friedman RA, Markowitz JC, Leon AC, Miller NL, Gniwesch L, Parides M 1996. Maintenance therapy for chronic depression: A controlled clinical trial of desipramine. Arch. Gen. Psychiatry 53, 769–774. Doi: 10.1001/archpsyc.1996.01830090013002 [DOI] [PubMed] [Google Scholar]
- Kupfer DJ, Ehler CL, Frank E, Grochocinski VJ, McEachran AB, Alhaji B 1994. Persistent effects of antidepressants: EEG sleep studies in depressed patients during maintenance treatment. Biol. Psychiatry 35, 781–793. Doi: 10.1016/0006-3223(94)91140-1 [DOI] [PubMed] [Google Scholar]
- Kupfer DJ, Frank E, Perel JM, Cornes C, Mallinger AG, Thase ME, McEachran AB, Grochocinski VJ 1992. Five-year outcome for maintenance therapies in recurrent depression. Arch. Gen. Psychiatry 49, 769–773. Doi: 10.1001/archpsyc.1992.01820100013002 [DOI] [PubMed] [Google Scholar]
- Manber R, Buysse DJ, Edinger J, Krystal A, Luther JF, Wisniewski SR, Trockel M, Kraemer HC, Thase ME 2016. Efficacy of Cognitive-Behavioral Therapy for Insomnia Combined with antidepressant pharmacotherapy in patients with comorbid depression and insomnia: A randomized controlled trial. J. Clin. Psychiatry 77(10):e1316–e1323. [DOI] [PubMed] [Google Scholar]
- Morin CM, Belleville G, Belanger L, Ivers H 2011. The Insomnia Severity Index: psychometric indicators to detect insomnia cases and evaluate treatment response. Sleep. 34, 601–608. Doi: 10.1093/sleep/34.5.601 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Musselman DL, Evans DL, Nemeroff CB 1998. The relationship of depression to cardiovascular disease: epidemiology, biology, and treatment. Arch. Gen, Psychiatry 55(7), pp.580–592. Doi: 10.1001/archpsyc.55.7.580 [DOI] [PubMed] [Google Scholar]
- Nierenberg AA, Husain MM, Trivedi MH, Fava M, Warden D, Wisniewski SR, Miyahara S, Rush AJ 2010. Residual symptoms after remission of MDD with citalopram and risk of relapse: A STAR* D report. Psychol. Med 40, 41–50. Doi: 10.1017/S0033291709006011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perlis ML, Giles DE, Buysse DJ, Tu X, Kupfer DJ 1997. Self-reported sleep disturbance as a prodromal symptom in recurrent depression. J. Affect. Disord 42, 209–212. Doi: 10.1016/S0165-0327(96)01411-5 [DOI] [PubMed] [Google Scholar]
- Pigeon WR, May PE, Perlis ML, Ward EA, Lu N, & Talbot NL 2009. The effect of interpersonal psychotherapy for depression on insomnia symptoms in a cohort of women with sexual abuse histories. J. Trauma Stress 22(6):634–638. DOI: 10.1002/jts.20456 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pigeon WR, Pinquart M Conner K 2012. Meta-analysis of sleep disturbance and suicidal thoughts and behaviors. J. Clin. Psychiatry 73(9):e1160–1167. https://psycnet.apa.org/doi/10.4088/JCP.11r07586 [DOI] [PubMed] [Google Scholar]
- Rapaport MH, Bose A, Zheng H 2004. Escitalopram continuation treatment prevents relapse of depressive episodes. J. Clin. Psychiatry 65, 44–49. Doi: 10.4088/JCP.v65n0107 [DOI] [PubMed] [Google Scholar]
- Reynolds CF III, Frank E, Houck PR, Mazumdar S, Dew MA, Cornes C, Buysse DJ, Begley A, Kupfer DJ 1997. Which elderly patients with remitted depression remain well with continued interpersonal psychotherapy after discontinuation of antidepressant medication? Am. J. Psychiatry 154, 958–62. DQI: 10.1176/ajp.154.7.958 [DOI] [PubMed] [Google Scholar]
- Reynolds CF III, Hoch CC, Buysse DJ, George CJ, Houck PR, Mazumdar S, Miller M, Pollock BG, Rifai H, Frank E 1991. Sleep in late-life recurrent depression. Changes during early continuation therapy with nortriptyline. Neuropsychopharmacology. 5, 85–96. [PubMed] [Google Scholar]
- Rock PL, Roiser JP, Riedel WJ, Blackwell AD 2014. Cognitive impairment in depression: a systematic review and meta-analysis. Psychol. Med 44(10):2029–2040. 10.1017/S0033291713002535 [DOI] [PubMed] [Google Scholar]
- Rush AJ, Armitage R, Gillin JC, Yonkers KA, Winokur A, Moldofsky H, Vogel GW, Kaplita SB, Fleming JB, Montplaisir J, Erman MK, Albala BJ, McQuade RD 1998. Comparative effects of nefazodone and fluoxetine on sleep in outpatients with major depressive disorder. Biol. Psychiatry 44, 3–14. Doi: 10.1016/S0006-3223(98)00092-4 [DOI] [PubMed] [Google Scholar]
- Rush AJ, Carmody T, Reimitz PE 2000. The Inventory of Depressive Symptomatology (IDS): clinician (IDS-C) and self-report (IDS-SR) ratings of depressive symptoms. Int. J. Methods Psychiatr. Res 9, 45–59. Doi: 10.1002/mpr.79 [DOI] [Google Scholar]
- Taylor DJ, Walters HM, Vittengl JR, Krebaum S, Jarrett RB 2010. Which depressive symptoms remain after response to cognitive therapy of depression and predict relapse and recurrence? J. Affect. Disord 123, 181–187. Doi: 10.1016/j.jad.2009.08.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thase ME, Simons AD, Reynolds CF 1996. Abnormal electroencephalographic sleep profiles in major depression: association with response to cognitive behavior therapy. Arch. Gen. Psychiatry 53, 99–108. Doi: 10.1001/archpsyc.1996.01830020013003 [DOI] [PubMed] [Google Scholar]
- Trauer JM, Qian MY, Doyle JS, Rajaratnam SMW, Cunnington D 2015. Cognitive behavioral therapy for chronic insomnia: A systematic review and meta-analysis. Ann. Intern. Med 163(3): 191–204. DOI: 10.7326/M14-2841 [DOI] [PubMed] [Google Scholar]
- Troxel WM, Kupfer DJ, Reynolds CF, Frank E, Thase M, Miewald J, Buysse DJ 2012. Insomnia and objectively measured sleep disturbances predict treatment outcome in depressed patients treated with psychotherapy or psychotherapy-pharmacotherapy combinations. J. Clin. Psychiatry 73, 478 Doi: 10.4088/JCP.11m07184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vancampfort D, Stubbs B, Mitchell AJ, De Hert M, Wampers M, Ward PB, Rosenbaum S Correll CU 2015. Risk of metabolic syndrome and its components in people with schizophrenia and related psychotic disorders, bipolar disorder and major depressive disorder: a systematic review and meta-analysis. World Psychiatry. 14(3):339–347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vittengl JR, Clark LA, Thase ME, Jarrett RB 2013. Nomothetic and idiographic symptom change trajectories in acute phase cognitive therapy for recurrent depression. J. Consult. Clin. Psychol 81, 615–626. Doi: 10.1037/a0032879 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang H, Sinicropi-Yao L, Chuzi S, Youn SJ, Clain A, Baer L, Chen Y, McGrath PJ, Fava M, Papakostas GI 2010. Residual sleep disturbance and risk of relapse during the continuation/maintenance phase treatment of major depressive disorder with the selective serotonin reuptake inhibitor fluoxetine. Ann. Gen. Psychiatry 9:10. doi: 10.1186/1744-859X-9-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young J, Beck AT 1980. Cognitive Therapy Scale Rating Manual Center for Cognitive Therapy, Philadelphia, PA. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.