Abstract
Background
Current treatment options for chronic pain and depression are largely medication-based, which may cause adverse side effects. Integrative Medical Group Visits (IMGV) combines mindfulness techniques, evidence based integrative medicine, and medical group visits, and is a promising adjunct to medications, especially for diverse underserved patients who have limited access to non-pharmacological therapies.
Objective
Determine the effectiveness of IMGV compared to a Primary Care Provider (PCP) visit in patients with chronic pain and depression.
Design
9-week single-blind randomized control trial with a 12-week maintenance phase (intervention—medical groups; control—primary care provider visit)
Setting
Academic tertiary safety-net hospital and 2 affiliated federally-qualified community health centers.
Participants
159 predominantly low income racially diverse adults with nonspecific chronic pain and depressive symptoms.
Interventions
IMGV intervention– 9 weekly 2.5 hour in person IMGV sessions, 12 weeks on-line platform access followed by a final IMGV at 21 weeks.
Measurements
Data collected at baseline, 9, and 21 weeks included primary outcomes depressive symptoms (Patient Health Questionnaire 9), pain (Brief Pain Inventory). Secondary outcomes included pain medication use and utilization.
Results
There were no differences in pain or depression at any time point. At 9 weeks, the IMGV group had fewer emergency department visits (RR 0.32, 95% CI: 0.12, 0.83) compared to controls. At 21 weeks, the IMGV group reported reduction in pain medication use (Odds Ratio: 0.42, CI: 0.18–0.98) compared to controls.
Limitations
Absence of treatment assignment concealment for patients and disproportionate group attendance in IMGV.
Conclusion
Results demonstrate that low-income racially diverse patients will attend medical group visits that focus on non-pharmacological techniques, however, in the attention to treat analysis there was no difference in average pain levels between the intervention and the control group.
Trial registration
clinicaltrials.gov NCT02262377.
Introduction
Chronic pain annually affects 25 million adults in the United States and is linked to significant disability and high medical utilization [1–3]. Treatment of chronic pain is complex due to safety concerns of prescription pain medications (e.g. opioids) and comorbid conditions such as depression and substance use [4,5]. Depression often complicates the treatment of chronic pain [6,7]. Even when pain medications are effective in reducing pain; they may not improve mental and functional status and may actually increase depression [8,9]. Furthermore, patients with chronic pain and depressive symptoms have increased use of medical care and higher risk of medical utilization [10–12].
Due to socioeconomic factors, treating chronic pain and depressive symptoms may be challenging in a low income, racially and ethnically diverse patients [13,14]. Disparities in access to prescription and non-prescription treatment for chronic pain and associated conditions may contribute to negative impact on economic (ability to work), emotional (social isolation), and daily functioning [15–17]. For example, minority patients with chronic pain receive less patient education, medications, surgery, and specialty referrals [14]. Access to non-pharmacological therapies is challenging as these therapies are often located far from low income neighborhoods, require an out of pocket payment, may not be covered by health insurance, and are less likely to be offered as a treatment to low-income or under-represented minority patients [18–23].
One such non-pharmacological treatment is Evidence Based Integrative Medicine (EBIM) which combines “mainstream medical therapies and complementary therapies for which there is high-quality scientific evidence of safety and effectiveness” [24,25]. EBIM addresses factors such as activity, social connection, nutrition, lifestyle modification, and stress, all of which play significant roles in chronic conditions [26]. Clinical studies on EBIM demonstrate health improvements in patients’ chronic pain and/or depressive symptoms [27]. For example, mind body techniques, such as Mindfulness Based Stress Reduction (MBSR), demonstrate benefits for individuals with chronic pain [8,9,28]. Several systematic reviews on mindfulness clinical trials for patients with chronic pain show improvement in pain scores and mental health status [5, 29–32].
In 2012, at Boston Medical Center, an urban safety-net hospital, the Integrative Medical Group Visit (IMGV) was developed to increase access to EBIM for patients in the outpatient setting. IMGV combines a medical group visit (MGV), principles of mindfulness, and EBIM [33,34]. We chose to use the medical group visit as the means to deliver EBIM for the following reasons: clinicians can bill insurance for the medical group visit, it increases access to EBIM, patients were introduced to EBIM in a trusted environment, and the medical group visits could be conducted in local neighborhood community health centers affiliated with BMC [35–37]. Medical group visits (shared medical appointments) are comprised of two clinicians who treat a group of eight to twelve patients at one time and include: individual medical attention, patient education, self-management, self-monitoring, and social support. MGVs are used to treat an increasing number of chronic illnesses and improve symptom management [38]. Current literature suggests that MGVs improve health-related quality of life, patient satisfaction, and reduce health care utilization [39,40].
In an uncontrolled study, the IMGV model demonstrated the potential for reducing pain and depressive symptoms [40]. Additionally, it was found to increase quality of life and reduce Emergency Department (ED) use [33]. However, it is unknown how the IMGV compares to a Primary Care Provider (PCP) visit in socioeconomically diverse patients with chronic pain and depressive symptoms [33,41–43]. This paper reports the main outcome findings of a single blind randomized controlled trial comparing the effectiveness of an IMGV in reducing pain, depressive symptoms, and pain medication use to a primary care visit. Our primary hypothesis was a greater reduction in pain, depressive symptoms, and medication use for participants randomized to IMGV compared with participants randomized to the control group. Additionally, this analysis examines who attended the IMGV and correlates of high versus low IMGV attendance.
Materials and methods
The study was approved by the Boston University Medical Campus Institutional Review Board (IRB) and the community health center’s (CHC) research committees (IRB Approval Number: H33096). We registered this randomized controlled trial (RCT) in the international trial register [ClinicalTrials.gov: Identifier NCT02262377]. For further detail please refer to our methods paper [34].
This RCT was conducted at an ambulatory primary care clinic at the Boston Medical Center (BMC) and two affiliated federally qualified Community Health Centers (CHC): Codman Square Health Center (CSHC) and DotHouse Health (DH). These practices serve low-income, racially and ethnically diverse populations living in the Boston area. Our inclusion criteria included: age 18 years or older, able to communicate in English language, score of ≥ 5 on the Patient Health Questionnaire-9 (PHQ-9), score of ≥ 4 on a 0–10 scale measuring daily chronic pain intensity for at least 12 weeks, and having a PCP located at the site where the IMGV was being held [43–47]. The exclusion criteria included: self-reported symptoms of psychosis or mania, active substance abuse (alcohol, cocaine or heroin use in the last 3 months), previous participation in an IMGV, a new pain treatment in the past month or plans to begin any new pain treatments in the next three months, active suicidality, any other severe disabling chronic medical or psychiatric co-morbidities preventing attendance to the IMGV, or no access to the internet during the study period [34].
Participants were recruited through their clinicians’ outpatient referral, clinicians’ letter to patients about the study, or self-referral. After being contacted by the research assistant (RA), patients then consented to be screened. If the eligibility was verified and there was patient written consent, the patient was next enrolled in the study.
This study is a single-blinded, two-arm randomized controlled trial. All participants (N = 155) who were consented and completed baseline assessments were randomized (1:1) to either intervention (IMGV) or control group. A randomization list was created using computer-generated permuted blocks with a block size of 6. We used the Studytrax database system to designate the treatment assignments. These were placed in opaque, sequentially numbered, sealed envelopes, which were only opened by a research assistant when a new enrolled participant received their treatment allocation. The investigators and biostatisticians were blinded to the treatment assignment. Patients were not blinded to allocation due to the group nature of the intervention. All patients in the control group were offered to access to the IMGV groups after study completion.
IMGV Intervention
The IMGV intervention includes three concurrent deliveries of the same self- management curriculum delivered with different formats–an in-person MGV, and two adjunct companion technologies available on a computer tablet provided to the intervention participant. The first technology was the Our Whole Lives (OWL), an e-Health toolkit platform, and the second technology was an Embodied Conversational Agent (ECA).
A detailed description of the IMGV self management intervention has previously been described [34]. The IMGV consists of a total of ten in-person medical group visits each lasting 2.5-hours conducted weekly from week 1 to week 9 (9 in-person sessions plus OWL/ECA). This is followed by a 12-week maintance phase where there is access to the technology only (OWL/ECA). A tenth and final in-person session is conducted at week 21.
At the start of the IMGV, participants measured their vital signs, moodstate, and pain levels. They then met individually with a trained physician (a co-facilatator) for a medical assessment. Two trained non-physician facilitators (see below) then led mindfulness practices. Patients were instructed in the principles of mindfulness and EBIM self-management techniques (such as acupressure and massage). Each week, the physician facilitated a discussion on health topics such as stress, insomnia, depression, chronic pain cycle, activity, and healthy food choices. Finally, the IMGV ended with a healthy meal, which mirrored the healthy nutrition topic in each session.
In addition to a physician, an experienced co-facilitator with training in mindfuness (certified MBSR instructor, yoga and meditation teacher) attended all groups. Facilitators were mentored via direct observation of two pilot group visits, one-on-one meetings, and phone calls by an experienced MBSR trained faculty.
To reinforce all content delivered in the in-person group, an internet-based platform called Our Whole Lives (OWL) delivered the same in-person curriculum. OWL could be accessed with a computer, smart phone, or tablet. The ECA, a female automated character, emulated the conversational behavior of an empathic coach [48]. The ECA (Gabby) reviewed all the content discussed in the IMGV with the participants outside of the in-person group. A Dell Venue 8 Pro tablet was distributed to all intervention participants in the first session of the group. Results of the use of technology will be published in an additional manuscript.
After the nine-week in-person group visit phase concluded, the intervention participants entered a 12-week maintenance phase. The intervention participants retained the study tablet and continued to have access to the ECA and the OWL website. At the end of the 21 weeks, there was one final in-person group visit.
A trained study RA directly observed all groups and assessed the facilitator’s adherence to the intervention components through a monitoring and evaluation checklist. These checklists were used to assess each MGV session at all sites during the study.
Prior to the start of the study, we provided continuing medical education training in evidence based chronic pain management at the study sites. We also provided access to the educational content on safe prescribing practices for chronic pain patients available on a website (http://mytopcare.org/) through small group presentations and/or Grand Round presentations at each study site for staff and clinical providers [49]. We did not collect data on who attended the training.
All participants randomized to the control group were asked to visit their PCP during the study period (baseline to 21 weeks). We verified a PCP clinical visit, via electronic medical record (EMR) documentation. We did not collect data on the duration or content of the visit.
Outcomes
Research assistants collected outcome data at baseline, 9 weeks, and 21 weeks. Self-reported data included: baseline demographics (age, gender, race, ethnicity, income, work status, education) and types of pain medications used in the last seven days.
Our primary outcomes included: 1) self-reported pain measured by the Brief Pain Inventory [(BPI) pain interference, pain severity, and average pain score in the last seven days] [45,50] and 2) depression level measured by the PHQ-9, a self-reported depression scale [49,50]. BPI pain interference, pain severity, and average pain are on a 0-10-point scale. The higher the score, the more severe the pain. PHQ-9 is on a 0-27-point scale. The higher the score, the more severe the depression.
Secondary outcomes included pain self-efficacy, self-reported pain medication use, health-related quality of life, patient activation, risk of opioid misuse, and ED use [51–60]. Pain self-efficacy was measured with the Pain Self-Efficacy Scale (PSEQ) and ranged from 0–60 points. High PSEQ scores are associated with higher confidence to function with pain [61]. For self-reported pain medication use, we used the Timeline Follow Back method to determine patient reported use of pain medications in the prior seven days [51, 62]. We categorized pain medications by opioids (MS Contin, Vicodin, Oxycodone, OxyContin, Tramadol, Tylenol with Codeine #3), Nonsteroidal Anti-inflammatory Drugs (NSAIDS: Ibuprofen, Naproxen, Aspirin), and other pain medication (Acetaminophen, Cyclobenzaprine, Gabapentin).
Health-related quality of life was measured using the Short form 12 Health Survey version 2 (SF-12). The SF-12 is composed of two component scores: Mental Component Summary (MCS) and Physical Component Summary (PCS) [63]. SF-12 scores ranged from 0–100 points, where a zero score indicates the lowest level of health and 100 indicates the highest level of health. Activation in patients was measured using Patient Activation Measure (PAM) [52,55]. PAM scores are transformed to a scale of 0–100 points. The risk of opioid misuse was measured using the Common Opioid Misuse Measure (COMM) [58, 64]. COMM is a 17-item assessment measure and scored based on a Likert 5-point scale from 0–4. The COMM cut-off score of 9 or above is a positive indicator for misusing medication.
We measured ED utilization for the 12 weeks prior to the study and throughout the study period through self-reported ED use at baseline, 9 weeks, and 21 weeks and through the EMR. After completing the 9 and 21-week data collection, patients received a $25 gift card incentive.
We assumed a two-sided alpha error = 0.05 and estimated a 20% dropout rate from baseline to 21-weeks. Based on previous literature, we defined a statistically significant change in effect size to include reductions in average pain from the BPI (1.5 points) and PHQ-9 (4 points) [65,66]. Although some debate exists on how to define a minimal clinically important change, many pain researchers consider changes in pain of more than 1–1.5 points to be clinically important [67]. A sample size of 62 participants per treatment group across all sites had an 80% power to detect a 1.5 difference in average pain and a sample size of 31 per treatment group had 80% power to detect a difference of 4 points in PHQ-9 score. Additionally, we defined a clinical meaningful result as a 1.5 reduction in average pain or 4-point reduction in PHQ-9 score [65,66].
Data analysis
We performed descriptive data analysis for baseline demographics. Means and standard deviations were calculated for continuous variables, and frequencies and percentages were calculated for categorical variables. To examine the success of randomization, we applied Pearson’s Chi-Square Test and Fisher’s Exact Test (categorical variable), and two-sample T-Test and Wilcoxon Rank-Sum Test (continuous variable) to compare the results between intervention and control at baseline, with a significance level of 0.1. Variables that were significantly different across study groups at baseline were considered confounders and were adjusted for in the following analysis. All data were analyzed using SAS 9.3. [68].
The primary analysis was intention-to-treat analysis. The ITT analysis included all participants who were randomized in the study, regardless of adherence to attending the IMGV or attending a PCP visit (control). To address missing data in all analyses, we used multiple imputation approach with 20 imputed data sets.
We performed the ITT analyses for the primary (pain and depressive symptoms) and secondary outcome variables. Descriptive statistics were calculated for all the outcome variables (Mean, SD, or N, %). Histograms were created to assess if the variables were normally distributed. Sensitivity analysis was performed on all multivariable regressions and logistic regressions.
For primary and secondary outcomes, we used multivariable regression models fit with generalized estimating equations (GEEs) to account for serially collected data, with an indicator for treatment assignment as the predictor of interest. We adjusted our models for potential confounders and assessed effect modification. For continuous and count variables we considered different regression models (Poisson, Negative Binomial, and Log Normal Model, as appropriate) to obtain the best fit for our outcomes. The models with lowest Akaike Information Criteria (AIC) were selected. Dichotomous outcomes (any pain medication use, opioid use, NSAIDS use, and other medication use) were fit with logistic regression. An interaction term of time and treatment was included in our models to assess for changes in the treatment over time.
On the advice of our patient advisory group and scientific advisory group, we conducted an exploratory per-protocol attendance analysis to understand how the “exposure to amount of health care”affected outcomes in those participants with no PCP visits during the study, low attendance to IMGV (1–4 sessions), medium attendance (5–6 sessions), and high attendance (7–10 sessions). We examined the baseline characteristics as well as longitudinal multivariable regressions, comparing intervention participants who attended different numbers of sessions to the control participants who did and did not attend PCP visits. The predictor of interest was treatment assignment, which indicated either the number of IMGV sessions attended (1–4 sessions, 5–6 sessions, and 7–10 sessions), or the control PCP visits (≥ 1 PCP visit, and no PCP visit). Poisson and negative binomial models were selected where AIC was minimized. Potential confounders were adjusted for in all models. A significance level of 0.05 was used, except where otherwise noted [68]. As we are performing multiple analyses, it is possible that we will see p-values that are below 0.05 by chance alone. Therefore, we do not strictly interpret statistical significance at the 0.05 level for analyses beyond the primary aims of the study and view these results as suggestive of areas that might merit further study.
Data on adverse events were reviewed and monitored on a quarterly basis by the PI. A Data Safety Monitoring Board (DSMB) reviewed all adverse events, data collection, and adherence to research protocol independently of study staff. All participants were included in the safety analyses using descriptive statistics.
Results
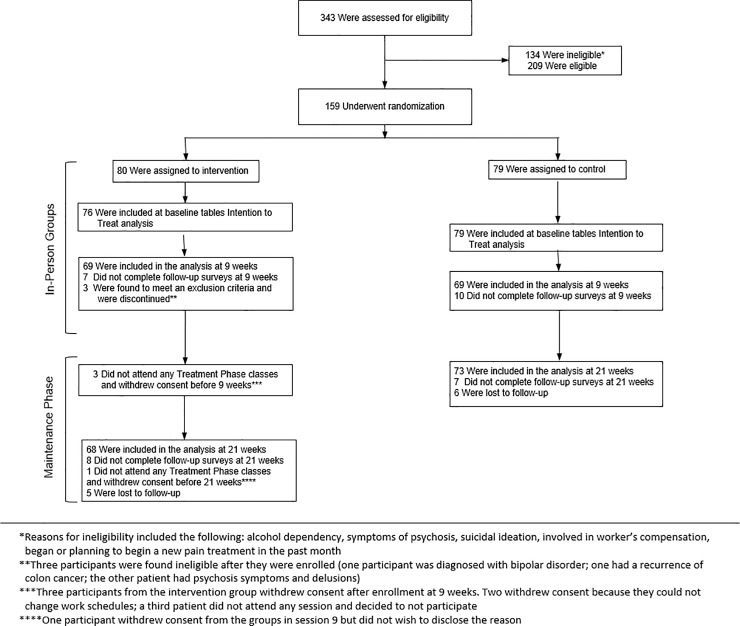
Screening, eligibility, randomization, and reasons of participants’ ineligibility after enrollment or withdrawal are given in Fig 1.
Fig 1. Participant flow in CONSORT diagram.
The recruitment began in November 2014 and finished in October 2016. Three hundred forty-three patients were assessed for eligibility, 209 patients were eligible, and 159 were enrolled and randomized to intervention (80) or control (79).
Four participants in intervention group were excluded from the analysis because they withdrew their consent after being enrolled. A total of 155 participants were included in the ITT analysis and baseline tables. After being enrolled in the study, three participants in the intervention were found to meet an exclusion criterion and were discontinued. These participants were included in the demographic descriptive analysis (Table 1), but not for 9- and 21-week ITT analysis.
Table 1. Baseline demographics for participants by group.
| Variable | Totals* (N = 155) |
Intervention (N = 76) |
Control (N = 79) |
P-Value | |||
|---|---|---|---|---|---|---|---|
| Mean (SD) | Mean (SD) | Mean (SD) | |||||
| Age (22–84) | 50.5 (12.3) | 50 (12.2) | 51 (12.4) | 0.62 | |||
| Gender | N | % | N | % | N | % | |
| Female | 134 | 86 | 64 | 84 | 70 | 89 | 0.42 |
| Male | 21 | 14 | 12 | 16 | 9 | 11 | |
| Race | |||||||
| White | 29 | 19 | 12 | 16 | 17 | 21.5 | 0.83 |
| Black | 87 | 56 | 44 | 58 | 43 | 54 | |
| Multiple race | 9 | 6 | 5 | 7 | 4 | 5 | |
| Unknown or Not Reported | 30 | 30 | 15 | 20 | 16 | 19 | |
| Ethnicity | |||||||
| Hispanic | 22 | 14 | 10 | 13 | 12 | 15 | 0.71 |
| Non-Hispanic | 133 | 86 | 66 | 87 | 67 | 85 | |
| Study Sites | |||||||
| BMC | 68 | 44 | 33 | 43 | 35 | 44 | 0.94 |
| DHHC | 40 | 26 | 19 | 25 | 21 | 27 | |
| CSHC | 47 | 30 | 24 | 32 | 23 | 29 | |
| Income | |||||||
| Less than $5K | 20 | 13 | 9 | 12 | 11 | 14 | 0.78 |
| $5K-$29.99K | 77 | 50 | 36 | 47 | 41 | 52 | |
| $30K and over | 13 | 8 | 6 | 8 | 7 | 9 | |
| Refused/DK/None | 45 | 29 | 25 | 33 | 20 | 25 | |
| Work Status | |||||||
| Full/Part time | 32 | 21 | 15 | 20 | 17 | 22 | 0.93 |
| Unemployed | 22 | 14 | 10 | 13 | 12 | 15 | |
| Retired/Home | 18 | 12 | 9 | 12 | 9 | 11 | |
| Disability | 66 | 42 | 32 | 42 | 34 | 43 | |
| Other | 17 | 11 | 10 | 13 | 7 | 9 | |
| Education Level | N | % | N | % | N | % | |
| < High school/some | 27 | 18 | 15 | 20 | 12 | 15 | 0.33 |
| High school degree | 53 | 34 | 22 | 29 | 31 | 39 | |
| Some college/AA degree | 53 | 34 | 30 | 39 | 23 | 29 | |
| College degree or > | 22 | 14 | 9 | 12 | 13 | 17 | |
*Four withdrew consent to use their data after randomization
Baseline characteristics
Baseline demographic characteristics are listed in Table 1. Of the 155 participants, the average age was 51 years old, 86% identified as female, 56% identified as black, 36% identified as “other” race, and 19% identified as white. There were no significant differences for baseline characteristics.
Common co-morbidities in the participants were hypertension (41%), obesity (37%), diabetes (23%), insomnia (26%), anxiety (28%), Post-Traumatic Stress Disorder (PTSD) (16%), and any substance use disorder (25%).
The baseline, 9 week, and 21 week measurements of the primary and secondary outcomes are listed in Tables 2–4. At baseline, the average PHQ-9 score for depressive symptoms was 12, which is characterized as moderate depression. Eighty-five percent of participants used pain medication in the last seven days at baseline (opioids: 37%, NSAIDS 48%, other medication: 43%). The average Physical Composite Score (PCS) was 34 and the average Mental Composite Score (MCS) was 36, compared to national average scores of 50. There were significant differences between the intervention and control group for patient activation measure (p = 0.051) and current opioid misuse measures (COMM) (p = 0.0495) at a significant level of 0.1, so they were adjusted for in all subsequent models.
Table 2. Baseline outcomes for all participants by intervention and control group.
| Variable (range) ^ | Intervention Baseline (N = 76) | Control Baseline (N = 79) |
|||
|---|---|---|---|---|---|
| Mean (SD) | Mean (SD) | P-value | |||
| Average pain (0–10) | 7 (1.9) | 7 (1.9) | 0.97 | ||
| BPI Interference (0–10) | 7 (2.2) | 6 (2.3) | 0.47 | ||
| BPI Severity (0–10) | 7 (1.9) | 7 (1.8) | 0.95 | ||
| PHQ (0–27) | 13 (5.6) | 11 (5.3) | 0.11 | ||
| Pain Self-Efficacy (0–60) | 30 (15.4) | 32 (13.5) | 0.47 | ||
| Patient Activation Measure (0–100) | 60 (15.6) | 56 (11.8) | 0.05* | ||
| SF-12 Physical Composite Score (0–100) | 33 (10.3) | 35 (10.6) | 0.12 | ||
| SF-12 Mental Composite Score (0–100) | 35 (9.5) | 36 (10.3) | 0.62 | ||
| Current Opioid Misuse Measure (0–64) | 11 (5.9) | 9 (5.8) | 0.05* | ||
| N | % | N | % | P-value | |
| Pain medication past 7 days | 67 | 88 | 65 | 82 | 0.30 |
| Emergency Department Use | 15 | 21 | 11 | 14 | 0.28 |
*Significant differences between the intervention and control group for PAM (p = 0.0513), COMM (p = 0.0495) at a significant level of 0.1, so they were adjusted for in all subsequent models.
^Continuous variables are summarized with mean (standard deviation)
Table 4. Outcomes for 21 weeks for all participants by intervention and control group.
| Outcomes for Specific Aim 1 (Reduction of Pain) | |||||||
| Variable (range) | Intervention Baseline (N = 68) | Control Baseline (N = 72) |
Total (N = 140) |
||||
| Mean (SD) | Mean (SD) | P-value | Mean (SD) | ||||
| Average pain (0–10) | 6 (2.0) | 6 (2.0) | 0.64 | 6 (2.0) | |||
| BPI Interference (0–10) | 6 (2.7) | 5 (2.7) | 0.046* | 5 (2.7) | |||
| BPI Severity (0–10) | 6 (2.3) | 6 (2.0) | 0.96 | 6 (2.1) | |||
| Outcome for Specific Aim 2 (Reduction of Depression) | |||||||
| PHQ (0–27) | 9 (5.4) | 10 (5.9) | 0.39 | 10 (5.7) | |||
| Outcomes for Specific Aim 3 (Increase of Pain Self-Efficacy and Reduced Use of Pain Medication) | |||||||
| Pain Self-Efficacy (0–60) | 34 (14.7) | 38 (13.5) | 0.10 | 36 (14.2) | |||
| n | % | n | % | P-value | n | % | |
| Pain medication past 7 days | 49 | 72 | 60 | 83 | 0.11 | 109 | 78 |
| Secondary Self Report Outcomes | |||||||
| Mean (SD) | Mean (SD) | P-value | Mean (SD) | ||||
| Perceived Stress Scale (0–16) | 7 (3.3) | 7 (3.4) | 0.83 | 7 (3.3) | |||
| Patient Activation Measure (0–100) | 62 (13.5) | 63 (16.2) | 0.69 | 62 (14.9) | |||
| SF-12 Physical Composite Score (0–100) | 33 (11.0) | 38 (9.7) | 0.006* | 36 (10.6) | |||
| SF-12 Mental Composite Score (0–100) | 41 (11.6) | 38 (11.3) | 0.19 | 40 (11.5) | |||
| Current Opioid Misuse Measure (0–64) | 9 (6.1) | 8 (5.2) | 0.28 | 8 (5.6) | |||
| n | % | n | % | P-value | n | % | |
| Emergency Department Use | 9 | 12 | 9 | 11 | 0.86 | 18 | 12 |
*indicates that the results are statistically significant
Table 3. Outcomes for 9 weeks for all participants by intervention and control group.
| Outcomes for Specific Aim 1 (Reduction of Pain) | |||||||
| Variable (range) | Intervention Baseline (N = 69) | Control Baseline (N = 69) |
Total (N = 138) |
||||
| Mean (SD) | Mean (SD) | P-value | Mean (SD) | ||||
| Average pain (0–10) | 6 (2.3) | 6 (2.0) | 0.61 | 6 (2.1) | |||
| BPI Interference (0–10) | 6 (2.8) | 5 (2.5) | 0.64 | 6 (2.7) | |||
| BPI Severity (0–10) | 6 (2.2) | 6 (2.2) | 0.94 | 6 (2.2) | |||
| Outcome for Specific Aim 2 (Reduction of Depression) | |||||||
| PHQ (0–27) | 11 (5.5) | 10 (5.7) | 0.15 | 10 (5.6) | |||
| Outcomes for Specific Aim 3 (Increase of Pain Self-Efficacy and Reduced Use of Pain Medication) | |||||||
| Pain Self-Efficacy (0–60) | 36 (15.7) | 34.8 (14.1) | 0.68 | 35 (14.8) | |||
| n | % | n | % | P-value | n | % | |
| Pain medication past 7 days | 53 | 77 | 54 | 78 | 0.84 | 107 | 78 |
| Secondary Self Report Outcomes | |||||||
| Mean (SD) | Mean (SD) | P-value | Mean (SD) | ||||
| Perceived Stress Scale (0–16) | 7 (3.3) | 7 (3.7) | 0.85 | 7 (3.5) | |||
| Patient Activation Measure (0–100) | 61 (13.6) | 62 (16.4) | 0.58 | 62 (15.0) | |||
| SF-12 Physical Composite Score (0–100) | 36 (10.1) | 37 (10.4) | 0.68 | 36 (10.2) | |||
| SF-12 Mental Composite Score (0–100) | 36 (9.9) | 39 (11.4) | 0.22 | 37 (10.7) | |||
| Current Opioid Misuse Measure (0–64) | 9 (6.4) | 8 (6.0) | 0.22 | 9 (6.2) | |||
| n | % | n | % | P-value | n | % | |
| Emergency Department Use | 6 | 8 | 13 | 16 | 0.13 | 19 | 13 |
In ITT analysis (Table 5), we found no clinically or statistically significant difference between two groups for average pain (RR: 1.03, 95% CI: 0.92, 1.15) or PHQ-9 score (RR: 1.09, 95% CI: 0.92, 1.28) at 9 weeks. For the primary outcomes at 21 weeks, there was no difference of average pain (RR: 0.98, 95% CI: 0.88, 1.08) and PHQ-9 score (RR: 0.89, 95% CI: 0.75, 1.06) for those in the intervention group compared with the control group. Participants who attended the intervention group, there was a 4-point reduction (baseline– 13 points / 21 weeks– 9 points) in PHQ-9 compared with the control group (baseline– 11 points / 21 weeks– 10 points). This translates into a clinically meaningful difference.
Table 5. Intention to treat results for outcome data.
| Week 9 Relative Risk (CI) |
Week 21 Relative Risk (CI) |
RR without time interaction term of 9 week and 21 weeks, if it is not significant | |
|---|---|---|---|
| Average painᵖ | 1.03 (0.92, 1.15) | 0.98 (0.88, 1.08) | 1.00 (0.92, 1.08) |
| BPI Interferenceᵖ | 1.00 (0.86, 1.16) | 1.17 (0.99,1.37) | 1.06 (0.96, 1.18) |
| BPI Severityᵖ | 1.00 (0.89, 1.13) | 1.01 (0.90, 1.14) | 1.01 (0.92, 1.10) |
| PHQ-9† | 1.09 (0.92, 1.28) | 0.89 (0.75, 1.06) | Interaction is significant |
| Pain Self Efficacy† | 1.09 (0.96, 1.25) | 0.93 (0.83, 1.05) | Interaction is significant |
| Patient Activation Measureᵖ | 0.99 (0.92, 1.07) | 1.00 (0.93, 1.08) | Interaction is significant |
| SF-12 Physical Composite Scoreᴸ | 1.01 (0.92, 1.12) | 0.86 (0.77, 0.97)* | Interaction is significant |
| SF-12 Mental Composite Scoreᴸ | 1.01 (0.95, 1.07) | 1.07 (1.01, 1.12)* | 1.02 (0.96, 1.08) |
| Current Opioid Misuse Measureᵖ | 1.14 (0.92, 1.42) | 1.13 (0.91, 1.40) | 1.20 (1.10, 1.42) |
| Pain medication in the last 7 days (Odds Ratio) |
0.75 (0.33, 1.68) | 0.42 (0.18, 0.98)* | Interaction is significant |
| ED useᵖ | 0.31 (0.10, 0.89)* | 0.85 (0.32, 2.22) | 0.72 (0.41,1.26) |
ᵖ Poisson Model was used for this outcome variable.
† Negative Binomial Model was used for this outcome variable.
ᴸ Log Normal Model was used for this outcome variable.
OR Logistic regression model was use for this outcome variable. The results are OR (95%CI).
* Results are statistically significant and 95% Confidence Intervals (CI) does not include the number 1. All models were adjusted for COMM, PAM and this table shows the “9 week” and “21 week” results. The control group as well as the baseline outcomes were set as reference groups.
For secondary outcomes, at 9 weeks there was no difference in PCS (RR: 1.01, 95% CI: 0.92, 1.12), but was lower at 21 weeks (RR = 0.86 (0.77, 0.97)). At 21 weeks, the intervention group had higher mental quality of life compared with the control group (RR: 1.07, 95% CI: 1.01, 1.12). Although not significant at 9 weeks, at 21 weeks the intervention group had a reduction in any pain medication use compared with the control (OR = 0.42 (0.18, 0.98)). We found that at 9 weeks, the intervention group had fewer ED visits (RR = 0.31 (0.10, 0.89)) (baseline intervention n = 15 reduced to n = 6) (baseline control n = 11 increased to n = 13) compared with control group, but this was not maintained at 21 weeks. There was no meaningful change in the pain severity and pain interreference between the intervention and the control group at 9 and 21 weeks.
Exploratory attendance to group visits
In terms of attendance, the minimum number of sessions attended was zero and the maximum was ten. The average number of sessions attended was 6.1 (S.D. = 2.9) and the mode is 5. Recorded reasons for no attendance included: lack of transportation, death of family or friends, work conflict, lack of child-care, weather, and doctor’s appointments. The most common reason participants missed a session was that they were “too sick or in “too much pain to come”. Participants in the intervention group who attended few (4 or less) sessions differed from those who had high attendance (Tables A and B in S1 Appendix). They were younger (mean 41 years old), more likely to be female (92%), and reported higher pain, pain severity, and depressive symptoms (PHQ-9 mean = 14.23) than those attending 5 or more groups. Participants in the control group who did not attend a primary care provider appointment during the study (n = 17) were different from the controls who did (n = 62). They were younger (42 years old compared with 54 years old) and used less pain medication than those who did see their PCP.
In the exploratory attendance multivariable regression analysis, we compared intervention participants with different attendance to the control participants who did not visit PCP. Among those who attended 5–6 sessions [RR: 0.80, 95%CI: 0.67, 0.97] or 7–10 sessions [RR: 0.87, 95%CI: 0.76, 1.00, p = 0.05] there was a reduction in average pain. Among participants who attended 5–6 sessions, there was a 28% reduction in PHQ-9 scores at 9 weeks [RR: 0.72, 95% CI: 0.54, 0.97] and a 33% reduction of PHQ-9 scores at 21 weeks [RR: 0.67, 95% CI: 0.47, 0.95] compared to control, which translated into a clinically meaningful result of 4.8 points difference. Participants who attended 7–10 sessions had a 30% reduction in PHQ-9 scores and reduced their opioid use from 42% to 28%.
Adverse events
Forty-one adverse events occurred in 13 participants in the control group and 19 participants in the intervention group. The most common adverse events were ED visits (11 in the intervention and 17 in the control group). There were two hospital admissions from both the intervention and the control group. Among the 41 adverse events, 40 were determined to be unrelated to the intervention. The one event determined to be due to the intervention was when a participant fell off a swivel chair during a group visit. This participant was not harmed.
Fidelity and evaluation data
Each group was scored with a monitoring and evaluation checklist by a research assistant for fidelity. The checklist monitored adherence to vitals, centering meditation, delivery of health topic, mind-body activity, and the review of home practice. The maximum possible fidelity score per group is 80. Across the seven cohorts the average fidelity score was 77.3.
Discussion
This RCT tested the effectiveness of a 21-week mind body self-management medical group visit in a socioeconomic diverse patient population with chronic pain and depressive symptoms and found no different in pain and depressive symptoms compared to primary care visits, with both groups experiencing improvement in symptoms. There was a significant reduction in pain medication use and increase in mental quality of life attributable to the intervention as well as a reduction in total ED visits, reproducing our previous findings of decreased ED utilization [69]. Although the primary outcome of pain and depressive symptoms were not different from a primary care visit, decreased ED use and pain medication use suggest IMGV may be helpful in patients with chronic pain and depression. The study further demonstrates that IMGVs are viable in urban outpatient clinics and CHCs (94% attended at least one IMGV, 72% attended half or more sessions.
Chronic pain places a burden on patients' lives with many patients also suffering from depression [70]. Clinical studies have shown a reduction in depressive symptoms as a secondary outcome [71–74]. In clinical trials on chronic pain and MGVs in low-income patients, our lack of significant reduction in pain is inconsistent with prior studies. For example, Geller et al. conducted a prospective cohort study of MGVs for 42 women in a low-income patient population and showed changes in bodily pain and mental health. [75,76]. Chao et al., conducted a prospective RCT of the effectiveness of a 7-week MGV or an educational booklet control condition in 45 older women with nonspecific chronic pelvic pain [77] and found a reduction in pain intensity immediately following the group sessions [78]. Our study incorporated participants with depressive symptoms and chronic pain, which increases comorbidity; therefore, this was a more difficult population to treat then in previous published studies above.
In the U.S., current treatment options for chronic pain are largely medication-based (opioids) despite mixed evidence of efficacy and increased risk of potentially dangerous side effects, including addiction and death [24,25, 79–83]. In this study, a statistically significant reduction in pain medication use occurred in the intervention compared with the control group. Other MGVS studies have found showed a reduction in pain medication use [71,84].
Chronic pain often leads to poor quality of life and frequent health care utilization [26]. The IMGV showed a significant reduction in ED visits between intervention and control groups at 9 weeks and a non-significant reduction at 21 weeks, suggesting that the active interaction with the clinician at the IMGV may offer opportunities to intervene on subacute issues prior to requiring an ED visit [69]. MGVs have consistently showed a reduction in ED visits, and suggest that the MGVs have the potential to reduce health care costs [85–90]. Literature also supports that pain education, present in this intervention, can contribute to lower healthcare utilization and may provide additional explanation for the reduction in ED visits [91]. The IMGV was helpful to patient’s mental quality of life at 21 weeks, which may be attributable to a reduction in isolation and increase in emotional support [76, 90, 92–93]. The IMGV was protective during the group visit because the participants had access to a clinician and social support.
Based on our previous attendance to clinical group visits, we did not anticipate the variety of different levels of attendance to the IMGV or to the PCP (control). Although asked to visit their PCP clinician, 17 control participants did not attend any PCP visits during the course of the study. Those in the control group who did not visit their PCP during the study were younger and more depressed with higher pain scores, consistent with trends seen elsewhere [94]. Participants who attended few MGVs were clinically different from those who had high attendance. Since low attendance participants had the highest average pain at baseline—this may have been a factor affecting their mobility and ability to attend a minimum number of groups. Not all patients are the right candidates for medical group visits, and it is important to determine who will come to a MGV and who will not [95]. To design the appropriate group intervention, it is important to recognize participants with low attendance and the factors that differentiate them from other participants in the study [96–98].
Limitations of the study
There are several limitations in this RCT that may have affected the outcomes of the study. For example, it is possible that 9 weeks of active in-person group visits was not long enough to see a significant change when comparing a routine primary care to medical group visit. In addition, at enrollment, some patients may have heightened pain and depressive symptoms, and as time went on, their scores may have regressed to the mean. We also used self-reported measurements for pain and depression, and these can change day-to-day. Another limitation is that we did not have the statistical power to conduct a multi-variable regression for reduction in opioids and NSAIDS because of our small sample size. We performed many analyses and it is possible that the results could appear significant by chance alone. We suggest that these results be taken as suggestive and hypothesis generating for future studies.
In conclusion, MGVs are one way to incorporate patient self-management, non-pharmacological techniques, pain education, and increase social connections into the health care system [99–101]. When comparing groups that attended MGVs or had a PCP visit, both showed a similar reduction in self-reported pain and depressive symptoms. However, our results suggest that IMGV is a feasible adjunct model of care for low-income diverse patients and is more effective than a PCP visit at reducing ED visits and pain medications.
Supporting information
(PDF)
(DOCX)
Abbreviations
- BMC
Boston Medical Center
- CHC
Community Health Center
- COMM
Current Opioid Misuse Measure
- CSHC
Codman Square Health Center
- DH
Dothouse Health Center
- DSMB
Data Safety Monitoring Board
- EBIM
Evidence-Based Integrative Medicine
- ECA
Embodied Conversational Agent/ Gabby
- ED
Emergency Department
- EMR
Electronic Medical Record
- MBSR
Mindfulness-Based Stress Reduction
- MGV
Medical Group Visits
- IMGV
Integrative Medical Group Visits
- IRB
Internal Review Board
- ITT
Intention to Treat
- NSAIDS
Nonsteroidal Anti-Inflammatory Drugs
- OWL
Our Whole Lives; an e-health toolkit
- PAM
Patient Activation Measure
- PCP
Primary Care Provider
- PHQ-9
Patient Health Questionnaire– 9 items
- PP
Per Protoco
- PSEQ
Pain Self-Efficacy Scale
- RA
research assistant
- RCT
Randomized Controlled Trial
Data Availability
Data cannot be shared publicly because of patient confidentiality. Data are available from the Boston Medical Center Institutional Data Access / Ethics Committee (contact via email) for researchers who meet the criteria for access to confidential data.
Funding Statement
This work was supported by Award AD - 1304-6218, Patient-Centered Outcomes Research Institute (PCORI) to PG. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Nahin RL. Estimates of Pain Prevalence and Severity in Adults: United States, 2012. J Pain. 2015; 769–780. 10.1016/j.jpain.2015.05.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ballantyne JC. Opioids for the Treatment of Chronic Pain: Mistakes Made, Lessons Learned, and Future Directions. Anesth Analg. 2017;125: 1769–1778. 10.1213/ANE.0000000000002500 [DOI] [PubMed] [Google Scholar]
- 3.Morasco BJ, Yarborough BJ, Smith NX, Dobscha SK, Deyo RA, Perrin NA, et al. Higher Prescription Opioid Dose is Associated With Worse Patient-Reported Pain Outcomes and More Health Care Utilization. J Pain Off J Am Pain Soc. 2017;18: 437–445. 10.1016/j.jpain.2016.12.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chou R, Côté P, Randhawa K, Torres P, Yu H, Nordin M, et al. The Global Spine Care Initiative: applying evidence-based guidelines on the non-invasive management of back and neck pain to low- and middle-income communities. Eur Spine J. 2018; 10.1007/s00586-017-5433-8 [DOI] [PubMed] [Google Scholar]
- 5.Qaseem A, Wilt TJ, McLean RM, Forciea MA, Clinical Guidelines Committee of the American College of Physicians. Noninvasive Treatments for Acute, Subacute, and Chronic Low Back Pain: A Clinical Practice Guideline From the American College of Physicians. Ann Intern Med. 2017;166: 514–530. 10.7326/M16-2367 [DOI] [PubMed] [Google Scholar]
- 6.Rayner L, Hotopf M, Petkova H, Matcham F, Simpson A, McCracken LM. Depression in patients with chronic pain attending a specialised pain treatment centre: prevalence and impact on health care costs. Pain. 2016;157: 1472–1479. 10.1097/j.pain.0000000000000542 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kapoor S, Thorn BE. Healthcare use and prescription of opioids in rural residents with pain. Rural Remote Health. 2014;14: 2879 [PubMed] [Google Scholar]
- 8.Burke A, Lam CN, Stussman B, Yang H. Prevalence and patterns of use of mantra, mindfulness and spiritual meditation among adults in the United States. BMC Complement Altern Med. 2017;17: 316 10.1186/s12906-017-1827-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Baker N. Using Cognitive Behavior Therapy and Mindfulness Techniques in the Management of Chronic Pain in Primary Care. Prim Care. 2016;43: 203–216. 10.1016/j.pop.2016.01.001 [DOI] [PubMed] [Google Scholar]
- 10.Wilson M, Roll J, Pritchard P, Masterson B, Howell D, Barbosa-Leiker C. Depression and pain interference among patients with chronic pain after ED encounters. J Emerg Nurs JEN Off Publ Emerg Dep Nurses Assoc. 2014;40: e55–61. 10.1016/j.jen.2013.03.011 [DOI] [PubMed] [Google Scholar]
- 11.Ernst FR, Mills JR, Berner T, House J, Herndon C. Opioid Medication Practices Observed in Chronic Pain Patients Presenting for All-Causes to Emergency Departments: Prevalence and Impact on Health Care Outcomes. J Manag Care Spec Pharm. 2015;21: 925–936. 10.18553/jmcp.2015.21.10.925 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Choi NG, Marti CN, Bruce ML, Kunik ME. Relationship between depressive symptom severity and emergency department use among low-income, depressed homebound older adults aged 50 years and older. BMC Psychiatry. 2012;12: 233 10.1186/1471-244X-12-233 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Newman AK, Van Dyke BP, Torres CA, Baxter JW, Eyer JC, Kapoor S, Thorn BE. The relationship of sociomographic and psychological variables with chronic pain variables in a low-income population. Pain. 2017. September; 158(9):1687–1696. 10.1097/j.pain.0000000000000964 [DOI] [PubMed] [Google Scholar]
- 14.Orhan C, Van Looveren E, Cagnie B, Mukhtar NB, Lenoir D, Meeus M. Are Pain Beliefs, Cognitions, and Behaviors Influenced by Race, Ethnicity, and Culture in Patients with Chronic Musculoskeletal Pain: A Systematic Review. Pain Physician. 2018. November;21(6):541–558. [PubMed] [Google Scholar]
- 15.Landefeld JC, Miaskowski C, Tieu L, Ponath C, Lee CT, Guzman D, et al. Characteristics and Factors Associated With Pain in Older Homeless Individuals: Results From the Health Outcomes in People Experiencing Homelessness in Older Middle Age (HOPE HOME) Study. J Pain Off J Am Pain Soc. 2017;18: 1036–1045. 10.1016/j.jpain.2017.03.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Naushad N, Dunn LB, Muñoz RF, Leykin Y. Depression increases subjective stigma of chronic pain. J Affect Disord. 2018;229: 456–462. 10.1016/j.jad.2017.12.085 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Darnall BD, Scheman J, Davin S, Burns JW, Murphy JL, Wilson AC, et al. Pain Psychology: A Global Needs Assessment and National Call to Action. Pain Med Malden Mass. 2016;17: 250–263. 10.1093/pm/pnv095 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Escoto KH, Milbury K, Nguyen N, Cho D, Roberson C, Wetter D, McNeill LH. Use of Complementary Health Practices in a Church-Based African American Cohort. J Altern Complement Med. 2018. 10.1016/j.jpain.2017.03.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Johnson CC, Sheffield KM, Brown RE. Mind-Body Therapies for African-American Women at Risk for Cardiometabolic Disease: A Systematic Review. Evid Based Complement Alternat Med. 2018. February 26;2018:5123217 10.1155/2018/5123217 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cheng T, D'Amico S, Luo M, Lestoquoy AS, Yinusa-Nyahkoon L, Laird LD, Gardiner PM. Health Disparities in Access to Nonpharmacologic Therapies in an Urban Community. J Altern Complement Med. 2019. January;25(1):48–60 10.1089/acm.2018.0217 [DOI] [PubMed] [Google Scholar]
- 21.Szanton SL, Wenzel J, Connolly AB, Piferi RL. Examining mindfulness-based stress reduction: perceptions from minority older adults residing in a low-income housing facility. BMC Complement Altern Med. 2011. May 31;11:44 10.1186/1472-6882-11-44 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kligler B, Buonora M, Gabison J, Jacobs E, Karasz A, McKee MD. “I Felt Like It Was God’s Hands Putting the Needles In”: A Qualitative Analysis of the Experience of Acupuncture for Chronic Pain in a Low-Income, Ethnically Diverse, and Medically Underserved Patient Population. J Altern Complement Med N Y N. 2015;21: 713–719. 10.1089/acm.2014.0376 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Giannitrapani KF, Ahluwalia SC, McCaa M, Pisciotta M, Dobscha S, Lorenz KA. Barriers to Using Nonpharmacologic Approaches and Reducing Opioid Use in Primary Care. Pain Med Malden Mass. 2017; 10.1093/pm/pnx220 [DOI] [PubMed] [Google Scholar]
- 24.Alford DP. Chronic back pain with possible prescription opioid misuse. JAMA. 2013;309: 919–925. 10.1001/jama.2013.522 [DOI] [PubMed] [Google Scholar]
- 25.Chou R, Turner JA, Devine EB, Hansen RN, Sullivan SD, Blazina I, et al. The effectiveness and risks of long-term opioid therapy for chronic pain: a systematic review for a National Institutes of Health Pathways to Prevention Workshop. Ann Intern Med. 2015;162: 276–286. 10.7326/M14-2559 [DOI] [PubMed] [Google Scholar]
- 26.Bonakdar RA. Integrative Pain Management. Med Clin North Am. 2017;101: 987–1004. 10.1016/j.mcna.2017.04.012 [DOI] [PubMed] [Google Scholar]
- 27.Chou R, Deyo R, Friedly J, Skelly A, Hashimoto R, Weimer M, Fu R, Dana T, Kraegel P, Griffin J, Grusing S, Brodt E. Noninvasive Treatments for Low Back Pain. Comparative Effectiveness Review No. 169. (Prepared by the Pacific Northwest Evidence-based Practice Center under Contract No. 290-2012-00014-I.) AHRQ Publication No. 16-EHC004-EF. Rockville, MD: Agency for Healthcare Research and Quality; February 2016. [PubMed]
- 28.Morone NE, Greco CM, Moore CG, Rollman BL, Lane B, Morrow LA, et al. A Mind-Body Program for Older Adults With Chronic Low Back Pain: A Randomized Clinical Trial. JAMA Intern Med. 2016;176: 329–337. 10.1001/jamainternmed.2015.8033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Majeed MH, Ali AA, Sudak DM. Mindfulness-based interventions for chronic pain: Evidence and applications. Asian J Psychiatr. 2018. February;32:79–83. 10.1016/j.ajp.2017.11.025 [DOI] [PubMed] [Google Scholar]
- 30.Skelly AC, Chou R, Dettori JR, Turner JA, Friedly JL, Rundell SD, Fu R, Brodt ED, Wasson N, Winter C, Ferguson AJR. Noninvasive Nonpharmacological Treatment for Chronic Pain: A Systematic Review [Internet]. Rockville (MD): Agency for Healthcare Research and Quality (US); 2018. June. [PubMed] [Google Scholar]
- 31.Anheyer D, Haller H, Barth J, Lauche R, Dobos G, Cramer H. Mindfulness-Based Stress Reduction for Treating Low Back Pain: A Systematic Review and Meta-analysis. Ann Intern Med. 2017;166: 799–807. 10.7326/M16-1997 [DOI] [PubMed] [Google Scholar]
- 32.Dowell D, Haegerich TM, Chou R. CDC guideline for prescribing opioids for chronic pain—United States, 2016. JAMA. 2016;315: 1624–1645. 10.1001/jama.2016.1464 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Gardiner P, Dresner D, Barnett KG, Sadikova E, Saper R. Medical group visits: a feasibility study to manage patients with chronic pain in an underserved urban clinic. Glob Adv Health Med. 2014;3: 20–26. 10.7453/gahmj.2014.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Gardiner P, Lestoquoy AS, Gergen-Barnett K, Penti B, White LF, Saper R, et al. Design of the integrative medical group visits randomized control trial for underserved patients with chronic pain and depression. Contemp Clin Trials. 2017;54: 25–35. 10.1016/j.cct.2016.12.013 [DOI] [PubMed] [Google Scholar]
- 35.Chen L, Crockett AH, Covington-Kolb S, Heberlein E, Zhang L, Sun X. Centering and Racial Disparities (CRADLE study): rationale and design of a randomized controlled trial of centering pregnancy and birth outcomes. BMC Pregnancy Childbirth. 2017. April 13;17(1):118 10.1186/s12884-017-1295-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kahkoska AR, Brazeau NF, Lynch KA, Kirkman MS, Largay J, Young LA, Buse JB. Implementation and Evaluation of Shared Medical Appointments for Type 2 Diabetes at a Free, Student-Run Clinic in Alamance County, North Carolina. J Med Educ Train. 2018;2(1). pii: 032 [PMC free article] [PubMed] [Google Scholar]
- 37.Srivastava G, Palmer KD, Ireland KA, McCarthy AC, Donovan KE, Manders AJ, McDougal J, Lenders CM, Apovian CM. Shape-Up and Eat Right Families Pilot Program: Feasibility of a Weight Management Shared Medical Appointment Model in African-Americans With Obesity at an Urban Academic Medical Center. Front Pediatr. 2018. April 12;6:101 10.3389/fped.2018.00101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Vaughan EM, Johnston CA, Arlinghaus KR, Hyman DJ, Foreyt JP. A Narrative Review of Diabetes Group Visits in Low-Income and Underserved Settings. Curr Diabetes Rev. 2018. November 12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Schneeberger D, Golubíc M, Moore HCF, Weiss K, Abraham J, Montero A, Doyle J, Sumego M, Roizen M. Focused Shared Medical Appointments to Improve Risk Factors for Chronic Diseases and Quality of Life in Breast Cancer Survivors. Lifestyle Medicine. [DOI] [PubMed] [Google Scholar]
- 40.Kirsh SR, Aron DC, Johnson KD, Santurri LE, Stevenson LD, Jones KR, et al. A realist review of shared medical appointments: How, for whom, and under what circumstances do they work? BMC Health Serv Res. 2017;17: 113-017–2064-z. 10.1186/s12913-017-2064-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Gardiner P, Crooks D, Johnson G, McCue K, Laird L, Haas N, Mitchell S. Qualitative Evaluation of an Integrative Medicine Group Visits Model of Care for Patients with Chronic Pain and Depression. Glob Adv Health Med. 2015. November 1;4(6): 65–72. 10.7453/gahmj.2015.125 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lestoquoy AS, Laird LD, Mitchell S, Gergen-Barnett K, Negash NL, McCue K, et al. Living with chronic pain: Evaluating patient experiences with a medical group visit focused on mindfulness and non-pharmacological strategies. Complement Ther Med. 2017;35: 33–38. 10.1016/j.ctim.2017.09.002 [DOI] [PubMed] [Google Scholar]
- 43.Cornelio-Flores O, Lestoquoy AS, Abdallah S, DeLoureiro A, Lorente K, Pardo B, et al. The Latino Integrative Medical Group Visit as a Model for Pain Reduction in Underserved Spanish Speakers. J Altern Complement Med N Y N. 2018;24: 125–131. 10.1089/acm.2017.0132 [DOI] [PubMed] [Google Scholar]
- 44.Wong SY-S, Chan FW-K, Wong RL-P, Chu M-C, Kitty Lam Y-Y, Mercer SW, et al. Comparing the effectiveness of mindfulness-based stress reduction and multidisciplinary intervention programs for chronic pain: a randomized comparative trial. Clin J Pain. 2011;27: 724–734. 10.1097/AJP.0b013e3182183c6e [DOI] [PubMed] [Google Scholar]
- 45.Mccue K, Shamekhi A, Crooks D, Bickmore T, Gergen-Barnett K, Johnson G, et al. A Feasibility Study to introduce an Embodied Conversational Agent (ECA) on a tablet computer into a group medical visit. American Public Health Association (APHA), Chicago, IL: APHA; 2015. [Google Scholar]
- 46.The PHQ-9: A New Depression Diagnostic and Severity Measure [Internet]. [cited 3 Apr 2018]. Available: https://www.healio.com/psychiatry/journals/psycann/2002-9-32-9/%7Bb9ab8f2c-53ce-4f76-b88e-2d5a70822f69%7D/the-phq-9-a-new-depression-diagnostic-and-severity-measure
- 47.Huang FY, Chung H, Kroenke K, Delucchi KL, Spitzer RL. Using the patient health questionnaire‐9 to measure depression among racially and ethnically diverse primary care patients. J Gen Intern Med. 2006;21: 547–552. 10.1111/j.1525-1497.2006.00409.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Gardiner PM, McCue KD, Negash LM, Cheng T, White LF, Yinusa-Nyahkoon L, et al. Engaging women with an embodied conversational agent to deliver mindfulness and lifestyle recommendations: A feasibility randomized control trial. Patient Educ Couns. 2017;100: 1720–1729. 10.1016/j.pec.2017.04.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Lasser KE, Shanahan C, Parker V, Beers D, Xuan Z, Heymann O, et al. A Multicomponent Intervention to Improve Primary Care Provider Adherence to Chronic Opioid Therapy Guidelines and Reduce Opioid Misuse: A Cluster Randomized Controlled Trial Protocol. J Subst Abuse Treat. 2016;60: 101–109. 10.1016/j.jsat.2015.06.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Cleeland CS, Ryan KM. Pain assessment: global use of the Brief Pain Inventory. Ann Acad Med Singapore. 1994;23: 129–138. [PubMed] [Google Scholar]
- 51.Sobell LC, Sobell MB. Timeline Follow-Back Measuring Alcohol Consumption. Humana Press, Totowa, NJ; 1992. pp. 41–72. 10.1007/978-1-4612-0357-5_3 [DOI] [Google Scholar]
- 52.Hibbard JH, Stockard J, Mahoney ER, Tusler M. Development of the Patient Activation Measure (PAM): conceptualizing and measuring activation in patients and consumers. Health Serv Res. 2004;39: 1005–1026. 10.1111/j.1475-6773.2004.00269.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Cohen S, Kamarck T, Mermelstein R. A Global Measure of Perceived Stress. J Health Soc Behav. 1983;24: 385–396. 10.2307/2136404 [DOI] [PubMed] [Google Scholar]
- 54.Brazier JE, Harper R, Jones NM, O’Cathain A, Thomas KJ, Usherwood T, et al. Validating the SF-36 health survey questionnaire: new outcome measure for primary care. BMJ. 1992;305: 160–164. 10.1136/bmj.305.6846.160 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Hibbard JH, Mahoney ER, Stockard J, Tusler M. Development and testing of a short form of the patient activation measure. Health Serv Res. 2005;40: 1918–1930. 10.1111/j.1475-6773.2005.00438.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Buysse DJ, Reynolds CF, Monk TH, Berman SR, Kupfer DJ. The Pittsburgh Sleep Quality Index: a new instrument for psychiatric practice and research. Psychiatry Res. 1989;28: 193–213. 10.1016/0165-1781(89)90047-4 [DOI] [PubMed] [Google Scholar]
- 57.Broadhead W, Gehlbach SH, De Gruy FV, Kaplan BH. The Duke-UNC Functional Social Support Questionnaire: Measurement of social support in family medicine patients. Med Care. 1988; 709–723. 10.1097/00005650-198807000-00006 [DOI] [PubMed] [Google Scholar]
- 58.Butler SF, Budman SH, Fanciullo GJ, Jamison RN. Cross validation of the current opioid misuse measure to monitor chronic pain patients on opioid therapy. Clin J Pain. 2010;26: 770–776. 10.1097/AJP.0b013e3181f195ba [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Centers for Disease Control and Prevention. National Health and Nutrition Examination Survey 2013–2014 [Internet]. 2014. Available: http://wwwn.cdc.gov/nchs/nhanes/search/nhanes13_14.aspx
- 60.Suzuki J, Zinser J, Klaiber B, Hannon M, Grassi H, Spinosa M, et al. Feasibility of Implementing Shared Medical Appointments (SMAs) for Office-Based Opioid Treatment With Buprenorphine: A Pilot Study. Subst Abuse. 2015;36: 166–169. 10.1080/08897077.2014.998400 [DOI] [PubMed] [Google Scholar]
- 61.Nicholas MK. The pain self‐efficacy questionnaire: Taking pain into account. Eur J Pain. 2007;11: 153–163. 10.1016/j.ejpain.2005.12.008 [DOI] [PubMed] [Google Scholar]
- 62.Fals-Stewart W, O’Farrell TJ, Freitas TT, McFarlin SK, Rutigliano P. The timeline followback reports of psychoactive substance use by drug-abusing patients: psychometric properties. J Consult Clin Psychol. 2000;68: 134–144. 10.1037//0022-006x.68.1.134 [DOI] [PubMed] [Google Scholar]
- 63.Cheak-Zamora NC, Wyrwich KW, McBride TD. Reliability and validity of the SF-12v2 in the medical expenditure panel survey. Qual Life Res Int J Qual Life Asp Treat Care Rehabil. 2009;18: 727–735. 10.1007/s11136-009-9483-1 [DOI] [PubMed] [Google Scholar]
- 64.Butler SF, Budman SH, Fernandez KC, Houle B, Benoit C, Katz N, et al. Development and validation of the Current Opioid Misuse Measure. Pain. 2007;130: 144–156. 10.1016/j.pain.2007.01.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.McMillan D, Gilbody S, Richards D. Defining successful treatment outcome in depression using the PHQ-9: a comparison of methods. J Affect Disord. 2010;127: 122–129. 10.1016/j.jad.2010.04.030 [DOI] [PubMed] [Google Scholar]
- 66.Furukawa TA. Assessment of mood: guides for clinicians. J Psychosom Res. 2010;68: 581–589. 10.1016/j.jpsychores.2009.05.003 [DOI] [PubMed] [Google Scholar]
- 67.Grotle M BJ, Vollestad NK. Concurrent comparison of responsiveness in pain and functional status measurements used for patients with low back pain. Spine Phila Pa 1976. 2004;29(21): E492–501. 10.1097/01.brs.0000143664.02702.0b [DOI] [PubMed] [Google Scholar]
- 68.SAS Institute, Cary, NC.
- 69.Gardiner P, Dresner D, Barnett KG, Sadikova E, Saper R. Medical group visits: a feasibility study to manage patients with chronic pain in an underserved urban clinic. Glob Adv Health Med. 2014. July;3(4):20–6. 10.7453/gahmj.2014.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Von Korff M, Crane P, Lane M, Miglioretti DL, Simon G, Saunders K, et al. Chronic spinal pain and physical-mental comorbidity in the United States: results from the national comorbidity survey replication. Pain. 2005;113: 331–339. 10.1016/j.pain.2004.11.010 [DOI] [PubMed] [Google Scholar]
- 71.Mehl-Madrona L, Mainguy B, Plummer J. Integration of Complementary and Alternative Medicine Therapies into Primary-Care Pain Management for Opiate Reduction in a Rural Setting. J Altern Complement Med. 2016;22: 621–626. 10.1089/acm.2015.0212 [DOI] [PubMed] [Google Scholar]
- 72.Taveira TH, Dooley AG, Cohen LB, Khatana SA, Wu WC. Pharmacist-led group medical appointments for the management of type 2 diabetes with comorbid depression in older adults. Ann Pharmacother. 2011;45: 1346–1355. 10.1345/aph.1Q212 [DOI] [PubMed] [Google Scholar]
- 73.Bohnert AS, Valenstein M, Bair MJ, Ganoczy D, McCarthy JF, Ilgen MA, et al. Association between opioid prescribing patterns and opioid overdose-related deaths. Jama. 2011;305: 1315–1321. 10.1001/jama.2011.370 [DOI] [PubMed] [Google Scholar]
- 74.Suzuki J, Zinser J, Klaiber B, Hannon M, Grassi H, Spinosa M, et al. Feasibility of Implementing Shared Medical Appointments (SMAs) for Office-Based Opioid Treatment With Buprenorphine: A Pilot Study. Subst Abuse. 2015;36: 166–169. 10.1080/08897077.2014.998400 [DOI] [PubMed] [Google Scholar]
- 75.Geller J, Dube E, and Kowaleski J. Establishing and Maintaining Successful Chronic Pain Group Medical Visits Using an Empowerment Model. [Internet]. 2010. Available: http://glfhc.org/images/final%20pain%20manual%202011.pdf [Google Scholar]
- 76.Geller JS, Orkaby A, Cleghorn GD. Impact of a group medical visit program on Latino health-related quality of life. EXPLORE J Sci Heal. 2011;7: 94–99. [DOI] [PubMed] [Google Scholar]
- 77.Chao MT, Abercrombie PD, Santana T, Duncan LG. Applying the RE-AIM framework to evaluate integrative medicine group visits among diverse women with chronic pelvic pain. Pain Manag Nurs. 2015;16: 920–929. 10.1016/j.pmn.2015.07.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Chao MT, Abercrombie PD, Duncan LG. Centering as a model for group visits among women with chronic pelvic pain. J Obstet Gynecol Neonatal Nurs. 2012;41: 703–710. 10.1111/j.1552-6909.2012.01406.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Turk DC, Wilson HD, Cahana A. Treatment of chronic non-cancer pain. The Lancet. 2011;377: 2226–2235. [DOI] [PubMed] [Google Scholar]
- 80.Ray WA, Chung CP, Murray KT, Hall K, Stein CM. Prescription of long-acting opioids and mortality in patients with chronic noncancer pain. JAMA. 2016;315: 2415–2423. 10.1001/jama.2016.7789 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Bohnert AS, Valenstein M, Bair MJ, Ganoczy D, McCarthy JF, Ilgen MA, et al. Association between opioid prescribing patterns and opioid overdose-related deaths. Jama. 2011;305: 1315–1321. 10.1001/jama.2011.370 [DOI] [PubMed] [Google Scholar]
- 82.Kennedy LC, Binswanger IA, Mueller SR, Levy C, Matlock DD, Calcaterra SL, et al. “Those Conversations in My Experience Don’t Go Well”: A Qualitative Study of Primary Care Provider Experiences Tapering Long-term Opioid Medications. Pain Med Malden Mass. 2017; 10.1093/pm/pnx276 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Institute of Medicine (US) Committee on Advancing PR. Relieving pain in America: a blueprint for transforming prevention, care, education, and research. Washington, D.C.: Washington, D.C.: National Academies Press; 2011. [PubMed] [Google Scholar]
- 84.Romanelli RJ, Dolginsky M, Byakina Y, Bronstein D, Wilson S. A Shared Medical Appointment on the Benefits and Risks of Opioids Is Associated With Improved Patient Confidence in Managing Chronic Pain. J Patient Exp. 2017;4: 144–151. 10.1177/2374373517706837 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Shaw S, Dresner D, Gardiner P, Barnett KG, Saper R. Integrative Medicine Group Visits and Emergency Department Utilization. J Altern Complement Med. 2014;20: A67–A68. 10.1089/acm.2014.5176.abstract [DOI] [Google Scholar]
- 86.Jaber R, Braksmajer A, Trilling JS. Group visits: a qualitative review of current research. J Am Board Fam Med JABFM. 2006;19: 276–290. 10.3122/jabfm.19.3.276 [DOI] [PubMed] [Google Scholar]
- 87.Smith CE, Piamjariyakul U, Wick JA, Spertus JA, Russell C, Dalton KM, et al. Multidisciplinary group clinic appointments: the Self-Management and Care of Heart Failure (SMAC-HF) trial. Circ Fail. 2014;7: 888–894. 10.1161/CIRCHEARTFAILURE.113.001246 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Levine MD, Ross TR, Balderson BH, Phelan EA. Implementing group medical visits for older adults at group health cooperative. J Am Geriatr Soc. 2010;58: 168–172. 10.1111/j.1532-5415.2009.02628.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Coleman EA, Eilertsen TB, Kramer AM, Magid DJ, Beck A, Conner D. Reducing emergency visits in older adults with chronic illness. A randomized, controlled trial of group visits. Eff Clin Pract ECP. 2001;4: 49–57. [PubMed] [Google Scholar]
- 90.Scott JC, Conner DA, Venohr I, Gade G, Mckenzie M, Kramer AM, et al. Effectiveness of a Group Outpatient Visit Model for Chronically Ill Older Health Maintenance Organization Members: A 2‐Year Randomized Trial of the Cooperative Health Care Clinic. J Am Geriatr Soc. 2004;52: 1463–1470. 10.1111/j.1532-5415.2004.52408.x [DOI] [PubMed] [Google Scholar]
- 91.Louw A, Zimney K, Puentedura EJ, Diener I. The efficacy of pain neuroscience education on musculoskeletal pain: a systematic review of the literature. Physiother Theory Pract. 2016;32(5): 332–355. 10.1080/09593985.2016.1194646 [DOI] [PubMed] [Google Scholar]
- 92.Kanter G, Komesu YM, Qaedan F, Jeppson PC, Dunivan GC, Cichowski SB, et al. Mindfulness-based stress reduction as a novel treatment for interstitial cystitis/bladder pain syndrome: a randomized controlled trial. Int Urogynecology J. 2016;27: 1705–1711. 10.1007/s00192-016-3022-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Seesing FM, Drost G, van der W, van Engelen B GM. Effects of shared medical appointments on quality of life and cost-effectiveness for patients with a chronic neuromuscular disease. Study protocol of a randomized controlled trial. BMC Neurol. 2011;11: 106–106. 10.1186/1471-2377-11-106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Nguyen DL, Dejesus RS, Wieland ML. Missed appointments in resident continuity clinic: patient characteristics and health care outcomes. J Grad Med Educ. 2011;3: 350–355. 10.4300/JGME-D-10-00199.1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Kirsh S, Watts S, Pascuzzi K, O’Day ME, Davidson D, Strauss G, et al. Shared medical appointments based on the chronic care model: a quality improvement project to address the challenges of patients with diabetes with high cardiovascular risk. Qual Saf Health Care. 2007;16: 349–353. 10.1136/qshc.2006.019158 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Edelman D, McDuffie J, Oddone E, Gierisch J, Nagi A, William JJ. Shared Medical Appointments for Chronic Medical Conditions: A Systematic Review [Internet]. Wash DC Dep Veterans Aff US. 2012 [PubMed] [Google Scholar]
- 97.Wright HR, Diamond JP. Service innovation in glaucoma management: using a Web-based electronic patient record to facilitate virtual specialist supervision of a shared care glaucoma programme. Br J Ophthalmol. 2015;99: 313–317. 10.1136/bjophthalmol-2014-305588 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Raymond JK. Models of Care for Adolescents and Young Adults with Type 1 Diabetes in Transition: Shared Medical Appointments and Telemedicine. Pediatr Ann. 2017;46: e193–e197. 10.3928/19382359-20170425-01 [DOI] [PubMed] [Google Scholar]
- 99.Gaynor CH, Vincent C, Safranek S, Illige M. FPIN’s clinical inquiries. Group medical visits for the management of chronic pain. Am Fam Physician. 2007;76(11):1704–1705. [PubMed] [Google Scholar]
- 100.Clancy DE, Brown SB, Magruder KM, Huang P. Group visits in medically and economically disadvantaged patients with type 2 diabetes and their relationships to clinical outcomes. Top Health Inf Manage. 2003;24(1):8–14. [PubMed] [Google Scholar]
- 101.Miller D, Zantop V, Hammer H, Faust S, Grumbach K. Group medical visits for low-income women with chronic disease: a feasibility study. J Womens Health 2002. 2004;13(2):217–225. 10.1089/154099904322966209 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(PDF)
(DOCX)
Data Availability Statement
Data cannot be shared publicly because of patient confidentiality. Data are available from the Boston Medical Center Institutional Data Access / Ethics Committee (contact via email) for researchers who meet the criteria for access to confidential data.