ABSTRACT
Cytosolic dynamin-related protein 1 (Drp1, also known as DNM1L) is required for both mitochondrial and peroxisomal fission. Drp1-dependent division of these organelles is facilitated by a number of adaptor proteins at mitochondrial and peroxisomal surfaces. To investigate the interplay of these adaptor proteins, we used gene-editing technology to create a suite of cell lines lacking the adaptors MiD49 (also known as MIEF2), MiD51 (also known as MIEF1), Mff and Fis1. Increased mitochondrial connectivity was observed following loss of individual adaptors, and this was further enhanced following the combined loss of MiD51 and Mff. Moreover, loss of adaptors also conferred increased resistance of cells to intrinsic apoptotic stimuli, with MiD49 and MiD51 showing the more prominent role. Using a proximity-based biotin labeling approach, we found close associations between MiD51, Mff and Drp1, but not Fis1. Furthermore, we found that MiD51 can suppress Mff-dependent enhancement of Drp1 GTPase activity. Our data indicates that Mff and MiD51 regulate Drp1 in specific ways to promote mitochondrial fission.
KEY WORDS: Mitochondria, Fission, Dynamin-related protein 1, GTPase, Apoptosis
Highlighted Article: MiD51 and Mff coordinate and regulate the action of Drp1 at the mitochondrial outer membrane during homeostatic mitochondrial fission, as assessed with gene-editing technology.
INTRODUCTION
Mitochondria are highly dynamic organelles whose movement and shape are governed by cytoskeletal interactions along with balanced fission and fusion events (Labbé et al., 2014; Lackner, 2014; Mishra and Chan, 2014; Richter et al., 2015; Roy et al., 2015). Mitochondrial fission and fusion is tuned to the bioenergetic state of the cell (Toyama et al., 2016; Wai et al., 2015), and is also important for quality control and mitochondrial inheritance (Ban-Ishihara et al., 2013; Lackner, 2013). Defects in fission and fusion have been linked with neurodegenerative diseases such as Alzheimer’s, Huntington’s and Parkinson’s diseases (Chen and Chan, 2009). Mitochondrial fission is mediated by dynamin-related protein 1 (Drp1, also known as DNM1L), a large cytosolic GTPase that is recruited to the surface of the organelle by the adaptors Mff, MiD49 (also known as MIEF2) and MiD51 (also known as MIEF1) (Gandre-Babbe and van der Bliek, 2008; Ingerman et al., 2005; Legesse-Miller et al., 2003; Otera et al., 2010; Palmer et al., 2011; Zhao et al., 2011). Whereas MiD49 and MiD51 are exclusively found on the mitochondrial outer membrane, Mff is additionally located on peroxisomes where it can also recruit Drp1 for fission (Koch et al., 2003; Otera et al., 2010; Palmer et al., 2013). Fis1, originally thought to be a Drp1 adaptor in both mammals and yeast (James et al., 2003; Mozdy et al., 2000; Stojanovski et al., 2004), has more recently been proposed to function in mitophagy (Shen et al., 2014; Yamano et al., 2014). However, the role of Fis1 in homeostatic fission is still controversial.
In addition to Drp1 and adaptors, mitochondrial fission also involves the endoplasmic reticulum (ER) and actin, which aid in the formation of constriction sites for Drp1 assembly with Mff (Friedman et al., 2011) and MiD proteins (Elgass et al., 2015). To date, it has not been clear whether these adaptors interact and/or perform separable roles in Drp1-mediated mitochondrial fission. Mitochondria also house and release pro-apoptotic molecules such as cytochrome c and, as such, are central to the regulation of the intrinsic cell death pathway (Martinou and Youle, 2011; Youle and Karbowski, 2005; Youle and Strasser, 2008). Whereas Drp1-dependent mitochondrial fragmentation is known to precede caspase activation, mitochondrial outer membrane permeabilization and cytochrome c release, it is not clear whether the adaptors used are the same as those employed for homeostatic fission (Youle and Karbowski, 2005).
Here, we report on the generation of a suite of Drp1-adaptor-knockout mouse embryonic fibroblasts (MEFs) and a Drp1-knockout MEF cell line to investigate the role of each adaptor protein and to further understand how these proteins interact and contribute to Drp1-mediated fission. Our findings indicate that loss of both MiD49 and MiD51, and MiD49 and MiD51 together with Mff confer resistance to cell death, with etoposide-treated cells retaining more cytochrome c in mitochondria than MEFs lacking only a single adaptor protein. In addition, proximity-based labeling techniques revealed that MiD51 and Mff are contiguous on the mitochondrial surface, suggesting that they interact at sites of Drp1-dependent mitochondrial fission. We also provide evidence that MiD51 and Mff can act concurrently in Drp1-mediated fission processes by regulating the rate of Drp1 GTP hydrolysis in opposing directions.
RESULTS
Characterization of gene-edited Drp1 adaptor MEFs
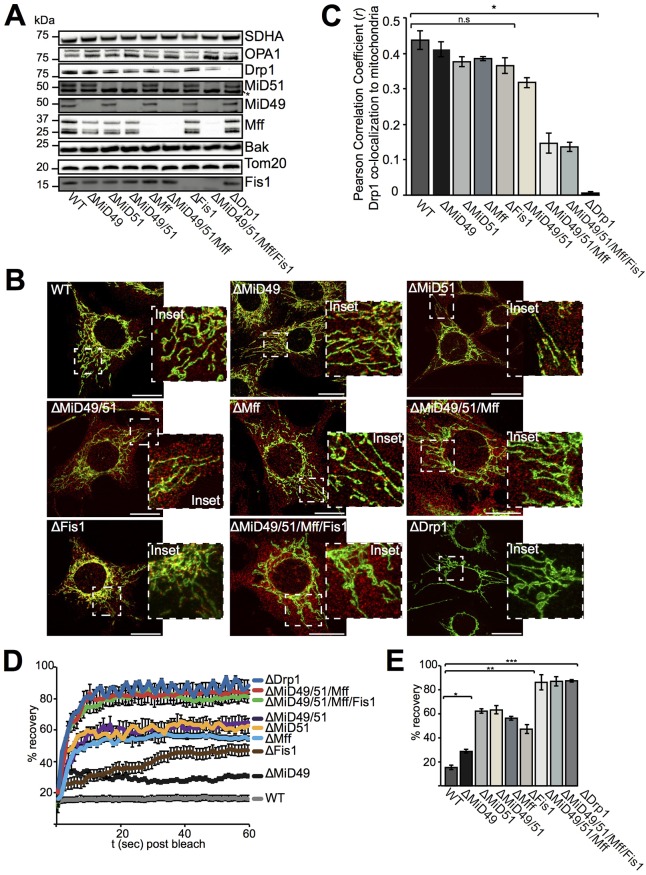
To understand the role played by adaptor proteins in Drp1 recruitment and subsequent mitochondrial fission, we generated a suite of single (ΔMiD49, ΔMiD51, ΔMff, ΔFis1 and ΔDrp1), double (ΔMiD49/MiD51), triple (ΔMiD49/MiD51/Fis1) and quadruple (ΔMiD49/MiD51/Mff/Fis1) MEF knockouts using gene-editing approaches. Genomic insertions and deletions (indels) resulting from gene-editing clearly showed disruptions to MiD51, Mff and Fis1 in cell lines (Fig. S1A–G). When observing the steady-state levels of morphology proteins in the absence of Drp1 adaptors, it appeared that loss of one or multiple adaptors did not affect the levels of the other adaptors still present (Fig. 1A). Although individual deletion of MiD49, MiD51, Mff or Fis1 did not appear to drastically change the mitochondrial morphology, or the extent to which Drp1 localizes to mitochondria, deletion of multiple adaptors increased mitochondrial connectivity and reduced Drp1 association with the mitochondrial outer membrane (Fig. 1B,C). [Residual Drp1 signal present on mitochondria lacking all adaptors (ΔMiD49/51/Mff/Fis1) might be due to non-specific signals or Drp1 involvement in ER–mitochondria contact-site formation or F-actin contacts at mitochondria.] Mitochondrial morphology and Drp1 correlation at mitochondria was not significantly different between ΔMiD49/51/Mff MEFs and ΔMiD49/51/Mff/Fis1 MEFs, supporting previous findings showing that Fis1 does not contribute significantly to mitochondrial fission in normal cell homeostasis (Otera et al., 2010).
Fig. 1.
Characterization of gene-edited MEF cell lines. (A) Gene-edited cell lines were analyzed for steady-state protein levels using antibodies as indicated. The asterisk denotes a non-specific band. (B) Gene-edited MEFs stained with anti-Tom20 (green) and anti-Drp1 (red) antibodies and imaged with confocal microscopy. The insets show a magnified view of the boxed region. Scale bars: 20 μm. (C) Pearson correlation (r) analysis showing the linear correlation where Drp1 colocalizes with Tom20-stained mitochondria. Data are mean±s.e.m. n=15. (D) FRAP analysis. For each indicated cell line, the percentage recovery was measured over 60 s following photobleaching. (E) Endpoint analysis of FRAP. Fluorescence recovery at 60 s postbleach from E. Data in D and E are mean±s.e.m., n=5. *P<0.05, **P<0.005, ***P<0.0005; n.s, non-significant (Student's t-test).
In order to study the connectivity of mitochondrial networks in the gene-edited MEFs, fluorescence recovery after photobleaching (FRAP) was employed using a matrix-targeted GFP that is fully diffusible. As can be seen (Fig. 1D,E), mitochondrial connectivity was most enhanced in ΔMiD49/51/Mff MEFs and ΔMiD49/51/Mff/Fis1 MEFs, reaching similar levels to that seen for ΔDrp1 MEFs. As well as affecting mitochondrial connectivity, deletion of Mff caused elongation of peroxisomes (Fig. 2A) as reported previously (Gandre-Babbe and van der Bliek, 2008; Otera et al., 2010). Additional deletion of MiD49, MiD51 and Fis1 did not further increase peroxisome length, suggesting that, of the known adaptor proteins, Mff, contributes most to peroxisome division (Fig. 2B; Fig. S2A). Loss of adaptor proteins did not seem to affect ER–mitochondrial contact sites, with mitochondrial constriction sites and ER tubules observed at similar levels in WT, ΔMff, ΔMiD49/51, ΔMiD49/51/Mff, ΔMiD49/51/Mff/Fis1 and ΔDrp1 MEFs (Fig. S2B,C). In addition, F-actin morphology did not seem to be noticeably affected in ΔMiD49/51/Mff or ΔMiD49/51/Mff/Fis1 MEFs (Fig. S2D).
Fig. 2.
Peroxosimal defects and rescue of knockout MEFs. (A) WT, ΔMff and ΔMiD49/51/Mff/Fis1 MEFs were stained with Hoechst 33258 (blue) and immunostained with antibodies against Pex14 (green) and cytochrome c (red) and imaged using confocal microscopy. Scale bars: 20 μm. (B) Peroxisome length was quantified from images in A and Fig. S2A and expressed as a fold change (mean±s.e.m.; n≥30,) over WT. *P<0.05 (Student's t-test). (C) ΔMiD49/51/Mff/Fis1 MEFs were transfected with either MiD49–GFP, MiD51–GFP, GFP–Fis1 or GFP–Mff. Cells were stained with Hoechst 33258 (blue) and immunostained for Tom20 (top panels) or cytochrome c (bottom panel) (red) and either Drp1 or Pex14 (gray) as indicated and imaged with confocal microscopy. Scale bars: 20 μm. Insets show a magnified view of the boxed region; in C, line-scans (15 µm) from the position indicated in the image are also shown to determine colocalization of GFP-tagged adaptors (green) with Drp1 (red).
To assess the role of the individual adaptors, ΔMiD49/51/Mff/Fis1 MEFs were transfected with plasmids expressing GFP fused to MiD49, MiD51, Mff or Fis1. Re-introduction of MiD49–GFP or MiD51–GFP into ΔMiD49/51/Mff/Fis1 MEFs was sufficient to fragment mitochondria and recruit the cytosolic pool of Drp1 to mitochondria (Fig. 2C). Expression of GFP–Mff rescued both the peroxisomal and mitochondrial elongation associated with the knockout of all adaptor proteins (Fig. 2C). Conversely, re-expression of GFP–Fis1 was not able to shift the morphology of mitochondria from a fused to a more reticular state, nor was it able to recruit Drp1 in the absence of MiD49, MiD51, Mff and Fis1 (Fig. 2C). These results indicate that MiD51, MiD49 and Mff can independently recruit fission-competent Drp1, whereas Fis1 is incapable of the recruitment.
Loss of Drp1 adaptors confers resistance to cell death
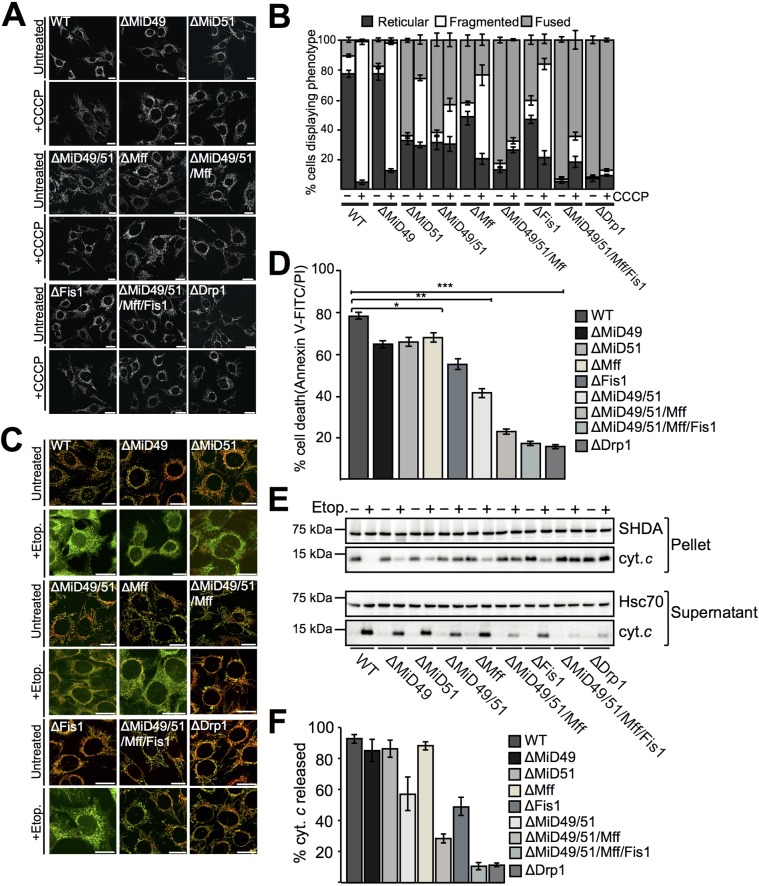
Cells harboring defects in mitochondrial fission (phenotypically presenting as elongated mitochondria) have been reported to be less sensitive to CCCP-induced mitochondrial fragmentation, presumably due to the lack of Drp1 recruited to mitochondria (Ishihara et al., 2009; Loson et al., 2013). To determine whether this is the case in gene-edited MEFs, we assessed their resistance to CCCP-induced fragmentation (Fig. 3A). Gene-edited MEFs were treated with 20 μM CCCP for 2 h and mitochondrial morphologies observed and quantified (Fig. 3A,B). ΔDrp1 MEFs were used as a positive control, as they are well documented to resist fragmentation upon uncoupling (Ishihara et al., 2009). ΔMiD49/51, ΔMiD49/51/Mff and ΔMiD49/51/Mff/Fis1 MEFs largely retained an interconnected mitochondrial network upon CCCP treatment, suggesting that functional Drp1 adaptors are required (Figs 3A,B). Furthermore, gene-edited MEFs were directly tested for sensitivity or resistance to apoptotic stimuli using etoposide (Fig. 3C,D). Imaging analysis of cells treated with etoposide (20 μM etoposide, 20 μM QVD, 8 h) revealed that ΔMiD49/51/Mff, ΔMiD49/51/Mff/Fis1 and ΔDrp1 MEFs preferentially retained cytochrome c (Fig. 3C). Although knockout of a single adaptor (MiD49, MiD51 or Mff), conferred some resistance to cell death [fluorescence-activated cell sorting (FACS) analysis; 20 μM etoposide for 16 h], the greatest resistance was observed in cell lines lacking multiple adaptors (ΔMiD49/51, ΔMiD49/51/Mff and ΔMiD49/51/Mff/Fis1; Fig. 3D). These findings are supported by biochemical data in which cell lines lacking MiD49 and MiD51, MiD49, MiD51 and Mff, and all four adaptors appear to retain more cytochrome c in the heavy membrane fraction than those lacking a single adaptor, with cytochrome c levels in the pellet fraction comparable to levels in ΔDrp1 MEFs (Fig. 3E,F).
Fig. 3.
Loss of MiD49 and MiD51 in MEFs confers resistance to cell death. (A) Gene-edited MEFs treated with 20 μM CCCP for 2 h, stained with antibodies against cytochrome c and imaged using confocal microscopy. Scale bars: 20 μm. (B) Cells from A were counted and mitochondrial morphology scored as indicated. Data are mean±s.e.m., n=3. (C) Cells were treated with 20 µM etoposide and 20 µM QVD for 8 h (+Etop.), immunostained for cytochrome c (green) and Tom20 (red) and imaged using confocal microscopy. Diffuse cytochrome c staining is observed in etoposide-treated WT and single-adaptor-knockout cells. Scale bars: 20 μm. (D) Gene-edited cells were treated with 20 μM etoposide for 16 h, stained with annexin-V–FITC and propidium iodide (PI) and analyzed by performing FACS. Data are mean±s.e.m., n=4. *P<0.05, **P<0.005, ***P<0.0005 (Student's t-test). (E) Gene-edited cells were treated with 20 μM etoposide for 16 h, then the heavy membrane and supernatant fractions were isolated and analyzed by western blotting with antibodies as indicated. (F) The percentage of cytochrome c released into the supernatant fraction upon etoposide treatment. Data are mean±s.e.m., n=4.
Proximity-based labeling analysis of MiD51, Mff and Fis1
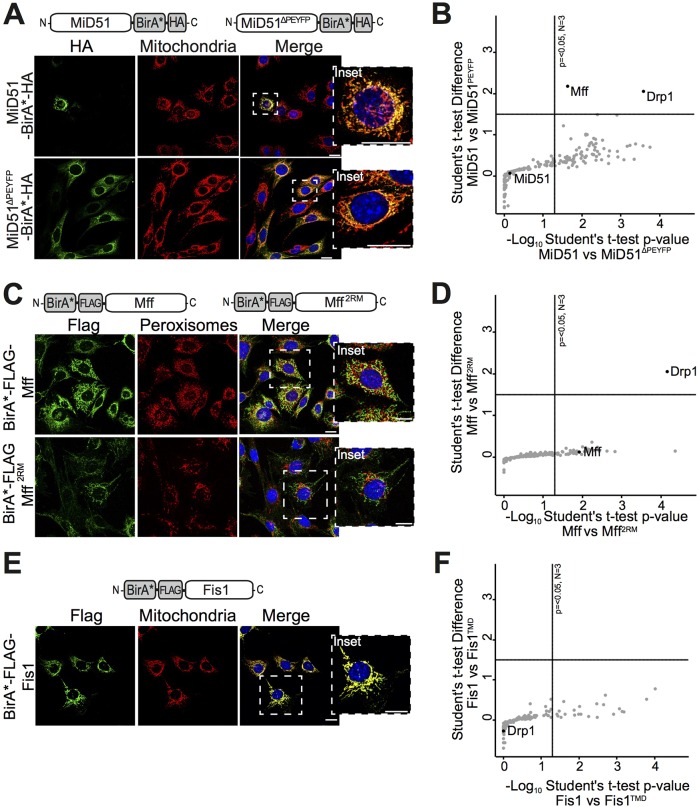
MiD51 and Mff can independently recruit Drp1 to mitochondria and promote mitochondrial fission (Koirala et al., 2013; Loson et al., 2013; Palmer et al., 2013). To address the involvement of other proteins in this process, we employed a proximity-based labeling technique, BioID (Roux et al., 2013), to determine proteins in close proximity to the adaptors. MiD51 was tagged with BirA* (MiD51–BirA*–HA) and stably expressed in ΔMiD51/MiD49 MEFs (as the MiD proteins are functional redundant, ΔMiD51/MiD49 MEFs were selected to ensure MiD49 did not inhibit protein interactions with MiD51–BirA*) (Fig. 4A). MiD51ΔPEYFP (MID51ΔPEYFP–BirA*–HA), which lacks the Drp1 recruitment loop (Richter et al., 2014), was also tagged with BirA* and used as a negative control. Both fusion proteins localized to mitochondria, where MiD51 had a punctate localization and induced mitochondrial fragmentation, in contrast to MiD51ΔPEYFP. To ensure that the expression of MiD51 construct was not too high as to induce a fission block (Palmer et al., 2013, 2011), we generated single-cell clones and attenuated the expression of MiD51–BirA* through a 4-hydroxytamoxifen (4-OHT)-inducible promoter. Protein levels were analyzed by western blotting to ensure that expression of the BirA* fusion proteins was not excessive when compared to endogenous levels (Fig. S3A). Although the levels of MiD51–BirA* were greater than endogenous levels, the fusion proteins were not at sufficient levels to cause the dominant-negative phenotype that is associated with MiD51 overexpression (resulting in elongated mitochondrial tubules). In fact, the levels of MiD51–BirA* caused mitochondrial fragmentation (Fig. 4A), which is associated with low-level MiD51 activity (Palmer et al., 2011). Expression of MiD51ΔPEYFP–BirA* was unable to alter the morphology of ΔMiD49/51 MEFs as it is fission incompetent. Biotin was added to the medium for labeling followed by quantitative label-free liquid-chromatography mass spectrometry (LC-MS) analysis of the purified biotinylated proteins (Table S1). Although many mitochondrial outer membrane proteins were detected, surprisingly only Drp1 and Mff were significantly enriched when comparing MiD51 against MiD51ΔPEYFP (Fig. 4B). This result supports other findings showing that MiD51 and Mff might assemble with Drp1 at the same fission foci (Elgass et al., 2015). Interestingly, no ER proteins were found to be enriched in this analysis.
Fig. 4.
BioID proximity-labeling analysis of MiD51, Mff and Fis1. (A) BirA*-tagged MiD51 and MiD51ΔPEYFP (schematics at top) were expressed in ΔMiD49/51 MEFs, stained with Hoechst 33258 (blue) and immunostained using antibodies against the HA tag (green) and Tom20 (red). (B) BioID proximity labeling and enrichment of proteins in BirA*–MiD51 relative to BirA*–MiD51ΔPEYFP. A Student's t-test was performed on the log-transformed LFQ intensities from three biological replicates of MiD51 and MiD51ΔPEYFP enrichments, and plotted against the difference between the mean log-transformed intensities. The thresholds for enriched were set at P≤0.05 and t-test difference at >1.5 (log10 LFQ intensities). (C) BirA*–Mff and BirA*–Mff2RM (schematics at top) were expressed in ΔMff MEF cells, stained with Hoechst 33258 (blue) and immunostained using anti-Flag (green) and anti-Pex14 (red) antibodies. (D) As for B, comparing relative enrichment between Mff and Mff2RM. (E) BirA*-tagged Fis1 (schematic at top) was expressed in ΔFis1 MEF cells, stained with Hoechst 33258 (blue) and immunostained for Flag (green) and Tom20 (red). (F) As for B, comparing relative enrichment between Fis1 and Fis1TMD. The insets show a magnified view of the boxed region. Scale bars: 20 μm.
Next, we applied this technique to Mff. We fused BirA*–FLAG to the N-terminus of Mff (BirA*–FLAG–Mff) and stably expressed it in ΔMff MEFs. A two-repeat mutant of Mff (Mff2RM) that does not recruit Drp1 (Strack and Cribbs, 2012) was also fused to BirA* and used as a control (BirA*–FLAG–Mff2RM). Both fusion proteins were localized to mitochondria and peroxisomes, and only the wild-type construct restored (Drp1-mediated) fission activity (Fig. 4C). We verified that the expression of the endogenous protein was comparable to that of the BirA* fusion proteins (Fig. S3B). We found that BirA*–Mff was able to rescue both the mitochondrial and peroxisomal phenotypes associated with the ΔMff MEFs, whereas the inactive mutant BirA*–Mff2RM was not. Biotin labeling followed by quantitative LC-MS analysis of biotinylated proteins (Table S2) revealed that BirA*–Mff significantly biotinylated Drp1 compared to the Mff2RM control (Fig. 4D). In this case, endogenous MiD51 was not detected in the samples. This might be due to the low levels of this protein in cells (Wang et al., 2012) and/or a poor ability to detect the protein by mass spectrometry approaches. To test this, the relative abundance of mitochondrial MiD49, MiD51, Mff and Fis1 was analyzed using recombinant proteins as a standard curve (Fig. S4A–D). MiD49 (1.5±0.5 ng) and MiD51 (3.2±0.5 ng) were found to exist in relatively low abundance in MEFs compared to that of Fis1 (18±8 ng) and Mff (386±108 ng) (mean±s.e.m.; n=3). Interestingly, the levels of the adaptors varied between other cell lines and tissues, potentially reflecting different regulatory requirements.
The role of Fis1 as a Drp1 adaptor has been questioned (Koirala et al., 2013; Otera et al., 2010). Given that the BioID approach successfully confirmed that Drp1 is in close proximity to Mff and MiD51, we reasoned that this was a valid approach to investigate whether Fis1 can do the same. BirA* was fused to the N-terminus of Fis1 (BirA*–FLAG–Fis1) and stably expressed in ΔFis1 MEFs. As can be seen (Fig. 4E), the fusion protein was localized to mitochondria. As a negative control, BirA* fused to the transmembrane domain of Fis1 was employed (BirA*–FLAG–Fis1TMD; here, there is no suitable non-binding mutant control) and was stably expressed in control MEFs. Although the levels of BirA*–Fis1 were greater than the endogenous Fis1 levels (Fig. S3C), the mitochondria remained in reticular form (Fig. 4E), indicating that the levels were not excessive. Biotin labeling followed by quantitative LC-MS analysis of biotinylated proteins revealed no enrichment of any proteins in Fis1–BirA*-expressing cells relative to control at the threshold levels seen for MiD51 and Mff (Fig. 4F; Table S3). One protein showing slight enrichment with Fis1–BirA* was TBC1D15, which has been shown to interact with Fis1 during mitophagy (Shen et al., 2014; Yamano et al., 2014). We hypothesize that because BioID was not performed under mitophagy-inducing conditions the association of TBC1D15 and Fis1 only occurred at background levels (Table S3). Overall, BioID followed by LC-MS supports that MiD51 and Mff are adaptors for Drp1 and they can exist in close proximity at the mitochondrial surface.
MiD51 and Mff exert opposing effects on Drp1 GTPase activity
As we could detect MiD51, Mff and Drp1 in close proximity, and these proteins might exist in the same scission foci (Elgass et al., 2015), we sought to investigate whether the two adaptors regulate Drp1 GTPase activity. To do this we designed a robust in vitro system of measuring Drp1 GTPase activity by tethering His-tagged cytosolic domains of adaptor proteins (MiD51ΔN118 and MffΔC20) on artificial liposomes containing a lipid with a charged Ni-chelating head group {6% 1,2-dioleoyl-sn-glycero-3-[(N-(5-amino-1-carboxypentyl)-iminodiacetic acid)succinyl] (DGS-NTA Ni2+) and 94% di-oleoyl phosphatidylcholine (DOPC)} termed Ni-liposomes. For both MiD51 and Mff, the His tags were located in place of the transmembrane region, mimicking the orientation of the mitochondrial-anchored native forms. In the presence of MiD51, Drp1 GTPase activity was substantially inhibited (Drp1, kcat=21.9 min−1 and Drp1+MiD51, kcat=4.9 min−1; Fig. 5A,B). The inhibition was dependent on the presence of the PEYFP loop of MiD51 needed for Drp1 recruitment (Richter et al., 2014) (Drp1+MiD51ΔPEYFP: kcat=20.3 min−1; Fig. 5A,B). In contrast to MiD51, and as recently reported (Clinton et al., 2015; Macdonald et al., 2015), Mff stimulated Drp1 GTPase activity (Drp1+Mff: kcat=40.4 min−1). The opposing effects of MiD51 and Mff on Drp1 GTPase activity suggest a divergence in the roles of the adaptors in fission foci. To analyze the effect of co-assembly between the adaptors, we monitored the GTPase activity of Drp1 following the addition of stoichiometric amounts (0.5 μM) of Mff or MiD51, and subsequently added the second adaptor 4.5 min later. When Mff was added after MiD51, it was unable to stimulate Drp1 GTPase activity (Fig. 5C). However, when MiD51 was added after Mff, stimulation of Drp1 GTPase activity was suppressed (Fig. 5D). Although MiD51 is able to bind ADP (Losón et al., 2014; Richter et al., 2014), addition of ADP to in vitro GTPase assays containing Mff and/or MiD51 did not alter the ability of these adaptors to promote or restrict the GTPase ability of Drp1 respectively (Fig. 5D). These results indicate that the two adaptors, MiD51 and Mff can regulate Drp1 GTPase activity on the lipid surface in opposing directions, and therefore have distinct and complementary roles in Drp1-mediated mitochondrial fission.
Fig. 5.
MiD51 and Mff have opposing effects on Drp1 GTPase activity. Drp1 GTPase activity stimulated by Ni-liposomes in the presence or absence of the indicated Drp1 adaptor variants was plotted by (A) linear regression, and the mean kcat values were calculated (B). (C) Drp1 GTPase activity stimulated by Ni-liposomes with the incorporation of Mff at 4.5 min as indicated. (D) Drp1 GTPase activity stimulated by Ni-liposomes with indicated adaptors added at 4.5 min and where indicated, 20 µM ADP (hatched lines) added at 8.5 min. Data mean±s.d. for n≥3.
DISCUSSION
Although it has been established that Mff, MiD49, and MiD51 recruit Drp1, how these adaptors interact with each other and other proposed molecules (including Fis1) in mitochondrial fission is not well understood. By creating MEFs that lack Drp1 adaptors, we were able to more precisely probe the importance of these proteins in Drp1-mediated fission. The levels of these proteins in MEFs appear to vary greatly, with Mff being the predominant adaptor at mitochondria. However, the concentrations of adaptors within actual fission foci has yet to be determined. Indeed, loss of any single adaptor (MiD49, MiD51 or Mff) or Fis1 did not appreciably affect the amount of Drp1 found at the mitochondrial surface. This is consistent with the redundant nature of the Drp1 adaptors regardless of the relative amount of endogenous adaptor present on mitochondria (Koirala et al., 2013; Otera et al., 2010; Palmer et al., 2013). Several studies have concluded that Mff is the major receptor for Drp1 (Liu and Chan, 2015; Loson et al., 2013; Otera et al., 2010); however, the degree of mitochondrial elongation in ΔMff MEFs was similar to that seen in ΔMiD51 or ΔMiD49/51 MEFs. Recently, it has been found that mice lacking Mff can live for up to 13 weeks and eventually die from cardiomyopathy due to mitochondrial fission defects (Chen et al., 2015). This phenotype is significantly milder than the embryonic lethality seen for Drp1-knockout mice (Ishihara et al., 2009; Wakabayashi et al., 2009), suggesting that mitochondrial fission can take place in other tissues lacking Mff (Chen et al., 2015). Mff might also play an important role in neurons given its ability to efficiently stimulate the GTPase activity of the brain-specific Drp1 isoform compared to the ubiquitously expressed isoform (Macdonald et al., 2015).
Recent data indicates that Mff and the MiDs can exist in the same fission foci that also contact the ER (Elgass et al., 2015; Friedman et al., 2011). In our BioID experiment using MiD51 fused with BirA*, we were able to confirm that both MiD51 and Mff are in close proximity to Drp1 under basal cellular conditions. Surprisingly, we did not detect members of the mitochondrial constriction machinery, including ER scaffolding proteins such as Syn17, INF2 and Spire1 in close proximity to MiD51 or Mff (Arasaki et al., 2015; Korobova et al., 2013; Manor et al., 2015). A potential explanation for this is that the criteria for enrichment in our BioID analysis was quite stringent because any proximity labeling seen in MiD51 or Mff mutants defective in Drp1 binding was treated as background. Thus, we might have biased against any interactions with adaptors that are independent of Drp1. Given this, when compared against the BioID experiments using Fis1 or BirA* that was associated with the mitochondrial outer membrane (Fis1TMD), we saw an enrichment of cytoskeletal components (including myosin and actin) in the Mff and MiD51 proximity-labeling experiments (data not shown). Current models suggest that actin–myosin fibers might assemble from the ER platform to engage at mitochondrial constriction sites (Ji et al., 2015; Korobova et al., 2013; Li et al., 2015). Thus, the relative distance of adaptor proteins from the ER machinery and the crowding of cytoskeletal proteins in-between might provide a substantial barrier to prevent activated biotin from cross-linking to ER components. Attempts to enhance biotinylation of other potential fission components by adding the mitochondrial uncoupler CCCP and shortening the time-frame of the biotin addition did not yield additional proteins to those previously detected (data not shown).
During apoptosis, Drp1 binding to mitochondria is increased and occurs concurrently with Bax translocation, upstream of caspase activation and cytochrome c release (Hoppins and Nunnari, 2012; Karbowski et al., 2002). What remains unclear, however, is whether the adaptors that mediate Drp1 recruitment under normal cell homeostasis perform the same role upon the initiation of apoptotic signaling (Scorrano, 2009). MiD49 and MiD51 appear to be important in the apoptotic recruitment of Drp1 to the mitochondrial surface, although apoptosis can still proceed in their absence. Like Drp1-knockout cells (Ishihara et al., 2009), cell lines lacking all known adaptors retained significantly more cytochrome c within mitochondria following apoptotic induction than MEFs lacking only a single adaptor. Recently, McBride and colleagues found that Drp1 oligomer formation facilitates cristae remodeling and Bax- and Bak-mediated cytochrome c release following apoptotic activation (Prudent et al., 2015). The apoptotic protection afforded by loss of adaptor proteins in cells might result from impaired formation of the Drp1 oligomer.
During manuscript revisions, Mihara and colleagues independently detailed the apoptotic resistance of Mff, and MiD49 and MiD51 gene-edited MEFs (Otera et al., 2016). Similar to results obtained here, MiD49 and MiD51 double-knockout MEFs retained more cytochrome c within mitochondria than either wild-type or single MiD-knockout MEFs (Otera et al., 2016). In contrast, MEFs lacking Mff were not resistant to cytochrome c release following actinomycin D treatment (Otera et al., 2016). Otera and colleagues (2016) concluded that loss of MiD49 and MiD51 impairs Opa1-dependent cristae remodeling during apoptosis. Although we also observed some apoptotic resistance in the ΔMiD49/51 MEFs, we saw greater resistance in ΔMiD49/51/Mff and ΔMiD49/51/Mff/Fis1 MEFs, suggesting an additive effect of Mff in apoptotic resistance and/or variable responses to different apoptotic stimuli.
The role of Fis1 as a bona fide Drp1 adaptor has been controversial, with recent reports downplaying the role of Fis1 in homeostatic fission, and assigning it a more pronounced role in stress or chemical-induced mitochondrial division and mitophagy (Shen et al., 2014; Yamano et al., 2014). Our results support Fis1 as not being intrinsically involved in homeostatic fission. Loss of Fis1 in MEFs did not induce obvious morphological changes to the mitochondrial or peroxisomal networks and unlike Mff or MiD51, the BioID approach showed no indication that Fis1 was in proximity to Drp1. However, ΔFis1 MEFs displayed some apoptotic resistance and the possibility exists that Fis1 acts during stress- or apoptotic-mediated fragmentation (Gomes and Scorrano, 2008; Shen et al., 2014; Wang et al., 2012).
It is not clear how the adaptors engage with Drp1 in fission and this is exacerbated by different conclusions made regarding the oligomeric state at which Drp1 is recognized. For example, Clinton and colleagues recently concluded that Mff recruits dimeric Drp1 for subsequent assembly (Clinton et al., 2015), whereas Liu and Chan concluded the opposite, namely that Drp1 oligomers are selectively recruited by Mff (Liu and Chan, 2015). Likewise, one study has found that MiD49 and MiD51 could not sequester assembly-defective forms of Drp1 (Palmer et al., 2011) whereas another study has shown that MiD49 and MiD51 could recruit assembly-deficient, dimeric Drp1 (Liu and Chan, 2015). Disparity in function between adaptors also lies in the opposing effects on Drp1 GTPase activity at the membrane – Drp1 GTPase activity is inhibited by MiD proteins whereas Mff stimulates it. This is consistent with other recent studies (Clinton et al., 2015; Macdonald et al., 2015). Taken together, these results present intriguing roles for MiD49, MiD51 and Mff – although they can both recruit Drp1 to mitochondria, they have functional divergence in regulating Drp1 GTPase activity. Although the inhibitory effect that MiD51 exerts on the GTPase activity of Drp1 was significantly stronger than the stimulatory effect seen by Mff, the stoichiometry of these molecules at mitochondrial fission sites and their mode of regulation (Chen et al., 2015; Loson et al., 2013; Palmer et al., 2013, 2011) is unclear. Based on these and other studies, a possible scenario is that MiD49 and MiD51 engages with, and facilitates, Drp1 assembly at the mitochondrial outer membrane by inhibiting GTPase activity. Given that Mff stimulates Drp1 GTPase activity, this points to its role in the constriction phase of fission (Francy et al., 2015). Because MiD51, Mff and Drp1 are present in close proximity, possibly in the same fission complex, the adaptors might also facilitate Drp1 cycling at the membrane. The transition from MiD51-dependent inhibition to Mff-dependent stimulation of Drp1 GTPase activity might be dependent on stoichiometric levels of the adaptors at the membrane. The presence of additional signals to mediate the transition is also likely. Although much detail is still to be worked out, the redundant nature of the adaptors indicates that Drp1 can function independently of either regulatory step or that additional proteins play similar roles. For example, actin has recently been shown to assemble with Drp1, and like Mff, stimulate the GTPase activity of Drp1 (Ji et al., 2015). Furthermore, sequestration of Drp1 at mitochondria upon MiD49 or MiD51 overexpression also recruits actin (Palmer et al., 2011). Additional proteins (including ER components) might substitute for the role of MiD49 and MiD51 in regulating Drp1 activity under some situations, including in non-chordate metazoans that only contain Mff.
MATERIALS AND METHODS
Generation of gene-edited MEFs
Mouse embryonic stem cell (ESC) lines containing a gene-trap disruption of smcr7 (MiD49) were obtained from the Knockout Mouse Project (KOMP) Repository (project ID: VG10452). The ESCs were injected into blastocysts to generate mouse lines in C57Bl/6N mice (Australian Phenomics Network). MEFs lacking MiD49 were generated from mid-gestation (E10.5 day) embryos from heterozygotes mating pairs. All experiments were performed in accordance with Animal Ethics Committee approval (Monash and La Trobe Universities).
TALEN pairs were generated against the following genes in the indicated genomic regions: Mff target site, exon 1, 5′-GCTGAGATGGCAGAA-3′; and Fis1 target site, exon 2, 5′-AGAATTTTGAAAGGAAATTTC-3′. ΔDrp1 MEFs were generated using the CRISPR/Cas9 system (Ran et al., 2013). The target site was determined through gene analysis using CHOPCHOP (Montague et al., 2014). The Drp1 target site was exon 1, 5′-GCAGGACGTCTTCAACACAG-3. TALEN pairs for MiD51 were as reported previously (Richter et al., 2014). TALEN and CRISPR/Cas9 cell lines were sorted through fluorescence (GFP and mCherry for TALEN pairs, GFP for CRISPR/Cas9) flow cytometry to give single cells to ensure clonality. Genomic verification of all cell lines was performed as reported previously (Stroud et al., 2013). Control MEFs were used as controls. The indels generated are shown in Fig. S1.
Cell culture, transfections and drug treatments
All MEF lines were cultured in Dulbecco's modified Eagle's medium (DMEM) containing 10% (v/v) fetal bovine serum (FBS) and penicillin–streptomycin, and were maintained at 37°C in an atmosphere of 5% CO2. Transient transfections of MEF cells were performed using Lipofectamine LTX with Plus Reagent or Lipofectamine 2000 (Invitrogen) according to manufacturer's instructions. Cell lines were routinely tested for mycoplasma contamination using PlasmaTest (InvivoGen).
Antibodies, chemicals and plasmids
Commercial antibodies used were anti-Drp1 (1:500, catalogue number: 611738, BD Biosciences), anti-Opa1 (1:500, catalogue number: 612606, BD Biosciences), anti-cytochrome c (1:500, native epitope: 556432, denatured epitope: 556433, BD Biosciences), anti-MiD51 (1:500, catalogue number 20164-1-AP, Proteintech), anti-Tom20 (1:500, catalogue number 11415, Santa Cruz Biotechnology) and anti-SDHA (1:500, catalogue number 14715, Abcam) antibodies. These antibodies have been validated previously (Palmer et al., 2013; Richter et al., 2014). Polyclonal antibodies against Fis1, Hsc70, Mff and MiD49 were raised in-house and have been reported previously (Richter et al., 2014; Stojanovski et al., 2004). Polyclonal antibodies were raised against Bak (lacking the transmembrane domain) and used at a dilution of 1:1000.
Carbonyl cyanide m-chlorophenyl hydrazone (CCCP) (Sigma) was used at a final concentration of 20 μM for 2 h at 37°C. Etoposide (Sigma) was used at a final concentration of 20 μM for 16 h at 37°C for FACS and western blot analysis, and for 8 h for mitochondrial morphology analysis. QVD (Sigma) was used at a final concentration of 20 µM.
MiD49–GFP, MiD51–GFP, mt-GFP, GFP–Mff, GFP–Fis1 and GFP–Sec61 plasmids have been previously reported (Elgass et al., 2015; Palmer et al., 2011; Stojanovski et al., 2004). MiD51 constructs were first cloned into pcDNA3.1 MCS-BirA(R118G)-HA (a gift from Kyle Roux, Sanford Children's Health Center, Sanford Research, Sioux Falls, SD; Addgene plasmid #36047). For lentiviral expression, open reading frames (ORFs) of mouse MiD51–BirA*–HA and mouse MiD51ΔPEYFP–BirA*–HA were cloned into the pF 5xUAS MCS SV40 vector using BglII and XbaI. For retroviral expression, ORFs of BirA*–Flag–human-Mff (isoform 8), BirA*–Flag–human-Mff 2RM (isoform 8) and BirA*–Flag–human-Fis1 were cloned into pMX-IRES-GFP vector using the EcoRI and SalI sites. For bacterial expression and purification, ORFs of MiD51 (119–463) and MiD51 (119–463)ΔPEYFP were cloned in pET10N vector using the NotI and BamHI sites, whereas ORFs of MffΔC20 were cloned into pET28a(+) using the NcoI and XhoI sites.
Proximity-labeling experiments using BioID
Generation, selection and induction of 4-hydroxy-tamoxifen-inducible MEF cell lines (for MiD51 constructs) were performed as previously described (Richter et al., 2014). Retrovirally transduced cells (for Mff and Fis1 constructs) were generated as previously described (Lazarou et al., 2015). Transduced cells expressing BirA*-tagged proteins were grown in two 15-cm tissue culture plates at 70–90% confluency in DMEM containing 10% (v/v) FBS and 50 µM biotin (Sigma-Aldrich) for the labeling time period. For each sample, three replicates were cultured. The cells were then harvested, and total protein levels of all replicates equalized by protein estimation using a BCA protein assay kit (Pierce). The cell pellet was frozen at −80°C. Pellets were resuspended and solubilized in 1 ml RIPA lysis buffer (50 mM Tris-HCl pH 7.5, 150 mM NaCl, 1% NP-40, 1 mM EDTA, 1 mM EGTA and 0.1% SDS), supplemented with 1× protease cocktail inhibitor (Roche), 0.5% sodium deoxycholate and 250 U of benzonase (Sigma-Aldrich). Lysis was performed at 4°C for 1 h with shaking. The lysates were sonicated on ice at 30% amplitude (3×10 s bursts with 2 s rest in between). The lysates were centrifuged for 30 min at 20,000 g for 30 min at 4°C. The cleared lysate was then incubated with Streptavidin-mag beads (Pierce) for 3 h at 4°C. The beads were separated using a magnet and washed two times with RIPA lysis buffer followed by two washes with TAP lysis buffer (50 mM HEPES-KOH pH 8.0, 100 mM KCl, 10% glycerol, 2 mM EDTA, 0.1% NP-40) and three 1 ml washes with 50 mM ammonium bicarbonate pH 8.0. Proteins bound to the beads were digested with 5 µg/ml trypsin at 37°C overnight. The supernatant was harvested and beads were washed two times with 50 mM ammonium bicarbonate pH 8.0, and washes were pooled together with the supernatant. The pooled tryptic digest was lyophilized and resuspended in 8 M urea, 50 mM ammonium bicarbonate and acidified with 1% (v/v) trifluoroacetic acid (TFA) prior to desalting on SDB-XC (Empore) StageTips as previously described (Rappsilber et al., 2007).
Mass spectrometry
Peptides were analyzed by online nano-high-pressure liquid chromatography (HPLC) electrospray ionization-tandem mass spectromery (MS/MS) on an LTQ-Orbitrap Elite Instrument (MiD51) or Q Exactive Plus (Mff and Fis1) connected to an Ultimate 3000 HPLC (Thermo-Fisher Scientific). For the MiD51 experiments, chromatography and mass spectrometry acquisition parameters were as previously described (Formosa et al., 2015). For Mff and Fis1 experiments, peptides reconstituted in 0.1% TFA and 2% acetonitrile (ACN) were loaded onto a trap column (Acclaim C18 PepMap nano Trap×2 cm, 100 μm I.D., 5-μm particle size and 300-Å pore size; Thermo-Fisher Scientific) at 15 μl/min for 3 min before switching the precolumn in line with the analytical column (Acclaim RSLC C18 PepMap Acclaim RSLC nanocolumn 75 μm×50 cm, PepMap100 C18, 3-μm particle size, 100-Å pore size; Thermo-Fisher Scientific). The separation of peptides was performed at 250 nl/min using a non-linear ACN gradient of buffer A (0.1% formic acid, 2% ACN) and buffer B (0.1% formic acid, 80% ACN), starting at 2.5% buffer B to 42.5% over 60 min. Data were collected in positive mode using a Data Dependent Acquisition m/z of 375–1800 as the scan range, and higher-energy collisional dissociation (HCD) for MS/MS of the 12 most intense ions with z ≥2. Other instrument parameters were: MS1 scan at 70,000 resolution (at 200 m/z), MS maximum injection time 50 ms, AGC target 3E6, normalized collision energy at 27% energy, isolation window of 1.8 Da, MS/MS resolution of 17,500, MS/MS AGC target of 1E5, MS/MS maximum injection time of 100 ms, minimum intensity set at 1E3 and dynamic exclusion set to 20 s. For data analysis, Thermo raw files were analyzed using the MaxQuant platform (version 1.5.3.17) searching against the Uniprot mouse database containing reviewed canonical isoforms in FASTA format (June 2015; 16,725 entries), the human sequences for bait proteins Mff and Fis1, and a database containing common contaminants, by the Andromeda search engine (Cox et al., 2011). Default settings for label-free quantification (LFQ) were used with modifications. Briefly, N-terminal acetylation and methionine oxidation were used as variable modifications. False discovery rates of 1% for proteins and peptides were applied by searching a reverse database, and ‘Re-quantify’ and ‘Match between runs’ options were enabled. Unique and razor peptides were used for quantification, using a minimum ratio count of one. ‘Label-free quantification’ was turned on, with the ‘LFQ min. ratio count’ set to one. Data analysis was performed using the Perseus software (version 1.5.2.6). Only proteins with a sequence coverage of >5%, more than one unique peptide and valid values in at least two out of three replicates (per experimental group) were considered for data analysis. A one-tailed Student's t-test was performed between control (MiD51PEYFP–BirA*, BirA*–Mff2RM or BirA*–Fis1TMD) and bait (MiD51–BirA*, BirA*–Mff or BirA*–Fis1) experimental groups.
Bacterial expression and purification
Drp1
Drp1 was purified with an N-terminal CBP tag and expressed in BL-21 CodonPlus expression cells (Agilent) following IPTG induction. Cells were lysed and affinity purified using calmodulin-agarose beads (G-Biosciences). Drp1 was further purified by ion-exchange chromatography using DEAE-Sepharose™ Fast Flow beads (GE Healthcare Life Sciences) to yield high-purity Drp1.
MiD51 variants
pET10N-MiD51 and pET10N-MiD51ΔPEYFP were expressed in BL-21 CodonPlus expression cells (Agilent) following IPTG induction. Cells were lysed and purified by Immobilized Metal Affinity Chromatography (IMAC) using Ni-NTA agarose beads (Qiagen) followed by removal of contaminating proteins by size-exclusion chromatography (Superdex™ 200 16/60 HiLoad; GE Healthcare Life Sciences) to yield high-purity MiD51 and MiD51ΔPEYFP.
MiD49
pQE-30-mouse MiD49ΔN50 was expressed in XL-1 Blue E. coli cells following IPTG induction. Cells were lysed and purified by IMAC using Ni-NTA agarose beads followed by removal of contaminating proteins by size-exclusion chromatography (Superdex™ 200 16/60 HiLoad; GE Healthcare Life Sciences) to yield high-purity MiD49.
Mff
pET28a(+)-Mff (isoform 8) was expressed in BL-21 Codon Plus E. coli cells following IPTG induction. Cells were lysed and purified by Immobilized Metal Affinity Chromatography (IMAC) using Ni-NTA agarose beads (Qiagen) followed by removal of contaminating ∼70-kDa migrating species by ion-exchange chromatography (mono Q 5/50 GL; GE Healthcare Life Sciences), to yield high-purity Mff.
GST–Fis1
pGEX-4T-1-Fis1ΔTMD was expressed in BL-21-CodonPlus expression cells following IPTG induction. Cells were lysed and purified by affinity purification using glutathione-agarose resin to yield high-purity GST–Fis1TMD.
GTPase assays
Stoichiometric amounts of Drp1 adaptors (0.5 µM) were pre-incubated on Ni-liposomes [6% DGS-NTA (Ni2+), 94% DOPC; Avanti Polar Lipids] liposomes for 15 min followed by Drp1 incubation for a further 15 min at room temperature before GTP was added to initiate the reaction at 37°C over a defined time using the GTPase assay previously described (Macdonald et al., 2014). Additional adaptors and variants were added at 4.5 min and 20 µm ADP added at 8.5 min when required.
Mitochondrial isolation and western blotting
Mitochondrial isolation and separation of proteins by Tris-Tricine SDS-PAGE was performed as previously described (Johnston et al., 2002). Signals were detected on PVDF membrane using ECL chemiluminescent substrate (BioRad) on a BioRadXRS+ ChemiDoc system with Image Lab software (BioRad).
Immunofluorescence assays
Cells were fixed with 4% (w/v) paraformaldehyde in PBS (pH 7.4), permeabilised with 0.2% (w/v) Triton X-100 in PBS and incubated with primary antibodies for 60 min at room temperature. Primary antibodies were labeled with either Alexa-Fluor-488, Alexa-Fluor-568- or Alexa-Fluor-647-conjugated anti-mouse-IgG or anti-rabbit-IgG secondary antibodies (Molecular Probes). F-actin was stained using a phalloidin–FITC conjugate (Thermo-Fisher). Hoechst 33258 (1 μg/ml) was used to stain nuclei.
Microscopy
Confocal microscopy was performed on either a Leica TCS SP5 5 channel confocal microscope or a Leica TCS SP8 equipped with HyD detectors using a 63× oil immersion objective. All images were processed using ImageJ (Schneider et al., 2012), MetaMorph software (Visitron Systems) or Leica AF processing software (Leica). Colocalization statistics were obtained using the image in its entirety (single planes) and are presented as Pearson correlation units (r). Images in all experimental groups were obtained using the same settings, except for detector gain adjustments on the Leica TCS SP5 microscope and accumulation time on the Leica TCS SP8 microscope. When required, z-sectioning was performed using 1-μm slices. Colocalization of GFP and Alexa Fluor 568 signals were determined using 15-μm linescans using Metamorph software (Visitron Systems).
Live-cell imaging
To detect ER–mitochondrial contact sites, cells were transfected with GFP–Sec61, stained with 25 nM Mito-Tracker Red (Thermo-Fisher) and imaged in DMEM containing 10% (v/v) FBS and maintained at 37°C under an atmosphere of 5% CO2. Fluorescence recovery after photobleaching (FRAP) was performed using the FRAP wizard on the Leica TCS SP5 confocal microscope. Cells were imaged in DMEM containing 10% (v/v) FBS and maintained at 37°C under an atmosphere of 5% CO2. Photobleaching of the GFP signal was achieved using a 488 nm laser at 100% power for 200 ms (sufficient to achieve 10–20% of the starting fluorescence signal). Subsequent recovery of the GFP signal was monitored over 60 s at 15% laser power.
Fluorescence-activated cell sorting
MEFs were harvested and resuspended in annexin-binding buffer (10 mM HEPES pH 7.4, 140 mM NaCl2, 25 mM CaCl2) and stained for 30 min with 250 μg/ml annexin-V–FITC and 50 μg/ml propidium iodide prior to analysis on a FACS Calibur (Becton Dickinson).
Statistical analysis
Data were generated from a minimum of three independent experiments. Statistical analysis was performed using Microsoft Excel or Prism. For all graphs and traces in Figs 1–3, Figs S2 and S3, error bars represent the mean±s.e.m. For Fig. 5, error bars represent the mean±s.d. All other data where possible and appropriate were analyzed using a Student's t-test. Significance is expressed as *P<0.05, **P<0.005, ***P<0.0005.
Supplementary Material
Acknowledgements
We thank Grant Dewson (Walter and Eliza Hall Institute, Melbourne, Australia), Gia Voeltz (University of Colorado, Boulder, CO) and Michael Lazarou (Monash University, Melbourne, Australia) for plasmids, Jason Mears (Case Western Reserve University, Cleveland, OH) for the pCAL-N-EK-hDrp1-3 plasmid, Stefan Strack (University of Iowa, Iowa City, IA) for Mff2RM cDNA and Heidi McBride for advice. We also thank the Monash Micro Imaging, FlowCore and Proteomics platforms at Monash University.
Footnotes
Competing interests
The authors declare no competing or financial interests.
Author contributions
L.D.O. generated and characterized gene-edited MEFs, A.P.S. performed BioID pulldowns and in vitro GTPase experiments, D.A.S. performed and analyzed mass spectrometry experiments, C.S.P. made gene-edited MEFs. M.T.R. conceived experiments. All authors analyzed results and wrote the manuscript.
Funding
This work was supported with funding from the National Health and Medical Research Council (NHMRC) [grant number 1049968]; and Australian Research Council [grant number DP160102176]. D.A.S. is a NHMRC Peter Doherty Fellow. R.R. was supported by an American Heart Association (AHA) Beginning Grant-in-Aid [grant number 13BGIA14810010].
Supplementary information
Supplementary information available online at http://jcs.biologists.org/lookup/suppl/doi:10.1242/jcs.185165/-/DC1
References
- Arasaki K., Shimizu H., Mogari H., Nishida N., Hirota N., Furuno A., Kudo Y., Baba M., Baba N., Cheng J. et al. (2015). A role for the ancient SNARE syntaxin 17 in regulating mitochondrial division. Dev. Cell 32, 304-317. 10.1016/j.devcel.2014.12.011 [DOI] [PubMed] [Google Scholar]
- Ban-Ishihara R., Ishihara T., Sasaki N., Mihara K. and Ishihara N. (2013). Dynamics of nucleoid structure regulated by mitochondrial fission contributes to cristae reformation and release of cytochrome c. Proc. Natl. Acad. Sci. USA 110, 11863-11868. 10.1073/pnas.1301951110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen H. and Chan D. C. (2009). Mitochondrial dynamics-fusion, fission, movement, and mitophagy-in neurodegenerative diseases. Hum. Mol. Genet. 18, R169-R176. 10.1093/hmg/ddp326 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen H., Ren S., Clish C., Jain M., Mootha V., McCaffery J. M. and Chan D. C. (2015). Titration of mitochondrial fusion rescues Mff-deficient cardiomyopathy. J. Cell Biol. 211, 795-805. 10.1083/jcb.201507035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clinton R. W., Francy C. A., Ramachandran R., Qi X. and Mears J. A. (2015). Dynamin-related protein 1 oligomerization in solution impairs functional interactions with membrane-anchored mitochondrial fission factor. J. Biol. Chem. 291, 478-492. 10.1074/jbc.M115.680025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cox J., Neuhauser N., Michalski A., Scheltema R. A., Olsen J. V. and Mann M. (2011). Andromeda: a peptide search engine integrated into the MaxQuant environment. J. Proteome Res. 10, 1794-1805. 10.1021/pr101065j [DOI] [PubMed] [Google Scholar]
- Elgass K. D., Smith E. A., LeGros M. A., Larabell C. A. and Ryan M. T. (2015). Analysis of ER-mitochondria contacts using correlative fluorescence microscopy and soft X-ray tomography of mammalian cells. J. Cell Sci. 128, 2795-2804. 10.1242/jcs.169136 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Formosa L. E., Mimaki M., Frazier A. E., McKenzie M., Stait T. L., Thorburn D. R., Stroud D. A. and Ryan M. T. (2015). Characterization of mitochondrial FOXRED1 in the assembly of respiratory chain complex I. Hum. Mol. Genet. 24, 2952-2965. 10.1093/hmg/ddv058 [DOI] [PubMed] [Google Scholar]
- Francy C. A., Alvarez F. J. D., Zhou L., Ramachandran R. and Mears J. A. (2015). The mechanoenzymatic core of dynamin-related protein 1 comprises the minimal machinery required for membrane constriction. J. Biol. Chem. 290, 11692-11703. 10.1074/jbc.M114.610881 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedman J. R., Lackner L. L., West M., DiBenedetto J. R., Nunnari J. and Voeltz G. K. (2011). ER tubules mark sites of mitochondrial division. Science 334, 358-362. 10.1126/science.1207385 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gandre-Babbe S. and van der Bliek A. M. (2008). The novel tail-anchored membrane protein Mff controls mitochondrial and peroxisomal fission in mammalian cells. Mol. Biol. Cell 19, 2402-2412. 10.1091/mbc.E07-12-1287 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gomes L. C. and Scorrano L. (2008). High levels of Fis1, a pro-fission mitochondrial protein, trigger autophagy. Biochim. Biophys. Acta 1777, 860-866. 10.1016/j.bbabio.2008.05.442 [DOI] [PubMed] [Google Scholar]
- Hoppins S. and Nunnari J. (2012). Mitochondrial dynamics and apoptosis--the ER connection. Science 337, 1052-1054. 10.1126/science.1224709 [DOI] [PubMed] [Google Scholar]
- Ingerman E., Perkins E. M., Marino M., Mears J. A., McCaffery J. M., Hinshaw J. E. and Nunnari J. (2005). Dnm1 forms spirals that are structurally tailored to fit mitochondria. J. Cell Biol. 170, 1021-1027. 10.1083/jcb.200506078 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishihara N., Nomura M., Jofuku A., Kato H., Suzuki S. O., Masuda K., Otera H., Nakanishi Y., Nonaka I., Goto Y.-i. et al. (2009). Mitochondrial fission factor Drp1 is essential for embryonic development and synapse formation in mice. Nat. Cell Biol. 11, 958-966. 10.1038/ncb1907 [DOI] [PubMed] [Google Scholar]
- James D. I., Parone P. A., Mattenberger Y. and Martinou J.-C. (2003). hFis1, a novel component of the mammalian mitochondrial fission machinery. J. Biol. Chem. 278, 36373-36379. 10.1074/jbc.M303758200 [DOI] [PubMed] [Google Scholar]
- Ji W.-k., Hatch A. L., Merrill R. A., Strack S. and Higgs H. N. (2015). Actin filaments target the oligomeric maturation of the dynamin GTPase Drp1 to mitochondrial fission sites. eLife 4, e11553 10.7554/elife.11553 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnston A. J., Hoogenraad J., Dougan D. A., Truscott K. N., Yano M., Mori M., Hoogenraad N. J. and Ryan M. T. (2002). Insertion and assembly of human tom7 into the preprotein translocase complex of the outer mitochondrial membrane. J. Biol. Chem. 277, 42197-42204. 10.1074/jbc.M205613200 [DOI] [PubMed] [Google Scholar]
- Karbowski M., Lee Y.-J., Gaume B., Jeong S.-Y., Frank S., Nechushtan A., Santel A., Fuller M., Smith C. L. and Youle R. J. (2002). Spatial and temporal association of Bax with mitochondrial fission sites, Drp1, and Mfn2 during apoptosis. J. Cell Biol. 159, 931-938. 10.1083/jcb.200209124 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koch A., Thiemann M., Grabenbauer M., Yoon Y., McNiven M. A. and Schrader M. (2003). Dynamin-like protein 1 is involved in peroxisomal fission. J. Biol. Chem. 278, 8597-8605. 10.1074/jbc.M211761200 [DOI] [PubMed] [Google Scholar]
- Koirala S., Guo Q., Kalia R., Bui H. T., Eckert D. M., Frost A. and Shaw J. M. (2013). Interchangeable adaptors regulate mitochondrial dynamin assembly for membrane scission. Proc. Natl. Acad. Sci. USA 110, E1342-E1351. 10.1073/pnas.1300855110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korobova F., Ramabhadran V. and Higgs H. N. (2013). An actin-dependent step in mitochondrial fission mediated by the ER-associated formin INF2. Science 339, 464-467. 10.1126/science.1228360 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Labbé K., Murley A. and Nunnari J. (2014). Determinants and functions of mitochondrial behavior. Annu. Rev. Cell Dev. Biol. 30, 357-391. 10.1146/annurev-cellbio-101011-155756 [DOI] [PubMed] [Google Scholar]
- Lackner L. L. (2013). Determining the shape and cellular distribution of mitochondria: the integration of multiple activities. Curr. Opin. Cell Biol. 25, 471-476. 10.1016/j.ceb.2013.02.011 [DOI] [PubMed] [Google Scholar]
- Lackner L. L. (2014). Shaping the dynamic mitochondrial network. BMC Biol. 12, 35 10.1186/1741-7007-12-35 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lazarou M., Sliter D. A., Kane L. A., Sarraf S. A., Wang C., Burman J. L., Sideris D. P., Fogel A. I. and Youle R. J. (2015). The ubiquitin kinase PINK1 recruits autophagy receptors to induce mitophagy. Nature 524, 309-314. 10.1038/nature14893 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Legesse-Miller A., Massol R. H. and Kirchhausen T. (2003). Constriction and Dnm1p recruitment are distinct processes in mitochondrial fission. Mol. Biol. Cell 14, 1953-1963. 10.1091/mbc.E02-10-0657 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li S., Xu S., Roelofs B. A., Boyman L., Lederer W. J., Sesaki H. and Karbowski M. (2015). Transient assembly of F-actin on the outer mitochondrial membrane contributes to mitochondrial fission. J. Cell Biol. 208, 109-123. 10.1083/jcb.201404050 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu R. and Chan D. C. (2015). The mitochondrial fission receptor Mff selectively recruits oligomerized Drp1. Mol. Biol. Cell 26, 4466-4477. 10.1091/mbc.E15-08-0591 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loson O. C., Song Z., Chen H. and Chan D. C. (2013). Fis1, Mff, MiD49, and MiD51 mediate Drp1 recruitment in mitochondrial fission. Mol. Biol. Cell 24, 659-667. 10.1091/mbc.E12-10-0721 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Losón O. C., Liu R., Rome M. E., Meng S., Kaiser J. T., Shan S.-o. and Chan D. C. (2014). The mitochondrial fission receptor MiD51 requires ADP as a cofactor. Structure 22, 367-377. 10.1016/j.str.2014.01.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macdonald P. J., Stepanyants N., Mehrotra N., Mears J. A., Qi X., Sesaki H. and Ramachandran R. (2014). A dimeric equilibrium intermediate nucleates Drp1 reassembly on mitochondrial membranes for fission. Mol. Biol. Cell 25, 1905-1915. 10.1091/mbc.E14-02-0728 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macdonald P. J., Francy C. A., Stepanyants N., Lehman L., Baglio A., Mears J. A., Qi X. and Ramachandran R. (2015). Distinct splice variants of dynamin-related protein 1 differentially utilize mitochondrial fission factor as an effector of cooperative GTPase activity. J. Biol. Chem., 291, 493-507. 10.1074/jbc.M115.680181 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manor U., Bartholomew S., Golani G., Christenson E., Kozlov M., Higgs H., Spudich J. and Lippincott-Schwartz J. (2015). A mitochondria-anchored isoform of the actin-nucleating spire protein regulates mitochondrial division. eLife 4, e08828 10.7554/eLife.08828 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinou J.-C. and Youle R. J. (2011). Mitochondria in apoptosis: Bcl-2 family members and mitochondrial dynamics. Dev. Cell 21, 92-101. 10.1016/j.devcel.2011.06.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mishra P. and Chan D. C. (2014). Mitochondrial dynamics and inheritance during cell division, development and disease. Nat. Rev. Mol. Cell Biol. 15, 634-646. 10.1038/nrm3877 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montague T. G., Cruz J. M., Gagnon J. A., Church G. M. and Valen E. (2014). CHOPCHOP: a CRISPR/Cas9 and TALEN web tool for genome editing. Nucleic Acids Res. 42, W401-W407. 10.1093/nar/gku410 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mozdy A. D., McCaffery J. M. and Shaw J. M. (2000). Dnm1p GTPase-mediated mitochondrial fission is a multi-step process requiring the novel integral membrane component Fis1p. J. Cell Biol. 151, 367-380. 10.1083/jcb.151.2.367 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Otera H., Wang C., Cleland M. M., Setoguchi K., Yokota S., Youle R. J. and Mihara K. (2010). Mff is an essential factor for mitochondrial recruitment of Drp1 during mitochondrial fission in mammalian cells. J. Cell Biol. 191, 1141-1158. 10.1083/jcb.201007152 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Otera H., Miyata N., Kuge O. and Mihara K. (2016). Drp1-dependent mitochondrial fission via MiD49/51 is essential for apoptotic cristae remodeling. J. Cell Biol. 212, 531-544. 10.1083/jcb.201508099 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palmer C. S., Osellame L. D., Laine D., Koutsopoulos O. S., Frazier A. E. and Ryan M. T. (2011). MiD49 and MiD51, new components of the mitochondrial fission machinery. EMBO Rep. 12, 565-573. 10.1038/embor.2011.54 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palmer C. S., Elgass K. D., Parton R. G., Osellame L. D., Stojanovski D. and Ryan M. T. (2013). MiD49 and MiD51 can act independently of Mff and Fis1 in Drp1 recruitment and are specific for mitochondrial fission. J. Biol. Chem. 288, 27584-27593. 10.1074/jbc.M113.479873 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prudent J., Zunino R., Sugiura A., Mattie S., Shore G. C. and McBride H. M. (2015). MAPL SUMOylation of Drp1 stabilizes an ER/mitochondrial platform required for cell death. Mol. Cell 59, 941-955. 10.1016/j.molcel.2015.08.001 [DOI] [PubMed] [Google Scholar]
- Ran F. A., Hsu P. D., Lin C.-Y., Gootenberg J. S., Konermann S., Trevino A. E., Scott D. A., Inoue A., Matoba S., Zhang Y. et al. (2013). Double nicking by RNA-guided CRISPR Cas9 for enhanced genome editing specificity. Cell 154, 1380-1389. 10.1016/j.cell.2013.08.021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rappsilber J., Mann M. and Ishihama Y. (2007). Protocol for micro-purification, enrichment, pre-fractionation and storage of peptides for proteomics using StageTips. Nat. Protoc. 2, 1896-1906. 10.1038/nprot.2007.261 [DOI] [PubMed] [Google Scholar]
- Richter V., Palmer C. S., Osellame L. D., Singh A. P., Elgass K., Stroud D. A., Sesaki H., Kvansakul M. and Ryan M. T. (2014). Structural and functional analysis of MiD51, a dynamin receptor required for mitochondrial fission. J. Cell Biol. 204, 477-486. 10.1083/jcb.201311014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richter V., Singh A. P., Kvansakul M., Ryan M. T. and Osellame L. D. (2015). Splitting up the powerhouse: structural insights into the mechanism of mitochondrial fission. Cell. Mol. Life Sci. 72, 3695-3707. 10.1007/s00018-015-1950-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roux K. J., Kim D. I. and Burke B. (2013). BioID: a screen for protein-protein interactions. Curr. Protoc. Protein Sci. 74, Unit 19 23 10.1002/0471140864.ps1923s74 [DOI] [PubMed] [Google Scholar]
- Roy M., Reddy P. H., Iijima M. and Sesaki H. (2015). Mitochondrial division and fusion in metabolism. Curr. Opin. Cell Biol. 33, 111-118. 10.1016/j.ceb.2015.02.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneider C. A., Rasband W. S. and Eliceiri K. W. (2012). NIH Image to ImageJ: 25 years of image analysis. Nat. Methods 9, 671-675. 10.1038/nmeth.2089 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scorrano L. (2009). Opening the doors to cytochrome c: changes in mitochondrial shape and apoptosis. Int. J. Biochem. Cell Biol. 41, 1875-1883. 10.1016/j.biocel.2009.04.016 [DOI] [PubMed] [Google Scholar]
- Shen Q., Yamano K., Head B. P., Kawajiri S., Cheung J. T. M., Wang C., Cho J.-H., Hattori N., Youle R. J. and van der Bliek A. M. (2014). Mutations in Fis1 disrupt orderly disposal of defective mitochondria. Mol. Biol. Cell 25, 145-159. 10.1091/mbc.E13-09-0525 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stojanovski D., Koutsopoulos O. S., Okamoto K. and Ryan M. T. (2004). Levels of human Fis1 at the mitochondrial outer membrane regulate mitochondrial morphology. J. Cell Sci. 117, 1201-1210. 10.1242/jcs.01058 [DOI] [PubMed] [Google Scholar]
- Strack S. and Cribbs J. T. (2012). Allosteric modulation of Drp1 mechanoenzyme assembly and mitochondrial fission by the variable domain. J. Biol. Chem. 287, 10990-11001. 10.1074/jbc.M112.342105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stroud D. A., Formosa L. E., Wijeyeratne X. W., Nguyen T. N. and Ryan M. T. (2013). Gene knockout using transcription activator-like effector nucleases (TALENs) reveals that human NDUFA9 protein is essential for stabilizing the junction between membrane and matrix arms of complex I. J Biol. Chem. 288, 1685-1690. 10.1074/jbc.C112.436766 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toyama E. Q., Herzig S., Courchet J., Lewis T. L. Jr., Loson O. C., Hellberg K., Young N. P., Chen H., Polleux F., Chan D. C. et al. (2016). AMP-activated protein kinase mediates mitochondrial fission in response to energy stress. Science 351, 275-281. 10.1126/science.aab4138 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wai T., Garcia-Prieto J., Baker M. J., Merkwirth C., Benit P., Rustin P., Rupérez F. J., Barbas C., Ibañez B. and Langer T. (2015). Imbalanced OPA1 processing and mitochondrial fragmentation cause heart failure in mice. Science 350, aad0116-1-11 10.1126/science.aad0116 [DOI] [PubMed] [Google Scholar]
- Wakabayashi J., Zhang Z., Wakabayashi N., Tamura Y., Fukaya M., Kensler T. W., Iijima M. and Sesaki H. (2009). The dynamin-related GTPase Drp1 is required for embryonic and brain development in mice. J. Cell Biol. 186, 805-816. 10.1083/jcb.200903065 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang K., Long B., Jiao J.-Q., Wang J.-X., Liu J.-P., Li Q. and Li P.-F. (2012). miR-484 regulates mitochondrial network through targeting Fis1. Nat. Commun. 3, 781 10.1038/ncomms1770 [DOI] [PubMed] [Google Scholar]
- Yamano K., Fogel A. I., Wang C., van der Bliek A. M. and Youle R. J. (2014). Mitochondrial Rab GAPs govern autophagosome biogenesis during mitophagy. eLife 3, e01612 10.7554/eLife.01612 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Youle R. J. and Karbowski M. (2005). Mitochondrial fission in apoptosis. Nat. Rev. Mol. Cell Biol. 6, 657-663. 10.1038/nrm1697 [DOI] [PubMed] [Google Scholar]
- Youle R. J. and Strasser A. (2008). The BCL-2 protein family: opposing activities that mediate cell death. Nat. Rev. Mol. Cell Biol. 9, 47-59. 10.1038/nrm2308 [DOI] [PubMed] [Google Scholar]
- Zhao J., Liu T., Jin S., Wang X., Qu M., Uhlén P., Tomilin N., Shupliakov O., Lendahl U. and Nister M. (2011). Human MIEF1 recruits Drp1 to mitochondrial outer membranes and promotes mitochondrial fusion rather than fission. EMBO J. 30, 2762-2778. 10.1038/emboj.2011.198 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.