Abstract
Background
Resting-state functional MRI (fMRI) studies have provided much evidence for abnormal intrinsic brain activity in schizophrenia, but results have been inconsistent.
Methods
We conducted a meta-analysis of whole-brain, resting-state fMRI studies that explored differences in amplitude of low-frequency fluctuation (ALFF) between people with schizophrenia (including first episode and chronic) and healthy controls.
Results
A systematic literature search identified 24 studies comparing a total of 1249 people with schizophrenia and 1179 healthy controls. Overall, patients with schizophrenia displayed decreased ALFF in the bilateral postcentral gyrus, bilateral precuneus, left inferior parietal gyri and right occipital lobe, and increased ALFF in the right putamen, right inferior frontal gyrus, left inferior temporal gyrus and right anterior cingulate cortex. In the subgroup analysis, patients with first-episode schizophrenia demonstrated decreased ALFF in the bilateral inferior parietal gyri, right precuneus and left medial prefrontal cortex, and increased ALFF in the bilateral putamen and bilateral occipital gyrus. Patients with chronic schizophrenia showed decreased ALFF in the bilateral postcentral gyrus, left precuneus and right occipital gyrus, and increased ALFF in the bilateral inferior frontal gyri, bilateral superior frontal gyrus, left amygdala, left inferior temporal gyrus, right anterior cingulate cortex and left insula.
Limitations
The small sample size of our subgroup analysis, predominantly Asian samples, processing steps and publication bias could have limited the accuracy of the results.
Conclusion
Our comprehensive meta-analysis suggests that findings of aberrant regional intrinsic brain activity during the initial stages of schizophrenia, and much more widespread damage with the progression of disease, may contribute to our understanding of the progressive pathophysiology of schizophrenia.
Introduction
Schizophrenia is one of the most severe psychiatric illnesses, typically associated with complex and diverse impairments in cognitive and affective behaviour and perceptual levels of processing, with a lifetime prevalence of 0.30% to 0.66% in the general population.1 Schizophrenia places a huge burden on patients, families and society, and continues to be a debilitatingly progressive and incurable condition. Schizophrenia is a prototypical disorder of brain connectivity that results in a classic collection of signs and symptoms, primarily starting with paranoid delusions and auditory hallucinations in adolescence or early adulthood.2–4 Although many studies of schizophrenia have been conducted over the last century, the pathophysiology of schizophrenia is yet to be fully illuminated.
Over the past decade, resting-state functional MRI (fMRI) has become a promising tool for the in vivo exploration of brain activity and connectivity, greatly enhancing our understanding of the pathophysiology of schizophrenia.5–13 In recent years, amplitude of low-frequency fluctuation (ALFF) has been used as an efficient and reliable neuroimaging marker in the exploration of resting-state regional brain activity in neuropsychiatric disorders, including schizophrenia.14–18 It is a whole-brain, resting-state fMRI analysis algorithm used to examine the total power of the local synchronization of spontaneous blood-oxygenation level–dependent signal fluctuations within a specific low-frequency range (0.01–0.08 Hz) at the single-voxel level.19 Decreases or increases in ALFF are thought to respectively reflect spontaneous hypo- or hyper-low-frequency fluctuations of neural activity in a certain voxel.20 Decreased ALFF may indicate a disease-related functional deprivation, while increased ALFF may reflect a compensatory mechanism to maintain normal cognitive performance. Alterations in ALFF in people with schizophrenia have been observed to correlate with both symptom burden21 and illness duration,22 which may be modulated using currently available treatments.23–25 However, findings from ALFF studies in schizophrenia have been inconsistent. Many studies have observed areas of decreased and increased ALFF in patients with schizophrenia relative to healthy controls,14,21,26,27 while several other studies have detected only decreased15,18 or increased23 ALFF. In addition, the affected brain regions identified in these studies were diverse: at times, different studies have reported increased or decreased ALFF in the same brain regions.14,17,27,28 Moreover, some neuroimaging studies have demonstrated that brain abnormalities in each phase (e.g., people likely to develop schizophrenia [ultrahigh risk], patients with first-episode schizophrenia and patients with chronic schizophrenia) were different in the development of schizophrenia. For patients with first-episode schizophrenia, brain abnormalities are thought to be related to genetic, prenatal and environmental factors;29 patients with chronic schizophrenia, during the progressive stages of illness, had more distractions involving the effects of antipsychotic medication and factors secondary to the illness (such as substance abuse or lifestyle), and brain abnormalities extended to wider areas of the brain.22,30 These results may reflect different neural pathophysiologies in the various stages of schizophrenia, so it is important to identify their separate neurobiological markers. Meta-analysis is an effective way of integrating the results of many studies in an unbiased way.
To our knowledge, no one has conducted an ALFF meta-analysis according to the different stages of schizophrenia. Only Xu and colleagues20 have performed a meta-analysis comparing ALFF differences between 86 patients with schizophrenia and 89 healthy controls using an activation likelihood estimation method. However, they combined the findings of 6 ALFF/fALFF studies and failed to separate patients with first-episode schizophrenia from those with chronic schizophrenia, probably because very few primary studies had been published at the time. Furthermore, they did not consider other confounding clinical variables such as drug treatments, illness duration, and Positive and Negative Syndrome Scale (PANSS) scores in their analysis. In this context, we considered it timely to conduct a meta-analysis to identify the most consistent and replicable ALFF changes in schizophrenia — particularly at different stages (i.e., first-episode and chronic). In the present study, we used seed-based d mapping (SDM), a well-established and validated meta-analytic tool for neuroimaging studies that has been widely used to detect the most spatially consistent structural and functional brain changes in a number of neuropsychiatric diseases.31–33 We also conducted meta-regression analyses to explore the potential associations between clinical variables (illness duration and PANSS scores) and ALFF changes. We hypothesized that all patients with schizophrenia, as well as patients with first-episode or chronic schizophrenia, would show distinct ALFF abnormalities in the large-scale brain networks.
Methods
Data sources, study selection and quality assessment
We conducted a comprehensive search of studies published between Jan. 1, 2000, and Apr. 24, 2018, using the PubMed, Embase, Web of Science, SinoMed, Chinese National Knowledge Infrastructure and WanFang databases and the Medical Subject Heading (MeSH) keywords “schizophrenia” OR “schizoaffective disorder” OR “schizophrenic disorder” OR “disorder, schizophrenic” OR “schizophrenic disorders” OR “dementia praecox”; AND “amplitude of low-frequency fluctuation” OR “ALFF” OR “low-frequency fluctuation” OR “LFF” OR “amplitude of low-frequency oscillation” OR “LFO.” We also checked the reference lists of the included studies and relevant review articles to identify additional relevant studies.
Studies that satisfied the following conditions were included in the meta-analysis: patients had been diagnosed with schizophrenia between the ages of 18 and 60 years; ALFF comparison of patients with schizophrenia versus healthy controls was conducted; 3-dimensional coordinates (Talairach or Montreal Neurological Institute) were reported for the whole-brain ALFF analysis; significant results were reported using thresholds for significance that were corrected for multiple comparisons or uncorrected with spatial extent thresholds; and the study was published as an original article (not as a letter or an abstract) in a peer-reviewed English- or Chinese-language journal.
Data sets were excluded if patients with schizophrenia were diagnosed with comorbid neurologic or psychiatric diseases (e.g., cognitive impairment or depression); the data were unavailable (e.g., missing neuroanatomical coordinates) even after contacting the authors by email or telephone; the data overlapped with those of another included publication; dynamic and fractional ALFF comparison of patients with schizophrenia versus healthy controls was conducted; a region-of-interest approach was used; ALFF comparison between patients with early-onset schizophrenia and healthy controls or first-degree relatives of patients with schizophrenia and healthy controls was conducted; or the ALFF study did not cover the complete band-pass data.
We used a 10-point checklist employed in previous meta-analyses of resting-state fMRI studies to assess the quality of each study selected for meta-analysis (Appendix 1, Table S1, available at jpn.ca/180245-a1).34,35 The literature search, study evaluation and study selection were conducted independently by 2 investigators (J.Y.G. and X.M.L.). Any discrepancies were resolved by a third investigator (Y.W.) for a final decision. The current study was conducted with reference to the Meta-analysis of Observational Studies in Epidemiology (MOOSE) guidelines for the meta-analyses of observational studies.36
Data analysis
Voxel-wise meta-analysis We conducted a meta-analysis of ALFF differences between patients with schizophrenia and healthy controls using SDM software (version 5.141 for Windows) in a standard process (www.sdmproject.com). The SDM approach uses effect sizes to combine reported peak coordinates that are extracted from databases with statistical parametric maps, and it recreates original maps of the effect size of ALFF difference between patients and healthy controls. We performed the analysis as described in the SDM tutorial and related publications37,38 and used MRIcron software (www.mricro.com/mricron/) to visualize SDM maps.
We describe the SDM approach briefly here. We first extracted peak coordinates and effect sizes (e.g., t values) for differences in ALFF between patients with schizophrenia and healthy controls from each data set. Then, we recreated a standard Montreal Neurological Institute map of the ALFF differences separately for each data set using an anisotropic Gaussian kernel. The mean map was finally generated by voxel-wise calculation of the random effects mean of the data set maps, weighted by the sample size, intra-data-set variability and between-data-set heterogeneity. To optimally balance false positives and negatives, we used the default SDM kernel size and thresholds (full width at half maximum [FWHM] = 20 mm; p = 0.005, uncorrected for false discovery rate; peak height Z = 1; cluster extent = 10 voxels).37,38 This FWHM kernel was intended to assign indicators of proximity to reported coordinates but not to smooth any image that was different in nature.
We first conducted the meta-analysis in all patients with schizophrenia; then, we did the same for the subgroup analysis of patients with first-episode (those with an illness duration of less than 2 years) and chronic (those with an illness duration of more than 2 years)22,39 schizophrenia. We also conducted the same analysis in medication-naïve patients with first-episode schizophrenia. We also applied the following analyses to the above 3 types of meta-analysis.
Jackknife sensitivity analysis
After preprocessing of the data, we performed a whole-brain voxel-based jackknife sensitivity analysis to test the robustness of the findings by iteratively repeating the same analysis, excluding one data set at a time.38,40 We did this to establish the extent to which the results could be replicated. If a brain region remained significant in all or most of the combinations of studies, we considered the finding to be highly replicable.
Analysis of heterogeneity and publication bias
We conducted a heterogeneity analysis using a random-effects model with Q statistics to explore unexplained between-study variability in the results. We obtained heterogeneous brain regions using the default SDM kernel size and thresholds (FWHM = 20 mm; p = 0.005, uncorrected for false discovery rate; peak height Z = 1; cluster extent = 10 voxels).37,38
We also performed Egger’s test using Stata/SE 12.0 software for Windows (Stata Corp LP) to assess possible publication bias by extracting the values from statistically significant relevant peaks between patients with schizophrenia and healthy controls.41 A p value of less than 0.05 was considered significant.
Meta-regression analyses
We carried out meta-regression analyses to explore the associations between the results of the analysis and clinical variables (e.g., illness duration, PANSS scores). The results were weighted by the square root of the sample size. To minimize the reporting of spurious relationships, we selected a more conservative threshold of p = 0.0005 as used in previous studies,38,42 requiring abnormalities to be detected both in the slope and in one of the extremes of the regressor, and discarding findings in regions other than those detected in the main analyses.
Results
Included studies and sample characteristics
After initially removing duplicates and reviewing the titles and abstracts of the 411 relevant documents found through the search strategy, we identified 51 studies as potentially eligible for inclusion. After a detailed review of full article texts, we excluded 27 studies: 10 studies investigated fALFF differences between patients with schizophrenia and healthy controls;43–52 2 studies reported the dynamic ALFF differences;53,54 2 did not cover the complete band-pass data;55,56 5 did not use whole-brain analysis;57–61 2 did not report whole-brain stereotactic coordinates;28,62 4 focused on first-degree relatives;63–66 and 2 investigated ALFF differences between patients with early-onset schizophrenia and healthy controls.67,68 In the end, 24 studies reporting 28 data sets that investigated ALFF differences between patients with schizophrenia and healthy controls were eligible for inclusion.14–18,21,23,25–28,69–80 Of these, 11 (13 data sets) were first-episode samples14,15,18,21,23,25,26,69,71,76,81 and 13 (15 data sets) were chronic samples.16,17,27,28,70,72–75,77–80 Of the first-episode samples, 69% were medication-naïve; the results for medication-naïve patients with first-episode schizophrenia are shown in Appendix 1, Figure S1 and Table S2.) A flow diagram depicting the identification and exclusion of studies is presented in Figure 1.
Fig. 1.
Flow diagram for the identification and exclusion of studies. ALFF = amplitude of low-frequency fluctuations; CNKI = Chinese National Knowledge Infrastructure; fALFF = fractional ALFF; MNI = Montreal Neurological Institute; ROI = region of interest.
The included data sets reported ALFF differences between 1249 patients with schizophrenia (583 with first-episode schizophrenia and 666 with chronic schizophrenia; 721 males and 528 females; mean age 28.93 yr; mean illness duration 70.23 mo) and 1179 healthy controls (636 males and 543 females; mean age 29.80 yr). We observed no significant differences between patients with schizophrenia and healthy controls with respect to age (standardized mean difference ~0; 95% confidence interval −4.066 to 3.082; t = −0.276; p = 0.78) or sex distribution (χ2 = 0.961; p = 0.33). Three of 24 included studies were performed on a 1.5 T scanner, and 21 were performed on a 3 T scanner. The demographic, clinical and imaging characteristics of the included studies, as well as quality scores, are summarized in Table 1.
Table 1.
Demographic, clinical and imaging characteristics of the included studies
| Study | Demographic characteristics | Clinical characteristics (patients with schizophrenia only) | Imaging characteristics | Quality score* | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
|
|
|
||||||||||||
| Participants (male), n | Mean age ± SD, yr | Illness duration, mo | PANSS total score | PANSS positive score | PANSS negative score | PANSS general psychopathology score | Scanner | Software | FWHM, mm | Threshold | ||||
|
|
|
|||||||||||||
| Schizophrenia | Healthy controls | Schizophrenia | Healthy controls | |||||||||||
| Samples from patients with first-episode schizophrenia | ||||||||||||||
| Chen et al.76 (2017) | 21 (12) | 25 (12) | 26.7 ± 9.4 | 25.8 ± 9.3 | 6.2 ± 5.9 | 83.5 ± 8.1 | 21.8 ± 6.6 | 20.1 ± 5.9 | 39.2 ± 8.9 | 3.0 T | SPM8, DPARSF | 4 | p < 0.001, uncorrected | 9.5 |
| Cui et al.26 (2016)† | 17 (10) | 19 (10) | 21.2 ± 3.9 | 23.8 ± 3.8 | 6.5 ± 6.0 | 106.2 ± 13.9 | 31.1 ± 7.1 | 25.5 ± 3.8 | 49.6 ± 9.3 | 3.0 T | DPARSF | 4 | pAlphaSim < 0.01 | 9.5 |
| 15 (8) | — | 22.5 ± 4.1 | 10.2 ± 18.2 | 88.1 ± 26.2 | 17.9 ± 9.3 | 22.7 ± 10.7 | 47.4 ± 10.4 | |||||||
| Guo et al.18 (2018) | 49 (30) | 50 (23) | 22.7 ± 4.6 | 23.5 ± 2.5 | 22.5 ± 6.7 | 91.3 ± 11.0 | 22.3 ± 5.3 | 22.8 ± 6.9 | 46.2 ± 6.8 | 3.0 T | SPM8, REST | 4 | pGRF < 0.05 | 9.5 |
| Huang et al.69 (2010) | 66 (30) | 66 (30) | 24.2 ± 8.4 | 24.5 ± 8.6 | 8.8 ± 14.1 | 107.2 ± 15.1 | 26.4 ± 5.2 | 20.7 ± 6.3 | 51.3 ± 9.2 | 3.0 T | SPM2, REST | 8 | pCORR < 0.05 | 9.5 |
| Jian et al.71 (2014) | 27 (18) | 22 (14) | 26.4 ± 8.3 | 26.6 ± 4.9 | 13.2 ± 8.3 | 96. 8 ± 12.0 | NA | NA | NA | 3.0 T | SPM8, REST | 4 | pAlphaSim < 0.05 | 9.5 |
| Lei et al.81 (2015) | 124 (61) | 102 (50) | 24.5 ± 6.7 | 24.8 ± 6.9 | 6.9 ± 8.5 | 88.4 ± 17.1 | 15.3 ± 4.3 | 15.7 ± 8.1 | NA | 3.0 T | DPARSF | 6 | pAlphaSim < 0.05 | 10 |
| Li et al.25 (2016) | 20 (6) | 16 (7) | 22.9 ± 8.5 | 22.4 ± 4.4 | 6.4 ± 13.6 | 101.6 ± 12.3 | 25.1 ± 5.7 | 18.8 ± 7.4 | 49.7 ± 7.5 | 3.0 T | SPM8, DPARSF | 8 | pAlphaSim < 0.05 | 9.5 |
| Li et al.21 (2017)† | 41 (23) | 42 (24) | 23.3 ± 6.9 | 23.3 ± 7.3 | 20.2 ± 14.0 | 86.2 ± 15.7 | 25.4 ± 6.2 | 16.5 ± 5.9 | 43.4 ± 9.5 | 1.5 T | REST | 8 | pFWE < 0.05 | 10 |
| 42 (25) | — | 22.9 ± 6.7 | 19.8 ± 13.0 | 85.8 ± 12.8 | 21.6 ± 4.9 | 23.2 ± 5.8 | 41.3 ± 6.4 | |||||||
| Liu et al.15 (2016) | 27 (15) | 27 (18) | 25.4 ± 5.9 | 27.4 ± 7.2 | 18.3 ± 15.8 | 85.8 ± 12.8 | 21.6 ± 4.9 | 23.2 ± 5.8 | 41.3 ± 6.4 | 1.5 T | REST | 8 | pFWE < 0.05 | 10 |
| Lui et al.23 (2010) | 66 (30) | 34 (13) | 24.6 ± 8.5 | 25.0 ± 8.0 | 7.8 ± 12.4 | 104.2 ± 13.9 | 26.9 ± 5.6 | 19.1 ± 6.2 | 49.9 ± 8.1 | 3.0 T | SPM2, REST | 8 | pFWE < 0.05 | 10 |
| Ren et al.14 (2013) | 100 (41) | 100 (41) | 24.3 ± 7.5 | 24.4 ± 7.6 | 6.25 ± 11.0 | 97.9 ± 17.8 | 25.1 ± 6.0 | 18.8 ± 7.7 | 47.6 ± 9.6 | 3.0 T | DPARSF | 8 | pAlphaSim < 0.05 | 9.5 |
| Samples from patients with chronic schizophrenia | ||||||||||||||
| Alonso-Solis et al.78 (2017)† | 19 (13) | 20 (13) | 40.1 ± 8.9 | 37.8 ± 7.4 | 193.3 ± 111.6 | NA | 17.9 ± 4.9 | 21.47 ± 5.9 | 34.2 ± 7.9 | 3.0 T | AFNI | 6 | pCORR < 0.05 | 10 |
| 14 (8) | — | 36.4 ± 7.1 | — | 96.0 ± 74.4 | NA | 11.4 ± 4.3 | 14.4 ± 4.7 | 27.4 ± 5.7 | ||||||
| Cui et al.75 (2016) | 40 (23) | 40 (23) | 27.9 ± 7.6 | 28.3 ± 10.0 | NA | NA | NA | NA | NA | 3.0 T | SPM8, DPARSF | 6 | pAlphaSim < 0.05 | 9.5 |
| Hoptman et al.16 (2010) | 29 (26) | 26 (19) | 36.5 ± 11.0 | 41.9 ± 10.9 | 156.0 ± 86.4 | 76.5 ± 16.6 | 18.4 ± 6.2 | 20.2 ± 6.2 | NA | 1.5 T | AFNI | 6 | p < 0.05, uncorrected | 9.5 |
| Li et al.79 (2017) | 11 (NA) | 10 (NA) | 42.2 ± 8.3 | 46.8 ± 9.1 | 219.2 ± 101.2 | 60.6 ± 9.4 | 12.8 ± 6.1 | 21.3 ± 4.5 | 26.2 ± 2.4 | 3.0 T | SPM8, REST | 6 | pAlphaSim < 0.05 | 8 |
| Liang et al.72 (2014) | 36 (NA) | 19 (NA) | 33.2 ± 9.9 | 30.0 ± 7.3 | 100.2 ± 84.5 | 80.4 ± 12.1 | 20.0 ± 4.0 | 15.8 ± 3.7 | NA | 3.0 T | SPM8, DPARSF | 4 | pAlphaSim < 0.01 | 8.5 |
| Liu et al.70 (2010) | 13 (8) | 18 (9) | 24.1 ± 4.4 | 25.4 ± 2.2 | 27.3 ± 15.7 | NA | 79.5 ± 16.9 | 79.5 ± 16.9 | NA | 3.0 T | SPM5 | 4 | p < 0.001, uncorrected | 9 |
| Lui et al.28 (2015) | 37 (22) | 59 (26) | 36.0 ± 14.0 | 38.0 ± 17.0 | 177.7 ± 143.9 | 71.2 ± 15.5 | 18.2 ± 4.8 | 18.3 ± 6.5 | 34.7 ± 7.4 | 3.0 T | SPM8, REST | 8 | pAlphaSim < 0.05 | 9 |
| Ma et al.74 (2016)† | 95 (51) | 99 (43) | 32.4 ± 6.1 | 30.9 ± 9.7 | 102.6 ± 89.2 | 72.6 ± 23.4 | 17.2 ± 7.3 | 20.9 ± 9.2 | 34.6 ± 10.6 | 3.0 T | SPM8 | 8 | pFWE < 0.05 | 8.5 |
| 95 (44) | 99 (56) | 34.9 ± 10.5 | 36.3 ± 11.5 | 135.1 ± 95.3 | 70.2 ± 22.9 | 16.5 ± 8.5 | 19.6 ± 8.6 | 34.2 ± 11.2 | ||||||
| Salvador et al.77 (2017) | 116 (81) | 122 (82) | 36.8 ± 11.1 | 36.51 ± 10.7 | 178.6 ± 143.4 | 69.2 ± 18.6 | 16.6 ± 5.6 | 19.6 ± 6.8 | 33.1 ± 9.2 | 1.5 T | FSL | NA | pFWE < 0.05 | 9.5 |
| Tan et al.80 (2017) | 32 (NA) | 32 (NA) | 29.4 ± 10.6 | 30.0 ± 10.4) | NA | NA | NA | NA | NA | 3.0 T | SPM8, REST | 6 | pAlphaSim < 0.05 | 9 |
| Turner et al.17 (2013) | 146 (111) | 160 (114) | 38.0 ± 11.3 | 37.0 ± 10.4 | 205.1 (24–456)‡ | 57.6 (37–88)‡ | 15.0 (8–25)‡ | 14.4 (7–29)‡ | 28.1 (18–43)‡ | 3.0 T | SPM5, REST | 8 | pFDR < 0.05 | 9 |
| Yang et al.73 (2014) | 9 (NA) | 9 (NA) | 23.2 ± 4.2 | NA | >60 | 83.9 ± 11.0 | 14.8 ± 2.6 | 27.7 ± 6.3 | NA | 3.0 T | SPM8, DPARSF | 4 | pAlphaSim < 0.05 | 8 |
| Yu et al.27 (2014) | 69 (NA) | 62 (NA) | 31.7 ± 9.6 | 29.9 ± 8.6 | 85.2 ± 78.0 | 52.9 ± 16.8 | 12.1 ± 4.7 | 13.4 ± 6.1 | 27.4 ± 9.6 | 3.0 T | SPM5, REST | NA | pFWE < 0.05 | 9.5 |
AFNI = analysis of functional neuroimage software; DPARSF = data processing assistant for resting-state fMRI software; FDR = false discovery rate; FSL = functional MRI of the brain’s software library; FWE = family-wise error; FWHM = full width at half maximum; GRF = Gaussian random field; NA = not available; PANSS = Positive and Negative Syndrome Scale; REST = the resting-state fMRI data analysis toolkit; SD = standard deviation; SPM = statistical parametric mapping.
Data are presented as mean ± SD unless otherwise indicated.
Quality score out of 10.
Two data sets included.
Range.
Voxel-wise meta-analysis
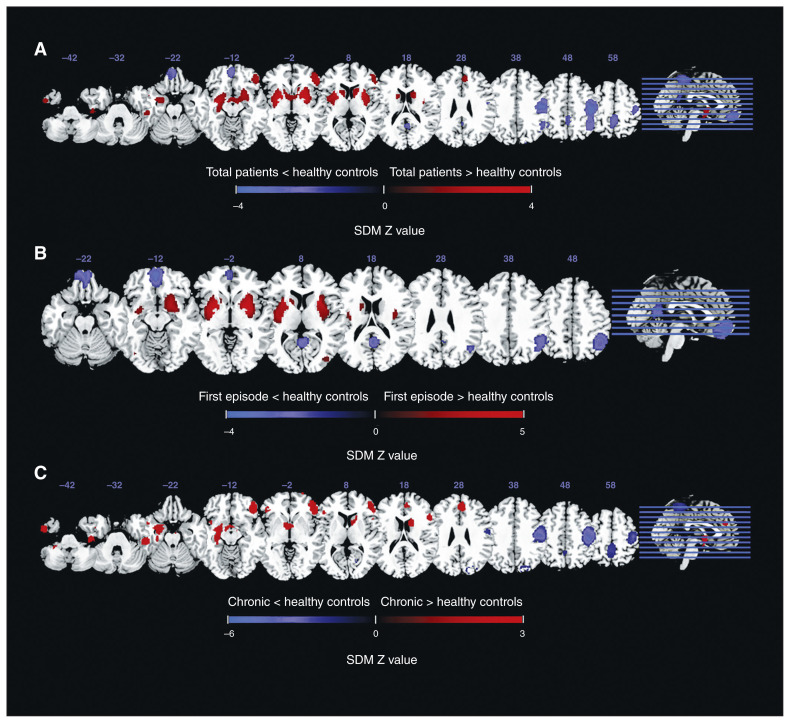
As illustrated in Figure 2, the meta-analysis brain map showed both decreased and increased ALFF in patients with schizophrenia relative to healthy controls. The total sample of patients with schizophrenia displayed decreased ALFF in the bilateral postcentral gyrus, bilateral precuneus, left inferior parietal gyri (IPG) and right occipital gyrus, and increased ALFF in the right putamen, right inferior frontal gyrus (IFG), left inferior temporal gyrus (ITG) and right anterior cingulate cortex (ACC).
Fig. 2.
Alterations in amplitude of low-frequency fluctuations (ALFF) in patients with schizophrenia compared with healthy controls. The panels reveal ALFF differences in (A) all patients with schizophrenia, (B) patients with first-episode schizophrenia and (C) patients with chronic schizophrenia, compared with healthy controls. Areas with decreased ALFF relative to healthy controls are displayed in blue, and areas with increased ALFF are displayed in red. The colour bar indicates the maximum and minimum seed-based d mapping (SDM) Z values.
In the subgroup analyses, we observed decreased ALFF in patients with first-episode schizophrenia mainly in the bilateral IPG, right precuneus and left medial prefrontal cortex (mPFC) and increased ALFF in the bilateral putamen and bilateral occipital gyrus. The results for medication-naïve patients with first-episode schizophrenia remained similar for the putamen, mPFC, angular gyrus/IPG and precuneus, but not for the occipital gyrus (Appendix 1, Figure S1 and Table S2). In patients with chronic schizophrenia, we detected decreased ALFF largely in the bilateral postcentral gyrus, left precuneus and right occipital gyrus, and increased ALFF in the bilateral IFG, bilateral superior frontal gyrus (SFG), left amygdala, left ITG, right ACC and left insula. The results for the SDM analysis are summarized in Table 2.
Table 2.
Clusters of ALFF differences in patients with schizophrenia (first episode, chronic and total) compared with healthy controls
| Sample | Cluster | Anatomic label | Brain network | Peak MNI coordinates, x, y, z | Voxels, n | SDM Z value | p value | Egger’s test (p value) | JK |
|---|---|---|---|---|---|---|---|---|---|
| Samples from all patients with schizophrenia (n = 28 data sets) | |||||||||
| Total patients > healthy controls | 1 | Right putamen | 26, 4, 2 | 4830 | 3.840 | < 0.001 | 0.38 | 28/28 | |
| 2 | Right IFG, BA 45 | SN | 50, 36, −4 | 841 | 2.654 | < 0.001 | 0.12 | 28/28 | |
| 3 | Left ITG, BA 20 | −50, 0, −36 | 233 | 2.015 | < 0.001 | 0.046 | 28/28 | ||
| 4 | Right ACC, BA32 | SN | 8, 40, 26 | 145 | 1.941 | < 0.001 | 0.06 | 26/28 | |
| 5 | Right IFG, BA 48 | SN | 48, 20, 14 | 41 | 1.698 | 0.002 | 0.20 | 26/28 | |
| Total patients < healthy controls | 6 | Right postcentral gyrus, BA 4 | SMN | 46, −20, 44 | 2615 | −3.791 | < 0.001 | 0.63 | 28/28 |
| 7 | Left precuneus, BA 5 | DMN | −2, −44, 60 | 641 | −2.239 | < 0.001 | 0.51 | 28/28 | |
| 8 | Left postcentral gyrus, BA 4 | SMN | −50, −18, 44 | 296 | −2.246 | < 0.001 | 0.71 | 27/28 | |
| 9 | Left IPG, BA 40 | DMN | −48, −54, 42 | 214 | −2.081 | 0.001 | 0.99 | 27/28 | |
| 10 | Right precuneus, BA 30 | DMN | 4, −54, 16 | 185 | −2.185 | < 0.001 | 0.10 | 26/28 | |
| 11 | Right SOG, BA 19 | VN | 26, −86, 34 | 37 | −1.844 | 0.003 | 0.028 | 27/28 | |
| 12 | Right calcarine gyrus, BA 17 | VN | 18, −68, 12 | 12 | −1.882 | 0.003 | 0.033 | 26/28 | |
| Samples from patients with first-episode schizophrenia (n = 13 data sets) | |||||||||
| First episode > healthy controls | 1 | Right putamen | 28, 6, 0 | 2223 | 4.830 | < 0.001 | 0.11 | 13/13 | |
| 2 | Left putamen | −22, 4, 0 | 1837 | 4.531 | < 0.001 | 0.10 | 13/13 | ||
| 3 | Right MOG, BA 19 | VN | 36, −82, 8 | 46 | 1.524 | 0.003 | 0.16 | 12/13 | |
| 4 | Left MOG, BA 19 | VN | −34, −72, −8 | 20 | 1.546 | 0.002 | 0.17 | 12/13 | |
| First episode < healthy controls | 5 | Left mPFC | DMN | −2, 54, −12 | 1609 | −3.478 | < 0.001 | 0.07 | 13/13 |
| 6 | Right IPG, BA 40 | DMN | 50, −48, 46 | 1294 | −2.854 | < 0.001 | 0.017 | 13/13 | |
| 7 | Right precuneus | DMN | 2, −52, 18 | 628 | −2.814 | < 0.001 | 0.013 | 13/13 | |
| 8 | Left IPG, BA 40 | DMN | −42, −52, 46 | 21 | −1.646 | 0.003 | 0.22 | 11/13 | |
| Samples from patients with chronic schizophrenia (n = 15 data sets) | |||||||||
| Chronic > healthy controls | 1 | Left amygdala | SN | −28, −6, −16 | 1558 | 2.647 | < 0.001 | 0.10 | 15/15 |
| 2 | Right IFG, BA 47 | SN | 50, 36, −10 | 1255 | 2.959 | < 0.001 | 0.06 | 15/15 | |
| 3 | Left ITG, BA 20 | −50, −28, −24 | 742 | 2.605 | < 0.001 | 0.31 | 15/15 | ||
| 4 | Right ACC, BA32 | SN | 8, 44, 28 | 384 | 2.499 | < 0.001 | 0.06 | 14/15 | |
| 5 | Left IFG, BA 47 | SN | −42, 38, −2 | 97 | 1.978 | 0.002 | 0.042 | 14/15 | |
| 6 | Left insula, BA 48 | SN | −40, 10, −10 | 20 | 1.859 | 0.002 | 0.020 | 12/15 | |
| 7 | Right SFG, BA 11 | 26, 68, 0 | 16 | 1.937 | 0.002 | 0.041 | 14/15 | ||
| 8 | Left SFG | −16, 70, 12 | 10 | 1.906 | 0.002 | 0.48 | 11/15 | ||
| Chronic < healthy controls | 9 | Right postcenral gyrus, BA 4 | SMN | 46, −20, 44 | 2517 | −5.160 | < 0.001 | 0.59 | 15/15 |
| 10 | Left postcentral gyrus, BA 4 | SMN | −50, −22, 44 | 747 | −2.893 | < 0.001 | 0.64 | 15/15 | |
| 11 | Left precuneus, BA 5 | DMN | −4, −48, 60 | 766 | −2.727 | < 0.001 | 0.55 | 15/15 | |
| 12 | Right cuneus cortex, BA 19 | VN | 18, −80, 40 | 175 | −2.200 | 0.001 | 0.15 | 15/15 | |
| 13 | Right calcarine gyrus, BA 18 | VN | 22, −70, 12 | 40 | −1.972 | 0.003 | 0.042 | 14/15 | |
ACC = anterior cingulate cortex; ALFF = amplitude of low-frequency fluctuations; BA = Brodmann area; DMN = default mode network; IFG = inferior frontal gyrus; IPG = inferior parietal gyri; ITG = inferior temporal gyrus; JK = Jackknife sensitivity analysis; MNI = Montreal Neurological Institute; MOG = middle occipital gyrus; mPFC = medial prefrontal cortex; SDM = seed-based d mapping; SFG = superior frontal gyrus; SMN = sensorimotor network; SN = salience network; SOG = superior occipital gyrus; VN = visual network.
Jackknife sensitivity analysis
A jackknife sensitivity analysis revealed that in all patients with schizophrenia, the most robust findings were for increased ALFF in the right putamen, right IFG and left ITG, and decreased ALFF in the right postcentral gyrus and left precuneus, replicable in all 28 data sets. Increased ALFF in the right ACC and right IFG and decreased ALFF in the right precuneus and right occipital gyrus also remained replicable, because they were significant in at least 26 of the 28 data sets.
In patients with first-episode schizophrenia, the most robust data were for increased ALFF in the bilateral putamen and decreased ALFF in the left SFG, right IPG and right precuneus, replicable in all 13 data sets. Increased ALFF in the bilateral occipital gyrus and decreased ALFF in the left IPG were replicated in at least 11 of the 13 data sets.
In patients with chronic schizophrenia, the most robust data were for increased ALFF in the left amygdala, right IFG and left ITG, and decreased ALFF in the bilateral postcentral gyrus, left precuneus and right occipital gyrus, replicable in all 15 data sets. Increased ALFF in the right ACC, left IFG, left insula and bilateral SFG, and decreased ALFF in the right occipital gyrus were replicated in at least 11 of the 15 data sets (Table 2).
Analysis of heterogeneity and publication bias
The analysis of heterogeneity revealed that some regions with altered ALFF showed significant unexplained between-study variability in all patients with schizophrenia (bilateral putamen, right precentral gyrus, right ITG, left gyrus rectus, right ACC, left middle occipital gyrus, left middle temporal gyrus, SFG and right superior occipital gyrus [SOG]); patients with first-episode schizophrenia (left gyrus rectus, bilateral putamen, right IPG, left middle frontal gyrus and right precuneus); and patients with chronic schizophrenia (right ITG, right caudate, middle temporal gyrus, left middle occipital gyrus, left SFG, right SOG, left Heschl gyrus, left IPG, right IFG and left precuneus; Appendix 1, Table S3).
The analysis of publication bias revealed that the Egger test was significant for the left ITG, right SOG and right calcarine gyrus in all patients with schizophrenia; for the right IPG and right precuneus in patients with first-episode schizophrenia; and for the left IFG, left insula, right SFG and right calcarine gyrus in patients with chronic schizophrenia.
Meta-regression analyses
In all patients with schizophrenia, meta-regression analysis indicated that a higher PANSS total score was correlated with greater increases in ALFF in the right putamen and SOG, and lesser decreases in the left ITG, right IFG and ACC. In patients with first-episode schizophrenia, PANSS total score was not associated with schizophrenia-related ALFF changes. In patients with chronic schizophrenia, higher PANSS total score exhibited lesser decreases in ALFF in the right IFG, ACC and left ITG. Mean illness duration was not associated with any schizophrenia-related ALFF changes. The results of the meta-regression analyses are presented in Table 3 and Appendix 1, Table S4 (subscale scores).
Table 3.
Meta-regression analyses: factors affecting ALFF in studies of patients with schizophrenia
| Factor | Anatomic label | Peak MNI coordinates, x, y, z | Voxels, n | SDM Z value | p value |
|---|---|---|---|---|---|
| All patients with schizophrenia | |||||
| Effects of illness duration | ALFF alterations in studies with higher illness duration | ||||
| None | |||||
| Effects of PANSS total score | ALFF alterations in studies with higher PANSS total score | ||||
| Right putamen | 32, −2, 4 | 235 | 3.516 | < 0.001 | |
| Right SOG, BA 19 | 16, −86, 36 | 55 | 3.055 | < 0.001 | |
| Right IFG, BA 45 | 48, 40, −4 | 471 | −3.924 | < 0.001 | |
| Right ACC, BA 32 | 8, 42, 26 | 90 | −3.283 | < 0.001 | |
| Right IFG, BA 48 | 52, 16, 20 | 31 | −2.861 | < 0.001 | |
| Left ITG, BA20 | −50, −24, −24 | 29 | −2.804 | < 0.001 | |
| Patients with first-episode schizophrenia | |||||
| Effects of illness duration | ALFF alterations in studies with higher illness duration | ||||
| None | |||||
| Effects of PANSS total score | ALFF alterations in studies with higher PANSS total score | ||||
| None | |||||
| Patients with chronic schizophrenia | |||||
| Effects of illness duration | ALFF alterations in studies with higher illness duration | ||||
| None | |||||
| Effects of PANSS total score | ALFF alterations in studies with higher PANSS total score | ||||
| Right IFG, BA 45 | 46, 38, −2 | 289 | −3.582 | < 0.001 | |
| Right ACC, BA 32 | 8, 42, 26 | 119 | −3.633 | < 0.001 | |
| Left ITG, BA 20 | −50, −24, −24 | 54 | −3.121 | < 0.001 | |
ACC = anterior cingulate cortex; ALFF = amplitude of low-frequency fluctuations; BA = Brodmann area; IFG = inferior frontal gyrus; ITG = inferior temporal gyrus; MNI = Montreal Neurological Institute; PANSS = Positive and Negative Syndrome Scale; SDM = seed-based d mapping; SOG = superior occipital gyrus.
Discussion
As far as we know, the current study is the first quantitative meta-analysis to combine whole-brain ALFF findings from resting-state fMRI studies in patients at different stages of schizophrenia in a voxel-wise manner. The results of our meta-analysis displayed decreased ALFF in the default mode network (DMN), sensorimotor network (SMN) and visual network (VN) regions, and increased ALFF in the putamen, salience network (SN) and frontotemporal regions in all patients with schizophrenia. In the subgroup analysis, patients with first-episode schizophrenia showed decreased ALFF in the DMN regions and increased ALFF in the putamen and VN regions; moreover, the results for the DMN and putamen regions remained consistent in medication-naïve patients with first-episode schizophrenia. In patients with chronic schizophrenia, decreased ALFF was largely detected in the DMN, SMN and VN regions and increased ALFF mainly in the SN and frontotemporal regions. These results overlapped somewhat with those of a previous study,20 except for an extremely significant increase of ALFF in the putamen in patients with first-episode schizophrenia and much more widespread brain functional damage in patients with chronic schizophrenia. Although the included studies showed some heterogeneity and publication bias, we verified the main results to be highly replicable and stable using jackknife sensitivity analysis. Meta-regression analyses demonstrated that in all patients with schizophrenia and patients with chronic schizophrenia, ALFF alterations involving the putamen, SOG, IFG, ACC and ITG were correlated with PANSS total score.
ALFF alterations in patients with first-episode schizophrenia
Our meta-analysis found consistent regions of decreased ALFF in the DMN in patients with schizophrenia, particularly in the first-episode stage, including the bilateral IPG, right precuneus and left mPFC, suggesting disruption in the DMN regions in the early course of the disorder. Furthermore, medication-naïve first-episode patients displayed decreased ALFF in the DMN, including the right angular gyrus/IPG, right precuneus and left mPFC. This finding was inconsistent with the results of a recent meta-analysis on ALFF abnormalities in first-episode and drug-naïve patients with schizophrenia, which reported decreased ALFF in the right cerebellar lobule VIII, left cerebellar lobule IX and right cerebellar Crus I.82 This inconsistency may be attributed to inadequate sample size (smaller than ours, 783 patients and 704 healthy controls) and participant demographic characteristics (including patients with early-onset schizophrenia, 13 to 18 years old), which may have weakened the efficacy of the meta-analysis. Our findings in the DMN were in similar regions to those of reduced grey matter volume in schizophrenia reported in a meta-analysis of voxel-based morphometry studies.83 The DMN (including the PCC/precuneus, mPFC and IPG) plays an important role in cognitive performance, such as autobiographical memory, decision-making, mental theory and monitoring of the surrounding environment.84 The DMN has recently attracted attention as a novel means of understanding the neural mechanisms of schizophrenia. Aberrant intrinsic connectivity and activity patterns of the DMN in schizophrenia have been documented in many reports and also associated with social cognition and social functioning impairments,13,85,86 executive deficits,87 self-referential processing abnormalities,88 dysfunction of autobiographical memory,89 auditory-verbal hallucinations90,91 and abnormal attentional control processing.92 As the core of the DMN, the mPFC is important for the affective value of reinforcers, decision-making, the generation and regulation of negative emotion, social cognition and expectation.93 The IPG, one of the major connection hubs, is associated with constructing mental scenes, episodic memory, spatial attention and language processing.94,95 The precuneus has been implicated in the neural processing of retrieval of autobiographical episodic memory, as well as self-referential tasks.96 In this meta-analysis, decreased ALFF in the precuneus was also found in patients with schizophrenia at different stages (first-episode and chronic). Our results forcefully identify for the first time the most reliable brain areas of ALFF alteration in the DMN that play critical roles in the pathophysiology of schizophrenia. These findings were not affected by illness duration, suggesting that such changes were independent of the disease stages that occur early in schizophrenia, and they might serve as potential neuroimaging biomarkers.
The current meta-analysis found increased ALFF in the putamen in patients with schizophrenia, particularly in those with first-episode schizophrenia (including medication-naïve patients). The putamen is a portion of the striatum, which includes 3 main functional subregions — sensory movement, cognition and limbic (or emotion/motivation) — playing a critical role in multiple aspects of motor function, executive/associative function and emotion/motivation.97 Structural and functional abnormalities of the putamen have been found in schizophrenia using MRI. The involvement of the putamen in speech production and language processing has been reported,98,99 and lower volume and hyperconnectivity in the putamen have been found in patients with schizophrenia with auditory–verbal hallucinations.100,101 Another study revealed that decreased ALFF in the left putamen was relatively specific to auditory–verbal hallucinations,26 and putamen volume loss may be a biological correlate of delusions in schizophrenia.102 The putamen also has widespread connections with the frontal cortex, involved in motor sequence performance and habitual instrumental behaviour; executive function and goal-directed behaviour; emotion processing; and reinforcement learning.103 In our meta-analysis, the increased cluster of putamen in patients with first-episode schizophrenia did not appear in patients with chronic schizophrenia; we speculate that increased ALFF in the putamen plays an important compensatory mechanism for maintaining normal cognitive performance in the early stages of schizophrenia.
Interestingly, our study observed an inconsistent activation in the VN regions (occipital gyrus, including BA 17, 18 and 19). We found increased ALFF in the patients with first-episode schizophrenia and decreased ALFF in patients with chronic schizophrenia, suggesting altered intrinsic activity in the VN. In schizophrenia, both structural and functional abnormalities in the VN have been previously reported. For example, the association of visual hallucinations with VN damage in schizophrenia has already been demonstrated, as well as a decrease in the overall volume of the occipital gyrus, and reduction in magnetization transfer ratio and fractional anisotropy in the white matter adjacent to visual processing regions in patients with schizophrenia.22,104,105 A recent fMRI study proved that patients with schizophrenia have decreased activation in the VN during performance of several cognitive tasks.104 Diminished strength of functional connectivity and reduced ALFF during the resting state in patients with schizophrenia have also been found throughout the VN.16,106 Therefore, it is possible that decreased ALFF in the VN in patients with chronic schizophrenia correlate with vision impairment, while increased ALFF in the VN in first-episode patients plays an important role in vision compensation for schizophrenia.
ALFF alterations in patients with chronic schizophrenia
Dividing the longitudinal course of a disease into different stages (ultra-high risk, first-episode and chronic schizophrenia) might reveal different dynamic and pathophysiological processes that occur in the brain at different times. Our meta-analysis approach involving data sets in chronic schizophrenia from different regions showed that ALFF differences compared with healthy controls and patients with first-episode schizophrenia became much more widespread, most prominently including decreased ALFF in the DNM (precuneus), SMN (postcentral gyrus) and VN (occipital gyrus), and increased ALFF in the SN (amygdala, insula, IFG and ACC) and frontotemporal regions (SFG, IFG and ITG). Decreased ALFF in the SMA and VN, and increased ALFF in the SN and frontotemporal regions, reflects a progressive imbalance between the primary and advanced functional areas, which might be related to abnormal function patterns in patients with chronic schizophrenia. However, it remains difficult to control for the effects of antipsychotic medication and factors secondary to the illness (such as substance abuse or lifestyle) on brain structure. Moreover, meta-regression analyses revealed that the ALFF in the ACC, IFG and ITG were correlated with PANSS total score, suggesting that the damage to SN and ITG activity was more serious with more severe clinical symptoms of schizophrenia.
The SMN is known to play a key role in the processing of colour, form perception, motion, stereopsis, depth, orientation and subserving motor control, action selection, preparation and motor execution, especially for internally generated movement.107,108 Schizophrenia commonly exhibits a variety of symptoms, such as psychomotor and fine-motor symptoms, as well as touch, temperature, nociception, tension and vibration abnormalities. Functional107,109 and structural110,111 abnormalities in the SMA have been reported that may be correlated with the increased involuntary movements, severe motor impairments and neurologic soft signs (describing neurologic abnormalities in sensory integration, motor regulation, sequencing of complex motor acts and primitive reflexes) that occur in the majority of patients with schizophrenia.112 Our finding, which was consistent with those of another meta-analysis20 and the findings described above, may provide further evidence that the SMN plays a key role in the psychomotor symptoms of schizophrenia. Decreased intrinsic activity in the bilateral postcentral gyrus may serve as a differentiating feature of chronic schizophrenia.
The current meta-analysis found increased ALFF in the SN (including the left amygdala, left insula, bilateral IFG [BA 47] and right ACC) in patients with chronic schizophrenia. The SN plays a central role in emotion processing and autonomic regulation. It has been proposed to function in the identification of internal and external stimuli (salience) and the shifting of brain function from DMN to central executive network activities.113 Several schizophrenia symptoms (e.g., hallucinations, delusions, disorganization and psychomotor poverty) and deficits in cognitive function may be related to SN dysfunction.114–116 Meta-analyses of neuroimaging studies in schizophrenia have reported reductions in the volume of the constituent regions of the SN,117 its internal coherence and its integration within the brain functional connectome.118 Decreased connectivity between the SN and the calcarine fissure was found, significantly correlated with PANSS score.10 Increased functional connectivity strength in the insula119 and ACC,120 and increased intra-SN temporal dynamics of the connectivity were also found,121 similar to our observation of comprehensive increases in the SN in ALFF studies of chronic and total patients with schizophrenia. Thus, the SN is unstable in schizophrenia, and this could play a functional compensation role for the systematic structural anomalies found in schizophrenia.
We also identified increased ALFF in the frontotemporal regions (including the bilateral SFG, IFG and left ITG) in patients with chronic schizophrenia. Our meta-regression analyses also revealed that the increased ALFF in the frontotemporal lobe was negatively correlated with PANSS total score, suggesting that the brain activity damage was more serious with the progression of disease. Task-based fMRI studies found an increase in oxy-hemoglobin in the frontal and temporal regions in patients with schizophrenia during the verbal fluency task.122 The frontal lobe, which includes the language-related areas in the IFG (Broca’s area), is critically involved in speech production and language processing.123 It is functionally connected with the temporal lobe to form a language network; its dysfunction has been correlated with delusions, blunted affect and thought disorders in schizophrenia.30 Our ALFF analysis of 666 patients with chronic schizophrenia showed increased ALFF changes in the IFG, but unlike the findings of Li and colleagues,30 the increased functional connectivity involving the IFG presented only in first-episode patients, suggesting that the IFG is responsible for the core pathogenesis in the entire course of schizophrenia. Further work using other structural, functional and metabolic methods are needed to examine this hypothesis.
Limitations
Our study had several limitations. First, given a lack of data, the sample size of our subgroup analysis on the first-episode and chronic patients was somewhat small, and some of the results should be interpreted with caution. As well, we selected a duration-based definition for first-episode schizophrenia of less than 2 years,22,39 while some studies have selected patients with disease durations of less than 1, 3 or 5 years to define first-episode schizophrenia.14,18,21 Given that a patient in the first-episode group may have a second episode during these 2 years, we looked back at the 11 articles included in the first-episode group, and none of the patients had experienced more than 1 episode during their illness duration. Moreover, we were unable to conduct subgroup meta-analyses of the studies of people at ultra-high risk of developing schizophrenia because of a limited number of data sets (only 4 studies). Second, our meta-analysis included mainly Asian samples (23/28 Asian, 3/28 European, 2/28 American), which may limit the application of these findings to other populations. Further efforts are needed to expand the ALFF method to other schizophrenia populations. Third, our meta-analysis is based on coordinates from studies rather than on raw data, limiting its accuracy.40 Further studies with large homogeneous samples are needed to verify our results. Fourth, the Egger test indicated a potential publication bias in the subgroup analysis; it will be important to validate our findings with an updated meta-analysis. Fifth, the sample included in the chronic schizophrenia group had a relatively wide age range, but the sample included in the first-episode schizophrenia group was mostly in their 20s. Thus, our results should be interpreted cautiously and regard age as a covariate when comparing first-episode with patients with chronic schizophrenia. Finally, we found substantial heterogeneity in our results for some regions with altered ALFF, a finding that may be attributed to the clinical diversity of the participants and the methodological and statistical diversity of the studies, partially limiting the interpretability and generalizability of the results.
Conclusion
Our comprehensive meta-analysis suggests that during the initial stages of schizophrenia (that is, patients first-episode schizophrenia), aberrant regional intrinsic brain activity predominantly involved the DMN, VN and putamen. With the progression of disease, brain activity abnormalities progressed over time. Patients with chronic schizophrenia demonstrated much more widespread brain functional damage, including the DMN, SN, SMN, VN and frontotemporal regions, contributing to our understanding of the progressive pathophysiology of schizophrenia. Further work is required to determine whether this pattern of altered ALFF can serve as a neuroimaging marker for schizophrenia.
Footnotes
Funding: The study was supported by grants from the National Natural Science Foundation of China (81671670, 81501456, 81471650 and 81801685); Planned Science and Technology Project of Guangdong Province, China (2014B020212022); Planned Science and Technology Project of Guangzhou, China (1563000653, 201508020004, 201604020007 and 201604020184); and National Natural Science Foundation of Guangdong Province, China (2018A030310003). The funding organizations played no role in study design; data collection, analysis or interpretation; writing; or decision to publish the manuscript.
Competing interests: The authors declare that they have no competing interests.
Contributors: L. Huang and Y. Wang designed the study. J. Gong and X. Luo acquired the data, which J. Wang, G. Chen, H. Huang and R. Huang analyzed. J. Gong wrote the article, which all authors reviewed. All authors approved the final version to be published and can certify that no other individuals not listed as authors have made substantial contributions to the paper.
References
- 1.McGrath J, Saha S, Chant D, et al. Schizophrenia: a concise overview of incidence, prevalence, and mortality. Epidemiol Rev. 2008;30:67–76. doi: 10.1093/epirev/mxn001. [DOI] [PubMed] [Google Scholar]
- 2.Fornito A, Zalesky A, Pantelis C, et al. Schizophrenia, neuro-imaging and connectomics. Neuroimage. 2012;62:2296–314. doi: 10.1016/j.neuroimage.2011.12.090. [DOI] [PubMed] [Google Scholar]
- 3.Insel TR. Rethinking schizophrenia. Nature. 2010;468:187–93. doi: 10.1038/nature09552. [DOI] [PubMed] [Google Scholar]
- 4.Kambeitz J, Kambeitz-Ilankovic L, Leucht S, et al. Detecting neuroimaging biomarkers for schizophrenia: a meta-analysis of multivariate pattern recognition studies. Neuropsychopharmacol. 2015;40:1742–51. doi: 10.1038/npp.2015.22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wang L, Metzak PD, Honer WG, et al. Impaired efficiency of functional networks underlying episodic memory-for-context in schizophrenia. J Neurosci. 2010;30:13171–9. doi: 10.1523/JNEUROSCI.3514-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bassett DS, Nelson BG, Mueller BA, et al. Altered resting state complexity in schizophrenia. Neuroimage. 2012;59:2196–207. doi: 10.1016/j.neuroimage.2011.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rubinov M, Bullmore E. Schizophrenia and abnormal brain network hubs. Dialogues Clin Neurosci. 2013;15:339–49. doi: 10.31887/DCNS.2013.15.3/mrubinov. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Penner J, Ford KA, Taylor R, et al. Medial prefrontal and anterior insular connectivity in early schizophrenia and major depressive disorder: a resting functional MRI evaluation of large-scale brain network models. Front Hum Neurosci. 2016;10:132. doi: 10.3389/fnhum.2016.00132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lang X, Wang L, Zhuo CJ, et al. Reduction of interhemispheric functional connectivity in sensorimotor and visual information processing pathways in schizophrenia. Chin Med J (Engl) 2016;129:2422–6. doi: 10.4103/0366-6999.191758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mueller F, Musso F, London M, et al. Pharmacological fMRI: effects of subanesthetic ketamine on resting-state functional connectivity in the default mode network, salience network, dorsal attention network and executive control network. Neuroimage Clin. 2018;19:745–57. doi: 10.1016/j.nicl.2018.05.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Shao J, Meng C, Tahmasian M, et al. Common and distinct changes of default mode and salience network in schizophrenia and major depression. Brain Imaging Behav. 2018;12:1708–19. doi: 10.1007/s11682-018-9838-8. [DOI] [PubMed] [Google Scholar]
- 12.McNabb CB, Sundram F, Soosay I, et al. Increased sensorimotor network connectivity associated with clozapine eligibility in people with schizophrenia. Psychiatry Res Neuroimaging. 2018;275:36–42. doi: 10.1016/j.pscychresns.2018.02.008. [DOI] [PubMed] [Google Scholar]
- 13.Fox JM, Abram SV, Reilly JL, et al. Default mode functional connectivity is associated with social functioning in schizophrenia. J Abnorm Psychol. 2017;126:392–405. doi: 10.1037/abn0000253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ren W, Lui S, Deng W, et al. Anatomical and functional brain abnormalities in drug-naive first-episode schizophrenia. Am J Psychiatry. 2013;170:1308–16. doi: 10.1176/appi.ajp.2013.12091148. [DOI] [PubMed] [Google Scholar]
- 15.Liu C, Xue Z, Palaniyappan L, et al. Abnormally increased and incoherent resting-state activity is shared between patients with schizophrenia and their unaffected siblings. Schizophr Res. 2016;171:158–65. doi: 10.1016/j.schres.2016.01.022. [DOI] [PubMed] [Google Scholar]
- 16.Hoptman MJ, Zuo XN, Butler PD, et al. Amplitude of low-frequency oscillations in schizophrenia: a resting state fMRI study. Schizophr Res. 2010;117:13–20. doi: 10.1016/j.schres.2009.09.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Turner JA, Damaraju E, van Erp TG, et al. A multi-site resting state fMRI study on the amplitude of low frequency fluctuations in schizophrenia. Front Neurosci. 2013;7:137. doi: 10.3389/fnins.2013.00137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Guo W, Zhang F, Liu F, et al. Cerebellar abnormalities in first-episode, drug-naive schizophrenia at rest. Psychiatry Res Neuroimaging. 2018;276:73–9. doi: 10.1016/j.pscychresns.2018.03.010. [DOI] [PubMed] [Google Scholar]
- 19.Zang YF, He Y, Zhu CZ, et al. Altered baseline brain activity in children with ADHD revealed by resting-state functional MRI. Brain Dev. 2007;29:83–91. doi: 10.1016/j.braindev.2006.07.002. [DOI] [PubMed] [Google Scholar]
- 20.Xu Y, Zhuo C, Qin W, et al. Altered spontaneous brain activity in schizophrenia: a meta-analysis and a large-sample study. Biomed Res Int. 2015;2015 doi: 10.1155/2015/204628. 204628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Li Z, Lei W, Deng W, et al. Aberrant spontaneous neural activity and correlation with evoked-brain potentials in first-episode, treatment naive patients with deficit and non-deficit schizophrenia. Psychiatry Res Neuroimaging. 2017;261:9–19. doi: 10.1016/j.pscychresns.2017.01.001. [DOI] [PubMed] [Google Scholar]
- 22.Zhao C, Zhu J, Liu X, et al. Structural and functional brain abnormalities in schizophrenia: a cross-sectional study at different stages of the disease. Prog Neuropsychopharmacol Biol Psychiatry. 2018;83:27–32. doi: 10.1016/j.pnpbp.2017.12.017. [DOI] [PubMed] [Google Scholar]
- 23.Lui S, Li T, Deng W, et al. Short-term effects of antipsychotic treatment on cerebral function in drug-naive first-episode schizophrenia revealed by “resting state” functional magnetic resonance imaging. Arch Gen Psychiatry. 2010;67:783–92. doi: 10.1001/archgenpsychiatry.2010.84. [DOI] [PubMed] [Google Scholar]
- 24.Turner JA, Chen H, Mathalon DH, et al. Reliability of the amplitude of low-frequency fluctuations in resting state fMRI in chronic schizophrenia. Psychiatry Res. 2012;201:253–5. doi: 10.1016/j.pscychresns.2011.09.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Li F, Lui S, Yao L, et al. Longitudinal changes in resting-state cerebral activity in patients with first-episode schizophrenia: a 1-year follow-up functional MR imaging study. Radiology. 2016;279:867–75. doi: 10.1148/radiol.2015151334. [DOI] [PubMed] [Google Scholar]
- 26.Cui LB, Liu K, Li C, et al. Putamen-related regional and network functional deficits in first-episode schizophrenia with auditory verbal hallucinations. Schizophr Res. 2016;173:13–22. doi: 10.1016/j.schres.2016.02.039. [DOI] [PubMed] [Google Scholar]
- 27.Yu R, Chien YL, Wang HL, et al. Frequency-specific alternations in the amplitude of low-frequency fluctuations in schizophrenia. Hum Brain Mapp. 2014;35:627–37. doi: 10.1002/hbm.22203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lui S, Yao L, Xiao Y, et al. Resting-state brain function in schizophrenia and psychotic bipolar probands and their first-degree relatives. Psychol Med. 2015;45:97–108. doi: 10.1017/S003329171400110X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Van Os J, Kapur S. Schizophrenia. Lancet. 2009;374:635–45. doi: 10.1016/S0140-6736(09)60995-8. [DOI] [PubMed] [Google Scholar]
- 30.Li T, Wang Q, Zhang J, et al. Brain-wide analysis of functional connectivity in first-episode and chronic stages of schizophrenia. Schizophr Bull. 2017;43:436–48. doi: 10.1093/schbul/sbw099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Luijten M, Schellekens AF, Kuhn S, et al. Disruption of reward processing in addiction: an image-based meta-analysis of functional magnetic resonance imaging studies. JAMA Psychiatry. 2017;74:387–98. doi: 10.1001/jamapsychiatry.2016.3084. [DOI] [PubMed] [Google Scholar]
- 32.Pan P, Zhan H, Xia M, et al. Aberrant regional homogeneity in Parkinson’s disease: a voxel-wise meta-analysis of resting-state functional magnetic resonance imaging studies. Neurosci Biobehav Rev. 2017;72:223–31. doi: 10.1016/j.neubiorev.2016.11.018. [DOI] [PubMed] [Google Scholar]
- 33.Iwabuchi SJ, Krishnadas R, Li C, et al. Localized connectivity in depression: a meta-analysis of resting state functional imaging studies. Neurosci Biobehav Rev. 2015;51:77–86. doi: 10.1016/j.neubiorev.2015.01.006. [DOI] [PubMed] [Google Scholar]
- 34.Chen ZQ, Du MY, Zhao YJ, et al. Voxel-wise meta-analyses of brain blood flow and local synchrony abnormalities in medication-free patients with major depressive disorder. J Psychiatry Neurosci. 2015;40:401–11. doi: 10.1503/jpn.140119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Shepherd AM, Matheson SL, Laurens KR, et al. Systematic meta-analysis of insula volume in schizophrenia. Biol Psychiatry. 2012;72:775–84. doi: 10.1016/j.biopsych.2012.04.020. [DOI] [PubMed] [Google Scholar]
- 36.Stroup DF, Berlin JA, Morton SC, et al. Meta-analysis of observational studies in epidemiology: a proposal for reporting. Meta-analysis Of Observational Studies in Epidemiology (MOOSE) group. JAMA. 2000;283:2008–12. doi: 10.1001/jama.283.15.2008. [DOI] [PubMed] [Google Scholar]
- 37.Lim L, Radua J, Rubia K. Gray matter abnormalities in childhood maltreatment: a voxel-wise meta-analysis. Am J Psychiatry. 2014;171:854–63. doi: 10.1176/appi.ajp.2014.13101427. [DOI] [PubMed] [Google Scholar]
- 38.Radua J, Rubia K, Canales-Rodriguez EJ, et al. Anisotropic kernels for coordinate-based meta-analyses of neuroimaging studies. Front Psychiatry. 2014;5:13. doi: 10.3389/fpsyt.2014.00013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Siegel SJ, Irani F, Brensinger CM, et al. Prognostic variables at intake and long-term level of function in schizophrenia. Am J Psychiatry. 2006;163:433–41. doi: 10.1176/appi.ajp.163.3.433. [DOI] [PubMed] [Google Scholar]
- 40.Radua J, Mataix-Cols D. Voxel-wise meta-analysis of grey matter changes in obsessive-compulsive disorder. Br J Psychiatry. 2009;195:393–402. doi: 10.1192/bjp.bp.108.055046. [DOI] [PubMed] [Google Scholar]
- 41.Radua J, Grau M, van den Heuvel OA, et al. Multimodal voxel-based meta-analysis of white matter abnormalities in obsessive-compulsive disorder. Neuropsychopharmacol. 2014;39:1547–57. doi: 10.1038/npp.2014.5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Radua J, Mataix-Cols D, Phillips ML, et al. A new meta-analytic method for neuroimaging studies that combines reported peak coordinates and statistical parametric maps. Eur Psychiatry. 2012;27:605–11. doi: 10.1016/j.eurpsy.2011.04.001. [DOI] [PubMed] [Google Scholar]
- 43.He Z, Deng W, Li M, et al. Aberrant intrinsic brain activity and cognitive deficit in first-episode treatment-naive patients with schizophrenia. Psychol Med. 2013;43:769–80. doi: 10.1017/S0033291712001638. [DOI] [PubMed] [Google Scholar]
- 44.Guo W, Su Q, Yao D, et al. Decreased regional activity of default-mode network in unaffected siblings of schizophrenia patients at rest. Eur Neuropsychopharmacol. 2014;24:545–52. doi: 10.1016/j.euroneuro.2014.01.004. [DOI] [PubMed] [Google Scholar]
- 45.Chyzhyk D, Grana M, Ongur D, et al. Discrimination of schizophrenia auditory hallucinators by machine learning of resting-state functional MRI. Int J Neural Syst. 2015;25:1550007. doi: 10.1142/S0129065715500070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Guo W, Liu F, Xiao C, et al. Dissociation of anatomical and functional alterations of the default-mode network in first-episode, drug-naive schizophrenia. Clin Neurophysiol. 2015;126:2276–81. doi: 10.1016/j.clinph.2015.01.025. [DOI] [PubMed] [Google Scholar]
- 47.Chang X, Luo C, Hou C, et al. Different effects of taking aripiprazole and risperidone on spontaneous brain activity in schizophrenics [in Chinese] Sichuan Mental Health. 2015;28:492–5. [Google Scholar]
- 48.Sui J, Pearlson GD, Du Y, et al. In search of multimodal neuroimaging biomarkers of cognitive deficits in schizophrenia. Biol Psychiatry. 2015;78:794–804. doi: 10.1016/j.biopsych.2015.02.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Fryer SL, Roach BJ, Wiley K, et al. Reduced amplitude of low-frequency brain oscillations in the psychosis risk syndrome and early illness schizophrenia. Neuropsychopharmacol. 2016;41:2388–98. doi: 10.1038/npp.2016.51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Guo W, Liu F, Chen J, et al. Hyperactivity of the default-mode network in first-episode, drug-naive schizophrenia at rest revealed by family-based case-control and traditional case-control designs. Medicine (Baltimore) 2017;96:e6223. doi: 10.1097/MD.0000000000006223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Iwabuchi SJ, Palaniyappan L. Abnormalities in the effective connectivity of visuothalamic circuitry in schizophrenia. Psychol Med. 2017;47:1300–10. doi: 10.1017/S0033291716003469. [DOI] [PubMed] [Google Scholar]
- 52.Meng X, Jiang R, Lin D, et al. Predicting individualized clinical measures by a generalized prediction framework and multimodal fusion of MRI data. Neuroimage. 2017;145:218–29. doi: 10.1016/j.neuroimage.2016.05.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Shen H, Li Z, Zeng LL, et al. Internetwork dynamic connectivity effectively differentiates schizophrenic patients from healthy controls. Neuroreport. 2014;25:1344–9. doi: 10.1097/WNR.0000000000000267. [DOI] [PubMed] [Google Scholar]
- 54.Fu Z, Tu Y, Di X, et al. Characterizing dynamic amplitude of low-frequency fluctuation and its relationship with dynamic functional connectivity: an application to schizophrenia. Neuroimage. 2018;180:619–31. doi: 10.1016/j.neuroimage.2017.09.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Meda SA, Wang Z, Ivleva EI, et al. Frequency-specific neural signatures of spontaneous low-frequency resting state fluctuations in psychosis: evidence from Bipolar-Schizophrenia Network on Intermediate Phenotypes (B-SNIP) consortium. Schizophr Bull. 2015;41:1336–48. doi: 10.1093/schbul/sbv064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Hare SM, Ford JM, Ahmadi A, et al. Modality-dependent impact of hallucinations on low-frequency fluctuations in schizophrenia. Schizophr Bull. 2017;43:389–96. doi: 10.1093/schbul/sbw093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Sui J, He H, Yu Q, et al. Combination of resting state fMRI, DTI, and sMRI data to discriminate schizophrenia by N-way MCCA + jICA. Front Hum Neurosci. 2013;7:235. doi: 10.3389/fnhum.2013.00235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Zhou Y, Wang Z, Zuo XN, et al. Hyper-coupling between working memory task-evoked activations and amplitude of spontaneous fluctuations in first-episode schizophrenia. Schizophr Res. 2014;159:80–9. doi: 10.1016/j.schres.2014.07.023. [DOI] [PubMed] [Google Scholar]
- 59.Wang X, Li F, Zheng H, et al. Breakdown of the striatal-default mode network loop in schizophrenia. Schizophr Res. 2015;168:366–72. doi: 10.1016/j.schres.2015.07.027. [DOI] [PubMed] [Google Scholar]
- 60.Fryer SL, Roach BJ, Ford JM, et al. Relating intrinsic low-frequency BOLD cortical oscillations to cognition in schizophrenia. Neuropsychopharmacol. 2015;40:2705–14. doi: 10.1038/npp.2015.119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.McHugo M, Rogers BP, Talati P, et al. Increased amplitude of low frequency fluctuations but normal hippocampal-default mode network connectivity in schizophrenia. Front Psychiatry. 2015;6:92. doi: 10.3389/fpsyt.2015.00092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Cui LB, Liu L, Guo F, et al. Disturbed brain activity in resting-state networks of patients with first-episode schizophrenia with auditory verbal hallucinations: a cross-sectional functional MR imaging study. Radiology. 2017;283:810–9. doi: 10.1148/radiol.2016160938. [DOI] [PubMed] [Google Scholar]
- 63.Tian L, Yan H, Zhao Q, et al. Amplitude of low-frequency fluctuations of the first-degree relatives of schizophrenia patients: a resting-state functional magnetic resonance imaging study [in Chinese] Chin J Psychiatry. 2014;47:137–41. [Google Scholar]
- 64.Tang Y, Chen K, Zhou Y, et al. Neural activity changes in unaffected children of patients with schizophrenia: a resting-state fMRI study. Schizophr Res. 2015;168:360–5. doi: 10.1016/j.schres.2015.07.025. [DOI] [PubMed] [Google Scholar]
- 65.Guo W, Song Y, Liu F, et al. Dissociation of functional and anatomical brain abnormalities in unaffected siblings of schizophrenia patients. Clin Neurophysiol. 2015;126:927–32. doi: 10.1016/j.clinph.2014.08.016. [DOI] [PubMed] [Google Scholar]
- 66.Wang Y, Wang D, Wei S, et al. A resting-state functional magnetic resonance imaging study in first-episode drug-naïve schizophrenia and offspring of schizophrenia patients [in Chinese] Chin J Nerv Ment Dis. 2016;42:342–6. [Google Scholar]
- 67.Lv D, Shao R, Liang Y, et al. FractionaI amplitude of low-frequency fluctuations in childhood and adolescence-onset schizophrenia: a resting state fMRI study [in Chinese] Nat Med J China. 2016;96:3479–84. doi: 10.3760/cma.j.issn.0376-2491.2016.43.008. [DOI] [PubMed] [Google Scholar]
- 68.Xiong Y, Ren Y, Cui X, et al. Resting state fMRI study of amplitude of low-frequency fluctuation in early onset schizophrenia [in Chinese] Chin J Nerv Ment Dis. 2016;42:272–6. [Google Scholar]
- 69.Huang XQ, Lui S, Deng W, et al. Localization of cerebral functional deficits in treatment-naive, first-episode schizophrenia using resting-state fMRI. Neuroimage. 2010;49:2901–6. doi: 10.1016/j.neuroimage.2009.11.072. [DOI] [PubMed] [Google Scholar]
- 70.Liu H, Fan G, Xu K, et al. Amplitude of low-frequency fluctuation study of resting-state functional MRI in schizophrenia [in Chinese] Chin J Med Imaging Technol. 2010;26:1659–62. [Google Scholar]
- 71.Jian F, Fang L, Xie S, et al. Study of resting-state spontaneous activity of local brain in first episode and untreated patients with schizophrenia [in Chinese] J Clin Psychiatry. 2014;24:21–3. [Google Scholar]
- 72.Liang J, Xie S. Changes of resting state functional magnetic resonance brain spontaneous low-frequency amplitude in male patients with paranoid schizophrenia [in Chinese] J Clin Psychiatry. 2014;24:77–9. [Google Scholar]
- 73.Yang F, Yang T, Kang C, et al. The change of resting state functional magnetic resonance imaging in chronic schizophrenia patients combined risperidone treatment [in Chinese] J Clin Psychiatry. 2014;24:73–6. [Google Scholar]
- 74.Ma X, Wang D, Zhou Y, et al. Sex-dependent alterations in resting-state cerebral blood flow, amplitude of low-frequency fluctuations and their coupling relationship in schizophrenia. Aust N Z J Psychiatry. 2016;50:334–44. doi: 10.1177/0004867415601728. [DOI] [PubMed] [Google Scholar]
- 75.Cui W, Zhou Q, Wang F, et al. Comparison of amplitude of low frequency fluctuation between schizophrenia and bipolar disorder patient: a resting-state functional magnetic resonance imaging study [in Chinese] J China Med Univ. 2016;45:977–81. [Google Scholar]
- 76.Chen F, Yao Q, Zhan D, et al. The abnormalities of amplitude of low-frequency fluctuation in the resting-state functional magnetic resonance imaging of first-episode medication-naive schizophrenia before and after antipsychotic drugs therapy [in Chinese] Chin J Psychiatry. 2017;50:133–8. [Google Scholar]
- 77.Salvador R, Landin-Romero R, Anguera M, et al. Non redundant functional brain connectivity in schizophrenia. Brain Imaging Behav. 2017;11:552–64. doi: 10.1007/s11682-016-9535-4. [DOI] [PubMed] [Google Scholar]
- 78.Alonso-Solis A, Vives-Gilabert Y, Portella MJ, et al. Altered amplitude of low frequency fluctuations in schizophrenia patients with persistent auditory verbal hallucinations. Schizophr Res. 2017;189:97–103. doi: 10.1016/j.schres.2017.01.042. [DOI] [PubMed] [Google Scholar]
- 79.Li J, Xu H, Yang H, et al. Different effects of taking risperidone and clozapine on spontaneous brain activity in schizophrenics [in Chinese] Sichuan Mental Health. 2017;30:27–31. [Google Scholar]
- 80.Tan Z, Chen W, Pang Y, et al. Applications of resting-state functional MRI in low-frequency fluctuation combined with voxel-based morphometry in schizophrenia [in Chinese] J Pract Med. 2017;33:2380–5. [Google Scholar]
- 81.Lei W, Li M, Deng W, et al. Sex-specific patterns of aberrant brain function in first-episode treatment-naive patients with schizophrenia. Int J Mol Sci. 2015;16:16125–43. doi: 10.3390/ijms160716125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Ding Y, Ou Y, Pan P, et al. Cerebellar structural and functional abnormalities in first-episode and drug-naive patients with schizophrenia: a meta-analysis. Psychiatry Res Neuroimaging. 2019;283:24–33. doi: 10.1016/j.pscychresns.2018.11.009. [DOI] [PubMed] [Google Scholar]
- 83.Bora E, Fornito A, Yucel M, et al. The effects of gender on grey matter abnormalities in major psychoses: a comparative voxelwise meta-analysis of schizophrenia and bipolar disorder. Psychol Med. 2012;42:295–307. doi: 10.1017/S0033291711001450. [DOI] [PubMed] [Google Scholar]
- 84.Whitfield-Gabrieli S, Ford JM. Default mode network activity and connectivity in psychopathology. Annu Rev Clin Psychol. 2012;8:49–76. doi: 10.1146/annurev-clinpsy-032511-143049. [DOI] [PubMed] [Google Scholar]
- 85.Lee WH, Doucet GE, Leibu E, et al. Resting-state network connectivity and metastability predict clinical symptoms in schizophrenia. Schizophr Res. 2018;201:208–16. doi: 10.1016/j.schres.2018.04.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Wang S, Zhan Y, Zhang Y, et al. Abnormal long- and short-range functional connectivity in adolescent-onset schizophrenia patients: a resting-state fMRI study. Prog Neuropsychopharmacol Biol Psychiatry. 2018;81:445–51. doi: 10.1016/j.pnpbp.2017.08.012. [DOI] [PubMed] [Google Scholar]
- 87.Zhou Y, Zeidman P, Wu S, et al. Altered intrinsic and extrinsic connectivity in schizophrenia. Neuroimage Clin. 2018;17:704–16. doi: 10.1016/j.nicl.2017.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Jiang Y, Duan M, Chen X, et al. Common and distinct dysfunctional patterns contribute to triple network model in schizophrenia and depression: a preliminary study. Prog Neuropsychopharmacol Biol Psychiatry. 2017;79:302–10. doi: 10.1016/j.pnpbp.2017.07.007. [DOI] [PubMed] [Google Scholar]
- 89.Repovs G, Barch DM. Working memory related brain network connectivity in individuals with schizophrenia and their siblings. Front Hum Neurosci. 2012;6:137. doi: 10.3389/fnhum.2012.00137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Lavigne KM, Woodward TS. Hallucination- and speech-specific hypercoupling in frontotemporal auditory and language networks in schizophrenia using combined task-based fMRI data: an fBIRN study. Hum Brain Mapp. 2018;39:1582–95. doi: 10.1002/hbm.23934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Thomas F, Moulier V, Valero-Cabre A, et al. Brain connectivity and auditory hallucinations: in search of novel noninvasive brain stimulation therapeutic approaches for schizophrenia. Rev Neurol (Paris) 2016;172:653–79. doi: 10.1016/j.neurol.2016.09.001. [DOI] [PubMed] [Google Scholar]
- 92.Antonucci LA, Taurisano P, Fazio L, et al. Association of familial risk for schizophrenia with thalamic and medial prefrontal functional connectivity during attentional control. Schizophr Res. 2016;173:23–9. doi: 10.1016/j.schres.2016.03.014. [DOI] [PubMed] [Google Scholar]
- 93.Hiser J, Koenigs M. The multifaceted role of the ventromedial prefrontal cortex in emotion, decision making, social cognition, and psychopathology. Biol Psychiatry. 2018;83:638–47. doi: 10.1016/j.biopsych.2017.10.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Caspers S, Schleicher A, Bacha-Trams M, et al. Organization of the human inferior parietal lobule based on receptor architectonics. Cereb Cortex. 2013;23:615–28. doi: 10.1093/cercor/bhs048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Sestieri C, Corbetta M, Romani GL, et al. Episodic memory retrieval, parietal cortex, and the default mode network: functional and topographic analyses. J Neurosci. 2011;31:4407–20. doi: 10.1523/JNEUROSCI.3335-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Buckner RL, Vincent JL. Unrest at rest: default activity and spontaneous network correlations. Neuroimage. 2007;37:1091–6. doi: 10.1016/j.neuroimage.2007.01.010. [DOI] [PubMed] [Google Scholar]
- 97.Obeso JA, Rodriguez-Oroz MC, Stamelou M, et al. The expanding universe of disorders of the basal ganglia. Lancet. 2014;384:523–31. doi: 10.1016/S0140-6736(13)62418-6. [DOI] [PubMed] [Google Scholar]
- 98.Price CJ. The anatomy of language: a review of 100 fMRI studies published in 2009. Ann N Y Acad Sci. 2010;1191:62–88. doi: 10.1111/j.1749-6632.2010.05444.x. [DOI] [PubMed] [Google Scholar]
- 99.Hugdahl K. Auditory hallucinations: a review of the ERC “VOICE” project. World J Psychiatry. 2015;5:193–209. doi: 10.5498/wjp.v5.i2.193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Hoffman RE, Fernandez T, Pittman B, et al. Elevated functional connectivity along a corticostriatal loop and the mechanism of auditory/verbal hallucinations in patients with schizophrenia. Biol Psychiatry. 2011;69:407–14. doi: 10.1016/j.biopsych.2010.09.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.van Tol MJ, van der Meer L, Bruggeman R, et al. Voxel-based gray and white matter morphometry correlates of hallucinations in schizophrenia: the superior temporal gyrus does not stand alone. Neuroimage Clin. 2014;4:249–57. doi: 10.1016/j.nicl.2013.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Huang X, Pu W, Li X, et al. Decreased left putamen and thalamus volume correlates with delusions in first-episode schizophrenia patients. Front Psychiatry. 2017;8:245. doi: 10.3389/fpsyt.2017.00245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Lin P, Wang X, Zhang B, et al. Functional dysconnectivity of the limbic loop of frontostriatal circuits in first-episode, treatment-naive schizophrenia. Hum Brain Mapp. 2018;39:747–57. doi: 10.1002/hbm.23879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Tohid H, Faizan M, Faizan U. Alterations of the occipital lobe in schizophrenia. Neurosciences (Riyadh) 2015;20:213–24. doi: 10.17712/nsj.2015.3.20140757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Palaniyappan L, Al-Radaideh A, Mougin O, et al. Combined white matter imaging suggests myelination defects in visual processing regions in schizophrenia. Neuropsychopharmacol. 2013;38:1808–15. doi: 10.1038/npp.2013.80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.van de Ven V, Rotarska JA, Oertel-Knochel V, et al. Reduced intrinsic visual cortical connectivity is associated with impaired perceptual closure in schizophrenia. Neuroimage Clin. 2017;15:45–52. doi: 10.1016/j.nicl.2017.04.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Wei Y, Chang M, Womer FY, et al. Local functional connectivity alterations in schizophrenia, bipolar disorder, and major depressive disorder. J Affect Disord. 2018;236:266–73. doi: 10.1016/j.jad.2018.04.069. [DOI] [PubMed] [Google Scholar]
- 108.Nachev P, Kennard C, Husain M. Functional role of the supplementary and pre-supplementary motor areas. Nat Rev Neurosci. 2008;9:856–69. doi: 10.1038/nrn2478. [DOI] [PubMed] [Google Scholar]
- 109.Krukow P, Jonak K, Karakula-Juchnowicz H, et al. Disturbed functional connectivity within the left prefrontal cortex and sensorimotor areas predicts impaired cognitive speed in patients with first-episode schizophrenia. Psychiatry Res Neuroimaging. 2018;275:28–35. doi: 10.1016/j.pscychresns.2018.03.001. [DOI] [PubMed] [Google Scholar]
- 110.Molina V, Galindo G, Cortes B, et al. Different gray matter patterns in chronic schizophrenia and chronic bipolar disorder patients identified using voxel-based morphometry. Eur Arch Psychiatry Clin Neurosci. 2011;261:313–22. doi: 10.1007/s00406-010-0183-1. [DOI] [PubMed] [Google Scholar]
- 111.James A, Hough M, James S, et al. Greater white and grey matter changes associated with early cannabis use in adolescent-onset schizophrenia (AOS) Schizophr Res. 2011;128:91–7. doi: 10.1016/j.schres.2011.02.014. [DOI] [PubMed] [Google Scholar]
- 112.Bombin I, Arango C, Buchanan RW. Significance and meaning of neurological signs in schizophrenia: two decades later. Schizophr Bull. 2005;31:962–77. doi: 10.1093/schbul/sbi028. [DOI] [PubMed] [Google Scholar]
- 113.Uddin LQ. Salience processing and insular cortical function and dysfunction. Nat Rev Neurosci. 2015;16:55–61. doi: 10.1038/nrn3857. [DOI] [PubMed] [Google Scholar]
- 114.White TP, Joseph V, Francis ST, et al. Aberrant salience network (bilateral insula and anterior cingulate cortex) connectivity during information processing in schizophrenia. Schizophr Res. 2010;123:105–15. doi: 10.1016/j.schres.2010.07.020. [DOI] [PubMed] [Google Scholar]
- 115.Palaniyappan L, Mallikarjun P, Joseph V, et al. Reality distortion is related to the structure of the salience network in schizophrenia. Psychol Med. 2011;41:1701–8. doi: 10.1017/S0033291710002205. [DOI] [PubMed] [Google Scholar]
- 116.Pu W, Li L, Zhang H, et al. Morphological and functional abnormalities of salience network in the early-stage of paranoid schizophrenia. Schizophr Res. 2012;141:15–21. doi: 10.1016/j.schres.2012.07.017. [DOI] [PubMed] [Google Scholar]
- 117.Haijma SV, Van Haren N, Cahn W, et al. Brain volumes in schizophrenia: a meta-analysis in over 18 000 subjects. Schizophr Bull. 2013;39:1129–38. doi: 10.1093/schbul/sbs118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Dong D, Wang Y, Chang X, et al. Dysfunction of large-scale brain networks in schizophrenia: a meta-analysis of resting-state functional connectivity. Schizophr Bull. 2018;44:168–81. doi: 10.1093/schbul/sbx034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Wang S, Zhan Y, Zhang Y, et al. Abnormal functional connectivity strength in patients with adolescent-onset schizophrenia: a resting-state fMRI study. Eur Child Adolesc Psychiatry. 2017;26:839–45. doi: 10.1007/s00787-017-0958-2. [DOI] [PubMed] [Google Scholar]
- 120.Woodward ND, Rogers B, Heckers S. Functional resting-state networks are differentially affected in schizophrenia. Schizophr Res. 2011;130:86–93. doi: 10.1016/j.schres.2011.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Wang X, Zhang W, Sun Y, et al. Aberrant intra-salience network dynamic functional connectivity impairs large-scale network interactions in schizophrenia. Neuropsychologia. 2016;93:262–70. doi: 10.1016/j.neuropsychologia.2016.11.003. [DOI] [PubMed] [Google Scholar]
- 122.Noda T, Nakagome K, Setoyama S, et al. Working memory and prefrontal/temporal hemodynamic responses during post-task period in patients with schizophrenia: a multi-channel near-infrared spectroscopy study. J Psychiatr Res. 2017;95:288–98. doi: 10.1016/j.jpsychires.2017.09.001. [DOI] [PubMed] [Google Scholar]
- 123.Greenlee JD, Oya H, Kawasaki H, et al. Functional connections within the human inferior frontal gyrus. J Comp Neurol. 2007;503:550–9. doi: 10.1002/cne.21405. [DOI] [PubMed] [Google Scholar]