Abstract
Background
Deep brain stimulation targeting the subcallosal cingulate gyrus (SCG DBS) improves the symptoms of treatment-resistant depression in some patients, but not in others. We hypothesized that there are pre-existing structural brain differences between responders and nonresponders to SCG DBS, detectable using structural MRI.
Methods
We studied preoperative, T1-weighted MRI scans of 27 patients treated with SCG DBS from 2003 to 2011. Responders (n = 15) were patients with a > 50% improvement in Hamilton Rating Scale for Depression score following 12 months of SCG DBS. Preoperative subcallosal cingulate gyrus grey matter volume was obtained using manual segmentation by a trained observer blinded to patient identity. Volumes of hippocampus, thalamus, amygdala, whole-brain cortical grey matter and white matter volume were obtained using automated techniques.
Results
Preoperative subcallosal cingulate gyrus, thalamic and amygdalar volumes were significantly larger in patients who went on to respond to SCG-DBS. Hippocampal volume did not differ between groups. Cortical grey matter volume was significantly smaller in responders, and cortical grey matter:white matter ratio distinguished between responders and nonresponders with high sensitivity and specificity.
Limitations
Normalization by intracranial volume nullified some between-group differences in volumetric measures.
Conclusion
There are structural brain differences between patients with treatment-resistant depression who respond to SCG DBS and those who do not. Specifically, the structural integrity of the subcallosal cingulate gyrus target region and its connected subcortical areas, and variations in cortical volume across the entire brain, appear to be important determinants of response. Structural MRI shows promise as a biomarker in deep brain stimulation for depression, and may play a role in refining patient selection for future trials.
Introduction
Deep brain stimulation (DBS) is a well-established therapy for patients with movement disorders such as Parkinson disease, essential tremor and dystonia.1 Recent attempts have been made to apply DBS to patients with refractory psychiatric disorders, including obsessive–compulsive disorder2 and treatment-resistant depression (TRD).3,4 In particular, DBS for TRD has generated considerable enthusiasm because of an anticipated increase in depression-related disease burden,5 and the immense socioeconomic costs of depression worldwide.6 Multiple small open-label trials of DBS in patients with TRD have been published to date, using various stimulation targets including the ventral capsule/ventral striatum,7 nucleus accumbens,8 medial forebrain bundle,9 inferior thalamic peduncle,10 lateral habenula11 and subcallosal cingulate gyrus with its subjacent white matter (referred to as the SCG from here on).12–15 These open-label studies largely report improvement in depression severity scores with long-term stimulation irrespective of target, although at present there are insufficient data to conclusively demonstrate the superiority of any one target over another.
To date, the therapeutic effectiveness of DBS for TRD has not yet been conclusively established in randomized clinical trials using sham stimulation and blinded outcome assessment. In fact, a recent trial of ventral capsule/ventral striatum DBS failed to show a significant difference in TRD response rate between active stimulation and sham groups at the end of a 16-week controlled phase.16 Similarly, a randomized, double-blind, sham-controlled trial of SCG DBS failed to demonstrate superior antidepressant efficacy in the active stimulation group at 6 months, and, following an interim futility analysis, the trial was stopped prematurely by its industry sponsor.17 However, clinically meaningful antidepressant responses were observed at long-term follow-up in patients who continued to receive open-label, active stimulation beyond the initial 6-month randomized phase (48% response, 25% remission at 2 years). As a result, SCG DBS for TRD is still considered to have promise, although it is clear that we lack a complete understanding of the factors that may influence antidepressant response.17 Indeed, the inherent heterogeneity of TRD makes it unlikely that all TRD patients will respond to DBS, and broadly offering DBS to all patients who meet criteria for a diagnosis of TRD may result in a substantial rate of treatment failure. Because DBS is an invasive and costly therapy with the potential for serious adverse effects, it would be useful to have reliable, objectively measured biomarkers of expected antidepressant response that could be measured before surgical intervention.
A small number of studies have examined potential factors influencing response to SCG DBS, including DBS electrode targeting,18,19 stimulation parameters,20 variations in specific electroencephalographic features21,22 and pre-DBS neurocognitive function.23 We hypothesized that variations in preoperative neuroanatomy might similarly moderate differences in response to SCG DBS; this hypothesis follows from previous findings suggesting that pretreatment hippocampal volume may predict medication treatment response in patients presenting with major depressive disorder.24 Anatomically, the SCG grey matter is composed of cortex belonging to Brodmann area 25, as well as the caudal portions of areas 24 and 32,25 and is cytoarchitectonically distinct from the more dorsal anterior cingulate region.26 Projections to and from the SCG have largely been inferred from anatomic studies in non-human primates, and a small number of diffusion tensor imaging tractography studies in humans (see Hamani and colleagues25 for a comprehensive review of SCG projections). In brief, the SCG has prominent connections with prefrontal, orbitofrontal and anterior cingulate cortices. Subcortically, strong afferent and efferent connections have consistently been found between the SCG and the hippocampus and amygdala, consistent with the key roles of these structures in emotional processing as components of the limbic system. Additionally, there are connections between the SCG and multiple thalamic and hypothalamic nuclei.
In this study, we applied quantitative morphometric analysis techniques to preoperatively acquired structural MRI scans from 27 SCG DBS patients (15 responders, 12 nonresponders) treated for TRD. Our prediction was that structural brain differences between responders and nonresponders would be detectable on MRI scans, and would reflect the relative integrity of the SCG and closely connected brain regions. Our approach involved 3 different analyses: measurement of SCG grey matter volume as a surrogate measure of stimulation target integrity; volumetric measurements of 3 key subcortical structures—the hippocampus, amygdala and thalamus—which are connected to the SCG region and which have been implicated in depression; and measurement of whole brain cortical grey and white matter volume.
Methods
Patient selection, DBS parameters and follow-up
This retrospective cohort study was approved by the research ethics boards of the University Health Network and the Centre for Addiction and Mental Health, Toronto, Canada. We searched the Toronto Western Hospital DBS program database to identify all patients who had undergone implantation of a bilateral DBS system targeted to the SCG between January 1, 2003, and December 31, 2011. In total, we identified 43 patients, all with a diagnosis of TRD. Of these patients, 8 had missing or poor-quality preoperative standard volumetric stereotactic T1-weighted MRI scans (see MRI acquisition and preprocessing, below). We then also excluded patients who were treated as part of a multicentre randomized controlled trial (n = 8), because trial patients routinely underwent a period of sham stimulation. This left a total of 27 patients for analysis. Waiver of informed consent for the present study was granted by the institutional research ethics board, because it involved only the retrospective use of de-identified imaging data; however, all patients had originally provided written informed consent to proceed with bilateral SCG DBS after having been made aware of the potential risks and benefits of the procedure.
All analyzed patients had been selected for SCG DBS based on standard inclusion and exclusion criteria at our centre.27 Of note, all patients had undergone at least 1 course of psychotherapy before SCG DBS implantation, but not all patients had undergone electroconvulsive therapy. Technical aspects of the bilateral SCG DBS implantation procedure have been detailed in previous publications.14,27 Similarly, guidelines for stimulation parameter selection have also been published previously.27 Responders were defined as patients who, after 12 months of SCG DBS, had achieved a 50% or greater reduction in Hamilton Rating Scale for Depression-17 (HRSD-17) scores compared with baseline. For details related to patient selection, DBS programming and psychiatric follow-up, see Appendix 1, Supplemental Materials, available at jpn.ca/180207-a1.
MRI acquisition and preprocessing
The MRI scans analyzed in this study were standard preoperative stereotactic scans obtained on the morning of DBS implantation surgery. All MRI scans were acquired on the same 1.5 T GE Signa EXCITE scanner (GE Healthcare). A T1-weighted, 3-dimensional spoiled gradient recalled volumetric sequence was obtained with the following parameters: repetition time 11.9 ms, echo time 5.0 ms, inversion time 300 ms, flip angle 20°, field of view 25.9 cm × 25.9 cm, matrix 256 × 256, reformatted into 1.4 mm axial slices (with 0.7 mm overlap to prevent staircase artifact). Effective voxel size was 0.5 × 0.5 × 1.4 mm = 0.35 mm3. All scans underwent N3 intensity nonuniformity correction28 and realignment to the anterior–posterior commissure plane using Medical Image Processing and Visualization (MIPAV) version 5.2.1 (National Institutes of Health). Images were resampled into isotropic 1 mm3 voxels using trilinear interpolation, as per our standard laboratory protocol.29
Volumetric analysis of SCG grey matter
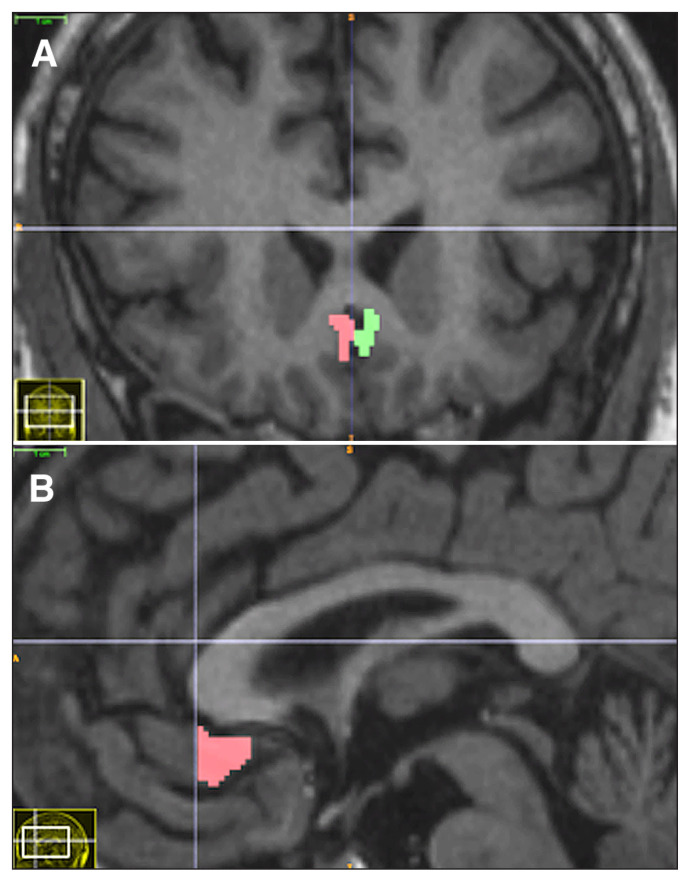
To assess whether the anatomic attributes of the stimulation target affect response to SCG DBS, we measured SCG grey matter volume on preoperative structural MRI scans. A trained observer blinded to patient identity and clinical outcome measured left and right SCG volumes by manual segmentation. All manual segmentations were performed using ITK-SNAP version 2.4.0 (www.itksnap.org). The SCG grey matter was segmented according to the protocol of Drevets and colleagues (Fig. 1).30 Segmentations were performed on coronal sections. The anterior boundary of the SCG was the anterior-most coronal slice containing the corpus callosum, and the posterior boundary was the anterior-most slice where the internal capsule no longer divided the striatum. All grey matter belonging to the first full gyrus below the corpus callosum was included in the SCG volume. Occasionally, the SCG appeared as 2 contiguous small double gyri instead of a single larger gyrus; double gyri were always included together in the total SCG volume. Segmented volumes were inspected on sagittal slices to ensure a consistent inferior boundary along the antero-posterior extent of the SCG (Fig. 1B).
Fig. 1.
(A) Coronal and (B) sagittal T1-weighted MRI scans showing the subcallosal cingulate gyrus region delineated by manual segmentation.
To assess inter-rater reliability, a second blinded observer independently segmented the SCG in 14 randomly selected patients. Reliability was quantified using the intraclass correlation coefficient. We report an absolute agreement standard for intraclass correlation coefficient, which for each structure represents the variance of the measurement, divided by the sum of the variance of the measurement and the variance over patients.31
Volumetric analysis of hippocampus, amygdala and thalamus
To test the hypothesis that the integrity of brain structures connected to the SCG may predict eventual response to SCG DBS, we measured the volumes of the hippocampus, amygdala and thalamus, which have strong afferent and efferent connections to the SCG.25 Volumetry of these structures was performed by automated segmentation using FSL-FIRST, part of the FSL toolkit32 (http://fsl.fmrib.ox.ac.uk/fsl/fslwiki/). FIRST is a model-based segmentation tool that uses shape- and appearance-based models constructed from manually segmented images.33 The manual labels are parameterized as surface meshes and modelled as a point distribution model. Deformable surfaces are used to automatically parameterize the volumetric labels using constraints to preserve vertex correspondence across the training data. Based on learned models, FIRST searches through linear combinations of shape modes of variation for the most probable shape instance given the observed intensities in a T1-weighted image. FIRST was implemented using FSL version 4.1.9. FIRST segmentations were performed using the run_first_all script according to the FIRST user guide (http://fsl.fmrib.ox.ac.uk/fsl/fslwiki/FIRST/UserGuide).
All segmentations generated by FIRST were visually inspected for gross errors by a single observer blinded to patient identity and clinical outcome.
Whole brain grey and white matter analysis
To assess whether global brain structure differences could influence response to SCG DBS in depression, we compared whole brain grey and white matter volumes between responders and nonresponders. Brain tissue volume, normalized for patient head size, was estimated with SIENAX,34 part of FSL.32 SIENAX starts by extracting brain and skull images from the single whole-head input data.35 The brain image is then affine-registered to MNI152 space36,37 (using the skull image to determine the registration scaling); this is primarily to obtain the volumetric scaling factor, to be used as a normalization for head size. Next, tissue-type segmentation with partial volume estimation is carried out38 to calculate the total volume of brain tissue (including separate estimates of volumes of grey matter, white matter, peripheral grey matter and cerebrospinal fluid). We ran SIENAX with the “-r” option to calculate cortical grey matter volumes (i.e., excluding subcortical and cerebellar grey matter volumes). We used cortical grey matter:white matter ratio as a composite global structural metric in comparisons between responders and nonresponders.
Normalization by intracranial volume
A common approach in quantitative volumetric imaging studies of the brain is to use intracranial volume (ICV) as a normalization factor when comparing the volumes of various brain structures and regions between groups.39 Theoretically, this ensures that head size does not confound between-group comparisons of structural volumes. A surrogate measure of ICV was determined for each patient using the scaling factor (VSCALING) generated by SIENAX, which represents the amount by which each patient’s skull volume must be multiplied to be transformed into a standard image space (MNI 152). A smaller scaling factor implies a larger ICV. All raw structural volumes were multiplied by the scaling factor and then subjected to further statistical analysis.
Statistical analysis
Given our small sample size, we compared the volumes of the SCG, hippocampus, amygdala and thalamus SCG DBS responders and nonresponders using the nonparametric Mann–Whitney test. Similarly, we compared cortical grey matter volume, whole brain white matter volume, and cortical grey matter:white matter ratio between responders and nonresponders using the Mann–Whitney test. All analyses were performed for both raw volumes and volumes normalized by SIENAX ICV scaling factor. To assess the discriminatory power of cortical grey matter:white matter ratio for response to SCG DBS, we constructed receiver operating characteristic curves, calculating the areas under the curves and the corresponding 95% confidence intervals using the method of DeLong and colleagues.40 We performed correlation analysis between structural variables of interest and HRSD-17 scores using Spearman correlation. In all statistical tests, we used p < 0.05 as the threshold for statistical significance. We performed Bonferroni correction to account for multiple comparisons in the volumetric analysis of the hippocampus, amygdala and thalamus (i.e., cut-off for significant p value 0.05/3 = 0.017). Given the exploratory nature of the study, the small sample size and the use of nonparametric statistics, we did not control for confounding variables in any analyses.
Results
Clinical and demographic variables
A summary comparison of clinical and demographic variables between responder and nonresponder groups is shown in Table 1. We found no differences for age at SCG DBS implantation; sex; HRSD-17 score; number of previous major depressive episodes; years since onset of major depressive disorder; duration of current depressive episode; number of antidepressant medication trials before DBS; or the proportion of patients previously treated with electroconvulsive therapy, treated with DBS during a first major depressive episode, previously treated with antipsychotics, or with a family history of major depressive disorder. Predictably, responders demonstrated a significantly larger median percentage improvement in HRSD-17 score than nonresponders after 1 year of stimulation (U = 7; p < 0.0001).
Table 1.
Clinical and demographic characteristics of nonresponders and responders to SCG DBS*
| Characteristic | Nonresponders (n = 12) | Responders (n = 15) | p value |
|---|---|---|---|
| Age, yr | 47.9 ± 7.7 | 46.6 ± 8.4 | NS |
| Male, n | 3 | 7 | NS |
| HRSD-17 baseline score | 25.2 ± 3.9 | 25.4 ± 3.7 | NS |
| Previous major depressive episodes, n | 3.9 ± 3.1 | 3.6 ± 2.8 | NS |
| Years since onset of MDD | 21.3 ± 9.6 | 18.1 ± 8.4 | NS |
| Duration of current major depressive episode, mo | 91.0 ± 56.3 | 53.5 ± 35.8 | NS |
| Trials of antidepressant medication before DBS, n | 4.2 ± 4.1 | 4.4 ± 3.6 | NS |
| Treated with ECT before DBS, n (%) | 5 (41.7) | 7 (46.7) | NS |
| Treated with DBS during first major depressive episode, n (%) | 1 (8.3) | 1 (6.7) | NS |
| Treated with antipsychotics at time of DBS, n (%) | 4 (33.3) | 5 (33.3) | NS |
| Family history of MDD, n (%) | 6 (50.0) | 7 (46.7) | NS |
| % improvement in HRSD-17 at 12 mo | 34.3 ± 18.3 | 69.2 ± 12.8 | < 0.001 |
DBS = deep brain stimulation; ECT = electroconvulsive therapy; HRSD-17 = Hamilton Rating Scale for Depression; MDD = major depressive disorder; NS = not significant; SCG = subcallosum cingulate gyrus; SD = standard deviation.
All values expressed as mean ± SD, except where otherwise indicated. Significance threshold p < 0.05. Mann–Whitney test used to compare continuous variables, Fisher exact test used for dichotomous variables.
Subcallosal cingulate gyrus volume
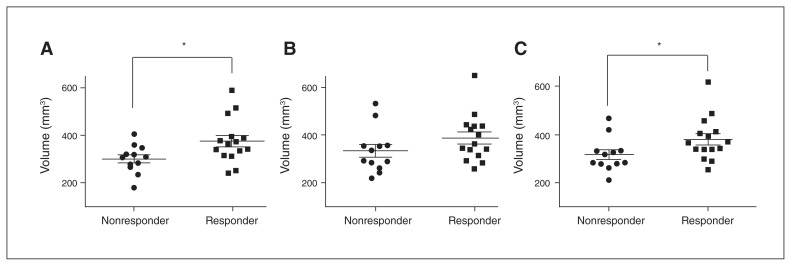
Inter-rater reliability for SCG volume was excellent, with an intraclass correlation coefficient of 0.91. Volumetric results for SCG are summarized in Fig. 2. The median volume of the left SCG was significantly larger in responders to SCG DBS than in nonresponders (Fig. 2A; U = 44.5; p = 0.028). Median volume of the average SCG was also significantly larger in responders (Fig. 2C; U = 42.0; p = 0.021). Overall, there was no difference in left versus right median SCG volume in either the responder or the nonresponder group.
Fig. 2.
Subcallosal cingulate gyrus volume in responders versus nonresponders to deep brain stimulation. (A) Left subcallosal cingulate gyrus volume. (B) Right subcallosal cingulate gyrus volume. (C) Average subcallosal cingulate gyrus volume. *p < 0.05, Mann–Whitney test.
Average SCG volume was not significantly correlated with percent change in HRSD-17 scores at 12 months.
Hippocampus, amygdala and thalamus volume
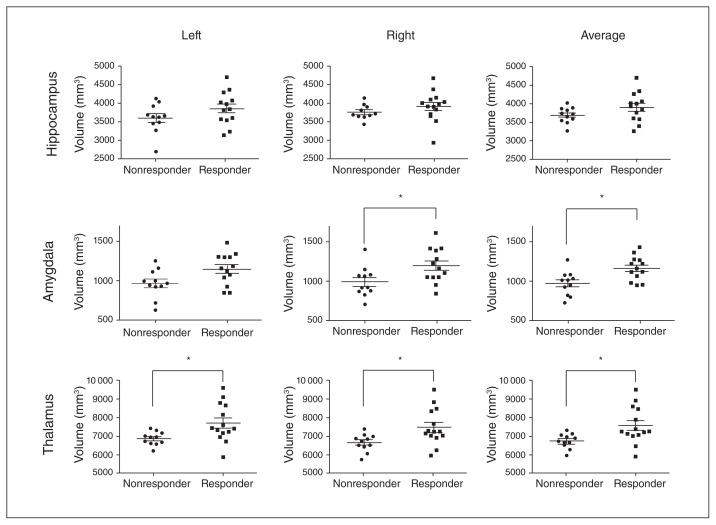
Fig. 3 summarizes the results of automated volumetry for the hippocampus, amygdala and thalamus across responders and nonresponders. Visual inspection by an observer blinded to patient identity and clinical outcome identified grossly erroneous hippocampal segmentations in 3 of 27 patients (2 responders, 1 nonresponder), amygdalar segmentations in 3 of 27 patients (2 responders, 1 nonresponder), and thalamic segmentations in 2 of 27 patients (1 responder, 1 nonresponder). These patients were excluded from subsequent between-group statistical comparisons.
Fig. 3.
Hippocampus, amygdala and thalamus volume in responders versus nonresponders to deep brain stimulation of the subcallosal cingulate gyrus. Each row shows volumetric results for a single structure; columns represent left, right and average volumes, respectively. *p < 0.002, Mann–Whitney test.
Left, right or average hippocampal volumes were not significantly different between responders and nonresponders. In contrast, average amygdala volume was significantly larger in responders compared with nonresponders (U = 28.00; p = 0.013) after correcting for multiple comparisons; on their own, left and right amygdala volumes showed strong trends toward being larger in responders, but these findings did not survive Bonferroni correction (p = 0.05 and p = 0.037, respectively). Left, right, and average thalamic volume were all significantly larger in responders after correction for multiple comparisons (U = 30.00, p = 0.011 for all).
We found a significant correlation between pre-DBS average thalamic volume and percent change in 12-month HRSD-17 scores (Spearman’s r = 0.54, p = 0.0057). We found no significant correlations between hippocampal or amygdala volume and percent change in HRSD-17 scores.
Whole brain grey and white matter analysis
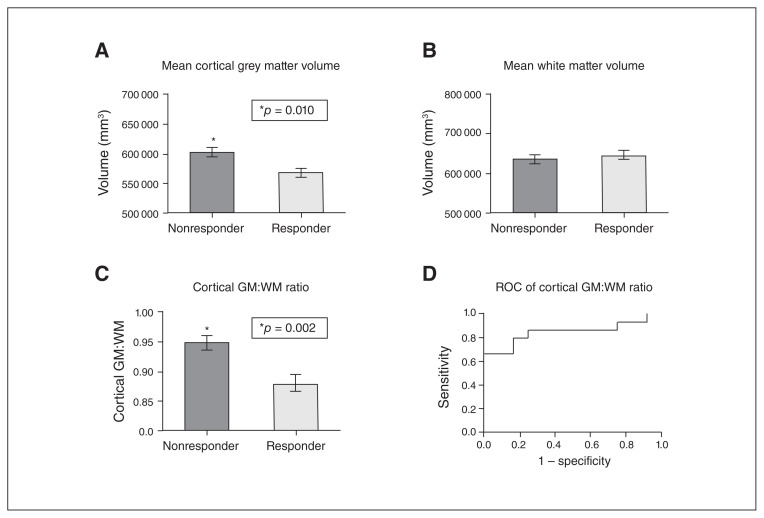
Total cortical grey matter volume was significantly lower in responders than in nonresponders (U = 37.00; p = 0.010), but there was no difference in total white matter volume between both groups (Fig. 4A, B). Cortical grey matter:white matter ratio was also significantly lower in responders (U = 27.00; p = 0.002, Fig. 4C). Overall, the discriminatory power of cortical grey matter:white matter ratio for response to SCG DBS was high: the area under the receiver operating characteristic curve for cortical grey matter:white matter ratio was 0.85 (Fig. 4D). Cortical grey matter volume and cortical grey matter:white matter ratio were both well correlated with percent improvement in HRSD-17 scores after 12 months of DBS (Spearman’s r = 0.39, p = 0.043 and Spearman’s r = 0.41, p = 0.036, respectively).
Fig. 4.
(A) Mean cortical grey matter volume in responders versus nonresponders to deep brain stimulation of the subcallosal cingulate gyrus. (B) Mean white matter volume in responders versus nonresponders. (C) Mean cortical grey matter:white matter ratio in responders versus nonresponders. (D) Receiver operating characteristic of cortical grey matter:white matter ratio, showing that cortical grey matter:white matter ratio can distinguish between responders and nonresponders with high sensitivity and specificity. All between-group statistical comparisons in A to C were performed using the Mann–Whitney test. GM = grey matter; WM = white matter.
Effect of normalization by intracranial volume
Normalization by ICV nullified any significant differences in SCG, hippocampus, amygdala and thalamus volume between responders and nonresponders. To further explore this finding, we compared median SIENAX VSCALING factor between responders and nonresponders and found that it was significantly smaller in responders (U = 23.00; p = 0.0019; Appendix 1, Figure S1). Put differently, responders had larger ICV than nonresponders on average. When we employed a different method for measuring ICV (Appendix 1, Supplemental Methods), we again found that ICV was significantly larger in responders than in nonresponders (U = 35.00; p = 0.0078; Appendix 1, Figure S2), with excellent correlation between ICV measurements by both methods (Appendix 1, Figure S3).
Discussion
In patients with TRD, we identified differences in preoperative brain structure between responders and nonresponders to SCG DBS therapy. Specifically, we found evidence of larger SCG, amygdalar and thalamic volume in responders, but no difference in hippocampal volume between groups. Preoperative thalamic volume was particularly well correlated with improvement in depression scores. At the brain-wide level, we observed a greater volume of cortical grey matter in nonresponders, and the cortical grey matter:white matter ratio performed well in discriminating between responders and nonresponders. Taken together, these novel findings suggest that neuroanatomical variability may influence eventual response to SCG DBS, as may clinical factors or DBS electrode position.
Cortical and subcortical volumetric predictors of response to SCG DBS
Several volumetric MRI studies have examined the SCG region — and in particular its grey matter volume — in unipolar and bipolar depression.30,41–50 Although the definition of the anatomic boundaries of the SCG varies across studies and effect sizes are variable, existing data suggest that SCG volume is decreased in unipolar depression, with more severe atrophy in the left SCG than in the right. Such SCG volume loss is observed in depressed patients as early as the initial episode of major depression and is more severe in patients with a family history of depression, suggesting that SCG atrophy may reflect susceptibility to — rather than a secondary consequence of — ongoing depression.50 Accordingly, our finding of reduced SCG volume in DBS nonresponders, particularly on the left, may suggest that nonresponders are neuroanatomically susceptible to TRD. Another possibility is that smaller SCG volume in nonresponders may reflect poorer integrity of the target region for DBS, leading to poorer clinical response. However, it has been argued that the putative SCG DBS target may actually be the SCG white matter, with preliminary evidence that optimal antidepressant effects require stimulation of the confluence of 3 white matter bundles connecting the SCG with the medial frontal cortex (via forceps minor and uncinate fasciculus), the rostral and dorsal cingulate cortex (via the cingulum bundle) and subcortical nuclei, respectively.19 Unfortunately, we did not acquire diffusion tensor imaging scans in our preoperative imaging protocol, so we were unable to assess the preoperative integrity of these key white matter bundles. The extent to which grey and white matter alterations in the SCG region are correlated, and how this relationship might influence DBS response, remains to be determined.
An association between depression and hippocampal atrophy has consistently been reported across several studies, including 5 separate meta-analyses of MRI volumetric studies in depression.51–55 The exact nature of the association remains uncertain. There is some evidence that a smaller hippocampus may predispose to depression,56 further supported by the association between smaller hippocampal volume and both a family history of depression and early-life adversity.57 Hippocampal atrophy is also correlated with longer overall duration of depressive illness,52,55 while hippocampal volume appears more significantly reduced in the depressed rather than the remitted state.53,56,58 Finally, smaller hippocampal volume appears to predict poorer response to antidepressant treatment.59 We found no differences in pre-DBS hippocampal volume between responders and nonresponders to SCG DBS in our population of TRD patients. It is possible that the similar proportion of patients with a family history of depression, similar duration of illness and similar duration of antidepressant therapy (see Table 1) may have attenuated any subtle inter-group pre-DBS variability in hippocampal volume. Furthermore, the hippocampus is a highly neuroplastic structure whose volume can be influenced by a multitude of factors, including aerobic exercise, glucocorticoid levels (elevated in depression), electroconvulsive therapy and mood-altering medications (notably lithium).60 These factors may have affected hippocampal volume to varying degrees across our patient population, possibly confounding any pre-DBS differences between responders and nonresponders.
We found significantly smaller thalamic and amygdalar volumes in nonresponders to SCG DBS. A recent meta-analysis of volumetric neuroimaging studies reported that the thalamus volume is consistently reduced in major depressive disorder.53 Thalamic atrophy is present even in early, treatment-naïve depression, suggesting that it is potentially a trait marker of susceptibility to depression.61 Further supporting this are recent data demonstrating that thalamic volume loss may be a key diagnostic feature (at the individual patient level) of pediatric unipolar depression, regardless of disease duration or extent of previous psychotropic medication use.62 There is also growing evidence that thalamic volume may be modulated by genetic factors known to be important in depression, including the Val66Met polymorphism in the brain-derived neurotrophic factor gene, as well as the serotonin transporter gene-linked polymorphism.63 Analogous to our SCG volume findings, thalamic atrophy in nonresponders may indicate a more neuroanatomically severe form of depression resistant to beneficial effects of SCG DBS. Amygdalar atrophy in nonresponders may reflect the same phenomenon, although the existing data on amygdalar volume in depression are variable and inconclusive,53,56,64–67 despite clear evidence for the key role of the amygdala in mood disorders.
Whole brain structural predictors of response to SCG DBS
Arguably, the most intriguing finding from our structural neuroimaging analysis was significantly reduced cortical grey matter volume and grey matter:white matter ratio in responders, with cortical grey matter:white matter ratio in particular revealing itself as a robust means of segregating responders from nonresponders. At first glance, these results appear counterintuitive, since many studies demonstrate significant thinning across various frontal cortical regions in patients with chronic depression, including the orbitofrontal cortex,68–70 dorsal anterior cingulate cortex,71 rostral middle frontal gyrus58,69 and dorsolateral prefrontal cortex.72 It also appears that some cortical regions — primarily in the frontal lobe but also encompassing the inferior temporal gyrus — may increase in thickness with successful medical therapy that leads to remission.58 However, recent work has shown increased thickness in the orbitofrontal cortex in people at increased familial risk of depression,73 and other studies have demonstrated increased orbitofrontal74 and cingulate/temporopolar cortical thickness in patients with first-episode, medication-naïve depression compared with controls.75 These findings support the notion that greater cortical thickness in certain brain regions may be a trait marker in depression, and therefore in line with the possibility, as suggested above, that nonresponders exhibit a neuroanatomically more severe form of depression. This neuroanatomical profile may also reflect a relative failure of cortical pruning during adolescent brain development in nonresponders, which has been linked to the development of depression.76 To our knowledge, there have been no previously published attempts to use the SIENAX tool in characterizing outcome or response to treatment. Most cortical thickness studies in depression have employed a vertex-based approach in which regionally specific clusters of difference in cortical thickness are reported between groups. However, this approach is insensitive to smaller variations in cortical thickness when averaged over larger regions or the entire brain.58 Our approach generates a simple scalar measure (i.e., cortical grey matter:white matter ratio) which can serve as a rapidly measured, automatically generated biomarker at the individual patient level.
The potential influence of ICV on our results is a key issue that merits discussion. Briefly, we found that median ICV — measured using 2 different methods — was significantly different between responders and nonresponders to SCG DBS. As a result, when normalizing by ICV — to correct for the potential confounding effect of head size and premorbid brain size differences between responders and nonresponders — significant between-group differences in volume of the SCG, amygdala and thalamus were abolished. Following a thorough literature search, we could not find any direct evidence of differences in average ICV between depressed patients and controls, or between responders and nonresponders to antidepressant therapy. However, there are some links between head size and inherited genetic polymorphisms (e.g., locus 17q2177,78) implicated in the development of depression. In our particular cohort, the ICV may have differed between response groups because of an uneven distribution of males and females in each group (i.e., the male:female ratio was higher in responders), although, strictly speaking, this did not meet the threshold for a statistically significant inter-group difference.
Accounting for ICV in volumetric MRI studies is a controversial topic. An important concern is that the specific method used to compute ICV can significantly influence between-group volumetric comparisons, with different ICV methods leading to divergent conclusions.79 Another concern is that the relationship between ICV and regional brain volumes is not necessarily linear, so that simple division of a structure’s volume by ICV — as performed in most studies — may be inappropriate.80 This limitation could be addressed by using ICV as a covariate in a general linear model; however, given our small sample size we chose to use nonparametric statistical methods that did not easily lend themselves to adjustment for covariates.81 For these reasons, we believe that our raw volumetric results should not necessarily be dismissed out of hand because of the effect of ICV normalization, although they will require validation in a larger study. It is also worth mentioning that cortical grey matter and associated cortical grey matter:white matter ratio results are already head size–corrected by the SIENAX algorithm, and are therefore unaffected by between-group differences in ICV.
Limitations
In addition to the influence of ICV on our volumetric results, there are several other reasons to interpret our results with caution. Most obviously, the sample size is small, which limits the robustness of any reported differences between responder and nonresponder groups. Both the small sample size and the obligatory use of nonparametric statistical methods precluded any meaningful attempt to control for confounding variables during inter-group comparisons. Second, MRI data were collected over a long period (8 yr), during which there were multiple software upgrades to the MRI scanner that could result in minor changes to image contrast, in turn influencing volumetric analysis (although the same scanner was used throughout the study). As well, all MRI data were resampled to generate isotropic voxels, which may have resulted in interpolation errors affecting image quality.82
Conclusion
Our findings suggest that TRD patients who do not respond to SCG DBS may exhibit a more neuroanatomically severe form of depression that may not necessarily be reflected in demographic or clinical features. Larger studies are required to determine if structural MRI can be used to improve patient selection in future trials of DBS in TRD.
Footnotes
Funding: T. Sankar’s work on this manuscript was supported by a Canadian Institutes of Health Research (CIHR) fellowship award. A. Lozano was supported by a Canada Research Chair in Neuroscience and the R.R. Tasker Chair in Functional Neurosurgery.
Competing interests: M. Chakravarty is a member of the JPN editorial board; he was not involved in the decision-making on this manuscript. P. Giacobbe and S. Kennedy have received honoraria from St. Jude Medical, Inc. H. Mayberg has received consulting and intellectual property fees from St. Jude Medical, Inc. C. Hamani is a consultant for St. Jude Medical, Inc. A. Lozano is a consultant to Medtronic, Inc., St. Jude Medical, Inc., and Boston Scientific, Inc.; serves on the scientific advisory board of Ceregene, Codman, Neurophage, Aleva and Alcyone Life Sciences; is co-founder of Functional Neuromodulation Inc.; and holds intellectual property in the field of deep brain stimulation. T. Sankar, N. Jawa, S. Li and S. Rizvi report no biomedical financial interests or other conflicts of interest.
Contributors: T. Sankar, C. Hamani and A. Lozano designed the study. T. Sankar, N. Jawa, S. Li, P. Giacobbe, S. Kennedy and S. Rizvi acquired the data, which T. Sankar, M. Chakravarty, P. Giacobbe, S. Kennedy and H. Mayberg analyzed. T. Sankar, M. Chakravarty, S. Kennedy and S. Rizvi wrote the article, which all authors reviewed. All authors approved the final version to be published and can certify that no other individuals not listed as authors have made substantial contributions to the paper.
References
- 1.Pizzolato G, Mandat T. Deep brain stimulation for movement disorders. Front Integr Neurosci. 2012;6:2. doi: 10.3389/fnint.2012.00002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mallet L, Polosan M, Jaafari N, et al. Subthalamic nucleus stimulation in severe obsessive-compulsive disorder. N Engl J Med. 2008;359:2121–34. doi: 10.1056/NEJMoa0708514. [DOI] [PubMed] [Google Scholar]
- 3.Giacobbe P, Kennedy SH. Deep brain stimulation for treatment-resistant depression: a psychiatric perspective. Curr Psychiatry Rep. 2006;8:437–44. doi: 10.1007/s11920-006-0048-5. [DOI] [PubMed] [Google Scholar]
- 4.Giacobbe P, Mayberg HS, Lozano AM. Treatment resistant depression as a failure of brain homeostatic mechanisms: implications for deep brain stimulation. Exp Neurol. 2009;219:44–52. doi: 10.1016/j.expneurol.2009.04.028. [DOI] [PubMed] [Google Scholar]
- 5.Lopez AD, Murray CC. The global burden of disease, 1990–2020. Nat Med. 1998;4:1241–3. doi: 10.1038/3218. [DOI] [PubMed] [Google Scholar]
- 6.Kessler RC. The costs of depression. Psychiatr Clin North Am. 2012;35:1–14. doi: 10.1016/j.psc.2011.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Malone DA, Jr, Dougherty DD, Rezai AR, et al. Deep brain stimulation of the ventral capsule/ventral striatum for treatment-resistant depression. Biol Psychiatry. 2009;65:267–75. doi: 10.1016/j.biopsych.2008.08.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Schlaepfer TE, Cohen MX, Frick C, et al. Deep brain stimulation to reward circuitry alleviates anhedonia in refractory major depression. Neuropsychopharmacology. 2008;33:368–77. doi: 10.1038/sj.npp.1301408. [DOI] [PubMed] [Google Scholar]
- 9.Schlaepfer TE, Bewernick BH, Kayser S, et al. Rapid effects of deep brain stimulation for treatment-resistant major depression. Biol Psychiatry. 2013;73:1204–12. doi: 10.1016/j.biopsych.2013.01.034. [DOI] [PubMed] [Google Scholar]
- 10.Jimenez F, Nicolini H, Lozano AM, et al. Electrical stimulation of the inferior thalamic peduncle in the treatment of major depression and obsessive compulsive disorders. World Neurosurg. 2013;80:S30 e17–25. doi: 10.1016/j.wneu.2012.07.010. [DOI] [PubMed] [Google Scholar]
- 11.Sartorius A, Kiening KL, Kirsch P, et al. Remission of major depression under deep brain stimulation of the lateral habenula in a therapy-refractory patient. Biol Psychiatry. 2010;67:e9–11. doi: 10.1016/j.biopsych.2009.08.027. [DOI] [PubMed] [Google Scholar]
- 12.Kennedy SH, Giacobbe P, Rizvi SJ, et al. Deep brain stimulation for treatment-resistant depression: follow-up after 3 to 6 years. Am J Psychiatry. 2011;168:502–10. doi: 10.1176/appi.ajp.2010.10081187. [DOI] [PubMed] [Google Scholar]
- 13.Lozano AM, Giacobbe P, Hamani C, et al. A multicenter pilot study of subcallosal cingulate area deep brain stimulation for treatment-resistant depression. J Neurosurg. 2012;116:315–22. doi: 10.3171/2011.10.JNS102122. [DOI] [PubMed] [Google Scholar]
- 14.Mayberg HS, Lozano AM, Voon V, et al. Deep brain stimulation for treatment-resistant depression. Neuron. 2005;45:651–60. doi: 10.1016/j.neuron.2005.02.014. [DOI] [PubMed] [Google Scholar]
- 15.Holtzheimer PE, Kelley ME, Gross RE, et al. Subcallosal cingulate deep brain stimulation for treatment-resistant unipolar and bipolar depression. Arch Gen Psychiatry. 2012;69:150–8. doi: 10.1001/archgenpsychiatry.2011.1456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dougherty DD, Rezai AR, Carpenter LL, et al. A randomized sham-controlled trial of deep brain stimulation of the ventral capsule/ventral striatum for chronic treatment-resistant depression. Biol Psychiatry. 2015;78:240–8. doi: 10.1016/j.biopsych.2014.11.023. [DOI] [PubMed] [Google Scholar]
- 17.Holtzheimer PE, Husain MM, Lisanby SH, et al. Subcallosal cingulate deep brain stimulation for treatment-resistant depression: a multisite, randomised, sham-controlled trial. Lancet Psychiatry. 2017;4:839–49. doi: 10.1016/S2215-0366(17)30371-1. [DOI] [PubMed] [Google Scholar]
- 18.Hamani C, Mayberg H, Snyder B, et al. Deep brain stimulation of the subcallosal cingulate gyrus for depression: anatomical location of active contacts in clinical responders and a suggested guideline for targeting. J Neurosurg. 2009;111:1209–15. doi: 10.3171/2008.10.JNS08763. [DOI] [PubMed] [Google Scholar]
- 19.Riva-Posse P, Choi KS, Holtzheimer PE, et al. Defining critical white matter pathways mediating successful subcallosal cingulate deep brain stimulation for treatment-resistant depression. Biol Psychiatry. 2014;76:963–9. doi: 10.1016/j.biopsych.2014.03.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ramasubbu R, Anderson S, Haffenden A, et al. Double-blind optimization of subcallosal cingulate deep brain stimulation for treatment-resistant depression: a pilot study. J Psychiatry Neurosci. 2013;38:325–32. doi: 10.1503/jpn.120160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Broadway JM, Holtzheimer PE, Hilimire MR, et al. Frontal theta cordance predicts 6-month antidepressant response to subcallosal cingulate deep brain stimulation for treatment-resistant depression: a pilot study. Neuropsychopharmacology. 2012;37:1764–72. doi: 10.1038/npp.2012.23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Quraan MA, Protzner AB, Daskalakis ZJ, et al. EEG power asymmetry and functional connectivity as a marker of treatment effectiveness in DBS surgery for depression. Neuropsychopharmacology. 2014;39:1270–81. doi: 10.1038/npp.2013.330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.McInerney SJ, McNeely HE, Geraci J, et al. Neurocognitive predictors of response in treatment resistant depression to subcallosal cingulate gyrus deep brain stimulation. Front Hum Neurosci. 2017;11:74. doi: 10.3389/fnhum.2017.00074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Frodl T, Meisenzahl EM, Zetzsche T, et al. Hippocampal and amygdala changes in patients with major depressive disorder and healthy controls during a 1-year follow-up. J Clin Psychiatry. 2004;65:492–9. doi: 10.4088/jcp.v65n0407. [DOI] [PubMed] [Google Scholar]
- 25.Hamani C, Mayberg H, Stone S, et al. The subcallosal cingulate gyrus in the context of major depression. Biol Psychiatry. 2011;69:301–8. doi: 10.1016/j.biopsych.2010.09.034. [DOI] [PubMed] [Google Scholar]
- 26.Johansen-Berg H, Gutman DA, Behrens TE, et al. Anatomical connectivity of the subgenual cingulate region targeted with deep brain stimulation for treatment-resistant depression. Cereb Cortex. 2008;18:1374–83. doi: 10.1093/cercor/bhm167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lozano AM, Mayberg HS, Giacobbe P, et al. Subcallosal cingulate gyrus deep brain stimulation for treatment-resistant depression. Biol Psychiatry. 2008;64:461–7. doi: 10.1016/j.biopsych.2008.05.034. [DOI] [PubMed] [Google Scholar]
- 28.Sled JG, Zijdenbos AP, Evans AC. A nonparametric method for automatic correction of intensity nonuniformity in MRI data. IEEE Trans Med Imaging. 1998;17:87–97. doi: 10.1109/42.668698. [DOI] [PubMed] [Google Scholar]
- 29.Sankar T, Chakravarty MM, Bescos A, et al. Deep brain stimulation influences brain structure in Alzheimer’s disease. Brain Stimulat. 2015;8:645–54. doi: 10.1016/j.brs.2014.11.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Drevets WC, Price JL, Simpson JR, Jr, et al. Subgenual prefrontal cortex abnormalities in mood disorders. Nature. 1997;386:824–7. doi: 10.1038/386824a0. [DOI] [PubMed] [Google Scholar]
- 31.Nugent AC, Luckenbaugh DA, Wood SE, et al. Automated subcortical segmentation using FIRST: test-retest reliability, interscanner reliability, and comparison to manual segmentation. Hum Brain Mapp. 2013;34:2313–29. doi: 10.1002/hbm.22068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Jenkinson M, Beckmann CF, Behrens TE, et al. FSL. Neuroimage. 2012;62:782–90. doi: 10.1016/j.neuroimage.2011.09.015. [DOI] [PubMed] [Google Scholar]
- 33.Patenaude B, Smith SM, Kennedy DN, Jenkinson M. A Bayesian model of shape and appearance for subcortical brain segmentation. Neuroimage. 2011;56:907–22. doi: 10.1016/j.neuroimage.2011.02.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Smith SM, Zhang Y, Jenkinson M, et al. Accurate, robust, and automated longitudinal and cross-sectional brain change analysis. Neuroimage. 2002;17:479–89. doi: 10.1006/nimg.2002.1040. [DOI] [PubMed] [Google Scholar]
- 35.Smith SM. Fast robust automated brain extraction. Hum Brain Mapp. 2002;17:143–55. doi: 10.1002/hbm.10062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Jenkinson M, Smith S. A global optimisation method for robust affine registration of brain images. Med Image Anal. 2001;5:143–56. doi: 10.1016/s1361-8415(01)00036-6. [DOI] [PubMed] [Google Scholar]
- 37.Jenkinson M, Bannister P, Brady M, et al. Improved optimization for the robust and accurate linear registration and motion correction of brain images. Neuroimage. 2002;17:825–41. doi: 10.1016/s1053-8119(02)91132-8. [DOI] [PubMed] [Google Scholar]
- 38.Zhang Y, Brady M, Smith S. Segmentation of brain MR images through a hidden Markov random field model and the expectation-maximization algorithm. IEEE Trans Med Imaging. 2001;20:45–57. doi: 10.1109/42.906424. [DOI] [PubMed] [Google Scholar]
- 39.Whitwell JL, Crum WR, Watt HC, et al. Normalization of cerebral volumes by use of intracranial volume: implications for longitudinal quantitative MR imaging. AJNR Am J Neuroradiol. 2001;22:1483–9. [PMC free article] [PubMed] [Google Scholar]
- 40.DeLong ER, DeLong DM, Clarke-Pearson DL. Comparing the areas under two or more correlated receiver operating characteristic curves: a nonparametric approach. Biometrics. 1988;44:837–45. [PubMed] [Google Scholar]
- 41.Botteron KN, Raichle ME, Drevets WC, et al. Volumetric reduction in left subgenual prefrontal cortex in early onset depression. Biol Psychiatry. 2002;51:342–4. doi: 10.1016/s0006-3223(01)01280-x. [DOI] [PubMed] [Google Scholar]
- 42.Coryell W, Nopoulos P, Drevets W, et al. Subgenual prefrontal cortex volumes in major depressive disorder and schizophrenia: diagnostic specificity and prognostic implications. Am J Psychiatry. 2005;162:1706–12. doi: 10.1176/appi.ajp.162.9.1706. [DOI] [PubMed] [Google Scholar]
- 43.Hastings RS, Parsey RV, Oquendo MA, et al. Volumetric analysis of the prefrontal cortex, amygdala, and hippocampus in major depression. Neuropsychopharmacology. 2004;29:952–9. doi: 10.1038/sj.npp.1300371. [DOI] [PubMed] [Google Scholar]
- 44.Bremner JD, Vythilingam M, Vermetten E, et al. Reduced volume of orbitofrontal cortex in major depression. Biol Psychiatry. 2002;51:273–9. doi: 10.1016/s0006-3223(01)01336-1. [DOI] [PubMed] [Google Scholar]
- 45.Hirayasu Y, Shenton ME, Salisbury DF, et al. Subgenual cingulate cortex volume in first-episode psychosis. Am J Psychiatry. 1999;156:1091–3. doi: 10.1176/ajp.156.7.1091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Sharma V, Menon R, Carr TJ, et al. An MRI study of subgenual prefrontal cortex in patients with familial and non-familial bipolar I disorder. J Affect Disord. 2003;77:167–71. doi: 10.1016/s0165-0327(02)00109-x. [DOI] [PubMed] [Google Scholar]
- 47.Sanches M, Sassi RB, Axelson D, et al. Subgenual prefrontal cortex of child and adolescent bipolar patients: a morphometric magnetic resonance imaging study. Psychiatry Res. 2005;138:43–9. doi: 10.1016/j.pscychresns.2004.11.004. [DOI] [PubMed] [Google Scholar]
- 48.Brambilla P, Nicoletti MA, Harenski K, et al. Anatomical MRI study of subgenual prefrontal cortex in bipolar and unipolar subjects. Neuropsychopharmacology. 2002;27:792–9. doi: 10.1016/S0893-133X(02)00352-4. [DOI] [PubMed] [Google Scholar]
- 49.Zimmerman ME, DelBello MP, Getz GE, et al. Anterior cingulate subregion volumes and executive function in bipolar disorder. Bipolar Disord. 2006;8:281–8. doi: 10.1111/j.1399-5618.2006.00298.x. [DOI] [PubMed] [Google Scholar]
- 50.Hajek T, Kozeny J, Kopecek M, et al. Reduced subgenual cingulate volumes in mood disorders: a meta-analysis. J Psychiatry Neurosci. 2008;33:91–9. [PMC free article] [PubMed] [Google Scholar]
- 51.Campbell S, Marriott M, Nahmias C, et al. Lower hippocampal volume in patients suffering from depression: a meta-analysis. Am J Psychiatry. 2004;161:598–607. doi: 10.1176/appi.ajp.161.4.598. [DOI] [PubMed] [Google Scholar]
- 52.Videbech P, Ravnkilde B. Hippocampal volume and depression: a meta-analysis of MRI studies. Am J Psychiatry. 2004;161:1957–66. doi: 10.1176/appi.ajp.161.11.1957. [DOI] [PubMed] [Google Scholar]
- 53.Kempton MJ, Salvador Z, Munafo MR, et al. Structural neuroimaging studies in major depressive disorder. Meta-analysis and comparison with bipolar disorder. Arch Gen Psychiatry. 2011;68:675–90. doi: 10.1001/archgenpsychiatry.2011.60. [DOI] [PubMed] [Google Scholar]
- 54.Koolschijn PC, van Haren NE, Lensvelt-Mulders GJ, et al. Brain volume abnormalities in major depressive disorder: a meta-analysis of magnetic resonance imaging studies. Hum Brain Mapp. 2009;30:3719–35. doi: 10.1002/hbm.20801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.McKinnon MC, Yucel K, Nazarov A, et al. A meta-analysis examining clinical predictors of hippocampal volume in patients with major depressive disorder. J Psychiatry Neurosci. 2009;34:41–54. [PMC free article] [PubMed] [Google Scholar]
- 56.Frodl T, Jager M, Smajstrlova I, et al. Effect of hippocampal and amygdala volumes on clinical outcomes in major depression: a 3-year prospective magnetic resonance imaging study. J Psychiatry Neurosci. 2008;33:423–30. [PMC free article] [PubMed] [Google Scholar]
- 57.Rao U, Chen LA, Bidesi AS, et al. Hippocampal changes associated with early-life adversity and vulnerability to depression. Biol Psychiatry. 2010;67:357–64. doi: 10.1016/j.biopsych.2009.10.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Phillips JL, Batten LA, Tremblay P, et al. Prospective, longitudinal study of the effect of remission on cortical thickness and hippocampal volume in patients with treatment-resistant depression. Int J Neuropsychopharmacol. 2015;18 doi: 10.1093/ijnp/pyv037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Colle R, Dupong I, Colliot O, et al. Smaller hippocampal volumes predict lower antidepressant response/remission rates in depressed patients: a meta-analysis. World J Biol Psychiatry. 2018;19:360–7. doi: 10.1080/15622975.2016.1208840. [DOI] [PubMed] [Google Scholar]
- 60.Fotuhi M, Do D, Jack C. Modifiable factors that alter the size of the hippocampus with ageing. Nat Rev Neurol. 2012;8:189–202. doi: 10.1038/nrneurol.2012.27. [DOI] [PubMed] [Google Scholar]
- 61.Zeng LL, Shen H, Liu L, et al. State-dependent and trait-related gray matter changes in nonrefractory depression. Neuroreport. 2015;26:57–65. doi: 10.1097/WNR.0000000000000301. [DOI] [PubMed] [Google Scholar]
- 62.Wu MJ, Wu HE, Mwangi B, et al. Prediction of pediatric unipolar depression using multiple neuromorphometric measurements: a pattern classification approach. J Psychiatr Res. 2015;62:84–91. doi: 10.1016/j.jpsychires.2015.01.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Jaworska N, MacMaster FP, Foster J, et al. The influence of 5-HTTLPR and Val66Met polymorphisms on cortical thickness and volume in limbic and paralimbic regions in depression: a preliminary study. BMC Psychiatry. 2016;16:61. doi: 10.1186/s12888-016-0777-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Mervaala E, Fohr J, Kononen M, et al. Quantitative MRI of the hippocampus and amygdala in severe depression. Psychol Med. 2000;30:117–25. doi: 10.1017/s0033291799001567. [DOI] [PubMed] [Google Scholar]
- 65.Frodl T, Meisenzahl E, Zetzsche T, et al. Enlargement of the amygdala in patients with a first episode of major depression. Biol Psychiatry. 2002;51:708–14. doi: 10.1016/s0006-3223(01)01359-2. [DOI] [PubMed] [Google Scholar]
- 66.Lange C, Irle E. Enlarged amygdala volume and reduced hippocampal volume in young women with major depression. Psychol Med. 2004;34:1059–64. doi: 10.1017/s0033291703001806. [DOI] [PubMed] [Google Scholar]
- 67.Frodl T, Meisenzahl EM, Zetzsche T, et al. Larger amygdala volumes in first depressive episode as compared to recurrent major depression and healthy control subjects. Biol Psychiatry. 2003;53:338–44. doi: 10.1016/s0006-3223(02)01474-9. [DOI] [PubMed] [Google Scholar]
- 68.Jarnum H, Eskildsen SF, Steffensen EG, et al. Longitudinal MRI study of cortical thickness, perfusion, and metabolite levels in major depressive disorder. Acta Psychiatr Scand. 2011;124:435–46. doi: 10.1111/j.1600-0447.2011.01766.x. [DOI] [PubMed] [Google Scholar]
- 69.Tu PC, Chen LF, Hsieh JC, et al. Regional cortical thinning in patients with major depressive disorder: a surface-based morphometry study. Psychiatry Res. 2012;202:206–13. doi: 10.1016/j.pscychresns.2011.07.011. [DOI] [PubMed] [Google Scholar]
- 70.Grieve SM, Korgaonkar MS, Koslow SH, et al. Widespread reductions in gray matter volume in depression. Neuroimage Clin. 2013;3:332–9. doi: 10.1016/j.nicl.2013.08.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Li M, Metzger CD, Li W, et al. Dissociation of glutamate and cortical thickness is restricted to regions subserving trait but not state markers in major depressive disorder. J Affect Disord. 2014;169:91–100. doi: 10.1016/j.jad.2014.08.001. [DOI] [PubMed] [Google Scholar]
- 72.Van Tol MJ, Li M, Metzger CD, et al. Local cortical thinning links to resting-state disconnectivity in major depressive disorder. Psychol Med. 2014;44:2053–65. doi: 10.1017/S0033291713002742. [DOI] [PubMed] [Google Scholar]
- 73.Peterson BS, Warner V, Bansal R, et al. Cortical thinning in persons at increased familial risk for major depression. Proc Natl Acad Sci U S A. 2009;106:6273–8. doi: 10.1073/pnas.0805311106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Qiu L, Lui S, Kuang W, et al. Regional increases of cortical thickness in untreated, first-episode major depressive disorder. Transl Psychiatry. 2014;4:e378. doi: 10.1038/tp.2014.18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Van Eijndhoven P, van Wingen G, Katzenbauer M, et al. Paralimbic cortical thickness in first-episode depression: evidence for trait-related differences in mood regulation. Am J Psychiatry. 2013;170:1477–86. doi: 10.1176/appi.ajp.2013.12121504. [DOI] [PubMed] [Google Scholar]
- 76.Paus T, Keshavan M, Giedd JN. Why do many psychiatric disorders emerge during adolescence? Nat Rev Neurosci. 2008;9:947–57. doi: 10.1038/nrn2513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Ikram MA, Fornage M, Smith AV, et al. Common variants at 6q22 and 17q21 are associated with intracranial volume. Nat Genet. 2012;44:539–44. doi: 10.1038/ng.2245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Ishitobi Y, Nakayama S, Yamaguchi K, et al. Association of CRHR1 and CRHR2 with major depressive disorder and panic disorder in a Japanese population. Am J Med Genet B Neuropsychiatr Genet. 2012;159B:429–36. doi: 10.1002/ajmg.b.32046. [DOI] [PubMed] [Google Scholar]
- 79.Nordenskjold R, Malmberg F, Larsson EM, et al. Intracranial volume estimated with commonly used methods could introduce bias in studies including brain volume measurements. Neuroimage. 2013;83:355–60. doi: 10.1016/j.neuroimage.2013.06.068. [DOI] [PubMed] [Google Scholar]
- 80.Barnes J, Ridgway GR, Bartlett J, et al. Head size, age and gender adjustment in MRI studies: a necessary nuisance? Neuroimage. 2010;53:1244–55. doi: 10.1016/j.neuroimage.2010.06.025. [DOI] [PubMed] [Google Scholar]
- 81.Wang L, Akritas MG. Testing for covariate effects in the fully nonparametric analysis of covariance model. J Am Stat Assoc. 2006;101:722–36. [Google Scholar]
- 82.Mahmoudzadeh AP, Kashou NH. Evaluation of interpolation effects on upsampling and accuracy of cost functions-based optimized automatic image registration. Int J Biomed Imaging. 2013;2013 doi: 10.1155/2013/395915. 395915. [DOI] [PMC free article] [PubMed] [Google Scholar]