Abstract
Background
Attentional bias modification (ABM) may lead to more adaptive emotion perception and emotion regulation. Understanding the neural basis of these effects may lead to greater precision for the development of future treatments. Task-related functional MRI (fMRI) after ABM training has not been investigated in depression so far. The main aim of this randomized controlled trial was to explore differences in brain activity after ABM training, in response to emotional stimuli.
Methods
A total of 134 people with previous depression, who had been treated for depression and had various degrees of residual symptoms, were randomized to 14 days of active ABM or a closely matched placebo training, followed by an fMRI emotion regulation task. The training procedure was a classical dot–probe task with emotional face stimuli. In the active ABM condition, the probes replaced the more positively valenced face of a given pair. As participants implicitly learned to predict the probe location, this would be likely to induce a more positive attentional bias. The placebo condition was identical, except for the contingency of the probe, which appeared equally behind positive and negative stimuli. We compared depression symptoms and subjective ratings of perceived negativity during fMRI between the training groups. We explored brain activation in predefined regions of interest and across the whole brain. We explored activation in areas associated with changes in attentional bias and degree of depression.
Results
Compared with the placebo group, the ABM group showed reduced activation in the amygdala and the anterior cingulate cortex when passively viewing negative images. We found no group differences in predefined regions of interest associated with emotion regulation strategies. Response in the temporal cortices was associated with the degree of change in attentional bias and the degree of depressive symptoms in ABM versus placebo.
Limitations
These findings should be replicated in other samples of patients with depression, and in studies using fMRI designs that allow analyses of within-group variability from baseline to follow-up.
Conclusion
Attentional bias modification training has an effect on brain function in the circuitry associated with emotional appraisal and the generation of affective states.
Clinicaltrials.gov identifier
Introduction
A number of effective treatments are available for major depressive disorder. However, following successful treatment, relapse is common: 50% to 70% of patients relapse within 5 years.1,2 Residual symptoms are among the strongest predictors of relapse in recurrent depression.3 Cognitive theories of depression posit that biased information processing for emotional stimuli plays a key role in depression development and relapse.4 Despite mixed findings, people with clinical depression and who previously had depression but are currently euthymic have repeatedly been reported to orient their attention toward negative faces rather than neutral or positive faces.5–10 Attentional biases and deficits in cognitive control may interfere with emotion regulation and mood state. Negative cognitive biases in depression are thought to be facilitated by increased influence from subcortical emotion processing regions, combined with attenuated top–down cognitive control.4,11 There is evidence for blunted responsiveness to positive stimuli and reward in depression.12–14 At the same time, researchers have linked depression to dysregulated attentional processing of both positive and negative stimuli.15–17
Computerized attentional bias modification (ABM) procedures are aimed at implicitly retraining biased attentional patterns.18 Although there is debate about the true effect size of ABM in depression,19,20 some studies have reported reduced depressive symptoms after successful modification of attentional bias.21–24 Antidepressant treatments have been shown to dampen negative biases before clinical effects on mood are measurable,25 and these observations have led to a cognitive neuropsychological theory suggesting that modifying negative biases in emotional information processing may play a causal role in antidepressant treatment effects.26,27 According to the theory, symptom improvement should consolidate over time. However, there is little empirical evidence of the long-term effects of ABM.
It has been suggested that attentional biases are an important component of explicit methods of emotion regulation.28 According to influential theories of emotion regulation,29,30 redirecting attention toward or away from emotionally arousing aspects of a situation enables people to increase or decrease the intensity of the emotions.31,32 Psychological models of reappraisal suggest that many of the cognitive control processes used to regulate attention, memory and thoughts in general are also used in the cognitive regulation of emotion.33 Thus, it has been hypothesized that one way ABM might work could be by improving emotion regulation.28,34
The neural basis of changes in attentional bias, which is believed to be the mechanism of change behind symptom improvement after ABM training, has not been investigated so far. The functional neurobiology of emotion perception distinguishes between structures that are critical for appraisal, generation of affective states and emotion regulation. The amygdala and insular cortex are particularly important in a ventral system linked to the emotional significance of stimuli and the production of affective states.35,36 The ventral anterior cingulate cortex (ACC) plays a major role in the automatic regulation of emotional responses. A dorsal system includes the dorsal ACC and prefrontal regions and is thought to be involved in effortful regulation of affective states and subsequent behaviour.35,37
The neural effects of a single session of ABM in healthy people include lateral prefrontal cortex reactivity toward emotional stimuli,38 indicating moderation of the dorsal neurocircuitry in emotion perception. One resting-state fMRI study in young women with subthreshold depression found differences between ABM and placebo in measures of spontaneous fluctuations in the right anterior insula and right middle frontal gyrus,39 areas critical for emotion generation and automatic regulation of emotional responses. In a study of people with depression, differences in resting-state connectivity between ABM and placebo were found in the middle frontal gyrus and dorsal ACC (a neural system important for cognitive control over emotions) along with changes in a network associated with sustained attention to visual information in the placebo group.40 Overall, these early results provide some evidence that ABM modifies function in emotional regulatory systems, although the small study sample sizes and variety of approaches used may underpin the absence of consistent effects across studies.41
To our knowledge, no study has investigated ABM-induced changes in emotion processing using fMRI in a large clinical sample after multiple training sessions. In this preregistered clinical trial, we used a sample of 134 participants previously treated for depression and with various degrees of residual symptoms. A major aim was to explore the neural effects of ABM in ventral and dorsal emotion-perception circuitry during an emotion regulation task. The preregistered hypotheses were that, compared with placebo: (1) the ABM group would show increased blood-oxygenation level–dependent (BOLD) signal in prefrontal cortical regions and (2) the ABM group would reveal decreased BOLD signal in the amygdala. We also measured fMRI response in prefrontal and subcortical regions of interest (ROIs) in a well-established emotion regulation circuitry based on previous studies. Finally, we examined how changes in attentional bias (the mechanism by which ABM is believed to work) and changes in symptoms differed between ABM- and placebo-treated groups.
Methods
Participants and screening procedures
Patients who had been treated for at least 1 previous episode of major depressive disorder were randomized into 2 treatment groups with either a positive ABM or a closely matched active placebo training condition. We performed block randomization (1:1) at inclusion to ensure equal numbers of participants and similar characteristics for the 2 groups. Participants were invited to be part of the fMRI study immediately after training and preferably within 1 week after ABM training. The current clinical trial (NCT02931487) was an extension of a larger double-blind, randomized controlled trial (NCT02658682) that included 321 patients with a history of depression. A total of 136 eligible participants between the ages of 18 and 65 years were enrolled for fMRI.
The main recruitment site was an outpatient clinic in the Department of Psychiatry, Diakonhjemmet Hospital, in Oslo. Participants were also recruited from other clinical sites and via social media. Individuals diagnosed with a current major depressive episode, current or former neurologic disorder, psychosis, bipolar spectrum disorder, substance use disorder, attention deficit disorder, or head trauma were excluded during prescreening. A history of an anxiety disorder was not an exclusion criterion. Informed consent was obtained before enrolment. The procedure was approved by the Regional Ethical Committee for Medical and Health Research for Southern Norway (2014/217/REK sor-ost D). Inclusion criteria were people who had experienced more than 1 major depressive episode (lifetime) that fulfilled the Mini International Neuropsychiatric Interview (MINI 6.0.0) A1a (depressed mood) and/or A2a (loss of interest or pleasure) criteria, more than 5 positive items on A3, and fulfilling the A5 criterion (DSM 296.30–296.36 recurrent/ICD-10 F33.x). To assess both self- and clinician-rated symptoms, we administered the Beck Depression Inventory (BDI-II)42 and the Hamilton Rating Scale for Depression (HAM-D),43 respectively. There was no upper or lower threshold for depression symptom scores. Raters were blind to the intervention.
Attentional bias modification procedure
The ABM task was a computerized visual dot–probe procedure developed by Browning and colleagues.21 A fixation cross was initially displayed, followed by 2 images (the stimuli) presented concurrently at the top and bottom of the computer screen. Following stimulus onset, a probe (1 or 2 dots) immediately appeared at the same location as 1 of the image stimuli and remained on the screen until the participant responded. Responses were collected via a button press from 1 of 2 buttons. The types of stimuli were pictures of emotional faces. The face stimuli had 3 valences — positive (happy), neutral, or negative (angry and fearful) — and were based on 4 databases: the Karolinska Directed Emotional Faces,44 NimStim,45 Matsumoto and Ekman’s Japanese and Caucasian Facial Expressions of Emotion,46 and Ekman Pictures of Facial Affect.47 A single session of the task involved 96 trials with equal numbers of the 3 stimulus pair types. In addition, equal numbers of trials were randomly presented for 500 or 1000 ms before the probe was displayed. In each trial of the task, stimuli from 2 valences were displayed in one of the following pairing types: positive–neutral, positive–negative and negative–neutral. In the ABM condition, probes were located behind positive stimuli in 87% of the trials (valid trials), and 13% of probes were located behind negative stimuli (invalid trials). Consequently, participants could implicitly learn to deploy their attention toward positive stimuli, and in this way develop a more positive attentional bias when completing the task. The neutral ABM placebo condition was identical except for the location of the probe, which was located behind the positive (valid trials) stimuli in 50% of the trials. Participants completed 2 sessions (192 trials) of ABM daily at home over 14 days (28 sessions in total) on identical notebook computers (14” HP EliteBook 840, 1600 × 900, 8 GB, Intel Core i5–4310U), which were set up and used exclusively for ABM training. The duration of each training session was approximately 5 minutes. We ensured adherence using a calendar system and daily reminders on SMS. We calculated adherence rates for the training procedure (percentage complete sessions) from the computers’ log files; mean adherence rates were 82.32%.
MRI scan acquisition
We conducted scanning on a 3 T Philips Ingenia whole-body scanner, with a 32 channel Philips SENSE head coil (Philips Medical Systems). We obtained functional images with a single-shot T2*-weighted echo planar imaging sequence (repetition time 2000 ms, slice echo time 30 ms, field of view 240 × 240 × 117, imaging matrix 80 × 80, flip angle 90°, 39 axial slices, interleaved at 3 mm thickness, no gap, voxel size 3 × 3 × 3 mm). The scanning session consisted of 340 volumes, synchronized to the onset of the experiment. We adjusted slice orientation to the line running from the anterior to the posterior commissure. We recorded a T1-weighted anatomic image with a voxel size of 1 × 1 × 1 mm for registration of the functional images (repetition time 8.5 ms, echo time 2.3 ms, field of view 256 × 256 × 184, flip angle 7°, 184 sagittal slices).
fMRI experimental procedure
The study used a modified emotion regulation experiment. Participants were scanned as they viewed sequences of negative and neutral images and carried out instructions to downregulate their emotional responses using a reappraisal strategy or allow themselves to attend to the pictures without trying to influence their emotional reactions. After each image, the participants provided a rating of the intensity of their emotional state using a visual analogue scale (VAS) that ranged from neutral to negative. Stimuli were selected from the International Affective Picture System48 and the Emotional Picture Set.49 Negative and neutral pictures were counterbalanced based on their normative valence and arousal ratings (see Appendix 1, available at jpn.ca/180118-a1 for more detail). Each trial started with a fixation cross, followed by a written instruction: “Attend” or “Regulate.” The instruction was presented for 2000 ms. Then, a negative or neutral image was presented for 6000 ms, followed by a rating screen time-locked to 6000 ms. Between stimuli, we used a temporal jitter randomized from 2000 to 8000 ms (mean interstimulus interval 3700 ms) to optimize statistical efficiency in the event-related design.50 The task consisted of blocks of 18 trials with a 20-second null trial between the 2 blocks. The procedure was completed in 2 independent runs during the scanning session, for 72 trials in total. In each block, 12 items were neutral and 24 items were negative, providing 3 counterbalanced experimental conditions: AttendNeutral, AttendNegative and RegulateNegative. The stimulus order in each block was interspersed pseudorandomly from 12 unique lists. The total duration of a single functional scanning run was approximately 11 minutes, and the total scan time was approximately 22 minutes. Stimuli were presented using E-Prime 2.0 software (Psychology Software Tools). An MRI-compatible monitor was placed at the end of the scanner behind the participant’s head. Participants watched the monitor using a mirror placed at the head coil. Responses were collected using a response grip with 2 response buttons. Physiologic data (heart and respiration curves) were recorded at 1000 Hz using a clinical monitoring unit digitized together with scanner pulses.
Training and instruction procedures
We used a written protocol with detailed instructions to introduce the emotion regulation experiment. The protocol was dictated for each participant by the researcher outside the MRI scanner in order to standardize the verbal instructions. The fMRI experiment had 3 in-scanner exercise trials before the scan started to familiarize participants with the instructions, timing, response buttons and VAS. The training procedure was repeated before the second run of the experiment.
Symptom change and subjective ratings of negativity
We analyzed changes in self-rated and clinician-rated symptoms using PASW 25.0 (IBM) and a repeated-measures analysis of variance (ANOVA), with intervention (ABM versus placebo training) as a fixed factor. The dependent variable was symptoms at baseline and 2 weeks’ follow-up (time). To investigate self-reported emotional reactivity (VAS scores) during fMRI, we added a factor based on the 3 experimental conditions (AttendNeutral, AttendNegative, RegulateNegative) and analyzed it using repeated-measures ANOVA.
fMRI analyses
Whole brain analysis used the AttendNegative > AttendNeutral contrast to test whether ABM influenced overall brain activity in response to passive viewing of emotional stimuli. We de-meaned clinician-rated (HAM-D) symptoms at baseline (subtracting the mean from the individual value) and used it as a covariate. Spatial smoothing full width at half maximum was set to 5 mm. We used Featquery for FMRI Expert Analysis Tool (FEAT) result interrogation. We extracted mean local percent signal change to explore individual distribution within significant clusters from FEAT. We performed interaction analysis to test whether areas in the brain responded differently in the ABM and placebo groups in relation to attentional bias and symptom change.
We performed ROI analyses using a small volume correction (SVC) method with a priori volumes based on coordinates from a recent meta-analysis on neuroimaging and reappraisal. The meta-analysis by Buhle and colleagues51 consisted of 48 neuroimaging studies of reappraisal, in which most studies involved downregulation of negative affect, and which reported 7 clusters related to emotion regulation that were consistently found in prefrontal cognitive control areas when contrasted with passive viewing of negative images. The clusters were situated in the left and right middle frontal gyrus, the right inferior frontal gyrus, the right medial frontal gyrus, the left and right superior temporal lobe, and the left middle temporal gyrus (Appendix 1). The bilateral amygdala was reported for the contrast comparing negative viewing to emotion regulation. Brain activation derived from passive viewing of negative images (as compared with passive viewing of neutral images) was not included in the results from the meta-analysis.51
We used binary spheres with a 5 mm radius, based on Montreal Neurological Institute (MNI) coordinates of peak voxels for the predefined regions. We created 2 single masks for the emotion regulation contrast (RegulateNegative > AttendNegative, AttendNegative > RegulateNegative). We combined the 7 cortical spheres and the 2 subcortical spheres into 2 single binary ROIs. We set the Z-threshold at 2.3 and the cluster p-threshold at 0.05. We extracted the mean local percent signal change from the 2 ROIs to explore individual distribution in significant clusters from FEAT. Again, clinician-rated (HAM-D) symptoms at baseline were de-meaned and used as a covariate in the ROI analysis.
fMRI data preprocessing and noise reduction
We used the FMRIB Software Library version (FSL version 6.00; www.fmrib.ox.ac.uk/fsl)52,53 to preprocess and analyze fMRI data. We carried out fMRI data processing using FEAT version 6.00, a part of FSL (FMRIB Software Library). In conjunction with FEAT FSL-PNM, we applied 34 explanatory variables to regress out physiologic noise from pulse and respiration.54 We carried out registration to high-resolution structural and/or standard space images using FLIRT.55,56 We then further refined registration from high-resolution structural to standard space using FNIRT nonlinear registration.57 We manually inspected all registrations to ensure proper alignment. We carried out time-series statistical analysis using FILM with local autocorrelation correction.58 We conducted linear registration with 12 degrees of freedom. The Z (Gaussianised T/F) statistic images were thresholded using clusters determined by Z > 2.3 and a (corrected) cluster significance threshold of p = 0.05.59 Two participants were excluded from the analyses due to signal loss caused by a technical problem with the head coil. We combined the time series from each participant’s 2 first-level runs using an intermediate fixed-effect model in FEAT before submission to second-level analysis. A total of 134 participants (64 from the ABM group and 70 from the placebo group) were included in the intermediate and higher-level FEAT analysis at the group level. We conducted nonparametric testing using FSL randomize60 with threshold-free cluster enhancement61 and 5000 permutations as an alternative to FEAT with a cluster-defining threshold.
Results
Sample participant characteristics can be found in Table 1.
Table 1.
Sample characteristics*
| Characteristic | Placebo (n = 70) | ABM (n = 64) | F | p value† |
|---|---|---|---|---|
| Age, yr | 39.65 ± 13.54 | 39.09 ± 12.80 | 0.061 | 0.81 |
| Female, no. | 44 | 47 | 1.717 | 0.20 |
| Education level, ISCED | 5.92 ± 1.20 | 5.85 ± 1.27 | 0.113 | 0.74 |
| SSRI medication, no.‡ | 23 | 22 | 0.035 | 0.86 |
| No. of major depressive episodes§ | 4.48 ± 5.30 | 4.79 ± 7.56 | 0.077 | 0.78 |
| Days between ABM and fMRI | 6.94 ± 8.70 | 6.65 ± 7.19 | 0.041 | 0.84 |
| Baseline symptoms | ||||
| Hamilton Rating Scale for Depression | 7.53 ± 4.69 | 9.56 ± 6.38 | 4.469 | 0.036 |
| Beck Depression Inventory II | 12.09 ± 8.66 | 17.12 ± 11.62 | 8.187 | 0.005 |
ABM = attentional bias modification; fMRI = functional MRI; ISCED = International Standard Classification of Education; SSRI = selective serotonin reuptake inhibitor.
Unless otherwise indicated, findings are presented as mean ± standard deviation.
Pearson χ2 test for dichotomous variables.
Any current use of an antidepressant belonging to the SSRI class.
According to the MINI International Neuropsychiatric Interview.
Symptom change after ABM
We found a statistically significant group × time interaction for rater-evaluated depression as measured by change in HAM-D. Follow-up comparisons for this significant interaction revealed lower symptoms of depression in the ABM group (F1,132 = 4.277, η2 = 0.03, p = 0.041). The means and standard deviations at baseline were 9.56 ± 6.38 for the ABM group and 7.53 ± 4.69 for the placebo group, and changed to 7.93 ± 5.90 and 7.77 ± 5.76, respectively, at 2 weeks’ follow-up.
We found no statistically significant effects for self-reported symptoms as measured by the BDI-II (F1,132 = 2.048, p = 0.16). The means and standard deviations at baseline were 17.12 ± 11.62 for the ABM group and 12.09 ± 8.66 for the placebo group, and changed to 13.25 ± 12.04 and 9.82 ± 8.72, respectively, at 2 weeks’ follow-up.
We found general symptom improvement in both the ABM and placebo groups as measured by the BDI-II from baseline to after training (F1,132 = 29.775; η2 = 0.18; p < 0.001). This finding was in accordance with results from the sample from which this smaller cohort was drawn.62
Subjective ratings of perceived negativity
We found a statistically significant difference between task conditions in self-reported emotional reactivity as measured by VAS scores during the fMRI experiment. The repeated-measures ANOVA showed that mean VAS scores were lowest when viewing neutral images (8.2 ± 7.8), followed by when patients were encouraged to regulate negative responses to negative images (40.8 ± 16.9), and highest for passive viewing of negative images (62.0 ± 15.3, F1,133 = 0.074, η2 = 0.93, p < 0.001). A post hoc test showed that the differences between the passive and regulated viewing conditions for negative stimuli was large and statistically significant (F1,133 = 202.81, η2 = 0.60, p < 0.001). The VAS ratings did not differ between the ABM and placebo groups (F1,133 = 0.993, p < 0.65).
Post-treatment differences between ABM- and placebo-treated groups from whole brain analyses
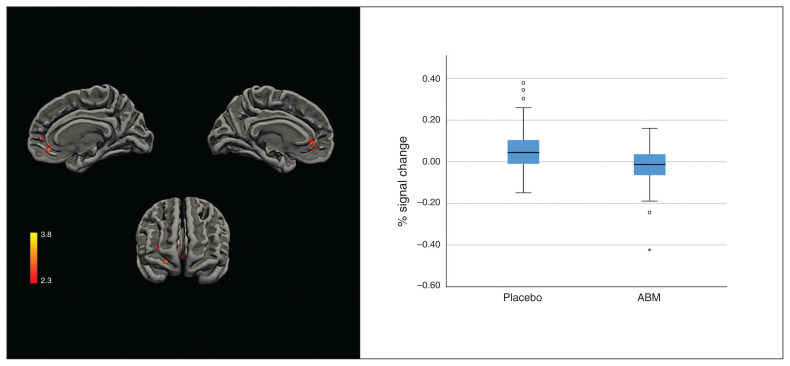
The RegulateNegative > AttendNegative and AttendNegative > RegulateNegative contrasts revealed no group differences. Compared with the ABM group, the AttendNegative > AttendNeutral contrast revealed greater activation in the placebo-treated group in a cluster in the pregenual ACC, the paracingulate and the medial cortex bilaterally, extending to the right frontal orbital cortex and the frontal pole. The peak activation for this cluster was found in the left frontal medial cortex (MNI coordinates x, y, z = −16, 36, −10; Z = 3.86, p = 0.001; Fig. 1).
Fig. 1.
Left: cluster activation (Z > 2.3) for placebo over attentional bias modification (ABM) for the AttendNegative > AttendNeutral contrast. Right: distribution of individual percentage signal changes over significant clusters; solid line indicates the median; box indicates the 50th percentile of the median; whiskers indicate 95% confidence intervals; dots indicate potential outliers (1.5 standard deviation [SD] outside the 50th percentile of the median); asterisk indicates an extreme outlier (3 SD outside the 50th percentile of the median).
Post-treatment differences between ABM- and placebo-treated groups within predefined ROIs
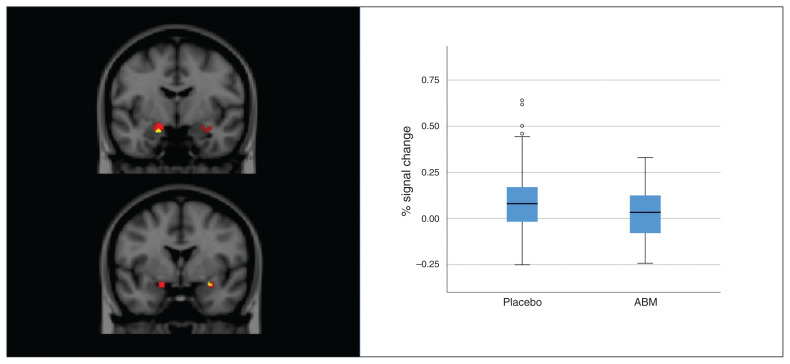
Analyses masked across predefined emotion regulation circuitry revealed more activation in the placebo-treated group than in the ABM group in the right amygdala (MNI x, y, z = −18, −6, −20; size = 8, Z = 2.89, p = 0.032) and left amygdala (MNI x, y, z = 28, 0, −16; size = 3, Z = 2.55, p = 0.040) for the passive viewing contrast (AttendNegative > AttendNeutral; Fig. 2).
Fig. 2.
Amygdala activation for the AttendNegative > AttendNeutral contrast. Left: red voxels represent mean amygdala activation across placebo and attentional bias modification (ABM) training groups. Yellow voxels are superimposed and represent peak voxels where the placebo group had more activation than the ABM group. Right: distribution of individual percentage signal changes over significant clusters; solid line indicates the median; box indicates the 50th percentile of the median; whiskers indicate 95% confidence intervals; dots indicate potential outliers (1.5 standard deviation outside the 50th percentile of the median).
We found no differences between the ABM and placebo groups for the emotion regulation contrasts. Across both groups, the regulate contrast (RegulateNegative > AttendNegative) revealed activation in 2 ROIs in the left inferior frontal gyrus (MNI x, y, z = −30, −2, −54; size = 75, Z = 10.5, p = 0.017) and right middle frontal gyrus (MNI x, y, z = 60, 26, 6; size = 29, Z = 5.48, p = 0.048). The opposite contrast ( AttendNegative > RegulateNegative) revealed increased bilateral amygdala activation in both the ABM and placebo groups. The largest cluster was in the left amygdala (MNI x, y, z = −18, 0, −14; size = 75, Z = 7.93, p = 0.006), and a smaller cluster was in the right amygdala (MNI x, y, z = 26, −2, −16; size = 13, Z = 4.05, p = 0.026; Appendix 1, Fig. S1).
Nonparametric testing using randomize did not reveal statistically significant cluster activation for the whole brain or for predefined ROIs.
Interaction with degree of attentional biases and symptom change
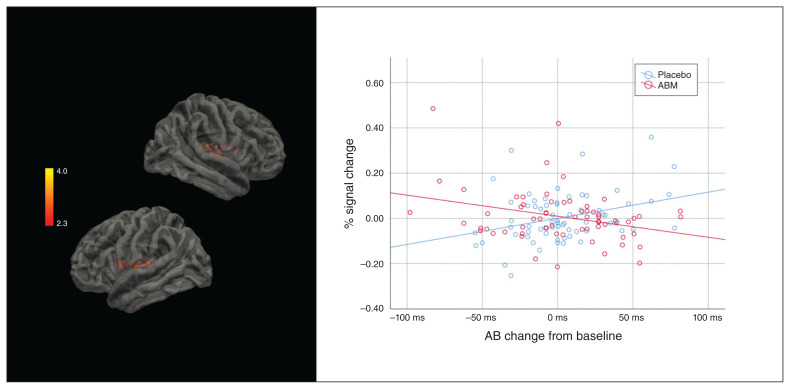
Two distinct clusters were associated with the interaction between passive viewing of negative images (AttendNegative > AttendNeutral), the intervention and the degree of attentional bias change (MNI x, y, z = 54, −24, 8; size = 1061, Z = 4.05, p < 0.001; and MNI x, y, z = −50, 0, 10; size = 547, Z = 3.44, p < 0.020). The degree of change in attentional bias was associated with a lower percent signal change in the ABM group, and a higher percent signal change in the placebo group. (Fig. 3).
Fig. 3.
Left: areas activated in association with the interaction between attentional bias (AB) and the intervention for AttendNegative > AttendNeutral. Right: regression lines and individual distribution in the attentional bias modification (ABM) and placebo conditions.
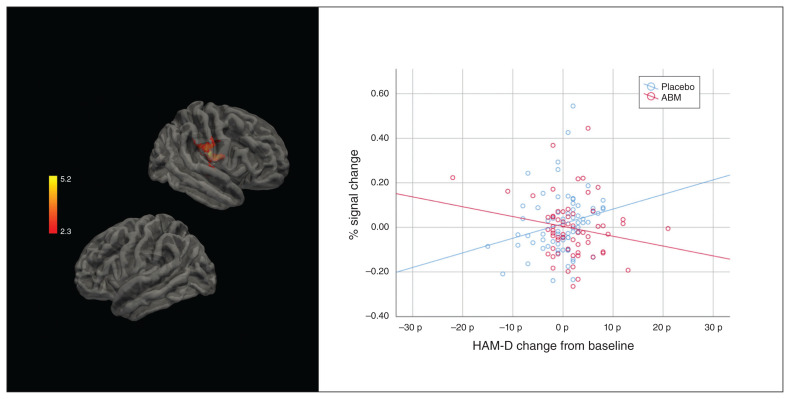
We found an interaction between passive viewing ( AttendNegative > AttendNeutral), the intervention and the degree of symptom change (HAM-D) in the right planum temporale and the insular cortex (MNI x, y, z = 50 −10 18; size = 872, Z = 5.28, p < 0.001). The degree of symptom change was associated with a lower percent signal change in the ABM group, and a higher percent signal change in the placebo group (Fig. 4).
Fig. 4.
Left: areas activated in association to the interaction between Hamilton Rating Scale for Depression (HAM-D) and the intervention for AttendNegative > AttendNeutral. Right: regression lines and individual distribution in the attentional bias modification (ABM) and placebo conditions.
Sensitivity analysis and nonparametric testing
Cluster thresholding determined by Z > 3.1 and a (corrected) cluster significance threshold of p = 0.001 did not reveal statistically significant cluster activation in the whole brain analysis or in SVCs. None of the reported results survived nonparametric testing using randomize (5000 permutations, threshold-free cluster enhancement).
Discussion
Our results revealed differences in brain activity between ABM and placebo training groups related to passive viewing of negative stimuli in areas consistently associated with emotional appraisal and the generation of affective states — areas in a circuitry known to be altered in depression.4,35,37 The placebo group showed more pronounced activation in the amygdala, midline structures and the pregenual ACC. We found no group differences related to explicit emotion regulation. Analysis of the mechanism of change showed that the degree of change in attentional bias was linearly linked to activity in the bilateral insular cortex. Symptom improvement after ABM was linearly associated with activation in the right insular cortex, an area involved in the generation of affective states.35
Analyses in predefined areas associated with effortful emotion regulation revealed activation in the left inferior frontal gyrus and the right middle frontal gyrus across groups.51 The amygdala was also more activated during passive viewing than explicit regulation of negative stimuli, but this did not differ between the ABM and placebo groups. In line with these results, we found no differences between ABM and placebo training as measured by subjective ratings (VAS) of perceived negativity. This finding was consistent with the primary outcomes from the clinical trial, which found an ABM effect restricted to blinded clinician-rated symptoms, but not self-rated symptoms.62 Together, these results may imply that the early effects of ABM are restricted to changes in emotion generation and appraisal, rather than more conscious forms of emotion regulation linked to the dorsal system.
A considerable number of meta-analyses using functional connectivity in depression have shown altered activity in areas that distinguished the ABM and placebo groups in the current study, including the insula and the ACC.63–67 Midline brain structures, including the pregenual ACC, have been linked to self-referential processing,68 hopelessness,69 anhedonia70 and impaired emotion processing71 and are identified in studies of functional connectivity in depression. Notably, the ACC and insula, together with the amygdala, are core areas of the salience network, which determines the significance of external stimuli. The salience network is thought to play a role in switching between task positive and negative networks72,73 and may play a role in symptom improvement after ABM, as found in this study.
The insula and amygdala are among the core brain areas that respond preferentially to negative stimuli in healthy people, and activation in the insula and ACC has repeatedly been reported across a range of experiments that use emotional tasks with cognitive demand and mental imagery.74,75 Neural responses to negative stimuli in the amygdala, insula and ACC are more pronounced in depressed patients than in healthy controls.76 Ma77 describes an emotional circuit including the insula, the bilateral amygdala and the ACC affected by antidepressant medication by decreasing activity toward negative stimuli and increasing activity toward positive stimuli. Antidepressants have been hypothesized to work by remediating negative affective biases (that is, targeting the same mechanism as when applying an ABM procedure).26,78,79 Similarly, the moderation of awareness toward negative stimuli via ABM (the hypothesized mechanism of change) may alter automatic emotional vigilance and arousal toward negative stimuli. This moderation may lead to altered parasympathetic responses via circuitry that involves the amygdala and ACC. The translation of these changes into improved subjective mood may take time as the individual learns to respond to this new and more positive social and emotional perspective of the world. However, neural correlates of early changes in the processing of emotional stimuli might be a marker of a process leading to symptom improvement. This model is consistent with cognitive theories of depression4,80 that the ABM procedure builds on. Accordingly, studies on cognitive behavioural therapy show that pregenual ACC is positively correlated with degree of symptom improvement.81–86 Moreover, given that the pregenual ACC is believed to play an important role in the downregulation of limbic hyper-reactivity, 37,87,88 the group difference found in this study may reflect more adaptive emotion processing after ABM.
Worldwide, there is a pressing demand for evidence-based treatments in mental health. It has been argued that psychotherapy research does not provide explanations for how or why even the most commonly used interventions produce change.89 In a recent statement from Lancet Psychiatry’s Commission on Treatments Research in Tomorrow’s Science, the authors argue that there is an acute need to improve treatment, and clinical trials should focus not only on efficacy, but also on identifying the underlying mechanisms by which treatments operate.90 The current study addresses such mechanisms by targeting changes in attentional biases, which is believed to be the mechanism that translates to symptom improvement after ABM.
The current study was based on a randomized controlled trial with a larger sample of patients that found an ABM effect on clinician-rated symptoms. It used a well validated emotion perception task and followed a stringent preregistered research protocol, which was a strength. This study exploited the link between a psychological mechanism, clinical measures, and underlying brain function as measured by fMRI, so the results should have translational potential. The current study is the largest study to investigate changes in emotion processing using fMRI after ABM training.
Limitations
A key limitation related to research design is that fMRI assessment after ABM does not allow for statistical modelling of within-individual variance from baseline to follow-up. We found an unexpected difference in symptom degrees at baseline that could have been associated with group differences in brain activation. We sought to address this possible confound by including symptom degree as a covariate in the fMRI analyses. The higher baseline symptom scores in the ABM group may still have represented a higher potential for change. The sample consisted of patients with previous depression and various degrees of residual symptoms; it needs to be replicated in studies with other patient groups. Brain activation related to ABM may also be conditionally mediated by multiple biological and environmental factors outside the scope of this study. Future studies should consider experimental designs that include passive viewing and upregulation for positive images to match for potential process and arousal effects. More precise insight into stimulus characteristics such as arousal and valence may help validate future ABM procedures. The risk of false-positive findings should have been reduced by a relatively large sample size, correction for physiologic artifacts and a study design in which only voxel activation over 2 individual runs was submitted to group-level analysis. However, the results did not survive nonparametric testing or stricter thresholding as suggested by Eklund and colleagues,91 but see also Carter and colleagues92 and Poldrack and colleagues.93 Further replication of the reported findings is warranted.
Conclusion
This study showed that ABM-associated differences in brain circuitry were linked to passive viewing but not to conscious regulation of emotional stimuli and represents, to our knowledge, the first experimental evidence of an ABM effect using task-based fMRI.
Acknowledgements
The authors thank their fMRI research assistant Dani Beck; the Division of Psychiatry, Diakonhjemmet Hospital, for help and support during the recruiting period; the Intervention Centre, OUS, for radiological assistance in MRI protocols, data acquisitions and screening for unexpected neuropathological findings; Tor Endestad for establishing invaluable infrastructure for MRI research in the department; and the external recruitment sites Unicare, Coperiosenteret AS, Torgny Syrstad, MD, Synergi Helse AS and Lovisenberg Hospital. This project is supported by the Southeastern Norway Regional Health Authority, grant number 2015052 (to NIL); the Research Council Norway, grant number 229135 (to NIL); and the Department of Psychology, University of Oslo. CJH is supported by the NIHR Oxford Health Biomedical Research Centre. The views expressed are those of the authors and not necessarily those of the NHS, the NIHR or the Department of Health.
Footnotes
Competing interests: N. Landro has received consultancy fees and travel expenses from Lundbeck. C. Harmer has received consultancy fees from Johnson and Johnson Inc, P1 vital and Lundbeck. M. Browning holds a part-time position at P1 vital Ltd. and owns shares in P1 vital products Ltd. M. Browning has also received travel expenses from Lundbeck and has acted as a consultant for J&J. No other competing interests declared.
Contributors: E. Hilland, N. Landro, C. Harmer, M. Browning and R. Jonassen designed the study. E. Hilland, L. Maglanoc and R. Jonassen acquired the data, which E. Hilland, N. Landro, C. Harmer, M. Browning and R. Jonassen analyzed. E. Hilland and R. Jonassen wrote the article, which all authors reviewed. All authors approved the final version to be published and can certify that no other individuals not listed as authors have made substantial contributions to the paper.
References
- 1.Brent DA, Brunwasser SM, Hollon SD, et al. Effect of a cognitive-behavioral prevention program on depression 6 years after implementation among at-risk adolescents: a randomized clinical trial. JAMA Psychiatry. 2015;72:1110–8. doi: 10.1001/jamapsychiatry.2015.1559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Fergusson DM, Boden JM, Horwood LJ. Recurrence of major depression in adolescence and early adulthood, and later mental health, educational and economic outcomes. Br J Psychiatry. 2007;191:335–42. doi: 10.1192/bjp.bp.107.036079. [DOI] [PubMed] [Google Scholar]
- 3.Mojtabai R. Residual symptoms and impairment in major depression in the community. Am J Psychiatry. 2001;158:1645–51. doi: 10.1176/appi.ajp.158.10.1645. [DOI] [PubMed] [Google Scholar]
- 4.Disner SG, Beevers CG, Haigh EA, et al. Neural mechanisms of the cognitive model of depression. Nat Rev Neurosci. 2011;12:467–77. doi: 10.1038/nrn3027. [DOI] [PubMed] [Google Scholar]
- 5.Joormann J, Gotlib IH. Selective attention to emotional faces following recovery from depression. J Abnorm Psychol. 2007;116:80–5. doi: 10.1037/0021-843X.116.1.80. [DOI] [PubMed] [Google Scholar]
- 6.Zvielli A, Vrijsen JN, Koster EH, et al. Attentional bias temporal dynamics in remitted depression. J Abnorm Psychol. 2016;125:768. doi: 10.1037/abn0000190. [DOI] [PubMed] [Google Scholar]
- 7.Peckham AD, McHugh RK, Otto MW. A meta-analysis of the magnitude of biased attention in depression. Depress Anxiety. 2010;27:1135–42. doi: 10.1002/da.20755. [DOI] [PubMed] [Google Scholar]
- 8.Everaert J, Grahek I, Duyck W, et al. Mapping the interplay among cognitive biases, emotion regulation, and depressive symptoms. Cogn Emotion. 2017;31:726–35. doi: 10.1080/02699931.2016.1144561. [DOI] [PubMed] [Google Scholar]
- 9.Armstrong T, Olatunji BO. Eye tracking of attention in the affective disorders: a meta-analytic review and synthesis. Clin Psychol Rev. 2012;32:704–23. doi: 10.1016/j.cpr.2012.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Clasen PC, Wells TT, Ellis AJ, et al. Attentional biases and the persistence of sad mood in major depressive disorder. J Abnorm Psychol. 2013;122:74. doi: 10.1037/a0029211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rock P, Roiser J, Riedel W, et al. Cognitive impairment in depression: a systematic review and meta-analysis. Psychol Med. 2014;44:2029–40. doi: 10.1017/S0033291713002535. [DOI] [PubMed] [Google Scholar]
- 12.McFarland BR, Klein DN. Emotional reactivity in depression: diminished responsiveness to anticipated reward but not to anticipated punishment or to nonreward or avoidance. Depress Anxiety. 2009;26:117–22. doi: 10.1002/da.20513. [DOI] [PubMed] [Google Scholar]
- 13.Pizzagalli DA, Iosifescu D, Hallett LA, et al. Reduced hedonic capacity in major depressive disorder: evidence from a probabilistic reward task. J Psychiatr Res. 2008;43:76–87. doi: 10.1016/j.jpsychires.2008.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Vrieze E, Pizzagalli DA, Demyttenaere K, et al. Reduced reward learning predicts outcome in major depressive disorder. Biol Psychiatry. 2013;73:639–45. doi: 10.1016/j.biopsych.2012.10.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Epstein J, Pan H, Kocsis JH, et al. Lack of ventral striatal response to positive stimuli in depressed versus normal subjects. Am J Psychiatry. 2006;163:1784–90. doi: 10.1176/ajp.2006.163.10.1784. [DOI] [PubMed] [Google Scholar]
- 16.Heller AS, Johnstone T, Shackman AJ, et al. Reduced capacity to sustain positive emotion in major depression reflects diminished maintenance of fronto-striatal brain activation. Proc Natl Acad Sci U S A. 2009;106:22445–50. doi: 10.1073/pnas.0910651106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Shestyuk AY, Deldin PJ, Brand JE, et al. Reduced sustained brain activity during processing of positive emotional stimuli in major depression. Biol Psychiatry. 2005;57:1089–96. doi: 10.1016/j.biopsych.2005.02.013. [DOI] [PubMed] [Google Scholar]
- 18.MacLeod C, Rutherford E, Campbell L, et al. Selective attention and emotional vulnerability: assessing the causal basis of their association through the experimental manipulation of attentional bias. J Abnorm Psychol. 2002;111:107. [PubMed] [Google Scholar]
- 19.Cristea IA, Kok RN, Cuijpers P. Efficacy of cognitive bias modification interventions in anxiety and depression: meta-analysis. Br J Psychiatry. 2015;206:7. doi: 10.1192/bjp.bp.114.146761. [DOI] [PubMed] [Google Scholar]
- 20.Grafton B, MacLeod C, Rudaizky D, et al. Confusing procedures with process when appraising the impact of cognitive bias modification on emotional vulnerability. Br J Psychiatry. 2017;211:266–71. doi: 10.1192/bjp.bp.115.176123. [DOI] [PubMed] [Google Scholar]
- 21.Browning M, Holmes EA, Charles M, et al. Using attentional bias modification as a cognitive vaccine against depression. Biol Psychiatry. 2012;72:572–9. doi: 10.1016/j.biopsych.2012.04.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yang W, Ding Z, Dai T, et al. Attention bias modification training in individuals with depressive symptoms: a randomized controlled trial. J Behav Ther Exp Psychiatry. 2015;49:101–11. doi: 10.1016/j.jbtep.2014.08.005. [DOI] [PubMed] [Google Scholar]
- 23.Wells TT, Beevers CG. Biased attention and dysphoria: manipulating selective attention reduces subsequent depressive symptoms. Cogn Emotion. 2010;24:719–28. [Google Scholar]
- 24.Baert S, De Raedt R, Schacht R, et al. Attentional bias training in depression: therapeutic effects depend on depression severity. J Behav Ther Exp Psychiatry. 2010;41:265–74. doi: 10.1016/j.jbtep.2010.02.004. [DOI] [PubMed] [Google Scholar]
- 25.Kaltenboeck A, Harmer C. The neuroscience of depressive disorders: a brief review of the past and some considerations about the future. Brain and Neuroscience Advances. 2018;2:1–6. doi: 10.1177/2398212818799269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Harmer CJ, Goodwin GM, Cowen PJ. Why do antidepressants take so long to work? A cognitive neuropsychological model of antidepressant drug action. Br J Psychiatry. 2009;195:102–8. doi: 10.1192/bjp.bp.108.051193. [DOI] [PubMed] [Google Scholar]
- 27.Harmer CJ, Duman RS, Cowen PJ. How do antidepressants work? New perspectives for refining future treatment approaches. Lancet Psychiatry. 2017;4:409–18. doi: 10.1016/S2215-0366(17)30015-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Todd RM, Cunningham WA, Anderson AK, et al. Affect-biased attention as emotion regulation. Trends Cogn Sci. 2012;16:365–72. doi: 10.1016/j.tics.2012.06.003. [DOI] [PubMed] [Google Scholar]
- 29.Gross JJ. Antecedent-and response-focused emotion regulation: divergent consequences for experience, expression, and physiology. J Pers Soc Psychol. 1998;74:224. doi: 10.1037//0022-3514.74.1.224. [DOI] [PubMed] [Google Scholar]
- 30.Koole SL. The psychology of emotion regulation: an integrative review. Cogn Emotion. 2009;23:4–41. [Google Scholar]
- 31.Sanchez A, Vazquez C, Gomez D, et al. Gaze-fixation to happy faces predicts mood repair after a negative mood induction. Emotion. 2014;14:85. doi: 10.1037/a0034500. [DOI] [PubMed] [Google Scholar]
- 32.Van Reekum CM, Johnstone T, Urry HL, et al. Gaze fixations predict brain activation during the voluntary regulation of picture-induced negative affect. Neuroimage. 2007;36:1041–55. doi: 10.1016/j.neuroimage.2007.03.052. [DOI] [PubMed] [Google Scholar]
- 33.Ochsner KN, Gross JJ. Cognitive emotion regulation insights from social cognitive and affective neuroscience. Curr Dir Psychol Sci. 2008;17:153–8. doi: 10.1111/j.1467-8721.2008.00566.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Van Bockstaele B, Notebaert L, MacLeod C, et al. The effects of attentional bias modification on emotion regulation. J Behav Ther Exp Psychiatry. 2019;62:38–48. doi: 10.1016/j.jbtep.2018.08.010. [DOI] [PubMed] [Google Scholar]
- 35.Phillips ML, Drevets WC, Rauch SL, et al. Neurobiology of emotion perception II: implications for major psychiatric disorders. Biol Psychiatry. 2003;54:515–28. doi: 10.1016/s0006-3223(03)00171-9. [DOI] [PubMed] [Google Scholar]
- 36.Etkin A, Buchel C, Gross JJ. The neural bases of emotion regulation. Nat Rev Neurosci. 2015;16:693. doi: 10.1038/nrn4044. [DOI] [PubMed] [Google Scholar]
- 37.Phillips ML, Ladouceur CD, Drevets WC. A neural model of voluntary and automatic emotion regulation: implications for understanding the pathophysiology and neurodevelopment of bipolar disorder. Mol Psychiatry. 2008;13:829–57. doi: 10.1038/mp.2008.65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Browning M, Holmes EA, Murphy SE, et al. Lateral prefrontal cortex mediates the cognitive modification of attentional bias. Biol Psychiatry. 2010;67:919–25. doi: 10.1016/j.biopsych.2009.10.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Li H, Wei D, Browning M, et al. Attentional bias modification (ABM) training induces spontaneous brain activity changes in young women with subthreshold depression: a randomized controlled trial. Psychol Med. 2016;46:909–20. doi: 10.1017/S003329171500238X. [DOI] [PubMed] [Google Scholar]
- 40.Beevers CG, Clasen PC, Enock PM, et al. Attention bias modification for major depressive disorder: effects on attention bias, resting state connectivity, and symptom change. J Abnorm Psychol. 2015;124:463–75. doi: 10.1037/abn0000049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wiers CE, Wiers RW. Imaging the neural effects of cognitive bias modification training. Neuroimage. 2017;151(Suppl C):81–91. doi: 10.1016/j.neuroimage.2016.07.041. [DOI] [PubMed] [Google Scholar]
- 42.Beck AT, Steer RA, Brown GK. Manual for the Beck Depression Inventory II. San Antonio, TX: Psychological Corporation; 1996. [Google Scholar]
- 43.Hamilton M. A rating scale for depression. J Neurol Neurosurg Psychiatry. 1960;23:56–62. doi: 10.1136/jnnp.23.1.56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Lundqvist D, Flykt A, Ohman A. The Karolinska directed emotional faces (KDEF) [CD ROM] Stockholm, Sweden: Department of Clinical Neuroscience, Psychology section, Karolinska Institutet; 1998. [Google Scholar]
- 45.Tottenham N, Borscheid A, Ellertsen K, et al. The NimStim face set. MacBrain.org; 2002. [accessed 2019 Jul 8]. Available: www.macbrain.org/faces/indexhtm. [Google Scholar]
- 46.Biehl M, Matsumoto D, Ekman P, et al. Matsumoto and Ekman’s Japanese and Caucasian Facial Expressions of Emotion (JACFEE): reliability data and cross-national differences. J Nonverbal Behav. 1997;21:3–21. [Google Scholar]
- 47.Ekman P. Pictures of facial affect. Palo Alto (CA): Consulting Psychologists Press; 1976. [Google Scholar]
- 48.Lang P, Bradley MM, Cuthbert BN. Technical Report A-8. Gainesville (FL): University of Florida; 2008. International affective picture system (IAPS): affective ratings of pictures and instruction manual. [Google Scholar]
- 49.Wessa M, Kanske P, Neumeister P, et al. EmoPics: Subjektive und psychophysiologische Evaluation neuen Bildmaterials fur die klinisch-bio-psychologische Forschung. Zeitschrift für Klinische Psychologie und Psychotherapie. 2010;39(Suppl 1/11):77. [Google Scholar]
- 50.Dale AM. Optimal experimental design for event-related fMRI. Hum Brain Mapp. 1999;8:109–14. doi: 10.1002/(SICI)1097-0193(1999)8:2/3<109::AID-HBM7>3.0.CO;2-W. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Buhle JT, Silvers JA, Wager TD, et al. Cognitive reappraisal of emotion: a meta-analysis of human neuroimaging studies. Cereb Cortex. 2014;24:2981–90. doi: 10.1093/cercor/bht154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Smith SM, Jenkinson M, Woolrich MW, et al. Advances in functional and structural MR image analysis and implementation as FSL. Neuroimage. 2004;23:S208–19. doi: 10.1016/j.neuroimage.2004.07.051. [DOI] [PubMed] [Google Scholar]
- 53.Jenkinson M, Beckmann CF, Behrens TE, et al. FSL. Neuroimage. 2012;62:782–90. doi: 10.1016/j.neuroimage.2011.09.015. [DOI] [PubMed] [Google Scholar]
- 54.Brooks JC, Beckmann CF, Miller KL, et al. Physiological noise modelling for spinal functional magnetic resonance imaging studies. Neuroimage. 2008;39:680–92. doi: 10.1016/j.neuroimage.2007.09.018. [DOI] [PubMed] [Google Scholar]
- 55.Jenkinson M, Bannister P, Brady M, et al. Improved optimization for the robust and accurate linear registration and motion correction of brain images. Neuroimage. 2002;17:825–41. doi: 10.1016/s1053-8119(02)91132-8. [DOI] [PubMed] [Google Scholar]
- 56.Jenkinson M, Smith S. A global optimisation method for robust affine registration of brain images. Med Image Anal. 2001;5:143–56. doi: 10.1016/s1361-8415(01)00036-6. [DOI] [PubMed] [Google Scholar]
- 57.Andersson JL, Jenkinson M, Smith S. Non-linear registration, aka spatial normalisation FMRIB technical report TR07JA2. Oxford, United Kingdom: FMRIB Analysis Group, University of Oxford; 2007. [Google Scholar]
- 58.Woolrich MW, Ripley BD, Brady M, et al. Temporal autocorrelation in univariate linear modeling of FMRI data. Neuroimage. 2001;14:1370–86. doi: 10.1006/nimg.2001.0931. [DOI] [PubMed] [Google Scholar]
- 59.Worsley K. Statistical analysis of activation images. In: Jezzard P, Matthews PM, Smith SM, editors. Functional MRI: an introduction to methods. Oxford, United Kingdom: Oxford University Press; 2001. pp. 251–70. [Google Scholar]
- 60.Winkler AM, Ridgway GR, Webster MA, et al. Permutation inference for the general linear model. Neuroimage. 2014;92:381–97. doi: 10.1016/j.neuroimage.2014.01.060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Smith SM, Nichols TE. Threshold-free cluster enhancement: addressing problems of smoothing, threshold dependence and localisation in cluster inference. Neuroimage. 2009;44:83–98. doi: 10.1016/j.neuroimage.2008.03.061. [DOI] [PubMed] [Google Scholar]
- 62.Jonassen R, Harmer CJ, Hilland E, et al. Effects of attentional bias modification on residual symptoms in depression. a randomized controlled trial. BMC Psychiatry. 2019;19:141. doi: 10.1186/s12888-019-2105-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Kaiser RH, Andrews-Hanna JR, Wager TD, et al. Large-scale network dysfunction in major depressive disorder: a meta-analysis of resting-state functional connectivity. JAMA Psychiatry. 2015;72:603–11. doi: 10.1001/jamapsychiatry.2015.0071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Greicius MD, Flores BH, Menon V, et al. Resting-state functional connectivity in major depression: abnormally increased contributions from subgenual cingulate cortex and thalamus. Biol Psychiatry. 2007;62:429–37. doi: 10.1016/j.biopsych.2006.09.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Wang L, Hermens D, Hickie I, et al. A systematic review of resting-state functional-MRI studies in major depression. J Affect Disord. 2012;142:6–12. doi: 10.1016/j.jad.2012.04.013. [DOI] [PubMed] [Google Scholar]
- 66.Avery JA, Drevets WC, Moseman SE, et al. Major depressive disorder is associated with abnormal interoceptive activity and functional connectivity in the insula. Biol Psychiatry. 2014;76:258–66. doi: 10.1016/j.biopsych.2013.11.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Hamilton, Furman DJ, Chang C, Thomason ME, et al. Default-mode and task-positive network activity in major depressive disorder: implications for adaptive and maladaptive rumination. Biol Psychiatry. 2011;70:327–33. doi: 10.1016/j.biopsych.2011.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Lemogne C, Gorwood P, Bergouignan L, et al. Negative affectivity, self-referential processing and the cortical midline structures. Soc Cogn Affect Neurosci. 2010;6:426–33. doi: 10.1093/scan/nsq049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Grimm S, Ernst J, Boesiger P, et al. Increased self-focus in major depressive disorder is related to neural abnormalities in subcortical-cortical midline structures. Hum Brain Mapp. 2009;30:2617–27. doi: 10.1002/hbm.20693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Walter M, Henning A, Grimm S, et al. The relationship between aberrant neuronal activation in the pregenual anterior cingulate, altered glutamatergic metabolism, and anhedonia in major depression. Arch Gen Psychiatry. 2009;66:478–86. doi: 10.1001/archgenpsychiatry.2009.39. [DOI] [PubMed] [Google Scholar]
- 71.Grimm S, Boesiger P, Beck J, et al. Altered negative BOLD responses in the default-mode network during emotion processing in depressed subjects. Neuropsychopharmacology. 2009;34:932. doi: 10.1038/npp.2008.81. [DOI] [PubMed] [Google Scholar]
- 72.Menon V, Uddin LQ. Saliency, switching, attention and control: a network model of insula function. Brain Struct Funct. 2010;214:655–67. doi: 10.1007/s00429-010-0262-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Sridharan D, Levitin DJ, Menon V. A critical role for the right fronto-insular cortex in switching between central-executive and default-mode networks. Proc Natl Acad Sci U S A. 2008;105:12569–74. doi: 10.1073/pnas.0800005105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Lindquist KA, Satpute AB, Wager TD, et al. The brain basis of positive and negative affect: evidence from a meta-analysis of the human neuroimaging literature. Cereb Cortex. 2015;26:1910–22. doi: 10.1093/cercor/bhv001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Phan KL, Wager T, Taylor SF, et al. Functional neuroanatomy of emotion: a meta-analysis of emotion activation studies in PET and fMRI. Neuroimage. 2002;16:331–48. doi: 10.1006/nimg.2002.1087. [DOI] [PubMed] [Google Scholar]
- 76.Hamilton, Paul J, Etkin A, Furman DJ, et al. Functional neuroimaging of major depressive disorder: a meta-analysis and new integration of baseline activation and neural response data. Am J Psychiatry. 2012;169:693–703. doi: 10.1176/appi.ajp.2012.11071105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Ma Y. Neuropsychological mechanism underlying antidepressant effect: a systematic meta-analysis. Mol Psychiatry. 2015;20:311. doi: 10.1038/mp.2014.24. [DOI] [PubMed] [Google Scholar]
- 78.Harmer CJ, O’Sullivan U, Favaron E, et al. Effect of acute antidepressant administration on negative affective bias in depressed patients. Am J Psychiatry. 2009;166:1178–84. doi: 10.1176/appi.ajp.2009.09020149. [DOI] [PubMed] [Google Scholar]
- 79.Warren MB, Pringle A, Harmer CJ. A neurocognitive model for understanding treatment action in depression. Philos Trans R Soc Lond B Biol Sci. 2015;370:20140213. doi: 10.1098/rstb.2014.0213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Beck AT. The evolution of the cognitive model of depression and its neurobiological correlates. Am J Psychiatry. 2008;165:969–77. doi: 10.1176/appi.ajp.2008.08050721. [DOI] [PubMed] [Google Scholar]
- 81.McGrath CL, Kelley ME, Dunlop BW, et al. Pretreatment brain states identify likely nonresponse to standard treatments for depression. Biol Psychiatry. 2014;76:527–35. doi: 10.1016/j.biopsych.2013.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Konarski JZ, Kennedy SH, Segal ZV, et al. Predictors of nonresponse to cognitive behavioural therapy or venlafaxine using glucose metabolism in major depressive disorder. J Psychiatry Neurosci. 2009;34:175. [PMC free article] [PubMed] [Google Scholar]
- 83.Siegle GJ, Carter CS, Thase ME. Use of FMRI to predict recovery from unipolar depression with cognitive behavior therapy. Am J Psychiatry. 2006;163:735–8. doi: 10.1176/ajp.2006.163.4.735. [DOI] [PubMed] [Google Scholar]
- 84.Siegle GJ, Thompson WK, Collier A, et al. Toward clinically useful neuroimaging in depression treatment: prognostic utility of subgenual cingulate activity for determining depression outcome in cognitive therapy across studies, scanners, and patient characteristics. Arch Gen Psychiatry. 2012;69:913–24. doi: 10.1001/archgenpsychiatry.2012.65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Dunlop BW, Rajendra JK, Craighead WE, et al. Functional connectivity of the subcallosal cingulate cortex and differential outcomes to treatment with cognitive-behavioral therapy or antidepressant medication for major depressive disorder. Am J Psychiatry. 2017;174:533–45. doi: 10.1176/appi.ajp.2016.16050518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Roiser JP, Elliott R, Sahakian BJ. Cognitive mechanisms of treatment in depression. Neuropsychopharmacology. 2012;37:117–36. doi: 10.1038/npp.2011.183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Gyurak A, Gross JJ, Etkin A. Explicit and implicit emotion regulation: a dual-process framework. Cogn Emotion. 2011;25:400–12. doi: 10.1080/02699931.2010.544160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Etkin A, Egner T, Kalisch R. Emotional processing in anterior cingulate and medial prefrontal cortex. Trends Cogn Sci. 2011;15:85–93. doi: 10.1016/j.tics.2010.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Kazdin AE. Mediators and mechanisms of change in psychotherapy research. Annu Rev Clin Psychol. 2007;3:1–27. doi: 10.1146/annurev.clinpsy.3.022806.091432. [DOI] [PubMed] [Google Scholar]
- 90.Holmes EA, Ghaderi A, Harmer CJ, et al. The Lancet Psychiatry Commission on psychological treatments research in tomorrow’s science. Lancet Psychiatry. 2018;5:237–86. doi: 10.1016/S2215-0366(17)30513-8. [DOI] [PubMed] [Google Scholar]
- 91.Eklund A, Nichols TE, Knutsson H. Cluster failure: why fMRI inferences for spatial extent have inflated false-positive rates. Proc Natl Acad Sci. 2016 doi: 10.1073/pnas.1602413113. 201602413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Carter CS, Lesh TA, Barch DM. Thresholds, power, and sample sizes in clinical neuroimaging. Biol Psychiatry. 2016;1:99–100. doi: 10.1016/j.bpsc.2016.01.005. [DOI] [PubMed] [Google Scholar]
- 93.Poldrack RA, Baker CI, Durnez J, et al. Scanning the horizon: towards transparent and reproducible neuroimaging research. Nat Rev Neurosci. 2017;18:115. doi: 10.1038/nrn.2016.167. [DOI] [PMC free article] [PubMed] [Google Scholar]