Abstract
Sepsis-associated acute kidney injury (S-AKI) is a frequent complication of the critically ill patient and is associated with unacceptable morbidity and mortality. Prevention of S-AKI is difficult because by the time patients seek medical attention, most have already developed acute kidney injury. Thus, early recognition is crucial to provide supportive treatment and limit further insults. Current diagnostic criteria for acute kidney injury has limited early detection; however, novel biomarkers of kidney stress and damage have been recently validated for risk prediction and early diagnosis of acute kidney injury in the setting of sepsis. Recent evidence shows that microvascular dysfunction, inflammation, and metabolic reprogramming are 3 fundamental mechanisms that may play a role in the development of S-AKI. However, more mechanistic studies are needed to better understand the convoluted pathophysiology of S-AKI and to translate these findings into potential treatment strategies and add to the promising pharmacologic approaches being developed and tested in clinical trials.
Keywords: epidemiology, inflammation, metabolic reprogramming, microvascular dysfunction, prevention, sepsis-associated acute kidney injury, sepsis-induced acute kidney injury, treatment
Sepsis-associated acute kidney injury (S-AKI) is a common complication in hospitalized and critically ill patients, which increases the risk of developing chronic comorbidities and is associated with extremely high mortality.1–4 As individual syndromes, sepsis and acute kidney injury (AKI) render the host susceptible to each other. Although sepsis is the most common contributing factor for developing AKI, AKI of any origin is associated with higher risk of developing sepsis.5 Sepsis has a complex and unique pathophysiology, which makes S-AKI a distinct syndrome from any other phenotype of AKI. Identifying the exact onset of injury in sepsis is nearly impossible, leading to difficulty in timely intervention for prevention of renal injury. In this review, we will focus on the definition of the syndrome, the role of biomarkers, and the recent advances in pathophysiology and treatment of S-AKI.
Limitations of current tools used to define and diagnose S-AKI
The diagnosis of AKI is currently based on an increase serum creatinine concentration and/or a decrease in urine output.6,7 As in other forms of AKI, serum creatinine can be an insensitive indicator of kidney injury, and oliguria can be nonspecific in S-AKI. However, in sepsis, oliguria appears to carry increased significance, and even by 3 to 5 hours, an association between oliguria and AKI may be detectable.8,9 Serum creatinine is also limited by the absence of baseline value in many patients, and a consensus is lacking as to the best way to handle this missing information.10,11
Although sepsis has been long-recognized as the leading cause of AKI in the critically ill, Mehta et al.5 found that 40% of critically ill patients develop sepsis after AKI, suggesting that AKI may increase the risk of sepsis. However, both sepsis and AKI are clinical diagnoses, and it is usually difficult to define the precise time either of these syndromes begin. Furthermore, as we shall discuss, sepsis and its treatment expose the kidney to injury. Thus, we will use S-AKI to acknowledge the uncertainty around the attribution of etiology.
The Third International Consensus Definitions for Sepsis and Septic Shock (Sepsis-3) was proposed recently.12 Thus, S-AKI is usually defined as AKI in the presence of sepsis without other significant contributing factors explaining AKI or characterized by the simultaneous presence of both Sepsis-3 and Kidney Disease: Improving Global Outcomes (KDIGO) criteria.13,14 In clinical practice, urine output criteria is often difficult to confirm outside the intensive care unit (ICU). Intensive monitoring of urine output is associated with (though not necessarily causally related to) improved survival in patients developing AKI,15 and once AKI has developed, urine output still has an important role for predicting the short- and long-term outcomes up to 1 year.16 Patients who reach maximum AKI stage by both serum creatinine and urine output criteria have highest rates of in-hospital renal replacement therapy (RRT), longer ICU and hospital stays, and increased mortality.16 However, there are several limitations to serum creatinine and urine output for the diagnosis of AKI. In animals, sepsis reduces muscle perfusion and thus the production of creatine falls, which blunts the increase in serum creatinine concentration and limits early detection of AKI.17 Together with dilutional effects secondary to aggressive fluid resuscitation in septic shock, AKI may be under-diagnosed. Diuretic administration might limit the use of oliguria and other urine indices for AKI diagnosis although intact tubular function is necessary for loop diuretics to work.18 Urine microscopy is one of the conventional methods widely used for detection of kidney disease. Patients with S-AKI had higher urine microscopy scores when compared with those with AKI from other causes.19 Urine microscopy was rather specific but poorly sensitive to detect worsening AKI—a score ≥3 had a sensitivity and specificity of 0.67 (95% confidence interval [CI]: 0.39–0.86) and 0.95 (95% CI: 0.84–0.99). Thus, urine sediment may help establish the cause of AKI and provide prognostic information, but it has poor sensitivity to detect AKI and worsening of AKI.
Epidemiology and outcomes of S-AKI
Remarkably, little is known about the epidemiology of S-AKI. This is perhaps due to a lack of coordinated epidemiology between sepsis criteria and AKI criteria and among researchers working in these areas. Even just the global incidence of sepsis is largely unknown. Extrapolating from rates in the United States, Adhikari et al.20 estimated up to 19 million cases worldwide per year, but the true incidence is presumably much greater. Because roughly 1 in 3 patients with sepsis will develop AKI,21 the annual global incidence of S-AKI might be approximately 6 million cases or nearly 1 per 1000 population. However, this number is low compared with estimates working backward from AKI incidence. Using a 500,000-patient database from western Pennsylvania, we found rates of AKI of 12%. Given that 35 million Americans are hospitalized each year, this puts the annual AKI rate at approximately 4.2 million for the United States and by extrapolation nearly 98 million worldwide. If only 10% had sepsis, the annual incidence would be just under 10 million or 1.4 per 1000 population. Others have reported AKI rates in hospitalized patients ranging from 5% to 31%.22–24
For patients in the ICU, sepsis is found in about 40% to 50% of patients with AKI in the ICU.1–4 A prospective cohort study including 1177 patients with sepsis across 198 ICUs in 24 European countries reported a 51% incidence of AKI with an ICU mortality rate of 41%.25 A retrospective study across China including 146,148 patients found AKI in 47.1% of sepsis cases.26 An ancillary analysis of a multicenter randomized controlled trial (RCT) in septic shock including 1243 patients, AKI was present at enrollment in the emergency department in 50.4% and another 18.7% developed subsequent AKI within 7 days.27,28 Two-thirds of patients with AKI were classified as stage 2 or 3.28 However, AKI is common even among patients without severe sepsis or shock: 34% of nonsevere community-acquired pneumonia developed AKI.21
Another aspect of the problem is a semantic or epistemological issue. Because sepsis is defined as an infection-associated organ dysfunction, infection plus AKI equals sepsis. Thus, we should really be asking about the rates of AKI in patients with infection or the proportion of AKI where the inciting event is infection. Current consensus guidelines for sepsis12 use the Sepsis-related Organ Failure Assessment score29 for quantifying organ dysfunction, including for the kidney rather than the KDIGO definition. Because the Sepsis-related Organ Failure Assessment score does not distinguish AKI from CKD nor adequately consider demographic differences in baseline creatinine, it cannot reliably assess infection-associated kidney dysfunction.
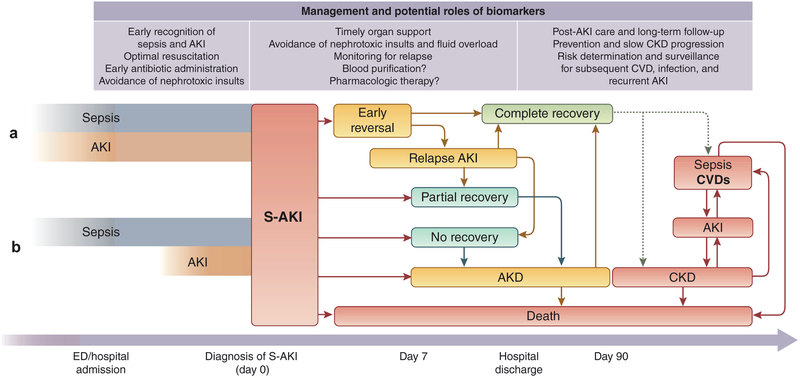
S-AKI is strongly associated with poor clinical outcomes. Among critically ill patients with AKI, S-AKI was associated with higher risk of in-hospital death (odds ratio: 1.48) and a longer hospital stay compared with AKI from any other causes (37 vs. 21 days).2 In-hospital RRT requirement was strongly associated with hospital mortality.30,31 Those who have renal recovery after S-AKI have dramatically improved survival. In 1 study, patients who had reversal of S-AKI within 24 hours after documentation of shock experienced a reduced in-hospital mortality (hazard ratio: 0.64).32 Relapse of AKI is also common after initial recovery (Figure 1). In our analysis of 16,968 ICU patients with moderate to severe AKI, 32% of patients with sepsis experienced AKI relapses during hospitalization after initial AKI reversal.30 The long-term outcomes of patients with S-AKI in terms of survival has shown to be determined by severity of AKI and recovery status at hospital discharge. Those with recovery, even partial, seem to have similar prognosis to those without AKI.28 About 40% of patients with moderate to severe S-AKI from nonsevere pneumonia experienced AKI recovery at hospital discharge and had comparable 3-year survival to those without AKI (28% vs. 23% mortality), whereas those with S-AKI who did not recover had the worst prognosis (44% mortality).31 After recovery from AKI, patients still carry the risk of developing chronic kidney disease (CKD), end-stage renal disease, and death (Figure 1).33 The severity of AKI, RRT requirement, and recovery status during hospitalization has been shown to determine the risk of progression to CKD.34,35 Over 1 year, CKD developed in 21%, 30%, and 79% of 105 survivors with AKI reversal, recovery, and nonrecovery, respectively.35
Figure 1 |. Clinical course and outcomes of sepsis-associated acute kidney injury (S-AKI).
The exact onset of kidney injury in sepsis is unknown. Patients who present with sepsis should be suspected for AKI, and, vice versa, those who present with AKI should be suspected for sepsis as well. AKI may present simultaneously with sepsis at hospital admission (a) or develop during hospitalization (b). In the latter case, it is still possible to prevent AKI by optimal resuscitation and appropriate sepsis treatment. Novel biomarkers have an established role in the early recognition of AKI at this point. Once S-AKI is diagnosed, close monitoring and timely organ support should be done together to prevent further kidney injury. However, S-AKI is still associated with an extremely high risk of in-hospital death. The survivors have various clinical trajectories and outcomes. S-AKI is able to reverse early during the first week after being documented and is associated with a good prognosis. Some patients may experience 1 or more episodes of relapse after the initial reversal of AKI during hospitalization. This emphasizes that close monitoring and avoidance of nephrotoxic insults are mandatory along the clinical course of S-AKI even after early reversal or recovery. Patients with complete recovery of S-AKI may be discharged with good health; however, they still carry the risk of chronic kidney disease (CKD) and other consequences, including recurrent sepsis (dotted lines). Those patients who do not completely recover by 7 days after being documented AKI will be classified as having acute kidney disease (AKD), which may recover later or progress to CKD and is associated with adverse long-term outcomes. Further research regarding the potential role of biomarkers for the prediction of renal recovery is needed. S-AKI survivors who are discharged from the hospital should be followed up in the long term with optimal care by a nephrologist to monitor progression to CKD and other long-term consequences. CVD, cardiovascular disease; ED, emergency department.
Pathophysiology of S-AKI
Sepsis is the most common cause of AKI in critically ill patients.4 Despite this, the pathophysiologic mechanisms of S-AKI are not well understood. Therefore, therapy remains reactive and nonspecific, and no available preventive therapies exist. Advances in the understanding of the pathogenesis of AKI have been slow and curbed due to the several technical, technological, and ethical restrictions that converge around this field.36 Hence, most of the current understanding of S-AKI have been extrapolated from animal models of sepsis, in vitro cellular studies, and postmortem observations in septic humans. These observations should be interpreted carefully because the response to sepsis in animal models may vary widely from that of humans.36 The National Institutes of Health in the United States have begun initiatives to address this knowledge deficit such as the Kidney Precision Medicine Project that aims to expand our understanding of AKI by obtaining kidney biopsies in patients with AKI. The prevailing pathophysiologic paradigm attributes S-AKI to the decreased global renal blood flow and secondary tubular epithelial cell death, or acute tubular necrosis.37 One reason for this belief was that the leading causes of AKI (e.g., sepsis, major surgery, heart failure, and hypovolemia) are all associated with hypoperfusion and shock,1 and ischemic injury can cause extensive cell death (e.g., acute tubular necrosis). However, it is becoming increasingly clear that ischemia-reperfusion injury is not the only mechanism of S-AKI, but rather multiple mechanisms must be at play.38–40 For instance, S-AKI may develop in the absence of renal hypoperfusion and clinical signs of hemodynamic instability21,41,42 and in the presence of normal or increased global renal blood flow.41,43–49 In addition, histopathological findings in postmortem human observations and harvested animal organs are not as severe as expected and do not correlate with functional alterations. A heterogeneous, focal, and patchy tubular injury, with minimal tubule-epithelial cell death (<5%), apical vacuolization, and minor focal mesangial expansion is observed in these samples.50–54 The controversy generated from these data indicates that multiple mechanisms should be at play in the pathogenesis of S-AKI and that the dissociation between structural and functional changes could also be the result of adaptive mechanisms in which cells prioritize survival at the expense of organ function.38 Regardless of the organ, 3 mechanisms are consistent during sepsis organ injury: inflammation,51,55,56 microcirculatory dysfunction,57 and metabolic reprogramming.58,59 A “unified theory” of S-AKI has been proposed in an attempt to place these various mechanisms into a coherent framework of synergic interaction.60 The inflammatory response is the host’s main defense mechanism from invading pathogens. However, as the new sepsis definition implies, a dysregulated inflammatory response may be responsible for organ dysfunction and poor outcome. During sepsis, inflammatory mediators including pathogen- and damage-associated molecular patterns are released in the intravascular compartment. These molecules bind membrane-bound pattern recognition receptors, such as Toll-like receptors, that are present on the surface of immune cells, initiating a downstream cascade of signals that will result in the synthesis and release of proinflammatory molecules. Renal tubular epithelial cells (TECs) also express Toll-like receptors, especially TLR2 and TLR4. When exposed to damage- or pathogen-associated molecular patterns filtered through the glomerulus or through neighboring peritubular capillaries, proximal TECs exhibit an increase in oxidative stress, production of reactive oxygen species, and mitochondrial injury (Figure 2).61–64 There is evidence that TECs may also initiate paracrine signaling, which may signal neighboring cells to deactivate in an attempt to minimize cell death at the expense of function. In addition, histological observations show that kidneys from septic animals, compared with nonseptic control animals, have increased infiltrating of monocytes in the glomeruli and into the peritubular area.52,65
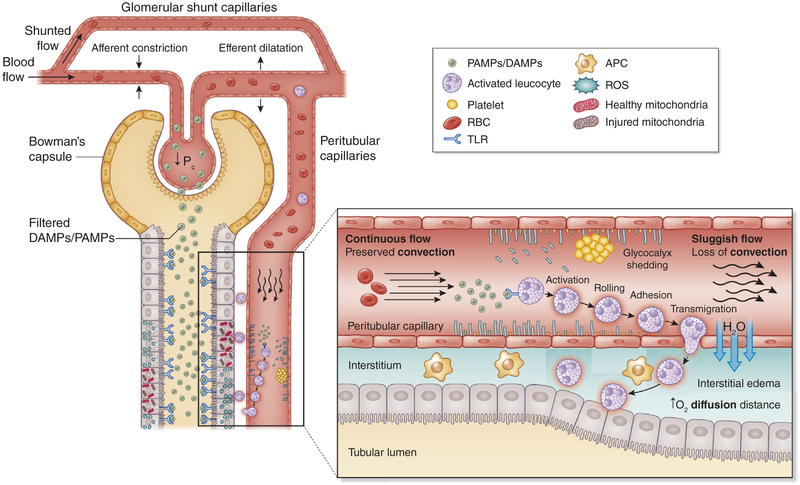
Figure 2 |. Microcirculatory and inflammatory alterations.
Sepsis-associated acute kidney injury can occur in the absence of overt signs of hypoperfusion and clinical signs of hemodynamic instability. Several theories involving microcirculatory, including hemodynamic, changes and inflammation have been proposed to explain the dissociation between the structural findings and the altered renal function observed during sepsis-associated acute kidney injury. Glomerular filtration rate is correlated with the glomerular blood flow and the intraglomerular pressure (Pc). Glomerular shunting and constriction of the efferent arteriole result in a Pc decrease with the subsequent decline in glomerular filtration rate and urine output. Pathogen-associated molecular patterns (PAMPs) and damage-associated molecular patterns (DAMPs) released after the invasion of infectious pathogens have the ability to bind to a family of receptors known as pattern recognition receptors, especially Toll-like receptors (TLRs), which are expressed on the surface of immune cells, endothelial cells, and tubular epithelial cells (TECs). These result in a downstream cascade of signals and an increased synthesis of proinflammatory cytokines, reactive oxygen species (ROS), oxidative stress, and endothelial activation. Endothelial activation promotes rolling and adhesion of leucocytes and platelets, resulting in increased risk of thrombi formation and flow continuity alterations (intermittent or no flow). Also, endothelial activation is associated with increased vascular permeability and leakage, causing interstitial edema and increasing oxygen diffusion distance to the TECs. In addition to these endothelia and flow alterations, DAMPs and PAMPs can also directly affect TECs. It has been demonstrated that TECs also expressed TLRs on their surface. DAMPs and PAMPs are small enough to be filtered in the glomeruli and then to be exposed to TLR present on the TEC surface, resulting in increased production of ROS, oxidative stress, and mitochondrial damage. APCs, antigen-presenting cells; RBCs, red blood cells.
Tissue perfusion is critical for the adequate functioning of any organ. Alterations in oxygen delivery and consumption are a consistent finding in most septic organ injuries,66 which may not be dependent on systemic circulatory abnormalities. Several experimental and clinical studies show that even in the absence of macrohemodynamic instability, microcirculatory alterations still develop during sepsis. These observations have led many investigators to consider that microcirculatory alterations should play a key role in the development of organ injury.67 S-AKI is characterized by profound heterogeneous changes in microcirculatory flow, whereby a decrease in the capillary density is associated with a decrease proportion of capillaries with continuous flow and an increase in the proportion of capillaries with intermittent and stop flow.56,57,66,68–70 Multiple mechanisms may lead to microcirculatory alterations such as endothelial injury, autonomic nervous system response, shedding of the glycocalyx, and activation of the coagulation cascade (Figure 2).57,71 Endothelial injury and shedding of the glycocalyx caused by inflammatory mediators enhances leucocyte and platelets rolling and adhesion with a concomitant reduction in blood flow velocity. Potential consequences are increased susceptibility to microthrombi formation and occlusion of capillaries and longer exposure of the TECs to inflammatory mediators of activated circulating inflammatory cells. Endothelial injury is also associated with vasodilation, increased vascular permeability, and endothelial leak. The resulting formation of peritubular interstitial edema can have a significant impact on the perfusion of TECs by increasing the diffusion distance of oxygen from capillaries to TECs and by increasing venous output pressures, thus altering convection.67,72–74 Microcirculatory hemodynamics may also play a key role during S-AKI. As glomerular filtration rate is independent of changes in renal blood flow and is determined by intraglomerular hydrostatic pressure, constriction of the renal afferent arteriole and dilation of the efferent arteriole have been proposed as a mechanism to explain a fall in intraglomerular pressure leading to loss of glomerular filtration rate (Figure 2).42,67,75,76 In addition, during sepsis, intrarenal blood flow redistribution occurs, driving blood flow away from the medulla.77 In addition, the existence of capillaries that bypass the glomerulus and connect the afferent directly to the efferent arterioles may explain in part the shunting of blood proposed during S-AKI.78,79 However, it is unclear how or when these accessory shunt pathways are opened and whether this occurs during sepsis. In summary, redistribution of blood flow and the increase in shunting are mechanisms that might explain the potential presence of heterogeneous areas at risk for ischemia during S-AKI.67,80
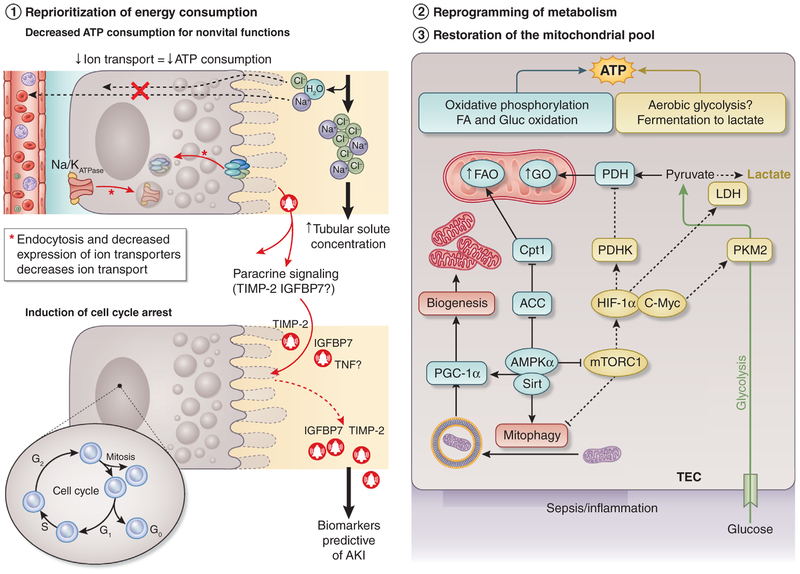
The paucity of TEC death during S-AKI may be explained by key metabolic adaptations that occur early during sepsis, whereby cell survival is prioritized at the expense of cell and organ function. Multiple theories have been proposed to explain the metabolic reprograming that TECs undergo during sepsis. Most of these point to a mitochondrial-mediated process, characterized by energy expenditure optimization, reprogramming of substrate utilization, and counteraction of proapoptotic triggers.50,54,58,59 Inflammation is associated with optimization of energy consumption, which implies a decrease in energy utilization in nonvital functions (e.g., protein synthesis or ion transport), and maintenance of energy utilization in sustaining vital cell functions (e.g., membrane Na+/K+ adenosine triphosphatase pump function) while avoiding cell death. During inflammation, the renal tubular expression of ion transporters is downregulated and tubular solute transport is decreased (Figure 3),81–85 which in the absence of cell death suggests reprioritization of energy expenditure through metabolic reprogramming is an adaptive mechanism for survival. It remains unclear how metabolic reprogramming occurs. However, evidence suggests that a key component depends on how the cell processes energy-containing substrates such as glucose and fatty acids (Figure 3). The consequences of using oxidative phosphorylation (OXPHOS) versus aerobic glycolysis (i.e., in the presence of sufficient oxygen to use OXPHOS, or Warburg metabolism) with fermentation to lactate as a last step seem to have profound effects on the ability of the cells and hosts to survive the septic insult. For instance, inhibition of aerobic glycolysis and induction of OXPHOS during sepsis result in improved survival and decreased susceptibility to develop AKI.86–88 Macrophages of animals exposed to inflammation exhibit a phasic shift, with an early switch toward aerobic glycolysis and a later return to OXPHOS.89 Based on this evidence and our preliminary data, we have proposed that TECs may exhibit a phasic switch between aerobic glycolysis and OXPHOS that is similar to Warburg metabolism (Figure 3).58,90 An integral component of metabolic reprogramming is the mitochondria, as these organelles house important molecular machinery necessary for substrate processing through OXPHOS among other functions. It is well known that sepsis induces significant mitochondrial injury and that activation of mitochondrial quality control processes such as mitophagy (specialized mechanism by which injured mitochondria are signaled and engulfed into autophagosomes within the cell) and biogenesis (the process of synthesis of new, functional mitochondria) also improve survival. Although preservation of a functional mitochondrial pool may confer protection through a myriad of mechanisms, it is clear that restoration of OXPHOS for substrate processing can only occur if functional mitochondria are available (Figure 3).
Figure 3 |. Metabolic reprogramming.
During sepsis-associated acute kidney injury (AKI), a reprioritization of energy occurs that seeks to meet metabolic vital needs prioritizing survival at the expense of cell function. Multiple highly consuming adenosine triphosphate (ATP) functions are downregulated to save energy, including protein synthesis and ion transportation, especially in the proximal tubular epithelial cells (TECs) and cellular replication. In addition to this shutdown of nonvital functions, experimental studies have suggested that TECs may reprogram their metabolism switching to aerobic glycolysis and oxidative phosphorylation to fulfill energy requirements during sepsis. Preservation of functional mitochondrial poll is necessary to carry out all the metabolic changes. During sepsis, mitochondria enter a series of quality control processes such as mitophagy and biogenesis to preserve the mitochondrial pool to confer protection and fulfill the necessary energetic requirements. ACC, acetyl coenzyme A carboxylase α; AMPKα, adenosine monophosphate kinase α; C-Myc, cell Myc gen; Cpt1, carnitine palmitoyltransferase 1; FA, frataxin; FAO, fatty acid oxidation; G0–G2, phases of the cell cycle; Gluc, glucose; GO, golgin; HIF-1α, hypoxia-inducible factor-1α; IGFBP7, insulin-like growth factor binding protein 7; LDH, lactic acid dehydrogenase; mTORC1, mammalian target of rapamycin complex 1; PDH, pyruvate dehydrogenase; PDHK, pyruvate dehydrogenase kinase; PGC-1α, peroxisome proliferator-activated receptor gamma coactivator-1α; PKM2, pyruvate kinase isozyme M2; Sirt, sirtuins; TIMP-2, tissue inhibitor of metalloproteinase-2; TNF, tumor necrosis factor.
One of the most energy-consuming processes cells undergo is replication (duplication of the entire genome, proteome, and lipidome of the cell). During the cell cycle, several checkpoints serve to evaluate whether the cell will have sufficient energy to replicate. If the answer is no, the cell will undergo cell cycle arrest to avoid cell death due to energy failure. Therefore, cell cycle arrest is another mechanism of downregulation of energy expenditure that TECs may invoke to reprogram metabolism and defend from the septic insult (Figure 3). In support of the relevance of this process in human sepsis, 2 markers of cell cycle arrest—the tissue inhibitor of metalloproteinase-2 (TIMP-2) and insulin-like growth factor binding protein 7 (IGFBP7)—have been found to be the best predictors of the development of S-AKI.91 Although several advances have been made in the last few years in the understanding of S-AKI and several possible therapeutic strategies have been proposed, more studies are necessary to clarify the mechanisms by which sepsis causes organ injury, especially AKI.
Role of biomarkers in S-AKI
Early recognition of AKI in the setting of sepsis is vital to provide optimal treatment and avoid further kidney injury. Equally, detection of AKI in the setting of infection is critical because it may define sepsis in a given patient (Figure 1). The use of injury (or stress) markers together with measures of function may provide more information than either alone.92 Table 1 summarizes some biomarkers studied in S-AKI.93–107
Table 1 |.
Biomarkers for S-AKI
| Biomarker | Source tested | Known functions | Potential use in S-AKI |
|---|---|---|---|
| Marker of tubular injury | |||
| NGAL | Urine, plasma | NGAL chelates labile Fe released from damaged tubules and prevents formation of hydroxy radicals; upregulates heme-oxygenase-1 | Higher plasma and urine NGAL levels in patients with S-AKI compared with AKI due to other causes.93 Plasma NGAL appeared to be useful for predicting renal recovery at hospital discharge in patients with S-AKI.94 |
| Special concern: plasma NGAL increase in systemic infection and inflammation without AKI; ability to distinguish AKI from CKD by NGAL was also limited by the low sensitivity and specificity values of NGAL | |||
| KIM-1 | Urine | A type-1 membrane glycoprotein; phosphatidylserine receptor; upregulated in tubular injury and activates immune cells; promotes apoptotic and necrotic cell clearance and remodeling of injured epithelia | Data for KIM-1 specifically used for S-AKI are limited. Urinary KIM-1 at 24 h predicted S-AKI with an AUC of 0.91; the nonsurvivors had significantly higher level of urinary KIM-1 at 24 and 48 h.95 |
| L-FABP | Urine | Cytoplasmic protein, transports free fatty acids to mitochondria and peroxisomes for metabolism; protects against damage caused by reactive oxygen species; upregulated during ischemia-reperfusion injury | Urinary L-FABP levels at admission were significantly higher in nonsurvivors than in survivors with S-AKI96 and may also reflect the severity of sepsis and monitor the effectiveness of polymyxin hemoperfusion treatment.97 |
| Marker of cell cycle arrest | |||
| [TIMP-2] • [IGFBP7] | Urine | TIMP-2 stimulates p27 expression, IGFBP7 increases the expression of p53 and p21, block cyclin-dependent protein kinase complexes on cell cycle promotion | Prediction of AKI with AUC of 0.80.91 Levels did not increase in nonrenal organ failures in sepsis [TIMP-2] • [IGFBP7].98 |
| Marker of endothelial injury | |||
| Angiopoietins | Plasma | Angiogenic factors for vascular development; Ang-1 has been found to be protective by stabilizing endothelium, while Ang-2 promotes vascular leak, which can worsen sepsis | In patients with S-AKI, plasma Ang-1 was significant lower than in patients with sepsis but without AKI.99 Higher levels of Ang-1 were associated with lower risk of AKI and higher levels of Ang-2 were associated with higher risk of AKI and are an independent predictor of 28-day mortality in ICU patients with AKI requiring RRT.100,101 |
| VE-cadherin | Plasma | An endothelial transmembrane glycoprotein that forms adherens junctions | Plasma VE-cadherin level at time of enrollment was associated with severe AKI requiring RRT (OR: 6.44 per log increase in plasma VE-cadherin; 95% CI: 1.126–36.847; P = 0.036).102 |
| Soluble thrombomodulin | Plasma | Thrombomodulin is a thrombin receptor that is expressed on the surface of endothelial cells and is released into the bloodstream when endothelial cells are activated. | Soluble thrombomodulin in a patient with sepsis at ICU admission is an independent predictor for S-AKI with AUC of 0.758.103 |
| Marker of inflammation | |||
| Interleukin-6 | Plasma | A cytokine with a wide range of biological activities; helps control the induction of the acute-phase response; a mediator for immunoglobulin class switching | Baseline interleukin-6 at admission predicted AKI in patients with severe sepsis.104 |
| sTREM-1 | Urine, plasma | TREM-1 is an activating receptor selectively expressed on the surface of neutrophils and monocytes and associated with the inflammatory response triggered by bacterial infection. (It is almost undetectable in noninfectious inflammation.) | sTREM-1 may be produced locally by the endothelial cells, tubular epithelial cells, or infiltrating inflammatory cells during acute tubular necrosis.105,106 In patients with sepsis, urine sTREM-1 at ICU admission predicted AKI at 48 h with AUC of 0.922.107 Diagnostic value for S-AKI: AUCs of 0.794 for plasma and 0.707 for urine; predictive value 24 h before S-AKI diagnosis: AUC of 0.746 for plasma and 0.778 for urine.105 |
Ang-1, angiopoietin-1; AUC, area under the receiver operating characteristic curve; CI, confidence interval; CKD, chronic kidney disease; IGFBP7, insulin-like growth factor-binding protein 7; KIM-1, kidney injury molecule-1; L-FABP, liver-type fatty acid binding protein; NGAL, neutrophil gelatinase-associated lipocalin; OR, odds ratio; RRT, renal replacement therapy; S-AKI, sepsis-associated acute kidney injury; sTREM-1, soluble triggering receptor expressed on myeloid cells 1; TIMP-2, tissue inhibitor of metalloproteinases-2; VE, vascular endothelial.
Neutrophil gelatinase-associated lipocalin (NGAL) has been extensively investigated in various AKI phenotypes. NGAL is released by activated neutrophils and various epithelial cells including renal TECs. In early studies, NGAL showed good sensitivity for prediction of AKI and also was a useful prognostic tool for RRT requirement and in-hospital mortality.108,109 Patients with S-AKI generally have higher detectable plasma and urinary NGAL levels than do patients with AKI from other causes.93 Plasma NGAL appeared to be useful for predicting renal recovery at hospital discharge in patients with S-AKI.94 Interpretation of NGAL in patients with sepsis should also consider potential nonrenal sources. Plasma NGAL may increase in systemic infection and inflammation without evidence for AKI. Some studies have found that NGAL failed to discriminate patients with an AKI from those with a non-AKI in the setting of sepsis.110–113 Distinguishing AKI from CKD by NGAL was also limited by low sensitivity and specificity.114,115
Urinary kidney injury molecule-1 (KIM-1) is another kidney damage biomarker and is upregulated in renal proximal TECs after ischemic and nephrotoxic injuries. A meta-analysis suggested that urinary KIM-1 was a good predictor of AKI (area under the curve of 0.86, sensitivity of 74%, specificity of 86%).116 Data for KIM-1 specifically used for S-AKI are scant. One prospective study showed that using urinary KIM-1 at 24 hours for prediction of early AKI in patients with sepsis had an area under the curve of 0.91, and the nonsurvivors had significantly higher level of urinary KIM-1 at 24 and 48 hours.95
Urinary liver-type fatty acid binding protein is upregulated and localized to the cytoplasmic region of proximal TECs, especially after hypoxic insults.97 In patients with S-AKI, urinary liver-type fatty acid binding protein levels at admission were significantly higher in nonsurvivors than in survivors.96 Urinary liver-type fatty acid binding protein levels may also reflect the severity of sepsis and monitor the effectiveness of polymyxin B hemoperfusion (PMX-HP) treatment.97
Urinary TIMP-2 and IGFBP7 are regulatory proteins involved in G1 cell cycle arrest, a protective mechanism during cellular stress. The product of urinary [TIMP-2]∙[IGFBP7] outperformed other biomarkers for prediction of AKI with area under the curve of 0.80 in a validation study including 728 patients, of which 20% had sepsis.91 Another 2 validation studies demonstrated consistent results.117,118 Importantly unlike many biomarkers, nonrenal organ failures in sepsis did not result in increased [TIMP-2]∙[IGFBP7].98 A test measuring urinary [TIMP-2]∙[IGFBP7] has regulatory approval in the United States, the European Union, and other parts of the world for AKI risk stratification.
Prevention of S-AKI
Prevention of S-AKI is usually impossible because most patients developing S-AKI will already have it at presentation.28 In general, early appropriate antibiotic administration and source control remain the backbone of sepsis treatment, which may also prevent further kidney injury. Delayed antibiotic administration in septic shock was associated with early AKI development.119 However, certain nephrotoxic agents involving in the treatment such as aminoglycosides, vancomycin particularly in combination with piperacillin-tazobactam,120 and amphotericin B, as well as diagnostic agents such as intravenous radiocontrast media should be used with caution to prevent kidney injury according to the KDIGO AKI guidelines.7 Strict therapeutic drug monitoring should be considered when applicable.
Fluid resuscitation and S-AKI.
Fluid resuscitation followed by vasopressor medications are cornerstones in the treatment of shock. Protocolized resuscitation has been recommended.7 However, 3 landmark clinical trials in patients with septic shock (Protocolized Care for Early Septic Shock [ProCESS], Australasian Resuscitation in Sepsis Evaluation [ARISE], and Protocolized Management in Sepsis [ProMISe]) consistently demonstrated no advantage of protocol-based management on mortality or need for RRT.27,121,122 An ancillary analysis of the ProCESS trial focused on renal outcomes up to 1 year and found that the use of early goal-directed therapy, alternative protocolized resuscitation, or usual care did not influence new AKI development, severity of AKI, fluid overload, RRT requirement, or renal recovery.28
Type of resuscitation fluid.
Isotonic crystalloid has been recommended for use in patients at risk of AKI.7 However, worsening renal function with chloride-rich solutions (e.g., 0.9% saline) in sepsis has been observed in animal and human studies. Observational studies involving ICU patients including those with septic shock have shown a reduction in AKI incidence and lower mortality when using crystalloid solutions with more physiological chloride concentrations (e.g., Ringer’s solution).123–125 A number of RCTs have investigated the benefit of so-called balanced crystalloid (chloride concentrations <110 mmol/l) compared with 0.9% saline on clinical outcomes. The 0.9% Saline Versus Plasma-Lyte 148 for Intensive Care Fluid Therapy (SPLIT) trial demonstrated no difference in rate of AKI or RRT between the use of saline and balanced crystalloid in ICU patients even in the subgroup with sepsis.126 However, exposure to study fluids was low (<2 l) and the population was generally low risk. By contrast, the much larger Saline Against Lactated Ringer’s or Plasma-Lyte in the Emergency Department (SALT-ED) and Isotonic Solutions and Major Adverse Renal Events (SMART) trials found fewer major adverse kidney events by 30 days (a composite of death, dialysis, and persistent renal dysfunction).127,128 Both trials, collectively enrolling nearly 30,000 patients, found about 1% absolute risk reduction for major adverse kidney events by 30 days in patients treated with balanced crystalloid. Importantly, sepsis was a preplanned subgroup analysis in the SMART trial and the 15% of patients with sepsis or septic shock experienced a much larger effect (odds ratio: 0.80, 95% CI: 0.67–0.94, P = 0.01) than the rest of the cohort. Thus, there is now substantial evidence that the use of balanced solutions should be used instead of saline especially in patients with sepsis.
All intravenous fluids can contribute to adverse renal and patient outcomes by fluid overload and renal edema. In a prospective observational study including 2526 ICU patients, daily and cumulative fluid balance over the first 3 days was higher in patients with AKI and fluid overload was an independent risk factor for AKI and AKI severity.129 Hydroxyethyl starch has been found to increase the risk of AKI, RRT requirement, and mortality.130–132 However, the signal is most evident when large volumes of hydroxyethyl starch are given to patients with sepsis130 and the long-term effects on the kidney in lower dose or in lower-risk patients is unclear.131 Similarly, gelatin, compared with crystalloid, in patients with sepsis may be associated with higher rates of AKI and need for RRT.133 Albumin solutions have generally been found to be safe in sepsis resuscitation;134,135 however, evidence that hyperoncotic albumin might deteriorate AKI and ICU outcomes is growing.136–138 A recent observational study including 11,152 patients with shock found that early exposure to hyperoncotic albumin in postoperative shock appeared to be significantly associated with AKI.138 Further studies are needed to better elucidate this risk and determine the mechanism of toxicity.
Vasopressors and target blood pressure to prevent S-AKI.
Norepinephrine is recommended as an agent of choice for septic shock treatment.139 Dopamine is not recommended for renal protection and is associated with more adverse events than norepinephrine is.139–141 Vasopressin does not appear to increase AKI risk142 and was even associated with lower RRT rates in an open-label trial.143 A large RCT in septic shock showed that a higher mean arterial blood pressure target of 80 to 85 mm Hg, compared with a target of 65 to 70 mm Hg, reduced use of RRT in a subgroup of patients with chronic hypertension. However, no survival benefit was observed.144
Timing of RRT initiation in S-AKI
Whereas a survival advantage for early initiation of RRT in patients with severe AKI has been seen in observational studies,145,146 RCTs have been less consistent (Table 2).147–149 The Artificial Kidney Initiation in Kidney Injury (AKIKI) trial, in which 80% of patients enrolled had sepsis, failed to demonstrate a benefit of early initiation of RRT.147 A post hoc analysis of patients with septic shock (56%) found similar results.150 A survival benefit at 90 days for early RRT initiation was shown in the Early Versus Late Initiation of Renal Replacement Therapy in Critically Ill Patients With Acute Kidney Injury (ELAIN) trial, but only 21% of the patients had sepsis.148 Early initiation resulted in a significantly reduced rate of major adverse kidney events, mortality, and enhanced renal recovery at 1-year follow-up.151 The recent Initiation of Dialysis Early Versus Delayed in the Intensive Care Unit (IDEAL-ICU) trial specifically focused on patients with septic shock and severe AKI, but it demonstrated no significant difference in 90-day mortality between patients receiving early versus delayed RRT initiation.149 Concerningly, 9% of the patients died between early and delayed initiation. These conflicting results may be explained by distinct study designs in heterogeneous settings. An ongoing clinical trial (Standard Versus Accelerated Initiation of Dialysis in Acute Kidney Injury [STARRT-AKI], ) may provide a more definitive answer.152
Table 2 |.
Timing for RRT in S-AKI
| Trial | Setting | Percentage of sepsis | Modality of RRT | Timing of RRT initiation | Outcome | |
|---|---|---|---|---|---|---|
| Early strategy | Delayed strategy | |||||
| Gaudry et al.147 (AKIKI) | Adults, ICU patients with AKI (multicenter) | 80% | IHD/CRRT | Median time 2 h (IQR: 1–3 h) after randomization or 4.3 h (IQR: 2.7–5.9 h) after documented KDIGO stage 3 AKI, N = 311 | Median time 57 h (IQR: 25–83 h) after randomization, N = 308 (51% received RRT) | No difference in 60-day mortality (48.5% vs. 49.7%), post hoc analysis in patients with septic shock (56%) showed similar results, more patients with CRBSI and hypophosphatemia in early group, adequate diuresis with no need for RRT were observed earlier in delayed group. |
| Zarbock et al.148 (ELAIN) | Adults, ICU patients, 47% with cardiac surgery (single center) | 21% | CVVHDF | Median time 6 h (IQR: 4–7 h) after documented KDIGO stage 2 AKI, N = 112 | Median time 25.5 h (IQR: 18.8–40.3 h) after documented KDIGO stage 2 AKI (within 12 h after KDIGO stage 3 AKI), N = 119 (91% received RRT) | Significantly lower 90-day mortality in early group (39.3% vs. 54.7%), no difference in renal recovery at 90 days, 95% of study population were surgical patients and small number of patients with sepsis. |
| In follow-up analysis found significantly reduced rate of MAKE365 (64.9% vs. 89.1%) and 1-year mortality (50.2% vs. 69.8%) in early group. | ||||||
| Barbar et al.149 (IDEAL-ICU) | Adults, patients with septic shock and severe AKI (multicenter) | 100% | IHD/CRRT | Median time 7.6 h (IQR: 4.4–11.5 h) after RIFLE-F, N = 246 | Median time 51.5 h (IQR: 34.6–59.5 h) after documented AKI or meet emergency RRT criteria (K >6.5 mmol/l, pH < 7.15, fluid overload), N = 242 (51% received RRT) | No difference in 90-day mortality (58% vs. 54%), more days of RRT and less RRT-free days in early group, more patients with hyperkalemia in delayed group, similar fluid balance, 29% of patients in delayed group had spontaneous recovery. |
| STARRT-AKI () | Adults, ICU patients with severe AKI (RIFLE-I, oliguria, pNGAL $400 ng/ml (multicenter) | N/A | IHD/SLED/CRRT | “Accelerated” initiation: RRT will be initiated within 12 h of fulfilling eligibility | “Standard” initiation: participants will be monitored over 7 days to identify indications for RRT (K ≥6.0 mmol/l, bicarbonate ≤10 mmol/l, PaO2/FiO2 <200 and bilateral infiltrates on CXR, persistent AKI >72 h after eligibility) | N/A |
AKIKI, Artificial Kidney Initiation in Kidney Injury trial; CRBSI, catheter-related bloodstream infection; CRRT, continuous renal replacement therapy; CVVHDF, continuous venovenous hemodiafiltration; CXR, chest x-ray; ELAIN, Early Versus Late Initiation of Renal Replacement Therapy in Critically Ill Patients With Acute Kidney Injury trial; ICU, intensive care unit; IDEAL-ICU, Initiation of Dialysis Early Versus Delayed in the Intensive Care Unit trial; IHD, intermittent hemodialysis; IQR, interquartile range; KDIGO, Kidney Disease: Improving Global Outcomes; MAKE365, major adverse kidney events by 365 days; N/A, not applicable; pNGAL, plasma neutrophil gelatinase-associated lipocalin; RIFLE, Risk, Injury, Failure, Loss of Kidney Function, End-Stage Kidney Disease classification; RRT, renal replacement therapy; S-AKI, sepsis-associated acute kidney injury; SLED, sustained low efficiency dialysis; STARRT-AKI, Standard Versus Accelerated Initiation of Dialysis in Acute Kidney Injury trial.
Experimental therapies for S-AKI
Blood purification.
Numerous techniques are available but clinical evidence is still limited (Table 3).153–172 The vast majority of studies in patients have not measured target solutes so it remains unclear whether negative trials failed to clear inflammatory mediators or were ineffective in terms of survival despite clearance. Cytokines are highly variable in patients even with septic shock,173 and high endogenous clearance means their levels are dynamic. Below are some modalities that have been used in recent trials.
Table 3 |.
Experimental therapies for S-AKI
| Treatment | Property/mechanism of action | Comment |
|---|---|---|
| Blood purification | ||
| High-volume hemofiltration | Convection enhances removal of large molecule proinflammatory cytokines | No survival benefit, increased the rate of hypophosphatemia and hypokalemia, reduced circulating cytokine level (see text) |
| High cutoff membrane | Large pore membrane (average 20 nm in diameter) allows removal of large molecule cytokines | Compared with conventional CRRT in patients with S-AKI, the use of high cutoff membrane significantly reduced circulating levels of inflammatory cytokines and improved hemodynamics, but no effect on mortality was observed.153–159 Excessive amounts of albumin were also lost. |
| Adsorptive therapy | ||
| Polymyxin B hemoperfusion | Synthetic membrane coated with polymyxin B that binds endotoxin | Improved hemodynamic parameter and monocyte and neutrophil function with controversy on survival benefit (see text) |
| CytoSorb | Porous polymer beads; adsorption of cytokines, myoglobin, free hemoglobin, bilirubin/bile acid | Reduce circulating IL-6, improve hemodynamics, no survival benefit160–162 |
| oXiris | Surface-treated AN69 membrane with PEI and coated with heparin; adsorption of endotoxin and cytokines | Reduced SOFA score at 48 h.163 Ongoing RCTs are investigating the effectiveness of this treatment (ENDoX, ; oXiris, ). |
| HA-330 | Neutral microporous resin; adsorption of cytokines, complements, free hemoglobin | Improved hemodynamics and organ function, shortened ICU stay, and reduced ICU mortality164 |
| LPS adsorbers | Synthetic polypeptide bound to porous polyethylene discs; adsorption of endotoxins | A case series in patients with gram-negative sepsis reported improvement of hemodynamics and decreased endotoxin level but no effect on survival.165–167 |
| CPFA | Combined plasma separation with adsorption and hemodialysis; removes inflammatory mediators | No survival benefit, technical issue (clotted), high cost.168 Additional RCTs are pending (COMPACT 2, ; ROMPA, ). |
| Pharmacologic therapy | ||
| Human recombinant alkaline phosphatase | Dephosphorylation of endotoxins and proinflammatory mediators such as extracellular adenosine triphosphate, which is released by mitochondria in response to inflammation and hypoxia | No significant improvement in short-term kidney function was found compared with placebo use. Mortality at day 28 was found to be reduced with AP.169 |
| Angiotensin II | Potent vasoconstrictor to increase blood pressure (effect on efferent arterioles > afferent arterioles); potent vasopressor, without inotropic or chronotropic properties | Pilot study in patients with catecholamine-resistant shock showed restored blood pressure and increase urine output.170 Subgroup analysis in patients with high-output shock with AKI requiring RRT showed angiotensin II associated with an improved survival and a higher rate of renal recovery.171 Ongoing RCT aims to evaluate the effect of angiotensin II on hemodynamics and urine output in patients with S-AKI (ASK-IT, ). |
| Levocarnitine | Enhance fatty acid entry into the mitochondria; antioxidant, anti-inflammatory, and antiapoptotic actions | Did not improved organ dysfunction in septic shock.172 Ongoing RCT aims to explore the effect of levocarnitine as an adjunctive treatment for patients with S-AKI (CarniSave, ) |
| Reltecimod (AB103) | CD28 antagonist prevents binding of bacterial superantigens to the CD28 T-cell receptor | Ongoing phase 2 RCT of reltecimod versus placebo in patients with S-AKI; aims to assess recovery from AKI and safety (Phase 2 Study of Reltecimod Versus Placebo in Patients With Sepsis-associated Acute Kidney Injury, ). |
AP, alkaline phosphatase; ASK-IT, Angiotensin in Septic Kidney Injury Trial; CarniSave, L-carnitine as an Adjunct Treatment for Septic Shock Patients With Acute Kidney Injury trial; COMPACT 2, Combining Plasma-Filtration and Adsorption 2 trial; CPFA, coupled plasma filtration adsorption; CRRT, continuous renal replacement therapy; ENDoX, Effects of a Polyethylene-Coated Membrane (oXiris) for Hemofiltration Versus Polymyxin B–Immobilized Fiber Column (Toraymyxin) for Hemoperfusion on Endotoxin Activity and Inflammatory Conditions in Septic Shock study; IL-6, interleukin-6; LPS, lipopolysaccharides; oXiris, Comparing Cytokines, Toxins Adsorbing oXiris Filter to ST150 Filter During CRRT in Patients With Septic Shock trial; PEI, polyethyleneimine; RCT, randomized controlled trial; ROMPA, Mortality Reduction in Septic Shock by Plasma Adsorption trial; RRT, renal replacement therapy; S-AKI, sepsis-associated acute kidney injury; SOFA, Sequential Organ Failure Assessment.
High-volume hemofiltration (HVHF) is defined as a continuous treatment with a convective dose >35 ml/kg per hour.174,175 Several small studies have investigated the role of HVHF (dose ranging from 40 to 100 ml/kg per hour) in patients with S-AKI and found variable results in terms of mortality.176–182 A multicenter RCT (Hemofiltration Study: High Volume in Intensive Care [IVOIRE]) randomized 140 patients with S-AKI for less than 24 hours to receive either HVHF (70 ml/kg per hour) or standard hemofiltration (35 ml/kg per hour) for 96 hours. No differences in 28-, 60-, or 90-day mortality; duration of RRT; or renal recovery were seen between the 2 groups.183 Another RCT that compared the treatment with HVHF between doses of 85 ml/kg per hour and 50 ml/kg per hour in S-AKI also found no differences in mortality or renal outcome.184 Meta-analysis of HVHF in patients with S-AKI from 4 trials showed no benefit of HVHF on 28-day survival, but did show increased rates of hypophosphatemia and hypokalemia.185 Some RCTs demonstrated an improvement of hemodynamics and organ function,186 as well as a reduction in circulating inflammatory cytokines, but these improvements did not influence the clinical outcomes in the high-dose arm.187 Recently, a systematic review of 8 clinical studies, including the landmark Veterans Affairs/National Institutes of Health Acute Renal Failure Trial Network (ATN)188 and Randomized Evaluation of Normal Versus Augmented Level of Replacement Therapy (RENAL)189 trials, demonstrated no significant difference between 20 to 25 ml/kg per hour and more intensive ($35 ml/kg per hour) RRT on 30-day mortality, renal recovery, or ICU and hospital length of stay (not limited to S-AKI).190 Clinicians should also be aware of the potential for increased antibiotic removal when high-intensity continuous renal replacement therapy is prescribed.191
PMX-HP has been used as adjunctive treatment for sepsis in Japan since 1994 and is claimed to remove circulating endotoxin. Multiple RCTs have been conducted to determine the efficacy of PMX-HP in sepsis and have shown conflicting results. A multicenter pilot trial in Europe enrolled 36 surgical patients with intra-abdominal sepsis and demonstrated that the treatment with 2-hour PMX-HP only once led to an improvement of left ventricular function and a decrease in RRT requirement.192 The Early Use of Polymyxin B Hemoperfusion in Abdominal Sepsis (EUPHAS) trial studied 64 patients with severe intra-abdominal sepsis and reported favorable results of treatment with PMX-HP for 2 consecutive days in terms of hemodynamics, organ functions, and 28-day survival.193 However, 2 subsequent, larger RCTs were negative. The Effects of Hemoperfusion With a Polymyxin B Membrane in Peritonitis With Septic Shock (ABDOMIX) trial including 243 patients with septic shock secondary to peritonitis compared standard therapy with 2 PMX-HP sessions plus standard therapy. Patients in the PMX-HP group had no improvement in survival or organ failure.194 The Safety and Efficacy of Polymyxin B Hemoperfusion for Septic Shock (EUPHRATES) trial enrolled 450 adults with septic shock from various sources of infection and an endotoxin activity assay level of ≥0.60 to receive either 2 PMX-HP sessions or sham hemoperfusion plus standard therapy. No significant difference in 28-day mortality or other secondary outcomes including RRT-free days was found between the groups.195 Based on the saturation effect of the polymyxin column in patients with very high circulating endotoxin that could reduce treatment efficacy, the post hoc subgroup analysis in patients with an endotoxin activity assay level of ≥0.6 to 0.89 found a benefit of PMX-HP in hemodynamic improvement, ventilator-free days, and mortality.196 Recently, the immunomodulation effect of PMX-HP has been reported in another RCT by improvement of monocyte and neutrophil functions in patients with sepsis without a benefit in mortality or renal outcome.197
Pharmacologic therapy.
A number of new drugs for AKI are currently being investigated,198 but relatively few are focused specifically on S-AKI. Several molecules have been studied in septic animal models to amend mitochondrial dysfunction, inflammation, and oxidative stress.199 The notable exceptions are reltecimod (AB103; AtoxBio, Durham, NC), which is under active investigation to improve recovery from AKI (Phase 2 Study of Reltecimod Versus Placebo in Patients With Sepsis-associated Acute Kidney Injury, ), alkaline phosphatase, angiotensin II, and levocarnitine (Table 3). Given that sepsis is the leading cause of AKI in the critically ill and given the dramatic effects from AKI on sepsis survival, it is our sincere hope that other companies will join the search for an S-AKI treatment.
Human recombinant alkaline phosphatase (AP) is an endogenous enzyme that confers renal protection during sepsis via the dephosphorylation of various compounds, including bacterial endotoxins and proinflammatory mediators such as extracellular adenosine triphosphate, which is released by mitochondria in response to inflammation and hypoxia.200 AP is depleted in the kidney following an ischemic insult in rats.201 In animal sepsis models, treatment with AP attenuated systemic inflammation, organ dysfunction, and improved survival.202 In 2 small clinical trials, administration of bovine AP significantly improved kidney function in patients with S-AKI.203,204 In septic shock, infusion of AP inhibits the upregulation of renal inducible nitric oxide synthase, leading to subsequent reduced nitric oxide metabolite production, and attenuated tubular secretion of glutathione S-transferase A1–1 (a marker of proximal tubular injury) in urine.203 An RCT evaluated this effect in 36 patients with S-AKI by intravenous infusion of AP (bolus injection followed by continuous infusion for 48 hours or placebo) starting within 48 hours of AKI onset and showed improvement of endogenous creatinine clearance, requirement for and duration of dialysis, decreased urinary biomarkers of renal injury (KIM-1 and interleukin-18), and inflammatory biomarkers from baseline to day 28 in patients receiving AP.204 Human recombinant AP is a highly stable, biologically active enzyme. The recent A Safety, Tolerability, Efficacy, and QoL Study of Human recAP in the Treatment of Patients With SA-AKI (STOP-AKI) trial is an international double-blind RCT conducted in 301 patients with S-AKI that aimed to investigate the optimal therapeutic dose, effect on kidney function, and adverse effects of human recombinant AP.169 AP was administered within 24 hours after S-AKI was documented and then 24 and 48 hours following the first dose. Improvement in endogenous creatinine clearance from days 1 to 7 were not different between groups. The 7-day period might have been too short for evaluation the effect of treatment on the kidney. Mortality, a secondary endpoint, was found to be reduced with AP.
Angiotensin II (ATII) is a potent vasoconstrictor acting via angiotensin II type 1 receptors and appears to cause vasoconstriction of efferent more than afferent arterioles, resulting in increasing glomerular perfusion pressure and filtration rate.205 Sepsis leads to relative scarcity of ATII.206 In addition, ATII is a potent vasopressor without inotropic or chronotropic properties. Unlike norepinephrine, ATII may preserve medullary perfusion and oxygenation.80,207 ATII infusion restored blood pressure, increased urine output and creatinine clearance despite decreased renal blood flow in septic animals.208 A pilot study in patients with catecholamine-resistant septic shock (Intravenous Angiotensin II for the Treatment of High-Output Shock [ATHOS]) found that ATII restored blood pressure and increased urine output.170 A recent RCT (ATHOS-3), which mainly examined the effect of intravenous ATII in patients with catecholamine-resistant high-output shock (80% were diagnosed with septic shock), showed increased blood pressure and decreased need for other vasopressors.209 Post hoc analysis in patients with AKI requiring RRT (n = 105) found a 23% risk reduction in 28-day mortality and more patients discontinued RRT in the ATII group.171 Thus, patients with S-AKI with shock may benefit from ATII. An ongoing Angiotensin in Septic Kidney Injury Trial (ASK-IT; ) aims to evaluate the effect of ATII on hemodynamics and urine output in patients with S-AKI.
Recovery from S-AKI and long-term follow-up
Evaluation of renal recovery after AKI has many concerns including assessment of baseline renal function, definition, and timing of recovery.210 Recently, the Acute Disease Quality Initiative developed consensus definitions for renal recovery after AKI.211 Many factors may determine recovery including preexisting renal functional reserve, severity, duration, and repetitive episodes of AKI.212 In sepsis, several factors may contribute to maladaptive repair leading to delayed recovery and progression to CKD. Persistent inflammation, fibrosis, and vascular dropout lead to persistent tissue ischemia and hypoxia and thus promote interstitial fibrosis.212,213 Pharmacologic treatments to ameliorate fibrosis and oxidative stress (e.g., hypoxia-inducible factor activation, Nuclear factor erythroid 2–related factor 2 (Nrf-2) activation, vascular endothelial growth factor) are still experimental and there is limited data in sepsis models.214–216 Thus, prevention, early recognition, and treatment of sepsis; avoidance of renal hypoperfusion and nephrotoxic insults; and close follow-up are the mainstay to promote renal recovery (Figure 1).
Long-term follow-up for development of CKD, recurrent sepsis, recurrent AKI, and cardiovascular consequences should be considered for all S-AKI survivors regardless of AKI severity. Early follow-up by a nephrologist after an AKI episode in those requiring RRT was associated with improved survival.217 However, observational studies have shown that the rate of nephrology referral for post-AKI follow-up is low.218,219 An RCT in AKI survivors comparing nephrologist follow-up versus usual care for 1 year is ongoing (Nephrologist Follow-up Versus Usual Care After an Acute Kidney Injury Hospitalization [FUSION]; ). The primary outcome is development of a major adverse kidney events.
Conclusion
S-AKI is the result of a dysregulated response of the host to infection. Patients who develop S-AKI have various clinical trajectories in which renal recovery is possible and is associated with improved outcomes. Clinicians need to clearly understand the clinical course of this complex syndrome to improve patient care both in the short and long terms. The value of biomarkers has been established and may be complementary to clinical judgment, functional tests, and current criteria to improve early detection, potentially guide management, and monitor recovery. Advances in understanding the pathophysiologic mechanisms have provided insight into potential new therapies; however, effective, specific interventions for prevention and treatment of S-AKI are still lacking. Future research must focus on better understanding the mechanisms leading to S-AKI and on bridging and streamlining the transition of knowledge from bench, big data-based population studies, and clinical trials.
ACKNOWLEDGMENTS
Support was received from the National Institutes of Health Grants UG3DK114861 (JAK), UL1TR001857 (JAK), and K08GM117310 (HG).
Footnotes
DISCLOSURE
JAK discloses grant support and consulting fees from Astute Medical, Baxter, bioMerieux, BioPorto, and NxStage. All the other authors declared no competing interests.
REFERENCES
- 1.Uchino S, Kellum JA, Bellomo R, et al. Acute renal failure in critically ill patients: a multinational, multicenter study. JAMA. 2005;294:813–818. [DOI] [PubMed] [Google Scholar]
- 2.Bagshaw SM, Uchino S, Bellomo R, et al. Septic acute kidney injury in critically ill patients: clinical characteristics and outcomes. Clin J Am Soc Nephrol. 2007;2:431–439. [DOI] [PubMed] [Google Scholar]
- 3.Bouchard J, Acharya A, Cerda J, et al. A prospective international multicenter study of AKI in the intensive care unit. Clin J Am Soc Nephrol. 2015;10:1324–1331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hoste EA, Bagshaw SM, Bellomo R, et al. Epidemiology of acute kidney injury in critically ill patients: the multinational AKI-EPI study. Intensive Care Med. 2015;41:1411–1423. [DOI] [PubMed] [Google Scholar]
- 5.Mehta RL, Bouchard J, Soroko SB, et al. Sepsis as a cause and consequence of acute kidney injury: program to improve care in acute renal disease. Intensive Care Med. 2011;37:241–248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bellomo R, Ronco C, Kellum JA, et al. Acute renal failure—definition, outcome measures, animal models, fluid therapy and information technology needs: the Second International Consensus Conference of the Acute Dialysis Quality Initiative (ADQI) Group. Crit Care. 2004;8: R204–R212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kidney Disease: Improving Global Outcomes (KDIGO) Acute Kidney Injury Work Group. KDIGO clinical practice guideline for acute kidney injury. Kidney Int Suppl. 2012;2:1–138. [Google Scholar]
- 8.Leedahl DD, Frazee EN, Schramm GE, et al. Derivation of urine output thresholds that identify a very high risk of AKI in patients with septic shock. Clin J Am Soc Nephrol. 2014;9:1168–1174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Prowle JR, Liu YL, Licari E, et al. Oliguria as predictive biomarker of acute kidney injury in critically ill patients. Crit Care. 2011;15:R172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Thongprayoon C, Cheungpasitporn W, Kittanamongkolchai W, et al. Optimum methodology for estimating baseline serum creatinine for the acute kidney injury classification. Nephrology (Carlton). 2015;20: 881–886. [DOI] [PubMed] [Google Scholar]
- 11.Thongprayoon C, Cheungpasitporn W, Harrison AM, et al. The comparison of the commonly used surrogates for baseline renal function in acute kidney injury diagnosis and staging. BMC Nephrol. 2016;17:6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Singer M, Deutschman CS, Seymour CW, et al. The Third International Consensus Definitions for Sepsis and Septic Shock (Sepsis-3). JAMA. 2016;315:801–810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bellomo R, Kellum JA, Ronco C, et al. Acute kidney injury in sepsis. Intensive Care Med. 2017;43:816–828. [DOI] [PubMed] [Google Scholar]
- 14.Godin M, Murray P, Mehta RL. Clinical approach to the patient with AKI and sepsis. Semin Nephrol. 2015;35:12–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jin K, Murugan R, Sileanu FE, et al. Intensive monitoring of urine output is associated with increased detection of acute kidney injury and improved outcomes. Chest. 2017;152:972–979. [DOI] [PubMed] [Google Scholar]
- 16.Kellum JA, Sileanu FE, Murugan R, et al. Classifying AKI by urine output versus serum creatinine level. J Am Soc Nephrol. 2015;26:2231–2238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Doi K, Yuen PS, Eisner C, et al. Reduced production of creatinine limits its use as marker of kidney injury in sepsis. J Am Soc Nephrol. 2009;20: 1217–1221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chawla LS, Davison DL, Brasha-Mitchell E, et al. Development and standardization of a furosemide stress test to predict the severity of acute kidney injury. Crit Care. 2013;17:R207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bagshaw SM, Haase M, Haase-Fielitz A, et al. A prospective evaluation of urine microscopy in septic and non-septic acute kidney injury. Nephrol Dial Transplant. 2012;27:582–588. [DOI] [PubMed] [Google Scholar]
- 20.Adhikari NK, Fowler RA, Bhagwanjee S, et al. Critical care and the global burden of critical illness in adults. Lancet. 2010;376:1339–1346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Murugan R, Karajala-Subramanyam V, Lee M, et al. Acute kidney injury in non-severe pneumonia is associated with an increased immune response and lower survival. Kidney Int. 2010;77:527–535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Selby NM, Crowley L, Fluck RJ, et al. Use of electronic results reporting to diagnose and monitor AKI in hospitalized patients. Clin J Am Soc Nephrol. 2012;7:533–540. [DOI] [PubMed] [Google Scholar]
- 23.Susantitaphong P, Cruz DN, Cerda J, et al. World incidence of AKI: a meta-analysis. Clin J Am Soc Nephrol. 2013;8:1482–1493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chertow GM, Burdick E, Honour M, et al. Acute kidney injury, mortality, length of stay, and costs in hospitalized patients. J Am Soc Nephrol. 2005;16:3365–3370. [DOI] [PubMed] [Google Scholar]
- 25.Vincent JL, Sakr Y, Sprung CL, et al. Sepsis in European intensive care units: results of the SOAP study. Crit Care Med. 2006;34:344–353. [DOI] [PubMed] [Google Scholar]
- 26.Xu X, Nie S, Liu Z, et al. Epidemiology and clinical correlates of AKI in Chinese hospitalized adults. Clin J Am Soc Nephrol. 2015;10:1510–1518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Pro CI, Yealy DM, Kellum JA, et al. A randomized trial of protocol-based care for early septic shock. N Engl J Med. 2014;370:1683–1693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kellum JA, Chawla LS, Keener C, et al. The effects of alternative resuscitation strategies on acute kidney injury in patients with septic shock. Am J Respir Crit Care Med. 2016;193:281–287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Vincent JL, Moreno R, Takala J, et al. , for the Working Group on Sepsis-Related Problems of the European Society of Intensive Care Medicine. The SOFA (Sepsis-related Organ Failure Assessment) score to describe organ dysfunction/failure. Intensive Care Med. 1996;22:707–710. [DOI] [PubMed] [Google Scholar]
- 30.Kellum JA, Sileanu FE, Bihorac A, et al. Recovery after acute kidney injury. Am J Respir Crit Care Med. 2017;195:784–791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Fiorentino M, Tohme FA, Wang S, et al. Long-term survival in patients with septic acute kidney injury is strongly influenced by renal recovery. PLoS One. 2018;13:e0198269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sood MM, Shafer LA, Ho J, et al. Early reversible acute kidney injury is associated with improved survival in septic shock. J Crit Care. 2014;29: 711–717. [DOI] [PubMed] [Google Scholar]
- 33.See EJ, Jayasinghe K, Glassford N, et al. Long-term risk of adverse outcomes after acute kidney injury: a systematic review and meta-analysis of cohort studies using consensus definitions of exposure. Kidney Int. 2019;95:160–172. [DOI] [PubMed] [Google Scholar]
- 34.Chawla LS, Amdur RL, Amodeo S, et al. The severity of acute kidney injury predicts progression to chronic kidney disease. Kidney Int. 2011;79:1361–1369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Chua HR, Wong WK, Ong VH, et al. Extended mortality and chronic kidney disease after septic acute kidney injury [e-pub ahead of print]. J Intensive Care Med. 10.1177/0885066618764617. Accessed July 5, 2019. [DOI] [PubMed] [Google Scholar]
- 36.Rosen S, Heyman SN. Difficulties in understanding human “acute tubular necrosis”: limited data and flawed animal models. Kidney Int. 2001;60:1220–1224. [DOI] [PubMed] [Google Scholar]
- 37.Schrier RW, Wang W. Acute renal failure and sepsis. N Engl J Med. 2004;351:159–169. [DOI] [PubMed] [Google Scholar]
- 38.Chua HR, Glassford N, Bellomo R. Acute kidney injury after cardiac arrest. Resuscitation. 2012;83:721–727. [DOI] [PubMed] [Google Scholar]
- 39.Zager RA. Partial aortic ligation: a hypoperfusion model of ischemic acute renal failure and a comparison with renal artery occlusion. J Lab Clin Med. 1987;110:396–405. [PubMed] [Google Scholar]
- 40.Cerchiari EL, Safar P, Klein E, et al. Visceral, hematologic and bacteriologic changes and neurologic outcome after cardiac arrest in dogs: the visceral post-resuscitation syndrome. Resuscitation. 1993;25: 119–136. [DOI] [PubMed] [Google Scholar]
- 41.Langenberg C, Wan L, Egi M, et al. Renal blood flow in experimental septic acute renal failure. Kidney Int. 2006;69:1996–2002. [DOI] [PubMed] [Google Scholar]
- 42.Prowle JR, Molan MP, Hornsey E, et al. Measurement of renal blood flow by phase-contrast magnetic resonance imaging during septic acute kidney injury: a pilot investigation. Crit Care Med. 2012;40:1768–1776. [DOI] [PubMed] [Google Scholar]
- 43.Brenner M, Schaer GL, Mallory DL, et al. Detection of renal blood flow abnormalities in septic and critically ill patients using a newly designed indwelling thermodilution renal vein catheter. Chest. 1990;98: 170–179. [DOI] [PubMed] [Google Scholar]
- 44.Langenberg C, Bellomo R, May C, et al. Renal blood flow in sepsis. Crit Care. 2005;9:R363–R374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Di Giantomasso D, May CN, Bellomo R. Norepinephrine and vital organ blood flow during experimental hyperdynamic sepsis. Intensive Care Med. 2003;29:1774–1781. [DOI] [PubMed] [Google Scholar]
- 46.Di Giantomasso D, Bellomo R, May CN. The haemodynamic and metabolic effects of epinephrine in experimental hyperdynamic septic shock. Intensive Care Med. 2005;31:454–462. [DOI] [PubMed] [Google Scholar]
- 47.Di Giantomasso D, May CN, Bellomo R. Vital organ blood flow during hyperdynamic sepsis. Chest. 2003;124:1053–1059. [DOI] [PubMed] [Google Scholar]
- 48.Ravikant T, Lucas CE. Renal blood flow distribution in septic hyperdynamic pigs. J Surg Res. 1977;22:294–298. [DOI] [PubMed] [Google Scholar]
- 49.Wan L, Bellomo R, May CN. The effect of normal saline resuscitation on vital organ blood flow in septic sheep. Intensive Care Med. 2006;32: 1238–1242. [DOI] [PubMed] [Google Scholar]
- 50.Takasu O, Gaut JP, Watanabe E, et al. Mechanisms of cardiac and renal dysfunction in patients dying of sepsis. Am J Respir Crit Care Med. 2013;187:509–517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Maiden MJ, Otto S, Brealey JK, et al. Structure and function of the kidney in septic shock. a prospective controlled experimental study. Am J Respir Crit Care Med. 2016;194:692–700. [DOI] [PubMed] [Google Scholar]
- 52.Lerolle N, Nochy D, Guerot E, et al. Histopathology of septic shock induced acute kidney injury: apoptosis and leukocytic infiltration. Intensive Care Med. 2010;36:471–478. [DOI] [PubMed] [Google Scholar]
- 53.Langenberg C, Gobe G, Hood S, et al. Renal histopathology during experimental septic acute kidney injury and recovery. Crit Care Med. 2014;42:e58–e67. [DOI] [PubMed] [Google Scholar]
- 54.Hotchkiss RS, Swanson PE, Freeman BD, et al. Apoptotic cell death in patients with sepsis, shock, and multiple organ dysfunction. Crit Care Med. 1999;27:1230–1251. [DOI] [PubMed] [Google Scholar]
- 55.Wang Z, Holthoff JH, Seely KA, et al. Development of oxidative stress in the peritubular capillary microenvironment mediates sepsis-induced renal microcirculatory failure and acute kidney injury. Am J Pathol. 2012;180:505–516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Seely KA, Holthoff JH, Burns ST, et al. Hemodynamic changes in the kidney in a pediatric rat model of sepsis-induced acute kidney injury. Am J Physiol Renal Physiol. 2011;301:F209–F217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.De Backer D, Donadello K, Taccone FS, et al. Microcirculatory alterations: potential mechanisms and implications for therapy. Ann Intensive Care. 2011;1:27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Gómez H, Kellum JA, Ronco C. Metabolic reprogramming and tolerance during sepsis-induced AKI. Nat Rev Nephrol. 2017;13:143–151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Singer M, De Santis V, Vitale D, et al. Multiorgan failure is an adaptive, endocrine-mediated, metabolic response to overwhelming systemic inflammation. Lancet. 2004;364:545–548. [DOI] [PubMed] [Google Scholar]
- 60.Gomez H, Ince C, De Backer D, et al. A unified theory of sepsis-induced acute kidney injury: inflammation, microcirculatory dysfunction, bioenergetics, and the tubular cell adaptation to injury. Shock. 2014;41: 3–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Hotchkiss RS, Karl IE. The pathophysiology and treatment of sepsis. N Engl J Med. 2003;348:138–150. [DOI] [PubMed] [Google Scholar]
- 62.Fry DE. Sepsis, systemic inflammatory response, and multiple organ dysfunction: the mystery continues. Am Surg. 2012;78:1–8. [PubMed] [Google Scholar]
- 63.Kalakeche R, Hato T, Rhodes G, et al. Endotoxin uptake by S1 proximal tubular segment causes oxidative stress in the downstream S2 segment. J Am Soc Nephrol. 2011;22:1505–1516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Dellepiane S, Marengo M, Cantaluppi V. Detrimental cross-talk between sepsis and acute kidney injury: new pathogenic mechanisms, early biomarkers and targeted therapies. Crit Care. 2016;20:61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Aslan A, van den Heuvel MC, Stegeman CA, et al. Kidney histopathology in lethal human sepsis. Crit Care. 2018;22:359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.De Backer D, Creteur J, Preiser JC, et al. Microvascular blood flow is altered in patients with sepsis. Am J Respir Crit Care Med. 2002;166:98–104. [DOI] [PubMed] [Google Scholar]
- 67.Post EH, Kellum JA, Bellomo R, et al. Renal perfusion in sepsis: from macro- to microcirculation. Kidney Int. 2017;91:45–60. [DOI] [PubMed] [Google Scholar]
- 68.Verdant CL, De Backer D, Bruhn A, et al. Evaluation of sublingual and gut mucosal microcirculation in sepsis: a quantitative analysis. Crit Care Med. 2009;37:2875–2881. [DOI] [PubMed] [Google Scholar]
- 69.Tiwari MM, Brock RW, Megyesi JK, et al. Disruption of renal peritubular blood flow in lipopolysaccharide-induced renal failure: role of nitric oxide and caspases. Am J Physiol Renal Physiol. 2005;289:F1324–F1332. [DOI] [PubMed] [Google Scholar]
- 70.Holthoff JH, Wang Z, Seely KA, et al. Resveratrol improves renal microcirculation, protects the tubular epithelium, and prolongs survival in a mouse model of sepsis-induced acute kidney injury. Kidney Int. 2012;81:370–378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Verma SK, Molitoris BA. Renal endothelial injury and microvascular dysfunction in acute kidney injury. Semin Nephrol. 2015;35:96–107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Dyson A, Bezemer R, Legrand M, et al. Microvascular and interstitial oxygen tension in the renal cortex and medulla studied in a 4-h rat model of LPS-induced endotoxemia. Shock. 2011;36:83–89. [DOI] [PubMed] [Google Scholar]
- 73.Almac E, Siegemund M, Demirci C, et al. Microcirculatory recruitment maneuvers correct tissue CO2 abnormalities in sepsis. Minerva Anestesiol. 2006;72:507–519. [PubMed] [Google Scholar]
- 74.Rajendram R, Prowle JR. Venous congestion: are we adding insult to kidney injury in sepsis? Crit Care. 2014;18:104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Martensson J, Bellomo R. Sepsis-induced acute kidney injury. Crit Care Clin. 2015;31:649–660. [DOI] [PubMed] [Google Scholar]
- 76.Singh P, Okusa MD. The role of tubuloglomerular feedback in the pathogenesis of acute kidney injury. Contrib Nephrol. 2011;174:12–21. [DOI] [PubMed] [Google Scholar]
- 77.Calzavacca P, Evans RG, Bailey M, et al. Cortical and medullary tissue perfusion and oxygenation in experimental septic acute kidney injury. Crit Care Med. 2015;43:e431–e439. [DOI] [PubMed] [Google Scholar]
- 78.Ljungqvist A. Ultrastructural demonstration of a connection between afferent and efferent juxtamedullary glomerular arterioles. Kidney Int. 1975;8:239–244. [DOI] [PubMed] [Google Scholar]
- 79.Casellas D, Mimran A. Shunting in renal microvasculature of the rat: a scanning electron microscopic study of corrosion casts. Anat Rec. 1981;201:237–248. [DOI] [PubMed] [Google Scholar]
- 80.Lankadeva YR, Kosaka J, Evans RG, et al. Intrarenal and urinary oxygenation during norepinephrine resuscitation in ovine septic acute kidney injury. Kidney Int. 2016;90:100–108. [DOI] [PubMed] [Google Scholar]
- 81.Mandel LJ, Balaban RS. Stoichiometry and coupling of active transport to oxidative metabolism in epithelial tissues. Am J Physiol. 1981;240: F357–F371. [DOI] [PubMed] [Google Scholar]
- 82.Bhargava P, Schnellmann RG. Mitochondrial energetics in the kidney. Nat Rev Nephrol. 2017;13:629–646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Schmidt C, Hocherl K, Schweda F, et al. Proinflammatory cytokines cause down-regulation of renal chloride entry pathways during sepsis. Crit Care Med. 2007;35:2110–2119. [DOI] [PubMed] [Google Scholar]
- 84.Good DW, George T, Watts BA 3rd. Lipopolysaccharide directly alters renal tubule transport through distinct TLR4-dependent pathways in basolateral and apical membranes. Am J Physiol Renal Physiol. 2009;297: F866–F874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Hsiao HW, Tsai KL, Wang LF, et al. The decline of autophagy contributes to proximal tubular dysfunction during sepsis. Shock. 2012;37:289–296. [DOI] [PubMed] [Google Scholar]
- 86.Yang L, Xie M, Yang M, et al. PKM2 regulates the Warburg effect and promotes HMGB1 release in sepsis. Nat Commun. 2014;5:4436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Escobar DA, Botero-Quintero AM, Kautza BC, et al. Adenosine monophosphate-activated protein kinase activation protects against sepsis-induced organ injury and inflammation. J Surg Res. 2015;194: 262–272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Opal SM, Ellis JL, Suri V, et al. Pharmacological SIRT1 activation improves mortality and markedly alters transcriptional profiles that accompany experimental sepsis. Shock. 2016;45:411–418. [DOI] [PubMed] [Google Scholar]
- 89.Frauwirth KA, Riley JL, Harris MH, et al. The CD28 signaling pathway regulates glucose metabolism. Immunity. 2002;16:769–777. [DOI] [PubMed] [Google Scholar]
- 90.Waltz P, Carchman E, Gomez H, et al. Sepsis results in an altered renal metabolic and osmolyte profile. J Surg Res. 2016;202:8–12. [DOI] [PubMed] [Google Scholar]
- 91.Kashani K, Al-Khafaji A, Ardiles T, et al. Discovery and validation of cell cycle arrest biomarkers in human acute kidney injury. Crit Care. 2013;17: R25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Murray PT, Mehta RL, Shaw A, et al. Potential use of biomarkers in acute kidney injury: report and summary of recommendations from the 10th Acute Dialysis Quality Initiative consensus conference. Kidney Int. 2014;85:513–521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Bagshaw SM, Bennett M, Haase M, et al. Plasma and urine neutrophil gelatinase-associated lipocalin in septic versus non-septic acute kidney injury in critical illness. Intensive Care Med. 2010;36:452–461. [DOI] [PubMed] [Google Scholar]
- 94.Srisawat N, Murugan R, Lee M, et al. Plasma neutrophil gelatinase-associated lipocalin predicts recovery from acute kidney injury following community-acquired pneumonia. Kidney Int. 2011;80: 545–552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Tu Y, Wang H, Sun R, et al. Urinary netrin-1 and KIM-1 as early biomarkers for septic acute kidney injury. Ren Fail. 2014;36:1559–1563. [DOI] [PubMed] [Google Scholar]
- 96.Doi K, Noiri E, Maeda-Mamiya R, et al. Urinary L-type fatty acid-binding protein as a new biomarker of sepsis complicated with acute kidney injury. Crit Care Med. 2010;38:2037–2042. [DOI] [PubMed] [Google Scholar]
- 97.Nakamura T, Sugaya T, Koide H. Urinary liver-type fatty acid-binding protein in septic shock: effect of polymyxin B-immobilized fiber hemoperfusion. Shock. 2009;31:454–459. [DOI] [PubMed] [Google Scholar]
- 98.Honore PM, Nguyen HB, Gong M, et al. Urinary tissue inhibitor of metalloproteinase-2 and insulin-like growth factor-binding protein 7 for risk stratification of acute kidney injury in patients with sepsis. Crit Care Med. 2016;44:1851–1860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Ebihara I, Hirayama K, Nagai M, et al. Angiopoietin balance in septic shock patients with acute kidney injury: effects of direct hemoperfusion with polymyxin B-immobilized fiber. Ther Apher Dial. 2016;20:368–375. [DOI] [PubMed] [Google Scholar]
- 100.Robinson-Cohen C, Katz R, Price BL, et al. Association of markers of endothelial dysregulation Ang1 and Ang2 with acute kidney injury in critically ill patients. Crit Care. 2016;20:207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Kumpers P, Hafer C, David S, et al. Angiopoietin-2 in patients requiring renal replacement therapy in the ICU: relation to acute kidney injury, multiple organ dysfunction syndrome and outcome. Intensive Care Med. 2010;36:462–470. [DOI] [PubMed] [Google Scholar]
- 102.Yu WK, McNeil JB, Wickersham NE, et al. Vascular endothelial cadherin shedding is more severe in sepsis patients with severe acute kidney injury. Crit Care. 2019;23:18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Katayama S, Nunomiya S, Koyama K, et al. Markers of acute kidney injury in patients with sepsis: the role of soluble thrombomodulin. Crit Care. 2017;21:229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Chawla LS, Seneff MG, Nelson DR, et al. Elevated plasma concentrations of IL-6 and elevated APACHE II score predict acute kidney injury in patients with severe sepsis. Clin J Am Soc Nephrol. 2007;2:22–30. [DOI] [PubMed] [Google Scholar]
- 105.Dai X, Zeng Z, Fu C, et al. Diagnostic value of neutrophil gelatinase-associated lipocalin, cystatin C, and soluble triggering receptor expressed on myeloid cells-1 in critically ill patients with sepsis-associated acute kidney injury. Crit Care. 2015;19:223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Su L, Xie L, Liu D. Urine sTREM-1 may be a valuable biomarker in diagnosis and prognosis of sepsis-associated acute kidney injury. Crit Care. 2015;19:281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Su LX, Feng L, Zhang J, et al. Diagnostic value of urine sTREM-1 for sepsis and relevant acute kidney injuries: a prospective study. Crit Care. 2011;15:R250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Haase M, Bellomo R, Devarajan P, et al. Accuracy of neutrophil gelatinase-associated lipocalin (NGAL) in diagnosis and prognosis in acute kidney injury: a systematic review and meta-analysis. Am J Kidney Dis. 2009;54:1012–1024. [DOI] [PubMed] [Google Scholar]
- 109.Haase M, Devarajan P, Haase-Fielitz A, et al. The outcome of neutrophil gelatinase-associated lipocalin-positive subclinical acute kidney injury: a multicenter pooled analysis of prospective studies. J Am Coll Cardiol. 2011;57:1752–1761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Di Nardo M, Ficarella A, Ricci Z, et al. Impact of severe sepsis on serum and urinary biomarkers of acute kidney injury in critically ill children: an observational study. Blood Purif. 2013;35:172–176. [DOI] [PubMed] [Google Scholar]
- 111.Wheeler DS, Devarajan P, Ma Q, et al. Serum neutrophil gelatinase-associated lipocalin (NGAL) as a marker of acute kidney injury in critically ill children with septic shock. Crit Care Med. 2008;36:1297–1303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Aydogdu M, Gursel G, Sancak B, et al. The use of plasma and urine neutrophil gelatinase associated lipocalin (NGAL) and cystatin C in early diagnosis of septic acute kidney injury in critically ill patients. Dis Markers. 2013;34:237–246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Bell M, Larsson A, Venge P, et al. Assessment of cell-cycle arrest biomarkers to predict early and delayed acute kidney injury. Dis Markers. 2015;2015:158658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Bolignano D, Lacquaniti A, Coppolino G, et al. Neutrophil gelatinase-associated lipocalin reflects the severity of renal impairment in subjects affected by chronic kidney disease. Kidney Blood Press Res. 2008;31:255–258. [DOI] [PubMed] [Google Scholar]
- 115.Bolignano D, Lacquaniti A, Coppolino G, et al. Neutrophil gelatinase-associated lipocalin (NGAL) and progression of chronic kidney disease. Clin J Am Soc Nephrol. 2009;4:337–344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Shao X, Tian L, Xu W, et al. Diagnostic value of urinary kidney injury molecule 1 for acute kidney injury: a meta-analysis. PLoS One. 2014;9: e84131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Bihorac A, Chawla LS, Shaw AD, et al. Validation of cell-cycle arrest biomarkers for acute kidney injury using clinical adjudication. Am J Respir Crit Care Med. 2014;189:932–939. [DOI] [PubMed] [Google Scholar]
- 118.Hoste EA, McCullough PA, Kashani K, et al. Derivation and validation of cutoffs for clinical use of cell cycle arrest biomarkers. Nephrol Dial Transplant. 2014;29:2054–2061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Bagshaw SM, Lapinsky S, Dial S, et al. Acute kidney injury in septic shock: clinical outcomes and impact of duration of hypotension prior to initiation of antimicrobial therapy. Intensive Care Med. 2009;35:871–881. [DOI] [PubMed] [Google Scholar]
- 120.Luther MK, Timbrook TT, Caffrey AR, et al. Vancomycin plus piperacillin-tazobactam and acute kidney injury in adults: a systematic review and meta-analysis. Crit Care Med. 2018;46:12–20. [DOI] [PubMed] [Google Scholar]
- 121.ARISE Investigators, ANZICS Clinical Trials Group, Peake SL, et al. Goal-directed resuscitation for patients with early septic shock. N Engl J Med. 2014;371:1496–1506. [DOI] [PubMed] [Google Scholar]
- 122.Mouncey PR, Osborn TM, Power GS, et al. Trial of early, goal-directed resuscitation for septic shock. N Engl J Med. 2015;372:1301–1311. [DOI] [PubMed] [Google Scholar]
- 123.Yunos NM, Bellomo R, Hegarty C, et al. Association between a chloride-liberal vs chloride-restrictive intravenous fluid administration strategy and kidney injury in critically ill adults. JAMA. 2012;308:1566–1572. [DOI] [PubMed] [Google Scholar]
- 124.Yunos NM, Bellomo R, Glassford N, et al. Chloride-liberal vs. chloride-restrictive intravenous fluid administration and acute kidney injury: an extended analysis. Intensive Care Med. 2015;41:257–264. [DOI] [PubMed] [Google Scholar]
- 125.Raghunathan K, Bonavia A, Nathanson BH, et al. Association between initial fluid choice and subsequent in-hospital mortality during the resuscitation of adults with septic shock. Anesthesiology. 2015;123: 1385–1393. [DOI] [PubMed] [Google Scholar]
- 126.Young P, Bailey M, Beasley R, et al. Effect of a buffered crystalloid solution vs saline on acute kidney injury among patients in the intensive care unit: the SPLIT randomized clinical trial. JAMA. 2015;314: 1701–1710. [DOI] [PubMed] [Google Scholar]
- 127.Self WH, Semler MW, Wanderer JP, et al. Balanced crystalloids versus saline in noncritically ill adults. N Engl J Med. 2018;378:819–828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Semler MW, Self WH, Wanderer JP, et al. Balanced crystalloids versus saline in critically ill adults. N Engl J Med. 2018;378:829–839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Wang N, Jiang L, Zhu B, et al. Fluid balance and mortality in critically ill patients with acute kidney injury: a multicenter prospective epidemiological study. Crit Care. 2015;19:371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Perner A, Haase N, Guttormsen AB, et al. Hydroxyethyl starch 130/0.42 versus Ringer’s acetate in severe sepsis. N Engl J Med. 2012;367:124–134. [DOI] [PubMed] [Google Scholar]
- 131.Myburgh JA, Finfer S, Bellomo R, et al. Hydroxyethyl starch or saline for fluid resuscitation in intensive care. N Engl J Med. 2012;367:1901–1911. [DOI] [PubMed] [Google Scholar]
- 132.Zarychanski R, Abou-Setta AM, Turgeon AF, et al. Association of hydroxyethyl starch administration with mortality and acute kidney injury in critically ill patients requiring volume resuscitation: a systematic review and meta-analysis. JAMA. 2013;309:678–688. [DOI] [PubMed] [Google Scholar]
- 133.Bayer O, Reinhart K, Kohl M, et al. Effects of fluid resuscitation with synthetic colloids or crystalloids alone on shock reversal, fluid balance, and patient outcomes in patients with severe sepsis: a prospective sequential analysis. Crit Care Med. 2012;40:2543–2551. [DOI] [PubMed] [Google Scholar]
- 134.Caironi P, Tognoni G, Masson S, et al. Albumin replacement in patients with severe sepsis or septic shock. N Engl J Med. 2014;370: 1412–1421. [DOI] [PubMed] [Google Scholar]
- 135.Finfer S, McEvoy S, Bellomo R, et al. Impact of albumin compared to saline on organ function and mortality of patients with severe sepsis. Intensive Care Med. 2011;37:86–96. [DOI] [PubMed] [Google Scholar]
- 136.Frenette AJ, Bouchard J, Bernier P, et al. Albumin administration is associated with acute kidney injury in cardiac surgery: a propensity score analysis. Crit Care. 2014;18:602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Schortgen F, Girou E, Deye N, et al. The risk associated with hyperoncotic colloids in patients with shock. Intensive Care Med. 2008;34:2157–2168. [DOI] [PubMed] [Google Scholar]
- 138.Udeh CI, You J, Wanek MR, et al. Acute kidney injury in postoperative shock: is hyperoncotic albumin administration an unrecognized resuscitation risk factor? Perioper Med (Lond). 2018;7:29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Rhodes A, Evans LE, Alhazzani W, et al. Surviving sepsis campaign: international guidelines for management of sepsis and septic shock: 2016. Intensive Care Med. 2017;43:304–377. [DOI] [PubMed] [Google Scholar]
- 140.De Backer D, Biston P, Devriendt J, et al. Comparison of dopamine and norepinephrine in the treatment of shock. N Engl J Med. 2010;362:779–789. [DOI] [PubMed] [Google Scholar]
- 141.De Backer D, Aldecoa C, Njimi H, et al. Dopamine versus norepinephrine in the treatment of septic shock: a meta-analysis*. Crit Care Med. 2012;40:725–730. [DOI] [PubMed] [Google Scholar]
- 142.Gordon AC, Russell JA, Walley KR, et al. The effects of vasopressin on acute kidney injury in septic shock. Intensive Care Med. 2010;36:83–91. [DOI] [PubMed] [Google Scholar]
- 143.Gordon AC, Mason AJ, Thirunavukkarasu N, et al. Effect of early vasopressin vs norepinephrine on kidney failure in patients with septic shock: the VANISH randomized clinical trial. JAMA. 2016;316:509–518. [DOI] [PubMed] [Google Scholar]
- 144.Asfar P, Meziani F, Hamel JF, et al. High versus low blood-pressure target in patients with septic shock. N Engl J Med. 2014;370:1583–1593. [DOI] [PubMed] [Google Scholar]
- 145.Seabra VF, Balk EM, Liangos O, et al. Timing of renal replacement therapy initiation in acute renal failure: a meta-analysis. Am J Kidney Dis. 2008;52:272–284. [DOI] [PubMed] [Google Scholar]
- 146.Karvellas CJ, Farhat MR, Sajjad I, et al. A comparison of early versus late initiation of renal replacement therapy in critically ill patients with acute kidney injury: a systematic review and meta-analysis. Crit Care. 2011;15: R72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Gaudry S, Hajage D, Schortgen F, et al. Initiation strategies for renal-replacement therapy in the intensive care unit. N Engl J Med. 2016;375: 122–133. [DOI] [PubMed] [Google Scholar]
- 148.Zarbock A, Kellum JA, Schmidt C, et al. Effect of early vs delayed initiation of renal replacement therapy on mortality in critically ill patients with acute kidney injury: the ELAIN randomized clinical trial. JAMA. 2016;315:2190–2199. [DOI] [PubMed] [Google Scholar]
- 149.Barbar SD, Clere-Jehl R, Bourredjem A, et al. Timing of renal-replacement therapy in patients with acute kidney injury and sepsis. N Engl J Med. 2018;379:1431–1442. [DOI] [PubMed] [Google Scholar]
- 150.Gaudry S, Hajage D, Schortgen F, et al. Timing of renal support and outcome of septic shock and acute respiratory distress syndrome: a post hoc analysis of the AKIKI randomized clinical trial. Am J Respir Crit Care Med. 2018;198:58–66. [DOI] [PubMed] [Google Scholar]
- 151.Meersch M, Kullmar M, Schmidt C, et al. Long-term clinical outcomes after early initiation of RRT in critically ill patients with AKI. J Am Soc Nephrol. 2018;29:1011–1019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Smith OM, Wald R, Adhikari NK, et al. Standard Versus Accelerated Initiation of Renal Replacement Therapy in Acute Kidney Injury (STARRT-AKI): study protocol for a randomized controlled trial. Trials. 2013;14:320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Morgera S, Rocktaschel J, Haase M, et al. Intermittent high permeability hemofiltration in septic patients with acute renal failure. Intensive Care Med. 2003;29:1989–1995. [DOI] [PubMed] [Google Scholar]
- 154.Morgera S, Slowinski T, Melzer C, et al. Renal replacement therapy with high-cutoff hemofilters: impact of convection and diffusion on cytokine clearances and protein status. Am J Kidney Dis. 2004;43:444–453. [DOI] [PubMed] [Google Scholar]
- 155.Morgera S, Haase M, Kuss T, et al. Pilot study on the effects of high cutoff hemofiltration on the need for norepinephrine in septic patients with acute renal failure. Crit Care Med. 2006;34:2099–2104. [DOI] [PubMed] [Google Scholar]
- 156.Haase M, Bellomo R, Baldwin I, et al. Hemodialysis membrane with a high-molecular-weight cutoff and cytokine levels in sepsis complicated by acute renal failure: a phase 1 randomized trial. Am J Kidney Dis. 2007;50:296–304. [DOI] [PubMed] [Google Scholar]
- 157.Chelazzi C, Villa G, D’Alfonso MG, et al. Hemodialysis with high cut-off hemodialyzers in patients with multi-drug resistant gram-negative sepsis and acute kidney injury: a retrospective, case-control study. Blood Purif. 2016;42:186–193. [DOI] [PubMed] [Google Scholar]
- 158.Kade G, Lubas A, Rzeszotarska A, et al. Effectiveness of high cut-off hemofilters in the removal of selected cytokines in patients during septic shock accompanied by acute kidney injury-preliminary study. Med Sci Monit. 2016;22:4338–4344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Villa G, Chelazzi C, Morettini E, et al. Organ dysfunction during continuous veno-venous high cut-off hemodialysis in patients with septic acute kidney injury: a prospective observational study. PLoS One. 2017;12:e0172039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Kogelmann K, Jarczak D, Scheller M, et al. Hemoadsorption by CytoSorb in septic patients: a case series. Crit Care. 2017;21:74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Friesecke S, Stecher SS, Gross S, et al. Extracorporeal cytokine elimination as rescue therapy in refractory septic shock: a prospective single-center study. J Artif Organs. 2017;20:252–259. [DOI] [PubMed] [Google Scholar]
- 162.Schadler D, Pausch C, Heise D, et al. The effect of a novel extracorporeal cytokine hemoadsorption device on IL-6 elimination in septic patients: a randomized controlled trial. PLoS One. 2017;12:e0187015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Shum HP, Chan KC, Kwan MC, et al. Application of endotoxin and cytokine adsorption haemofilter in septic acute kidney injury due to Gram-negative bacterial infection. Hong Kong Med J. 2013;19:491–497. [DOI] [PubMed] [Google Scholar]
- 164.Huang Z, Wang SR, Su W, et al. Removal of humoral mediators and the effect on the survival of septic patients by hemoperfusion with neutral microporous resin column. Ther Apher Dial. 2010;14:596–602. [DOI] [PubMed] [Google Scholar]
- 165.Yaroustovsky M, Abramyan M, Popok Z, et al. Preliminary report regarding the use of selective sorbents in complex cardiac surgery patients with extensive sepsis and prolonged intensive care stay. Blood Purif. 2009;28:227–233. [DOI] [PubMed] [Google Scholar]
- 166.Ala-Kokko TI, Laurila J, Koskenkari J. A new endotoxin adsorber in septic shock: observational case series. Blood Purif. 2011;32:303–309. [DOI] [PubMed] [Google Scholar]
- 167.Adamik B, Zielinski S, Smiechowicz J, et al. Endotoxin elimination in patients with septic shock: an observation study. Arch Immunol Ther Exp (Warsz). 2015;63:475–483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Livigni S, Bertolini G, Rossi C, et al. Efficacy of coupled plasma filtration adsorption (CPFA) in patients with septic shock: a multicenter randomised controlled clinical trial. BMJ Open. 2014;4:e003536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Pickkers P, Mehta RL, Murray PT, et al. Effect of human recombinant alkaline phosphatase on 7-day creatinine clearance in patients with sepsis-associated acute kidney injury: a randomized clinical trial. JAMA. 2018;320:1998–2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Chawla LS, Busse L, Brasha-Mitchell E, et al. Intravenous Angiotensin II for the Treatment of High-Output Shock (ATHOS trial): a pilot study. Crit Care. 2014;18:534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Tumlin JA, Murugan R, Deane AM, et al. Outcomes in patients with vasodilatory shock and renal replacement therapy treated with intravenous angiotensin II. Crit Care Med. 2018;46:949–957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Jones AE, Puskarich MA, Shapiro NI, et al. Effect of levocarnitine vs placebo as an adjunctive treatment for septic shock: the Rapid Administration of Carnitine in Sepsis (RACE) randomized clinical trial. JAMA Netw Open. 2018;1:e186076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Kellum JA, Pike F, Yealy DM, et al. Relationship between alternative resuscitation strategies, host response and injury biomarkers, and outcome in septic shock: analysis of the protocol-based care for early septic shock study. Crit Care Med. 2017;45:438–445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Villa G, Neri M, Bellomo R, et al. Nomenclature for renal replacement therapy and blood purification techniques in critically ill patients: practical applications. Crit Care. 2016;20:283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Ankawi G, Neri M, Zhang J, et al. Extracorporeal techniques for the treatment of critically ill patients with sepsis beyond conventional blood purification therapy: the promises and the pitfalls. Crit Care. 2018;22:262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Joannes-Boyau O, Rapaport S, Bazin R, et al. Impact of high volume hemofiltration on hemodynamic disturbance and outcome during septic shock. ASAIO J. 2004;50:102–109. [DOI] [PubMed] [Google Scholar]
- 177.Piccinni P, Dan M, Barbacini S, et al. Early isovolaemic haemofiltration in oliguric patients with septic shock. Intensive Care Med. 2006;32:80–86. [DOI] [PubMed] [Google Scholar]
- 178.Cornejo R, Downey P, Castro R, et al. High-volume hemofiltration as salvage therapy in severe hyperdynamic septic shock. Intensive Care Med. 2006;32:713–722. [DOI] [PubMed] [Google Scholar]
- 179.Cole L, Bellomo R, Journois D, et al. High-volume haemofiltration in human septic shock. Intensive Care Med. 2001;27:978–986. [DOI] [PubMed] [Google Scholar]
- 180.Boussekey N, Chiche A, Faure K, et al. A pilot randomized study comparing high and low volume hemofiltration on vasopressor use in septic shock. Intensive Care Med. 2008;34:1646–1653. [DOI] [PubMed] [Google Scholar]
- 181.Honore PM, Jamez J, Wauthier M, et al. Prospective evaluation of short-term, high-volume isovolemic hemofiltration on the hemodynamic course and outcome in patients with intractable circulatory failure resulting from septic shock. Crit Care Med. 2000;28:3581–3587. [DOI] [PubMed] [Google Scholar]
- 182.Ratanarat R, Brendolan A, Piccinni P, et al. Pulse high-volume haemofiltration for treatment of severe sepsis: effects on hemodynamics and survival. Crit Care. 2005;9:R294–R302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Joannes-Boyau O, Honore PM, Perez P, et al. High-Volume Versus Standard-Volume Haemofiltration for Septic Shock Patients With Acute Kidney Injury (IVOIRE study): a multicentre randomized controlled trial. Intensive Care Med. 2013;39:1535–1546. [DOI] [PubMed] [Google Scholar]
- 184.Zhang P, Yang Y, Lv R, et al. Effect of the intensity of continuous renal replacement therapy in patients with sepsis and acute kidney injury: a single-center randomized clinical trial. Nephrol Dial Transplant. 2012;27: 967–973. [DOI] [PubMed] [Google Scholar]
- 185.Clark E, Molnar AO, Joannes-Boyau O, et al. High-volume hemofiltration for septic acute kidney injury: a systematic review and meta-analysis. Crit Care. 2014;18:R7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Chung KK, Coates EC, Smith DJ Jr, et al. High-volume hemofiltration in adult burn patients with septic shock and acute kidney injury: a multicenter randomized controlled trial. Crit Care. 2017;21:289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Park JT, Lee H, Kee YK, et al. High-dose versus conventional-dose continuous venovenous hemodiafiltration and patient and kidney survival and cytokine removal in sepsis-associated acute kidney injury: a randomized controlled trial. Am J Kidney Dis. 2016;68:599–608. [DOI] [PubMed] [Google Scholar]
- 188.Palevsky PM, Zhang JH, O’Connor TZ, et al. Intensity of renal support in critically ill patients with acute kidney injury. N Engl J Med. 2008;359: 7–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Bellomo R, Cass A, Cole L, et al. Intensity of continuous renal-replacement therapy in critically ill patients. N Engl J Med. 2009;361: 1627–1638. [DOI] [PubMed] [Google Scholar]
- 190.Fayad AI, Buamscha DG, Ciapponi A. Intensity of continuous renal replacement therapy for acute kidney injury. Cochrane Database Syst Rev. 2016;10:CD010613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Shaw AR, Chaijamorn W, Mueller BA. We underdose antibiotics in patients on CRRT. Semin Dial. 2016;29:278–280. [DOI] [PubMed] [Google Scholar]
- 192.Vincent JL, Laterre PF, Cohen J, et al. A pilot-controlled study of a polymyxin B-immobilized hemoperfusion cartridge in patients with severe sepsis secondary to intra-abdominal infection. Shock. 2005;23: 400–405. [DOI] [PubMed] [Google Scholar]
- 193.Cruz DN, Antonelli M, Fumagalli R, et al. Early use of polymyxin B hemoperfusion in abdominal septic shock: the EUPHAS randomized controlled trial. JAMA. 2009;301:2445–2452. [DOI] [PubMed] [Google Scholar]
- 194.Payen DM, Guilhot J, Launey Y, et al. Early use of polymyxin B hemoperfusion in patients with septic shock due to peritonitis: a multicenter randomized control trial. Intensive Care Med. 2015;41:975–984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Dellinger RP, Bagshaw SM, Antonelli M, et al. Effect of targeted polymyxin B hemoperfusion on 28-day mortality in patients with septic shock and elevated endotoxin level: the EUPHRATES randomized clinical trial. JAMA. 2018;320:1455–1463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Klein DJ, Foster D, Walker PM, et al. Polymyxin B hemoperfusion in endotoxemic septic shock patients without extreme endotoxemia: a post hoc analysis of the EUPHRATES trial. Intensive Care Med. 2018;44: 2205–2212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Srisawat N, Tungsanga S, Lumlertgul N, et al. The effect of polymyxin B hemoperfusion on modulation of human leukocyte antigen DR in severe sepsis patients. Crit Care. 2018;22:279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Kellum JA, Fuhrman DY. The handwriting is on the wall: there will soon be a drug for AKI. Nat Rev Nephrol. 2019;15:65–66. [DOI] [PubMed] [Google Scholar]
- 199.Chen GD, Zhang JL, Chen YT, et al. Insulin alleviates mitochondrial oxidative stress involving upregulation of superoxide dismutase 2 and uncoupling protein 2 in septic acute kidney injury. Exp Ther Med. 2018;15:3967–3975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Chen H, Busse LW. Novel therapies for acute kidney injury. Kidney Int Rep. 2017;2:785–799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Khundmiri SJ, Asghar M, Khan F, et al. Effect of reversible and irreversible ischemia on marker enzymes of BBM from renal cortical PT subpopulations. Am J Physiol. 1997;273:F849–F856. [DOI] [PubMed] [Google Scholar]
- 202.Peters E, Masereeuw R, Pickkers P. The potential of alkaline phosphatase as a treatment for sepsis-associated acute kidney injury. Nephron Clin Pract. 2014;127:144–148. [DOI] [PubMed] [Google Scholar]
- 203.Heemskerk S, Masereeuw R, Moesker O, et al. Alkaline phosphatase treatment improves renal function in severe sepsis or septic shock patients. Crit Care Med. 2009;37:417–423. [DOI] [PubMed] [Google Scholar]
- 204.Pickkers P, Heemskerk S, Schouten J, et al. Alkaline phosphatase for treatment of sepsis-induced acute kidney injury: a prospective randomized double-blind placebo-controlled trial. Crit Care. 2012;16:R14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Denton KM, Anderson WP, Sinniah R. Effects of angiotensin II on regional afferent and efferent arteriole dimensions and the glomerular pole. Am J Physiol Regul Integr Comp Physiol. 2000;279: R629–R638. [DOI] [PubMed] [Google Scholar]
- 206.Bucher M, Ittner KP, Hobbhahn J, et al. Downregulation of angiotensin II type 1 receptors during sepsis. Hypertension. 2001;38:177–182. [DOI] [PubMed] [Google Scholar]
- 207.Lankadeva YR, Kosaka J, Evans RG, et al. Urinary oxygenation as a surrogate measure of medullary oxygenation during angiotensin II therapy in septic acute kidney injury. Crit Care Med. 2018;46:e41–e48. [DOI] [PubMed] [Google Scholar]
- 208.Wan L, Langenberg C, Bellomo R, et al. Angiotensin II in experimental hyperdynamic sepsis. Crit Care. 2009;13:R190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Khanna A, English SW, Wang XS, et al. Angiotensin II for the treatment of vasodilatory shock. N Engl J Med. 2017;377:419–430. [DOI] [PubMed] [Google Scholar]
- 210.Forni LG, Darmon M, Ostermann M, et al. Renal recovery after acute kidney injury. Intensive Care Med. 2017;43:855–866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Chawla LS, Bellomo R, Bihorac A, et al. Acute kidney disease and renal recovery: consensus report of the Acute Disease Quality Initiative (ADQI) 16 Workgroup. Nat Rev Nephrol. 2017;13:241–257. [DOI] [PubMed] [Google Scholar]
- 212.Chawla LS, Eggers PW, Star RA, et al. Acute kidney injury and chronic kidney disease as interconnected syndromes. N Engl J Med. 2014;371: 58–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.Basile DP, Bonventre JV, Mehta R, et al. Progression after AKI: understanding maladaptive repair processes to predict and identify therapeutic treatments. J Am Soc Nephrol. 2016;27:687–697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214.Kapitsinou PP, Jaffe J, Michael M, et al. Preischemic targeting of HIF prolyl hydroxylation inhibits fibrosis associated with acute kidney injury. Am J Physiol Renal Physiol. 2012;302:F1172–F1179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215.Leonard EC, Friedrich JL, Basile DP. VEGF-121 preserves renal microvessel structure and ameliorates secondary renal disease following acute kidney injury. Am J Physiol Renal Physiol. 2008;295: F1648–F1657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216.Liu M, Reddy NM, Higbee EM, et al. The Nrf2 triterpenoid activator, CDDO-imidazolide, protects kidneys from ischemia-reperfusion injury in mice. Kidney Int. 2014;85:134–141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217.Harel Z, Wald R, Bargman JM, et al. Nephrologist follow-up improves all-cause mortality of severe acute kidney injury survivors. Kidney Int. 2013;83:901–908. [DOI] [PubMed] [Google Scholar]
- 218.Siew ED, Peterson JF, Eden SK, et al. Outpatient nephrology referral rates after acute kidney injury. J Am Soc Nephrol. 2012;23:305–312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219.Kirwan CJ, Blunden MJ, Dobbie H, et al. Critically ill patients requiring acute renal replacement therapy are at an increased risk of long-term renal dysfunction, but rarely receive specialist nephrology follow-up. Nephron. 2015;129:164–170. [DOI] [PubMed] [Google Scholar]