Abstract
Purpose
To describe the 4-year metabolic follow-up results from the BLAST study.
Materials and Methods
Baseline hemoglobin A1c (HbA1c), weight, and waist circumference (WC) data were recorded in 185 men recruited for the BLAST randomised controlled trial (RCT) and erectile function (EF) scores were also available in an additional 48 men screened for the RCT. Intra/inter-group associations between these parameters and testosterone replacement therapy (TRT) were assessed at 1) end of the RCT (30 weeks), 2) open-label phase (82 weeks), and 3) final assessment via non-parametric statistics.
Results
Improvement in HbA1c and weight at the end of the RCT and open-label phase in men on TRT was not maintained long-term. The convergence in HbA1c could have been due to incentivised care with HbA1c targets. Interestingly those on TRT at final assessment required fewer anti-diabetic agents. The weight increase in routine care may have been due to changes in diabetes medication or an increase in lean muscle mass. WC continued to decrease in men on TRT indicating possible reduction in visceral fat. Improvement in EF scores continued with long-term TRT, this was abolished when TRT was discontinued.
Conclusions
This study hints at benefits in glycaemic control, weight and WC, and long-term RCTs studying mechanisms of benefit and clinical outcomes are necessary. Our results also show that EF scores continued to improve with long-term TRT, even beyond the 6 months that we previously reported in the BLAST RCT.
Keywords: Diabetes mellitus, type 2; Erectile dysfunction; Hypogonadism; Testosterone
INTRODUCTION
Hypogonadism (HG), characterised by low serum total testosterone (TT) levels (<12 nmol/L) and sexual symptoms, is prevalent in about 70% of men with type 2 diabetes (T2DM) [1]. Testosterone replacement therapy (TRT) has been associated with improved dyslipidaemia, weight, body mass index (BMI), waist circumference (WC), visceral fat mass, lean muscle mass, insulin levels, insulin resistance and inflammatory markers in men with and without T2DM [2,3]. The BLAST (Burntwood Lichfield Atherstone Sutton Tamworth) randomised controlled trial (RCT) carried out by our research group studied the association between TRT and changes in metabolic parameters and sexual function over 30 weeks [4,5]. It was seen that hemoglobin A1c (HbA1c), WC, and BMI improved at the end of the study when concomitant treatments were not altered. Erectile function (EF) was seen to improve in men with baseline TT<8 nmol/L [6,7]. Since then we followed-up the 857 men screened for the BLAST RCT (mean follow-up of 3.8±1.2 years) and showed that all-cause mortality was significantly and independently reduced with TRT and phosphodiesterase 5-inhibitor (PDE5I) use, the analysis adjusted for age and statin treatment [8,9].
In this analysis we aim to determine if the improvements seen and reported after 30 weeks of TRT in the BLAST RCT were maintained long-term when other treatments were potentially altered. We describe the effects of TRT over a mean 3.8 years of routine care on 1) HbA1c, weight, and WC in 185 men with baseline data taking part in the BLAST RCT, and 2) international index of erectile function (IIEF) EF scores in the above men as well as a further 48 men screened for the BLAST RCT with documented baseline IIEF EF scores.
MATERIALS AND METHODS
1. Patients and treatment
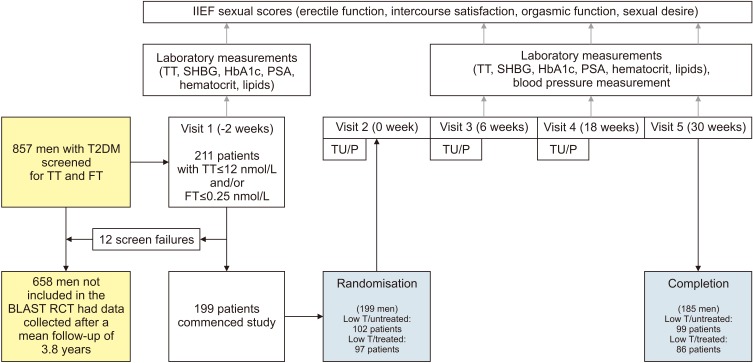
Eight hundred and fifty-seven men with T2DM from patient registers of 5 English Midlands practices were screened for TT and free testosterone (FT) during April 2007 to April 2009 as part of the BLAST RCT, a double blind randomised placebo-controlled study designed to investigate the effects of long acting testosterone undecanoate (TU) (1,000 mg) on sexual function scores and metabolic parameters [4,5]. The study included 199 men with TT≤12 nmol/L or FT≤0.25 nmol/L, with a confirmatory morning measurement taken in accordance with European Association of Urology guidelines, after a minimum of 2 weeks [10]. The 30 weeks of the RCT was followed by a 52-week open-label extension period with free TU provided. At that stage, a clinical decision was made by the health care practitioner as to whether TRT provided at NHS expense, was to be continued or not. Between April to September 2014, data were collected from the most recent diabetes attendance to assess changes from baseline in the 857 men screened for the BLAST RCT. Recruitment and study details of the 199 patients who took part in the BLAST RCT are shown in Fig. 1.
Fig. 1. Patient recruitment for the BLAST randomised controlled trial (RCT). Of the 857 men screened 199 men took part in the BLAST RCT evaluating changes in metabolic parameters and sexual function scores after 30 weeks of testosterone replacement therapy compared with placebo. IIEF: international index of erectile function, TT: total testosterone, SHBG: sex hormone-binding globulin, HbA1c: hemoglobin A1c, PSA: prostate specific antigen, T2DM: type 2 diabetes, FT: free testosterone, TU: testosterone undecanoate, P: placebo, T: testosterone.
Inclusion criteria for the BLAST intervention study were men aged 18 to 80 years with an initial finding of either a TT (on 2 separate occasions) ≤12 nmol/L or FT≤0.18 nmol/L with symptoms of HG defined by the Ageing Male Symptom score. Exclusion criteria included any past history of testosterone therapy, history of prostate, breast or hepatic cancer, abnormal digital rectal examination, severe symptoms of prostate hypertrophy or elevated prostate-specific antigen>4 ng/mL or haematocrit≥55%.
2. Study groups
The following men with data of weight, WC, HbA1c, and IIEF EF scores (Table 1).
Table 1. Baseline characteristics of the study group when classified by patient groups based on TRT.
| Baseline characteristic | Low testosterone/untreated | Low testosterone/treated | Low testosterone/treated/stopped | Low testosterone/treated/continuous |
|---|---|---|---|---|
| BLAST cohort: HbA1c, weight, and WC analyses | ||||
| Total patient | 99 | 86 | 48 | 38 |
| Age (y) | 61.9±9.4 | 61.7±10.6 | 64.4±9.0 | 58.3±10.8 |
| Follow-up (y) | 4.0±0.7 | 3.9±1.0 | 4.1±0.8 | 3.6±1.3 |
| HbA1c (%) | 7.5±1.2 | 7.6±1.1 | 7.6±1.1 | 7.6±1.4 |
| Weight (kg) | 100.4±17.8 | 100.2±18.8 | 96.6±18.6 | 104.7±17.7 |
| WC (cm) | 113.6±12.9 | 114.4±12.6 | 112.4±11.8 | 116.9±12.5 |
| BLAST cohort+men with IIEF EF data: IIEF EF analyses | ||||
| Total patient | 110 | 123 | 53 | 70 |
| IIEF EF | 11.7/10 (2–20) | 12.5/10 (4–22) | 12.2/10 (4–20) | 12.8/14 (5–24) |
Values are presented as nu±standard deviation, or mean/median (range).
TRT: testosterone replacement therapy, HbA1c: hemoglobin A1c, WC: waist circumference, IIEF: ational index of erectile function, EF: erectile function.
1) Patients taking part in the BLAST RCT, where data on HbA1c, weight, and WC were available during the 30-week trial period. All patients had TT≤12 nmol/L (346 ng/dL) or FT≤0.25 nmol/L (7.2 ng/dL). The randomised groups were termed:
(1) Low testosterone/untreated
(2) Low testosterone/treated (further stratified after 82 weeks [open-label period] into low testosterone/treated/stopped and low testosterone/treated/continuous based on whether TRT was discontinued or not)
2) Patients screened for the BLAST study with data on baseline EF scores obtained via IIEF EF questionnaires. This included the men taking part in the BLAST RCT and 48 men (part of the BLAST screened population) who had IIEF EF scores at baseline and at other times during follow-up. The men were stratified into the following groups:
(1) Low testosterone/untreated
(2) Low testosterone/treated (further stratified after 82 weeks [open-label period] into low testosterone/treated/stopped and low testosterone/treated/continuous based on whether TRT was discontinued or not)
3. Laboratory methods
Fasting morning TT was measured using the validated Roche common platform immunoassay. Serum sex hormone-binding globulin was analysed using a Roche Modular automated analyzer (Roche Diagnostics, Burgess Hill, UK). FT was calculated using the equations of Vermeulen et al [11]. HbA1c was measured in whole blood using a Tosoh G7 ion exchange high performance liquid chromatography analyzer (Tosoh Bioscience Ltd., Redditch, UK) the method standardized using International Federation of Clinical Chemistry reference material and the results were adjusted to give derived National Glycohaemoglobin Standardisation Programme units (%). All available laboratory data from the total cohort was obtained from primary and secondary care databases.
4. Ethics statement
The BLAST RCT were approved by the West Midlands Regional Ethics Committee (reference: 08/H1208/30), the National Institute for Health Research (Birmingham and the Black Country Comprehensive Local Research Park–RM&G reference: 1268), and Warwickshire Primary Care Trust (reference: WAR230909) with the long-term follow-up approved as an audit by all the appropriate Primary Care Trust Ethics Committees. The study is included in the European Union Clinical Trials Register (EudraCT 2008-000931-16) and conducted in accordance with the revised guidelines of the World Medical Association Declaration of Helsinki (https://www.wma.net/policies-post/wma-declaration-of-helsinki-ethical-principles-for-medical-research-involving-human-subjects/).
5. Statistics
Stata ver. 8 (StataCorp, College Station, TX, USA) was used for statistical analyses with age, follow-up, HbA1c, weight, WC analysed via non-parametric tests (sign-rank and rank-sum) in view of baseline values not being normally distributed. Differences in the IIEF EF scores were also analysed using non-parametric tests due to the scores being ordinal. Differences in drugs used for glycaemic control at final assessment between the patient groups were checked by chi square analysis.
RESULTS
Baseline age was similar in the low testosterone/treated (mean age, 61.7 years) and low testosterone/untreated (mean age, 61.9 years; p=0.67; rank-sum test) men randomised in the BLAST RCT. No significant difference in baseline age was observed with the addition of the 48 men with IIEF EF scores from the BLAST screening programme (low testosterone/treated: mean age, 61.2 years; low testosterone/untreated: mean age, 62.4 years; p=0.12; rank-sum test). Follow-up was similar in the groups randomised in the BLAST RCT (low testosterone/treated: mean follow-up, 3.9 years; low testosterone/untreated: mean follow-up, 4.0 years; p=0.47; rank-sum test). Follow-up was significantly different between the groups when the men from the BLAST screening were included (low testosterone/treated: mean follow-up, 3.4 years; low testosterone/untreated: mean follow-up, 3.9 years; p=0.010; rank-sum test).
1. Analysis of hemoglobin A1c, weight, and waist circumference
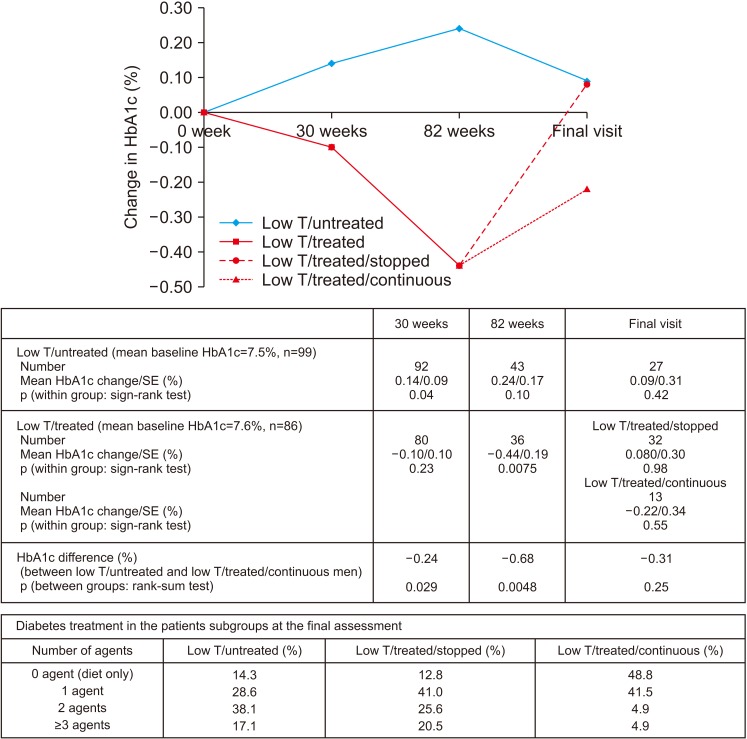
Fig. 2 shows the baseline HbA1c, and changes observed during the follow-up. At the end of 30 weeks (p=0.029, rank-sum test) and open-label phase (p=0.0048, rank-sum test), HbA1c was lower in the low testosterone/treated men compared to the low testosterone/untreated counterparts. Importantly, men in the low testosterone/treated group saw significant reduction HbA1c of 0.44% (p=0.0075, sign-rank test) at the end of the open-label phase. However, at final assessment, following a period of guideline-based diabetes care in primary care, no difference in HbA1c was observed; either within the low testosterone/untreated, low testosterone/treated/stopped and low testosterone/treated/continuous groups or between the low testosterone/untreated and low testosterone/treated/continuous groups. Fig. 2 also shows that the number of drugs used to control diabetes in the patient groups at final assessment varied (p<0.0001, chi-square). In the low testosterone/untreated group 14.3%, 28.6%, 38.1%, and 17.1% of men were on 0, 1, 2, and ≥3 drugs, respectively. The corresponding figures for the low testosterone/treated/stopped and low testosterone/treated/continuous groups were 12.8%, 41.0%, 25.6%, 20.5% and 48.8%, 41.5%, 4.9%, 4.9%, respectively. Thus, fewer men in the low testosterone/treated/continuous group were on ≥3 drugs for glycaemic control.
Fig. 2. Changes in hemoglobin A1c (HbA1c) (low testosterone [low T]/treated and low T/untreated men) during the 30-week BLAST randomised controlled trial, open-label (30–82 weeks), and normal care (post 82 weeks) phases. The low T/treated group were further stratified into low T/treated/stopped and low T/treated/continuous groups depending on whether testosterone replacement therapy was continued or not. The number of drugs prescribed to control diabetes in each group is also shown. SE: standard error.
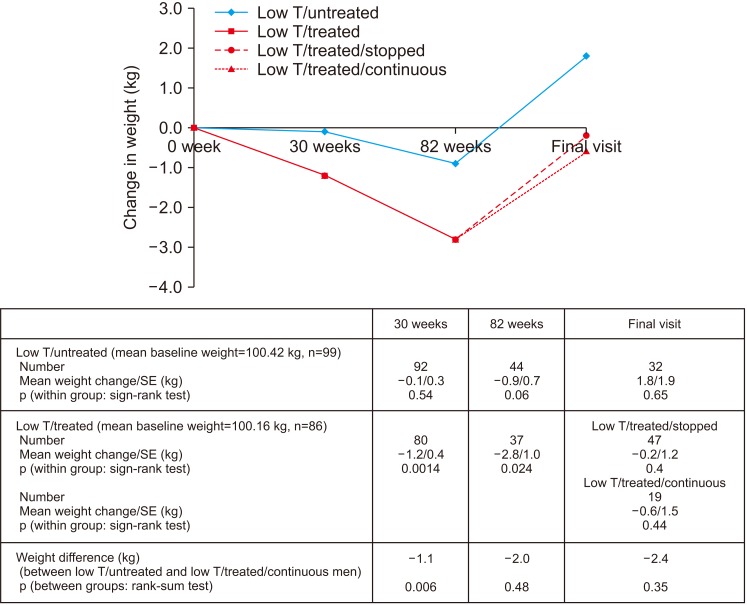
Fig. 3 shows significant reductions in weight in the low testosterone/treated group at the end of 30 weeks (p=0.0014, sign-rank test) and the open-label phase (p=0.024, sign-rank test). Weight reduction was significantly greater in the low testosterone/treated men (p=0.006, rank-sum test) than their low testosterone/untreated counterparts after 30 weeks, this significance was lost at the end of the open-label phase. No significant weight change was seen in the low testosterone/untreated men at any of the time points. At final assessment no difference in weight was observed within any of the individual groups (low testosterone/untreated, low testosterone/treated/stopped and low testosterone/treated/continuous) or between the low testosterone/untreated and low testosterone/treated/continuous groups.
Fig. 3. Change in weight (low testosterone [low T]/treated and low T/untreated men) during the 30-week BLAST randomised controlled trial, open-label (30–82 weeks), and normal care (post 82 weeks) phases. The low T/treated group were further stratified into low T/treated/stopped and low T/treated/continuous groups depending on whether testosterone replacement therapy was continued or not. SE: standard error.
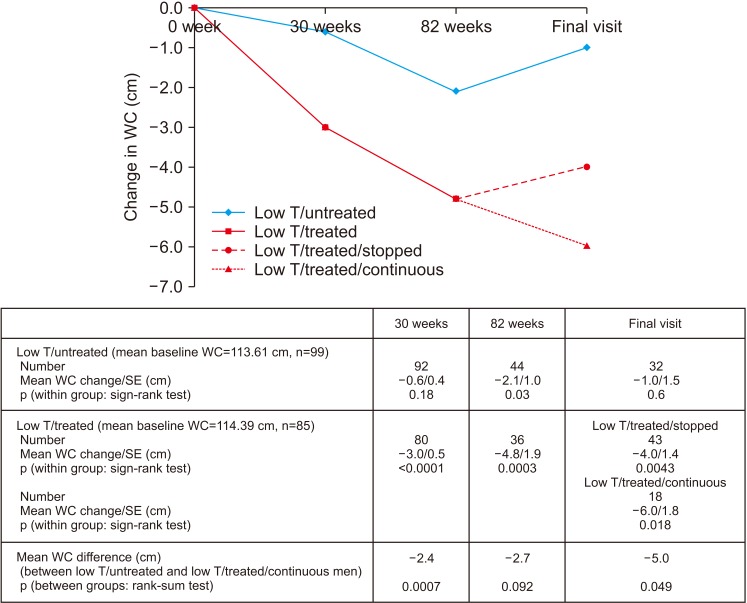
A significant reduction in WC (p<0.0001, sign-rank test) was observed in the low testosterone/treated men after 30 weeks of treatment with TU and this was significantly different (p=0.0007, rank-sum test) from that seen in the low testosterone/untreated men (Fig. 4). No change was seen in the Low testosterone/untreated arm treated with placebo. Reduction in WC (p=0.0003, sign-rank test) continued in the low testosterone/treated men during the open-label phase of the trial up to 82 weeks. Interestingly, the low testosterone/untreated men in the BLAST RCT showed a non-significant decrease in WC after the 30-week trial period and a significant decrease of 2.1 cm after the 82 weeks open-label phase (p=0.030, sign-rank test). However, at final assessment no change in WC was apparent in these men. However, WC significantly decreased at final assessment compared to baseline values in both the low testosterone/treated/stopped (mean WC decrease, 4.0 cm; p=0.0043; sign-rank test) and low testosterone/treated/continuous men (mean WC decrease, 6.0 cm; p=0.018; sign-rank test).
Fig. 4. Change in waist circumference (WC) (low testosterone [low T]/treated and low T/untreated men) during the 30-week BLAST randomised controlled trial, open-label (30–82 weeks), and normal care (post 82 weeks) phases. The low T/treated group were further stratified into low T/treated/stopped and low T/treated/continuous groups depending on whether testosterone replacement therapy was continued or not. SE: standard error.
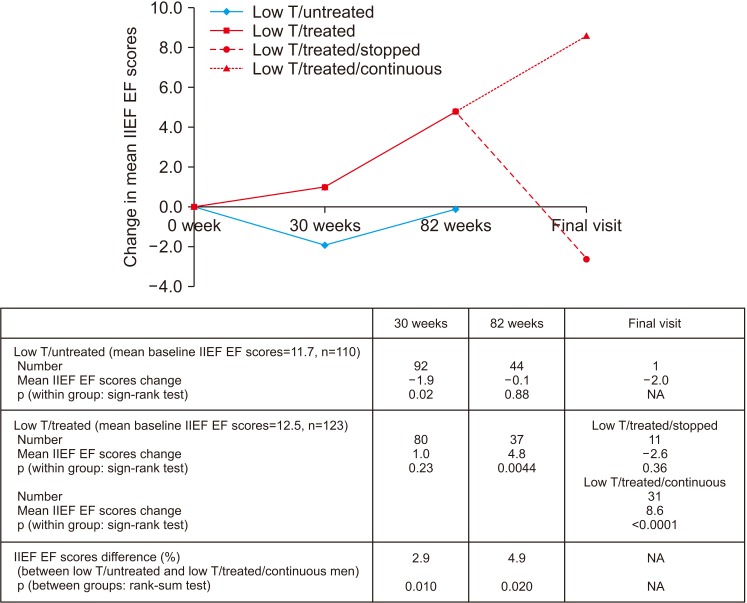
2. Analysis of erectile function
We could not statistically study within-group IIEF EF change in the low testosterone/untreated group post open-label phase as EF data was not routinely collected in primary care. Fig. 5 shows that IIEF EF significantly improved in the low testosterone/treated men during the BLAST RCT compared to the low testosterone/untreated men at 30 weeks (mean change, +2.9; p=0.010; rank-sum test) and at the end of the open-label phase (mean change, +4.9; p=0.020; rank-sum test). Interestingly intra-group analysis of the low testosterone/treated men showed significant improvements at the end of the open-label phase (mean change, +4.8; p=0.0044; sign-rank test). At the final assessment IIEF EF was significantly higher (mean IIEF EF difference, 8.6; p<0.0001; sign-rank test) in the 32 men who continued TRT (low testosterone/treated/continuous). Further, change in IIEF EF was significantly improved in the 32 men continued TRT compared to the 11 men in whom the TRT was discontinued (p=0.0012, rank-sum test). The significant increase in IIEF EF in the low testosterone/treated/continuous men appeared independent of PDE5I use; mean IIEF EF increase in the 20 men on TRT and PDE5I (9.1 [p=0.0001, sign-rank test]), mean IIEF EF increase in the 12 men on TRT, but not on PDE5I (7.7 [p=0.021, sign-rank test]). Contrastingly no significant change was observed in the 11 men in whom TRT was discontinued (low testosterone/treated/stopped).
Fig. 5. Changes in international index of erectile function (IIEF) erectile function (EF) (low testosterone [low T]/treated and low T/untreated men) during the 30 week BLAST randomised controlled trial (RCT), open-label (30–82 weeks), and normal care (post 82 weeks) phases in men recruited to the BLAST RCT and those screened with baseline IIEF EF scores. The low T/treated group were further stratified into low T/treated/stopped and low T/treated/continuous groups. SE: standard error.
3. Adverse events
We investigated adverse events in the 637 men screened for the BLAST RCT with TT≤12 nmol/L or FT≤0.18 nmol/L. There was no apparent difference in non-fatal Major Adverse Cardiac Events between the groups; 26 (19.8/1,000 years) and 10 (15.2/1,000 years) events in low testosterone/untreated and low testosterone/treated groups, respectively. Similarly prostaterelated outcomes were low and similar in the screened groups; 10 in low testosterone/untreated (7.6/1,000 years) and 4 in low testosterone/treated (6.1/1,000 years).
DISCUSSION
This is the first longitudinal long-term study (mean follow-up, 3.8 years) to describe the effect of TRT in men with T2DM undergoing routine primary care management of glycaemic control. Published data from registry studies with up to 8 years of TRT suggest progressive weight loss, BMI and WC reduction with improved glycaemic control in men with T2DM or pre-diabetes [2,3]. We were unable to wholly confirm all these findings at final assessment following routine care, although at the end of the open-label phase significant reductions in HbA1c, weight, and WC were observed.
Several studies have considered the impact of testosterone therapy on metabolism. Conclusions from meta-analyses of RCTs are that TRT improves lean muscle mass and moderately lowers insulin resistance [12,13]. Effects on HbA1c are variable, but some studies have reported benefit in men with poorly controlled diabetes [5]. Results appear improved when therapy was combined with appropriate diet and lifestyle intervention [14]. Variable results in lipid profiles have been demonstrated [12,13]. Similarly, some studies have shown reduction in inflammatory markers with others showing little or no change [13,15]. Interestingly some long term longitudinal studies for up to 10 years from a single centre have shown improvements in WC, BMI, and HbA1c [2,3].
Instead of providing clarification, meta-analyses have led to confusion possibly due to clustering of trials with differing duration and inclusion criteria [12,16]. Although RCTs could be considered to provide robust high-level evidence, many were of short duration, often between 12 to 26 weeks (mean, 34 weeks) [16]. Further, considerable heterogeneity in trial populations and treatment formulations existed [17,18].
In our study there are possible reasons for the initial HbA1c decrease not being maintained at long-term follow-up. T2DM is a progressive pathology [19] and over the follow-up any within-group improvement could be lost. Further, the care of diabetes in primary care is target driven and incentivised in England [20]. It is possible that the trend in HbA1c that we observed might be the result of ‘target driven’ treatment, where patients with HbA1c above target were treated actively whereas those below the target were left alone until their levels crossed the target. Thus, both groups could be expected to converge. This phenomenon was observed in a longitudinal study by Clarke et al [21] when patients with diabetes were followed-up over 9 years of target driven care. This phenomenon is important for long-term interventional studies conducted within a ‘target driven’ health care system. Our findings hint that men continued TRT required fewer drugs to control diabetes, this potentially could have a significant impact on healthcare.
Our data shows that weight appeared to fall significantly in the men on TRT until the end of the open-label phase, this being followed by a rise. Once again there are many possible reasons. Many agents used to treat T2DM are associated with weight gain; sulphonylureas, thiazolidinediones, and insulin. Although we had data on therapeutic agents the patients were prescribed at final assessment, treatment duration and baseline therapy were unavailable. It could be that measurement of weight and BMI miss the more significant improvement in lean muscle mass associated with increased muscle strength, physical fitness and reduced frailty [22]. Frailty, defined as “an age-related state of vulnerable health with a serious impact on physical dependence and quality of life” was strongly related to increased mortality and hospital admission [23]. The gathering evidence has led to the American College of Endocrinology recommending testosterone measurement in all men with T2DM, BMI>30 cm/kg2, or WC>102 cm [24]. Further, the recent American Urology Association Guidelines (2018) [25] recommends testosterone measurement in men with either T2DM or erectile dysfunction, adding that men with low testosterone be warned that they are at increased cardiovascular risk. In contrast the guidelines of the Endocrine Society (2018) [15] do not recommend screening of these groups or informing them that they may be of higher cardiovascular risk.
Significant reduction in WC was observed in men commenced on TRT. Fig. 4 hints that when TRT was discontinued the reduction in WC ceases. This is an important finding as central obesity is a major driver of the metabolic syndrome [26]. Thus, it is best to consider changes in weight and WC together. Studies have indicated that TRT is associated with a reduction in visceral fat and increase in lean muscle mass [12,14]. The testosterone trial confirmed positive benefits from TRT in terms of walking distance, physical function, mood and vitality, all of which might result in frailty in ageing men [27]. Further, a meta-analysis by Corona et al [13] involving 59 RCTs involving 3,029 treated and 2,049 controls men concluded that TRT was associated with reduced fat mass and increased lean muscle mass. They also suggested that TRT improved insulin resistance and glycaemic control, this association more marked in men with metabolic diseases.
There have been suggestions that the initial approach in obese men with HG should be weight loss strategies rather than TRT [12]. A recent double blind RCT found that, in such men, intensive diet and exercise combined with placebo reduced both fat and lean mass, whereas in the testosterone group, the weight loss was almost exclusively body fat [14]. Overall weight and BMI reduction was similar in both groups, but men treated with testosterone markedly increased their physical activity and grip strength. These findings alone suggest that TRT may play an important part in preservation of lean muscle mass, contributing to reduced frailty and improved metabolic health.
The progressive improvement in EF at final visit (nearly 4 years), independent of PDE5I use is perhaps the finding that has greatest practical benefit, suggesting that guidelines recommending short duration TRT would result in sub-optimal benefit [28]. It was also suggested that PDE5I and not TRT should be the front-line treatment in men with erectile dysfunction [27]. There is a case for combining both agents due to the benefit on EF being independent. Further, this independence extended to reduced all-cause mortality [8,9]. We previously reported that the benefits in EF following TRT were seen almost exclusively in men with baseline TT levels<8 nmol/L or FT<0.18 nmol/L [6,7], although patient numbers prohibited us from analysing this sub-group separately in this paper. A recent meta-analysis by Ponce et al [29] concluded that TRT improved sexual desire, EF, sexual satisfaction but increased erythrocytosis. However, there have been other studies demonstrating inconclusive results. Some of these have included a majority of men with minimally reduced testosterone levels with short-term follow-up [12]. Perhaps these variable results were due to a failure to appreciate the threshold for symptoms and signs of HG described by Zitzmann et al [30] or the importance of treatment duration. It is important that meta-analyses should stratify their cohort by such factors influencing outcomes.
Strengths and limitations
This study is a long-term follow-up of the BLAST RCT/screened population which demonstrated important findings during the RCT. The strengths of the long-term findings are that they represent what can be expected in routine care as opposed to the controlled RCT scenario which cannot be expected to continue long-term. However, there are study limitations stemming from the transition from RCT to routine care. There were considerable data missing, this could have been due to data not being recorded or patient care being transferred to other primary care practices. Thus, bias could have been introduced at the final assessment. We would have also wished to have good quality data on baseline treatment and duration of diabetes. Regardless our findings appear consistent with background data and the findings of the preceding RCT.
CONCLUSIONS
Our study provides some interesting findings, although adequately powered, long-term prospective confirmatory studies are required. It would be interesting to see if our finding of fewer agents needed to achieve HbA1c targets in men on TRT is confirmed. Further, the relationship between weight and WC changes must be investigated mechanistically as it could reflect improvement in lean muscle mass and visceral fat. Our findings also clearly demonstrate that improvement in EF continues long-term and premature cessation of TRT in men without adverse effects must be avoided in both routine care and RCTs.
ACKNOWLEDGEMENTS
The study was supported by a grant from Bayer to cover practice expenses. The sponsor had no role in the design of the study, statistical analysis, findings or preparation of manuscripts.
Administrative, technical, or material support was provided by Mrs Sally Hackett and Mrs Alice Blakey.
Footnotes
Disclosures: Professor Geoffrey Hackett has received honoraria for acting as a speaker for Bayer plc who provided the grant. Professor Sudarshan Ramachandran has received educational grants to attend meetings and honoraria for serving as a speaker for Besins Health Care Ltd. Professor Geoffrey Hackett has spoken at various national and international meetings on testosterone and PDE5I treatments in men. These companies and activities had no influence on this project. And other authors have no potential conflicts of interest to disclose.
Author Contribution: Full access to all the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis: Hackett G, Ramachandran S. Study concept and design: Hackett G, Cole N. Analysis and interpretation of data: Hackett G, Ramachandran S, Mulay A, Strange RC. Drafting of the manuscript: Hackett G, Cole N, Mulay A, Strange RC, Ramachandran S.
References
- 1.Hackett G, Cole N, Deshpande A, Popple M, Kennedy D, Wilkinson P. Biochemical hypodonadism and type 2 diabetes in primary care. Br J Diab Vasc Dis. 2009;9:226–231. [Google Scholar]
- 2.Saad F, Haider A, Doros G, Traish A. Long-term treatment of hypogonadal men with testosterone produces substantial and sustained weight loss. Obesity (Silver Spring) 2013;21:1975–1981. doi: 10.1002/oby.20407. [DOI] [PubMed] [Google Scholar]
- 3.Traish AM, Haider A, Doros G, Saad F. Long-term testosterone therapy in hypogonadal men ameliorates elements of the metabolic syndrome: an observational, long-term registry study. Int J Clin Pract. 2014;68:314–329. doi: 10.1111/ijcp.12319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hackett G, Cole N, Bhartia M, Kennedy D, Raju J, Wilkinson P, et al. The response to testosterone undecanoate in men with type 2 diabetes is dependent on achieving threshold serum levels (the BLAST study) Int J Clin Pract. 2014;68:203–215. doi: 10.1111/ijcp.12235. [DOI] [PubMed] [Google Scholar]
- 5.Hackett G, Cole N, Bhartia M, Kennedy D, Raju J, Wilkinson P. Testosterone replacement therapy improves metabolic parameters in hypogonadal men with type 2 diabetes but not in men with coexisting depression: the BLAST study. J Sex Med. 2014;11:840–856. doi: 10.1111/jsm.12404. [DOI] [PubMed] [Google Scholar]
- 6.Hackett G, Cole N, Saghir A, Jones P, Strange RC, Ramachandran S. Testosterone undecanoate improves sexual function in men with type 2 diabetes and severe hypogonadism: results from a 30-week randomized placebo-controlled study. BJU Int. 2016;118:804–813. doi: 10.1111/bju.13516. [DOI] [PubMed] [Google Scholar]
- 7.Hackett G, Cole N, Saghir A, Jones P, Strange RC, Ramachandran S. Testosterone replacement therapy: improved sexual desire and erectile function in men with type 2 diabetes following a 30-week randomized placebo-controlled study. Andrology. 2017;5:905–913. doi: 10.1111/andr.12399. [DOI] [PubMed] [Google Scholar]
- 8.Hackett G, Heald AH, Sinclair A, Jones PW, Strange RC, Ramachandran S. Serum testosterone, testosterone replacement therapy and all-cause mortality in men with type 2 diabetes: retrospective consideration of the impact of PDE5 inhibitors and statins. Int J Clin Pract. 2016;70:244–253. doi: 10.1111/ijcp.12779. [DOI] [PubMed] [Google Scholar]
- 9.Hackett G, Jones PW, Strange RC, Ramachandran S. Statin, testosterone and phosphodiesterase 5-inhibitor treatments and age related mortality in diabetes. World J Diabetes. 2017;8:104–111. doi: 10.4239/wjd.v8.i3.104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dohle GR, Arver S, Bettocchi C, Kliesch S, Punab M, de Ronde W. Guidelines on Male Hypogonadism [Internet] Arnhem: European Association of Urology; c2014. [cited 2012 Mar 6]. Available from: https://uroweb.org/wp-content/uploads/18-Male-Hypogonadism_LR.pdf. [Google Scholar]
- 11.Vermeulen A, Verdonck L, Kaufman JM. A critical evaluation of simple methods for the estimation of free testosterone in serum. J Clin Endocrinol Metab. 1999;84:3666–3672. doi: 10.1210/jcem.84.10.6079. [DOI] [PubMed] [Google Scholar]
- 12.Grossmann M, Hoermann R, Wittert G, Yeap BB. Effects of testosterone treatment on glucose metabolism and symptoms in men with type 2 diabetes and the metabolic syndrome: a systematic review and meta-analysis of randomized controlled clinical trials. Clin Endocrinol (Oxf) 2015;83:344–351. doi: 10.1111/cen.12664. [DOI] [PubMed] [Google Scholar]
- 13.Corona G, Giagulli VA, Maseroli E, Vignozzi L, Aversa A, Zitzmann M, et al. Therapy of endocrine disease: testosterone supplementation and body composition: results from a meta-analysis study. Eur J Endocrinol. 2016;174:R99–R116. doi: 10.1530/EJE-15-0262. [DOI] [PubMed] [Google Scholar]
- 14.Ng Tang Fui M, Prendergast LA, Dupuis P, Raval M, Strauss BJ, Zajac JD, et al. Effects of testosterone treatment on body fat and lean mass in obese men on a hypocaloric diet: a randomised controlled trial. BMC Med. 2016;14:153. doi: 10.1186/s12916-016-0700-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bhasin S, Brito JP, Cunningham GR, Hayes FJ, Hodis HN, Matsumoto AM, et al. Testosterone therapy in men with hypogonadism: an Endocrine Society clinical practice guideline. J Clin Endocrinol Metab. 2018;103:1715–1744. doi: 10.1210/jc.2018-00229. [DOI] [PubMed] [Google Scholar]
- 16.Corona G, Maseroli E, Rastrelli G, Isidori AM, Sforza A, Mannucci E, et al. Cardiovascular risk associated with testosterone-boosting medications: a systematic review and meta-analysis. Expert Opin Drug Saf. 2014;13:1327–1351. doi: 10.1517/14740338.2014.950653. [DOI] [PubMed] [Google Scholar]
- 17.Corona G, Rastrelli G, Morgentaler A, Sforza A, Mannucci E, Maggi M. Meta-analysis of results of testosterone therapy on sexual function based on international index of erectile function scores. Eur Urol. 2017;72:1000–1011. doi: 10.1016/j.eururo.2017.03.032. [DOI] [PubMed] [Google Scholar]
- 18.Borst SE, Yarrow JF. Injection of testosterone may be safer and more effective than transdermal administration for combating loss of muscle and bone in older men. Am J Physiol Endocrinol Metab. 2015;308:E1035–E1042. doi: 10.1152/ajpendo.00111.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.McEwan P, Bennett H, Qin L, Bergenheim K, Gordon J, Evans M. An alternative approach to modelling HbA1c trajectories in patients with type 2 diabetes mellitus. Diabetes Obes Metab. 2017;19:628–634. doi: 10.1111/dom.12865. [DOI] [PubMed] [Google Scholar]
- 20.DHSC 2003. Investing in General Practice: Implementing the New GMS Contract [Internet] London: Department of Health Social Care; [cited 2018 May 5]. Available from: http://www.nhsemployers.org/~/media/Employers/Documents/SiteCollectionDocuments/gms_contract_cd_130209.pdf. [Google Scholar]
- 21.Clarke EL, Richardson JR, Bhartia M, Kennedy DM, Milles JJ, Ramachandran S. Convergence of HbA1c values towards target in 272 primary care patients following nine years of target-driven care. Qual Prim Care. 2013;21:287–292. [PubMed] [Google Scholar]
- 22.Srinivas-Shankar U, Roberts SA, Connolly MJ, O'Connell MD, Adams JE, Oldham JA, et al. Effects of testosterone on muscle strength, physical function, body composition, and quality of life in intermediate-frail and frail elderly men: a randomized, double-blind, placebo-controlled study. J Clin Endocrinol Metab. 2010;95:639–650. doi: 10.1210/jc.2009-1251. [DOI] [PubMed] [Google Scholar]
- 23.Saad F, Röhrig G, von Haehling S, Traish A. Testosterone deficiency and testosterone treatment in older men. Gerontology. 2017;63:144–156. doi: 10.1159/000452499. [DOI] [PubMed] [Google Scholar]
- 24.Garvey WT, Mechanick JI, Brett EM, Garber AJ, Hurley DL, Jastreboff AM, et al. Reviewers of the AACE/ACE Obesity Clinical Practice Guidelines. AACE/ACE guidelines: American Association of Clinical Endocrinologists and American College of Endocrinology clinical practice guidelines for comprehensive medical care of patients with obesity. Endocr Pract. 2016;22:842–884. doi: 10.4158/EP161356.ESGL. [DOI] [PubMed] [Google Scholar]
- 25.Mulhall JP, Trost LW, Brannigan RE, Kurtz EG, Redmon JB, Chiles KA, et al. Evaluation and management of testosterone deficiency: AUA guideline. J Urol. 2018;200:423–432. doi: 10.1016/j.juro.2018.03.115. [DOI] [PubMed] [Google Scholar]
- 26.Shipman KE, Strange RC, Ramachandran S. Use of fibrates in the metabolic syndrome: a review. World J Diabetes. 2016;7:74–88. doi: 10.4239/wjd.v7.i5.74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Snyder PJ, Bhasin S, Cunningham GR, Matsumoto AM, Stephens-Shields AJ, Cauley JA, et al. Effects of testosterone treatment in older men. N Engl J Med. 2016;374:611–624. doi: 10.1056/NEJMoa1506119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bhasin S, Cunningham GR, Hayes FJ, Matsumoto AM, Snyder PJ, Swerdloff RS, et al. Testosterone therapy in men with androgen deficiency syndromes: an Endocrine Society clinical practice guideline. J Clin Endocrinol Metab. 2010;95:2536–2559. doi: 10.1210/jc.2009-2354. [DOI] [PubMed] [Google Scholar]
- 29.Ponce OJ, Spencer-Bonilla G, Alvarez-Villalobos N, Serrano V, Singh-Ospina N, Rodriguez-Gutierrez R, et al. The efficacy and adverse events of testosterone replacement therapy in hypogonadal men: a systematic review and meta-analysis of randomized, placebo-controlled trials. J Clin Endocrinol Metab. 2018;103:1745–1754. doi: 10.1210/jc.2018-00404. [DOI] [PubMed] [Google Scholar]
- 30.Zitzmann M, Faber S, Nieschlag E. Association of specific symptoms and metabolic risks with serum testosterone in older men. J Clin Endocrinol Metab. 2006;91:4335–4343. doi: 10.1210/jc.2006-0401. [DOI] [PubMed] [Google Scholar]