Abstract
Recent epidemiological research has indicated that men have increased health risks due to biological and social factors. Research in the area of men's health has been focused on disease events and subsequent disabilities. In future aging societies, more attention should be paid to the importance of men's health because a decreased quality of life and increased social burden are impending unless proper maneuvers are taken to slow the development and progression of morbidity through the use of preventive strategies. The adoption of a healthier lifestyle and the early identification and management of risk factors are very important and can be an initiative for prevention and for slowing the progression of morbidities with related quality of life issues. Males are rather vulnerable in terms of health, and conscious and active efforts are required to promote their health in an aging society. Here, we hope to shed light on the influence of lifestyle modifications and their clinical implications on men's health.
Keywords: Exercise, Healthy aging, Healthy lifestyle, Men's health, Testosterone
INTRODUCTION
Men's health tends to be focused on disease events and subsequent disabilities. Men have been reported to have more risks for health problems due to biological, environmental and social factors compared to women [1,2]. In fact, the average lifespan of men is known to be shorter than that of women, both under normal circumstances and during periods of severe famines and epidemics [3]. However, the importance of men's health has been less noticed and more diffusely recognized than women's health, which may be attributed partly to the fact that there is no definite period of drastic hormonal change in men [4].
The health paradigm has been to rule out issues, except for diseases, and to take a passive, wait-and-see approach [5]. However, the future paradigm of medicine should use the concepts of rule in and disease prevention. In the future aging society, more attention should be paid to the importance of men's health because of its effects on decreased quality of life and increased social burden, unless proper maneuvers are taken to slow the development and progression of morbidities using preventive strategies [6]. Thus, the adoption of healthier lifestyles and the early identification and management of risk factors are very important and can be an initiative for prevention as well as for slowing the progression of morbidities with related quality of life issues.
In this regard, we propose five key habits for a healthy lifestyle in men, especially after midlife. A healthy lifestyle must be considered from the perspective of health issues that are unique to men, as well as those that can be applied to the general population. This review aims to shed light on the influence of lifestyle modifications and their clinical implications for men's health. In this review, we suggest the five healthy lifestyle changes necessary for good health for men, using the following ‘ABCDE’ mnemonic: 1. Avoidance of the smoking habit and the moderation of alcohol consumption; 2. Body weight, with waist circumference management; 3. Control of blood pressure, glucose and cholesterol and the maintenance of cognitive health; 4. Doing regular exercise and keeping physically active; and 5. Emotional health maintenance, including stress management and good sleep. These maneuvers may have significant clinical implications for men's health, both in terms of general factors and in terms of factors that are unique to men.
LIFESTYLE AND CLINICAL IMPLICATIONS
1. Avoidance of smoking habits and moderation of alcohol consumption
Before adding good habits to the lifestyle, the avoidance of bad habits is a better choice and is a prerequisite to good health, especially for men. The importance of quitting smoking cannot be overstated [7]. It would be helpful to examine the physiological changes caused by smoking cessation during the first month. Blood oxygen increases within the first 12 hours after smoking cessation. After one week of smoking cessation, the circulation improves; additionally, after one month, lung function significantly recovers. In the long run, it has been reported that smoking cessation before the age of 35 would virtually prevent life-shortening [8]. Approximately 10 years after smoking cessation, stroke mortality declines by 42%, mortality from myocardial infarction is reduced by 57%, and cancer-related mortality is decreased by 23%, especially mortality due to lung cancer, which is decreased by 79% [9,10]. In addition, the chronic obstructive pulmonary disease hospitalization rate is also reduced by 34% [11]. In this context, the recommendation for lung cancer screening in Korea suggests that a low-dose chest computed tomography should be administered every year in high-risk patients aged 55 to 75 years who have a smoking history of 30 packs per year or more, and follow-up observations are suggested for 15 years after smoking cessation [12]. The incidence of erectile dysfunction, which is a men's health issue, is two times higher in smokers than in nonsmokers [13,14], although cigarette smoking may have a positive effect on testosterone levels [15]. It has been reported that the prevalence of erectile dysfunction is reduced after smoking cessation and becomes similar to that of nonsmokers. Smoking causes erectile dysfunction through a mechanism where either nicotine itself or nicotinic byproducts, such as free radicals, inhibit the production of nitric oxide, which is important for penile erection.
Chronic alcohol consumption not only is a cardinal risk factor for chronic liver disease but also is a trigger for gastrointestinal carcinogenesis and metabolic alterations [16,17]. As for men's health issue, alcohol dependence has been related with sexual dysfunction and erectile dysfunction [18,19]. However, moderate alcohol consumption has beneficial effects for vascular health, including raising high density lipoprotein cholesterol levels and improving insulin sensitivity, and may confer some protection on erectile dysfunction with the effect of androgen balance in men [18,20]. There has been no global consensus on the recommended safe limit of alcohol consumption. However, some guidelines have been provided by epidemiological studies or governmental health agencies. The limit of alcohol consumption that allows the maintenance of adequate blood pressure has been suggested to be approximately 30 g/d [21]. Some studies have indicated that alcohol consumption of 50 g/d or more is associated with an increased risk of over 50% of developing colorectal cancer [22]. In the US governmental guidelines, moderate alcohol ingestion has been defined as drinking up to two drinks/ d for men [23]. However, individual responses to alcohol vary and could be influenced by various factors, including age, ethnicity, body mass index (BMI), medications, alcohol use behavior, and the polymorphism of alcohol metabolism.
2. Body weight with waist circumference management
Obesity is associated with various disorders, such as metabolic syndrome, type 2 diabetes mellitus, cardiovascular disease (CVD) and some cancers, resulting in increased medical expenditures and substantial public health burdens [24,25,26]. The prevalence of overweight and obesity, defined as a BMI ≥23 kg/m2 for overweight and a BMI ≥25 kg/m2 for obesity, showed an upward trend among Korean men in an epidemiological study using the 1998 to 2009 Korean National Health and Nutrition Examination Survey (KNHANES), whereas the prevalence of overweight and obesity slightly decreased among women starting in 2001 [27]. In particular, metabolic syndrome represents a cluster of cardiometabolic risk factors, including central obesity, elevated blood pressure, impaired glucose metabolism, and atherogenic dyslipidemia [28], which can be implicated in erectile dysfunction, especially among individuals with CVD [29]. Based on the 2007–2008 KNHANES data, the higher the incomes of men are, the higher the prevalence of metabolic syndrome, while few women had metabolic syndrome [30]. Health awareness and lifestyle associated with socioeconomic status may be reflected in this result.
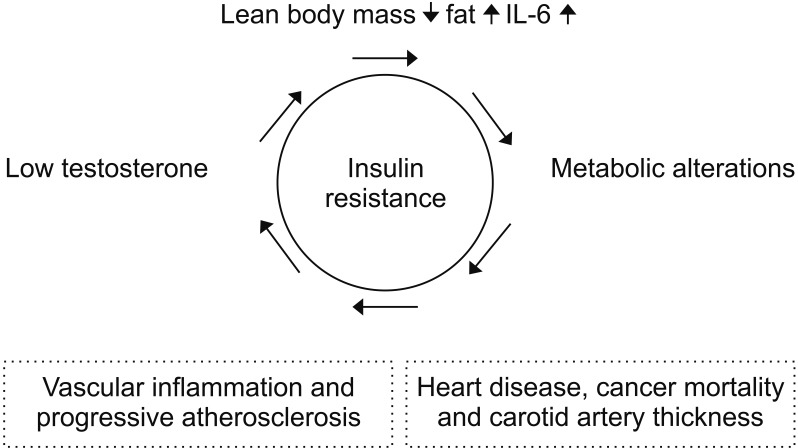
Although testosterone deficiency is common in the individuals with metabolic syndrome, the relationship between male hormone deficiency and metabolic alterations are controversial, as it is not clear whether there is a causal relationship or which issue develops first [31]. However, there have been many reports that hormone deficiency and metabolic alterations interact and can affect major health problems, such as CVD and cancer mortality (Fig. 1). Testosterone in men increases lean body mass and reduces fat, leptin, and interleukin-6, which may help improve insulin resistance [32,33,34]. Fasting plasma glucose and male hormones were inversely related in male participants, both with and without diabetes [35]. As the fasting blood glucose level increases, aldose reductase can be activated, which, in turn, increases the sorbitol concentration [36]. Directly, increased sorbitol can lead to higher levels of advanced glycation end-products and osmotic damage to lenses, liver, and kidneys. Indirectly, when aldose reductase is activated, the consumption of nicotinamide adenine dinucleotide phosphate (NADPH) may reduce the production of nitric oxide, which is important for vascular health in men, and it may decrease glutathione, which is a powerful antioxidant. Actually, weight reduction in overweight and obese men has some beneficial effect on maintaining erectile function and increasing testosterone levels [37,38]. However, sarcopenic change due to muscle mass reduction, has been closed related to low testosterone levels as well as metabolic alterations [39,40].
Fig. 1. Schematic view of the interrelationship between low testosterone levels and metabolic alterations and their clinical implications. IL: interleukin.
For men, estrogen, as well as testosterone, can be a hidden health issue. Previous studies have demonstrated that mortality tends to be U-shaped when estradiol concentrations are divided into quintiles in males [41]. Tivesten et al [42] also found that male hormones correlated inversely with mortality and that female hormones correlated with mortality, showing a reverse J shape in the same subjects. Estrogen in men is primarily produced by the aromatization of testosterone in adipocytes; therefore, it is necessary to maintain a proper weight with a proper waist circumference.
3. Control of blood pressure, glucose and cholesterol and the maintenance of cognitive health
Data from the US Centers for Disease Control and Prevention in 2013 shows that the incidence of brain degenerative diseases, such as Alzheimer's disease, is increasing, as are those for cancer and CVDs, which are well-known causes of older men's deaths. The CVD has been identified as a leading cause of premature mortality among middle-aged and elderly individuals in both developing and developed countries. In fact, CVD and many cancers share some of the same pathogenic factors, such as chronic inflammation and metabolic alterations, including elevated blood pressure, high blood glucose, and dyslipidemia [43]. Imagine a man in his mid-50s who does not smoke, has a mild degree of hyperlipidemia and prehypertension but does not take any medication. He has a moderate risk, with his 10 year-heart attack risk exceeding 10%, according to the calculator provided by the American Heart Association and American College of Cardiology. Recently, the use of statins or aspirin has been proposed to prevent future CVD morbidity and mortality based on the Framingham risk estimation of a 10-year CVD event [44,45]. Thus, it is imperative to have adequate prevention and control of metabolic alterations even before getting diagnosed with the disease.
In addition, more care and attention appears to be required for cognitive health, especially in regards to vascular dementia and Alzheimer's disease. The proportion of dementia in elderly people will increase from 9.8% in 2015 to 15.1% in 2050, and dementia management costs will exceed defense costs based on reports from the National Institute of Dementia in Korea. In 2017, the Lancet Commission on Dementia reported that it is possible to reduce the incidence of dementia by approximately 35% if only nine lifestyle factors, including the control of blood pressure and glucose levels, smoking cessation, exercise, and education, are controlled in advance [46]. A pilot study of patients with mild cognitive impairment has shown the preservation and partial enhancement of brain gray matter in a group that received omega-3 fatty acid supplementation, aerobic exercise, and cognitive stimulation compared with a group that received omega-3 fatty acid supplementation and no aerobic exercise [47]. A previous cohort study that followed 2,315 Finnish middle-aged men for 20 years showed that Alzheimer's disease incidence was reduced by 20% in a group that experienced the sauna 2 to 3 times a week and was reduced by 65% in a group that experienced the sauna 4 to 7 times a week compared to a group that experienced the sauna only one time a week [48]. The authors explained that relaxation, in addition to the circulation effects of the sauna, has a preventive effect. Also, low endogenous testosterone levels have been associated with reduced cognitive function in older men and testosterone replacement therapy may improve some aspects of cognitive function [49,50].
4. Doing regular exercise and keeping physically active
Many studies in the field of sports medicine have been conducted using a salivary hormone test to examine the immediate hormonal changes after exercise. A recent meta-analysis of studies performed to assess the hormone changes within 30 minutes after aerobic and resistance exercise showed a significant increase in testosterone [51]. In addition, the standard difference in means increased by 0.891 and 1.061 for aerobic and resistance exercise, respectively, compared to the baseline. However, the salivary testosterone to cortisol ratio, which is a surrogate anabolic indicator after exercise, showed no significant positive correlation.
With regards to the long-term effects of exercise, the baseline conditioning state and exercise intensity should be considered simultaneously. In a study that examined elderly men with a sedentary lifestyle, a 6-week period of conditioning exercises was followed by a 6-week period of high-intensity interval training [52]. Male hormones were measured at baseline, during the 6th week, and during the 12th week. Total testosterone increased at 6 weeks and 12 weeks, and free testosterone increased significantly only after 12 weeks. Unlike the acute phase reaction to exercise, serum cortisol did not increase but instead decreased slightly. Another study to evaluate major indicators before and after a 12-week exercise program for obese men showed that BMI, waist circumference, and lipid profile improved, and insulin levels decreased at 12 weeks [38]. Moreover, testosterone increased significantly, and central systolic blood pressure decreased. Exercise may have a positive effect on the metabolic index through the recovery of male hormones, as well as weight reduction. Furthermore, exercise can not only improve the efficacy of the testosterone replacement therapy, but also help to maintain the efficacy of the treatment [53,54]. For older men with sarcopenia or frailty, physical activity, especially resistance exercise, should be considered as a key intervention in multidisciplinary approach [55,56].
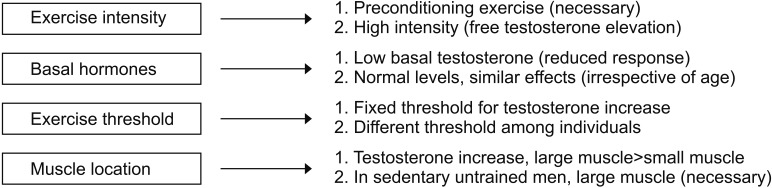
Let us take a closer look at the effects of exercise on male hormones (Fig. 2). First, in terms of exercise intensity, preconditioning exercise increases total testosterone and sex hormone-binding globulin, but it tends to raise free testosterone slightly. However, high-intensity exercise, in addition to preconditioning exercise, significantly increases free testosterone [52]. Second, in terms of the testosterone concentration, the lower the total testosterone levels are when you start an exercise, the fewer synergistic effects are observed after exercise. Men with a normal range of male hormones, even in the elderly, showed synergistic effects of male hormones after exercise that were similar to those observed in young men with a eugonadal state [57]. Third, in the case of the correlation between the exercise threshold and male hormones, male hormones did not increase when only 2 sets of 8 to 12 reps of arm exercise were performed. When both arm and leg exercises were performed at the same frequency, male hormones increased immediately after exercise [58]. This result suggests that there is a threshold of exercise required to promote the secretion of male hormones. Fourth, after the exercise, the acute phase reaction of male hormones peaked almost immediately after exercise and returned to the baseline level after 15 to 30 minutes. Some studies have reported that male hormone elevation can be maintained for up to 1 hour depending on the exercise session [57]. Fifth, for men who do not typically exercise, an exercise involving large muscle groups is essential to increase male hormones [58]. Last, cortisol after exercise decreases for low-intensity exercise but increases for moderate intensity exercise and tends to maintain its levels for longer than the rise time for male hormones. This may be a physiologic response to glycogenolysis and gluconeogenesis after exercise [57].
Fig. 2. Key considerations for proper exercise for men, with respect to male hormones.
5. Emotional health maintenance, including stress management and good sleep
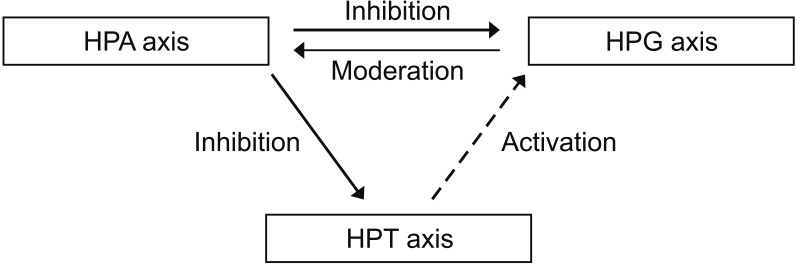
Emotional health should not be underestimated for men's health and must be assessed from an integrated hormonal standpoint. If men are under stressful conditions, the activation of the hypothalamus-pituitary-adrenal (HPA) axis inhibits testosterone, as observed in women; however, testosterone tends to relieve the stress pathway in men, unlike in women (Fig. 3). In animal studies using mice, it has been reported that male hormone levels decreased, and stress hormones increased in a gonadectomy group [59]. In terms of thyroid hormones, an activated HPA axis can inhibit the hypothalamus-pituitary-thyroid (HPT) axis, whereas low testosterone levels in men may decrease tissue levels of triiodothyronine without changing the levels of thyroid-stimulating hormone [60,61].
Fig. 3. Plausible integrative hormonal interactions among the hypothalamus-pituitary-adrenal-thyroid-gonad axis (HPA-T-G) axis.
According to the World Health Organization, depression is one of the largest contributors to years lived with a disability, with rates increasing 18% over the last decade. A depressive mood is very closed linked to lifestyle issues, such as impaired sleep, reduced physical activity and poor diet [62]. HPA imbalances, immune-inflammation and imbalances and neurotransmitter imbalances have been suggested as the underlying mechanisms. In particular, sleep disturbances have been related to vulnerability to stress, dysregulated appetite, insulin resistance, heart disease, and emotional problems, such as anxiety and depression.
CONCLUSIONS
While previous studies have identified reasons why women live longer than men in terms of sociological factors, recent epidemiological studies have indicated that biological factors could also have important effects. Males are rather vulnerable in terms of health, and conscious and active efforts are needed to promote their health in an aging society.
In this review, we summarized the five healthy lifestyle changes necessary for good health for men, which may have significant clinical implications for men's health, both in terms of general factors and in terms of factors that are unique to men.
Footnotes
Disclosure: The authors have no potential conflicts of interest to disclose.
- Research conception & design: Park B, Lee YJ.
- Data acquisition & drafting of the manuscript: Park B.
- Critical revision of the manuscript: Lee YJ.
- Approval of final manuscript: all authors.
References
- 1.Austad SN. Why women live longer than men: sex differences in longevity. Gend Med. 2006;3:79–92. doi: 10.1016/s1550-8579(06)80198-1. [DOI] [PubMed] [Google Scholar]
- 2.Barford A, Dorling D, Davey Smith G, Shaw M. Life expectancy: women now on top everywhere. BMJ. 2006;332:808. doi: 10.1136/bmj.332.7545.808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zarulli V, Barthold Jones JA, Oksuzyan A, Lindahl-Jacobsen R, Christensen K, Vaupel JW. Women live longer than men even during severe famines and epidemics. Proc Natl Acad Sci U S A. 2018;115:E832–E840. doi: 10.1073/pnas.1701535115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Tsujimura A. The relationship between testosterone deficiency and men's health. World J Mens Health. 2013;31:126–135. doi: 10.5534/wjmh.2013.31.2.126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Park B. Functional approach to chronic fatigue and energy homeostasis in perspective of adrenal hormones. J Korean Inst Funct Med. 2018;1:32–38. [Google Scholar]
- 6.Park BJ, Shim JY, Lee YJ, Lee JH, Lee HR. Inverse relationship between bioavailable testosterone and subclinical coronary artery calcification in non-obese Korean men. Asian J Androl. 2012;14:612–615. doi: 10.1038/aja.2012.19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mackay J. The global epidemiology of tobacco and related chronic diseases. Public Health. 2012;126:199–201. doi: 10.1016/j.puhe.2012.01.027. [DOI] [PubMed] [Google Scholar]
- 8.Centers for Disease Control and Prevention (CDC) Quitting smoking among adults: United States, 2001–2010. MMWR Morb Mortal Wkly Rep. 2011;60:1513–1519. [PubMed] [Google Scholar]
- 9.Song YM, Cho HJ. Risk of stroke and myocardial infarction after reduction or cessation of cigarette smoking: a cohort study in Korean men. Stroke. 2008;39:2432–2438. doi: 10.1161/STROKEAHA.107.512632. [DOI] [PubMed] [Google Scholar]
- 10.Song YM, Sung J, Cho HJ. Reduction and cessation of cigarette smoking and risk of cancer: a cohort study of Korean men. J Clin Oncol. 2008;26:5101–5106. doi: 10.1200/JCO.2008.17.0498. [DOI] [PubMed] [Google Scholar]
- 11.Godtfredsen NS, Vestbo J, Osler M, Prescott E. Risk of hospital admission for COPD following smoking cessation and reduction: a Danish population study. Thorax. 2002;57:967–972. doi: 10.1136/thorax.57.11.967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jang SH, Sheen S, Kim HY, Yim HW, Park BY, Kim JW, et al. The Korean guideline for lung cancer screening. J Korean Med Assoc. 2015;58:291–301. [Google Scholar]
- 13.DeLay KJ, Haney N, Hellstrom WJ. Modifying risk factors in the management of erectile dysfunction: a review. World J Mens Health. 2016;34:89–100. doi: 10.5534/wjmh.2016.34.2.89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.McVary KT, Carrier S, Wessells H. Smoking and erectile dysfunction: evidence based analysis. J Urol. 2001;166:1624–1632. [PubMed] [Google Scholar]
- 15.Zhao J, Leung JY, Lin SL, Schooling CM. Cigarette smoking and testosterone in men and women: a systematic review and meta-analysis of observational studies. Prev Med. 2016;85:1–10. doi: 10.1016/j.ypmed.2015.12.021. [DOI] [PubMed] [Google Scholar]
- 16.Park B, Lee HR, Lee YJ. Alcoholic liver disease: focus on prodromal gut health. J Dig Dis. 2016;17:493–500. doi: 10.1111/1751-2980.12375. [DOI] [PubMed] [Google Scholar]
- 17.Park BJ, Lee YJ, Lee HR. Chronic liver inflammation: clinical implications beyond alcoholic liver disease. World J Gastroenterol. 2014;20:2168–2175. doi: 10.3748/wjg.v20.i9.2168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jiann BP. Effect of alcohol consumption on the risk of erectile dysfunction. Urol Sci. 2010;21:163–168. [Google Scholar]
- 19.Arackal BS, Benegal V. Prevalence of sexual dysfunction in male subjects with alcohol dependence. Indian J Psychiatry. 2007;49:109–112. doi: 10.4103/0019-5545.33257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sarkola T, Eriksson CJ. Testosterone increases in men after a low dose of alcohol. Alcohol Clin Exp Res. 2003;27:682–685. doi: 10.1097/01.ALC.0000060526.43976.68. [DOI] [PubMed] [Google Scholar]
- 21.Moreira LB, Fuchs FD, Moraes RS, Bredemeier M, Duncan BB. Alcohol intake and blood pressure: the importance of time elapsed since last drink. J Hypertens. 1998;16:175–180. doi: 10.1097/00004872-199816020-00007. [DOI] [PubMed] [Google Scholar]
- 22.Fedirko V, Tramacere I, Bagnardi V, Rota M, Scotti L, Islami F, et al. Alcohol drinking and colorectal cancer risk: an overall and dose-response meta-analysis of published studies. Ann Oncol. 2011;22:1958–1972. doi: 10.1093/annonc/mdq653. [DOI] [PubMed] [Google Scholar]
- 23.US Department of Health and Human Services, US Department of Agriculture. Dietary guidelines for Americans, 2015–2020. Washington D.C.: US Department of Health and Human Services, US Department of Agriculture; 2015. [Google Scholar]
- 24.Després JP, Lemieux I. Abdominal obesity and metabolic syndrome. Nature. 2006;444:881–887. doi: 10.1038/nature05488. [DOI] [PubMed] [Google Scholar]
- 25.Logue J, Murray HM, Welsh P, Shepherd J, Packard C, Macfarlane P, et al. Obesity is associated with fatal coronary heart disease independently of traditional risk factors and deprivation. Heart. 2011;97:564–568. doi: 10.1136/hrt.2010.211201. [DOI] [PubMed] [Google Scholar]
- 26.Park MH, Falconer C, Viner RM, Kinra S. The impact of childhood obesity on morbidity and mortality in adulthood: a systematic review. Obes Rev. 2012;13:985–1000. doi: 10.1111/j.1467-789X.2012.01015.x. [DOI] [PubMed] [Google Scholar]
- 27.Kang HT, Shim JY, Lee HR, Park BJ, Linton JA, Lee YJ. Trends in prevalence of overweight and obesity in Korean adults, 1998-2009: the Korean National Health and Nutrition Examination Survey. J Epidemiol. 2014;24:109–116. doi: 10.2188/jea.JE20130017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Park B, Lee YJ. Metabolic syndrome and its components as risk factors for prolonged corrected QT interval in apparently healthy Korean men and women. J Clin Lipidol. 2018;12:1298–1304. doi: 10.1016/j.jacl.2018.07.004. [DOI] [PubMed] [Google Scholar]
- 29.Sanchez E, Pastuszak AW, Khera M. Erectile dysfunction, metabolic syndrome, and cardiovascular risks: facts and controversies. Transl Androl Urol. 2017;6:28–36. doi: 10.21037/tau.2016.10.01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Park SJ, Kang HT, Nam CM, Park BJ, Linton JA, Lee YJ. Sex differences in the relationship between socioeconomic status and metabolic syndrome: the Korean National Health and Nutrition Examination Survey. Diabetes Res Clin Pract. 2012;96:400–406. doi: 10.1016/j.diabres.2011.12.025. [DOI] [PubMed] [Google Scholar]
- 31.Salam R, Kshetrimayum AS, Keisam R. Testosterone and metabolic syndrome: the link. Indian J Endocrinol Metab. 2012;16 Suppl 1:S12–S19. doi: 10.4103/2230-8210.94248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lambert CP, Sullivan DH, Evans WJ. Effects of testosterone replacement and/or resistance training on interleukin-6, tumor necrosis factor alpha, and leptin in elderly men ingesting megestrol acetate: a randomized controlled trial. J Gerontol A Biol Sci Med Sci. 2003;58:165–170. doi: 10.1093/gerona/58.2.m165. [DOI] [PubMed] [Google Scholar]
- 33.Sih R, Morley JE, Kaiser FE, Perry HM, 3rd, Patrick P, Ross C. Testosterone replacement in older hypogonadal men: a 12-month randomized controlled trial. J Clin Endocrinol Metab. 1997;82:1661–1667. doi: 10.1210/jcem.82.6.3988. [DOI] [PubMed] [Google Scholar]
- 34.Wittert GA, Chapman IM, Haren MT, Mackintosh S, Coates P, Morley JE. Oral testosterone supplementation increases muscle and decreases fat mass in healthy elderly males with low-normal gonadal status. J Gerontol A Biol Sci Med Sci. 2003;58:618–625. doi: 10.1093/gerona/58.7.m618. [DOI] [PubMed] [Google Scholar]
- 35.Shin JY, Park EK, Park BJ, Shim JY, Lee HR. High-normal glucose levels in non-diabetic and pre-diabetic men are associated with decreased testosterone levels. Korean J Fam Med. 2012;33:152–156. doi: 10.4082/kjfm.2012.33.3.152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Chung SS, Ho EC, Lam KS, Chung SK. Contribution of polyol pathway to diabetes-induced oxidative stress. J Am Soc Nephrol. 2003;14:S233–S236. doi: 10.1097/01.asn.0000077408.15865.06. [DOI] [PubMed] [Google Scholar]
- 37.Wing RR, Rosen RC, Fava JL, Bahnson J, Brancati F, Gendrano Iii IN, et al. Effects of weight loss intervention on erectile function in older men with type 2 diabetes in the Look AHEAD trial. J Sex Med. 2010;7:156–165. doi: 10.1111/j.1743-6109.2009.01458.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kumagai H, Zempo-Miyaki A, Yoshikawa T, Tsujimoto T, Tanaka K, Maeda S. Lifestyle modification increases serum testosterone level and decrease central blood pressure in overweight and obese men. Endocr J. 2015;62:423–430. doi: 10.1507/endocrj.EJ14-0555. [DOI] [PubMed] [Google Scholar]
- 39.Zhang H, Lin S, Gao T, Zhong F, Cai J, Sun Y, et al. Association between sarcopenia and metabolic syndrome in middleaged and older non-obese adults: a systematic review and meta-analysis. Nutrients. 2018;10:E364. doi: 10.3390/nu10030364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Shin MJ, Jeon YK, Kim IJ. Testosterone and sarcopenia. World J Mens Health. 2018;36:192–198. doi: 10.5534/wjmh.180001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Jankowska EA, Rozentryt P, Ponikowska B, Hartmann O, Kustrzycka-Kratochwil D, Reczuch K, et al. Circulating estradiol and mortality in men with systolic chronic heart failure. JAMA. 2009;301:1892–1901. doi: 10.1001/jama.2009.639. [DOI] [PubMed] [Google Scholar]
- 42.Tivesten A, Vandenput L, Labrie F, Karlsson MK, Ljunggren O, Mellström D, et al. Low serum testosterone and estradiol predict mortality in elderly men. J Clin Endocrinol Metab. 2009;94:2482–2488. doi: 10.1210/jc.2008-2650. [DOI] [PubMed] [Google Scholar]
- 43.Koene RJ, Prizment AE, Blaes A, Konety SH. Shared risk factors in cardiovascular disease and cancer. Circulation. 2016;133:1104–1114. doi: 10.1161/CIRCULATIONAHA.115.020406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.US Preventive Services Task Force. Aspirin for the prevention of cardiovascular disease: U.S. Preventive Services Task Force recommendation statement. Ann Intern Med. 2009;150:396–404. doi: 10.7326/0003-4819-150-6-200903170-00008. [DOI] [PubMed] [Google Scholar]
- 45.Goff DC, Jr, Lloyd-Jones DM, Bennett G, Coady S, D'Agostino RB, Sr, Gibbons R, et al. 2013 ACC/AHA guideline on the assessment of cardiovascular risk: a report of the American College of Cardiology/American Heart Association Task Force on practice guidelines. J Am Coll Cardiol. 2014;63:2935–2959. doi: 10.1016/j.jacc.2013.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Livingston G, Sommerlad A, Orgeta V, Costafreda SG, Huntley J, Ames D, et al. Dementia prevention, intervention, and care. Lancet. 2017;390:2673–2734. doi: 10.1016/S0140-6736(17)31363-6. [DOI] [PubMed] [Google Scholar]
- 47.Köbe T, Witte AV, Schnelle A, Lesemann A, Fabian S, Tesky VA, et al. Combined omega-3 fatty acids, aerobic exercise and cognitive stimulation prevents decline in gray matter volume of the frontal, parietal and cingulate cortex in patients with mild cognitive impairment. Neuroimage. 2016;131:226–238. doi: 10.1016/j.neuroimage.2015.09.050. [DOI] [PubMed] [Google Scholar]
- 48.Laukkanen T, Kunutsor S, Kauhanen J, Laukkanen JA. Sauna bathing is inversely associated with dementia and Alzheimer's disease in middle-aged Finnish men. Age Ageing. 2017;46:245–249. doi: 10.1093/ageing/afw212. [DOI] [PubMed] [Google Scholar]
- 49.Beauchet O. Testosterone and cognitive function: current clinical evidence of a relationship. Eur J Endocrinol. 2006;155:773–781. doi: 10.1530/eje.1.02306. [DOI] [PubMed] [Google Scholar]
- 50.Jung HJ, Shin HS. Effect of testosterone replacement therapy on cognitive performance and depression in men with testosterone deficiency syndrome. World J Mens Health. 2016;34:194–199. doi: 10.5534/wjmh.2016.34.3.194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Hayes LD, Grace FM, Baker JS, Sculthorpe N. Exercise-induced responses in salivary testosterone, cortisol, and their ratios in men: a meta-analysis. Sports Med. 2015;45:713–726. doi: 10.1007/s40279-015-0306-y. [DOI] [PubMed] [Google Scholar]
- 52.Hayes LD, Herbert P, Sculthorpe NF, Grace FM. Exercise training improves free testosterone in lifelong sedentary aging men. Endocr Connect. 2017;6:306–310. doi: 10.1530/EC-17-0082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Cho DY, Yeo JK, Cho SI, Jung JE, Yang SJ, Kong DH, et al. Exercise improves the effects of testosterone replacement therapy and the durability of response after cessation of treatment: a pilot randomized controlled trial. Asian J Androl. 2017;19:602–607. doi: 10.4103/1008-682X.184269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Heufelder AE, Saad F, Bunck MC, Gooren L. Fifty-two-week treatment with diet and exercise plus transdermal testosterone reverses the metabolic syndrome and improves glycemic control in men with newly diagnosed type 2 diabetes and subnormal plasma testosterone. J Androl. 2009;30:726–733. doi: 10.2164/jandrol.108.007005. [DOI] [PubMed] [Google Scholar]
- 55.Iolascon G, Di Pietro G, Gimigliano F, Mauro GL, Moretti A, Giamattei MT, et al. Physical exercise and sarcopenia in older people: position paper of the Italian Society of Orthopaedics and Medicine (OrtoMed) Clin Cases Miner Bone Metab. 2014;11:215–221. [PMC free article] [PubMed] [Google Scholar]
- 56.Park BJ, Lee YJ. Integrative approach to elderly frailty. Korean J Fam Med. 2010;31:747–754. [Google Scholar]
- 57.O'Leary CB, Hackney AC. Acute and chronic effects of resistance exercise on the testosterone and cortisol responses in obese males: a systematic review. Physiol Res. 2014;63:693–704. doi: 10.33549/physiolres.932627. [DOI] [PubMed] [Google Scholar]
- 58.Hansen S, Kvorning T, Kjaer M, Sjøgaard G. The effect of short-term strength training on human skeletal muscle: the importance of physiologically elevated hormone levels. Scand J Med Sci Sports. 2001;11:347–354. doi: 10.1034/j.1600-0838.2001.110606.x. [DOI] [PubMed] [Google Scholar]
- 59.Viau V. Functional cross-talk between the hypothalamic-pituitary-gonadal and -adrenal axes. J Neuroendocrinol. 2002;14:506–513. doi: 10.1046/j.1365-2826.2002.00798.x. [DOI] [PubMed] [Google Scholar]
- 60.Harris AR, Vagenakis AG, Braverman LE. Sex-related differences in outer ring monodeiodination of thyroxine and 3,3′,5′-triiodothyronine by rat liver homogenates. Endocrinology. 1979;104:645–652. doi: 10.1210/endo-104-3-645. [DOI] [PubMed] [Google Scholar]
- 61.Miyashita K, Murakami M, Iriuchijima T, Takeuchi T, Mori M. Regulation of rat liver type 1 iodothyronine deiodinase mRNA levels by testosterone. Mol Cell Endocrinol. 1995;115:161–167. doi: 10.1016/0303-7207(95)03689-x. [DOI] [PubMed] [Google Scholar]
- 62.Lopresti AL, Hood SD, Drummond PD. A review of lifestyle factors that contribute to important pathways associated with major depression: diet, sleep and exercise. J Affect Disord. 2013;148:12–27. doi: 10.1016/j.jad.2013.01.014. [DOI] [PubMed] [Google Scholar]