Abstract
Immunoprecipitation with autoantibodies to the insulin receptor derived from patients with extreme insulin resistance and acanthosis nigricans revealed that the receptor is comprised of two subunits of 135 kDa (α subunit) and 95 kDa (β subunit) and that insulin induces the rapid phosphorylation of the β subunit in intact cells. Incubation of a highly purified insulin receptor preparation with [γ-32P]ATP also resulted in tyrosine phosphorylation of the β subunit in an insulin-dependent manner, suggesting that the receptor itself is a tyrosine-specific protein kinase. Furthermore, a Japanese boy with insulin resistance and acanthosis nigricans was found to be heterozygous for a mutation of the insulin receptor gene that resulted in the replacement of glycine-996 with valine in the ATP binding site of the receptor. Expression of the mutant receptor in cultured cells revealed it to be deficient in tyrosine kinase activity and mediation of insulin action, suggesting that the tyrosine kinase activity of the insulin receptor is essential for insulin action in vivo.
Keywords: protein kinase, phosphotyrosine, gene mutation
Introduction
Insulin is a peptide hormone produced by pancreatic β cells that regulates carbohydrate, lipid, and protein metabolism as the principal anabolic hormone of the body. An increase in blood glucose concentration thus triggers the secretion of insulin from β cells into the blood, and the released hormone then stimulates glucose uptake from the blood into liver, fat, and skeletal muscle, thereby restoring the blood glucose level to the normal range. Insulin is administered in medicine to lower the blood glucose concentration of individuals with diabetes who produce insufficient amounts of the endogenous hormone.
The first step of insulin action, like that for other peptide hormones, is binding of the hormone to specific receptors on the plasma membrane of target cells. In 1971, two research groups demonstrated the existence of the insulin receptor by detecting the specific binding of 125I-labeled insulin to the plasma membrane of adipose tissue or liver cells.1,2) The interaction between insulin and its receptor under both normal and pathophysiological conditions was subsequently characterized in more detail,3–5) but its molecular basis remained poorly understood.
Several approaches were adopted to determine the structure of the insulin receptor. One group solubilized and purified the insulin receptor with the use of insulin-agarose affinity chromatography. The eluate from the insulin affinity column was found to contain two major protein bands with apparent molecular masses of 135 and 45 kDa on sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) under reducing conditions.6) A second group synthesized photoreactive insulin derivatives and found that these derivatives labeled two protein bands of 130 and 90 kDa in liver plasma membrane preparations, again as detected by SDS-PAGE under reducing conditions.7) And a third group covalently linked 125I-labeled insulin to its receptor with the use of bifunctional cross-linking agents such as disuccinimidyl suberate, which revealed that the receptor contained at least one subunit with a molecular mass of 125 kDa on SDS-PAGE under reducing conditions.8) All three groups thus appeared to identify an insulin binding subunit with an apparent molecular mass of 125 to 135 kDa on reducing SDS-PAGE. Their findings were discrepant, however, with regard to the size or even presence of an additional subunit of the receptor.
Structure of the insulin receptor
In July 1979, I joined the Diabetes Branch of the National Institute of Arthritis, Metabolism, and Digestive Diseases (NIAMDD) at the U.S. National Institutes of Health (NIH) in Bethesda, Maryland, as a Fogarty International Fellow. When my mentor, Ronald Kahn, took sabbatical leave to study cell biology in Paris, I was left to plan my research project by myself. Almost a full year passed without any promising idea for the project, although I spent a lot of time reading scientific papers and thinking. However, I eventually came up with the idea that it might be possible to isolate the insulin receptor by taking advantage of autoantibodies to the receptor that had been identified in patients with extreme insulin resistance and acanthosis nigricans,4) given that I was aware that several such autoantibody preparations with a high titer were available in the Diabetes Branch.
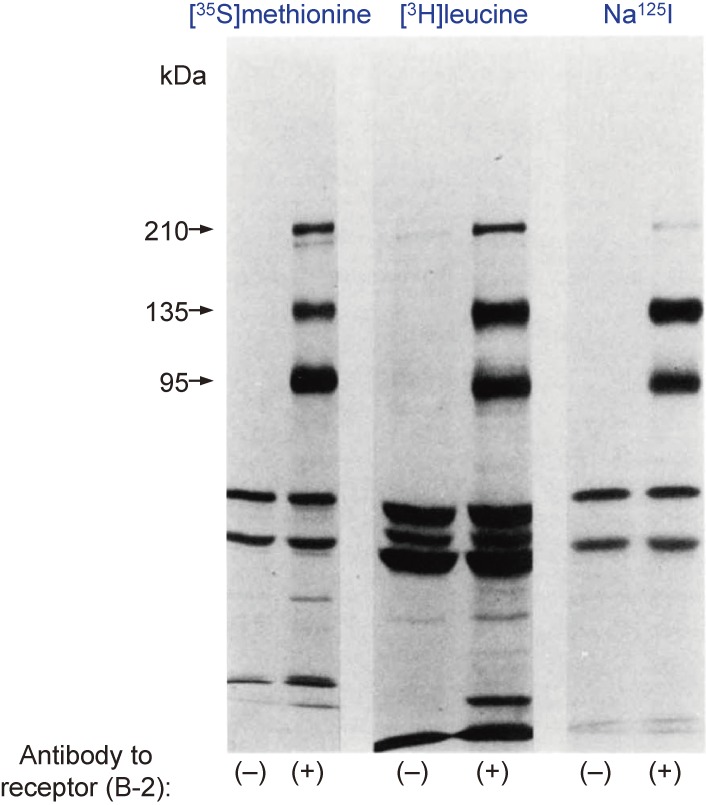
In pursuit of this idea, and with the help of Kenneth Yamada at the nearby National Cancer Institute, I developed a method to identify insulin receptor subunits by immunoprecipitation with antibodies to the insulin receptor after biosynthetic or surface labeling of intact cells and subsequent analysis of the immunoprecipitates by SDS-PAGE and autoradiography. I worked together with Emmanuel Van Obberghen and Jose Hedo, who were interested in the immunoprecipitation method. We first confirmed that the receptor cross-linked with 125I-labeled insulin was immunoprecipitated with a panel of autoantibodies to the insulin receptor derived from patients.9) Application of the immunoprecipitation method to cells biosynthetically labeled with [35S]methionine, [3H]leucine, [3H]glucosamine, [3H]galactose, [3H]fucose, or [3H]mannose indicated that the receptor consists of two major glycoprotein subunits with apparent molecular masses of 135 kDa (α subunit) and 95 kDa (β subunit) and a minor glycoprotein with an apparent molecular mass of 210 kDa (γ subunit) on SDS-PAGE under reducing conditions (Fig. 1).10–13)
Figure 1.
Labeling of insulin receptor subunits. IM-9 human lymphocytes were labeled either biosynthetically with [35S]methionine or [3H]leucine, or externally with Na125I and lactoperoxidase. The cells were then solubilized with Trion X-100, and glycoproteins were purified by chromatography with wheat germ agglutinin–agarose and then subjected to immunoprecipitation with control serum or serum containing antibodies to the insulin receptor (B-2). The immunoprecipitates were analyzed by SDS-PAGE after reduction with dithiothreitol, and the gel was then dried and subjected to autoradiography. Modified with permission from ref. 13.
Peptide mapping of the [35S]methionine-labeled receptor showed that the α and β subunits were distinct, whereas similarities were apparent between many of the proteolytic fragments of the γ subunit and those of the α or β subunits. Surface labeling of cultured cells with Na125I and lactoperoxidase also revealed that all three putative subunits were surface proteins (Fig. 1). On the basis of our and previous findings, the α subunit appeared to be the insulin binding subunit, whereas the β subunit was possibly an effector subunit and the γ subunit possibly a precursor. We also confirmed the existence of disulfide-linked higher molecular weight complexes containing α, β, and γ subunits or α and β subunits (possibly α2β2).13) Later we found that a component of the receptor with an apparent molecular mass of 190 kDa undergoes proteolytic cleavage to generate the α and β subunits. We therefore postulated that the γ subunit corresponds to this precursor that has escaped cleavage and is expressed at the plasma membrane.14)
Immunoprecipitation with autoantibodies to the insulin receptor found in patients with extreme insulin resistance and acanthosis nigricans thus revealed the basic structure of the insulin receptor, and we were convinced that we would also be able to detect covalent modifications of the receptor with this method.
Phosphorylation of the insulin receptor
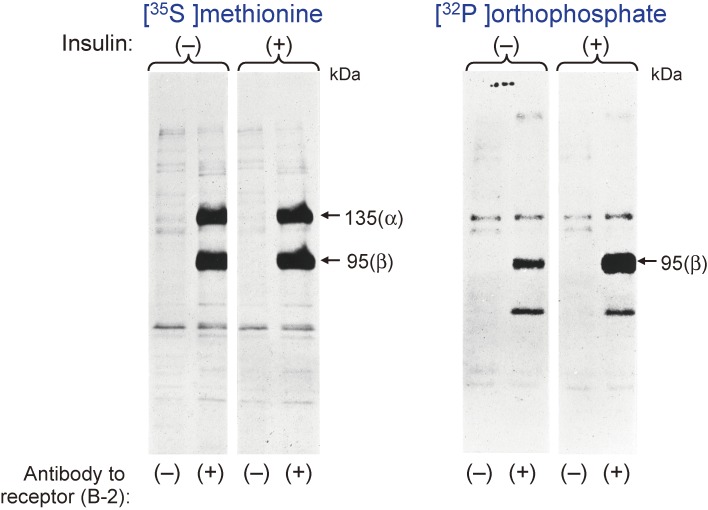
Anders Karlsson, a thyroidologist at Uppsala University in Sweden, came to the Diabetes Branch for sabbatical leave. He was interested in my research and we worked together. We decided to examine whether the insulin receptor is phosphorylated or not, given that phosphorylation had been identified as a reversible covalent modification that contributes to the rapid regulation of protein function and that insulin had recently been found to affect the phosphorylation state of cellular proteins.15,16) We labeled cultured cells with [32P]orthophosphate and then isolated the insulin receptor subunits by immunoprecipitation and reducing SDS-PAGE. In May 1981, we found that the β subunit of the receptor is phosphorylated and that exposure of the cells to insulin for as little as 1 min stimulated this phosphorylation in a concentration-dependent manner, suggesting that phosphorylation of the β subunit is an early event in insulin action (Fig. 2).17)
Figure 2.
Labeling of insulin receptor subunits with [32P]orthophosphate. Rat hepatoma (Fao) cells were labeled biosynthetically with [35S]methionine or [32P]orthophosphate and then incubated in the absence or presence of 1 µM insulin for 15 min. Cell lysates were then prepared and subjected to immunoprecipitation with antibodies to the insulin receptor (B-2) followed by SDS-PAGE under reducing conditions.
The next obvious question concerned the identity of the kinase enzyme responsible for phosphorylation of the β subunit of the insulin receptor. To address this question, we examined whether insulin stimulates phosphorylation of the β subunit in a solubilized plasma membrane fraction. We found that insulin indeed stimulated the incorporation of 32P from [γ-32P]ATP into the β subunit of the receptor in a solubilized plasma membrane fraction of the liver in the presence of Mn2+ and in a specific and concentration-dependent manner.18)
We next set out to identify the phosphorylated amino acids of the β subunit of the insulin receptor. Phosphotyrosine had just been identified in 1979, with this rare protein modification having been detected in cells after transformation with an RNA tumor virus19,20) or after stimulation with growth factors such as epidermal growth factor21) and platelet-derived growth factor.22) Given that insulin also functions as a growth factor, we thought that insulin might stimulate the tyrosine phosphorylation of its own receptor. Indeed, we found that phosphotyrosine was the only phosphorylated amino acid detected in the β subunit of the insulin receptor in the basal state, and that insulin induced a marked increase in the amount of phosphotyrosine in the β subunit in a cell-free system.18)
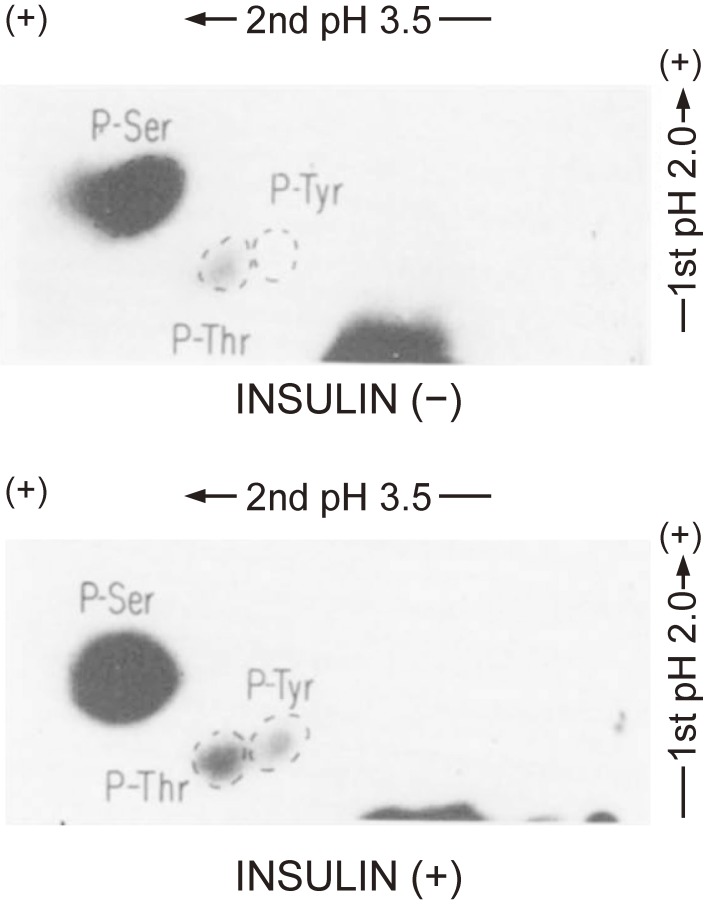
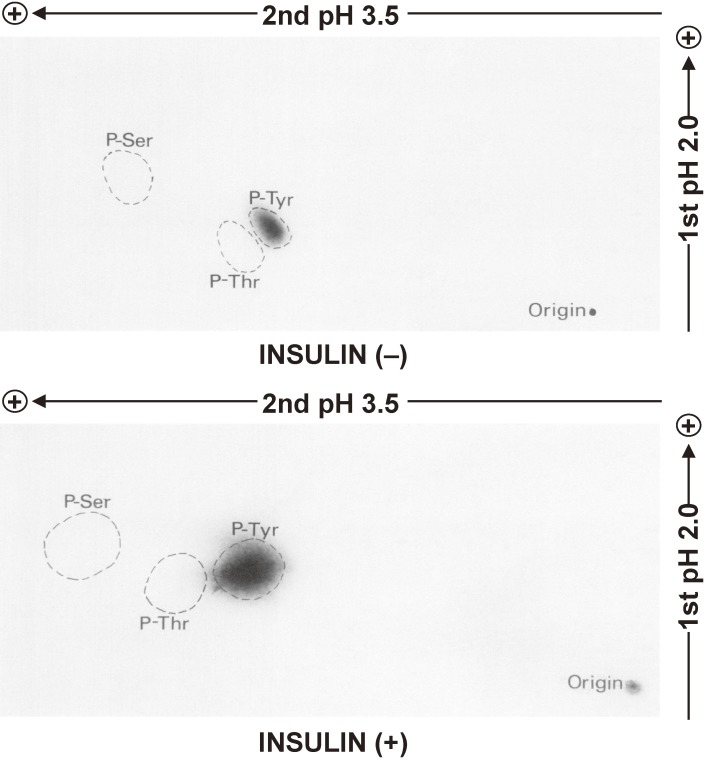
We also examined the phosphoamino acids of the β subunit of the insulin receptor in intact cells. We detected phosphoserine and phosphothreonine in the β subunit of intact cells under basal conditions as well as an increase in the amount of phosphoserine and the appearance of phosphotyrosine after insulin stimulation (Fig. 3).23)
Figure 3.
Identification of phosphoamino acids in the β subunit of the insulin receptor in intact cells. Rat hepatoma (Fao) cells were labeled biosynthetically with [32P]orthophosphate and then incubated with or without 1 µM insulin for 15 min, after which the insulin receptor was isolated by immunoprecipitation with specific antibodies followed by SDS-PAGE under reducing conditions. The band corresponding to the 95-kDa immunoreactive protein (β subunit) was excised from the gel, and the protein was eluted by electrophoresis and subjected to hydrolysis with 6 M HCl for 2 h at 110 ℃. The resulting hydrolysate was analyzed by two-dimensional electrophoresis and autoradiography. The positions of spots corresponding to phosphoserine (P-Ser), phosphotyrosine (P-Tyr), and phosphothreonine (P-Thr) are indicated. Modified with permission from ref. 23.
Our results thus suggested that a tyrosine-specific protein kinase present in the plasma membrane mediates the insulin-induced phosphorylation of the β subunit of the insulin receptor, and that such tyrosine phosphorylation may be the primary phosphorylation event that occurs in response to insulin binding, with the phosphorylation of serine requiring an additional component (or components) absent from a cell-free system. We therefore next examined whether the insulin receptor itself might be a tyrosine-specific protein kinase.
Insulin receptor kinase hypothesis
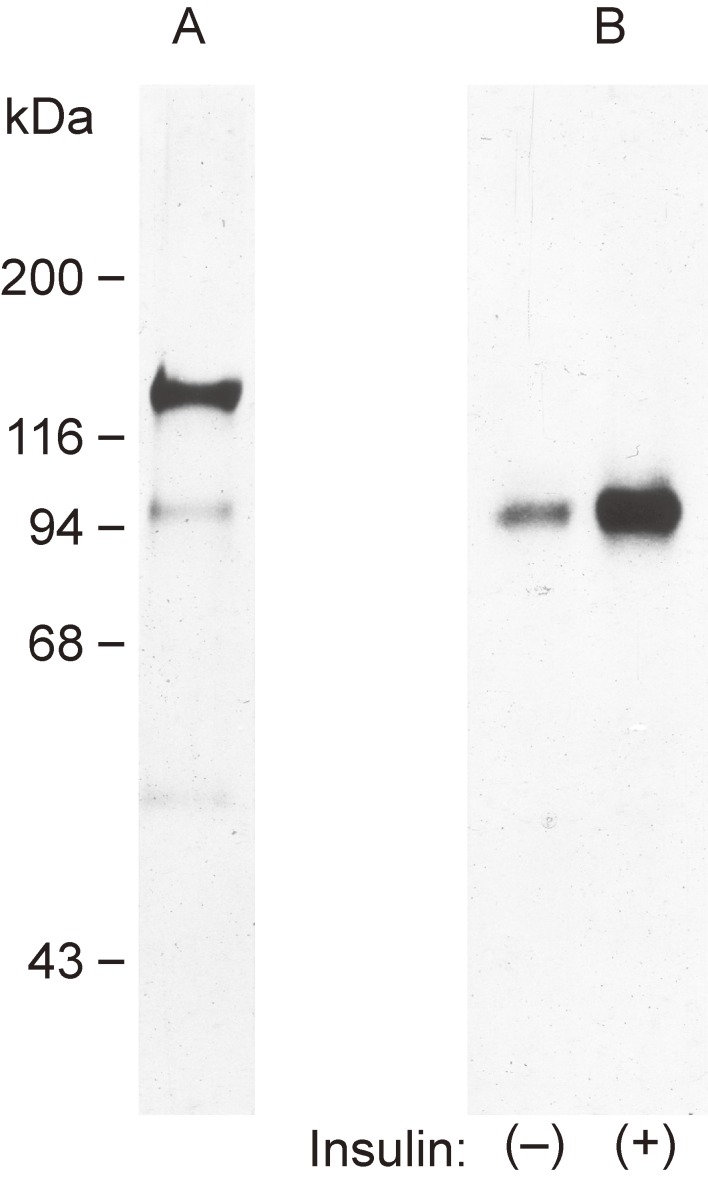
Around that time, Yoko Fujita-Yamaguchi and colleagues at the City of Hope Research Institute in Duarte, California, obtained a highly purified insulin receptor preparation from human placenta by sequential affinity chromatography on wheat germ agglutinin and insulin-agarose. This preparation had an insulin binding capacity of 4.7 pmol per milligram of protein and thus approached theoretical maximal purity.24) Silver staining of this highly purified receptor after SDS-PAGE under reducing conditions revealed only the α and β subunits as well as a faint band corresponding to a protein with an apparent molecular mass of 52 kDa (Fig. 4), the latter of which was thought to be a degradation product of the receptor given that it was immunoprecipitated by antibodies to the insulin receptor. Incubation of this preparation with [γ-32P]ATP in the presence of Mn2+ resulted in the phosphorylation of the β subunit. Furthermore, exposure of the receptor preparation to insulin induced a three- to fivefold increase in the extent of 32P incorporation into the β subunit in a concentration-dependent manner (Fig. 4). Phosphoamino acid analysis by two-dimensional electrophoresis revealed only phosphotyrosine in receptor preparations incubated under basal or insulin-stimulated conditions (Fig. 5). Furthermore, insulin-induced phosphorylation of the β subunit of the receptor was also apparent on incubation of immunoprecipitates prepared from a sample of the highly purified receptor with antibodies to the insulin receptor. These data thus suggested that a tyrosine-specific protein kinase activity was associated with the purified insulin receptor and was probably intrinsic to the receptor itself.25) Two other groups reached the same conclusion by demonstrating the existence of an ATP binding site on the β subunit of the insulin receptor by affinity labeling with [α-32P]ATP.26,27) We also found that the highly purified receptor catalyzed the incorporation of 32P from [γ-32P]ATP not only into the β subunit of the receptor but also into artificial substrates. Insulin induced a three- to fivefold increase in the extent of phosphorylation of these substrates, suggesting that insulin stimulates the kinase activity of its own receptor.28) On the basis of these various data, we proposed that some or even all of the actions of insulin may be mediated by activation of a tyrosine-specific protein kinase intrinsic to the insulin receptor (Fig. 6).25,28)
Figure 4.
Phosphorylation of the β subunit of a highly purified insulin receptor preparation. Lane A shows silver staining of the highly purified human receptor after SDS-PAGE under reducing conditions. Lanes B show the incorporation of 32P from [γ-32P]ATP into the highly purified receptor after incubation with or without 0.1 µM insulin for 30 min. The incorporation was detected by SDS-PAGE under reducing conditions followed by autoradiography. Modified with permission from ref. 25.
Figure 5.
Identification of phosphoamino acids in the β subunit of the highly purified insulin receptor preparation. The purified receptor was incubated with [γ-32P]ATP in the absence or presence of 0.1 µM insulin for 30 min and then subjected to hydrolysis for analysis of phosphoamino acids by two-dimensional electrophoresis and autoradiography. Modified with permission from ref. 25.
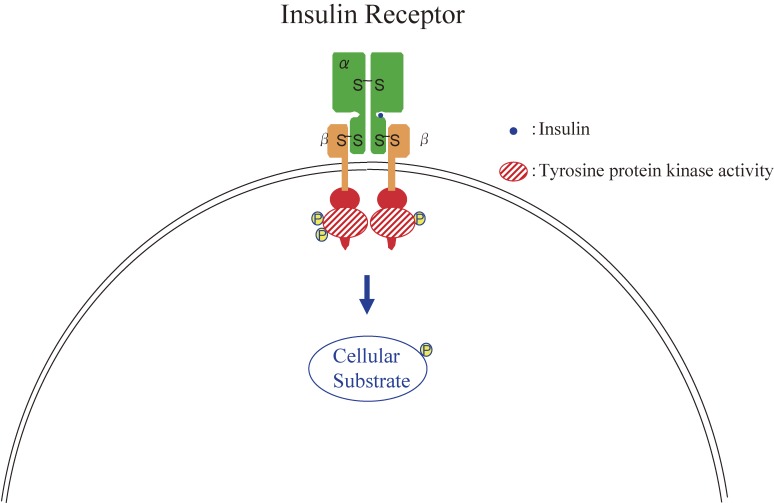
Figure 6.
Structure and function of the insulin receptor.
I returned to the Third Department of Internal Medicine at The University of Tokyo in November 1982 and continued my research on the mechanism of insulin action. At that time, the chemical mediator hypothesis of insulin action was prominent. This hypothesis was based on findings with broken cell preparations suggesting that insulin triggers the release from the plasma membrane of one or more low molecular weight factors that are able to regulate the activity of insulin-sensitive enzymes.29–32) Given that there was no conclusive method to show that the protein tyrosine kinase activity of the insulin receptor is necessary for insulin action, our proposal was not immediately accepted by researchers in the field.
Cloning of the insulin receptor cDNA
In 1985, two groups succeeded in cloning of the human insulin receptor cDNA,33,34) the isolation and sequencing of which provided primary structural information about the receptor protein. As had been predicted, this information indicated that the α subunit of the receptor contains the insulin binding site and that the β subunit contains the tyrosine kinase domain with the presumed ATP binding region.
The cloning of the receptor cDNA also made it possible to answer the critical question of whether the tyrosine kinase activity of the insulin receptor is required for insulin action by the application of site-directed mutagenesis. Indeed, two groups constructed mutant versions of the human insulin receptor in which alanine, methionine, or arginine was substituted for a key lysine residue in the ATP binding domain. These mutant receptors lacked kinase activity and did not mediate the stimulation by insulin of deoxyglucose uptake, ribosomal S6 kinase activity, endogenous substrate phosphorylation, glycogen synthesis, or thymidine incorporation into DNA when expressed in cultured cells.35,36) These data thus suggested that the tyrosine-specific protein kinase activity of the receptor is indeed required for some if not all of the effects of insulin in cultured cells.
Insulin receptor mutation in a human patient
In 1986, Nobuo Matsuura of Hokkaido University referred a patient, a Japanese boy with the syndrome of insulin resistance and acanthosis nigricans, to our hospital. The boy was not obese but had a glucose tolerance typical of a diabetic patient and was extremely hyperinsulinemic. He was negative for both insulin autoantibodies and insulin receptor autoantibodies. However, the number of insulin receptors expressed on Epstein-Barr virus-transformed lymphoblasts derived from the patient was at the lower limit of normal. We therefore examined the tyrosine kinase activity of the insulin receptor in these lymphoblasts and found that it was reduced by 50% to 80%.37) These data suggested that the patient had a defect in the tyrosine kinase activity of the receptor. To identify the molecular basis of this defect, we decided to construct a cDNA library from polyadenylated RNA obtained from the patient’s lymphoblasts. For this purpose, in December 1987, I returned to the Diabetes Branch at NIH for 3 months. With the help of members of the Diabetes Branch including Takashi Kadowaki and Simeon Taylor, I succeeded in preparing a cDNA library and obtained positive clones after screening with an insulin receptor cDNA probe.
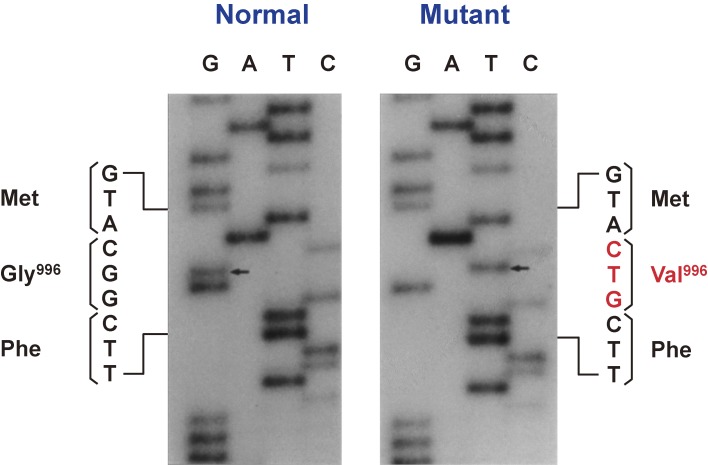
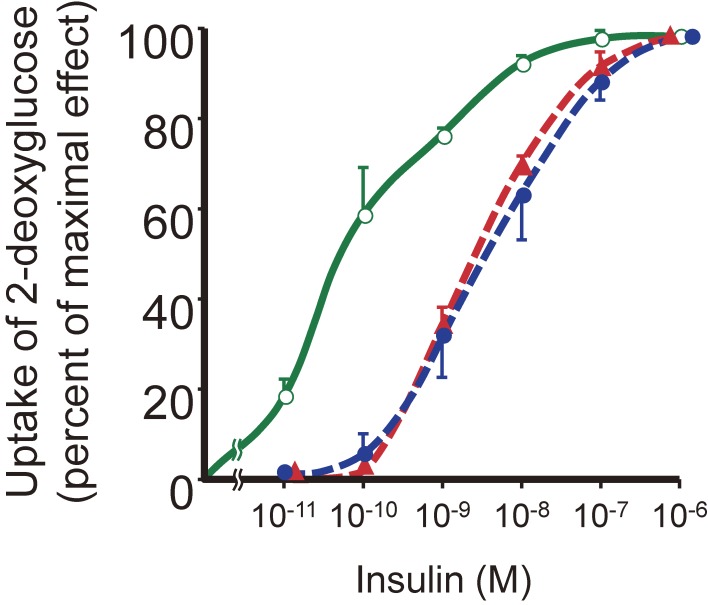
After I returned to Tokyo, we determined the nucleotide sequence of the positive clones and found that G was replaced by T at position 3116, which resulted in the conversion of codon 996 from GGC (glycine) to GTC (valine), in one of the patient’s insulin receptor gene alleles (Fig. 7).38) Glycine-996 corresponds to the third glycine in the conserved Gly–X–Gly–X–X–Gly (where X represents any amino acid) motif that is present in the ATP binding site of protein kinases, with the first and third glycines having been suggested to support a sharp turn in the polypeptide chain in this region. To assess the significance of the Val996 mutation, we expressed both wild-type and mutant forms of the insulin receptor in Chinese hamster ovary (CHO) cells by transfection with the corresponding cDNAs. Although the Val996 mutation did not affect the binding of 125I-labeled insulin to the receptor, the extent of insulin-induced receptor autophosphorylation was reduced by more than 90% in the mutant protein, suggesting that the large side chain of valine at position 996 disrupts the three-dimensional structure of the ATP binding site in the kinase domain (Fig. 8).38) Furthermore, the kinase-impaired mutant receptor did not mediate the stimulation of deoxyglucose uptake by insulin in CHO cells (Fig. 9). Together, these findings suggested that the tyrosine-specific kinase activity of the insulin receptor is required for the ability of the receptor to mediate insulin action in vivo.
Figure 7.
Partial nucleotide sequence of the two alleles of the insulin receptor gene of a patient with insulin resistance and acanthosis nigricans. In the mutant allele, codon 996 is GTC instead of GGC, which results in the substitution of valine for glycine at position 996. Modified with permission from ref. 38.
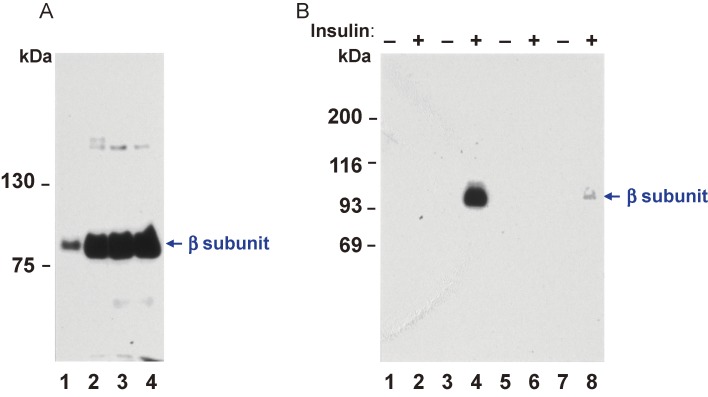
Figure 8.
Expression of wild-type and mutant human insulin receptor cDNAs in CHO cells. (A) Immunoblot analysis with antibodies to the insulin receptor of partially purified insulin receptors from nontransfected cells (lane 1), from cells transfected with cDNA for the wild-type human insulin receptor (Gly996) (lane 2), and from two cell lines transfected with cDNA for the Val996 mutant form of the receptor (lanes 3 and 4). (B) Autophosphorylation of partially purified insulin receptors from nontransfected cells (lanes 1 and 2) or cells transfected with wild-type (lanes 3 and 4) or mutant (lanes 5 to 8) human insulin receptor cDNAs as in (A). CHO cells were solubilized and insulin receptors were partially purified on wheat germ agglutinin-agarose. Receptors were incubated in the absence (odd lanes) or presence (even lanes) of 0.1 µM insulin for 30 min and then were phosphorylated in the presence of [γ-32P]ATP and MnCl2 as previously descrived.18) Insulin receptors were then immunoprecipitated and analyzed by SDS-PAGE and autoradiography. Modified with permission from ref. 38.
Figure 9.
Insulin-stimulated uptake of 2-deoxyglucose in CHO cells expressing wild-type or mutant human insulin receptor cDNAs. Nontransfected cells ( ) or cells transfected with cDNAs for wild-type (
) or cells transfected with cDNAs for wild-type ( ) or Gly996→Val mutant (
) or Gly996→Val mutant ( ) forms of the human insulin receptor were assayed for 2-deoxyglucose uptake in the presence of various insulin concentrations.
) forms of the human insulin receptor were assayed for 2-deoxyglucose uptake in the presence of various insulin concentrations.
Postscript
More than 35 years have passed since we proposed that some or even all of the actions of insulin might be dependent on signaling from the insulin receptor mediated by activation of an intrinsic tyrosine-specific protein kinase. Although this notion was not immediately embraced, the data accumulated over the intervening years have supported our proposal.
Acknowledgements
I am grateful to the late Dr. Kinori Kosaka, who introduced me to research into the insulin receptor; to Drs. Ron Kahn, Jesse Roth, Phil Gorden, and Fumimaro Takaku for their advice and encouragement; and to all my collaborators and colleagues over the years for their discussion and helpful comments.
Profile
Masato Kasuga was born in Tokyo on 4 February 1948 and graduated from the Faculty of Medicine at The University of Tokyo with an M.D. degree in 1973. After a residency in medicine at The University of Tokyo Hospital, he joined the Third Department of Internal Medicine in the Faculty of Medicine at The University of Tokyo as a research fellow to examine the relation between insulin binding and insulin effects in isolated adipocytes under the supervision of Dr. Yasuo Akanuma and Prof. Kinori Kosaka. In 1978, he earned a Doctor of Medical Science (Ph.D.) degree from The University of Tokyo. The next year, he moved to the National Institutes of Health (NIH) in Bethesda, Maryland, U.S.A., as a Fogarty International Fellow in the Diabetes Branch of the National Institute of Arthritis, Metabolism, and Digestive Diseases (NIAMDD). His research at NIH was performed under the supervision of scientists including Drs. C. Ronald Kahn, Ken Yamada, Jesse Roth, and Phil Gorden, and it revealed that the insulin receptor is a tyrosine-specific protein kinase. In 1981, he moved to the Joslin Diabetes Center in Boston to continue his research with Dr. C. Ronald Kahn. He returned to the Third Department of Internal Medicine at The University of Tokyo as an Assistant Professor in 1983 and established his own research group. His group showed that a patient with severe insulin resistance harbored a heterozygous mutation of the insulin receptor that impaired its tyrosine kinase activity. In 1990, he moved to Kobe University School of Medicine to become Professor and Chairman of the Second Department of Internal Medicine. He and his group analyzed postreceptor mechanisms of insulin action by engineering the expression of dominant negative mutants of signaling proteins in cultured cells. They thus showed that phosphatidylinositol (PI) 3-kinase associated with insulin receptor substrate (IRS), but not the Grb2-Sos complex or the phosphatase SHP-2, was important for mediating the metabolic actions of insulin in such cells. They further analyzed insulin signaling downstream of PI 3-kinase and demonstrated important roles for the protein kinase Akt and other molecules.
From 1998 onward, Dr. Kasuga’s laboratory gradually shifted its focus to the mechanisms of insulin action in the whole body. By studying genetically manipulated mice, he and his colleagues showed that PI 3-kinase and its downstream effector PDK1 (phosphoinositide-dependent kinase 1) play a key role in mediating the metabolic actions of insulin in the liver. They uncovered a new insulin signaling pathway by which the hormone regulates hepatic glucose production via a central action that results in the interleukin-6–dependent activation of the transcription factor STAT3 in the liver. They also showed that PDK1 is important for maintenance of pancreatic β cell mass, and that the accumulation of the cell cycle inhibitor p27Kip1 contributes to β cell failure during the development of type 2 diabetes. Furthermore, they found that macrophage infiltration into adipose tissue promoted by the chemokine MCP-1 underlies the development of obesity-induced insulin resistance, and they identified both Dok1 as an important molecule in the development of adipocyte hypertrophy associated with diet-induced obesity as well as FSP27 as a key protein in the formation of unilocular lipid droplets in white adipose tissue.
In 2008, Dr. Kasuga moved to Tokyo as Director-General of the Research Institute at the International Medical Center of Japan. In 2012, he became President of the National Center for Global Health and Medicine in Tokyo. In 2018, he was appointed Director of the Institute for Adult Disease of Asahi Life Foundation.
Masato Kasuga and his colleagues have performed seminal research defining the molecular mechanisms of insulin action and their impairment in type 2 diabetes. He has published more than 300 peer-reviewed research papers. For these achievements, he has received many awards including the Erwin Von Bälz Preis from Boehringer Ingelheim, the Medical Award of the Japan Medical Association, the Medical Award of Takeda Science Foundation, the Medal with Purple Ribbon, Claude Bernard Medal, and a Lectureship from the European Association for the Study of Diabetes, and the Manpei Suzuki International Prize for Diabetes Research.
References
- 1).Cuatrecasas P. (1971) Insulin–receptor interactions in adipose tissue cells: Direct measurement and properties. Proc. Natl. Acad. Sci. U.S.A. 68, 1264–1268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2).Freychet P., Roth J., Neville D.M., Jr. (1971) Insulin receptors in the liver: Specific binding of (125I)insulin to the plasma membrane and its relation to insulin bioactivity. Proc. Natl. Acad. Sci. U.S.A. 68, 1833–1837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3).Kahn C.R., Neville D.M., Jr., Roth J. (1973) Insulin-receptor interaction in the obese-hyperglycemic mouse. A model of insulin resistance. J. Biol. Chem. 248, 244–250. [PubMed] [Google Scholar]
- 4).Flier J.S., Kahn C.R., Roth J., Bar R.S. (1975) Antibodies that impair insulin receptor binding in an unusual diabetic syndrome with severe insulin resistance. Science 190, 63–65. [DOI] [PubMed] [Google Scholar]
- 5).Kasuga M., Akanuma Y., Iwamoto Y., Kosaka K. (1977) Effects of fasting and refeeding on insulin receptors and glucose metabolism in rat adipocytes. Endocrinology 100, 1384–1390. [DOI] [PubMed] [Google Scholar]
- 6).Jacobs S., Hazum E., Shechter Y., Cuatrecasas P. (1979) Insulin receptor: Covalent labeling and identification of subunits. Proc. Natl. Acad. Sci. U.S.A. 76, 4918–4921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7).Yip C.C., Yeung C.W., Moule M.L. (1980) Photoaffinity labeling of insulin receptor proteins of liver plasma membrane preparations. Biochemistry 19, 70–76. [DOI] [PubMed] [Google Scholar]
- 8).Pilch P.F., Czech M.P. (1980) The subunit structure of the high affinity insulin receptor. Evidence for a disulfide-linked receptor complex in fat cell and liver plasma membranes. J. Biol. Chem. 255, 1722–1731. [PubMed] [Google Scholar]
- 9).Kasuga M., van Obberghen E., Yamada K.M., Harrison L.C. (1981) Autoantibodies against the insulin receptor recognize the insulin binding subunits of an oligomeric receptor. Diabetes 30, 354–357. [DOI] [PubMed] [Google Scholar]
- 10).Van Obberghen E., Kasuga M., Le Cam A., Hedo J.A., Itin A., Harrison L.C. (1981) Biosynthetic labeling of insulin receptor: Studies of subunits in cultured human IM-9 lymphocytes. Proc. Natl. Acad. Sci. U.S.A. 78, 1052–1056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11).Hedo J.A., Kasuga M., Van Obberghen E., Roth J., Kahn C.R. (1981) Direct demonstration of glycosylation of insulin receptor subunits by biosynthetic and external labeling: Evidence for heterogeneity. Proc. Natl. Acad. Sci. U.S.A. 78, 4791–4795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12).Kasuga M., Kahn C.R., Hedo J.A., Van Obberghen E., Yamada K.M. (1981) Insulin-induced receptor loss in cultured human lymphocytes is due to accelerated receptor degradation. Proc. Natl. Acad. Sci. U.S.A. 78, 6917–6921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13).Kasuga M., Hedo J.A., Yamada K.M., Kahn C.R. (1982) The structure of insulin receptor and its subunits. Evidence for multiple nonreduced forms and a 210,000 possible proreceptor. J. Biol. Chem. 257, 10392–10399. [PubMed] [Google Scholar]
- 14).Hedo J.A., Kahn C.R., Hayashi M., Yamada K.M., Kasuga M. (1983) Biosynthesis and glycosylation of the insulin receptor. Evidence for a single polypeptide precursor of the two major subunits. J. Biol. Chem. 258, 10020–10026. [PubMed] [Google Scholar]
- 15).Avruch J., Leone G.R., Martin D.B. (1976) Effects of epinephrine and insulin on phosphopeptide metabolism in adipocytes. J. Biol. Chem. 251, 1511–1515. [PubMed] [Google Scholar]
- 16).Smith C.J., Wejksnora P.J., Warner J.R., Rubin C.S., Rosen O.M. (1979) Insulin-stimulated protein phosphorylation in 3T3-L1 preadipocytes. Proc. Natl. Acad. Sci. U.S.A. 76, 2725–2729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17).Kasuga M., Karlsson F.A., Kahn C.R. (1982) Insulin stimulates the phosphorylation of the 95,000-dalton subunit of its own receptor. Science 215, 185–187. [DOI] [PubMed] [Google Scholar]
- 18).Kasuga M., Zick Y., Blithe D.L., Crettaz M., Kahn C.R. (1982) Insulin stimulates tyrosine phosphorylation of the insulin receptor in a cell-free system. Nature 298, 667–669. [DOI] [PubMed] [Google Scholar]
- 19).Hunter T., Sefton B.M. (1980) Transforming gene product of Rous sarcoma virus phosphorylates tyrosine. Proc. Natl. Acad. Sci. U.S.A. 77, 1311–1315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20).Collett M.S., Purchio A.F., Erikson R.L. (1980) Avian sarcoma virus-transforming protein, pp60src shows protein kinase activity specific for tyrosine. Nature 285, 167–169. [DOI] [PubMed] [Google Scholar]
- 21).Ushiro H., Cohen S. (1980) Identification of phosphotyrosine as a product of epidermal growth factor-activated protein kinase in A-431 cell membranes. J. Biol. Chem. 255, 8363–8365. [PubMed] [Google Scholar]
- 22).Ek B., Westermark B., Wasteson A., Heldin C.H. (1982) Stimulation of tyrosine-specific phosphorylation by platelet-derived growth factor. Nature 295, 419–420. [DOI] [PubMed] [Google Scholar]
- 23).Kasuga M., Zick Y., Blith D.L., Karlsson F.A., Häring H.U., Kahn C.R. (1982) Insulin stimulation of phosphorylation of the β subunit of the insulin receptor. Formation of both phosphoserine and phosphotyrosine. J. Biol. Chem. 257, 9891–9894. [PubMed] [Google Scholar]
- 24).Fujita-Yamaguchi Y., Choi S., Sakamoto Y., Itakura K. (1983) Purification of insulin receptor with full binding activity. J. Biol. Chem. 258, 5045–5049. [PubMed] [Google Scholar]
- 25).Kasuga M., Fujita-Yamaguchi Y., Blithe D.L., Kahn C.R. (1983) Tyrosine-specific protein kinase activity is associated with the purified insulin receptor. Proc. Natl. Acad. Sci. U.S.A. 80, 2137–2141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26).Roth R.A., Cassell D.J. (1983) Insulin receptor: evidence that it is a protein kinase. Science 219, 299–301. [DOI] [PubMed] [Google Scholar]
- 27).Van Obberghen E., Rossi B., Kowalski A., Gazzano H., Ponzio G. (1983) Receptor-mediated phosphorylation of the hepatic insulin receptor: Evidence that the Mr 95,000 receptor subunit is its own kinase. Proc. Natl. Acad. Sci. U.S.A. 80, 945–949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28).Kasuga M., Fujita-Yamaguchi Y., Blithe D.L., White M.F., Kahn C.R. (1983) Characterization of the insulin receptor kinase purified from human placental membranes. J. Biol. Chem. 258, 10973–10980. [PubMed] [Google Scholar]
- 29).Larner J., Galasko G., Cheng K., DePaoli-Roach A.A., Huang L., Daggy P., et al. (1979) Generation by insulin of a chemical mediator that controls protein phosphorylation and dephosphorylation. Science 206, 1408–1410. [DOI] [PubMed] [Google Scholar]
- 30).Kiechle F.L., Jarett L., Kotagal N., Popp D.A. (1981) Partial purification from rat adipocyte plasma membranes of a chemical mediator which simulates the action of insulin on pyruvate dehydrogenase. J. Biol. Chem. 256, 2945–2951. [PubMed] [Google Scholar]
- 31).Seals J.R., Czech M.P. (1980) Evidence that insulin activates an intrinsic plasma membrane protease in generating a secondary chemical mediator. J. Biol. Chem. 255, 6529–6531. [PubMed] [Google Scholar]
- 32).Saltiel A.R., Siegel M.I., Jacobs S., Cuatrecasas P. (1982) Putative mediators of insulin action: Regulation of pyruvate dehydrogenase and adenylate cyclase activities. Proc. Natl. Acad. Sci. U.S.A. 79, 3513–3517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33).Ullrich A., Bell J.R., Chen E.Y., Herrera R., Petruzzelli L.M., Dull T.J., et al. (1985) Human insulin receptor and its relationship to the tyrosine kinase family of oncogenes. Nature 313, 756–761. [DOI] [PubMed] [Google Scholar]
- 34).Ebina Y., Ellis L., Jarnagin K., Edery M., Graf L., Clauser E., et al. (1985) The human insulin receptor cDNA: The structural basis for hormone-activated transmembrane signalling. Cell 40, 747–758. [DOI] [PubMed] [Google Scholar]
- 35).Ebina Y., Araki E., Taira M., Shimada F., Mori M., Craik C.S., et al. (1987) Replacement of lysine residue 1030 in the putative ATP-binding region of the insulin receptor abolishes insulin- and antibody-stimulated glucose uptake and receptor kinase activity. Proc. Natl. Acad. Sci. U.S.A. 84, 704–708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36).Chou C.K., Dull T.J., Russell D.S., Gherzi R., Lebwohl D., Ullrich A., et al. (1987) Human insulin receptors mutated at the ATP-binding site lack protein tyrosine kinase activity and fail to mediate postreceptor effects of insulin. J. Biol. Chem. 262, 1842–1847. [PubMed] [Google Scholar]
- 37).Yamamoto R., Shiba T., Tobe K., Shibasaki Y., Koshio O., Izumi T., et al. (1990) Defect in tyrosine kinase activity of the insulin receptor from a patient with insulin resistance and acanthosis nigricans. J. Clin. Endocrinol. Metab. 70, 869–878. [DOI] [PubMed] [Google Scholar]
- 38).Odawara M., Kadowaki T., Yamamoto R., Shibasaki Y., Tobe K., Accili D., et al. (1989) Human diabetes associated with a mutation in the tyrosine kinase domain of the insulin receptor. Science 245, 66–68. [DOI] [PubMed] [Google Scholar]