Abstract
Oxidative protein folding in the endoplasmic reticulum (ER) is a significant source of hydrogen peroxide (H2O2). For correct protein folding the redox state of the ER must be efficiently regulated. As such, several mechanisms with varying degrees of overlap manage the redox state of the ER. H2O2 also functions as a second messenger playing a role in most aspects of cellular physiology and pathology, requiring tight control of the concentration and flux of H2O2. Bestetti et al. have demonstrated a role for Aquaporin 11 in transport of H2O2 out of the ER.
Keywords: Endoplasmic reticulum, Hydrogen peroxide, Aquaporins
Graphical abstract
Highlights
-
•
Protein folding is a major source of H2O2 in the endoplasmic reticulum (ER).
-
•
Aquaporin-11 mediates H2O2 transport across the ER membrane.
-
•
HyPer is sensitive to pH.
The role of Hydrogen peroxide (H2O2) as a messenger molecule has progressively become more studied over the past two decades. The ability of H2O2 to regulate the spatial and temporal organization of cellular events, such as differentiation and development, as well as responses to environmental stimuli, necessitates a detailed understanding of its regulation and trafficking. The various roles of H2O2 are possible through tight spatial and temporal regulation of its subcellular concentration. One well studied mechanism by which cells are able to create localized high concentrations of H2O2 is through Nadph oxidase isoforms that have been shown to colocalize to specific cellular machinery [1,2]. Another important source of H2O2 occurs during the folding of proteins in the endoplasmic reticulum (ER). This process involves protein disulfide isomerase (PDI) working in concert with ER oxidoreductin 1 (Ero 1) to catalyze the formation of disulfide bonds. Molecular oxygen is the ultimate electron acceptor in this process and yields one H2O2 molecule for every disulfide bond formed [3]. In highly active cells there are rapid increases in H2O2 within the ER; if uncontrolled this increased ROS load would lead to ER stress activating the unfolded protein response (UPR), leading to apoptosis. To prevent ROS overload the ER needs a robust antioxidant mechanism. The most abundant reducing agent in the cell is reduced glutathione (GSH), however, while the ratio of reduced to oxidized (GSSG) glutathione is in excess of 100:1 in the cytosol [4], such a highly reducing environment would be unfavorable for disulfide bond formation. Indeed, the ER maintains a GSH:GSSG ratio closer to 3:1 [5].
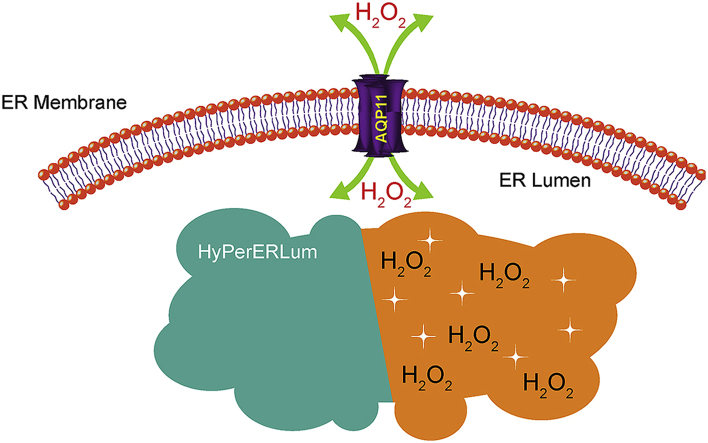
Bestetti et al.. in this current issue of Redox Biology demonstrate that aquaporins play a major role in transporting H2O2 out of the ER [6]. Aquaporins (AQP) are transmembrane channels that were originally described as being either predominantly water permeable or glycerol and water permeable [7]. Since those studies several small uncharged solutes have been described as substrates for AQPs including nitric oxide [8], carbon dioxide [9], ammonia [10,11], and H2O2. In mammals AQP1 [12], AQP3 [13,14], AQP8 [15,16], and AQP9 [17] have been demonstrated to facilitate H2O2 transport across membranes. These aquaporins modulate cell signaling cascades by creating localized increases in H2O2. H2O2 in turn inhibits phosphatases thereby amplifying kinase mediated signaling [15]. Indeed, silencing of AQP8 blunts the epidermal growth factor induced influx of H2O2 as well as the accumulation of tyrosine-phosphorylated proteins. AQP8 silencing decreases H2O2 transit into the ER in digitonin permeabilized cells [15]. This transit was measured using the florescent H2O2 sensor HyPer with an ER targeting domain; however, the localization of AQP8 was not interrogated in this system. AQP8 null mice develop normally and are phenotypically normal except for enlarged testicles [18]. In contrast to this, AQP11 mice die before weaning due to advance polycystic kidney disease, characterized by increased ER stress inducing apoptosis and vacuolization [[19], [20], [21]]. Bestetti et al. interrogate the role of AQP11 in ER H2O2 transit [6]. They demonstrate that AQP11 colocalizes to the ER, interestingly, they also report some degree of colocalization to the mitochondrial-associated membrane (MAM) which are signaling hubs with essential functions in regulating signal transduction. By adding tags on either terminus of recombinant AQP11 they were able to elegantly demonstrate the conformation of APQ11 within the ER membrane; by utilizing HyPer localized to cytosol, mitochondria, or the ER (graphical abstract) they demonstrate the specificity of AQP11 to ER H2O2 transport. They further demonstrate AQP8 primarily localizes to the plasma membrane suggesting that although it may contribute to ER H2O2 transport it is likely this effect occurs while in transit to the plasma membrane.
Unfortunately, redox sensitive dyes are plagued with various caveats and pH sensitivity is the one caveat to consider when using HyPer. Weller et al., in 2014 went so far as to suggest that any use of HyPer should be performed in the presence of a pH indicator such as SypHer [22]. However, Bestetti et al. have performed extra experiments that suggest the movement of H2O2 through AQP11 is a real observation. The ER is a pH neutral location, although accumulation of H2O2 could decrease the pH. In this manuscript, however, exogenous H2O2 was shown to increase HyPer signal, DTT (reducing conditions) influenced HyPer signal, and cells with low basal HyPer signal were used, allowing a more robust ability to measure smaller swings in H2O2 accumulation [6].
Taken together these data demonstrate a central role of AQP11 mediated H2O2 transit into and out of the ER and suggest a mechanism to prevent H2O2 toxicity during protein folding. The potential that AQP11 associates with the MAM and can allow movement of H2O2 is another exciting aspect of this manuscript suggesting a role for this channel in several pathologies. Dysfunctional MAMs have been implicated in diseases ranging from neurodegenerative diseases [23], aging [24], neoplasia [25], to heart failure [26].
Declaration of competing interest
The authors have no conflicts of interest to declare.
Acknowledgements
This work was supported by NIH R01 HL139755 awarded to Christopher B. Pattillo.
References
- 1.Chen K. Regulation of ROS signal transduction by NADPH oxidase 4 localization. J. Cell Biol. 2008;181(7):1129–1139. doi: 10.1083/jcb.200709049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ushio-Fukai M. Localizing NADPH oxidase-derived ROS. Sci. STKE. 2006;2006(349):re8. doi: 10.1126/stke.3492006re8. [DOI] [PubMed] [Google Scholar]
- 3.Tu B.P., Weissman J.S. Oxidative protein folding in eukaryotes: mechanisms and consequences. J. Cell Biol. 2004;164(3):341–346. doi: 10.1083/jcb.200311055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zitka O. Redox status expressed as GSH:GSSG ratio as a marker for oxidative stress in paediatric tumour patients. Oncol Lett. 2012;4(6):1247–1253. doi: 10.3892/ol.2012.931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dixon B.M. Assessment of endoplasmic reticulum glutathione redox status is confounded by extensive ex vivo oxidation. Antioxidants Redox Signal. 2008;10(5):963–972. doi: 10.1089/ars.2007.1869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bestetti S. Human aquaporin-11 guarantees efficient transport of H2O2 across the endoplasmic reticulum membrane. Redox Biol. 2019;28:101326. doi: 10.1016/j.redox.2019.101326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bienert G.P., Chaumont F. Aquaporin-facilitated transmembrane diffusion of hydrogen peroxide. Biochim. Biophys. Acta. 2014;1840(5):1596–1604. doi: 10.1016/j.bbagen.2013.09.017. [DOI] [PubMed] [Google Scholar]
- 8.Herrera M., Hong N.J., Garvin J.L. Aquaporin-1 transports NO across cell membranes. Hypertension. 2006;48(1):157–164. doi: 10.1161/01.HYP.0000223652.29338.77. [DOI] [PubMed] [Google Scholar]
- 9.Uehlein N. The tobacco aquaporin NtAQP1 is a membrane CO2 pore with physiological functions. Nature. 2003;425(6959):734–737. doi: 10.1038/nature02027. [DOI] [PubMed] [Google Scholar]
- 10.Loque D. Tonoplast intrinsic proteins AtTIP2;1 and AtTIP2;3 facilitate NH3 transport into the vacuole. Plant Physiol. 2005;137(2):671–680. doi: 10.1104/pp.104.051268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jahn T.P. Aquaporin homologues in plants and mammals transport ammonia. FEBS Lett. 2004;574(1–3):31–36. doi: 10.1016/j.febslet.2004.08.004. [DOI] [PubMed] [Google Scholar]
- 12.Al Ghouleh I. Aquaporin 1, Nox 1, and Ask 1 mediate oxidant-induced smooth muscle cell hypertrophy. Cardiovasc. Res. 2013;97(1):134–142. doi: 10.1093/cvr/cvs295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Miller E.W., Dickinson B.C., Chang C.J. Aquaporin-3 mediates hydrogen peroxide uptake to regulate downstream intracellular signaling. Proc. Natl. Acad. Sci. U. S. A. 2010;107(36):15681–15686. doi: 10.1073/pnas.1005776107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hara-Chikuma M. Chemokine-dependent T cell migration requires aquaporin-3-mediated hydrogen peroxide uptake. J. Exp. Med. 2012;209(10):1743–1752. doi: 10.1084/jem.20112398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bertolotti M. Tyrosine kinase signal modulation: a matter of H2O2 membrane permeability? Antioxidants Redox Signal. 2013;19(13):1447–1451. doi: 10.1089/ars.2013.5330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Marchissio M.J. Mitochondrial aquaporin-8 knockdown in human hepatoma HepG2 cells causes ROS-induced mitochondrial depolarization and loss of viability. Toxicol. Appl. Pharmacol. 2012;264(2):246–254. doi: 10.1016/j.taap.2012.08.005. [DOI] [PubMed] [Google Scholar]
- 17.Watanabe S. Aquaporin-9 facilitates membrane transport of hydrogen peroxide in mammalian cells. Biochem. Biophys. Res. Commun. 2016;471(1):191–197. doi: 10.1016/j.bbrc.2016.01.153. [DOI] [PubMed] [Google Scholar]
- 18.Yang B. Phenotype analysis of aquaporin-8 null mice. Am. J. Physiol. Cell Physiol. 2005;288(5):C1161–C1170. doi: 10.1152/ajpcell.00564.2004. [DOI] [PubMed] [Google Scholar]
- 19.Morishita Y. Disruption of aquaporin-11 produces polycystic kidneys following vacuolization of the proximal tubule. Mol. Cell. Biol. 2005;25(17):7770–7779. doi: 10.1128/MCB.25.17.7770-7779.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Atochina-Vasserman E.N. Aquaporin 11 insufficiency modulates kidney susceptibility to oxidative stress. Am. J. Physiol. Renal. Physiol. 2013;304(10):F1295–F1307. doi: 10.1152/ajprenal.00344.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Okada S. Aquaporin-11 knockout mice and polycystic kidney disease animals share a common mechanism of cyst formation. FASEB J. 2008;22(10):3672–3684. doi: 10.1096/fj.08-111872. [DOI] [PubMed] [Google Scholar]
- 22.Weller J. Response properties of the genetically encoded optical H2O2 sensor HyPer. Free Radic. Biol. Med. 2014;76:227–241. doi: 10.1016/j.freeradbiomed.2014.07.045. [DOI] [PubMed] [Google Scholar]
- 23.Pinton P. Mitochondria-associated membranes (MAMs) and pathologies. Cell Death Dis. 2018;9(4):413. doi: 10.1038/s41419-018-0424-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Janikiewicz J. Mitochondria-associated membranes in aging and senescence: structure, function, and dynamics. Cell Death Dis. 2018;9(3):332. doi: 10.1038/s41419-017-0105-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Morciano G. Role of mitochondria-associated ER membranes in calcium regulation in cancer-specific settings. Neoplasia. 2018;20(5):510–523. doi: 10.1016/j.neo.2018.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zhang K. Mitochondria-associated endoplasmic reticulum membranes (MAMs) involve in the regulation of mitochondrial dysfunction and heart failure. Acta Biochim. Biophys. Sin. 2018;50(6):618–619. doi: 10.1093/abbs/gmy044. [DOI] [PubMed] [Google Scholar]