Abstract
It has been shown that anti-inflammatory cytokines interleukin-35 (IL-35) and IL-10 could inhibit acute endothelial cell (EC) activation, however, it remains unknown if and by what pathways IL-35 and IL-10 could block atherogenic lipid lysophosphatidylcholine (LPC)-induced sustained EC activation; and if mitochondrial reactive oxygen species (mtROS) can differentiate mediation of EC activation from trained immunity (innate immune memory). Using RNA sequencing analyses, biochemical assays, as well as database mining approaches, we compared the effects of IL-35 and IL-10 in LPC-treated human aortic ECs (HAECs). Principal component analysis revealed that both IL-35 and IL-10 could similarly and partially reverse global transcriptome changes induced by LPC. Gene set enrichment analyses showed that while IL-35 and IL-10 could both block acute EC activation, characterized by upregulation of cytokines/chemokines and adhesion molecules, IL-35 is more potent than IL-10 in suppressing innate immune signatures upregulated by LPC. Surprisingly, LPC did not induce the expression of trained tolerance itaconate pathway enzymes but induced trained immunity enzyme expressions; and neither IL-35 nor IL-10 was found to affect LPC-induced trained immunity gene signatures. Mechanistically, IL-35 and IL-10 could suppress mtROS, which partially mediate LPC-induced EC activation and innate immune response. Therefore, anti-inflammatory cytokines could reverse mtROS-mediated acute and innate immune trans-differentiation responses in HAECs, but it could spare metabolic reprogramming and trained immunity signatures, which may not fully depend on mtROS. Our characterizations of anti-inflammatory cytokines in blocking mtROS-mediated acute and prolonged EC activation, and sparing trained immunity are significant for designing novel strategies for treating cardiovascular diseases, other inflammatory diseases, and cancers.
Keywords: Interleukin-35, Interleukin-10, Endothelial cell activation, Mitochondrial ROS, Trained immunity, Atherosclerosis
Abbreviations: ECs, endothelial cells; HAECs, human aortic endothelial cells; LPC, lysophosphatidylcholine; ROS, reactive oxygen species; MtROS, mitochondrial reactive oxygen species; CVDs, cardiovascular diseases
1. Introduction
Cardiovascular disease (CVD) remains a leading cause of fatality in the United States and worldwide. As a type of chronic autoimmune disease, the development of atherosclerosis is propagated by aberrant immune responses, which mediate the initiation, development, and ultimate lethal thrombotic events such as myocardial infarction, stroke, and peripheral artery disease [1]. We and the others have reported previously that CVD stressors and dangers, such as hyperlipidemia, promote the development of atherosclerosis via several distinct immune-related mechanisms [[2], [3], [4], [5], [6], [7], [8], [9], [10], [11], [12], [13], [14], [15], [16], [17]]. Among these, the role of anti-inflammatory cytokines in the development of atherosclerosis and hyperlipidemia-induced EC activation remains poorly understood.
The innate and adaptive immune cells play essential roles in regulating immune responses by differentiating into various functional subsets [10,11,[18], [19], [20], [21], [22], [23], [24]]. The majority of immune functions of these immune subsets are carried out by secreting various proinflammatory and anti-inflammatory cytokines. Anti-inflammatory cytokines have been found to play essential roles in suppressing atherogenesis [[24], [25], [26]]. It has been shown that CD4+Foxp3+ regulatory T cells (Treg)-related anti-inflammatory cytokines, such as interleukin 35 (IL-35), interleukin 10 (IL-10), and transforming growth factor-β (TGF-β), protect against atherosclerosis development in murine models [[27], [28], [29]]. IL-35 is a recently-characterized Treg-generated anti-inflammatory cytokine, which is a heterodimeric protein with two subunits, IL-35A (IL-12A) and Epstein-Barr virus induced 3 (EBI3) [[30], [31], [32], [33]]. Our previous reports indicated that unlike TGF-β, which is a housekeeping anti-inflammatory cytokine, IL-35 is not constitutively expressed, but rather is induced by proinflammatory stimuli and during early atherosclerosis development [29,30]; and IL-35 inhibits EC activation [29,34,35]. Besides those cytokines secreted by Treg, anti-inflammatory cytokines secreted by other cell types, such as macrophages and mesenchymal stem cells, also play crucial roles in regulating immune response [36,37]. In particular, IL-37 is a member of the IL-1 family of cytokines, which has emerged as a natural suppressor of innate and adaptive inflammatory responses [38,39]. However, the issue of how IL-35 shares its immunosuppressive duty with other anti-inflammatory cytokines such as IL-10 in blocking hyperlipidemia stimuli-induced EC activation remains unknown [40].
As we and others reported, lysophosphatidylcholine (LPC) is a newly proposed conditional danger-associated molecular pattern, which is a group of bioactive, proinflammatory lipid molecules [41,42]. LPC has been critically involved in the development of atherosclerosis [43]. By serving as one type of hyperlipidemia stimuli, LPC could acutely activate ECs by upregulating the expressions of EC adhesion molecules and proinflammatory cytokine genes [44], which mediate the adhesion, migration, and trans-endothelial crossing of leukocytes into the intima [45], contributing to atherosclerosis development [2,4]. We reported six important mechanisms underlying LPC-induced EC activation including: 1) caspase-1/inflammasome activation [2,44,46], 2) proton-leaked facilitated, and ATP generation-uncoupled mitochondrial reactive oxygen species (mtROS) generation [[47], [48], [49], [50]]; 3) mtROS-mediated histone 3 lysine 14 (H3K14) acetylation [29]-mediated EC activation; 4) LPC-induced circular RNAs as EC activation checkpoints [39); 5) differential bindings of acetylated H3K14 in the genomic regions of EC activation effectors versus that of trained immunity genes [51]; and 6) LPC also induced innate immune transdifferentiation and trained immunity memory signatures [51], resulting in prolonged EC activation response [52]. However, whether IL-35 and IL-10 could suppress LPC-induced innate immune reprogramming and trained immunity responses remains unclear.
Using RNA-sequencing (RNA-Seq) analyses and database mining approaches, we found that both IL-35 and IL-10 could block mtROS-mediated acute EC activation. In addition, IL-35 is more potent than IL-10 in suppressing innate immune transdifferentiation signatures in human aortic ECs (HAECs). Furthermore, neither IL-35 nor IL-10 could inhibit the expression of LPC-induced trained immunity enzymes in HAECs. Therefore, we have uncovered that hyperlipidemia-induced trained immunity (innate immune memory) is spared by Treg-secreted anti-inflammatory cytokines. Our analyses of the roles of anti-inflammatory cytokines in suppressing prolonged EC activation and our new working model of two-step innate immune transdifferentiation of ECs are significant for future development of novel anti-inflammatory therapeutics against CVDs, other inflammatory diseases, and cancers.
2. Materials and methods
2.1. Chemicals and cytokines
All the chemicals were from Sigma-Aldrich (St. Louis, MO) unless otherwise indicated. LPC (16:0) was purchased from Avanti Polar Lipids, Inc (#855675P; Alabaster, Alabama). Recombinant human IL-35 (#ALX-522-140-C010) was purchased from Enzo (Farmingdale, NY). Recombinant human IL-10 (#217-IL-005) and TGF-β (#240-B-002) were purchased from R&D systems (Minneapolis, MN). For mtROS measurement, MitoSOX Red Mitochondrial Superoxide Indicator (#M36008; Life technologies, Carlsbad, CA) was used.
2.2. Human samples
All the experiment procedures were performed in accordance with protocols approved by the Institutional Review Board at Temple University, which confirmed to the National Institutes of Health Guidelines. All the studies involving human subjects abide by the Declaration of Helsinki principles.
2.3. Cell culture
HAECs (Lonza, CC2535; Walkersville, MD) were cultured in the M199 medium (Hyclone laboratories, Logan, UT) supplemented with 15% fetal bovine serum (FBS; HyClone, Logan, UT); endothelial cell growth supplement (ECGS, 50 μg/mL); BD Biosciences, San Jose, CA); heparin (50 μg/mL); and 1% penicillin, streptomycin, and amphotericin (PSA; Invitrogen, Carlsbad, CA). HAECs were grown on 0.2% gelatin-coated flasks, plates, or dishes and experiments were performed at passage 9.
2.4. Fluorescence activated cell sorting (FACS)
For mtROS measurement: After staining with MitoSOX, HAECs were incubated at 37 °C for 10 min (min) and washed with PBS twice afterwards. After LPC treatment, cells were washed once with ice-cold PBS and Trypsin-EDTA was added to detach cells. Trypsinization was terminated by adding FACS buffer (2% FBS in PBS) and cells were collected by centrifugation. After re-suspension in 0.2 ml FACS buffer, samples were subjected to flow cytometry analysis, where fluorescence emissions were measured by FACSCalibur machine (BD, Franklin Lakes, NJ).
2.5. RNA-Seq
RNA-Seq was performed by BGI (Shenzhen, China). Total RNAs were extracted from samples, then mRNA and non-coding RNAs were enriched by removing rRNA from the total RNAs. By using the fragmentation buffer, the mRNAs were fragmented into short fragments (about 200–500 nucleotides (nt)), then the first-strand cDNA was synthesized by random hexamer-primer using the fragments as templates; and dTTP was substituted by dUTP during the synthesis of the second strand. Short fragments were purified and resolved with elution buffer for end reparation and single nucleotide A (adenine) addition. After that, the short fragments were connected with adapters, then the second strand was degraded finally using UNG (Uracil-N-Glycosylase) [2]. After agarose gel electrophoresis, the suitable fragments were selected for the PCR amplification as templates. During the quality control steps, Agilent 2100 Bioanaylzer (https://www.genomics.agilent.com/en/Bioanalyzer-System/2100-Bioanalyzer-Instruments/?cid=AG-PT-106) and ABI StepOnePlus Real-Time PCR System (https://www.thermofisher.com/order/catalog/product/4376600) were used in quantification and qualification of the sample library. At last, the library was sequenced by Illumina HiSeq4000 using PE100 strategy. Primary sequencing data that produced by Illumina Hiseq4000 called as raw reads, were filtered into clean reads by removing adaptors contained and low quality reads by BGI (Shenzhen, China) in-house software. Reference annotation based assembly method was used to reconstruct the transcripts by Tophat (v2.0.10) + Cufflinks (v2.1.1), while background noise was reduced by using FPKM (Fragments Per Kilobase Million) and coverage threshold. Data were deposited as E-MTAB-6604 at ArrayExpress.
2.6. Sequencing data analyses
Data analysis was carried out using the statistical computing environment, R, the Bioconductor suite of packages for R, and RStudio (https://www.rstudio.com/) [53]. Raw data were background subtracted, variance stabilized, and normalized by robust spline normalization. Differentially expressed genes were identified by linear modeling and Bayesian statistics using the Limma package. Pathway analysis was performed using Gene Set Enrichment Analysis (GSEA, http://software.broadinstitute.org/gsea/index.jsp). Briefly, GSEA is a computational method that determines whether a priori defined set of genes shows statistically significant, concordant differences between two biological states. GSEA does not focus on only significantly/highly changed genes, but examines all the genes that belongs to a certain biological process instead.
Statistical analyses — Data were expressed as the mean ± standard error of the mean (SEM). For comparisons between two groups, two-tailed Student t-test was used for evaluation of statistical significance or, when the data were not normally distributed, a nonparametric Mann-Whitney U test was used. For comparisons across multiple groups, one-way ANOVA with Bonferroni post-test adjustment was used or, when the data were not normally distributed, the data were analyzed using one-way ANOVA with the Kruskal-Wallis test, followed by pairwise comparison using the Dunn test. For linear regression tests, simple linear regression analyses were performed using GraphPad Prism (https://www.graphpad.com/scientific-software/prism/) to determine coefficient of determination and p value. Data shown were representative of two to three independent experiments. NS, not significant; *, significant with p < 0.05; ***, p < 0.001.
3. Results
3.1. IL-35 is more potent than IL-10 in suppressing LPC-induced ICAM-1 gene expression
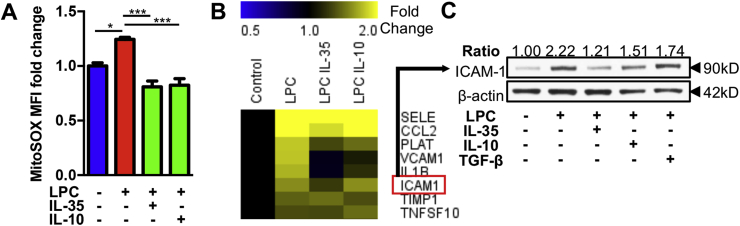
We have reported previously that IL-35 could suppress LPC-induced human aortic endothelial activation by inhibiting mtROS [48]. In this study, we hypothesized that anti-inflammatory cytokines including IL-35 and IL-10 are different in their scales to inhibit EC activation. To test this hypothesis, we firstly measured the production of mtROS and found that both IL-35 and IL-10, when used in the same dosage, similarly blocked LPC-induced mtROS in HAECs (Fig. 1A). Nevertheless, by using QIAGEN Endothelial Cell Biology PCR array, we found that IL-35 was more potent in suppressing the expression of proinflammatory cytokines and adhesion molecule genes including interleukin 1 beta (IL1B) and intercellular adhesion molecule 1 (ICAM1) (Fig. 1B and Supplemental Table 1). Moreover, IL-35 was more potent than IL-10 and TGF-β in suppressing ICAM-1 protein expression (Fig. 1C). Taken together, our results indicate that IL-35 is a more potent anti-inflammatory cytokine than IL-10 in suppressing LPC-induced and mtROS-mediated EC activation.
Fig. 1.
IL-35 and IL-10 suppress lysophosphatidylcholine (LPC)-induced human aortic endothelial cell (HAEC) activation by inhibiting mitochondrial reactive oxygen species (mtROS), adhesion molecules (ICAM1 and VCAM1), and proinflammatory cytokine IL1B; IL-35 is more potent than IL-10 in suppressing LPC-induced ICAM-1 expression. HAEC were stimulated by LPC (10 μM) with or without IL-35/IL-10/TGF-β (10 ng/mL) for 1 h (A) or 18 h (B&C), mtROS measurement using flow cytometry (n = 4 in each group) (A), Human Endothelial Cell Biology RT2 Profiler PCR Array (B; 3 pooled samples in each group), and Western Blot (C) were performed. Of note, transforming growth factor-β (TGF-β) served as a ‘house-keeping anti-inflammatory cytokine” control (see our paper, PMID: 22438968). For all panels, data are expressed as mean ± SEM and data are representative of at least two independent experiments. ***p < 0.001, *p < 0.05. MitoSOX is a fluorogenic dye to specifically measure mtROS in live cells. LPC, lysophosphatidylcholine; HAEC, human aortic endothelial cells; mtROS: mitochondrial reactive oxygen species.
3.2. IL-35 and IL-10 similarly and partially reverse the effects of LPC on global gene expression in HAECs
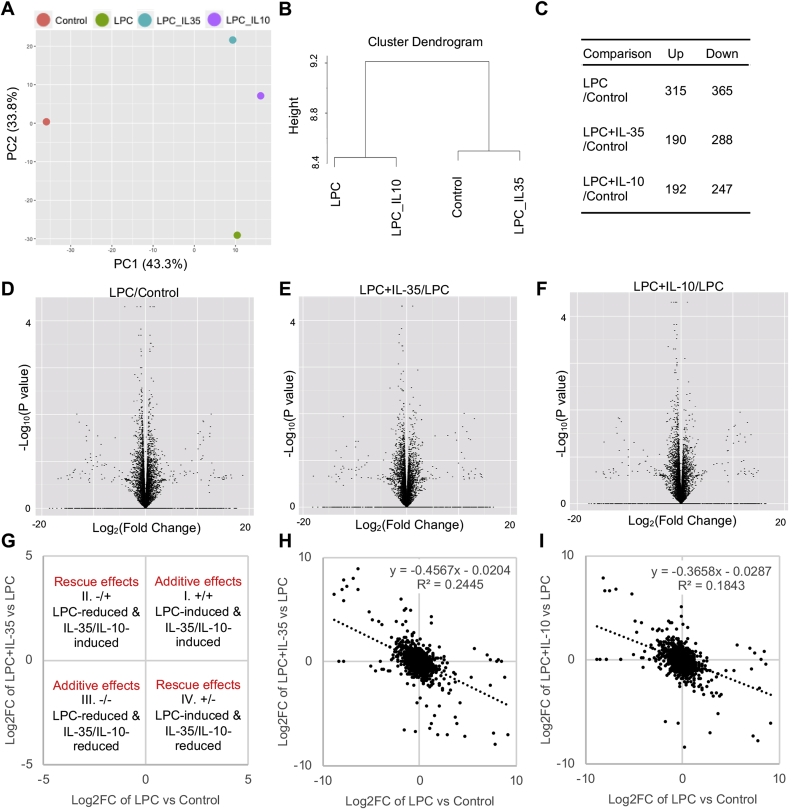
Next, we hypothesized that IL-35 and IL-10 could reverse LPC-induced global gene expression in HAECs. To test this hypothesis, we performed RNA sequencing (RNA-Seq) of 1) control, 2) LPC-treated, 3) LPC plus IL-35-treated, and 4) LPC plus IL-10-treated HAECs. When Principal component (PC) analysis (PCA) was performed, we found that IL-35-treated, and IL-10-treated group clustered together, indicating that IL-35 and IL-10 had similar effects in regulating global gene expression in HAECs (Fig. 2A). Moreover, although both IL-35 and IL-10 could completely reverse the effects of LPC on PC2, which accounted for 33.8% of the differences among groups, neither IL-35 nor IL-10 could block LPC-induced PC1 changes, which accounted for 43.3% of the differences (Fig. 2A) (also see others’ introduction on PCA https://www.sciencedirect.com/topics/engineering/principal-component-analysis). Nevertheless, when cluster dendrogram analysis was performed, we found that IL-35 was more potent than IL-10 in reversing the effects of LPC on HAECs (Fig. 2B). In addition, while IL-35 and IL-10 could upregulate similar numbers of genes, IL-35 downregulated 41 more genes than IL-10 in LPC-treated cells (Fig. 2C). When examining all the significantly changed genes, we found that although LPC could upregulate and downregulate roughly similar numbers of genes in HAECs, IL-35 and IL-10 were mainly inhibitory in regulating gene expressions (Figs. 2D to 2F). To further determine the relationship between LPC-changed genes and IL-35/IL-10-changed genes, we plotted fold change by fold change (FC/FC) plot (Fig. 2G), which showed that both IL-35 and IL-10 could partially reverse LPC-changed genes in HAECs (Fig. 2H and I). Thus, both IL-35 and IL-10 could partially reverse LPC-induced global gene expression in HAECs.
Fig. 2.
IL-35 and IL-10 similarly and partially reverse the modulation effects of LPC on global gene expressions in HAECs. HAECs were treated with LPC (10 μM), with or without IL-35/IL-10 (10 ng/mL) for 18 h, RNA sequencing (RNA-Seq) experiments were performed. A. Principal component analyses (PCA) of the RNA-Seq data. B. Cluster analysis. C. Summary of the number of changed genes (by 1.4-fold) by LPC, IL-35, and IL-10. D to F. Volcano plots showing the genes that are changed by LPC (D), IL-35 (E), and IL-10 (F). G. Principle of the fold change by fold change (FC/FC) plot. H & I. FC/FC plot showing the relationship between LPC-induced genes and the genes that are regulated by IL-35 or IL-10.
3.3. IL-35 blocks innate immune signatures in HAECs
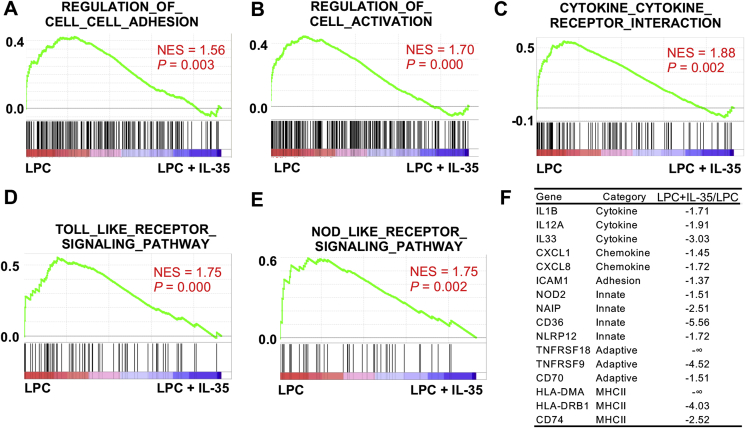
We have previously reported that LPC could induce innate immune signatures in HAECs, resulting in prolonged EC activation [54]. To examine the hypothesis that IL-35 and IL-10 could block innate immune signatures in HAECs, we performed gene set enrichment analysis (GSEA) [55]. We found that IL-35 not only significantly blocked LPC-induced gene expressions of EC adhesion molecules, activation marker genes, and cytokine/cytokine receptor genes, which collectively define acute EC activation (Fig. 3A and C), but also significantly reversed innate immune signature pathways including Toll-like receptor and Nod-like receptor pathways, indicating that IL-35 could block LPC-induced innate immune transdifferentiation in HAECs (Fig. 3D and E), as defined in our previous report [56]. When looking at individual genes, we found that IL-35 not only blocked the expressions of acute EC activation marker genes such as IL1B, IL33 [57], IL12A, CXCL1, CXCL8, and ICAM-1, but also inhibited the gene expression of innate immune signature genes including nucleotide-binding oligomerization domain-containing protein 2 (NOD2), neuronal apoptosis inhibitory protein (NAIP), CD36 (also known as platelet glycoprotein 4, fatty acid translocase, and scavenger receptor class B member 3), nucleotide-binding domain and leucine-rich repeat containing (NLR) family pyrin domain containing 12 (NLRP12), tumor necrosis factor receptor superfamily member 18 (TNFRSF18), TNFRSF9, CD70 (a ligand for CD27), human leukocyte antigen (HLA)-DMA, HLA-DRB1, and CD74 (also known as HLA class II histocompatibility antigen gamma chain, and HLA-DR antigens-associated invariant chain) (Fig. 3F).
Fig. 3.
IL-35 blocks LPC-induced upregulation of innate immune signatures in HAECs. HAECs were treated with LPC (10 μM), with or without IL-35 (10 ng/mL) for 18 h, RNA sequencing (RNA-Seq) experiments were performed. A to E. Gene set enrichment analysis (GSEA) plots showing the significantly enriched pathways that were downregulated by IL-35 in HAECs. F. Representative cytokines, chemokines, adhesion molecules, innate, and adaptive gene molecules that were decreased by IL-35. NES, normalized enrichment score.
3.4. IL-35 is more effective than IL-10 in blocking innate immune signatures in HAECs
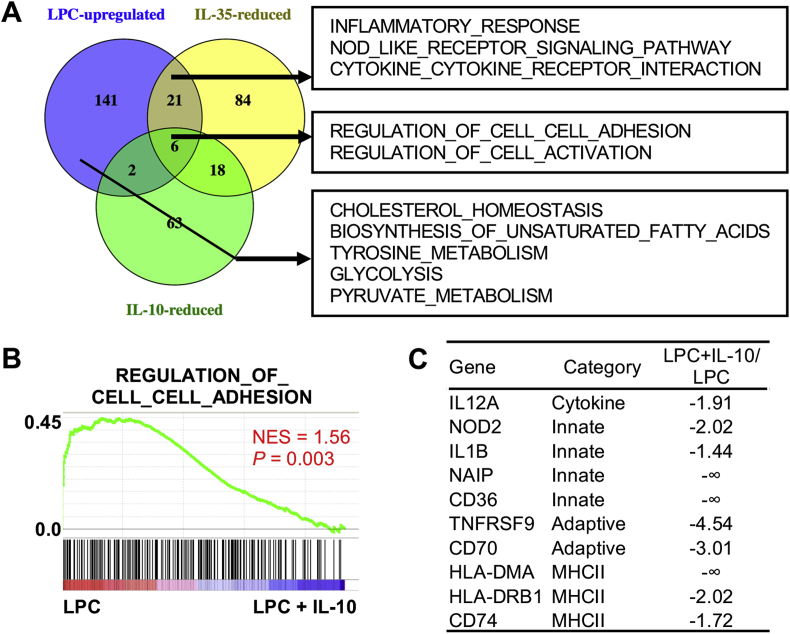
In contrast to IL-35, we found that IL-10 reversed much less gene set signatures in HAECs, and could not significantly reverse the signatures of inflammatory response, Nod-like receptor signaling pathways, and cytokine-cytokine receptor interaction, although both IL-35 and IL-10 could block adhesion molecule and cell activation signatures (Fig. 4A and B). Nevertheless, when looking at individual genes, IL-10 did inhibit some innate immune signatures, as IL-35 did (Fig. 4C). Moreover, LPC downregulated IL-35 receptor IL35RB2 (IL12RB2) in HAECs, which could be rescued by IL-35. In contrast, neither LPC nor IL-10 had any effects on IL-10 receptor subunits (Supplemental Table 2). Taken together, our results showed that IL-35 is more potent than IL-10 in blocking innate immune signatures and in amplifying its own response pathways in HAECs.
Fig. 4.
IL-35 is more effective than IL-10 in blocking LPC-induced innate immune signature genes in HAECs, while neither IL-35 nor IL-10 could block LPC-induced reprogramming of key immunometabolic pathways in HAECs, such as cholesterol homeostasis, biosynthesis of unsaturated fatty acids, tyrosine metabolism, glycolysis, and pyruvate metabolism. HAECs were treated with LPC (10 μM), with or without IL-10 (10 ng/mL) for 18 h, RNA sequencing (RNA-Seq) experiments were performed. A. Venn diagram of the Gene set enrichment analysis (GSEA) pathways that are upregulated by LPC, downregulated by IL-35, and downregulated by IL-10 in HAECs. B. Representative GSEA plot from the downregulated pathways by IL-10. C. Representative cytokines, adhesion molecules, innate, and adaptive gene molecules that are decreased by IL-10. NES, normalized enrichment score.
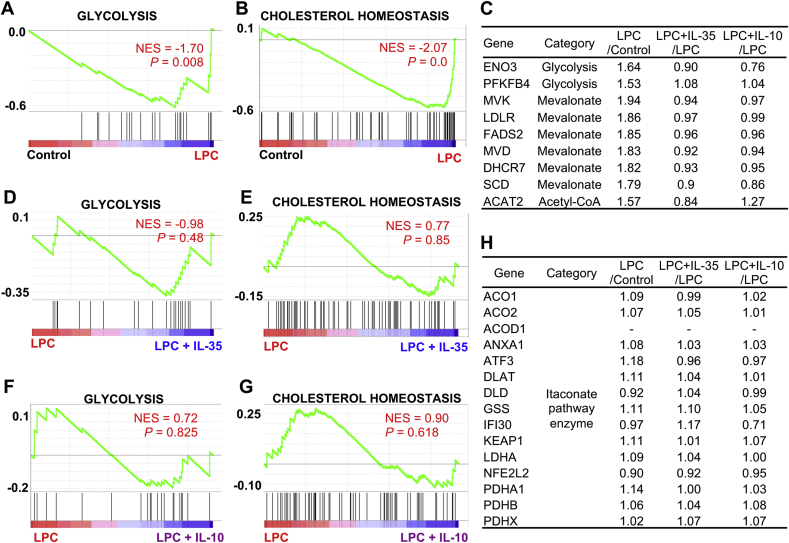
3.5. Proatherogenic LPC does not induce the expression of trained tolerance itaconate pathway enzymes when inducing trained immunity enzymes; and IL-35 and IL-10 spare metabolic reprogramming and trained immunity signatures
Reprograming of the expressions of metabolic pathways such as cholesterol metabolism, glycolysis, acetyl-CoA metabolic enzymes are prominent features of trained immunity responses whereas the regulations of itaconate pathway enzymes are prominent features of trained tolerance responses [58,59]. Since we recently reported that LPC could induce trained immunity signature gene expressions in HAECs [51], we hypothesized that IL-35 and IL-10 might affect this response. Our results showed that LPC significantly induced the expressions of metabolic signature genes such as cholesterol homeostasis, glycolysis, and acetyl-CoA metabolic pathway in HAECs (Fig. 4, Fig. 5C; Supplemental Table 3). Importantly, neither IL-35 nor IL-10 could reverse LPC-induced the expressions of metabolic reprogramming and trained immunity signature genes (Fig. 4, Fig. 5G; Supplemental Table 3). Similarly, when we cross-examined the upregulated trained immunity genes from β-glucan-treated human monocytes [60] through database mining for a comparison, we found that there was no overlap between trained immunity genes from human monocytes and IL-35/IL-10-inhibited genes in HAECs (Supplemental Fig. 1), suggesting that non-inhibition of trained immunity genes by IL-35/IL-10 is not limited to ECs. Next, we examined published transcriptome studies, which studied the effects of IL-35 loss-of-function/gain-of-function in T cells [61,62]; and found that IL-35 did not affect the expressions of trained immunity signatures in T cells as well (Supplemental Fig. 2). Moreover, LPC, IL-35, and IL-10 did not change the trained tolerance itaconate pathway enzyme gene expressions [59,63] (Fig. 5H). Taken together, our results indicate that proatherogenic LPC does not induce the expression of trained tolerance itaconate pathway enzymes when inducing trained immunity enzymes; that IL-35 and IL-10 do not affect LPC-induced metabolic reprogramming and trained immunity signatures in HAECs, and that IL-35 also did not affect trained immunity signatures in immune cells as well.
Fig. 5.
IL-35 and IL-10 do not reverse LPC-induced upregulation of trained immunity signature enzymes in HAECs. HAECs were treated with LPC (10 μM), with or without IL-35/IL-10 (10 ng/mL) for 18 h, RNA sequencing (RNA-Seq) experiments were performed. A & B. Gene set enrichment analysis (GSEA) plots showing that glycolysis and cholesterol homeostasis pathways are upregulated by LPC in HAECs. C. Representative gene expression changes by LPC, IL-35, and IL-10. D & E. GSEA plots showing that glycolysis and cholesterol homeostasis pathways are not affected by IL-35 in HAECs. F & G. GSEA plots showing that glycolysis and cholesterol homeostasis pathways are not changed by IL-10 in HAECs. H. Representative gene expression changes by LPC, IL-35, and IL-10. NES, normalized enrichment score.
3.6. IL-35/IL-10-inhibited ROS partially mediate LPC-induced EC activation and innate immunity response, while ROS are not involved in trained immunity response
Since we found that both IL-35 and IL-10 could inhibit LPC-induced mtROS (Fig. 1A) and innate immunity response (Fig. 3, Fig. 4), we hypothesized that IL-35/IL-10 could block LPC-induced innate immunity response by ROS-related mechanisms. To test this hypothesis, we analyzed the relationships between the genes that are significantly upregulated in anti-oxidant transcription factor nuclear factor erythroid 2–related factor 2 (Nrf2) knockout cells (ROS-upregulated genes) [64] and LPC-upregulated genes, IL-35-inhibited genes, and IL-10-inhibited genes in HAECs. We identified 10 ROS-upregulated genes were induced by LPC. Among these 10 genes, IL-35 and IL-10 could both block 3 genes, including IGFBP5, TRAF1, and IL1B, while CXCL1 was only blocked by IL-35 (Supplemental Fig. 3). We further hypothesized that ROS might be involved in mediating innate immunity response, but not trained immunity response. To test this hypothesis, we analyzed the RNA-Seq results from lipopolysaccharide (LPS)-treated human monocytes (Innate immunity response) and β-glucan-treated human monocytes (trained immunity response) [60]. We found that 10 innate immunity response genes are ROS target genes, while none of the trained immunity response genes are ROS target genes (Supplemental Fig. 4). Notably, there was very little overlap between innate immunity genes and trained immunity genes in human monocytes, even the two datasets were collected from the same RNA-Seq experiments (Supplemental Fig. 4), suggesting that innate immunity and trained immunity engage distinct cellular signaling programs in ECs and other immune cells. Reciprocally, when looking at the effects of innate immunity/trained immunity genes on ROS production, we found that neither LPS-induced innate immunity response nor β-glucan-induced trained immunity response has any effect on the expressions of ROS-regulating enzymes or upstream regulators (Supplemental Table 4; Supplemental Fig. 5). Taken together, these results helped to explain why IL-35/IL-10 could block LPC-induced innate immune response, but spare trained immunity response in HAECs: IL-35/IL-10-inhibited ROS are located in the upstream in the regulatory pathways and partially mediate LPC-induced EC activation and innate immune response, whereas ROS are not significantly involved in trained immunity (innate immune memory) responses.
4. Discussion
Atherosclerosis is a chronic inflammatory pathological process, which drives the development of CVDs such as myocardial infarction, stroke, and peripheral artery disease. Despite of our significant knowledge of the important roles the immune system plays during atherosclerosis development, there are still limited options of anti-inflammatory therapeutic strategies that could prevent or treat atherosclerotic CVDs [65]. We have previously reported that LPC could induce innate immune signatures and trained immunity responses in HAECs, resulting in prolonged EC activation [51,52]. In addition, we have previously reported that IL-35 is induced during early atherosclerosis; and it could block LPC-induced EC activation by inhibiting mtROS-mediated site-specific acetylation of histone 3 lysine 14 (H3K14) [29]. In the current study, we compared the effects of two anti-inflammatory cytokines IL-35 and IL-10 in LPC-induced EC activation and investigated their roles in LPC-induced innate immune transdifferentiation and trained immunity responses. We have made the following findings: 1) IL-35 and IL-10 similarly block LPC-induced mtROS generation in HAECs, while IL-35 is more potent than IL-10 in blocking LPC-induced and mtROS-mediated ICAM1 gene expression; 2) IL-35 and IL-10 partially reverse LPC-induced global transcriptome changes in HAECs; 3) IL-35 is more effective than IL-10 in blocking LPC-induced expressions of innate immune signature genes in HAECs; 4) IL-35 could amplify its own signaling response in HAECs by upregulating IL-35 receptor subunit IL35RB2 (IL12RB2) gene expression; 5) LPC does not induce trained tolerance itaconate pathway enzyme expression but induces the expressions of trained immunity pathway enzymes; and neither IL-35 nor IL-10 could block LPC-induced the expressions of metabolic and trained immunity signature genes in HAECs; and 6) IL-35 does not block the expressions of trained immunity signature genes in T cells.
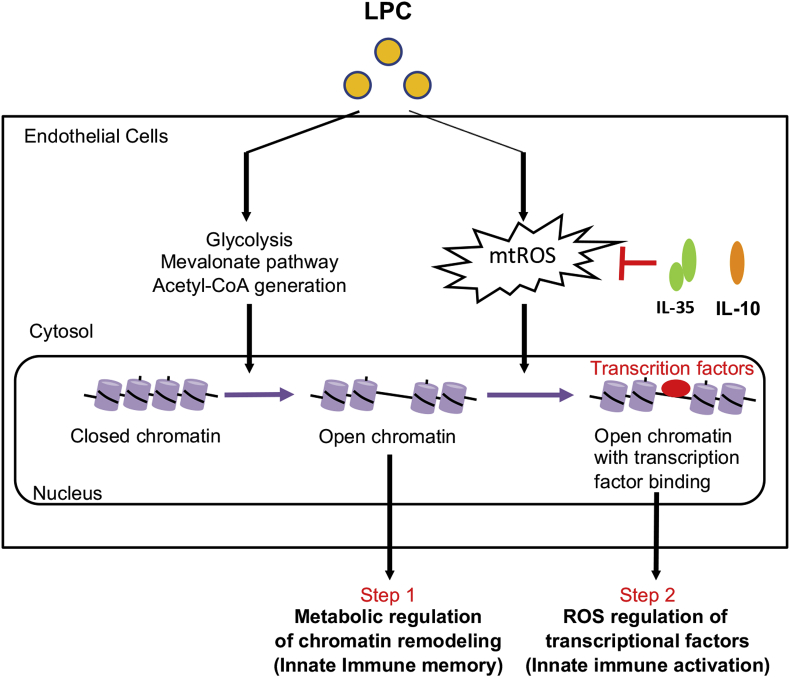
To summarize, we propose a new working model of how Treg and other cell-generated anti-inflammatory cytokines IL-35 and IL-10 share their duties in inhibiting acute and prolonged human aortic EC activation by hyperlipidemia stimuli LPC: LPC could induce innate immune transdifferentiation in ECs, which is characterized by enhanced expression of adhesion molecules, cytokines, chemokines, DAMP receptors, costimulatory molecules, and MHC class II. This process is characterized by two steps: In the first step, LPC induce metabolic reprogramming, including upregulation of glycolysis, mevalonate enzymes, and acetyl-CoA generation, leading to chromatin remodeling in the genomic regions encoding innate immune signature genes [51] and innate immune memory without induction of trained tolerance itaconate pathway enzymes. In the second step, LPC-induced mtROS are responsible for the H3K14 acetylation-mediated “opening of chromatin” [29] and recruitment of pro-inflammatory transcription factors such as AP-1 [47] to the genomic regions encoding EC activation genes, leading to innate immune activation [52]. While IL-35 and IL-10 could suppress LPC-induced innate immune activation via inhibiting mtROS, they could not suppress LPC-induced metabolic reprogramming and trained innate immune memory response (Fig. 6). Of note, since many types of ROS have been identified as we recently summarized [66,67], the future work will be needed to determine which types of ROS regulate innate immune activations, metabolic remodeling, and trained immunity as well as trained tolerance in ECs and other cell types. Our findings have provided an important insight on the relationship between ROS-mediated endothelial activation programs and ROS-independent trained immunity/trained tolerance in aortic ECs; and our results are significant for future design of novel anti-inflammatory therapies against CVDs, other inflammatory diseases, and cancers.
Fig. 6.
Anti-inflammatory cytokines IL-35 and IL-10 block LPC-induced innate immune activation, but not immune memory in HAECs. LPC could induce innate immune transdifferentiation in HAECs, which is characterized by enhanced expression of adhesion molecules, cytokines, chemokines, danger-associated molecular pattern (DAMP) receptors, costimulatory molecules, and MHC class II. This process is characterized by two steps: In the first step, LPC induce metabolic reprogramming, including upregulation of glycolysis, mevalonate enzymes, and acetyl-CoA generation, leading to chromatin remodeling in the genomic regions encoding innate immune signature genes and innate immune memory. In the second step, LPC-induced mtROS are responsible for the recruitment of pro-inflammatory transcription factors, leading to innate immune activation. While IL-35 and IL-10 could suppress LPC-induced innate immune activation via inhibiting mtROS, they could not suppress LPC-induced metabolic reprogramming and trained innate immune memory response.
Funding sources
This work was partially supported by the National Institutes of Health Grants to XFY and HW.
Declaration of competing interest
None.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.redox.2019.101373.
Appendix A. Supplementary data
The following are the Supplementary data to this article:
References
- 1.Packard R.R., Lichtman A.H., Libby P. Innate and adaptive immunity in atherosclerosis. Semin. Immunopathol. 2009;31:5–22. doi: 10.1007/s00281-009-0153-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Yin Y., Li X., Sha X., Xi H., Li Y.F., Shao Y., Mai J., Virtue A., Lopez-Pastrana J., Meng S., Tilley D.G., Monroy M.A., Choi E.T., Thomas C.J., Jiang X., Wang H., Yang X.F. Early hyperlipidemia promotes endothelial activation via a caspase-1-sirtuin 1 pathway. Arterioscler. Thromb. Vasc. Biol. 2015;35:804–816. doi: 10.1161/ATVBAHA.115.305282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Shao Y., Cheng Z., Li X., Chernaya V., Wang H., Yang X.F. Immunosuppressive/anti-inflammatory cytokines directly and indirectly inhibit endothelial dysfunction- a novel mechanism for maintaining vascular function. J. Hematol. Oncol. 2014;7:80. doi: 10.1186/s13045-014-0080-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Li X., Fang P., Li Y., Kuo Y.M., Andrews A.J., Nanayakkara G., Johnson C., Fu H., Shan H., Du F., Hoffman N.E., Yu D., Eguchi S., Madesh M., Koch W.J., Sun J., Jiang X., Wang H., Yang X. Mitochondrial reactive oxygen species mediate lysophosphatidylcholine-induced endothelial cell activation. Arterioscler. Thromb. Vasc. Biol. 2016;36:1090–1100. doi: 10.1161/ATVBAHA.115.306964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Li X., Fang P., Yang W.Y., Chan K., Lavallee M., Xu K., Gao T., Wang H., Yang X. Mitochondrial ROS, uncoupled from ATP synthesis, determine endothelial activation for both physiological recruitment of patrolling cells and pathological recruitment of inflammatory cells. Can. J. Physiol. Pharmacol. 2016:1–6. doi: 10.1139/cjpp-2016-0515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Combadiere C., Potteaux S., Rodero M., Simon T., Pezard A., Esposito B., Merval R., Proudfoot A., Tedgui A., Mallat Z. Combined inhibition of CCL2, CX3CR1, and CCR5 abrogates Ly6C(hi) and Ly6C(lo) monocytosis and almost abolishes atherosclerosis in hypercholesterolemic mice. Circulation. 2008;117:1649–1657. doi: 10.1161/CIRCULATIONAHA.107.745091. [DOI] [PubMed] [Google Scholar]
- 7.Fang P., Zhang D., Cheng Z., Yan C., Jiang X., Kruger W.D., Meng S., Arning E., Bottiglieri T., Choi E.T., Han Y., Yang X.F., Wang H. Hyperhomocysteinemia potentiates hyperglycemia-induced inflammatory monocyte differentiation and atherosclerosis. Diabetes. 2014;63:4275–4290. doi: 10.2337/db14-0809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fang P., Li X., Shan H., Saredy J.J., Cueto R., Xia J., Jiang X., Yang X.F., Wang H. Ly6C(+) inflammatory monocyte differentiation partially mediates hyperhomocysteinemia-induced vascular dysfunction in type 2 diabetic db/db mice. Arterioscler. Thromb. Vasc. Biol. 2019;39:2097–2119. doi: 10.1161/ATVBAHA.119.313138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ait-Oufella H., Salomon B.L., Potteaux S., Robertson A.K., Gourdy P., Zoll J., Merval R., Esposito B., Cohen J.L., Fisson S., Flavell R.A., Hansson G.K., Klatzmann D., Tedgui A., Mallat Z. Natural regulatory T cells control the development of atherosclerosis in mice. Nat. Med. 2006;12:178–180. doi: 10.1038/nm1343. [DOI] [PubMed] [Google Scholar]
- 10.Xiong Z., Yan Y., Song J., Fang P., Yin Y., Yang Y., Cowan A., Wang H., Yang X.F. Expression of TCTP antisense in CD25(high) regulatory T cells aggravates cuff-injured vascular inflammation. Atherosclerosis. 2009;203:401–408. doi: 10.1016/j.atherosclerosis.2008.07.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yang W.Y., Shao Y., Lopez-Pastrana J., Mai J., Wang H., Yang X.F. Pathological conditions re-shape physiological Tregs into pathological Tregs. Burns Trauma. 2015;3 doi: 10.1186/s41038-015-0001-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Du F., Zhou J., Gong R., Huang X., Pansuria M., Virtue A., Li X., Wang H., Yang X.F. Endothelial progenitor cells in atherosclerosis. Front. Biosci. 2012;17:2327–2349. doi: 10.2741/4055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Li Y.F., Ren L.N., Guo G., Cannella L.A., Chernaya V., Samuel S., Liu S.X., Wang H., Yang X.F. Endothelial progenitor cells in ischemic stroke: an exploration from hypothesis to therapy. J. Hematol. Oncol. 2015;8:33. doi: 10.1186/s13045-015-0130-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ferrer L.M., Monroy A.M., Lopez-Pastrana J., Nanayakkara G., Cueto R., Li Y.F., Li X., Wang H., Yang X.F., Choi E.T. Caspase-1 plays a critical role in accelerating chronic kidney disease-promoted neointimal hyperplasia in the carotid artery. Journal of cardiovascular translational research. 2016;9:135–144. doi: 10.1007/s12265-016-9683-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Monroy M.A., Fang J., Li S., Ferrer L., Birkenbach M.P., Lee I.J., Wang H., Yang X.F., Choi E.T. Chronic kidney disease alters vascular smooth muscle cell phenotype. Front Biosci (Landmark Ed) 2015;20:784–795. doi: 10.2741/4337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ferrer L.M., Monroy A.M., Lopez-Pastrana J., Nanayakkara G., Cueto R., Li Y.F., Li X., Wang H., Yang X.F., Choi E.T. Caspase-1 plays a critical role in accelerating chronic kidney disease-promoted neointimal hyperplasia in the carotid artery. J. Cardiovasc. Transl. Res. 2016;9:135–144. doi: 10.1007/s12265-016-9683-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Virtue A., Johnson C., Lopez-Pastrana J., Shao Y., Fu H., Li X., Li Y.F., Yin Y., Mai J., Rizzo V., Tordoff M., Bagi Z., Shan H., Jiang X., Wang H., Yang X.F. MicroRNA-155 deficiency leads to decreased atherosclerosis, increased white adipose tissue obesity, and non-alcoholic fatty liver disease: a novel mouse model OF obesity paradox. J. Biol. Chem. 2017;292:1267–1287. doi: 10.1074/jbc.M116.739839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mai J., Wang H., Yang X.F. Th 17 cells interplay with Foxp3+ Tregs in regulation of inflammation and autoimmunity. Front. Biosci. 2010;15:986–1006. doi: 10.2741/3657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fang P., Li X., Dai J., Cole L., Camacho J.A., Zhang Y., Ji Y., Wang J., Yang X.F., Wang H. Immune cell subset differentiation and tissue inflammation. J. Hematol. Oncol. 2018;11:97. doi: 10.1186/s13045-018-0637-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Xu K., Yang W.Y., Nanayakkara G.K., Shao Y., Yang F., Hu W., Choi E.T., Wang H., Yang X. gaTa3, hDac6, and Bcl6 regulate FOXP3+ Treg Plasticity and Determine Treg conversion into either novel antigen-Presenting cell-like Treg or Th1-Treg. Front. Immunol. 2018;9:45. doi: 10.3389/fimmu.2018.00045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mai J., Nanayakkara G., Lopez-Pastrana J., Li X., Li Y.F., Wang X., Song A., Virtue A., Shao Y., Shan H., Liu F., Autieri M.V., Kunapuli S.P., Iwakura Y., Jiang X., Wang H., Yang X.F. Interleukin-17A promotes aortic endothelial cell activation via transcriptionally and post-translationally activating p38 mitogen-activated protein kinase (MAPK) pathway. J. Biol. Chem. 2016;291:4939–4954. doi: 10.1074/jbc.M115.690081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Xiong Z., Song J., Yan Y., Huang Y., Cowan A., Wang H., Yang X.F. Higher expression of Bax in regulatory T cells increases vascular inflammation. Front. Biosci. 2008;13:7143–7155. doi: 10.2741/3217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yan Y., Xiong Z., Zhang S., Song J., Huang Y., Thornton A.M., Wang H., Yang X.F. CD25high T cells with a prolonged survival inhibit development of diabetes. Int. J. Immunopathol. Pharmacol. 2008;21:767–780. doi: 10.1177/039463200802100401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Yang X.F., Yin Y., Wang H. Vascular inflammation and atherogenesis are activated via receptors for pamps and suppressed by regulatory T cells. Drug Discov. Today Ther. Strat. 2008;5:125–142. doi: 10.1016/j.ddstr.2008.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ait-Oufella H., Taleb S., Mallat Z., Tedgui A. Recent advances on the role of cytokines in atherosclerosis. Arterioscler. Thromb. Vasc. Biol. 2011;31:969–979. doi: 10.1161/ATVBAHA.110.207415. [DOI] [PubMed] [Google Scholar]
- 26.Pastrana J.L., Sha X., Virtue A., Mai J., Cueto R., Lee I.A., Wang H., Yang X.F. Regulatory T cells and atherosclerosis. J. Clin. Exp. Cardiol. 2012:2. doi: 10.4172/2155-9880.S12-002. 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Caligiuri G., Rudling M., Ollivier V., Jacob M.P., Michel J.B., Hansson G.K., Nicoletti A. Interleukin-10 deficiency increases atherosclerosis, thrombosis, and low-density lipoproteins in apolipoprotein E knockout mice. Mol. Med. 2003;9:10–17. [PMC free article] [PubMed] [Google Scholar]
- 28.Singh N.N., Ramji D.P. The role of transforming growth factor-beta in atherosclerosis. Cytokine Growth Factor Rev. 2006;17:487–499. doi: 10.1016/j.cytogfr.2006.09.002. [DOI] [PubMed] [Google Scholar]
- 29.Li X., Shao Y., Sha X., Fang P., Kuo Y.M., Andrews A.J., Li Y., Yang W.Y., Maddaloni M., Pascual D.W., Luo J.J., Jiang X., Wang H., Yang X. IL-35 (Interleukin-35) suppresses endothelial cell activation by inhibiting mitochondrial reactive oxygen species-mediated site-specific acetylation of H3K14 (histone 3 lysine 14) Arterioscler. Thromb. Vasc. Biol. 2018;38:599–609. doi: 10.1161/ATVBAHA.117.310626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Li X., Mai J., Virtue A., Yin Y., Gong R., Sha X., Gutchigian S., Frisch A., Hodge I., Jiang X., Wang H., Yang X.F. IL-35 is a novel responsive anti-inflammatory cytokine--a new system of categorizing anti-inflammatory cytokines. PLoS One. 2012;7 doi: 10.1371/journal.pone.0033628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Collison L.W., Vignali D.A. Interleukin-35: odd one out or part of the family? Immunol. Rev. 2008;226:248–262. doi: 10.1111/j.1600-065X.2008.00704.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Collison L.W., Workman C.J., Kuo T.T., Boyd K., Wang Y., Vignali K.M., Cross R., Sehy D., Blumberg R.S., Vignali D.A. The inhibitory cytokine IL-35 contributes to regulatory T-cell function. Nature. 2007;450:566–569. doi: 10.1038/nature06306. [DOI] [PubMed] [Google Scholar]
- 33.Choi J., Leung P.S., Bowlus C., Gershwin M.E. IL-35 and autoimmunity: a comprehensive perspective. Clin. Rev. Allergy Immunol. 2015;49:327–332. doi: 10.1007/s12016-015-8468-9. [DOI] [PubMed] [Google Scholar]
- 34.Sha X., Meng S., Li X., Xi H., Maddaloni M., Pascual D.W., Shan H., Jiang X., Wang H., Yang X.F. Interleukin-35 inhibits endothelial cell activation by suppressing MAPK-AP-1 pathway. J. Biol. Chem. 2015;290:19307–19318. doi: 10.1074/jbc.M115.663286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Li X., Fang P., Yang W.Y., Wang H., Yang X. IL-35, as a newly proposed homeostasis-associated molecular pattern, plays three major functions including anti-inflammatory initiator, effector, and blocker in cardiovascular diseases. Cytokine. 2019;122:154076. doi: 10.1016/j.cyto.2017.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Banchereau J., Pascual V., O'Garra A. From IL-2 to IL-37: the expanding spectrum of anti-inflammatory cytokines. Nat. Immunol. 2012;13:925–931. doi: 10.1038/ni.2406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Caraffa A., Gallenga C.E., Kritas S.K., Ronconi G., Conti P. Impact of mast cells in systemic lupus erythematosus: can inflammation be inhibited? J. Biol. Regul. Homeost. Agents. 2019;33:669–673. [PubMed] [Google Scholar]
- 38.Gallenga C.E., Pandolfi F., Caraffa A., Kritas S.K., Ronconi G., Toniato E., Martinotti S., Conti P. Interleukin-1 family cytokines and mast cells: activation and inhibition. J. Biol. Regul. Homeost. Agents. 2019;33:1–6. [PubMed] [Google Scholar]
- 39.Gugliandolo A., Caraffa A.L., Gallenga C.E., Kritas S.K., Ronconi G., Trubiani O., Conti P., Di Emidio P., Mazzon E. Mesenchymal stem cells and IL-37: a powerful combination. J. Biol. Regul. Homeost. Agents. 2019;33:1019–1022. [PubMed] [Google Scholar]
- 40.Shao Y., Cheng Z., Li X., Chernaya V., Wang H., Yang X.-f. Immunosuppressive/anti-inflammatory cytokines directly and indirectly inhibit endothelial dysfunction-a novel mechanism for maintaining vascular function. J. Hematol. Oncol. 2014;7:80. doi: 10.1186/s13045-014-0080-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wang X., Li Y.F., Nanayakkara G., Shao Y., Liang B., Cole L., Yang W.Y., Li X., Cueto R., Yu J., Wang H., Yang X.F. Lysophospholipid receptors, as novel conditional danger receptors and homeostatic receptors modulate inflammation-novel paradigm and therapeutic potential. Journal of cardiovascular translational research. 2016;9:343–359. doi: 10.1007/s12265-016-9700-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Shao Y., Nanayakkara G., Cheng J., Cueto R., Yang W.Y., Park J.Y., Wang H., Yang X. Lysophospholipids and their receptors serve as conditional DAMPs and DAMP receptors in tissue oxidative and inflammatory injury. Antioxidants Redox Signal. 2017;28:10. doi: 10.1089/ars.2017.7069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Li Y.F., Li R.S., Samuel S.B., Cueto R., Li X.Y., Wang H., Yang X.F. Lysophospholipids and their G protein-coupled receptors in atherosclerosis. Front Biosci (Landmark Ed) 2016;21:70–88. doi: 10.2741/4377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Lopez-Pastrana J., Ferrer L.M., Li Y.F., Xiong X., Xi H., Cueto R., Nelson J., Sha X., Li X., Cannella A.L., Imoukhuede P.I., Qin X., Choi E.T., Wang H., Yang X.F. Inhibition of caspase-1 activation in endothelial cells improves angiogenesis: a novel therapeutic potential for ischemia. J. Biol. Chem. 2015;290:17485–17494. doi: 10.1074/jbc.M115.641191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Vestweber D. How leukocytes cross the vascular endothelium. Nat. Rev. Immunol. 2015;15:692–704. doi: 10.1038/nri3908. [DOI] [PubMed] [Google Scholar]
- 46.Yin Y., Pastrana J.L., Li X., Huang X., Mallilankaraman K., Choi E.T., Madesh M., Wang H., Yang X.F. Inflammasomes: sensors of metabolic stresses for vascular inflammation. Front Biosci (Landmark Ed) 2013;18:638–649. doi: 10.2741/4127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Li X., Fang P., Li Y., Kuo Y.-M., Andrews A.J., Nanayakkara G., Johnson C., Fu H., Shan H., Du F., Hoffman N.E., Yu D., Eguchi S., Madesh M., Koch W.J., Sun J., Jiang X., Wang H., Yang X. Mitochondrial reactive oxygen species mediate lysophosphatidylcholine-induced endothelial cell Activation Highlights. Arterioscler. Thromb. Vasc. Biol. 2016;36:1090–1100. doi: 10.1161/ATVBAHA.115.306964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Li X., Fang P., Yang W.Y., Chan K., Lavallee M., Xu K., Gao T., Wang H., Yang X. Mitochondrial ROS, uncoupled from ATP synthesis, determine endothelial activation for both physiological recruitment of patrolling cells and pathological recruitment of inflammatory cells. Can. J. Physiol. Pharmacol. 2017;95:247–252. doi: 10.1139/cjpp-2016-0515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Cheng J., Nanayakkara G., Shao Y., Cueto R., Wang L., Yang W.Y., Tian Y., Wang H., Yang X. Mitochondrial proton leak plays a critical role in pathogenesis of cardiovascular diseases. Adv. Exp. Med. Biol. 2017;982:359–370. doi: 10.1007/978-3-319-55330-6_20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Nanayakkara G.K., Wang H., Yang X. Proton leak regulates mitochondrial reactive oxygen species generation in endothelial cell activation and inflammation - a novel concept. Arch. Biochem. Biophys. 2019;662:68–74. doi: 10.1016/j.abb.2018.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Lu Y., Sun Y., Drummer C.t., Nanayakkara G.K., Shao Y., Saaoud F., Johnson C., Zhang R., Yu D., Li X., Yang W.Y., Yu J., Jiang X., Choi E.T., Wang H., Yang X. Increased acetylation of H3K14 in the genomic regions that encode trained immunity enzymes in lysophosphatidylcholine-activated human aortic endothelial cells - novel qualification markers for chronic disease risk factors and conditional DAMPs. Redox Biol. 2019;24:101221. doi: 10.1016/j.redox.2019.101221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Li X., Wang L., Fang P., Sun Y., Jiang X., Wang H., Yang X.F. Lysophospholipids induce innate immune transdifferentiation of endothelial cells, resulting in prolonged endothelial activation. J. Biol. Chem. 2018;293:11033–11045. doi: 10.1074/jbc.RA118.002752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Beiting D.P., Hidano S., Baggs J.E., Geskes J.M., Fang Q., Wherry E.J., Hunter C.A., Roos D.S., Cherry S. The orphan nuclear receptor TLX is an enhancer of STAT1-mediated transcription and immunity to toxoplasma gondii. PLoS Biol. 2015;13 doi: 10.1371/journal.pbio.1002200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Sun Y., Johnson C., Zhou J., Wang L., Li Y.F., Lu Y., Nanayakkara G., Fu H., Shao Y., Sanchez C., Yang W.Y., Wang X., Choi E.T., Li R., Wang H., Yang X.F. Uremic toxins are conditional danger- or homeostasis-associated molecular patterns. Front Biosci (Landmark Ed) 2018;23:348–387. doi: 10.2741/4595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Subramanian A., Tamayo P., Mootha V.K., Mukherjee S., Ebert B.L., Gillette M.A., Paulovich A., Pomeroy S.L., Golub T.R., Lander E.S., Mesirov J.P. Gene set enrichment analysis: a knowledge-based approach for interpreting genome-wide expression profiles. Proc. Natl. Acad. Sci. U. S. A. 2005;102:15545–15550. doi: 10.1073/pnas.0506580102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Li X., Wang L., Fang P., Sun Y., Jiang X., Wang H., Yang X.-F. Lysophospholipids induce innate immune transdifferentiation of endothelial cells, resulting in prolonged endothelial activation. J. Biol. Chem. 2018:002752. doi: 10.1074/jbc.RA118.002752. jbc.RA118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Liew F.Y., Girard J.P., Turnquist H.R. Interleukin-33 in health and disease. Nat. Rev. Immunol. 2016;16:676–689. doi: 10.1038/nri.2016.95. [DOI] [PubMed] [Google Scholar]
- 58.Dominguez-Andres J., Joosten L.A., Netea M.G. Induction of innate immune memory: the role of cellular metabolism. Curr. Opin. Immunol. 2019;56:10–16. doi: 10.1016/j.coi.2018.09.001. [DOI] [PubMed] [Google Scholar]
- 59.Dominguez-Andres J., Novakovic B., Li Y., Scicluna B.P., Gresnigt M.S., Arts R.J.W., Oosting M., Moorlag S., Groh L.A., Zwaag J., Koch R.M., Ter Horst R., Joosten L.A.B., Wijmenga C., Michelucci A., van der Poll T., Kox M., Pickkers P., Kumar V., Stunnenberg H., Netea M.G. The itaconate pathway is a central regulatory node linking innate immune tolerance and trained immunity. Cell Metabol. 2019;29:211–220. doi: 10.1016/j.cmet.2018.09.003. e215. [DOI] [PubMed] [Google Scholar]
- 60.Saeed S., Quintin J., Kerstens H.H., Rao N.A., Aghajanirefah A., Matarese F., Cheng S.C., Ratter J., Berentsen K., van der Ent M.A., Sharifi N., Janssen-Megens E.M., Ter Huurne M., Mandoli A., van Schaik T., Ng A., Burden F., Downes K., Frontini M., Kumar V., Giamarellos-Bourboulis E.J., Ouwehand W.H., van der Meer J.W., Joosten L.A., Wijmenga C., Martens J.H., Xavier R.J., Logie C., Netea M.G., Stunnenberg H.G. Epigenetic programming of monocyte-to-macrophage differentiation and trained innate immunity. Science. 2014;345:1251086. doi: 10.1126/science.1251086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Collison L.W., Chaturvedi V., Henderson A.L., Giacomin P.R., Guy C., Bankoti J., Finkelstein D., Forbes K., Workman C.J., Brown S.A., Rehg J.E., Jones M.L., Ni H.T., Artis D., Turk M.J., Vignali D.A. IL-35-mediated induction of a potent regulatory T cell population. Nat. Immunol. 2010;11:1093–1101. doi: 10.1038/ni.1952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Sawant D.V., Yano H., Chikina M., Zhang Q., Liao M., Liu C., Callahan D.J., Sun Z., Sun T., Tabib T., Pennathur A., Corry D.B., Luketich J.D., Lafyatis R., Chen W., Poholek A.C., Bruno T.C., Workman C.J., Vignali D.A.A. Adaptive plasticity of IL-10(+) and IL-35(+) Treg cells cooperatively promotes tumor T cell exhaustion. Nat. Immunol. 2019;20:724–735. doi: 10.1038/s41590-019-0346-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Mulder W.J.M., Ochando J., Joosten L.A.B., Fayad Z.A., Netea M.G. Therapeutic targeting of trained immunity. Nat. Rev. Drug Discov. 2019;18:553–566. doi: 10.1038/s41573-019-0025-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Kobayashi E.H., Suzuki T., Funayama R., Nagashima T., Hayashi M., Sekine H., Tanaka N., Moriguchi T., Motohashi H., Nakayama K., Yamamoto M. Nrf2 suppresses macrophage inflammatory response by blocking proinflammatory cytokine transcription. Nat. Commun. 2016;7:11624. doi: 10.1038/ncomms11624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Back M., Hansson G.K. Anti-inflammatory therapies for atherosclerosis. Nat. Rev. Cardiol. 2015;12:199–211. doi: 10.1038/nrcardio.2015.5. [DOI] [PubMed] [Google Scholar]
- 66.Zhang L., Wang X., Cueto R., Effi C., Zhang Y., Tan H., Qin X., Ji Y., Yang X., Wang H. Biochemical basis and metabolic interplay of redox regulation. Redox Biol. 2019;26:101284. doi: 10.1016/j.redox.2019.101284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Li X., Fang P., Mai J., Choi E.T., Wang H., Yang X.F. Targeting mitochondrial reactive oxygen species as novel therapy for inflammatory diseases and cancers. J. Hematol. Oncol. 2013;6:19. doi: 10.1186/1756-8722-6-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.