Abstract
For several years, research from around the world has suggested that the neuroactive steroid (3α,5α)-3-hydroxypregnan-20-one (allopregnanolone) may have therapeutic potential for treatment of various stress-related diseases including post-traumatic stress disorder (PTSD), depression, alcohol use disorders (AUDs), as well as neurological and psychiatric conditions that are worsened in the presence of stress, such as multiple sclerosis, schizophrenia, and seizure disorders. In this review, we make the argument that the pleiotropic actions of allopregnanolone account for its ability to promote recovery in such a wide variety of illnesses. Likewise, the allopregnanolone precursors, pregnenolone and progesterone, share many actions of allopregnanolone. Of course, pregnenolone and progesterone lack direct effects on GABAA receptors, but these compounds are converted to allopregnanolone in vivo. This review presents a theoretical framework for understanding how endogenous neurosteroids that regulate 1) γ-aminobutyric acid (GABA)A receptors, 2) corticotropin releasing factor (CRF) and 3) pro-inflammatory signaling in the innate immune system and brain could play a key role in both the prevention and treatment of stress-related disease. We further discuss cautions and limitations of allopregnanolone or precursor therapy as well as the need for more clinical studies.
Keywords: Allopregnanolone, Pregnenolone, Progesterone, CRF, GABAergic transmission, Toll-like receptors
Abbreviations
- AP-1
activator protein-1
- ACTH
adrenocorticotropic hormone
- AUD
alcohol use disorder
- 3α,5α-THDOC
allotetrahydrodeoxycorticosterone
- BNST
bed nucleus of striaterminalis
- CNS
central nervous system
- CRF
corticotropin releasing factor
- CRFR
CRF receptors
- CREB
cyclic AMP response element binding protein
- GABA
γ-aminobutyric acid
- HMGB1
high motility group box 1
- HPA
hypothalamic-pituitary-adrenal
- IRF
interferon regulatory factor
- KCC2
K+/Cl− co-transporter
- LPS
Lipopolysaccharide
- MD-2
lymphocyte antigen 96
- MCP-1
monocyte chemoattractant protein 1
- MyD88
myeloid differentiation response protein 88
- NFκB
nuclear factor kappa-light-chain-enhancer of activated B cells
- PVN
paraventricular nucleus
- PTSD
post-traumatic stress disorder
- TRIF
TIR-domain-containing adapter-inducing interferon-β
- TLRs
toll-like receptors
- TRAF6
tumor necrosis factor receptor associated factor 6
- TNF-α
tumor necrosis factor α
- THIP
4,5,6,7-tetrahydroisoxazolo(5,4-c)pyridin-3-ol
- 17 PA
[3α,5α]17-phenylandrost-16-en-3-ol
1. Introduction
This review primarily addresses the rationale for using the neuroactive steroid allopregnanolone for treatment of various stress-related diseases based on its pleiotropic actions upon γ-aminobutyric acid (GABA)A receptors, corticotropin releasing factor (CRF) signaling and pro-inflammatory signaling in the innate immune system. We present theories that stress-related disease results from 1) the loss of central nervous system (CNS) inhibition due to adaptations in GABAA receptor-mediated neurotransmission across brain, 2) excessive CRF signaling that dysregulates the hypothalamic-pituitary-adrenal (HPA) axis and extrahypothalamic CRF circuits across brain, and 3) excessive neuroimmune signaling through Toll-like receptors (TLRs) in the innate immune system and the brain. Next, we review the evidence that allopregnanolone counteracts these stress-induced adaptations to restore GABAergic inhibition, moderate CRF signaling across brain and inhibit pro-inflammatory TLR signaling in the innate immune system and brain. Pregnenolone and progesterone are precursors of allopregnanolone that increase its concentration in serum and brain and also directly inhibit inflammatory signaling through TLR receptors. We argue that all of these endogenous neurosteroids may represent protective factors that prevent the development of stress-related disease vulnerability and could be valuable therapeutics as well. The pleiotropic actions of these neurosteroids provide an attractive strategy to simultaneously address three major domains of stress-related disease in its treatment. In addition, this rationale summarizes evidence that allopregnanolone, pregnenolone or progesterone address many of the co-occurring symptoms of stress-related neuropsychiatric conditions, including anxiety, depression, sleep disturbance, and pain, all of which may facilitate the management of disease.
2. The impact of stress on GABAergic transmission
An abundance of evidence shows that GABAergic transmission is impacted by stress at multiple levels. Clinical studies of stress-related psychiatric disorders suggest alterations in GABAergic transmission, including decreased GABA levels, reductions in the density of GABAergic interneurons and changes in GABAA receptor subunit expression (Baer et al., 2000; Sanacora et al., 2004; Hasler et al., 2007; Rajkowska et al., 2007; Sequeira et al., 2009; Luscher et al., 2011). In agreement, numerous preclinical studies have highlighted the role of GABAergic transmission in the HPA response to stress, and how chronic stress exposure disrupts these regulatory responses. Local GABAergic neurons in the paraventricular nucleus (PVN) of the hypothalamus and in adjacent forebrain areas send a GABAergic inhibitory signal to CRF neurons in response to various stressors (Cullinan et al., 2008). This regulatory response is disrupted under stress conditions. Indeed, several paradigms of acute stress (inescapable tail-shock, mild foot-shock, carbon dioxide inhalation, forced swim, handling) induce a rapid and reversible downregulation of GABAergic transmission in several brain regions of male rats, as assessed by binding assays of GABA and GABAA receptor modulators, as well as GABA-stimulated chloride flux in synaptoneurosomes (Drugan et al., 1989; Biggio et al., 2007). However, sex and species differences in the effects of stress on GABAA receptor function have also been highlighted (Akinci and Johnston, 1993; Chadda and Devaud, 2004).
Another mechanism that contributes to the stress-induced loss of GABAergic inhibition involves a dephosphorylation and downregulation of the K+/Cl− co-transporter KCC2, which has been reported to occur in the hypothalamus of male rats and mice following acute stress; such changes switch the GABAergic transmission on CRF neurons from inhibitory to excitatory, resulting in an ineffective inhibition of the HPA axis (Hewitt et al., 2009; Sarkar et al., 2011). A similar mechanism occurs in the hippocampus of male mice following chronic stress, which contributes to increased seizures susceptibility (MacKenzie and Maguire, 2015).
Acute and chronic stress differentially alter GABAA receptor subunit expression in male rats, depending on brain region and species examined (Orchinik et al., 1995; Verkuyl et al., 2004; Poulter et al., 2010; Jacobson-Pick et al., 2012). However, both acute and chronic stress increase the expression of extrasynaptic α4/δ subunit containing GABAA receptors, with a subsequent increase in tonic inhibition in granule and pyramidal cell neurons of the hippocampus of male rats and mice (Serra et al., 2006, 2008; Maguire and Mody, 2007). Extrasynaptic α4/δ GABAA receptors are more sensitive to the actions of endogenous 3α,5α-reduced neuroactive steroids (Belelli and Lambert, 2005), and it has been suggested that such neuroactive steroids may promote the physiological response to stress by increasing the firing of CRF neurons via δ subunit containing GABAA receptors (Sarkar et al., 2011). Indeed, selective deletion of the δ subunit from CRF neurons, with subsequent loss of tonic GABAergic inhibition, blunts the HPA response to acute stress as well as anxiety- and depressive-like behaviors in male mice (Lee et al., 2014).
Under chronic stress, a loss of GABAergic inhibition in cortical and limbic brain regions has been reported in male rats and mice, which includes impairment of GABAergic interneurons, decreased expression of glutamic acid decarboxylase (the main enzyme necessary for GABA synthesis in GABAergic interneurons), decreased expression of vesicular and plasma membrane transporters for GABA, and reduced density and function of GABAergic synapses (Verkuyl et al., 2004; Czeh et al., 2015, 2018; Ma et al., 2016, 2019).
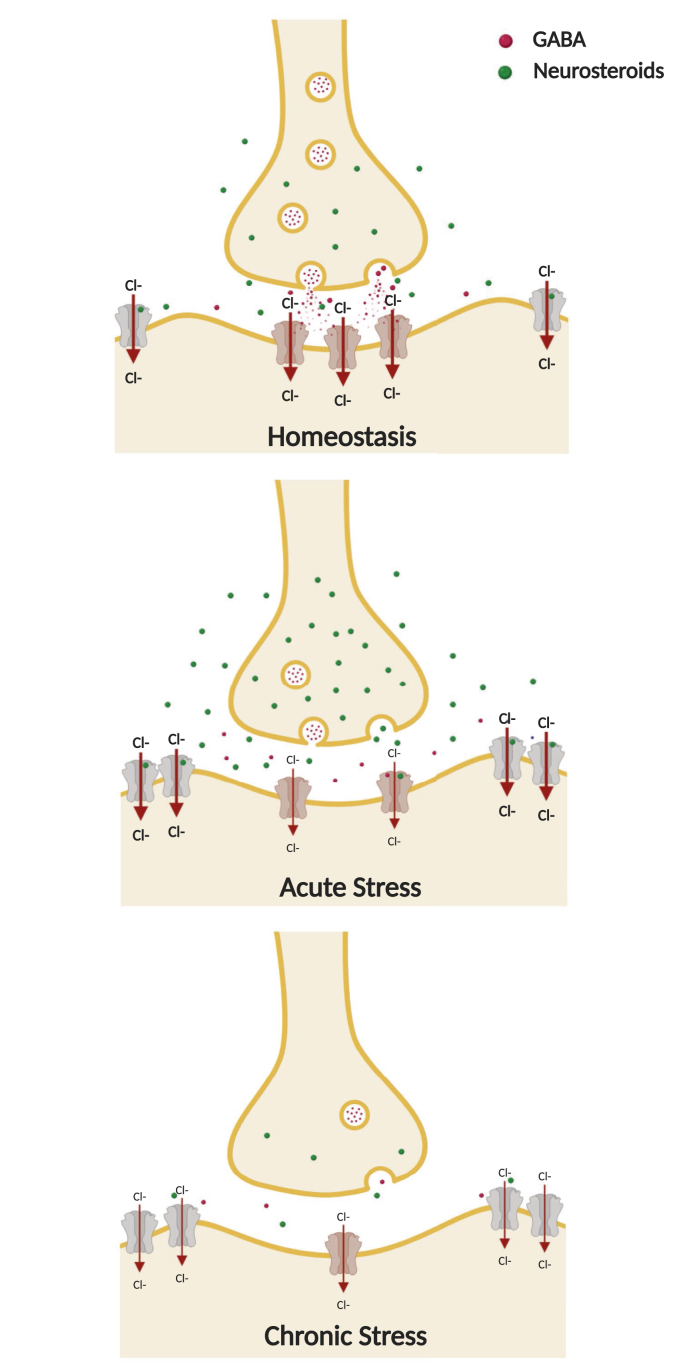
Exposure to early life stress also impairs GABAergic transmission in adulthood. Rats subjected to maternal separation stress early in life show increased neophobia and acoustic startle responses in adulthood, along with reduced expression of benzodiazepine-sensitive GABAA receptors in the frontal cortex, amygdala, locus coeruleus and the nucleus tractus solitarius (Caldji et al., 2000). Further, adolescent mice subjected to fragmented maternal care during the postnatal period showed an altered excitatory/inhibitory balance in the hypothalamus which results in impaired inhibitory actions of 3α,5α-reduced neuroactive steroids upon GABAA receptors in CRF-releasing neurons (Gunn et al., 2013). It has been suggested that stress during development may impair the physiological fluctuations in neuroactive steroid levels (Grobin and Morrow, 2001) and the GABA switch (from a depolarizing to a hyperpolarizing action) (Cherubini et al., 1991) that occur during postnatal development (Gunn et al., 2015). These adaptations are depicted schematically in Fig. 1.
Fig. 1.
Schematic representation of the impact of acute and chronic stress on GABAergic transmission. Acute stress causes a reduction of GABA release, but an increase of GABAergic neuroactive steroids and extrasynaptic α4/δ GABAA receptors. Chronic stress decreases GABA, neuroactive steroid levels and the synaptic GABAA receptors, but increases extrasynaptic α4/δ GABAA receptors, leading to a dramatic reduction of GABAergic transmission. Allopregnanolone, through binding with extrasynaptic α4/δ GABAA receptors, may restore homeostasis by increasing GABAergic tone by the enhancement of tonic inhibition. (Created with Biorender.com).
3. The impact of stress on CRF signaling
CRF signaling plays a major role in the coordination of the physiological/neuroendocrine response to stress via activation of the HPA axis. While glucocorticoid negative feedback plays a predominant role under basal conditions, the activity of CRF neurons is controlled by neural afferents from the brain stem and limbic system, as well as peripheral hormonal influences, during stress. The afferents inputs release various neurotransmitters such as norepinephrine, glutamate, GABA, angiotensin II, CRF itself and other peptides in the PVN. These molecules interact with their receptors located in the CRF neurons and regulate CRF synthesis, release and intracellular signal-transduction pathways. Hypothalamic CRF neurons receive inhibitory afferents from hippocampus and, indirectly, from prefrontal cortex, as well as excitatory inputs from central amygdala and from the bed nucleus of stria terminalis (BNST), indirectly (Ziegler and Herman, 2000; Ulrich-Lai and Herman, 2009). In basal conditions, the CRF neurons are under the inhibitory influence of GABAergic interneurons located in the PVN (Cullinan et al., 2008). During stress, the main neurotransmitters released in PVN are norepinephrine and glutamate (Herman et al., 2002). In stress-related psychiatric disorders, chronic stress disrupts the equilibrium between excitatory/inhibitory inputs, causing a decrease in inhibition and an increase in excitatory signals that results in hyperactivity of the HPA axis.
CRF and its receptors (CRFR1/2) are widely distributed across the brain and by binding its high affinity receptor, CRFR1, CRF is able to coordinate autonomic and behavioral stress responses. Thereby, dysregulation of the CRF/CRFR1 system has been linked to stress-related psychiatric disorders such as depression and anxiety (Nemeroff, 1998; Reul and Holsboer, 2002; de Kloet et al., 2005; Binder and Nemeroff, 2010). Hyperactivity of the HPA axis, and consequently impaired negative feedback, have been reported in patients affected by depression, postpartum depression, PTSD and AUDs (Bixo et al., 1997; Adinoff et al., 2005a, 2005b, 2005c; Girdler and Klatzkin, 2007; Girdler et al., 2012; Baumeister et al., 2014; Schule et al., 2014; Rasmusson et al., 2017).
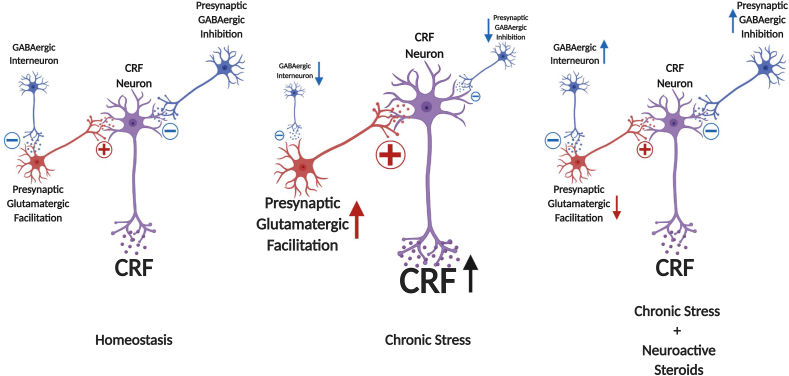
Supporting the role of CRFhyperactivity in stress-related neuropsychopathologies, studies in rodents show that CRFinjections into the ventral hippocampus increase anxiety-like behavior in rats (Pentkowski et al., 2009). Furthermore, central and basolateral amygdala infusion of CRF increases anxiety-related and freezing behavior and reduces social interaction in rats (Swiergiel et al., 1993; Sajdyk et al., 1999). Intra-BNST administration of CRF dose-dependently increases anxiety-like behavior in rats (Sahuque et al., 2006). In nucleus accumbens, CRF regulates appetitive behavior through regulation of dopamine release. In this case, severe stress produces a persistent dysregulation of CRF-dopamine interactions, causing a switch from appetitive to aversive behavioral actions (Lemos et al., 2012). In ventral tegmental area, the dysregulation of the CRF/CRFR1/2 system has been correlated with AUDs and other substance use disorders (Grieder et al., 2014; Zorrilla et al., 2014; Henckens et al., 2016). Finally, modification in the noradrenergic system of the locus coeruleus caused by CRF hyperactivity has been proposed to underlie pathological hyperarousal observed in numerous stress-related psychiatric disorders (Bissette et al., 2003). The circuit adaptations described here are shown in Fig. 2 along with the hypothetical role of neuroactive steroids in restoring excessive CRF signaling in the brain.
Fig. 2.
Hypothetical representation of neural regulation of CRF neurons in hypothalamic and extrahypothalamic regions. CRF neurons are regulated by the balance of glutamatergic and GABAergic regulation in many brain areas. Chronic stress increases CRF release in the hypothalamus by decreasing GABAergic and increasing glutamatergic inputs that control CRF neurons. Allopregnanolone may restore homeostasis by increasing GABAergic inputs and, indirectly, decreasing glutamatergic signals, leading to a restoration in CRF signaling throughout the brain. (Created with Biorender.com).
4. The impact of stress on pro-inflammatory neuroimmune signaling
Neuroimmune signaling through TLRs is an important contributor to various inflammatory systemic, neurological and psychiatric conditions, including traumatic hemorrhagic shock (Reino et al., 2011), AUDs (He and Crews, 2008; Qin et al., 2008; Crews et al., 2017a, Crews et al., 2017b), other addictions (Lacagnina et al., 2017), depression (Dantzer et al., 2008; Bhattacharya et al., 2016), traumatic brain injury (He et al., 2004a) and epilepsies (Maroso et al., 2011). The innate immune cells in the brain, microglia, as well as neurons and other glial cells, signal via TLRs to promote innate immune gene expression and produce pro-inflammatory cytokines and chemokines in a progressive and feed-forward fashion (Pavlov and Tracey, 2017) that is particularly long-lasting in the brain (Crews et al., 2017a, Crews et al., 2017b).
4.1. Evidence for stress-induced activation of TLR signaling
TLRs recognize all types of microbes that invade the human body as well as endogenous signals that are released in response to tissue damage, cellular stress and psychological stress. The activation of TLRs can protect against external invasions of pathogens to fight infection, but they can also arise in response to tissue injury such as ischemia (Aguirre et al., 2013), cellular oxidative stress (Akhter et al., 2019) and psychological stressors such as chronic restraint (Zhang et al., 2008). Using TLR4-deficient mice, Zhang and colleagues demonstrated that activation of TLR4 is responsible for the stress-induced increase in pro-inflammatory cytokines tumor necrosis factor (TNF-α) and interleukin-1β, as well as a decrease in the anti-inflammatory cytokines interferon-γ and interleukin-2. Moreover, TLR4 contributed to the lymphocyte reduction induced by chronic stress (Zhang et al., 2008).
The activation of TLRs in response to endogenous signals can be harmful and often causes more problems than the initial cell or tissue injury (Hennessy et al., 2010). This process is known as pro-inflammatory innate immune signaling in the periphery and neuroimmune signaling in the brain. One example of stress activation of TLR signaling is evident from recent studies showing that the endogenous TLR4 agonist endotoxin is increased in circulation following acute alcohol and restraint stress in rats (Walter et al., 2017). The effect is similar in magnitude to the elevation of cortisol in the circulation and may represent an important connection between stress and TLR4 signaling. Furthermore, TLR activity is known to be enhanced by CRF in both macrophages (Tsatsanis et al., 2006) and brain (Whitman et al., 2013), indicating another mechanism of stress enhancement of pro-inflammatory signaling.
Ethanol exposure is one example of a chronic stressor that activates the HPA axis with acute exposure and blunts the HPA axis with chronic exposure (Adinoff et al., 2005b, 2005c). Ethanol increases the expression of TLR2, TLR3, TLR4, and TLR7, and members of the pathways activated by these pathogen-activated receptors (Crews et al., 2017a, Crews et al., 2017b). TLR signaling initiates with complex formation and activation of myeloid differentiation response protein (MyD88)-dependent and independent pathways. These include transcription factors nuclear factor kappa-light-chain-enhancer of activated B cells (NFκB), cyclic AMP response element binding protein (CREB), activator protein-1 (AP-1), and interferon regulatory factor (IRF) that translocate to the nucleus and control expression of chemokines and cytokines. Ethanol activates the TLR-MyD88-NFκB pathways as well as the TLR3-regulated TIR-domain-containing adapter-inducing interferon-β (TRIF) pathway. Intermittent alcohol drinking during adolescence upregulates the expression of TLR4 in the adult hippocampus, TLR3 and TLR4 in the adult prefrontal cortex, and TLR1-TLR8 in the adult cerebellum (Breese and Knapp, 2016; Knapp et al., 2016; Harper et al., 2017), suggesting distinct immune activation and function at distinct brain sites.
Production of pro-inflammatory cytokines appears to be involved in altered brain activity (Gruol, 2013). Indeed, neuroimmune signaling via the classic pro-inflammatory cytokines in brain enhances glutamatergic transmission and reduces GABAergic transmission (Stellwagen et al., 2005; Ferguson et al., 2008; Stuck et al., 2012; Pribiag and Stellwagen, 2013), rather than produce other signs of inflammation such as swelling or macrophage infiltration. On the other hand, TLR7 signaling in brain leads to neurodegeneration, involving the production of high motility group box 1 (HMGB1) and the miRNA let-7 (Coleman et al., 2017). Together, the production of pro-inflammatory signaling and the resultant shift in the excitatory/inhibitory balance in the brain likely contribute to the pathological effects of stress.
The following sections will describe the evidence that neurosteroids may restore losses in GABAergic transmission, CRF overactivation and excessive TLR signaling. The combination of these pleiotropic actions may be key to their therapeutic benefits.
5. Evidence that GABAergic neuroactive steroids abrogate the loss of GABAergic inhibition in stress-related disease
Neuroactive steroids that are 3α,5α-reduced derivatives of progesterone, deoxycorticosterone, dehydroepiandrosterone and testosterone, are endogenous positive modulators of all GABAA receptor subtypes (Puia et al., 1991; Kokate et al., 1994; Belelli et al., 2002; Reddy and Rogawski, 2002; Herd et al., 2007). Allopregnanolone and allotetrahydrodeoxycorticosterone (3α,5α-THDOC) exhibit high potency at most subtypes of GABAA receptors (Puia et al., 1990, 1993), although they are more potent at some extrasynaptic GABAA receptors containing the δ subunit (Belelli and Lambert, 2005; Carver and Reddy, 2013). For example, low concentrations of 3α,5α-THDOC, which have no effect on phasic conductance mediated by synaptic GABAA receptors, selectively enhance tonic currents mediated by δ subunit containing GABAA receptors in mouse dentate gyrus and cerebellar granule cells (Stell et al., 2003). Likewise, allopregnanolone and the synthetic neuroactive steroids SGE-516 and ganaxolone also increase tonic currents in dentate gyrus granule cells (Modgil et al., 2017). Although the neuroactive steroid binding sites are located at the interface of the α and β subunits of synaptic receptors (Hosie et al., 2006), the δ subunit is thought to be responsible for the enhanced sensitivity to neuroactive steroids. In fact, δ subunit knockout mice have blunted tonic currents along with reduced sensitivity to neuroactive steroid modulation of GABAA receptors (Mihalek et al., 1999; Spigelman et al., 2003; Stell et al., 2003). Furthermore, α4 subunit knockout mice also display a loss of δ subunits and blunted tonic inhibition (Chandra et al., 2006), along with reduced GABAergic potentiation by alphaxalone (Liang et al., 2008).
Neuroactive steroids might represent a useful therapeutic approach to restore deficits in GABAergic inhibition observed in patients with stress-related disease. This argument is supported by data from animal models and the effects of neuroactive steroids in human conditions that involve the loss of inhibitory control in the CNS. Some examples are described below.
5.1. Anxiolytic and antidepressant actions in animals and humans
Administration of allopregnanolone exerts anxiolytic-like and antidepressant-like effects in rodents (Crawley et al., 1986; Bitran et al., 1991; Khisti et al., 2000) and humans (Kanes et al., 2017; Meltzer-Brody et al., 2018), which may be important for prevention and recovery from stress-related disorders and AUDs. Specifically, intravenous infusion of Brexanolone, a proprietary formulation of allopregnanolone by Sage Therapeutics, exerts a rapid effect in counteracting postpartum anxiety and depression. Women with moderate to severe postpartum depression showed a quick improvement in agitation, anxiety and depressive symptoms during a 60 hour constant intravenous infusion of the drug, an effect that lasted more than a month (Kanes et al., 2017; Meltzer-Brody et al., 2018). Its rapid and long-lasting effects represent a major breakthrough for the treatment of depression and raise the possibility that its precursor steroids that share its pleotropic actions may have benefits as well. While it is unknown if the therapeutic effects of Brexanolone for postpartum depression are dependent on its GABAergic activity, this mechanism seems likely to contribute to these actions. Previous evidence has documented that GABA inhibition is compromised in patients with depression, since levels of GABA are reduced in plasma, cerebrospinal fluid and occipital cortex of depressed subjects, while GABAA receptors and physiological activity are also diminished (Sanacora and Saricicek, 2007; Croarkin et al., 2011). The recent studies with Brexanolone represent important clinical evidence supporting the idea that restoration of GABAergic inhibition may be therapeutic for depression. Of course, this possibility does not preclude contributions from other mechanisms of allopregnanolone action on CRF and pro-inflammatory signaling to be described below.
5.2. Anticonvulsive effects in ethanol dependent rats
The GABAergic neuroactive steroids have potent anticonvulsant effects in several animal models (Reddy and Rogawski, 2012). For instance, acute ethanol-induced elevations of allopregnanolone contribute to its anticonvulsant effects in male rats (VanDoren et al., 2000). By contrast, alcohol dependence reduces GABAergic inhibition across brain, including the sensitivity to GABA- and ethanol-induced anticonvulsant actions, thus leading to increased seizure susceptibility. In fact, allopregnanolone or 3α,5α-THDOC administration before seizure induction blocks the increased seizure susceptibility induced by ethanol withdrawal in dependent male and female rats, with female rats being more sensitive to the protective effects of neuroactive steroids (Devaud et al., 1995, 1998). Chronic intermittent ethanol exposure, which causes a reduction in hippocampal allopregnanolone content, also decreases seizure threshold in rats, an effect that is reversed by alphaxalone administration (Cagetti et al., 2004). Ethanol dependence produces tolerance to ethanol and cross-tolerance to benzodiazepines and barbiturates, including the anticonvulsant effect of benzodiazepines (Devaud et al., 1996). However, ethanol-dependent rats are sensitized to the anticonvulsant effects of allopregnanolone and 3α,5α-THDOC, and their GABAA receptors also have enhanced sensitivity to the actions of neuroactive steroids (Devaud et al., 1996; Cagetti et al., 2004). Thus, GABAergic neuroactive steroids may be therapeutic during ethanol withdrawal, and their use may have advantages over benzodiazepine therapy since benzodiazepines exhibit cross-tolerance with ethanol and promote the loss of GABAA receptors in the CNS.
5.3. Effectiveness in status epilepticus
Status epilepticus is a neurological life-threatening condition characterized by continuous or recurring seizures with loss of consciousness for more than 30 min. Patients with this condition do not respond to the classical benzodiazepine therapy because seizures are likely to alter GABAA receptors subunit expression and trafficking (Rogawski et al., 2013). In fact, preclinical studies in rat models of status epilepticus showed that benzodiazepine sensitive synaptic GABAA receptors are internalized, causing a reduction in synaptic inhibition (Naylor et al., 2005), while benzodiazepine insensitive extrasynaptic α4/δ GABAA receptors do not internalize and are functional (Goodkin et al., 2008), thus representing a putative pharmacological target for status epilepticus. Given that GABAergic neuroactive steroids have potent anticonvulsant effects in several animal models (Devaud et al., 1995; Reddy and Rogawski, 2012), and potent modulatory actions at extrasynaptic GABAA receptors (Belelli and Lambert, 2005), they may be valuable therapeutic agents. Indeed, allopregnanolone and its synthetic 3β-methyl analog ganaxolone both proved effective in a mouse model of treatment-resistant status epilepticus (Rogawski et al., 2013; Zolkowska et al., 2018). Furthermore, case reports suggested that intravenous allopregnanolone may be therapeutic for status epilepticus in a number of individuals (Broomall et al., 2014; Vaitkevicius et al., 2017). However, a clinical trial (NCT02477618) evaluating the efficacy of Brexanolone did not show any improvement for the treatment of super refractory status epilepticus in human patients, according to the company news release (https://investor.sagerx.com/news-releases/news-release-details/sage-therapeutics-reports-top-line-results-phase-3-status-trial), although its intravenous administration was well tolerated among adults and children (Vaitkevicius et al., 2017). Dosing of allopregnanolone and variations in metabolism among patients are issues that may have impacted the outcome of this study, since some patients clearly responded to the therapy. Another clinical trial is ongoing to evaluate the efficacy of intravenous ganaxolone as adjunctive therapy to treat status epilepticus (NCT03350035).
5.4. Potential actions in sleep disturbances
Both synaptic and extrasynaptic GABAA receptors contribute to regulate sleep and wakefulness. Benzodiazepines, traditionally used to treat insomnia, typically act on synaptic α1-2-3-5βγ2 GABAA receptors mediating phasic inhibition. Benzodiazepines shorten latency to sleep and increase sleep continuity (Winsky-Sommerer, 2009). In addition, endogenous neuroactive steroids also exert sedative-hypnotic actions (Mendelson et al., 1987; Lancel et al., 1997), similar to other exogenous modulators (gaboxadol or 4,5,6,7-tetrahydroisoxazolo (5,4-c)pyridin-3-ol (THIP)) of extrasynaptic α4βδ GABAA receptors (Belelli and Lambert, 2005). Indeed, tonic inhibition mediated by α4βδ GABAA receptors in the thalamus and cortex, by adjusting the excitability of neuronal circuitries, plays an important role in modulating the magnitude and frequency of network oscillation characterizing waking and sleep (Winsky-Sommerer, 2009). Sleep disturbances are reported in stress-related disorders (Lo Martire et al., 2019) and sleep deprivation is itself a stressor that disrupts homeostasis (McEwen and Karatsoreos, 2015). In agreement, blunted neuroactive steroids responses to stress were associated to poor sleep quality in pregnant women (Crowley et al., 2016). Thus, administration of neuroactive steroids might ameliorate deficits in sleep quality in stress-related disorders by restoring the GABAergic tone.
In summary, deficits in inhibitory transmission in the CNS are hallmarks of stress-related disease. The neuroactive steroid allopregnanolone is capable of correcting deficits in inhibitory neurotransmission and has demonstrated clinical efficacy for treatment of anxiety, depression and seizures in both animal models and early human clinical trials. Importantly, allopregnanolone exhibited no evidence of toxicity or untoward effects in any study to date. The overlap and co-morbidity of these conditions should not be ignored and based on these observations, the potential for allopregnanolone therapy warrants immediate consideration. The allopregnanolone precursors pregnenolone and progesterone lack direct effects on GABAA receptors (Purdy et al., 1990), but these steroids are rapidly converted to allopregnanolone in vivo (Marx et al., 2007; Porcu et al., 2009; Milivojevic et al., 2016), and therefore may also share the ability to abrogate the loss of inhibitory transmission in stress-related disease.
6. Evidence that allopregnanolone restores deficits precipitated by aberrant CRF signaling
Acute stress rapidly induces HPA axis activation with subsequent release of corticosterone, as well as allopregnanolone (Purdy et al., 1991). The increase in allopregnanolone content, which occurs approximately 30 min after acute stress, is thought to represent a homeostatic mechanism to restore the GABAergic inhibition upon the hypothalamic PVN, thus shutting down HPA axis activity (Biggio et al., 2007; Gunn et al., 2015). Both corticosterone and allopregnanolone exert a negative feedback upon the hypothalamus and pituitary. Specifically, allopregnanolone counteracts the anxiety-like behavior induced by CRF administration, although it does not appear to be involved in basal CRF release (Patchev et al., 1994). Moreover, allopregnanolone or 3α,5α-THDOC administration before stress attenuates the stress-induced increase in adrenocorticotropic hormone (ACTH) and corticosterone (Owens et al., 1992; Patchev et al., 1996). In agreement, intracerebroventricular administration of allopregnanolone antiserum enhanced the corticosterone response to stress in prepubertal and adult rats, without affecting its basal levels (Guo et al., 1995). In addition, systemic administration of allopregnanolone to adult male non-stressed rats increased hypothalamic CRFcontent as well as serum ACTH and corticosterone (Naert et al., 2007), supporting a regulatory role for this neuroactive steroid in HPAfunction, whereby allopregnanolone may increase hormone levels in basal conditions and decrease them in stress-induced perturbations to restore homeostasis. Systemic administration of pregnenolone, dehydroepiandrosterone and their sulfate metabolites also increased hypothalamic CRF and serum ACTH and corticosterone (Naert et al., 2007). All these effects were rapid and likely mediated by a direct action of neuroactive steroids on neurotransmission in the hypothalamus that regulate HPA axis activation.
Several lines of both clinical and basic science evidence suggest that neuroactive steroids may restore homeostasis in CRF signaling both at the hypothalamic and extrahypothalamic circuit levels. The anxiolytic effects of allopregnanolone are likely to be related to both hypothalamic and extrahypothalamic CRF levels since CRF circuits are tightly coupled to anxiety-like behaviors in rodents (vide supra). Some examples follow.
Affective disorders, including major depression, postpartum depression, PTSD and AUDs are characterized, among other features, by neuroendocrine alterations at the HPA axis level (Bixo et al., 1997; Adinoff et al., 2005a, 2005b, 2005c; Girdler and Klatzkin, 2007; Girdler et al., 2012; Baumeister et al., 2014; Schule et al., 2014; Rasmusson et al., 2017). These alterations generally involve excessive baseline cortisol and suppression of the HPA axis response to stress. Considering the ability of allopregnanolone to regulate the HPA axis at the level of the hypothalamus, it is possible that restoration of HPA axis balance is an important component of treatment. Indeed, the remarkable clinical efficacy of Brexanolone in the treatment of postpartum depression may be related to this property of allopregnanolone. While further studies are needed to determine the mechanism(s) of the antidepressant actions of Brexanolone, the rapid and long-lasting efficacy following a short course of 60 hours of treatment suggests a type of reset that is consistent with normalization of HPA axis function.
Furthermore, although classical treatments for depression such as selective serotonin reuptake inhibitors require several weeks to produce therapeutic actions, increased neurosteroidogenesis is a likely mechanism involved in their therapeutic actions. Several studies have shown that administration of antidepressant drugs restores neuroactive steroid concentrations in both patients and rodents (Uzunov et al., 1996; Romeo et al., 1998; Uzunova et al., 1998; Marx et al., 2006; Schule et al., 2014). Indeed, serotonin reuptake inhibitors promote the conversion of 5α-dihydroprogesterone to allopregnanolone via a direct effect on the neurosteroidogenic enzyme 3α-hydroxysteroid dehydrogenase (Griffin and Mellon, 1999). Thus, allopregnanolone may have great potential for the treatment of stress-related psychiatric disorders in general and specifically in those associated with dysregulation of the HPA axis.
PTSD patients show elevated CRF levels (Bremner et al., 1997), along with a dysregulated HPA axis function that leads to glucocorticoid hypersensitivity (Castro-Vale et al., 2016). In addition, concentrations of GABAergic neuroactive steroids are altered in PTSD patients: a reduction in cerebrospinal levels of allopregnanolone has been reported in premenopausal women and men, and such levels were negatively correlated with PTSD symptoms (Rasmusson et al., 2006, 2017). Indeed, both preclinical and clinical evidence suggests a role for allopregnanolone in PTSD (Rasmusson et al., 2017). Clinical trials are ongoing (NCT03799562; NCT00560781) to test the effectiveness of the precursor pregnenolone in targeting PTSD symptoms.
Social isolation in animal models of affective disorders each induce alterations in CRF and HPA axis function and each of these conditions is responsive to treatment with allopregnanolone or pregnenolone. Social isolation from weaning to adulthood is a model of chronic stress and PTSD that markedly decreases brain and plasma allopregnanolone levels in rats and mice (Serra et al., 2000; Dong et al., 2001). A blunted HPA axis activity has been hypothesized to account for such decrease (Biggio et al., 2014). In agreement, socially isolated male rats also show a reduction in basal ACTH and corticosterone levels (Serra et al., 2005; Pisu et al., 2016). However, socially isolated male rats are more sensitive to the steroidogenic effects of stress, as shown by the greater increase in allopregnanolone content following acute stress (Serra et al., 2000, 2003), suggesting that social isolation actually induces an hyperresponsiveness of the HPA axis. In agreement, central administration of CRF increased plasma corticosterone levels to a greater extent in socially isolated rats, compared to group-housed controls (Serra et al., 2005). Likewise, exposure to acute stress induced a greater and prolonged increase in CRFand ACTH content in socially isolated rats (Boero et al., 2018), supporting a dysregulation of the HPAaxis. Indeed, social isolation also impairs the HPA negative feedback regulation, as suggested by the blunted corticosterone response to dexamethasone challenge, observed in socially isolated vs. group-housed male rats (Serra et al., 2005). Importantly, administration of allopregnanolone either from the onset of social isolation, or following six weeks of isolation, prevents or normalizes the corticosterone response to the dexamethasone suppression test, as well as depression- and anxiety-like behavior in male rats (Evans et al., 2012). Since allopregnanolone normalizes the HPA axis and behavioral abnormalities after social isolation, it could have similar effects in other conditions that dysregulate the HPA axis and produce abnormal anxiety and depression.
7. Evidence that neurosteroids inhibit TLR4 signaling and the production of pro-inflammatory molecules
Recent studies indicate that pregnenolone and allopregnanolone inhibit pro-inflammatory signaling by TLR4 receptors in cultured macrophage cells (RAW264.7 cells) and the brain of alcohol-preferring P rats that exhibit innate TLR4 activation (Balan et al., 2019). In the mouse macrophage cell line, lipopolysaccharide (LPS) increased the entire pathway of pro-inflammatory markers of TLR4 activation, and all of these effects were completely inhibited by both allopregnanolone and pregnenolone at 0.5 and 1.0 μM doses. Pregnenolone was more potent than allopregnanolone and was not converted to allopregnanolone in the cells. The mechanism of neuroactive steroid inhibition of TLR4 signaling appears to involve blockade of TLR4/lymphocyte antigen 96 (MD-2) protein interactions in RAW246.7 cells, as both neurosteroids blocked the co-immunoprecipitation of TLR4 with MD-2, a requisite step in the activation of the TLR4/MD-2/MyD88-dependent signaling pathway. These data suggest a novel role for both pregnenolone and allopregnanolone to inhibit TLR signaling in the innate immune system (Balan et al., 2019).
Systemic allopregnanolone administration (15 mg/kg, I.P.) also reduced expression of the TLR4 activation marker tumor necrosis factor receptor associated factor 6 (TRAF6), CRF, and the pro-inflammatory cytokine monocyte chemoattractant protein 1 (MCP-1) in the P rat brain, although the effects were less pronounced than the cells. In addition, allopregnanolone inhibited TLR4 binding to its activation partners in the brain: the GABAA receptor α2 subunit protein and MyD88, both of which are required for TLR4 activation. The data suggest that inhibition of pro-inflammatory neuroimmune signaling contributes to the protective effects of allopregnanolone in immune cells and brain, apparently involving a blockade of protein-protein interactions that initiate TLR4-dependent signaling. Inhibition of pro-inflammatory TLR4 activation represents a new mechanism of allopregnanolone action in both the periphery and the brain (Balan et al., 2019). A similar blockade of TLR signaling by pregnenolone and its derivatives were found via degradation of TLR2 and TLR4-associated proteins (Murugan et al., 2019). Furthermore, other studies have shown that progesterone inhibits pro-inflammatory signaling following traumatic brain injury in rats (He et al., 2004a; Sayeed and Stein, 2009). Because multiple TLRs signal through the same molecular pathways, it is possible that the neurosteroids inhibit other pro-inflammatory pathways as well (He et al., 2004a; Sayeed and Stein, 2009; Murugan et al., 2019). Further studies are needed to address this possibility.
In light of the hypotheses that activation of neuroimmune signaling contributes to many stress-related psychiatric conditions, the ability of allopregnanolone to inhibit TLR4 signaling in both macrophages and brain suggests that it may have therapeutic utility in the prevention or treatment of these disorders. Neuroactive steroids might represent a useful therapeutic approach to arrest detrimental neuroimmune signaling, observed in patients. This argument is supported by data from animal models of chronic stress and effects of neuroactive steroids in other conditions that involve excessive neuroimmune activation in the CNS. Some examples are described.
7.1. Therapeutic effects of pregnenolone, progesterone or allopregnanolone in multiple neuroinflammatory diseases
The endogenous neurosteroids pregnenolone, progesterone, and allopregnanolone have protective activity in neurological and psychiatric conditions that involve pro-inflammatory signaling. Significantly, progesterone and/or allopregnanolone have shown efficacy in clinical studies of traumatic brain injury (Wright et al., 2007; Stein and Sayeed, 2019), cocaine craving (Fox et al., 2013; Milivojevic et al., 2016), as well as postpartum depression (Kanes et al., 2017; Meltzer-Brody et al., 2018). Further, allopregnanolone has therapeutic activity in animal models of traumatic brain injury (He et al., 2004b), multiple sclerosis (Schumacher et al., 2007; Noorbakhsh et al., 2014), and Alzheimer's disease (Brinton, 2013). This growing body of inflammatory conditions that respond to intervention with these neurosteroids supports the idea that inhibition of neuroimmune signaling may be an important component of their actions.
Pain is considered a neuroinflammatory condition. Preclinical studies suggest that neuroactive steroids play an important role in both inflammatory and neuropathic pain. Intracerebroventricular administration of allopregnanolone has analgesic effects in naïve male rats (Kavaliers and Wiebe, 1987). Likewise, systemic and intrathecal administration of allopregnanolone, or its precursor progesterone, exerts anti-nociceptive properties in several models of neuropathic (see (Gonzalez et al., 2019) for review) and inflammatory pain (Ocvirk et al., 2008). Thus, administration of neuroactive steroids might be beneficial to ameliorate pain symptoms. Indeed, a recent clinical study in 92 veterans showed that pregnenolone administration for 4 weeks reduced chronic pain symptoms by 20% while the patients in the placebo control group reported a decrease in pain symptoms of 4%. In addition, low back symptoms were inversely related to serum neuroactive steroid levels -https://www.ajmc.com/conferences/sobp-2018/new-drug-discoveries-aim-to-help-veterans-others-with-chronic-pain. However, other evidence in humans linking neuroactive steroids to pain is controversial: low serum allopregnanolone content was associated with either increased pain tolerance in healthy volunteers (Mechlin et al., 2007), or increased self-reported pain symptoms in another study of war veterans (Naylor et al., 2016). Therefore, allopregnanolone and pregnenolone should be further studied for inhibition of inflammatory signaling associated with pain.
Depression is increasingly linked to neuroinflammation or more specifically to the presence of pro-inflammatory markers in patients (Dantzer et al., 2008; Bhattacharya et al., 2016; Vichaya et al., 2019). Indeed, several studies show that inflammatory markers are elevated in women with postpartum depression (Kendall-Tackett, 2007; Corwin et al., 2008), suggesting that neuroimmune activation may contribute to symptoms of depression in these women. As presented in Sections 6, 5.1 of this review, the remarkable, rapid and long-lasting antidepressant effect of Brexanolone infusion for postpartum depression may involve inhibitory effects on neuroinflammation as well as restoration of GABAergic inhibition and normalization of CRF signaling. The ability of allopregnanolone to inhibit the activation of TLR signaling in the innate immune system could reduce pro-inflammatory signaling that contributes to these therapeutic effects. While this idea remains speculative, there is substantial evidence for the opposing condition where elevation of inflammatory signaling is well known to promote depression in humans and depression-like behavior in animal models (Fu et al., 2010; Dantzer et al., 2011; Painsipp et al., 2011).
In summary, several lines of evidence suggest that the ability of allopregnanolone and pregnenolone to inhibit TLR activation may be linked to their therapeutic actions in numerous neuropsychiatric conditions that involve inflammatory signaling. The innate immune system plays a key role in this process and neuroactive steroids may be a critical factor that moderates the pathological effects of stress on inflammation that contribute to disease.
8. Cautions and limitations of neuroactive steroid therapeutics
Considering the plethora of evidence for neuroactive steroid actions that might serve to support the recovery from stress-related conditions, it is surprising that more human laboratory and clinical studies have not been conducted using neuroactive steroids or their precursors. Caution has prevailed due to the widespread perception that GABAergic neuroactive steroids are similar to benzodiazepines, despite the fact that the actions of neuroactive steroids are clearly distinct from benzodiazepines as described in the preceding sections. Even among researchers, however, there have been controversies regarding their risks and potential for use in human disease. Here we address the issues that are most compelling and propose a rational approach to the introduction of neuroactive steroid therapy for stress-related disease.
8.1. Lack of potent and specific inhibitors of neuroactive steroid effects hampers conclusions on their role in CNS function and disease
To date, no potent and specific antagonist of neuroactive steroid effects on GABAA receptors has been found. Several candidate antagonist molecules have been reported, including the 3β-enantiomers of certain pregnane steroids (Wang et al., 2002), the 3α,5β- or 3α,5α-reduced metabolites of cortisol (Penland and Morrow, 2004), and the steroid analog [3α,5α]17-phenylandrost-16-en-3-ol (17PA) (Mennerick et al., 2004; Kelley et al., 2007). However, none of these compounds exhibit sufficient potency, efficacy, or specificity to provide useful tools for the interrogation of neuroactive steroid actions.
Numerous investigators have employed the inhibition of steroid synthesis to interrogate the role of neuroactive steroids in disease models. The strategy has been to use finasteride to block the formation of allopregnanolone in order to determine its role in normal brain function and pathology. Endogenous allopregnanolone is formed by the reduction of progesterone in two steps. The classical pathway indicates that 5α-reductase converts progesterone to 5α-dihydroprogesterone and then 3α-hydroxysteroid dehydrogenase converts 5α-dihydroprogesterone to allopregnanolone (see the biosynthetic pathway in (Porcu et al., 2009)). However, an alternative pathway also exists where 3α-hydroxysteroid dehydrogenase converts progesterone to 3α-hydroxyprogesterone (also known as 3α-hydroxy-4-pregnen-20-one). This neuroactive steroid is also GABAergic and displays equal potency and efficacy as allopregnanolone and 3α,5α-THDOC in the Cl− flux assay of effects on GABAA receptors (Morrow et al., 1987, 1990). 3α-Hydroxyprogesterone is found in brain in amounts that are approximately equal to allopregnanolone (Wiebe et al., 1997; Griffin and Mellon, 2001) and in male testes (Wiebe, 1982), but its physiologic and behavioral effects are largely unknown. In the presence of finasteride, this steroid would not be further metabolized to allopregnanolone. In addition, progesterone is converted by 5β-reductase to 5β-dihydroprogesterone and then to pregnanolone (3α,5β-3-hydroxypregnan-20-one). This steroid is also an equipotent GABAergic allosteric modulator that is present in human serum in approximately the same quantity as allopregnanolone. Although it is normally undetectable in rodents, the administration of pregnenolone to rats induces the appearance of pregnanolone (Porcu et al., 2009). If 5α-reductase is inhibited or subjected to genetic deletion, pregnenolone and progesterone are increased and the role of 3α-hydroxyprogesterone and pregnanolone as substitute neuroactive steroids must be considered. If investigators presume that finasteride blocks the formation of allopregnanolone, and there is no compensation by other steroids, the interpretation of data may lead to inappropriate conclusions. In light of this consideration, the interpretation of effects of these interventions are very difficult at best. This unsolved conundrum in the field has resulted in data that promotes uncertainty and greater caution in the use of allopregnanolone for therapeutics.
8.2. Neuroactive steroid therapeutics may interact with socially acceptable drugs like nicotine or alcohol and/or lead to untoward effects including allopregnanolone dependence
It is known that ethanol and nicotine can increase GABAergic neuroactive steroids (VanDoren et al., 2000; Porcu et al., 2003, 2010; Concas et al., 2006) and these steroids may interact with ethanol or nicotine in ways that might be deleterious, such as when driving or operating machinery. Since addictions are chronic and characterized by both the loss of control of one's behavior as well as the tendency to relapse, the concern over these potential interactions is understandable and might be fully justified. Alternatively, it is possible that patients with addictions may have different responses to allopregnanolone and/or pregnenolone compared to average healthy subjects. This might occur if patients are suffering from deficient GABAergic transmission, CRF regulation, and/or neuroimmune overactivity. In this case, the administration of the neuroactive steroids may normalize biological function and interactions with ethanol would not be unmanageable. In diabetes, for example, the administration of insulin normalizes metabolic function rather than causing excess energy production that would be detrimental in normal healthy control subjects. In every clinical trial reported to date with allopregnanolone or pregnenolone for neuropsychiatric conditions, there were no serious adverse effects in the patients (vide supra). Nonetheless, until we know more about neuroactive steroid effects in patients with diseases that might respond to therapy with allopregnanolone, trials could be limited to intravenous infusions of allopregnanolone in a medically supervised setting, drug interactions could be tested in laboratory settings ,and oral treatments could be given for short periods to assess patient responses.
The rapid and long-lasting antidepressant effect of Brexanolone in patients with postpartum depression (Meltzer-Brody et al., 2018) suggests that allopregnanolone therapy might be useful for generalized depression, PTSD, alcohol or cocaine detoxification and might last up to a month or longer such that other treatments such as cognitive behavioral therapies could have a greater impact. Neuroactive steroid therapy might be considered like other anti-inflammatory therapies that are administered once monthly to control inflammatory conditions, such as the TNF-α antagonist, Remicade. The allopregnanolone precursors pregnenolone and progesterone may also be effective and further studies are clearly warranted. However, pregnenolone and progesterone also produce other metabolites that could have unexpected effects and more studies are needed to justify the use of these steroids in stress-related disease at this time.
Other animal studies have suggested that progesterone withdrawal and therefore by analogy, withdrawal from allopregnanolone can lead to anxiety, GABAA receptor dysregulation and dysfunction (Costa et al., 1995; Moran et al., 1998; Moran and Smith, 1998; Smith et al., 1998). The data suggests a danger from rapid allopregnanolone withdrawal in human subjects as well. Again, this clinical concern has not borne out in any of the clinical studies conducted to date. Nonetheless, withdrawal signs should be monitored and prevented by gradual tapering if therapy is discontinued. However, therapy for chronic conditions may be life-long, as in the case of cardiovascular disease, diabetes and many other conditions.
9. Final conclusion
We have previously suggested that allopregnanolone may be a protective factor in normal healthy controls that helps to prevent the development of AUDs (Morrow, 2007). We now suggest that it might also help to prevent many other stress-related diseases that involve the dysregulation of GABAergic transmission, excessive CRF signaling, and neuroimmune activation. Allopregnanolone is an endogenous factor that helps to maintain normal CNS inhibition, behavioral control, HPA axis homeostasis, and prevents the activation of neuroimmune signaling through various TLR receptors that initiate systemic inflammatory responses and excessive brain excitability. Acute stress and acute ethanol exposure increase neurosteroid levels in circulation along with endotoxin and cortisol/corticosterone. The concurrent production of pregnenolone, progesterone and allopregnanolone helps to ensure the return to hypothalamic and extrahypothalamic CRF and HPA axis homeostasis and prevents the activation of TLR signaling and thus the production of pro-inflammatory molecules. This is a healthy response to alcohol and stress, and it may explain why most people can manage the stress of life without issues.
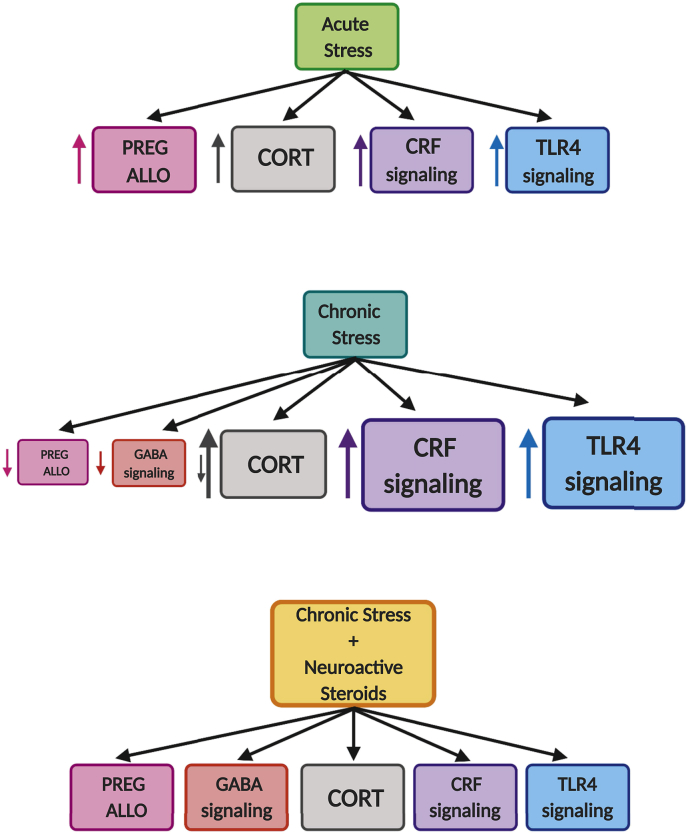
With chronic stress, the neurosteroids are depleted in serum and/or brain, HPA axis and CRF dysregulation ensue, GABAA receptor function is dysregulated, and markers of neuroinflammation are elevated. In addition, there may be tolerance to the effects of acute stress challenge on the production of allopregnanolone. At this point, behavioral manifestations of disease are evident with anxiety, dysphoria and possibly drug craving/intake to relieve the symptoms of the adaptations caused by chronic exposure to stress. Fig. 3 illustrates how allopregnanolone can restore homeostasis of GABA inhibition, CRF signaling, and neuroimmune activation following chronic stress exposure.
Fig. 3.
Theoretical framework of the multi-system dysregulation caused by stress and the potential restoration of homeostasis by neuroactive steroids. Acute stress causes an increase in neuroactive steroids, CRF, glucocorticoids and proinflammatory neuroimmune signaling. Chronic stress induces a decrease of neuroactive steroids and GABAergic signaling while increasing CRF and TLR4 neuroimmune signaling. Neuroactive steroids may restore homeostasis by increasing GABAA receptor-mediated inhibition, decreasing CRF, and stabilizing glucocorticoids release and deleterious TLR4 signaling, all of which contribute to the etiology of stress-related psychiatric disorders. (PREG = pregnenolone; ALLO = allopregnanolone; CORT = cortisol/corticosterone). (Created with Biorender.com).
Here we propose that inadequate GABAergic function, along with excessive CRF and neuroimmune signaling is central to stress-related disorders, and therefore patients would respond to treatment with allopregnanolone or its precursors pregnenolone/progesterone in a favorable manner. The pleiotropic actions of allopregnanolone are likely key to its therapeutic potential. Pregnenolone and progesterone have inhibitory properties on TLR signaling, and they are rapidly converted to allopregnanolone in vivo and thus may share its beneficial pleiotropic actions. Furthermore, these precursors are readily available and less expensive to obtain. It is time for clinical studies to address and to compare the potential of these neurosteroids in stress-related conditions.
Funding
This review was supported by the NIAAA-R01-AA024095 (ALM), and the UNC Bowles Center for Alcohol Studies.
A. L. Morrow has filed a worldwide patent on the use of allopregnanolone and pregnenolone for inflammatory conditions.
A. L. Morrow has received research funding from Sage Therapeutics.
Declaration of competing interest
All other authors report no conflict of interest.
References
- Adinoff B., Junghanns K., Kiefer F., Krishnan-Sarin S. Suppression of the HPA axis stress-response: implications for relapse. Alcohol Clin. Exp. Res. 2005;29(7):1351–1355. doi: 10.1097/01.ALC.0000176356.97620.84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adinoff B., Krebaum S.R., Chandler P.A., Ye W., Brown M.B., Williams M.J. Dissection of hypothalamic-pituitary-adrenal axis pathology in 1-month-abstinent alcohol-dependent men, part 1: adrenocortical and pituitary glucocorticoid responsiveness. Alcohol Clin. Exp. Res. 2005;29(4):517–527. doi: 10.1097/01.ALC.0000158940.05529.0A. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adinoff B., Krebaum S.R., Chandler P.A., Ye W., Brown M.B., Williams M.J. Dissection of hypothalamic-pituitary-adrenal axis pathology in 1-month-abstinent alcohol-dependent men, part 2: response to ovine corticotropin-releasing factor and naloxone. Alcohol Clin. Exp. Res. 2005;29(4):528–537. doi: 10.1097/01.ALC.0000158939.25531.EE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aguirre A., Maturana C.J., Harcha P.A., Saez J.C. Possible involvement of TLRs and hemichannels in stress-induced CNS dysfunction via mastocytes, and glia activation. Mediat. Inflamm. 2013;2013 doi: 10.1155/2013/893521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akhter N., Madhoun A., Arefanian H., Wilson A., Kochumon S., Thomas R., Shenouda S., Al-Mulla F., Ahmad R., Sindhu S. Oxidative stress induces expression of the toll-like receptors (TLRs) 2 and 4 in the human peripheral blood mononuclear cells: implications for metabolic inflammation. Cell. Physiol. Biochem. 2019;53(1):1–18. doi: 10.33594/000000117. [DOI] [PubMed] [Google Scholar]
- Akinci M.K., Johnston G.A.R. Sex differences in the effects of acute swim stress on binding to GABAA receptors in mouse brain. J. Neurochem. 1993;60:2212–2216. doi: 10.1111/j.1471-4159.1993.tb03507.x. [DOI] [PubMed] [Google Scholar]
- Baer K., Essrich C., Balsiger S., Wick M.J., Harris R.A., Fritschy J.M., Luscher B. Rescue of gamma2 subunit-deficient mice by transgenic overexpression of the GABAA receptor gamma2S or gamma2L subunit isoforms. Eur. J. Neurosci. 2000;12(7):2639–2643. doi: 10.1046/j.1460-9568.2000.00159.x. [DOI] [PubMed] [Google Scholar]
- Balan I., Beattie M.C., O'Buckley T.K., Aurelian L., Morrow A.L. Endogenous neurosteroid (3⍺,5⍺)3-hydroxypregnan-20-one inhibits toll-like-4 receptor activation and pro-inflammatory signaling in macrophages and brain. Sci. Rep. 2019;9(1):1220. doi: 10.1038/s41598-018-37409-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baumeister D., Lightman S.L., Pariante C.M. The interface of stress and the HPA Axis in behavioural phenotypes of mental illness. Curr. Top. Behav. Neurosci. 2014;18:13–24. doi: 10.1007/7854_2014_304. [DOI] [PubMed] [Google Scholar]
- Belelli D., Casula A., Ling A., Lambert J.J. The influence of subunit composition on the interaction of neurosteroids with GABAA receptors. Neuropharmacology. 2002;43(4):651–661. doi: 10.1016/s0028-3908(02)00172-7. [DOI] [PubMed] [Google Scholar]
- Belelli D., Lambert J.J. Neurosteroids: endogenous regulators of the GABAA receptor. Nat. Rev. Neurosci. 2005;6(7):565–575. doi: 10.1038/nrn1703. [DOI] [PubMed] [Google Scholar]
- Bhattacharya A., Derecki N.C., Lovenberg T.W., Drevets W.C. Role of neuro-immunological factors in the pathophysiology of mood disorders. Psychopharmacology (Berlin) 2016;233(9):1623–1636. doi: 10.1007/s00213-016-4214-0. [DOI] [PubMed] [Google Scholar]
- Biggio G., Concas A., Follesa P., Sanna E., Serra M. Stress, ethanol, and neuroactive steroids. Pharmacol. Ther. 2007;116(1):140–171. doi: 10.1016/j.pharmthera.2007.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biggio G., Pisu M.G., Biggio F., Serra M. Allopregnanolone modulation of HPA axis function in the adult rat. Psychopharmacology (Berlin) 2014;231(17):3437–3444. doi: 10.1007/s00213-014-3521-6. [DOI] [PubMed] [Google Scholar]
- Binder E.B., Nemeroff C.B. The CRF system, stress, depression and anxiety-insights from human genetic studies. Mol. Psychiatry. 2010;15(6):574–588. doi: 10.1038/mp.2009.141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bissette G., Klimek V., Pan J., Stockmeier C., Ordway G. Elevated concentrations of CRF in the locus coeruleus of depressed subjects. Neuropsychopharmacology. 2003;28(7):1328–1335. doi: 10.1038/sj.npp.1300191. [DOI] [PubMed] [Google Scholar]
- Bitran D., Hilvers R.J., Kellogg C.K. Anxiolytic effects of 3⍺-hydroxy-5⍺[ß]-pregnan-20-one: endogenous metabolites of progesterone that are active at the GABAA receptor. Brain Res. 1991;561:157–161. doi: 10.1016/0006-8993(91)90761-j. [DOI] [PubMed] [Google Scholar]
- Bixo M., Andersson A., Winblad B., Purdy R.H., Backstrom T. Progesterone, 5⍺-pregnan-3,20-dione and 3⍺-hydroxy-5⍺-pregnane-20-one in specific regions of the human female brain in different endocrine states. Brain Res. 1997;764:173–178. doi: 10.1016/s0006-8993(97)00455-1. [DOI] [PubMed] [Google Scholar]
- Boero G., Pisu M.G., Biggio F., Muredda L., Carta G., Banni S., Paci E., Follesa P., Concas A., Porcu P., Serra M. Impaired glucocorticoid-mediated HPA axis negative feedback induced by juvenile social isolation in male rats. Neuropharmacology. 2018;133:242–253. doi: 10.1016/j.neuropharm.2018.01.045. [DOI] [PubMed] [Google Scholar]
- Breese G.R., Knapp D.J. Persistent adaptation by chronic alcohol is facilitated by neuroimmune activation linked to stress and CRF. Alcohol. 2016;52:9–23. doi: 10.1016/j.alcohol.2016.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bremner J.D., Licinio J., Darnell A., Krystal J.H., Owens M.J., Southwick S.M., Nemeroff C.B., Charney D.S. Elevated CSF corticotropin-releasing factor concentrations in posttraumatic stress disorder. Am. J. Psychiatry. 1997;154(5):624–629. doi: 10.1176/ajp.154.5.624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brinton R.D. Neurosteroids as regenerative agents in the brain: therapeutic implications. Nat. Rev. Endocrinol. 2013;9(4):241–250. doi: 10.1038/nrendo.2013.31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Broomall E., Natale J.E., Grimason M., Goldstein J., Smith C.M., Chang C., Kanes S., Rogawski M.A., Wainwright M.S. Pediatric super-refractory status epilepticus treated with allopregnanolone. Ann. Neurol. 2014;76(6):911–915. doi: 10.1002/ana.24295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cagetti E., Pinna G., Guidotti A., Baicy K., Olsen R.W. Chronic intermittent ethanol (CIE) administration in rats decreases levels of neurosteroids in hippocampus, accompanied by altered behavioral responses to neurosteroids and memory function. Neuropharmacology. 2004;46(4):570–579. doi: 10.1016/j.neuropharm.2003.10.001. [DOI] [PubMed] [Google Scholar]
- Caldji C., Francis D., Sharma S., Plotsky P.M., Meaney M.J. The effects of early rearing environment on the development of GABAA and central benzodiazepine receptor levels and novelty-induced fearfulness in the rat. Neuropsychopharmacology. 2000;22(3):219–229. doi: 10.1016/S0893-133X(99)00110-4. [DOI] [PubMed] [Google Scholar]
- Carver C.M., Reddy D.S. Neurosteroid interactions with synaptic and extrasynaptic GABA(A) receptors: regulation of subunit plasticity, phasic and tonic inhibition, and neuronal network excitability. Psychopharmacology. 2013;230(2):151–188. doi: 10.1007/s00213-013-3276-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castro-Vale I., van Rossum E.F., Machado J.C., Mota-Cardoso R., Carvalho D. Genetics of glucocorticoid regulation and posttraumatic stress disorder--What do we know? Neurosci. Biobehav. Rev. 2016;63:143–157. doi: 10.1016/j.neubiorev.2016.02.005. [DOI] [PubMed] [Google Scholar]
- Chadda R., Devaud L.L. Sex differences in effects of mild chronic stress on seizure risk and GABAA receptors in rats. Pharmacol. Biochem. Behav. 2004;78(3):495–504. doi: 10.1016/j.pbb.2004.03.022. [DOI] [PubMed] [Google Scholar]
- Chandra D., Jia F., Liang J., Peng Z., Suryanarayanan A., Werner D.F., Spigelman I., Houser C.R., Olsen R.W., Harrison N.L., Homanics G.E. GABAA receptor ⍺4 subunits mediate extrasynaptic inhibition in thalamus and dentate gyrus and the action of gaboxadol. Proc. Natl. Acad. Sci. U. S. A. 2006;103(41):15230–15235. doi: 10.1073/pnas.0604304103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cherubini E., Gaiarsa J.L., Ben-Ari Y. GABA: an excitatory transmitter in early postnatal life. Trends Neurosci. 1991;14:515–519. doi: 10.1016/0166-2236(91)90003-d. [DOI] [PubMed] [Google Scholar]
- Coleman L.G., Jr., Zou J., Crews F.T. Microglial-derived miRNA let-7 and HMGB1 contribute to ethanol-induced neurotoxicity via TLR7. J. Neuroinflammation. 2017;14(1):22. doi: 10.1186/s12974-017-0799-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Concas A., Sogliano C., Porcu P., Marra C., Brundu A., Biggio G. Neurosteroids in nicotine and morphine dependence. Psychopharmacology (Berlin) 2006;186(3):281–292. doi: 10.1007/s00213-005-0111-7. [DOI] [PubMed] [Google Scholar]
- Corwin E.J., Johnston N., Pugh L. Symptoms of postpartum depression associated with elevated levels of interleukin-1 beta during the first month postpartum. Biol. Res. Nurs. 2008;10(2):128–133. doi: 10.1177/1099800408323220. [DOI] [PubMed] [Google Scholar]
- Costa A.M., Spence K.T., Smith S.S., ffRench-Mullen J. Withdrawal from the endogenous steroid progesterone results in GABAA currents insensitive to benzodiazepine modulation in rat CA1 hippocampus. J. Neurophysiol. 1995;74:464–469. doi: 10.1152/jn.1995.74.1.464. [DOI] [PubMed] [Google Scholar]
- Crawley J.N., Glowa J.R., Majewska M.D., Paul S.M. Anxiolytic activity of an endogenous adrenal steroid. Brain Res. 1986;398:382–385. doi: 10.1016/0006-8993(86)91500-3. [DOI] [PubMed] [Google Scholar]
- Crews F.T., Lawrimore C.J., Walter T.J., Coleman L.G., Jr. The role of neuroimmune signaling in alcoholism. Neuropharmacology. 2017;122:56–73. doi: 10.1016/j.neuropharm.2017.01.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crews F.T., Walter T.J., Coleman L.G., Jr., Vetreno R.P. Toll-like receptor signaling and stages of addiction. Psychopharmacology (Berlin) 2017;234(9–10):1483–1498. doi: 10.1007/s00213-017-4560-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Croarkin P.E., Levinson A.J., Daskalakis Z.J. Evidence for GABAergic inhibitory deficits in major depressive disorder. Neurosci. Biobehav. Rev. 2011;35(3):818–825. doi: 10.1016/j.neubiorev.2010.10.002. [DOI] [PubMed] [Google Scholar]
- Crowley S.K., O'Buckley T.K., Schiller C.E., Stuebe A., Morrow A.L., Girdler S.S. Blunted neuroactive steroid and HPA axis responses to stress are associated with reduced sleep quality and negative affect in pregnancy: a pilot study. Psychopharmacology (Berlin) 2016;233(7):1299–1310. doi: 10.1007/s00213-016-4217-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cullinan W.E., Ziegler D.R., Herman J.P. Functional role of local GABAergic influences on the HPA axis. Brain Struct. Funct. 2008;213(1–2):63–72. doi: 10.1007/s00429-008-0192-2. [DOI] [PubMed] [Google Scholar]
- Czeh B., Vardya I., Varga Z., Febbraro F., Csabai D., Martis L.S., Hoigaard K., Henningsen K., Bouzinova E.V., Miseta A., Jensen K., Wiborg O. Long-term stress disrupts the structural and functional integrity of GABAergic neuronal networks in the medial prefrontal cortex of rats. Front. Cell. Neurosci. 2018;12 doi: 10.3389/fncel.2018.00148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Czeh B., Varga Z.K.K., Henningsen K., Kovacs G.L., Miseta A., Wiborg O. Chronic stress reduces the number of GABAergic interneurons in the adult rat Hippocampus, dorsal-ventral and region-specific differences. Hippocampus. 2015;25(3):393–405. doi: 10.1002/hipo.22382. [DOI] [PubMed] [Google Scholar]
- Dantzer R., O'Connor J.C., Freund G.G., Johnson R.W., Kelley K.W. From inflammation to sickness and depression: when the immune system subjugates the brain. Nat. Rev. Neurosci. 2008;9(1):46–56. doi: 10.1038/nrn2297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dantzer R., O'Connor J.C., Lawson M.A., Kelley K.W. Inflammation-associated depression: from serotonin to kynurenine. Psychoneuroendocrinology. 2011;36(3):426–436. doi: 10.1016/j.psyneuen.2010.09.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Kloet E.R., Joels M., Holsboer F. Stress and the brain: from adaptation to disease. Nat. Rev. Neurosci. 2005;6(6):463–475. doi: 10.1038/nrn1683. [DOI] [PubMed] [Google Scholar]
- Devaud L.L., Fritschy J.-M., Morrow A.L. Influence of gender on chronic ethanol-induced alternations of GABAA receptors in rats. Brain Res. 1998;796:222–230. doi: 10.1016/s0006-8993(98)00357-6. [DOI] [PubMed] [Google Scholar]
- Devaud L.L., Purdy R.H., Finn D.A., Morrow A.L. Sensitization of gamma-aminobutyric acidA receptors to neuroactive steroids in rats during ethanol withdrawal. J. Pharmacol. Exp. Ther. 1996;278(2):510–517. [PubMed] [Google Scholar]
- Devaud L.L., Purdy R.H., Morrow A.L. The neurosteroid, 3α-hydroxy-5α-pregnan-20-one, protects against bicuculline-induced seizures during ethanol withdrawal in rats. Alcohol Clin. Exp. Res. 1995;19:350–355. doi: 10.1111/j.1530-0277.1995.tb01514.x. [DOI] [PubMed] [Google Scholar]
- Dong E., Matsumoto K., Uzunova V., Sugaya I., Takahata H., Nomura H., Watanabe H., Costa E., Guidotti A. Brain 5-alpha-dihydroprogesterone and allopregnanolone synthesis in a mouse model of protracted social isolation. Proc. Natl. Acad. Sci. U. S. A. 2001;98:2849–2854. doi: 10.1073/pnas.051628598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drugan R.C., Morrow A.L., Weizman R., Weizman A., Deutsch S.I., Crawley J.N., Paul S.M. Stress-induced behavioral depression in the rat is associated with a decrease in GABA receptor-mediated chloride ion flux and brain benzodiazepine receptor occupancy. Brain Res. 1989;487:45–51. doi: 10.1016/0006-8993(89)90938-4. [DOI] [PubMed] [Google Scholar]
- Evans J., Sun Y., McGregor A., Connor B. Allopregnanolone regulates neurogenesis and depressive/anxiety-like behaviour in a social isolation rodent model of chronic stress. Neuropharmacology. 2012;63(8):1315–1326. doi: 10.1016/j.neuropharm.2012.08.012. [DOI] [PubMed] [Google Scholar]
- Ferguson A.R., Christensen R.N., Gensel J.C., Miller B.A., Sun F., Beattie E.C., Bresnahan J.C., Beattie M.S. Cell death after spinal cord injury is exacerbated by rapid TNF alpha-induced trafficking of GluR2-lacking AMPARs to the plasma membrane. J. Neurosci. 2008;28(44):11391–11400. doi: 10.1523/JNEUROSCI.3708-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox H.C., Sofuoglu M., Morgan P.T., Tuit K.L., Sinha R. The effects of exogenous progesterone on drug craving and stress arousal in cocaine dependence: impact of gender and cue type. Psychoneuroendocrinology. 2013;38(9):1532–1544. doi: 10.1016/j.psyneuen.2012.12.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fu X., Zunich S.M., O'Connor J.C., Kavelaars A., Dantzer R., Kelley K.W. Central administration of lipopolysaccharide induces depressive-like behavior in vivo and activates brain indoleamine 2,3 dioxygenase in murine organotypic hippocampal slice cultures. J. Neuroinflammation. 2010;7:43. doi: 10.1186/1742-2094-7-43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Girdler S.S., Klatzkin R. Neurosteroids in the context of stress: implications for depressive disorders. Pharmacol. Ther. 2007;116(1):125–139. doi: 10.1016/j.pharmthera.2007.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Girdler S.S., Lindgren M., Porcu P., Rubinow D.R., Johnson J.L., Morrow A.L. A history of depression in women is associated with an altered GABAergic neuroactive steroid profile. Psychoneuroendocrinology. 2012;37(4):543–553. doi: 10.1016/j.psyneuen.2011.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzalez S.L., Meyer L., Raggio M.C., Taleb O., Coronel M.F., Patte-Mensah C., Mensah-Nyagan A.G. Allopregnanolone and progesterone in experimental neuropathic pain: former and new insights with a translational perspective. Cell. Mol. Neurobiol. 2019;39(4):523–537. doi: 10.1007/s10571-018-0618-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodkin H.P., Joshi S., Mtchedlishvili Z., Brar J., Kapur J. Subunit-specific trafficking of GABA(A) receptors during status epilepticus. J. Neurosci. 2008;28(10):2527–2538. doi: 10.1523/JNEUROSCI.3426-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grieder T.E., Herman M.A., Contet C., Tan L.A., Vargas-Perez H., Cohen A., Chwalek M., Maal-Bared G., Freiling J., Schlosburg J.E., Clarke L., Crawford E., Koebel P., Repunte-Canonigo V., Sanna P.P., Tapper A.R., Roberto M., Kieffer B.L., Sawchenko P.E., Koob G.F., van der Kooy D., George O. VTA CRF neurons mediate the aversive effects of nicotine withdrawal and promote intake escalation. Nat. Neurosci. 2014;17(12):1751–1758. doi: 10.1038/nn.3872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griffin L.D., Mellon S.H. Selective serotonin reuptake inhibitors directly alter activity of neurosteroidogenic enzymes. Proc. Natl. Acad. Sci. U.S.A. 1999;96(23):13512–13517. doi: 10.1073/pnas.96.23.13512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griffin L.D., Mellon S.H. Biosynthesis of the neurosteroid 3 alpha-hydroxy-4-pregnen-20-one (3 alpha hp), a specific inhibitor of FSH release. Endocrinology. 2001;142(11):4617–4622. doi: 10.1210/endo.142.11.8477. [DOI] [PubMed] [Google Scholar]
- Grobin A.C., Morrow A.L. 3α-Hydroxy-5α-pregnan-20-one levels and GABAA receptor-mediated 36Cl- flux across development in rat cerebral cortex. Brain Res. Dev. Brain Res. 2001;131(1–2):31–39. doi: 10.1016/s0165-3806(01)00242-5. [DOI] [PubMed] [Google Scholar]
- Gruol D.L. Neuroimmune regulation of neurophysiology in the cerebellum. Cerebellum. 2013;12(3):307–309. doi: 10.1007/s12311-012-0445-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gunn B.G., Cunningham L., Cooper M.A., Corteen N.L., Seifi M., Swinny J.D., Lambert J.J., Belelli D. Dysfunctional astrocytic and synaptic regulation of hypothalamic glutamatergic transmission in a mouse model of early-life adversity: relevance to neurosteroids and programming of the stress response. J. Neurosci. 2013;33(50):19534–19554. doi: 10.1523/JNEUROSCI.1337-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gunn B.G., Cunningham L., Mitchell S.G., Swinny J.D., Lambert J.J., Belelli D. GABAA receptor-acting neurosteroids: a role in the development and regulation of the stress response. Front. Neuroendocrinol. 2015;36:28–48. doi: 10.1016/j.yfrne.2014.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo A.-L., Petraglia F., Criscuolo M., Ficarra G., Nappi R.E., Palumbo M.A., Trentini G.P., Purdy R.H., Genazzani A.R. Evidence for a role of neurosteroids in modulation of diurnal changes and acute stress-induced corticosterone secretion in rats. Gynecol. Endocrinol. 1995;9:1–7. doi: 10.3109/09513599509160184. [DOI] [PubMed] [Google Scholar]
- Harper K.M., Knapp D.J., Park M.A., Breese G.R. Age-related differences in anxiety-like behavior and amygdalar CCL2 responsiveness to stress following alcohol withdrawal in male Wistar rats. Psychopharmacology (Berlin) 2017;234(1):79–88. doi: 10.1007/s00213-016-4439-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hasler G., van der Veen J.W., Tumonis T., Meyers N., Shen J., Drevets W.C. Reduced prefrontal glutamate/glutamine and gamma-aminobutyric acid levels in major depression determined using proton magnetic resonance spectroscopy. Arch. Gen. Psychiatr. 2007;64(2):193–200. doi: 10.1001/archpsyc.64.2.193. [DOI] [PubMed] [Google Scholar]
- He J., Crews F.T. Increased MCP-1 and microglia in various regions of the human alcoholic brain. Exp. Neurol. 2008;210(2):349–358. doi: 10.1016/j.expneurol.2007.11.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He J., Evans C.O., Hoffman S.W., Oyesiku N.M., Stein D.G. Progesterone and allopregnanolone reduce inflammatory cytokines after traumatic brain injury. Exp. Neurol. 2004;189(2):404–412. doi: 10.1016/j.expneurol.2004.06.008. [DOI] [PubMed] [Google Scholar]
- He J., Hoffman S.W., Stein D.G. Allopregnanolone, a progesterone metabolite, enhances behavioral recovery and decreases neuronal loss after traumatic brain injury. Restor. Neurol. Neurosci. 2004;22(1):19–31. [PubMed] [Google Scholar]
- Henckens M.J., Deussing J.M., Chen A. Region-specific roles of the corticotropin-releasing factor-urocortin system in stress. Nat. Rev. Neurosci. 2016;17(10):636–651. doi: 10.1038/nrn.2016.94. [DOI] [PubMed] [Google Scholar]
- Hennessy E.J., Parker A.E., O'Neill L.A. Targeting Toll-like receptors: emerging therapeutics? Nat. Rev. Drug Discov. 2010;9(4):293–307. doi: 10.1038/nrd3203. [DOI] [PubMed] [Google Scholar]
- Herd M.B., Belelli D., Lambert J.J. Neurosteroid modulation of synaptic and extrasynaptic GABAA receptors. Pharmacol. Ther. 2007;116(1):20–34. doi: 10.1016/j.pharmthera.2007.03.007. [DOI] [PubMed] [Google Scholar]
- Herman J.P., Tasker J.G., Ziegler D.R., Cullinan W.E. Local circuit regulation of paraventricular nucleus stress integration: glutamate-GABA connections. Pharmacol. Biochem. Behav. 2002;71(3):457–468. doi: 10.1016/s0091-3057(01)00681-5. [DOI] [PubMed] [Google Scholar]
- Hewitt S.A., Wamsteeker J.I., Kurz E.U., Bains J.S. Altered chloride homeostasis removes synaptic inhibitory constraint of the stress axis. Nat. Neurosci. 2009;12(4):438–443. doi: 10.1038/nn.2274. [DOI] [PubMed] [Google Scholar]
- Hosie A.M., Wilkins M.E., da Silva H.M., Smart T.G. Endogenous neurosteroids regulate GABAA receptors through two discrete transmembrane sites. Nature. 2006;444:486–489. doi: 10.1038/nature05324. [DOI] [PubMed] [Google Scholar]
- Jacobson-Pick S., Audet M.C., McQuaid R.J., Kalvapalle R., Anisman H. Stressor exposure of male and female juvenile mice influences later responses to stressors: modulation of GABAA receptor subunit mRNA expression. Neuroscience. 2012;215:114–126. doi: 10.1016/j.neuroscience.2012.04.038. [DOI] [PubMed] [Google Scholar]
- Kanes S., Colquhoun H., Gunduz-Bruce H., Raines S., Arnold R., Schacterle A., Doherty J., Epperson C.N., Deligiannidis K.M., Riesenberg R., Hoffmann E., Rubinow D., Jonas J., Paul S., Meltzer-Brody S. Brexanolone (SAGE-547 injection) in post-partum depression: a randomised controlled trial. Lancet. 2017;390(10093):480–489. doi: 10.1016/S0140-6736(17)31264-3. [DOI] [PubMed] [Google Scholar]
- Kavaliers M., Wiebe J.P. Analgesic effects of the progesterone metabolite, 3 alpha-hydroxy-5 alpha-pregnan-20-one, and possible modes of action in mice. Brain Res. 1987;415(2):393–398. doi: 10.1016/0006-8993(87)90228-9. [DOI] [PubMed] [Google Scholar]
- Kelley S.P., Alan J.K., O'Buckley T.K., Mennerick S., Krishnan K., Covey D.F., Morrow A.L. Antagonism of neurosteroid modulation of native gamma-aminobutyric acid receptors by (3alpha,5alpha)-17-phenylandrost-16-en-3-ol. Eur. J. Pharmacol. 2007;572(2–3):94–101. doi: 10.1016/j.ejphar.2007.06.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kendall-Tackett K. A new paradigm for depression in new mothers: the central role of inflammation and how breastfeeding and anti-inflammatory treatments protect maternal mental health. Int. Breastfeed. J. 2007;2:6. doi: 10.1186/1746-4358-2-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khisti R.T., Chopde C.T., Jain S.P. Antidepressant-like effect of the neurosteroid 3α-hydroxy-5α-pregnan-20-one in mice forced swim test. Pharmacol., Biochem. Behav. 2000;67(1):137–143. doi: 10.1016/s0091-3057(00)00300-2. [DOI] [PubMed] [Google Scholar]
- Knapp D.J., Harper K.M., Whitman B.A., Zimomra Z., Breese G.R. Stress and withdrawal from chronic ethanol induce selective changes in neuroimmune mRNAs in differing brain sites. Brain Sci. 2016;6(3) doi: 10.3390/brainsci6030025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kokate T.G., Svensson B.E., Rogawski M.A. Anticonvulsant activity of neurosteroids: correlation with gamma-aminobutyric acid-evoked chloride current potentiation. J. Pharmacol. Exp. Ther. 1994;270:1223–1229. [PubMed] [Google Scholar]
- Lacagnina M.J., Rivera P.D., Bilbo S.D. Glial and neuroimmune mechanisms as critical modulators of drug use and abuse. Neuropsychopharmacology. 2017;42(1):156–177. doi: 10.1038/npp.2016.121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lancel M., Faulhaber J., Schiffelholz T., Romeo E., di Michele F., Holsboer F., Rupprecht R. Allopregnanolone affects sleep in a benzodiazepine-like fashion. J. Pharmacol. Exp. Ther. 1997;282:1213–1218. [PubMed] [Google Scholar]
- Lee V., Sarkar J., Maguire J. Loss of Gabrd in CRH neurons blunts the corticosterone response to stress and diminishes stress-related behaviors. Psychoneuroendocrinology. 2014;41:75–88. doi: 10.1016/j.psyneuen.2013.12.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lemos J.C., Wanat M.J., Smith J.S., Reyes B.A., Hollon N.G., Van Bockstaele E.J., Chavkin C., Phillips P.E. Severe stress switches CRF action in the nucleus accumbens from appetitive to aversive. Nature. 2012;490(7420):402–406. doi: 10.1038/nature11436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang J., Suryanarayanan A., Chandra D., Homanics G.E., Olsen R.W., Spigelman I. Functional consequences of GABAA receptor α4 subunit deletion on synaptic and extrasynaptic currents in mouse dentate granule cells. Alcohol Clin. Exp. Res. 2008;32(1):19–26. doi: 10.1111/j.1530-0277.2007.00564.x. [DOI] [PubMed] [Google Scholar]
- Lo Martire V., Caruso D., Palagini L., Zoccoli G., Bastianini S. Stress & sleep: a relationship lasting a lifetime. Neurosci. Biobehav. Rev. 2019 doi: 10.1016/j.neubiorev.2019.08.024. [DOI] [PubMed] [Google Scholar]
- Luscher B., Shen Q., Sahir N. The GABAergic deficit hypothesis of major depressive disorder. Mol. Psychiatry. 2011;16(4):383–406. doi: 10.1038/mp.2010.120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma K., Xu A., Cui S., Sun M.R., Xue Y.C., Wang J.H. Impaired GABA synthesis, uptake and release are associated with depression-like behaviors induced by chronic mild stress. Transl. Psychiatry. 2016;6(10) doi: 10.1038/tp.2016.181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma K., Zhang H., Wang S., Wang H., Wang Y., Liu J., Song X., Dong Z., Han X., Zhang Y., Li H., Rahaman A., Wang S., Baloch Z. The molecular mechanism underlying GABAergic dysfunction in nucleus accumbens of depression-like behaviours in mice. J. Cell Mol. Med. 2019 doi: 10.1111/jcmm.14596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacKenzie G., Maguire J. Chronic stress shifts the GABA reversal potential in the hippocampus and increases seizure susceptibility. Epilepsy Res. 2015;109:13–27. doi: 10.1016/j.eplepsyres.2014.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maguire J., Mody I. Neurosteroid synthesis-mediated regulation of GABA(A) receptors: relevance to the ovarian cycle and stress. J. Neurosci. 2007;27(9):2155–2162. doi: 10.1523/JNEUROSCI.4945-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maroso M., Balosso S., Ravizza T., Liu J., Bianchi M.E., Vezzani A. Interleukin-1 type 1 receptor/Toll-like receptor signalling in epilepsy: the importance of IL-1beta and high-mobility group box 1. J. Intern. Med. 2011;270(4):319–326. doi: 10.1111/j.1365-2796.2011.02431.x. [DOI] [PubMed] [Google Scholar]
- Marx C.E., Keefe R.S., Leimone L.A., Hamer R.M., Bradford D.W., Dunn L., Payne V.M., Naylor J.C., Savitz A.J., Strauss J.L., Lieberman J.A., Shampine L.J. Proof-of-concept trial with the neurosteroid pregnenolone targeting neurocognitive and negative symptoms in schizophrenia. Biol. Psychiatry. 2007;61:13S. doi: 10.1038/npp.2009.26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marx C.E., Shampine L.J., Khisti R.T., Trost W.T., Bradford D.W., Grobin A.C., Massing M.W., Madison R.D., Butterfield M.I., Lieberman J.A., Morrow A.L. Olanzapine and fluoxetine administration and coadministration increase rat hippocampal pregnenolone, allopregnanolone and peripheral deoxycorticosterone: implications for therapeutic actions. Pharmacol. Biochem. Behav. 2006;84(4):609–617. doi: 10.1016/j.pbb.2006.07.032. [DOI] [PubMed] [Google Scholar]
- McEwen B.S., Karatsoreos I.N. Sleep deprivation and circadian disruption stress, allostasis, and allostatic load. Sleep Med. Clin. 2015;10(1):1. doi: 10.1016/j.jsmc.2014.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mechlin B., Morrow A.L., Maixner W., Girdler S.S. The relationship of allopregnanolone immunoreactivity and HPA-axis measures to experimental pain sensitivity: evidence for ethnic differences. Pain. 2007;131(1–2):142–152. doi: 10.1016/j.pain.2006.12.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meltzer-Brody S., Colquhoun H., Riesenberg R., Epperson C.N., Deligiannidis K.M., Rubinow D.R., Li H., Sankoh A.J., Clemson C., Schacterle A., Jonas J., Kanes S. Brexanolone injection in post-partum depression: two multicentre, double-blind, randomised, placebo-controlled, phase 3 trials. Lancet. 2018;392(10152):1058–1070. doi: 10.1016/S0140-6736(18)31551-4. [DOI] [PubMed] [Google Scholar]
- Mendelson W.B., Martin J.V., Perlis M., Wagner R., Majewska M.D., Paul S.M. Sleep induction by an adrenal steroid in the rat. Psychopharmacology. 1987;93:226–229. doi: 10.1007/BF00179939. [DOI] [PubMed] [Google Scholar]
- Mennerick S., He Y., Jiang X., Manion B.D., Wang M., Shute A., Benz A., Evers A.S., Covey D.F., Zorumski C.F. Selective antagonism of 5alpha-reduced neurosteroid effects at GABA(A) receptors. Mol. Pharmacol. 2004;65(5):1191–1197. doi: 10.1124/mol.65.5.1191. [DOI] [PubMed] [Google Scholar]
- Mihalek R.M., Banerjee P.K., Korpi E.R., Quinlan J.J., Firestone L.L., Mi Z.P., Lagenaur C., Tretter V., Sieghart W., Anagnostaras S.G., Sage J.R., Fanselow M.S., Guidotti A., Spigelman I., Li Z., DeLorey T.M., Olsen R.W., Homanics G.E. Attenuated sensitivity to neuroactive steroids in γ-aminobutyrate type A receptor delta subunit knockout mice. Proc. Natl. Acad. Sci. U. S. A. 1999;96(22):12905–12910. doi: 10.1073/pnas.96.22.12905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milivojevic V., Fox H.C., Sofuoglu M., Covault J., Sinha R. Effects of progesterone stimulated allopregnanolone on craving and stress response in cocaine dependent men and women. Psychoneuroendocrinology. 2016;65:44–53. doi: 10.1016/j.psyneuen.2015.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Modgil A., Parakala M.L., Ackley M.A., Doherty J.J., Moss S.J., Davies P.A. Endogenous and synthetic neuroactive steroids evoke sustained increases in the efficacy of GABAergic inhibition via a protein kinase C-dependent mechanism. Neuropharmacology. 2017;113(Pt A):314–322. doi: 10.1016/j.neuropharm.2016.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moran M.H., Goldberg N., Smith S.S. Progesterone withdrawal II: insensitivity to the sedative effects of a benzodiazepine. Brain Res. 1998;807:91–100. doi: 10.1016/s0006-8993(98)00781-1. [DOI] [PubMed] [Google Scholar]
- Moran M.H., Smith S.S. Progesterone withdrawal I: pro-convulsant effects. Brain Res. 1998;807:84–90. doi: 10.1016/s0006-8993(98)00782-3. [DOI] [PubMed] [Google Scholar]
- Morrow A.L. Recent developments in the significance and therapeutic relevance of neuroactive steroids - introduction to the special issue. Pharmacol. Ther. 2007;116(1):1–6. doi: 10.1016/j.pharmthera.2007.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morrow A.L., Pace J.R., Purdy R.H., Paul S.M. Characterization of steroid interactions with γ-aminobutyric acid receptor-gated chloride ion channels: evidence for multiple steroid recognition sites. Mol. Pharmacol. 1990;37:263–270. [PubMed] [Google Scholar]
- Morrow A.L., Suzdak P.D., Paul S.M. Steroid hormone metabolites potentiate GABA receptor-mediated chloride ion flux with nanomolar potency. Eur. J. Pharmacol. 1987;142:483–485. doi: 10.1016/0014-2999(87)90094-x. [DOI] [PubMed] [Google Scholar]
- Murugan S., Jakka P., Namani S., Mujumdar V., Radhakrishnan G. The neurosteroid pregnenolone promotes degradation of key proteins in the innate immune signaling to suppress inflammation. J. Biol. Chem. 2019;294(12):4596–4607. doi: 10.1074/jbc.RA118.005543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naert G., Maurice T., Tapia-Arancibia L., Givalois L. Neuroactive steroids modulate HPA axis activity and cerebral brain-derived neurotrophic factor (BDNF) protein levels in adult male rats. Psychoneuroendocrinology. 2007;32(8–10):1062–1078. doi: 10.1016/j.psyneuen.2007.09.002. [DOI] [PubMed] [Google Scholar]
- Naylor D.E., Liu H., Wasterlain C.G. Trafficking of GABA(A) receptors, loss of inhibition, and a mechanism for pharmacoresistance in status epilepticus. J. Neurosci. 2005;25(34):7724–7733. doi: 10.1523/JNEUROSCI.4944-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naylor J.C., Kilts J.D., Szabo S.T., Dunn C.E., Keefe F.J., Tupler L.A., Shampine L.J., Morey R.A., Strauss J.L., Hamer R.M., Wagner H.R., Workgroup M., Marx C.E. Allopregnanolone levels are inversely associated with self-reported pain symptoms in U.S. Iraq and Afghanistan-era veterans: implications for biomarkers and therapeutics. Pain Med. 2016;17(1):25–32. doi: 10.1111/pme.12860. [DOI] [PubMed] [Google Scholar]
- Nemeroff C.B. The neurobiology of depression. Sci. Am. 1998;278(6):42–49. doi: 10.1038/scientificamerican0698-42. [DOI] [PubMed] [Google Scholar]
- Noorbakhsh F., Baker G.B., Power C. Allopregnanolone and neuroinflammation: a focus on multiple sclerosis. Front. Cell. Neurosci. 2014;8:134. doi: 10.3389/fncel.2014.00134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ocvirk R., Pearson Murphy B.E., Franklin K.B., Abbott F.V. Antinociceptive profile of ring A-reduced progesterone metabolites in the formalin test. Pain. 2008;138(2):402–409. doi: 10.1016/j.pain.2008.01.019. [DOI] [PubMed] [Google Scholar]
- Orchinik M., Weiland N.G., McEwen B.S. Chronic exposure to stress levels of corticosterone alters GABAA receptor subunit mRNA levels in rat hippocampus. Mol. Brain Res. 1995;34:29–37. doi: 10.1016/0169-328x(95)00118-c. [DOI] [PubMed] [Google Scholar]
- Owens M.J., Ritchie J.C., Nemeroff C.B. 5α-Pregnane-3α,21-diol-20-one (THDOC) attenuates mild stress-induced increases in plasma corticosterone via a non-glucocorticoid mechanism: comparison with alprazolam. Brain Res. 1992;573:353–355. doi: 10.1016/0006-8993(92)90788-b. [DOI] [PubMed] [Google Scholar]
- Painsipp E., Kofer M.J., Sinner F., Holzer P. Prolonged depression-like behavior caused by immune challenge: influence of mouse strain and social environment. PLoS One. 2011;6(6) doi: 10.1371/journal.pone.0020719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patchev V.K., Holsboer F., Almeida O.F.X. Neuroactive steroids and the behavioral and endocrine response to stress. In: Genazzani A.R., Petraglia F., Purdy R.H., editors. The Brain: Source and Target for Sex Steroid Hormones. The Parthenon Group; New York: 1996. pp. 93–102. [Google Scholar]
- Patchev V.K., Shoaib M., Holsboer F., Almeida O.F.X. The neurosteroid tetrahydroprogesterone counteracts corticotropin-releasing hormone-induced anxiety and alters the release and gene expression of corticotropin-releasing hormone in the rat hypothalamus. Neuroscience. 1994;62:265–271. doi: 10.1016/0306-4522(94)90330-1. [DOI] [PubMed] [Google Scholar]
- Pavlov V.A., Tracey K.J. Neural regulation of immunity: molecular mechanisms and clinical translation. Nat. Neurosci. 2017;20(2):156–166. doi: 10.1038/nn.4477. [DOI] [PubMed] [Google Scholar]
- Penland S., Morrow A.L. 3α,5β-Reduced cortisol exhibits antagonist properties on cerebral cortical GABAA receptors. Eur. J. Pharmacol. 2004;506:129–132. doi: 10.1016/j.ejphar.2004.11.007. [DOI] [PubMed] [Google Scholar]
- Pentkowski N.S., Litvin Y., Blanchard D.C., Vasconcellos A., King L.B., Blanchard R.J. Effects of acidic-astressin and ovine-CRF microinfusions into the ventral hippocampus on defensive behaviors in rats. Horm. Behav. 2009;56(1):35–43. doi: 10.1016/j.yhbeh.2009.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pisu M.G., Garau A., Boero G., Biggio F., Pibiri V., Dore R., Locci V., Paci E., Porcu P., Serra M. Sex differences in the outcome of juvenile social isolation on HPA axis function in rats. Neuroscience. 2016;320:172–182. doi: 10.1016/j.neuroscience.2016.02.009. [DOI] [PubMed] [Google Scholar]
- Porcu P., O'Buckley T.K., Alward S.E., Marx C.E., Shampine L.J., Girdler S.S., Morrow A.L. Simultaneous quantification of GABAergic 3α,5α/3α,5β neuroactive steroids in human and rat serum. Steroids. 2009;74:463–473. doi: 10.1016/j.steroids.2008.12.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Porcu P., O'Buckley T.K., Alward S.E., Song S.C., Grant K.A., de Wit H., Morrow A.L. Differential effects of ethanol on serum GABAergic 3alpha,5alpha/3alpha,5beta neuroactive steroids in mice, rats, cynomolgus monkeys, and humans. Alcohol Clin. Exp. Res. 2010;34(3):432–442. doi: 10.1111/j.1530-0277.2009.01123.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Porcu P., Sogliano C., Cinus M., Purdy R.H., Biggio G., Concas A. Nicotine-induced changes in cerebrocortical neuroactive steroids and plasma corticosterone concentrations in the rat. Pharmacol. Biochem. Behav. 2003;74(3):683–690. doi: 10.1016/s0091-3057(02)01065-1. [DOI] [PubMed] [Google Scholar]
- Poulter M.O., Du L., Zhurov V., Merali Z., Anisman H. Plasticity of the GABA(A) receptor subunit cassette in response to stressors in reactive versus resilient mice. Neuroscience. 2010;165(4):1039–1051. doi: 10.1016/j.neuroscience.2009.11.028. [DOI] [PubMed] [Google Scholar]
- Pribiag H., Stellwagen D. TNF-alpha downregulates inhibitory neurotransmission through protein phosphatase 1-dependent trafficking of GABA(A) receptors. J. Neurosci. 2013;33(40):15879–15893. doi: 10.1523/JNEUROSCI.0530-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Puia G., Ducic I., Vicini S., Costa E. Does neurosteroid modulatory efficacy depend on GABAA receptor subunit composition? Recept. Channels. 1993;1:135–142. [PubMed] [Google Scholar]
- Puia G., Santi M., Vicini S., Pritchett D.B., Purdy R.H., Paul S.M., Seeburg P.H., Costa E. Neurosteroids act on recombinant human GABAA receptors. Neuron. 1990;4:759–765. doi: 10.1016/0896-6273(90)90202-q. [DOI] [PubMed] [Google Scholar]
- Puia G., Vicini S., Seeburg P.H., Costa E. Influence of recombinant GABAA receptor subunit composition on the action of allosteric modulators of GABA-gated Cl- currents. Mol. Pharmacol. 1991;39:691–696. [PubMed] [Google Scholar]
- Purdy R.H., Morrow A.L., Blinn J.R., Paul S.M. Synthesis, metabolism, and pharmacological activity of 3α-hydroxy steroids which potentiate GABA-receptor-mediated chloride ion uptake in rat cerebral cortical synaptoneurosomes. J. Med. Chem. 1990;33:1572–1581. doi: 10.1021/jm00168a008. [DOI] [PubMed] [Google Scholar]
- Purdy R.H., Morrow A.L., Moore P.H., Jr., Paul S.M. Stress-induced elevations of gamma-aminobutyric acid type A receptor-active steroids in the rat brain. Proc. Natl. Acad. Sci. U. S. A. 1991;88:4553–4557. doi: 10.1073/pnas.88.10.4553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qin L., He J., Hanes R.N., Pluzarev O., Hong J.S., Crews F.T. Increased systemic and brain cytokine production and neuroinflammation by endotoxin following ethanol treatment. J. Neuroinflammation. 2008;5:10. doi: 10.1186/1742-2094-5-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rajkowska G., O'Dwyer G., Teleki Z., Stockmeier C.A., Miguel-Hidalgo J.J. GABAergic neurons immunoreactive for calcium binding proteins are reduced in the prefrontal cortex in major depression. Neuropsychopharmacology. 2007;32(2):471–482. doi: 10.1038/sj.npp.1301234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rasmusson A.M., Marx C.E., Pineles S.L., Locci A., Scioli-Salter E.R., Nillni Y.I., Liang J.J., Pinna G. Neuroactive steroids and PTSD treatment. Neurosci. Lett. 2017;649:156–163. doi: 10.1016/j.neulet.2017.01.054. [DOI] [PubMed] [Google Scholar]
- Rasmusson A.M., Pinna G., Paliwal P., Weisman D., Gottschalk C., Charney D., Krystal J., Guidotti A. Decreased cerebrospinal fluid allopregnanolone levels in women with posttraumatic stress disorder. Biol. Psychiatry. 2006;60(7):704–713. doi: 10.1016/j.biopsych.2006.03.026. [DOI] [PubMed] [Google Scholar]
- Reddy D.S., Rogawski M.A. Stress-induced deoxycorticosterone-derived neurosteroids modulate GABAA receptor function and seizure susceptibility. J. Neurosci. 2002;22(9):3795–3805. doi: 10.1523/JNEUROSCI.22-09-03795.2002. 20026274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reddy D.S., Rogawski M.A. Neurosteroids - endogenous regulators of seizure susceptibility and role in the treatment of epilepsy. In: Noebels J.L., Avoli M., Rogawski M.A., Olsen R.W., Delgado-Escueta A.V., editors. Jasper's Basic Mechanisms of the Epilepsies. 2012. Bethesda (MD) [Google Scholar]
- Reino D.C., Pisarenko V., Palange D., Doucet D., Bonitz R.P., Lu Q., Colorado I., Sheth S.U., Chandler B., Kannan K.B., Ramanathan M., Xu D.Z., Deitch E.A., Feinman R. Trauma hemorrhagic shock-induced lung injury involves a gut-lymph-induced TLR4 pathway in mice. PLoS One. 2011;6(8) doi: 10.1371/journal.pone.0014829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reul J.M., Holsboer F. Corticotropin-releasing factor receptors 1 and 2 in anxiety and depression. Curr. Opin. Pharmacol. 2002;2(1):23–33. doi: 10.1016/s1471-4892(01)00117-5. [DOI] [PubMed] [Google Scholar]
- Rogawski M.A., Loya C.M., Reddy K., Zolkowska D., Lossin C. Neuroactive steroids for the treatment of status epilepticus. Epilepsia. 2013;54(Suppl. 6):93–98. doi: 10.1111/epi.12289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romeo E., Ströhle A., Spalletta G., di Michele F., Hermann B., Holsboer F., Pasini A., Rupprecht R. Effects of antidepressant treatment on neuroactive steroids in major depression. Am. J. Psychiatry. 1998;155:910–913. doi: 10.1176/ajp.155.7.910. [DOI] [PubMed] [Google Scholar]
- Sahuque L.L., Kullberg E.F., McGeehan A.J., Kinder J.R., Hicks M.P., Blanton M.G., Janak P.H., Olive M.F. Anxiogenic and aversive effects of corticotropin-releasing factor (CRF) in the bed nucleus of the stria terminalis in the rat: role of CRF receptor subtypes. Psychopharmacology (Berlin) 2006;186(1):122–132. doi: 10.1007/s00213-006-0362-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sajdyk T.J., Schober D.A., Gehlert D.R., Shekhar A. Role of corticotropin-releasing factor and urocortin within the basolateral amygdala of rats in anxiety and panic responses. Behav. Brain Res. 1999;100(1–2):207–215. doi: 10.1016/s0166-4328(98)00132-6. [DOI] [PubMed] [Google Scholar]
- Sanacora G., Gueorguieva R., Epperson C.N., Wu Y.T., Appel M., Rothman D.L., Krystal J.H., Mason G.F. Subtype-specific alterations of gamma-aminobutyric acid and glutamate in patients with major depression. Arch. Gen. Psychiatr. 2004;61(7):705–713. doi: 10.1001/archpsyc.61.7.705. [DOI] [PubMed] [Google Scholar]
- Sanacora G., Saricicek A. GABAergic contributions to the pathophysiology of depression and the mechanism of antidepressant action. CNS Neurol. Disord. - Drug Targets. 2007;6(2):127–140. doi: 10.2174/187152707780363294. [DOI] [PubMed] [Google Scholar]
- Sarkar J., Wakefield S., MacKenzie G., Moss S.J., Maguire J. Neurosteroidogenesis is required for the physiological response to stress: role of neurosteroid-sensitive GABAA receptors. J. Neurosci. 2011;31(50):18198–18210. doi: 10.1523/JNEUROSCI.2560-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sayeed I., Stein D.G. Progesterone as a neuroprotective factor in traumatic and ischemic brain injury. Prog. Brain Res. 2009;175:219–237. doi: 10.1016/S0079-6123(09)17515-5. [DOI] [PubMed] [Google Scholar]
- Schule C., Nothdurfter C., Rupprecht R. The role of allopregnanolone in depression and anxiety. Prog. Neurobiol. 2014;113:79–87. doi: 10.1016/j.pneurobio.2013.09.003. [DOI] [PubMed] [Google Scholar]
- Schumacher M., Guennoun R., Stein D.G., De Nicola A.F. Progesterone: therapeutic opportunities for neuroprotection and myelin repair. Pharmacol. Ther. 2007;116(1):77–106. doi: 10.1016/j.pharmthera.2007.06.001. [DOI] [PubMed] [Google Scholar]
- Sequeira A., Mamdani F., Ernst C., Vawter M.P., Bunney W.E., Lebel V., Rehal S., Klempan T., Gratton A., Benkelfat C., Rouleau G.A., Mechawar N., Turecki G. Global brain gene expression analysis links glutamatergic and GABAergic alterations to suicide and major depression. PLoS One. 2009;4(8) doi: 10.1371/journal.pone.0006585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Serra M., Mostallino M.C., Talani G., Pisu M.G., Carta M., Mura M.L., Floris I., Maciocco E., Sanna E., Biggio G. Social isolation-induced increase in α4 and δ subunit gene expression is associated with a greater efficacy of ethanol on steroidogenesis and GABAA receptor function. J. Neurochem. 2006;98:122–133. doi: 10.1111/j.1471-4159.2006.03850.x. [DOI] [PubMed] [Google Scholar]
- Serra M., Pisu M.G., Floris I., Biggio G. Social isolation-induced changes in the hypothalamic-pituitary-adrenal axis in the rat. Stressforskningsrapporter. 2005;8(4):259–264. doi: 10.1080/10253890500495244. [DOI] [PubMed] [Google Scholar]
- Serra M., Pisu M.G., Floris I., Cara V., Purdy R.H., Biggio G. Social isolation-induced increase in the sensitivity of rats to the steroidogenic effect of ethanol. J. Neurochem. 2003;85(1):257–263. doi: 10.1046/j.1471-4159.2003.01680.x. [DOI] [PubMed] [Google Scholar]
- Serra M., Pisu M.G., Littera M., Papi G., Sanna E., Tuveri F., Usala L., Purdy R.H., Biggio G. Social isolation-induced decreases in both the abundance of neuroactive steroids and GABAA receptor function in rat brain. J. Neurochem. 2000;75(2):732–740. doi: 10.1046/j.1471-4159.2000.0750732.x. [DOI] [PubMed] [Google Scholar]
- Serra M., Pisu M.G., Mostallino M.C., Sanna E., Biggio G. Changes in neuroactive steroid content during social isolation stress modulate GABAA receptor plasticity and function. Brain Res. Rev. 2008;57(2):520–530. doi: 10.1016/j.brainresrev.2007.06.029. [DOI] [PubMed] [Google Scholar]
- Smith S.S., Gong Q.H., Hsu F.-C., Markowitz R.S., Ffrench-Mullen J.M.H., Li X. GABAA receptor α4 subunit suppression prevents withdrawal properties of an endogenous steroid. Nature. 1998;392:926–930. doi: 10.1038/31948. [DOI] [PubMed] [Google Scholar]
- Spigelman I., Li Z., Liang J., Cagetti E., Samzadeh S., Mihalek R.M., Homanics G.E., Olsen R.W. Reduced inhibition and sensitivity to neurosteroids in hippocampus of mice lacking the GABA(A) receptor delta subunit. J. Neurophysiol. 2003;90(2):903–910. doi: 10.1152/jn.01022.2002. [DOI] [PubMed] [Google Scholar]
- Stein D.G., Sayeed I. Repurposing and repositioning neurosteroids in the treatment of traumatic brain injury: a report from the trenches. Neuropharmacology. 2019;147:66–73. doi: 10.1016/j.neuropharm.2018.04.006. [DOI] [PubMed] [Google Scholar]
- Stell B.M., Brickley S.G., Tang C.Y., Farrant M., Mody I. Neuroactive steroids reduce neuronal excitability by selectively enhancing tonic inhibition mediated by δ subunit-containing GABAA receptors. Proc. Natl. Acad. Sci. U. S. A. 2003;100(24):14439–14444. doi: 10.1073/pnas.2435457100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stellwagen D., Beattie E.C., Seo J.Y., Malenka R.C. Differential regulation of AMPA receptor and GABA receptor trafficking by tumor necrosis factor-alpha. J. Neurosci. 2005;25(12):3219–3228. doi: 10.1523/JNEUROSCI.4486-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stuck E.D., Christensen R.N., Huie J.R., Tovar C.A., Miller B.A., Nout Y.S., Bresnahan J.C., Beattie M.S., Ferguson A.R. Tumor necrosis factor alpha mediates GABA(A) receptor trafficking to the plasma membrane of spinal cord neurons in vivo. Neural Plast. 2012;2012:261345. doi: 10.1155/2012/261345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swiergiel A.H., Takahashi L.K., Kalin N.H. Attenuation of stress-induced behavior by antagonism of corticotropin-releasing factor receptors in the central amygdala in the rat. Brain Res. 1993;623(2):229–234. doi: 10.1016/0006-8993(93)91432-r. [DOI] [PubMed] [Google Scholar]
- Tsatsanis C., Androulidaki A., Alissafi T., Charalampopoulos I., Dermitzaki E., Roger T., Gravanis A., Margioris A.N. Corticotropin-releasing factor and the urocortins induce the expression of TLR4 in macrophages via activation of the transcription factors PU.1 and AP-1. J. Immunol. 2006;176(3):1869–1877. doi: 10.4049/jimmunol.176.3.1869. [DOI] [PubMed] [Google Scholar]
- Ulrich-Lai Y.M., Herman J.P. Neural regulation of endocrine and autonomic stress responses. Nat. Rev. Neurosci. 2009;10(6):397–409. doi: 10.1038/nrn2647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uzunov D.P., Cooper T.B., Costa E., Guidotti A. Fluoxetine-elicited changes in brain neurosteroid content measured by negative ion mass fragmentography. Proc. Natl. Acad. Sci. U. S. A. 1996;93:12599–12604. doi: 10.1073/pnas.93.22.12599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uzunova V., Sheline Y., Davis J.M., Rasmusson A., Uzunov D.P., Costa E., Guidotti A. Increase in the cerebrospinal fluid content of neurosteroids in patients with unipolar major depression who are receiving fluoxetine or fluvoxamine. Proc. Natl. Acad. Sci. U. S. A. 1998;95:3239–3244. doi: 10.1073/pnas.95.6.3239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaitkevicius H., Husain A.M., Rosenthal E.S., Rosand J., Bobb W., Reddy K., Rogawski M.A., Cole A.J. First-in-man allopregnanolone use in super-refractory status epilepticus. Ann. Clin. Transl. Neurol. 2017;4(6):411–414. doi: 10.1002/acn3.408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- VanDoren M.J., Matthews D.B., Janis G.C., Grobin A.C., Devaud L.L., Morrow A.L. Neuroactive steroid 3α-hydroxy-5α-pregnan-20-one modulates electrophysiological and behavioral actions of ethanol. J. Neurosci. 2000;20(5):1982–1989. doi: 10.1523/JNEUROSCI.20-05-01982.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verkuyl J.M., Hemby S.E., Joels M. Chronic stress attenuates GABAergic inhibition and alters gene expression of parvocellular neurons in rat hypothalamus. Eur. J. Neurosci. 2004;20(6):1665–1673. doi: 10.1111/j.1460-9568.2004.03568.x. [DOI] [PubMed] [Google Scholar]
- Vichaya E.G., Laumet G., Christian D.L., Grossberg A.J., Estrada D.J., Heijnen C.J., Kavelaars A., Dantzer R. Motivational changes that develop in a mouse model of inflammation-induced depression are independent of indoleamine 2,3 dioxygenase. Neuropsychopharmacology. 2019;44(2):364–371. doi: 10.1038/s41386-018-0075-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walter T.J., Vetreno R.P., Crews F.T. Alcohol and stress activation of microglia and neurons: brain regional effects. Alcohol Clin. Exp. Res. 2017 doi: 10.1111/acer.13511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang M., He Y., Eisenman L.N., Fields C., Zeng C.M., Mathews J., Benz A., Fu T., Zorumski E., Steinbach J.H., Covey D.F., Zorumski C.F., Mennerick S. 3β-hydroxypregnane steroids are pregnenolone sulfate-like GABAA receptor antagonists. J. Neurosci. 2002;22(9):3366–3375. doi: 10.1523/JNEUROSCI.22-09-03366.2002. 20026342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitman B.A., Knapp D.J., Werner D.F., Crews F.T., Breese G.R. The cytokine mRNA increase induced by withdrawal from chronic ethanol in the sterile environment of brain is mediated by CRF and HMGB1 release. Alcohol Clin. Exp. Res. 2013;37(12):2086–2097. doi: 10.1111/acer.12189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiebe J.P. Identification of a unique sertoli cell steroid as 3⍺-hydroxy-4-pregnen-20-one (3⍺-dihydroprogesterone: 3⍺-DHP) Steroids. 1982;39:259–278. doi: 10.1016/0039-128x(82)90146-5. [DOI] [PubMed] [Google Scholar]
- Wiebe J.P., Boushy D., Wolfe M. Synthesis, metabolism and levels of the neuroactive steroid, 3α-hydroxy-4-pregnen-20-one (3αHP), in rat pituitaries. Brain Res. 1997;764:158–166. doi: 10.1016/s0006-8993(97)00452-6. [DOI] [PubMed] [Google Scholar]
- Winsky-Sommerer R. Role of GABAA receptors in the physiology and pharmacology of sleep. Eur. J. Neurosci. 2009;29(9):1779–1794. doi: 10.1111/j.1460-9568.2009.06716.x. [DOI] [PubMed] [Google Scholar]
- Wright D.W., Kellermann A.L., Hertzberg V.S., Clark P.L., Frankel M., Goldstein F.C., Salomone J.P., Dent L.L., Harris O.A., Ander D.S., Lowery D.W., Patel M.M., Denson D.D., Gordon A.B., Wald M.M., Gupta S., Hoffman S.W., Stein D.G. ProTECT: a randomized clinical trial of progesterone for acute traumatic brain injury. Ann. Emerg. Med. 2007;49(4):391–402. doi: 10.1016/j.annemergmed.2006.07.932. [DOI] [PubMed] [Google Scholar]
- Zhang Y., Woodruff M., Zhang Y., Miao J., Hanley G., Stuart C., Zeng X., Prabhakar S., Moorman J., Zhao B., Yin D. Toll-like receptor 4 mediates chronic restraint stress-induced immune suppression. J. Neuroimmunol. 2008;194(1–2):115–122. doi: 10.1016/j.jneuroim.2007.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ziegler D.R., Herman J.P. Local integration of glutamate signaling in the hypothalamic paraventricular region: regulation of glucocorticoid stress responses. Endocrinology. 2000;141(12):4801–4804. doi: 10.1210/endo.141.12.7949. [DOI] [PubMed] [Google Scholar]
- Zolkowska D., Wu C.Y., Rogawski M.A. Intramuscular allopregnanolone and ganaxolone in a mouse model of treatment-resistant status epilepticus. Epilepsia. 2018;59(Suppl. 2):220–227. doi: 10.1111/epi.13999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zorrilla E.P., Logrip M.L., Koob G.F. Corticotropin releasing factor: a key role in the neurobiology of addiction. Front. Neuroendocrinol. 2014;35(2):234–244. doi: 10.1016/j.yfrne.2014.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]