Summary
Fumonisin B1 (FB1) and Alternaria alternate f. sp. lycopersici (AAL)‐toxin are classified as sphinganine analog mycotoxins (SAMTs), which induce programmed cell death (PCD) in plants and pose health threat to humans who consume the contaminated crop products. Herein, Fumonisin B1 Resistant41 ( FBR41), a dominant mutant allele, was identified by map‐based cloning of Arabidopsis FB1‐resistant mutant fbr41, then ectopically expressed in AAL‐toxin sensitive tomato (Solanum lycopersicum) cultivar. FBR41‐overexpressing tomato plants exhibited less severe cell death phenotype upon AAL‐toxin treatment. Analysis of free sphingoid bases showed that both fbr41 and FBR41‐overexpressing tomato plants accumulated less sphinganine and phytosphingosine upon FB1 and AAL‐toxin treatment, respectively. Alternaria stem canker is a disease caused by AAL and responsible for severe economic losses in tomato production, and FBR41‐overexpressing tomato plants exhibited enhanced resistance to AAL with decreased fungal biomass and less cell death, which was accompanied by attenuated accumulation of free sphingoid bases and jasmonate (JA). Taken together, our results indicate that FBR41 is potential in inhibiting SAMT‐induced PCD and controlling Alternaria stem canker in tomato.
Keywords: Alternaria alternata f. sp. lycopersici , AAL‐toxin, Fumonisin B1 Resistant41, programmed cell death, sphinganine analog mycotoxin, tomato
Introduction
Programmed cell death (PCD) is an active suicide process that leads to selective removal of unwanted or severely damaged cells, which occurs during development, senescence, as well as response to abiotic and biotic stress (Das et al., 2010). Necrotrophic pathogens that obtain nutrients from the dead cells have developed a strategy to successfully elicit PCD of their hosts by secreting mycotoxins (Walton, 1996). Fumonisin B1 (FB1) and Alternaria alternate f. sp. lycopersici (AAL)‐toxin are two well‐studied mycotoxins, produced by Fusarium species and Alternaria alternate, respectively (Abbas et al., 1994). They are classified as sphinganine analog mycotoxins (SAMTs) because of their structural similarity to sphinganine, the backbone precursor of sphingolipids.
The underlying mechanism of SAMT‐induced PCD is associated with the competitive inhibition of ceramide synthase, which suppresses the conversion of sphinganine, phytosphingosine and other free sphingoid bases to complex ceramides. The resultant accumulation of free sphingoid bases acts as a second message to activate transduction pathways of PCD elicitation (Lachaud et al., 2013; Saucedo‐Garcia et al., 2011; Shi et al., 2007). De novo biosynthesis of free sphingoid bases is initiated by serine palmitoyltransferase (SPT), which catalyses the condensation of serine and palmitoyl‐CoA to form intermediate 3‐ketosphingosine. The product of this reaction is then reduced to sphinganine, the simplest free sphingoid base (Chen et al., 2010; Lynch and Dunn, 2004). Further modification of sphinganine is conducted by the addition of a hydroxyl group at C4 to yield phytosphingosine and/or by introduction of double bonds at C4 and C8 to produce other free sphingoid bases (Chen et al., 2010; Lynch and Dunn, 2004). Different from a soluble homodimer in some bacterial, SPT is an endoplasmic reticulum (ER)‐localized heterodimer comprised of long chain base 1 (LCB1) and LCB2 subunits in all known eukaryotes (Chen et al., 2006; Dietrich et al., 2008; Ikushiro et al., 2001; Tamura et al., 2001). The catalytic lysine residue that binds with pyridoxal 5′ phosphate is located in LCB2 subunit and LCB1 is able to stabilize LCB2 (Hanada, 2003; Tamura et al., 2001). In addition, another smaller subunit, termed the small subunit of SPT (ssSPT), also interacts with the LCB1/LCB2 subunits. The ssSPTs usually enhance SPT activity by stabilizing SPT complex, which has been shown in Arabidopsis with ssSPT overexpression leading to increased SPT activity and ssSPT suppression resulting in reduced SPT activity (Kimberlin et al., 2013). There is one AtLCB1 (At4g36480) gene and two homologous LCB2 genes, AtLCB2a (At5g23680) and AtLCB2b (At3g48780) in Arabidopsis. The expression of AtLCB2a is usually higher than that of AtLCB2b (Dietrich et al., 2008). Partial suppression of AtLCB1 led to enhanced resistance to FB1, which was accompanied by decreased accumulation of free sphingoid bases (Shi et al., 2007). A null mutant of AtLCB2a also displayed FB1‐resistant phenotype and attenuated accumulation of free sphingoid bases (Saucedo‐Garcia et al., 2011). These results link the function of SPT as a PCD regulator to free sphingoid bases.
AAL is the causal agent of Alternaria stem canker in tomato in several parts of the world (Grogan et al.,1975; Kohmoto et al., 1982; Malathrakis, 1983), leading to serious economic losses in tomato production. The disease symptom occurs in the form of dark‐brown concentric cankers on stems and necrosis spots on leaves (Grogan et al., 1975). AAL pathogenicity depends on the production of AAL‐toxin, as toxin‐deficient AAL mutants neither colonize nor cause symptoms in susceptible tomato cultivars (Akamatsu et al., 1997). Resistance to AAL and AAL‐toxin has been reported to be controlled by the single co‐dominant Alternaria stem canker (Asc) locus (Brandwagt et al., 2000; Mesbah et al., 1999). The Asc‐1 gene isolated from resistant tomato genotype could salvage the transportation of glycosilphosphatidylinositol anchored proteins from ER to Golgi through production of alternative ceramides (Brandwagt et al., 2000). Quantitative analysis of free sphingoid bases demonstrated that the tomato with resistant Asc/Asc genotype accumulated about half the levels of sphinganine and phytosphingosine than the one with susceptible asc/asc genotype when treated with AAL‐toxin (Abbas et al., 1994), suggesting that Asc could prevent the build‐up of free sphingoid bases induced by AAL‐toxin, thus enhancing the resistance of tomato to AAL‐toxin.
Phytohormone signalling pathways have a critical role in regulation of plant defence against pathogen attack, among which jasmonate (JA)‐dependent pathway is usually effective against necrotrophic pathogens and salicylic acid (SA)‐dependent responses counteract biotrophic pathogens (Glazebrook, 2005). However, in tomato–AAL interaction system, JA promotes the susceptibility of tomato to AAL. Disease development and in planta growth of AAL were decreased in JA‐deficient mutants and increased in prosystemin‐overexpressing transgenic lines (35S::prosystemin) which constitutively accumulate high level of JA. Exogenous application of methyl jasmonate (MeJA) restored disease symptom of JA‐deficient mutants to wild‐type (WT) level and led to increased disease symptom in WT (Egusa et al., 2009; Jia et al., 2013). In contrast to JA signalling pathway, SA‐dependent pathway has been shown to enhance the resistance of tomato to AAL. Pretreatment of SA greatly decreased lesion occurrence after AAL infection, and transgenic plants defective in SA accumulation (NahG) exhibited more expanding lesions with higher amount of fungal than WT (Jia et al., 2013).
Although several molecular components and pathways involved in the resistance of plants to SAMT‐induced PCD and SAMT‐producing pathogens have been identified in model plants, their application in crop production is limited, and the related molecular mechanisms remain to be further elucidated. Herein, we have identified a dominant mutant allele, Fumonisin B1 Resistant41 (FBR41) by map‐based cloning of FB1‐resistant mutant in Arabidopsis. Ectopic overexpression of FBR41 conferred an increased insensitivity to AAL‐toxin and mediated resistance to Alternaria stem canker in tomato. The results provide a potential strategy for controlling SAMT contamination and diseases caused by SAMT‐producing pathogens in crops.
Results
Identification, phenotypic and genetic analysis of fbr41 mutant
Approximately 15 000 ethyl methanesulfonate (EMS)‐mutagenized Arabidopsis M2 seeds were germinated on half‐strength MS medium containing 1 μm FB1, and a FB1‐resistant mutant, designated as fumonisin b1 resistant 41 (fbr41), was isolated (Figure 1a). Then, the mutant was self‐fertilized, and the resulting progeny (M3) were all resistant to FB1.
Figure 1.
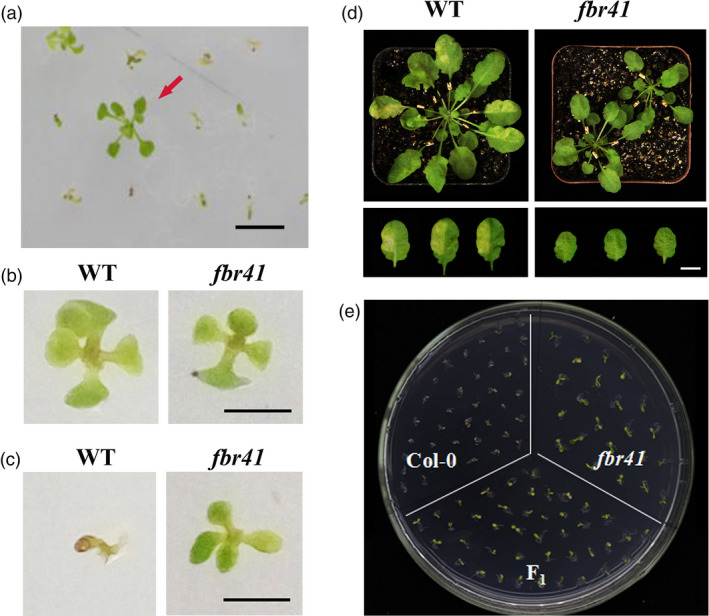
Isolation, phenotypic and genotypic characterization of fumonisin B1 (FB1)‐resistant mutant fbr41. (a) Isolation of FB1‐resistant mutant fbr41. An ethyl methanesulfonate (EMS)‐generated mutant population seeds were sown on half‐strength MS medium containing 1.5 μm FB1, and FB1‐resistant individual (indicated by red arrow) was isolated for further identification. (b,c) Phenotypes of wild‐type (Col‐0) and fbr41 seedlings grown on half‐strength MS medium containing 0.15% methanol (v/v) (b) or 1.5 μm FB1 (c), photographs were taken 14 days after sowing. (d) Typical phenotypes of FB1‐induced programmed cell death. Six‐week‐old WT and fbr41 were infiltrated with 10 μm FB1 (left half of treated leaves) and 1% (v/v) methanol as control (right half of treated leaves), then photographed at 3 days postinjection (DPI). (e) FB1 resistance of F1 progeny derived from crosses between fbr41 and Col‐0 plants. Scale bars = 1 cm (a–d).
To further analyse the sensitivity of fbr41 to FB1, the mutant and WT (Col‐0) were grown on half‐strength MS medium containing 1.5 μm FB1 or infiltrated with 10 μm FB1 solution on leaves. FB1 seriously inhibited the growth of WT but hardly affected the growth of fbr41 (Figure 1c). When rosette leaves of six‐week‐old plants were infiltrated with 10 μm FB1 solution, the FB1‐treated leaves of WT exhibited hypersensitive response‐like lesions at 3 days postinfiltration. In contrast, no obvious lesions were observed on the leaves of fbr41 plant (Figure 1d). Of note, fbr41 displayed dwarf phenotype in the absence of FB1 (Figure 1b,d), while had no defects in reproductive growth.
When fbr41 was backcrossed with parent Col‐0, F1 progeny were resistant to FB1 (Figure 1e), and F2 population derived from self‐fertilized F1 plants segregated in a 3:1 ratio (FB1‐resistant: sensitive = 535:182, χ2 = 0.637, P = 0.73). These results suggest that the FB1‐resistant phenotype of fbr41 is controlled by a single dominant Mendelian locus.
Map‐based cloning of FBR41
A map‐based cloning strategy was used to isolate the FBR41 locus in a population generated from a cross between fbr41 and Arabidopsis ecotype Landsberg erecta.
A total of 23 pairs of simple sequence length polymorphism (SSLP) markers (Table S2) were selected for first‐pass mapping of FBR41, and FBR41 was first mapped to the bottom arm of chromosome 3 in the interval between molecular markers T24C20 and T18N14 (Figure 2a). Subsequent fine mapping of the gene was performed in a total of 2500 FB1‐resistant individuals with cleaved amplified polymorphic sequences (CAPS) markers (Table S3), and FBR41 was delimited to an interval flanked by molecular markers 18005CAPS and 18175CAPS.
Figure 2.
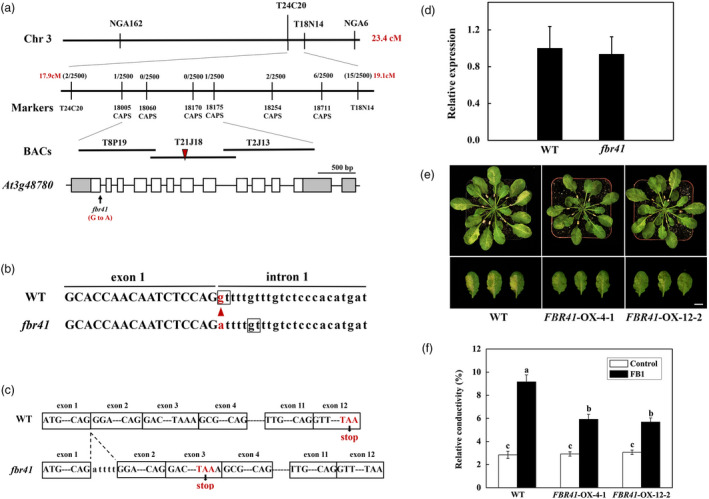
Map‐based cloning of FBR41. (a) Map‐based cloning of FBR41 locus. An arrowhead indicates a G‐to‐A substitution in At3g48780 locus in fbr41. Grey boxes and white boxes indicate UTR and exons, respectively, and line segments represent introns. BACs, bacterial artificial chromosomes. (b) Genomic DNA sequence of At3g48780 in wild type (WT) and fbr41. The triangle shows the mutation in the splicing junction of the exon 1 and the intron 1 in At3g48780. The nucleotides in boxes indicate the 5′ splice donor sites. (c) A five base‐pair insertion (ATTTT) between exon 1 and exon 2, and a premature stop codon at exon 3 in the At3g48780 transcripts in fbr41. (d) Quantitative RT‐PCR analysis of the At3g48780 transcripts in WT and fbr41. (e) Phenotypes of FBR41‐overexpressing plants upon FB1 treatment. Photographs were taken at 3 days postinjection (DPI). Scale bar = 1 cm. (f) Quantitative measurements of relative conductivity in treated leaves at 3 DPI. Six representative leaves from three plants were collected and pooled as one sample. Data shown are means ± SD of three biological replicates. Different letters denote a statistically significant difference from control‐infiltrated WT (one‐way ANOVA, P < 0.05, Tukey's test).
Within the identified region, the causative mutation was predicted according to the annotation and function displayed in Cereon tair database (http://arabidopsis.org/). Among these genes, we identified a gene encoding AtLCB2b subunit of SPT (At3g48780), which catalyses the first step of sphingolipid biosynthesis and is involved in SAMT‐induced PCD based on previous studies (Saucedo‐Garcia et al., 2011; Shi et al., 2007; Spassieva et al., 2002). Therefore, we examined genomic DNA sequence of At3g48780 in fbr41. A nucleotide substitution from G to A was identified at the splicing junction of the first exon and the first intron in At3g48780 (Figure 2b), which was predicted to cause splicing error of At3g48780 pre‐mRNA. To check the transcription of At3g48780 in fbr41 mutant, we amplified and sequenced the full‐length At3g48780 complementary DNA (cDNA) in fbr41. A five base‐pair (ATTTT) insertion between the first and second exon was observed in the At3g48780 transcript in fbr41 (Figure 2b), which was predicted to introduce a premature stop codon and produce a putative protein with the first 42 amino acids of the annotated 489 amino acids of WT protein plus 52 additional amino acids before a premature stop codon (Figure S2). Furthermore, we conducted quantitative RT‐PCR to examine if the expression level of At3g48780 was altered in fbr41. As shown in Figure 2d, there was no difference between WT and fbr41 in the abundance of At3g48780 transcripts (Figure 2d).
FB1‐resistant phenotype is controlled by a dominant locus, to confirm that the G‐to‐A transition in At3g48780 is responsible for the dominant phenotype, transgenic plants expressing the mutant At3g48780 allele under CaMV 35S promoter (35S::FBR41) was generated in the WT background, and their phenotypes were observed. As expected, transgenic lines presented similar FB1‐resistant phenotypes with fbr41, with fewer lesions than WT after FB1 infiltration (Figure 2e). Accordingly, the extent of tissue damage as indicated by relative conductivity in transgenic plants leaves upon FB1 infiltration was significantly lower than that of WT (Figure 2f). Therefore, it is convincible that the FB1‐resistant phenotype of fbr41 is the result of a single nucleotide mutation in At3g48780.
FBR41 attenuates the increase in free sphingoid bases upon FB1 treatment
As FBR41 corresponds to AtLCB2b, which encodes an essential subunit of SPT, we supposed that the FB1‐resistant phenotype of fbr41 and FBR41‐overexpressing lines is potentially related to altered accumulation of free sphingoid bases, the putative downstream products of SPT (Figure 3a). To confirm the assumption, we compared the accumulation of specific free sphingoid bases in fbr41, FBR41‐overexpressing lines and WT with or without FB1 treatment. Total free sphingoid bases were selectively extracted from leaves of 6‐week‐old plants and quantitatively analysed by LC‐MS/MS. Individual free sphingoid base was identified by the retention of corresponding standard. Free sphingoid bases analysed included sphinganine and phytosphingosine, which were abundant and most affected by FB1 according to previous studies (Saucedo‐Garcia et al., 2011; Shi et al., 2007). The results showed that the levels of free sphingoid bases in fbr41 or transgenic lines under control condition were low and showed no significant difference compared to WT (Figure 3b), suggesting that the mutation did not affect basal levels of free sphingoid bases. Dramatic increase in free sphingoid bases was observed at 6 h after FB1 exposure, in agreement with the previous reports (Saucedo‐Garcia et al., 2011; Shi et al., 2007). However, the contents of sphinganine and phytosphingosine in fbr41 were 30% and 74% of those in WT, respectively (Figure 3b). FB1‐treated FBR41‐overexpressing lines also produced low levels of sphinganine and phytosphingosine, similar to those exhibited by fbr41 (Figure 3b). The findings demonstrate that increased resistance to FB1 in fbr41 as well as FBR41‐overexpressing lines is associated with attenuated accumulation of free sphingoid bases.
Figure 3.
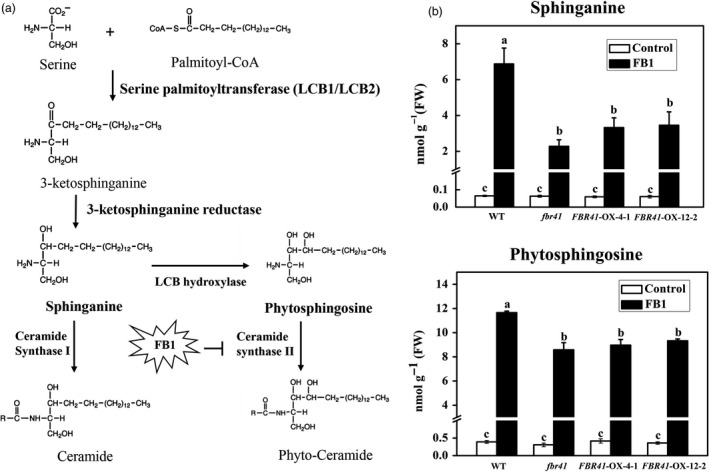
Biosynthetic pathway of free sphingoid bases and their contents in wild‐type (WT), fbr41 and FBR41‐overexpressing plants. (a) Biosynthesis of free sphingoid bases. De novo synthesis of free sphingoid bases is initiated through condensation of serine and palmitoyl‐CoA, which is catalysed by serine palmitoyltransferase (SPT). The product of SPT is immediately reduced to the simplest free sphingoid base sphinganine, which is further modified by the addition of a hydroxyl group at C4 to yield phytosphingosine. Both sphinganine and phytosphingosine can be converted into ceramide and phyto‐ceramide, respectively, by ceramide synthase. Fumonisin B1 (FB1) is the potential competitive inhibitor of ceramide synthase II, leading to accumulation of phytosphinsoine as well as sphinganine. (b) Contents of free sphingoid bases in WT, fbr41 mutant and FBR41‐overexpressing transgenic lines. Six‐week‐old plants were injected with 10 μm FB1 or 1% methanol (v/v, control), and samples were harvested at 6 h postinjection. Data shown are means ± SD of three biological replicates. Different letters denote a statistically significant difference among treatment (one‐way ANOVA, P < 0.05, Tukey's test). FW, fresh weight.
FBR41 impacts the in vivo interaction between AtLCB1 and AtLCB2a
In Arabidopsis, AtLCB1 interacts with AtLCB2 to form a functional SPT (Chen et al., 2006; Dietrich et al., 2008; Shi et al., 2007). FBR41 is a mutant allele of AtLCB2b, encoding an abnormal protein with 94 amino acids (Figure S2). To test whether FBR41 interacts with AtLCB1, luciferase complementation imaging (LCI) assay was conducted, in which FBR41 and AtLCB1 were fused to the C‐terminal fragment (FBR41‐Cluc) and N‐terminal fragment (AtLCB1‐Nluc) of the firefly luciferase (LUC) enzyme. Co‐expression of FBR41‐Cluc with AtLCB1‐Nluc resulted in strong luminescence signal in the leaves of N. benthamiana, whereas co‐expression of AtLCB1‐Nluc/Cluc, FBR41‐Cluc/Nluc and an unfused Nluc/Cluc pair, as three negative controls, showed only background levels of luminescence (Figure 4a,b). Western blot confirmed the similar expression levels of Nluc‐ and Cluc‐fusion proteins in different treatments (Figure 4c), suggesting that the strong luminescence output detected from co‐expressed AtLCB1‐Nluc/FBR41‐Cluc was not resulted from high levels of AtLCB1‐Nluc and FBR41‐Cluc proteins, but rather dominated by the specific interaction between AtLCB1 and FBR41. The results demonstrate that FBR41 could interact with AtLCB1 in vivo.
Figure 4.
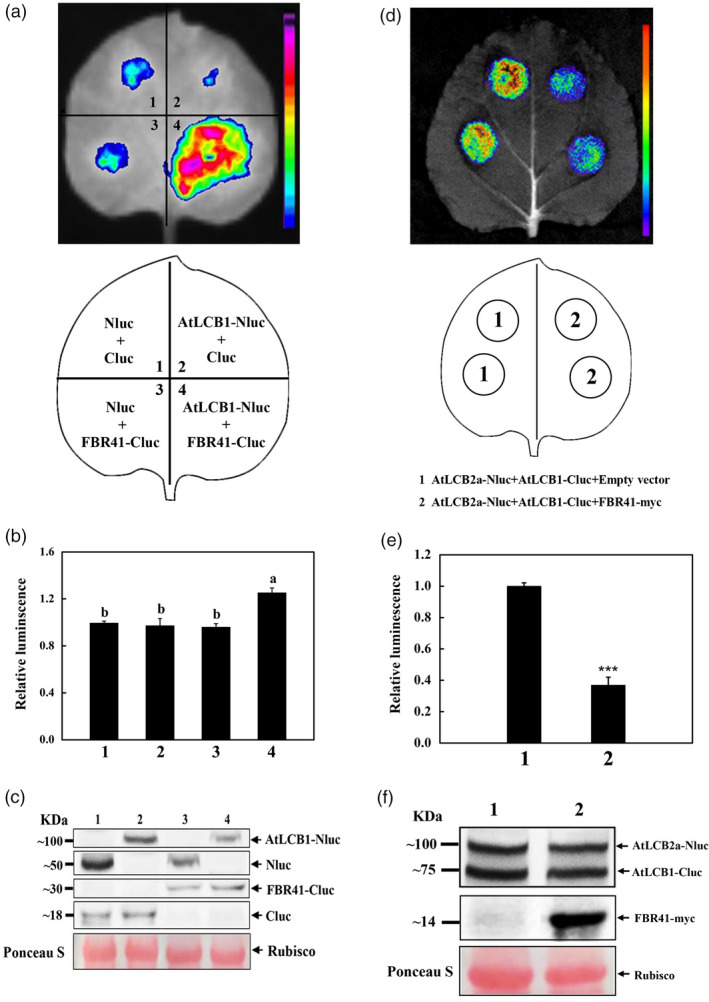
FBR41 disrupts the in vivo interaction between AtLCB1 and AtLCB2a (a) Luciferase complementation imaging (LCI) assays showing the interaction between FBR41 and AtLCB1. Agrobacterium harbouring AtLCB1‐Nluc and FBR41‐Cluc constructs were co‐infiltrated into N. benthamiana leaves. Luminescence was monitored at 3 days postinfiltration. The Nluc/Cluc, AtLCB1/Cluc and Nluc/FBR41‐Cluc pairs were used as negative control. (b) Quantitative analysis of luminescence intensity in different treatments as indicated in (a). Data are means ± SD of three biological replicates. Different letters denote a statistically significant difference from each other (one‐way ANOVA, P < 0.05, Tukey's test). (c) Western blot showing the expression levels of Nluc‐ or Cluc‐fusion proteins in agroinfiltrated leaf samples shown in (a). Ponceau S staining shows the equal loading of total proteins. (d) LCI assays showing that FBR41 disrupts the interaction between AtLCB1 and AtLCB2a. Agrobacterium carrying AtLCB1‐Nluc/AtLCB2a‐Cluc plus FBR41‐myc were co‐infiltrated into N. benthamiana leaves. Luminescence was monitored at 3 days postinfiltration. The AtLCB1‐Nluc/AtLCB2a‐Cluc/empty vector group was used as control. (e) Quantitative measurement of luminescence intensity in different treatments as indicated in (d). Data are means ± SD of three biological replicates. Asterisks denote statistically significant differences compared with control (Student's t‐test, ***P < 0.001). (f) Western blot showing the expression levels of AtLCB2a‐Nluc, AtLCB1‐Cluc and FBR41‐myc in agroinfiltrated leaf samples shown in (d). Ponceau S staining shows the equal loading of total proteins.
The in vivo interaction between FBR41 and AtLCB1 promoted us to speculate that FBR41 might impact the interaction between AtLCB1 and AtLCB2. To verify the assumption, we transiently expressed FBR41 with AtLCB1‐Cluc/AtLCB2a‐Nluc pair in N. benthamiana leaves and examined whether the introduction of FBR41 could impact the in vivo interaction between AtLCB1 and AtLCB2a. As shown in Figure 4d, a strong luminescence signal was obtained when co‐expressing AtLCB2a‐Nluc/AtLCB1‐Cluc plus empty vector (control), indicating the in vivo interaction between AtLCB1 and AtLCB2a. However, co‐expression of AtLCB2a‐Nluc/AtLCB1‐Cluc plus FBR41 produced substantially reduced luminescence output, compared with that of control (Figure 4d,e). Western blot showed that the expression levels of the AtLCB2a‐Nluc and AtLCB1‐Cluc were similar between AtLCB2a‐Nluc/AtLCB1‐Cluc/empty vector and AtLCB2a‐Nluc/AtLCB1‐Cluc/FBR41‐myc groups (Figure 4f), suggesting that FBR41 did not impact the expression of AtLCB1 and AtLCB2a, but rather disrupted the in vivo interaction between AtLCB1 and AtLCB2a.
Generation of FBR41‐overexpressing tomato plants
Based on the results that FBR41 mediates resistance to FB1 in Arabidopsis, we further explored the role of FBR41 in SAMT resistance in phylogenetically distant tomato plants. A construct containing FBR41 driven by cauliflower mosaic virus (CaMV) 35S promoter was introduced into AAL‐toxin sensitive tomato cultivar. Nine independent transgenic lines were obtained (Figure 5a), and five lines were confirmed to carry single transgenic insertion based on segregation analysis on T1 seeds (kanamycin resistance: kanamycin sensitive = 3:1). Homozygous FBR41 lines were selected from self‐pollinated FBR41‐overexpressing plants of T1 generation and verified by semi‐quantitative RT‐PCR (Figure 5b).
Figure 5.
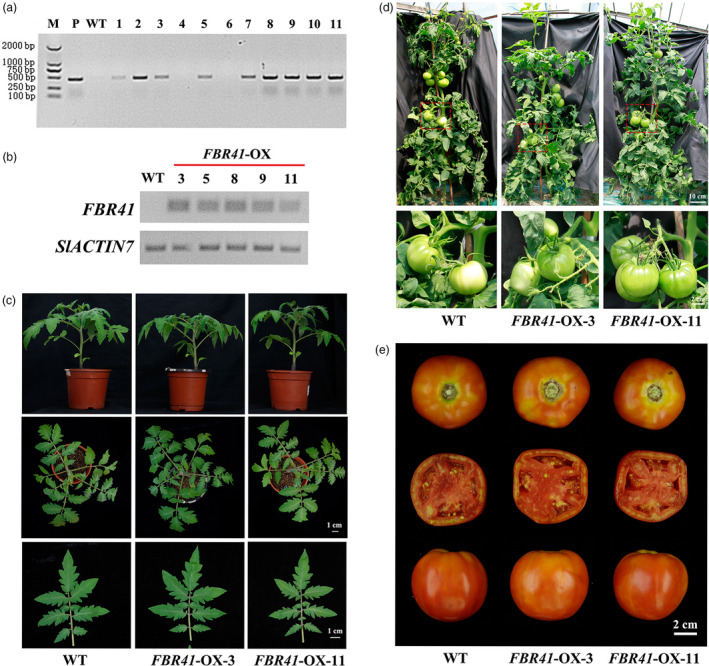
Genotyping and phenotyping analysis of FBR41‐overexpressing tomato plants. (a) PCR analysis of putative T0 transgenic plants using primers of FBR41‐F and MYC ‐R. A 0.44‐kb DNA fragment was amplified to identify positive transgenic plants. M: DL 2000 marker; P: positive control; WT: wild‐type; Lane 1–11: putative T0 transgenic lines. (b) Semi‐quantitative RT‐PCR analysis of homozygous transgenic plants containing single transgene insertions. (c–e) Effect of FBR41 overexpression on plant height, morphology, leaf size (c), mature green fruits (d) and ripen fruits (e). In (c), the top and middle row shows 5‐week‐old tomato plants grown in phytotron and the bottom row shows 4th leaf of tomato plants. In (d), the top panel shows 4‐month‐old tomato plants grown in field and the dotted line represents the mature green (MG) tomato fruits. The bottom panel shows the magnified pictures of MG tomato fruits.
We randomly chose two homozygous FBR41‐overexpressing lines for analysis of phenotypes. Plant height, morphology and leaf size of FBR41‐overexpressing plants were indistinguishable from WT (Figure 5c). Furthermore, no significant differences between transgenic plants and WT were observed for flowering time, fruit development and yield (Table 1; Figure 5d,e). These results show that FBR41 have no negative pleiotropic effects on plant growth and development.
Table 1.
Effect of FBR41 on tomato flowering, fruit ripening and yield
| Parameter | Wild type | FBR41‐OX‐3 | FBR41‐OX‐11 |
|---|---|---|---|
| Flowering time (days) | 59.7 ± 1.6 a | 60.5 ± 1.6 a | 59.2 ± 1.2 a |
| Days from anthesis to fruit ripen | 73.4 ± 1.8 a | 74.6 ± 2.1 a | 75.8 ± 1.7 a |
| Ripen fruits per plant | 10.0 ± 1.1 a | 10.3 ± 1.8 a | 9.5 ± 1.3 a |
| Fruit weight (g) | 133.33 ± 11 a | 129.29 ± 9.1 a | 131.43 ± 11.38 a |
| Fruit yield per plant (g) | 1277.7 ± 42.9 a | 1290 ± 45.4 a | 1238 ± 41.1 a |
Data shown are means ± SD for 16 plants grown in glasshouse. Means denoted by the same letter did not differ significantly according to Tukey's test (P < 0.05).
Ectopic expression of FBR41 enhances resistance to AAL‐toxin‐induced cell death in tomato
To investigate the effect of FBR41 on resistance to AAL‐toxin‐induced cell death in tomato, detached leaflets of FBR41‐overexpressing plants and WT were treated with 0.2 μm AAL‐toxin, and PCD symptom was observed continuously. Visible black necrotic lesions, the typical AAL‐toxin‐induced symptom as previously reported (Mesbah et al., 2000; Zhang et al., 2011), were observed on the leaflets of sensitive tomato cultivars at 48 h after toxin treatment (Figure 6a). However, toxin‐treated leaflets of FBR41‐overexpressing plants displayed minor necrotic lesions and lower relative conductivity compared to WT (Figure 6a,b). As different transgenic lines exhibited similar disease symptom (Figure 6a), we chose FBR41‐OX‐3 as a representative transgenic line for further analysis.
Figure 6.
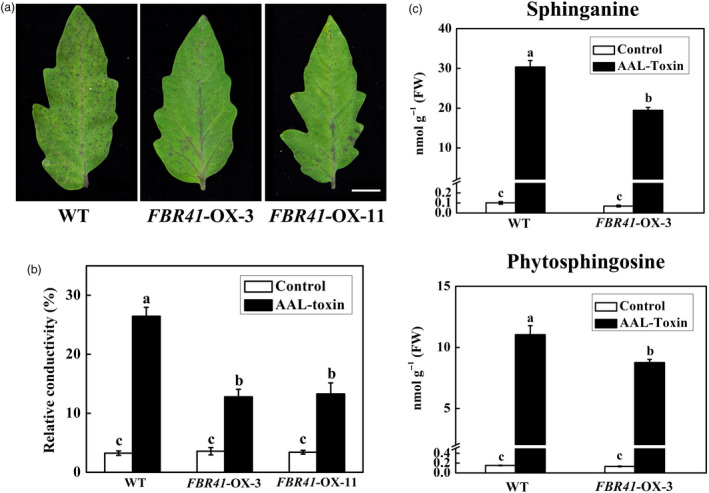
Effect of FBR41 overexpression on resistance to AAL‐toxin and free sphingoid bases contents in tomato leaflets. (a) Symptom of detached leaflets of wild‐type (WT) and transgenic plants after AAL‐toxin treatment. Detached leaflets of 5‐week‐old plants were incubated with 0.2 μm AAL‐toxin under continuous light at 25 °C for 48 h and photographed. Scale bar = 1 cm. (b,c) Relative conductivity (b) and free sphingoid bases content (c) in leaflets of WT and transgenic plants after 48 h of AAL‐toxin or control treatment. Data shown are means ± SD of three biological replicates. Different letters denote a statistically significant difference among treatments (one‐way ANOVA, P < 0.05, Tukey's test). FW, fresh weight.
Considering FBR41 alters the accumulation of free sphingoid bases upon FB1 treatment in Arabidopsis, we also analysed these compounds in leaflets of FBR41‐overexpressing tomato plants. The levels of sphinganine and phytosphingosine were low with no obvious difference between FBR41‐overexpressing plants and WT under control (Figure 6c). Marked increase in sphinganine and phytosphingosine was attained after AAL‐toxin treatment in both FBR41‐overexpressing plants and WT at 48 h of toxin treatment (Figure 6c). However, the levels of sphingosine and phytosphingosine in FBR41‐overexpressing tomato leaflets were 64% and 79% of those in WT, respectively (Figure 6c). The results provide a link between enhanced resistance to AAL‐toxin‐induced PCD and attenuated accumulation of free sphingoid bases in FBR41‐overexpressing tomato plants.
Ectopic expression of FBR41 enhances resistance to Alternaria stem canker in tomato
AAL‐toxin is a host‐specific pathogenicity factor of necrotrophic pathogen AAL, which causes Alternaria stem canker and leaf necrosis on susceptible tomato cultivars. FBR41‐overexpressing tomato plants were more resistant to AAL‐toxin; we then further tested the resistance of them to Alternaria stem canker. Typical necrotic lesions began to develop on leaves at approximately 3 days postinoculation (DPI) of AAL. As the disease progressed, the necrotic lesions enlarged and the infected leaves wilted and eventually died. Fewer lesions were observed in FBR41‐overexpressing tomato plants at 3 DPI, compared with WT plants (Figure 7a; Figure S5). To quantify the extent of tissue damage and fungal biomass of AAL‐infected leaves, electrolyte leakage and amount of fungal DNA were measured, respectively. Significantly lower relative conductivity (Figure 7b) and less fungal DNA (Figure 7d) were found in leaves of FBR41‐overexpressing plants at 3 DPI when compared with the corresponding WT, which is consistence with the phenotypic assay. Along with the infection, we also found that leaves gradually lost chlorophyll and became yellow, which was consistent with previous studies (Meena et al., 2016). However, delayed degradation of total chlorophyll was observed in FBR41‐overexpressing plants upon AAL infection, compared to WT plants (Figure 7c). In sum, FBR41 enhances resistance to Alternaria stem canker caused by AAL in tomato.
Figure 7.
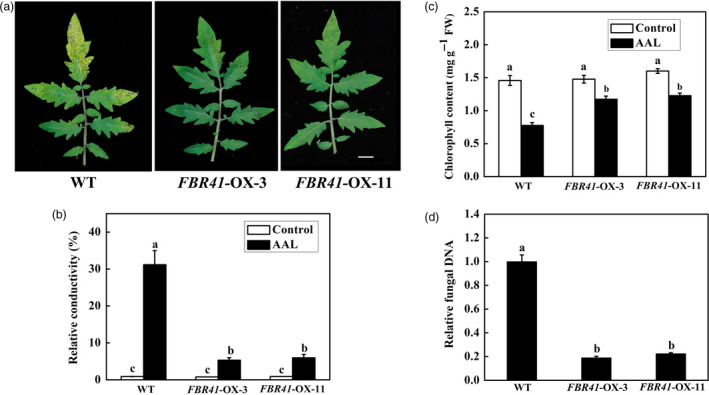
FBR41‐overexpressing tomato plants show resistance to Alternaria stem canker. (a) The symptom of wild‐type (WT) and FBR41‐overexpressing tomato plants after AAL inoculation. Five‐week‐old plants were inoculated with AAL, and representative 4th leaves were photographed at 3 days postinoculation (DPI). Scale bar = 1 cm. (b–d) Relative conductivity (b), total chlorophyll content (c) and relative fungal DNA amount (d) in leaves of WT and transgenic plants at 3 DPI. In (d), the amount of fungal DNA was quantified by quantitative RT‐PCR using ALT1‐specific primers. Data shown are means ± SD of three biological replicates. Different letters denote a statistically significant difference among treatment (one‐way ANOVA, P < 0.05, Tukey's test). FW, fresh weight.
Further analysis of the free sphingoid bases showed that both sphinganine and phytosphingosine were significantly induced by AAL inoculation in FBR41‐overexpressing tomato plants and WT (Figure 8a). There was no significant difference in free sphingoid bases between FBR41‐overexpressing plants and WT without AAL inoculation (Figure 8a). However, the levels of sphinganine and phytosphingosine in FBR41‐overexpressing tomato leaves at 3 DPI were 59% and 68% of those in WT, respectively (Figure 8a), which might account for the less foliar symptom in FBR41‐overexpressing plants.
Figure 8.
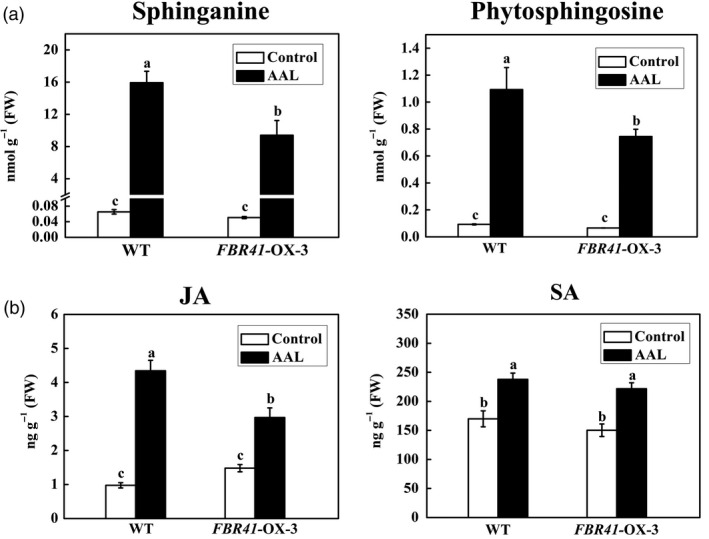
Contents of free sphingoid bases and phytohormones in wild‐type (WT) and FBR41‐overexpressing tomato plants under Alternaria alternata f. sp. lycopersici (AAL) infection. (a,b) The contents of free sphingoid bases (a) and jasmonate (JA) and salicylic acid (SA) (b) in WT and FBR41‐overexpressing tomato plants with AAL treatment or control (0.1% Tween 20) at 3 days postinoculation (DPI). Data shown are means ± SD of three biological replicates. Different letters denote a statistically significant difference among treatments (one‐way ANOVA, P < 0.05, Tukey's test).
Phytohormones JA and SA are commonly known to modulate the susceptibility of tomato to AAL (Egusa et al., 2009; Jia et al., 2013); therefore, we also determined the levels of both phytohormones in FBR41‐overexpressing tomato plants and WT. As shown in Figure 8b, there was no significant difference in endogenous SA content between FBR41‐overexpressing plants and WT with or without AAL treatment. Although the level of JA in FBR41‐overexpressing plants was similar to that in WT under control treatment, FBR41‐overexpressing plants accumulated less JA than WT after AAL inoculation. Considering AAL utilizes JA signalling pathway for successful invasion into the host as previously reported (Egusa et al., 2009; Jia et al., 2013), the inhibition of JA accumulation might account for less fungal biomass in FBR41‐overexpressing plants.
Discussion
Based on the fact that micromolar levels of FB1 can efficiently inhibit growth of Arabidopsis seedlings, screening of FB1‐resistant mutants has been used to identify genes involved in FB1‐induced PCD. In this study, we have characterized FBR41 through map‐based cloning of FB1‐resistant mutant in Arabidopsis, which carries a nucleotide substitution in the gene encoding AtLCB2b subunit of SPT. Different from previously reported mutant alleles by forward genetic approaches, all of which are recessive (Table S1), FBR41 is the first dominant FB1‐resistant mutant allele identified so far.
As the first step enzyme in sphingolipid biosynthesis, SPT plays a critical role in regulation of SAMT‐induced PCD. Chemical treatment with myriocin, a potent SPT inhibitor, partially relieved the susceptibility of Arabidopsis to FB1, as well as tomato to AAL‐toxin (Brandwagt et al., 2002; Shi et al., 2007). SPT functions as a heterodimer comprised of LCB1 and LCB2 subunits in Arabidopsis, mutations in either subunit may affect its enzymatic activity, thus relieving SAMT‐induced PCD. Both fbr11‐1 (a weak mutant allele in AtLCB1) and Atlcb2a‐1 (a T‐DNA insertion mutant) failed to induce PCD when challenged by FB1 (Saucedo‐Garcia et al., 2011; Shi et al., 2007). In addition, a smaller subunit of SPT termed ssSPT, which interacts with LCB1/LCB2 subunits, has been demonstrated to strongly influence the activity of SPT, and the sensitivity to FB1 was increased by ssSPT overexpression and reduced by ssSPT‐RNA interference in Arabidopsis (Kimberlin et al., 2013).
The involvement of free sphingoid bases, the downstream products of SPT, in FB1‐induced PCD has been widely reported, and the diminished SPT activity led to attenuated accumulation of these molecules (Saucedo‐Garcia et al., 2011; Shi et al., 2007). Among the free sphingoid bases species, the most representative ones are sphinganine and phytosphingosine, which showed gradual and significant increase starting at early time of FB1 exposure (Saucedo‐Garcia et al., 2011; Shi et al., 2007). In the case of fbr11‐1 and Atlcb2a‐1, although the absence of AtLCB1 or AtLCB2a subunit did not affect the basal levels of free sphingoid bases, it resulted in less increase in free sphingoid bases upon FB1 treatment than the WT (Saucedo‐Garcia et al., 2011; Shi et al., 2007). In present study, we found that FBR41 had a mutation in AtLCB2b encoding an essential subunit of SPT. Similar to fbr11‐1 and Atlcb2a‐1, fbr41 produced lower levels of sphinganine and phytosphingosine after FB1 treatment compared with the WT (Figure 3b). One possibility is that SPT activity is reduced due to the loss of function in AtLCB2b. However, this possibility could be ruled out because Arabidopsis has two functionally redundant genes (AtLCB2a and AtLCB2b) that encode functional isoforms of LCB2 subunits, the expression level of AtLCB2a is higher than AtLCB2b (Dietrich et al., 2008; Saucedo‐Garcia et al., 2011), suggesting that AtLCB2a plays a dominant role in maintaining SPT activity. Moreover, the null mutant of AtLCB2b‐1 did not display resistance to FB1 (Figure S1). Another possibility might be that FBR41 affects the combination of functional subunits, possibly through competing with functional AtLCB2 subunit for binding AtLCB1, yielding a dysfunctional AtLCB1/FBR41 heterodimer. The following clues are in support of this assumption. Firstly, FBR41 is a dominant mutant allele as the F1 progeny from fbr41 and WT was resistant to FB1 (Figure 1e). Additionally, the conserved pyridoxal phosphate‐binding motif and lys residue in AtLCB2, which are essential for SPT function, are absent in FBR41 (Figure S2). Furthermore, luciferase complementation assays show that FBR41 interacts with AtLCB1 in vivo (Figure 4a–c), and FBR41 competes with AtLCB2a for binding to AtLCB1 (Figure 4d–f). Finally, we measured the SPT activity of microsomes prepared from leaves of WT and fbr41 mutant. The data showed that in planta SPT activity in fbr41 was approximately 70% of that in WT, either in untreated (Figure S3a) or in FB1‐treated (Figure S3b) condition. Based on above evidence, it is concluded that FBR41 might produce a dysfunctional SPT by competing with functional AtLCB2a subunit in AtLCB1 binding, thus attenuating the accumulation of free sphingoid bases and enhancing the resistance to FB1 (Figure S4).
In addition to regulation of SAMT‐induced PCD, SPT is also involved in plant growth and development (Chen et al., 2006; Dietrich et al., 2008; Teng et al., 2008). Suppression of AtLCB1 and silencing of AtLCB2b in an Atlcb2a mutant background resulted in growth retardation, suggesting the crucial role of SPT in plant vegetative growth (Chen et al., 2006; Dietrich et al., 2008). In current survey, reduced plant size was observed in fbr41 and FBR41‐overexpressing Arabidopsis compared to WT (Figures 1d and 2d). Such findings are explicable, because SPT is suppressed in fbr41 (Figure S3a), theoretically accompanied by decreased level of free sphingoid bases. To maintain the free sphingoid base pool, plants may restrict their growth. This possibility is supported by the fact that basal levels of free sphingoid bases in fbr41 or FBR41‐overexpressing Arabidopsis plants were similar to that in WT (Figure 3b). However, no obvious growth retardation was observed in FBR41‐overexpressing tomato plants (Figure 5c), demonstrating a divergent effect of FBR41 on plant vegetative growth in different species. It is probable that plant growth is marginally altered in FBR41‐overexpressing tomato plants, which is difficult to distinguish from the WT plants. Also, we cannot rule out the possibility that FBR41‐overexpressing tomato plants might adopt other strategy, instead of restricting vegetative growth, to replenish the reduced availability of free sphingoid bases.
The potential application of FBR41 in crop improvement for enhanced resistance to SAMT‐induced cell death was verified by ectopic overexpression of FBR41 in phylogenetically distant tomato plants. FBR41 positively regulates the resistance to AAL‐toxin in tomato (Figure 6a) without pleiotropic effects on tomato growth and development (Table 1; Figure 5c–e). AAL‐toxin‐induced PCD is associated with the accumulation of free sphingoid bases, and prevention of this process can efficiently alleviate cell death. Chemical treatment with myriocin inhibited the activity of SPT, partially relieving the elevation of free sphingoid bases, therefore enhancing the resistance of tomato to AAL‐toxin (Spassieva et al., 2002). The Asc‐1 partially restored the block on sphingolipid synthesis, thereby preventing the AAL‐toxin‐induced cell death (Brandwagt et al., 2000, 2002; Spassieva et al., 2002). In FBR41‐overexpressing tomato plants, we also observed a suppressed elevation in free sphingoid bases upon AAL‐toxin treatment, which might partially account for enhanced resistance to AAL‐toxin‐induced PCD (Figure 6b).
Tomato stem canker is a devastating disease worldwide and causes severe economic losses for tomato producers. The necrotrophic pathogen AAL is the causal agent of the disease (Grogan et al.,1975; Kohmoto et al., 1982; Malathrakis, 1983). In AAL‐tomato interaction systems, AAL has evolved some pathogenic strategies to plunder the host. Previous reports have revealed the participation of JA signalling pathway in susceptibility of tomato to AAL (Egusa et al., 2009; Jia et al., 2013). In present study, FBR41‐overexpressing tomato plants exhibited reduced accumulation of JA in response to AAL (Figure 8b), which might prevent the invasion of AAL into the host, thus leading to less fungal biomass and reduced disease symptom (Figure 7a–c). After successful invasion, AAL secretes a host‐specific pathogenicity factor AAL‐toxin, which causes marked accumulation of free sphingoid bases and induces cell death of the host (Abbas et al., 1994; Wang et al., 1996; Zhang et al., 2011). However, elevation of free sphingoid bases in FBR41‐overexpressing tomato plants after AAL infection was attenuated compared to that in WT plants (Figure 8a). This might explain the less extent of cell death in FBR41‐overexpressing plants. Taken together, FBR41 might play a double role in improvement of tomato for enhanced resistance to Alternaria stem canker: inhibition of JA biosynthesis to prevent pathogen invasion and attenuation of free sphingoid base accumulation to suppress cell death.
During the whole lifespan, plants are exposed to broad range of pathogens, including necrotrophic and biotrophic pathogens according to their lifestyles. Pseudomonas syringae pv. tomato DC3000 (Pst DC3000) is a common type of (hemi) biotrophic pathogen, which causes bacterial speck disease in tomato. Interestingly, FBR41‐overexpressing tomato plants displayed increased susceptibility to Pst DC3000 (Figure S6), indicating that FBR41 functions differently in resistance against necrotrophic and biotrophic pathogens, but the underlying mechanism remains to be addressed in further studies.
SAMT contamination in certain agricultural commodities has been a growing concern for animal and human health, and SAMT‐producing pathogens are a serious threat to crop production (Wang et al., 2006). As a dominant gene, FBR41 is easier to be manipulated to inhibit pathogen invasion and reduce SAMT‐induced cell death, therefore specifically reducing mycotoxin contamination in agricultural products and controlling the diseases caused by SAMT‐producing pathogens.
Experimental procedures
Plant materials and growth conditions
Arabidopsis thaliana plants in Columbia (Col‐0) ecotype were used in this study. fbr41 was identified from an EMS‐generated mutant population; the details were described in Methods S1. Previously characterized T‐DNA insertional mutants Atlcb2a‐1 (SALK_061472) and Atlcb2b‐1 (SALK_110242) (Dietrich et al., 2008; Teng et al., 2008) were kindly provided by Dr Jianru Zuo (Chinese Academy of Sciences, Beijing, China).
AAL‐sensitive tomato (Solanum lycopersicum) cultivar was obtained from Tomato Genetics Resource Center (University of California, Davis, CA), which was used for FBR41 transformation.
Arabidopsis seeds were surface sterilized and imbibed in water at 4 °C for 3 days, then sown on half‐strength MS medium containing 1% (w/v) sucrose and 0.7% (w/v) agar and cultivated at 22 °C under a 10‐h light/14‐h dark photoperiod. After 2 weeks, seedlings were transferred to pots containing a mixture of peat: vermiculite: perlite (6:3:1, v/v/v) and maintained in the same condition for 4 weeks.
Tomato seeds were germinated on wet filter paper and transferred to the aperture disc filled with a mixture of peat: vermiculite (2:1, v/v). The seedlings were grown under a 16‐h light/8‐h dark photoperiod with the temperature of 26 °C in phytotron. After 3 weeks, seedlings were transplanted into plastic pot filled with the same substrate formula, which were watered every two days and fertilized at regular intervals. The plants were allowed to grow either in phytotron for characterization of plant height, morphology and leaf size and resistance to AAL‐toxin and AAL or in the glasshouse for evaluation of flowering time, fruit development and yield.
Nicotiana benthamiana were grown in the same conditions as tomato seedlings, and plants at 5–6 leaf stages were used for transient transformation.
Mapping and cloning of FBR41
SSLP markers evenly distributed on chromosomes were selected to determine the approximate location of the mutation. For fine mapping, a total of 2500 FB1‐resistant individuals from F2 generations of a cross between fbr41 and Landsberg erecta were used for PCR‐based mapping with CAPS markers. The candidate gene within mapping interval was amplified using genomic DNA of fbr41 and WT as template and sequenced. Primers used in this study were listed in Tables S2–S4.
Plasmid construction and plant transformation
To generate the 35S::FBR41‐myc construct, the coding sequence (CDS) of FBR41 was amplified from fbr41 cDNA (Primers listed in Table S4) and cloned into pQBV3 entry vector and combined with Gateway binary vector pGWB17 (35S promotor, c‐4myc) (Nakagawa et al., 2007). The resulting vectors were transformed into Agrobacterium tumefaciens strain GV3101 and LBA4404 for Arabidopsis and tomato transformation, respectively.
Arabidopsis transformation was performed by a floral dip procedure (Clough and Bent, 1998). Transformants were selected on half‐strength MS agar medium containing Kanamycin. Homozygous T3 transgenic lines were used for phenotype characterization.
The details of Agrobacterium‐mediated tomato transformation were described in Methods S2. To identify positive T0 transgenic plants, PCR analysis was performed using genomic DNA as template and gene‐specific forward primer FBR41‐F and vector‐specific reverse primer MYC‐R (Table S4). The number of T‐DNA inserts was assumed by the separation rate at about 3:1 in T1 generation on selective medium (half‐strength MS agar medium containing 50 mg/L kanamycin). Five independent transgenic lines with single copy were selected, and their seeds (T2 generation) were sown on selective medium to identify homozygous lines.
RNA isolation and gene expression analysis
Total RNA was extracted from plant leaves using TRIzol reagent (Invitrogen, Carlsbad, CA) according to the manufacturer's protocols. Genomic DNA was removed with RNA‐free DNase I (Takara Bio, Otsu, Shiga, Japan). RNA (1 μg) was reverse‐transcribed into cDNA with PrimeScript™ RT Reagent Kit (Takara) following the manufacturer's instructions. Quantitative RT‐PCR was performed as previously described (Miao et al., 2013). Semi‐quantitative RT‐PCR was performed using FBR41‐specific primers and SlACTIN7 as an internal control. PCR condition was 94 °C for 2 min followed by 30 cycles of 98 °C for 10 s, 50 °C for 30 s and 68 °C for 20 s. PCR fragments were analysed on 1.5% agarose gel and visualized with a UV transilluminator. The primers used for gene expression analysis are listed in Table S4.
Extraction and quantification of free sphingoid bases, JA and SA
Measurement of free sphingoid bases was performed as previously described (Bielawski et al., 2009) with minor modifications. Briefly, approximately 500 mg of rosette leaves from 6‐week‐old plants was homogenized and fortified with the internal standards (C17 base D‐erythro‐sphingosine, Avanti Polar Lipids, Alabaster, AL), which was extracted with ethyl acetate/isopropanol/H2O (6:3:1, v/v/v) solvent. After evaporation, the samples were dissolved in 0.5 mL methanol and analysed by Agilent 6460 Triple Quadrupole LC/MS/MS system (Agilent, Palo Alto, CA) using an Agilent ZORBAX SB‐C18 (150 × 2.1 mm, 3.5 μm particle size column, with 2 mm ammonium acetate containing 0.1% formic acid/methanol mobile phase system). Peaks for target analytes and internal standards were collected and processed with the Agilent Masshunter Quantitative Analysis software. Quantitative analyses of free sphingoid bases were based on the analyte specific calibration curves, generated by plotting the analyte/internal standard peak area ratio against analyte concentrations. SPT is a key enzyme of biosynthetic pathway of free sphingoid bases, and measurement of its activity was described in Methods S4.
Extraction and quantification of JA and SA was performed as previously reported (Pan et al., 2008) with minor modifications. In brief, approximately 0.3 g leaves were ground into powder in liquid nitrogen, and 1.5 mL of isopropanol/H2O/concentrated HCL (2:1:0.005, v/v/v) solvent with 10 ng internal standard (D5‐JA, C/D/N Isotopes, Pointe‐Claire, PQ, Canada; D4‐SA, Toronto Research Chemicals, Toronto, ON, Canada) was added, followed by agitation for 30 min at 4 °C. 1.5 mL of CH2Cl2 was added, followed by agitation for 30 min at room temperature and then centrifugation at 12 000 g for 5 min. After centrifugation, the supernviaould‐like liquid was collected, evaporated and dissolved in 200 μL of methanol/H2O (3:2, v/v) solvent. The samples were analysed by Agilent 6460 Triple Quadrupole LC/MS/MS system. Peaks for JA/SA molecule and internal standard were collected and processed with Agilent Masshunter Quantitative Analysis software. The amount of JA and SA was determined by comparison of response to the corresponding internal standard.
Luciferase complementation imaging (LCI) assays
The LCI assay was performed as previously described (Chen et al., 2008) with minor modifications. To assay the interaction between FBR41 and AtLCB1, FBR41 CDS and AtLCB1 CDS were amplified by PCR with primers containing appropriate restriction site and inserted into pCAMBIA‐nLUC (35S promotor, c‐Nluc) and pCAMBIA‐cLUC (35S promotor, n‐Cluc), respectively. To assay the impact of FBR41 on the interaction of AtLCB1 and AtLCB2a, AtLCB1 CDS and AtLCB2a CDS were amplified by PCR and cloned into pCAMBIA‐cLUC and pCAMBIA‐nLUC, respectively. Meanwhile, the 35S::FBR41‐myc construct was used for transient transformation and its corresponding empty vector was used as control. The primers used for vector construction were listed in Table S4. Agrobacterium strain GV3101 harbouring the indicated constructs were grown in Luria–Bertani (LB) medium at 28 °C overnight and then transferred to new LB medium supplemented with 10 mm MES (pH 5.6) and 40 μm acetosyringone (1:100, v/v) for 16 h. After centrifugation, the cell pellets were resuspended with agroinfiltration buffer (10 mm MgCl2, 0.2 mm acetosyringone) to an OD600 of 1.5. Equal volumes of Agrobacterium suspension were mixed and infiltrated into N. benthamiana leaves. After infiltration, plants were incubated at 26 °C for 72 h with 16‐h light/8‐h dark before the luciferase activity measurement. Low‐light cooled CCD imaging apparatus NightOWL II LB983 (Belthold, Bad Wildbad, Germany) with Indigo software was used to capture the luciferase image. The leaves were sprayed with 0.5 mm D‐luciferin (Promega, Madison, WI) and placed in darkness for 5 min before luminescence detection. Relative luminescence was used for the comparison of luciferase activity.
Western blot
Protein extraction was carried out as previously described (An et al., 2017). The agroinfiltrated parts of N. benthamiana leaves were harvested, and the leaf tissue was ground in liquid nitrogen resuspended in protein extraction buffer containing 50 mm Tris–HCl (pH 7.5), 150 mm NaCl, 0.1% Triton X‐100, 0.2% Nonidet P‐40, 0.6 mm PMSF, 20 mm MG132, 5 μm DTT and protease inhibitor mixture (Roche, Rotkreuz, Switzerland), and placed on ice for 30 min. After centrifuge (4 °C, 12 000 g , 20 min), the supernatants were collected and total protein concentration was quantified using Bradford method (Beyotime, Haimen, China) following the manufacturer's protocols.
For Western blot analysis, samples were denatured using SDS loading buffer, separated by 10% SDS‐PAGE and then transferred onto a polyvinylidene fluoride membrane (Millipore, Billenca, MA). For detection of nLUC‐ or cLUC‐fusion protein, the membrane was incubated with the rabbit anti‐firefly luciferase antibody (Abcam, Cambridge, UK), which reacts with both N‐terminal fragment and C‐terminal fragment of LUC, followed by a horseradish peroxidase (HRP)‐conjugated goat anti‐rabbit IgG secondary antibody (Abmart, Arlington, MA). For detection of FBR41‐myc protein, a mouse anti‐c‐MYC antibody (Abmart) was used as a primary antibody and a HRP‐conjugated goat anti‐mouse IG was used as the secondary antibody. After antibody incubation, the membrane was visualized using an enhanced chemiluminescence (ELC) substrate kit (Fdbio science, Hangzhou, China) according to the manufacturer's instructions.
Abiotic and biotic stress treatment
For FB1 treatment, Arabidopsis seedlings were grown on half‐strength MS medium containing 1.5 μL FB1 (Sigma‐Aldrich, St. Louis, MO) or 0.15% methanol as control. Arabidopsis leaves were infiltrated with 10 μL FB1 solution as previously described (Zhao et al., 2015). AAL‐toxin treatment of detached tomato leaflets was performed as previously described (Zhang et al., 2011). AAL toxin was a gift from Dr Liangcheng Du (Department of Chemistry, University of Nebraska, Lincoln, NE, USA).
AAL infection was performed according to Jia et al. (2013) with minor modifications. In brief, leaves of 5‐week‐old tomato plants were sprayed with AAL spore suspensions containing approximately 1.0 × 106 spores/mL or 0.1% (v/v) Tween 20 as control, then maintained at high humidity at 26 °C and a 16‐h light/8‐h dark photoperiod. Disease symptoms emerged about 3–4 days after infection. Pseudomonas syringae pv tomato DC3000 infection assays were described in Methods S3.
Measurement of cell death, fungal biomass and chlorophyll content
Cell death was measured with an electrolyte leakage assay according to Gechev et al. (2004). Measurement of fungal biomass was performed as previously described (Jia et al., 2013). The primer pairs DeH‐F and E8T7 (Table S4) designed for the amplification of AAL‐toxin biosynthesis gene (ALT1) were used for detecting AAL. Estimation of chlorophylls was carried out according to the procedure of Arnon (1949).
Statistical analysis
Statistical analysis was performed using the SPSS package program version 19.0.0 (SPSS Inc., Chicago, IL). Data were analysed by one‐way ANOVA followed by Turkey's test at a 95% confidence level (P < 0.05) or Student's t‐test at 99.9% confidence level (P < 0.001). The values were reported as means with standard deviation (SD) for all the results.
Conflict of interest
The authors declare that they have no conflict of interests.
Author contributions
QW, JW, YZ and ZS planned and designed the research. ZS, YZ, SC, FM, HL and SH performed the experiments and analysed data. CL provided EMS‐generated mutants and guided map‐based cloning. ZS, QW, LL, YZ and JW wrote the manuscript. ZS and YZ contributed equally.
Supporting information
Figure S1 Lesion formation upon fumonisin B1 (FB1) injection in fbr41 and null mutants for AtLCB2a and AtLCB2b.
Figure S2 Comparison of amino acid sequences of FBR41, AtLCB2b and AtLCB2a.
Figure S3 In planta SPT activity in wild‐type (WT) and fbr41 mutant.
Figure S4 Proposed model considering the role of FBR41 in the resistance to sphinganine analog mycotoxin (SAMT)‐induced cell death.
Figure S5 The disease symptoms of wild‐type (WT) and FBR41‐overexpressing transgenic plants after AAL inoculation.
Figure S6 FBR41‐overexpressing tomato exhibited decreased resistance against Pseudomonas syringae pv. tomato DC 3000 (Pst DC3000).
Table S1 Fumonisin B1‐resistant mutants identified by forward genetic screening in Arabidopsis.
Table S2 List of SSLP markers used for first‐pass mapping.
Table S3 List of CAPS markers used for fine mapping.
Table S4 Primers used in this study.
Methods S1 Screening of the fumonisin B1‐resistant mutant.
Methods S2 Tomato transformation.
Methods S3 Pseudomonas syringae pv tomato DC3000 infection assays.
Methods S4 Microsome preparation and SPT assay.
Acknowledgements
We are grateful to Tomato Genetics Resource Center (University of California, Davis, CA, USA) for providing AAL‐sensitive tomato cultivar, Dr Jianru Zuo (Chinese Academy of Sciences, Beijing, China) for providing T‐DNA insertional mutants Atlcb2a‐1 (SALK_061472) and Atlcb2b‐1 (SALK_110242), Dr Liangcheng Du (University of Nebraska, Lincoln, NE, USA) for providing AAL‐toxin, and Dr Steffen Abel (Leibniz‐Institute of Plant Biochemistry, Halle, Germany) for critical reading. This work was supported by the Ministry of Agriculture of China (2016ZX08009003‐001), National Natural Science Foundation of China (31200230, 31601746) and Zhejiang Provincial Natural Science Foundation of China (LZ15C150001).
Contributor Information
Jiansheng Wang, Email: wangjs@mail.zaas.ac.cn.
Qiaomei Wang, Email: qmwang@zju.edu.cn.
References
- Abbas, H.K. , Tanaka, T. , Duke, S.O. , Porter, J.K. , Wray, E.M. , Hodges, L. , Sessions, A.E. et al. (1994) Fumonisin‐ and AAL‐Toxin‐induced disruption of sphingolipid metabolism with accumulation of free sphingoid bases. Plant Physiol. 106, 1085–1093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akamatsu, H. , Itoh, Y. , Kodama, M. , Otani, H. and Kohmoto, K. (1997) AAL‐Toxin‐deficient mutants of Alternaria alternata tomato pathotype by restriction enzyme‐mediated integration. Phytopathology, 87, 967–972. [DOI] [PubMed] [Google Scholar]
- An, C. , Li, L. , Zhai, Q. , You, Y. , Deng, L. , Wu, F. , Chen, R. et al. (2017) Mediator subunit MED25 links the jasmonate receptor to transcriptionally active chromatin. Proc. Natl Acad. Sci. USA, 114, E8930–E8939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arnon, D.I. (1949) Copper enzymes in isolated chloroplasts. Polyphenoloxidase in beta vulgaris. Plant Physiol. 24, 1–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bielawski, J. , Pierce, J.S. , Snider, J. , Rembiesa, B. , Szulc, Z.M. and Bielawska, A. (2009) Comprehensive quantitative analysis of bioactive sphingolipids by high‐performance liquid chromatography‐tandem mass spectrometry. Methods Mol. Biol. 579, 443–467. [DOI] [PubMed] [Google Scholar]
- Brandwagt, B.F. , Mesbah, L.A. , Takken, F.L. , Laurent, P.L. , Kneppers, T.J. , Hille, J. and Nijkamp, H.J. (2000) A longevity assurance gene homolog of tomato mediates resistance to Alternaria alternata f. sp. lycopersici toxins and fumonisin B1. Proc. Natl Acad. Sci. USA, 97, 4961–4966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brandwagt, B.F. , Kneppers, T.J. , Nijkamp, H.J. and Hille, J. (2002) Overexpression of the tomato Asc‐1 gene mediates high insensitivity to AAL toxins and fumonisin B1 in tomato hairy roots and confers resistance to Alternaria alternata f. sp. lycopersici in Nicotiana umbratica plants. Mol. Plant Microbe Interact. 15, 35–42. [DOI] [PubMed] [Google Scholar]
- Chen, M. , Han, G. , Dietrich, C.R. , Dunn, T.M. and Cahoon, E.B. (2006) The essential nature of sphingolipids in plants as revealed by the functional identification and characterization of the Arabidopsis LCB1 subunit of serine palmitoyltransferase. Plant Cell, 18, 3576–3593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen, H. , Zou, Y. , Shang, Y. , Lin, H. , Wang, Y. , Cai, R. , Tang, X. et al. (2008) Firefly luciferase complementation imaging assay for protein‐protein interactions in plants. Plant Physiol. 146, 368–376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen, M. , Cahoon, E.B. , Saucedo‐García, M. , Plasencia, J. and Gavilanes‐Ruíz, M. (2010) Plant sphingolipids: structure, synthesis and function. In Lipids in Photosynthesis: Essential and Regulatory Functions ( Wada, H. and Murata, N. , eds), pp. 77–115. Dordrecht: Springer Netherlands. [Google Scholar]
- Clough, S.J. and Bent, A.F. (1998) Floral dip: a simplified method for Agrobacterium‐mediated transformation of Arabidopsis thaliana . Plant J. 16, 735–743. [DOI] [PubMed] [Google Scholar]
- Das, A. , Kawai‐Yamada, M. and Uchimiya, H. (2010) Programmed cell death in plants. In Abiotic Stress Adaptation in Plants: Physiological, Molecular and Genomic Foundation ( Pareek, A. , Sopory, S.K. and Bohnert, H.J. , eds), pp. 371–383. Dordrecht: Springer Netherlands. [Google Scholar]
- Dietrich, C.R. , Han, G. , Chen, M. , Berg, R.H. , Dunn, T.M. and Cahoon, E.B. (2008) Loss‐of‐function mutations and inducible RNAi suppression of Arabidopsis LCB2 genes reveal the critical role of sphingolipids in gametophytic and sporophytic cell viability. Plant J. 54, 284–298. [DOI] [PubMed] [Google Scholar]
- Egusa, M. , Ozawa, R. , Takabayashi, J. , Otani, H. and Kodama, M. (2009) The jasmonate signaling pathway in tomato regulates susceptibility to a toxin‐dependent necrotrophic pathogen. Planta, 229, 965–976. [DOI] [PubMed] [Google Scholar]
- Gechev, T.S. , Gadjev, I.Z. and Hille, J. (2004) An extensive microarray analysis of AAL‐toxin‐induced cell death in Arabidopsis thaliana brings new insights into the complexity of programmed cell death in plants. Cell. Mol. Life Sci. 61, 1185–1197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glazebrook, J. (2005) Contrasting mechanisms of defense against biotrophic and necrotrophic pathogens. Annu. Rev. Phytopathol. 43, 205–227. [DOI] [PubMed] [Google Scholar]
- Grogan, R.G. , Kimble, K.A. and Misaghi, I. (1975) A stem canker disease of tomato caused by Alternaria alternata f. sp. lycopersici . Phytopathology, 65, 880–886. [Google Scholar]
- Hanada, K. (2003) Serine palmitoyltransferase, a key enzyme of sphingolipid metabolism. Biochim. Biophys. Acta, 1632, 16–30. [DOI] [PubMed] [Google Scholar]
- Ikushiro, H. , Hayashi, H. and Kagamiyama, H. (2001) A water‐soluble homodimeric serine palmitoyltransferase from Sphingomonas paucimobilis EY2395T strain. Purification, characterization, cloning, and overproduction. J. Biol. Chem. 276, 18249–18256. [DOI] [PubMed] [Google Scholar]
- Jia, C. , Zhang, L. , Liu, L. , Wang, J. , Li, C. and Wang, Q. (2013) Multiple phytohormone signalling pathways modulate susceptibility of tomato plants to Alternaria alternata f. sp. lycopersici . J. Exp. Bot. 64, 637–650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kimberlin, A.N. , Majumder, S. , Han, G. , Chen, M. , Cahoon, R.E. , Stone, J.M. , Dunn, T.M. et al. (2013) Arabidopsis 56‐amino acid serine palmitoyltransferase‐interacting proteins stimulate sphingolipid synthesis, are essential, and affect mycotoxin sensitivity. Plant Cell, 25, 4627–4639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kohmoto, K. , Verma, V.S. , Nishimura, S. , Tagami, M. and Scheffer, R.P. (1982) New outbreak of Alternaria stem canker of tomato in Japan and production of host‐selective toxins by the causal fungus. Anim. Behav. 72, 1405–1416. [Google Scholar]
- Lachaud, C. , Prigent, E. , Thuleau, P. , Grat, S. , Da Silva, D. , Briere, C. , Mazars, C. et al. (2013) 14‐3‐3‐regulated Ca(2+)‐dependent protein kinase CPK3 is required for sphingolipid‐induced cell death in Arabidopsis . Cell Death Differ. 20, 209–217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lynch, D.V. and Dunn, T.M. (2004) An introduction to plant sphingolipids and a review of recent advances in understanding their metabolism and function. New Phytol. 161, 677–702. [DOI] [PubMed] [Google Scholar]
- Malathrakis, N.E. (1983) Alternaria stem canker of tomato in Greece. Phytopathol. Mediterr. 22, 33–38. [Google Scholar]
- Meena, M. , Zehra, A. , Dubey, M.K. , Aamir, M. , Gupta, V.K. and Upadhyay, R.S. (2016) Comparative evaluation of biochemical changes in tomato (Lycopersicon esculentum Mill.) infected by Alternaria alternata and its toxic metabolites (tea, AOH, and AME). Front. Plant Sci. 7, 1408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mesbah, L.A. , Kneppers, T.J. , Takken, F.L. , Laurent, P. , Hille, J. and Nijkamp, H.J. (1999) Genetic and physical analysis of a YAC contig spanning the fungal disease resistance locus Asc of tomato (Lycopersicon esculentum). Mol. Gen. Genet. 261, 50–57. [DOI] [PubMed] [Google Scholar]
- Mesbah, L.A. , van der Weerden, G.M. , Nijkamp, H.J.J. and Hille, J. (2000) Sensitivity among species of Solanaceae to AAL toxins produced by Alternaria alternata f.sp lycopersici . Plant. Pathol. 49, 734–741. [Google Scholar]
- Miao, H. , Wei, J. , Zhao, Y. , Yan, H. , Sun, B. , Huang, J. and Wang, Q. (2013) Glucose signalling positively regulates aliphatic glucosinolate biosynthesis. J. Exp. Bot. 64, 1097–1109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakagawa, T. , Kurose, T. , Hino, T. , Tanaka, K. , Kawamukai, M. , Niwa, Y. , Toyooka, K. et al. (2007) Development of series of gateway binary vectors, pGWBs, for realizing efficient construction of fusion genes for plant transformation. J. Biosci. Bioeng. 104, 34–41. [DOI] [PubMed] [Google Scholar]
- Pan, X. , Welti, R. and Wang, X. (2008) Simultaneous quantification of major phytohormones and related compounds in crude plant extracts by liquid chromatography‐electrospray tandem mass spectrometry. Phytochemistry, 69, 1773–1781. [DOI] [PubMed] [Google Scholar]
- Saucedo‐Garcia, M. , Guevara‐Garcia, A. , Gonzalez‐Solis, A. , Cruz‐Garcia, F. , Vazquez‐Santana, S. , Markham, J.E. , Lozano‐Rosas, M.G. et al. (2011) MPK6, sphinganine and the LCB2a gene from serine palmitoyltransferase are required in the signaling pathway that mediates cell death induced by long chain bases in Arabidopsis . New Phytol. 191, 943–957. [DOI] [PubMed] [Google Scholar]
- Shi, L. , Bielawski, J. , Mu, J. , Dong, H. , Teng, C. , Zhang, J. , Yang, X. et al. (2007) Involvement of sphingoid bases in mediating reactive oxygen intermediate production and programmed cell death in Arabidopsis . Cell Res. 17, 1030–1040. [DOI] [PubMed] [Google Scholar]
- Spassieva, S.D. , Markham, J.E. and Hille, J. (2002) The plant disease resistance gene Asc‐1 prevents disruption of sphingolipid metabolism during AAL‐toxin‐induced programmed cell death. Plant J. 32, 561–572. [DOI] [PubMed] [Google Scholar]
- Tamura, K. , Mitsuhashi, N. , Hara‐Nishimura, I. and Imai, H. (2001) Characterization of an Arabidopsis cDNA encoding a subunit of serine palmitoyltransferase, the initial enzyme in sphingolipid biosynthesis. Plant Cell Physiol. 42, 1274–1281. [DOI] [PubMed] [Google Scholar]
- Teng, C. , Dong, H. , Shi, L. , Deng, Y. , Mu, J. , Zhang, J. , Yang, X. et al. (2008) Serine palmitoyltransferase, a key enzyme for de novo synthesis of sphingolipids, is essential for male gametophyte development in Arabidopsis . Plant Physiol. 146, 1322–1332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walton, J.D. (1996) Host‐selective toxins: agents of compatibility. Plant Cell, 8, 1723–1733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang, H. , Li, J. , Bostock, R.M. and Gilchrist, D.G. (1996) Apoptosis: a functional paradigm for programmed plant cell death induced by a host‐selective phytotoxin and invoked during development. Plant Cell, 8, 375–391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang, Q. , Wang, J. , Yu, F. , Zhu, X. , Kathia, Z.R. and Du, L. (2006) Mycotoxin fumonisins: health impacts and biosynthetic mechanism. Prog. Nat. Sci. 16, 7–15. [Google Scholar]
- Zhang, L. , Jia, C. , Liu, L. , Zhang, Z. , Li, C. and Wang, Q. (2011) The involvement of jasmonates and ethylene in Alternaria alternata f. sp. lycopersici toxin‐induced tomato cell death. J. Exp. Bot. 62, 5405–5418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao, Y. , Wang, J. , Liu, Y. , Miao, H. , Cai, C. , Shao, Z. , Guo, R. et al. (2015) Classic myrosinase‐dependent degradation of indole glucosinolate attenuates fumonisin B1‐induced programmed cell death in Arabidopsis . Plant J. 81, 920–933. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1 Lesion formation upon fumonisin B1 (FB1) injection in fbr41 and null mutants for AtLCB2a and AtLCB2b.
Figure S2 Comparison of amino acid sequences of FBR41, AtLCB2b and AtLCB2a.
Figure S3 In planta SPT activity in wild‐type (WT) and fbr41 mutant.
Figure S4 Proposed model considering the role of FBR41 in the resistance to sphinganine analog mycotoxin (SAMT)‐induced cell death.
Figure S5 The disease symptoms of wild‐type (WT) and FBR41‐overexpressing transgenic plants after AAL inoculation.
Figure S6 FBR41‐overexpressing tomato exhibited decreased resistance against Pseudomonas syringae pv. tomato DC 3000 (Pst DC3000).
Table S1 Fumonisin B1‐resistant mutants identified by forward genetic screening in Arabidopsis.
Table S2 List of SSLP markers used for first‐pass mapping.
Table S3 List of CAPS markers used for fine mapping.
Table S4 Primers used in this study.
Methods S1 Screening of the fumonisin B1‐resistant mutant.
Methods S2 Tomato transformation.
Methods S3 Pseudomonas syringae pv tomato DC3000 infection assays.
Methods S4 Microsome preparation and SPT assay.


