Abstract
A major frontier in the post-genomic era is the investigation of the control of coordinated growth and three-dimensional form. Dynamic remodeling of complex organs in regulative embryogenesis, regeneration, and cancer reveals that cells and tissues make decisions that implement complex anatomical outcomes. It is now essential to understand not only the genetics that specifies cellular hardware but also the physiological software that implements tissue-level plasticity and robust morphogenesis. Here, we review recent discoveries about the endogenous mechanisms of bioelectrical communication among non-neural cells that enables them to cooperate in vivo. We discuss important advances in bioelectronics, as well as computational and pharmacological tools that are enabling the taming of biophysical controls toward applications in regenerative medicine and synthetic bioengineering.
Subject Areas: Biotechnology, Bioelectronics, Developmental Biology, Bioelectrical Engineering, Electronic Materials, Biomedical Materials
Biotechnology; Bioelectronics; Developmental Biology; Bioelectrical Engineering; Electronic Materials; Biomedical Materials
Introduction
Rapid advances in genomics are revealing the mechanisms (and their evolutionary origin) underlying cellular hardware: the complement of proteins that a given cell deploys. However, fundamental gaps in our understanding remain, with respect to the software: the algorithms by which cells accomplish specific goals under a range of diverse environmental perturbations (Pezzulo and Levin, 2016). Single cells, including microscopic paramecia and the giant Acetabularia (a plant-like alga that is > 10 cm tall), achieve an extremely highly patterned invariant morphology without benefit of cell–cell communication or stem cell differentiation. Some animals, such as the salamander, are able to regenerate whole limbs, eyes, and other organs throughout its lifespan (as can single cells such as Stentor) (Birnbaum and Alvarado, 2008). As remarkable as their ability to create new patterned tissue upon injury is the fact that regeneration stops when the correct pattern has been completed. How does this system know what a correct pattern looks like (Pezzulo and Levin, 2015)? The ability to regenerate the same structure and then stop at the appropriate time requires single cells to cooperate, measuring and acting on structural information on a much larger scale (Pezzulo and Levin, 2015). Embryos can build a smaller, but morphologically identical animal body, even when >50% of the cells are removed (Cooke, 1981), and pieces cut from planarian flatworms grow precisely the needed organs, no more and no less, at the correct wound surfaces, while rescaling themselves to perfect proportions (Saló et al., 2009).
This sort of anatomical homeostasis (the ability to maintain a large-scale target morphology under a range of unpredictable damage/intervention) highlights the essential role of decision-making algorithms implemented in living tissue (Friston et al., 2015, Manicka and Levin, 2019). Genomic and biochemical signals are clearly important but are not the whole story. It has long been known that cancer cells can be reprogrammed by embryonic and regenerative environments into normal histogenesis (Hendrix et al., 2007, Mintz and Illmensee, 1975), whereas asexually reproducing planaria execute 100% perfect anatomical regeneration despite an incredibly messy genome (including mixoploidy) resulting from 400 million years of somatic inheritance and accumulation of mutations [reviewed in Levin et al. (2018)]. An exciting frontier is emerging around the basic questions of how genome-specified hardware implements robust, goal-directed, tissue-level behavior among cell collectives. In addition to its impact on our understanding of evolution, developmental cell biology, and multicellularity, this area has crucial implications for biomedicine. If we understood the information processing pathways that enable cells to harness chemistry, physics, and computation toward specific outcomes, transformative approaches could be implemented to repair birth defects, regenerate organs after traumatic injury or aging, reprogram tumors, and build artificial living machines to desired spec (Levin, 2011).
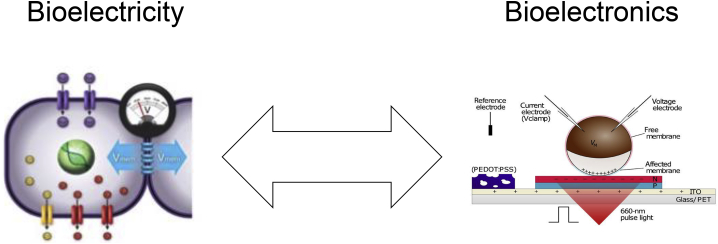
It is increasingly becoming clear that important outcomes can be induced not only by rewiring the hardware (i.e., genomic editing and gene therapy) but also by providing specific inputs into the cellular decision-making networks. Complementing decades of focus on transcriptionally modulated chemical signals, recent work has begun to explore and exploit the role of physical forces and microenvironment in regulating cell behavior. Due to space limitations, we do not cover tensile forces and biomechanics, which have been expertly reviewed elsewhere (Beloussov, 2012, Davidson, 2012, Miller and Davidson, 2013, Navis and Bagnat, 2015, von Dassow and Davidson, 2011) but instead focus on developmental bioelectricity: ways in which non-neural cellular networks process information via electrical dynamics (Levin and Martyniuk, 2018, Mathews and Levin, 2018). Advances in novel materials, computational platforms, and bioengineering tools are greatly facilitating the discovery of fundamental new biology and novel applications in biomedicine and synthetic biology. Here, we present recent advances in target bioelectric circuits and bioelectronic devices that can stimulate them (Figure 1).
Figure 1.
Bioelectric Signaling Can Communicate with Bioelectronic Devices to Affect Cell Function and Drive Growth and Patterning
Targeting Endogenous Bioelectric Circuits: Ion Channel Targets
As in neurons, the bioelectric state of all other cells is set by the combined functionality of ion channels and pumps, which determine resting potential and electrical synapses known as gap junctions, which selectively propagate those states through the network (Figure 2). Indeed, this system of coordination is evolutionarily ancient, having an origin in coordination in bacterial biofilms (Humphries et al., 2017, Prindle et al., 2015) but extending also to algae (Bentrup and Jaffe, 1968, Jaffe, 1966, Robinson et al., 1981). Thus, ion channels and gap junctions represent critical targets for manipulation of endogenous bioelectric signaling and the downstream changes in gene expression, cytoskeletal structure, and cell behavior. Several recent applications have highlighted the potential for rational effects at the cell and tissue/organ level by manipulating endogenous bioelectric pathways (Bates, 2015, Funk, 2013, Mathews and Levin, 2017, McLaughlin and Levin, 2018).
Figure 2.
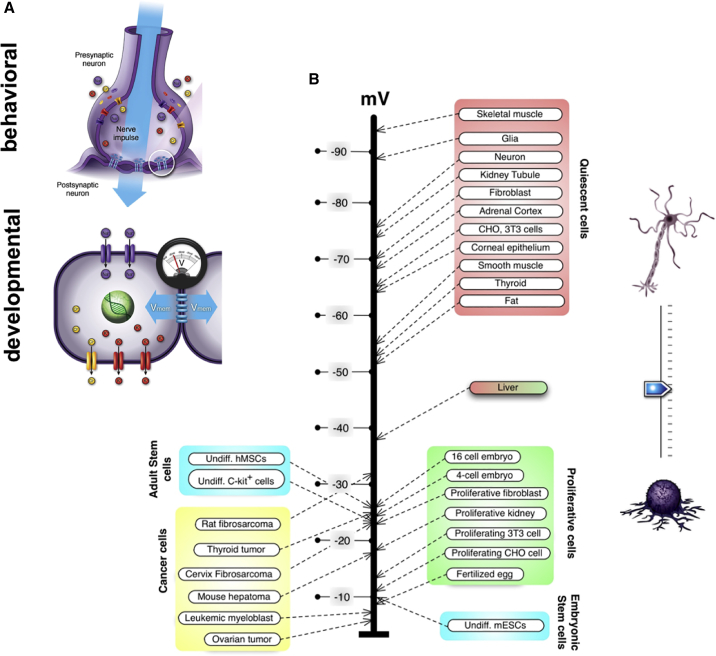
Bioelectric Signaling at the Cellular Level
(A) All cells, including non-neural cells, possess a resting membrane potential that is defined by the activity of its ion channels and pumps and the concentrations of various ions inside and outside of the cell. Cells communicate their individual bioelectric states via gap junctions—electrical synapses—to their neighbors, and the resulting bioelectric network guides individual and tissue-level cell behaviors. Images by Jeremy Guay of Peregrine Creative Inc.
(B) Individual cell states are determined by Vmem; with highly plastic (embryonic, stem, and cancer) cells being relatively depolarized while mature, terminally differentiated cells are hyperpolarized. Data taken from (Binggeli and Weinstein, 1986).
Manipulating Ion Channels Leads to Changes in Morphogenesis
One of the functions of transmembrane potential (Vmem) gradients is to provide prepatterns for the creation of various complex organs (Vandenberg et al., 2011) (Figure 3). For example, the voltage distribution across the nascent face is the reason why mutations in the Kir2.1 result in channelopathies not only of the heartbeat but also of craniofacial development (Adams et al., 2016). Importantly, it is now known that a key step in this process, stem cell differentiation, is regulated by bioelectric state. Using small molecule drugs to target ion channels of mesenchymal stem cells has revealed that Vmem can induce (Sundelacruz et al., 2008) and in fact override bioelectric chemical inducers (Sundelacruz et al., 2013a, Sundelacruz et al., 2013b). Interestingly, the downstream genetic targets of bioelectric state change are highly conserved between mammalian mesenchymal stem cells (MSCs) and regenerating tissues in frog and axolotl (Pai et al., 2016) (including pathways such as bone morphogenetic proteins [BMPs], Ca2+ signaling, integrins, and histone deacetylase), consistent with the evolutionarily ancient origins of bioelectrical signaling.
Figure 3.
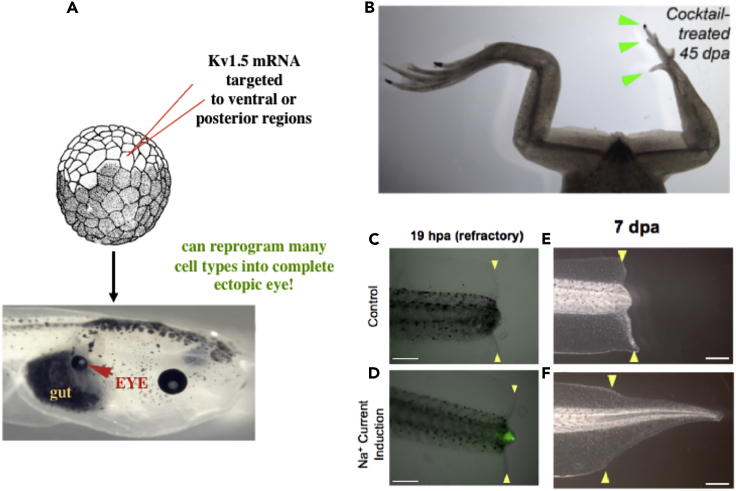
Bioelectric Signaling Instructs Growth and Form
(A) Ion channels that set an eye-specific bioelectric prepattern, injected as mRNA into cells of a frog embryo, can reprogram regions of the body (such as the gut) to form an eye, including its internal layers (panel taken with permission from [Pai et al., 2012]).
(B–F) (B) An ionophore applied to the leg amputation of a froglet induces the growth of a leg with toenails and toes, during stages where limbs do not normally regenerate (panel taken with permission from [Tseng and Levin, 2013]). The same ionophore can be used to trigger tail regeneration. Normal amputated tadpole tails (C) at refractory stages do not regenerate (E); however, if an influx of sodium (D) is induced, regeneration of a tail results (F). Scale bar in panels C–F is 500 μm. Panels taken with permission from Tseng et al. (2010).
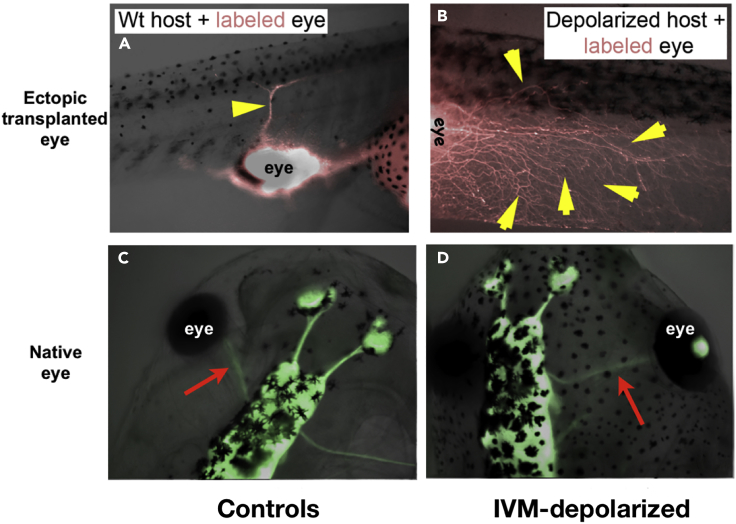
Beyond the single-cell level, bioelectric mechanisms are an important aspect of the microenvironment that enables cells to make decisions based on the states of their neighbors. Galvanotaxis, or migration in electric fields generated by epithelia, has now been observed across taxa, from amebae (Gao et al., 2015) and fungi (Brown et al., 2019, Gow and Morris, 1995) to human cells (Feng et al., 2012, Kim et al., 2017, Ren et al., 2019), and is being exploited for wound healing applications (Reid et al., 2011, Reid and Zhao, 2014, Wang and Zhao, 2010). Classic work by Lionel Jaffe examined bioelectrics in coordinating cell activity via transcellular currents in models such as amebas, pollen tubes, and various kinds of eggs and embryos (Borgens et al., 1979a, Jaffe and Robinson, 1978, Jaffe and Nuccitelli, 1977, Nuccitelli and Jaffe, 1975, Nuccitelli and Jaffe, 1976, Nuccitelli et al., 1975, Nuccitelli et al., 1977, Robinson and Jaffe, 1973, Robinson and Jaffe, 1976, Weisenseel et al., 1975). In addition to electric fields, cells also sense static charges (Beech, 1997, Strilic et al., 2010, Weihua et al., 2005) and resting potentials. For example, transplanted eye primordia normally grow one optic nerve that connects to the host. However, if the cells in the host cells are depolarized, for example by targeting chloride channels via the compound ivermectin (Figure 4), a massive hyperinnervation occurs (Blackiston et al., 2015). Characterization of the transduction mechanism revealed the involvement of serotonergic signaling directed by the differential resting potential between neighboring cells, which has also been implicated in bioelectric transformation to melanoma (Blackiston et al., 2011). This made it possible to identify small molecule drugs that improve the vision resulting from eye implants into otherwise blind animals (Blackiston et al., 2017). Remarkably, the native innervation is unchanged by such treatments, indicating that only nerve that is ectopic (recognizes that it is in the wrong location) reads and reacts to this environmental cue. Investigation into how nerves detect whether they are in their correct place, and making the decision of reading the surrounding cells' Vmem or not, is a major area for future investigation and will be greatly facilitated by emerging technology that implements spatial control of ionic environment with which to confront cells (Deng et al., 2016, Selberg et al., 2018).
Figure 4.
Bioelectric Controls of Ectopic Innervation
(A) Ectopic eye labeled with red fluorescent protein normally makes one optic nerve that reaches toward the spinal cord.
(B–D) (B) When the host body is depolarized—for example, by the application of the chloride channel opener ivermectin (IVM)—massive hyper-innervation occurs. Remarkably, the normal innervation (C) is not altered in these animals (compare C to D), showing that only the ectopic nerve is paying attention to the surrounding cells' bioelectric topography and suggesting the use of local depolarization as a method to augment innervation of transplanted organs. Panels taken with permission from Blackiston and Levin (2013).
Targeting Bioelectric States Repairs Birth Defects
Because of its central role in morphogenesis of a variety of body systems, bioelectrical signaling has also been exploited to understand, and counteract, the effects of teratogens and mutations in birth defects of the brain (Pai et al., 2015b). Nicotine and mutations of the critical neurogenesis gene Notch both perturb the normal bioelectric pattern observed in the nascent ectoderm. The bioelectric distribution is normally hyperpolarized in the middle of the tissue and depolarized at the edges, and this is critical—if the pattern is flattened in either direction, gene expression and brain patterning become completely abnormal. Artificially reinforcing this pattern, by activation of HCN2 channels to hyperpolarize the middle region and depolarize the lateral regions rescues normal gene expression, anatomy, and even learning rate (behavioral IQ) despite the exposure to strong teratogens and Notch mutation (Pai et al., 2018). In addition to the obvious biomedical implications, these data shed light on the basic biology of organogenesis: the borders of the nascent brain are set by the differences in Vmem between cell groups, showing how developmental compartments are set up by physiological gradients. Work is ongoing in our laboratory to understand how these differences (properties on a multi-scale level) are readout and transduced into changes of gene expression and cell behavior, even at a distance (Pai et al., 2015a).
Bioelectric Modulation for Regenerative Repair
Beyond embryogenesis, bioelectrics has been studied in regeneration, a process in which some species are able to re-grow complex organs following amputation (Birnbaum and Alvarado, 2008). Classic data revealed a role for trans-epithelial bioelectric gradients: shunting these reduced regenerative ability (Borgens et al., 1979b, Borgens et al., 1979c), whereas inducing them could cause a degree of limb regeneration in otherwise non-regenerative species (Becker and Spadaro, 1972, Bodemer, 1964, Borgens et al., 1977). The endogenous electric field appears to be critical for migration of cells toward the wound and for inducing innervation, which is known to augment regenerative capacity (Kumar and Brockes, 2012). Applied electric field approaches are now being pursued in rodents with modern markers to understand immune system and stem cell involvement in the process (Leppik et al., 2015, Leppik et al., 2018, Mobini et al., 2017, Oliveira et al., 2019). Complementing the electric field and its directionality vector are the gradients of resting potentials in the wound region, which regulate proliferation and trigger organ-forming cascades. Managing the time course of these gradients in vivo has been used to induce tail (including spinal cord and muscle) regeneration via Notch and fibroblast growth factor (FGF) signaling pathways under non-regenerative conditions via ionophores that enable control of wound bioelectric state (Tseng et al., 2010). The ability to control resting potential and other aspects of the wound will be a critical component of future studies investigating the role of neurons and immune system cells as first responders to the ionic signaling that reveals the location of damage and guides downstream repair cascades (Ferreira et al., 2016, Ferreira et al., 2018, Zhu et al., 2019).
Fundamental Biology Revealed by Bioelectric Experiments
A few important lessons about basic biology are starting to emerge from the recent applications of molecular physiology methods to the control of anatomical patterning. First, it is critical to note that the driver in these cases is not a specific channel protein or gene: unlike in many biochemical pathways, the “pressure point” on the system that affords optimal control is a physiological macrostate, the resting potential of the cell. The same outcome can be induced by a range of different ion channels, as long as the resulting resting potential pattern is correct. For example, in tail and spinal cord regeneration, the endogenous V-ATPase pump can be shut down and replaced with a yeast proton pump that has no structural or sequence homology (Adams et al., 2007). This, however, rescues the regenerative ability, because the tail-building module is driven by a bioelectric state of the wound. Because ion channels can be opened and closed post-translationally, cells can be in exactly the same bioelectric state despite highly divergent gene expression; conversely, cells with identical transcriptomic or proteomic profiles can be in very different bioelectric states. This means that physiomic profiling using dyes (Adams and Levin, 2012, Deal et al., 2016, Kulkarni et al., 2018) or genetically encoded voltage reporters (Mutoh et al., 2012, St-Pierre et al., 2015) is an essential complement to biochemical profiling. It also means that in contexts such as cancer, where bioelectrics is critical for progression and metastasis, it is incorrect to think of ion channel oncogenes as simple targets to be turned on or off—strategies must be formulated for the control of Vmem, which may require differential control of multiple channels in diverse cell types and microenvironments (Cervera et al., 2016a, Kale et al., 2015, Moore et al., 2017).
Indeed, many ion channels and gap junctions are themselves voltage sensitive, which can lead to the formation of multi-protein circuits with complex system-level behaviors (Cervera et al., 2016a, Cervera et al., 2016b, Cervera et al., 2018a, Cervera et al., 2018b, Cervera et al., 2018c, Cervera et al., 2019, Palacios-Prado and Bukauskas, 2009). Some of these circuits establish robustness (resistance of cells to induced Vmem modulation), whereas others implement positive feedback loops that result in a kind of memory where changes to resting potential are reinforced for long-term physiological effects that last long after the stimulus is removed. Thus, it is essential to use predictive computational models to understand spatiotemporal behavior of bioelectric circuits. New open-source computational tools are coming on-line (Cervera et al., 2018c, Pietak and Levin, 2016, Pietak and Levin, 2017), which facilitate bio-realistic simulation of physiological and biochemical networks. These enable the prediction of single cell- and tissue-level dynamics and allow testing of candidate strategies for inducing repair such as of brain defects (Pai et al., 2018).
An important component of tissue-level bioelectric dynamics is the fact that instructive states are not local. For example, amputation (but not puncture injury) of a leg in froglets results in rapid (∼30-s long) bioelectric signal in the contralateral (untouched) leg, which reveals the location of the damage (proximodistal position along the limb) (Busse et al., 2018). This kind of phenomenon is general and is not merely an indicator (readout) but is functionally instructive. For example, the size of the developing brain can be determined by bioelectric state of ventral cells destined to be gut (Pai et al., 2015a), whereas the normalization of induced tumors can be achieved by hyperpolarizing channels expressed on the opposite side of the animal (Chernet et al., 2015, Chernet and Levin, 2014). As in the nervous system, bioelectrical networks enable coordination and long-range integration of information, which strongly suggests that future applications need to take into account far more than tumor or wound tissue, or even just the immediately adjacent microenvironment. Remarkably, such long-range controls via bioelectrics had been predicted by H. S. Burr as far back as the 1930s (Burr, 1941, Burr et al., 1938, Burr et al., 1940). However, current molecular insight into the mechanisms of long-range communication (via calcium waves, gap junction-based transport of neurotransmitters, and diffusion of second messengers such as butyrate [Chernet and Levin, 2014]) can be exploited for surrogate site diagnosis and treatment of difficult-to-reach regions.
A final emerging theme is that of modularity: simple triggers induce a self-limiting, complex cascade of signaling and morphogenesis that results in the formation of a coherent structure. For example, expression of some ion channels in the frog embryo induces a whole eye to be formed in aberrant locations, such as in the gut or on the tail (Pai et al., 2012). Similarly, recent work showed that a transient (24-h) treatment of amputated adult frog legs with a hormone (progesterone) that induces, among other pathways, bioelectrical remodeling leads to more than 12 months of limb regeneration and patterning (Herrera-Rincon et al., 2018). Similarly brief (48-h) reduction of electrical connectivity, or three-hour depolarization, in planarian flatworms permanently converts them to a double-headed phenotype that continues to regenerate entire ectopic heads in future rounds of regeneration in plain water (Durant et al., 2019, Oviedo et al., 2010). In all cases, a brief trigger induces a coherent morphogenetic event, not an out-of-control tumor, which stops growing at the correct time. Modularity has important implications for both evolution and biomedicine; identifying such “subroutine calls” in vivo is an important task for the field. Bioelectrical triggers for complex organs facilitate evolvability because relatively small physiological or genetic changes that result in differential Vmem patterns can readily induce major changes to body anatomy. Another implication is that once discovered, triggers can be used to induce organ formation long before we have the knowledge about how to make a complex structure or have the technical ability to manually assemble it from stem cell progeny.
Current and Future Techniques for Modulating Ion Channel Signaling
Most of the molecular work unraveling the roles of bioelectric signals in development and regeneration has utilized misexpression of well-characterized ion channel constructs, to gain specificity and mechanistic insight (Mathews and Levin, 2018, Pai et al., 2017, Pai et al., 2018, Pitcairn et al., 2017). Indeed, recent advances in optogenetics have enabled light-induced regeneration of spinal cord and tail (Adams et al., 2013, Adams et al., 2014, Adams et al., 2016, Chernet et al., 2016). However, the biomedicine is likely to be most rapidly advanced by methods not requiring genome editing or gene therapy. Therefore, the immense toolkit of ion channel drugs, many of which are already approved for human use in cardiac arrhythmias, epilepsy, and other indications, can be re-purposed as electroceuticals in regenerative and anti-neoplastic (Cairns et al., 2018, Lobikin et al., 2015, Ozkucur et al., 2015, Tuszynski et al., 2017). An additional exciting set of directions however concerns new materials for sensing and actuation of bioelectrical signals in vivo.
Devices and Materials for Uncovering New Biology
Beginning with Galvani's studies on animal electricity (Galvani, 1972), bioelectronics has merged electronic devices with living systems. Advances in microfabrication and novel materials have now reduced the size of the bioelectronic interface from macroscale contacts capable of stimulating entire muscles to devices that can communicate with individual cells (Zhou et al., 2017) and membrane proteins (Misra et al., 2009, Noy, 2011, Soto-Rodriguez et al., 2016). This broad range of length scales makes these bioelectronic devices ideal means to interrogate and actuate bioelectric circuits.
Biosensors
To interrogate bioelectric circuit, bioelectronic sensors can sense electric fields, ionic concentrations, and biological markers (Fang et al., 2015, Loffler et al., 2017, Rivnay et al., 2014, Simon et al., 2016, Someya et al., 2016, Tian et al., 2018). These many types of bioelectronic sensors have been described in prior reviews (Bollella et al., 2017, Rim et al., 2017, Rivnay et al., 2014, Zhang and Lieber, 2016, Zhu et al., 2016). A comprehensive and detailed description is beyond the scope of this work. Here we will focus on cell-sensors for extracellular and intracellular recordings, or group of cells, for both extracellular detection and measurement of Vmem (Abbott et al., 2018, Tian and Lieber, 2019).
Extracellular Recordings
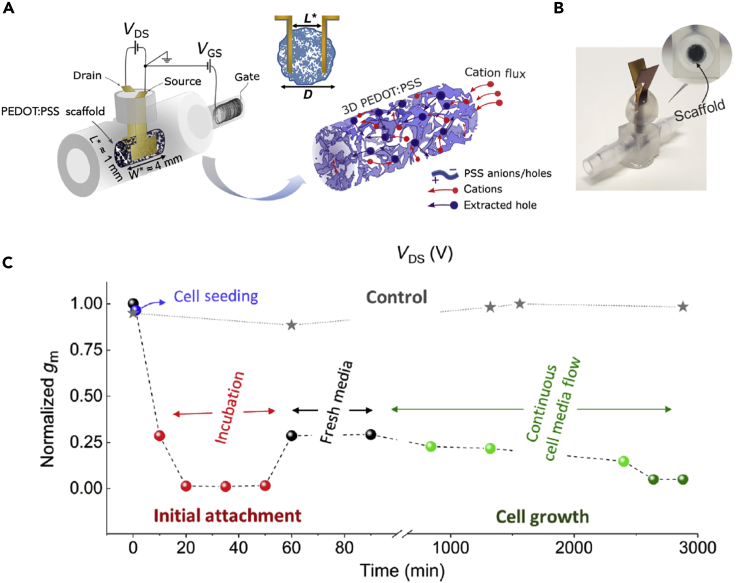
Transistor type biosensors afford signal amplification and large-scale multiplexing (Kaisti et al., 2016). A common transistor biosensor platform is the organic electrochemical transistor (OECT). A typical OECT is made by a mixture of the polymers poly(3,4-ethylenedioxythiophene):poly(styrenesulfonate) (PEDOT:PSS) that are combined with selected enzymes or antibodies to detect molecules of interest (Strakosas et al., 2015, Strakosas et al., 2017). The main advantage of the OECT platform is its inherent sensitivity to ionic species in solution that spans from the fact that the conducting channel conducts both electrons and ions (Berggren and Richter-Dahlfors, 2007, Rivnay et al., 2016, Rivnay et al., 2018). Mixed conductivity is essential for interfacing with bioelectric circuits because electric fields and electric currents in biological systems are mostly associated with ionic concentration gradients and ionic currents rather than electrons. Water, which is ubiquitous in biological systems, is a poor electronic conductor, but ions move freely in the liquid. Therefore, the OECT platform effectively acts as a transducer between the two types of charge carriers. This transduction mechanism can be used to assess cell health and response of barrier tissue to foreign substances (Jimison et al., 2012). The layer of cells grown on top of the OECT serves as a barrier between the OECT and the electrolyte. A healthy barrier layer with intact tight junctions between the cells does not allow the passage of ions into the OECT channel when a gate potential is applied so that the modulation in the channel current is small. Upon introduction of H2O2, tight junctions are damaged and the OECT response is increased. Recently, OECT have been interfaced with three-dimensional tissue with tubistors (Pitsalidis et al., 2018). The tubistor is the first example of a bioelectronic device in three dimensions. The tubistor affords a more natural interface with cells of many types of tissue and the 3D tubular geometry is ideal for flow of nutrients and for mimicking tubular types of tissues such as vascular, gastrointestinal, and kidneys. With the tubistor, Owens and co-authors have monitored in vitro the progression of cell cultures growing into a 3D conducting scaffold (Figure 5). Given the sensitivity of OECT to ionic species and external electric fields, a similar approach can be used to monitor bioelectric circuits.
Figure 5.
The Tubistor, the First Example of a Bioelectronic Device in Three Dimensions
(A) Schematic representation of the tubistor. A 3D Pedot:PSS scaffold is placed inside a microfluidic channel that comprises a source and drain contact across which VDS is applied and an electrochemical gate that modulates the conductivity of the Pedot:PSS between source and drain, (B) picture of the tubistor, (C) different stages of cell growth affect the transconductance (gm) of the tubistor that can be used to monitor cell activity (reproduced from Pitsalidis et al., 2018).
Intracellular Recording
The advent of nanomaterials and microfabrication strategies has allowed bioelectronics devices to interrogate the intracellular environment of single cells for recording of Vmem. These recordings are built on the well-developed technique of patch clamping that is described in detail elsewhere (Annecchino and Schultz, 2018). Most of the bioelectronic work has revolved around measuring spatiotemporal mapping of Vmem of electrically excitable cells, such as neurons and cardiomyocytes (Chang Liao et al., 2015), but the functionality developed with these devices can be easily expanded to other non-electrically excitable cells to monitor bioelectric circuits. Silicon nanowires with nanoscale dimensions and high aspect ratios are suitable for crossing the cell membrane and connecting the intracellular domain with minimal or no damage to the cell. These nanowires are synthesized with spatially controlled electrical properties so that a nanoscale field effect transistor is created on an individual nanowire by varying the doping levels (Tian et al., 2010). These nanowires are synthesized with a kink, so that they can be mounted on the tip of an atomic force microscope probe that is used to insert the nanowire into the individual cell. Recordings of action potential of cardiomyocytes are more precise when collected inside the cell with the silicon nanowire than when recorded in the extracellular space (Tian and Lieber, 2019).
In addition to intracellular recordings, bioelectronic devices are able to monitor the current response of membrane ion channels in artificial lipid bilayers. For example, gramicidin, bacteriorhodopsin, alamethicin, and ATPase are connected directly with carbon nanotubes (Huang et al., 2010), silicon nanowires (Misra et al., 2009), and organic field effect transistors (Angione et al., 2012) to develop biosensors with increased functionality. In these biosensors, the ion transport across the channels affects the electrostatic environment of the semiconducting material (nanotube, nanowire, conductive polymer) and is recorded as a change in current between the source and the drain electrodes. In this fashion, transport across both passive (gramicidin, alametachin) and active (rhodopsin, ATPase) is monitored as a function of extracellular environment. For example, Noy has recorded the activity of the enzyme ATPase using silicon nanowire transistors as a function of ATP concentration in solution (Misra et al., 2009). The Rolandi group has placed an artificial lipid bilayer on Pd contacts to monitor directly the transport of H+ across the ion channels (gA) and alamethicin (ALM). In this scenario, the Pd electrode serves as a transducer between the H+ current across the ion channels and the electronic current in the devices, thus effectively coupling the biological and electronic worlds (Hemmatian et al., 2016). With these devices, H+ current across the gA and ALM channels is monitored and modulated with a potential difference applied across the cell membrane mimic. As expected, gA acts as a H+ passive channel, whereas ALM H+ transport is voltage gated and ALM only opens above the threshold voltage of approximately 70 mV. Additionally, rhodopsin integrated with these H+−selective bioelectronic devices leads to light-driven H+ transport across the cell membrane (Soto-Rodriguez et al., 2016).
Bioactuators
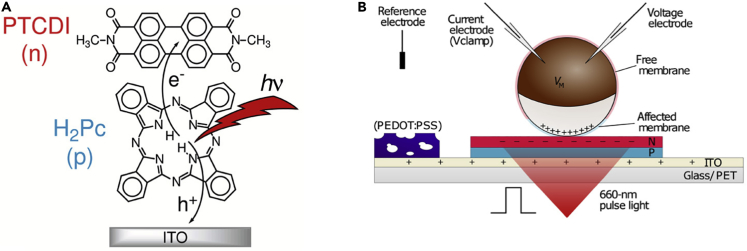
In the early experiments of Galvani and modern medical technologies, such as pace-makers and neuronal implants, bioelectric signaling to cells occurs through an electronic impulse at the electrodes that affects the action potential of electrically excitable cells. There have been many comprehensive reviews on neuronal (Bettinger, 2018) and peripheral nerve stimulation (Pavlov and Tracey, 2019), electroceuticals using electronic stimulations (Famm et al., 2013), and the use of electric fields for bone growth (Khalifeh et al., 2018) and wound repair (Zhao, 2009). Here, we will focus on bioelectronic devices that are able to deliver directly biophysical and chemical signals targeting specific cell functions. These signals include the electrophoretic delivery of ions and small molecules, such as neurotransmitters, to modify cell behavior (Arbring Sjöström et al., 2018). Organic electronic ion pumps composed of PEDOT:PSS are able to pump ions and small charged molecules from a reservoir well through both an ionically conductive poly-ion exchange membrane and a PEDOT:PSS electrode into bulk solution (Isaksson et al., 2007, Jonsson et al., 2016, Owens and Malliaras, 2011). This architecture pumps different charged species, such as monovalent cations, gamma-aminobutyric acid (GABA), and acetylcholine (Jonsson et al., 2015). Delivery of the neurotransmitter GABA+ into the extracellular f≥lku6luid surrounding hyperactive neurons reduces epileptic activity (Williamson et al., 2015). These organic ion pumps are able to deliver multiple ions, as well as charged molecules, but their delivery efficiency becomes smaller with increasing molecule size. Simon et al. have recently demonstrated electrophoretic delivery of molecules such as acetylcholine using capillary fibers (Poxson et al., 2019). Delivery of larger molecules is enabled by a dendrimer-based ionic polymer with controlled porosity that allows for transit of larger molecules. Recently, Glowacki and co-workers (Jakešová et al., 2019) have demonstrated optoelectronic control of single cells by merging organic photocapacitors with Xenopus laevis oocytes. They performed electrophysiological recordings while illuminating the organic photocapacitors, showing that they are able to photoinduce transient changes between 20 and 110 mV with a time constant between 5 μs and 5 ms. These transients induce the opening of potassium channels, indicating that this device can directly control membrane protein conductivities (Figure 6).
Figure 6.
Schematic Representation of Organic Photocapacitor Working Principle and the Experimental Setup
(A) A photon absorbed causes electron hole separation in the polymer blend.
(B) A cell placed upon the organic photocapacitor detects a change in membrane potential when the light is turned out due to the charge separation occurring on the surface of the organic photocapacitor.
Another strategy for controlling delivery of ions is to use PdHx contacts that transfer H+ across the solution contact interface for control of pH (Deng et al., 2016, Miyake et al., 2015). Using PdHx contacts, Miyake et al. have used bioelectronics to monitor the output of the enzymatic biochemical reaction of glucose oxidation that reduces pH and have created with this reaction an enzymatic AND gate and an enzymatic flip-flop circuit (Miyake et al., 2015). In turn, Deng et al. have used the pH control spanning from the PdHx bioelectronics devices to affect the rate and light output of the bioluminescent reaction of luciferin with luciferase (Deng et al., 2016). This reaction is faster and brighter at high pH and thus can be turned on or off using bioelectronics pH control.
A variety of nanomaterials have been developed for reading and writing bioelectric states in tissue. Particles that alter resting potential of cells by contact (Jayaram et al., 2017, Rana et al., 2016, Shin et al., 2013, Warren and Payne, 2015) offer the possibility of spatially targeted interventions without the use of transgenes. Complementing these are fluorescent nanoparticles that report the concentration of specific ions such as sodium (Dubach et al., 2009, Dubach et al., 2011, Dubach et al., 2011b). An important future use of these reagents will be to understand the link between bioelectric signals regulated by K+, Na+, and Cl− channels and the still poorly understood but likely important flow of electrons in living tissues (Sanjuan-Alberte et al., 2018, Sanjuan-Alberte and Rawson, 2019).
Opportunities and Next Steps
Proteomic and physiological profiling, together with drug screens (Sullivan and Levin, 2018), are revealing the molecular sources of relevant ion fluxes. Similarly, the transduction of changes in Vmem into second messenger pathways is now understood at the cellular level: voltage-regulated neurotransmitter transporters, calcium channels, KRAS clustering, and butyrate-regulated histone deacetylases convert bioelectric events into transcriptional responses [reviewed in Levin et al. (2017)]. Thus, at the scale of a single cell, bioelectric pathways are being decoded at high molecular resolution. However, the major knowledge gap concerns the tissue-level bioelectric code: the mapping between spatiotemporal distribution of ion currents, electric fields, and patterns of resting potential and the resulting morphogenetic outcomes (Levin and Martyniuk, 2018). Computational modeling of bioelectric circuits is beginning to reveal their memory, self-organizing, symmetry-breaking, and robustness properties (Cervera et al., 2015, Cervera et al., 2016a, Cervera et al., 2016b, Cervera et al., 2018a, Cervera et al., 2018b, Cervera et al., 2018c, Cervera et al., 2019, Garcia-Morales et al., 2017).
To understand how specific patterns of bioelectric state drive the creation and remodeling of complex organs, experiments will require the ability to read and write bioelectric states in tissues in vivo. Thus, newly developed ionogenic materials provide an important complement to optogenetics (Adams et al., 2013, Adams et al., 2016, Chernet et al., 2016), to interrogate the control of growth and form by ionic signals. This is especially relevant for model systems such as planaria, in which transient bioelectric changes can permanently change the regenerative pattern to which their stem cell progeny construct (Durant et al., 2017, Durant et al., 2019) but are not amenable to introduction of foreign optogenetic ion channels.
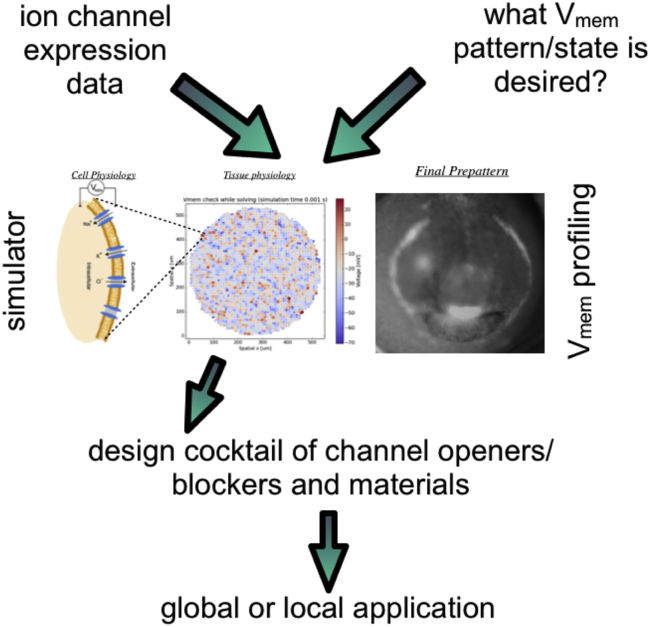
Bioelectronic devices presage the development of strategies to both monitor and guide regeneration, repair, and immune response modulation via knowledge of the bioelectronic code. To achieve the guided self-assembly needed for patterning in vivo or in bioengineering and synthetic morphology contexts (McNamara et al., 2016, McNamara et al., 2018), closed-loop control would be desirable using strategies from biological control and machine learning that have been proposed to converge synthetic biology with bioelectronics (Selberg et al., 2018). Integration with optogenetic and microfluidic platforms is essential, as these are already beginning to interrogate cellular perception and decision-making mechanisms (Bugaj et al., 2017, Perkins et al., 2019). Likewise, new bioelectronic materials will be increasingly incorporated into wearable bioreactors used for induction of complex regenerative repair (Golding et al., 2016, Hechavarria et al., 2010, Herrera-Rincon et al., 2018). Together, newly developed quantitative physiological predictive platforms (Pietak and Levin, 2016, Pietak and Levin, 2017) and dynamically controlled ionogenic materials will enable the complex and nonlinear behavior of bioelectric circuits in tissues to be understood in health and disease states (Figure 7). The discovery and deployment of novel bioelectric signaling interventions are likely to have a major impact as an alternative to genomic editing and gene therapy for a wide range of applications in birth defects, regeneration, cancer, immunology, and synthetic morphology.
Figure 7.
Bioelectric Strategies for Therapeutics
Data on ion channel expression in human tissues, together with knowledge of which bioelectric states are desired for healthy patterning and physiology, can be fed into simulators of the bioelectric circuits that enable predictions of the effects of possible ionic perturbations. The resulting suggested combinations of ion channel drugs and ion-releasing materials can be deployed in vivo or in vitro to manipulate cell and tissue-level behaviors in therapeutic or bioengineering contexts.
Acknowledgments
M.L. gratefully acknowledges support from the Allen Discovery Center program through The Paul G. Allen Frontiers Group (12171), the Templeton World Charity Foundation (TWCF0089/AB55), and the Bailey Family Foundation. M.L and M.R. acknowledge funding from the Defense Advanced Research Projects Agency (DARPA), Army Research Office under Cooperative Agreement Number W911NF-18-2-0104.
Author Contributions
All authors wrote and edited this review article.
References
- Abbott J., Ye T., Ham D., Park H. Optimizing nanoelectrode arrays for scalable intracellular electrophysiology. Acc. Chem. Res. 2018;51:600–608. doi: 10.1021/acs.accounts.7b00519. [DOI] [PubMed] [Google Scholar]
- Adams D.S., Lemire J.M., Kramer R.H., Levin M. Optogenetics in Developmental Biology: using light to control ion flux-dependent signals in Xenopus embryos. Int. J. Dev. Biol. 2014;58:851–861. doi: 10.1387/ijdb.140207ml. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adams D.S., Levin M. Measuring resting membrane potential using the fluorescent voltage reporters DiBAC4(3) and CC2-DMPE. Cold Spring Harb. Protoc. 2012;2012:459–464. doi: 10.1101/pdb.prot067702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adams D.S., Masi A., Levin M. H+ pump-dependent changes in membrane voltage are an early mechanism necessary and sufficient to induce Xenopus tail regeneration. Development. 2007;134:1323–1335. doi: 10.1242/dev.02812. [DOI] [PubMed] [Google Scholar]
- Adams D.S., Tseng A.S., Levin M. Light-activation of the Archaerhodopsin H(+)-pump reverses age-dependent loss of vertebrate regeneration: sparking system-level controls in vivo. Biol. Open. 2013;2:306–313. doi: 10.1242/bio.20133665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adams D.S., Uzel S.G., Akagi J., Wlodkowic D., Andreeva V., Yelick P.C., Devitt-Lee A., Pare J.F., Levin M. Bioelectric signalling via potassium channels: a mechanism for craniofacial dysmorphogenesis in KCNJ2-associated Andersen-Tawil Syndrome. J. Physiol. 2016;594:3245–3270. doi: 10.1113/JP271930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Angione M.D., Cotrone S., Magliulo M., Mallardi A., Altamura D., Giannini C., Cioffi N., Sabbatini L., Fratini E., Baglioni P. Interfacial electronic effects in functional biolayers integrated into organic field-effect transistors. Proc. Natl. Acad. Sci. U S A. 2012;109:6429–6434. doi: 10.1073/pnas.1200549109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Annecchino L.A., Schultz S.R. Progress in automating patch clamp cellular physiology. Brain Neurosci. Adv. 2018;2 doi: 10.1177/2398212818776561. 2398212818776561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arbring Sjöström T., Berggren M., Gabrielsson E.O., Janson P., Poxson D.J., Seitanidou M., Simon D.T. A decade of iontronic delivery devices. Adv. Mater. Technol. 2018;3:1700360. [Google Scholar]
- Bates E. Ion channels in development and cancer. Annu. Rev. Cell Dev. Biol. 2015;31:231–247. doi: 10.1146/annurev-cellbio-100814-125338. [DOI] [PubMed] [Google Scholar]
- Becker R.O., Spadaro J.A. Electrical stimulation of partial limb regeneration in mammals. Bull. N. Y. Acad. Med. 1972;48:627–641. [PMC free article] [PubMed] [Google Scholar]
- Beech J.A. Bioelectric potential gradients may initiate cell cycling: ELF and zeta potential gradients may mimic this effect. Bioelectromagnetics. 1997;18:341–348. doi: 10.1002/(sici)1521-186x(1997)18:5<341::aid-bem1>3.0.co;2-2. [DOI] [PubMed] [Google Scholar]
- Beloussov L.V. Morphogenesis as a macroscopic self-organizing process. BioSystems. 2012;109:262–279. doi: 10.1016/j.biosystems.2012.05.003. [DOI] [PubMed] [Google Scholar]
- Bentrup F.W., Jaffe L.F. Analyzing the "group effect": rheotropic responses of developing fucus eggs. Protoplasma. 1968;65:25–35. doi: 10.1007/BF01666369. [DOI] [PubMed] [Google Scholar]
- Berggren M., Richter-Dahlfors A. Organic bioelectronics. Adv. Mater. 2007;19:3201–3213. [Google Scholar]
- Bettinger C.J. Recent advances in materials and flexible electronics for peripheral nerve interfaces. Bioelectron. Med. 2018;4:6. doi: 10.1186/s42234-018-0007-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Binggeli R., Weinstein R. Membrane potentials and sodium channels: hypotheses for growth regulation and cancer formation based on changes in sodium channels and gap junctions. J. Theor. Biol. 1986;123:377–401. doi: 10.1016/s0022-5193(86)80209-0. [DOI] [PubMed] [Google Scholar]
- Birnbaum K.D., Alvarado A.S. Slicing across kingdoms: Regeneration in plants and animals. Cell. 2008;132:697–710. doi: 10.1016/j.cell.2008.01.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blackiston D., Adams D.S., Lemire J.M., Lobikin M., Levin M. Transmembrane potential of GlyCl-expressing instructor cells induces a neoplastic-like conversion of melanocytes via a serotonergic pathway. Dis. Model. Mech. 2011;4:67–85. doi: 10.1242/dmm.005561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blackiston D.J., Anderson G.M., Rahman N., Bieck C., Levin M. A novel method for inducing nerve growth via modulation of host resting potential: gap junction-mediated and serotonergic signaling mechanisms. Neurotherapeutics. 2015;12:170–184. doi: 10.1007/s13311-014-0317-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blackiston D.J., Levin M. Ectopic eyes outside the head in Xenopus tadpoles provide sensory data for light-mediated learning. J. Exp. Biol. 2013;216:1031–1040. doi: 10.1242/jeb.074963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blackiston D.J., Vien K., Levin M. Serotonergic stimulation induces nerve growth and promotes visual learning via posterior eye grafts in a vertebrate model of induced sensory plasticity. NPJ Regen. Med. 2017;2:8. doi: 10.1038/s41536-017-0012-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bodemer C.W. Evocation of regrowth phenomena in anuran limbs by electrical stimulation of the nerve supply. Anat. Rec. 1964;148:441–457. doi: 10.1002/ar.1091480303. [DOI] [PubMed] [Google Scholar]
- Bollella P., Fusco G., Tortolini C., Sanzo G., Favero G., Gorton L., Antiochia R. Beyond graphene: electrochemical sensors and biosensors for biomarkers detection. Biosens. Bioelectron. 2017;89:152–166. doi: 10.1016/j.bios.2016.03.068. [DOI] [PubMed] [Google Scholar]
- Borgens R.B., Vanable J.W., Jr., Jaffe L. Small artificial currents enhance Xenopus limb regeneration. J. Exp. Zool. 1979;207:217–226. [Google Scholar]
- Borgens R.B., Vanable J.W., Jr., Jaffe L.F. Bioelectricity and regeneration. I. Initiation of frog limb regeneration by minute currents. J. Exp. Zool. 1977;200:403–416. doi: 10.1002/jez.1402000310. [DOI] [PubMed] [Google Scholar]
- Borgens R.B., Vanable J.W., Jr., Jaffe L.F. Reduction of sodium dependent stump currents disturbs urodele limb regeneration. J. Exp. Zool. 1979;209:377–386. doi: 10.1002/jez.1402090304. [DOI] [PubMed] [Google Scholar]
- Borgens R.B., Vanable J.W., Jr., Jaffe L.F. Role of subdermal current shunts in the failure of frogs to regenerate. J. Exp. Zool. 1979;209:49–56. doi: 10.1002/jez.1402090106. [DOI] [PubMed] [Google Scholar]
- Brown A.J.P., Gow N.A.R., Warris A., Brown G.D. Memory in fungal pathogens promotes immune evasion, colonisation, and infection. Trends Microbiol. 2019;27:219–230. doi: 10.1016/j.tim.2018.11.001. [DOI] [PubMed] [Google Scholar]
- Bugaj L.J., O'Donoghue G.P., Lim W.A. Interrogating cellular perception and decision making with optogenetic tools. J. Cell Biol. 2017;216:25–28. doi: 10.1083/jcb.201612094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burr H.S. Changes in the field properties of mice with transplanted tumors. Yale J. Biol. Med. 1941;13:783–788. [PMC free article] [PubMed] [Google Scholar]
- Burr H.S., Smith G.M., Strong L.C. Electrometric studies of tumors in mice induced by the external application of benzpyrene. Yale J. Biol. Med. 1940;12:711–717. [PMC free article] [PubMed] [Google Scholar]
- Burr H.S., Strong L.C., Smith G.M. Bio-electric correlates of methylcolanthrene-induced tumors in mice. Yale J. Biol. Med. 1938;10:539–544. [PMC free article] [PubMed] [Google Scholar]
- Busse S.M., McMillen P.T., Levin M. Cross-limb communication during Xenopus hindlimb regenerative response: non-local bioelectric injury signals. Development. 2018;145 doi: 10.1242/dev.164210. [DOI] [PubMed] [Google Scholar]
- Cairns D.M., Giordano J.E., Conte S., Levin M., Kaplan D.L. Ivermectin promotes peripheral nerve regeneration during wound healing. ACS Omega. 2018;3:12392–12402. doi: 10.1021/acsomega.8b01451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cervera J., Alcaraz A., Mafe S. Bioelectrical signals and ion channels in the modeling of multicellular patterns and cancer biophysics. Sci. Rep. 2016;6:20403. doi: 10.1038/srep20403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cervera J., Manzanares J.A., Mafe S. Electrical coupling in ensembles of nonexcitable cells: modeling the spatial map of single cell potentials. J. Phys. Chem. B. 2015;119:2968–2978. doi: 10.1021/jp512900x. [DOI] [PubMed] [Google Scholar]
- Cervera J., Manzanares J.A., Mafe S. Cell-cell bioelectrical interactions and local heterogeneities in genetic networks: a model for the stabilization of single-cell states and multicellular oscillations. Phys. Chem. Chem. Phys. 2018;20:9343–9354. doi: 10.1039/C8CP00648B. [DOI] [PubMed] [Google Scholar]
- Cervera J., Manzanares J.A., Mafe S., Levin M. Synchronization of bioelectric oscillations in networks of nonexcitable cells: from single-cell to multicellular states. J. Phys. Chem. B. 2019;123:3924–3934. doi: 10.1021/acs.jpcb.9b01717. [DOI] [PubMed] [Google Scholar]
- Cervera J., Meseguer S., Mafe S. The interplay between genetic and bioelectrical signaling permits a spatial regionalisation of membrane potentials in model multicellular ensembles. Sci. Rep. 2016;6:35201. doi: 10.1038/srep35201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cervera J., Meseguer S., Mafe S. Intercellular connectivity and multicellular bioelectric oscillations in nonexcitable cells: a biophysical model. ACS Omega. 2018;3:13567–13575. doi: 10.1021/acsomega.8b01514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cervera J., Pietak A., Levin M., Mafe S. Bioelectrical coupling in multicellular domains regulated by gap junctions: a conceptual approach. Bioelectrochemistry. 2018;123:45–61. doi: 10.1016/j.bioelechem.2018.04.013. [DOI] [PubMed] [Google Scholar]
- Chang Liao M.L., de Boer T.P., Mutoh H., Raad N., Richter C., Wagner E., Downie B.R., Unsold B., Arooj I., Streckfuss-Bomeke K. Sensing cardiac electrical activity with a cardiac myocyte-targeted optogenetic voltage indicator. Circ. Res. 2015;117:401–412. doi: 10.1161/CIRCRESAHA.117.306143. [DOI] [PubMed] [Google Scholar]
- Chernet B.T., Adams D.S., Lobikin M., Levin M. Use of genetically encoded, light-gated ion translocators to control tumorigenesis. Oncotarget. 2016;7:19575–19588. doi: 10.18632/oncotarget.8036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chernet B.T., Fields C., Levin M. Long-range gap junctional signaling controls oncogene-mediated tumorigenesis in Xenopus laevis embryos. Front. Physiol. 2015;5:519. doi: 10.3389/fphys.2014.00519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chernet B.T., Levin M. Transmembrane voltage potential of somatic cells controls oncogene-mediated tumorigenesis at long-range. Oncotarget. 2014;5:3287–3306. doi: 10.18632/oncotarget.1935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooke J. Scale of body pattern adjusts to available cell number in amphibian embryos. Nature. 1981;290:775–778. doi: 10.1038/290775a0. [DOI] [PubMed] [Google Scholar]
- Davidson L.A. Epithelial machines that shape the embryo. Trends Cell Biol. 2012;22:82–87. doi: 10.1016/j.tcb.2011.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deal P.E., Kulkarni R.U., Al-Abdullatif S.H., Miller E.W. Isomerically pure tetramethylrhodamine voltage reporters. J. Am. Chem. Soc. 2016;138:9085–9088. doi: 10.1021/jacs.6b05672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deng Y., Miyake T., Keene S., Josberger E.E., Rolandi M. Proton mediated control of biochemical reactions with bioelectronic pH modulation. Sci. Rep. 2016;6:24080. doi: 10.1038/srep24080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dubach J.M., Balaconis M.K., Clark H.A. Fluorescent nanoparticles for the measurement of ion concentration in biological systems. J. Vis. Exp. 2011:2896. doi: 10.3791/2896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dubach J.M., Das S., Rosenzweig A., Clark H.A. Visualizing sodium dynamics in isolated cardiomyocytes using fluorescent nanosensors. Proc. Natl. Acad. Sci. U S A. 2009;106:16145–16150. doi: 10.1073/pnas.0905909106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dubach J.M., Lim E., Zhang N., Francis K.P., Clark H. In vivo sodium concentration continuously monitored with fluorescent sensors. Integr. Biol. (Camb) 2011;3:142–148. doi: 10.1039/c0ib00020e. [DOI] [PubMed] [Google Scholar]
- Durant F., Bischof J., Fields C., Morokuma J., LaPalme J., Hoi A., Levin M. The role of early bioelectric signals in the regeneration of planarian anterior/posterior polarity. Biophys. J. 2019;116:948–961. doi: 10.1016/j.bpj.2019.01.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Durant F., Morokuma J., Fields C., Williams K., Adams D.S., Levin M. Long-term, stochastic editing of regenerative anatomy via targeting endogenous bioelectric gradients. Biophys. J. 2017;112:2231–2243. doi: 10.1016/j.bpj.2017.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Famm K., Litt B., Tracey K.J., Boyden E.S., Slaoui M. A jump-start for electroceuticals. Nature. 2013;496:159. doi: 10.1038/496159a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fang Y., Li X.M., Fang Y. Organic bioelectronics for neural interfaces. J. Mater. Chem. C. 2015;3:6424–6430. [Google Scholar]
- Feng J.F., Liu J., Zhang X.Z., Zhang L., Jiang J.Y., Nolta J., Zhao M. Guided migration of neural stem cells derived from human embryonic stem cells by an electric field. Stem Cells. 2012;30:349–355. doi: 10.1002/stem.779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferreira F., Luxardi G., Reid B., Zhao M. Early bioelectric activities mediate redox-modulated regeneration. Development. 2016;143:4582–4594. doi: 10.1242/dev.142034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferreira F., Raghunathan V., Luxardi G., Zhu K., Zhao M. Early redox activities modulate Xenopus tail regeneration. Nat. Commun. 2018;9:4296. doi: 10.1038/s41467-018-06614-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friston K., Levin M., Sengupta B., Pezzulo G. Knowing one’s place: a free-energy approach to pattern regulation. J. R. Soc. Interface. 2015;12 doi: 10.1098/rsif.2014.1383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Funk R. Ion gradients in tissue and organ biology. Biol. Syst. 2013;2:1. [Google Scholar]
- Galvani A.L. Classic - effects of artificial electricity on muscular motion. Clin. Orthop. Relat. Res. 1972;88:2–10. [PubMed] [Google Scholar]
- Gao R., Zhao S., Jiang X., Sun Y., Gao J., Borleis J., Willard S., Tang M., Cai H., Kamimura Y. A large-scale screen reveals genes that mediate electrotaxis in Dictyostelium discoideum. Sci. Signal. 2015;8:ra50. doi: 10.1126/scisignal.aab0562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia-Morales V., Manzanares J.A., Mafe S. Weakly coupled map lattice models for multicellular patterning and collective normalization of abnormal single-cell states. Phys. Rev. E. 2017;95:042324. doi: 10.1103/PhysRevE.95.042324. [DOI] [PubMed] [Google Scholar]
- Golding A., Guay J.A., Herrera-Rincon C., Levin M., Kaplan D.L. A tunable silk hydrogel device for studying limb regeneration in adult xenopus laevis. PLoS One. 2016;11:e0155618. doi: 10.1371/journal.pone.0155618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gow N.A., Morris B.M. The electric fungus. Bot. J. Scotland. 1995;47:263–277. [Google Scholar]
- Hechavarria D., Dewilde A., Braunhut S., Levin M., Kaplan D.L. BioDome regenerative sleeve for biochemical and biophysical stimulation of tissue regeneration. Med. Eng. Phys. 2010;32:1065–1073. doi: 10.1016/j.medengphy.2010.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hemmatian Z., Keene S., Josberger E., Miyake T., Arboleda C., Soto-Rodriguez J., Baneyx F., Rolandi M. Electronic control of H+ current in a bioprotonic device with Gramicidin A and Alamethicin. Nat. Commun. 2016;7:12981. doi: 10.1038/ncomms12981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hendrix M.J., Seftor E.A., Seftor R.E., Kasemeier-Kulesa J., Kulesa P.M., Postovit L.M. Reprogramming metastatic tumour cells with embryonic microenvironments. Nat. Rev. Cancer. 2007;7:246–255. doi: 10.1038/nrc2108. [DOI] [PubMed] [Google Scholar]
- Herrera-Rincon C., Golding A.S., Moran K.M., Harrison C., Martyniuk C.J., Guay J.A., Zaltsman J., Carabello H., Kaplan D.L., Levin M. Brief local application of progesterone via a wearable bioreactor induces long-term regenerative response in adult xenopus hindlimb. Cell Rep. 2018;25:1593–1609.e7. doi: 10.1016/j.celrep.2018.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang S.-C.J., Artyukhin A.B., Misra N., Martinez J.A., Stroeve P.A., Grigoropoulos C.P., Ju J.-W.W., Noy A. Carbon nanotube transistor controlled by a biological ion pump gate. Nano Lett. 2010;10:1812–1816. doi: 10.1021/nl100499x. [DOI] [PubMed] [Google Scholar]
- Humphries J., Xiong L., Liu J., Prindle A., Yuan F., Arjes H.A., Tsimring L., Suel G.M. Species-independent attraction to biofilms through electrical signaling. Cell. 2017;168:200–209.e12. doi: 10.1016/j.cell.2016.12.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Isaksson J., Kjall P., Nilsson D., Robinson N.D., Berggren M., Richter-Dahlfors A. Electronic control of Ca2+ signalling in neuronal cells using an organic electronic ion pump. Nat. Mater. 2007;6:673–679. doi: 10.1038/nmat1963. [DOI] [PubMed] [Google Scholar]
- Jaffe L.A., Robinson K.R. Membrane-potential of unfertilized sea-urchin egg. Dev. Biol. 1978;62:215–228. doi: 10.1016/0012-1606(78)90103-3. [DOI] [PubMed] [Google Scholar]
- Jaffe L.F. Electrical currents through the developing fucus egg. Proc. Natl. Acad. Sci. U S A. 1966;56:1102–1109. doi: 10.1073/pnas.56.4.1102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaffe L.F., Nuccitelli R. Electrical controls of development. Ann. Rev. Biophys. Bioeng. 1977;6:445–476. doi: 10.1146/annurev.bb.06.060177.002305. [DOI] [PubMed] [Google Scholar]
- Jakešová M., Silverå Ejneby M., Đerek V., Schmidt T., Gryszel M., Brask J., Schindl R., Simon D.T., Berggren M., Elinder F. Optoelectronic control of single cells using organic photocapacitors. Sci. Adv. 2019;5:eaav5265. doi: 10.1126/sciadv.aav5265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jayaram D.T., Luo Q., Thourson S.B., Finlay A.H., Payne C.K. Controlling the resting membrane potential of cells with conducting polymer microwires. Small. 2017;13 doi: 10.1002/smll.201700789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jimison L.H., Tria S.A., Khodagholy D., Gurfinkel M., Lanzarini E., Hama A., Malliaras G.G., Owens R.M. Measurement of barrier tissue integrity with an organic electrochemical transistor. Adv. Mater. 2012;24:5419–5423. doi: 10.1002/adma.201202612. [DOI] [PubMed] [Google Scholar]
- Jonsson A., Inal S., Uguz I., Williamson A., Kergoat L., Rivnay J., Khodagholy D., Berggren M., Bernard C., Malliaras G.G. Bioelectronic neural pixel: chemical stimulation and electrical sensing at the same site. Proc. Natl. Acad. Sci. U S A. 2016;113:E6903. doi: 10.1073/pnas.1604231113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jonsson A., Song Z., Nilsson D., Meyerson B.A., Simon D.T., Linderoth B., Berggren M. Therapy using implanted organic bioelectronics. Sci. Adv. 2015;1:e1500039. doi: 10.1126/sciadv.1500039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaisti M., Boeva Z., Koskinen J., Nieminen S., Bobacka J., Levon K. Hand-held transistor based electrical and multiplexed chemical sensing system. ACS Sensors. 2016;1:1423–1431. [Google Scholar]
- Kale V.P., Amin S.G., Pandey M.K. Targeting ion channels for cancer therapy by repurposing the approved drugs. Biochim. Biophys. Acta. 2015;1848:2747–2755. doi: 10.1016/j.bbamem.2015.03.034. [DOI] [PubMed] [Google Scholar]
- Khalifeh J.M., Zohny Z., MacEwan M., Stephen M., Johnston W., Gamble P., Zeng Y., Yan Y., Ray W.Z. Electrical stimulation and bone healing: a review of current technology and clinical applications. IEEE Rev. Biomed. Eng. 2018;11:217–232. doi: 10.1109/RBME.2018.2799189. [DOI] [PubMed] [Google Scholar]
- Kim M.S., Lee M.H., Kwon B.J., Seo H.J., Koo M.A., You K.E., Kim D., Park J.C. Control of neonatal human dermal fibroblast migration on poly(lactic-co-glycolic acid)-coated surfaces by electrotaxis. J. Tissue Eng. Regen. Med. 2017;11:862–868. doi: 10.1002/term.1986. [DOI] [PubMed] [Google Scholar]
- Kulkarni R.U., Vandenberghe M., Thunemann M., James F., Andreassen O.A., Djurovic S., Devor A., Miller E.W. In vivo two-photon voltage imaging with sulfonated rhodamine dyes. ACS Cent. Sci. 2018;4:1371–1378. doi: 10.1021/acscentsci.8b00422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar A., Brockes J.P. Nerve dependence in tissue, organ, and appendage regeneration. Trends Neurosci. 2012;35:691–699. doi: 10.1016/j.tins.2012.08.003. [DOI] [PubMed] [Google Scholar]
- Leppik L., Zhihua H., Mobini S., Thottakkattumana Parameswaran V., Eischen-Loges M., Slavici A., Helbing J., Pindur L., Oliveira K.M.C., Bhavsar M.B. Combining electrical stimulation and tissue engineering to treat large bone defects in a rat model. Sci. Rep. 2018;8:6307. doi: 10.1038/s41598-018-24892-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leppik L.P., Froemel D., Slavici A., Ovadia Z.N., Hudak L., Henrich D., Marzi I., Barker J.H. Effects of electrical stimulation on rat limb regeneration, a new look at an old model. Sci. Rep. 2015;5:18353. doi: 10.1038/srep18353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levin M. The wisdom of the body: future techniques and approaches to morphogenetic fields in regenerative medicine, developmental biology and cancer. Regen. Med. 2011;6:667–673. doi: 10.2217/rme.11.69. [DOI] [PubMed] [Google Scholar]
- Levin M., Martyniuk C.J. The bioelectric code: an ancient computational medium for dynamic control of growth and form. Biosystems. 2018;164:76–93. doi: 10.1016/j.biosystems.2017.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levin M., Pezzulo G., Finkelstein J.M. Endogenous bioelectric signaling networks: exploiting voltage gradients for control of growth and form. Annu. Rev. Biomed. Eng. 2017;19:353–387. doi: 10.1146/annurev-bioeng-071114-040647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levin M., Pietak A.M., Bischof J. Planarian regeneration as a model of anatomical homeostasis: recent progress in biophysical and computational approaches. Semin. Cell Dev Biol. 2018;87:125–144. doi: 10.1016/j.semcdb.2018.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lobikin M., Lobo D., Blackiston D.J., Martyniuk C.J., Tkachenko E., Levin M. Serotonergic regulation of melanocyte conversion: a bioelectrically regulated network for stochastic all-or-none hyperpigmentation. Sci. Signal. 2015;8:ra99. doi: 10.1126/scisignal.aac6609. [DOI] [PubMed] [Google Scholar]
- Loffler S., Melican K., Nilsson K.P.R., Richter-Dahlfors A. Organic bioelectronics in medicine. J. Intern. Med. 2017;282:24–36. doi: 10.1111/joim.12595. [DOI] [PubMed] [Google Scholar]
- Manicka S., Levin M. The Cognitive Lens: a primer on conceptual tools for analysing information processing in developmental and regenerative morphogenesis. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2019;374:20180369. doi: 10.1098/rstb.2018.0369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mathews J., Levin M. Gap junctional signaling in pattern regulation: physiological network connectivity instructs growth and form. Dev. Neurobiol. 2017;77:643–673. doi: 10.1002/dneu.22405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mathews J., Levin M. The body electric 2.0: recent advances in developmental bioelectricity for regenerative and synthetic bioengineering. Curr. Opin. Biotechnol. 2018;52:134–144. doi: 10.1016/j.copbio.2018.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLaughlin K.A., Levin M. Bioelectric signaling in regeneration: mechanisms of ionic controls of growth and form. Dev. Biol. 2018;433:177–189. doi: 10.1016/j.ydbio.2017.08.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McNamara H.M., Dodson S., Huang Y.L., Miller E.W., Sandstede B., Cohen A.E. Geometry-dependent arrhythmias in electrically excitable tissues. Cell Syst. 2018;7:359–370.e6. doi: 10.1016/j.cels.2018.08.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McNamara H.M., Zhang H., Werley C.A., Cohen A.E. Optically controlled oscillators in an engineered bioelectric tissue. Phys. Rev. X. 2016;6:031001. [Google Scholar]
- Miller C.J., Davidson L.A. The interplay between cell signalling and mechanics in developmental processes. Nat. Rev. Genet. 2013;14:733–744. doi: 10.1038/nrg3513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mintz B., Illmensee K. Normal genetically mosaic mice produced from malignant teratocarcinoma cells. Proc. Natl. Acad. Sci. U S A. 1975;72:3585–3589. doi: 10.1073/pnas.72.9.3585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Misra N., Martinez J.A., Huang S.C.J., Wang Y.M., Stroeve P., Grigoropoulos C.P., Noy A. Bioelectronic silicon nanowire devices using functional membrane proteins. Proc. Natl. Acad. Sci. U S A. 2009;106:13780–13784. doi: 10.1073/pnas.0904850106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyake T., Josberger E.E., Keene S., Deng Y.X., Rolandi M. An enzyme logic bioprotonic transducer. APL Mater. 2015;3 [Google Scholar]
- Mobini S., Leppik L., Thottakkattumana Parameswaran V., Barker J.H. In vitro effect of direct current electrical stimulation on rat mesenchymal stem cells. PeerJ. 2017;5:e2821. doi: 10.7717/peerj.2821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore D., Walker S.I., Levin M. Cancer as a disorder of patterning information: computational and biophysical perspectives on the cancer problem. Converg. Sci. Phys. Oncol. 2017;3:043001. [Google Scholar]
- Mutoh H., Akemann W., Knopfel T. Genetically engineered fluorescent voltage reporters. ACS Chem. Neurosci. 2012;3:585–592. doi: 10.1021/cn300041b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Navis A., Bagnat M. Developing pressures: fluid forces driving morphogenesis. Curr. Opin. Genet. Dev. 2015;32:24–30. doi: 10.1016/j.gde.2015.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noy A. Bionanoelectronics. Adv. Mater. 2011;23:807–820. doi: 10.1002/adma.201003751. [DOI] [PubMed] [Google Scholar]
- Nuccitelli R., Jaffe L. Ionic components of current pulses which cross growing tips of pelvetia embryos. Biophys. J. 1975;15:A123. [Google Scholar]
- Nuccitelli R., Jaffe L.F. Ionic components of current pulses generated by developing fucoid eggs. Dev. Biol. 1976;49:518–531. doi: 10.1016/0012-1606(76)90193-7. [DOI] [PubMed] [Google Scholar]
- Nuccitelli R., Poo M.M., Jaffe L.F. Amebas drive electrical currents through themselves. J. Cell Biol. 1975;67:A311. [Google Scholar]
- Nuccitelli R., Poo M.M., Jaffe L.F. Relations between ameboid movement and membrane-controlled electrical currents. J. Gen. Physiol. 1977;69:743–763. doi: 10.1085/jgp.69.6.743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oliveira K.M.C., Barker J.H., Berezikov E., Pindur L., Kynigopoulos S., Eischen-Loges M., Han Z., Bhavsar M.B., Henrich D., Leppik L. Electrical stimulation shifts healing/scarring towards regeneration in a rat limb amputation model. Sci. Rep. 2019;9:11433. doi: 10.1038/s41598-019-47389-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oviedo N.J., Morokuma J., Walentek P., Kema I.P., Gu M.B., Ahn J.M., Hwang J.S., Gojobori T., Levin M. Long-range neural and gap junction protein-mediated cues control polarity during planarian regeneration. Dev. Biol. 2010;339:188–199. doi: 10.1016/j.ydbio.2009.12.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Owens R.M., Malliaras G.G. Organic electronics at the interface with biology. MRS Bull. 2011;35:449–456. [Google Scholar]
- Ozkucur N., Quinn K.P., Pang J.C., Du C., Georgakoudi I., Miller E., Levin M., Kaplan D.L. Membrane potential depolarization causes alterations in neuron arrangement and connectivity in cocultures. Brain Behav. 2015;5:24–38. doi: 10.1002/brb3.295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pai V.P., Aw S., Shomrat T., Lemire J.M., Levin M. Transmembrane voltage potential controls embryonic eye patterning in Xenopus laevis. Development. 2012;139:313–323. doi: 10.1242/dev.073759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pai V.P., Lemire J.M., Chen Y., Lin G., Levin M. Local and long-range endogenous resting potential gradients antagonistically regulate apoptosis and proliferation in the embryonic CNS. Int. J. Dev. Biol. 2015;59:327–340. doi: 10.1387/ijdb.150197ml. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pai V.P., Lemire J.M., Pare J.F., Lin G., Chen Y., Levin M. Endogenous gradients of resting potential instructively pattern embryonic neural tissue via notch signaling and regulation of proliferation. J. Neurosci. 2015;35:4366–4385. doi: 10.1523/JNEUROSCI.1877-14.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pai V.P., Martyniuk C.J., Echeverri K., Sundelacruz S., Kaplan D.L., Levin M. Genome-wide analysis reveals conserved transcriptional responses downstream of resting potential change in Xenopus embryos, axolotl regeneration, and human mesenchymal cell differentiation. Regeneration (Oxf) 2016;3:3–25. doi: 10.1002/reg2.48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pai V.P., Pietak A., Willocq V., Ye B., Shi N.Q., Levin M. HCN2 Rescues brain defects by enforcing endogenous voltage pre-patterns. Nat. Commun. 2018;9:998. doi: 10.1038/s41467-018-03334-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pai V.P., Willocq V., Pitcairn E.J., Lemire J.M., Pare J.F., Shi N.Q., McLaughlin K.A., Levin M. HCN4 ion channel function is required for early events that regulate anatomical left-right patterning in a nodal and lefty asymmetric gene expression-independent manner. Biol. Open. 2017;6:1445–1457. doi: 10.1242/bio.025957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palacios-Prado N., Bukauskas F.F. Heterotypic gap junction channels as voltage-sensitive valves for intercellular signaling. Proc. Natl. Acad. Sci. U S A. 2009;106:14855–14860. doi: 10.1073/pnas.0901923106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pavlov V.A., Tracey K.J. Bioelectronic medicine: updates, challenges and paths forward. Bioelectron. Med. 2019;5:1. doi: 10.1186/s42234-019-0018-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perkins M.L., Benzinger D., Arcak M., Khammash M. Cell-in-the-loop pattern formation with optogenetically emulated cell-to-cell signaling. bioRxiv. 2019:679597. doi: 10.1038/s41467-020-15166-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pezzulo G., Levin M. Re-membering the body: applications of computational neuroscience to the top-down control of regeneration of limbs and other complex organs. Integr. Biol. (Camb) 2015;7:1487–1517. doi: 10.1039/c5ib00221d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pezzulo G., Levin M. Top-down models in biology: explanation and control of complex living systems above the molecular level. J. R. Soc. Interface. 2016;13 doi: 10.1098/rsif.2016.0555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pietak A., Levin M. Exploring instructive physiological signaling with the bioelectric tissue simulation engine (BETSE) Front. Bioeng. Biotechnol. 2016;4:55. doi: 10.3389/fbioe.2016.00055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pietak A., Levin M. Bioelectric gene and reaction networks: computational modelling of genetic, biochemical and bioelectrical dynamics in pattern regulation. J. R. Soc. Interface. 2017;14 doi: 10.1098/rsif.2017.0425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pitcairn E., Harris H., Epiney J., Pai V.P., Lemire J.M., Ye B., Shi N.Q., Levin M., McLaughlin K.A. Coordinating heart morphogenesis: a novel role for Hyperpolarization-activated cyclic nucleotide-gated (HCN) channels during cardiogenesis in Xenopus laevis. Commun. Integr. Biol. 2017;10:e1309488. doi: 10.1080/19420889.2017.1309488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pitsalidis C., Ferro M.P., Iandolo D., Tzounis L., Inal S., Owens R.M. Transistor in a tube: a route to three-dimensional bioelectronics. Sci. Adv. 2018;4:eaat4253. doi: 10.1126/sciadv.aat4253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poxson D.J., Gabrielsson E.O., Bonisoli A., Linderhed U., Abrahamsson T., Matthiesen I., Tybrandt K., Berggren M., Simon D.T. Capillary-fiber based electrophoretic delivery device. ACS Appl. Mater. Interfaces. 2019;11:14200–14207. doi: 10.1021/acsami.8b22680. [DOI] [PubMed] [Google Scholar]
- Prindle A., Liu J., Asally M., Ly S., Garcia-Ojalvo J., Suel G.M. Ion channels enable electrical communication in bacterial communities. Nature. 2015;527:59–63. doi: 10.1038/nature15709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rana M.A., Yao N.S., Mukhopadhyay S., Zhang F.M., Warren E., Payne C. Modeling the effect of nanoparticles & the bistability of transmembrane potential in non-excitable cells. P. Amer. Contr. Conf. 2016:400–405. https://ieeexplore.ieee.org/document/752494 [Google Scholar]
- Reid B., Graue-Hernandez E.O., Mannis M.J., Zhao M. Modulating endogenous electric currents in human corneal wounds–a novel approach of bioelectric stimulation without electrodes. Cornea. 2011;30:338–343. doi: 10.1097/ICO.0b013e3181f7f2de. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reid B., Zhao M. The electrical response to injury: molecular mechanisms and wound healing. Adv. Wound Care (New Rochelle) 2014;3:184–201. doi: 10.1089/wound.2013.0442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ren X., Sun H., Liu J., Guo X., Huang J., Jiang X., Zhang Y., Huang Y., Fan D., Zhang J. Keratinocyte electrotaxis induced by physiological pulsed direct current electric fields. Bioelectrochemistry. 2019;127:113–124. doi: 10.1016/j.bioelechem.2019.02.001. [DOI] [PubMed] [Google Scholar]
- Rim T., Kim K., Cho H., Jeong W., Yoon J.-S., Kim Y., Meyyappan M., Baek C.-K. Electrical characteristics of doped silicon nanowire channel field-effect transistor biosensors. IEEE Sensors J. 2017;17:667–673. [Google Scholar]
- Rivnay J., Inal S., Collins B.A., Sessolo M., Stavrinidou E., Strakosas X., Tassone C., Delongchamp D.M., Malliaras G.G. Structural control of mixed ionic and electronic transport in conducting polymers. Nat. Commun. 2016;7:11287. doi: 10.1038/ncomms11287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rivnay J., Inal S., Salleo A., Owens R.M., Berggren M., Malliaras G.G. Organic electrochemical transistors. Nat. Rev. Mater. 2018;3:17086. [Google Scholar]
- Rivnay J., Owens R.M., Malliaras G.G. The rise of organic bioelectronics. Chem. Mater. 2014;26:679–685. [Google Scholar]
- Robinson K.R., Jaffe L.A., Brawley S.H. Electro-physiological properties of fucoid algal eggs during fertilization. J. Cell Biol. 1981;91:A179. [Google Scholar]
- Robinson K.R., Jaffe L.F. Ion movements in a developing fucoid egg. Dev. Biol. 1973;35:349–361. doi: 10.1016/0012-1606(73)90029-8. [DOI] [PubMed] [Google Scholar]
- Robinson K.R., Jaffe L.F. Calcium gradients and egg polarity. J. Cell Biol. 1976;70:A37. [Google Scholar]
- Saló E., Abril J.F., Adell T., Cebrià F., Eckelt K., Fernandez-Taboada E., Handberg-Thorsager M., Iglesias M., Molina M.D., Rodriguez-Esteban G. Planarian regeneration: achievements and future directions after 20 years of research. Int. J. Dev. Biol. 2009;53:1317–1327. doi: 10.1387/ijdb.072414es. [DOI] [PubMed] [Google Scholar]
- Sanjuan-Alberte P., Alexander M.R., Hague R.J.M., Rawson F.J. Electrochemically stimulating developments in bioelectronic medicine. Bioelectron. Med. 2018;4:1. doi: 10.1186/s42234-018-0001-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanjuan-Alberte P., Rawson F.J. Engineering the spark into bioelectronic medicine. Ther. Deliv. 2019;10:139–142. doi: 10.4155/tde-2019-0008. [DOI] [PubMed] [Google Scholar]
- Selberg J., Gomez M., Rolandi M. The potential for convergence between synthetic biology and bioelectronics. Cell Syst. 2018;7:231–244. doi: 10.1016/j.cels.2018.08.007. [DOI] [PubMed] [Google Scholar]
- Shin E.H., Li Y., Kumar U., Sureka H.V., Zhang X., Payne C.K. Membrane potential mediates the cellular binding of nanoparticles. Nanoscale. 2013;5:5879–5886. doi: 10.1039/c3nr01667f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simon D.T., Gabrielsson E.O., Tybrandt K., Berggren M. Organic bioelectronics: bridging the signaling gap between biology and technology. Chem. Rev. 2016;116:13009–13041. doi: 10.1021/acs.chemrev.6b00146. [DOI] [PubMed] [Google Scholar]
- Someya T., Bao Z.N., Malliaras G.G. The rise of plastic bioelectronics. Nature. 2016;540:379–385. doi: 10.1038/nature21004. [DOI] [PubMed] [Google Scholar]
- Soto-Rodriguez J., Hemmatian Z., Josberger E.E., Rolandi M., Baneyx F. A palladium-binding deltarhodopsin for light-activated conversion of protonic to electronic currents. Adv. Mater. 2016;28:6581–6585. doi: 10.1002/adma.201600222. [DOI] [PubMed] [Google Scholar]
- St-Pierre F., Chavarha M., Lin M.Z. Designs and sensing mechanisms of genetically encoded fluorescent voltage indicators. Curr. Opin. Chem. Biol. 2015;27:31–38. doi: 10.1016/j.cbpa.2015.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strakosas X., Bongo M., Owens R.M. The organic electrochemical transistor for biological applications. J. Appl. Polym. Sci. 2015;132 [Google Scholar]
- Strakosas X., Huerta M., Donahue M.J., Hama A., Pappa A.-M., Ferro M., Ramuz M., Rivnay J., Owens R.M. Catalytically enhanced organic transistors for in vitro toxicology monitoring through hydrogel entrapment of enzymes. J. Appl. Polym. Sci. 2017;134 [Google Scholar]
- Strilic B., Eglinger J., Krieg M., Zeeb M., Axnick J., Babal P., Muller D.J., Lammert E. Electrostatic cell-surface repulsion initiates lumen formation in developing blood vessels. Curr. Biol. 2010;20:2003–2009. doi: 10.1016/j.cub.2010.09.061. [DOI] [PubMed] [Google Scholar]
- Sullivan K.G., Levin M. Inverse drug screening of bioelectric signaling and neurotransmitter roles: illustrated using a xenopus tail regeneration assay. Cold Spring Harb. Protoc. 2018;2018 doi: 10.1101/pdb.prot099937. pdb prot099937. [DOI] [PubMed] [Google Scholar]
- Sundelacruz S., Levin M., Kaplan D.L. Membrane potential controls adipogenic and osteogenic differentiation of mesenchymal stem cells. PLoS One. 2008;3:e3737. doi: 10.1371/journal.pone.0003737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sundelacruz S., Levin M., Kaplan D.L. Depolarization alters phenotype, maintains plasticity of predifferentiated mesenchymal stem cells. Tissue Eng. A. 2013;19:1889–1908. doi: 10.1089/ten.tea.2012.0425.rev. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sundelacruz S., Li C., Choi Y.J., Levin M., Kaplan D.L. Bioelectric modulation of wound healing in a 3D in vitro model of tissue-engineered bone. Biomaterials. 2013;34:6695–6705. doi: 10.1016/j.biomaterials.2013.05.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tian B., Lieber C.M. Nanowired bioelectric interfaces. Chem. Rev. 2019;119:9136–9152. doi: 10.1021/acs.chemrev.8b00795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tian B., Xu S., Rogers J.A., Cestellos-Blanco S., Yang P., Carvalho-de-Souza J.L., Bezanilla F., Liu J., Bao Z., Hjort M. Roadmap on semiconductor–cell biointerfaces. Phys. Biol. 2018;15:031002. doi: 10.1088/1478-3975/aa9f34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tian B.Z., Cohen-Karni T., Qing Q., Duan X.J., Xie P., Lieber C.M. Three-dimensional, flexible nanoscale field-effect transistors as localized bioprobes. Science. 2010;329:830–834. doi: 10.1126/science.1192033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tseng A., Levin M. Cracking the bioelectric code: Probing endogenous ionic controls of pattern formation. Commun. Integr. Biol. 2013;6:1–8. doi: 10.4161/cib.22595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tseng A.S., Beane W.S., Lemire J.M., Masi A., Levin M. Induction of vertebrate regeneration by a transient sodium current. J. Neurosci. 2010;30:13192–13200. doi: 10.1523/JNEUROSCI.3315-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tuszynski J., Tilli T.M., Levin M. Ion channel and neurotransmitter modulators as electroceutical approaches to the control of cancer. Curr. Pharm. Des. 2017;23:4827–4841. doi: 10.2174/1381612823666170530105837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vandenberg L.N., Morrie R.D., Adams D.S. V-ATPase-dependent ectodermal voltage and pH regionalization are required for craniofacial morphogenesis. Dev. Dyn. 2011;240:1889–1904. doi: 10.1002/dvdy.22685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- von Dassow M., Davidson L.A. Physics and the canalization of morphogenesis: a grand challenge in organismal biology. Phys. Biol. 2011;8:045002. doi: 10.1088/1478-3975/8/4/045002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang E.T., Zhao M. Regulation of tissue repair and regeneration by electric fields. Chin. J. Traumatol. 2010;13:55–61. [PubMed] [Google Scholar]
- Warren E.A., Payne C.K. Cellular binding of nanoparticles disrupts the membrane potential. RSC Adv. 2015;5:13660–13666. doi: 10.1039/C4RA15727C. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weihua Z., Tsan R., Schroit A.J., Fidler I.J. Apoptotic cells initiate endothelial cell sprouting via electrostatic signaling. Cancer Res. 2005;65:11529–11535. doi: 10.1158/0008-5472.CAN-05-2718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weisenseel M.H., Nuccitelli R., Jaffe L.F. Large electrical currents traverse growing pollen tubes. J. Cell Biol. 1975;66:556–567. doi: 10.1083/jcb.66.3.556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williamson A., Rivnay J., Kergoat L., Jonsson A., Inal S., Uguz I., Ferro M., Ivanov A., Sjostrom T.A., Simon D.T. Controlling epileptiform activity with organic electronic ion pumps. Adv. Mater. 2015;27:3138–3144. doi: 10.1002/adma.201500482. [DOI] [PubMed] [Google Scholar]
- Zhang A., Lieber C.M. Nano-bioelectronics. Chem. Rev. 2016;116:215–257. doi: 10.1021/acs.chemrev.5b00608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao M. Electrical fields in wound healing—an overriding signal that directs cell migration. Semin. Cell Dev. Biol. 2009;20:674–682. doi: 10.1016/j.semcdb.2008.12.009. [DOI] [PubMed] [Google Scholar]
- Zhou W., Dai X.C., Lieber C.M. Advances in nanowire bioelectronics. Rep. Prog. Phys. 2017;80:016701. doi: 10.1088/0034-4885/80/1/016701. [DOI] [PubMed] [Google Scholar]
- Zhu H., Li L., Zhou W., Shao Z., Chen X. Advances in non-enzymatic glucose sensors based on metal oxides. J. Mater. Chem. B. 2016;4:7333–7349. doi: 10.1039/c6tb02037b. [DOI] [PubMed] [Google Scholar]
- Zhu K., Takada Y., Nakajima K., Sun Y., Jiang J., Zhang Y., Zeng Q., Takada Y., Zhao M. Expression of integrins to control migration direction of electrotaxis. FASEB J. 2019;33:9131–9141. doi: 10.1096/fj.201802657R. [DOI] [PMC free article] [PubMed] [Google Scholar]