Abstract
Ibrutinib has revolutionized the treatment of chronic lymphoid malignancies. Despite its success, ibrutinib has been linked with several reports of invasive fungal infections. We present a case of CNS aspergillosis in a CLL patient on first line ibrutinib therapy. We summarize existing case reports and case series of invasive aspergillosis in patients on ibrutinib, the pathogenesis of invasive aspergillosis, and discuss the clinical controversies regarding anti-fungal prophylaxis in this population.
Keywords: Aspergillus, Ibrutinib, Central nervous system (CNS), Chronic lymphocytic leukemia (CLL), Invasive fungal infection (IFI)
1. Introduction
Ibrutinib, an irreversible Bruton's tyrosine kinase (Btk) inhibitor, has dramatically improved progression-free and overall survival in patients with a variety of lymphoid malignancies [1,2]. In the midst of its therapeutic success, however, several cases of invasive fungal infections (IFI) in patients receiving ibrutinib have emerged [[3], [4], [5]]. We present a case of central nervous system (CNS) aspergillosis in a patient with chronic lymphocytic leukemia (CLL) on ibrutinib who had not received any previous or concurrent corticosteroid, chemo- or immunotherapy and was successfully managed with this life-threatening CNS infection.
2. Case
A 62-year-old man with newly diagnosed CLL who started ibrutinib therapy one month ago (day 0) presented with fevers, aphasia and a new brain lesion.
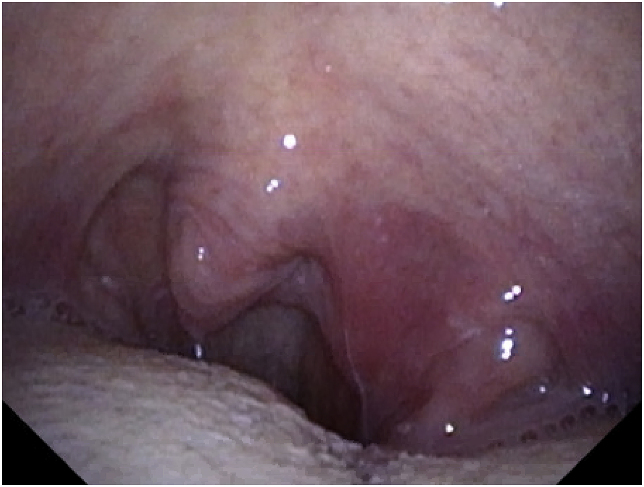
The patient was treatment-naïve prior to starting the ibrutinib and had no significant past medical history up until his CLL diagnosis. He was doing well on this treatment until he developed a sore throat and neck swelling on day +23. He was seen by an otolaryngologist at that time who performed laryngoscopy and visualized a left peritonsillar abscess (Fig. 1). An aspiration of the left peritonsillar abscess was attempted, but no purulence was aspirated. Post-procedure, the patient was instructed to take amoxicillin-clavulanate for 10 days.
Fig. 1.
Left peritonsillar abscess.
When nearing the end of the antibiotic therapy, the patient began to have intermittent fevers and progressive word finding difficulty (day +34). His symptoms progressed to confusion and profound expressive aphasia (day +36). He was subsequently admitted to the hospital on day +36 for work-up. Upon arrival to Duke University Medical Center, the patient was poorly cooperative with the exam and was confused. He was unable to state his name, location or the date. His cranial nerves were intact, and his strength symmetric throughout. However, the patient exhibited an unsteady gait, moderate dysarthria and significant word finding difficulties. He had difficulty reading words but recognized pictures. A non-contrast head computed tomography (CT) demonstrated a large 3.2 cm (cm) hyperdense mass in the left cerebral hemisphere with surrounding vasogenic edema, a mass effect on the left lateral ventricle and small hyperdense masses in the right cerebral hemisphere. The patient was started on dexamethasone and levetiracetam. Laboratory analysis was remarkable for a mild leukopenia (WBC 3.2 × 10^9/L) (Table 1). Noninvasive infectious markers were unrevealing (Table 2).
Table 1.
Laboratory tests.
| Patient Values | Reference Range | |
|---|---|---|
| Complete blood count | ||
| White blood cells | 3.2 × 10^9/L | 3.2–9.8 × 10^9/L |
| Neutrophils (%) | 60 | 37–80 |
| Bands (%) | 1 | 0–6 |
| Basophils (%) | 1 | 0–2 |
| Eosinophils (%) | 1 | 0–7 |
| Lymphocytes (%) | 34 | 10–50 |
| Monocytes (%) | 3 | 0–12 |
| Hemoglobin (g/dL) | 11.6 | 13.7–17.3 |
| Mean corpuscular volume (fL) | 229 | 80–98 |
| Hematocrit (%) | 32.3 | 39–49 |
| Platelets (x10^9/L) | 175 | 150–450 |
| Basic metabolic panel | ||
| Sodium (mmol/L) | 136 | 135–145 |
| Potassium (mmol/L) | 4.1 | 3.5–5.0 |
| Chloride (mmol/L) | 101 | 98–108 |
| Carbon dioxide (mmol/L) | 26 | 21–30 |
| Urea nitrogen (mg/dL) | 11 | 7–20 |
| Creatinine (mg/dL) | 0.6 | 0.6–1.3 |
| Glucose (mg/dL) | 105 | 70–140 |
| Hepatic panel | ||
| Aspartate aminotransferase (U/L) | 20 | 15–41 |
| Alanine aminotransferase (U/L) | 16 | 17–63 |
| Alkaline phosphatase (U/L) | 51 | 24–110 |
| Total bilirubin (mg/dL) | 0.6 | 0.4–1.5 |
| Albumin (g/dL) | 4.0 | 3.5–4.8 |
Table 2.
Microbiological studies.
| Test | Result |
|---|---|
| Blood cultures | No growth |
| Serum Aspergillus galactomannan | <0.5 index (not detected) |
| Serum beta-D glucan | <31 pg/mL (not detected) |
| Serum cryptococcal antigen | Negative |
| HIV antibody/antigen | Negative |
| Toxoplasma IgG | Negative |
| Toxoplasma IgM | Negative |
| Toxoplasma PCR (whole blood) | Negative |
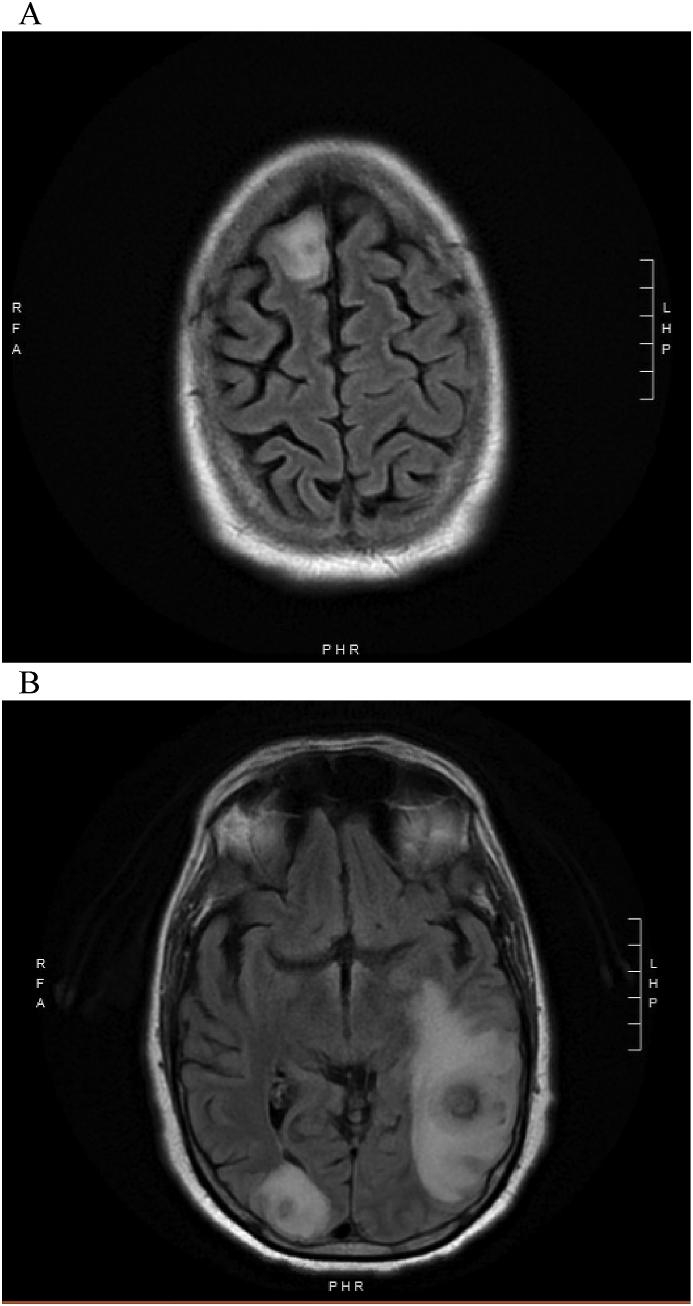
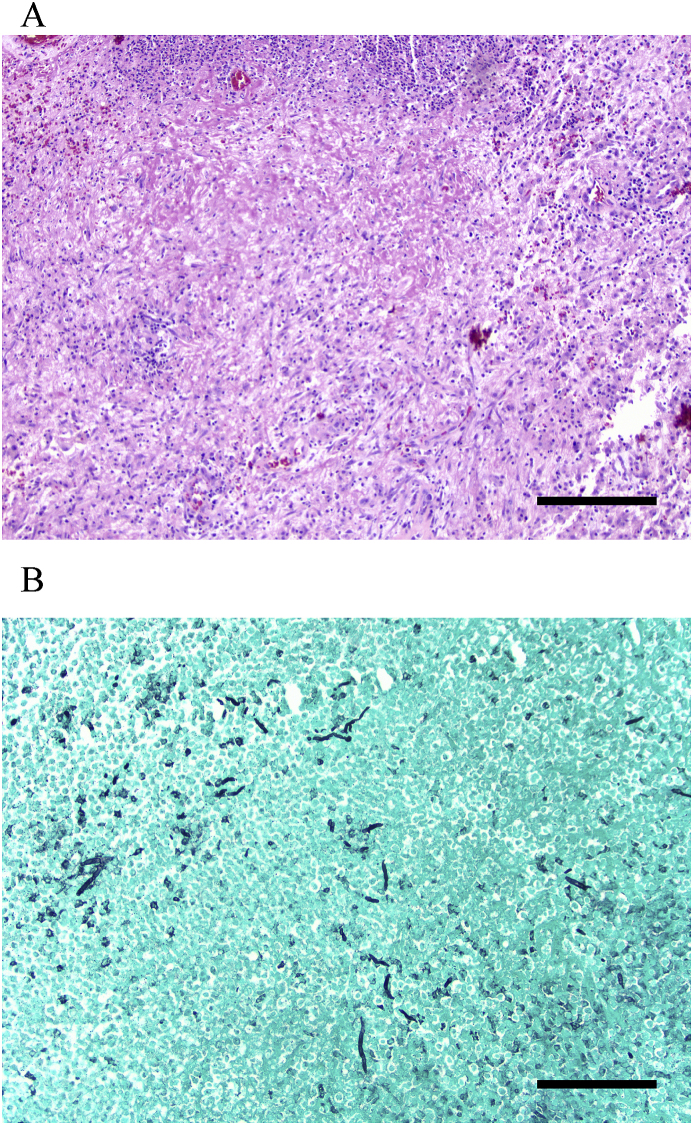
A magnetic resonance imaging (MRI) of the brain with and without contrast demonstrated three enhancing mass lesions with surrounding vasogenic edema. Specifically, there was a 3.2 cm round heterogeneous mass noted in the left parieto-temporal region, a 12 mm (mm) mass in the right occipital lobe and an oval shaped 8 mm mass in the right frontal lobe (Fig. 2A–B). The patient subsequently underwent brain biopsy (day +41), and the pathology revealed necrosis, acute inflammation and granulation tissue consistent with an abscess and a Gomori methenamine-silver (GMS) stain highlighting septate hyphae [Fig. 3A and B]. Cultures from the biopsy grew Aspergillus fumigatus species complex (identification based on morphologic criteria) on day +43. Notably, a chest CT was also obtained and demonstrated a new spiculated lung nodule within the right lower lobe measuring 1.5 × 1.7 cm with surrounding ground glass opacity.
Fig. 2.
A–B: MRI Axial T2 Flair of the brain at the time of diagnosis of CNS aspergillosis.
Fig. 3.
A–B: Pathology slides from the brain biopsy. H&E stain demonstrates necrosis, acute inflammation and granulation tissue, consistent with an abscess (3A); GMS stain highlights fungal hyphae (3B). Scale bar = 200 μm in (A) and (B).
The patient was treated with 1 year of voriconazole therapy (300mg by mouth every 12 hours) with brief combination echinocandin (micafungin 100mg intravenously every 24 hours) upfront for 2 weeks. Ibrutinib was discontinued upon presentation of symptoms and was held for the entire duration of aspergillosis treatment. Voriconazole dosing was adjusted based on trough levels which were obtained every 7–14 days throughout his treatment. Overall his voriconazole troughs were within the desired target range.
Serial CT and MRI imaging of the chest and brain, respectively, demonstrated an excellent response to antifungal therapy with imaging at the close of 1 year of therapy with no suggestion of residual infection. Fortunately, the patient's underlying CLL remained stable during this period, and he did not necessitate additional therapy with the exception of infrequent doses of granulocyte colony stimulating factor for intermittent mild neutropenia. However, he experienced multiple toxicities on voriconazole therapy including gastrointestinal disturbances, significant photosensitivity and nail changes. The patient also had a history of non-melanoma skin cancers; hence, the decision was made to transition to isavuconazole for secondary fungal prophylaxis after completion of 1 year of voriconazole therapy. The transition to isavuconazole also occurred alongside impending plans by the oncology team to initiate venetoclax as his next line of CLL therapy.
3. Discussion
We present a case of CNS aspergillosis in a 62-year-old man with CLL who had initiated ibrutinib less than one month prior to the diagnosis. To our knowledge, we present the first case of CNS aspergillosis in a patient on ibrutinib monotherapy who had not received prior corticosteroid, chemo- or biologic therapy for a chronic lymphoid malignancy.
Since the introduction of ibrutinib to treat hematologic malignancies, multiple reports of IFIs emerged prompting larger studies to investigate the incidence of IFI with ibrutinib in the hematologic malignancy population. The prevalence reported in these studies ranged from 2.4% [6] to 4.2% [7], and the majority of these IFIs were due to Aspergillus with a trend towards CNS involvement.
Aspergillus activates Btk in macrophages which in turn leads to downstream macrophage calcineurin-NFAT signaling to recruit neutrophils to the site of the Aspergillus infection [8]. However, when Btk is inhibited such as in the setting of ibrutinib, the downstream NFAT and NFΚβ response is impaired, resulting in the lack of neutrophil recruitment [9]. Additionally, Blez, et al. have found that neutrophils harvested from patients treated with ibrutinib have significantly reduced neutrophil oxidative burst and absent IL-8 secretion in the setting of Aspergillus stimulation [10]. With an ibrutinib-impaired impaired innate immune system, the host cannot contain or clear Aspergillus infection, and the hyphae may spread and invade other organs via hematogenous dissemination.
Most cases of IFI in patients on ibrutinib therapy, including the one discussed in this report, have presented within weeks after starting ibrutinib [Table 3]. It is possible that Aspergillus may infect the CNS through hematogenous dissemination or direct extension (e.g. secondary to sinusitis, mastoiditis, trauma or surgery). Importantly, CNS infection is not common in invasive aspergillosis, only occurring in 2.7–6% of cases [11,12]. In patients on ibrutinib, however, a striking proportion (40–41%) of invasive Aspergillus cases involve the CNS [6,13]. The reasoning for this apparent dichotomy is incompletely understood; however, there are a few possible explanations for this [1]: Aspergillus species produce mycotoxins that can alter the blood-brain barrier, destroy neural cells, and evade phagocytosis and conidial opsonization and propagate CNS infection [2,14] ibrutinib-affected macrophages may transmit Aspergillus spores across the blood-brain barrier establishing CNS infection [6]; and [3] given its good CNS penetration, ibrutinib may inhibit CNS macrophages or microglial cells [5]. Further research is needed to understand the neural disposition of invasive aspergillosis in patients treated with ibrutinib.
Table 3.
Summary of CNS aspergillosis cases in patients treated with ibrutinib.
| Study | Patient | Prior Oncologic Treatment | Aspergillus Species | Time from Ibrutinib Initiation to Diagnosis of CNS Aspergillosis (months) | Antifungal Treatment | Patient Outcome at Time of Publication |
|---|---|---|---|---|---|---|
| Gaye et al. [17] | Patient 1: CLL | Obinituzumab, chlorambucil, rituximab, bendamustine | A. fumigatus | <1 | Voriconazole; ibrutinib discontinued | Alive |
| Patient 2: CLL | Corticosteroids | A. fumigatus | 2 | Voriconazole and amphotericin B; ibrutinib continued | Alive | |
| Ruchlemer et al. [18] | Patient 1: CLL | Corticosteroids | NR | 1 | NR | Dead |
| Patient 2: CLL | Corticosteroids | NR | 2 | LAMB and voriconazole; ibrutinib discontinued | Alive | |
| Patient 3: CLL | Corticosteroids | NR | 2 | NR | Alive | |
| Jain et al. [19] | CLL | Rituximab | NR | 2 | NR | Deceased |
| Baron et al. [3] | CLL | Fludarabine, CP, rituximab | A. nidulans | <1 | Voriconazole; ibrutinib discontinued | Alive |
| Beresford et al. [20] | CLL | Fludarabine, CP, bendamustine, rituximab | A. fumigatus | 12 | Voriconazole subsequently switched to posaconazole; ibrutinib discontinued | Alive |
| Ghez et al. [13] | 11 patients | Median number of prior therapies: 2 | NR | Median 3 monthsb | NR | NR: Total mortality 52% for all patients with IFI |
| Ruchlemer et al. [6] | 9 patients; CLLa or NHL | Median number of prior therapies: 3 (no patients were treatment-naïve prior to starting ibrutinib) | NR | Median 1.5 monthsb | NR | NR: Total mortality 69% for all patients with IFI (including those with non-CNS disease) |
| Lionakis et al. [14] | Patient 1: PCNSL | Corticosteroids | A. fumigatus | <1 | NR | Deceased |
| Patient 2: PCNSL | 4 prior therapies, Corticosteroids, on TEDDi-R | A. fumigatus | <1 | Deceased | ||
| Patient 3: PCNSL | 4 cycles TEDDi-R | A. fumigatus | 3 | Deceased | ||
| Patient 4: PCNSL | Corticosteroids; 1 cycle TEDDi-R | A. fumigatus | <1 | Alive | ||
| Present Case | 62yo M CLL | None | A. fumigatus | <1 | Voriconazole, ibrutinib discontinued | Alive |
CLL chronic lymphocytic leukemia; NR not reported; LAMB liposomal amphotericin B; NHL Non-Hodgkin Lymphoma; PCNSL Primary CNS Lymphoma; CP cyclophosphamide, TEDDi-R Temozolomide, Etoposide, Doxil, Dexamethasone, Ibrutinib, and Rituximab.
2 of the patients included in this study were also included in reference [21].
Analysis included all patients with IFI on ibrutinib regardless of site of infection.
Notably, A. fumigatus is the predominant fungal species involved in proven CNS infection (33.3%–82.6%) [12,15] and was the causative pathogen in the present case. Further investigation is needed into the virulence of these fungi as to how they invade the CNS so quickly with Btk inhibition by ibrutinib, and how virulence may be associated with adverse clinical outcomes.
Our case adds to the accumulating body of evidence that ibrutinib carries a substantial increased risk of invasive aspergillosis. It should be emphasized that despite our patient's positive clinical outcome, CNS aspergillosis is frequently fatal even with mold-active therapy [15]. The attributable mortality rate of CNS aspergillosis in patients taking ibrutinib is a devastating 52–69% [6]. The precise treatment strategy and its length remain uncertain and empirical. Given these grave mortality statistics, two important clinical questions arose from our patient's case. First, should ibrutinib should be held during the entire treatment of the CNS aspergillosis? While there is little evidence to guide such decision making, it is often the practice to do so [6]. We immediately stopped ibrutinib in our patient in hopes of optimizing his innate immune system's capacity to control his infection. We continued to hold it for the entire duration of his antifungal treatment, and fortunately, his CLL remained stable. Second, should patients on ibrutinib receive primary antifungal prophylaxis? This question is nuanced in that antifungal inhibition of the cytochrome P450 isoenzyme CYP3A4 can raise levels of ibrutinib [16] and prove challenging to titrate and monitor. Future studies are needed to identify patients on ibrutinib who are at greatest risk for IFI and should receive primary antifungal prophylaxis.
Declaration of competing interest
JP discloses the following consulting/advisory committee/research grants: Astellas, Merck, F2G, Cidara, Pfizer, Scynexis, Viamet, Amplyx, Vical, Matinas, Minnetronix. DB discloses the following consulting/advisory committee/research grants: AbbVie, ArQule, AstraZeneca, BeiGene, DTRM, Genentech, Juno, MEI Pharma, Novartis, Pharmacyclics, Teva, TG Therapeutics, Tolero, Verastem.
JM, EE, SW and JS have nothing to disclose.
Acknowledgements
The authors are grateful to the patient and his family for allowing us to participate in his care and for their permission to publish his case in the literature. EME is supported by a training grant from National Institute of Allergy and Infectious Diseases (NIAID; Grant Number 5T32AI100851).
References
- 1.Burger J.A., Tedeschi A., Barr P.M., Robak T., Owen C., Ghia P. Ibrutinib as initial therapy for patients with chronic lymphocytic leukemia. N. Engl. J. Med. 2015;373(25):2425–2437. doi: 10.1056/NEJMoa1509388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chanan-Khan A., Cramer P., Demirkan F., Fraser G., Silva R.S., Grosicki S. Ibrutinib combined with bendamustine and rituximab compared with placebo, bendamustine, and rituximab for previously treated chronic lymphocytic leukaemia or small lymphocytic lymphoma (HELIOS): a randomised, double-blind, phase 3 study. Lancet Oncol. 2016;17(2):200–211. doi: 10.1016/S1470-2045(15)00465-9. [DOI] [PubMed] [Google Scholar]
- 3.Baron M., Zini J.M., Challan Belval T., Vignon M., Denis B., Alanio A. Fungal infections in patients treated with ibrutinib: two unusual cases of invasive aspergillosis and cryptococcal meningoencephalitis. Leuk. Lymphoma. 2017;58(12):2981–2982. doi: 10.1080/10428194.2017.1320710. [DOI] [PubMed] [Google Scholar]
- 4.Chamilos G., Lionakis M.S., Kontoyiannis D.P. Call for action: invasive fungal infections associated with ibrutinib and other small molecule kinase inhibitors targeting immune signaling pathways. Clin. Infect. Dis. 2018;66(1):140–148. doi: 10.1093/cid/cix687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lionakis M.S., Dunleavy K., Roschewski M., Widemann B.C., Butman J.A., Schmitz R. Inhibition of B Cell receptor signaling by ibrutinib in primary CNS lymphoma. Cancer Cell. 2017;31(6):833–843. doi: 10.1016/j.ccell.2017.04.012. e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ruchlemer R., Ben-Ami R., Bar-Meir M., Brown J.R., Malphettes M., Mous R. Ibrutinib associated invasive fungal diseases in patients with CLL and non-Hodgkin lymphoma: an observational study. Mycoses. 2019;62(12):1140–1147. doi: 10.1111/myc.13001. [DOI] [PubMed] [Google Scholar]
- 7.Varughese T., Taur Y., Cohen N., Palomba M.L., Seo S.K., Hohl T.M. Serious infections in patients receiving ibrutinib for treatment of lymphoid cancer. Clin. Infect. Dis. 2018;67(5):687–692. doi: 10.1093/cid/ciy175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Herbst S., Shah A., Mazon Moya M., Marzola V., Jensen B., Reed A. Phagocytosis-dependent activation of a TLR9-BTK-calcineurin-NFAT pathway co-ordinates innate immunity to Aspergillus fumigatus. EMBO Mol. Med. 2015;7(3):240–258. doi: 10.15252/emmm.201404556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bercusson A., Colley T., Shah A., Warris A., Armstrong-James D. Ibrutinib blocks Btk-dependent NF-kB and NFAT responses in human macrophages during Aspergillus fumigatus phagocytosis. Blood. 2018;132(18):1985–1988. doi: 10.1182/blood-2017-12-823393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Blez D., Blaize M., Soussain C., Boissonnas A., Meghraoui-Kheddar A., Menezes N. Ibrutinib induces multiple functional defects in the neutrophil response against Aspergillus fumigatus. Haematologica. 2019 doi: 10.3324/haematol.2019.219220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Patterson T.F., Kirkpatrick W.R., White M., Hiemenz J.W., Wingard J.R., Dupont B. Invasive aspergillosis. Disease spectrum, treatment practices, and outcomes. I3 Aspergillus Study Group. Medicine (Baltim.) 2000;79(4):250–260. doi: 10.1097/00005792-200007000-00006. [DOI] [PubMed] [Google Scholar]
- 12.Steinbach W.J., Marr K.A., Anaissie E.J., Azie N., Quan S.P., Meier-Kriesche H.U. Clinical epidemiology of 960 patients with invasive aspergillosis from the PATH Alliance registry. J. Infect. 2012;65(5):453–464. doi: 10.1016/j.jinf.2012.08.003. [DOI] [PubMed] [Google Scholar]
- 13.Ghez D., Calleja A., Protin C., Baron M., Ledoux M.P., Damaj G. Early-onset invasive aspergillosis and other fungal infections in patients treated with ibrutinib. Blood. 2018;131(17):1955–1959. doi: 10.1182/blood-2017-11-818286. [DOI] [PubMed] [Google Scholar]
- 14.Patel R., Hossain M.A., German N., Al-Ahmad A.J. Gliotoxin penetrates and impairs the integrity of the human blood-brain barrier in vitro. Mycotoxin Res. 2018;34(4):257–268. doi: 10.1007/s12550-018-0320-7. [DOI] [PubMed] [Google Scholar]
- 15.Kourkoumpetis T.K., Desalermos A., Muhammed M., Mylonakis E. Central nervous system aspergillosis: a series of 14 cases from a general hospital and review of 123 cases from the literature. Medicine (Baltim.) 2012;91(6):328–336. doi: 10.1097/MD.0b013e318274cd77. [DOI] [PubMed] [Google Scholar]
- 16.Woyach J.A. Ibrutinib and Aspergillus: a Btk-targeted risk. Blood. 2018;132(18):1869–1870. doi: 10.1182/blood-2018-08-865659. [DOI] [PubMed] [Google Scholar]
- 17.Gaye E., Le Bot A., Talarmin J.P., Le Calloch R., Belaz S., Dupont M. Cerebral aspergillosis: an emerging opportunistic infection in patients receiving ibrutinib for chronic lymphocytic leukemia? Med. Maladies Infect. 2018;48(4):294–297. doi: 10.1016/j.medmal.2018.01.003. [DOI] [PubMed] [Google Scholar]
- 18.Ruchlemer R., Ben Ami R., Lachish T. Ibrutinib for chronic lymphocytic leukemia. N. Engl. J. Med. 2016;374(16):1593–1594. doi: 10.1056/NEJMc1600328. [DOI] [PubMed] [Google Scholar]
- 19.Jain P., Keating M.J., Wierda W.G., Sivina M., Thompson P.A., Ferrajoli A. Long-term follow-up of treatment with ibrutinib and rituximab in patients with high-risk chronic lymphocytic leukemia. Clin. Cancer Res. 2017;23(9):2154–2158. doi: 10.1158/1078-0432.CCR-16-1948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Beresford R., Dolot V., Foo H. Cranial aspergillosis in a patient receiving ibrutinib for chronic lymphocytic leukemia. Med. Mycol. Case Rep. 2019;24:27–29. doi: 10.1016/j.mmcr.2019.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]