Abstract
Visceral leishmaniasis caused by Leishmania donovani is regarded as mostly anthroponotic, but a role for animal reservoir hosts in transmission has been suggested in East Africa. Field studies in this region have shown the presence of this parasite in several mammalian species, including rodents of the genera Arvicanthis and Mastomys. Further, the natural reservoirs of Leishmania (Mundinia) sp. causing human cutaneous disease in Ghana, West Africa, are unknown. This study assessed the potential role of the Sub-Saharan rodents Arvicanthis neumanni, A. niloticus and Mastomys natalensis as hosts of L. donovani and L. sp. from Ghana, based on experimental infections of animals and xenodiagnoses. The distribution and load of parasites were determined post mortem using qPCR from the blood, skin and viscera samples. The attractiveness of Arvicanthis and Mastomys to Phlebotomus orientalis was tested by pair-wise comparisons. None of the animals inoculated with L. donovani were infectious to P. orientalis females, although, in some animals, parasites were detected by PCR even 30 weeks post infection. Skin infections were characterized by low numbers of parasites while high parasite burdens were present in spleen, liver and lymph nodes only. Therefore, wild Arvicanthis and Mastomys found infected with L. donovani, should be considered parasite sinks rather than parasite reservoirs. This is indirectly supported also by results of host choice experiments with P. orientalis in which females preferred humans over both Arvicanthis and Mastomys, and their feeding rates on rodents ranged from 1.4 to 5.8% only. Therefore, the involvement of these rodents in transmission of L. donovani by P. orientalis is very unlikely. Similarly, poor survival of Leishmania parasites in the studied rodents and negative results of xenodiagnostic experiments do not support the involvement of Arvicanthis and Mastomys spp. in the transmission cycle of L. sp. from Ghana.
Keywords: Visceral leishmaniasis, Reservoir hosts, Xenodiagnosis, Grass rats, Multimammate mice, Mundinia
Graphical abstract
Highlights
-
•
Three species of Sub-Saharan rodents were infected and xenodiagnosed.
-
•
Skin infections in Arvicanthis and Mastomys were characterized by low parasite loads.
-
•
None of animals inoculated with leishmania were infectious to sand flies.
-
•
Phlebotomus orientalis females significantly preferred man over rodents.
-
•
Rodents are unlikely to be involved in the circulation of the two studied Leishmania in nature.
1. Introduction
Leishmania (Kinetoplastida: Trypanosomatidae) are parasites alternating between blood feeding sand flies (Diptera: Psychodidae) and vertebrate hosts, including humans and various other mammals. Visceral leishmaniasis (VL) is a severe disease caused by parasites of the L. donovani complex; specifically by L. donovani in Asia and East Africa and by L. infantum in Asia, the Middle East, Europe and Latin America. VL caused by L. infantum is typically a zoonosis, where domestic dogs serve as principal reservoirs, although high prevalence of infection has been reported in some other mammalian species (reviewed by Quinnell and Courtenay, 2009). In contrast, VL caused by L. donovani is regarded as mostly anthroponotic; however, in East Africa a role for animal reservoir hosts has been suggested for many years and various findings suggest that reservoir animals may contribute to the transmission of L. donovani during initial outbreaks (reviewed by Ashford, 2000). In Sudan, high levels of seroprevalence and confirmed infections with L. donovani were found in dogs (Dereure et al., 2003), and serious risk of infection in some uninhabited areas indicates a strong probability that L. donovani can be zoonotic (Elnaiem et al., 1998). Indeed, previous field studies on wild mammals in East Africa have shown the presence of L. donovani in small carnivores (Elnaiem et al., 2001; Hoogstraal and Heyneman, 1969) and several rodent species: Arvicanthis niloticus (Hoogstraal and Heyneman, 1969; Elnaiem et al., 2001; El-Hassan et al., 1993; Chance et al., 1978), Mastomys natalensis (Elnaiem et al., 2001), Acomys sp. (Hoogstraal and Heyneman, 1969; Chance et al., 1978) and Rattus rattus (Hoogstraal and Heyneman, 1969; Chance et al., 1978). Recently, our team identified L. donovani in southern Ethiopia, using PCR and DNA sequencing of the ITS1 gene, in Arvicanthis sp., Mastomys erythroleucus and Gerbilliscus nigricaudus (Kassahun et al., 2015).
A Leishmania parasite causing human cutaneous disease in Ghana was identified as a member of the L. enriettii species complex in 2015 (Kwakye-Nuako et al., 2015). The species complex was classified as a new subgenus Mundinia one year later (Espinosa et al., 2016). The five known members of this subgenus (L. enriettii, L. macropodum, L. martiniquensis, L. orientalis and L. sp. originating from Ghana) are geographically widely dispersed and vary substantially in their potential to cause human disease. Although medically important, the biology of these Leishmania species is poorly understood and information on their natural reservoir hosts as well as vector species is scarce. Identification of reservoir hosts and vector species of Ghanaian Leishmania species is, therefore, a significant research challenge.
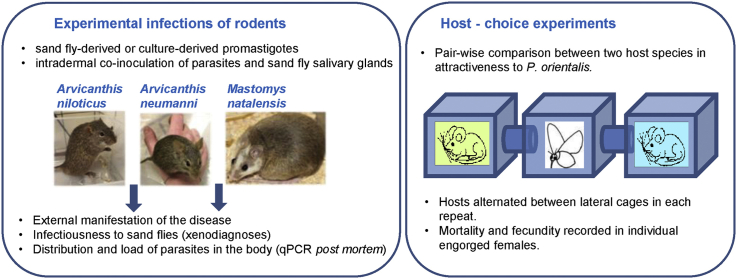
Dense populations of the rodents of the genera Arvicanthis and Mastomys live in close vicinity of humans in Sub-Saharan Africa. We recently studied the susceptibility of Arvicanthis neumanni (Neumann's Grass Rat), A. niloticus (Nile Grass Rat) and Mastomys natalensis (Natal Multimammate Mouse) to Leishmania major and demonstrated that the latter may serve as a reservoir host for this parasite (Sadlova et al., 2019). This study is a follow-up analysis of the host competence of these three rodent species for L. donovani and L. (M.) sp. from Ghana. Arvicanthis niloticus and M. natalensis are widespread in almost all Sub-Saharan Africa (Granjon and Ducroz, 2013; Leirs, 2013) while Arvicanthis neumanni with a range restricted to East Africa (absent in Ghana) was tested only for L. donovani. The response of these rodents to infection and their ability to infect sand flies were evaluated by experimental infections and xenodiagnosis. In addition, feeding rates of P. orientalis on these rodents were tested by host-choice experiments.
2. Materials and methods
2.1. Sand flies, parasites and rodents
The colonies of P. orientalis (originating from Ethiopia) and P. duboscqi (originating from Senegal) were maintained in the insectaries of the Department of Parasitology, Charles University in Prague, under standard conditions as described previously (Volf and Volfova, 2011). Two Sub-Saharan Leishmania strains were used: L. donovani strain MHOM/ET/2010/GR374 (a human isolate from Ethiopia) and L. (M.) sp. strain MHOM/GH/2012/GH5; LV757 (a human isolate from Ghana; Kwakye-Nuako et al., 2015). Promastigotes were cultured in M199 medium (Sigma) containing 10% heat-inactivated fetal bovine serum (FBS, Gibco) supplemented with 1% BME (Basal Medium Eagle, Sigma) vitamins, 2% sterile human urine and 250 μg/ml amikacin (Amikin, Bristol-Myers Squibb). To obtain higher densities of L. donovani metacyclic forms in the stationary-phase culture for rodent infections, a mixture of salts (sodium urate 10 mg/ml, uric acid 10 mg/ml and cysteic acid 50 mg/ml, all Sigma) was added to the medium according to Howard et al. (1987). Breeding colonies of A. neumanni, A. niloticus and M. natalensis were established and maintained at the animal facility of the Department of Parasitology as described previously (Sadlova et al., 2019).
2.2. Rodent infections and xenodiagnosis
Two methods were used for infecting rodents with L. donovani – infections initiated with sand fly-derived Leishmania according to Sadlova et al. (2015) and infections initiated with culture-derived promastigotes. For the first method, P. orientalis females, experimentally infected with L. donovani as described previously (Sadlova et al., 2017), were dissected on day 8 post bloodmeal (PBM), when mature infections with accumulation of metacyclic forms in the thoracic midguts (TMG) were observed. Pools of 100 freshly dissected TMG with a good density of parasites were homogenized in 50 μl of saline.
The natural vector of L. sp. from Ghana is not known; therefore, only culture-derived promastigotes were used for rodent infections with this Leishmania species. For this purpose, stationary-phase promastigotes (day 7 post inoculation) were washed twice in saline and counted using a Burker apparatus. Pools of 108 promastigotes were resuspended in 50 μl of saline.
Dissected salivary glands (SG) of P. orientalis (for L. donovani infections) and P. duboscqi females (for infections with L. sp. from Ghana) were pooled in sterile saline (10 glands per 10 μl of saline) and stored at −20 °C. Prior to mice inoculation, SG were disintegrated by 3 successive immersions into liquid nitrogen and added to both types (sand fly - and culture-derived) of promastigote suspensions.
Rodents were infected as described previously (Sadlova et al., 2019). Briefly, 5.5 μl of the suspension of parasites and SG were injected intradermally into the ear pinnae. The inoculum of culture-derived promastigotes comprised 107 parasites with 73% of metacyclic forms in L. donovani and 33–42% of metacyclic forms in L. sp. from Ghana. The inoculum of sand fly-derived L. donovani was 3–6 × 104 with 65–90% of metacyclic forms; the proportions of metacyclic forms were identified on Giemsa stained smears based on morphological criteria described previously (Sadlova et al., 2010). Animals were checked weekly for external signs of the disease until week 20–30 post infection (p.i.) when they were sacrificed.
Five to seven-day-old P. orientalis females (natural vectors of L. donovani) were allowed to feed on the site of L. donovani inoculation (ear pinnae) of anaesthetized rodents at 5 weeks-intervals as described previously for L. major infections (Sadlova et al., 2019). Fed sand fly females were separated and maintained at 26 °C on 50% sucrose until day 10–12 PBM, when females were dissected and their guts examined under the light microscope for presence and quality of Leishmania infections. As natural vectors for xenodiagnoses of L. sp. from Ghana are not available, animals infected with this Leishmania species were exposed to the unnatural vector P. duboscqi which supports the infection only until defecation of blood remnants on day 4–5 PBM (JS and TB, unpublished data). Therefore, parasite presence was determined on day 3 PBM, allowing multiplication of parasites before loss of infections, using PCR in pools of 5 whole female bodies.
2.3. Tissue sampling and quantitative PCR
Rodents were euthanized by cervical dislocation under anesthesia. Samples from both ears (inoculated and contralateral), both ear-draining lymph nodes, spleen, liver, paws and tail were stored at −20 °C for qPCR. Extraction of total DNA from rodent tissues (on equal weight samples) and sand flies was performed using a DNA tissue isolation kit (Roche Diagnostics, Indianapolis, IN) according to the manufacturer's instructions. Quantitative PCR (Q-PCR) for detection and quantification of Leishmania parasites was performed in a Bio-Rad iCycler & iQ Real-Time PCR Systems using the SYBR Green detection method (SsoAdvanced™ Universal SYBR®, Bio-Rad, Hercules, CA). Infectious loads in rodent tissues were scored using a scoring table considering the number of parasites detected: ˂ 1000 was evaluated as low parasite loads; 1000–10000 as medium parasite loads; ˃ 10000 as high parasite loads.
2.4. Host choice experiments and assessment of mortality and fecundity of sand flies fed on different hosts
Pair-wise comparisons between two types of hosts were performed using the same equipment as described previously (Sadlova et al., 2019). Briefly, 200 P. orientalis females were placed into the central cage from a row of three connected small cages and left for habituation for 20 min. Then, anaesthetized animals were placed or the forearm of a human volunteer positioned in each of the lateral cages for 1 h, after which sand flies were collected. Hosts alternated between lateral cages in each repeat and each pair of hosts was tested four times. Experiments were conducted in darkness at 24–26 °C. Fed females were maintained under the same conditions as the colony and their mortality was recorded for 4 days post-feeding. Then, females were introduced individually into small glass vials lined with wet filter papers and covered with fine mesh. A small ball of cotton wool soaked with 50% sugar solution was placed on the mesh. The numbers of eggs oviposited were compared to determine the effect of the host blood source host on P. orientalis fecundity (Killick-Kendrick and Killick-Kendrick, 1991).
2.5. Statistical analysis
Statistical analyses were carried out using R software (http://cran.r-project.org/). The differences in feeding preferences, mortality and fecundity of P. orientalis females fed on different host species were analysed by the Chi-square test. The differences in numbers of eggs laid by females fed on different hosts were tested by the nonparametric Mann Whitney U test. The relationships between weight and groups (infected and non-infected animals) and time were tested by fitting multilevel linear regression models (package “nlme”), taking into account the correlation between repeated measures of the same animal over time. The model used included interaction term between groups (categorical variable) and time (continuous independent variable).
2.6. Animal experimentation guidelines
Animals were maintained and handled in the animal facility of Charles University in Prague in accordance with institutional guidelines and Czech legislation (Act No. 246/1992 and 359/2012 coll. on Protection of Animals against Cruelty in present statutes at large), which complies with all relevant European Union and international guidelines for experimental animals. All experiments were approved by the Committee on the Ethics of Laboratory Experiments of the Charles University in Prague and were performed under permit no. MSMT-10270/2015–5 of the Ministry of the Education, Youth and Sports of the Czech Republic. Investigators were certified for experimentation with animals by the Ministry of Agriculture of the Czech Republic.
3. Results
3.1. Experimental infections with L. donovani
In total, 8 M. natalensis, 7 A. neumanni and 10 A. niloticus were infected by L. donovani; all M. natalensis, all A. neumanni and five A. niloticus with sand fly-derived Leishmania, while five A. niloticus were infected with culture-derived Leishmania. Two animals of each species served as controls inoculated with sterile saline.
None of the inoculated animals developed lesions or other external signs of the disease throughout the entire experiment. The weight of animals did not differ significantly between infected animals and uninfected controls (P = 0.126, P = 0.446 and P = 0.382 in A. niloticus, A. neumanni and M. natalensis, respectively) (S1 Table). All 100, 194 and 251 P. orientalis females used at different times p.i. for xenodiagnoses on M. natalensis, A. neumanni and A. niloticus, respectively, were Leishmania-negative.
Nevertheless, PCR performed at the end of the experiment, on week 30 p.i., revealed presence of L. donovani in different tissues and organs of infected rodents; the quantities of parasites were subsequently determined using qPCR. High parasite loads were found in the lymph node draining the inoculated ear of one A. neumanni and low parasite loads in forepaws and the inoculated ear of three A. niloticus (Table 1). The parasite loads in M. natalensis were the highest among tested rodent species. Half of the animals maintained L. donovani until week 30 p.i., with parasites localized mostly in liver, spleen and paws. An inoculated ear was positive in one specimen and draining lymph nodes in two animals. High parasite burdens were detected only in the spleen and liver. No parasites were detected in the right ear, tail or blood (Table 1).
Table 1.
Presence, amount and location of L. donovani DNA determined by qPCR in various tissue of individual animals. IE, inoculated ear; CE, contralateral ear; DN-IE, draining lymph nodes of the inoculated ear; DN-CE, draining lymph nodes of the contralateral ear; FP, forepaws; HP, hindpaws; T, tail; L, liver; S, spleen; B, blood; -, negative results, +, <1000 parasites; ++, 1000–10000 parasites; +++, >10000 parasites.
| Rodent species | Individual marks of animals | IE | CE | DN-IE | DN-CE | FP | HP | T | L | S | B |
|---|---|---|---|---|---|---|---|---|---|---|---|
| A. niloticus | A1 | – | – | – | – | – | – | – | – | – | – |
| A2 | – | – | – | – | + | – | – | – | – | – | |
| A3 | – | – | – | – | – | – | – | – | – | – | |
| A4 | – | – | – | – | – | – | – | – | – | – | |
| A5 | – | – | – | – | – | – | – | – | – | – | |
| A6 | – | – | – | – | – | – | – | – | – | – | |
| A7 | + | – | – | – | + | – | – | – | – | – | |
| A8 | – | – | – | – | + | – | – | – | – | – | |
| A9 | – | – | – | – | – | – | – | – | – | – | |
| A10 | – | – | – | – | – | – | – | – | – | – | |
| M. natalensis | M1 | – | – | – | – | – | – | – | – | – | – |
| M2 | – | – | – | – | + | ++ | – | ++ | +++ | – | |
| M3 | – | – | ++ | – | + | + | – | ++ | +++ | – | |
| M4 | – | – | – | – | – | – | – | – | – | – | |
| M5 | – | – | ++ | + | + | – | – | +++ | +++ | – | |
| M6 | + | – | – | – | – | – | – | – | – | – | |
| M7 | – | – | – | – | – | – | – | – | – | – | |
| M8 | – | – | – | – | – | – | – | – | – | – |
3.2. Experimental infections with L. sp. from Ghana
Six A. niloticus (3 males and 3 females) and six female M. natalensis were inoculated with culture-derived promastigotes, but one M. natalensis died before the end of the experiment. Six animals of each species were used as controls inoculated with sterile saline. No external signs of infection were apparent on infected animals throughout the experiment. The weight of infected A. niloticus did not differ significantly from control animals (P = 0.98) but in M. natalensis, infected animals gained significantly less weight than controls (P = 0.01) (S2 Table).
Xenodiagnoses with P. duboscqi did not reveal Leishmania presence in sand flies tested by PCR – all 95 and 87 pools of sand flies used for xenodiagnoses on inoculated ears of A. niloticus and M. natalensis, respectively, on different week p.i., were negative. By the end of the experiment, on week 25 p.i., PCR failed to detect Leishmania DNA in any A. niloticus. In M. natalensis, the presence of Leishmania was confirmed in a single animal on week 20 p.i., with localization in the inoculated ear and its draining lymph nodes, the contralateral ear and forepaws. Based on qPCR, all these tissues possessed only low parasite loads.
3.3. Host choice experiments with P. orientalis
Two potential rodent hosts were offered to P. orientalis females in each pair-wise comparison, and each host combination was tested four times with hosts alternating between lateral cages. Phlebotomus orientalis females showed very low feeding rate on all tested rodents: 1.4–5.8% of females took bloodmeals during experiments (Table 2). No difference was observed in attractiveness of A. niloticus (the bigger species) and A. neumanni (the smaller species), or between Mastomys and Arvicanthis (represented by A. neumanni). On the other hand, significant preferences were observed when human forearm was offered as an alternative to rodents: sand flies preferred human forearms to both Arvicanthis and Mastomys (Table 2). Engorged females that took blood meals on different hosts were further followed for comparison of mortality and fecundity. Mortality (until day 4 PBM) ranged between 15.2% and 54.5%, but was not significantly influenced by host types (Table 2). Four days PBM, females were allowed to oviposit in small glass vials where they were kept individually. Blood source did not influence significantly either the proportion of females laying eggs or the numbers of eggs laid by individual females. Median numbers of eggs per female were 36–53, depending on the experiment (Table 2).
Table 2.
Feeding preferences, mortality and fecundity of P. orientalis females fed on different host species. The between-species differences were tested by the Chi-squared test. The differences in numbers of eggs laid were tested by the nonparametric Mann Whitney U test.
| Host combination | Host | N (%) of fed sand flies | Significance of between-species differences | Mortality post feeding: N dying/N (%) | Significance of between-species differences | Fecundity N eggs-lying/N (%) | Significance of between-species differences | Number of eggs Median (Min, Max) | Significance of between-species differences |
|---|---|---|---|---|---|---|---|---|---|
| A. neumanni vs. A. niloticus | A. neumanni | 22 (1.4%) | χ2 = 1.231 P = 0.267 |
12/22 (54.5%) | χ2 = 0.746 P = 0.483 |
10/10 (100%) | χ2 = 0.405 P = 0.600 |
36 (5, 60) | P = 1.000 |
| A. niloticus | 30 (2.0%) | 15/30 (50.0%) | 14/15 (93.3%) | 38 (2, 70) | |||||
| A. neumanni vs. M. natalensis | A. neumanni | 46 (2.3%) | χ2 = 0.095 P = 0.758 |
7/46 (15.2%) | χ2 = 0.259 P = 0.192 |
37/39 (94.9%) | χ2 = 0.115 P = 0.115 |
49 (1, 67) | P = 0.966 |
| M. natalensis | 49 (2.4%) | 12/49 (24.5%) | 31/37 (83.8%) | 48 (1, 75) | |||||
| A. neumanni vs. man | A. neumanni | 17 (3,4%) | χ2 = 14.0625 P = 0.0002 |
7/17 (41.2%) | χ2 = 0.922 P = 0.577 |
10/10 (100%) | χ2 = 0.376 P = 0.527 |
50 (26, 64) | P = 0.552 |
| man | 47 (9.4%) | 20/47 (42.6%) | 25/27 (92.3%) | 53 (2, 78) | |||||
| M. natalensis vs. man | M. natalensis | 29 (5.8) | χ2 = 5.582 P = 0.0182 |
7/29 (24.1%) | χ2 = 0.989 P = 0.597 |
19/22 (86.4%) | χ2 = 0.718 P = 0.510 |
52 (15, 66) | P = 0.918 |
| man | 50 (10.0) | 12/50 (24.0%) | 34/38 (89.5%) | 51 (5, 76) |
4. Discussion
Zoonotic transmission of L. donovani has long been suggested in Sudan, based on repeated outbreaks of the disease in people who had visited uninhabited areas and findings of the parasite in wild animals. However, despite a considerable effort over many years no reservoir host has been proven (reviewed by Ashford, 2000; Ashford, 1996). Here we undertook testing of the reservoir role of rodents in the genera Arvicanthis and Mastomys, as L. donovani parasites were repeatedly found in these rodent species during several field studies performed in East Africa (El-Hassan et al., 1993; Elnaiem et al., 2001), since studies in the 1960s when the highest prevalence was found in A. niloticus in the southern part of Sudan (Hoogstraal and Heyneman, 1969). Recently, the presence of L. donovani was reported in Arvicanthis and Mastomys in south-western Ethiopia (Kassahun et al., 2015).
The results of our laboratory study are in accordance with the above cited field studies, since we confirmed that L. donovani parasites survive in some individuals of Arvicanthis and Mastomys for several months. Parasites were localized not only in the viscera, but in some animals also in external organs - ears and paws, therefore, theoretically available for transmission by biting sand flies. However, all xenodiagnoses performed on ears of animals were negative. This is in contrast with results of our previous study performed with the same Ethiopian L. donovani strain on BALB/c mice (Sadlova et al., 2015). Mice were infected using the same method – intradermal inoculation of sand fly derived parasites in the ear pinnae. Mice did not show any signs of disease in our previous study, but 19% of the P. orientalis females that fed at the site of inoculation, became infected (Sadlova et al., 2015).
Parasites of the Mundinia subgenus from Ghana still lack their scientific name and there is no current information on their natural reservoir hosts and vector species. Our experimental infections of A. niloticus and M. natalensis, rodents present in endemic localities, did not confirm their host competence for this parasite. Leishmania were mostly completely lost and survived only in a single M. natalensis till week 20 p.i. In this animal, Leishmania disseminated to different tissues (draining lymph nodes of the inoculated ear, the contralateral ear and forepaws), but were present only in low parasite loads.
Generally, a failure of infectivity to sand flies can be explained by absence or low numbers of parasites in peripheral blood or in the skin. In dogs infected with L. infantum, high parasite numbers in skin (and blood) have been shown to be the best markers of infectiousness to sand fly vectors (Courtenay et al., 2014; Borja et al., 2016). In our study, high parasite loads were present only in the spleen and liver of M. natalensis and lymph nodes of A. neumanni infected with L. donovani, these being visceral organs where the parasites were not accessible to sand fly borne transmission. Skin samples derived from whole ears and paws revealed 10–700 amastigotes, compared with 14–80 thousand present in lymph nodes and viscera. A similar phenomenon was observed in the study of host competence of Mastomys and Arvicanthis for L. major – the L. major strains which produced poor skin infections in animals were not infectious to sand flies (Sadlova et al., 2019).
The main vector of L. donovani in Sudan and Ethiopia is Phlebotomus (Larroussius) orientalis (Ashford, 2000). Host choice experiments reported here showed that this sand fly species was not strongly attracted to rodents. Females clearly preferred humans as a source of blood meals. Preference of P. orientalis for humans and large domestic animals was also observed during field studies with animal baited traps and blood meal analyses in Ethiopia (Gebresilassie et al., 2015a, 2015b; Yared et al., 2019). Similarly, the very low engorgement rates of P. orientalis on rodents in our experiments (1.4–5.8%) are in accordance with the results of the study of Gebresilassie et al. (2015b), where only 1.08% of females took blood meals on small wild animals compared to 30.53% feeding on larger domestic animals. The likelihood of L. donovani being maintained in rodents in areas where P. orientalis acts as the main vector is, therefore, very low.
Taken together, the results of this study suggest that rodents infected with L. donovani in East Africa most probably represent parasite sinks on which the infected sand fly occasionally feed but which do not contribute to vector infection and transmission to the next host (Chaves et al., 2007). True animal reservoirs of L. donovani in East Africa must be confirmed with further studies, and good candidates may be small carnivores like the Egyptian mongooses, 14% of which were found infected in Dinder National Park (Elnaiem et al., 2001), or Senegal genet (Genetta G. senegalensis), Sudanese serval (Felis serval phillipsi) or White-tailed mongooses (Ichneumia albicauda) reported to be infected with L. donovani in southern Sudan and Ethiopia, respectively (Hoogstraal and Heyneman, 1969; Kassahun et al., 2015). For the Mundinia parasites causing cutaneous leishmaniasis in Ghana we do not have any indication from the field for identification of the reservoir host; therefore, any such findings would be highly valuable to enable further experimental research.
In conclusion, the results of this laboratory study do not support the involvement of Arvicanthis and Mastomys spp. in the transmission cycle of L. donovani in East Africa nor L. sp. in Ghana. In contrast, these rodent species most probably do comprise important reservoir hosts of L. major in this region (Sadlova et al., 2019).
Declaration of competing interest
None.
Acknowledgements
We would like to thank to Prof. Asrat Hailu and the late Prof. Teshome Gebre-Michael, Addis Ababa University, Ethiopia, for kindly providing the L. donovani isolate and the progeny of the P. orientalis colony and Dr. Jan Votypka, Charles University, Czech Republic, for help with molecular methods. This study was funded by the Czech Science Foundation GACR (grant number 17-01911S), GA UK (grant number 288217) and ERD Funds, project CePaViP (CZ.02.1.01/0.0/0.0/16_019/0000759).
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.ijppaw.2019.12.002.
Appendix A. Supplementary data
The following are the Supplementary data to this article:
References
- Ashford R.W. Leishmaniasis reservoirs and their significance in control. Clin. Dermatol. 1996;14:523–532. doi: 10.1016/0738-081x(96)00041-7. [DOI] [PubMed] [Google Scholar]
- Ashford R.W. The leishmaniases as emerging and reemerging zoonoses. Int. J. Parasitol. 2000;30:1269–1281. doi: 10.1016/s0020-7519(00)00136-3. [DOI] [PubMed] [Google Scholar]
- Borja L.S., de Sousa O.M.F., da Silva Solcà M., Bastos L.A., Bordoni M., Magalhães J.T., Larangeira D.F., Barrouin-Melo S.M., Fraga D.B.M., Veras P.S.T. Parasite load in the blood and skin of dogs naturally infected by Leishmania infantum is correlated with their capacity to infect sand fly vectors. Vet. Parasitol. 2016;229:110–117. doi: 10.1016/j.vetpar.2016.10.004. [DOI] [PubMed] [Google Scholar]
- Chance M.L., Schnur L.F., Thomas S.C., Peters W. Biochemical and serological taxonomy of Leishmania from Ethiopian zoogeographical region of Africa. Ann. Trop. Med. Parasitol. 1978;72:533–542. doi: 10.1080/00034983.1978.11719357. [DOI] [PubMed] [Google Scholar]
- Chaves L.F., Hernandez M.J., Dobson A.P., Pascual M. Sources and sinks: revisiting the criteria for identifying reservoirs for American cutaneous leishmaniasis. Trends Parasitol. 2007;23:311–316. doi: 10.1016/j.pt.2007.05.003. [DOI] [PubMed] [Google Scholar]
- Courtenay O., Carson C., Calvo-Bado L., Garcez L.M., Quinnell R.J. Heterogeneities in Leishmania infantum infection: using skin parasite burdens to identify highly infectious dogs. PLoS Neglected Trop. Dis. 2014;8:26. doi: 10.1371/journal.pntd.0002583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dereure J., El-safi S.H., Bucheton B., Boni M., Mohamed M., Davoust B., Pratlong F., Feugier E., Lambert M., Dessein A., Dedet J. Visceral leishmaniasis in eastern Sudan: parasite identification in humans and dogs; host-parasite relationships. Microbes Infect. 2003;5:1103–1108. doi: 10.1016/j.micinf.2003.07.003. [DOI] [PubMed] [Google Scholar]
- El-Hassan A.M., Zijlstra E.E., Meredith S.E.O., Ghalib H.W., Ismail A. Identification of Leishmania donovani using a polymerase chain reaction in patient and animal material obtained from an area of endemic kala-azar in the Sudan. Acta Trop. 1993;55:87–90. doi: 10.1016/0001-706x(93)90051-c. [DOI] [PubMed] [Google Scholar]
- Elnaiem D.A., Ward R.D., Hassan H.K., Miles M.A., Frame I.A. Infection rates of Leishmania donovani in Phlebotomus orientalis from a focus of visceral leishmaniasis in eastern Sudan. Ann. Trop. Med. Parasitol. 1998;92:229–232. doi: 10.1080/00034989860085. [DOI] [PubMed] [Google Scholar]
- Elnaiem D.A., Hassan M.M., Maingon R., Nureldin G.H., Mekawi A.M., Miles M., Ward R.D. The egyptian mongoose, Herpestes ichneumon, is a possible reservoir host of visceral leishmaniasis in eastern Sudan. Parasitology. 2001;122:531–536. doi: 10.1017/s0031182001007594. [DOI] [PubMed] [Google Scholar]
- Espinosa O.A., Serrano M.G., Camargo E.P., Teixeira M.M.G., Shaw J.J. An appraisal of the taxonomy and nomenclature of trypanosomatids presently classified as Leishmania and Endotrypanum. Parasitology. 2016;145(Special Issue 4):430–442. doi: 10.1017/S0031182016002092. [DOI] [PubMed] [Google Scholar]
- Gebresilassie A., Abbasi I., Aklilu E., Yared S., Kirstein O.D., Moncaz A., Tekie H., Balkew M., Warburg A., Hailu A., Gebre-michael T. Host-feeding preference of Phlebotomus orientalis (Diptera: Psychodidae) in an endemic focus of visceral leishmaniasis in northern Ethiopia. Parasites Vectors. 2015;8:270. doi: 10.1186/s13071-015-0883-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gebresilassie A., Yared S., Aklilu E., Kirstein O.D., Moncaz A., Tekie H., Balkew M., Warburg A., Hailu A., Gebre-Michael T. Host choice of Phlebotomus orientalis (Diptera: Psychodidae) in animal baited experiments: a field study in Tahtay Adiyabo district, northern Ethiopia. Parasites Vectors. 2015;8:1–10. doi: 10.1186/s13071-015-0807-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Granjon L., Ducroz J.-F. Genus Arvicanthis Grass rats. In: Happold D.C.D., editor. Mammals of Africa: Volume III. Bloomsbury Publishing; London: 2013. pp. 379–380. [Google Scholar]
- Hoogstraal H., Heyneman D. Leishmaniasis in the Sudan republic. 30. Final epidemiological report. Am. J. Trop. Med. Hyg. 1969;18:1091–1210. [Google Scholar]
- Howard M.K., Sayers G., Miles M.A. Leishmania donovani metacyclic promastigotes: transformation in vitro, lectin agglutination, complement resistance, and infectivity. Exp. Parasitol. 1987;64:147–156. doi: 10.1016/0014-4894(87)90138-x. [DOI] [PubMed] [Google Scholar]
- Kassahun A., Sadlova J., Dvorak V., Kostalova T., Rohousova I., Frynta D., Aghova T., Yasur-Landau D., Lemma W., Hailu A., Baneth G., Warburg A., Volf P., Votypka J. Detection of Leishmania donovani and L. tropica in Ethiopian wild rodents. Acta Trop. 2015;145:39–44. doi: 10.1016/j.actatropica.2015.02.006. [DOI] [PubMed] [Google Scholar]
- Killick-Kendrick M., Killick-Kendrick R. The initial establishment of sandfly colonies. Parassitologia. 1991;33:313–320. [PubMed] [Google Scholar]
- Kwakye-Nuako G., Mosore M.T., Duplessis C., Bates M.D., Puplampu N., Mensah-Attipoe I., Desewu K., Afegbe G., Asmah R.H., Jamjoom M.B., Ayeh-Kumi P.F., Boakye D.A., Bates P.A. First isolation of a new species of Leishmania responsible for human cutaneous leishmaniasis in Ghana and classification in the Leishmania enriettii complex. Int. J. Parasitol. 2015;45:679–684. doi: 10.1016/j.ijpara.2015.05.001. [DOI] [PubMed] [Google Scholar]
- Leirs H. Genus Mastomys multimammate mice. In: Happold D.C.D., editor. Mammals of Africa: Volume III. Bloomsbury Publishing; London: 2013. pp. 460–471. [Google Scholar]
- Quinnell R.J., Courtenay O. Transmission, reservoir hosts and control of zoonotic visceral leishmaniasis. Parasitology. 2009;136:1915–1934. doi: 10.1017/S0031182009991156. [DOI] [PubMed] [Google Scholar]
- Sadlova J., Price H.P., Smith B.A., Votypka J., Volf P., Smith D.F. The stage-regulated HASPB and SHERP proteins are essential for differentiation of the protozoan parasite Leishmania major in its sand fly vector. Phlebotomus papatasi. Cell. Microbiol. 2010;12 doi: 10.1111/j.1462-5822.2010.01507.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sadlova J., Seblova V., Votypka J., Warburg A., Volf P. Xenodiagnosis of Leishmania donovani in BALB/c mice using Phlebotomus orientalis: a new laboratory model. Parasites Vectors. 2015;8:158. doi: 10.1186/s13071-015-0765-x. Article number. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sadlova J., Myskova J., Lestinova T., Votypka J., Yeo M., Volf P. Leishmania donovani development in Phlebotomus argentipes: comparison of promastigote and amastigote-initiated infections. Parasitology. 2017;144:403–410. doi: 10.1017/S0031182016002067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sadlova J., Vojtkova B., Hrncirova K., Lestinova T., Spitzova T., Becvar T., Votypka J., Bates P., Volf P. Host competence of African rodents Arvicanthis neumanni, A. niloticus and Mastomys natalensis for Leishmania major. Int. J. Parasitol. Parasites Wildl. 2019;8:118–126. doi: 10.1016/j.ijppaw.2019.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volf P., Volfova V. Establishment and maintenance of sand fly colonies. J. Vector Ecol. 2011;36:1–9. doi: 10.1111/j.1948-7134.2011.00106.x. [DOI] [PubMed] [Google Scholar]
- Yared S., Gebresilassie A., Abbasi I., Aklilu E., Kirstein O.D., Balkew M., Brown A.S., Clouse R.M., Warburg A., Hailu A., Gebre-michael T. A molecular analysis of sand fly blood meals in a visceral leishmaniasis endemic region of northwestern Ethiopia reveals a complex host-vector system. Heliyon. 2019;5 doi: 10.1016/j.heliyon.2019.e02132. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.