Abstract
Epilepsy is the fourth most prevalent brain disorder affecting millions of people of all ages. Epilepsy is divided into six categories different in etiology and molecular mechanisms; however, their common denominator is the inability to maintain ionic homeostasis. Antiepileptic drugs have a broad spectrum of action and high toxicity to the whole organism. In many cases, they could not penetrate the blood-brain barrier (BBB) and reach corresponding targets. Nucleic acid aptamers are a new and promising class of antiepileptic drugs as they are non-toxic, specific, and able to regulate the permeability of ion channels or inhibit inflammatory proteins. In this review, we summarize the mechanisms of epileptogenesis and its interconnection with the BBB and show the potential of aptamers for antiepileptic treatment.
Main Text
Epilepsy is the most prevalent chronic neurological disorder, affecting at least 50 million people worldwide.1 Epilepsy is characterized by spontaneous recurrent seizures or epileptic attacks with a fixed and prolonged nature. This condition is accompanied by tonic convulsions of the respiratory musculature, aspiration of saliva and blood from the oral cavity, and arrhythmia of breathing.2 Epilepsy is not deadly, but it is an extremely nasty disease. Unpredictability of seizures and physiological stress associated with it significantly worsen the quality of the patient’s life and the lives of people in the patient’s life.
The International League Against Epilepsy (ILAE) has defined epilepsy as a disorder of the brain resulting in the predisposition to generate epileptic seizures characterized by its psychosocial consequences. In a more practical sense, an epilepsy diagnosis requires: (1) at least two unprovoked (or reflex) seizures occurring over 24 h; (2) one unprovoked (or reflex) seizure and a probability of further seizures similar to the general recurrence risk (at least 60%) after two unprovoked seizures, occurring over the next 10 years; and (3) diagnosed epilepsy syndrome.3
Progression of the disease generally consists of evolving pathologic modifications such as exacerbation of spontaneous seizures (e.g., an increase in their frequency, duration, or generalization), development of drug-resistant seizures, worsening of neuropathology, and onset of comorbidities.4
What Is Epileptogenesis?
Epileptogenesis is the process of structural and functional changes that transforms normal cells in the brain to one that can generate abnormal neuronal activity resulting in seizures.5 These changes include neurodegeneration, neurogenesis, gliosis, axonal damage or sprouting, dendritic plasticity, blood-brain barrier (BBB) damage, recruitment of inflammatory cells into brain tissue, reorganization of the extracellular matrix, and reorganization of the molecular architecture of individual neuronal cells.6
Epileptogenesis arises in the neuroglial cells of the brain. An epileptic neuron is characterized by its inability to maintain appropriate membrane potential across its cell membrane and, thus, its tendency to depolarize.7 It also causes changes in glial physiology and in the homeostatic environment.8 Neuronal excitability during epileptogenesis alters progressively and leads to critical interconnections and structural changes even before the first spontaneous seizure occurs.9 Each seizure represents a rapid loss of homeostatic equilibrium, with altered energy and molecular gradients and corresponding interruption of normal behavior and consciousness.8
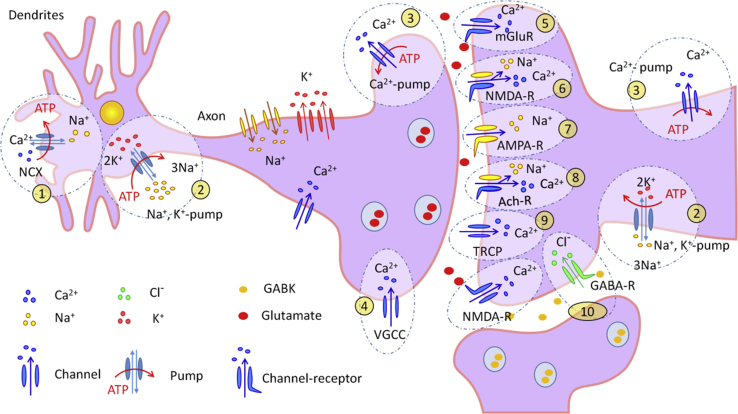
Epilepsy is divided into six categories: structural, genetic, infectious, metabolic, immune, and unknown.10 All categories differ in etiology and mechanisms; however, their common denominator is the inability to maintain ionic homeostasis.11 Epileptogenesis may occur as a result of the malfunction of molecular structures responsible for maintenance of ionic homeostasis (Table 1). For example, during an epileptic seizure, the concentration of sodium (I) cations in neurons increases 5.5 times,12 the calcium (II) ion concentration increases 10 times,13,14 and the chloride concentration increases almost 4 times compared to normal physiological values.15 The most common culprits are summarized in Figure 1.
Table 1.
Molecular Structures Involved in Regulation of Ionic Homeostasis
| Number | Molecular Structures | Name | Functions | References |
|---|---|---|---|---|
| 1 | ion channels | voltage-gated potassium, sodium, and calcium channels; Na+/Ca2+ exchangers; chloride channels | provide a controlled movement of K+, Na+, Ca2+, and Cl− or free movement of Ca2+ and Na+ cations by a voltage-dependent mechanism | 87, 88, 89, 90, 91, 92 |
| 2 | ATPases | Na+/K+-ATPase; plasma membrane Ca2+ ATPase; sarco/endoplasmic reticulum Ca2+-ATPase | provide an energy-dependent or a volatile controlled movement of K+, Na+, and Ca2+ against a concentration gradient necessary to restore ion homeostasis after an action potential | 91,93,94 |
| 3 | receptors linked with ion channels | receptors for acetylcholine, N-methyl-D-aspartate, α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid kainate, metabotropic glutamate, and gamma-aminobutyric acid | provide a controlled movement K+, Na+, Ca2+, Cl- by receptor-mediated mechanism | 95, 96, 97, 98, 99, 100 |
| 4 | aquaporins | a family of allosterically regulated proteins | forming ion channels in the cytoplasmic membrane for water passing | 101 |
Figure 1.
Mechanisms for Maintaining Ionic Homeostasis in Neurons
1, Na+/Ca2+ exchanger (NCX). 2, Na+ and K+ pump. 3, Ca2+ pump. 4, voltage-gated calcium channel (VGCC). 5, metabotropic glutamate receptor (mGluR). 6, N-methyl-D-aspartate receptor (NMDA-R). 7, α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid receptor (AMPA-R). 8, acetylcholine receptor (Ach-R). 9, transient receptor potential channel (TRCP). 10, gamma-aminobutyric acid receptor (GABA-R).
Ion channels play a crucial role in epileptogenesis, providing transport of Na+, Ca2+, and K+16 as well as excitatory neurotransmitters—glutamate and aspartate and the main inhibitory neurotransmitter gamma-aminobutyric acid.17 The primary hypothesis for the pathogenesis of epilepsy is the imbalance between the excitatory (glutamate and aspartate) and inhibitory (gamma-amino-butyric acid (GABA), taurine, glycine, noradrenaline, dopamine, and serotonin) mediators.18,19 Other reasons for seizures are contravention of ion transport caused by mutations in neuronal receptors20 and different genetic mutations.21, 22, 23
The Blood-Brain Barrier and Its Functions
The BBB is a physical and metabolic barrier between the brain and the systemic circulation that serves to protect and regulate the microenvironment of the brain.24 The unique structure and morphology of the BBB limit the penetration of pathogens, toxic chemicals, and metabolic products into neurons, thereby maintaining the integrity of vital tissues.25 The BBB also limits the delivery of drugs for the treatment of neurological diseases and disorders in the brain tissue.26 The anatomical structure of the BBB consists of dense contacts of endotheliocytes. In addition to endotheliocytes, the BBB contains pericytes, astrocytes, and microglia.27 Above the endotheliocytes, there is a fiber layer of fibronectin and laminin with a significant amount of proteoglycans.28
The protective structure of the BBB includes 3 main systems:27, 28, 29
-
1.
The enzymatic barrier of the BBB, which prevents the passage of drugs and xenobiotics from entering the endothelial cells of capillaries. The enzymatic barrier line is formed by alkaline phosphatase, adenosine triphosphatase, nucleoside diphosphatase, acetylcholinesterase, 5′ nucleotides, and others.
-
2.
The cell barrier, which is formed by the cells of the BBB and the intercellular contacts. Endothelial cells limit the passage of water-soluble substances and control endocytosis and pinocytosis.
-
3.
The ABC system of protein carriers, which include about 48 vectors classified into 7 families (ABC-A, -B, -C, -D, -E, -F, and G). Among them, the most studied transporter is the ABCB1 encoded P-glycoprotein (P-gp or MDR1), as well as ABBC4 and ABCG2 (BCR-protein).
Substances can cross the BBB in two different ways. The first is paracellular transport, which involves the passage of substances between endothelial cells through tight junctions. The second involves the transfer of substances with the help of passive and active transport and is called transcellular transfer. Passive transport of molecules occurs through the endothelial cell through a concentration or electrochemical gradient, with no expenditure of energy.30 This transfer is regulated by physicochemical properties, such as molecular weight, electrical charge, and lipophilicity and is usually limited to small lipophilic molecules that are less than 500 Da. Active transport goes along a more complex mechanism against the gradient already with the expenditure of energy. The permeability of tight contacts directly depends on the metabolism of endothelial cells; therefore, substances do not pass well by this type of transport.31
In addition to the aforementioned transport systems, there are various less lipophilic mechanisms that are necessary for the passage of nutrients and proteins. These include mediated carriers, receptor-mediated transcytosis, and adsorption-mediated transcytosis.32
Connection of the Blood-Brain Barrier and Epilepsy
The BBB is disrupted in various neurological disorders such as ischemia, diseases correlated with inflammation in nervous tissue, multiple sclerosis, Alzheimer’s disease, and epilepsy (Figure 2). In epilepsy, acute hypertension that occurs during seizure activity can lead to the BBB opening.33,34 It was shown that, in animal models, BBB leakage happens within minutes after status epilepticus and lasts for several hours or days.35,36 In human, the increased BBB permeability can persist for several weeks, months, or even years, which may contribute to enhanced excitability.37
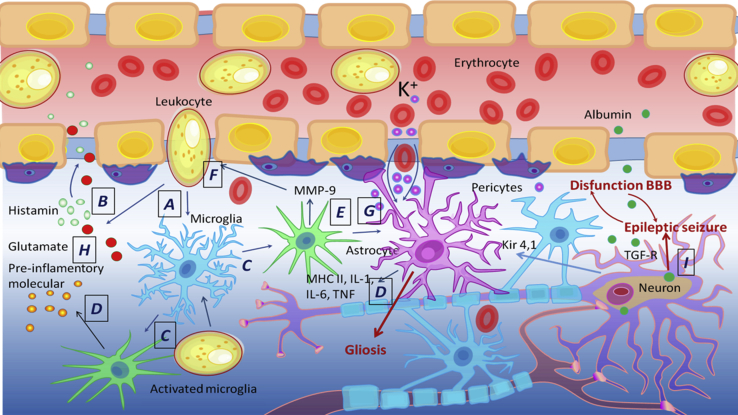
Figure 2.
Blood-Brain Barrier in Epilepsy
(A–F) Disruption of the BBB induces blood cells to enter the brain (A); leukocytes cause inflammation, secrete histamine (B), and activate microglia and astrocytes (C); activated microglia and astrocytes increase inflammation (D), activate metalloproteinase MMP-9 (E), and increase the BBB permeability (F). (G and H) Disruption increases the content of intracellular potassium (G) and glutamate (H) ions. (I) Disruption increases the entry of albumin into the brain, absorbed by neurons and astrocytes through TGF-β receptors, as a result of which Kir 4.1 permeability of potassium channels in astrocytes is reduced. Systemic inflammatory disorders cause an accumulation of inflammatory mediators and contribute to the destruction of the BBB. All processes together increase the excitability of neurons and stimulate epileptimorphic seizures, and the excessive activation of astrocytes stimulates gliosis.
Epileptic seizures contribute to the BBB leakage. However, some evidence also suggests that BBB failure may also be an etiological factor contributing to the development of seizures.38 Marchi et al.39 monitored the onset of seizures in patients undergoing osmotic disruption of the BBB followed by intra-arterial chemotherapy to treat primary brain lymphomas. They found that focal motor seizures occurred immediately after BBB disruption in 25% of procedures and originated contralaterally to the hemisphere of BBB disruption, whereas no seizures were observed when only intra-arterial chemotherapy was administered without BBB disruption. Opening of the BBB is likely to occur after a single seizure in the chronic epileptic phase, and transient opening of the BBB by hyperosmotic treatment could aggravate epileptic seizures, often in a persistent manner.36 One of the mechanisms of BBB leakage involvement in epileptogenesis describes extravasation of albumin from the circulation into the brain neuropil that leads to astrocytic transformation and dysfunction.38 It is estimated that albumin present in brain tissue may bind to neurons, astrocytes, and microglial cells, particularly shortly after traumatic brain injury and status epilepticus.37 Thus, it is extremely important to have an objective picture of the condition of the BBB and nervous tissue in order to select an adequate tactic of treatment and rehabilitation.
How Epilepsy Is Treated
Epilepsy is considered to be resolved for individuals past the applicable age of an age-dependent epilepsy syndrome or those who have remained seizure-free for the past 10 years, with no use of seizure medicines for the past 5 years.40 Unfortunately, 6% to 69% of patients fail to respond to standard medical and surgical therapies and continue to experience debilitating refractory seizures.41 These patients are classified as having drug-resistant epilepsy (DRE), a diagnosis with poor prognostic implications that include premature death, physical injury, psychosocial dysfunction, and reduced quality of life.42 In 2010, the ILAE proposed a formal consensus definition of DRE. The definition includes two levels of categorization: level 1 provides a general scheme to categorize response to each therapeutic intervention (i.e., response to antiepileptic drug [AED] trials), while level 2 provides a core definition of DRE using a set of essential criteria based on the categorization of response from level 1. The ILAE defined DRE as the failure of adequate trials of two tolerated, appropriately chosen, and used AED schedules (whether as monotherapy or in combination) to achieve sustained freedom from seizures.43
Therapy of epilepsy is also a problem for patients responding to treatment, as more than a third of available AEDs still contribute to recurrent seizures and undesirable side effects.44 Up to 40% of patients with epilepsy are women of fertile age. For mothers with epilepsy, it is especially dangerous that they have to take AEDs in large doses during pregnancy. The use of AEDs (e.g., valproate) can lead to embryonic disorders, which leads to structural defects of almost all body systems, as well as mental disability.45 Therefore, the main interests in new AED development are providing these unmet needs of superior treatment efficacy and tolerability without compromising short- and long-term patient safety.46
Although many factors may contribute to the variability of clinical outcomes in individual patients, unpredictability may, at least in part, result from genetic variation. The influence of genes on the outcome of drug treatment is a rapidly evolving field termed “pharmacogenetics” over 40 years ago by the German geneticist Friedrich Vogel.47 The ultimate goal of pharmacogenetics is to use the genetic makeup of an individual to predict drug response and efficacy as well as potential adverse drug events.48 The concept of “personalized” medicine is receiving much attention, and expectations have been raised that pharmacogenetics may be an important tool to optimize the treatment of epilepsy for the individual patient. As outlined earlier, treatment of epilepsy with AEDs is complicated by the unpredictability of efficacy, adverse drug reactions (ADRs), and optimal doses in individual patients. At least in part, this unpredictability may result from individual genes whose variations exert a measurable influence on the effect of a given drug.49
In general, as with other drugs, the absorption and distribution—and, hence, clinical efficacy—of AEDs depend on their physicochemical characteristics such as lipophilicity, solubility, molecular weight, and ionic state. Most AEDs are sufficiently lipophilic to penetrate cellular membranes by passive diffusion.50 However, drug efflux transporters at the gastrointestinal tract and the BBB may limit absorption and brain uptake of AEDs, so that genetic variation in the expression and functionality of such transporters may determine clinical outcomes. For drugs such as AEDs that act on targets in the brain, sufficient penetration through the BBB is a prerequisite for therapeutic efficacy.
Delivery of Antiepileptic Drugs through BBB
Most AEDs are quite lipophilic, so they can easily penetrate through the brain capillary endothelial cells that form the BBB.50 However, efflux transporters such as P-glycoprotein (Pgp), which are located at the apical (luminal) membrane of brain capillary endothelial cells and protect the brain from intoxication by lipophilic xenobiotics, may restrict the brain uptake of AEDs and mediate extrusion of AEDs from the brain.51 Because most AEDs are only weak substrates for Pgp, the basal (constitutive) expression of Pgp at the BBB is unlikely to restrict brain penetration of AEDs to any clinically important extent. However, intrinsic or acquired overexpression of Pgp in the BBB may critically limit drug penetration into the brain, leading to resistance against all AEDs that are substrates of Pgp.50 Such Pgp overexpression can result from the effects of disease or drug treatment on Pgp expression or from ABCB1 polymorphisms and might explain the clinical observation that patients with DRE are usually resistant to a broad range of AEDs with different mechanisms of action.51
Increased expression of Pgp and other drug efflux transporters has been determined in epileptogenic brain tissue of patients with DRE and in rodent models of DRE. In 2003, Siddiqui et al.52 reported the C3435T polymorphism in the ABCB1 gene as being associated with resistance to multiple AEDs, leading to the suggestion that drug resistance in epilepsy might be genetically determined, which could open new therapeutic avenues. In a follow-up study,53 the association of AED resistance with the C3435T polymorphism was confirmed in a larger group of patients, and intronic sites that are strongly associated with the C3435T polymorphism were identified. Almost all previous studies on gene variation that might affect AED distribution have dealt with polymorphisms in the ABCB1 gene that encodes Pgp. However, there are polymorphisms in other drug efflux transporters, such as members of the human multidrug resistance protein (MRP) family that may affect the distribution of AEDs and need further investigation.
Once an AED has successfully passed the BBB, its next step is to reach its molecular target in the brain. Although currently available AEDs appear to be directed against a relatively small number of targets—mainly ion channels or other components of the synaptic machinery—matters are complicated by the fact that many AEDs seem to work through multiple mechanisms, some of which are still unresolved.54 Recent efforts have revealed interesting genetic polymorphisms in some of these AED targets. However, it has to be stressed that, at present, we still know very little about the functionality of such polymorphisms and to what extent such variations have a clinically relevant impact on AED treatment.
As knowledge about mechanisms of seizure generation has improved, there has been a concurrent evolution in our thinking about seizure-related definitions and indications for the initiation of treatment. Several next-generation drug therapies with more specific targets have also become available. Taken together, there have been significant improvements in care options. The search for new ways of transporting of AEDs through the BBB to target neurons/neuronal circuits would allow us to achieve good safety and a high efficiency of antiepileptic therapy.
Restoring BBB in Epilepsy
Since it is proved that, the participation of the BBB plays a crucial role in the development of epilepsy reduction of BBB leakage may be a novel therapeutic approach.36,37 To date, there are already some known ways to restore the BBB based on the inhibition of some receptors and pathways.
One of them is the inhibition of albumin-mediated transforming growth factor β (TGF-β) signaling.55 The TGF-β family of proteins is a group of cytokines that play a role in intercellular communication and regulate cellular processes, including cell growth, migration, differentiation, apoptosis, inflammation, and expression of extracellular matrix proteins.55, 56, 57 TGF-β signaling is activated by albumin and induces astrocytic transformation and inflammatory signaling and delays the downregulation of GABA-related genes.55,58,59 These lead to the downregulation of potassium channels, glutamate transporters, and gap junction proteins, resulting in accumulation of extracellular potassium and glutamate upon neuronal activation, further increasing network excitability.55
Bar-Klein et al.55 have shown that the Food and Drug Administration (FDA)-approved angiotensin II type 1 receptor (AT1) antagonist losartan may be used as a potential TGF-β signaling blocker. Losartan decreases BBB permeability in hypertensive rats60,61 and prevents epilepsy following BBB dysfunction.55
One more way to restore BBB function is the inhibition of proinflammatory cytokine interleukin (IL)-1β. Brain inflammation is considered a crucial etiopathogenetic mechanism of epilepsy. Seizures initiate brain inflammation in glia and promote BBB damage that is independent of either leukocytes or bloodborne inflammatory molecules.62 IL-1β is an etiologic trigger for BBB breakdown, and its serum elevation occurs before the onset of status epilepticus (SE). SE is determined as a continuous seizure continuing more than 30 min. Intravenous administration of IL-1 receptor antagonists (IL-1ras) prevented pilocarpine-induced seizures in animals. Animals pretreated with IL-1ras exhibited significant reduction of SE and BBB damage.63
Using a mouse model of epilepsy, Fabene et al.64 showed that seizures induce elevated expression of vascular cell adhesion molecules and enhance the leukocyte rolling and the arrest in brain vessels mediated by the leukocyte mucin P-selectin glycoprotein ligand-1 (PSGL-1) and leukocyte integrins α4β1 and αLβ2. Inhibition of leukocyte-vascular interactions either with blocking antibodies or genetic manipulations in mice deficient in functional PSGL-1 reduced seizures. The blockade of leukocyte-vascular adhesion by anti-α4 interine and PSGL-1 or FucT-VII deficiency prevented BBB leakage.64
Aptamers as Potential Antiepileptic Drugs
A key step in the progression of epileptogenesis is the dysregulation of ion and anion channels and the channels associated with them, followed by depolarization of neuron membrane. Another problem is inflammation leading to chronic epilepsy and selective permeability of the BBB. Obviously, an ideal drug for epilepsy treatment should meet a number of requirements such as minimal toxicity, an ability to regulate membrane potential, and an ability to restore the BBB.
Aptamers are non-toxic and able to ensure address delivery, so they may have the potential to be a good drug. The ability to easily modify aptamers by attaching functional groups such as biotin, carboxyl, thiol, and so forth65, 66, 67 allows the creation of aptamer complexes with different drugs and nanomaterials.67 Due to their small size, aptamers have a better ability to penetrate tissues compared with antibodies,68 which makes it possible to use the aptamers for targeted drug delivery. Modification with 2′-O-methylribonucleotides gives aptamers nuclease resistance and allows their use in vivo.65 Aptamers, functional DNA or RNA molecules, can be selected to bind almost any receptor. Aptamers interact with receptors through non-covalent interactions, hydrogen bonding, electrostatic interactions, and van der Waals forces. Since different receptors play an important role in the development of epileptogenesis, aptamers can be used to inhibit or activate a particular receptor of interest.
To date, there are already a number of drugs based on aptamers that have been introduced into the clinic and a large number of publications and studies of aptamers that have potential clinical application. The main problem in the development of aptamer-based antiepileptic drugs is the right choice of a target and following validation of the selected aptamers. A general outline of aptamer-based drug development includes three main steps: (1) aptamer selection, (2) development of an aptamer-based drug, and (3) preclinical and clinical studies of the drug. Here, we draw attention to the aptamer selection using selection of ligands by exponential enrichment (SELEX). SELEX consists of several repeating rounds of three major steps: (1) incubation of a library or a pool of oligonucleotides with a target; (2) partitioning the aptamer-target complex from free oligonucleotides; and (3) amplification of the bound aptamers by PCR.
There are a variety of SELEX methods of obtaining aptamers to biological targets. When a target is a whole cell, cell-SELEX is the right choice.67,69 Cell-SELEX can be used without much knowledge of a molecular profile of the cell. The aptamers selected by this method can recognize unknown cell proteins at their native states and posttranslational modifications. Protein identity can be established after protein pulldown and the following mass spectrometry analysis. Cell-SELEX allows us to discover new biomarkers and find a molecular fingerprint of disease cells. When a target is known, ligand-guided selection of target-specific aptamers (LIGS) is recommended.70 It allows us to obtain aptamers against the desired receptor on a cell in its native conformation and modification. The principle of the method is based on competitive displacement of aptamers from the receptor complex by the excess of a monoclonal antibody or a corresponding ligand. Both methods worked well for selection of the aptamers to epilepsy-related targets.
Aptamers for Epilepsy Therapy, BBB Penetration, and Targeted Drug Delivery
Several aptamers for epilepsy therapy have been discovered. Two classes of RNA aptamers that bind to the membrane GABA in rat brain have been selected by Cui et al.71 The GABA receptor (GABA-R) belongs to a superfamily of membrane-bound proteins that regulate signal transmission between cells in the nervous system. It is the target of convulsants such as picrotoxin and is mutated in some forms of epilepsy. Class I and class II of RNA aptamers have different consensus sequences and different binding affinities for the receptor. The class I molecules have a higher affinity for the closed-channel form than for the open-channel receptor form and inhibit GABA-R; the class II aptamers bind with equal or higher affinity to the open-channel form and alleviate picrotoxin inhibition.
Other work describes the aptamer selection for cell surface signaling receptor TrkB. TrkB is a neurotrophin receptor that is widely expressed in the developing and mature mammalian CNS. Signaling via TrkB pathway has a diverse role in the CNS; in particular, it is known to contribute to plasticity. Excessive activation of TrkB is sufficient to induce both epilepsy and neuropathic pain.72 One of the selected aptamers, C4-3, was characterized with the recombinant protein binding assay; cell-based signaling and functional assays; and in vivo, a seizure model in mice. C4-3 binds the extracellular domain of TrkB with high affinity (a dissociation constant, KD ∼2 nM) and exhibits potent TrkB partial agonistic activity and neuroprotective effects in cultured cortical neurons. In mice, C4-3 activates TrkB upon infusion into the hippocampus; systemic administration of C4-3 potentiates kainic-acid-induced seizure development. C4-3 may potentially be a useful therapeutic agent for neurodegenerative diseases in which reduced TrkB activation has been implicated.72
Excessive activation of AMPA (α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid) receptors (AMPA-Rs), a subtype of glutamate ion channels, is associated with various neurological diseases, including epilepsy. AMPA-Rs are activated by glutamate, the major excitatory neurotransmitter, which is thought to play a key role in inducing seizures by initiating and synchronizing glutamatergic transmission.73 AMPA-R antagonists have been investigated for antiepileptic activity both preclinically and clinically, with mixed success.74 In this way, the inhibition of AMPA-R may be used in epilepsy treatment. One such inhibitor is the AN58 aptamer, which exhibits nanomolar affinity for the GluR2 and GluR4 AMPA-Rs and blocks their activity. Huang et al.75 selected RNA aptamers for AMPA-Rs and showed their potential opportunity for the treatment of epilepsy. It was shown that the isolated aptamer is a non-competitive inhibitor that selectively binds the open-channel conformation of AMPA-Rs with nanomolar affinity. The potency and selectivity of the non-competitive aptamer match small-molecule inhibitors. The results, therefore, demonstrate the utility of this approach to develop water-soluble, highly potent, and conformation-selective non-competitive inhibitors of AMPA-Rs.75
One of the known causes of epilepsy is excessive activation of N-methyl-D-aspartate (NMDA) receptors (NMDA-Rs). To reduce the activity of NMDA-Rs, various antagonists with proved effectiveness could be used.76 However, these antagonists have serious side effects, one of which is impaired cognitive function.77,78 Aptamers have several advantages over traditional inhibitors and are promising for future treatment of epilepsy. For example, Lee et al.79 obtained F-modified RNA aptamers with a high affinity for NMDA-Rs and selectively inhibiting GluN2A with little effect on AMPA-Rs and kainate receptors.
Cellular mechanisms of seizure generation are mainly caused by excessive entry of Na+ and Ca2+ neurons under the influence of the neurotransmitters glutamate and aspartate, which cause membrane depolarization and neuron excitation. The mGluRs, AMPA-Rs, kainate receptors, and NMDA-Rs are subtypes of the glutamate ion channels. The AMPA-Rs and kainate receptors have structural similarities, and the excessive activity of these receptors is associated with epilepsy; therefore, suppressing the activity of ionotropic glutamate receptors may be therapeutically beneficial. Huang et al.75 obtained an RNA aptamer to the open-channel conformation of the GluA2 AMPA receptor subunit and showed that the full-length 101-nt aptamer selectively inhibits AMPAR with an inhibitor constant, Ki, of ∼5 μM.
Violation of ion homeostasis can cause changes in functions of ion channels without any mutations in genes. Researchers have shown that some forms of epilepsy may be associated with the formation of new ion channels. The formation of ion channels is a well-known phenomenon.80 Recently, it has been assumed that one of the causes of impaired ion homeostasis during epilepsy may be the formation of such channels de novo.11 Thereby, inhibition of pore formation can restore impaired ion homeostasis and prevent epileptogenesis.
Non-specific ion channels may be formed by amyloidogenic proteins—in particular, α-synuclein. Lashuel et al.81 described the ability of α-synuclein to form annular protofibrils, which might incorporate into membrane, form pores, and disturb membrane permeabilization, leading to cell dysfunction. DNA aptamers F5R1 and F5R2 obtained by Zheng et al.82 that are associated with α-synuclein (with KD in the nanomolar range) with high affinity and specificity may be used for the prevention of pore formation de novo. Both aptamers effectively reduce α-synuclein aggregation in vitro in cells and contribute to the degradation of α-synuclein in lysosomes.
As noted, the BBB plays an important role in the progression of epilepsy. It was found that one of the reasons for the violation of the BBB is the activation of metalloproteinase, which degrades the extracellular matrix.83 Obviously, the suppression of metalloproteinase activity may contribute to the restoration of the broken BBB. Aptamers to metalloproteinases may become good candidates for repairing the BBB disrupted by the degradation of the extracellular matrix.84
It was shown that aptamers can penetrate the BBB by itself and may be used for targeted delivery of other therapeutic aptamers in brain. RNA aptamers penetrating the BBB of mice were selected in vivo by Cheng et al.85 To obtain aptamers, an RNA library 40 nt long, resistant to nucleases, was used. The library was injected into the tail vein of the mouse; then, after 1–3 h, the mouse was perfused with phosphate buffer, and the brain was removed. RNA aptamers were extracted, amplified, and injected into the tail vein of the next mouse. After the 12th round of selection, negative selection was performed for the mouse serum. In total, 22 rounds of aptamer selection were carried out, after which three sequences were selected after sequencing. It was shown that RNA aptamers had the ability to penetrate mouse BBB, initially binding to endothelial cells.85
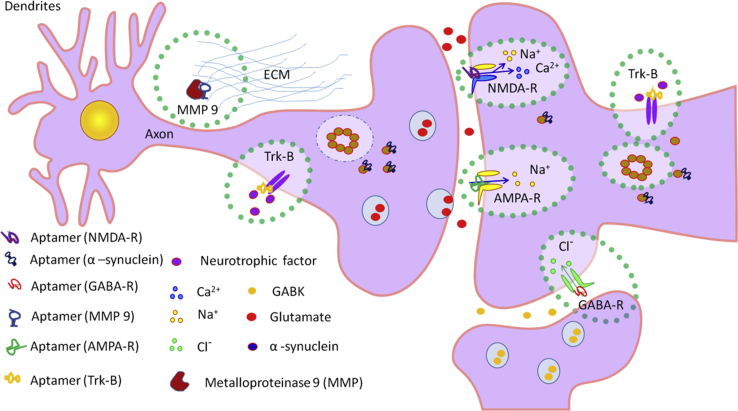
The possibility of targeted delivery of therapeutic aptamers to the brain was shown by Macdonald et al.86 An aptamer for transferrin was used as an agent that binds to the epithelial cell adhesion molecule. This generated a proof of concept that, on the one hand, the bifunctional aptamer can overcome the blood-brain barrier and that, on the other hand, it can specifically target brain disorders. The initial fusion of the two sequences enhanced the binding affinity of both aptamers while maintaining specificity. Additionally, mutations were introduced into both binding loops to determine their effect on aptamer specificity. The ability of the aptamer to transcytose the BBB was then confirmed in vivo following a 1-nmol injection. This study has shown that, through the fusion of two aptamer sequences, a bifunctional aptamer can be generated that has the potential to be developed for the specific treatment of brain disorders.86 The potential possibility of using aptamers for the treatment of epilepsy and targeted delivery of aptamers through the BBB for the treatment of diseases of the CNS is summarized in Table 2 and Figure 3.
Table 2.
Aptamers for Epilepsy Therapy
| Aptamer and Sequence | Target Cells | Protein Target | Application | References |
|---|---|---|---|---|
| F-modified RNA aptamers | neuron | GABA-R | aptamers control the opening of the GABA-R | 71 |
| C4-3: 5′−TCGGGCGAGTCGTCTGUCGUAUUAUCCGCUGCACGCCCGCATCGTCCTCCC-3′ | neuron | TrkB | C4-3 binds the extracellular domain of TrkB with high affinity (KD = ~2 nM) and exhibits potent TrkB partial agonistic activity | 72 |
| AN58: 5′-GGGCGAAUUCAACUGCCAUCUAGGCAGUAACCAGGAGUUAGUAGGACAAGUUUCGUCC-3′ | neuron | AMPA-R | the aptamer is a noncompetitive AMPA-R inhibitor | 75,102 |
| FN1040: 5′-ACGCUACUGUGAGUGUUGUGAUGGCGGCUGAACGAUCGAAACGGAACUGU-3′ | neuron | NMDA-R | the aptamer is an NMDA-R inhibitor | 103 |
| F5R1: 5′-ATCGAGTGTGTACGGGGTCCGGTAGGGTGGCGAGGTCTTCCTGTCGTAGCAGGATCCA-3′; and F5R2: 5′-ATCGAGTGGACGAGTGCCTCCGGTACGAGCTGTCTGATGGGTTTGCGCGCAGGATCCA-3′ | neuron | α-synuclein | aptamers could effectively reduce α-syn aggregation in vitro and in cells and target the α-syn to intracellular degradation through the lysosomal pathway | 82 |
| 8F14A: 5′-TCG TAT GGC ACG GGG TTG GTG TTG GGT TGG-3′ | – | MMP-9 | aptamer inhibits activity metalloproteinase 9 | 84 |
Figure 3.
Existed Aptamers and Their Epilepsy-Related Protein Targets
Discussion
In most cases, epilepsy is caused by violations in mechanisms of membrane potential regulation that cannot be compensated by different reasons. This leads to the depolarization of neuron membrane and status epilepticus, or spontaneous seizures. Normally, in healthy organisms, violation in the mechanisms of maintaining biologically relevant parameters is compensated by other mechanisms. For example, the pathologically high permeability of cell membrane for Na+ ions followed by membrane depolarization is compensated by the high activity of Na+- and K+-ATPase; thereby, membrane potential remains unchanged.
The etiological variety of epilepsy hampered the addressed influence on a pathological target. Consequently, all drugs for the therapy have a broad spectrum of action and high toxicity effect on the whole organism. Moreover, in some cases, the AEDs used do not penetrate the BBB and could not reach the desirable target. Recent studies have shown a close link between the development of epilepsy and the BBB. Epileptic seizures result from membrane depolarization, which causes BBB leakage that, in turn, leads to increased neuron excitability, stimulation of epileptimorphic seizures, and gliosis followed by chronic epilepsy. Therefore, treatment of epilepsy should be comprehensive and influence both the BBB and brain systems. For effective epilepsy treatment, AEDs should be non-toxic, be specific, penetrate the BBB, and restore the BBB.
Aptamers that function, on the one hand, to regulate a membrane potential and stop the seizure and, on the other hand, to treat inflammation and restore the BBB may be a good alternative for the standard drugs, due to their specificity and non-toxicity. Such kinds of drug may include a pool of different aptamers to different targets as well. To date, aptamers for GABA-R, TrkB, AMPA-R, NMDA-R, α-synuclein, and MMP-9 have been selected. AMPA-R, NMDA-R, GABA-R, TrkB, and α-synuclein are located on neurons and carry out active or passive transport of ions; MMP-9 is located in extracellular space and participates in the development of inflammation. Addressed influence of aptamers on these targets may be part of the strategy for epilepsy treatment.
However, to prescribe an adequate treatment, the full picture of epilepsy for each patient should be described. Future studies should be divided in two different ways: (1) development of new diagnostic methods of epilepsy to determine unknown mechanism leading to seizures and (2) development of AEDs for known targets to compensate membrane polarization by known mechanisms. Aptamers may be perspective candidates for both directions of the investigation. To summarize this review, we list here the most promising potential targets for the selection of aptamers as anti-epileptic drug candidates.
-
1.
To block an epileptic seizure and restore membrane potential: Na+/Ca2+ exchangers; Na+ and K+ pumps; Ca2+ pumps; voltage-gated calcium channels; mGluRs; NMDA-Rs; AMPA-Rs; Ach-Rs; transient receptor potential channels; and GABA-Rs.
-
2.
To restore the BBB and decrease inflammation: albumin-mediated TGF-β; AT1; proinflammatory cytokine IL-1β; PSGL-1; and leukocyte integrins α4β1 and αLβ2.
Contributor Information
Maxim V. Berezovski, Email: maxim.berezovski@uottawa.ca.
Anna S. Kichkailo, Email: aszamay@gmail.com.
References
- 1.Duncan J.S., Sander J.W., Sisodiya S.M., Walker M.C. Adult epilepsy. Lancet. 2006;367:1087–1100. doi: 10.1016/S0140-6736(06)68477-8. [DOI] [PubMed] [Google Scholar]
- 2.Brooks-Kayal A.R., Bath K.G., Berg A.T., Galanopoulou A.S., Holmes G.L., Jensen F.E., Kanner A.M., O’Brien T.J., Whittemore V.H., Winawer M.R. Issues related to symptomatic and disease-modifying treatments affecting cognitive and neuropsychiatric comorbidities of epilepsy. Epilepsia. 2013;54(Suppl 4):44–60. doi: 10.1111/epi.12298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Fisher R.S., Cross J.H., French J.A., Higurashi N., Hirsch E., Jansen F.E., Lagae L., Moshé S.L., Peltola J., Roulet Perez E. Operational classification of seizure types by the International League Against Epilepsy: position paper of the ILAE Commission for Classification and Terminology. Epilepsia. 2017;58:522–530. doi: 10.1111/epi.13670. [DOI] [PubMed] [Google Scholar]
- 4.Vezzani A., Pascente R., Ravizza T. Biomarkers of epileptogenesis: the focus on glia and cognitive dysfunctions. Neurochem. Res. 2017;42:2089–2098. doi: 10.1007/s11064-017-2271-3. [DOI] [PubMed] [Google Scholar]
- 5.Beamer E., Fischer W., Engel T. The ATP-gated P2X7 receptor as a target for the treatment of drug-resistant epilepsy. Front. Neurosci. 2017;11:21. doi: 10.3389/fnins.2017.00021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Pitkänen A., Lukasiuk K. Molecular and cellular basis of epileptogenesis in symptomatic epilepsy. Epilepsy Behav. 2009;14(Suppl 1):16–25. doi: 10.1016/j.yebeh.2008.09.023. [DOI] [PubMed] [Google Scholar]
- 7.Pitkänen A., Lukasiuk K. Mechanisms of epileptogenesis and potential treatment targets. Lancet Neurol. 2011;10:173–186. doi: 10.1016/S1474-4422(10)70310-0. [DOI] [PubMed] [Google Scholar]
- 8.Boison D., Sandau U.S., Ruskin D.N., Kawamura M., Jr., Masino S.A. Homeostatic control of brain function – new approaches to understand epileptogenesis. Front. Cell. Neurosci. 2013;7:109. doi: 10.3389/fncel.2013.00109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Engel J., Jr. Classification is not EZ. Epileptic Disord. 2005;7:317–320. [PubMed] [Google Scholar]
- 10.Brodie M.J., Zuberi S.M., Scheffer I.E., Fisher R.S. The 2017 ILAE classification of seizure types and the epilepsies: what do people with epilepsy and their caregivers need to know? Epileptic Disord. 2018;20:77–87. doi: 10.1684/epd.2018.0957. [DOI] [PubMed] [Google Scholar]
- 11.Surguchov A., Surgucheva I., Sharma M., Sharma R., Singh V. Pore-forming proteins as mediators of novel epigenetic mechanism of epilepsy. Front. Neurol. 2017;8:3. doi: 10.3389/fneur.2017.00003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rose C.R., Konnerth A. NMDA receptor-mediated Na+ signals in spines and dendrites. J. Neurosci. 2001;21:4207–4214. doi: 10.1523/JNEUROSCI.21-12-04207.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Pumain R., Heinemann U. Stimulus- and amino acid-induced calcium and potassium changes in rat neocortex. J. Neurophysiol. 1985;53:1–16. doi: 10.1152/jn.1985.53.1.1. [DOI] [PubMed] [Google Scholar]
- 14.Rose C.R., Konnerth A. Exciting glial oscillations. Nat. Neurosci. 2001;4:773–774. doi: 10.1038/90464. [DOI] [PubMed] [Google Scholar]
- 15.Ellender T.J., Raimondo J.V., Irkle A., Lamsa K.P., Akerman C.J. Excitatory effects of parvalbumin-expressing interneurons maintain hippocampal epileptiform activity via synchronous afterdischarges. J. Neurosci. 2014;34:15208–15222. doi: 10.1523/JNEUROSCI.1747-14.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Villa C., Combi R. Potassium channels and human epileptic phenotypes: an updated overview. Front. Cell. Neurosci. 2016;10:81. doi: 10.3389/fncel.2016.00081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Pitkänen A., Kharatishvili I., Karhunen H., Lukasiuk K., Immonen R., Nairismägi J., Gröhn O., Nissinen J. Epileptogenesis in experimental models. Epilepsia. 2007;48(Suppl 2):13–20. doi: 10.1111/j.1528-1167.2007.01063.x. [DOI] [PubMed] [Google Scholar]
- 18.Nissinen J., Halonen T., Koivisto E., Pitkänen A. A new model of chronic temporal lobe epilepsy induced by electrical stimulation of the amygdala in rat. Epilepsy Res. 2000;38:177–205. doi: 10.1016/s0920-1211(99)00088-1. [DOI] [PubMed] [Google Scholar]
- 19.Rakhade S.N., Jensen F.E. Epileptogenesis in the immature brain: emerging mechanisms. Nat. Rev. Neurol. 2009;5:380–391. doi: 10.1038/nrneurol.2009.80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lerche H., Jurkat-Rott K., Lehmann-Horn F. Ion channels and epilepsy. Am. J. Med. Genet. 2001;106:146–159. doi: 10.1002/ajmg.1582. [DOI] [PubMed] [Google Scholar]
- 21.Pal D.K., Pong A.W., Chung W.K. Genetic evaluation and counseling for epilepsy. Nat. Rev. Neurol. 2010;6:445–453. doi: 10.1038/nrneurol.2010.92. [DOI] [PubMed] [Google Scholar]
- 22.Wang J., Lin Z.J., Liu L., Xu H.Q., Shi Y.W., Yi Y.H., He N., Liao W.P. Epilepsy-associated genes. Seizure. 2017;44:11–20. doi: 10.1016/j.seizure.2016.11.030. [DOI] [PubMed] [Google Scholar]
- 23.Wei F., Yan L.M., Su T., He N., Lin Z.J., Wang J., Shi Y.W., Yi Y.H., Liao W.P. Ion channel genes and epilepsy: functional alteration, pathogenic potential, and mechanism of epilepsy. Neurosci. Bull. 2017;33:455–477. doi: 10.1007/s12264-017-0134-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Huber J.D., Egleton R.D., Davis T.P. Molecular physiology and pathophysiology of tight junctions in the blood-brain barrier. Trends Neurosci. 2001;24:719–725. doi: 10.1016/s0166-2236(00)02004-x. [DOI] [PubMed] [Google Scholar]
- 25.Ballabh P., Braun A., Nedergaard M. The blood-brain barrier: an overview: structure, regulation, and clinical implications. Neurobiol. Dis. 2004;16:1–13. doi: 10.1016/j.nbd.2003.12.016. [DOI] [PubMed] [Google Scholar]
- 26.Cardoso F.L., Brites D., Brito M.A. Looking at the blood-brain barrier: molecular anatomy and possible investigation approaches. Brain Res. Brain Res. Rev. 2010;64:328–363. doi: 10.1016/j.brainresrev.2010.05.003. [DOI] [PubMed] [Google Scholar]
- 27.Pardridge W.M. Blood-brain barrier delivery. Drug Discov. Today. 2007;12:54–61. doi: 10.1016/j.drudis.2006.10.013. [DOI] [PubMed] [Google Scholar]
- 28.Angelow S., Yu A.S. Claudins and paracellular transport: an update. Curr. Opin. Nephrol. Hypertens. 2007;16:459–464. doi: 10.1097/MNH.0b013e32820ac97d. [DOI] [PubMed] [Google Scholar]
- 29.Schlosshauer B., Steuer H. Comparative anatomy, physiology and in vitro models of the blood–brain and blood–retina barrier. Curr. Med. Chem.-Cent. Nerv. Syst. Agents. 2002;2:175–186. [Google Scholar]
- 30.Burnette R., Marrero D. Comparison between passive transport of protein across excised nude mouse skin. J. Pharm. Sci. 1994;75:738–743. doi: 10.1002/jps.2600750803. [DOI] [PubMed] [Google Scholar]
- 31.Pardridge W.M. The blood-brain barrier: bottleneck in brain drug development. NeuroRx. 2005;2:3–14. doi: 10.1602/neurorx.2.1.3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Abbott N.J., Patabendige A.A., Dolman D.E., Yusof S.R., Begley D.J. Structure and function of the blood-brain barrier. Neurobiol. Dis. 2010;37:13–25. doi: 10.1016/j.nbd.2009.07.030. [DOI] [PubMed] [Google Scholar]
- 33.Johansson B., Nilsson B. The pathophysiology of the blood-brain barrier dysfunction induced by severe hypercapnia and by epileptic brain activity. Acta Neuropathol. 1977;38:153–158. doi: 10.1007/BF00688563. [DOI] [PubMed] [Google Scholar]
- 34.Petito C.K., Schaefer J.A., Plum F. Ultrastructural characteristics of the brain and blood-brain barrier in experimental seizures. Brain Res. 1977;127:251–267. doi: 10.1016/0006-8993(77)90539-x. [DOI] [PubMed] [Google Scholar]
- 35.Michalak Z., Sano T., Engel T., Miller-Delaney S.F., Lerner-Natoli M., Henshall D.C. Spatio-temporally restricted blood-brain barrier disruption after intra-amygdala kainic acid-induced status epilepticus in mice. Epilepsy Res. 2013;103:167–179. doi: 10.1016/j.eplepsyres.2012.10.006. [DOI] [PubMed] [Google Scholar]
- 36.van Vliet E.A., da Costa Araújo S., Redeker S., van Schaik R., Aronica E., Gorter J.A. Blood-brain barrier leakage may lead to progression of temporal lobe epilepsy. Brain. 2007;130:521–534. doi: 10.1093/brain/awl318. [DOI] [PubMed] [Google Scholar]
- 37.van Vliet E.A., Aronica E., Gorter J.A. Blood-brain barrier dysfunction, seizures and epilepsy. Semin. Cell Dev. Biol. 2015;38:26–34. doi: 10.1016/j.semcdb.2014.10.003. [DOI] [PubMed] [Google Scholar]
- 38.Friedman A., Kaufer D., Heinemann U. Blood-brain barrier breakdown-inducing astrocytic transformation: novel targets for the prevention of epilepsy. Epilepsy Res. 2009;85:142–149. doi: 10.1016/j.eplepsyres.2009.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Marchi N., Angelov L., Masaryk T., Fazio V., Granata T., Hernandez N., Hallene K., Diglaw T., Franic L., Najm I., Janigro D. Seizure-promoting effect of blood-brain barrier disruption. Epilepsia. 2007;48:732–742. doi: 10.1111/j.1528-1167.2007.00988.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Jobst B.C. Consensus over individualism: validation of the ILAE definition for drug resistant epilepsy. Epilepsy Curr. 2015;15:172–173. doi: 10.5698/1535-7511-15.4.172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kwan P., Schachter S.C., Brodie M.J. Drug-resistant epilepsy. N. Engl. J. Med. 2011;365:919–926. doi: 10.1056/NEJMra1004418. [DOI] [PubMed] [Google Scholar]
- 42.Téllez-Zenteno J.F., Ronquillo L.H., Wiebe S. Sudden unexpected death in epilepsy: evidence-based analysis of incidence and risk factors. Epilepsy Res. 2005;65:101–115. doi: 10.1016/j.eplepsyres.2005.05.004. [DOI] [PubMed] [Google Scholar]
- 43.Kwan P., Arzimanoglou A., Berg A.T., Brodie M.J., Allen Hauser W., Mathern G., Moshé S.L., Perucca E., Wiebe S., French J. Definition of drug resistant epilepsy: consensus proposal by the ad hoc Task Force of the ILAE Commission on Therapeutic Strategies. Epilepsia. 2010;51:1069–1077. doi: 10.1111/j.1528-1167.2009.02397.x. [DOI] [PubMed] [Google Scholar]
- 44.Brodie M.J., Barry S.J., Bamagous G.A., Norrie J.D., Kwan P. Patterns of treatment response in newly diagnosed epilepsy. Neurology. 2012;78:1548–1554. doi: 10.1212/WNL.0b013e3182563b19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Meador K.J., Baker G.A., Browning N., Cohen M.J., Clayton-Smith J., Kalayjian L.A., Kanner A., Liporace J.D., Pennell P.B., Privitera M. Foetal antiepileptic drug exposure and verbal versus non-verbal abilities at three years of age. Brain. 2011;134:396–404. doi: 10.1093/brain/awq352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Chung S.S., Kelly K., Schusse C. New and emerging treatments for epilepsy: review of clinical studies of lacosamide, eslicarbazepine acetate, ezogabine, rufinamide, perampanel, and electrical stimulation therapy. J. Epilepsy Res. 2011;1:35–46. doi: 10.14581/jer.11008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Vogel F. Moderne probleme der humangenetik. Med. Kinderheilkunde. 1959;12:52–125. [Google Scholar]
- 48.Löscher W., Klotz U., Zimprich F., Schmidt D. The clinical impact of pharmacogenetics on the treatment of epilepsy. Epilepsia. 2009;50:1–23. doi: 10.1111/j.1528-1167.2008.01716.x. [DOI] [PubMed] [Google Scholar]
- 49.Lewis L.D. Personalized drug therapy; the genome, the chip and the physician. Br. J. Clin. Pharmacol. 2005;60:1–4. doi: 10.1111/j.1365-2125.2005.02457.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Löscher W., Potschka H. Role of drug efflux transporters in the brain for drug disposition and treatment of brain diseases. Prog. Neurobiol. 2005;76:22–76. doi: 10.1016/j.pneurobio.2005.04.006. [DOI] [PubMed] [Google Scholar]
- 51.Kwan P., Brodie M.J. Potential role of drug transporters in the pathogenesis of medically intractable epilepsy. Epilepsia. 2005;46:224–235. doi: 10.1111/j.0013-9580.2005.31904.x. [DOI] [PubMed] [Google Scholar]
- 52.Siddiqui A., Kerb R., Weale M.E., Brinkmann U., Smith A., Goldstein D.B., Wood N.W., Sisodiya S.M. Association of multidrug resistance in epilepsy with a polymorphism in the drug-transporter gene ABCB1. N. Engl. J. Med. 2003;348:1442–1448. doi: 10.1056/NEJMoa021986. [DOI] [PubMed] [Google Scholar]
- 53.Soranzo N., Cavalleri G.L., Weale M.E., Wood N.W., Depondt C., Marguerie R., Sisodiya S.M., Goldstein D.B. Identifying candidate causal variants responsible for altered activity of the ABCB1 multidrug resistance gene. Genome Res. 2004;14:1333–1344. doi: 10.1101/gr.1965304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Rogawski M.A., Löscher W. The neurobiology of antiepileptic drugs. Nat. Rev. Neurosci. 2004;5:553–564. doi: 10.1038/nrn1430. [DOI] [PubMed] [Google Scholar]
- 55.Bar-Klein G., Cacheaux L.P., Kamintsky L., Prager O., Weissberg I., Schoknecht K., Cheng P., Kim S.Y., Wood L., Heinemann U. Losartan prevents acquired epilepsy via TGF-β signaling suppression. Ann. Neurol. 2014;75:864–875. doi: 10.1002/ana.24147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Massagué J. How cells read TGF-beta signals. Nat. Rev. Mol. Cell Biol. 2000;1:169–178. doi: 10.1038/35043051. [DOI] [PubMed] [Google Scholar]
- 57.Shi Y., Massagué J. Mechanisms of TGF-beta signaling from cell membrane to the nucleus. Cell. 2003;113:685–700. doi: 10.1016/s0092-8674(03)00432-x. [DOI] [PubMed] [Google Scholar]
- 58.Cacheaux L.P., Ivens S., David Y., Lakhter A.J., Bar-Klein G., Shapira M., Heinemann U., Friedman A., Kaufer D. Transcriptome profiling reveals TGF-beta signaling involvement in epileptogenesis. J. Neurosci. 2009;29:8927–8935. doi: 10.1523/JNEUROSCI.0430-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.David Y., Cacheaux L.P., Ivens S., Lapilover E., Heinemann U., Kaufer D., Friedman A. Astrocytic dysfunction in epileptogenesis: consequence of altered potassium and glutamate homeostasis? J. Neurosci. 2009;29:10588–10599. doi: 10.1523/JNEUROSCI.2323-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Kaya M., Kalayci R., Küçük M., Arican N., Elmas I., Kudat H., Korkut F. Effect of losartan on the blood-brain barrier permeability in diabetic hypertensive rats. Life Sci. 2003;73:3235–3244. doi: 10.1016/j.lfs.2003.06.014. [DOI] [PubMed] [Google Scholar]
- 61.Kucuk M., Kaya M., Kalayci R., Cimen V., Kudat H., Arican N., Elmas I., Korkut F. Effects of losartan on the blood-brain barrier permeability in long-term nitric oxide blockade-induced hypertensive rats. Life Sci. 2002;71:937–946. doi: 10.1016/s0024-3205(02)01772-1. [DOI] [PubMed] [Google Scholar]
- 62.Librizzi L., Noè F., Vezzani A., de Curtis M., Ravizza T. Seizure-induced brain-borne inflammation sustains seizure recurrence and blood-brain barrier damage. Ann. Neurol. 2012;72:82–90. doi: 10.1002/ana.23567. [DOI] [PubMed] [Google Scholar]
- 63.Marchi N., Fan Q., Ghosh C., Fazio V., Bertolini F., Betto G., Batra A., Carlton E., Najm I., Granata T., Janigro D. Antagonism of peripheral inflammation reduces the severity of status epilepticus. Neurobiol. Dis. 2009;33:171–181. doi: 10.1016/j.nbd.2008.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Fabene P.F., Navarro Mora G., Martinello M., Rossi B., Merigo F., Ottoboni L., Bach S., Angiari S., Benati D., Chakir A. A role for leukocyte-endothelial adhesion mechanisms in epilepsy. Nat. Med. 2008;14:1377–1383. doi: 10.1038/nm.1878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Da Pieve C., Williams P., Haddleton D.M., Palmer R.M., Missailidis S. Modification of thiol functionalized aptamers by conjugation of synthetic polymers. Bioconjug. Chem. 2010;21:169–174. doi: 10.1021/bc900397s. [DOI] [PubMed] [Google Scholar]
- 66.Kang W.J., Chae J.R., Cho Y.L., Lee J.D., Kim S. Multiplex imaging of single tumor cells using quantum-dot-conjugated aptamers. Small. 2009;5:2519–2522. doi: 10.1002/smll.200900848. [DOI] [PubMed] [Google Scholar]
- 67.Sefah K., Shangguan D., Xiong X., O’Donoghue M.B., Tan W. Development of DNA aptamers using cell-SELEX. Nat. Protoc. 2010;5:1169–1185. doi: 10.1038/nprot.2010.66. [DOI] [PubMed] [Google Scholar]
- 68.Levy-Nissenbaum E., Radovic-Moreno A.F., Wang A.Z., Langer R., Farokhzad O.C. Nanotechnology and aptamers: applications in drug delivery. Trends Biotechnol. 2008;26:442–449. doi: 10.1016/j.tibtech.2008.04.006. [DOI] [PubMed] [Google Scholar]
- 69.Fang X., Tan W. Aptamers generated from cell-SELEX for molecular medicine: a chemical biology approach. Acc. Chem. Res. 2010;43:48–57. doi: 10.1021/ar900101s. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Zumrut H.E., Ara M.N., Fraile M., Maio G., Mallikaratchy P. Ligand-guided selection of target-specific aptamers: a screening technology for identifying specific aptamers against cell-surface proteins. Nucleic Acid Ther. 2016;26:190–198. doi: 10.1089/nat.2016.0611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Cui Y., Rajasethupathy P., Hess G.P. Selection of stable RNA molecules that can regulate the channel-opening equilibrium of the membrane-bound gamma-aminobutyric acid receptor. Biochemistry. 2004;43:16442–16449. doi: 10.1021/bi048667b. [DOI] [PubMed] [Google Scholar]
- 72.Huang Y.Z., Hernandez F.J., Gu B., Stockdale K.R., Nanapaneni K., Scheetz T.E., Behlke M.A., Peek A.S., Bair T., Giangrande P.H., McNamara J.O., 2nd RNA aptamer-based functional ligands of the neurotrophin receptor, TrkB. Mol. Pharmacol. 2012;82:623–635. doi: 10.1124/mol.112.078220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Scharfman H.E. The neurobiology of epilepsy. Curr. Neurol. Neurosci. Rep. 2007;7:348–354. doi: 10.1007/s11910-007-0053-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Hanada T., Hashizume Y., Tokuhara N., Takenaka O., Kohmura N., Ogasawara A., Hatakeyama S., Ohgoh M., Ueno M., Nishizawa Y. Perampanel: a novel, orally active, noncompetitive AMPA-receptor antagonist that reduces seizure activity in rodent models of epilepsy. Epilepsia. 2011;52:1331–1340. doi: 10.1111/j.1528-1167.2011.03109.x. [DOI] [PubMed] [Google Scholar]
- 75.Huang Z., Han Y., Wang C., Niu L. Potent and selective inhibition of the open-channel conformation of AMPA receptors by an RNA aptamer. Biochemistry. 2010;49:5790–5798. doi: 10.1021/bi100690k. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Aarsland D., Ballard C., Walker Z., Bostrom F., Alves G., Kossakowski K., Leroi I., Pozo-Rodriguez F., Minthon L., Londos E. Memantine in patients with Parkinson’s disease dementia or dementia with Lewy bodies: a double-blind, placebo-controlled, multicentre trial. Lancet Neurol. 2009;8:613–618. doi: 10.1016/S1474-4422(09)70146-2. [DOI] [PubMed] [Google Scholar]
- 77.Calabresi P., Centonze D., Cupini L.M., Costa C., Pisani F., Bernardi G. Ionotropic glutamate receptors: still a target for neuroprotection in brain ischemia? Insights from in vitro studies. Neurobiol. Dis. 2003;12:82–88. doi: 10.1016/s0969-9961(02)00016-5. [DOI] [PubMed] [Google Scholar]
- 78.Traynelis S.F., Wollmuth L.P., McBain C.J., Menniti F.S., Vance K.M., Ogden K.K., Hansen K.B., Yuan H., Myers S.J., Dingledine R. Glutamate receptor ion channels: structure, regulation, and function. Pharmacol. Rev. 2010;62:405–496. doi: 10.1124/pr.109.002451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Lee G., MacLean D.M., Ulrich H., Zhao X., Aronowski J., Jayaraman V. RNA based antagonist of NMDA receptors. ACS Chem. Neurosci. 2014;5:559–567. doi: 10.1021/cn500041k. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Zakharov S.D., Hulleman J.D., Dutseva E.A., Antonenko Y.N., Rochet J.C., Cramer W.A. Helical alpha-synuclein forms highly conductive ion channels. Biochemistry. 2007;46:14369–14379. doi: 10.1021/bi701275p. [DOI] [PubMed] [Google Scholar]
- 81.Lashuel H.A., Hartley D., Petre B.M., Walz T., Lansbury P.T., Jr. Neurodegenerative disease: amyloid pores from pathogenic mutations. Nature. 2002;418:291. doi: 10.1038/418291a. [DOI] [PubMed] [Google Scholar]
- 82.Zheng Y., Qu J., Xue F., Zheng Y., Yang B., Chang Y., Yang H., Zhang J. Novel DNA aptamers for Parkinson’s disease treatment inhibit α-synuclein aggregation and facilitate its degradation. Mol. Ther. Nucleic Acids. 2018;11:228–242. doi: 10.1016/j.omtn.2018.02.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Rempe R.G., Hartz A.M.S., Soldner E.L.B., Sokola B.S., Alluri S.R., Abner E.L., Kryscio R.J., Pekcec A., Schlichtiger J., Bauer B. Matrix metalloproteinase-mediated blood-brain barrier dysfunction in epilepsy. J. Neurosci. 2018;38:4301–4315. doi: 10.1523/JNEUROSCI.2751-17.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Scarano S., Dausse E., Crispo F., Toulmé J.J., Minunni M. Design of a dual aptamer-based recognition strategy for human matrix metalloproteinase 9 protein by piezoelectric biosensors. Anal. Chim. Acta. 2015;897:1–9. doi: 10.1016/j.aca.2015.07.009. [DOI] [PubMed] [Google Scholar]
- 85.Cheng C., Chen Y.H., Lennox K.A., Behlke M.A., Davidson B.L. In vivo SELEX for Identification of brain-penetrating aptamers. Mol. Ther. Nucleic Acids. 2013;2:e67. doi: 10.1038/mtna.2012.59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Macdonald J., Henri J., Goodman L., Xiang D., Duan W., Shigdar S. Development of a bifunctional aptamer targeting the transferrin receptor and epithelial cell adhesion molecule (EpCAM) for the treatment of brain cancer metastases. ACS Chem. Neurosci. 2017;8:777–784. doi: 10.1021/acschemneuro.6b00369. [DOI] [PubMed] [Google Scholar]
- 87.Zuberi S.M., Eunson L.H., Spauschus A., De Silva R., Tolmie J., Wood N.W., McWilliam R.C., Stephenson J.B., Kullmann D.M., Hanna M.G. A novel mutation in the human voltage-gated potassium channel gene (Kv1.1) associates with episodic ataxia type 1 and sometimes with partial epilepsy. Brain. 1999;122:817–825. doi: 10.1093/brain/122.5.817. [DOI] [PubMed] [Google Scholar]
- 88.Spampanato J., Kearney J.A., de Haan G., McEwen D.P., Escayg A., Aradi I., MacDonald B.T., Levin S.I., Soltesz I., Benna P. A novel epilepsy mutation in the sodium channel SCN1A identifies a cytoplasmic domain for beta subunit interaction. J. Neurosci. 2004;24:10022–10034. doi: 10.1523/JNEUROSCI.2034-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Zamponi G.W., Lory P., Perez-Reyes E. Role of voltage-gated calcium channels in epilepsy. Pflugers Arch. 2010;460:395–403. doi: 10.1007/s00424-009-0772-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Vennekens R., Menigoz A., Nilius B. TRPs in the brain. Rev. Physiol. Biochem. Pharmacol. 2012;163:27–64. doi: 10.1007/112_2012_8. [DOI] [PubMed] [Google Scholar]
- 91.Raimondo J.V., Burman R.J., Katz A.A., Akerman C.J. Ion dynamics during seizures. Front. Cell. Neurosci. 2015;9:419. doi: 10.3389/fncel.2015.00419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.N’Gouemo P. Probing the role of the sodium/calcium exchanger in pentylenetetrazole-induced generalized seizures in rats. Brain Res. Bull. 2013;90:52–57. doi: 10.1016/j.brainresbull.2012.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Du M., Li J., Wang R., Wu Y. The influence of potassium concentration on epileptic seizures in a coupled neuronal model in the hippocampus. Cogn Neurodyn. 2016;10:405–414. doi: 10.1007/s11571-016-9390-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Bravo-Martínez J., Delgado-Coello B., García D.E., Mas-Oliva J. Analysis of plasma membrane Ca2+-ATPase gene expression during epileptogenesis employing single hippocampal CA1 neurons. Exp. Biol. Med. (Maywood) 2011;236:409–417. doi: 10.1258/ebm.2011.010342. [DOI] [PubMed] [Google Scholar]
- 95.Becchetti A., Aracri P., Meneghini S., Brusco S., Amadeo A. The role of nicotinic acetylcholine receptors in autosomal dominant nocturnal frontal lobe epilepsy. Front. Physiol. 2015;6:22. doi: 10.3389/fphys.2015.00022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Liu X., Yan B., Wang R., Li C., Chen C., Zhou D., Hong Z. Seizure outcomes in patients with anti-NMDAR encephalitis: a follow-up study. Epilepsia. 2017;58:2104–2111. doi: 10.1111/epi.13929. [DOI] [PubMed] [Google Scholar]
- 97.Russo E., Gitto R., Citraro R., Chimirri A., De Sarro G. New AMPA antagonists in epilepsy. Expert Opin. Investig. Drugs. 2012;21:1371–1389. doi: 10.1517/13543784.2012.705277. [DOI] [PubMed] [Google Scholar]
- 98.Crépel V., Mulle C. Physiopathology of kainate receptors in epilepsy. Curr. Opin. Pharmacol. 2015;20:83–88. doi: 10.1016/j.coph.2014.11.012. [DOI] [PubMed] [Google Scholar]
- 99.Funke M.G., Costa Mda.S., Amado D., Cavalheiro E.A., Naffah-Mazzacoratti Mda.G. Calcium homeostasis and temporal lobe epilepsy. Arq. Neuropsiquiatr. 2003;61(Suppl 1):8–14. [PubMed] [Google Scholar]
- 100.Engelborghs S., D’Hooge R., De Deyn P.P. Pathophysiology of epilepsy. Acta Neurol. Belg. 2000;100:201–213. [PubMed] [Google Scholar]
- 101.Binder D.K., Nagelhus E.A., Ottersen O.P. Aquaporin-4 and epilepsy. Glia. 2012;60:1203–1214. doi: 10.1002/glia.22317. [DOI] [PubMed] [Google Scholar]
- 102.Huang Z., Pei W., Jayaseelan S., Shi H., Niu L. RNA aptamers selected against the GluR2 glutamate receptor channel. Biochemistry. 2007;46:12648–12655. doi: 10.1021/bi701036p. [DOI] [PubMed] [Google Scholar]
- 103.Huang Z., Wen W., Wu A., Niu L. Chemically modified, α-amino-3-hydroxy-5-methyl-4-isoxazole (AMPA) receptor RNA aptamers designed for in vivo use. ACS Chem. Neurosci. 2017;8:2437–2445. doi: 10.1021/acschemneuro.7b00211. [DOI] [PubMed] [Google Scholar]