Short abstract
Background
A high burden of non-communicable diseases (NCDs) is contributing to high mortality and morbidity in India. Recent advancements in digital health interventions, including mHealth, eHealth, and telemedicine, have facilitated patient-centered care for NCDs.
Objective
This systematic review aims to evaluate the current evidence on digital interventions for people living with NCDs in India and the outcomes of those interventions.
Methods
We adopted PRISMA guidelines and systematically reviewed articles from MEDLINE, CINAHL, PsycINFO, ERIC, and Scopus databases with following criteria: journal articles presenting digital intervention(s) used by people with at least one of the NCDs, reporting health outcomes following the intervention, studies conducted in India among Indian population.
Results
Among 1669 articles retrieved from multiple sources, only 13 articles met our criteria. Most (n = 7) studies were conducted in southern states of India; eight studies included patients with diabetes, followed by neuropsychiatric disorders and other NCDs. Five studies recruited participants from tertiary hospitals; six interventions used text-messaging for delivering health services, and 10 studies reported randomized controlled trials. All the studies reported positive health outcomes following the intervention, including better self-management, increased patient–provider communication, improved medication adherence, and reduced disease symptoms. Most studies scored moderate to high in quality assessment checklist of Downs and Black.
Conclusion
Current evidence suggests a low number of interventions with positive outcomes. Future research should explore avenues of advanced technologies ensuring equitable and sustainable development of digital health interventions for people living with NCDs in India.
Keywords: Non-communicable diseases, chronic diseases, mhealth, telemedicine, digital health, India
Introduction
The World Health Organization (WHO) defines non-communicable diseases (NCDs) as chronic conditions of long duration resulting from a combination of genetic, physiological, behavioral, and environmental factors.1 Nearly 63% of all deaths can be attributable to NCDs, making this the leading cause of mortality in the world.2 While more than 36 million people die each year due to NCDs, about 80% of NCD deaths occur in low and middle-income countries.2 India is a highly populous country with a population of more than 1.3 billion which is facing an immense burden of NCDs.3 Demographic and epidemiological transitions over the past few decades have resulted in a shift in the national burden of diseases from infectious diseases to NCDs.3,4 In 2017, India State-Level Disease Burden Initiative Collaborators reported nationwide variations of disease burden, which highlights that the prevalence of and mortality due to NCDs have increased across all the states between 1990 to 2016.5 About 8.3% of all deaths and 5% of total disability-adjusted life years (DALYs) in 2016 were due to the contribution of cancer, which has doubled since 1990.6 In addition to contributing to mortalities and morbidities, NCDs also affect the national economy enormously. For example, the economic losses from heart disease, stroke, and diabetes were estimated to be US$54 billion in 2015.7 Considering the population health and associated challenges, it is essential to explore potential avenues to alleviate the high burden of NCDs in India. Providing conventional care for NCDs in a large population can be difficult because patients living with chronic conditions need continuous monitoring and prolonged treatment.8 As the country suffers from a lack of adequate healthcare infrastructure and a severe scarcity of human resources for health,9 conventional healthcare delivery methods involving face-to-face doctor–patient interactions might not be available to most of the people in India. Moreover, critical challenges such as long distance from the nearest health center, lack of transportation to health facilities, lack of awareness about health services, and high cost of seeking care can affect timely diagnosis and treatment of NCDs.10 These problems necessitate interventions for making healthcare for NCDs more available, accessible, and acceptable to the patients who need them the most. Furthermore, chronic diseases make individuals vulnerable to mental and physical stress.11 Empowering patients and their caregivers can help in addressing health problems through enhanced participation and adherence to optimal care at the community level.12
In the era of digital technologies, many mHealth and eHealth interventions have been developed to provide a wide range of healthcare services to people living with different health conditions.13–15 Different devices and platforms, including mobile phone, website, software, wearable devices, and tablet computers, using online and offline digital technology platforms, are increasingly being used in healthcare; these are collectively termed as digital health.16,17 The WHO recognizes the potential of digital health interventions to achieve universal health coverage and ensure high-quality care to individuals.17 These interventions can provide preventive, diagnostic, therapeutic, and palliative care using digital platforms such as mobile phone, portable computing devices, internet-based applications, and social media interfaces.18 Many such interventions are designed and implemented for people living with NCDs. For example, a systematic review by Larson et al. evaluated nine telehealth interventions for patients receiving cancer care.19 They found telehealth interventions to be similarly effective as usual care in terms of improving quality of life among study participants. This study highlighted opportunities to increase access to effective telehealth services at a lower cost. Another meta-analysis of 35 randomized controlled trials has reported beneficial effects of internet-based interventions for patients with type 2 diabetes mellitus.20 Similar studies have been conducted among patients with other NCDs, including cardiovascular diseases,21 stroke,22 osteoarthritis,23 depression,24 and chronic obstructive pulmonary disease (COPD).25 Such digital interventions facilitate better symptom assessment, self-management, reduction of symptom distress, awareness of health conditions, patient–provider communication, timely care-seeking, follow-up and referral, treatment adherence, and improved quality of living among the patients living with NCDs.26–28
However, the application of such advanced technological innovations is relatively low among the low and middle-income countries, who share the major proportion of the global burden of NCDs but have limited resources and opportunities to leverage the benefits of digital health.29,30 In India, the use of digital technologies is gaining momentum in recent years.31 However, most of these interventions are being implemented within hospitals, where the health workforce has access to them.31,32 One systematic review by Bassi et al. reported the application of mHealth interventions in strengthening the overall health system in India with a primary focus on health service delivery.33 These applications are mostly used by formal healthcare providers to diagnose diseases, report health conditions to the healthcare institutions, and make decisions for delivering care.
In addition to the available evidence on the systems-level mHealth interventions, it is essential to understand how digitalization is promoting health among individuals suffering from chronic conditions. The use of digital interventions has not been examined at the user level, which highlights a critical knowledge gap about patient-centered applications of digital health technologies for NCD patients in India. Evidence on how digital technologies are being used by people living with NCDs and how such interventions may contribute to health outcomes may inform large-scale adoption of evidence-based interventions among the NCD-affected population. This systematic review contributes to this knowledge gap. The objective of this article is to systematically evaluate the current evidence on digital health interventions used by people living with NCDs in India, and assess the outcomes of those interventions to inform the future development and implementation.
Methods
Search strategy
We conducted this systematic review following the Preferred Reporting Items for Systematic Reviews and Meta-Analysis (PRISMA) guidelines.34 We searched published studies in MEDLINE, SCOPUS, CINAHL, PsycINFO, and ERIC databases using specific keywords, both as subject headings and general keywords, with appropriate Boolean operators, as shown in Table 1. These keywords were structured for searching literature on digital interventions for NCDs in the context of India, published since the inception of these databases. Four authors developed the working protocol for review and search strategy. All the databases were searched on 30 May 2019 for the last time.
Table 1.
Keywords for database searching.
| Search query (title, abstract, keywords, subject headings) | ||||||||
|---|---|---|---|---|---|---|---|---|
| digital OR mHealth OR eHealth OR internet OR online OR website OR mobile health OR electronic health OR telemedicine OR telehealth OR text messag* OR chat* OR social media OR facebook OR twitter OR whatsapp | AND | intervention OR program* OR plan OR initiative OR policy OR strateg* OR application | AND | non-communicable disease OR cardiac OR mental or diabet* OR arthritis OR poor health OR medicine OR chronic OR disease* OR illness OR sickness OR disability* OR disorder OR medical OR surgical OR psychiatric OR behavioral OR clinical OR mortalit* OR morbidit* OR health condition | AND | treatment outcomes or patient-reported outcomes or health outcomes or effects or impacts or consequences | AND | India |
Literature screening, inclusion and exclusion criteria
Articles found through database searching and additional sources were screened using Rayaan QCRI and RefWorks tools, which are online platforms for conducting systematic reviews and reference management, respectively.35,36 Three authors independently conducted the screening process. This step was a blinded process; conflicts that appeared in independent evaluations were discussed, and a consensus was made in the presence of two more authors.
We screened the literature and included articles in this review if they were: (a) empirical studies published in a peer-reviewed journal, (b) studies conducted among Indian population living in India, (c) studies presenting at least one health intervention, (d) the intervention was delivered through mobile phone, computer, internet, tablet, social media, and any digital medium, (e) interventions focused on at least one NCD among the intervention population, (f) the intervention was accessible to and utilized by the patients or caregivers, (g) studies which reported any health or health-related outcomes among the participants, and (h) studies published in the English language only.
Articles were excluded from this systematic review if they conflicted with any of the above-mentioned inclusion criteria.
Data extraction
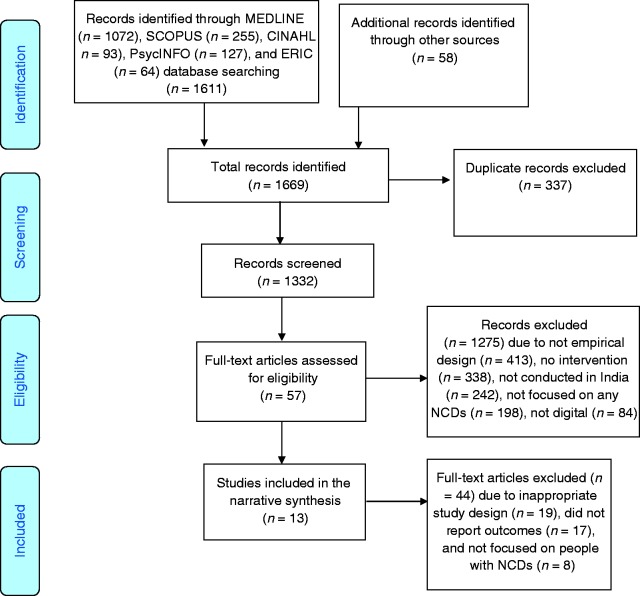
We found 1611 articles through searching five databases: MEDLINE (n = 1072), Scopus (n = 255), CINAHL (n = 93), PsycINFO (n = 127), and ERIC (n = 64). Also, we found 58 additional articles from reference search, consultations with domain experts, hand searching, and other sources (Figure 1). Therefore, the total number of articles primarily considered in this review was 1669. Further, 337 duplicate articles were removed, and the titles and abstracts of the remaining 1332 articles were evaluated based on the described inclusion or exclusion criteria. After this step, we removed 1275 articles that did not meet our criteria. The reasons for exclusion at this stage were not having an empirical design (n = 413), no intervention reported (n = 338), not conducted in India (n = 242), not focused on any NCDs (n = 198), and the reported intervention did not use any digital platform (n = 84). At the next stage we evaluated the full texts of the 57 remaining articles, among which 44 were excluded due to inappropriate study design (n = 19), did not report outcomes (n = 17), and not focused on people living with NCDs (n = 8). Finally, the remaining 13 articles were recruited for this systematic review. Furthermore, two authors re-evaluated the full texts of the finally recruited articles and extracted data in a pre-designed codebook. The codebook included following domains for data extraction: (a) location and time of the study, (b) description and components of the intervention, (c) types of NCDs among the study participants, (d) sampling and recruitment strategies, (e) intervention strategy, (f) design of the evaluation study, and (g) outcomes after the interventions.
Figure 1.
PRISMA flow diagram of the systematic review.
The coded data were reviewed by the other three authors to identify conflicts and resolve these based on consensus. Further, we included the key findings in the respective domain extracted from individual studies in a tabulated format. Finally, a brief narrative description of the interventions, characteristics of the study population, study or evaluation design, and the outcomes following the interventions are presented in the results.
Study quality assessment
In this systematic review, we used the Downs and Black checklist for assessing the methodological quality of both non-randomized and randomized intervention studies.37 Three authors evaluated the studies independently. The results were further reviewed, and an agreement was made in the presence of three more authors. This checklist has a total of 27 items in five sub-scales for evaluating different methodological domains of the published studies. The first sub-scale has 10 items for assessing the quality of reporting (e.g. “Are the main findings of the study clearly described?”). The second sub-scale has three items for checking external validity (e.g. “Were the subjects asked to participate representative of the entire population from which they were recruited?”). The third and fourth sub-scales examine internal validity by assessing biases in three items (e.g. “Were subjects randomized to intervention groups?”) and confounding in six items. The fourth sub-scale evaluated confounding in six items (e.g. “Were the patients in different intervention groups (trials and cohort studies) or were the cases and controls (case-control studies) recruited from the same population?”). The last sub-scale evaluates the power of the study on a range of 0–5 (e.g. “Did the study have sufficient power to detect a clinically important effect where the probability value for a difference being due to chance is less than 5%?”) Most items can be scored as “0” for “no” or “unable to decide” or “1” for “yes”, except one item in the reporting sub-scale (which can be score between 0 to 2) and the last sub-scale for power (scoring ranged between 0 to 5). Therefore, a study can receive a maximum score of 32 in this 27-item checklist.
Results
Out of 1669 studies derived from multiple databases and additional sources, 13 articles met our inclusion criteria (Table 2). Key findings from these studies are discussed below.
Table 2.
Overview of digital interventions for people living with NCDs in India.
| Source | Study location and period (if reported) | Sample size | Sample characteristics and recruitment strategy | Non-communicable disease | Description of the intervention | Study design and follow-up | Outcomes |
|---|---|---|---|---|---|---|---|
| Kesavadev et al., 2012.47 | Kerala; study period was not specified. | n = 1000 | Mean age was 53.2 years, 64% were male, educated; recruited from a diabetic research center. | Diabetes mellitus. | Participants received self-management of diabetes, glucometer usage, and briefed about the DTMS (Diabetes Telemedicine Management System). They had three telemedicine options to report their information via phone call, email, and a secure website and obtain advice regarding their treatment from a team of multi-disciplinary healthcare providers. | Cohort study; follow-up after 6 months. | Mean HbA1c reduced from 8.5 ± 1.4% to 6.3 ± 0.6% (p < 0.0001); 84% reported no hypoglycemia; patients also showed statistically significant reduction (p < 0.01) in LDL, Total Cholesterol and Fasting Blood Glucose. |
| Sharan et al., 2012.50 | Study location and period were not specified. | n = 29 (intervention = 14, control = 15). | Postoperative cerebral palsy children; age 8.88–10.38 years; the recruitment process was not specified. | Cerebral palsy. | Nintendo Wii and Wii fit were used for virtual reality-based
training to provide the opportunity to play games and practice
balance in the comfort of one's living room. Children played these games every three alternative days for three weeks under the supervision of physiotherapists. |
Randomized control trial; follow-up assessment was conducted after providing the treatment to both groups. | Balance and manual ability improved in both the groups
significantly (Balance: study: t-2.28,
p < 0.05; control: t-3.5,
p < 0.01. Manual ability: study: t-5.58, p < 0.001; control: t-7.06, p < 0.001). Pediatric balance score (PBS) improvement was significantly greater in the study group. (t-t-2.02, p < 0.05) compared with the control participants. Also, the level of participation, motivation, cooperation and satisfaction of the child were significantly higher among the study group. |
| Ramachandran et al., 2013.46 | Tamil Nadu and Andhra Pradesh; 2009–2012. | n = 537 (intervention = 271, control = 266). | Mean age was 46.1 (SD 4.6) and 45.9 (SD 4.8) years in the control and intervention group, respectively; recruited from public and private industrial units in Southwest India. | Impaired glucose tolerance and diabetes mellitus. | The intervention group received mobile phone messages at a regular interval, containing personalized information about healthy lifestyle, cues to start physical activity and a healthy diet, strategies to avoid relapse, and motivation to continue. | Prospective, parallel-group, randomized controlled trial; follow-up at 6-months. | Intention to treat analysis revealed that, the cumulative incidence of type 2 diabetes was lower in the intervention group than in control: 50 (18%) participants in the intervention group developed T2D whereas 73 (27%) in the control group (hazard ratio 0·64, 95% CI 0·45–0·92; p = 0·015). The number needed to treat to prevent one case of type 2 diabetes was 11 (95% CI 6–55). |
| Radhakrishnan et al., 2014.49 | Bengaluru, Karnataka; 2010–2011. | n = 61 (intervention = 33 and control = 28). | Mean age 53 and 46 years for group 1 and 2 respectively; recruited from a tertiary care hospital. | Metabolic syndrome. | Both the control and intervention group were enrolled in an aerobic exercise program and asked to perform it at least 30 min on 5 days per week. The intervention group received personalized text messages two times per week, which reminded them to exercise and contained all the necessary information regarding it. | Randomized controlled trial; follow-up at 12 weeks. | The intervention group showed significant improvement in – pulse wave velocity, aortic pulse pressure, aortic diastolic pressure, vitality, and fasting blood glucose. 16% of participants in both groups were able to reverse metabolic syndrome. |
| Kaur et al., 2015.48 | Punjab; study period was not specified. | n = 120. | Mean age was 49, 54, and 51 years in Group A, B, and C respectively, most participants (55% and above in three groups) were from a rural area; recruited from the outpatient department of a tertiary care teaching hospital. | Diabetes mellitus. | Patients were randomly allocated to three groups: Rare mode (patients advised 3-monthly follow-ups on OPD basis); Moderate mode (patients advised monthly OPD visits); and frequent mode (patients advised monthly OPD along with weekly telephonic consultation). | Three-arm randomized controlled trial; follow-up after 3 months. | There was a net decrease in adverse events, with an increase in
the frequency of follow-up. Changes in HbA1c suggested a
positive impact of weekly telephonic consultation. Higher follow-up was associated with a better lipid profile (higher HDL and lower TG) and improvement in QoL pertaining physical health domain. |
| Patnaik et al., 2015.38 | Study location was not specified;2012–2013. | n = 100 (test 50; and control 50). | Mean age was 54 (SD 11.5) years; 64% (control)-66% (intervention) were male; 39% were educated at the graduate or higher level; recruited from an endocrinology outpatient department of a tertiary care hospital. | Diabetes mellitus and stress. | Test group received counseling using printed materials and computers. Additionally, the researchers contacted them every 3 weeks for 3 months, and they've also received weekly SMS containing educational tips. The control group received standard care in the form of printed materials. | Randomized controlled trial; follow-up after 3 months. | The level of stress in the intervention group reduced (17.05) whereas the level of stress increased (20.7) in the control group. |
| Jha et al., 2016.43 | Delhi; 2014–2015. | n = 109 (intervention = 39 and control = 70). | Mean age 52.55 (SD 10.65) years, 74.3% male; recruited from a tertiary care hospital. | Diabetes mellitus. | Alongside the scheduled visit to their endocrinologists, patients in the intervention group received weekly telephonic follow-up by a team of physicians/ health educators to assess their glycemic situation and solve their issues. Patients in the intervention group also had access to educational video and received daily tips for managing T2D informs of SMS/Emails. Control group received conventional care. | Two-arm cohort study follow-up 5 months. | Statistically significant reduction in HbA1c (p = 0.001) was observed. Knowledge scores on Diabetes and QOL indices improved significantly in the intervention group. In both groups, FBS and 2 Hr PP showed a trend toward improvement but did not reach to the point of statistical significance. |
| Kleinman et al., 2017.39 | Gujarat and Tamil Nadu; 2015 | n = 91 | Mean age was 48.4 years (SD 9.2), 30% were female, 29.6% had a university education or higher; recruited from three diabetic clinics. | Diabetes mellitus | Intervention group received access to a mobile phone app and phone plan stipend. This app sent reminders, addressed the problems they faced and was a platform to communicate directly with the providers. Control group received usual care. | Two-armed, open-label, randomized clinical trial; follow-up after 6 months | Participants in the intervention group had better medication adherence comparing the control group. (39% vs.12.5%, p = 0.03); frequency of blood glucose self-testing was higher in the intervention group. (39% vs. 10.5%, p = 0.01). |
| Singh et al., 2017.40 | Bengaluru, Karnataka; 2014–2015. | n = 214 (Intervention = 106, control = 108). | Male 55.66% (intervention), 63.88% control; around; 28.3–28.7% were illiterate; recruited from community-based mental health clinic. | Neuropsychiatric disorders including schizophrenia depression neurological disorders, alcohol use disorder. | Initially, the intervention group received an SMS 1 day prior to their appointment. At the next level, all the patients who had missed previously scheduled appointments were called, the reason for missing the appointment was asked and requested for a follow-up. | Randomized controlled trial with stepped-up design; follow-up after 6 months. | The rate of reaching the appointment was significantly higher in the intervention group comparing the control one (62.26% vs. 45.37%). Further, 66 out of 88 (75%) patients who missed previous appointments came back for follow-up. |
| Kumar et ai., 2018.41 | Himachal Pradesh; 2015–2016. | n = 955 (intervention = 479 and control = 476). | Mean age 57–57.5 years, female 61.8–68.5% (intervention and control); recruited from primary secondary and private healthcare facilities. | Diabetic mellitus. | Tailored SMS in plain English language was selected from a message bank developed following the Indian guideline for the management of DM were sent to the patients in the intervention group twice a week for 12 months. Control group received usual care. | Randomized controlled trial; follow-up at 0–6th month = Baseline, 7th–12th month = End line. | Fasting blood glucose (FBG) intervention group declined from163.7 to 152.8 mg/dl (p = 0.019). FBG decline in the control group was not significant 150.5 to 149.2 mg/dl (p = 0.859). The intervention was significantly effective (OR:107; CI:1.2–2.6). |
| Menon et al., 2018.42 | Puducherry; 2015–2017. |
n = 132 (intervention = 62, control = 70). |
Mean age 37.1% and 38.7%, and male 54.8% and 40% in case and control, respectively; recruited from a teaching cum tertiary care hospital. | Bipolar I disorder. | The intervention group received identical biweekly text reminders to increase medication adherence along with TAU (treatment as usual) for the first three months and TAU only for the last 3 months. Control group only received TAU for 6 months. | Open-label, Rater-blinded Randomized controlled trial; follow-up at, 3-month intervention endpoint and 3 months post-intervention. | SMS intervention improved both medication adherence and attitude toward medication at intervention endpoint. But only medication adherence was maintained 3 months after the intervention. It did not improve QoL outcomes. |
| Mehrotra et al., 2018.45 | Bengaluru, Karnataka; study period was not specified. | n = 78. | Male 51.3%; 66.63% aged below 35 years, 85.9% graduates or above; recruited from online and offline community outreach programs. | Depression. | PUSH-D (practice and Use Self Help) for depression includes-a comprehensive coverage of therapeutic strategies of CBT, interpersonal therapy, supportive psychotherapy, and positive psychology. | Single-group pre–post study with 2 months follow-up. | Participants who completed all 10 essential zone sections showed a significant reduction in depression and improvement in functioning and increase in the standard well-being measurement score. Participants who completed up to five essential sections showed similar results. Gains were maintained at the follow-up. |
| Goruntla et al., 2019.44 | Andhra Pradesh; 2016–2017. | n = 330 (intervention = 165, control = 165). | Mean age 58.5 (SD 8.5) years, 51.8% male, 76.4% with no education; recruited from a outpatient department of a secondary care referral hospital. | Diabetes mellitus. | The intervention group was provided with face-to-face counseling regarding knowledge, education and non-pharmacological strategies related to DM management along with daily SMS reminders for medication intake 30 min prior due time and aerobic exercise in the morning for 6 months. Control group received usual care from their physician. | Opel-labeled randomized controlled trial; first follow-up after 3 months and second follow-up after 6 months. | The intervention group had significant medication adherence at 6 months follow-up. (p < 0.01), reduction in HbA1c (7.79 ± 0.67 to 6.91 ± 0.83), SBP (136.75 ± 20.09 to 126.23 ± 18.22), LDL (104.14 ± 26.23 to 98.29 ± 20.87), which was significant (p < 0.01). No significant improvement was noticed in the control group. |
Study period and location
Among the 13 studies included in this review, nine studies mentioned the study period during which they were conducted. The first study reporting digital intervention for NCDs was conducted by Patnaik et al. in 2012.38 Most of the studies (n = 5) were conducted around 2015.39–43 The most recent study, by Goruntla et al., was conducted in 2017.44 Studies were conducted in different states of India; however, most studies (n = 7) were from southern states. The highest number of studies (n = 3) were conducted in Bengaluru city of Karnataka state.38,40,45 There were two studies each from Tamil Nadu39,46 and Andhra Pradesh,44,46 and one each from Kerala,47 Himachal Pradesh,41 Punjab,48 Puducherry,42 Gujarat,39 and Delhi.43
Study design, recruitment strategy, and participants
There were only two cohort studies,43,47 and one pre–post study.45 All the remaining studies (n = 10) were randomized control trials. In most of the studies (n = 5) the participants were recruited from tertiary care hospitals.38,42,43,48,49 The study conducted by Kleinman et al. recruited participants from multiple diabetes clinics and secondary care hospitals.39 In the rest of the studies, participants were recruited from various sources including primary care hospital, mental health clinic, community outreach program, diabetes research center, and industrial workplace.
Among the included studies, the sample size ranged from 29 to 1000 participants. The majority of them (n = 8) recruited patients with diabetes. Three studies recruited patients with mental health disorders.40,42,45 One study recruited children with cerebral palsy,50 and another recruited participants with metabolic syndrome.49 In most of the studies (n = 9) the majority of the participants were males, in the middle age group (n = 11), with minimal literacy level to understand instructions related to the interventions (n = 10). More than half of the studies (n = 7) included participants with access to mobile phones.
Quality assessment of the included studies
Methodological quality was assessed for all the recruited studies (Table 3). Scores in five sub-scales were added to calculate the total score in the scale of 32. The mean score of the studies was 22.76 (range 19–26). The mean score for randomized controlled trials and studies with non-randomized designs were 23 and 22, respectively. The studies were further evaluated and labeled in three groups: low quality (score 18 or below, n = 0), moderate quality (score 19 to 22, n = 5), and high quality (score 23 or above, n = 9). Among the studies recruited in this review, four studies40,47,49,50 had moderate quality and the remaining nine studies had high quality.38,39,41–46,48
Table 3.
Quality assessment of the intervention studies.
| Authors and year of publication | Reporting (_/11) | External validity (_/3) | Internal Validity – Confounding (_/6) | Internal Validity – Bias (_/7) | Power (_/5) | Total score (_/32) |
|---|---|---|---|---|---|---|
| Kesavadev et al., 2012 | 6 | 2 | 2 | 4 | 5 | 19 |
| Sharan et al., 2012 | 6 | 2 | 3 | 4 | 5 | 20 |
| Ramachandaran et al., 2013 | 8 | 1 | 4 | 5 | 5 | 23 |
| Radhakrishnan et al., 2014 | 7 | 1 | 3 | 4 | 5 | 20 |
| Kaur et al., 2015 | 9 | 2 | 4 | 4 | 5 | 24 |
| Patnaik et al., 2015 | 8 | 2 | 4 | 4 | 5 | 23 |
| Jha et al., 2016 | 8 | 3 | 3 | 5 | 5 | 24 |
| Kleinman et al., 2017 | 8 | 2 | 5 | 6 | 5 | 26 |
| Singh et al., 2017 | 6 | 2 | 4 | 3 | 5 | 20 |
| Kumar et al., 2018 | 9 | 2 | 4 | 5 | 5 | 25 |
| Menon et al., 2018 | 8 | 2 | 4 | 5 | 5 | 24 |
| Mehrotra et al., 2018 | 8 | 2 | 3 | 5 | 5 | 23 |
| Goruntla et al., 2018 | 9 | 2 | 4 | 5 | 5 | 25 |
Characteristics of the interventions
Among the included studies, six studies focused on self-management of the disease.38,39,41,43,45,50 For example, Mehrotra et al. focused on self-management of depression using the principles of cognitive behavioral therapy (CBT).45 Moreover, four studies focused on increasing patient–provider communication.39,43,47,48 For example, Kesavadesv et al. provided three options (email, telephone calls, and a secured website) for the patients to communicate with a team of healthcare providers.47 Five studies focused on educating the participants about their disease.38,43,44,46,47 Kesavadev et al. and Goruntla et al. provided face-to-face educational session alongside digital interventions,44,47 whereas Jha et al. provided educational videos for the participants.43 Studies conducted by Ramachandaran et al.46 and Patnaik et al.38 reported SMS-based intervention to educate the participants about lifestyle modification. Furthermore, three studies focused on increasing follow-up.40,43,47 For example, Sing et al. provided reminders before the scheduled appointment for follow-up.40 They also called the participants who had missed their appointments, requested for follow-up, and noted the reasons for missing the same. Two studies offered counseling services to patients.38,44 For example, Goruntla et al. provided face-to-face counseling regarding knowledge, education, and non-pharmacological strategies related to diabetes mellitus management for the participants.44 Two interventions sent reminders to increase medication adherence among the patients including SMS reminders prior to their medication intake.42,44 Moreover, two interventions focused on increasing physical activity among the participants.44,49 For example, Radhakrishnan et al. encouraged participants to engage in aerobic exercise and sent reminders to exercise biweekly.49
Although majority of the studies (n = 10) used one digital medium to deliver the intervention, the remaining studies (n = 3) used multiple methods to deliver the interventions to the participants. Among the interventions with a single component, the majority (n = 6) used SMS to deliver the intervention.38,41,42,44,46,49 For example, Kumar et al, sent biweekly SMSs to participants in the intervention group containing messages regarding self-management of diabetes.41 Two interventions were delivered via mobile apps to send reminders, address patients’ problems, and increase communication with the providers.39,45 Kaur et al. was the only study to use telephonic consultation as the only component to increase the follow-up visits by the patients.48 Another intervention by Sharan et al. used a virtual reality-based training program to help children with cerebral palsy to receive rehabilitation training at home.50 There were a few (n = 3) studies with multiple modes of delivery.40,43,47 For example, Kesavadev et al.47 provided three options for reporting the blood glucose level—phone, email and a secure website—and Jha et al. provided the facility for telephonic follow-up, SMS reminders, and access to educational videos.43
The characteristics of the intervention studies were diverse irrespective of the study design. The non-randomized studies used telemedicine technologies to promote symptom management and follow-up.43,45,47 Similar contents and approaches to promote healthier behavior, lifestyle modification, management of health conditions, medication adherence, and communication with providers were reported among intervention studies that used a randomized controlled trial design.38–42,44,46,48–50
Outcomes of the interventions
All the studies reported outcomes specific to the NCD of interest in the respective studies. These outcomes included changes in biomarkers for specific diseases, improvements in follow-up and adherence to medication, improved health conditions in terms of patient-reported symptoms, satisfaction and increased participation in the offered health services. Reduction in the HbA1c level and improvement in the lipid profile was reported by most of the studies (n = 5).39,43,44,47,48 For example, Kesavadev et al. reported a significant decrease in the HbA1c level and decrease in low-density lipoprotein.47
Five studies reported improvement in disease symptoms.38,45,46,49,50 For example, Sharan et al. reported improvement in balance, manual ability, and Pediatric Balance Score among the children in the intervention group.50 Mehrotra et al. reported a reduction in the depression symptoms among the participants who completed all essential sessions.45 Three studies reported improvement in medication adherence.39,42,44 For example, Menon et al. reported improvement in medication adherence and positive change in attitude among the participants in the intervention group.42
Moreover, two studies reported improvement in the follow-up.40,47 For example, Singh et al. reported that the rate of reaching the appointment was significantly higher in the intervention group and 75% patients who missed previous appointments came back for follow-up.40 Furthermore, three studies mentioned that participants were highly satisfied with the interventions.39,48,50 For example, Sharan et al. reported that level of participation, motivation, and satisfaction were significantly higher among children in the intervention group.50
According to the study design, both randomized and non-randomized studies reported effectiveness in terms of health outcomes of interest in respective studies. Three non-randomized studies reported improved adherence, psychosomatic health outcomes, and better quality of living.42,45,47Among the randomized studies, the study participants reported varying levels of improvements in physical abilities,50 management of impaired glycemic profile and diabetes,41,44,46,48,49 adherence to therapies and more frequent follow-up visits,39,42,44 and reduction in chronic physical and mental health conditions.38,40,49
Discussion
To our knowledge, this is the first systematic review of digital interventions for people living with NCDs in India. In this review, we found studies presenting different digital interventions for varying diagnoses of NCDs, ranging from cerebral palsy among children to diabetes among adult participants. A majority of the studies (n = 10) were randomized in design, two were cohort studies, and one used pre–post design. The target population, NCDs of interest, design and content of the intervention, and outcomes following the intervention were diverse across the included studies. Such heterogeneity in the interventions studies does not allow us to draw inferences on comparative outcomes between the studies. However, within-study variations in terms of outcomes of interest can provide analytical perspectives on how those interventions contributed to better health outcomes. Both among the randomized and non-randomized studies, the interventions attempted to increase access to different health services and deliver health-related information or self-management instructions to the participants. In these processed, digitalization played a pivotal role in establishing unidirectional and bi-directional communication between the providers and people living with NCDs. For example, the randomized clinical trial study by Kleinman et al. recruited patients with diabetes and the intervention group was offered mobile app to communicate with the providers, which allowed them to adopt self-management and lifestyle modification.39 This study found a marked increase in medication adherence and self-testing of blood glucose, highlighting how strengthened communication and care through the digital platform resulted in better health outcomes. Similarly, the mobile-based digital intervention by Goruntla et al. found higher adherence and improved outcomes in blood glucose level, blood pressure, and lipid profile.44 This evidence highlights that frequent reminders, instructions, and counseling may enhance treatment consumption and health outcomes among the study participants. While all the interventions in this review resulted in varying improvements in different health outcomes, none of them reported any adverse effect or unintended consequences following the implementation of the interventions. Despite the low number of studies, the current evidence shows various benefits of using digital health interventions among people living with NCDs. Furthermore, these interventions provide several critical insights on how India is leveraging the avenues of digital health and how future directions can be adopted to improve population health outcomes in the realm of NCDs.
First, the number of digital health interventions for NCDs is relatively low for India, which shares a major proportion of the global burden of NCDs. Like many other low- and middle-income countries, digital health interventions for NCDs have not reached their fullest potential in India, as seen in many developed nations.51 However, investing in digital health can reduce the cost of direct care, enhance access to health services, and improve health outcomes for resource-constrained countries.52 Future policymaking, research, and implementation should seek opportunities to adopt more evidence-based digital approaches to improve NCD care in the context of India.
Second, most of intervention studies were conducted in southern states of India, whereas we did not find any study from north-eastern states. These states share a similar burden of NCD compared with the national scenario.5 Therefore, unequal distribution of studies highlights potential disparities in the development and implementation of digital health interventions across Indian states. This necessitates careful attention from researchers and practitioners to consider the adoption of evidence-based interventions and development of culturally tailored digital interventions for those states where digital health is in a developing stage.
Third, most of the studies recruited participants from hospitals, clinics, research centers, and community sources located in urban areas. Such urban-centered growth of digital interventions for NCDs may not help to improve population health outcomes, as 68.84% of India’s total population live in rural areas.53 Moreover, the rural population in India often suffers from a lack of health infrastructure, scarcity of expert physicians, and lack of access to health services.10 These places may have a higher need for technological advancements like digital health services to bridge the gaps of healthcare. In recent years, mHealth interventions have been implemented for community health services; for example, maternal and child health services by “Accredited Social Health Workers (ASHA)” in many states in India.54 However, the benefits of the digital revolution in healthcare remain beyond the reach of the rural population suffering from NCDs. This is an area where future research and interventions should be focused, to alleviate the urban–rural health disparities in the digital era.
Fourth, most of the interventions were designed and implemented for diabetes, followed by neuropsychiatric disorders, whereas none of the included studies focused on cancer, cardiovascular diseases, or COPD, which constitute a large proportion of NCDs in India.6,55,56 People living with these diseases would need varying support which can be delivered successfully through digital interventions. For example, many studies have shown how mHealth and telemedicine interventions can help cancer patients or survivors.57–59 Moreover, studies have also shown that the use of digital self-management and exercise-based interventions can improve health outcomes among patients suffering from COPD. These opportunities should be explored in the context of India to improve patient-centered care in those unexplored areas of NCDs.
Fifth, most of the included studies discussed the implementation of digital interventions for localized patient populations in the respective study locations, whereas none of the studies have shown a large-scale adoption of the interventions at the community, state, regional, or national level. Moreover, little is known about the sustainability of the interventions presented in this review. Most studies reported health outcomes at the follow-up evaluations; however, it cannot be concluded that the interventions have brought sustainable health practices and outcomes among the participants. Moreover, digital interventions would require availability and accessibility to digital devices and services such as internet and messaging.60 It cannot be assumed that the participants will continue to use the interventions once the study has ended. Future research in digital health in India should overcome such challenges and expand the outreach of health services for NCDs in a sustainable manner. A few potential strategies to do so may include subsidizing digital health devices and services for large-scale and sustainable adoption,61 incentivizing healthcare providers to encourage digital health interventions complementing conventional care,62 and development of low-cost and culturally appropriate digital interventions for different NCDs, ensuring optimal compliance among the users.63
Sixth, NCD multimorbidity is an emerging global health challenge in which individuals suffer from co-existence of more than one NCD.64 Earlier studies conducted in India have shown a high prevalence of multimorbidity,65,66 which needs extensive planning and optimal management of the patients. Studies conducted in other countries have shown that digital interventions can help in managing multimorbidity among NCD patients.67–69 However, most of the studies in this review had participants with diabetes, among which the burden of multiple morbidities was not evaluated explicitly. Only one study has reported an intervention for addressing psychological stress among diabetic patients.38 This evidence highlights the need for examining how multimorbidity exists among people reporting one NCD, and how multiple digital health services can be delivered for those patients more efficiently. Such development of multifaceted digital interventions would require interoperability among healthcare providers offering specialized services in multiple domains of NCDs.70,71 As the burden of NCDs has been increasing in India, scholars and practitioners should consider such strategies to ensure optimal, multipronged, and holistic NCD care in the future.
Seventh, technological aspects of digital interventions should be evaluated in the context of global advancements. Artificial technologies such as machine learning and deep learning are increasingly being used for optimizing the performance of digital health interventions and customizing health services as per the unique requirements for individual patients.72–74 Such personalization of health services may facilitate precision healthcare delivery for patients with NCDs. While such innovations are contingent upon availability of big data including genomic, behavioral, epidemiological, and environmental data on a given population, these advancements in digital health should be acknowledged to unlock the potential of precision public health in resource-constrained contexts.75 Current evidence in our review has shown text-messaging and internet-based applications in most of the studies in India. Understanding global trends in health information technologies may help in developing digital health interventions with advanced technologies in the future.
Eighth, the safety and security of personal health data is a major concern in the age of digitalization. In developed countries such as the United States, protected health information stored or exchanged through electronic systems is secured under regulatory measures such as the “Health Insurance Portability and Accountability Act - HIPPA.”76 Despite such measures, breach of protected health information is a public health challenge in the United States.77 In India, Clinical Establishments (Registration and Regulation) Act – 2010 and the Clinical Establishments (Central Government) Rules 2012 have been instituted to regulate health information services across the nation;78 however, little is known about the scope of these measures in the era of rapidly evolving digital health. As electronic health records and other digital health applications are still under development, future innovations in digital health in India should be conceptualized maintaining compliance with legislative and regulatory measures. Although such measures would help digitalized health systems in general, they are likely to provide more protection to people with NCDs with a higher risk of data breaches, as they are more likely to use digital health services for managing chronic conditions across their lifespan.
Ninth, the participation of the patients and their informal caregivers is critical for effective development and successful implementation of digital interventions for NCDs.79,80 Many factors, including literacy about digital health and activation of the users, are essential for increasing adoption of digital health interventions.61,81 In most of the studies included in this review, the participants were recruited through direct communication, which may be expensive and time-consuming. Also, the findings of successful interventions should be widely communicated with people living with NCDs for better adoption of such interventions. The researchers and healthcare strategists should consider e-recruitment strategies, with culturally appropriate and context-specific behavior change communication approaches to increase participation in and adoption of such interventions among NCD patients.
Lastly, addressing the above-mentioned challenges and advancing digital health for NCDs would require collegial efforts of key stakeholders including physicians, other healthcare professionals, researchers working on NCDs, specialists working on health information technologies, health services organizations, and policymakers.61,82 The National Health Policy 2017 recognized the role of digital health technologies and envisioned establishing National Digital Health Authority to develop, deploy, and regulate digital health across the continuum of care.83 It is critical to acknowledge the high burden of NCDs in India and incorporate specific provisions in these policy discourses to create an enabling environment within which multiple stakeholders can collaborate and work together to promote digital healthcare for NCDs.
Limitations
This systematic review has several limitations. First, this systematic review carries the limitations within the included studies. We acknowledge that there are publication biases within the studies as our team could see only the published studies with positive findings in most cases. This limits the process of learning about those interventions which did not work or were not published in the journals. Second, there could be limitations within the process of conducting the systematic review. We searched major databases with a plausible search strategy, and searched further to obtain more articles from additional sources. Therefore, we could not retrieve any literature stored in databases we did not search, which may be another limitation of this review. Third, the number of studies and sample sizes within those studies are relatively low, which may limit generalizing the findings for wide range of NCDs in a large country like India. However, we strictly followed the PRISMA guidelines and conducted each phase of the review in the presence of two or more reviewers to eliminate potential biases as well as other limitations in the process of conducting this systematic review. Future evidence synthesis should maintain strict methodologies and address the limitations of this review to better inform evidence on digital interventions for people living with NCDs.
Conclusion
To conclude, our systematic review shows a low yet gradually increasing number of digital health interventions for people living with NCDs in India. Most of the intervention studies have shown a wide range of positive health outcomes following the interventions, which highlight the potential for alleviating health problems among individuals suffering from different NCDs. Nevertheless, our review also presents the gaps alongside the current evidence, which may affect the overall development of digital health interventions for NCDs in the context of India. These gaps and challenges are critical for the large-scale adoption of evidence-based interventions, as well as for developing and implementing newer interventions for people living with NCDs. Future research and practice should engage key stakeholders and address issues highlighted in this review to advance patient-centered NCD care in the digital age in India.
Supplemental Material
Supplemental material, DHJ896153 Supplemental Material for Digital interventions for people living with non-communicable diseases in India: A systematic review of intervention studies and recommendations for future research and development by Md Mahbub Hossain, Samia Tasnim, Rachit Sharma, Abida Sultana, Araish Farzana Shaik, Farah Faizah, Ravneet Kaur, Madhuri Uppuluri, Mitali Sribhashyam and Sudip Bhattacharya in Digital Health
Acknowledgements
We would like to thank Shah Akib Sarwar, Md Nazmus Sakib, and Arif Arman for their kind support in conducting this study.
Contributorship
MMH and ST conceived the study. MMH, RS, AS, and SB developed the review protocol and search strategy. MMH, RS, and AS conducted the primary screening; which was further reviewed by AF and FF. ST and RK conducted the data extraction from screened literature; which was further reviewed by MMH and RS. Quality assessment was conducted independently by AF, MU, MS; which was finally reviewed by MMH, ST, and FF. RK, MU, and MS conducted the background literature review. The first draft of the manuscript was written by MMH, ST, and AS. Further, it was further critically reviewed and edited by RS, AF, FF, and SB. All the authors reviewed and finalized the submitted version of the manuscript.
Conflicting interests
The authors have no conflicts of interest to declare.
Ethical approval
Not required.
Funding
The authors received no financial support for the research, authorship, and/or publication of this article.
Guarantor
MMH
ORCID iD
Md Mahbub Hossain https://orcid.org/0000-0002-7059-7768
Peer review
This manuscript was reviewed by reviewers, the authors have elected for these individuals to remain anonymous.
Supplemental material
Supplemental material for this article is available online.
References
- 1.World Health Organization (WHO). Noncommunicable diseases, https://www.who.int/news-room/fact-sheets/detail/noncommunicable-diseases (2018, accessed 8 November 2019).
- 2.World Health Organization (WHO). World Health Organization | Deaths from NCDs. WHO, https://www.who.int/gho/ncd/mortality_morbidity/ncd_total_text/en/ ( 2015, accessed 30 July 2019).
- 3.Nethan S, Sinha D, Mehrotra R. Non communicable disease risk factors and their trends in India. Asian Pac J Cancer Prev 2017; 18: 2005–2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Barik D, Arokiasamy P. Rising health expenditure due to non-communicable diseases in India: An outlook. Front Public Heal 2016; 4: 268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.India State-Level Disease Burden Initiative Collaborators L, Dandona R, Kumar GA, et al. Nations within a nation: Variations in epidemiological transition across the states of India, 1990–2016 in the Global Burden of Disease Study. Lancet (London, England) 2017; 390: 2437–2460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.India State-Level Disease Burden Initiative Cancer Collaborators PK, Mathur P, Nandakumar A, et al. The burden of cancers and their variations across the states of India: the Global Burden of Disease Study 1990-2016. Lancet Oncol 2018; 19: 1289–1306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sinha R, Pati S. Addressing the escalating burden of chronic diseases in India: Need for strengthening primary care. J Fam Med Prim Care 2017; 6: 701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Budnik LT, Adam B, Albin M, et al. Diagnosis, monitoring and prevention of exposure-related non-communicable diseases in the living and working environment: DiMoPEx-project is designed to determine the impacts of environmental exposure on human health. J Occup Med Toxicol 2018; 13: 6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Golechha M. Healthcare agenda for the Indian government. Indian J Med Res 2015; 141: 151–153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Balarajan Y, Selvaraj S, Subramanian SV. Health care and equity in India. Lancet (London, England) 2011; 377: 505–515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Salleh MR. Life event, stress and illness. Malays J Med Sci 2008; 15: 9. [PMC free article] [PubMed] [Google Scholar]
- 12.Chen J, Mullins CD, Novak P, et al. Personalized strategies to activate and empower patients in health care and reduce health disparities. Health Educ Behav 2016; 43: 25–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Saleh S, Farah A, El Arnaout N, et al. mHealth use for non-communicable diseases care in primary health: patients’ perspective from rural settings and refugee camps. J Public Health (Bangkok) 2018; 40: ii52–ii63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Majumdar A, Kar SS, S GK, et al. mHealth in the prevention and control of non-communicable diseases in India: Current possibilities and the way forward. J Clin Diagn Res 2015; 9: LE06–LE10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bloomfield GS, Vedanthan R, Vasudevan L, et al. Mobile health for non-communicable diseases in Sub-Saharan Africa: A systematic review of the literature and strategic framework for research. Global Health 2014; 10: 49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.U.S. Food and Drug Administration. Digital Health | FDA, https://www.fda.gov/medical-devices/digital-health (accessed 8 November 2019).
- 17.World Health Organization (WHO). WHO releases first guideline on digital health interventions, https://www.who.int/news-room/detail/17-04-2019-who-releases-first-guideline-on-digital-health-interventions (2019, accessed 30 July 2019).
- 18.Bhavnani SP, Narula J, Sengupta PP. Mobile technology and the digitization of healthcare. Eur Heart J 2016; 37: 1428–1438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Larson JL, Rosen AB, Wilson FA. The effect of telehealth interventions on quality of life of cancer patients: A systematic review and meta-analysis. Telemed e-Health 2018; 24: 397–405. [DOI] [PubMed] [Google Scholar]
- 20.Shen Y, Wang F, Zhang X, et al. Effectiveness of internet-based interventions on glycemic control in patients with type 2 diabetes: Meta-analysis of randomized controlled trials. J Med Internet Res 2018; 20: e172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jin K, Khonsari S, Gallagher R, et al. Telehealth interventions for the secondary prevention of coronary heart disease: A systematic review and meta-analysis. Eur J Cardiovasc Nurs 2019; 18: 260–271. [DOI] [PubMed] [Google Scholar]
- 22.Zhai Y, Zhu W, Hou H, et al. Efficacy of telemedicine for thrombolytic therapy in acute ischemic stroke: A meta-analysis. J Telemed Telecare 2015; 21: 123–130. [DOI] [PubMed] [Google Scholar]
- 23.Schäfer AGM, Zalpour C, von Piekartz H, et al. The efficacy of electronic health-supported home exercise interventions for patients with osteoarthritis of the knee: Systematic review. J Med Internet Res 2018; 20: e152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Deady M, Choi I, Calvo RA, et al. eHealth interventions for the prevention of depression and anxiety in the general population: A systematic review and meta-analysis. BMC Psychiatry 2017; 17: 310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Alwashmi M, Hawboldt J, Davis E, et al. The effect of smartphone interventions on patients with chronic obstructive pulmonary disease exacerbations: A systematic review and meta-analysis. JMIR mHealth uHealth 2016; 4: e105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Whiteman KL, Naslund JA, DiNapoli EA, et al. Systematic review of integrated general medical and psychiatric self-management interventions for adults with serious mental illness. Psychiatr Serv 2016; 67: 1213–1225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lee J-A, Choi M, Lee SA, et al. Effective behavioral intervention strategies using mobile health applications for chronic disease management: A systematic review. BMC Med Inform Decis Mak 2018; 18: 12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Pai ALH, McGrady M. Systematic review and meta-analysis of psychological interventions to promote treatment adherence in children, adolescents, and young adults with chronic illness. J Pediatr Psychol 2014; 39: 918–931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Cho Y-M, Lee S, Islam SMS, et al. Theories applied to m-health interventions for behavior change in low- and middle-income countries: A systematic review. Telemed J E Health 2018; 24: 727–741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hurt K, Walker RJ, Campbell JA, et al. mHealth interventions in low and middle-income countries: A systematic review. Glob J Health Sci 2016; 8: 183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Orton M, Agarwal S, Muhoza P, et al. Strengthening delivery of health services using digital devices. Glob Heal Sci Pract 2018; 6: S61–S71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Long L-A, Pariyo G, Kallander K. Digital technologies for health workforce development in low- and middle-income countries: A scoping review. Glob Heal Sci Pract 2018; 6: S41–S48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bassi A, John O, Praveen D, et al. Current status and future directions of mhealth interventions for health system strengthening in India: Systematic review. JMIR mHealth uHealth 2018; 6: e11440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Liberati A, Altman DG, Tetzlaff J, et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate healthcare interventions: Explanation and elaboration. BMJ 2009; 339: b2700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ouzzani M, Hammady H, Fedorowicz Z, et al. Rayyan—a web and mobile app for systematic reviews. Syst Rev 2016; 5: 210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hendren C. RefWorks. J Med Libr Assoc 2004; 92(1): 111–113. [Google Scholar]
- 37.Downs SH, Black N. The feasibility of creating a checklist for the assessment of the methodological quality both of randomised and non-randomised studies of health care interventions. J Epidemiol Community Heal 1998; 52: 377–384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Patnaik L, Joshi A, Sahu T. Mobile phone-based education and counseling to reduce stress among patients with diabetes mellitus attending a tertiary care hospital of India. Int J Prev Med 2015; 6: 37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kleinman NJ, Shah A, Shah S, et al. Improved medication adherence and frequency of blood glucose self-testing using an m-health platform versus usual care in a multisite randomized clinical trial among people with type 2 diabetes in India. Telemed J E Health 2017; 23: 733–740. [DOI] [PubMed] [Google Scholar]
- 40.Singh G, Manjunatha N, Rao S, et al. Use of mobile phone technology to improve follow-up at a community mental health clinic: A randomized control trial. Indian J Psychol Med 2017; 39: 276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kumar D, Raina S, Sharma SB, et al. Effectiveness of randomized control trial of mobile phone messages on control of fasting blood glucose in patients with type-2 diabetes mellitus in a Northern State of India. Indian J Public Health 2018; 62: 224–226. [DOI] [PubMed] [Google Scholar]
- 42.Menon V, Selvakumar N, Kattimani S, et al. Therapeutic effects of mobile-based text message reminders for medication adherence in bipolar I disorder: Are they maintained after intervention cessation? J Psychiatr Res 2018; 104: 163–168. [DOI] [PubMed] [Google Scholar]
- 43.Jha S, Dogra S, Yadav A, et al. A prospective observational study to assess the effectiveness of an electronic health (E-health) and mobile health (M-health) platform versus conventional care for the management of diabetes mellitus. Int J Diabetes Dev Ctries 2016; 36: 529–534. [Google Scholar]
- 44.Goruntla N, Mallela V and, Devanna N. Impact of pharmacist-directed counseling and message reminder services on medication adherence and clinical outcomes in type 2 diabetes mellitus. J Pharm Bioallied Sci 2019; 11: 69–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Mehrotra S, Sudhir P, Rao G, et al. Development and pilot testing of an internet-based self-help intervention for depression for Indian users. Behav Sci (Basel) 2018; 8: 36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ramachandran A, Snehalatha C, Ram J, et al. Effectiveness of mobile phone messaging in prevention of type 2 diabetes by lifestyle modification in men in India: A prospective, parallel-group, randomised controlled trial. Lancet Diabetes Endocrinol 2013; 1: 191–198. [DOI] [PubMed] [Google Scholar]
- 47.Kesavadev J, Shankar A, Pillai PBS, et al. Cost-effective use of telemedicine and self-monitoring of blood glucose via diabetes tele management system (DTMS) to achieve target glycosylated hemoglobin values without serious symptomatic hypoglycemia in 1,000 subjects with type 2 diabetes mellitus—A. Diabetes Technol Ther 2012; 14: 772–776. [DOI] [PubMed] [Google Scholar]
- 48.Kaur R, Kajal KS, Kaur A, et al. Telephonic consultation and follow-up in diabetics: Impact on metabolic profile, quality of life, and patient compliance. N Am J Med Sci 2015; 7: 199–207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Radhakrishnan J, Swaminathan N, Pereira N, et al. Effect of an IT-supported home-based exercise programme on metabolic syndrome in India. J Telemed Telecare 2014; 20: 250–258. [DOI] [PubMed] [Google Scholar]
- 50.Sharan D, Ajeesh PS, Rameshkumar R, et al. Virtual reality based therapy for post operative rehabilitation of children with cerebral palsy. Work 2012; 41: 3612–3615. [DOI] [PubMed] [Google Scholar]
- 51.Feroz A, Kadir MM, Saleem S. Health systems readiness for adopting mhealth interventions for addressing non-communicable diseases in low- and middle-income countries: A current debate. Glob Health Action 2018; 11: 1496887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Lewis T, Synowiec C, Lagomarsino G, et al. E-health in low- and middle-income countries: Findings from the Center for Health Market Innovations. Bull World Health Organ 2012; 90: 332–340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Government of India. Census of India 2011, http://censusindia.gov.in/2011-prov-results/paper2/data_files/india/Rural_Urban_2011.pdf (2011, accessed 30 July 2019).
- 54.Prinja S, Bahuguna P, Gupta A, et al. Cost effectiveness of mHealth intervention by community health workers for reducing maternal and newborn mortality in rural Uttar Pradesh, India. Cost Eff Resour Alloc 2018; 16: 25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.India State-Level Disease Burden Initiative CVD Collaborators D, Jeemon P, Sharma M, et al. The changing patterns of cardiovascular diseases and their risk factors in the states of India: The Global Burden of Disease Study 1990-2016. Lancet Glob Heal 2018; 6: e1339–e1351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.India State-Level Disease Burden Initiative CRD Collaborators S, Kumar GA, Dhaliwal RS, et al. The burden of chronic respiratory diseases and their heterogeneity across the states of India: The Global Burden of Disease Study 1990-2016. Lancet Glob Heal 2018; 6: e1363–e1374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Seiler A, Klaas V, Tröster G, et al. eHealth and mHealth interventions in the treatment of fatigued cancer survivors: A systematic review and meta-analysis. Psychooncology 2017; 26: 1239–1253. [DOI] [PubMed] [Google Scholar]
- 58.Escriva Boulley G, Leroy T, Bernetière C, et al. Digital health interventions to help living with cancer: A systematic review of participants’ engagement and psychosocial effects. Psychooncology 2018; 27: 2677–2686. [DOI] [PubMed] [Google Scholar]
- 59.Roberts AL, Fisher A, Smith L, et al. Digital health behaviour change interventions targeting physical activity and diet in cancer survivors: A systematic review and meta-analysis. J Cancer Surviv 2017; 11: 704–719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Fairburn CG, Patel V. The impact of digital technology on psychological treatments and their dissemination. Behav Res Ther 2017; 88: 19–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Van Velthoven MH, Cordon C. Sustainable adoption of digital health innovations: Perspectives from a stakeholder workshop. J Med Internet Res 2019; 21: e11922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Huang F, Blaschke S, Lucas H. Beyond pilotitis: Taking digital health interventions to the national level in China and Uganda. Global Health 2017; 13: 49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Hearn J, Ssinabulya I, Schwartz JI, et al. Self-management of non-communicable diseases in low- and middle-income countries: A scoping review. PLoS One 2019; 14: e0219141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Jindai K, Nielson CM, Vorderstrasse BA, et al. Multimorbidity and functional limitations among adults 65 or older, NHANES 2005–2012. Prev Chronic Dis 2016; 13: 160174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Pati S, Swain S, Metsemakers J, et al. Pattern and severity of multimorbidity among patients attending primary care settings in Odisha, India. PLoS One 2017; 12: e0183966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Pati S, Agrawal S, Swain S, et al. Non communicable disease multimorbidity and associated health care utilization and expenditures in India: Cross-sectional study. BMC Health Serv Res 2014; 14: 451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Melchiorre MG, Lamura G, Barbabella F, et al. eHealth for people with multimorbidity: Results from the ICARE4EU project and insights from the “10 e’s” by Gunther Eysenbach. PLoS One 2018; 13: e0207292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Bousquet J, Arnavielhe S, Bedbrook A, et al. MASK 2017: ARIA digitally-enabled, integrated, person-centred care for rhinitis and asthma multimorbidity using real-world-evidence. Clin Transl Allergy 2018; 8: 45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Irfan Khan A, Gill A, Cott C, et al. mHealth Tools for the self-management of patients with multimorbidity in primary care settings: Pilot study to explore user experience. JMIR mHealth uHealth 2018; 6: e171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Boonstra A, Broekhuis M. Barriers to the acceptance of electronic medical records by physicians from systematic review to taxonomy and interventions. BMC Health Serv Res 2010; 10: 231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Ross J, Stevenson F, Dack C, et al. Developing an implementation strategy for a digital health intervention: An example in routine healthcare. BMC Health Serv Res 2018; 18: 794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Kuziemsky C, Maeder A, John O, et al. Role of artificial intelligence within the telehealth domain. Yearb Med Inform. Epub ahead of print 25 April 2019. DOI: 10.1055/s-0039-1677897. [DOI] [PMC free article] [PubMed]
- 73.Eggerth A, Hayn D, Schreier G. Medication management needs information and communications technology‐based approaches, including telehealth and artificial intelligence. Br J Clin Pharmacol 2019; bcp.14045. [DOI] [PMC free article] [PubMed]
- 74.Qi J, Wu F, Li L, et al. Artificial intelligence applications in the telecommunications industry. Expert Syst 2007; 24: 271–291. [Google Scholar]
- 75.Prosperi M, Min JS, Bian J, et al. Big data hurdles in precision medicine and precision public health. BMC Med Inform Decis Mak 2018; 18: 139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Cohen IG, Mello MM. HIPAA and protecting health information in the 21st century. JAMA 2018; 320: 231. [DOI] [PubMed] [Google Scholar]
- 77.McCoy TH, Perlis RH. Temporal trends and characteristics of reportable health data breaches, 2010-2017. JAMA 2018; 320: 1282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Government of India. Report of the National Consultative Workshop on Clinical Establishments Act. 2014, http://www.searo.who.int/india/publications/national_stakeholder_consultation_1.pdf (accessed 30 July 2019).
- 79.Gagnon M-P, Ndiaye MA, Larouche A, et al. Optimising patient active role with a user-centred eHealth platform (CONCERTO+) in chronic diseases management: A study protocol for a pilot cluster randomised controlled trial. BMJ Open 2019; 9: e028554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Krishnan A, Ekowati R, Baridalyne N, et al. Evaluation of community-based interventions for non-communicable diseases: Experiences from India and Indonesia. Health Promot Int 2011; 26: 276–289. [DOI] [PubMed] [Google Scholar]
- 81.Effy V, Tobias H, Afua A, et al. Digital health: meeting the ethical and policy challenges. Swiss Med Wkly 2018; 148: 14571. [DOI] [PubMed] [Google Scholar]
- 82.Adjekum A, Blasimme A, Vayena E. Elements of trust in digital health systems: Scoping review. J Med Internet Res 2018; 20: e11254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Government of India. National Health Policy 2017, https://mohfw.gov.in/sites/default/files/9147562941489753121.pdf (2017, accessed 30 July 2019).
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, DHJ896153 Supplemental Material for Digital interventions for people living with non-communicable diseases in India: A systematic review of intervention studies and recommendations for future research and development by Md Mahbub Hossain, Samia Tasnim, Rachit Sharma, Abida Sultana, Araish Farzana Shaik, Farah Faizah, Ravneet Kaur, Madhuri Uppuluri, Mitali Sribhashyam and Sudip Bhattacharya in Digital Health