Abstract
Given the recent in vitro discovery that the free soluble oligosaccharide of GM1 is the bioactive portion of GM1 for neurotrophic functions, we investigated its therapeutic potential in the B4galnt1+/− mice, a model of sporadic Parkinson’s disease. We found that the GM1 oligosaccharide, systemically administered, reaches the brain and completely rescues the physical symptoms, reduces the abnormal nigral α-synuclein content, restores nigral tyrosine hydroxylase expression and striatal neurotransmitter levels, overlapping the wild-type condition. Thus, this study supports the idea that the Parkinson’s phenotype expressed by the B4galnt1+/− mice is due to a reduced level of neuronal ganglioside content and lack of interactions between the oligosaccharide portion of GM1 with specific membrane proteins. It also points to the therapeutic potential of the GM1 oligosaccharide for treatment of sporadic Parkinson’s disease.
Subject terms: Diseases of the nervous system, Preclinical research
Introduction
Gangliosides are key components of the neuronal plasma membranes1–6, where they participate to determine the membrane organization and to modulate protein functions, in many cases by interacting with their oligosaccharide moiety. Over the course of physiological aging, the ganglioside metabolic pathways (especially that of GM1) undergo progressive decline due to reduction of membrane ganglioside content and composition7,8, which can lead to progressive neurological dysfunction.
Accumulating evidence is pointing to a central role for subnormal levels of gangliosides as putative initiators of sporadic Parkinson’s disease (sPD) pathogenesis9–12. sPD patients present a reduction of GM1 ganglioside in the substantia nigra9, in the occipital cortex13 and various peripheral tissues, suggesting a GM1 systemic deficiency that would correlate with the systemic symptoms of sPD12. In neuromelanin-containing neurons in the sPD substantia nigra the reduced ganglioside content is accompanied by a significant deficient expression in two genes involved in ganglioside synthesis: B3GALT4 and ST3GAL210. Several neuronal functions can become gradually compromised as the membrane GM1 content diminishes below a certain threshold with aging14 and/or under epigenetic influences. In particular, it has been hypothesized that the reduced level of plasma membrane GM1 in PD neurons can trigger the neurodegenerative process by a failure in neurotrophic signaling (i.e. GDNF)13 together with a reduction of GM1 interaction with α-synuclein (α-syn) that prevents aggregation of the latter12,15,16. Accordingly, as the population continues to age with a progressive decline of a-series gangliosides (GM1 and GD1a, its metabolic precursor, via plasma membrane bound sialidase Neu3), it can be expected that the number and percentage of persons developing sPD will multiply.
GM1 replacement therapy has shown modest but significant success in a monocentric controlled, delayed start trial in treated sPD patients17, acting as symptomatic and potentially disease modifier, since a partial restoration of dopamine (DA) transporter functional level in the striatum of GM1-treated subjects was reported18. Despite these suggestive positive evidences, the use of GM1 in clinical trials is severely hampered due to its low capacity to reach brain neurons. Gangliosides are amphiphilic compounds and in water solutions form micellar aggregates displaying very low aggregation concentration. The critical micellar concentration of GM1 is about 10−9 M19. Thus independently by the ganglioside concentration, the monomer concentration cannot be over 10−9 M. Only monomers are capable to insert into the cell membranes20,21 using their lipid moiety, the ceramide. Accordingly, a very minor quantity of injected GM1 overcomes the blood brain barrier and reaches the neurons. Thus to obtain a therapeutic effect, GM1 is injected in great amount increasing the possibility to inject significant amounts of contaminants22,23. The risk of GM1 protein contamination, due to its animal origin, and the completely disproved, but still discussed, relationship with Guillain-Barre syndrome24–27 inhibit serious consideration of GM1 therapeutic use.
The consequences of partial removal of GM1 and the more complex gangliosides, obtained from the heterozygous disruption of the B4galnt1 gene (GM2/GD2 synthase), was a condition sufficient for these mice to develop PD phenotype: α-syn elevation and aggregation within central (CNS) and peripheral nervous (PNS) lesions, striatal degeneration and growing motor dysfunction6,9,12,28,29. Interestingly, B4galnt1+/− mice subjected to GM1 replacement therapy by administration of semi-synthetic and brain-permeable GM1 derivative coded LIGA20 (with a dichloroacetyl group linked to the sphingosine amino group instead of the acyl chain) showed reversal of the pathological phenotype9. This result suggests that specific plasma membrane oligosaccharide–protein triggered pathways are required for the correct neuronal homeostasis.
Many studies on GM1 neurotrophic properties have been carried out using in vitro culture of neuroblastoma and pheochromocytoma cell lines, which differentiate into neuron like cells following GM1 exogenous administration30–33. The differentiative properties of GM1 have been associated to its monomeric insertion into the plasma membrane and to its interaction/modulation with membrane protein receptors, such as TrkA and RET, membrane ion channels and integrins11,12,34.
We recently reported that the soluble GM1 oligosaccharide administered to neuroblastoma cells replicates the neurotrophic and neuroprotective properties of the GM1 ganglioside35–37. The GM1 oligosaccharide added to the cell culture medium activates the TrkA auto-phosphorylation followed by the downstream MAPK signaling35–37. Molecular modelling suggested the formation of a very stable trimeric complex between GM1 oligosaccharide, TrkA and NGF35.
In this paper, we describe the results obtained by administering the soluble oligosaccharide of ganglioside GM1 to the heterozygous B4galnt1+/− mouse, animal model of PD. During 4 weeks of oligosaccharide systemic administration, animals showed reversal of motor impairment, reduction of the aberrant levels of α-syn in the substantia nigra pars compacta (SNpc), recovery of nigral tyrosine hydroxylase (TH) expression and striatal DA level. These results are in favor of the development of a new human therapy of PD based on the administration of the GM1 soluble oligosaccharide.
Results
Identification of the [3H]OligoGM1 in the brain of treated WT mice
To understand if the OligoGM1 could reach the CNS, we administered [3H]OligoGM1 to wild-type (WT) mice. Mice were intraperitoneally (IP) injected with [3H]OligoGM1 (20 mg/kg plus 13 × 106 dmp) and, 24 h following injection, brains were submitted to water soluble compounds and analyzed for the radioactivity and tritium labeled oligosaccharide contents. As shown in Fig. 1A, about 20% (±3.25 × 106 dpm) of the total injected radioactivity (1.3 × 107 dpm) was found associated to the brain. As reported (Fig. S1 of Supplementary) the large amount of radioactivity associated to the brain was non-volatile radioactivity, meaning that it is not associated to tritiated water generating upon the saccharide catabolism but rather it is associated to [3H]OligoGM1.
Figure 1.
OligoGM1 penetrates into the brain. (A) Radioactivity associated to the brain mouse after injection of 1.3 × 107 dpm [3H]OligoGM1. Data are expressed as mean ± SEM of five independent experiments (n = 5). (B) Representative image of the HPTLC separation of the radioactive material contained in the brain (n = 5). Lane 1: standard [3H]OligoGM1; Lane 2: soluble extract from brain homogenate derived from [3H]OligoGM1 injected mouse (24 h). Result is representative of those obtained for all the animals analyzed. HPTLC plates were developed with chloroform/methanol/0.2% CaCl2, 30:50:13 by vol. Tritium was detected with Beta-Imager 2000 instrument (Biospace) using an acquisition time of 16 h.
To further verify that the radioactivity was indeed corresponding to intact OligoGM1, we verify its metabolic stability by high-performance thin-layer chromatography (HPTLC) autoradiography using a BetaIMAGER TRACER system (Biospace lab) and visualized using M3 vision software. As shown in Fig. 1B, metabolically stable [3H]OligoGM1 was found in the brain. No other radioactive compounds were identified considering that the detection limit of the instrument (BetaIMAGER TRACER system, Biospace lab) is 15 spotted dpm.
Restored motor activity in B4galnt1+/− mice treated with OligoGM1
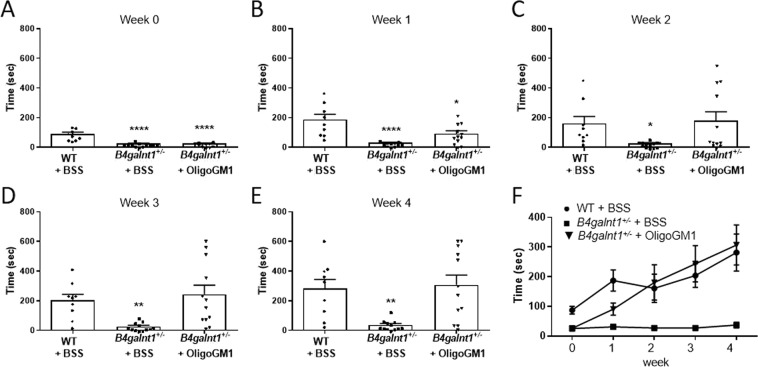
OligoGM1 (20 mg/Kg) was daily IP injected to B4galnt1+/− mice for 4 weeks. To evaluate its effect on motor coordination, balance and fine motor movement, grip duration and irritant removal tests were assessed weekly9,13,28. Before starting the treatments, the physical impairment of animals was tested to record the basal state for both “grip” and “irritant removal” tests, in comparison with WT animals (Figs. 2 and 3). The graphs depicted in Fig. 2 and in Fig. 3 clearly show a significant recovery in motor behavior of B4galnt1+/− treated mice. Moreover, mice were tested with pole climbing for motor coordination and balance (Fig. 4) but only at the end of treatment to avoid that the learning ability could influence the success of the test. Figures 2–4 show that the treated B4galnt1+/− mice rapidly recovered motor functions, some of them immediately after the first week of injections. OligoGM1 treated PD animals showed motor functions comparable with those of WT animals following 4 weeks of daily treatment. No significant difference was found between male and female mice in response to the treatment (Fig. S2 of Supplementary).
Figure 2.
OligoGM1 effect on motor balance and coordination measured by grip duration test. (A–E) B4galnt1+/− mice at 270 days of age were IP injected with 20 mg/kg OligoGM1 (n = 12) or with BSS (n = 12) daily for 4 weeks. WT mice (n = 9) of the same age were IP injected with BSS daily for 4 weeks. Data are expressed as mean ± SEM [one-way ANOVA followed by Bonferroni’s multiple comparisons test]. (A) Week 0, F(2,30) = 31.36, ****p < 0.0001 B4galnt1+/− + BSS and B4galnt1+/− + OligoGM1 compared with WT. (B) Week 1, F(2,30) = 13.30, ****p < 0.0001 B4galnt1+/− + BSS compared with WT and *p < 0.05 B4galnt1+/− + OligoGM1 compared with WT. (C) Week 2, F(2,30) = 3.842, *p < 0.05 B4galnt1+/− + BSS compared with WT and B4galnt1+/− + OligoGM1. (D) Week 3, F(2,30) = 7.546, **p < 0.01 B4galnt1+/− + BSS compared with WT and B4galnt1+/− + OligoGM1. (E) Week 4, F(2,30) = 8.630, **p < 0.01 B4galnt1+/− + BSS compared with WT and B4galnt1+/− + OligoGM1; (F) overview of the 4 weeks’ treatment (●WT + BSS; ■ B4galnt1+/− + BSS; ▼ B4galnt1+/− + OligoGM1).
Figure 3.
OligoGM1 effect on fine motor movement (sensorimotor) measured by irritant removal test. (A–E) B4galnt1+/− mice at 270 days of age were IP injected with 20 mg/kg OligoGM1 (n = 12) or with BSS (n = 12) daily for 4 weeks. WT mice (n = 9) of the same age were IP injected with BSS daily for 4 weeks. Data are expressed as mean ± SEM [one-way ANOVA followed by Bonferroni’s multiple comparisons test]. (A) Week 0, F(2,21) = 3.710, *p < 0.05 B4galnt1+/− + BSS and B4galnt1+/− + OligoGM1 compared with WT. (B) Week 1, no significant difference between B4galnt1+/− + BSS or + OligoGM1 compared with WT. (C) Week 2, F(2,19) = 5.256, *p < 0.05 B4galnt1+/− + BSS compared with WT and B4galnt1+/− + OligoGM1. (D) Week 3, F(2,30) = 6.783, *p < 0.05 B4galnt1+/− + BSS compared with WT and **p < 0.01 B4galnt1+/− + BSS compared with B4galnt1+/− + OligoGM1. (E) Week 4, F(2,19) = 6.027, **p < 0.01 B4galnt1+/− + BSS compared with WT and B4galnt1+/− + OligoGM1; (F) overview of the 4 weeks’ treatment (●WT + BSS; ■ B4galnt1+/− + BSS; ▼ B4galnt1+/− + OligoGM1).
Figure 4.

OligoGM1 effect on motor balance and coordination measured by pole climbing test. B4galnt1+/− mice at 270 days of age were IP injected with 20 mg/kg OligoGM1 (n = 12) or with BSS (n = 12) daily for 4 weeks. WT mice (n = 9) of the same age were IP injected with BSS daily for 4 weeks. Data are expressed as mean ± SEM: F(2,52) = 10.37, ***p < 0.001 B4galnt1+/− + BSS compared with WT and B4galnt1+/− + OligoGM1 [one-way ANOVA followed by Bonferroni’s multiple comparisons test].
Reduction in a-series gangliosides in B4galnt1+/− mice
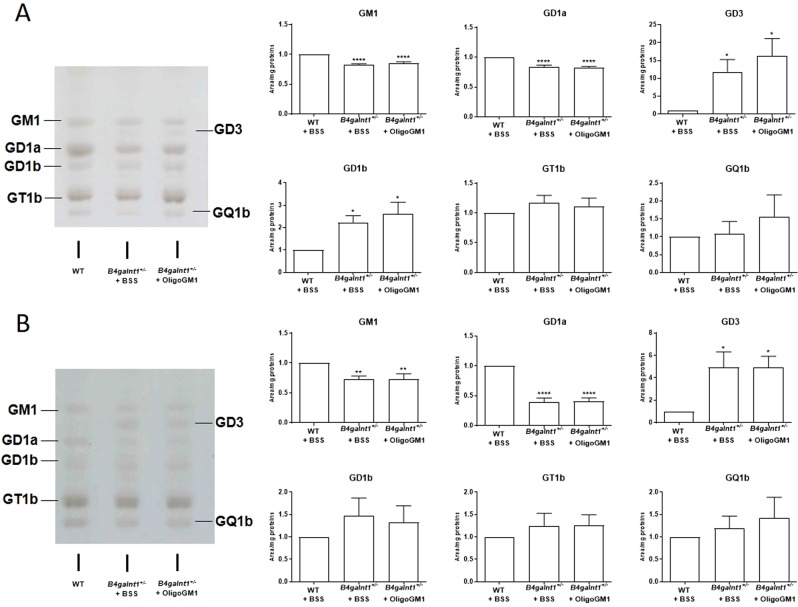
Following the 4 weeks of treatment, the ganglioside pattern and content in mice brain cortex and cerebellum were analyzed following brain lipid extraction, ganglioside purification, ganglioside separation by HPTLC and ganglioside revelation by colorimetric detection39–41. WT and B4galnt1+/− mice treated with balanced saline solution (BSS), and B4galnt1+/− treated with the OligoGM1 were analyzed.
The B4galnt1+/− mouse brains had reduced amount of GM1 and GD1a with respect to WTs in both brain regions examined (Fig. 5). In parallel, we found a fair accumulation of GD3 in cerebellum and cerebral cortex of B4galnt1+/− mice with respect to WTs (Fig. 5). With the exception of GD1b, which was elevated in the cortex of B4galnt1+/− mice, the content of b-series gangliosides was not affected, suggesting that the amount of GD3 ganglioside available results in an optimal substrate level for the galactosaminyl transferase. No differences in ganglioside pattern and content were found in the brain of B4galnt1+/− animals treated for 4 weeks with saline solution or OligoGM1, indicating that the treatment is not capable to act on the metabolic pathway of gangliosides (Fig. S3 of Supplementary).
Figure 5.
Ganglioside content in brain cortex (A) and cerebellum (B). Ganglioside patterns resolved by HPTLC in the presence of specific standards and revealed by Ehrlich spray reagent. On the left: HPTLC representative image. On the right: relative quantitation of the intensity of each ganglioside band normalized on the protein content. Patterns are representative of those obtained for all the animals analyzed (WT, n = 5; B4galnt1+/− + BSS, n = 8 B4galnt1+/− + OligoGM1, n = 8). Data are express as fold increase or decrease over WT of mean ± SEM: (A) GM1: F(2,21) = 23.54 ****p < 0.0001, GD1a: F(2,21) = 18.35 ****p < 0.0001, GD3: F(2,21) = 5.178, GD1b F(2,21) = 5.762 *p < 0.05 WT vs B4galnt1+/− (one-way ANOVA, followed by Bonferroni’s post-hoc test); (B) GM1: F(2,21) = 6.948 **p < 0.01, GD1a: F(2,21) = 46.01 ****p < 0.0001, GD3: F(2,18) = 5.479 *p < 0.05 WT vs B4galnt1+/− (one-way ANOVA, followed by Bonferroni’s post-hoc test).
These results confirm the reduction of gangliosides GM1 and GD1a in the B4galnt1+/− brain mice9 and show that the recovery of motor functions in B4galnt1+/− animals following oligosaccharide treatment was not associated to recovery of gangliosides GM1 and GD1a expression, due to activation of residual galactosaminyl transferase enzyme.
Reduction of the aberrant α-syn content in the SNpc of B4galnt1+/− mice treated with OligoGM1
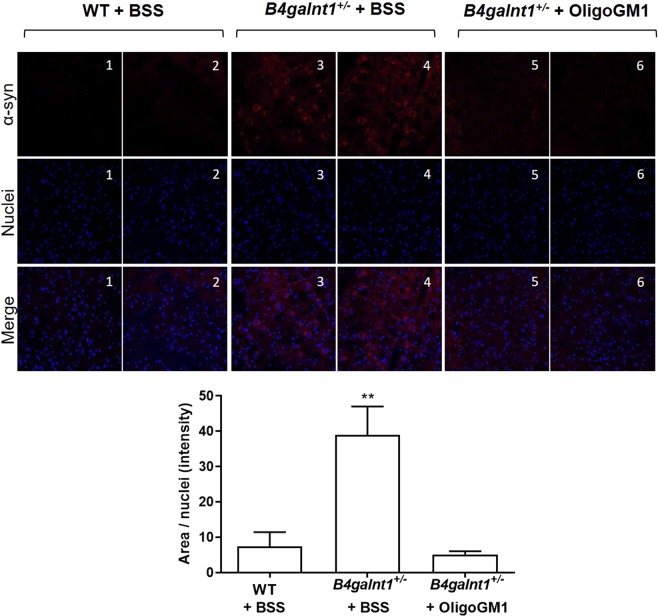
A key feature of the B4galnt1+/− mice was marked elevation of α-syn in the SNpc9. To understand if the restored motor activity was accompanied by biochemical rescue of α-syn physiological levels, we performed fluorescent immunohistochemistry (IHC) analysis of nigral α-syn. We confirmed the increased content of α-syn in the B4galnt1+/− mice (Fig. 6), reproducing what was previously reported9. Interestingly, the OligoGM1 treated B4galnt1+/− mice were found with significantly reduced levels of α-syn overall reaching the WT situation (Fig. 6). Although we did not quantify the level of Ser129-phosphorylated α-syn, we found by IHC the positivity of α-syn to the Ser129-phosphorylation in the untreated B4galnt1+/− mice at the level of SNpc (Fig. S4, panel A of Supplementary). Importantly, the positive signal was less intense in the OligoGM1 treated B4galnt1+/− and, as expected, in WT mice (Fig. S4, panel A of Supplementary). Finally, immunoblotting analysis showed levels of Ser129-phosphorylated α-syn in OligoGM1 treated animals similar to those of WT mice, and lower compared to saline-treated animals (Fig. S4, panel B of Supplementary).
Figure 6.
α-syn content within substantia nigra. On the top: representative fluorescent IHC images of α-syn (red) and nuclei (blue) immunolabeling in the SNpc. (1–2) WT + BSS (n = 4); (3–4) B4galnt1+/− + BSS, (n = 4) (5–6) B4galnt1+/− + OligoGM1, n = 4). On the bottom: quantification of α-syn normalized on nuclei area (n = 4 for group; 8–10 brain sections/animal). Data are expressed as mean ± SEM (F(2,32) = 7.7, **p < 0.01 B4galnt1+/− + BSS compared with WT and B4galnt1+/− + OligoGM1; one-way ANOVA followed by Bonferroni’s multiple comparisons test).
Recovery of the TH expression in the SNpc substantia nigra of B4galnt1+/− mice treated with OligoGM1
It has been reported that the B4galnt1 heterozygous disruption causes the degeneration of TH expressing neurons within the SNpc as well as the reduction of TH expression level9. To verify if OligoGM1 could restore the TH expression, we performed fluorescent IHC analysis on SNpc. As previously reported9, we found a decrease level of TH expression in B4galnt1+/− mice, which was rescued by OligoGM1 treatment, with restoration to WT levels (Fig. 7A). Additionally, immunoblotting evaluation confirmed a significant recovery of nigral TH expression in B4galnt1+/− mice treated with OligoGM1 compared to the WT level (Fig. 7B).
Figure 7.
TH expression in the substantia nigra. (A) On the top: representative fluorescent IHC images of TH+ neurons (red) and nuclei (blue) immunolabeling in the SNpc. (1–2) WT + BSS (n = 4); (3–4) B4galnt1+/− + BSS, (n = 4) (5–6) B4galnt1+/− + OligoGM1, n = 4). On the bottom: quantification of the TH+ neurons normalized on nuclei area (n = 4 for group; 8–10 brain/sections animal). Data are expressed as mean ± SEM (F(2,32) = 17.26, ****p < 0.001 B4galnt1+/− + BSS compared with WT and B4galnt1+/− + OligoGM1; one-way ANOVA followed by Bonferroni’s multiple comparisons test). (B) On the left: representative immunoblotting images of TH and calnexin are shown after cropping (full length images of blot are presented as Supplementary Fig. S8). On the right: Semi-quantitative analysis of nigral TH expression related to calnexin level (n = 4). Data are expressed as fold increase or decrease over the control (WT) of the mean ± SEM (F(2,8) = 10.34, **p < 0.01 B4galnt1+/− + BSS compared with WT and B4galnt1+/− + OligoGM1; one-way ANOVA followed by Bonferroni’s multiple comparisons test).
Restoration of striatal neurotransmitter levels in B4galnt1+/− mice treated with OligoGM1
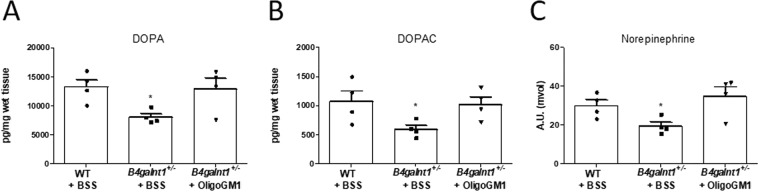
The homozygous B4galnt1−/− mice were previously found to have decreased levels of DA as well as its metabolite, 3,4-dihydroxyphenylacetic acid (DOPAC), in the striatum28. Thus, in order to further validate our findings and to determine whether the OligoGM1 could rescue the pathological phenotype by promoting the synthesis of striatal neurotransmitters, we performed neurochemical analysis with electrochemical detection high performance liquid chromatography (ED-HPLC). We confirmed that striatal levels of DA, DOPAC and norepinephrine are diminished in BSS treated B4galnt1+/− mice with respect to WT mice (Fig. 8, Fig. S5 of Supplementary). Importantly, we found that the levels of DA, DOPAC and of norepinephrine were rescued in OligoGM1 treated B4galnt1+/− mice (Fig. 8, Fig. S5 of Supplementary).
Figure 8.
Striatal neurotransmitter content. (A,B) Striatal tissue DA and DOPAC content are expressed as pico-grams per mg of wet weight of tissue. Data are expressed as mean ± SEM: DA, F(2,9) = 4.61, *p < 0.05 B4galnt1+/− + BSS compared with WT and B4galnt1+/− + OligoGM1; DOPAC, F(2,9) = 4.834, *p < 0.05 B4galnt1+/− + BSS compared with WT and B4galnt1+/− + OligoGM1 (n = 4, one-way ANOVA followed by Bonferroni’s multiple comparisons test). (C) Striatal tissue norepinephrine content data are expressed as arbitrary units (mvolt). Data are expressed as mean ± SEM (n = 4, F(2,9) = 4.915, *p < 0.05 B4galnt1+/− + BSS compared with WT and B4galnt1+/− + OligoGM1; one-way ANOVA followed by Bonferroni’s multiple comparisons test).
Discussion
Unlike familial forms, sPD has no cogent theory that summarizes the disparate data into a compelling narrative regarding its etiology42. Although various hypotheses have been suggested, none has successfully reconciled the collected data that explain the diverse central and peripheral manifestations of PD. α-Syn is generally considered to have a central role in PD, but as yet there is no explanation as to what causes its elevation and resultant aggregation in sporadic PD coinciding with progressive impairment of the neuronal dopaminergic system12.
Recently, a theory has emerged that defines a central role for ganglioside GM1 which levels diminish with aging7,8 and/or under epigenetic influences; it was proposed that in some individuals, presumably those starting life with lower levels, GM1 falls below a threshold level necessary to maintain TH neuron viability thereby gradually leading to sPD12. This would be the consequence of the numerous essential neuronal functions of GM114,23. Considering the cases reported so far, sPD patients show GM1 deficiency in the substantia nigra9, the occipital cortex13, and in various peripheral tissues12, suggesting a systemic GM1 deficiency. Moreover, a significantly deficient expression in genes involved in ganglioside synthesis (B3GALT4, ST3GAL2) was reported in neuromelanin-containing neurons (but not in other neuronal types) in the substantia nigra of sPD subjects10. These findings suggest a ganglioside systemic deficiency that would correlate with the systemic symptoms of sPD.
It has been hypothesized that subnormal levels of a-series ganglioside (GM1/GD1a) can gradually compromise several neuronal functions14,23. In particular, the reduced level of plasma membrane GM1 in sPD neurons can trigger the neurodegenerative process by a failure in trophic signaling via GDNF13 together with a reduction of clearance promoting the α-syn accumulation6,14,43. Most relevant in regard to neurodegeneration are neurotrophic factors whose receptors require GM1 association to facilitate lifelong survival of neurons. An example of this was the finding that GM1 binds with high-affinity to TrkA, the NGF receptor, and promotes tyrosine autophosphorylation: the starting event leading to neurite elongation34,44–47. Moreover, a similar GM1 partnership has been reported for the GDNF receptor, comprising RET receptor and GPI-anchored co-receptor GFRα12,13. Deficiency of RET phosphorylation, an indicator of failed GDNF signaling, was identified in the substantia nigra of sPD subjects and also in the GM1-deficient B4Galnt1+/− mice; in the latter GDNF signaling was restored by LIGA20, a membrane-permeable analog of GM113. In addition, GM1 displays binding to the soluble α-syn, and the interaction between GM1 and α-syn has been proposed to prevent α-syn aggregation and deposition15,16. Structural studies have shown the existence of α-syn region defined as the ganglioside binding region15. In vitro experiments revealed that N-alpha-acetylation of α-syn strengthened the interaction with GM1 and its resistance to aggregation16. In addition to this, free α-syn inhibits Nurr1, necessary for the expression and functioning of GDNF signaling48. Significantly, application of GM1 to a rat α-syn model of PD reduced α-syn aggregation43.Thus, the reduction of brain GM1 is claimed to be responsible for the onset of sPD.
GM1 replacement therapy has shown modest but significant success in a monocentric controlled, delayed start trial in treated sPD patients, acting as symptomatic and potentially disease modifier17; significantly, partial restoration of DA transporter functional level in the striatum of GM1-treated subjects was reported18. These results strongly support the idea that the loss of GM1-sustained trophism is the crucial point in sPD pathogenesis, so much so that restoring its brain level ameliorates symptoms and slows the disease progression. However, the great amount of GM1 necessary to obtain results, its animal origin and its discussed immunogenicity22,23 have questioned GM1 as a suitable drug for human therapy.
Beneficial results were gained in B4galnt1 GM1-deficient mice treated with a semi-synthetic, brain-permeable GM1 ganglioside analog, LIGA209,12,13. This GM1 analogue was prepared by FIDIA-Farmaceutici S.p.a. (Italy) as membrane permeable ganglioside analog capable to traverse the blood brain barrier. In those mice, LIGA20 treatment could also reduce accumulation of α-syn in substantia nigra9,12. Most important in this study is the fact that the use of a more hydrophilic GM1 derivative, modified on the ceramide moiety with a dichloroacetyl group instead of the C18 acyl chain, but keeping the entire oligosaccharide chain intact, still maintains trophic potential, supporting the hypothesis that only specific plasma membrane oligosaccharide–protein triggered pathway is required for the correct neuronal homeostasis9,12 and not the lipid moiety. Human therapy based on that particular compound cannot be approached due to the toxicity of the dichloroacetyl moiety.
It was notable that plasma membrane GM1 increase by intraventricular injection of Vibrio cholerae sialidase exerts a neuroprotective effect on the damaged nigrostriatal DA system of MPTP mice49. This enzyme removes sialic acid residues from polysialogangliosides (e.g. GD1a) in the brain, thus increasing plasma membrane GM1. In this scenario, the idea emerges that plasma membrane GM1 oligosaccharide decline could be the trigger for sustained pathways impairment as the etiopathogenetic target event of PD.
Recently, we showed in neuronal cells that, within the entire GM1 molecule, the oligosaccharide chain is the actual moiety responsible for GM1 neurotrophic properties35–37. GM1 oligosaccharide directly interacts with the NGF receptor TrkA, leading to ERK1/2 downstream pathway activation, to cell differentiation and to sustained neuroprotection from MPTP toxicity. Proteomic analysis revealed a broad spectrum of molecular events prompted by the OligoGM1 involving calcium regulation, antioxidant mechanism, mitochondrial bioenergetics, and anti-inflammatory response36.
Considering all our in vitro evidence about the OligoGM1 neurotrophic potential, we wondered if the OligoGM1 could be used to replace GM1 functions using the B4galnt1+/− mouse as in vivo approach. This model, obtained by the heterozygous disruption of the B4galnt1 gene (GM2/GD2 synthase) causing partial deletion of GM1, represents an accurate recapitulation of the human sPD condition. These mice, with reduced brain content of GM1 and GD1a (especially in the SNpc9) with respect to the WT condition, develop progressively the sPD phenotype: alteration of neurotrophic signaling, SNpc α-syn elevation and aggregation within CNS and PNS lesions, loss of TH-expressing neurons in the SNpc, striatal degeneration, and increasing motor dysfunction9,12,13,28.
Firstly, we verified the capacity of the OligoGM1 to cross the blood brain barrier and stably reach the CNS, postponing a detailed study on its pharmacological distribution to the future. This study demonstrated recovery of normal motor function in the B4galnt1+/− mice by OligoGM1 treatment, as shown with three movement tests. Similarly, neuropathological lesions were largely resolved by OligoGM1 in relation to nigral TH-expressing neurons and neurotransmitter (DA, norepinephrine) levels in the striatum. OligoGM1-treated animals also showed a significant reduction of the abnormal α-syn content compared to untreated animals at the level of SNpc. Although we performed a limited examination regarding the toxic phosphorylated form of α-syn, which specifically represents the dominant pathological modification of α-syn in familial and sporadic Lewy body diseases, as well as in transgenic animal models of synucleinopathies50–52, we found a reduction expression in the α-syn phosphorylation (Ser129) in OligoGM1-treated B4galnt1+/− mice compared to untreated mice. Now, follow-up studies are necessary to study in detail the relationship between OligoGM1 administration and α-syn phosphorylation and aggregation.
Importantly, we did not find any difference in ganglioside content between OligoGM1 and BSS treated B4galnt1+/− mice, suggesting that the OligoGM1 is not able to influence the ganglioside metabolic pathway.
Although our study does not explain the exact mechanism by which the OligoGM1 rescues the PD phenotype, it strongly supports the idea that it could account for the GM1 neurotrophic function. Our hypothesis is that the sPD pathogenesis is fundamentally due to plasma membrane GM1 deficiency that causes the alteration/loss of molecular interaction between the oligosaccharide portion of GM1 and specific proteins, giving rise to the gradual impairment of several functions. We suggest that in sPD neurons, the reduced level of GM1, and hence of its oligosaccharide, could trigger the neurodegenerative process by a failure in trophic signals together with a reduction of α-syn clearance.
The idea that the age-dependent GM1 deficiency could be the trigger for idiopathic PD is attractive, not only because it is supported by preclinical data, but also because it recognizes that GM1 functions as a key node in a network of regulated processes and its deficiency would affect the neuronal homeostasis in multi-modal ways12,14. The GM1 decrease could drive trophic signaling failure and α-syn accumulation, leading in turn to glia/microglia activation, oxidative stress, mitochondria dysfunction and excitotoxicity, resulting in the onset of cell death. Our recent data36 showed that GM1 oligosaccharide activates a broad range of neuroprotective biochemical pathways in neuroblastoma cells and prevents their death caused by toxic molecules. The latter findings, together with the results of the present work, concur in setting a reasonable basis for considering GM1 oligosaccharide as an agent that overcomes GM1 pharmacological limits and could display significant therapeutic benefit for sPD.
Materials and Methods
Materials
Commercial chemicals were of the highest purity available, common solvents were distilled before use and water was doubly distilled in a glass apparatus.
Paraformaldehyde (PFA), BSS, sucrose, fetal calf serum, Triton X-100, bovine serum albumin, trypan blue, perchloric acid, sodium acetate, sodium octyl sulfate, EDTA, DA, DOPAC and DAPI were from Sigma-Aldrich (St. Louis, MO, USA). Phosphate buffered saline (PBS) was from EuroClone. 4–20% Mini-PROTEAN TGX Precast Protein Gels, Turbo Polyvinylidene difluoride (PVDF) Mini-Midi membrane and DC protein assay kit were from Bio-Rad (Hercules, CA, USA). HPTLC plates were from Merck (Frankfurt, Germany). Optima cutting temperature (OCT) was from VWR (Radnor, PA, USA). Dako fluorescent mounting medium was from Dako (Glostrup, Denmark). Chemiluminescent kit for western blot was from Cyanagen (Bologna, Italy). Ultima gold was from PerkinElmer (Waltham, MA, USA).
GM1 ganglioside and GM1 oligosaccharide preparation
GM1 ganglioside was purified from the total ganglioside mixture extracted from fresh pig brains collected at the slaughterhouse of the Galbani company (Melzo, Italy), according to the procedure developed previously53. The ganglioside mixture, 5 g as sialic acid, was dissolved in prewarmed (36 °C) 500 mL of 0.05 M sodium acetate, 1 mM CaCl2 buffer, pH 5.5. Vibrio cholerae sialidase (1 unit) was added to the solution every 12 h54. Incubation at 36 °C and under magnetic stirring was maintained for two days, and the solution dialyzed at 23 °C for 4 days against 10 L of water changed 5 times a day. The sialidase treated ganglioside mixture was subjected to 150 cm × 2 cm silica gel 100 column chromatography equilibrated and eluted with chloroform-methanol-water, 60:35:5 by vol. The fractions containing GM1, identified by TLC, were pooled, dried and submitted to a further column chromatographic purification using the above experimental conditions. Fractions containing pure GM1 were collected and dried. The residue was dissolved in chloroform-methanol (2:1 v/v) and precipitated by adding 4 volumes of cold acetone. After centrifugation (15,000 × g) the GM1 pellet was separated from the acetone, dried, dissolved in 50 mL of deionized water and lyophilized giving 1350 mg of white powder which was stored at −20 °C.
GM1 containing tritium at position 6 of external galactose was prepared by enzymatic oxidation with galactose oxidase followed by reduction with sodium boro[3H]hydride55.
The oligosaccharides II3Neu5Ac-Gg4 and II3Neu5Ac-[6-3H(IVGal)]Gg4 (OligoGM1 and [3H]OligoGM1, respectively) were prepared by ozonolysis followed by alkaline degradation56 of GM1 and [3H]GM1, respectively. Minor changes for the alkaline degradation were introduced. Briefly, GM1 ganglioside or [3H]GM1 gangliosides was dissolved in the minimum required methanol and slowly saturated with and maintained under ozone at 23 °C for 6 h under continuous stirring. The solvent was then evaporated under vacuum and the residue brought immediately to pH 10.5–11.0 by addition of triethylamine. After solvent evaporation, GM1 oligosaccharide or [3H]GM1 oligosaccharide was purified by flash chromatography using chloroform/methanol/2-propanol/water 60:35:5:5 v/v/v/v as eluent. GM1 oligosaccharide was dissolved in methanol and stored at 4 °C.
NMR, Mass Spectrometry and HPTLC analyses showed a purity over 99% for the prepared OligoGM1 (Fig. S6 of Supplementary Files). HPTLC followed by radio-imaging showed a purity over 98% for the [3H]OligoGM1 (Fig. 1B).
Animal
All animal procedures were approved by the Rutgers University and conducted in accordance with Institutional Animal Care and Use Committee guidelines. Mice were handled following the Ethical Guidelines for Treatment of Laboratory Animals of Rutgers University.
Animals were housed with a 12 h light/12 h dark cycle in groups of five in plastic cages with ad libitum access to food and water.
A breeding pair of heterozygotes with disrupted gene for B4galnt1 (C57BL/6 background), created by Dr. Richard Proia and coworkers57, was provided as gift by Dr. Ronald Schnaar (Johns Hopkins University, Baltimore, MD, USA). Generation of B4galnt1+/− − C57BL/6 J heterozygous mice has been described previously9. All mice were backcrossed for 10 generations in a C57BL/6J background. For all experiments, WT mice with the same genetic background bred and housed in the same animal facility were used as controls. All mice were 8–9 months old at the start of the experiments. Animals were genotyped as previously reported58. Both male (35 to 45 g) and female (25 to 35 g) mice have been included in the study to maintain the variability introduced by sex factors, reported for PD59.
Animals treatment
OligoGM1 was dissolved in BSS at a concentration of 4 mg/mL and was injected intraperitoneal (IP) at 20 mg/kg body weight, daily for 4 weeks.
Since it is already known that systemically administered GM1 fails to cross the blood brain barrier60, in our experiments we chose an arbitrary and lower dose of the oligosaccharide molecule with respect to the quantity of the lipid (GM1) used in peripheral neurodegenerative experiments in the past (30 mg/kg)61.
B4galnt1+/− mice were randomly assigned to receive daily BSS (n = 12, female n = 6, male n = 6) or OligoGM1 (n = 12, female n = 6, male n = 6). No animal was excluded from analysis. At the conclusion of the study (treatment and parallel behavioral testing), animals were euthanized, and randomly divided for the biochemical analysis.
For IHC analysis, four brain for each group [WT + BSS n = 4 (2 male and 2 female); B4galnt1+/− + BSS n = 4 (2 male and 2 female); B4galnt1+/− + OligoGM1, n = 4 (2 male and 2 female)] were fixed in 4% PFA. For neurochemical, ganglioside and western blotting studies the same animals were used to collect different brain area. Specifically:
for neurochemical analysis striatal samples (both sides) were rapidly removed and immediately frozen for later analysis from four brain for each group [WT + BSS n = 4 (2 male and 2 female); B4galnt1+/− + BSS n = 4 (2 male and 2 female); B4galnt1+/− + OligoGM1 n = 4 (2 male and 2 female)].
for ganglioside analysis, cortex and cerebellum samples were rapidly collected and immediately frozen for later analysis [WT + BSS n = 5 (3 male and 2 female), B4galnt1+/− + BSS n = 8 (4 male and 4 female), B4galnt1+/− + OligoGM1, n = 8 (4 male and 4 female)];
for western blotting substantia nigra samples were rapidly removed and immediately frozen for later analysis from four brain for each group [WT + BSS n = 4 (2 male and 2 female); B4galnt1+/− + BSS n = 4 (2 male and 2 female); B4galnt1+/− + OligoGM1 n = 4 (2 male and 2 female)].
Behavioral experimental procedures
The sample size was predetermined using G*Power software and a priori analysis9,28. The effect size was determined for each experiment considering the difference we expected to see with respect to control group and the standard deviations that we derived from previous published data. Before starting behavioral experiments, each animal was handled for five days. Each experiment was performed during the light cycle and in a separate behavioral testing room after a 30-min-period of acclimation. During the test performing the rater was blind as to the experimental condition of the animal.
Grip duration test was used to test motor coordination and balance38. Briefly, each mouse was suspended by its forepaws on a horizontal rounded metal bar (diameter 3 mm) positioned 50 cm above a foam pillow and the mouse’s tail is gently held by experimenter to prevent climbing with hind legs9,13,62. The hang time to fall is measured up to 600 s for each trial in three consecutive trials, with a rest interval of 30 min. The hang times were then averaged for each mouse. Mice were tested for grip duration before starting the treatment to measure the basal state and once a week over the treatments.
Irritant removal test was assessed to determine motor response to sensory stimulus9,13,38,63. Each mouse is restrained and a small piece of adhesive is applied on the snout using forceps. The mouse is placed in a cage and a timer is set to record how long it takes each mouse to remove the sticker, typically with their forepaws. The maximum amount of time each mouse is tested is 2 min (120 s), and if a mouse fails to remove the sticker it scores 120 s. The testing was performed over 5 trials with mice having a short break between trials and mean of times were considered for subsequent analyses. The base line was measured before starting the OligoGM1 administration and, throughout treatment mice were weekly tested.
Pole climbing test was used to test motor coordination and balance9,13,38. A wooden pole (diameter 8 mm, height 55 cm) was tilted at 45° angle with respect to the floor. The times spent by each mouse to ascend, turn around and descend the pole is measured. Mice were trained two times 2 hours before the test to allow familiarization with the equipment. Mice were tested three times and the times were averaged for each mouse. The pole climbing test was performed at day 27 of treatment, to prevent learning from compromising the success of the test.
Neurochemical analysis
The levels of DA, DOPAC and norepinephrine were determined by measuring their tissue content in the striatum of animals after 4 weeks of treatment as previously reported64,65. Briefly, mice were anesthetized with IP injection of ketamine (100 mg/kg)/xylazine (10 mg/kg), and were decapitated. Striatal tissue was rapidly dissected on ice, weighed and frozen at −80 °C for future analysis. Upon thawing, each striatum sample (both side) was homogenized on ice with a mechanical stirrer (Mechanical Stirrer – Heavy duty, ISOLAB, Laborgerate GmbH, electronic speed settled at 300 rpm) for 30 sec in 0.1 M perchloric acid and 100 μM EDTA (20 μL/mg wet tissue). Homogenates were centrifuged at 14,000 × g for 20 min. Catecholamine content (sample vol. = 20 μL) of the resulting supernatants were quantified by HPLC–ED. The HPLC-ED system used was HTEC-500 (EiCOM USA, San Diego, CA, USA) that was equipped with a reverse-phase column (Varian, Brownlee, RP-18, Velosep, 3 μm, C18, 100 Å), and graphite working electrode (WE-3G, EiCOM USA, San Diego, CA, USA). Neurotransmitters were separated on a Velosep RP-18 column (100 × 3.2 mm; Applied Biosystems, Inc., Foster City, CA, USA) and quantified by measuring oxidative current at a glassy carbon wall-jet electrode (WE-GC model; Amuza Neuroscience) set at + 400 mV versus an Ag/AgCl electrode coupled to a potentiostat (RE-500; Amuza Neuroscience). The mobile phase consisted in 0.1 M sodium acetate buffer, pH 4.2, 0.1 mM EDTA, 1.2 mM sodium octyl sulfate, 8.0% (v/v) methanol. A solvent delivery pump (model HTEC-510; Amuza Neuroscience) delivered the mobile phase at 0.7 mL/min. Retention time was used to identify elements of interest, which were quantified on the basis of the peak height of oxidative current. The detection limit of the assay was 0.7 pg DA/sample.
Fluorescent immunohistochemistry (IHC), imaging and analysis
Mice were deeply anesthetized with IP injection of ketamine (100 mg/kg)/xylazine (10 mg/kg), and transcardially perfused with 0.1 M PBS (23 °C, pH 7.4), followed by freshly prepared ice-cold 4% PFA in PBS. Whole brains were isolated, post-fixed overnight in 4% PFA at 4 °C and soaked in cryoprotective solution (30% sucrose in PBS) until tissue sinking. Brains were snap frozen in pre-cooled isopentane upon OCT embedding and stored at −80 °C, prior to cryostat sectioning. Tissues were sectioned (16 μm) using cryostat (MC 5050 Semi-automatic Cryostat, Histo-Line Laboratories) after OCT compound embedding in dry ice. Sections through the rostro-caudal extent of the substantia nigra were collected and every fifth section from substantia nigra was processed for TH and α-syn IHC analysis. SNpc was used for the IHC detection of TH and α-syn, as specifically showed in Fig. 7S of Supplementary Files. For immunofluorescence, 16 μm slices were treated for 1 h with citrate buffer and heat in a water bath at 80 °C. Then the slices were put in a blocking solution (1 h, 23 °C) containing 10% fetal calf serum and 0.25% Triton X-100 in PBS. After blocking, samples were incubated with the primary antibody diluted with the same solution overnight at 23 °C. The slices were then incubated with the secondary antibody (1 h, 23 °C), followed by nuclei staining with DAPI66 (1:5000, 15 min, 23 °C).
Primary antibodies for the following epitopes were used: anti-TH Rabbit pAb (1:500; Merck Millipore Cat# 657012-100UL, RRID:AB_10681344), and anti-α-syn Mouse IgG1 (1:1000; BD Biosciences Cat# 610787, RRID:AB_398108). The following secondary antibodies were used: goat anti-Rabbit IgG (H + L) antibody (1:500; Alexa Fluor 488, Thermo Fisher Scientific Cat# A-11008, RRID:AB_143165) and goat anti-Mouse IgG1 antibody (1:500; Alexa Fluor 568, Thermo Fisher Scientific Cat# A-21124, RRID:AB_2535766).
Confocal images were acquired using a Leica TCS SP5 laser scanning confocal microscope (Leica Microsystems, GmbH) using a HCX PL APO 40× (NA 1.25) Oil immersion objective. Tiff images were then imported and analyzed using ImageJ software (ImageJ, NIH http://rsb.info.nih.gov/ij/).
Immunoblotting analysis
Freshly collected brains from PBS perfused mice were employed and the midbrain cut into 250 μm coronal sections with a vibratome sectioning system in cold PBS. The entire substantia nigra region, including pars compacta and reticulata, was isolated by dissecting under the microscope. The pooled sections were extracted with 1 mL/100 mg tissue of Cell Lysis Buffer (Cell Signaling, Danvers, MA). Aliquots containing 30 μg protein were denatured with Laemmli sample buffer (final concentration 0.1 M DTT, 63 mM Tris-HCl, 10% glycerol v/v, 2% SDS w/v, 0.01% blue bromophenol v/v), boiled at 100 °C for 5 min, separated on 4–20% polyacrylamide gels, and transferred to PVDF membranes using the Trans-Blot Turbo Transfer System (Bio-Rad). PVDF membranes were blocked with 5% milk (w/v) in TBS-0.1% tween (v/v) at 23 °C for 1 hour under gently shaking. TH and calnexin levels were assayed incubating the PVDF membranes respectively with primary anti-TH mouse antibody (1:2000 in 5% milk (w/v) in TBS-0.1% tween (v/v); Santa Cruz Biotechnology Cat# sc-25269, RRID:AB_628422), and anti-calnexin mouse antibody (1:1000 in 5% BSA (w/v) in TBS-0.1% tween (v/v), BD Biosciences Cat# 610524, RRID:AB_397884) overnight at 4 °C under gently shaking. Following, the reaction with secondary HRP-conjugated goat anti-mouse IgG (H + L) antibody (1:2000 in 5% milk (w/v) in TBS-0.1% tween (v/v), Thermo Fischer Scientific, Cat# RRID: AB_228307) at 23 °C for 1 in agitation and luminol detection (Cyanagen) were performed. Finally, the TH signal was normalized to calnexin signal. The data acquisition and analysis were performed using Alliance Uvitec (Cleaver Scientific Ltd, UK).
Ganglioside analysis
Cortex and cerebellum were collected from WT and B4galnt1+/− mice treated with BSS or OligoGM1 and subjected to lyophilization. The extraction of total lipids from lyophilized tissue was carried out with the solvent system chloroform/methanol/water in proportion of 20:10:1 by vol (1.5 mL/50 mg dry tissue) mixing samples at 23 °C in a thermomixer (Eppendorf) at 1400 rpm for 15 min. Total lipid extract was separated from the pellet by centrifugation at 13000 × g for 15 min, followed by a second and third extractions with chloroform/methanol, 2:1 by vol39–41,67. Total lipid extracts were subjected to a two phase partitioning by adding 20% water resulting in the separation of an aqueous phase containing gangliosides and in an organic phase containing all the other lipids after centrifugation at 13000 × g for 15 min at 23 °C68. Following a dialysis step to remove salts, gangliosides contained in the dialyzes aqueous phase, corresponding to 300 μg of tissue proteins, were resolved by mono-dimensional HPTLC using the solvent system chloroform/methanol/0.2% calcium chloride 50:42:11 by vol69–72. Following solvent evaporation, gangliosides were recognized by specific detection spraying with Ehrlich reagent, which is specific for sialic acid, and heating at 120 °C for 15 min. The relative amount of each ganglioside, taking into account its sialic acid content, was determined by densitometry using ImageJ software (NIH, Bethesda, USA; http://rsbweb.nih.gov/ij/).
[3H]OligoGM1 penetration into the brain
To assesses the capability of OligoGM1 to reach the brain, five WT mice (n = 5, male) weighing 25 g each were IP injected with [3H]OligoGM1 (specific radioactivity: 2.2 Ci/mmol). A total of 6.5 × 107 dpm of [3H]OligoGM1 was gently dried under nitrogen and dissolved in BSS containing 2.5 mg of cold OligoGM1. Each mouse was injected with 1.3 × 107 dpm of [3H]OligoGM1 and 0.5 mg of cold OligoGM1 corresponding to 20 mg/Kg of OligoGM1. Following 24 h from the injection, animals were euthanized by heart perfusion with saline solution to remove the blood73. Immediately the brain, without cerebellum, was collected, weighted (±350 mg) and homogenized with 1.4 mL of cold distilled water (rescue 100 mg/400 mL) by 1 min treatment with a mechanical stirrer (Mechanical Stirrer – Heavy duty, Isolab, Laborgerate GmbH, electronic speed settled at 1200 rpm) at 4 °C (cold room).
Determination of radioactivity
Brain homogenates were submitted to determination of radioactivity by liquid scintillation counting. Specifically, 100 μL of brain homogenate were combined with 5 mL of ULTIMA GOLD liquid (PerkinElmer), shaken and counted for 20 minutes by Liquid Scintillation Analyzer (TRI-CARB 2100TR, Packard). For each brain homogenate three samples were counted (n = 3). Counting of tritium was carried with a program calculating the ratio of counts between two regions of energy spectrum to detect spectral shift and thus quenching. In the case of tritium, the regions are typically set from 0 to 18.6 keV in Region A (counting cpm A) and 2 to 18.6 in Region B (counting cpm B). Based on the efficiency of the instrument, usually around 70%, cpm A and cpm B are converted automatically (by a mathematic integration) into dpm. In our case the efficiency of the instrument is 63.09% (H3 eff 0–18.6 keV) and cmp were transformed to dpm according to the curve determined with unquenched standard (#6008500/1215–111) provided by PerkinElmer. The radioactivity background was automatically subtracted from each sample using as a blank the brain homogenate from an untreated animal.
The value of the radioactivity determined in such way corresponded to volatile and non-volatile radioactivity (total radioactivity)73. To establish the specific amount of volatile and non-volatile radioactivity 100 μL of brain homogenate (3 samples from each brain, n = 3) were dried under nitrogen flux and resuspended in the same initial volume of cold distilled water (100 μL) and counted for radioactivity content as above. The difference between the value obtained from non-dry homogenate (total radioactivity) and dry homogenate corresponded to non-volatile radioactivity (Fig. S1 of Supplementary).
We recall that the OligoGM1 is tritium labeled at position 6 of external galactose. Any possible galactosidase activity can result in external galactose removal leading to loss of radioactivity from the penta-saccharide. Radiolabeled galactose may enter in hepatocytes and may be transformed into glucose. This latter enters in the glycolysis pathway finally producing tritiated water. We cannot exclude that a very minor portion of released galactose enters in glycoconjugate biosynthesis, but the largest part of non-volatile radioactivity corresponds to the original oligosaccharide, while the largest part of volatile radioactivity corresponds to water.
Metabolic stability
To verify that the non-volatile radioactivity indeed correspond to OligoGM1, 600 μL of the homogenized mixture was centrifuged at 12000 × g for 10 min at 4 °C and the supernatant containing the soluble fraction was lyophilized. The precipitate was solubilized in 600 μl of distilled water and counted for radioactivity content liquid scintillation counting as above. The lyophilized material was suspended in 600 μL of methanol. 60 μL of the methanol solution (1/10 of the starting material) was separated by HPTLC using the solvent system chloroform/methanol/0.2% calcium chloride 30:50:13 (v/v/v). The [3H]OligoGM1 was visualized with Beta-Imager 2000 instrument (Biospace) using an acquisition time of 16 h and identified using pure [3H]OligoGM1 as standard.
Protein determination
Protein concentration of samples was assessed using a DC protein assay kit according to manufacturer’s instructions, using bovine serum albumin as standard74.
Statistical analysis
Data are expressed as mean ± SEM and were analyzed for significance by one-way or two-way ANOVA test followed by Bonferroni’s multiple comparisons post-hoc test. The analysis was performed with Prism software (GraphPad Software v8, Inc. La Jolla, CA, USA). In all cases statistical significance was set at p < 0.05. All data are available upon request.
Other analytical methods
NMR spectra were recorded with a Bruker AVANCE-500 spectrometer at a sample temperature of 298 K. NMR spectra were recorded in CDCl3 or CD3OD and calibrated using the trimethylsilyl signal as internal reference.
Ganglioside and oligosaccharide mass spectrometry analyses were performed in positive ESI-MS. MS spectra were recorded on a Thermo Quest Finnigan LCQTM DECA ion trap mass spectrometer, equipped with a Finnigan ESI interface; data were processed by Finnigan Xcalibur software system (Thermo Fisher Scientific, Waltham, MA, USA).
All reactions were monitored by HPTLC on silica gel 60 plates.
Supplementary information
Acknowledgements
The authors acknowledge Euro-BioImaging (www.eurobioimaging.eu) for providing access to imaging technologies and services via the Italian Node (ALEMBIC, Milano, Italy). This work was supported by University of Milan departmental funds RV_TAR16SSONN_M to SS and Fond PSR2017_RONDELLI-CHIRICOZZI to EC, and by Rutgers Medical School grant and by Jolla Pharmaceutical Company to RWL. The authors declare that they have no conflict of interest.
Author contributions
E.C., G.W., S.A. and S.P. performed in vivo experiment. E.C., S.A. and R.A. performed neurochemical analysis of neurotransmitters. E.C., M.F., E.D.B., G.V. and S.C. performed fluorescent IHC, imaging and analysis of α-syn and TH signaling. M.V. and G.L. performed ganglioside analysis; L.M., M.G.C. and P.F. synthesized OligoGM1; DYP synthesized [3H]OligoGM1. E.C., G.L. and M.F. performed immunoblotting analysis. E.C., M.M. and N.L. performed statistical analysis. E.C., S.S. and R.W.L. designed the experiments. E.C., S.S. and R.W.L. wrote the paper. All authors edited the paper.
Data availability
The datasets generated and analyzed during the current study are available from the corresponding authors on reasonable request.
Competing interests
All the authors declare no competing interests in relation to the work described. The funder had no role in the conceptualization, design, data collection, analysis, decision to publish, or preparation of the manuscript.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Elena Chiricozzi, Email: elena.chiricozzi@unimi.it.
Sandro Sonnino, Email: sandro.sonnino@unimi.it.
Supplementary information
is available for this paper at 10.1038/s41598-019-55885-2.
References
- 1.Aureli M, Grassi S, Prioni S, Sonnino S, Prinetti A. Lipid membrane domains in the brain. BBA Molecular and Cell Biology of Lipids. 2015;1851:1006–1016. doi: 10.1016/j.bbalip.2015.02.001. [DOI] [PubMed] [Google Scholar]
- 2.Aureli M, et al. Neuronal membrane dynamics as fine regulator of sphingolipid composition. Glycoconj. J. 2018;35:397–402. doi: 10.1007/s10719-018-9841-8. [DOI] [PubMed] [Google Scholar]
- 3.Sandhoff R, Schulze H, Sandhoff K. Ganglioside metabolism in health and disease. Prog. Mol. Biol. Transl. Sci. 2018;156:1–62. doi: 10.1016/bs.pmbts.2018.01.002. [DOI] [PubMed] [Google Scholar]
- 4.Grassi S, Chiricozzi E, Mauri L, Sonnino S, Prinetti A. Sphingolipids and neuronal degeneration in lysosomal storage disorders. J. Neurochem. 2018;148:600–611. doi: 10.1111/jnc.14540. [DOI] [PubMed] [Google Scholar]
- 5.Sonnino S, et al. Gangliosides in membrane organization. Prog. Mol. Biol. Transl. Sci. 2018;156:83–120. doi: 10.1016/bs.pmbts.2017.12.007. [DOI] [PubMed] [Google Scholar]
- 6.Ledeen RW, Wu G. Gangliosides of the nervous system. Methods Mol. Bio. 2018;1804:19–55. doi: 10.1007/978-1-4939-8552-4_2. [DOI] [PubMed] [Google Scholar]
- 7.Svennerholm L, et al. Human brain gangliosides: developmental changes from early fetal stage to advanced age. BBA. 1989;1005:109–117. doi: 10.1016/0005-2760(89)90175-6. [DOI] [PubMed] [Google Scholar]
- 8.Svennerholm L, Bostrom K, Junbjer B, Olsson L. Membrane lipids of adult human brain: lipid composition of frontal and temporal lobe in subjects of age 20 to 100 years. J. Neurochem. 1994;63:1802–1811. doi: 10.1046/j.1471-4159.1994.63051802.x. [DOI] [PubMed] [Google Scholar]
- 9.Wu G, Lu ZH, Kulkarni N, Ledeen RW. Deficiency of ganglioside GM1 correlates with Parkinson’s disease in mice and humans. J. Neurosci. Res. 2012;90:1997–2008. doi: 10.1002/jnr.23090. [DOI] [PubMed] [Google Scholar]
- 10.Schneider Jay S. Altered expression of genes involved in ganglioside biosynthesis in substantia nigra neurons in Parkinson’s disease. PLOS ONE. 2018;13(6):e0199189. doi: 10.1371/journal.pone.0199189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Forsayeth J, Hadaczek P. Ganglioside metabolism and Parkinson’s disease. Front. Neurosci. 2018;12:45. doi: 10.3389/fnins.2018.00045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ledeen RW, Wu G. Gangliosides, α-synuclein, and Parkinson’s disease. Prog. Mol. Biol. Transl. Sci. 2018;156:435–454. doi: 10.1016/bs.pmbts.2017.12.009. [DOI] [PubMed] [Google Scholar]
- 13.Hadaczek P, et al. GDNF signaling implemented by GM1 ganglioside; failure in Parkinson’s disease and GM1-deficient murine model. Exp. Neurol. 2015;263:177–189. doi: 10.1016/j.expneurol.2014.10.010. [DOI] [PubMed] [Google Scholar]
- 14.Ledeen RW, Wu G. The multi-tasked life of GM1 ganglioside, a true factotum of nature. Trends Biochem. Sci. 2015;40:407–408. doi: 10.1016/j.tibs.2015.04.005. [DOI] [PubMed] [Google Scholar]
- 15.Martinez Z, Zhu M, Han S, Fink AL. GM1 specifically interacts with alpha-synuclein and inhibits fibrillation. Biochemistry. 2007;46:1868–1877. doi: 10.1021/bi061749a. [DOI] [PubMed] [Google Scholar]
- 16.Bartels, T., Kim, N. C., Luth, E. S. & Selkoe, D. J. N-alpha-acetylation of a-synuclein increases its helical folding propensity, gm1 binding specificity and resistance to aggregation. PLoS ONE. 9(7) (2014). [DOI] [PMC free article] [PubMed]
- 17.Schneider JS, et al. A randomized, controlled, delayed start trial of GM1 ganglioside in treated Parkinson’s disease patients. J. Neurol. Sci. 2013;324:140–148. doi: 10.1016/j.jns.2012.10.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Schneider JS, et al. GM1 ganglioside in Parkinson’s disease: pilot study of effects on dopamine transporter binding. J. Neurol. Sci. 2015;356:118–123. doi: 10.1016/j.jns.2015.06.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sonnino S, Cantù L, Corti M, Acquotti D, Venerando B. Aggregative properties of gangliosides in solution. Chem. Phys. Lipids. 1994;71:21–45. doi: 10.1016/0009-3084(94)02304-2. [DOI] [PubMed] [Google Scholar]
- 20.Radsak K, Schwarzmann G, Wiegandt H. Studies on the cell association of exogenously added sialo-glycolipids. Hoppe Seylers Z Physiol. Chem. 1982;363:263–272. doi: 10.1515/bchm2.1982.363.1.263. [DOI] [PubMed] [Google Scholar]
- 21.Chigorno V, et al. Association of gangliosides to fibroblasts in culture: a study performed with GM1(14C)-labelled at the sialic acid acetyl group. Glycoconj. J. 1975;2:279–291. doi: 10.1007/BF01049274. [DOI] [Google Scholar]
- 22.Zhang Wenchao, Krafft Paul R., Wang Tianlong, Zhang John H., Li Li, Tang Jiping. Pathophysiology of Ganglioside GM1 in Ischemic Stroke: Ganglioside GM1: A Critical Review. Cell Transplantation. 2019;28(6):657–661. doi: 10.1177/0963689718822782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Aureli M, et al. GM1 ganglioside: past studies and future potential. Mol. Neurobiol. 2015;53:1824–1842. doi: 10.1007/s12035-015-9136-z. [DOI] [PubMed] [Google Scholar]
- 24.Wu, X. et al. More severe manifestations and poorer short-term prognosis of ganglioside-associated Guillain-Barré syndrome in northeast china. PLoS ONE. 9(8) (2014). [DOI] [PMC free article] [PubMed]
- 25.Aureli, M. et al. Gangliosides and Guillain-Barrè Syndrome: The Facts, 20 Years after the Withdrawal of the ganglioside-Based Medications. In GBS100: Celebrating a century of progresses in Guillain-Barrè syndrome. (ed. Goodfellow J. and Willison H.) 344–348 (Peripheral Nerve Society) (2016).
- 26.Sonnino S, et al. Serum antibodies to glycan in peripheral neuropathies. Mol. Neurobiol. 2017;54:1564–1567. doi: 10.1007/s12035-016-9775-8. [DOI] [PubMed] [Google Scholar]
- 27.Shi M, Zhu J, Deng H. Clinical characteristics of intravenous injection of monosialotetrahexosyl ganglioside sodium-related guillain-barre syndrome. Front. Neurol. 2019;10:225. doi: 10.3389/fneur.2019.00225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wu G, Lu ZH, Kulkarni N, Amin R, Ledeen RW. Mice lacking major brain gangliosides develop parkinsonism. Neurochem. Res. 2011;36:1706–1714. doi: 10.1007/s11064-011-0437-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Vidal-Martinez G, et al. FTY720 improves behavior, increases brain derived neurotrophic factor levels and reduces α-synuclein pathology in parkinsonian GM2+/− mice. Neuroscience. 2019;15:1–10. doi: 10.1016/j.neuroscience.2019.05.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Facci L, et al. Promotion of neuritogenesis in mouse neuroblastoma cells by exogenous gangliosides. Relationship between the effect and the cell association of ganglioside GM1. J. Neurochem. 1984;42:299–305. doi: 10.1111/j.1471-4159.1984.tb02678.x. [DOI] [PubMed] [Google Scholar]
- 31.Mutoh T, Tokuda A, Inokuchi J, Kuriyama M. Glucosylceramide synthase inhibitor inhibits the action of nerve growth factor in PC12 cells. J. Biol. Chem. 1998;273:26001–26007. doi: 10.1074/jbc.273.40.26001. [DOI] [PubMed] [Google Scholar]
- 32.Valperta R, et al. Induction of axonal differentiation by silencing plasma membrane-associated sialidase Neu3 in neuroblastoma cells. J. Neurochem. 2007;100:708–719. doi: 10.1111/j.1471-4159.2006.04279.x. [DOI] [PubMed] [Google Scholar]
- 33.Zakharova IO, et al. GM1 ganglioside activates ERK1/2 and Akt downstream of Trk tyrosine kinase and protects PC12 cell against hydrogen peroxide toxicity. Neurochem. Res. 2014;39:2262–2275. doi: 10.1007/s11064-014-1428-6. [DOI] [PubMed] [Google Scholar]
- 34.Mutoh T, Tokuda A, Miyadai T, Hamaguchi M, Fujiki N. Ganglioside GM1 binds to the Trk proteins and regulates receptor function. Proc. Natl. Acad. Sci. 1995;92:5087–5091. doi: 10.1073/pnas.92.11.5087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Chiricozzi E, et al. Role of the GM1 ganglioside oligosaccharide portion in the TrkA–dependent neurite sprouting in neuroblastoma cells. J. Neurochem. 2017;143:645–659. doi: 10.1111/jnc.14146. [DOI] [PubMed] [Google Scholar]
- 36.Chiricozzi E, et al. The Neuroprotective Role of the GM1 oligosaccharide, II3Neu5Ac-Gg4, in neuroblastoma cells. Mol. Neurobiol. 2019;56:6673–6702. doi: 10.1007/s12035-019-1556-8. [DOI] [PubMed] [Google Scholar]
- 37.Chiricozzi E, et al. GM1 promotes TrkA-mediated neuroblastoma cell differentiation by occupying a plasma membrane domain different from TrkA. J. Neurochem. 2019;149:231–241. doi: 10.1111/jnc.14685. [DOI] [PubMed] [Google Scholar]
- 38.Sukoff Rizzo SJ, Crawley JN. Behavioral phenotyping assays for genetic mouse models of neurodevelopmental, neurodegenerative, and psychiatric disorders. Annu. Rev. Anim. Biosci. 2017;5:371–389. doi: 10.1146/annurev-animal-022516-022754. [DOI] [PubMed] [Google Scholar]
- 39.Prinetti A, et al. Secondary alterations of sphingolipid metabolism in lysosomal storage diseases. Neurochem. Res. 2011;36:1654–1668. doi: 10.1007/s11064-010-0380-3. [DOI] [PubMed] [Google Scholar]
- 40.Chiricozzi E, et al. Chaperone therapy for GM2 gangliosidosis: effects of pyrimethamine on β-hexosaminidase activity in Sandhoff fibroblasts. Mol. Neurobiol. 2014;50:159–167. doi: 10.1007/s12035-013-8605-5. [DOI] [PubMed] [Google Scholar]
- 41.Samarani M, et al. A lysosome-plasma membrane-sphingolipid axis linking lysosomal storage to cell growth arrest. FASEB J. 2018;32:5685–5702. doi: 10.1096/fj.201701512RR. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Stern M, Lang A, Poewe W. Toward a redefinition of Parkinson’s disease. Movement Disorders. 2012;27:54–56. doi: 10.1002/mds.24051. [DOI] [PubMed] [Google Scholar]
- 43.Schneider JS, et al. GM1 ganglioside modifies α-synuclein toxicity and is neuroprotective in a rat α-synuclein model of parkinson’s disease. Sci. Reports. 2019;9:8362. doi: 10.1038/s41598-019-42847-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ferrari G, Anderson BL, Stephens RM, Kaplan DR, Greene LA. Prevention of apoptotic neuronal death by GM1 ganglioside. Involvement of Trk neurotrophin receptors. J. Biol. Chem. 1995;270:3074–3080. doi: 10.1074/jbc.270.7.3074. [DOI] [PubMed] [Google Scholar]
- 45.Farooqui T, Franklin T, Pearl DK, Yates AJ. Ganglioside GM1 enhances induction by nerve growth factor of a putative dimer of TrkA. J. Neurochem. 1997;68:2348–2355. doi: 10.1046/j.1471-4159.1997.68062348.x. [DOI] [PubMed] [Google Scholar]
- 46.Rabin SJ, Bachis A, Mocchetti I. Gangliosides activate Trk receptors by inducing the release of neurotrophin. J. Biol. Chem. 2002;51:49466–49472. doi: 10.1074/jbc.M203240200. [DOI] [PubMed] [Google Scholar]
- 47.Duchemin AM, Ren Q, Mo L, Neff NH, Hadjiconstantinou M. GM1 ganglioside induces phosphorylation and activation of Trk and Erk in brain. J. Neurochem. 2002;81:696–707. doi: 10.1046/j.1471-4159.2002.00831.x. [DOI] [PubMed] [Google Scholar]
- 48.Decressac M, et al. α-Synuclein-induced down-regulation of Nurr1 disrupts GDNF signaling in nigral dopamine neurons. Sci. Transl. Med. 2012;4:163–156. doi: 10.1126/scitranslmed.3004676. [DOI] [PubMed] [Google Scholar]
- 49.Schneider, J. S., Seyfried, T. N., Chiu, H. S. & Kidd, S. K. Intraventricular sialidase administration enhances gm1 ganglioside expression and is partially neuroprotective in a mouse model of Parkinson’s disease. PLoS One10(12) (2015). [DOI] [PMC free article] [PubMed]
- 50.Oueslati A. Implication of Alpha-Synuclein Phosphorylation at S129 in Synucleinopathies: What Have We Learned in the Last Decade? J. Parkinsons Dis. 2016;6:39–51. doi: 10.3233/JPD-160779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Fujiwara H, et al. alpha-Synuclein is phosphorylated in synucleinopathy lesions. Nat. Cell Biol. 2002;4:160–164. doi: 10.1038/ncb748. [DOI] [PubMed] [Google Scholar]
- 52.Anderson JP, et al. Phosphorylation of Ser-129 is the dominant pathological modification of alpha-synuclein in familial and sporadic Lewy body disease. J. Biol. Chem. 2006;281:29739–52. doi: 10.1074/jbc.M600933200. [DOI] [PubMed] [Google Scholar]
- 53.Tettamanti G, Bonali F, Marchesini S, Zambotti V. A new procedure for the extraction, purification and fractionation of brain gangliosides. Biochim. Biophys Acta. 1973;296:160–170. doi: 10.1016/0005-2760(73)90055-6. [DOI] [PubMed] [Google Scholar]
- 54.Acquotti D, Cantu L, Ragg E, Sonnino S. Geometrical and conformational properties of ganglioside GalNAc-GD1a, IV4GalNAcIV3Neu5AcII3Neu5AcGgOse4Cer. Eur. J. Biochem. 1994;225:271–288. doi: 10.1111/j.1432-1033.1994.00271.x. [DOI] [PubMed] [Google Scholar]
- 55.Sonnino S, Nicolini M, Chigorno V. Preparation of radiolabeled gangliosides. Glycobiology. 1996;6:479–487. doi: 10.1093/glycob/6.5.479-e. [DOI] [PubMed] [Google Scholar]
- 56.Wiegandt H, Bücking HW. Carbohydrate components of extraneuronal gangliosides from bovine and human spleen, and bovine kidney. Eur. J. Biochem. 1970;15:287–292. doi: 10.1111/j.1432-1033.1970.tb01006.x. [DOI] [PubMed] [Google Scholar]
- 57.Liu Y, et al. A genetic model of substrate deprivation therapy for a glycosphingolipid storage disorder. J. Clin. Invest. 1999;103:497–505. doi: 10.1172/JCI5542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Wu G, Xie X, Lu ZH, Ledeen RW. Cerebellar neurons lacking complex gangliosides degenerate in the presence of depolarizing levels of potassium. Proc. Natl. Acad. Sci. USA. 2001;98:307–312. doi: 10.1073/pnas.98.1.307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Seyfried TN, et al. Sex-related abnormalities in substantia nigra lipids in Parkinson’s disease. ASN Neuro. 2018;10:1–10. doi: 10.1177/1759091418781889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Magistretti PJ, et al. Gangliosides: Treatment Avenues in Neurodegenerative Disease. Front. Neurol. 2019;10:859. doi: 10.3389/fneur.2019.00859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Chentanez V, et al. Ganglioside GM1 (porcine) ameliorates paclitaxel-induced neuropathy in rats. J. Med. Assoc. Thai. 2009;92:50–7. [PubMed] [Google Scholar]
- 62.Sedelis M, et al. MPTP susceptibility in the mouse: behavioral, neurochemical, and histological analysis of gender and strain differences. Behav. Genet. 2000;30:171–182. doi: 10.1023/A:1001958023096. [DOI] [PubMed] [Google Scholar]
- 63.Lu XH, et al. Bacterial artificial chromosome transgenic mice expressing a truncated mutant parkin exhibit age-dependent hypokinetic motor deficits, dopaminergic neuron degeneration, and accumulation of proteinase K-resistant alpha-synuclein. J Neurosci. 2009;29:1962–1976. doi: 10.1523/JNEUROSCI.5351-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Zackheim J, Abercrombie ED. Thalamic regulation of striatal acetylcholine efflux is both direct and indirect and qualitatively altered in the dopamine-depleted striatum. Neuroscience. 2005;131:423–436. doi: 10.1016/j.neuroscience.2004.11.006. [DOI] [PubMed] [Google Scholar]
- 65.Assini R, Abercrombie ED. Zolpidem ameliorates motor impairments in the unilaterally 6-hydroxydopamine-lesioned rat. Eur. J. Neurosci. 2018;48:1896–1905. doi: 10.1111/ejn.14075. [DOI] [PubMed] [Google Scholar]
- 66.Chiaretti S, Astro V, Chiricozzi E, de Curtis I. Effects of the scaffold proteins liprin-α1, β1 and β2 on invasion by breast cancer cells. Biol. Cell. 2016;108:65–75. doi: 10.1111/boc.201500063. [DOI] [PubMed] [Google Scholar]
- 67.Malekkou Anna, Samarani Maura, Drousiotou Anthi, Votsi Christina, Sonnino Sandro, Pantzaris Marios, Chiricozzi Elena, Zamba-Papanicolaou Eleni, Aureli Massimo, Loberto Nicoletta, Christodoulou Kyproula. Biochemical Characterization of the GBA2 c.1780G>C Missense Mutation in Lymphoblastoid Cells from Patients with Spastic Ataxia. International Journal of Molecular Sciences. 2018;19(10):3099. doi: 10.3390/ijms19103099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Folch J, Lees M, Sloane Stanley GH. A simple method for the isolation and purification of total lipids from animal tissues. J. Biol. Chem. 1957;226:497–509. [PubMed] [Google Scholar]
- 69.Massimo A, et al. Current and Novel Aspects on the Non-lysosomal β-Glucosylceramidase GBA2. Neurochem. Res. 2016;41:210–220. doi: 10.1007/s11064-015-1763-2. [DOI] [PubMed] [Google Scholar]
- 70.Loberto N, et al. Methods for assay of ganglioside catabolic enzymes. Methods Mol. Biol. 2018;1804:383–400. doi: 10.1007/978-1-4939-8552-4_18. [DOI] [PubMed] [Google Scholar]
- 71.Chiricozzi E, Mauri L, Ciampa MG, Prinetti A, Sonnino S. On the use of cholera toxin. Glycoconj. J. 2018;35:161–163. doi: 10.1007/s10719-018-9818-7. [DOI] [PubMed] [Google Scholar]
- 72.Schiumarini D, et al. Evidence for the involvement of lipid rafts and plasma membrane sphingolipid hydrolases in pseudomonas aeruginosa infection of cystic fibrosis bronchial epithelial cells. Mediators Inflamm. 2017;103:445–456. doi: 10.1155/2017/1730245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Orlando P, et al. The fate of tritium labeled GM1 ganglioside injected in mice. Pharmacol. Res. Com. 1979;11:759–773. doi: 10.1016/S0031-6989(79)80047-8. [DOI] [PubMed] [Google Scholar]
- 74.Aureli M, et al. Ionizing radiation increase the activity of cell surface glycohydrolases and plasma membrane ceramide content. Glycoconj. J. 2012;29:585–597. doi: 10.1007/s10719-012-9385-2. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets generated and analyzed during the current study are available from the corresponding authors on reasonable request.