Abstract
Protein sources are the second most important component in poultry diets. Due to the fluctuation in price of soybean meal (SBM) and persistent increase in feed prices, nutritionists have been exploring alternative protein sources. Replacement of SBM with alternative protein sources in poultry diets could reduce human-livestock competition for soybean and support the production of more animal protein. However, the use of alternative protein sources is limited to low inclusion due to the presence of anti-nutritional factors (ANF) such as glucosinolates (rapeseed meal), gossypol (cottonseed meal), non-starch polysaccharides (NSP) in lupin flour, high fibre (palm kernel cake), total phenolic contents and phytic acid (canola meal) known to impair animal performance, nutrient digestibility and feed utilization. As a processing technique, solid-state fermentation (SSF) has been researched for a long time in the food industry. An important objective of SSF is the production of enzymes, organic acids and other metabolites of economic importance. In recent times, SSF has been employed to enhance nutrient bioavailability, inhibit gut pathogenic bacteria and reduce ANF in plant protein sources resulting in improved nutrient digestibility, thereby improving performance and gut health of broiler chickens. Unlike pigs, there is still a dearth of information on feeding solid-state fermented feed ingredients to broiler chickens. This review aims to describe the nutritional value of the solid-state fermented products of rapeseed meal, canola meal, cottonseed meal, palm kernel cake and lupin flour on performance and intestinal health of broiler chickens.
Keywords: Anti-nutritional factor, Broiler chicken, Nutritional value, Protein source, Solid-state fermentation
1. Introduction
Soybean meal (SBM) is widely used in poultry diets due to its high crude protein and amino acid composition (Sun et al., 2013b). However, the persistent fluctuation in price and availability of SBM over the years has resulted to nutritionists finding alternative ways to sustain poultry production for the future. Moreover, there is an increasing demand for animal protein (meat and egg) by the ever-growing human population. Thus, under-utilized protein feed ingredients may be explored to meet the future protein requirements. A wide range of feed ingredients known to have good nutritional qualities for poultry feeding has been previously reported (Ravindran and Blair, 1992, Iji et al., 2017, Olarotimi and Adu, 2017). Among these are alternative protein sources such as rapeseed meal, canola meal, cottonseed meal, palm kernel cake and lupin flour. However, the anti-nutritional factors (ANF) present in these alternative protein sources may interfere with nutrient availability, cause toxicity and reduce animal performance (Ravindran and Blair, 1992). These limitations prevent them from gaining wider acceptance in the poultry industry. A promising method that can be applied to improve the nutritive quality of these alternative protein sources is solid-state fermentation (SSF). Solid-state fermentation is a bioprocessing technology that has a long history of application in the food and fermentation industries. In Asia, SSF is used for producing of foods such as bread, cheese, koji, miso, soy sauce, tempeh, natto and sufu (Cheng et al., 2009, Behera and Ray, 2016, Soccol et al., 2017). Considering the last century and in recent times, the application of SSF has resulted in producing important biochemical and value-added products such as amino acids, enzymes, organic acids, antibiotic (pharmaceutical), textile and biofuels among many others (Pandey, 1992, Pandey, 2003, Soccol and Vandenberghe, 2003, Thomas et al., 2013). Reviews on SSF of agro-industrial by-products for animal feeding have been previously published (Ramachandran et al., 2007, Ajila et al., 2012). The use of fermented feed products has been a useful strategy and common practice in pig production for many years (Canibe and Jensen, 2012). Dietary inclusion of solid-state fermented feed did not have any negative effect on performance and nutrient digestibility in growing-finishing pigs (Hu et al., 2016). Interestingly, there is an increasing interest in incorporating solid-state fermented feeds in broiler diets due to the benefits of improving performance and gut health (Alshelmani et al., 2016, Alshelmani et al., 2017a). The nutritional features of the aforementioned alternative protein sources have been evaluated by several authors, mainly with a view to assessing the implication of their use in poultry diets. However, until now little information has been published on their nutrient composition after SSF. For future utilization of solid-state fermented feed ingredients, it is important to understand their feeding value when incorporated in poultry feed. This review aims to describe the effect of SSF on the nutrient composition of rapeseed meal, canola meal, cottonseed meal, palm kernel cake and lupin flour. In addition, the feeding value of the solid-state fermented products of the aforementioned protein sources in terms of performance, intestinal microflora and intestinal morphology of broiler chickens will be discussed.
2. Solid-state fermentation
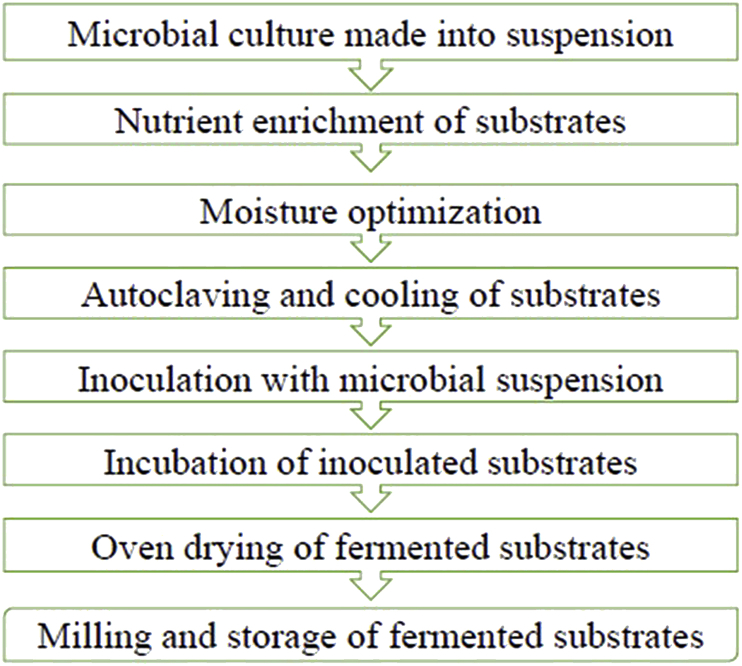
Solid-state fermentation is one of 2 main types of fermentation techniques, the other being submerged fermentation (Subramaniyam and Vimala, 2012). The process of SSF involves microorganisms growing on solid materials under controlled conditions in the absence of free water. The required moisture is in an absorbed state within the solid matrix (Pandey, 1992, Pandey, 2003, Krishna, 2005), however, the substrate must possess enough moisture to enhance the growth and metabolic activity of microorganisms. The quality of solid-state fermented products depends on conditions such as initial moisture, particle size, pH, temperature, media composition, operational system, mixing, sterilization, water activity, inoculum density, agitation, aeration, extraction of product and downstream process (Renge et al., 2012). These conditions need to be properly selected and carefully optimized (Fig. 1) when designing an effective SSF system (Abu Yazid et al., 2017) in order to achieve a good yield.
Fig. 1.
Schematic representation of steps involved in solid-state fermentation of substrates.
Various kinds of microorganisms are used in SSF. Due to the minimal moisture required, only a limited number of microorganisms such as yeast and filamentous fungi can grow well under SSF conditions (López-Pérez and Viniegra-González, 2016). Although, there are indications of the successful use of some bacteria strains in producing bioproducts from SSF (Couto and Sanromán, 2006, Singhania et al., 2009, Martins et al., 2011). As it has been recommended, the potential of SSF is to provide the selected microorganism with a suitable environment that is similar to its natural habitat (Thomas et al., 2013). Under this simulated condition, the microbes can modify the chemical or physico-chemical properties of the substrates as well as degrading the substrate by producing a wide range of enzymes (Table 1).
Table 1.
Reports on solid-state fermentation (SSF) of plant protein sources and enzymes involved.
| Substrate | Microorganism | Enzymes | Bioreactor | Duration and temperature | Reference |
|---|---|---|---|---|---|
| Rapeseed meal | Lactobacillus fermentum, Enterococcus faecium, Saccharomyces cerevisiae, Bacillus subtilis | Phytase, cellulase, protease, lipase, xylanase, glucanase, amylase, laccase, pectinase, invertase | Multi-layer polythene bag | 30 d at 30 ± 3 °C | Chiang et al. (2010) |
| Lactobacillus plantarum, Bacillus subtilis | Phytase, cellulase, protease, amylase, lipase, pectinase, xylanase, glucanase | Plastic bag | 3 wk (under anaerobic conditions) | Xu et al. (2011) | |
| Lactobacillus fermentum, Bacillus subtilis | Phytase, cellulase, protease, amylase, lipase, pectinase, xylanase, glucanase | Multi-layer polythene bag | 30 d at 30 ± 2 °C | Xu et al. (2012) | |
| Aspergillus niger | Lipase, protease, cellulase, xylanase, phytase | Flask | 3 d at 30 °C | Shi et al. (2015) | |
| Aspergillus niger | Lipase, protease, cellulase, xylanase, phytase | Bed-packed incubator | 3 d at 32 °C | Shi et al. (2016a) | |
| Aspergillus niger | Lipase, protease, cellulase, xylanase, phytase | Bed-packed incubator | 3 d at 34 °C | Shi et al. (2016b) | |
| Bacillus subtilis, Candida utilis, Enterococcus faecalis | Xylanase, protease, lipase, cellulase, pectinase, amylase, ligninase, phytase | Electro-heating standing-temperature cultivator | 3 d at 30 °C | Hu et al. (2016) | |
| Canola meal | Lactobacillus salivarius | α-galactosidase, β-galactosidase, α-glucosidase, β-glucosidase, phytase, cellulase, xylanase, glucanase | Plastic barrel | 30 d at 28 to 32 °C | Ahmed et al. (2014) |
| Lactobacillus salivarius | α-galactosidase, β-galactosidase, α-glucosidase, β-glucosidase, phytase, cellulase, xylanase, glucanase | 0.5 L Schott bottles | 30 d at 28 to 32 °C | Aljuobori et al. (2014) | |
| Lactobacillus salivarius | α-galactosidase, β-galactosidase, α-glucosidase, β-glucosidase, phytase, cellulase, xylanase, glucanase | 0.5 L Schott bottles | 30 d at 28 to 32 °C | Aljuobori et al. (2017) | |
| Camelina meal | Saccharomyces cerevisiae | Invertase, phytase, β-glucanase | Flask | 3 to 7 d at 25 °C | Olukomaiya et al. (2019) |
| Cottonseed meal | Bacillus subtilis BJ-1 | Protease, lipase, amylase, xylanase | Bed-packed incubator | 48 h | Sun et al. (2013a) |
| Bacillus subtilis BJ-1 | Protease, lipase, amylase, xylanase | Plastic container | 48 h at 30 °C | Sun et al. (2013b) | |
| Bacillus subtilis BJ-1 | Protease, lipase, amylase, xylanase | Plastic container | 48 h at 30 °C | Tang et al. (2012) | |
| Candida utilis | Amylase, pectinase, cellulase, xylanase, ligninase, protease, lipase, phytase | – | 24 h at 30 °C | Xiong et al. (2016) | |
| Bacillus subtilis ST-141, Saccharomycetes N5 | Protease, lipase, amylase, xylanase | Stainless steel fermentation tank | 48 h at 30 °C | Wang et al. (2017) | |
| Palm kernel cake | Aspergillus wentii | Lipase, amylase, dextranase, cellulase, pectinase, β-glucosidase, mannanase, xylanase | – | 48 h at 30 °C | Muangkeow and Chinajariyawong (2009) |
| Aspergillus wentii | Lipase, amylase, dextranase, cellulase, pectinase, β-glucosidase, mannanase, xylanase | – | 48 h at 30 °C | Muangkeow and Chinajariyawong (2013) | |
| Cellulolytic bacteria | Cellulase, xylanase, mannanase | Flask | 4 to 7 d at 30 °C | Alshelmani et al. (2013) | |
| Cellulolytic and hemicellulolytic bacteria | Cellulase, xylanase, mannanase | Flask | 12 d | Alshelmani et al. (2014) | |
| Paenibacillus polymyxa | Cellulase, xylanase, mannanase | Flask | 9 d at 30 °C | Alshelmani et al. (2016) | |
| Paenibacillus polymyxa | Cellulase, xylanase, mannanase | Flask | 9 d at 30 °C | Alshelmani et al. (2017a) | |
| Cellulolytic bacteria (Paenibacillus polymyxa and Paenibacillus curdlanolyticus) | Cellulase, xylanase, mannanase | Flask | 4 to 7 d at 30 °C | Alshelmani et al. (2017b) | |
| Lupin flour | Lactic acid bacteria (Lactobacillus sakei, Pediococcus acidilactici, Pediococcus pentosaceus) | α-galactosidase, β-galactosidase, α-glucosidase, β-glucosidase, phytase, cellulase, xylanase, glucanase | – | 24 h at 30 °C for Lactobacillus sakei, at 32 °C for Pediococcus acidilactici and at 35 °C for Pediococcus pentosaceus | Bartkiene et al. (2015) |
| Lactic acid bacteria (Lactobacillus sakei, Pediococcus acidilactici, Pediococcus pentosaceus) | α-galactosidase, β-galactosidase, α-glucosidase, β-glucosidase, phytase, cellulase, xylanase, glucanase | – | 24 h at 30 °C for Lactobacillus sakei, at 32 °C for Pediococcus acidilactici and at 35 °C for Pediococcus pentosaceus | Krunglevičiūtė et al. (2016) |
Other than enzymes, metabolites such as organic acids are also produced. Citric acid, oxalic acid and lactic acid can be secreted by Aspergillus niger, Aspergillus oryzae and Lactobacillus strains, respectively (Couto and Sanromán, 2006). As previously mentioned, the use of agroindustrial by-products or residues as feedstock in SSF is highly preferred. They play crucial roles in the production of high value animal feed because: 1) they are abundant, 2) less competitive between humans and livestock, 3) readily available at minimal cost for further processing, 4) possess suitable nutrient composition, and 5) can aid microbial development during SSF (Ajila et al., 2012). The value of these by-products are improved, they act as a physical support and serve as sources of carbon and nutrients for microbial growth and enzyme production (Farinas, 2015). On the other hand, their use facilitate solid waste management since they are abundant and mostly underutilized (Singhania et al., 2009).
In comparison to submerged fermentation, notable advantages of SSF include use of minimal moisture, low-cost media, better oxygen circulation, reduced capital cost, high productivity, reduced energy consumption, generation of low wastewater and less efforts in downstream processing (Singhania et al., 2009, Chen and He, 2012, Mussatto et al., 2012). The low technology involved makes it possible to be carried out on farms. In addition, the enzymes produced are less susceptible to problems of inhibition by the substrate and are more stable in terms of the effects of temperature and pH (Barrios-González, 2012). However, SSF has its own disadvantages namely: difficulties on scale up, heat build-up, difficulty to control the process parameters (moisture, temperature, nutrient content, etc.) and higher impurity product (Couto and Sanromán, 2006).
3. Effect of solid-state fermentation on the nutrient composition of plant protein sources
Generally, available literature confirms the feasibility of improving the nutrient composition of alternative protein sources with SSF. In particular, no decline in nutritional profile has been reported (Table 2). Most papers have described similar or even better nutrient composition in solid-state fermented protein sources compared to their unfermented forms or conventional SBM (Xu et al., 2011, Tang et al., 2012). The composition and nutritional availability of products obtained after SSF can vary based on the nature of feed, feed source, method of oil extraction, processing, storage parameters, microorganisms used and SSF conditions.
Table 2.
The effects of solid-state fermentation on the nutrient composition of alternative plant protein sources.
| Substrate | Microorganism | Results | Reference |
|---|---|---|---|
| Rapeseed meal | Lactobacillus fermentum, Enterococcus faecium, Saccharomyces cerevisiae, Bacillus subtilis | Increased crude protein and decreased isothiocyanates | Chiang et al. (2010) |
| Lactobacillus plantarum and Bacillus subtilis | Increased crude protein, increased crude fat and decreased isothiocyanate | Xu et al. (2011) | |
| Lactobacillus fermentum and Bacillus subtilis | Increased crude protein, increased crude fat, increased peptide and decreased isothiocyanate | Xu et al. (2012) | |
| Bacillus subtilis, Candida utilis, Enterococcus faecalis | Increased crude protein, increased crude fat, reduced crude fibre, increased peptide, decreased ANF (glucosinolates and its derivatives, phytic acid and tannins) | Hu et al. (2016) | |
| Aspergillus niger | Increased crude protein, increased ash, increased calcium, increased phosphorus, reduced crude fat, increased peptide and decreased ANF (glucosinolate and its derivatives, and phytic acid) | Shi et al. (2016b) | |
| Canola meal | Lactobacillus salivarius | Increased crude protein, increased crude fat, reduced crude fibre and decreased glucosinolates | Ahmed et al. (2014) |
| Lactobacillus salivarius | Increased crude protein, increased crude fat, reduced crude fibre and decreased glucosinolates | Aljuobori et al. (2014) | |
| Lactobacillus salivarius | Increased crude protein, increased crude fat, reduced crude fibre and decreased glucosinolates | Aljuobori et al. (2017) | |
| Cottonseed meal | Bacillus subtilis BJ-1 | Increased crude protein, reduced crude fibre, increased ash and decreased free gossypol | Tang et al. (2012) |
| Bacillus subtilis BJ-1 | Increased crude protein, reduced crude fat, increased phosphorus and decreased free gossypol | Sun et al. (2013a) | |
| Bacillus subtilis BJ-1 | Increased crude protein, reduced crude fat, reduced crude fibre, increased phosphorus and decreased free gossypol | Sun et al. (2013b) | |
| Candida utilis | Increased crude protein, reduced crude fibre, increased crude ash and reduced free gossypol | Xiong et al. (2016) | |
| Bacillus subtilis ST-141, Saccharomycetes N5 | Increased crude protein, reduced crude fibre, increased crude ash and reduced free gossypol | Wang et al. (2017) | |
| Palm kernel cake | Aspergillus wentii | Increased crude protein, increased crude fat, reduced crude fibre, increased ash, reduced ADF, reduced NDF, reduced hemicellulose and increased phosphorus | Muangkeow and Chinajariyawong (2009) |
| Paenibacillus polymyxa and Paenibacillus curdlanolyticus | Reduced crude protein, increased ash, increased fibre (ADF, NDF, hemicellulose) and reduced cellulose | Alshelmani et al., 2017a, Alshelmani et al., 2017b | |
| Lupin flour | Lactobacillus acidophilus, Lactobacillus aporogenes and Lactobacillus kefiri | Increased crude protein, reduced crude fat, reduced crude fibre, increased phosphorus and reduced ANF (phytic acid and tannins) | Van Vo et al. (2015) |
ANF = anti-nutritional factors; ADF = acid detergent fibre; NDF = neutral detergent fibre.
3.1. Rapeseed meal and canola meal
After oil extraction, the residue obtained (rapeseed meal) from rapeseed is used as a supplemental protein source for livestock. Rapeseed meal contains 34% to 38% crude protein depending upon the amount of hull and processing method. However, the presence of glucosinolates limits its utilization especially for young animals (Tripathi and Mishra, 2007). The genetically modified rapeseed breed, canola contains less than 2% erucic acid and less than 30 μg/g glucosinolate content (Bell, 1993). Canola meal is the by-product of canola seed crushing after oil extraction process. It contains up to 40% crude protein, a well-balanced amino acid composition (Newkirk, 2009), available energy (2,000 kcal/kg apparent metabolizable energy [AME]) but higher fibre content (12%) compared to soybean meal (Wickramasuriya et al., 2015). The high fibre, high indigestible carbohydrate content and glucosinolates even at a low concentration are some of the factors limiting the use of canola meal in chicken diets (Kocher et al., 2000). The enzyme, myrosinase breaks down glucosinolates to release a range of derivatives. The most common products are isothiocyanates which reduce feed intake, impair performance, antioxidative capacity and immune function in animals (Tripathi and Mishra, 2007). In order to maximize the nutritional quality of rapeseed meal and canola meal for wider acceptability, these ANF need to be minimized.
3.1.1. Effect of solid-state fermentation on the nutrient composition of rapeseed meal and canola meal
Solid-state fermentation has been used to increase the bioavailability of nutrients and reduce the level of ANF of rapeseed meal. A wide variety of microorganisms have been used for the SSF of rapeseed for nutritional enhancement predominantly Lactobacillus, Bacillus and Aspergillus strains. Aspergillus has the ability to produce enzymes such as hemicellulase, pectinase, protease, amylase, lipase, tannase (Pinto et al., 2001, Mathivanan et al., 2006) and phytase (Fujita et al., 2003). In the case of bacteria, Lactobacillus produces phytase, cellulase, xylanase and glucanase enzymes (Taheri et al., 2009). Bacillus can synthesize cellulase and phytase (Sun et al., 2012). The work of Chiang et al. (2010) on SSF of rapeseed meal with Lactobacillus fermentum, Enterococcus faecium, Saccharomyces cerevisiae and Bacillus subtilis established the importance of SSF in nutrient quality improvement and also created a path for further research work on rapeseed meal. Beneficial effects of SSF on rapeseed meal have been documented (Xu et al., 2012, Hu et al., 2016). Solid-state fermentation resulted in a reduction in the dry matter (DM) content of rapeseed meal (Chiang et al., 2010). The decline in DM content may be due to the consumption of carbohydrate by the microorganisms. Solid-state fermentation using Lactobacillus plantarum and Bacillus subtilis (Xu et al., 2011) increased crude protein from 37.1% to 58.4%. Likewise, the inoculation with Bacillus subtilis, Candida utilis and Enterococcus faecalis showed an increase in crude protein content from 42.11% to 44.63% (Hu et al., 2016). The increase in crude protein may be attributed to a decline in DM content as well as production of extra microbial protein during SSF. Altogether, SSF could be a promising approach for improving protein content for poultry diets. Other than crude protein, crude fat is also a component of interest in broiler diet. The lipid content of 5% or less is recommended for feed formulation as a higher level can adversely affect nutrient consumption, either through regulatory mechanisms that control consumption or restricted ability to oxidize fatty acids (Bringel et al., 2011). Solid-state fermentation using L. plantarum and Bacillus subtilis increased crude fat content of rapeseed meal (Xu et al., 2011). A similar result was observed by Xu et al. (2012) when L. fermentum and Bacillus subtilis were used. The increase in fat content may partially be attributed to the decrease in carbohydrate content during SSF. Unlike these studies, Shi et al. (2016a) reported a decline in crude fat when SSF of rapeseed meal was conducted using A. niger. Overall, factors such as the feed source, oil processing methods and microorganisms used may be responsible for the discrepancies in crude fat content of solid-state fermented rapeseed meal (SSFRM). Solid-state fermentation using Bacillus subtilis, C. utilis and E. faecalis reduced crude fibre content of rapeseed meal (Hu et al., 2016). Shi et al. (2016b) also reported that SSF with A. niger resulted in decreased fibre fractions (NDF, ADF, hemicellulose and lignin). Reduction in crude fibre can be attributed to fibre-degrading enzymes (hemicellulase and cellulase) produced by the microorganisms during SSF. It has been demonstrated that the inclusion of moderate amounts of fibre sources in the diet improves digestive organ development (Hetland et al., 2005), thus improving nutrient digestibility (Jiménez-Moreno et al., 2009) and performance (González-Alvarado et al., 2010) in animals. It was observed that SSF also increased the size of small peptides (less than 600 Da) in rapeseed meal inoculated with Bacillus subtilis, C. utilis and E. faecalis (Hu et al., 2016). Shi et al. (2016a) had previously reported an increase in small peptide after SSF with A. niger. Increase in the amount of small peptide might be due to partial digestion of large-size peptides in rapeseed meal by protease secreted by microorganisms during SSF. Aside from nutritional improvement of rapeseed, SSF also reduced ANF present in rapeseed meal. Solid-state fermentation with L. fermentum, E. faecium, S. cerevisiae and Bacillus subtilis decreased the amount of isothiocyanate (a derivative of glucosinolate) from 119 to 14 mmol/kg after 30 d (Chiang et al., 2010). Xu et al. (2011) also documented a reduction in the levels of isothiocyanate from 72.7 to 14.1 mmol/kg after SSF. Likewise, there were reductions in glucosinolates from 41.91 to 23.86 μmol/g, isothiocyanate from 2.48 to 1.10 mg/g, oxazolidithione from 1.88 to 1.04 mg/g and phytic acid from 2.66% to 0.37% in the report of Shi et al. (2016b). Tannins also reduced from 1.32% to 0.84% in the report of Hu et al. (2016). The reduction in glucosinolates may be due to utilization of glucose and the sulphur moieties of these compounds by microbial enzymes (Vig and Walia, 2001, Tripathi and Mishra, 2007). The reduction in phytic acid may be attributed to microbial enzymes (phytase) produced during SSF. Although only limited data are available, SSF with bacteria improved the nutritional quality and degraded ANF in canola meal. Solid-state fermentation with Lactobacillus salivarius resulted in increased crude protein content from 41.2% to 42.2%, reduced crude fibre by 15.8% and decreased glucosinolates from 22 to 13.6 μmol/g (Ahmed et al., 2014). This observation is similar to the findings of Aljuobori et al. (2014) and Aljuobori et al. (2017). These findings indicate that SSF is a feasible process majorly controlled by microbial enzymes capable of degrading ANF and improving the nutritional value of SSFRM and solid-state fermented canola meal (SSFCM).
3.1.2. Effect of solid-state fermented rapeseed meal and canola meal on performance of broiler chickens
The beneficial effects of the dietary inclusion of SSFRM in broiler diet are summarized in Table 3. Feeding trials with rapeseed meal treated with L. fermentum, E. faecium, S. cerevisiae and Bacillus subtilis showed improved body weight gain (BWG) and feed conversion ratio (FCR) in broiler chickens (Chiang et al., 2010). Xu et al. (2012) fed broiler chickens with SSFRM diets inoculated with L. fermentum and Bacillus subtilis. In their study, there was no difference in performance among SBM, 5% SSFRM and 10% SSFRM groups during all feeding periods. Over the 42-d growth period, broilers fed diets with 15% SSFRM had a significantly poorer FCR and BWG compared to broilers fed SBM, 5% SSFRM and 10% SSFRM based-diets. In an earlier study, Xu et al. (2011) had reported that rapeseed meal inoculated with L. plantarum and Bacillus subtilis could replace SBM at 33%, 67% and 100% concentrations in diets of ducklings at the ages of 15 to 45 d with no negative effect on BWG and FCR. Likewise, Shi et al. (2016a) reported that average daily gain (ADG) and FCR of pigs fed SSFRM treated with A. niger were superior compared to pigs fed unfermented rapeseed meal and did not differ from SBM (control). These improvements in performance can be attributed to reductions in ANF (glucosinolates and other compounds) during SSF, upgrade in nutrient quality during SSF and increase in lactic acid bacteria (LAB) population improving gut health of broiler chickens.
Table 3.
The effects of solid-state fermented rapeseed meal and canola meal on performance, intestinal microflora and intestinal morphology of broiler chickens.
| Substrate | Microorganism | Results | Reference |
|---|---|---|---|
| Rapeseed meal | Lactobacillus fermentum, Enterococcus faecium, Saccharomyces cerevisiae, Bacillus subtilis | Improved BWG and FCR compared to broilers fed unfermented rapeseed meal but did not differ from the SBM control. Increased LAB counts in the colon and caecal digesta when compared to broilers fed the control and unfermented rapeseed meal diets. Improved villus height and VH:CD ratio in the ileum and jejunum. | Chiang et al. (2010) |
| Lactobacillus fermentum, Bacillus subtilis | Had no negative effect on performance of broilers at 10% inclusion in place of SBM, but BWG decreased at 15% inclusion. Up to 10% inclusion improved VH:CD ratio in the jejunum of broilers | Xu et al. (2012) | |
| Bacillus subtilis, Candida utilis, Enterococcus faecalis | Increased villus height and VH:CD ratio, decreased crypt depth and intestinal wall thickness in the duodenum, jejunum, ileum and caecum in broilers | Hu et al. (2016) | |
| Canola meal | Lactobacillus salivarius | Inclusion rates higher than 10% reduced performance during 1 to 28 d, but no detrimental effects was observed when used up to 30% during 29 to 35 d under unheated and heated conditions | Aljuobori et al. (2014) |
BWG = body weight gain; FCR = feed conversion ratio; LAB = lactic acid bacteria; VH:CD ratio = villus height to crypt depth ratio; SBM = soybean meal.
Little information was found about the effect of SSFCM on performance of broiler chickens. Aljuobori et al. (2017) fed canola meal treated with L. salivarius at 10%, 20% and 30% inclusion levels to broiler chickens under heat stress for 35 d. They observed that the dietary inclusion of 20% and 30% SSFCM in broiler diet during d 1 to 28 induced a significant decline in BWG compared to those fed SBM. However, the inclusion of SSFCM up to 30% did not show any adverse effect on BWG, FCR and FI from d 29 to 35 under both heated and unheated conditions. Despite the reduction in glucosinolate and fibre, broiler chickens can not be fed more than 10% SSFCM during the starter phase without adverse effect on performance. However, during d 29 to 35, broilers can be fed up to 30% SSFCM without any negative effect on performance under unheated and heated conditions. More studies are needed to gain further insights into the potential effect of SSFCM on broiler performance.
3.1.3. Effect of solid-state fermented rapeseed meal and canola meal on nutrient digestibility in broiler chickens
The digestibility of crude protein and amino acids is necessary for the calculation of proper nutrient balance in diets of broiler chickens. As most of the feed trials previously reported on SSFRM did not include nutrient digestibility data, there exists scare information in the literature. In vitro total amino acid and essential amino acid digestibility of rapeseed cake inoculated with A. niger was improved by 5.87% and 6.69% units, respectively compared to unfermented rapeseed cake (Shi et al., 2015). This may be associated with the growth of A. niger which has ability to secrete many extracellular degradation enzymes, especially protease. In the report of Chiang et al. (2010), broilers fed SSFRM had higher total tract apparent digestibility coefficients for DM, energy and calcium than birds fed unfermented rapeseed meal on d 42. Although, there was an increase in nutrient digestibility of rapeseed meal, however, the mechanism is not clearly understood.
To the best of our knowledge, there is very little information on the effect of SSFCM on nutrient digestibility in broiler chickens. Only one study to date determined the ileal amino acid digestibility of SSFCM in broiler chickens. Ahmed et al. (2014) conducted a digestibility trial on 42-d old broiler chickens fed either canola meal or SSFCM as the sole source of energy and protein. The crude protein digestibility of canola meal inoculated with L. salivarius was superior increasing from 77.44% to 81.56% compared to unfermented canola meal. The amino acid digestibility coefficients in SSFCM significantly improved when compared to unfermented canola meal. This may be due to the secretion of enzymes (cellulase, phytase, xylanase) by the microorganisms converting fibre materials into monosaccharides. To gain more understanding on how broiler chickens will respond to dietary SSFRM and SSFCM, there is need for more studies to be conducted on their nutrient digestibility.
3.1.4. Effect of solid-state fermented rapeseed meal and canola meal on intestinal microflora of broiler chickens
The intestinal microbiota plays a key role in nutrition, microbiology, immunology and physiology of a host animal. In pig nutrition, fermented feed improves gut microbial ecosystems by lowering the population of enterobacteria such as coliform bacteria and salmonella in the gut (Canibe and Jensen, 2012). Beneficial microbial population also produce short chain fatty acids and thus, reduce intestinal pH. This also creates a competitive platform serving as a natural defense barrier against infections and pathogenic bacteria. Fermented feeds are characterized by high number of LAB and high lactic acid concentration (Niba et al., 2009). During the starter and finisher phases, the total number of LAB in the colon and caeca of birds fed 10% SSFRM diets were significantly higher than those fed same quantity of unfermented rapeseed meal or SBM (Chiang et al., 2010). The unique features of low pH, high LAB population, low enterobacteria population (Salmonella and coliform bacteria) and high lactic or acetic acid concentration indicate the beneficial effect of SSFRM on the intestinal microflora of broiler chickens. There is, however, need for more studies on intestinal microflora of broiler chickens fed SSFRM and SSFCM. This is required to gain more insight into their feeding value in broiler chickens.
3.1.5. Effect of solid-state fermented rapeseed meal on intestinal morphology of broiler chickens
Intestinal mucosa is very functional in the digestion and absorption of dietary nutrients which influence broiler performance and health. The intestinal villus is the main site of nutrient absorption. The villus epithelial cells are functional cells of digestion and absorption which are very favourable in enhancing enzyme activity and nutrient absorption (Hu et al., 2016). It was observed that villus height and the villus height to crypt depth ratio in the ileum and jejunum improved in broiler chickens fed diets containing SSFRM compared to birds fed SBM control diet (Chiang et al., 2010). Similar observation was seen in the study of Xu et al. (2012). Furthermore, Hu et al. (2016) reported that dietary inclusion of SSFRM enhanced small intestinal structure and function in broiler chickens compared to unfermented rapeseed meal. These findings suggest that feeding SSFRM can influence the growth of intestinal epithelial cells, increase the surface area of the intestinal mucosa for nutrient absorption and improve the efficiency of nutrient utilization in broiler chickens.
3.2. Cottonseed meal
Cottonseed meal is the by-product of oil extraction from cottonseeds. It contains 22.2% to 56.2% crude protein and 7.4 to 11.99 MJ/kg metabolizable energy (Nagalakshmi et al., 2007). However, the use of cottonseed meal in poultry diet is limited due to its gossypol and low lysine levels compared to SBM (Lordelo et al., 2007). Free gossypol binds to lysine and inhibit its availability in cottonseed meal (Mahmood et al., 2011). Free gossypol could reduce growth performance and increase mortality in broiler chickens (Henry et al., 2001).
3.2.1. Effect of solid-state fermentation on the nutrient composition of cottonseed meal
Bacillus strains have predominantly been used for the SSF of cottonseed meal. Bacillus subtilis can produce highly active protease, lipase and amylase to degrade complex carbohydrates in plant, increase nutrient digestibility and provide more nutrition to animals (Li et al., 2014). Studies (Tang et al., 2012, Sun et al., 2013a, Sun et al., 2013b) have ascertained the efficiency of SSF in improving the nutritional value and reducing ANF in cottonseed meal. Dry matter content was reduced when cottonseed meal was treated with Bacillus subtilis ST-141 and Saccharomycetes N5 (Wang et al., 2017). The decline in DM content can be attributed to the consumption of carbohydrate by the microorganisms. Solid-state fermentation using Bacillus subtilis BJ-1 increased crude protein content from 46.5% to 50.5% (Tang et al., 2012). Similarly, crude protein increased from 49.8% to 51% when cottonseed meal was inoculated with Bacillus subtilis ST-141 and Saccharomycetes N5 (Wang et al., 2017). Increase in crude protein may be attributed to a decline in DM content. Solid-state fermentation using Bacillus subtilis BJ-1 reduced the crude fat content of cottonseed meal (Sun et al., 2013a, Sun et al., 2013b). Reduction in crude fat content may be attributed to the lipase producing ability of Bacillus subtilis, thus degrading lipids into small particles. Solid-state fermentation using C. utilis reduced crude fibre content (Xiong et al., 2016). Inoculation with Bacillus subtilis ST-141 and Saccharomycetes N5 also reduced crude fibre content of cottonseed in the work of Wang et al. (2017). The decrease in crude fibre can be attributed to fibre-degrading enzymes secreted by the microorganisms. In addition to nutritional improvement of cottonseed meal, SSF significantly reduced the concentration of free gossypol (an ANF). Tang et al. (2012) reported a reduction in free gossypol from 0.82 to 0.21 g/kg in solid-state fermented cottonseed meal (SSCSM). Similarly, free gossypol decreased from 90 to 30 mg/kg in the study of Xiong et al. (2016). The reduction in free gossypol may be due to the binding of free gossypol to protein or amino acids produced by microorganisms or microbial enzymes degrading gossypol or perhaps by both mechanisms during SSF.
3.2.2. Effect of solid-state fermented cottonseed meal on performance of broiler chickens
As mentioned earlier, beneficial effects of SSCSM in broiler chickens (Tang et al., 2012, Sun et al., 2013b, Xiong et al., 2016, Wang et al., 2017) have been evaluated and documented. Table 4 summarizes the works of various research groups who have evaluated the effects of feeding SSCSM to broiler chickens. Over a period of 42 d, the BWG of broilers fed 8% SSCSM was superior to those fed SBM, 4% and 12% SSCSM diets (Tang et al., 2012). Similarly, broiler chickens fed diet containing 4% and 8% SSCSM had higher BWG than those fed with SBM or 12% SSCSM diets at 21 d (Sun et al., 2013b). Improved performance may be associated with the improved nutrient quality of cottonseed meal in addition to the elimination of ANF after SSF. In contrast, Wang et al. (2017) showed that between d 1 to 42 and d 22 to 42, ADG and ADFI were not significantly different in broilers fed SBM, cottonseed meal and SSCSM diets. However, broilers fed another SSCSM-based diet containing 55.3 mg/kg free gossypol had significantly decreased ADG and increased FCR. The reason for this is not clearly understood as the free gossypol content in the diet was lower compared to cottonseed meal-based diets. The authors presumed that this may be associated with other ANF (malvalic and sterculic acid) which may contribute to reduced performance. Also, lysine–arginine antagonism may have influenced amino acid digestibility of amino acid, thus, a negative effect on performance.
Table 4.
The effects of solid-state fermented cottonseed meal on performance, intestinal microflora and intestinal morphology of broiler chickens.
| Microorganism | Results | Reference |
|---|---|---|
| Bacillus subtilis BJ-1 | Improved the BWG and FI of broilers at 8% dietary inclusion | Tang et al. (2012) |
| Bacillus subtilis BJ-1 | Had no detrimental effect on performance as compared to control when included at 80 g/kg of diet. No effect on intestinal microbial diversity of broiler chickens but shifted intestinal microbiota with a more homogenous population and increased colonization of LAB. | Sun et al. (2013a) |
| Bacillus subtilis BJ-1 | Inclusion at 40 and 80 g/kg improved BWG. Increased LAB counts in the caecal digesta of broilers. Decreased coliform bacteria counts. Increased villus height in the duodenum, elevated villus height and VH:CD ratio in the jejunum. Improvement in jejunal villus height of broilers. Improved the activities of amylase and protease in the intestine | Sun et al. (2013b) |
| Candida utilis | Inclusion at 155 g/kg improved ADG and FCR higher than in broilers fed cottonseed meal but did not differ from SBM control | Xiong et al. (2016) |
| Bacillus subtilis ST-141, Saccharomycetes N5 | Over 42 d, no detrimental effect on ADG among SBM, cottonseed meal and SSF cottonseed meal (diet 1) groups. Decreased ADG in broilers fed SSF cottonseed meal (diet 2). Increased LAB counts in the caecal digesta of broilers fed SSF cottonseed meal diets compared with cottonseed meal diet | Wang et al. (2017) |
BWG = body weight gain; FI = feed intake; LAB = lactic acid bacteria; VH:CD ratio = villus height to crypt depth ratio; ADG = average daily gain; FCR = feed conversion ratio; SBM = soybean meal.
3.2.3. Effect of solid-state fermented cottonseed meal on intestinal microflora of broiler chickens
The microbial community and its activity in the intestine of broiler chickens are influenced by diet composition. In this respect, the dietary inclusion of SSCSM increased LAB population in the caecal digesta of broiler chickens at d 21 and 42. Coliform bacteria counts in the caeca also decreased at d 21 (Sun et al., 2013b). Similar observation was noted in the work of Wang et al. (2017). In a molecular study, the dietary inclusion of SSCSM did not affect the intestinal microbial diversity of broiler chickens but shifted intestinal microbiota with a more homogenous population and increased colonization of LAB (Sun et al., 2013a). The effect of SSCSM on the intestinal microflora of broiler chickens is most likely due to the existence of LAB (either live or dead cells) after SSF.
3.2.4. Effect of solid-state fermented cottonseed meal on intestinal morphology of broiler chickens
In the study of Sun et al. (2013b), the dietary inclusion of SSCSM increased villus height in the duodenum and linearly elevated villus height and the villus height to crypt depth ratio in the jejunum at d 21. Dietary inclusion of SSCSM improved the activities of amylase and protease in the intestine. In addition, a comparable improvement was noted in jejunal villus height at d 42. The result indicates that the absorptive function in the jejuna and duodena of broilers fed SSCSM diets was higher compared to those in the control group. This finding suggests that feeding SSCSM results in improved intestinal morphology and healthier animals which can be attributed to: 1) a direct relationship between intestinal microflora and gut health. The increased LAB population could inhibit the colonization of pathogens such as coliform bacteria and reduce the incidence of their negative effects on intestinal structure and tissue, 2) the breakdown of large proteins into small peptides after SSF of cottonseed meal and 3) the reduction of free gossypol in SSCSM (Jazi et al., 2017, Özdoğan et al., 2012). The positive effects of using SSCSM as an alternative protein source is evident as it could improve gut health and performance of broiler chickens.
3.3. Palm kernel cake
Palm kernel cake (or meal) is the by-product of oil extraction from kernels of oil palm. Palm kernel cake has been used as an energy source for ruminants and non-ruminants because of its palatability and crude protein content of about 14.5% to 19.24% (Sundu et al., 2006, Ezieshi and Olomu, 2007). The limitation of using palm kernel cake in feeding monogastric animal, especially poultry is due to poor amino acid balance, high indigestible fibre, coarse texture, grittiness, possible deterioration under unhygienic conditions and high concentrations of NSP such as mannan, xylan and cellulose (Alimon, 2004, Sundu et al., 2006, Alshelmani et al., 2013, Alshelmani et al., 2014). The indigestible portions are actually NSP comprising 78% β-mannan, 12% cellulose, 3% arabinoxylans and 3% water-insoluble glucoxylans (Sundu et al., 2006). Due to the adverse effects such as reduced feed intake and indigestion observed in poultry, the dietary inclusion of palm kernel cake has not exceeded 40% (Alimon, 2004, Sundu et al., 2006).
3.3.1. Effect of solid-state fermentation on the nutrient composition of palm kernel cake
Different strains that have been reported in the SSF of palm kernel cake include A. niger (Ong et al., 2004, Iluyemi et al., 2006, Oliveira et al., 2018), Aspergillus wentii (Muangkeow and Chinajariyawong, 2013), Sclerotium rolfsii, Trichoderma spp. (Iluyemi et al., 2006), Paenibacillus polymyxa and Paenibacillus curdlanolyticus (Alshelmani et al., 2014, Alshelmani et al., 2016). The beneficial effects of SSF as a processing technique in improving the nutritive value on palm kernel cake are well documented (Iluyemi et al., 2006, Ramin et al., 2010, Alshelmani et al., 2013, Alshelmani et al., 2014, Alshelmani et al., 2017b). A significant increase in crude protein content from 18.76% to 32.79% was obtained by growing Trichoderma longiobrachiatum in palm kernel cake under SSF conditions (Iluyemi et al., 2006). After 10 d of SSF, both A. niger and Rhizopus oryzae treatments increased the crude protein content in palm kernel cake from 18% to 27% (Ramin et al., 2010). The protein increase takes into account the reduction in the weight of substrate during culturing, which results from the utilization of the carbon content by the microorganisms. The increase in crude protein content may be due to the capability of the microorganisms to produce proteolytic enzymes. Furthermore, Alshelmani et al. (2017b) reported a significant decrease in NDF, ADF, crude fibre, hemicellulose and cellulose content of palm kernel cake after SSF with P. polymyxa and P. curdlanolyticus. Previous studies (Alshelmani et al., 2013, Alshelmani et al., 2014) have confirmed this observation. The reduction in NDF, ADF, crude fibre, hemicellulose and cellulose content may be related to the background of these bacterial cultures as they are capable of producing different types of cellulolytic and hemicellulolytic enzymes such as cellulase, xylanase and mannanase. Solid-state fermentation using A. niger, A. oryzae and Aspergillus awamori reduced the crude fat content of palm kernel cake in relation to its unfermented form (Oliveira et al., 2018). This may be due to lipid hydrolysis catalyzed by lipase produced by the fungi during the SSF process. It is noteworthy that there may be variations in reported nutrient composition of SSF palm kernel cake. Other than the type of microorganisms used and SSF conditions, Obese et al. (2001) suggested that the technique used for oil extraction or the processing efficiency can influence the composition of palm kernel cake. In this regard, this will influence the nutrient composition of the final substrate obtained after SSF.
3.3.2. Effect of solid-state fermented palm kernel cake on performance, nutrient digestibility, intestinal microflora and intestinal morphology of broiler chickens
Amino acids and metabolizable energy are 2 important parameters to be considered when formulating broiler diet especially with fibrous feed ingredient as palm kernel cake. Table 5 summarizes the effects of feeding solid-state palm kernel cake (SSPKC) to broiler chickens. A significant improvement in nutrient digestibility was found in broilers fed diets with 10% or 15% SSPKC compared to those fed with unfermented palm kernel cake (Alshelmani et al., 2016). In a latter study, the apparent ileal digestibility of crude protein and amino acid was significantly increased in SSPKC compared to the unfermented palm kernel cake (Alshelmani et al., 2017b). This improvement in crude protein and amino acid digestibility in SSPKC may be attributed to the reduction of NDF, ADF, crude fibre, hemicellulose, cellulose and NSP during SSF. Although true amino acid digestibility increased, but there was reduction in metabolizable energy values (AME, nitrogen-corrected AME [AMEn], true metabolizable energy [TME] and nitrogen-corrected TME [TMEn]) in broilers fed palm kernel meal treated with A. wentii compared to unfermented palm kernel meal group (Muangkeow and Chinajariyawong, 2009). This might possibly be due to the microorganism using up the energy source of the substrate especially nitrogen free extract (NFE) during SSF. Dietary inclusion of SSPKC up to 15% improved the performance of broiler chickens in a 42-d feeding trial (Alshelmani et al., 2017a). The improvement in broiler performance could be attributed to the degradation of NDF, ADF, crude fibre and NSP by cellulolytic bacteria during the SSF process. However, the use of SSPKC above 20% in starter broiler diets reduced feed intake, BWG and feed efficiency when compared to those fed SBM in the study of Muangkeow and Chinajariyawong (2013). The inclusion of up to 30% SSPKC at the finisher phase can be effective without negative effects. The poor performance observed at the starter phase was attributed to low crude protein digestibility, low nitrogen retention and increased crude fibre content as the dietary inclusion of SSPKC increased. Ileal enterobacteria counts were significantly lower for broilers fed with 15% SSPKC compared to those fed SBM or different levels of palm kernel cake at 21 d. Lactic acid bacteria count was significantly higher for broilers fed with 15% SSPKC than those fed SBM at 42 d (Alshelmani et al., 2016). An increase in LAB count can depress the growth of enteropathogens in the gut. It has been reported that high levels of crude fibre in poultry diets decreases the surface area, width and height of intestinal villi associated with nutrient digestion (Kalmendal et al., 2011). Despite the degradation in NDF, ADF, crude fibre and NSP content of palm kernel after SSF, there were no significant differences in villus height and crypt depth of the small intestine (duodenum, jejunum and ileum) of broiler chickens fed diets containing SBM, palm kernel cake and SSPKC at the starter or finisher phases (Alshelmani et al., 2016). The reduction in ANF by SSF might not have been sufficient to induce any growth-promoting effects in the intestine of broiler chickens. These observations suggest that 15% SSPKC can be included in starter broiler diets while up to 30% can be used at the finisher phase without negative effects on performance.
Table 5.
The effects of solid-state fermented palm kernel cake on performance, intestinal microflora and intestinal morphology of broiler chickens.
| Microorganism | Results | Reference |
|---|---|---|
| Aspergillus wentii | Increased true amino acid digestibility and reduced metabolizable energy values in broiler chickens | Muangkeow and Chinajariyawong (2009) |
| Aspergillus wentii | Dry matter digestibility, nitrogen retention and apparent metabolizable energy of SSF palm kernel meal based diets were lower than the control diets. Dietary inclusion above 20% reduced FI, BWG and feed efficiency in starter broilers while up to 30% at the finisher phase was effective | Muangkeow and Chinajariyawong (2013) |
| Paenibacillus polymyxa | Improved nutrient digestibility at 10% and 15% dietary inclusion compared to palm kernel cake group. Inclusion at 15% increased LAB counts and decreased Enterobacteriaceae counts in the intestine of broilers compared to other dietary treatments | Alshelmani et al. (2016) |
| Paenibacillus polymyxa | Inclusion at 10% and 15% improved BWG and FCR, higher than in broilers fed palm kernel cake but did not differ from SBM group | Alshelmani et al. (2017a) |
| Paenibacillus polymyxa and Paenibacillus curdlanolyticus | Improved apparent ileal digestibility of crude protein and amino acid digestibility compared to unfermented palm kernel cake | Alshelmani et al. (2017b) |
FI = feed intake; BWG = body weight gain; LAB = lactic acid bacteria; FCR = feed conversion ratio.
3.4. Lupin flour
Lupin is a leguminous crop whose seeds contains 28% to 48% crude protein in DM which depends on species and climatic conditions (White et al., 2007, Písaříková and Zralý, 2009). Lupin flour is obtained when lupin seeds in raw form are either ground through a roller mill (Koivunen et al., 2016) or hammer mill (Nalle et al., 2011, Nalle et al., 2012) or grinder (Zaworska et al., 2017). Despite breeding improvement, the inclusion of lupin flour at high levels in poultry diets remains daunting due to NSP, tannins and protease inhibitors which adversely affects nutrient digestibility and performance of non-ruminants (Jezierny et al., 2010).
3.4.1. Effect of solid-state fermentation on the nutrient composition of lupin flour
Fermented lupin is gaining more attention as a diet component in monogastric animal feeding. Fermentation can contribute to the improvement of nutritional value of lupin by increasing nutrient availability and effective degradation of ANF (Kasprowicz-Potocka et al., 2015, Kasprowicz-Potocka et al., 2016, Zaworska et al., 2017). Limited information was found on SSF of lupin flour as most papers used the generic term (fermentation) in their titles. Fermentation of lupin has been carried out using bacteria (Van Vo et al., 2015, Bartkiene et al., 2018), filamentous fungi (Jiménez-Martínez et al., 2007, Ortega-David and Rodriguez-Stouvenel, 2014) and different yeast strains (Kasprowicz-Potocka et al., 2015, Kasprowicz-Potocka et al., 2016, Ilham et al., 2017). Among the notable yeast strains used, S. cerevisiae is preferred due to its ability to produce enzymes such as β-glucanase (Cenamor et al., 1987), phytase (Ries and Alves Macedo, 2011) and invertase (Arumugam et al., 2014). The nutrient composition of fermented lupin flour appears to be variable possibly due to differences in microorganisms and fermentation conditions. An increase in crude protein content from 38.23% to 41.32% was obtained by using a combination of S. cerevisiae, E. faecium, L. plantarum, Lactobacillus buchneri and Lactobacillus casei (Zaworska et al., 2017). Likewise, a treatment with S. cerevisiae increased the crude protein content from 38.59% to 50.08% (Ilham et al., 2017). Increase in the crude protein is mostly connected with reduced non-structural carbohydrates in the biomass. Crude fat content was reduced after fermentation with Lactobacillus spp. (Van Vo et al., 2015). Similar observation was noted by Zaworska et al. (2016) when lupin flour was fermented with a combined mixture of S. cerevisiae, E. faecium, L. plantarum, L. buchneri and L. casei. Changes in the content of crude fat may be the results of increase or reduction in nitrogen-free extracts (NFE) or protein fractions. Furthermore, Van Vo et al. (2015) reported a decrease in crude fibre content of lupin flour after fermentation with Lactobacillus spp. However, in another study (Zaworska et al., 2016), crude fibre, NDF and ADF contents increased in lupin flour fermented with a multipreparation of S. cerevisiae, E. faecium, L. plantarum, L. buchneri and L. casei bacteria. Structural carbohydrates are quite resistant to direct yeast digestion, but cellulose, hemicellulose and pectin can be metabolized by some non-yeast enzymes (Bekatorou et al., 2006). Raffinose family oligosaccharides (RFO) are not broken down in the gut of non-ruminants due to the absence of α-1,6-galactosidase enzyme in the small intestine. As a result, RFO move down to the lower gut where they serve as potential substrate for bacterial fermentation. However, in the process, flatulence-inducing gases are produced leading to abdominal pains, interference with nutrient absorption and diarrhoea (Kumar et al., 2010). Fermentation with C. utilis (Kasprowicz-Potocka et al., 2015) and different yeast strains (Kasprowicz-Potocka et al., 2016) completely removed RFO in lupin flour. These findings are consistent with the result of Zaworska et al. (2016) and Zaworska et al. (2017) when fermentation was carried out with a multipreparation of S. cerevisiae, E. faecium, L. plantarum, L. buchneri and L. casei. During fermentation, different types of hydrolases are produced which degrade the oligosaccharides into simple sugars. Studies using yeast, bacteria or both (Kasprowicz-Potocka et al., 2015, Kasprowicz-Potocka et al., 2016, Van Vo et al., 2015, Zaworska et al., 2016, Zaworska et al., 2017, Ilham et al., 2017) reported a decrease in phytic acid content after fermentation. Likewise, tannin content of lupin flour was reduced in the study of Van Vo et al. (2015). The observed changes indicate that fermentation increases the activity of native enzymes (phytase and tannase) which can disintegrate insoluble organic complexes with minerals. High tannin content produces an anti-nutritional effect on nutrient digestibility and utilization in animals (Alemawor et al., 2009); therefore, its decrease by fermentation would enhance the nutritional quality of lupin flour. In general, these observations confirm the effectiveness of fermentation in degrading ANF in lupin flour.
3.4.2. Effect of solid-state fermented lupin flour on performance of broiler chickens
In practice, fermentation of lupin flour appears to be possible with promising results. However, no study to date has documented performance data of broiler chickens fed either fermented or solid-state fermented lupin flour as a protein feed ingredient. In addition, only few studies have focused on the use of fermented lupin flour in animal nutrition. The feeding value of fermented lupin flour in different group of animals is presented in Table 6. In the study of Bartkiene et al. (2013), diets containing lupin flour fermented with Pediococcus acidilactici resulted in better BWG and feed utilization in Wistar rats in relation to the unfermented control. Similarly, the use of lupin flour fermented with C. utilis increased the apparent protein digestibility, body mass gain and protein efficiency ratio in rats in comparison with raw lupin flour (Kasprowicz-Potocka et al., 2015). In another digestibility trial, Zaworska et al. (2017) fermented lupin flour using a combined preparation of S. cerevisiae, E. faecium, L. plantarum, L. buchneri and L. casei. They observed a significant improvement in the digestibility of crude protein and many essential amino acids in pigs, but there was no data on performance characteristics. Improvement in protein digestibility in different animals studied may be attributed to increment in crude protein content and reduction in ANF (e.g. phytic acid and RFO) of fermented lupin flour. For the purpose of this review, further studies are needed to gain more insights into the potential effects of solid-state fermented lupin flour on broiler performance and intestinal health.
Table 6.
The effects of feeding fermented lupin flour on performance and intestinal health of different animals.
| Animal | Microorganisms used | Enzymes | Results | Reference |
|---|---|---|---|---|
| Wistar rats | Pediococcus acidilactici KTU05-7 | Amylase, β-galactosidase, protease, laccase, cellulase | Improved body weight and BWG. Reduced activities of α-glucosidase, β-galactosidase and β-glucuronidase in the caecum. High LAB and bifidobacteria population in the gut. Low pH in caecum and colon. Inhibition of Escherichia coli | Bartkiene et al. (2013) |
| Wistar rats | Candida utilis | Phytase, cellulase, ligninase, pectinase, xylanase | Increased the apparent protein digestibility, body mass gain and protein efficiency ratio in rats in comparison to raw seeds | Kasprowicz-Potocka et al. (2015) |
| Rats | Saccharomyces cerevisiae, Enterococcus faecium, Lactobacillus plantarum, Lactobacillus buchneri and Lactobacillus casei | Phytase, cellulase, protease, lipase, xylanase, glucanase, amylase, laccase, pectinase, invertase | Improved apparent total tract digestibility of dietary protein and BWG of protein intake in rats. Low acidity of the caecum digesta | Zaworska et al. (2016) |
| Pigs | Saccharomyces cerevisiae, Enterococcus faecium, Lactobacillus plantarum, Lactobacillus buchneri and Lactobacillus casei | Phytase, cellulase, protease, lipase, xylanase, glucanase, amylase, laccase, pectinase, invertase | Improved crude protein and amino acid digestibility. No effect on microbial status of the ileal digesta | Zaworska et al. (2017) |
BWG = body weight gain; LAB = lactic acid bacteria.
4. Conclusions
Solid-state fermentation as a low-cost bioprocessing technique for improving the nutrient quality of alternative protein sources such as rapeseed meal, canola meal, cottonseed meal, palm kernel cake and lupin flour is technically feasible. It is evident that the dietary inclusion of the aforementioned SSF plant protein sources can improve performance, nutrient digestibility, intestinal microflora and intestinal morphology of broiler chickens. The overall variation in nutrient composition observed in the literature indicates that composition of SSF protein sources depend on source of feed, processing methods, type of microorganism and SSF conditions. As there is limited data on the use of SSF alternative protein sources in broiler nutrition, more studies on feeding value especially nutrient digestibility and utilization are needed. This will contribute to the competitiveness of SSF alternative protein sources and serve as a promising channel for the poultry industry to meet the globally increasing demand for animal protein in a sustainable way.
Conflicts of interest
We declare that we have no financial and personal relationships with other people or organizations that can inappropriately influence our work, there is no professional or other personal interest of any nature or kind in any product, service and/or company that could be construed as influencing the content of this paper.
Acknowledgments
The authors are sincerely grateful for the support of the University of Queensland, Brisbane, Australia. The scholarship support through the Australian Government Research Training Program Scholarship provided to Oladapo O. Olukomaiya during his PhD study at the University of Queensland, Brisbane, Australia is gratefully acknowledged.
Footnotes
Peer review under responsibility of Chinese Association of Animal Science and Veterinary Medicine.
References
- Abu Yazid N., Barrena R., Komilis D., Sánchez A. Solid-state fermentation as a novel paradigm for organic waste valorization: a review. Sustainability. 2017;9:224. [Google Scholar]
- Ahmed A., Zulkifli I., Farjam A.S., Abdullah N., Liang J.B., Awad E.A. Effect of solid state fermentation on nutrient content and ileal amino acids digestibility of canola meal in broiler chickens. Ital J Anim Sci. 2014;13:3293. [Google Scholar]
- Ajila C.M., Brar S.K., Verma M., Tyagi R.D., Godbout S., Valéro J.R. Bio-processing of agro-byproducts to animal feed. Crit Rev Biotechnol. 2012;32:382–400. doi: 10.3109/07388551.2012.659172. [DOI] [PubMed] [Google Scholar]
- Alemawor F., Dzogbefia V.P., Oddoye E.O., Oldham J.H. Effect of Pleurotus ostreatus fermentation on cocoa pod husk composition: influence of fermentation period and Mn2+ supplementation on the fermentation process. Afr J Biotechnol. 2009;8(9):1950–1958. [Google Scholar]
- Alimon A. The nutritive value of palm kernel cake for animal feed. Palm Oil Dev. 2004;40:12–14. [Google Scholar]
- Aljuobori A., Abdullah N., Zulkifli I., Soleimani A., Liang J., Oskoueian E. Lactobacillus salivarius fermentation reduced glucosinolate and fibre in canola meal. J Food Res. 2014;3:95–102. [Google Scholar]
- Aljuobori A., Idrus Z., Soleimani A.F., Abdullah N., Juan Boo L. Response of broiler chickens to dietary inclusion of fermented canola meal under heat stress condition. Ital J Anim Sci. 2017;16:546–551. [Google Scholar]
- Alshelmani M.I., Loh T.C., Foo H.L., Lau W.H., Sazili A.Q. Characterization of cellulolytic bacterial cultures grown in different substrates. Sci World J. 2013;2013 doi: 10.1155/2013/689235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alshelmani M.I., Loh T.C., Foo H.L., Lau W.H., Sazili A.Q. Biodegradation of palm kernel cake by cellulolytic and hemicellulolytic bacterial cultures through solid state fermentation. Sci World J. 2014;2014 doi: 10.1155/2014/729852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alshelmani M.I., Loh T.C., Foo H.L., Sazili A.Q., Lau W.H. Effect of feeding different levels of palm kernel cake fermented by Paenibacillus polymyxa ATCC 842 on nutrient digestibility, intestinal morphology and gut microflora in broiler chickens. Anim Feed Sci Technol. 2016;216:216–224. [Google Scholar]
- Alshelmani M.I., Loh T.C., Foo H.L., Sazili A.Q., Lau W.H. Effect of feeding different levels of palm kernel cake fermented by Paenibacillus polymyxa ATCC 842 on broiler growth performance, blood biochemistry, carcass characteristics and meat quality. Anim Prod Sci. 2017;57:839–848. [Google Scholar]
- Alshelmani M.I., Loh T.C., Foo H.L., Sazili A.Q., Lau W.H. Effect of solid state fermentation on nutrient content and ileal amino acids digestibility of palm kernel cake in broiler chickens. Indian J Anim Sci. 2017;87:1135–1140. [Google Scholar]
- Arumugam G., Sadiq A.M., Nagalingam M., Panneerselvam A. Production of invertase enzymes from Saccharomyces cerevisiae strain isolated from sugarcane and grape juices. Eur J Exp Biol. 2014;4(5):29–32. [Google Scholar]
- Barrios-González J. Solid-state fermentation: physiology of solid medium, its molecular basis and applications. Process Biochem. 2012;47:175–185. [Google Scholar]
- Bartkiene E., Juodeikiene G., Vidmantiene D., Zdunczyk Z., Zdunczyk P., Juskiewicz J., Cizeikiene D., Matusevicius P. Influence of diets to Wistar rats supplemented with soya, flaxseed and lupine products treated by lactofermentation to improve their gut health. Int J Food Sci Nutr. 2013;64(6):730–739. doi: 10.3109/09637486.2013.775230. [DOI] [PubMed] [Google Scholar]
- Bartkiene E., Krungleviciute V., Juodeikiene G., Vidmantiene D., Maknickiene Z. Solid state fermentation with lactic acid bacteria to improve the nutritional quality of lupin and soya bean. J Sci Food Agric. 2015;95:1336–1342. doi: 10.1002/jsfa.6827. [DOI] [PubMed] [Google Scholar]
- Bartkiene E., Sakiene V., Bartkevics V., Rusko J., Lele V., Juodeikiene G., Wiacek C., Braun P.G. Lupinus angustifolius L. lactofermentation and protein isolation: effects on phenolic compounds and genistein, antioxidant properties, trypsin inhibitor activity and protein digestibility. Eur Food Res Technol. 2018:1521–1531. [Google Scholar]
- Behera S.S., Ray R.C. Solid state fermentation for production of microbial cellulases: recent advances and improvement strategies. Int J Biol Macromol. 2016;86:656–669. doi: 10.1016/j.ijbiomac.2015.10.090. [DOI] [PubMed] [Google Scholar]
- Bekatorou A., Parianos C., Koutinas A. Production of food grade yeasts. Food Technol Biotechnol. 2006;44:407–415. [Google Scholar]
- Bell J. Factors affecting the nutritional value of canola meal: a review. Can J Anim Sci. 1993;73:689–697. [Google Scholar]
- Bringel L., Neiva J.N.M., de Araújo V.L., Bomfim M.A.D., Restle J., Ferreira A.C.H., Lôbo R.N. Consumo, digestibilidade e balanço de nitrogênio em borregos alimentados com torta de dendê em substituição à silagem de capim-elefante. Rev Bras Zootec. 2011;40:1975–1983. [Google Scholar]
- Canibe N., Jensen B.B.J. Fermented liquid feed - microbial and nutritional aspects and impact on enteric diseases in pigs. Anim Feed Sci Technol. 2012;173:17–40. [Google Scholar]
- Cenamor R., Molina M., Galdona J., Sánchez M., Nombela C. Production and secretion of Saccharomyces cerevisiae β-glucanases: differences between protoplast and periplasmic enzymes. An Microbiol. 1987;133(3):619–628. [Google Scholar]
- Chen Hz, He Q. Value-added bioconversion of biomass by solid-state fermentation. J Chem Technol Biotechnol. 2012;87:1619–1625. [Google Scholar]
- Cheng Y.-Q., Hu Q., Li L.-T., Saito M., Yin L.-J. Production of sufu, a traditional Chinese fermented soybean food, by fermentation with Mucor flavus at low temperature. Food Sci Technol Res. 2009;15:347–352. [Google Scholar]
- Chiang G., Lu W., Piao X., Hu J., Gong L., Thacker P. Effects of feeding solid-state fermented rapeseed meal on performance, nutrient digestibility, intestinal ecology and intestinal morphology of broiler chickens. Asian-Australas J Anim Sci. 2010;23:263–271. [Google Scholar]
- Couto S.R., Sanromán M.A. Application of solid-state fermentation to food industry—a review. J Food Eng. 2006;76:291–302. [Google Scholar]
- Ezieshi E.V., Olomu J.M. Nutritional evaluation of palm kernel meal types: 1. Proximate composition and metabolizable energy values. Afr J Biotechnol. 2007;6:2484–2486. [Google Scholar]
- Farinas C.S. Developments in solid-state fermentation for the production of biomass-degrading enzymes for the bioenergy sector. Renew Sustain Energy Rev. 2015;52:179–188. [Google Scholar]
- Fujita J., Shigeta S., Yamane Y.-I., Fukuda H., Kizaki Y., Wakabayashi S., Ono K. Production of two types of phytase from Aspergillus oryzae during industrial koji making. J Biosci Bioeng. 2003;95(5):460–465. doi: 10.1016/s1389-1723(03)80045-2. [DOI] [PubMed] [Google Scholar]
- González-Alvarado J.M., Jiménez-Moreno E., González-Sánchez D., Lázaro R., Mateos G.G. Effect of inclusion of oat hulls and sugar beet pulp in the diet on productive performance and digestive traits of broilers from 1 to 42 days of age. Anim Feed Sci Technol. 2010;162:37–46. [Google Scholar]
- Henry M.H., Pesti G.M., Bakalli R., Lee J., Toledo R.T., Eitenmiller R.R., Phillips R.D. The performance of broiler chicks fed diets containing extruded cottonseed meal supplemented with lysine. Poultry Sci. 2001;80:762–768. doi: 10.1093/ps/80.6.762. [DOI] [PubMed] [Google Scholar]
- Hetland H., Svihus B., Choct M. Role of insoluble fiber on gizzard activity in layers. J Appl Poult Res. 2005;14:38–46. [Google Scholar]
- Hu Y., Wang Y., Li A., Wang Z., Zhang X., Yun T., Qiu L., Yin Y. Effects of fermented rapeseed meal on antioxidant functions, serum biochemical parameters and intestinal morphology in broilers. Food Agric Immunol. 2016;27:182–193. [Google Scholar]
- Iji P.A., Toghyani M., Ahiwe E.U., Omede A.A. Burleigh Dodds Science Publishing Limited; 2017. Alternative sources of protein for poultry nutrition; pp. 1–33. [Google Scholar]
- Ilham I., Hapsari F., Fotedar R. Growth, enzymatic glutathione peroxidase activity and biochemical status of juvenile barramundi (Lates calcarifer) fed dietary fermented lupin meal supplemented with organic selenium. Aquacult Res. 2017;49:151–164. doi: 10.1007/s10695-016-0331-2. [DOI] [PubMed] [Google Scholar]
- Iluyemi F., Hanafi M., Radziah O., Kamarudin M. Fungal solid state culture of palm kernel cake. Bioresour Technol. 2006;97:477–482. doi: 10.1016/j.biortech.2005.03.005. [DOI] [PubMed] [Google Scholar]
- Jazi V., Boldaji F., Dastar B., Hashemi S.R., Ashayerizadeh A. Effects of fermented cottonseed meal on the growth performance, gastrointestinal microflora population and small intestinal morphology in broiler chickens. Br Poult Sci. 2017;58(4):402–408. doi: 10.1080/00071668.2017.1315051. [DOI] [PubMed] [Google Scholar]
- Jezierny D., Mosenthin R., Bauer E. The use of grain legumes as a protein source in pig nutrition: a review. Anim Feed Sci Technol. 2010;157:111–128. [Google Scholar]
- Jiménez-Moreno E., González-Alvarado J.M., Lázaro R., Mateos G.G. Effects of type of cereal, heat processing of the cereal, and fiber inclusion in the diet on gizzard pH and nutrient utilization in broilers at different ages. Poultry Sci. 2009;88:1925–1933. doi: 10.3382/ps.2009-00193. [DOI] [PubMed] [Google Scholar]
- Jiménez-Martínez C., Hernández-Sánchez H., Dávila-Ortiz G. Diminution of quinolizidine alkaloids, oligosaccharides and phenolic compounds from two species of Lupinus and soybean seeds by the effect of Rhizopus oligosporus. J Sci Food Agric. 2007;87:1315–1322. [Google Scholar]
- Kalmendal R., Elwinger K., Holm L., Tauson R. High-fibre sunflower cake affects small intestinal digestion and health in broiler chickens. Br Poult Sci. 2011;52:86–96. doi: 10.1080/00071668.2010.547843. [DOI] [PubMed] [Google Scholar]
- Kasprowicz-Potocka M., Borowczyk P., Zaworska A., Nowak W., Frankiewicz A., Gulewicz P. The effect of dry yeast fermentation on chemical composition and protein characteristics of blue lupin seeds. Food Technol Biotechnol. 2016;54:360–366. doi: 10.17113/ftb.54.03.16.4459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kasprowicz-Potocka M., Zaworska A., Frankiewicz A., Nowak W., Gulewicz P., Zduńczyk Z., Juśkiewicz J. The nutritional value and physiological properties of diets with raw and Candida utilis-fermented lupin seeds in rats. Food Technol Biotechnol. 2015;53(3):286–297. doi: 10.17113/ftb.53.03.15.3979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kocher A., Choct M., Porter M.D., Broz J. The effects of enzyme addition to broiler diets containing high concentrations of canola or sunflower meal. Poultry Sci. 2000;79:1767–1774. doi: 10.1093/ps/79.12.1767. [DOI] [PubMed] [Google Scholar]
- Koivunen E., Partanen K., Perttilä S., Palander S., Tuunainen P., Valaja J. Digestibility and energy value of pea (Pisum sativum L.), faba bean (Vicia faba L.) and blue lupin (narrow-leaf)(Lupinus angustifolius) seeds in broilers. Anim Feed Sci Technol. 2016;218:120–127. [Google Scholar]
- Krishna C. Solid-state fermentation systems—an overview. Crit Rev Biotechnol. 2005;25:1–30. doi: 10.1080/07388550590925383. [DOI] [PubMed] [Google Scholar]
- Krunglevičiūtė V., Starkutė V., Bartkienė E., Bartkevics V., Juodeikienė G., Vidmantienė D., Maknickienė Z. Design of lupin seeds lactic acid fermentation - changes of digestibility, amino acid profile and antioxidant activity. Vet Zootech Lith. 2016;73:47–53. [Google Scholar]
- Kumar V., Rani A., Goyal L., Dixit A.K., Manjaya J.G., Dev J., Swamy M. Sucrose and raffinose family oligosaccharides (RFOs) in soybean seeds as influenced by genotype and growing location. J Agric Food Chem. 2010;58:5081–5085. doi: 10.1021/jf903141s. [DOI] [PubMed] [Google Scholar]
- Li W.F., Bai J., Li Y., Qin Y., Yu D.Y. Effects of Bacillus subtilis on meat quality, nutrient digestibility and serum biochemical index of broilers. Chin J Vet Sci. 2014;34:1682–1685. [Google Scholar]
- López-Pérez M., Viniegra-González G. Production of protein and metabolites by yeast grown in solid state fermentation: present status and perspectives. J Chem Technol Biotechnol. 2016;91:1224–1231. [Google Scholar]
- Lordelo M., Calhoun M., Dale N., Dowd M., Davis A. Relative toxicity of gossypol enantiomers in laying and broiler breeder hens. Poultry Sci. 2007;86:582–590. doi: 10.1093/ps/86.3.582. [DOI] [PubMed] [Google Scholar]
- Mahmood F., Khan M.Z., Khan A., Muhammad G., Javed I. Lysine induced modulation of toxico-pathological effects of cottonseed meal in broiler breeder males. Pakistan J Zool. 2011;43(2):357–365. [Google Scholar]
- Martins S., Mussatto S.I., Martínez-Avila G., Montañez-Saenz J., Aguilar C.N., Teixeira J.A. Bioactive phenolic compounds: production and extraction by solid-state fermentation. A review. Biotechnol Adv. 2011;29:365–373. doi: 10.1016/j.biotechadv.2011.01.008. [DOI] [PubMed] [Google Scholar]
- Mathivanan R., Selvaraj P., Nanjappan K. Feeding of fermented soybean meal on broiler performance. Int J Poult Sci. 2006;5:868–872. [Google Scholar]
- Muangkeow N., Chinajariyawong C. Determination of true amino acid digestibility and metabolizable energy in fermented palm kernel meal with Aspergillus wentii TISTR 3075 for chickens. Walailak J Sci Technol. 2009;6:231–241. [Google Scholar]
- Muangkeow N., Chinajariyawong C. Diets containing fermented palm kernel meal with Aspergillus wentii TISTR 3075 on growth performance and nutrient digestibility of broiler chickens. Walailak J Sci Technol. 2013;10:131–147. [Google Scholar]
- Mussatto S.I., Ballesteros L.F., Martins S.L.F., Teixeira J.A. InTech; 2012. Use of agro-industrial wastes in solid-state fermentation processes. [Google Scholar]
- Nagalakshmi D., Rao S.V.R., Panda A.K., Sastry V.R. Cottonseed meal in poultry diets: a review. J Poult Sci. 2007;44:119–134. [Google Scholar]
- Nalle C.L., Ravindran V., Ravindran G. Nutritional value of narrow-leafed lupin (Lupinus angustifolius) for broilers. Br Poult Sci. 2011;52:775–781. doi: 10.1080/00071668.2011.639343. [DOI] [PubMed] [Google Scholar]
- Nalle C.L., Ravindran V., Ravindran G. Nutritional value of white lupins (Lupinus albus) for broilers: apparent metabolisable energy, apparent ileal amino acid digestibility and production performance. Animal. 2012;6:579–585. doi: 10.1017/S1751731111001686. [DOI] [PubMed] [Google Scholar]
- Newkirk R.W. 4th ed. Canadian International Grains Institute; Winnipeg, Manitoba: 2009. Canola meal feed industries guide. [Google Scholar]
- Niba A.T., Beal J.D., Kudi A.C., Brooks P.H. Potential of bacterial fermentation as a biosafe method of improving feeds for pigs and poultry. Afr J Biotechnol. 2009;8:1758–1767. [Google Scholar]
- Obese F., Osafo E., Okai D. Evaluation of the feeding value of palm press fibre using in vitro digestibility techniques. Trop Anim Health Prod. 2001;33:165–172. doi: 10.1023/a:1005293732378. [DOI] [PubMed] [Google Scholar]
- Olarotimi O.J., Adu O.A. Potentials of non-conventional protein sources in poultry nutrition. Arch Zootec. 2017;66(225):453–459. [Google Scholar]
- Oliveira A.C., Amorim G.M., Azevêdo J.A.G., Godoy M.G., Freire D.M. Solid-state fermentation of co-products from palm oil processing: production of lipase and xylanase and effects on chemical composition. Biocatal Biotransform. 2018;36:381–388. [Google Scholar]
- Olukomaiya O.O., Fernando W.C., Mereddy R., Zhang D., Li X., Sultanbawa Y. Phytic acid reduction in canola and camelina meals by fungal fermentation for potential broiler feeding. In: Roberts J., editor. Proceedings of the Australian poultry science symposium. 30th Annual Australian Poultry Science Symposium; Sydney, NSW, Australia: 2019. p. 203. February 17-20 2019. [Google Scholar]
- Ong L.G.A., Abd-Aziz S., Noraini S., Karim M., Hassan M. Enzyme production and profile by Aspergillus Niger during solid substrate fermentation using palm kernel cake as substrate. Appl Biochem Biotechnol. 2004;118:73–79. doi: 10.1385/abab:118:1-3:073. [DOI] [PubMed] [Google Scholar]
- Ortega-David E., Rodriguez-Stouvenel A. Bioprocessing of lupin cotyledons (Lupinus mutabilis) with Rhizopus oligosporus for reduction of quinolizidine alkaloids. J Food Process Technol. 2014;5:1000323. [Google Scholar]
- Özdoğan M., Wellmann K., Paksuz E. Effect of gossypol on blood serum parameters and small intestinal morphology of male broilers. J Anim Physiol Anim Nutr. 2012;96(1):95–101. doi: 10.1111/j.1439-0396.2010.01126.x. [DOI] [PubMed] [Google Scholar]
- Pandey A. Recent process developments in solid-state fermentation. Process Biochem. 1992;27:109–117. [Google Scholar]
- Pandey A. Solid-state fermentation. Biochem Eng J. 2003;13:81–84. doi: 10.1016/s1369-703x(00)00065-6. [DOI] [PubMed] [Google Scholar]
- Pinto G.A., Leite S.G., Terzi S.C., Couri S. Selection of tannase-producing Aspergillus Niger strains. Braz J Microbiol. 2001;32:24–26. [Google Scholar]
- Písaříková B., Zralý Z. Nutritional value of lupine in the diets for pigs (a review) Acta Vet. 2009;78:399–409. [Google Scholar]
- Ramachandran S., Singh S.K., Larroche C., Soccol C.R., Pandey A. Oil cakes and their biotechnological applications–A review. Biochem Eng J. 2007;98:2000–2009. doi: 10.1016/j.biortech.2006.08.002. [DOI] [PubMed] [Google Scholar]
- Ramin M., Alimon A., Ivan M. Effects of fungal treatment on the in vitro digestion of palm kernel cake. Livest Res Rural Dev. 2010;22 [Google Scholar]
- Ravindran V., Blair R. Feed resources for poultry production in Asia and the Pacific. II. Plant protein sources. World’s Poult Sci J. 1992;48:205–231. [Google Scholar]
- Renge V.C., Khedkar S.V., Nandurkar N.R. Enzyme synthesis by fermentation method: a review. Sci Rev Chem Comm. 2012;2:585–590. [Google Scholar]
- Ries E.F., Alves Macedo G. Improvement of phytase activity by a new Saccharomyces cerevisiae strain using statistical optimization. Enzyme res. 2011;2011 doi: 10.4061/2011/796394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi C., He J., Wang J., Yu J., Yu B., Mao X., Zheng P., Huang Z., Chen D. Effects of Aspergillus Niger fermented rapeseed meal on nutrient digestibility, growth performance and serum parameters in growing pigs. Anim Sci J. 2016;87(4):557–563. doi: 10.1111/asj.12457. [DOI] [PubMed] [Google Scholar]
- Shi C., He J., Yu J., Yu B., Huang Z., Mao X., Zheng P., Chen D. Solid state fermentation of rapeseed cake with Aspergillus Niger for degrading glucosinolates and upgrading nutritional value. J Anim Sci Biotechnol. 2015;6:13. doi: 10.1186/s40104-015-0015-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi C., He J., Yu J., Yu B., Mao X., Zheng P., Huang Z., Chen D. Physicochemical properties analysis and secretome of Aspergillus Niger in fermented rapeseed meal. PLoS One. 2016;11:e0153230. doi: 10.1371/journal.pone.0153230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singhania R.R., Patel A.K., Soccol C.R., Pandey A. Recent advances in solid-state fermentation. Biochem Eng J. 2009;44:13–18. [Google Scholar]
- Soccol C.R., da Costa E.S.F., Letti L.A.J., Karp S.G., Woiciechowski A.L., de Souza Vandenberghe L.P. Recent developments and innovations in solid state fermentation. Biotechnol Res. 2017;1:52–71. [Google Scholar]
- Soccol C.R., Vandenberghe L.P. Overview of applied solid-state fermentation in Brazil. Biochem Eng J. 2003;13:205–218. [Google Scholar]
- Subramaniyam R., Vimala R. Solid state and submerged fermentation for the production of bioactive substances: a comparative study. Int J Sci Nat. 2012;3:480–486. [Google Scholar]
- Sun H., Tang J.W., Yao X.H., Wu Y.F., Wang X., Feng J. Improvement of the nutritional quality of cottonseed meal by Bacillus subtilis and the addition of papain. Int J Agric Biol. 2012;14:563–568. [Google Scholar]
- Sun H., Tang J.W., Fang C.L., Yao X.H., Wu Y.F., Wang X., Feng J. Molecular analysis of intestinal bacterial microbiota of broiler chickens fed diets containing fermented cottonseed meal. Poultry Sci. 2013;92:392–401. doi: 10.3382/ps.2012-02533. [DOI] [PubMed] [Google Scholar]
- Sun H., Tang J.W., Yao X.H., Wu Y.F., Wang X., Feng J. Effects of dietary inclusion of fermented cottonseed meal on growth, cecal microbial population, small intestinal morphology, and digestive enzyme activity of broilers. Trop Anim Health Prod. 2013;45:987–993. doi: 10.1007/s11250-012-0322-y. [DOI] [PubMed] [Google Scholar]
- Sundu B., Kumar A., Dingle J. Palm kernel meal in broiler diets: effect on chicken performance and health. World's Poult Sci J. 2006;62:316–325. [Google Scholar]
- Taheri H., Moravej H., Tabandeh F., Zaghari M., Shivazad M. Screening of lactic acid bacteria toward their selection as a source of chicken probiotic. Poultry Sci. 2009;88:1586–1593. doi: 10.3382/ps.2009-00041. [DOI] [PubMed] [Google Scholar]
- Tang J.W., Sun H., Yao X.H., Wu Y.F., Wang X., Feng J. Effects of replacement of soybean meal by fermented cottonseed meal on growth performance, serum biochemical parameters and immune function of yellow-feathered broilers. Asian-Australas J Anim Sci. 2012;25:393. doi: 10.5713/ajas.2011.11381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas L., Larroche C., Pandey A. Current developments in solid-state fermentation. Biochem Eng J. 2013;81:146–161. [Google Scholar]
- Tripathi M.K., Mishra A.S. Glucosinolates in animal nutrition: a review. Anim Feed Sci Technol. 2007;132:1–27. [Google Scholar]
- Van Vo B., Bui D.P., Nguyen H.Q., Fotedar R. Optimized fermented lupin (Lupinus angustifolius) inclusion in juvenile barramundi (Lates calcarifer) diets. Aquaculture. 2015;444:62–69. [Google Scholar]
- Vig A.P., Walia A. Beneficial effects of Rhizopus oligosporus fermentation on reduction of glucosinolates, fibre and phytic acid in rapeseed (Brassica napus) meal. Bioresour Technol. 2001;78:309–312. doi: 10.1016/s0960-8524(01)00030-x. [DOI] [PubMed] [Google Scholar]
- Wang Y., Deng Q., Song D., Wang W., Zhou H., Wang L., Li A. Effects of fermented cottonseed meal on growth performance, serum biochemical parameters, immune functions, antioxidative abilities and cecal microflora in broilers. Food Agric Immunol. 2017;28(4):725–738. [Google Scholar]
- White C.L., Staines V., Staines Mv. A review of the nutritional value of lupins for dairy cows. Aust J Agric Res. 2007;58:185–202. [Google Scholar]
- Wickramasuriya S.S., Yi Y.-J., Yoo J., Kang N.K., Heo J.M. A review of canola meal as an alternative feed ingredient for ducks. J Anim Sci Technol. 2015;57:29. doi: 10.1186/s40781-015-0062-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiong J., Wang Z., Miao L., Meng F., Wu L. Growth performance and toxic response of broilers fed diets containing fermented or unfermented cottonseed meal. J Anim Feed Sci. 2016;25:348–353. [Google Scholar]
- Xu F.Z., Li L., Xu J., Qian K., Zhang Z., Liang Z. Effects of fermented rapeseed meal on growth performance and serum parameters in ducks. Asian-Australas J Anim Sci. 2011;24:678–684. [Google Scholar]
- Xu F.Z., Zeng X.G., Ding X.L. Effects of replacing soybean meal with fermented rapeseed meal on performance, serum biochemical variables and intestinal morphology of broilers. Asian-Australas J Anim Sci. 2012;25:1734–1741. doi: 10.5713/ajas.2012.12249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zaworska A., Frankiewicz A., Kasprowicz-Potocka M. The influence of narrow-leafed lupin seed fermentation on their chemical composition and ileal digestibility and microbiota in growing pigs. Arch Anim Nutr. 2017;71:285–296. doi: 10.1080/1745039X.2017.1329130. [DOI] [PubMed] [Google Scholar]
- Zaworska A., Kasprowicz-Potocka M., Frankiewicz A., Zduńczyk Z., Juśkiewicz J. Effects of fermentation of narrow-leafed lupine (L. angustifolius) seeds on their chemical composition and physiological parameters in rats. J Anim Feed Sci. 2016;25:326–334. [Google Scholar]