Abstract
Xanthones from a tropical fruit of Garcinia mangostana L. is known to possess a wide spectrum of pharmacologic properties, including antioxidant, anti-bacterial, anti-inflammatory, and antidiabetic activities. The current study aimed to assess the possible protective effects of xanthones against lead acetate (PbAc)-induced chronic kidney disease (CKD). To accomplish, in vitro antioxidant assays of xanthones, in vivo oxidative stress parameters, histopathology, inflammatory parameters were evaluated using PbAc-induced IRC male mice. The study was supported by in silico molecular docking of respective organ receptor protein-ligand interaction. Results revealed that xanthones potentially scavenged the DPPH, superoxide, hydroxyl, and nitric oxide radicals. Oxidative stress, kidney dysfunction, inflammatory markers, and kidney apoptosis increased by PbAc were attenuated with the co-treatment of xanthones. The treatment remarkably improved the tissue architecture. Of note, in silico prediction of activity study showed that protective role of xanthones could be due to its efficacy to activate the Nrf-2, regulate the intracellular [Ca2+], as well as downregulate the NF-kB, MAPK pathway. In a nutshell, xanthones could be a potential candidate for the management of PbAc-induced kidney damage.
Keywords: Xanthones, Lead (Pb), Chronic kidney disease (CKD), Oxidative stress, Inflammation, Apoptosis
Graphical abstract
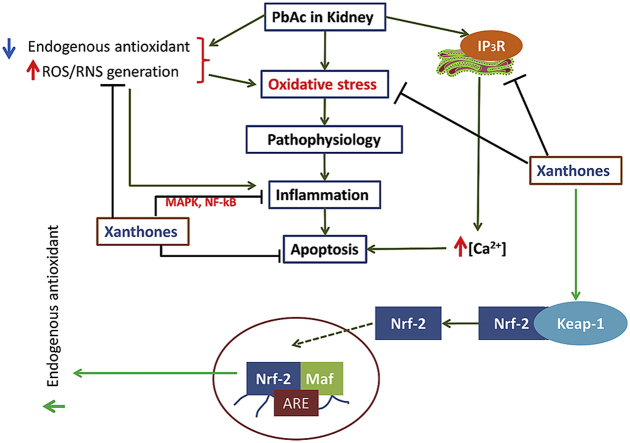
Protective role of xanthones from Garcinia mangostana fruit pericarp against PbAc-induced CKD.
Highlights
-
•
Xanthones potentially scavenged the DPPH, superoxide, hydroxyl, and nitric oxide radicals.
-
•
Xanthone attenuated the oxidative stress, kidney dysfunction, inflammatory markers, and kidney apoptosis increased by PbAc.
-
•
Computational model showed upregulation of of Nrf-2, intracellular [Ca2+], anddownregulation of NF-kB, MAPK pathway.
1. Introduction
Mangosteen (Garcinia mangostana L.), a juicy white pulp with sweet and slightly acidic in taste fruit, has a pericarp of radish or dark brown color and found in the different region of Asia including Thailand, India, Malaysia, Sri Lanka, and other countries. Nowadays in Thailand, xanthones from mangosteen pericarp is being used with tea for refreshment. Traditionally, mangosteen pericarp has been used to treat various diseases such as skin disease, bacterial infection, diarrhoea, wound healing, and inflammation [1]. Xanthone (9-xanthenone or dibenzo-c-pyrone), a phenol class compound, have been identified in the pericarp of this fruit where α-mangostin, β-mangostin, γ-mangostin are abundant [1]. A growing body of pharmacological researches revealed that xanthones possess potential antioxidant, anticancer, anti-diabetes, cardioprotective, and hepatoprotective activities [[2], [3], [4], [5]].
Lead (Pb) poisoning became an epidemic at Michigan State, USA through drinking water and continuous health complexity of people made it as national as well as global concern. Indeed, the possible causes of Pb intoxication include contamination of food, water, soil, cosmetics, house dust, and paint [6]. Accumulated evidence reported that Pb intoxication can causes gastrointestinal, hematological, reproductive, immunomodulatory, neurological, and renal disorders on the basis of the extent of exposure [[7], [8], [9], [10], [11], [12]]. Pb executes its noxious effect on major organs of the body, especially on kidney by means of oxidative stress-an imbalance between oxidant and antioxidant. This condition could arise the following abnormalities such as dysregulation of intracellular [Ca2+] homeostasis, displacement of essential metal ion from protein, and mitochondrial damage. As a result, outnumbers of oxidants brings damage to DNA, protein, and cell, therefore apoptosis of cells. Pb also intervenes the cellular permeability by modifying tight junction protein in the kidney. As a manifestation of kidney damage, increased protein urea and decreased kidney function along with the mutilated structure of nephron have been recorded in various studies, which later on turned into chronic kidney disease (CKD) [13]. CKD can be defined as kidney damage with substantial albuminuria and kidney dysfunction persistently for a three month period [14]. Previously, it had been found that antioxidant compounds specially xanthones ameliorated the PbAc-induced cognitive impairment of neurotoxic mice by reducing oxidative stress parameters and modulating acetycholinesterase (AChE) dysfunction [15]. Therefore, this study first time focuses on the protective effect of xanthones derivative from G. mangostana against PbAc-induced CKD.
2. Materials and methods
2.1. Xanthones extract preparation
The xanthones powder from Garcinia mangostana fruit pericarp was kindly provided by the Research excellent center for innovation and health products, Walailak University, Thailand. Other than xanthones, the powder contains carbohydrate, protein, fibre in a concentration of 77.2, 1.4, 10 gm per 100 gm of powder. In addition, the ORAC value was recorded as 79.46 unit per serving (20 g). According to recent study on xanthone, isolated xanthone (100 mg) from MVR contained α-mangostin (69.01%), γ -mangostin (17.85%), gartanin (4.13%), 8-deoxygartanin (2.95%), garcinon E (2.84%), and other xanthones (3.22%) [16]. Xanthones extract was prepared freshly prior to each experiment.
2.2. Determination of total phenolic content, antioxidant capacity and free radicals scavenging activities of xanthones
2.2.1. Total phenolic content of xanthones
Total phenolic content of the xanthones aqueous extract was determined as previously described method of Gulcin, 2005 [17]. Briefly, 12.5 μL of different concentrations (0.1, 0.25, 0.5, 1 mg/mL) of xanthones extract and distilled water (as blank) were added in the 96 well microplate. Then, 12.5 μL of Folin-Ciocalteu's phenol reagent was added to each well. After 5 min, 125 μL Na2CO3 solution (~7.5%) was added to the mixture and incubated at room temperature for 30 min. The absorbance was recorded at 765 nm using a microplate reader (Multiskan GO, Thermo Fisher Scientific, Finland). Gallic acid with different concentrations ranging from 0 to 100 mg/L was used to construct the calibration curve. A dose-response linear regression was generated from Gallic acid standard curve and the phenolic content in the samples was expressed as milligram of Gallic acid equivalents per milligram of dry weight (mg of GAE/gdw).
2.2.2. Total antioxidant capacity (ABTS assay) of xanthones
ABTSo+ scavenging activity was determined according to the method of Re et al., 1999 [18]. ABTSo+ was produced by reacting 7 mM of ABTS in water with 4.9 mM of K2S2O8 and stored in the condition dark at room temperature for 12–18 h before use. Then, 180 μL of ABTSo+ (absorbance range, 0.750 ± 0.025) solution was added to 20 μL of xanthones extract (concentrations range, 0.1–1 mg/mL). Finally, absorbance was recorded at 734 nm after 3 min of mixing against distilled water (blank). The values were expressed as mM of Trolox equivalent per gram of dry weight (mM of TEAC/gdw).
2.2.3. Free radical scavenging activities of xanthones
DPPH, superoxide, hydroxyl, nitric oxide radicals scavenging activities were evaluated according to the previously published report of Rana and Tangpong, 2017 [19], whereas IC50 was calculated and it's defined the concentration of the sample required to scavenge the 50% of radicals.
3. Animals
3.1. Animal treatment
Male ICR mice (body weight 30–33 g, eight-week-old age), were obtained from the National animal research center, Bangkok, Thailand. Mice were caged and housed at room temperature (23 ± 2 °C) and humidity-controlled environment (55 ± 5%) with a 12 h light/dark cycle was maintained from 6 a.m. to 6 p.m. in the experimental lab of the Natural Product Utilization Unit of Walailak University. The cages were cleaned, washed, sterilized with 70% methanol and filled with sterilized wooden made bed twice in a week. Mice were acclimatized for a week with free access to food and water prior to the experiment. All the instructions for care and use of mice and the protocol were approved by the Animal Care and Use Committee (ACUC) of Walailak University.
Treatment strategy was conducted according to our previous study, whereas xanthones attenuated the PbAc-induced cognitive dysfunction [15]. 42 mice were separated into seven groups (n = 6). Each group received the experimental solution once a day at between 8.30 – 9.30 a.m. via oral gavages for 38 days as follows:
Group 1: Sodium acetate (1% in drinking water) + Normal salineGroup 2: PbAc (1% in drinking water) + Normal salineGroup 3: Sodium acetate (1% in drinking water) +Xanthones 200 mg/kg BWGroup 4: PbAc (1% in drinking water) + Xanthones 100 mg/kg BWGroup 5: PbAc (1% in drinking water) + Xanthones 200 mg/kg BWGroup 6: PbAc (1% in drinking water) + Vitamin E 100 mg/kg BWGroup 7: Sodium acetate (1% in drinking water) + Vitamin E vehicle (mineral oil).
3.2. Blood collection and tissue sample preparation
Mice were anesthetized using Sodium Nembutal (65 mg/kgBW) and then sacrificed for collecting blood via left ventricle puncture. Afterward, the blood samples were kept in a 2.5 mL tube containing K3EDTA. Then, organs were thoroughly perfused with cold PBS (pH 7.4, 4 °C) to eradicate blood content from the tissue sample and stored at −30 °C for further use.
Kidney tissues were minced in cold PBS containing a mixture of protease inhibitors (leupeptin, pepstatin, and aprotinin) and homogenized (Sonics VCX70, USA). Thereafter, tissue suspensions were centrifuged at 15,000×g for 15 min at 4 °C. The supernatant was separated and stored at −30 °C for further enzymatic analysis.
A portion of kidney was homogenized in cold lysis buffer containing PMSF and kept on ice. There after centrifuge at 15,000×g for 15 min, 4 °C.The resulted supernatant was then collected emerging quickly the eppendorf at liquid nitrogen first, then preserve at −80 °C for Western blot analysis.
3.3. Determination of relative kidney weight
The weight of the left kidney and body weight was recorded and calculated relative kidney weight using the following formula [20]:
3.4. Determination of Pb concentration in blood and kidney sample
Pb content in whole blood and tissue samples were determined using an atomic absorption spectrometry (AAS) with flameless atomization in a graphite furnace (Zeeman Atomic Absorption Spectrophotometer Z-5000, Hitachi, Tokyo, Japan) as previously described by Tangpong and Satarug, 2010 [21]. Briefly, samples were diluted at a ratio of 1:5 using a diluent-containing 0.2% of Triton-X 100 and (NH4)2HPO4 in double deionized water. The absorption wavelength was 283.3 nm. Pb levels in samples were calculated using a calibration curve of standard Pb. The result was recorded as μg/dL for blood samples and μg/g protein for kidney samples, respectively.
3.5. Evaluation of kidney function parameters
Blood urea nitrogen (BUN) and creatinine are widely used to evaluate kidney function. A bioassay system reagent kit (Hayward, CA, USA) was used to evaluate the BUN and creatinine was determined using automatic chemistry analyzer (KONE Lab20, Tokyo, Japan).
3.6. Determination of lipid peroxidation in RBC, plasma and kidney tissue
Thiobarbituric acid reactive substances (TBARS), a secondary by-product of lipid peroxidation (LPO), is widely investigated as a marker to assess the magnitude of oxidative stress. TBARS assay in RBC, plasma and kidney tissue was evaluated according to the protocols of Ceci et al., 2014; Goulart et al., 2005; Jain, 1989 with slight modification [[22], [23], [24]]. Briefly, 100 μL of samples (RBC, plasma, and kidney homogenate) were mixed with 20% of trichloroacetic acid (TCA) and centrifuged at 3000 rpm for 15 min. Then, 15% of thiobarbituric acid (TBA) was added to the supernatant. Afterward, the mixture was boiled at 100 °C in a water bath for 30 min. After centrifugation at 3000 rpm for 15 min, the supernatant was collected and measured the absorbance at 595 nm. The lipid peroxide levels were compared with a standard curve of Malondialdehyde (1, 1, 3, 3-tetraethoxypropane). The results were expressed as μM/g Hb for RBC, μM/L for plasma, and μM/g protein for kidney sample.
3.7. Determination of antioxidant enzyme activities in kidney sample
Superoxide dismutase (SOD) activity was determined using a slightly modified method of Marklund et al. [25]. Briefly, 1 mL of pyrogallol solution (0.2 mM) was mixed with an equal volume of Tris-EDTA buffer (pH 8.2) to auto-oxidize the pyrogallol. After that, immediately 50 μL of sample was mixed with reaction mixture to inhibit the autoxidation. Then, the absorbance was measured at 420 nm. SOD activity was expressed as units/mL, while one unit of SOD activity defines the amount of enzyme required to cause 50% inhibition of pyrogallol autoxidation.
Catalase activity was determined according to the protocol of Weydert and Cullen, 2010 [26]. The absorbance of the reaction mixture was measured at 240 nm using an UV–vis spectrophotometer (JASC0 V-630, Japan). Catalase activity was expressed as Katal/mg of protein.
3.8. Determination of inflammatory markers using ELISA assay and Western blot analysis
Plasma TNF-α level was determined using a colorimetric sandwich enzyme-linked immunosorbent assay (ELISA) kit (R&D Systems, Minneapolis, MN, USA) protocol. Inflammation markers of kidney tissues were determined using Western blot analysis. Briefly, protein levels were determined using Bradford reagent (Bio-Rad, USA) and run through SDS-polyacrylamide gel electrophoresis at 100 V (Bio-Rad Laboratories, Inc., California, USA). Then, proteins were transferred to a nitrocellulose membrane (Roche Diagnostics Corporation, Indianapolis, IN, USA) at 100 V, 4 °C for 2 h. After that, the membrane was blocked with TBST solution (20 mM Tris–HCl, pH 7.4, 150 mM NaCl, 0.02% Tween 20 containing in 5% skimmed milk) for 1 h. Afterward, the membrane was incubated with the primary antibody of TNF-α, COX-2, and iNOS (Cell Signaling Technology, Massachusetts, USA) for overnight at 4 °C. Next, the membrane was washed with TBST solution (3 × 5 min) and incubated with peroxidase-conjugated secondary antibody (Cell Signaling Technology, Massachusetts, USA) at room temperature. After washing with TBST solution (3 × 5 min), the band of protein was detected by enhanced chemiluminescence detection kit (Bio-Rad, USA) and the result was expressed as the ratio with the β-actin band (Cell Signaling Technology, Massachusetts, USA) [27].
3.9. Determination of kidney tissues histology
Kidney tissues were fixed with 4% formaldehyde, embedded on paraffin and sectioned using a microtome to obtained 6 μm section. After deparaffinization and hydration, the sections were stained with hematoxylin and eosin, examined and photographed under a light microscope (Olympus EX51, Olympus Corporation, Tokyo, Japan).
3.10. Assessment of renal apoptosis by TUNEL assay
Frozen kidney tissues were sectioned into 7 μm using microtome (Leica Microsystem CM1950, Wetzlar, Germany). Thereafter, the whole assay was conducted according to the protocol supplied by “In situ apoptosis detection kit (Abcam, Cambridge, UK)” to investigate the extent of apoptosis in the kidney section. TUNEL positive cell was counted and expressed as a number of cells per 100 fields.
4. In silico studies of xanthones
4.1. In silico prediction of activity spectra (PASS) analysis
According to the literature survey, α-mangostin, β-mangostin, γ-mangostin are predominant among phytoconstituents present in the pericarp of the mangosteen fruits [1]. These three phytoconstituents were analyzed to predict antioxidant and anti-inflammatory effect, Nrf-2, Hemeoxygenase and catalase stimulating and Ca-regulatory activity using by online PASS analysis program (Way2Drug). This program predicts more than 4000 kinds of biological activity based on the structure of compound, i.e. structure activity relationship (SAR). The analyzed data were presented as Probable activity (Pa) and Probable inactivity (Pi) with the values in between 1.000 to 0.000 [28]. Compounds which exhibited higher Pa value than Pi (Pa > Pi) was considered as biological active and Pa>0.2 was regarded to have pharmacological potential.
4.2. In silico molecular docking study
The molecular docking study was accomplished following the description of Uddin et al., 2018 [28] using a Mestro module of Scodinger suit v11. Briefly, crystal structures of proteins were retrieved from the protein data bank as PDB format and imported into protein preparation wizard of Mestro v11. These protein structures were then preprocessed allowing the bond order, addition of hydrogen, creation of disulfide bond. Finally, the minimization was carried out by applying the OPLS5 force field, setting maximum heavy atom root-mean-square-deviation (RMSD) to 0.30 Å.
To prepare the ligand, 2D structures of α-, β-, γ-mangostin compounds were saved from PubChem as SDF format. Later on, input into ligprep wizard of Mestro v11. Then, the 3D structures were prepared with default setting by applying OPLS5 force field, pH 7.0 ± 2.0 to generate the ionization states, allowing possible 32 stereoisomers per ligand.
Following prepared protein was then processed to receptor grid generation by applying OPLS5 force field. Using the ligand docking module of Mestro v11, the grid generated structure of protein and ligand are allowed to dock (flexible docking). And, the score of docking was then exported as excel file. The lowest value (negative) of the docking score indicates the best docking.
5. Statistical analysis
Data were expressed as mean ± standard error mean (SEM). Variable among the groups were analyzed by One-way analysis of variance (ANOVA) followed by Newman–Keuls, post hoc test using GraphPad Prism software (version 5, USA). The statistically significant level was set up at p < 0.05.
6. Results
6.1. Total phenolic content, antioxidant capacity, and free radical scavenging activities of xanthones
The results of phenolic content, antioxidant capacity, and different reactive species scavenging activities by xanthones are presented in Table 1. The result showed that the total phenolic content of xanthones was 92 ± 5.0 mg GAE/g of extract while the total antioxidant capacity was 665 ± 0.0 mM TEAC/g of extract. Xanthones also had the ability to scavenge different reactive free radicals. Antioxidative effects of xanthones in terms of inhibition concentrations (IC50) to scavenge the radicals from DPPH, superoxide, hydroxyl and nitric oxide were found to be 0.693 ± 0.01 mg/mL, 0.463 ± 0.03 mg/mL, 1.214. ± 0.00 mg/mL, and 1.65 ± 0.03 mg/mL, respectively.
Table 1.
Phenolic content, total antioxidant capacity and scavenging activities of xanthones on different free radical.
| Aqueous extract | TPC (mg GAE/g extract) | TAC (mM TEAC/g extract) | DPPH |
Superoxide |
Hydroxyl |
Nitric oxide |
|---|---|---|---|---|---|---|
| IC50 (mg/mL) | ||||||
| Xanthones derivative | 92 ± 5.0 | 665 ± 0.0 | 0.693 ± 0.01 | 0.463 ± 0.03 | 1.214 ± 0.00 | 1.65 ± 0.03 |
TPC = Total phenolic content; TAC = Total antioxidant capacity. The values are express as mean ± SEM of three independent experiments.
6.2. Effect of xanthones on relative kidney weight and Pb deposition
The relative kidney weight is summarized in Table 2 which demonstrated that Pb caused a significant (p < 0.05) increment of relative kidney weight with reduced body weight. Pb treatment significantly (p < 0.05) increased the Pb concentration, both in the blood (62.35 ± 3.92 μg/dL) and tissue (49.97 ± 4.32 μg/g protein), which was managed to be reduced by the co-treatment with xanthones. Surprisingly, xanthones and vitamin E failed to lower the increased Pb deposition in a significant manner.
Table 2.
Xanthones significantly reduced relative kidney weight but failed to reduced aggregated PbAc from the body.
| Groups | Body weight (g) | Kidney weight (g) | Ratio (%) | Blood Pb (μg/dL) | Kidney Pb (μg/g protein) |
|---|---|---|---|---|---|
| Untreated control | 41.742 ± 0.80 | 0.33 ± 0.01 | 0.80 ± 0.03 | 1.65 ± 0.11 | 1.83 ± 0.19 |
| Pb (1% in drinking water) | 39.96 ± 1.24a | 0.43 ± 0.01a | 1.09 ± 0.05a | 62.35 ± 3.92a | 49.97 ± 4.32a |
| xanthones (200 mg/kgBW) | 43.27 ± 0.86 | 0.41 ± 0.01 | 0.95 ± 0.01 | 1.69 ± 0.08 | 1.37 ± 0.26 |
| Pb + xanthones (100 mg/kgBW) | 38.11 ± 0.98b | 0.34 ± 0.02b | 0.90 ± 0.04b | 59.12 ± 3.59a | 48.45 ± 3.14a |
| Pb + xanthones (200 mg/kgBW) | 39.14 ± 0.98b | 0.33 ± 0.02b | 0.85 ± 0.06b | 58.96 ± 4.25a | 46.50 ± 2.96a |
| Pb + Vit E (100 mg/kgBW) | 37.22 ± 1.00b | 0.37 ± 0.01b | 1.00 ± 0.06 | 60.89 ± 2.45a | 48.90 ± 2.18a |
| Vehicle control | 42.60 ± 0.80 | 0.32 ± 0.01b | 0.77 ± 0.04b | 1.69 ± 0.21b | 1.56 ± 0.11b |
Values are means ± SEM (n = 6). aSignificant difference at p < 0.05 compared with untreated control group. bSignificant difference at p < 0.05 compared with Pb-treated only.
6.3. Effect of xanthones on kidney function parameters
Biochemical analyses showed that Pb interfered the kidney function parameters particularly BUN and creatinine. Lead treated mice were found to show significantly higher (p < 0.05) BUN and creatinine values, compared with the normal control group, which were significantly (p < 0.05) and dose-dependently modulated by a 38-day xanthone administration (Table 3).
Table 3.
Xanthones effectively restored kidney function.
| Groups | BUN (mg/dL) | Creatinine (mg/dL) |
|---|---|---|
| Untreated control | 30.19 ± 2.01 | 0.23 ± 0.03 |
| Pb (1% in drinking water) | 39.04 ± 3.40a | 0.45 ± 0.05a |
| xanthones (200 mg/kgBW) | 29.65 ± 1.65b | 0.23 ± 0.03b |
| Pb + xanthones (100 mg/kgBW) | 28.38 ± 1.46b | 0.26 ± 0.09b |
| Pb + xanthones (200 mg/kgBW) | 26.96 ± 1.76b | 0.24 ± 0.03b |
| Pb + Vit E (100 mg/kgBW) | 26.33 ± 2.02b | 0.27 ± 0.06b |
| Vehicle control | 27.96 ± 2.44b | 0.26 ± 0.07b |
Values are means ± SEM (n = 6). a Significant difference at p < 0.05 with respect to untreated control group. bSignificant difference at p < 0.05 with respect Pb treated only.
6.4. Effect of xanthones on oxidative stress-related parameters
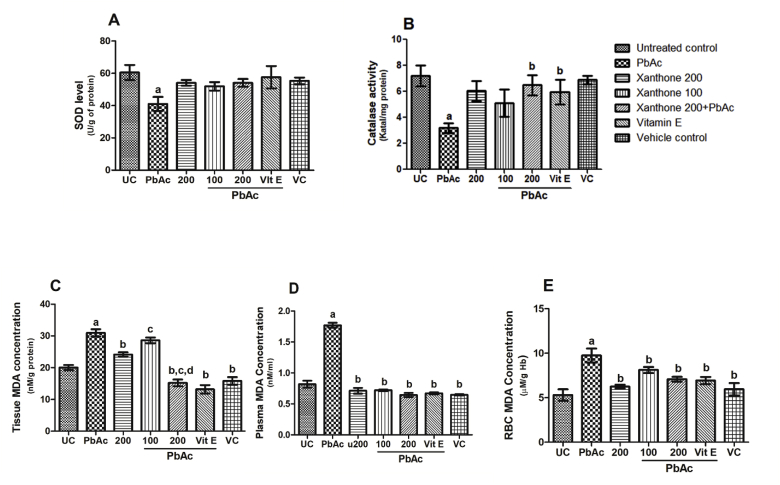
The lipid peroxidation (LPO), considered as oxidative stress marker, was significantly (p < 0.05) increased in RBC, plasma and kidney samples of Pb induced mice compared to normal control. Reduced activity of antioxidative enzymes SOD and CAT due to PbAc treatment has been reversed significantly (p < 0.05) with xanthone treatment. A 38 days treatment of PbAc-treated mice with xanthones significantly (p < 0.05) reduced the TBARS level in different samples than that of PbAc-treated mice (Fig. 1).
Fig. 1.
Xanthones attenuated oxidative stress parameters in PbAc-induced kidney toxicity. (A) SOD level in kidney (B) Catalase activity in kidney, (C) (D) (E) MDA concertation in tissue, Plasma, and RBC, respectively. Here, aP < 0.05 compared with untreated normal control group, bP < 0.05 compared with PbAc group, cP < 0.05 compared with the xanthone 200 only and dP < 0.05 compared with Pb + xanthone 100.
6.5. Effect of xanthones on inflammatory parameters
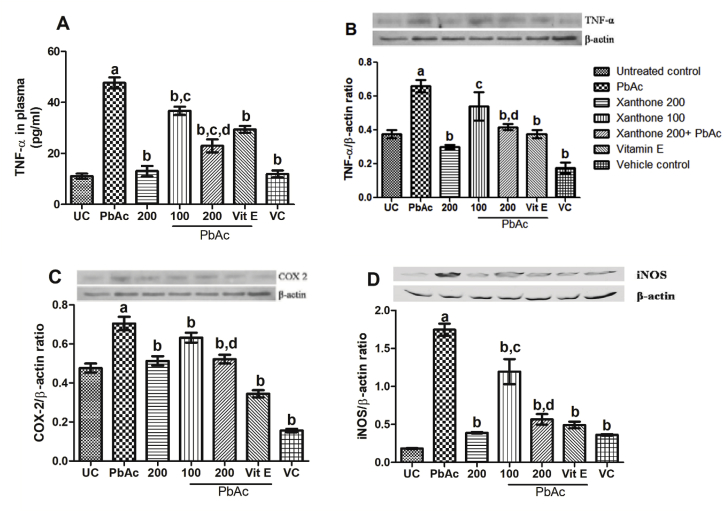
Fig. 2 revealed that PbAc significantly (p < 0.05) induced the inflammation in normal mice compared to untreated normal control group. Co-treatment with xanthones or Vitamin E reduced the plasma TNF-α concentration (Fig. 2A) and protein expression of TNF-α, COX-2, and iNOS in kidney tissue (Fig. 2B, C, 2D). No significant difference for the expressions of TNF-α, COX-2, and iNOS was noted in normal control group and xanthone alone.
Fig. 2.
Inflammation biomarkers quantification by ELISA assay and Western blot analysis. (A) TNF-α level in plasma. (B), (C), (D) protein expressions and quantification of TNF-α, COX-2, and iNOS in kidney, respectively. Data are presented as mean ± SEM (n = 3). aP < 0.05 compared with untreated normal control group, bP < 0.05 compared with PbAc group, cP < 0.05 compared with the xanthone 200 only and dP < 0.05 compared with Pb + xanthone 100.
6.6. Effect of xanthones on renal pathology and apoptosis
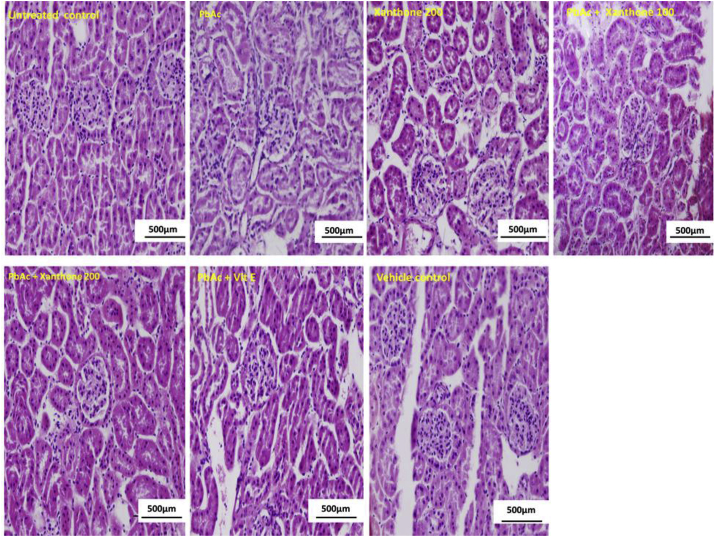
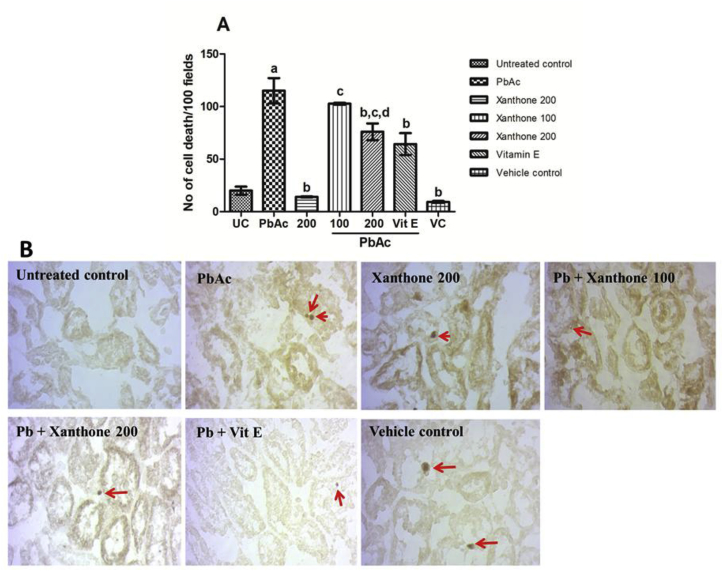
Alterations of tissue architecture were scored as severe, moderate and mild damage by PbAc treatment. Fig. 3 and Table 4 displays that co-treatment of xanthones or vitamin E restores the PbAc induced severe hydropic tubular degeneration, glomerular damage, and inflammatory cellular infiltration. The histopathological screening also showed that PbAc significantly (p < 0.05) induced the cellular apoptosis reflected by a higher number of TUNEL positive cells (Fig. 4). Co-treatment with xanthones significantly (p < 0.05) decreased the TUNEL positive cell number compared to PbAc treated group.
Fig. 3.
Photographs (H&E staining) of the protective role of xanthones in PbAc-induced kidney toxicity (original magnification x200). Histological alteration of kidney mainly observed in the cortex region, both in proximal tubule (PT) and distal tubule (DT) and there was presence of tubular necrosis, cloudy swelling of PT, shrinkage of both DT, PT following Pb toxicity. Disturbed renal corpuscle is also observed in PbAc-treated mice. Co-treatment with xanthones (100 and 200 mg/kgBW) and as Vitamin E reversed to normal condition. No toxic effect was observed within xanthones alone (200 mgkg/BW) and vehicle control groups.
Table 4.
Effect of xanthones on the histopathological scoring of different treatment groups.
| Groups | Hydropic degeneration changes in tubules | Glomerular damage | Inflammatory cellular infiltration |
|---|---|---|---|
| Untreated control | 0 | 0 | 0 |
| Pb (1% in drinking water) | + + + | + + + | + + |
| xanthones (200 mg/kgBW) | 0 | 0 | 0 |
| Pb + xanthones (100 mg/kgBW) | + + | + + | + |
| Pb + xanthones (200 mg/kgBW) | 0 | + | 0 |
| Pb + Vit E (100 mg/kgBW) | + | 0 | 0 |
| Vehicle control | 0 | 0 | 0 |
Here, 0: absent; +: mild; ++: moderate; +++: severe.
Fig. 4.
Xanthones co-treatment reduced the TUNEL positive cell in the kidney. (A) Quantification of TUNEL positive cell in kidney section counted in a hundred fields. (B) TUNEL positive cell in kidney tissue sectioned at 7 μm and the red arrow indicates the TUNEL positive cell (original magnification x200). Data are represent as mean ± SEM (n = 3). Here, aP < 0.05 compared with untreated normal control group, bP < 0.05 compared with PbAc group, cP < 0.05 compared with the xanthone 200 only and dP < 0.05 compared with Pb + xanthone 100.
6.7. PASS analysis and docking study
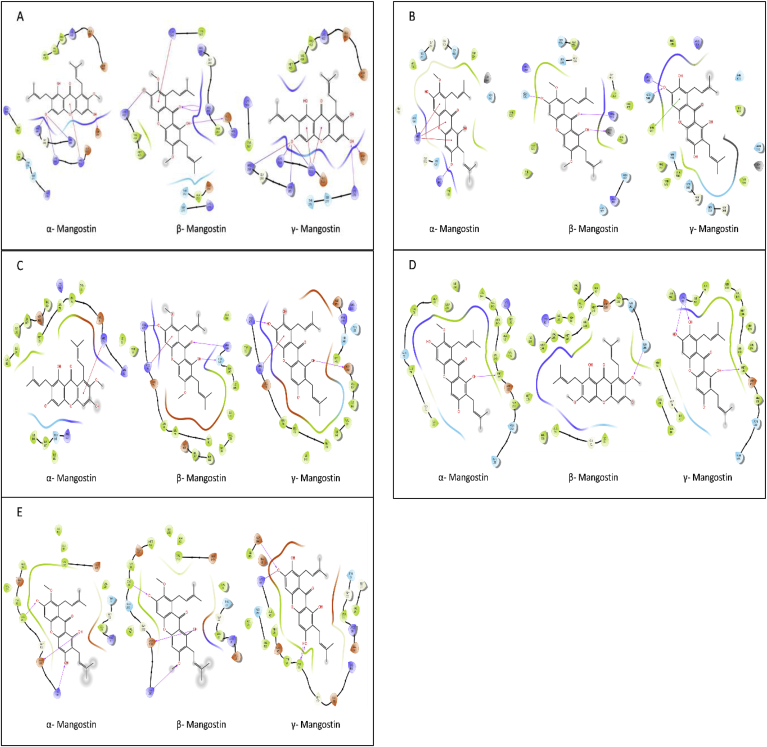
In silico docking studies had revealed the possible mode of interactions of three (α-, β-, γ-mangostin) constituents. According to the docking result, these phytoconstituents showed strong binding affinity with the receptors of JNK (PDB: 3OY1) and NFkB (PDB:4KIK) (Table 5 and Fig. 5). PASS prediction of three abundant phytoconstituents (α-, β-, γ-mangostin) had shown the probability to be effective as Nrf-2 and catalase stimulant, HOX1 expression enhancer, Ca-regulator, potent antioxidant and anti-inflammatory agent (Table 6). Based on sophisticated virtual screening and analysis of docking score, it could be predicted that xanthones exerted protective activity against oxidative stress and inflammation.
Table 5.
α,β,γ - mangostin attenuates oxidative stress and inflammation interacting with IP3R, KEAP-1, p38 kinase, JNK and NF-kB.
| Compound names | IP3R (PDB:3UJ0) |
KEAP-1 (PDB:5CGJ) |
p38 kinase (PDB:2YIS) |
JNK (PDB:3OY1) |
NF-kB (PDB:4KIK) |
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Docking score | Glide e model | Glide energy | Docking score | Glide e model | Glide energy | Docking score | Glide e model | Glide energy | Docking score | Glide e model | Glide energy | Docking score | Glide e model | Glide energy | |
| α -mangostin | −3.357 | −45.267 | −37.929 | −5.63 | −61.434 | −44.618 | −4.569 | −41.359 | −40.702 | −6.707 | −67.529 | −48.892 | −7.187 | −68.143 | −48.554 |
| β -mangostin | −3.171 | −44.084 | −37.195 | −4.616 | −55.464 | −43.602 | −4.149 | −49.645 | −41.21 | −6.535 | −67.363 | −50.166 | −6.606 | −68.279 | −51.382 |
| γ -mangostin | −3.38 | −45.657 | −38.001 | −4.917 | −56.047 | −43.873 | −5.004 | −45.676 | −38.879 | −6.941 | −71.599 | −50.986 | −7.183 | −72.28 | −51.774 |
Fig. 5.
Molecular docking of the α-, β-, γ-mangostin with IP3R (PDB: 3UJ0), KEAP-1 (PDB: 5CGJ), p38 kinase (PDB:2YIS), JNK (PDB:3OY1), nuclear factor kappa-B kinase subunit beta (PDB:4KIK). Here, color of residual indicate the chemical nature where, Asp, Glu: acidic (orange); Leu, Trp, Ala, Val, Ile Met, Cys, Pro, Tyr, Phe: hydrophobic (Green); Lys, Hip, Arg: basic (purple); Gly &water: grey; Thr, Gln, Ser, Asn, Hie, His, Hid: polar (blue) and line between receptor and ligand dictates: pink lines—H-bonds to the protein backbone; Orange line -pi-cation interactions; Green line—pi-pi stacking interactions and colored line near ligand is protein pocket.
Table 6.
Drug likeliness properties and orally availability of α,β,γ - mangostin.
| Compound name | Pubchem CID | Drug-likeliness properties |
Orally availability |
|||||
|---|---|---|---|---|---|---|---|---|
| MW | HB donor | HB acceptor | QPlogPo/w | QPlogS | QP PCaco | Primary Metabolite | ||
| α-mangostin | 5281650 | 410.466 | 4 | 6.25 | 2.60 | −6.074 | 1570.68 | 4 |
| β-mangostin | 5495925 | 424.493 | 4 | 6.50 | 2.831 | −6.132 | 2578.04 | 3 |
| γ-mangostin | 5464078 | 396.439 | 4 | 4.75 | 2.776 | −6.181 | 823.15 | 6 |
Drug-likeliness properties and orally availability of these three xanthone derivatives were analyzed using QikProp from Maestro 11 (schrodinger). Here, M.W- Molecular weight, HB donor-number of hydrogen bonds that would be donated by the solute to water molecules HB acceptor-number of hydrogen bonds that would be accepted by the solute from water molecule, QPlogPo/w- Predicted octanol/water partition coefficient, QPlogS-Predicted aqueous solubility, QPPCaco Predicted apparent Caco-2 cell permeability in nm/sec. Compounds possessed at least three of the criteria {MW- <500, HB donor- ≤5, HB acceptor- ≤10, QPlogPo/w- (−)2.0–6.5} would be considered to have drug-like properties. In addition, orally availability of drug would be considered if compound follows rule of three (QPlogS > −5.7, QP PCaco > 22 nm/s, Primary Metabolites < 7).
7. Discussion
Chronic kidney disease (CKD) has emerged as a common health burden worldwide [29] and about 11–13% of people are at stage 3 condition [30]. Kidney plays a pivotal role to excrete the waste product through urine. Thereby, kidney is the most vulnerable target organ to Pb following accumulation. Moreover, CKD is associated with Pb-toxicity [31] and also regarded as a risk factor for metabolic diseases such as diabetes, hypertension, and others [32]. The possible mechanism of kidney toxicity exerts via developing oxidative stress, which in turn causes the lipid peroxidation [6]. Indeed, CKD is characterized by renal injury initiated via the development of oxidative stress, oxidant/free radicals. In the long run, these reactive radicals triggered inflammation process and renal damage that reduce kidney performance [33]. In our current study, the interrelationship among the Pb accumulation, increased kidney/body weight ratio, kidney dysfunction, histopathology, and inflammation had been established, while xanthones reversed these abnormalities without efficient noxious effects. Present data revealed that PbAc treatment significantly (P < 0.05) decreased body weight and increased kidney weight. This anomaly was in agreement with the study of Dhkil et al. [20]. The weight loss of mice could be due to the interference of Pb on the central nervous system (CNS), which in turn develop the anorexia. Co-treatment with xanthones improved the body weight and ratio of kidney/body weight in a dose-dependent manner, possibly via improving cognitive function, as reported in the previous study of Phyu and Tangpong [15].
Accumulation studies suggested that elevated Pb content attributes to oxidative stress, kidney dysfunction [34], which are consistent with our present data. Augmented LPO marker (TBARS), kidney dysfunction, declined antioxidant status, along with Pb accumulation in blood and kidney suggested that “oxidative stress” was an underlying mechanism of kidney damage. In accordance with the previous study [35], xanthones co-treatment remarkably reduced the oxidative stress but failed to limit the Pb accumulation. These results demonstrate its inability as a chelating agent, a form of antioxidant activity [36]. Analyzed histopathology and TUNNEL assay photograph revealed that PbAc caused extensive damage to kidney including mutilated glomerulus, tubular swelling & necrosis, and infiltration of inflammatory cells with a high number of apoptotic cell compare to untreated control and xanthone 200mg/kgBW groups, respectively. These tubular cells alteration is might be due to the interrupted ion pump transport by Pb, which leads to hydropic changes and exerted tubular swelling and necrosis [39]. Coherent to histopathology, Pb also induced the apoptosis possibly through ROS production and TNF-α mediated caspase-3 activation [20,37]. In contrast, co-treatment of xanthones protected nephron's architecture and integrity by reducing cellular apoptosis as well as reversing above mentioned biochemical and enzymatic indices in a dose-response manner without exerting any potential adverse effect.
In Pb-induced group, infiltration of inflammatory cells was observed in interstitial space, which is believed to influence inflammation-mediated apoptosis via extrinsic pathway. Furthermore, activation of JNK-MAPK pathway contributes to ROS-mediated inflammation in Pb-intoxicated mice [37]. In addition, excessive ROS act as a signaling molecule to activate NF-kB, which in turn translocate to the nucleus to regulate the gene encoding inflammation-associated molecules such as TNF-α, COX-2, and iNOS [38]. Compared to untreated normal control, a sharp rise of pro-inflammatory cytokines TNF-α was observed in the circulation of PbAc-treated group. Additionally, the protein expression of TNF-α, COX-2, iNOS in tissue were also elevated in PbAc-treatment. To treat CKD patient, phenolic content or antioxidants are also recommended to suppress the oxidative stress and inflammation [39]. Our current study also uncovered that xanthones and Vitamin E effectively lessened inflammation similar to that of Wang et al., 2016 [40]. Likewise the therapeutic role of antioxidants to CKD, they are also efficient to protect kidney from PbAc [36,41] either by quenching free radicals, chain breaking mechanism against ROS [42], triggering nuclear factor E2–related factor 2 (Nrf2)–mediated activation of antioxidant response element (ARE) [38,43], or activating heme oxygenase 1 (HO-1) gene [49]. In general, Nrf2 in basal condition is bound with Kelch-like ECH-associated protein (KEAP-1), which makes nrf-2 susceptible to proteosomal degradation. Following stress or stimuli, the Nrf-2-Keap-1 complex breaks down and form the phosphorylated nrf-2 and therby translocate into the nucleus. In addition, nrf-2 bind with small musculoaponeurotic fibrosarcoma (sMaf) and antioxidant response element (ARE), and thus upregulate the ARE-driven antioxidant enzyme and other cytoprotective enzymes (e.g. SOD, CAT) [44]. Regarding antioxidant activity of xanthones, it possesses a considerable amount of phenolic content and antioxidant capacity. Besides, it was efficient enough to quench the DPPH, hydroxyl radical, superoxide, nitric oxide free radicals. All of these properties could reflect its antioxidant activity along with probable activation of nrf-2 pathway.
Considering the mechanism of action as described in our recently published review [6], we hypothesized that attenuation of kidney pathophysiology by xanthones was achieved through suppressing insositol-3-phosphapates (IP3R) receptor to modulate the intracellular [Ca2+], inflammation, and activation nrf-2. Taking these facts into account, structure-based prediction on possible biological activity (PASS analysis) of major xanthones (α-, β-, γ-mangostin) were analyzed. Analyzed results of PASS analysis suggested that α-, β-, γ-mangostin possess possible activity with higher a Pa value considering Ca-regulation, anti-inflammation, and induction of Phase II antioxidant enzymes. To look insight of mechanism and portray the binding pattern with residuals, we studied the molecular docking using Schrodinger suite v11. In silico docking and simulation studies of xanthones on active side (such as IP3R, p38, JNK, IKK, and KEAP1) confirmed the Pb-induced kidney pathophysiological alterations and its modulatory effect by xanthones. Suppression of IP3R could minimize the mitochondrial damage and apoptosis [6], whereas inhibition of pro-inflammatory mediator (TNF-α, COX-2), MAPK (c-Jun N-terminal kinases (JNKs), p38 family of kinases), NF-κB (IKK) pathways could suppress the inflammation and inflammation-induced apoptosis. Furthermore, escape of nrf-2 from Keap-1-nrf-2 complex prior to proteasomal degradation could trigger the ARE-driven enzymes, which directly [36] or indirectly [45] increase the activity of antioxidant enzymes (SOD, CAT), thus reduces the oxidative stress. Additionally, the drug likeliness properties and the rule of three reflected that α-, β-, γ-mangostin could be regarded as the orally administrable drug.
In conclusion, above findings reveal that cumulative attribution of α-, β-, γ-mangostin that present in xanthones could protect from Pb-induced kidney by reducing oxidative stress, suppressing inflammation mediator and apoptosis, which is similar to well-known antioxidants. The possible protective mechanism could be due to a part of the antioxidant activity (not by the chelating action) including nrf2–mediated antioxidant response (i.e. SOD, CAT, HO-1, etc.), and inhibition of NF-kB, MAPK pathways. Thus, the current findings demonstrated that xanthones could be a potential candidate for the management of heavy metal toxicity by suppressing oxidative stress and inflammation. Further studies are also required to isolate the abundant phytoconstituents and to evaluate the mechanistic pathways.
Ethical approval
The study was approved by the Animal Care and Use Committee (ACUC) of Walailak University, with Animal Ethics Approval Certificate Number 002/2013. All animal experiments comply with the ARRIVE guidelines and carried out following National Institutes of Health guide for the care and use of Laboratory Animals.
Consent for publication
All authors have agreed to publish all materials belongs to this article.
Availability of data and materials
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Funding
Funding was done by Walailak University (WU59122) to conduct the experiments.
Author's contribution
JT was involved in designing the experiment, editing the manuscript with critically evaluating the data for maintaining the integrity of works, and approved the manuscript for submission. MNR contributed to design the experiment, conduct the experiments, analyze data and manuscript writing, MAR has supplemented the idea, revised and rearranged the manuscript, redimensioned the interpretation and restructured the data setting.
Declaration of competing interest
The authors declare that they have no competing interests.
Acknowledgment
Authors are thankful to Mohammed Sohel Chowdhury, Department of Pharmacy, International Islamic University Chittagong, Bangladesh, for his technical support regarding Schrodinger Software.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.bbrep.2019.100718.
Contributor Information
Jitbanjong Tangpong, Email: rjitbanj@wu.ac.th.
Md Atiar Rahman, Email: atiar@cu.ac.bd.
List of Abbreviations
- PbAc
lead acetate
- ROS
reactive oxygen species
- BW
body weight
- TNF-α
Tumor necrosis factor-alpha
- iNOS
Inducible nitric oxide synthase
- COX-2
Cyclooxygenase-2
- ELISA
enzyme-linked immunosorbent assay
- BUN
Blood urea nitrogen
- TBARS
Thiobarbituric acid reactive substances
- TBST
Tris-phosphate buffer saline with Tween 20
- TBS
Tris phosphate saline
- H-E
Hematoxylin-Eosin
- TUNEL
Terminal deoxynucleotidyl transferase dUTP nick end labeling
Appendix A. Supplementary data
The following is the Supplementary data to this article:
References
- 1.Pedraza-Chaverri J., Cárdenas-Rodríguez N., Orozco-Ibarra M., Pérez-Rojas J.M. Medicinal properties of mangosteen (Garcinia mangostana) Food Chem. Toxicol. 2008;46:3227–3239. doi: 10.1016/j.fct.2008.07.024. [DOI] [PubMed] [Google Scholar]
- 2.Taher M., Tg Zakaria T.M.F.S., Susanti D., Zakaria Z.A. Hypoglycaemic activity of ethanolic extract of Garcinia mangostana Linn. in normoglycaemic and streptozotocin-induced diabetic rats. BMC Complement Altern. Med. 2016;16:135. doi: 10.1186/s12906-016-1118-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Devi Sampath P., Vijayaraghavan K. Cardioprotective effect of α-mangostin, a xanthone derivative frommangosteen on tissue defense system against isoproterenol-induced myocardial infarction in rats. J. Biochem. Mol. Toxicol. 2007;21:336–339. doi: 10.1002/jbt.20199. [DOI] [PubMed] [Google Scholar]
- 4.Shan T., Ma Q., Guo K., Liu J., Li W., Wang F., Wu E. Xanthones from mangosteen extracts as natural chemopreventive agents: potential anticancer drugs. Curr. Mol. Med. 2011;11:666–677. doi: 10.2174/156652411797536679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wang A., Liu Q., Ye Y., Wang Y., Lin L. Identification of hepatoprotective xanthones from the pericarps of Garcinia mangostana, guided with tert-butyl hydroperoxide induced oxidative injury in HL-7702 cells. Food Funct. 2015;6:3013–3021. doi: 10.1039/c5fo00573f. [DOI] [PubMed] [Google Scholar]
- 6.Nasiruddin M., Tangpong J., Rahman M. Toxicodynamics of Lead , Cadmium , Mercury and Arsenic- induced kidney toxicity and treatment strategy : a mini review. Toxicol. Rep. 2018;5:704–713. doi: 10.1016/j.toxrep.2018.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.United Nations Environment Programme . World Health Organization; 1995. International Labour Organisation.; World Health Organization.; International Program On Chemical Safety. Inorganic Lead. [Google Scholar]
- 8.Vega-Dienstmaier J.M., Salinas-Piélago J.E., Gutiérrez-Campos M. del R., Mandamiento-Ayquipa R.D., Yara-Hokama M. del C., Ponce-Canchihuamán J., Castro-Morales J. Lead levels and cognitive abilities in Peruvian children. Rev. Bras. Psiquiatr. 2006;28:33–39. doi: 10.1590/s1516-44462006000100008. doi:/S1516-44462006000100008. [DOI] [PubMed] [Google Scholar]
- 9.Tong S., von Schirnding Y.E., Prapamontol T. Environmental lead exposure: a public health problem of global dimensions. Bull. World Health Organ. 2000;78:1068–1077. [PMC free article] [PubMed] [Google Scholar]
- 10.Phyu M.P., Tangpong J. Protective effect of Thunbergia laurifolia (Linn.) on lead induced acetylcholinesterase dysfunction and cognitive impairment in mice. BioMed Res. Int. 2013;2013:186098. doi: 10.1155/2013/186098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Patrick L. Lead toxicity part II: the role of free radical damage and the use of antioxidants in the pathology and treatment of lead toxicity. Altern. Med. Rev. 2006;11:114–127. [PubMed] [Google Scholar]
- 12.Ademuyiwa O., Ugbaja R.N., Rotimi S.O., Abam E., Okediran B.S., Dosumu O.A., Onunkwor B.O. Erythrocyte acetylcholinesterase activity as a surrogate indicator of lead-induced neurotoxicity in occupational lead exposure in Abeokuta, Nigeria. Environ. Toxicol. Pharmacol. 2007;24:183–188. doi: 10.1016/j.etap.2007.05.002. [DOI] [PubMed] [Google Scholar]
- 13.Muntner P., He J., Vupputuri S., Coresh J., Batuman V. Blood lead and chronic kidney disease in the general United States population: results from NHANES III. Kidney Int. 2003;63:1044–1050. doi: 10.1046/j.1523-1755.2003.00812.x. [DOI] [PubMed] [Google Scholar]
- 14.Thomas R., Kanso A., Sedor J.R. Chronic kidney disease and its complications. Prim. Care. 2008;35 doi: 10.1016/j.pop.2008.01.008. 329–vii. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Phyu M.P., Tangpong J. Neuroprotective effects of xanthone derivative of Garcinia mangostana against lead-induced acetylcholinesterase dysfunction and cognitive impairment. Food Chem. Toxicol. 2014;70:151–156. doi: 10.1016/j.fct.2014.04.035. [DOI] [PubMed] [Google Scholar]
- 16.Karim N., Rahman A., Chanudom L., Thongsom M., Tangpong J. Mangosteen vinegar rind from Garcinia mangostana prevents high‐fat diet and streptozotocin‐induced type II diabetes nephropathy and apoptosis. J. Food Sci. 2019:1750–3841. doi: 10.1111/1750-3841.14511. 00. 14511. [DOI] [PubMed] [Google Scholar]
- 17.Gülçin İ. The antioxidant and radical scavenging activities of black pepper (Piper nigrum) seeds. Int. J. Food Sci. Nutr. 2005;56:491–499. doi: 10.1080/09637480500450248. [DOI] [PubMed] [Google Scholar]
- 18.Re R., Pellegrini N., Proteggente A., Pannala A., Yang M., Rice-Evans C. Antioxidant activity applying an improved ABTS radical cation decolorization assay. Free Radic. Biol. Med. 1999;26:1231–1237. doi: 10.1016/s0891-5849(98)00315-3. [DOI] [PubMed] [Google Scholar]
- 19.Rana M.N., Tangpong J. In vitro free radical scavenging and ANTI-GENOTOXIC activities OF thunbergia laurifolia aqueous leaf extract. J. Heal. Res. 2017;31:127–133. [Google Scholar]
- 20.Dkhil M.A., Al-Khalifa M.S., Al-Quraishy S., Zrieq R., Abdel Moneim A.E. Indigofera oblongifolia mitigates lead-acetate-induced kidney damage and apoptosis in a rat model. Drug Des. Dev. Ther. 2016;10:1847–1856. doi: 10.2147/DDDT.S105511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tangpong J., Satarug S. Alleviation of lead poisoning in the brain with aqueous leaf extract of the Thunbergia laurifolia (Linn.) Toxicol. Lett. 2010;198:83–88. doi: 10.1016/j.toxlet.2010.04.031. [DOI] [PubMed] [Google Scholar]
- 22.Ceci R., Beltran Valls M.R., Duranti G., Dimauro I., Quaranta F., Pittaluga M., Sabatini S., Caserotti P., Parisi P., Parisi A., Caporossi D. Oxidative stress responses to a graded maximal exercise test in older adults following explosive-type resistance training. Redox Biol. 2014;2:65–72. doi: 10.1016/j.redox.2013.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Jain S.K. Hyperglycemia can cause membrane lipid peroxidation and osmotic fragility in human red blood cells. J. Biol. Chem. 1989;264:21340–21345. [PubMed] [Google Scholar]
- 24.Goulart M., Batoréu M.C., Rodrigues A.S., Laires A., Rueff J. Lipoperoxidation products and thiol antioxidants in chromium exposed workers. Mutagenesis. 2005;20:311–315. doi: 10.1093/mutage/gei043. [DOI] [PubMed] [Google Scholar]
- 25.Marklund S., Marklund G. Involvement of the superoxide anion radical in the autoxidation of pyrogallol and a convenient assay for superoxide dismutase. Eur. J. Biochem. 1974;47:469–474. doi: 10.1111/j.1432-1033.1974.tb03714.x. [DOI] [PubMed] [Google Scholar]
- 26.Weydert C.J., Cullen J.J. Measurement of superoxide dismutase, catalase and glutathione peroxidase in cultured cells and tissue. Nat. Protoc. 2010;5:51–66. doi: 10.1038/nprot.2009.197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Yadav S.K., Adhikary B., Chand S., Maity B., Bandyopadhyay S.K., Chattopadhyay S. Molecular mechanism of indomethacin-induced gastropathy. Free Radic. Biol. Med. 2012;52:1175–1187. doi: 10.1016/j.freeradbiomed.2011.12.023. [DOI] [PubMed] [Google Scholar]
- 28.Uddin M.J., Reza A.S.M.A., Abdullah-Al-Mamun M., Kabir M.S.H., Nasrin M.S., Akhter S., Arman M.S.I., Rahman M.A. Antinociceptive and anxiolytic and sedative effects of methanol extract of anisomeles indica: an experimental assessment in mice and computer aided models. Front. Pharmacol. 2018;9 doi: 10.3389/fphar.2018.00246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Chronic Kidney Disease World kidney day. http://www.worldkidneyday.org/faqs/chronic-kidney-disease/ Available online.
- 30.Hill N.R., Fatoba S.T., Oke J.L., Hirst J.A., Callaghan A.O., Lasserson D.S., Hobbs F.D.R. vols. 1–18. 2016. (Global Prevalence of Chronic Kidney Disease – A Systematic Review and Meta-Analysis). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Jayasumana C., Gunatilake S., Siribaddana S. Simultaneous exposure to multiple heavy metals and glyphosate may contribute to Sri Lankan agricultural nephropathy. BMC Nephrol. 2015;16:103. doi: 10.1186/s12882-015-0109-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Leff T., Stemmer P., Tyrrell J., Jog R. 2018. Diabetes and Exposure to Environmental Lead (Pb) pp. 1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zhang H., Tsao R. Dietary polyphenols, oxidative stress and antioxidant and anti-inflammatory effects. Curr. Opin. Food Sci. 2016;8:33–42. [Google Scholar]
- 34.BaSalamah M.A., Abdelghany A.H., El-Boshy M., Ahmad J., Idris S., Refaat B. Vitamin D alleviates lead induced renal and testicular injuries by immunomodulatory and antioxidant mechanisms in rats. Sci. Rep. 2018;8:4853. doi: 10.1038/s41598-018-23258-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Reckziegel P., Dias V.T., Benvegnú D.M., Boufleur N., Barcelos R.C.S., Segat H.J., Pase C.S., dos Santos C.M.M., Flores É.M.M., Bürger M.E. Antioxidant protection of gallic acid against toxicity induced by Pb in blood, liver and kidney of rats. Toxicol. Rep. 2016;3:351–356. doi: 10.1016/j.toxrep.2016.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Abdel Moneim A.E., Dkhil M.A., Al-Quraishy S. The protective effect of flaxseed oil on lead acetate-induced renal toxicity in rats. J. Hazard Mater. 2011;194:250–255. doi: 10.1016/j.jhazmat.2011.07.097. [DOI] [PubMed] [Google Scholar]
- 37.Wang H., Li D., Hu Z., Zhao S., Zheng Z., Li W. Protective effects of green tea polyphenol against renal injury through ROS-mediated JNK-MAPK pathway in lead exposed rats. Mol. Cells. 2016;39:508–513. doi: 10.14348/molcells.2016.2170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Nair M.P., Mahajan S., Reynolds J.L., Aalinkeel R., Nair H., Schwartz S.A., Kandaswami C. The flavonoid quercetin inhibits proinflammatory cytokine (tumor necrosis factor alpha) gene expression in normal peripheral blood mononuclear cells via modulation of the NF-kappa beta system. Clin. Vaccine Immunol. 2006;13:319–328. doi: 10.1128/CVI.13.3.319-328.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Modaresi A., Nafar M., Sahraei Z. Oxidative stress in chronic kidney disease. 2015;9:165–179. [PubMed] [Google Scholar]
- 40.Wang H., Li D., Zhao S., Zheng Z., Li W. Protective effects of green tea polyphenol against renal injury through ROS-mediated JNK-MAPK pathway in lead exposed rats. Mol. Cells. 2016;39:508–513. doi: 10.14348/molcells.2016.2170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sudjarwo S.A., Eraiko K., Sudjarwo G.W. Koerniasari Protective effects of piperine on lead acetate induced-nephrotoxicity in rats. Iran. J. Basic Med. Sci. 2017;20:1227–1231. doi: 10.22038/IJBMS.2017.9487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Antonio-García M.T., Massó-Gonzalez E.L. Toxic effects of perinatal lead exposure on the brain of rats: involvement of oxidative stress and the beneficial role of antioxidants. Food Chem. Toxicol. 2008;46:2089–2095. doi: 10.1016/j.fct.2008.01.053. [DOI] [PubMed] [Google Scholar]
- 43.Ye F., Li X., Li L., Lyu L., Yuan J., Chen J. The role of Nrf2 in protection against Pb-induced oxidative stress and apoptosis in SH-SY5Y cells. Food Chem. Toxicol. 2015;86:191–201. doi: 10.1016/j.fct.2015.10.009. [DOI] [PubMed] [Google Scholar]
- 44.Balogun E., Hoque M., Gong P., Killeen E., Green C.J., Foresti R., Alam J., Motterlini R. Curcumin activates the haem oxygenase-1 gene via regulation of Nrf2 and the antioxidant-responsive element. Biochem. J. 2003;371:887–895. doi: 10.1042/BJ20021619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Zhao H., Jiang Z., Chang X., Xue H., Yahefu W., Zhang X. 4-hydroxyphenylacetic acid prevents acute APAP-induced liver injury by increasing phase II and antioxidant enzymes in mice. Front. Pharmacol. 2018;9:1–10. doi: 10.3389/fphar.2018.00653. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.