Abstract
A link between chronic inflammation and carcinogenesis has been depicted in many organ systems. Helicobacter pylori is the most prevalent bacterial pathogen, induces chronic gastritis and is associated with more than 90% of cases of gastric cancer (GC). However, the introduction of nucleotide sequencing techniques and the development of biocomputional tools have surpassed traditional culturing techniques and opened a wide field for studying the mucosal and luminal composition of the bacterial gastric microbiota beyond H. pylori. In studies applying animal models, a potential role in gastric carcinogenesis for additional bacteria besides H. pylori has been demonstrated. At different steps of gastric carcinogenesis, changes in bacterial communities occur. Whether these microbial changes are a driver of malignant disease or a consequence of the histologic progression along the precancerous cascade, is not clear at present. It is hypothesized that atrophy, as a consequence of chronic gastric inflammation, alters the gastric niche for commensals that might further urge the development of H. pylori-induced GC.
Here, we review the current state of knowledge on gastric bacteria other than H. pylori and on their synergism with H. pylori in gastric carcinogenesis.
Keywords: gastric bacterial microbiome, H. pylori, gastric cancer, microbiota, carcinogenesis
Introduction
Helicobacter pylori colonizes the human stomach, a unique ecological niche not amenable for colonization by other bacteria. The bacterium is an obligate pathogen, induces chronic gastritis and has been recognized as ‘definite carcinogen’ by the World Health Organization since 1994. Lines of evidence for its role in the development of gastric cancer (GC) were extended and updated in 2012,1 and H. pylori is now considered to be the most prevalent carcinogenic bacterium endangering human health.
The carcinogenic gastric cascade initiated by H. pylori is detailed in the Correa sequence of histological changes.2 Several bacterial virulence factors, host genetic make-up and facilitating ambient, predominantly nutritional, factors concur in this uneventful process. Robust clinical trials have demonstrated the beneficial effect of GC prevention by H. pylori eradication and thus completely proved the carcinogenic role of the bacterium. The role of bacteria other than H. pylori has now moved into focus in the study of gastric diseases. The introduction of nucleotide sequencing techniques and the development of biocomputional tools have surpassed traditional culturing techniques and opened a wide field for studying the mucosal and luminal composition of the gastric microbiota. A close connection between gastric microbiota and the bacterial composition in adjacent ecological niches such as the oral cavity and the duodenum has been demonstrated in recent studies.3 A potential role in gastric carcinogenesis for bacteria other than H. pylori has been shown in several experimental animal models. However, whether the microbial changes observed in GC are a driver of disease or a consequence of the histologic progression through the precancerous cascade, is not clear at present.
The link between H. pylori and GC development has been established using data from epidemiological, basic and translational studies (Table 1). Although GC development is without doubt a result of a complex interplay between host, bacterial and environmental factors, noncardia adenocarcinomas are attributed to H. pylori with an odds ratio (OR) of 21.0.4,5
Table 1.
Key epidemiological studies supporting the link between H. pylori infection and gastric carcinogenesis.
| Author | Study design | Result |
|---|---|---|
| Hansson6 | Case control study | The prevalence of H. pylori seropositivity was significantly higher (p = 0.002) among patients with GC than control patients. The OR was 2.60 (95% CI, 1.35–5.02). |
| Ekstrom4 | Population based case-control study | Based on IgG ELISA and CagA seropositivity the OR for noncardia GC among H. pylori-positive subjects is 21 (95% CI, 8.3–53.4) |
| Uemura7 | Prospective endoscopic follow-up study | GC develops in persons infected with H. pylori but not in uninfected persons. There is an increased risk for GC in patients with severe gastric atrophy, corpus-predominant gastritis or intestinal metaplasia. |
| Wong8 | Prospective, randomized, placebo-controlled, population-based primary prevention study | In H. pylori infected individuals without precancerous lesions, eradication of H. pylori significantly decreases the development of GC. |
| Fuccio9 | Meta-analysis | H. pylori eradication is a primary chemo-preventive strategy of GC. |
| Fukase10 | Multi-centre, open-label, randomized controlled trial | Eradication of H. pylori after endoscopic resection of early GC has the potential to prevent the development of metachronous gastric carcinoma. |
| Ma11 | Prospective randomized controlled trial | GC was diagnosed in 3.0% of subjects who received H. pylori treatment and in 4.6% of those who received placebo (OR = 0.61, 95% CI = 0.38–0.96, p = 0.032). GC deaths occurred among 1.5% of subjects assigned H. pylori treatment and among 2.1% of those assigned placebo (HR of death = 0.67, 95% CI = 0.36–1.28). |
| Li12 | Prospective randomized controlled trial | Treatment was associated with a statistically significant decrease in GC incidence (OR = 0.36; 95% CI = 0.17–0.79) and mortality (HR = 0.26; 95% CI = 0.09–0.79) at ages 55 years and older and with a statistically significant decrease in incidence among those with intestinal metaplasia or dysplasia at baseline (odds ratio = 0.56; 95% CI = 0.34–0.91). |
| Doorakkers13 | Population based cohort study | Eradication treatment for H. pylori seems to counteract the development of gastric adenocarcinoma and noncardia gastric adenocarcinoma in this Western population. |
CI, confidence interval; ELISA, enzyme-linked immunosorbent assay; GC, gastric cancer; HR, hazard ratio; OR, odds ratio.
In this paper, we review the current state of knowledge on gastric bacteria other than H. pylori on the one hand, and on their synergism with H. pylori in gastric carcinogenesis on the other.
Infection-associated cancer: a global burden
Chronic infections are major risk factors triggering carcinogenesis in several organs.26 About 15% of all diagnosed cancer cases are attributable to infections.27 The International Agency for Research on cancer (IARC) has classified a total of 11 infectious agents as group 1 carcinogens, but only H. pylori belongs to the domain bacteria.28 This knowledge and awareness open doors for prevention and therapy of chronic infections to decrease the incidence of infection-associated cancer. Socioeconomic conditions have a major impact on regional differences in risk patterns for infection driven carcinogenesis. While in high and very high developed regions, H. pylori is the most relevant infectious carcinogenetic agent, in low and very low developed countries, HHV8, HPV, HBV and HCV play a more dominant role in infection-associated cancers.29
Screen-and-treat strategies to prevent GC by H. pylori eradication are cost-effective in countries with a high prevalence of GC, but may be extended to countries with an intermediate GC risk. 13,30,31
Helicobacter pylori in gastric carcinogenesis
The vast majority of noncardia adenocarcinomas is attributed to H. pylori. The risk of cardia cancer attributed to H. pylori is variable and requires stratification according to the precise topographic location.32 The risk of cancer arising from H. pylori infection is identical for GC of both the intestinal and diffuse type.31,33,34
H. pylori was initially classified as a carcinogen based solely on large and well-performed epidemiological studies. The evidence has later been extended and strengthened by in vitro and in vivo studies.35,36 Bacterial virulence, host susceptibility genes and environmental factors such as nutritional factors are recognized to be part of a complex interplay involved in GC development.5
Different allotypes of bacterial virulence factors such as CagA and VacA are associated with an increased GC risk. CagA is translocated into the host cell by the type IV secretion system and acts as a classic oncogene.5 On the host side, polymorphisms and epigenetic alterations in genes encoding factors involved in the inflammatory immune response to the infection, including gene alterations in both the adaptive and the innate immune system such as interleukins (IL1β, IL8), transcription factors (CDX2, RUNX3, TLR1) and DNA repair enzymes, play a crucial role.37–40
H. pylori eradication therapy is effective in preventing GC.8,9,41–43 In intestinal type GC, carcinogenesis develops stepwise with the transition from chronic atrophic gastritis to intestinal metaplasia, dysplasia to invasive neoplasia (Correa Cascade).2 Wether eradication of H. pylori has the potential to stop or even reverse this process and prevent carcinogenesis at any stage of this cascade or if there is a point of no return has been studied intensively (Figure 1). More recently, even in patients being treated for early GC, H. pylori eradication was shown to be still effective in a subset of patients by minimizing the risk of metachronous GC.44,45 Several studies have identified patients with severe atrophic gastritis with and without intestinal metaplasia to be at high risk of developing GC.46,47 Thus, guidelines recommend that patients with severe gastric atrophy, corpus-predominant gastritis or intestinal metaplasia should be regularly followed up by surveillance endoscopies.31,48,49
Figure 1.
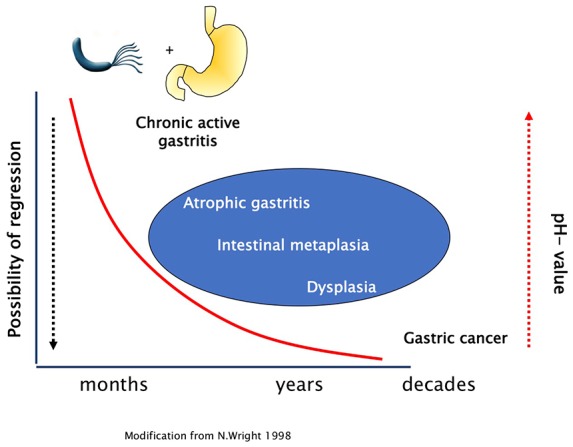
Stepwise carcinogenesis in intestinal type gastric cancer.
Later stages of the mucosal damage due to H. pylori-induced inflammation might enhance the carcinogenic effect of other risk factors such as other gastric microbiota, salt intake or tobacco smoking.
Gastric bacteria other than Helicobacter pylori and their role in gastric carcinogenesis
Despite the selective advantage of H. pylori to survive in the acidic gastric environment, other bacteria, either as resident community or as transient microbes, interact with the gastric mucosa. Studies based on culturing techniques have demonstrated bacteria other than H. pylori in conditions of hypo/achlorhydria that carry an oncogenic potential through their nitrosamine forming functions.3,33 The availability of high-throughput sequencing permits completely new insights into the gastric microbiota composition (Table 2).
Table 2.
Key findings from pivotal studies on other bacteria than H. pylori in gastric carcinogenesis.
| Author | Study design | Methods | Key findings |
|---|---|---|---|
| Sharma14 | Prospective interventional study | Ten healthy volunteers before, during, and after treatment with
omeprazole 30 mg daily for 2 weeks. Culture of gastric juice. |
Significant increases in the bacterial count and the nitrite and N-nitrosamine concentrations in the gastric juice after PPI (p < 0.001). |
| Mowat15 | Prospective interventional study | Gastric juice pH; nitrite, and colonization by other bacteria
were examined before and during omeprazole treatment in subjects
with and without HP infection. Culture. |
During omeprazole, H. pylori-positive subjects had a higher intragastric pH (7.8 versus 3.0; p < 0.00001) and greater colonization with non-HP species (5 × 107 versus 5 × 105 CFU/mL; p < 0.05) including nitrosating species. |
| Sanduleanu16 | Cohort-study | 145 patients on continuous acid inhibition (PPI or H2RA) and 75
dyspeptic patients without acid inhibition. Fasting gastric
juice was obtained for pH measurement and bacteriological
culture. Gastric biopsy specimens were examined for detection of
HP and of non-HP bacteria. Culture and histology. |
Both luminal and mucosal growth of non-HP bacteria were significantly greater in HP-positive than –negative patients taking PPI (p < 0.05 for both). Luminal growth of non-HP flora increased with the intragastric pH level. |
| Schulz17 | Cohort study | Saliva, gastric and duodenal aspirates as well as gastric and
duodenal biopsies from 24 patients (m:9, f:15, mean age
52.2 ± SD 14.5 years). RNA was extracted and the V1–V2 region of the retrotranscribed bacterial 16S rRNA amplified and sequenced on the Illumina MiSeq platform. |
687 bacterial phylotypes that belonged to 95 genera and 11 phyla were observed. Each individual comprised a unique microbiota composition that was consistent across the different niches. The stomach fluid enriched for specific microbiota components. |
| Lertpiriyapong18 | Mouse model | Gastric colonization with ASF and HP were correlated with
pathology, immune response and mRNA expression for
proinflammatory and cancer-related genes in germ-free (GF), HP
monoassociated (mHp), restricted ASF (rASF; 3 species), and
specific pathogen-free (complex IF), hypergastrinemic INS-GAS
mice 7 months postinfection. Quantitative PCR (qPCR) for ASF and HP levels |
Colonisation with HP and a restricted microbiota consisting of
only three species of commensal bacteria promoted gastric cancer
in gnotobiotic male INS-GAS mice to a similar extent as mice
colonized with complex microbiota. Gastric colonization with complex microbiota lowered gastric HP colonization in both male and female INS-GAS mice. |
| Jo19 | Cohort study | Gastric microbiota of 63 antral mucosal and 18 corpus mucosal
samples bar-coded 454 pyrosequencing of the 16S rRNA gene (targeting the V1–V3 regions, on GS Junior Sequencing System) with focus on bacteria other than Hp, especially nitrosating or nitrate-reducing bacteria (NB). |
The number of NB other than Hp (non-Hp-NB) was two times higher in the cancer groups than in the control groups, but it did not reach statistical significance. |
| Ferreira20 | Retrospective cohort study | 54 patients with gastric carcinoma and 81 patients with chronic
gastritis 16S rRNA gene profiling, using next-generation sequencing (targeting the V5–V6 hypervariable regions, sequenced in an Ion PGM Torrent platform). Associations between the most relevant taxa and clinical diagnosis validated by real-time quantitative PCR |
The gastric carcinoma microbiota was characterized by reduced microbial diversity, by decreased abundance of Hp and by the enrichment of other bacterial genera, mostly represented by intestinal commensals. The combination of these taxa into a microbial dysbiosis index revealed that dysbiosis has excellent capacity to discriminate between gastritis and gastric carcinoma. The functional composition of the total gastric carcinoma microbiota had increased nitrate reductase functions. |
| Wang21 | Cohort study | 315 patients (212 patients with chronic gastritis, 103 patients
with gastric cancer) bacterial load of gastric mucosa was determined using quantitative PCR amplicons of the 16S rRNA gene (variable V1–V3 region) from 12 patients were pyrosequenced on a 454 GS-FLX system. |
The amount of bacteria in gastric mucosa was estimated to be 6.9 × 10 per gram tissue on average. It was higher in HP-infected patients (7.80 ± 0.71) compared with those uninfected (7.59 ± 0.57, p = 0.005). An increased bacterial load up to 7.85 ± 0.70 was detected in gastric cancer compared with chronic gastritis (p = 0.001). |
| Yang22 | Matched case control study | 20 individuals from 2 towns (one with high, the other with low
incidence of gastric cancer), matched for age and sex a fragment of the conserved 16S rDNA gene was amplified with a set of broad range primers recognizing highly conserved sequence motifs, the amplicons were sequenced using Roche 454 FLX + technology |
The gastric microbiota composition was highly variable between individuals, but showed a significant correlation with the town of origin. Multiple OTUs were detected exclusively in either Tumaco or Túquerres. Two operational taxonomic units (OTUs), Leptotrichia wadei and a Veillonella sp., were significantly more abundant in Túquerres, and 16 OTUs, including a Staphylococcus sp. were significantly more abundant in Tumaco |
| Coker23 | Cohort study | 81 cases including superficial gastritis (SG), atrophic
gastritis (AG), intestinal metaplasia (IM) and GC from Xi’an,
China 16S rRNA gene analysis (targeting V4 hypervariable regions) of gastric mucosal samples sequencing on Illumina MiSeq platform |
significant mucosa microbial dysbiosis in IM and GC subjects, with significant enrichment of 21 and depletion of 10 bacterial taxa in GC compared with SG (q < 0.05) |
| Liu24 | Retrospective cohort study | 276 GC patients without preoperative chemotherapy, and 230 normal, 247 peritumoral and 229 tumoral tissues obtained for gastric microbiota analysis targeting V3–V4 regions of 16S rRNA gene by MiSeq sequencing | GC-specific stomach microhabitats, not GC stages or types, determine the composition and diversity of the gastric microbiota. Bacterial richness was decreased in peritumoral and tumoral microhabitats, and the correlation network of abundant gastric bacteria was simplified in tumoral microhabitat. HP, Prevotella copri and Bacteroides uniformis were significantly decreased, whereas Prevotella melaninogenica, Streptococcus anginosus and Propionibacterium acnes were increased in tumoral microhabitat. Higher HP colonisation influenced the overall structure of the gastric microbiota in normal and peritumoral microhabitats. |
| Ling25 | Retrospective cohort study | 64 GC patients without preoperative chemotherapy, and 60 normal,
61 peritumoral and 59 tumoral tissues were obtained for gastric
mucosal microbiota analysis and immunohistochemistry
analysis. Illumina sequencing-with PCR primers 319F/806R, targeting the V3–V4 regions of 16S rRNA gene |
diversity, composition and function of gastric mucosal microbiota changed more significantly in tumoral tissues than those in normal and peritumoral ones. Several nonabundant genera such as Stenotrophomonas and Selenomonas were positively correlated with BDCA2+pDCs and Foxp3+Tregs, respectively, while Comamonas and Gaiella were negatively correlated with BDCA2+pDCs and Foxp3+ Tregs |
AG, atrophic gastritis; ASF, Altered Schaedler’s flora; CFU, colony forming units; GC, gastric cancer; HP, Helicobacter pylori; IM, intestinal metaplasia; PPI, proton pump inhibitor; SG, superficial gastritis.
From a historical viewpoint, initial studies on this topic applied culturing methods to stomach aspirates and documented the presence of members of the phyla Firmicutes, Proteobacteria and Bacteroidetes, which are also dominating in the whole human gastrointestinal tract.34,35 However, there is a dominance of anaerobic bacteria, which are difficult to culture, in the gastrointestinal tract. Recent comparative studies applying next-generation sequencing (NGS) revealed that the active bacterial community in the gastric mucosa comprises more than 600 bacterial phylotypes and that mainly microbiota communities from the oral cavity are acquired with the use of aspirates from the stomach. The mucosa-associated gastric microbial community is dominated by Helicobacter spp. that additionally significantly impact on duodenal and oral communities.36
Several animal studies have demonstrated a potential role in gastric carcinogenesis for additional bacteria besides H. pylori. In the absence of intestinal bacteria in germ-free, but H. pylori-infected, INS-GAS mice, a reduction in the development of preliminary carcinogenic stages such as atrophic gastritis or intestinal metaplasia was observed. Mice cocolonised with altered Schaedler flora, including ASF356 Clostridium species, ASF361 Lactobacillus murinus and ASF519 Bacteroides species or intestinal flora and H. pylori developed the most severe pathology.37 These results led to the hypothesis that atrophy as consequence of inflammation alters the gastric niche for commensals that might further urge the development of H. pylori-induced GC.
With a clinical approach, gastric biopsies from patients living in an area of Colombia with high risk for the development of GC were compared with matched samples from a distant area with a 25-fold lower risk of GC. Sequencing analysis revealed two significantly more abundant taxa in the high-risk region (Leptotrichia wadei and Veillonella spp.) whereas Staphylococcus spp. were more abundant in the low-risk region. However, a high interindividual variability between all individuals was detected and conclusive interpretation of the findings is not yet possible.38
A comparison of gastric microbiota in mucosal biopsies of 54 patients with gastric carcinoma and 81 patients with chronic gastritis by 16S rRNA gene profiling revealed that patients with GC had significantly decreased microbial diversity. Patients with GC presented with an overpresentation of non-Helicobacter Proteobacteria. The authors conclude that colonization with bacteria other than H. pylori, namely gut commensals, contributes to altering the equilibrium between the resident gastric microbiota and the host, and that this dysbiotic microbial community may augment the risk for H. pylori-related cancer.39 Another study also evaluated gastric biopsies from cancer patients compared with chronic active gastritis patients. On the phylum level, no significant differences were detected, which was discussed to be a consequence of reduced acid secretion leading to reduced bacteriocidic capacity of the stomach in patients with GC or advanced stages of gastritis due to the loss of parietal cells.40 Additionally, a quantitative PCR was used to compare the bacterial load of biopsies in more extensive groups (n = 212 chronic gastritis and n = 103 GC). A significantly increased bacterial load per gram tissue was found in the cancer group, also indicating a loss of hostile conditions in cancerous lesions.
In a 16S rRNA gene analysis of gastric mucosal samples from 81 cases including superficial gastritis (SG), atrophic gastritis, intestinal metaplasia and GC from China, which was validated in a Mongolian cohort, significant mucosa microbial dysbiosis in subjects with intestinal metaplasia and GC was observed with significant enrichment of 21 and depletion of 10 bacterial taxa in GC compared with SG [q < 0.05 after adjusting p-values for multiple comparisons by the false discovery rate (FDR) method]. Important roles for P. stomatis, D. pneumosintes, S. exigua, P. micra and S. anginosus in gastric cancer progression were suggested.41 Another study evaluated 33 individuals including subjects with H. pylori-associated chronic gastritis, gastric intestinal metaplasia, gastric adenocarcinoma and H. pylori-negative controls. Microbiota in the stomach were analyzed by Illumina MiSeq platform targeting the 16 S rDNA from gastric biopsies. A strong negative correlation between H. pylori relative abundance and bacterial diversity was observed. In samples from patients with GC, this inverse association was weak. These samples tended to have lower bacterial diversity compared with other samples with similar H. pylori levels. After H. pylori eradication therapy, bacterial diversity increased, and the relative abundance of other bacteria to levels similar to individuals without H. pylori was restored.50
A comparison of gastric microbiota composition between normal tissue, peritumoral and tumoral tissues in a cohort of 276 patients with GC with an approach targeting the 16S rRNA gene by MiSeq sequencing, bacterial richness was lowered in peritumoral and tumoral microhabitats. Additionally, the tumoral microhabitat presented with a simplified correlation network of abundant gastric bacteria. H. pylori (HP), Prevotella copri and Bacteroides uniformis were less prevalent, whereas Prevotella melaninogenica, Streptococcus anginosus and Propionibacterium acnes were more abundant in tumoral microhabitat.43 The same group focused on the tumor-immune environment in relation to gastric microbiota in GC patients in a second analysis and depicted a correlation of regulatory T cells and plasmacytoid dendritic cells within the tumor microenvironment with gastric microbiota dysbiosis.44
Nitrate-reducing bacteria are considered to aggravate gastric carcinogenesis in addition to H. pylori infection. Therefore, Jo and colleagues analyzed the bacterial composition in gastric biopsies of different sites, and divided the cohort into four subgroups (cancer ±, H. pylori ±). Using pyrosequencing of the 16S rRNA gene with special focus on nitrate-reducing bacteria, no significant differences were detected.45 Later, one of the studies mentioned above revealed increased nitrate reductase functions promoting the reduction of nitrate to nitrite in addition to increased nitrite reductase functions promoting the reduction of nitrite to nitric oxide in gastric carcinoma microbiota in comparison to microbiota in chronic gastritis.39
Other authors bring up the hypothesis of a potential role of biofilm formation in the development of GC, but convincing data to support this assumption is still lacking.51,52
However, the analysis of gastric microbiota with sequencing approaches is impeded by the high content of human DNA in mucosal samples, which confounds microbial identification. This can in part be overcome if whole genome sequencing approaches are applied combined with intensive human DNA filtering methods. A study using whole genome sequencing, however, revealed highly consistent results in terms of microbiome profiling of endoscopic biopsy samples with qPCR quantification of H. pylori and universal 16S bacterial quantification.53 Furthermore, a comparison of published sequencing data on the bacterial gastric microbiome is further hampered by methodological differences in published studies with respect to the target of analysis (bacterial RNA or DNA), amplification and extraction methods.54
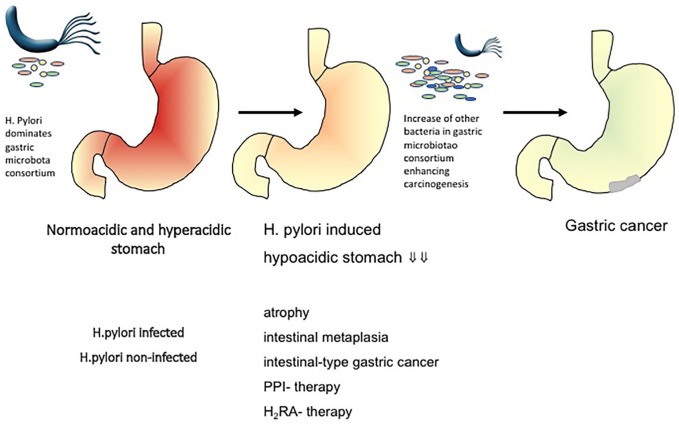
Summarizing current knowledge, the hypothesis is raised that the microenvironment modification, as consequence of chronic atrophic gastritis with atrophy and reduced acidity, results in H. pylori substitution by a cancer-prone microbiota. As a consequence, it is discussed that H. pylori infection is exclusively linked to a premalignant phase of chronic gastritis, but that the tremendous shifts in gastric microbiota composition in later stages play a more relevant role in carcinogenesis itself. This might have implications for clinical management (Figure 2).55 The complex interplay between the bacterial gastric microbiota, other players of the microbiome and the host and its immune system in gastric carcinogenesis, however, is far from being fully understood.
Figure 2.
Hypothesis on the impact of other gastric microbiota on gastric cancer development (adopted from Schulz and colleagues).3
Role of eradication therapy in gastric cancer prevention and interplay with gastric microbiota
Eradication of H. pylori is associated with a reduced risk of GC in Asian populations, but also in Western populations with lower incidence of the disease.11,56 Whether this is a consequence of the eradication of the carcinogen or of the alterations of the whole gastric microbiota following eradication in the short and long term, cannot be answered at this time. A study in 10 asymptomatic young adults compared the structure of the gastric microbiota before and after bismuth quadruple therapy. It demonstrated an increased alpha diversity after eradication over time with an increase in Lactobacillus and Bifidobacterium, which are assumed to act beneficially.57 This is in line with findings from a study mentioned before revealing that H. pylori infection results in alterations of gastric microbiota and reduction in bacterial diversity, which can be restored by eradication treatment.50
Conclusion
To date, not a single published trial provides convincing evidence for a strong involvement of bacteria other than H. pylori in human gastric carcinogenesis, albeit several studies have revealed differences in gastric microbiota composition between healthy individuals, patients with chronic gastritis and GC patients. The functional role of the changes in bacterial microbiota composition observed in advanced gastritis still needs to be elucidated. Additionally, a proportion of GC is associated with EBV infection.58 Incorporation of the virome and mycome into the complex picture of gastric carcinogenesis is a further challenge.
Future studies not only need to differentiate between active resident and transient bacteria, to prove their mucosal adherence or intracellular localization but to also take the whole complexity of microbiota into account. Additionally, the host response to the alterations observed needs to be characterized.
Footnotes
Funding: The author(s) received no financial support for the research, authorship, and publication of this article.
Conflict of interest statement: The authors declare that there is no conflict of interest.
ORCID iD: Christian Schulz  https://orcid.org/0000-0003-1841-1337
https://orcid.org/0000-0003-1841-1337
Contributor Information
Christian Schulz, Department of Medicine II, University Hospital, LMU Munich, Marchioninistraße 15, Munich 81377, Germany.
Kerstin Schütte, Department of Internal Medicine and Gastroenterology, Niels-Stensen-Kliniken, Marienhospital Osnabrück, Osnabrück, Germany; Department of Gastroenterology, Hepatology and Infectious Diseases, Otto-von-Guericke University, Magdeburg, Germany.
Julia Mayerle, Department of Medicine II, University Hospital, LMU Munich, Germany.
Peter Malfertheiner, Department of Medicine II, University Hospital, LMU Munich, Germany; Department of Gastroenterology, Hepatology and Infectious Diseases, Otto-von-Guericke University, Magdeburg, Germany.
References
- 1. IARC Working Group on the Evaluation of Carcinogenic Risks to Humans. Personal habits and indoor combustions. Volume 100 E. A review of human carcinogens. IARC Monogr Eval Carcinog Risks Hum 2012; 100: 1–538. [PMC free article] [PubMed] [Google Scholar]
- 2. Correa P. A human model of gastric carcinogenesis. Cancer Res 1988; 48: 3554–3560. [PubMed] [Google Scholar]
- 3. Schulz C, Schutte K, Malfertheiner P. Helicobacter pylori and other gastric microbiota in gastroduodenal pathologies. Dig Dis 2016; 34: 210–216. [DOI] [PubMed] [Google Scholar]
- 4. Ekstrom AM, Held M, Hansson LE, et al. Helicobacter pylori in gastric cancer established by CagA immunoblot as a marker of past infection. Gastroenterology 2001; 121: 784–791. [DOI] [PubMed] [Google Scholar]
- 5. Malfertheiner P, Link A, Selgrad M. Helicobacter pylori: perspectives and time trends. Nat Rev Gastroenterol Hepatol 2014; 11: 628–638. [DOI] [PubMed] [Google Scholar]
- 6. Hansson LE, Engstrand L, Nyren O, et al. Helicobacter pylori infection: independent risk indicator of gastric adenocarcinoma. Gastroenterology 1993; 105: 1098–1103. [DOI] [PubMed] [Google Scholar]
- 7. Uemura N, Okamoto S, Yamamoto S, et al. Helicobacter pylori infection and the development of gastric cancer. N Engl J Med 2001; 345: 784–789. [DOI] [PubMed] [Google Scholar]
- 8. Wong BC, Lam SK, Wong WM, et al. Helicobacter pylori eradication to prevent gastric cancer in a high-risk region of China: a randomized controlled trial. JAMA 2004; 291: 187–194. [DOI] [PubMed] [Google Scholar]
- 9. Fuccio L, Zagari RM, Eusebi LH, et al. Meta-analysis: can Helicobacter pylori eradication treatment reduce the risk for gastric cancer? Ann Intern Med 2009; 151: 121–128. [DOI] [PubMed] [Google Scholar]
- 10. Fukase K, Kato M, Kikuchi S, et al. Effect of eradication of Helicobacter pylori on incidence of metachronous gastric carcinoma after endoscopic resection of early gastric cancer: an open-label, randomised controlled trial. Lancet 2008; 372: 392–397. [DOI] [PubMed] [Google Scholar]
- 11. Ma JL, Zhang L, Brown LM, et al. Fifteen-year effects of Helicobacter pylori, garlic, and vitamin treatments on gastric cancer incidence and mortality. J Natl Cancer Inst 2012; 104: 488–492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Li WQ, Ma JL, Zhang L, et al. Effects of Helicobacter pylori treatment on gastric cancer incidence and mortality in subgroups. J Natl Cancer Inst 2014; 106. pii: dju116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Doorakkers E, Lagergren J, Engstrand L, et al. Helicobacter pylori eradication treatment and the risk of gastric adenocarcinoma in a Western population. Gut 2018; 67: 2092–2096. [DOI] [PubMed] [Google Scholar]
- 14. Sharma BK, Santana IA, Wood EC, et al. Intragastric bacterial activity and nitrosation before, during, and after treatment with omeprazole. Br Med J (Clin Res Ed) 1984; 289: 717–719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Mowat C, Williams C, Gillen D, et al. Omeprazole, Helicobacter pylori status, and alterations in the intragastric milieu facilitating bacterial N-nitrosation. Gastroenterology 2000; 119: 339–347. [DOI] [PubMed] [Google Scholar]
- 16. Sanduleanu S, Jonkers D, De Bruine A, et al. Non-Helicobacter pylori bacterial flora during acid-suppressive therapy: differential findings in gastric juice and gastric mucosa. Aliment Pharmacol Ther 2001; 15: 379–388. [DOI] [PubMed] [Google Scholar]
- 17. Schulz C, Schutte K, Koch N, et al. The active bacterial assemblages of the upper GI tract in individuals with and without Helicobacter infection. Gut 2018; 67: 216–225. [DOI] [PubMed] [Google Scholar]
- 18. Lertpiriyapong K, Whary MT, Muthupalani S, et al. Gastric colonisation with a restricted commensal microbiota replicates the promotion of neoplastic lesions by diverse intestinal microbiota in the Helicobacter pylori INS-GAS mouse model of gastric carcinogenesis. Gut 2014; 63: 54–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Jo HJ, Kim J, Kim N, et al. Analysis of gastric microbiota by pyrosequencing: minor role of bacteria other than Helicobacter pylori in the gastric carcinogenesis. Helicobacter 2016; 21: 364–374. [DOI] [PubMed] [Google Scholar]
- 20. Ferreira RM, Pereira-Marques J, Pinto-Ribeiro I, et al. Gastric microbial community profiling reveals a dysbiotic cancer-associated microbiota. Gut 2018; 67: 226–236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Wang L, Zhou J, Xin Y, et al. Bacterial overgrowth and diversification of microbiota in gastric cancer. Eur J Gastroenterol Hepatol 2016; 28: 261–266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Yang I, Woltemate S, Piazuelo MB, et al. Different gastric microbiota compositions in two human populations with high and low gastric cancer risk in Colombia. Sci Rep 2016; 6: 18594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Coker OO, Dai Z, Nie Y, et al. Mucosal microbiome dysbiosis in gastric carcinogenesis. Gut 2018; 67: 1024–1032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Liu X, Shao L, Liu X, et al. Alterations of gastric mucosal microbiota across different stomach microhabitats in a cohort of 276 patients with gastric cancer. EBioMedicine 2019; 40: 336–348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Ling Z, Shao L, Liu X, et al. Regulatory T cells and plasmacytoid dendritic cells within the tumor microenvironment in gastric cancer are correlated with gastric microbiota dysbiosis: a preliminary study. Front Immunol 2019; 10: 533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. de Martel C, Ferlay J, Franceschi S, et al. Global burden of cancers attributable to infections in 2008: a review and synthetic analysis. Lancet Oncol 2012; 13: 607–615. [DOI] [PubMed] [Google Scholar]
- 27. Plummer M, de Martel C, Vignat J, et al. Global burden of cancers attributable to infections in 2012: a synthetic analysis. Lancet Glob Health 2016; 4: e609–e616. [DOI] [PubMed] [Google Scholar]
- 28. Bouvard V, Baan R, Straif K, et al. A review of human carcinogens–Part B: biological agents. Lancet Oncol 2009; 10: 321–322. [DOI] [PubMed] [Google Scholar]
- 29. Parkin DM. The global health burden of infection-associated cancers in the year 2002. Int J Cancer 2006; 118: 3030–3044. [DOI] [PubMed] [Google Scholar]
- 30. Doorakkers E, Lagergren J, Engstrand L, et al. Eradication of Helicobacter pylori and gastric cancer: a systematic review and meta-analysis of cohort studies. J Natl Cancer Inst 2016; 108. pii: djw132. [DOI] [PubMed] [Google Scholar]
- 31. Malfertheiner P, Megraud F, O’Morain CA, et al. Management of Helicobacter pylori infection – the maastricht V/Florence consensus report. Gut 2017; 66: 6–30. [DOI] [PubMed] [Google Scholar]
- 32. Bornschein J, Dingwerth A, Selgrad M, et al. Adenocarcinomas at different positions at the gastro-oesophageal junction show distinct association with gastritis and gastric preneoplastic conditions. Eur J Gastroenterol Hepatol 2015; 27: 492–500. [DOI] [PubMed] [Google Scholar]
- 33. Abrams JA, Gonsalves L, Neugut AI. Diverging trends in the incidence of reflux-related and Helicobacter pylori-related gastric cardia cancer. J Clin Gastroenterol 2013; 47: 322–327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Fock KM, Graham DY, Malfertheiner P. Helicobacter pylori research: historical insights and future directions. Nat Rev Gastroenterol Hepatol 2013; 10: 495–500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Watanabe T, Tada M, Nagai H, et al. Helicobacter pylori infection induces gastric cancer in mongolian gerbils. Gastroenterology 1998; 115: 642–648. [DOI] [PubMed] [Google Scholar]
- 36. Wroblewski LE, Peek RM, Jr, Wilson KT. Helicobacter pylori and gastric cancer: factors that modulate disease risk. Clin Microbiol Rev 2010; 23: 713–739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Mayerle J, den Hoed CM, Schurmann C, et al. Identification of genetic loci associated with Helicobacter pylori serologic status. JAMA 2013; 309: 1912–1920. [DOI] [PubMed] [Google Scholar]
- 38. Hou L, El-Omar EM, Chen J, et al. Polymorphisms in Th1-type cell-mediated response genes and risk of gastric cancer. Carcinogenesis 2007; 28: 118–123. [DOI] [PubMed] [Google Scholar]
- 39. Companioni O, Bonet C, Munoz X, et al. Polymorphisms of Helicobacter pylori signaling pathway genes and gastric cancer risk in the European prospective investigation into cancer-eurgast cohort. Int J Cancer 2014; 134: 92–101. [DOI] [PubMed] [Google Scholar]
- 40. Crusius JB, Canzian F, Capella G, et al. Cytokine gene polymorphisms and the risk of adenocarcinoma of the stomach in the European prospective investigation into cancer and nutrition (EPIC-EURGAST). Ann Oncol 2008; 19: 1894–1902. [DOI] [PubMed] [Google Scholar]
- 41. Lee YC, Chiang TH, Chou CK, et al. Association between Helicobacter pylori eradication and gastric cancer incidence: a systematic review and meta-analysis. Gastroenterology 2016; 150: 1113–1124.e5. [DOI] [PubMed] [Google Scholar]
- 42. Take S, Mizuno M, Ishiki K, et al. The effect of eradicating Helicobacter pylori on the development of gastric cancer in patients with peptic ulcer disease. Am J Gastroenterol 2005; 100: 1037–1042. [DOI] [PubMed] [Google Scholar]
- 43. Malfertheiner P, Sipponen P, Naumann M, et al. Helicobacter pylori eradication has the potential to prevent gastric cancer: a state-of-the-art critique. Am J Gastroenterol 2005; 100: 2100–2115. [DOI] [PubMed] [Google Scholar]
- 44. Malfertheiner P. Helicobacter pylori treatment for gastric cancer prevention. N Engl J Med 2018; 378: 1154–1156. [DOI] [PubMed] [Google Scholar]
- 45. Choi IJ, Kook MC, Kim YI, et al. Helicobacter pylori therapy for the prevention of metachronous gastric cancer. N Engl J Med 2018; 378: 1085–1095. [DOI] [PubMed] [Google Scholar]
- 46. Rugge M, Meggio A, Pravadelli C, et al. Gastritis staging in the endoscopic follow-up for the secondary prevention of gastric cancer: a 5-year prospective study of 1755 patients. Gut 2019; 68: 11–17. [DOI] [PubMed] [Google Scholar]
- 47. Rugge M, Genta RM, Fassan M, et al. OLGA gastritis staging for the prediction of gastric cancer risk: a long-term follow-up study of 7436 patients. Am J Gastroenterol 2018; 113: 1621–1628. [DOI] [PubMed] [Google Scholar]
- 48. Dinis-Ribeiro M, Areia M, de Vries AC, et al. Management of precancerous conditions and lesions in the stomach (MAPS): guideline from the European society of gastrointestinal endoscopy (ESGE), European helicobacter study group (EHSG), European society of pathology (ESP), and the sociedade portuguesa de endoscopia digestiva (SPED). Endoscopy 2012; 44: 74–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Pimentel-Nunes P, Libanio D, Marcos-Pinto R, et al. Management of epithelial precancerous conditions and lesions in the stomach (MAPS II): European society of gastrointestinal endoscopy (ESGE), European helicobacter and microbiota study group (EHMSG), European society of pathology (ESP), and sociedade portuguesa de endoscopia digestiva (SPED) guideline update 2019. Endoscopy 2019; 51: 365–388. [DOI] [PubMed] [Google Scholar]
- 50. Li TH, Qin Y, Sham PC, et al. Alterations in gastric microbiota after H. Pylori eradication and in different histological stages of gastric carcinogenesis. Sci Rep 2017; 7: 44935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Hathroubi S, Servetas SL, Windham I, et al. Helicobacter pylori biofilm formation and its potential role in pathogenesis. Microbiol Mol Biol Rev 2018; 82. pii: e00001-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Rizzato C, Torres J, Kasamatsu E, et al. Potential role of biofilm formation in the development of digestive tract cancer with special reference to Helicobacter pylori infection. Front Microbiol 2019; 10: 846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Zhang C, Cleveland K, Schnoll-Sussman F, et al. Identification of low abundance microbiome in clinical samples using whole genome sequencing. Genome Biol 2015; 16: 265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Pichon M, Burucoa C. Impact of the gastro-intestinal bacterial microbiome on Helicobacter-associated diseases. Healthcare (Basel) 2019; 7 pii: E34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. de Assumpcao PP, Araujo TMT, de Assumpcao PB, et al. Suicide journey of H. pylori through gastric carcinogenesis: the role of non-H. pylori microbiome and potential consequences for clinical practice. Eur J Clin Microbiol Infect Dis 2019; 38: 1591–1597. [DOI] [PubMed] [Google Scholar]
- 56. Doorakkers E, Lagergren J, Engstrand L, et al. Reply to: Helicobacter pylori eradication treatment and the risk of gastric adenocarcinoma in a Western population. Gut. Epub ahead of print 21 May 2019. DOI: 10.1136/gutjnl-2019-319000. [DOI] [PubMed] [Google Scholar]
- 57. He C, Peng C, Wang H, et al. The eradication of Helicobacter pylori restores rather than disturbs the gastrointestinal microbiota in asymptomatic young adults. Helicobacter 2019; 24: e12590. [DOI] [PubMed] [Google Scholar]
- 58. Cancer Genome Atlas Research Network. Comprehensive molecular characterization of gastric adenocarcinoma. Nature 2014; 513: 202–209. [DOI] [PMC free article] [PubMed] [Google Scholar]