Abstract
Alzheimer’s disease (AD) is the most common neurodegenerative disease and its diagnosis has classically been based on clinical symptoms. Recently, a biological rather than a syndromic definition of the disease has been proposed that is based on biomarkers that reflect neuropathological changes. In AD, there are two main biomarker categories, namely neuroimaging and fluid biomarkers [cerebrospinal fluid (CSF) and blood]. As a complex and multifactorial disease, AD biomarkers are important for an accurate diagnosis and to stage the disease, assess the prognosis, test target engagement, and measure the response to treatment. In addition, biomarkers provide us with information that, even if it does not have a current clinical use, helps us to understand the mechanisms of the disease. In addition to the pathological hallmarks of AD, which include amyloid-β and tau deposition, there are multiple concomitant pathological events that play a key role in the disease. These include, but are not limited to, neurodegeneration, inflammation, vascular dysregulation or synaptic dysfunction. In addition, AD patients often have an accumulation of other proteins including α-synuclein and TDP-43, which may have a pathogenic effect on AD. In combination, there is a need to have biomarkers that reflect different aspects of AD pathogenesis and this will be important in the future to establish what are the most suitable applications for each of these AD-related biomarkers. It is unclear whether sex, gender, or both have an effect on the causes of AD. There may be differences in fluid biomarkers due to sex but this issue has often been neglected and warrants further research. In this review, we summarize the current state of the principal AD fluid biomarkers and discuss the effect of sex on these biomarkers.
Keywords: Alzheimer’s disease, biomarkers, blood, cerebrospinal fluid, gender, neurodegeneration, sex
Introduction
Alzheimer’s disease (AD) accounts for 60–80% of all cases of dementia worldwide. There are some rare familial AD cases that are caused by inherited mutations in the APP, PSEN1, and PSEN2 genes, but most cases are ‘sporadic’ or late-onset. The main risk factors for late-onset AD are aging and carrying the allele ε4 of Apolipoprotein E (APOE-ε4).1
According to the latest guidelines of the National Institute of Aging and Alzheimer Association (NIA-AA),2 the term ‘Alzheimer’s disease’ is applied whenever there is biomarker evidence of pathological deposits of the amyloid-β peptide (Aβ plaques) and tau neurofibrillary tangles (NFTs). The AT(N) classification summarizes biomarkers into three groups, ‘A’ refers to aggregated amyloid-β (Aβ), ‘T’ refers to aggregated tau, and ‘N’ refers to neurodegeneration.3 Currently, the most widely accepted biomarkers for each of the categories are amyloid positron emission tomography (PET), cerebrospinal fluid (CSF) Aβ42 and CSF Aβ 42/40 (A), tau PET and CSF phosphorylated tau (p-tau) (T), and structural magnetic resonance imaging, fluorodeoxyglucose (FDG) PET, CSF total tau (t-tau), and neurofilament light (NfL) (N).
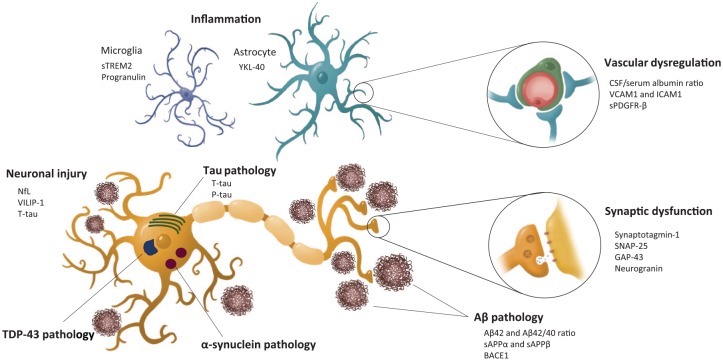
Although Aβ and tau deposits define AD pathology, a significant degree of copathology is common in patients with AD. In addition, multiple pathological processes co-occur in AD, including inflammation and innate immune response, synaptic and vascular dysfunction, or blood-brain barrier (BBB) impairment (Figure 1). Successful treatment of AD may require the targeting of several pathological processes. Therefore, there is a clear need for suitable biomarkers to track pathological processes and detect concomitant pathologies, diagnose more accurately, assess prognosis, monitor treatment response, test target engagement, and to stratify participants in research studies and clinical trials. CSF biomarkers are widely used in research and clinical practice but lumbar puncture, despite being a well tolerated procedure with a very low rate of adverse effects, is still an invasive procedure.4 Therefore, increasing efforts have been made in recent years to develop biomarkers in more accessible biological matrices including urine, saliva, and blood.5,6 The development of blood biomarkers has become a priority in the AD field and several new developments have occurred over the last few months.
Figure 1.
Pathological events in Alzheimer’s disease and their corresponding fluid biomarkers.
Aβ, β-amyloid; BACE1, β-site amyloid precursor protein cleavage enzyme 1; GAP-43, Growth-associated protein 43; ICAM-1, intercellular adhesion molecule-1; NfL, neurofilament light; p-tau, phosphorylated tau; sAPP, soluble N-terminal fragment of APP; SNAP-25, synaptosome-associated protein 25; sPDGFR-β, soluble platelet-derived growth factor receptor-β; sTREM2, soluble triggering receptor expressed on myeloid cells 2; TDP-43, transactive response DNA-binding protein 43; t-tau, total tau; VCAM-1, vascular cell adhesion molecule-1; VILIP-1, visinin-like protein 1; YKL-40, chitinase 3-like protein 1.
In this review, we will examine the current status of AD biomarkers in CSF and blood (Table 1 and Figure 1). Because recent evidence suggests that sex, gender, or both can have an influence on different aspects of AD, we also briefly discuss their effects on fluid biomarkers.
Table 1.
Summary of the main AD fluid biomarkers.
| Change in AD versus
controls |
|||
|---|---|---|---|
| Pathological mechanism | Biomarker | CSF | Serum/plasma |
| Aβ pathology | Aβ42 or Aβ42/40 | ↓ | ↓ |
| BACE1 | ≈ | ↑ | |
| sAPPα and sAPPβ | ≈ | ○ | |
| Tau pathology | t-tau | ↑ | ↑ |
| p-tau | ↑ | ↑ | |
| Neuronal injury | NfL | ↑ | ↑ |
| VILIP-1 | ↑ | ↑ | |
| Synaptic dysfunction | Neurogranin | ↑ | = |
| GAP-43 | ↑ | ○ | |
| Synaptotagmin-1 | ↑ | ↓ | |
| SNAP-25 | ↑ | ○ | |
| Vascular dysregulation | CSF/serum albumin ratio | NA | ↑ |
| VCAM1/ICAM1 | ↑ | ↑ | |
| sPDGFR-β | ○ | ○ | |
| Inflammation | sTREM2 | ↑ | = |
| Progranulin | ≈ | ≈ | |
| YKL-40 | ≈ | = | |
| IP-10 | ≈ | ≈ | |
| GFAP | ≈ | ↑ | |
| MCP-1 | = | ≈ | |
| TDP-43 pathology | TDP-43 | ≈ | ↑ |
| α–synuclein pathology | α-synuclein | ≈ | = |
Overview of the main AD-related fluid biomarkers. The table depicts the changes observed in AD (prodromal, dementia, or both) compared with controls as follows: ↑ increased or ↓ decreased levels in most or all studies; = no changes; ≈ inconsistent results; ○ unknown; NA not applicable. To complete these table, we reviewed all studies available in AlzBiomarker (https://www.alzforum.org/alzbiomarker) for GFAP, MCP-1, neurogranin, NfL, sAPPα, sAPPβ, sTREM2, VILIP-1, YKL-40. For Aβ, t-tau, and p-tau we reviewed those studies in AlzBiomarker with a total number of patients >300. We also included other biomarkers not available in AlzBiomarker that we, however, found relevant for AD.
Aβ, β-amyloid; BACE1, β-site amyloid precursor protein cleavage enzyme 1; CSF, cerebrospinal fluid; GAP-43, Growth-associated protein 43; GFAP, Glial fibrillary acidic protein; ICAM-1, intercellular adhesion molecule-1; IP-10, interferon-inducible protein-10; MCP-1, Monocyte chemoattractant protein-1; NA, not applicable; NfL, neurofilament light; p-tau, phosphorylated tau; sAPP, soluble N-terminal fragment of APP; SNAP-25, synaptosome-associated protein 25; sPDGFR-β, soluble platelet-derived growth factor receptor-β; sTREM2, soluble triggering receptor expressed on myeloid cells 2; TDP-43, transactive response DNA-binding protein 43; t-tau, total tau; VCAM-1, vascular cell adhesion molecule-1; VILIP-1, visinin-like protein 1; YKL-40, chitinase 3-like protein 1.
Aβ pathology
Aβ peptides are generated physiologically by the sequential cleavage of the amyloid-β precursor protein (APP), a type 1 transmembrane protein. APP can be cleaved by two pathways. The amyloidogenic pathway comprises the cleavage by the β-secretase enzyme (β-site APP-cleaving enzyme 1, BACE1) generating the β soluble N-terminal fragment of APP (sAPPβ) and the APP carboxy-terminal fragment (βCTF). βCTF is subsequently cleaved in the membrane by γ-secretase and results in Aβ peptides of variable lengths and the amyloid precursor intracellular domain (AICD). The 40-amino acid form (Aβ40) is the predominant type in the brain in physiological conditions, but the 42-amino acid form (Aβ42) is the predominant type in amyloid plaques.7 The AICD may act as a transcriptional regulator of several target genes and is thought to contribute to AD pathology.8,9 The nonamyloidogenic pathway comprises the cleavage by α-secretase resulting in the α soluble N-terminal fragment of APP (sAPPα) and the APP carboxy-terminal fragment (αCTF). α CTF is cleaved by γ-secretase that generates a p3 fragment and an AICD fragment.7,10,11 Of note, Aβ pathology is highly associated with the APOE genotype. Individuals carrying APOE-ε4 develop more amyloid plaques when they age.12,13 The APOE-ε4 allele decreases the brain clearance of Aβ, triggering its aggregation.14 In addition, the APOE-protein can bind Aβ, but its affinity differs between the three APOE isoforms. Thus, it is important to interpret amyloid-related biomarkers in the context of the APOE genotype.15,16
Aβ peptides
CSF Aβ42, in combination with t-tau and p-tau, constitutes the widely accepted CSF signature for AD diagnosis. CSF Aβ42 has a high diagnostic accuracy to discriminate between AD and controls and, in addition, it is helpful for distinguishing AD from other neurodegenerative diseases. More importantly, CSF Aβ42 allows us to identify AD in its preclinical stage. CSF Aβ42 accurately predicts disease progression in cognitively unimpaired individuals and in those with mild cognitive impairment (MCI).17 In contrast with amyloid PET, CSF Aβ42 plateaus early in the disease progression.18,19 Despite the high agreement between amyloid PET and CSF Aβ42, there are some conflicting results that may be due to the fact that CSF Aβ42 reflects a biological process preceding the aggregation of Aβ detected with amyloid PET.20 Recently, optimal centiloid cut-offs have been defined maximizing the agreement between the PET and CSF levels of Aβ42.21
However, the use of CSF Aβ42 can be limited by the impact of pre-analytical factors and by interindividual differences in Aβ production. These limitations can be partially overcome by normalizing Aβ42 values with Aβ40. Aβ40 does not change in AD and its concentration in CSF is 10-times higher than that of CSF Aβ42 and can, therefore, be used as a ‘proxy’ of total Aβ levels. The CSF Aβ42/Aβ40 ratio appears to be a better predictor of amyloid PET positivity in prodromal AD and performs better at distinguishing AD from other dementias than CSF Aβ42 alone.22,23 Similar to CSF Aβ40, CSF Aβ38 does not vary between AD and controls and the CSF Aβ42/Aβ38 ratio shows a comparable predictive potential to that of the CSF Aβ42/Aβ40 ratio.22
Increasing efforts are focusing on the measurement of blood Aβ. Most previous studies demonstrated inconsistent results and a lack of correlation between CSF and blood Aβ.24–26 That was probably due to the fact that Aβ concentration in blood is low27 and Aβ measurements were affected by matrix effects. However, recent studies using ultrasensitive assays are very promising. Aβ has been measured in blood using the Simoa platform,28,29 immunoprecipitation coupled with mass spectrometry (IP-MS)30–32 and stable isotope labeling kinetics followed by IP-MS.33. These studies have shown that blood Aβ42, Aβ42/Aβ40 ratio, or both, correlate with those in CSF and with amyloid PET.31–33 They have also shown that Aβ42, Aβ42/Aβ40 ratio, or both differ between AD and controls or other diagnostic groups, although the magnitude of the difference is smaller than that found in CSF. The xMAP technology has been used to measure Aβ42/Aβ40. While a study demonstrated that Aβ42/Aβ40 could not predict AD,34 other studies showed that pretreatment of plasma with a mixture of protease and phosphatase inhibitors may help to detect amyloid positive individuals.35,36 Despite these promising results, data presented at the Alzheimer’s Association International Conference (2019) showed that the different technologies used to measure blood Aβ have a poor correlation between them. This warrants further investigation to clarify whether these methods are measuring different Aβ pools or whether there are other technical issues involved.
Aβ oligomers have been measured in CSF and blood using different techniques including ELISA, single-molecule fluorescence microscopy, and protein misfolding cyclic amplification assays. However, results have not been consistent due to multiple methodological difficulties.37
Other Aβ pathology biomarkers
Soluble fragment sAPPα or sAPPβ can be readily measured in CSF, but results are conflicting with regards to differences between AD and controls, or between progressive MCI and stable MCI.38–41 However, sAPPβ may be used as a target engagement biomarker of BACE inhibitors. BACE1 activity, or its protein levels, can be measured in CSF but there are some inconsistencies in the results for AD patients. Most studies showed an increase on BACE1 (either activity or protein levels) in AD compared with controls.41–43 In addition, patients with MCI appear to have higher levels of BACE1 activity and protein levels compared with controls or AD patients, and BACE1 has been shown to be a good progression marker in MCI patients.44 However, other studies have reported different results, including no differences in BACE1 activity between controls, MCI and AD, or even decreased CSF levels in AD compared with controls.45,46 Probably, the main advantage of measuring BACE1 is as a biomarker of target engagement for BACE1 inhibitors. Available studies testing plasma BACE1 have shown inconsistent results regarding the potential of elevated BACE1 to differentiate AD patients from controls and to predict progression to AD among MCI patients.47
Tau biomarkers
NFTs containing hyperphosphorylated tau are, in combination with Aβ plaques, the pathological hallmarks of AD pathology.48,49 Tau inclusions, however, are found in a wide range of neurodegenerative diseases including progressive supranuclear palsy (PSP), corticobasal degeneration (CBD), some types of frontotemporal lobar degeneration (FTLD-tau), and chronic traumatic encephalopathy. All diseases with tau deposits are collectively known as ‘tauopathies’ and they differ in the isoform of tau and phosphorylations present in the aggregates.50
t-tau and p-tau
It has been very well established that CSF t-tau and p-tau levels are increased in AD compared with controls. CSF t-tau is considered to be a marker of the intensity of neuronal injury and neurodegeneration in AD. Therefore, t-tau has a clear diagnostic value for differentiating AD from normal aging but it can be elevated in a number of other neurological diseases. Of note, however, t-tau does not equally increase in all neurological diseases. It dramatically increases in Creutzfeldt–Jacob disease (CJD), and in acute stroke51 but, in contrast, it only marginally increases (or it does not change or can decrease) in other neurodegenerative diseases including Parkinson’s disease (PD), FTLD, PSP, and CBD.52 It has been previously suggested that the increased t-tau in CSF was a result of leakage from damaged neurons. However, this theory has been recently challenged and it has been demonstrated that there is an active secretion of tau that may differ between physiological and pathological conditions and it is especially sensitive to underlying Aβ pathology.53,54 In addition, it is important to note that t-tau is measured using antibodies against the mid-domain, and there are other truncated tau forms in CSF that are being investigated.
In contrast, CSF p-tau is considered to be a biomarker of tau deposition in AD. In fact, CSF p-tau appears normal or only slightly increased in most of the other neurological diseases. Thus, CSF p-tau is a more specific biomarker for AD. In the recently published AT(N) system,2 CSF t-tau and p-tau, account for neurodegeneration (N) and tau pathology (T), respectively. However, these two biomarkers are highly correlated and it is difficult to determine which component of each biomarker reflects neurodegeneration and tau pathology. Phosphorylation at threonine 181 (p-tau181) is the most widely used target as a p-tau biomarker in the clinical setting and increases very early in the AD continuum, even before tau PET becomes positive. Other tau species are increased in AD, including those phosphorylated in other mid-domain residues (threonine 231, serine 199, and 231) and C-terminal residues (Serine 396 and 404).55 Of note, the abundance of several other phosphorylation sites in tau differs in the brain and CSF of individuals with and without AD.56
In addition to being validated diagnostic markers of AD, CSF t-tau and p-tau are useful biomarkers to stage the disease, predict its prognosis, and monitor drug response. Tau deposition follows disease progression more effectively and has a stronger correlation with cognitive status than Aβ.57,58 However, longitudinal studies have shown that CSF t-tau and p-tau, and other neurodegeneration markers, may decrease in advanced stages of the disease. CSF t-tau and p-tau have been shown to be good predictors of progression from cognitively unimpaired to MCI and progression to AD dementia.17 CSF t-tau and p-tau are normally monitored in clinical trials, but the association between these biomarkers and the therapeutic response remains unclear.
Similarly to blood Aβ, the introduction of ultrasensitive immunoassays now allows the reliable measurement of tau in blood. Before this, most studies showed considerable variability in results.26 Using the ultrasensitive techniques, several groups have shown an increase in blood t-tau in AD compared with controls,59–62 but the overlapping values between groups precludes their use as a diagnostic tool. In addition, plasma t-tau predicts cognitive decline and the risk of dementia.59,60,63 The measurement of blood p-tau is technically challenging but promising results are being produced. Higher plasma or serum p-tau181 have been described in AD versus controls using different platforms, including MSD,64 Simoa,65 label-free real time surface plasmon resonance technology,66 and immunomagnetic reduction (IMR).67
Neuronal injury
NfL
Neurofilaments are intermediate filaments that are abundant in neuronal axons. Among the neurofilaments, NfL has the most promising results as a biomarker. NfL leaks into the CSF after neuronal and axonal damage. CSF NfL increases in multiple neurological diseases and is widely accepted as a nonspecific biomarker of axonal injury.68–70 Of note, this increase is more prominent in the neurological diseases where there is axonal degeneration, white matter injury, or both, including amyotrophic lateral sclerosis (ALS), FTLD, and CJD.
The development of ultrasensitive techniques enables the accurate measurement of NfL in blood. Blood NfL levels closely correlate with those of CSF.71,72 Therefore, blood NfL can be considered to be a noninvasive proxy of CSF NfL. Plasma or serum NfL levels are increased in AD and MCI compared with controls and in other neurological diseases.72–75 More importantly, blood and CSF NfL levels increase many years before the symptom onset in autosomal-dominant AD,76–78 and in Down syndrome.79 In addition, CSF and blood NfL are associated with disease severity markers including brain atrophy, glucose hypometabolism, and cognitive impairment, which favors its use as a disease staging biomarker.71,80 Finally, increasing agreement favors the use of NfL, instead of t-tau, as an independent marker of neurodegeneration ‘N’ in the AT(N) classification for AD.3
Visinin-like protein 1
Visinin-like protein 1 (VILIP-1) is a calcium sensor protein that is highly expressed in neurons.81 Intracellular VILIP-1 expression is decreased in AD, especially in the entorhinal cortex.82 VILIP-1 in CSF is correlated with t-tau and p-tau, supporting the theory that VILIP-1 is a neuronal injury biomarker.83 Most studies, except one84 have shown that VILIP-1 is increased in AD compared with controls,26,83,84. Further studies have suggested that it could help to differentiate AD from other dementias.85–87 In addition, VILIP-1 has been shown to predict cognitive impairment and atrophy rates and to identify the MCI individuals that will progress to AD dementia.84,87–89 Longitudinal measurements in late-onset AD and autosomal-dominant AD have shown that the initial increase in CSF VILIP-1 is ameliorated as dementia develops, similar to what happens with other neural injury markers including CSF t-tau.90,91 Studies on blood VILIP-1 are limited, but a significant increase in plasma VILIP-1 has been reported in AD compared with controls.87 In autosomal-dominant AD mutation carriers, VILIP-1 was also found to be increased but this finding did not not reach statistical significance.90
Synaptic dysfunction
Synapse dysfunction and eventual synapse loss is an early process in AD pathogenesis.92,93 Pathological studies have demonstrated that synapse loss is closely associated with cognitive impairment.94,95 Synaptic biomarkers can be classified as presynaptic (axonal) and postsynaptic (dendritic) biomarkers. The main presynaptic biomarkers are synaptotagmin-1, synaptosomal-associated protein 25 (SNAP-25), and growth-associated protein 43 (GAP-43), and the main postsynaptic biomarker is neurogranin.
Synaptotagmin-1
Synaptotagmin-1 is a calcium sensor protein located in the presynaptic plasma membrane that is involved in the exocytosis of synaptic vesicles and the release of neurotransmitters.96 Synaptotagmin-1 was first detected in the CSF in the late 1990s.97 A later study, using mass spectrometry, showed that CSF synaptotagmin-1 was increased in both MCI and dementia due to AD. Of interest, higher levels were found in MCI due to AD.98
SNAP-25
SNAP-25 is a component of the SNAP receptor (SNARE) complex located in the synaptic vesicles where it plays a key role in their exocytosis.99 CSF SNAP-25 is significantly increased in prodromal and AD dementia compared with controls.100 To date, to the best of our knowledge, no data is available on blood SNAP-25 in AD.
GAP-43
GAP-43 is a presynaptic protein involved in neuronal development and synaptogenesis in the adult brain. It is mainly expressed in the hippocampus, entorhinal cortex, neocortex, cerebellum, and olfactory bulb.101,102 CSF GAP-43 is increased in AD patients compared with controls and, importantly, compared with other neurodegenerative diseases. Therefore, it is a potential specific biomarker for AD-associated synaptic dysfunction.103,104 There is a lack of studies on blood GAP-43 in AD.
Neurogranin
Neurogranin is a postsynaptic protein that is highly expressed in the dendritic spines of the hippocampus, amygdala, caudate, and putamen. It binds the calcium-binding protein calmodulin (CaM) and regulates calcium signaling and synaptic plasticity.105 CSF neurogranin is increased in AD and MCI. It is also increased in progressive compared with stable MCI, and predicts cognitive decline in cognitively unimpaired individuals.26,68,106–108 More importantly, CSF neurogranin is specific for AD, reinforcing the view that synapses, in particular, are affected in AD. In addition, high levels of CSF neurogranin are found in CJD.109 Unexpectedly, a study in the Alzheimer’s Disease Neuroimaging Initiative (ADNI) cohort found a longitudinal decrease of CSF neurogranin in AD patients.91 Studies of blood neurogranin are limited and they have not reported significant differences between AD and controls.26
Emerging synaptic biomarkers
Synaptic markers are a current area of intensive research in the AD field and some interesting results are being produced. Among the most promising synaptic markers are the neural pentraxin 2 (NPTX2) and the synaptic vesicle glycoprotein 2A (SV2A). NPTX2 is specifically localized in excitatory synapses and binds the AMPA glutamate receptors. CSF NPTX2 decreases in AD patients and is correlated with cognitive status and hippocampal volume.110 Individuals with lower levels of CSF NPTX2 have a faster cognitive decline. SV2A is the specific target for the antiepileptic drug levetiracetam and PET tracers that bind SV2A have been developed. CSF SV2A decreases in AD and FTLD, but not in Lewy body dementia or vascular dementia (VaD), and is inversely correlated with CSF p-tau and t-tau and cognitive function (Nicholas Ashton; Alzheimer’s Association International Conference 2019). In addition, proteomic analyses of CSF have identified new synaptic proteins that are altered in the very early stages of the disease,111 or that differ between individuals that have a fast cognitive decline and those that are cognitively stable.112
Vascular dysregulation
Cerebrovascular disease and AD share multiple risk factors and concomitant cerebrovascular disease is more frequent in AD than in any other neurodegenerative disease.113 In fact, vascular dysregulation has been proposed as a causative factor for AD114 and, in addition, recent studies have proposed that vascular dysfunction is the earliest event in the sequence of pathogenic events that occur in AD.115,116
Alterations in the integrity of the blood-brain barrier (BBB) are related to the pathogenicity of AD and other dementias.117,118 From the fluid biomarker perspective, the CSF/serum albumin ratio has been the standard measure of BBB function in clinical routine practice.119,120 However, measurement of CSF/serum albumin ratio in AD has produced conflicting results and the meta-analysis by Olsson and colleagues found a minor but statistically significant increase in AD compared with controls.26 A large population study from the Swedish Dementia Registry observed an increase in the CSF/serum albumin ratio in late-onset AD that was even higher in DLB, VaD, and mixed dementia. The CSF/serum albumin ratio was associated with CSF NfL but not with AD CSF core biomarkers, suggesting that BBB damage is not a specific feature of AD.121 Other biomarkers that have been proposed to reflect BBB integrity include tight junction proteins, the proinflammatory cytokine CypA, MMP-9, and blood-derived fibrinogen and plasminogen.122 In addition, the CSF soluble platelet-derived growth factor receptor-β (sPDGFR-β) has been proposed as a marker of pericyte breakdown.117,123
The endothelial markers, vascular cell adhesion molecule-1 (VCAM-1), and intercellular adhesion molecule-1 (ICAM-1) have been studied in AD. Previous investigations of CSF and blood VCAM-1, and ICAM-1 have produced conflicting results, but a recent study found that the CSF levels of these biomarkers were increased in preclinical, prodromal, and dementia stages of AD, and correlated with CSF tau, Aβ, cortical thinning, and subsequent cognitive deterioration in nondemented patients.124 In addition, the same study found that an increase in CSF IL-15 and Flt-1, which are proteins also related to vascular dysfunction and inflammation. Blood levels of VCAM-1 are increased in AD and vascular dementia patients compared with elderly controls.125,126
Inflammation
Inflammation and innate immunity play a crucial role in AD pathogenesis from the very early stages.127–129 Misfolded and aggregated Aβ and tau proteins bind to pattern recognition receptors on microglia and astroglia and trigger an innate immune response.127 This inflammatory response is heterogeneous and may have a beneficial effect (e.g. clearance of Aβ and tau deposits, removal of cellular debris)130 or, in contrast, a detrimental one (e.g. accelerated neurodegeneration).131 In addition, the pattern of neuroinflammatory response may change throughout the AD continuum, exerting opposite effects at different stages.132 Several inflammatory-related biomarkers are available but only help to determine partial aspects of the complex innate immune response. Thus, there is a need to develop and validate biomarkers, or combinations of them, that reflect different patterns of inflammatory response along the Alzheimer’s continuum. Unfortunately, results on inflammatory-related biomarkers are inconsistent between studies.133,134 In this study we review the most promising ones.
Soluble triggering receptor expressed on myeloid cells 2
Triggering receptor expressed on myeloid cells 2 (TREM2) is an innate immune receptor of the immunoglobulin family that is expressed on the plasma membrane of myeloid lineage cells, including microglial cells in the central nervous system (CNS). TREM2 has multiple roles in microglia including migration, proliferation, cytokine release, phagocytosis, lipid sensing, APOE binding, and shielding of amyloid plaq- ues.135–141 Homozygous loss-of-function mutations in TREM2 cause Nasu–Hakola disease and FTD-like syndrome, that are rare neurodegenerative diseases characterized by an early-onset frontal syndrome and, in Nasu–Hakola disease, by a concomitant bone involvement.142,143 Low frequency coding variants in TREM2 have also been associated with an increased risk for AD144,145 and other neurodegenerative diseases. TREM2 is a type-1 transmembrane protein that is shed at the C-terminus of histidine 157 by ADAM10 and 17, and its soluble ectodomain (sTREM2) is released into the extracellular space138,146 and can be measured in CSF and blood.138,147 CSF sTREM2 may reflect the amount of TREM2 competent signaling on the surface of microglia and can, therefore, be used as a marker of the TREM2-mediated microglia response. However, to the best of our knowledge, it is still not known whether sTREM2 has a specific biological function other than acting as a decoy receptor opposing full-length TREM2 signaling.
Most studies have reported increased levels of CSF sTREM2 in AD versus controls,128,148–155 although this was not found in all studies.138,156 Further studies have demonstrated that CSF sTREM2 dynamically changes throughout the AD continuum and reaches a peak in the later asymptomatic stages and early symptomatic stages of late-onset AD and autosomal-dominant AD.128,150,151,155 CSF sTREM2 is closely associated with tau-related neurodegeneration but not with Aβ pathology.150 In addition, CSF sTREM2 increases in MS and other neuroinflammatory diseases,147,157 HIV1 infections,158 in dementia patients with delirium,159 and in those patients with a biomarker profile for suspected non-Alzheimer pathology (SNAP).150,151 This suggests that a TREM2-mediated microglial response occurs whenever there is neural injury, and not only in AD. Of note, CSF sTREM2 normalizes in MS after treatment with natalizumab or mitoxantrone,157 which indicates that CSF sTREM2 may be used to test treatment response. In contrast, CSF sTREM2 has not been shown to be increased in HD,160 FTLD,138,161 or in repetitive head impacts.162
Despite the changes found in CSF sTREM2 in AD, there is a considerable overlap in the values between AD and controls that precludes its use as a diagnostic marker. However, there is one situation where sTREM2 may be useful for diagnostic purposes. Both CSF and blood sTREM2 are undetectable in patients with Nasu–Hakola disease and FTD-like syndrome.138,156 Individuals bearing low frequency TREM2 coding variants, in contrast, may have increased, decreased, or unchanged sTREM2 levels compared with noncarriers of these variants.148,150,153,163
A major question in this field is whether TREM2 has a beneficial or a detrimental effect. Human neuroimaging cross-sectional and longitudinal studies have shown that higher CSF sTREM2 is associated with increased gray matter volume and reduced diffusivity in early AD.164,165 More importantly, a recent longitudinal study in the ADNI cohort has demonstrated that in individuals with biomarker evidence of amyloid and tau pathology, regardless of the clinical syndrome, higher CSF sTREM2 at baseline is associated with an attenuated decline in memory and global cognition at follow-up.130 In addition, common variants of the MS4A gene are associated with increased CSF sTREM2 concentrations. Of interest, these variants are additionally associated with reduced AD risk and delayed age at onset of disease.163 Altogether, this suggests a beneficial effect of TREM2 function.
In contrast with CSF sTREM2, blood sTREM2 has been considerably less studied. No changes have been found in plasma or serum sTREM2 in MS, neuroinflammatory diseases, AD, FTD, or PD.138,147,148,153 Of interest, higher serum sTREM2 levels predict a higher risk of developing dementia in the Japanese population.166
Progranulin
Progranulin is a secreted glycoprotein mainly expressed in activated microglia and, to a lesser extent, in neurons in the CNS. It contains 7.5 tandem repeats, that form the granulin domains. Proteolytic processing of full-length progranulin leads to the formation of individual granulins that are released into the extracellular space.167,168 Among other biological functions, progranulin and the granulins are involved in the modulation of neuroinflammation. Progranulin is best known for its relation to FTLD because loss-of-function mutations of the progranulin gene (GRN) cause FTLD-TDP. GRN mutation carriers have decreased blood and CSF progranulin levels. However, progranulin is related to AD because some GRN variants increase the risk for AD169,170 and studies in mice models have shown that progranulin has an impact in β-amyloid and tau pathology.171–173 In addition, these GRN variants have an effect on blood and CSF progranulin levels but to a lesser extent than the FTLD-related mutations.170,174,175
Unlike FTLD, most studies had reported no differences in CSF progranulin levels between AD, MCI, and controls.174–177 However, a larger cross-sectional study carried out in the ADNI cohort demonstrated that CSF progranulin increases over the course of autosomal-dominant AD and late-onset AD and is associated with cognitive decline and markers of neurodegeneration.178 Of note, higher CSF progranulin was associated with higher sTREM2 in AD but not in healthy controls.178 This suggests that, when there is underlying pathology, microglia secrete sTREM2 and PGRN which, interestingly, may have opposing effects.179 Results for blood progranulin are not very promising because no changes in AD have been found,175,180 and blood progranulin levels do not reflect progranulin levels in the brain.174 Overall, CSF and blood progranulin are only useful diagnostic markers to screen GRN mutations in FTLD, but not in AD.
YKL-40
YKL-40, which is also known as chitinase 3-like protein 1 (CHI3L1) or human cartilage glycoprotein 39 (hCGP-39) is upregulated in several inflammatory diseases and cancers and its biological function may be related to remodeling during inflammation.181 In the CNS there is an increasing amount of evidence suggesting that YKL-40 is predominantly an astroglial protein.181,182 In AD, CSF YKL-40 levels are associated with tau pathology and neuroimaging variables including cortical thickness in amyloid positive patients and gray matter volume in APOE-ε4 carriers.183–185
Several studies have found an increase in CSF YKL-40 in AD patients compared with controls and these results have been confirmed by a recent meta-analysis.26,183 CSF YKL-40 increases with disease progression as demonstrated in longitudinal studies91,186 and it is positively correlated with biomarkers of neurodegeneration. Some studies have even reported a significant increase of CSF YKL-40 in preclinical AD.39,185 CSF YKL-40 can predict progression from cognitively unimpaired to MCI183 and from MCI to AD dementia.84 Increases in CSF YKL-40 are not specific for AD and can be found in other neurological diseases.187–189 Of interest, CSF YKL-40 levels are unchanged or even decreased in PD without dementia.190–192
A few studies have assessed YKL-40 in blood. There may be a trend for elevated plasma YKL-40 in AD compared with controls.183,193 Overall, CSF YKL-40 might have a limited diagnostic value, but it can be useful for tracking astroglial activation, assessing response to treatments targeting neuroinflammation, and the identification of subgroups of patients or disease staging based on inflammatory processes.
Other inflammation markers
Several other inflammatory biomarkers for AD have been studied but with heterogeneous and inconsistent results.133 CSF human interferon-inducible protein-10 (IP-10) appears to increase in MCI and mild AD, although this is not consistent across studies.192,194 In addition, plasma IP-10 has shown conflicting results.115,195 Glial fibrillary acidic protein (GFAP) in CSF does not differ in AD compared with controls in most studies.26 However, a recent study found increased CSF and serum GFAP in AD patients and a significant correlation between serum GFAP and cognitive decline.196 Monocyte chemoattractant protein-1 (MCP-1) is slightly elevated in CSF of AD patients, and no differences are found in the blood.26 Other inflammatory markers have been reported to be elevated in AD.133,197 In contrast, serum sirtuin1 decreases with aging, making this reduction more pronounced in AD patients.198 Sirtuin1, a NAD-dependent protein deacetylase nuclear receptor, has been proposed as a regulator of aging, metabolic and inflammatory processes involved in AD.199–201 Overall, most AD-related inflammatory biomarkers do not demonstrate a clear diagnosis potential, but they can be useful in tracking different patterns of inflammatory response to AD pathology.
TDP-43 pathology
Transactive response DNA-binding protein of 43 kDa (TDP-43) is a DNA and RNA-binding protein that is found phosphorylated in the ubiquitin inclusions in most cases of ALS and in FTLD-TDP.202,203 Remarkably, TDP-43 deposits are found in elderly people and in 20–50% of patients with AD.204–209 TDP-43 copathology in AD is probably not a mere bystander but it is associated with memory loss and brain hippocampal atrophy.207,210,211 Recently, a new entity called ‘limbic-predominant age-related TDP-43 encephalopathy’ (LATE) has been described.212 The neuropathological changes of LATE are characterized by deposits of TDP-43 in the amygdala, hippocampus, and middle frontal gyrus, and is sometimes accompanied by hippocampal sclerosis. LATE usually presents with an amnestic syndrome and can, therefore, mimic AD. A few studies have investigated TDP-43 in AD. Plasma TDP-43 levels may indicate the amount of TDP-43 pathology in AD213. Another study showed that plasma TDP-43 is increased in AD and progressive MCI.214 The availability of TDP-43 biomarkers would not only be useful to diagnose ALS and FTLD-TDP but also to detect the TDP-43 pathology burden in AD. They would help to discriminate between LATE and other neurodegenerative diseases. However, the use of TDP-43 as a biomarker is restricted by the technical difficulties to specifically detect the protein.215
α-synuclein pathology
α-synuclein is the major component of Lewy bodies and Lewy neurites, which are intracellular inclusions that constitute the pathological hallmark of PD and DLB.216 In addition, α-synuclein aggregates can be found in multiple system atrophy (MSA), predominantly in oligodendrocytes and neurons.217 In combination, PD, DLB, and MSA are referred to as α-synucleinopathies. Of interest, over half of AD patients (including late-onset AD, autosomal-dominant AD, and Down syndrome) have Lewy pathology, especially in the amygdala.218,219 The pathological contribution of α-synuclein in AD is unclear, but it may promote β-amyloid and tau aggregation.220,221 CSF α-synuclein has mainly been studied as a biomarker for α-synucleinopathies and most studies have observed a decrease in CSF α-synuclein levels in PD and DLB compared with controls or AD.222–225 In AD, CSF α-synuclein is slightly higher compared with controls.26,226 CSF α-synuclein is higher in those MCI patients that rapidly progress to AD.227 Of interest, a set of AD patients have high CSF p-tau but low CSF α-synuclein, and it has been speculated that this may reflect AD patients with Lewy bodies. In CJD, CSF α-synuclein is significantly increased.222,228 In blood, no differences have been found in plasma α-synuclein between AD and controls,229,230 but one study observed that plasma α-synuclein increased in PD compared with controls.231 Of note, most studies on α-synuclein have measured the full-length protein and research is being carried out to detect α-synuclein fragments and oligomers in biological fluids. It is also important to note that α-synuclein biomarkers may simply reflect age-related copathologies rather than specific AD pathologies.
Sex differences in fluid AD biomarkers
There is an increasing body of evidence that sex, gender, or both have an effect on the epidemiology, pathogenesis, clinical presentation, course, and treatment response in AD. This is, however, a complex topic because multiple social and biological factors are involved. We will focus on sex differences, although gender (i.e. social and cultural differences rather than biological) have an impact on AD.232,233 The prevalence of AD dementia is higher in women than in men234 and women appear to have a faster cognitive decline and brain atrophy rate once symptoms have started.235 Some AD risk factors (e.g. depression and sleep disorders) are more prevalent in women232,236 and APOE-ε4 confers a greater AD risk in women than men.237 In addition, early menopause is associated with a higher risk of cognitive decline and AD neuropathology.238,239 Thus, understanding the role of sex in AD is crucial to develop a precision medicine approach in AD.232
Although sex is generally included as a covariable in biomarker studies, only a few studies specifically focus on the effect of sex in biomarkers. Table 2 reviews the main differences on AD-related biomarkers between women and men. In most studies, no sex differences have been found in AD core biomarkers.240–244 However, a few studies found higher basal levels of t-tau in women compared with men in the whole cohort,243 or solely in the AD group,245 which is consistent with the fact that women have higher levels of tau pathology compared with men in pathological and PET studies.246,247 In addition, the association between APOE-ε4 and CSF t-tau and p-tau is stronger in women than in men.240,243 Although most studies did not find differences in Aβ42 between women and men, one study demonstrated a modifying effect of gender and APOE on the evolution of CSF Aβ42 linked to age. Specifically, women carrying an APOE-ε4 allele have stable CSF Aβ42 levels until the age of 50 years old but they then show a faster decline.242
Table 2.
Sex differences in AD-related fluid biomarkers.
| CSF | Plasma/Serum | |||
|---|---|---|---|---|
| Aβ42 or Aβ42/40 | F = M | No differences found88,240–244 | ○ | unknown |
| sAPPα and sAPPβ | F ⩾ M | Most studies have not assessed it. One study found higher levels in AD women.248 | ○ | unknown |
| t-tau | F ⩾ M | Most studies have not reported differences. A few showed higher levels in women (controls, MCI, and AD)243,245 | F = M | Most studies have not assessed it. One study reported no differences59 |
| p-tau | F = M | Most studies have not reported differences240–243,249 | ○ | unknown |
| NfL | F ⩽ M | Most studies have not assessed it. A few had reported no differences,69,250 but a recent study found higher levels in men.186 | F = M | Only a few studies available and no differences found72,78 |
| VILIP-1 | F = M | Most studies have not assessed it. A study reported no sex differences in controls and AD88 | ○ | unknown |
| VCAM1 | F ⩽ M | Most studies have not assessed it. A few have found higher levels in men.124,242 | F ⩽ M | One study found higher levels in men.125. |
| ICAM1 | F ⩽ M | Most studies have not assessed it. One study found higher levels in men171 | ○ | unknown |
| sTREM2 | F ⩽ M | Most studies have not reported differences.150,151 One study found higher levels in men (controls and AD)148 | F = M | Only one study available and did not find differences148 |
| Progranulin | F ⩽ M | A number of studies have reported higher levels in men (controls, MCI, and AD)174,175,178 | F ⩾ M | Very few studies available. One study found higher levels in women while another did not find differences174,175 |
| YKL-40 | F = M | A number of studies have assessed it and no differences have been reported183,186,251–253 | F = M | Few studies have assessed it and reported no differences183,193 |
| MCP-1 | F = M | Most studies have not assessed it. One study reported no differences251 | F = M | Most studies have not assessed it. A few have reported no differences194,254 |
| α -synuclein | F = M | Most studies have not assessed it. Few have reported no differences226,255,256 | ○ | unknown |
The table depicts the differences found in AD-related biomarkers between women and men in AD (prodromal, dementia, or both), the controls or both. ○ indicates unknown. We reviewed all studies available in AlzBiomarker for GFAP, MCP-1, neurogranin, NfL, sAPPα, sAPPβ, sTREM2, VILIP-1, YKL-40, and α -synuclein. For Aβ, t-tau, and p-tau we reviewed those studies in AlzBiomarker with a total number of individuals >300. CSF/Serum Albumin ratio, GFAP, and neurogranin are not included in the table because there is no data available on sex differences either in CSF or blood. In addition, we included biomarkers where there is evidence of sex differences.
Aβ, β-amyloid; CSF, cerebrospinal fluid; ICAM-1, intercellular adhesion molecule-1; MCP-1, Monocyte chemoattractant protein-1; NfL, neurofilament light; p-tau, phosphorylated tau; sAPP, soluble N-terminal fragment of APP; sTREM2, soluble triggering receptor expressed on myeloid cells 2; t-tau, total tau; VILIP-1, visinin-like protein 1; YKL-40, chitinase 3-like protein 1.
With regard to other biomarkers, the data available on sex differences is limited. A recent study including patients along the AD continuum found higher CSF NfL levels in men than in women.186 CSF sAPPα and sAPPβ have been reported to be higher in women with AD,248 and CSF E-selectin and ICAM1 and VCAM1 are higher in men.124,242 Inflammatory biomarkers are the markers that appear to be more influenced by sex. CSF progranulin is consistently higher in men than in women174,175,178 but, surprisingly, plasma or serum progranulin are higher in women. In addition, CSF sTREM2 may be slightly higher in men, although the difference is not as pronounced as in CSF progranulin.148
Conclusion
Overall, the effect of sex and gender on AD and, in particular, on AD fluid biomarkers, requires further research because it will help to improve the understanding of the disease and, especially, in the design of preventive and therapeutic strategies. The Women’s Brain Project and the Alzheimer Precision Medicine Initiative have recently published some recommendations to improve the design, analysis, and reporting of sex and gender differences in future studies.232
Acknowledgments
The authors would like to thank Elsa Velasco for the scientific illustrations included in this review. We also recognize and have heartfelt gratitude to Mrs Blanca Brillas for her outstanding and continued support to the Pasqual Maragall Foundation that makes possible a future without Alzheimer’s.
Footnotes
Funding: The authors received no financial support for the research, authorship, and/or publication of this article.
Conflict of interest statement: JLM received advisory honoraria from Alergan, Roche diagnostics, Genentech, Novartis, Lundbeck, Oryzon, Biogen, Lilly, Janssen, Green Valley, MSD, Eisai, Alector and Raman Health. He also received research support from the EU/EFPIA Innovative Medicines Initiative Joint Undertaking AMYPAD grant agreement n° 115952; the EU/EFPIA Innovative Medicines Initiative Joint Undertaking EPAD grant agreement n° 115736; and ‘la Caixa’ Foundation. MSC received funding from the European Union’s Horizon 2020 Research and Innovation Program under the Marie Sklodowska-Curie action grant agreement No 752310.
ORCID iD: José Luís Molinuevo  https://orcid.org/0000-0003-0485-6001
https://orcid.org/0000-0003-0485-6001
Contributor Information
Marta Milà-Alomà, Barcelonaβeta Brain Research Center (BBRC), Pasqual Maragall Foundation, Barcelona, Spain; IMIM (Hospital del Mar Medical Research Institute), Barcelona.
Marc Suárez-Calvet, Barcelonaβeta Brain Research Center (BBRC), Pasqual Maragall Foundation, Barcelona, Spain; IMIM (Hospital del Mar Medical Research Institute), Barcelona; Department of Neurology, Hospital del Mar, Barcelona.
José Luís Molinuevo, Scientific Director, Alzheimer’s Prevention Program, Barcelonaβeta Brain Research Center, Wellington 30, Barcelona, 08005, Spain; IMIM (Hospital del Mar Medical Research Institute), Barcelona; CIBER Fragilidad y Envejecimiento Saludable, Madrid, Spain; Universitat Pompeu Fabra, Barcelona, Spain.
References
- 1. Liu CC, Liu CC, Kanekiyo T, et al. Apolipoprotein E and Alzheimer disease: risk, mechanisms and therapy. Nat Rev Neurol 2013; 9: 106–118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Jack CR, Bennett DA, Blennow K, et al. NIA-AA research framework: toward a biological definition of Alzheimer’s disease. Alzheimers Dement 2018; 14: 535–562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Jack CR, Bennett DA, Blennow K, et al. A/T/N: an unbiased descriptive classification scheme for Alzheimer disease biomarkers. Neurology 2016; 87: 539–547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Engelborghs S, Niemantsverdriet E, Struyfs H, et al. Consensus guidelines for lumbar puncture in patients with neurological diseases. Alzheimers Dement 2017; 8: 111–126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Shi L, Baird AL, Westwood S, et al. A decade of blood biomarkers for Alzheimer’s disease research: an evolving field, improving study designs, and the challenge of replication. J Alzheimers Dis 2018; 62: 1181–1198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Zetterberg H. Blood-based biomarkers for Alzheimer’s disease: an update. J Neurosci Methods 2019; 319: 2–6. [DOI] [PubMed] [Google Scholar]
- 7. Haass C, Kaether C, Thinakaran G, et al. Trafficking and proteolytic processing of APP. Cold Spring Harb Perspect Med 2012; 2: a006270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Flammang B, Pardossi-Piquard R, Sevalle J, et al. Evidence that the amyloid-β protein precursor intracellular domain, AICD, derives from β-secretase-generated C-terminal fragment. J Alzheimers Dis 2012; 30: 145–153. [DOI] [PubMed] [Google Scholar]
- 9. Goodger ZV, Rajendran L, Trutzel A, et al. Nuclear signaling by the APP intracellular domain occurs predominantly through the amyloidogenic processing pathway. J Cell Sci 2009; 122: 3703–3714. [DOI] [PubMed] [Google Scholar]
- 10. Sastre M, Steiner H, Fuchs K, et al. Presenilin-dependent γ-secretase processing of β-amyloid precursor protein at a site corresponding to the S3 cleavage of Notch. EMBO Rep 2001; 2: 835–841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Lichtenthaler SF, Haass C, Steiner H. Regulated intramembrane proteolysis-lessons from amyloid precursor protein processing. J Neurochem 2011; 117: 779–796. [DOI] [PubMed] [Google Scholar]
- 12. Rebeck GW, Reiter JS, Strickland DK, et al. Apolipoprotein E in sporadic Alzheimer’s disease: allelic variation and receptor interactions. Neuron 1993; 11: 575–580. [DOI] [PubMed] [Google Scholar]
- 13. Morris JC, Roe CM, Xiong C, et al. APOE predicts amyloid-beta but not tau Alzheimer pathology in cognitively normal aging. Ann Neurol 2010; 67: 122–131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Castellano JM, Kim J, Stewart FR, et al. Human apoE isoforms differentially regulate brain amyloid-β peptide clearance. Sci Transl Med 2011; 3: 89ra57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Ashford JW, Salehi A, Furst A, et al. Imaging the Alzheimer brain. J Alzheimers Dis 2011; 26: 1–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Kim S, Swaminathan S, Shen L, et al. Genome-wide association study of CSF biomarkers Abeta1–42, t-tau, and p-tau181p in the ADNI cohort. Neurology 2011; 76: 69–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Ferreira D, Rivero-Santana A, Perestelo-Pérez L, et al. Improving CSF biomarkers performance for predicting progression from mild cognitive impairment to Alzheimers disease by considering different confounding factors: a meta-analysis. Front Aging Neurosci 2014; 6: 287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Blennow K, Zetterberg H, Minthon L, et al. Longitudinal stability of CSF biomarkers in Alzheimer’s disease. Neurosci Lett 2007; 419: 18–22. [DOI] [PubMed] [Google Scholar]
- 19. Toledo JB, Xie SX, Trojanowski JQ, et al. Longitudinal change in CSF Tau and Aβ biomarkers for up to 48 months in ADNI. Acta Neuropathol 2013; 126: 659–670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Palmqvist S, Mattsson N, Hansson O. Cerebrospinal fluid analysis detects cerebral amyloid-β accumulation earlier than positron emission tomography. Brain 2016; 139: 1226–1236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Salvadó G, Molinuevo JL, Brugulat-Serrat A, et al. Centiloid cut-off values for optimal agreement between PET and CSF core AD biomarkers. Alzheimers Res Ther 2019; 11: 27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Janelidze S, Zetterberg H, Mattsson N, et al. CSF Aβ42/Aβ40 and Aβ42/Aβ38 ratios: better diagnostic markers of Alzheimer disease. Ann Clin Transl Neurol 2016; 3: 154–165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Lewczuk P, Matzen A, Blennow K, et al. Cerebrospinal fluid Aβ42/40 corresponds better than Aβ42 to amyloid PET in Alzheimer’s disease. J Alzheimers Dis 2017; 55: 813–822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Hansson O, Zetterberg H, Vanmechelen E, et al. Evaluation of plasma Aβ40 and Aβ42 as predictors of conversion to Alzheimer’s disease in patients with mild cognitive impairment. Neurobiol Aging 2010; 31: 357–367. [DOI] [PubMed] [Google Scholar]
- 25. Lui JK, Laws SM, Li Q-X, et al. Plasma amyloid-β as a biomarker in Alzheimer’s disease: the AIBL study of aging. J Alzheimers Dis 2010; 20: 1233–1242. [DOI] [PubMed] [Google Scholar]
- 26. Olsson B, Lautner R, Andreasson U, et al. CSF and blood biomarkers for the diagnosis of Alzheimer’s disease: a systematic review and meta-analysis. Lancet Neurol 2016; 15: 673–684. [DOI] [PubMed] [Google Scholar]
- 27. Portelius E, Westman-Brinkmalm A, Zetterberg H, et al. Determination of β-amyloid peptide signatures in cerebrospinal fluid using immunoprecipitation-mass spectrometry. J Proteome Res 2006; 5: 1010–1016. [DOI] [PubMed] [Google Scholar]
- 28. Janelidze S, Stomrud E, Palmqvist S, et al. Plasma β-amyloid in Alzheimer’s disease and vascular disease. Sci Rep 2016; 6: 26801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Verberk IMW, Slot RE, Verfaillie SCJ, et al. Plasma amyloid as prescreener for the earliest Alzheimer pathological changes. Ann Neurol 2018; 84: 648–658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Kaneko N, Nakamura A, Washimi Y, et al. Novel plasma biomarker surrogating cerebral amyloid deposition. Proc Jpn Acad Ser B 2014; 90: 353–364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Nakamura A, Kaneko N, Villemagne VL, et al. High performance plasma amyloid-β biomarkers for Alzheimer’s disease. Nature 2018; 554: 249–254. [DOI] [PubMed] [Google Scholar]
- 32. Schindler SE, Bollinger JG, Ovod V, et al. High-precision plasma β-amyloid 42/40 predicts current and future brain amyloidosis. Neurology. Epub ahead of print 1 August 2019. DOI: 10.1212/WNL.0000000000008081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Ovod V, Ramsey KN, Mawuenyega KG, et al. Amyloid β concentrations and stable isotope labeling kinetics of human plasma specific to central nervous system amyloidosis. Alzheimers Dement 2017; 13: 841–849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Lövheim H, Elgh F, Johansson A, et al. Plasma concentrations of free amyloid β cannot predict the development of Alzheimer’s disease. Alzheimers Dement 2017; 13: 778–782. [DOI] [PubMed] [Google Scholar]
- 35. Park JC, Han SH, Cho HJ, et al. Chemically treated plasma Aβ is a potential blood-based biomarker for screening cerebral amyloid deposition. Alzheimers Res Ther 2017; 9: 20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Park JC, Han SH, Yi D, et al. Plasma tau/amyloid-β1-42 ratio predicts brain tau deposition and neurodegeneration in Alzheimer’s disease. Brain 2019; 142: 771–786. [DOI] [PubMed] [Google Scholar]
- 37. Schuster J, Funke SA. Methods for the specific detection and quantitation of amyloid-β oligomers in cerebrospinal fluid. J Alzheimers Dis 2016; 53: 53–67. [DOI] [PubMed] [Google Scholar]
- 38. Alcolea D, Carmona-Iragui M, Suárez-Calvet M, et al. Relationship between β-secretase, inflammation and core cerebrospinal fluid biomarkers for Alzheimer’s disease. J Alzheimers Dis 2014; 42: 157–167. [DOI] [PubMed] [Google Scholar]
- 39. Alcolea D, Martínez-Lage P, Sánchez-Juan P, et al. Amyloid precursor protein metabolism and inflammation markers in preclinical Alzheimer disease. Neurology 2015; 85: 626–633. [DOI] [PubMed] [Google Scholar]
- 40. Olsson A, Höglund K, Sjögren M, et al. Measurement of α- and β-secretase cleaved amyloid precursor protein in cerebrospinal fluid from Alzheimer patients. Exp Neurol 2003; 183: 74–80. [DOI] [PubMed] [Google Scholar]
- 41. Zetterberg H, Andreasson U, et al. Elevated cerebrospinal fluid BACE1 activity in incipient Alzheimer disease. Arch Neurol 2008; 65: 1102–1107. [DOI] [PubMed] [Google Scholar]
- 42. Mulder SD, van der Flier WM, Verheijen JH, et al. BACE1 activity in cerebrospinal fluid and its relation to markers of AD pathology. J Alzheimers Dis 2010; 20: 253–260. [DOI] [PubMed] [Google Scholar]
- 43. Ewers M, Cheng X, Zhong Z, et al. Increased CSF-BACE1 activity associated with decreased hippocampus volume in Alzheimer’s disease. J Alzheimers Dis 2011; 25: 373–381. [DOI] [PubMed] [Google Scholar]
- 44. Zhong Z, Ewers M, Teipel S, et al. Levels of β-secretase (BACE1) in cerebrospinal fluid as a predictor of risk in mild cognitive impairment. Arch Gen Psychiatry 2007; 64: 718. [DOI] [PubMed] [Google Scholar]
- 45. Perneczky R, Alexopoulos P. Cerebrospinal fluid BACE1 activity and markers of amyloid precursor protein metabolism and axonal degeneration in Alzheimer’s disease. Alzheimers Dement 2014; 10: S425–S429.e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Savage MJ, Holder DJ, Wu G, et al. Soluble BACE-1 activity and sAβPPβ concentrations in Alzheimer’s disease and age-matched healthy control cerebrospinal fluid from the Alzheimer’s disease neuroimaging initiative-1 baseline cohort. J Alzheimers Dis 2015; 46: 431–440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Shen Y, Wang H, Sun Q, et al. Increased plasma beta-secretase 1 may predict conversion to Alzheimer’s disease dementia in individuals with mild cognitive impairment. Biol Psychiatry 2018; 83: 447–455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Grundke-Iqbal I, Iqbal K, Tung YC, et al. Abnormal phosphorylation of the microtubule-associated protein tau (tau) in Alzheimer cytoskeletal pathology. Proc Natl Acad Sci U S A 1986; 83: 4913–4917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Ksiezak-Reding H, Binder LI, Yen SH. Immunochemical and biochemical characterization of tau proteins in normal and Alzheimer’s disease brains with Alz 50 and Tau-1. J Biol Chem 1988; 263: 7948–7953. [PubMed] [Google Scholar]
- 50. Lee VM-Y, Goedert M, Trojanowski JQ. Neurodegenerative tauopathies. Annu Rev Neurosci 2001; 24: 1121–1159. [DOI] [PubMed] [Google Scholar]
- 51. Schraen-Maschke S, Sergeant N, Dhaenens C-M, et al. Tau as a biomarker of neurodegenerative diseases. Biomark Med 2008; 2: 363–384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. van Harten AC, Kester MI, Visser PJ, et al. Tau and p-tau as CSF biomarkers in dementia: a meta-analysis. Clin Chem Lab Med 2011; 49: 353–366. [DOI] [PubMed] [Google Scholar]
- 53. Karch CM, Jeng AT, Goate AM. Extracellular tau levels are influenced by variability in tau that is associated with tauopathies. J Biol Chem 2012; 287: 42751–42762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Sato C, Barthélemy NR, Mawuenyega KG, et al. Tau kinetics in neurons and the human central nervous system. Neuron 2018; 97: 1284–1298.e7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Schöll M, Maass A, Mattsson N, et al. Biomarkers for tau pathology. Mol Cell Neurosci 2019; 97: 18–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Barthélemy NR, Mallipeddi N, Moiseyev P, et al. Tau phosphorylation rates measured by mass spectrometry differ in the intracellular brain vs. extracellular cerebrospinal fluid compartments and are differentially affected by Alzheimer’s disease. Front Aging Neurosci 2019; 11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Aschenbrenner AJ, Gordon BA, Benzinger TLS, et al. Influence of tau PET, amyloid PET, and hippocampal volume on cognition in Alzheimer disease. Neurology 2018; 91: e859– e866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Nelson PT, Alafuzoff I, Bigio EH, et al. Correlation of Alzheimer disease neuropathologic changes with cognitive status: a review of the literature. J Neuropathol Exp Neurol 2012; 71: 362–381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Mattsson N, Zetterberg H, Janelidze S, et al. Plasma tau in Alzheimer disease. Neurology 2016; 87: 1827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Pase MP, Beiser AS, Himali JJ, et al. Assessment of plasma total tau level as a predictive biomarker for dementia and related endophenotypes. JAMA Neurol 2019; 76: 598–606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Zetterberg H, Wilson D, Andreasson U, et al. Plasma tau levels in Alzheimer’s disease. Alzheimers Res Ther 2013; 5: 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Fossati S, Ramos Cejudo J, Debure L, et al. Plasma tau complements CSF tau and P-tau in the diagnosis of Alzheimer’s disease. Alzheimers Dement 2019; 11: 483–492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Mielke MM, Hagen CE, Wennberg AMV, et al. Association of plasma total tau level with cognitive decline and risk of mild cognitive impairment or dementia in the Mayo Clinic study on aging. JAMA Neurol 2017; 74: 1073–1080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Mielke MM, Hagen CE, Xu J, et al. Plasma phospho-tau181 increases with Alzheimer’s disease clinical severity and is associated with tau- and amyloid-positron emission tomography. Alzheimers Dement 2018; 14: 989–997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Tatebe H, Kasai T, Ohmichi T, et al. Quantification of plasma phosphorylated tau to use as a biomarker for brain Alzheimer pathology: pilot case-control studies including patients with Alzheimer’s disease and down syndrome. Mol Neurodegener 2017; 12: 63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Shekhar S, Kumar R, Rai N, et al. Estimation of tau and phosphorylated tau181 in serum of Alzheimer’s disease and mild cognitive impairment patients. PLoS One 2016; 11: e0159099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Yang CC, Chiu MJ, Chen TF, et al. Assay of plasma phosphorylated tau protein (threonine 181) and total tau protein in early-stage Alzheimer’s disease. J Alzheimers Dis 2018; 61: 1323–1332 [DOI] [PubMed] [Google Scholar]
- 68. Bos I, Vos S, Verhey F, et al. Cerebrospinal fluid biomarkers of neurodegeneration, synaptic integrity, and astroglial activation across the clinical Alzheimer’s disease spectrum. Alzheimers Dement 2019; 15: 644–654. [DOI] [PubMed] [Google Scholar]
- 69. Sjögren M, Blomberg M, Jonsson M, et al. Neurofilament protein in cerebrospinal fluid: a marker of white matter changes. J Neurosci Res 2001; 66: 510–516. [DOI] [PubMed] [Google Scholar]
- 70. Skillbäck T, Farahmand B, Bartlett JW, et al. CSF neurofilament light differs in neurodegenerative diseases and predicts severity and survival. Neurology 2014; 83: 1945–1953. [DOI] [PubMed] [Google Scholar]
- 71. Mattsson N, Cullen NC, Andreasson U, et al. Association between longitudinal plasma neurofilament light and neurodegeneration in patients with Alzheimer disease. JAMA Neurol 2019; 76: 791–799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Mattsson N, Andreasson U, Zetterberg H, et al. ; Alzheimer’s Disease Neuroimaging Initiative. Association of plasma neurofilament light with neurodegeneration in patients with Alzheimer disease. JAMA Neurol 2017; 74: 557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Byrne LM, Rodrigues FB, Blennow K, et al. Neurofilament light protein in blood as a potential biomarker of neurodegeneration in Huntington’s disease: a retrospective cohort analysis. Lancet Neurol 2017; 16: 601–609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Rojas JC, Bang J, Lobach IV, et al. CSF neurofilament light chain and phosphorylated tau 181 predict disease progression in PSP. Neurology 2018; 90: e273– e281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Preische O, Schultz SA, Apel A, et al. Serum neurofilament dynamics predicts neurodegeneration and clinical progression in presymptomatic Alzheimer’s disease. Nat Med 2019; 25: 277–283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Weston PSJ, Poole T, Ryan NS, et al. Serum neurofilament light in familial Alzheimer disease: a marker of early neurodegeneration. Neurology 2017; 89: 2167–2175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Weston PSJ, Poole T, O’Connor A, et al. Longitudinal measurement of serum neurofilament light in presymptomatic familial Alzheimer’s disease. Alzheimers Res Ther 2019; 11: 19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Gisslén M, Price RW, Andreasson U, et al. Plasma concentration of the neurofilament light protein (NFL) is a biomarker of CNS injury in HIV infection: a cross-sectional study. EBioMedicine 2016; 3: 135–140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Fortea J, Carmona-Iragui M, Benejam B, et al. Plasma and CSF biomarkers for the diagnosis of Alzheimer’s disease in adults with down syndrome: a cross-sectional study. Lancet Neurol 2018; 17: 860–869. [DOI] [PubMed] [Google Scholar]
- 80. Sánchez-Valle R, Heslegrave A, Foiani MS, et al. Serum neurofilament light levels correlate with severity measures and neurodegeneration markers in autosomal dominant Alzheimer’s disease. Alzheimers Res Ther 2018; 10: 113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Braunewell KH, Szanto AJK. Visinin-like proteins (VSNLs): interaction partners and emerging functions in signal transduction of a subfamily of neuronal Ca2+ -sensor proteins. Cell Tissue Res 2009; 335: 301–316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Braunewell KH, Riederer P, Spilker C, et al. Abnormal localization of two neuronal calcium sensor proteins, visinin-like proteins (vilips)-1 and -3, in neocortical brain areas of Alzheimer disease patients. Dement Geriatr Cogn Disord 2001; 12: 110–116. [DOI] [PubMed] [Google Scholar]
- 83. Lee JM, Blennow K, Andreasen N, et al. The brain injury biomarker VLP-1 is increased in the cerebrospinal fluid of Alzheimer disease patients. Clin Chem 2008; 54: 1617–1623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Kester MI, Teunissen CE, Sutphen C, et al. Cerebrospinal fluid VILIP-1 and YKL-40, candidate biomarkers to diagnose, predict and monitor Alzheimer’s disease in a memory clinic cohort. Alzheimers Res Ther 2015; 7: 59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Babić Leko M, Borovečki F, Dejanović N, et al. Predictive value of cerebrospinal fluid visinin-like protein-1 levels for Alzheimer’s disease early detection and differential diagnosis in patients with mild cognitive impairment. J Alzheimers Dis 2016; 50: 765–778. [DOI] [PubMed] [Google Scholar]
- 86. Luo X, Hou L, Shi H, et al. CSF levels of the neuronal injury biomarker visinin-like protein-1 in Alzheimer’s disease and dementia with Lewy bodies. J Neurochem 2013; 127: 681–690. [DOI] [PubMed] [Google Scholar]
- 87. Tarawneh R, D’Angelo G, Macy E, et al. Visinin-like protein-1: diagnostic and prognostic biomarker in Alzheimer disease. Ann Neurol 2011; 70: 274–285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Tarawneh R, Lee JM, Ladenson JH, et al. CSF VILIP-1 predicts rates of cognitive decline in early Alzheimer disease. Neurology 2012; 78: 709–719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Tarawneh R, Head D, Allison S, et al. Cerebrospinal fluid markers of neurodegeneration and rates of brain atrophy in early Alzheimer disease. JAMA Neurol 2015; 72: 656–665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Fagan AM, Xiong C, Jasielec MS, et al. Longitudinal change in CSF biomarkers in autosomal-dominant Alzheimer’s disease. Sci Transl Med 2014; 6: 226ra30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Sutphen CL, McCue L, Herries EM, et al. Longitudinal decreases in multiple cerebrospinal fluid biomarkers of neuronal injury in symptomatic late onset Alzheimer’s disease. Alzheimers Dement 2018; 14: 869–879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Arendt T. Synaptic degeneration in Alzheimer’s disease. Acta Neuropathol 2009; 118: 167–179. [DOI] [PubMed] [Google Scholar]
- 93. Masliah E, Mallory M, Alford M, et al. Altered expression of synaptic proteins occurs early during progression of Alzheimer’s disease. Neurology 2001; 56: 127–129. [DOI] [PubMed] [Google Scholar]
- 94. DeKosky ST, Scheff SW. Synapse loss in frontal cortex biopsies in Alzheimer’s disease: correlation with cognitive severity. Ann Neurol 1990; 27: 457–464. [DOI] [PubMed] [Google Scholar]
- 95. Terry RD, Masliah E, Salmon DP, et al. Physical basis of cognitive alterations in Alzheimer’s disease: synapse loss is the major correlate of cognitive impairment. Ann Neurol 1991; 30: 572–580. [DOI] [PubMed] [Google Scholar]
- 96. Südhof TC, Rizo J. Synaptotagmins: C2-domain proteins that regulate membrane traffic. Neuron 1996; 17: 379–388. [DOI] [PubMed] [Google Scholar]
- 97. Davidsson P, Jahn R, Bergquist J, et al. Synaptotagmin, a synaptic vesicle protein, is present in human cerebrospinal fluid. Mol Chem Neuropathol 1996; 27: 195–210. [DOI] [PubMed] [Google Scholar]
- 98. Öhrfelt A, Brinkmalm A, Dumurgier J, et al. The pre-synaptic vesicle protein synaptotagmin is a novel biomarker for Alzheimer’s disease. Alzheimers Res Ther 2016; 8: 41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Sutton RB, Fasshauer D, Jahn R, et al. Crystal structure of a SNARE complex involved in synaptic exocytosis at 2.4 Å resolution. Nature 1998; 395: 347–353. [DOI] [PubMed] [Google Scholar]
- 100. Brinkmalm A, Brinkmalm G, Honer WG, et al. SNAP-25 is a promising novel cerebrospinal fluid biomarker for synapse degeneration in Alzheimer’s disease. Mol Neurodegener 2014; 9: 53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101. Neve RL, Finch EA, Bird ED, et al. Growth-associated protein GAP-43 is expressed selectively in associative regions of the adult human brain. Proc Natl Acad Sci U S A 1988; 85: 3638–3642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102. De la Monte SM, Federoff HJ, Ng SC, et al. GAP-43 gene expression during development: persistence in a distinctive set of neurons in the mature central nervous system. Brain Res Dev Brain Res 1989; 46: 161–168. [DOI] [PubMed] [Google Scholar]
- 103. Sandelius Å, Portelius E, Källén Å, et al. Elevated CSF GAP-43 is Alzheimer’s disease specific and associated with tau and amyloid pathology. Alzheimers Dement 2019; 15: 55–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104. Remnestål J, Just D, Mitsios N, et al. CSF profiling of the human brain enriched proteome reveals associations of neuromodulin and neurogranin to Alzheimer’s disease. Proteomics Clin Appl 2016; 10: 1242–1253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105. Xia Z, Storm DR. The role of calmodulin as a signal integrator for synaptic plasticity. Nat Rev Neurosci 2005; 6: 267–276. [DOI] [PubMed] [Google Scholar]
- 106. Thorsell A, Bjerke M, Gobom J, et al. Neurogranin in cerebrospinal fluid as a marker of synaptic degeneration in Alzheimer’s disease. Brain Res 2010; 1362: 13–22. [DOI] [PubMed] [Google Scholar]
- 107. Kester MI, Teunissen CE, Crimmins DL, et al. Neurogranin as a cerebrospinal fluid biomarker for synaptic loss in symptomatic Alzheimer disease. JAMA Neurol 2015; 72: 1275–1280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108. Tarawneh R, D’Angelo G, Crimmins D, et al. Diagnostic and prognostic utility of the synaptic marker neurogranin in Alzheimer disease. JAMA Neurol 2016; 73: 561–571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109. Blennow K, Diaz-Lucena D, Zetterberg H, et al. CSF neurogranin as a neuronal damage marker in CJD: a comparative study with AD. J Neurol Neurosurg Psychiatry 2019; 90: 846–853. [DOI] [PubMed] [Google Scholar]
- 110. Xiao MF, Xu D, Craig MT, et al. NPTX2 and cognitive dysfunction in Alzheimer’s disease. Elife 2017; 6: 1–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111. Lleó A, Núñez-Llaves R, Alcolea D, et al. Changes in synaptic proteins precede neurodegeneration markers in preclinical Alzheimer’s disease cerebrospinal fluid. Mol Cell Proteomics 2019; 18: 546–560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112. Wingo AP, Dammer EB, Breen MS, et al. Large-scale proteomic analysis of human brain identifies proteins associated with cognitive trajectory in advanced age. Nat Commun 2019; 10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113. Love S, Miners JS. Cerebrovascular disease in ageing and Alzheimer’s disease. Acta Neuropathol 2016; 131: 645–658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114. Iadecola C. Neurovascular regulation in the normal brain and in Alzheimer’s disease. Nat Rev Neurosci 2004; 5: 347–360. [DOI] [PubMed] [Google Scholar]
- 115. Iturria-Medina Y, Sotero RC, Toussaint PJ, et al. ; Alzheimer’s Disease Neuroimaging Initiative. Early role of vascular dysregulation on late-onset Alzheimer’s disease based on multifactorial data-driven analysis. Nat Commun 2016; 7: 11934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116. Sweeney MD, Montagne A, Sagare AP, et al. Vascular dysfunction: the disregarded partner of Alzheimer’s disease. Alzheimers Dement 2019; 15: 158–167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117. Montagne A, Barnes SR, Sweeney MD, et al. Blood-brain barrier breakdown in the aging human hippocampus. Neuron 2015; 85: 296–302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118. Nation DA, Sweeney MD, Montagne A, et al. Blood–brain barrier breakdown is an early biomarker of human cognitive dysfunction. Nat Med 2019; 25: 270–276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119. Tibbling G, Link H, Öhman S. Principles of albumin and IgG analyses in neurological disorders. I. Establishment of reference values. Scand J Clin Lab Invest 1977; 37: 385–390. [DOI] [PubMed] [Google Scholar]
- 120. Reiber H, Peter JB. Cerebrospinal fluid analysis: disease-related data patterns and evaluation programs. J Neurol Sci 2001; 184: 101–122. [DOI] [PubMed] [Google Scholar]
- 121. Skillbäck T, Delsing L, Synnergren J, et al. CSF/serum albumin ratio in dementias: a cross-sectional study on 1861 patients. Neurobiol Aging 2017; 59: 1–9. [DOI] [PubMed] [Google Scholar]
- 122. Sweeney MD, Sagare AP, Zlokovic BV. Cerebrospinal fluid biomarkers of neurovascular dysfunction in mild dementia and Alzheimer’s disease. J Cereb Blood Flow Metab 2015; 35: 1055–1068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123. Sagare AP, Sweeney MD, Makshanoff J, et al. Shedding of soluble platelet-derived growth factor receptor-β from human brain pericytes. Neurosci Lett 2015; 607: 97–101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124. Janelidze S, Mattsson N, Stomrud E, et al. CSF biomarkers of neuroinflammation and cerebrovascular dysfunction in early Alzheimer disease. Neurology 2018; 91: e867– e877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125. Zuliani G, Cavalieri M, Galvani M, et al. Markers of endothelial dysfunction in older subjects with late onset Alzheimer’s disease or vascular dementia. J Neurol Sci 2008; 272: 164–170. [DOI] [PubMed] [Google Scholar]
- 126. Ewers M, Mielke MM, Hampel H. Blood-based biomarkers of microvascular pathology in Alzheimer’s disease. Exp Gerontol 2010; 45: 75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127. Heneka MT, Carson MJ, El Khoury J, et al. Neuroinflammation in Alzheimer’s disease. Lancet Neurol 2015; 14: 388–405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128. Suárez-Calvet M, Araque Caballero MÁ, Kleinberger G, et al. Early changes in CSF sTREM2 in dominantly inherited Alzheimer’s disease occur after amyloid deposition and neuronal injury. Sci Transl Med 2016; 8: 369ra178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129. Schöll M, Carter SF, Westman E, et al. Early astrocytosis in autosomal dominant Alzheimer’s disease measured in vivo by multi-tracer positron emission tomography. Sci Rep 2015; 5: 16404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130. Ewers M, Franzmeier N, Suárez-Calvet M, et al. Increased soluble TREM2 in cerebrospinal fluid is associated with reduced cognitive and clinical decline in Alzheimer’s disease. Sci Transl Med 2019; 11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131. Cherry JD, Olschowka JA, O’Banion MK. Neuroinflammation and M2 microglia: the good, the bad, and the inflamed. J Neuroinflammation 2014; 11: 98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132. Fan Z, Brooks DJ, Okello A, et al. An early and late peak in microglial activation in Alzheimer’s disease trajectory. Brain 2017; 140: 792–803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133. Brosseron F, Krauthausen M, Kummer M, et al. Body fluid cytokine levels in mild cognitive impairment and Alzheimer’s disease: a comparative overview. Mol Neurobiol 2014; 50: 534–544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134. Shen XN, Niu LD, Wang YJ, et al. Inflammatory markers in Alzheimer’s disease and mild cognitive impairment: a meta-analysis and systematic review of 170 studies. J Neurol Neurosurg Psychiatry 2019; 90: 590–598. [DOI] [PubMed] [Google Scholar]
- 135. Atagi Y, Liu CC, Painter MM, et al. Apolipoprotein E is a ligand for triggering receptor expressed on myeloid cells 2 (TREM2). J Biol Chem 2015; 290: 26043–26050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136. Bailey CC, DeVaux LB, Farzan M. The triggering receptor expressed on myeloid cells 2 binds apolipoprotein E. J Biol Chem 2015; 290: 26033–26042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137. Kleinberger G, Brendel M, Mracsko E, et al. The FTD-like syndrome causing TREM2 T66M mutation impairs microglia function, brain perfusion, and glucose metabolism. EMBO J 2017; 36: 1837–1853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138. Kleinberger G, Yamanishi Y, Suárez-Calvet M, et al. TREM2 mutations implicated in neurodegeneration impair cell surface transport and phagocytosis. Sci Transl Med 2014; 6: 243ra86. [DOI] [PubMed] [Google Scholar]
- 139. Mazaheri F, Snaidero N, Kleinberger G, et al. TREM2 deficiency impairs chemotaxis and microglial responses to neuronal injury. EMBO Rep 2017; 18: 1186–1198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140. Parhizkar S, Arzberger T, Brendel M, et al. Loss of TREM2 function increases amyloid seeding but reduces plaque-associated ApoE. Nat Neurosci 2019; 22: 191–204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141. Zhong L, Chen X-F, Wang T, et al. Soluble TREM2 induces inflammatory responses and enhances microglial survival. J Exp Med 2017; 214: 597–607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142. Guerreiro R, Bilgic B, Guven G, et al. A novel compound heterozygous mutation in TREM2 found in a Turkish frontotemporal dementia-like family. Neurobiol Aging 2013; 34: 2890.e1– 2890.e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143. Paloneva J, Manninen T, Christman G, et al. Mutations in two genes encoding different subunits of a receptor signaling complex result in an identical disease phenotype. Am J Hum Genet 2002; 71: 656–662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144. Guerreiro R, Wojtas A, Bras J, et al. TREM2 variants in Alzheimer’s disease. N Engl J Med 2013; 368: 117–127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145. Jonsson T, Stefansson H, Steinberg S, et al. Variant of TREM2 associated with the risk of Alzheimer’s disease. N Engl J Med 2013; 368: 107–116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146. Schlepckow K, Kleinberger G, Fukumori A, et al. An Alzheimer-associated TREM2 variant occurs at the ADAM cleavage site and affects shedding and phagocytic function. EMBO Mol Med 2017; 9: 1356–1365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147. Piccio L, Buonsanti C, Cella M, et al. Identification of soluble TREM-2 in the cerebrospinal fluid and its association with multiple sclerosis and CNS inflammation. Brain 2008; 131: 3081–3091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148. Piccio L, Deming Y, Del-Águila JL, et al. Cerebrospinal fluid soluble TREM2 is higher in Alzheimer disease and associated with mutation status. Acta Neuropathol 2016; 131: 925–933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149. Heslegrave A, Heywood W, Paterson R, et al. Increased cerebrospinal fluid soluble TREM2 concentration in Alzheimer’s disease. Mol Neurodegener 2016; 11: 3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150. Suárez-Calvet M, Morenas-Rodríguez E, Kleinberger G, et al. Early increase of CSF sTREM2 in Alzheimer’s disease is associated with tau related-neurodegeneration but not with amyloid-β pathology. Mol Neurodegener 2019; 14: 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151. Suárez-Calvet M, Kleinberger G, Araque Caballero MÁ, et al. sTREM2 cerebrospinal fluid levels are a potential biomarker for microglia activity in early-stage Alzheimer’s disease and associate with neuronal injury markers. EMBO Mol Med 2016; 8: 466–476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152. Liu D, Cao B, Zhao Y, et al. Soluble TREM2 changes during the clinical course of Alzheimer’s disease: a meta-analysis. Neurosci Lett 2018; 686: 10–16. [DOI] [PubMed] [Google Scholar]
- 153. Bekris LM, Khrestian M, Dyne E, et al. Soluble TREM2 and biomarkers of central and peripheral inflammation in neurodegenerative disease. J Neuroimmunol 2018; 319: 19–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154. Nordengen K, Kirsebom BE, Henjum K, et al. Glial activation and inflammation along the Alzheimer’s disease continuum. J Neuroinflammation 2019; 16: 46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155. Rauchmann BS, Schneider-Axmann T, Alexopoulos P, et al. ; Alzheimer’s Disease Neuroimaging Initiative. CSF soluble TREM2 as a measure of immune response along the Alzheimer’s disease continuum. Neurobiol Aging 2019; 74: 182–190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156. Henjum K, Almdahl IS, Årskog V, et al. Cerebrospinal fluid soluble TREM2 in aging and Alzheimer’s disease. Alzheimers Res Ther 2016; 8: 17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157. Öhrfelt A, Axelsson M, Malmeström C, et al. Soluble TREM-2 in cerebrospinal fluid from patients with multiple sclerosis treated with natalizumab or mitoxantrone. Mult Scler 2016; 22: 1587–1595. [DOI] [PubMed] [Google Scholar]
- 158. Gisslén M, Heslegrave A, Veleva E, et al. CSF concentrations of soluble TREM2 as a marker of microglial activation in HIV-1 infection. Neurol Neuroimmunol Neuroinflamm 2019; 6: e512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159. Henjum K, Quist-Paulsen E, Zetterberg H, et al. CSF sTREM2 in delirium: relation to Alzheimer’s disease CSF biomarkers Aβ42, t-tau and p-tau. J Neuroinflammation 2018; 15: 304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160. Byrne LM, Rodrigues FB, Johnson EB, et al. Cerebrospinal fluid neurogranin and TREM2 in Huntington’s disease. Sci Rep 2018; 8: 4260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161. Woollacott IOC, Nicholas JM, Heslegrave A, et al. Cerebrospinal fluid soluble TREM2 levels in frontotemporal dementia differ by genetic and pathological subgroup. Alzheimers Res Ther 2018; 10: 79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162. Alosco ML, Tripodis Y, Fritts NG, et al. Cerebrospinal fluid tau, Aβ, and sTREM2 in Former National Football League Players: modeling the relationship between repetitive head impacts, microglial activation, and neurodegeneration. Alzheimers Dement 2018; 14: 1159–1170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163. Deming Y, Filipello F, Cignarella F, et al. The MS4A gene cluster is a key modulator of soluble TREM2 and Alzheimer’s disease risk. Sci Transl Med 2019; 11: eaau2291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164. Gispert JD, Suárez-Calvet M, Monté GC, et al. Cerebrospinal fluid sTREM2 levels are associated with gray matter volume increases and reduced diffusivity in early Alzheimer’s disease. Alzheimers Dement 2016; 12: 1259–1272. [DOI] [PubMed] [Google Scholar]
- 165. Falcon C, Monté-Rubio GC, Grau-Rivera O, et al. CSF glial biomarkers YKL40 and sTREM2 are associated with longitudinal volume and diffusivity changes in cognitively unimpaired individuals. NeuroImage Clin 2019; 23: 101801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166. Ohara T, Hata J, Tanaka M, et al. Serum soluble triggering receptor expressed on myeloid cells 2 as a biomarker for incident dementia: the Hisayama Study. Ann Neurol 2019; 85: 47–58. [DOI] [PubMed] [Google Scholar]
- 167. Bhandari V, Palfree RG, Bateman A. Isolation and sequence of the granulin precursor cDNA from human bone marrow reveals tandem cysteine-rich granulin domains. Proc Natl Acad Sci U S A 1992; 89: 1715–1719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168. Bateman A, Belcourt D, Bennett H, et al. Granulins, a novel class of peptide from leukocytes. Biochem Biophys Res Commun 1990; 173: 1161–1168. [DOI] [PubMed] [Google Scholar]
- 169. Brouwers N, Sleegers K, Engelborghs S, et al. Genetic variability in progranulin contributes to risk for clinically diagnosed Alzheimer disease. Neurology 2008; 71: 656–664. [DOI] [PubMed] [Google Scholar]
- 170. Rademakers R, Eriksen JL, Baker M, et al. Common variation in the miR-659 binding-site of GRN is a major risk factor for TDP43-positive frontotemporal dementia. Hum Mol Genet 2008; 17: 3631–3642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171. Minami SS, Min SW, Krabbe G, et al. Progranulin protects against amyloid β deposition and toxicity in Alzheimer’s disease mouse models. Nat Med 2014; 20: 1157–1164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172. Van Kampen JM, Kay DG. Progranulin gene delivery reduces plaque burden and synaptic atrophy in a mouse model of Alzheimer’s disease. PLoS One 2017; 12: e0182896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173. Takahashi H, Klein ZA, Bhagat SM, et al. Opposing effects of progranulin deficiency on amyloid and tau pathologies via microglial TYROBP network. Acta Neuropathol 2017; 133: 785–807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174. Nicholson AM, Finch NA, Thomas CS, et al. Progranulin protein levels are differently regulated in plasma and CSF. Neurology 2014; 82: 1871–1878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175. Morenas-Rodríguez E, Cervera-Carles L, Vilaplana E, et al. Progranulin protein levels in cerebrospinal fluid in primary neurodegenerative dementias. J Alzheimers Dis 2015; 50: 539–546. [DOI] [PubMed] [Google Scholar]
- 176. Wilke C, Gillardon F, Deuschle C, et al. Cerebrospinal fluid progranulin, but not serum progranulin, is reduced in GRN-negative frontotemporal dementia. Neurodegener Dis 2017; 17: 83–88. [DOI] [PubMed] [Google Scholar]
- 177. Körtvélyessy P, Gukasjan A, Sweeney-Reed CM, et al. Progranulin and amyloid-β levels: relationship to neuropsychology in frontotemporal and Alzheimer’s disease. J Alzheimers Dis 2015; 46: 375–380. [DOI] [PubMed] [Google Scholar]
- 178. Suárez-Calvet M, Capell A, Araque Caballero MÁ, et al. CSF progranulin increases in the course of Alzheimer’s disease and is associated with sTREM2, neurodegeneration and cognitive decline. EMBO Mol Med 2018; 10: e9712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179. Götzl JK, Brendel M, Werner G, et al. Opposite microglial activation stages upon loss of PGRN or TREM2 result in reduced cerebral glucose metabolism. EMBO Mol Med 2019; 11: e9711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180. Wilke C, Gillardon F, Deuschle C, et al. Serum levels of progranulin do not reflect cerebrospinal fluid levels in neurodegenerative disease. Curr Alzheimer Res 2016; 13: 654–662. [DOI] [PubMed] [Google Scholar]
- 181. Bonneh-Barkay D, Bissel SJ, Kofler J, et al. Astrocyte and macrophage regulation of YKL-40 expression and cellular response in neuroinflammation. Brain Pathol 2012; 22: 530–546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182. Querol-Vilaseca M, Colom-Cadena M, Pegueroles J, et al. YKL-40 (Chitinase 3-like I) is expressed in a subset of astrocytes in Alzheimer’s disease and other tauopathies. J Neuroinflammation 2017; 14: 118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183. Craig-Schapiro R, Perrin RJ, Roe CM, et al. YKL-40: a novel prognostic fluid biomarker for preclinical Alzheimer’s disease. Biol Psychiatry 2010; 68: 903–912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184. Alcolea D, Vilaplana E, Pegueroles J, et al. Relationship between cortical thickness and cerebrospinal fluid YKL-40 in predementia stages of Alzheimer’s disease. Neurobiol Aging 2015; 36: 2018–2023. [DOI] [PubMed] [Google Scholar]
- 185. Gispert JD, Monté GC, Suárez-Calvet M, et al. The APOE ε4 genotype modulates CSF YKL-40 levels and their structural brain correlates in the continuum of Alzheimer’s disease but not those of sTREM2. Alzheimer’s Dement 2017; 6: 50–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186. Lleó A, Alcolea D, Martínez-Lage P, et al. Longitudinal cerebrospinal fluid biomarker trajectories along the Alzheimer’s disease continuum in the BIOMARKAPD study. Alzheimers Dement 2019; 15: 742–753. [DOI] [PubMed] [Google Scholar]
- 187. Alcolea D, Irwin DJ, Illán-Gala I, et al. Elevated YKL-40 and low sAPPβ:YKL-40 ratio in antemortem cerebrospinal fluid of patients with pathologically confirmed FTLD. J Neurol Neurosurg Psychiatry 2019; 90: 180–186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188. Comabella M, Fernández M, Martin R, et al. Cerebrospinal fluid chitinase 3-like 1 levels are associated with conversion to multiple sclerosis. Brain 2010; 133: 1082–1093. [DOI] [PubMed] [Google Scholar]
- 189. Illán-Gala I, Alcolea D, Montal V, et al. CSF sAPPβ, YKL-40, and NfL along the ALS-FTD spectrum. Neurology 2018; 91: e1619–e1628. [DOI] [PubMed] [Google Scholar]
- 190. Hall S, Janelidze S, Surova Y, et al. Cerebrospinal fluid concentrations of inflammatory markers in Parkinson’s disease and atypical parkinsonian disorders. Sci Rep 2018; 8: 13276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191. Olsson B, Constantinescu R, Holmberg B, et al. The glial marker YKL-40 is decreased in synucleinopathies. Mov Disord 2013; 28: 1882–1885. [DOI] [PubMed] [Google Scholar]
- 192. Wennström M, Surova Y, Hall S, et al. The inflammatory marker YKL-40 Is elevated in cerebrospinal fluid from patients with Alzheimer’s but not Parkinson’s disease or dementia with lewy bodies. PLoS One 2015; 10: e0135458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193. Choi J, Lee H-W, Suk K. Plasma level of chitinase 3-like 1 protein increases in patients with early Alzheimer’s disease. J Neurol 2011; 258: 2181–2185. [DOI] [PubMed] [Google Scholar]
- 194. Galimberti D, Schoonenboom N, Scheltens P, et al. Intrathecal chemokine synthesis in mild cognitive impairment and Alzheimer disease. Arch Neurol 2006; 63: 538–543. [DOI] [PubMed] [Google Scholar]
- 195. Galimberti D, Venturelli E, Fenoglio C, et al. IP-10 serum levels are not increased in mild cognitive impairment and Alzheimer’s disease. Eur J Neurol 2007; 14: e3–e4. [DOI] [PubMed] [Google Scholar]
- 196. Oeckl P, Halbgebauer S, Anderl-Straub S, et al. Glial fibrillary acidic protein in serum is increased in Alzheimer’s disease and correlates with cognitive impairment. J Alzheimers Dis 2019; 67: 481–488. [DOI] [PubMed] [Google Scholar]
- 197. Lai KSP, Liu CS, Rau A, et al. Peripheral inflammatory markers in Alzheimer’s disease: a systematic review and meta-analysis of 175 studies. J Neurol Neurosurg Psychiatry 2017; 88: 876–882. [DOI] [PubMed] [Google Scholar]
- 198. Julien C, Tremblay C, Emond V, et al. Sirtuin 1 reduction parallels the accumulation of tau in Alzheimer disease. J Neuropathol Exp Neurol 2009; 68: 48–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199. Vachharajani VT, Liu T, Wang X, et al. Sirtuins link inflammation and metabolism. J Immunol Res 2016; 2016: 8167273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200. Kida Y, Goligorsky MS. Sirtuins, cell senescence, and vascular aging. Can J Cardiol 2016; 32: 634–641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201. Stamatovic SM, Martinez-Revollar G, Hu A, et al. Decline in Sirtuin-1 expression and activity plays a critical role in blood-brain barrier permeability in aging. Neurobiol Dis 2019; 126: 105–116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202. Neumann M, Sampathu DM, Kwong LK, et al. Ubiquitinated TDP-43 in frontotemporal lobar degeneration and amyotrophic lateral sclerosis. Science 2006; 314: 130–133. [DOI] [PubMed] [Google Scholar]
- 203. Cairns NJ, Neumann M, Bigio EH, et al. TDP-43 in familial and sporadic frontotemporal lobar degeneration with ubiquitin inclusions. Am J Pathol 2007; 171: 227–240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204. Amador-Ortiz C, Lin WL, Ahmed Z, et al. TDP-43 immunoreactivity in hippocampal sclerosis and Alzheimer’s disease. Ann Neurol 2007; 61: 435–445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205. Chang XL, Tan MS, Tan L, et al. The role of TDP-43 in Alzheimer’s disease. Mol Neurobiol 2016; 53: 3349–3359. [DOI] [PubMed] [Google Scholar]
- 206. Chen-Plotkin AS, Lee VMY, Trojanowski JQ. TAR DNA-binding protein 43 in neurodegenerative disease. Nat Rev Neurol 2010; 6: 211–220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207. Josephs KA, Whitwell JL, Knopman DS, et al. Abnormal TDP-43 immunoreactivity in AD modifies clinicopathologic and radiologic phenotype. Neurology 2008; 70: 1850–1857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208. Tremblay C, St-Amour I, Schneider J, et al. Accumulation of transactive response DNA binding protein 43 in mild cognitive impairment and Alzheimer disease. J Neuropathol Exp Neurol 2011; 70: 788–798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209. Uryu K, Nakashima-Yasuda H, Forman MS, et al. Concomitant TAR-DNA-binding protein 43 pathology is present in Alzheimer disease and corticobasal degeneration but not in other tauopathies. J Neuropathol Exp Neurol 2008; 67: 555–564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210. Josephs KA, Whitwell JL, Weigand SD, et al. TDP-43 is a key player in the clinical features associated with Alzheimer’s disease. Acta Neuropathol 2014; 127: 811–824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211. Wilson RS, Yu L, Trojanowski JQ, et al. TDP-43 pathology, cognitive decline, and dementia in old age. JAMA Neurol 2013; 70: 1418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212. Nelson PT, Dickson DW, Trojanowski JQ, et al. Limbic-predominant age-related TDP-43 encephalopathy (LATE): consensus working group report. Brain 2019; 142: 1503–1527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213. Foulds P, McAuley E, Gibbons L, et al. TDP-43 protein in plasma may index TDP-43 brain pathology in Alzheimer’s disease and frontotemporal lobar degeneration. Acta Neuropathol 2008; 116: 141–146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214. Williams SM, Schulz P, Rosenberry TL, et al. Blood-based oligomeric and other protein variant biomarkers to facilitate pre-symptomatic diagnosis and staging of Alzheimer’s disease. J Alzheimers Dis 2017; 58: 23–35. [DOI] [PubMed] [Google Scholar]
- 215. Goossens J, Vanmechelen E, Trojanowski JQ, et al. TDP-43 as a possible biomarker for frontotemporal lobar degeneration: a systematic review of existing antibodies. Acta Neuropathol Commun 2015; 3: 15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216. Spillantini MG, Crowther RA, Jakes R, et al. Alpha-Synuclein in filamentous inclusions of Lewy bodies from Parkinson’s disease and dementia with lewy bodies. Proc Natl Acad Sci U S A 1998; 95: 6469–6473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217. Spillantini MG, Crowther RA, Jakes R, et al. Filamentous alpha-synuclein inclusions link multiple system atrophy with Parkinson’s disease and dementia with Lewy bodies. Neurosci Lett 1998; 251:205–208. [DOI] [PubMed] [Google Scholar]
- 218. Hamilton RL. Lewy bodies in Alzheimer’s disease: a neuropathological review of 145 cases using alpha-synuclein immunohistochemistry. Brain Pathol 2000; 10: 378–384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219. Lippa CF, Fujiwara H, Mann DM, et al. Lewy bodies contain altered alpha-synuclein in brains of many familial Alzheimer’s disease patients with mutations in presenilin and amyloid precursor protein genes. Am J Pathol 1998; 153: 1365–1370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220. Giasson BI, Forman MS, Higuchi M, et al. Initiation and synergistic fibrillization of tau and alpha-synuclein. Science 2003; 300: 636–640. [DOI] [PubMed] [Google Scholar]
- 221. Marsh SE, Blurton-Jones M. Examining the mechanisms that link β-amyloid and α-synuclein pathologies. Alzheimers Res Ther 2012; 4: 11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222. Mollenhauer B, Cullen V, Kahn I, et al. Direct quantification of CSF α-synuclein by ELISA and first cross-sectional study in patients with neurodegeneration. Exp Neurol 2008; 213: 315–325. [DOI] [PubMed] [Google Scholar]
- 223. Mollenhauer B, Locascio JJ, Schulz-Schaeffer W, et al. α-Synuclein and tau concentrations in cerebrospinal fluid of patients presenting with parkinsonism: a cohort study. Lancet Neurol 2011; 10: 230–240. [DOI] [PubMed] [Google Scholar]
- 224. Kang JH, Irwin DJ, Chen-Plotkin AS, et al. Association of cerebrospinal fluid β-amyloid 1-42, T-tau, P-tau181, and α-synuclein levels with clinical features of drug-naive patients with early Parkinson disease. JAMA Neurol 2013; 70: 1277–1287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 225. Kang JH, Mollenhauer B, Coffey CS, et al. CSF biomarkers associated with disease heterogeneity in early Parkinson’s disease: the Parkinson’s Progression Markers Initiative study. Acta Neuropathol 2016; 131: 935–949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 226. Shi M, Bradner J, Hancock AM, et al. Cerebrospinal fluid biomarkers for Parkinson disease diagnosis and progression. Ann Neurol 2011; 69: 570–580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 227. Berge G, Sando SB, Albrektsen G, et al. Alpha-synuclein measured in cerebrospinal fluid from patients with Alzheimer’s disease, mild cognitive impairment, or healthy controls: a two year follow-up study. BMC Neurol 2016; 16: 180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 228. Kasai T, Tokuda T, Ishii R, et al. Increased α-synuclein levels in the cerebrospinal fluid of patients with Creutzfeldt-Jakob disease. J Neurol 2014; 261: 1203–1209. [DOI] [PubMed] [Google Scholar]
- 229. Shi M, Zabetian CP, Hancock AM, et al. Significance and confounders of peripheral DJ-1 and alpha-synuclein in Parkinson’s disease. Neurosci Lett 2010; 480: 78–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 230. Chang KA, Shin KY, Nam E, et al. Plasma soluble neuregulin-1 as a diagnostic biomarker for Alzheimer’s disease. Neurochem Int 2016; 97: 1–7. [DOI] [PubMed] [Google Scholar]
- 231. Lee PH, Lee G, Park HJ, et al. The plasma alpha-synuclein levels in patients with Parkinson’s disease and multiple system atrophy. J Neural Transm 2006; 113: 1435–1439. [DOI] [PubMed] [Google Scholar]
- 232. Ferretti MT, Iulita MF, Cavedo E, et al. Sex differences in Alzheimer disease: the gateway to precision medicine. Nat Rev Neurol 2018; 14: 457–469. [DOI] [PubMed] [Google Scholar]
- 233. Nebel RA, Aggarwal NT, Barnes LL, et al. Understanding the impact of sex and gender in Alzheimer’s disease: a call to action. Alzheimers Dement 2018; 14: 1171–1183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 234. Alzheimer’s Association. 2017 Alzheimer’s disease facts and figures. Alzheimers Dement 2017; 13: 325–373. [Google Scholar]
- 235. Lin KA, Choudhury KR, Rathakrishnan BG, et al. Marked gender differences in progression of mild cognitive impairment over 8 years. Alzheimers Dement 2015; 1: 103–110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 236. Mallampalli MP, Carter CL. Exploring sex and gender differences in sleep health: a society for women’s health research report. J Women’s Heal 2014; 23: 553–562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 237. Neu SC, Pa J, Kukull W, et al. Apolipoprotein E genotype and sex risk factors for Alzheimer disease. JAMA Neurol 2017; 74: 1178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 238. Bove R, Secor E, Chibnik LB, et al. Age at surgical menopause influences cognitive decline and Alzheimer pathology in older women. Neurology 2014; 82: 222–229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 239. Rocca WA, Bower JH, Maraganore DM, et al. Increased risk of cognitive impairment or dementia in women who underwent oophorectomy before menopause. Neurology 2007; 69: 1074–1083. [DOI] [PubMed] [Google Scholar]
- 240. Altmann A, Tian L, Henderson VW, et al. Sex modifies the APOE-related risk of developing Alzheimer disease. Ann Neurol 2014; 75: 563–573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 241. Hohman TJ, Thambisetty M, Jefferson AL, et al. Sex-specific genetic predictors of Alzheimer’s disease biomarkers. Acta Neuropathol 2018; 136: 857–872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 242. Li G, Shofer JB, Petrie EC, et al. Cerebrospinal fluid biomarkers for Alzheimer’s and vascular disease vary by age, gender, and APOE genotype in cognitively normal adults. Alzheimers Res Ther 2017; 9: 1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 243. Hohman TJ, Dumitrescu L, Barnes LL, et al. Sex-specific association of apolipoprotein e with cerebrospinal fluid levels of tau. JAMA Neurol 2018; 75: 989–998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 244. Paraskevas GP, Kapaki E, Papageorgiou SG, et al. CSF biomarker profile and diagnostic value in vascular dementia. Eur J Neurol 2009; 16: 205–211. [DOI] [PubMed] [Google Scholar]
- 245. Sunderland T, Linker G, Mirza N, et al. Decreased β-amyloid1-42 and increased tau levels in cerebrospinal fluid of patients with Alzheimer disease. JAMA 2003; 289: 2094–2103. [DOI] [PubMed] [Google Scholar]
- 246. Buckley RF, Mormino EC, Rabin JS, et al. Sex differences in the association of global amyloid and regional tau deposition measured by positron emission tomography in clinically normal older adults. JAMA Neurol 2019; 76: 542–551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 247. Filon JR, Intorcia AJ, Sue LI, et al. Gender differences in Alzheimer disease: brain atrophy, histopathology burden, and cognition. J Neuropathol Exp Neurol 2016; 75: 748–754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 248. Alexopoulos P, Tsolakidou A, Roselli F, et al. Clinical and neurobiological correlates of soluble amyloid precursor proteins in the cerebrospinal fluid. Alzheimers Dement 2012; 8: 304–311. [DOI] [PubMed] [Google Scholar]
- 249. Itoh N, Arai H, Urakami K, et al. Large-scale, multicenter study of cerebrospinal fluid tau protein phosphorylated at serine 199 for the antemortem diagnosis of Alzheimer’s disease. Ann Neurol 2001; 50: 150–156. [DOI] [PubMed] [Google Scholar]
- 250. Sjögren M, Rosengren L, Minthon L, et al. Cytoskeleton proteins in CSF distinguish frontotemporal dementia from AD. Neurology 2000; 54: 1960–1964. [DOI] [PubMed] [Google Scholar]
- 251. Mattsson N, Tabatabaei S, Johansson P, et al. Cerebrospinal fluid microglial markers in Alzheimer’s disease: elevated chitotriosidase activity but lack of diagnostic utility. Neuromolecular Med 2011; 13: 151–159. [DOI] [PubMed] [Google Scholar]
- 252. Teunissen CE, Elias N, Koel-Simmelink MJ, et al. Novel diagnostic cerebrospinal fluid biomarkers for pathologic subtypes of frontotemporal dementia identified by proteomics. Alzheimers Dement 2016; 2: 86–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 253. Olsson B, Hertze J, Lautner R, et al. Microglial markers are elevated in the prodromal phase of Alzheimer’s disease and vascular dementia. J Alzheimers Dis 2013; 33: 45–53. [DOI] [PubMed] [Google Scholar]
- 254. Fenoglio C, Galimberti D, Lovati C, et al. MCP-1 in Alzheimer’s disease patients: A-2518G polymorphism and serum levels. Neurobiol Aging 2004; 25: 1169–1173. [DOI] [PubMed] [Google Scholar]
- 255. Kapaki E, Paraskevas GP, Emmanouilidou E, et al. The diagnostic value of CSF α-synuclein in the differential diagnosis of dementia with Lewy bodies vs. normal s and patients with Alzheimer’s disease. PLoS One 2013; 8: e81654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 256. Korff A, Liu C, Ginghina C, et al. ; Alzheimer’s Disease Neuroimaging Initiative. α-Synuclein in cerebrospinal fluid of Alzheimer’s disease and mild cognitive impairment. J Alzheimers Dis 2013; 36: 679–688. [DOI] [PMC free article] [PubMed] [Google Scholar]