Short abstract
With the emergence of immuno-oncology, new therapeutic agents that modulate immune activation and regulation are being used to treat cancer patients with durable response. It is well known that following T-cell receptor (TCR) activation, many co-receptors can augment or suppress the TCR signal, and therapeutically targeting these co-receptors has proven effective. The B7-CD28 family is comprised of such immune-regulatory receptors, and antibodies against its members programmed cell death protein 1 (PD-1), programmed death-ligand 1 (PD-L1), and cytotoxic T-lymphocyte-associated protein 4 (CTLA-4) have revolutionized cancer treatment. These therapies promote an immune response against tumor cells, which demonstrated better long-term survival and tolerability compared to traditional cancer treatments. In this review we describe the history of the expanding B7-CD28 family, and by comparison of sequence and structure reveal that it is a non-traditional family. The family has grown to include proteins that share low sequence identity, generally grouped by regulation of immune response, which utilize the common immunoglobulin fold. This low level of commonality has provided additional challenges to the drug discovery process as the mechanisms and therapeutic potency between family members can vary greatly.
Impact statement
Immunotherapy as a field has dramatically expanded in the last decade in the area of oncology with efficacy demonstrated by PD-1, PD-L1, and CTLA-4 blockade. With all three “checkpoint blockade” receptors being in the B7-CD28 family, there has been increased interest in targeting other members in this family due to redundancy in immune regulation, i.e., the combination of therapeutic agents targeting multiple co-inhibitory receptors may yield additional antitumor efficacy. Therefore significant resources are being dedicated to developing additional B7-CD28 treatment options.
Keywords: Protein–protein interactions, immunology/molecular, oncology, structural biology, immunotherapy, immuno-oncology
Introduction
An effective, durable, and non-self-immune response is essential for maintaining human health. To achieve this balance and be adaptable, the immune system consists of various cell types that work in concert to evoke the appropriate response. For example, the B-cell response focuses predominantly on extracellular antigens, whereas the CD8 T-cell response is largely focused on antigens presented by major histocompatibility complexes. Both responses rely on initial antigen recognition, then subsequent activity tuning through co-stimulatory and inhibitory receptors, along with cytokine signaling. The discovery of these receptors, and subsequent targeting of inhibitory receptors for therapeutic benefits has been tremendously impactful and was highlighted by the 2018 Nobel Prize in Physiology or Medicine, which was awarded to James Allison and Tasuku Honjo. This new class of checkpoint inhibitors enhances the T-cell response to tumor antigens1 and tends to be more durable than traditional chemotherapy.2 This pioneering work has generated much interest in the B7-CD28 family, and reviews published in recent years provide details about the functionality of the B7 and CD28 co-receptor pathways.3–5 In this review we discuss the chronology leading to the current definition of the B7-CD28 family, the sequence identity and structure characteristics of the family, and how its non-classical characteristics provide challenges to drug discovery.
Chronology of B7-CD28 discoveries led to an expanding family
The discovery of B7 resulted from efforts to find antibodies against antigens to allow researchers to classify distinct B lymphocytes. With its members loosely related by sequence and function, the B7-CD28 family has gradually expanded over decades. The initial discovery of B1 (CD20) in 19806 was quickly followed by B2 (CD21),7 B3 (CD22),8 B4 (CD19),9 B5,10 and finally B7 (CD80).11 CD28, previously identified as a T-cell activator,12 was shown to bind CD80 by demonstrating CD80:CD28-mediated cell adhesion could be blocked by anti-CD80 and anti-CD28 antibodies.13
Not long after, a second receptor competing with CD28 for CD80, cytotoxic T-lymphocyte-associated protein 4 (CTLA-4), was identified.14 CTLA-4 had previously been described as homologous in sequence and structure to CD28 and both are located on chromosome 2,15 clearly showing familial relationship, but the biological role of CTLA-4 was unknown. The picture became more complicated in 1993 when four papers were published within a day of each other describing a second member of the B7 family, B7-2 (CD86), which also bound both CD28 and CTLA-4.16 Interestingly, while they have limited sequence similarity, CD80 and CD86 are located on chromosome 3.17 Whereas CD80 is expressed on activated antigen presenting cells (APC), CD86 is constitutively expressed on monocytes and dendritic cells, and on activated B-cells.18 Meanwhile, work progressed describing the biological role of CTLA-4, where it was proposed as a T-cell co-stimulatory molecule19 or a suppressing molecule.20 It was definitively shown in mid-1995 that CTLA-4 outcompetes CD28 for CD80/CD86 binding and inhibits T-cell activation.21,22 In early 1999, inducible T-cell co-stimulator (ICOS), a third CD28-related molecule was identified and shown to be inducible on activated T-cells; ICOS induces interleukin (IL)-10 but not IL-2 as seen with CD28 activation.23 With three related co-stimulatory molecules that modulate T-cell response (CD28, CTLA-4, and ICOS) and two identified ligands (CD80 and CD86), characterization of the B7-CD28 family seemed relatively clear.
However, the addition of a third B7 member, B7-H1 (programmed cell death protein 1 [PD-L1]), complicated the definition of the family,24 and expanded possibilities of what might be included. PD-L1 was initially identified as a co-stimulatory ligand for T-cells, but was later shown to be a suppressive when its receptor programmed cell death protein 1 (PD-1) was discovered.25 PD-1 is located on chromosome 2, but not in the locus shared by CD28, CTLA-4, and ICOS. Furthermore, PD-L1 is located on chromosome 9, whereas CD80 and CD86 are on chromosome 3. This series of additions broadened the family, with PD-L1 and PD-1 situated at new chromosomal locations and not interacting with any previously identified members. Additions to the family continued as more immunoglobulin (Ig) domain-containing molecules were found, usually involving APC:T-cell interactions, but sometimes only broadly related by structure and function. The B7 family grew to include B7-H2 (ICOSL),26 B7-H3 (CD276),27 B7-DC (PD-L2),28 B7-H4 (B7x),29 B7-H5 (VISTA),30 B7-H6 (NCRLG1),31 and B7-H7 (HHLA2).32 Concurrently, NKp30 and CD28 homolog (CD28H; TMIGD2) were identified as receptors for B7-H6 and B7-H7, respectively, thus enlarging the CD28 family. Recently, VSIG-3 has been proposed as a receptor for B7-H5.33 Receptors for B7-H3 and B7-H4, and if they are CD28-like (i.e. Ig domains), have yet to be determined. Through this chronology, we see that the family has its origins in B-cell characterization, where a series of discoveries and additions led to the creation of a family without distinctive features to separate itself from other Ig-containing receptors that also affect the immune system.
Sequence homology
A defining characteristic of the B7-CD28 family members is their ability to modulate the immune response. However, traditional characteristics that often define a family such as conserved signaling mechanisms, sequence homology, structural-similarity, or chromosome location do not apply across members of this family, except an Ig domain that is broadly utilized in immune regulation. Lacking these distinguishing protein features makes it difficult not only to clearly define the family, but also difficult to predict potential undiscovered members. Interestingly, B7-H1 (PD-L1) was initially identified as an IgV and IgC containing protein with low sequence identity to CD80, broadly expressed and affecting T-cells,24 though with more limited protein expression.34 We suppose that this classification may have led to the growth in the B7-CD28 family as other Ig-containing “family members” were discovered. Indeed, by initially naming PD-L1 as B7-H1, PD-1 entered a group containing CD28, CTLA-4, and ICOS, which are clearly related molecules that interact with CD80, CD86, and ICOSL, and share a conserved interface,35 but PD-1 and PD-L1 do not interact with these members.
Most families of homologous proteins have significant sequence identity, as they potentially derive from a common ancestor, gaining diversity through gene duplication and subsequent mutations. Proteins with more than 30% sequence identity have a higher likelihood of being structurally homologous (90%), and below 25% identity the chance of being a homolog falls to 10%.36 With high sequence identity comes more likelihood of being structurally similar, thus potentially conserving any structure–function relationships. However, B7-CD28 members have low sequence identity when quantified by globally aligning their full-length sequences (Figure 1(a)). The overall average sequence identities among human B7 and CD28 members from this alignment is 20% and 18%, respectively. This is comparable to the 17% sequence identity shared by all Ig domain sequences (as annotated in PFAM),37 indicative of a level of identity resulting from structural conservation alone.
Figure 1.
Full sequence alignment of available B7 and CD28 members. (a) Global alignment of human (h), Rhesus macaque (rh), and Mus musculus (m) B7 members. (b) Global alignment of human, rhesus, and mouse CD28 members. Number represents percent identity. Alignment performed using ClustalOmega.
The first B7 member discovered was CD80, which shares 24% identity with CD86, its closest human homolog (Figure 1(a)). The rest of the human members have an average of 20% sequence identity compared to CD80. Comparing each member to all others, human B7-H3 has the highest average sequence identity of 23%, and B7-H5 has the lowest at 17%. Overall, the two most similar human proteins are B7-H1 and B7-DC (37%), and the two least similar are B7-H4 and B7-H5 (14%). Globally, B7 members have five pairs with sequence identity above 25%, suggesting little homology a priori beyond that of the Ig superfamily (IgSF). Intracellularly, the cytoplasmic tails vary in length from 1 (B7-H4) to 171 (B7-H6) residues, there is little evidence a common motif in the tail is widely utilized, and understanding their role in biology is ongoing.38
A similar trend can be seen when comparing CD28 members (Figure 1(b)), which have an overall average sequence identity of 18%. CD28 and CTLA-4, the two initial members, share 30% identity (Figure 1(b)). Homology falls to 15–23% comparing other human members to CD28. ICOS has the lowest average sequence identity compared to other CD28 members at 14%, and CD28 has the highest at 22%. Pairwise, CD28 and CTLA-4 share the highest identity as mentioned, while PD-1 and ICOS only have 8% identity, the lowest across all comparisons. Besides the initial members, CD28 and CTLA-4, no other receptor pair has identity greater than 25%. Intracellularly, the cytoplasmic tail range from 38 to 111 residues, with many members having tyrosine signaling motifs.5
The low sequence homology of B7-CD28 members is in contrast to a more traditional family like butyrophilin. Butyrophilin members have been described as B7-like,39 as they modulate the immune response and contain Ig domains. The family is clustered on chromosome 6, has an overall 42% sequence identity (unpublished analysis), and typically contains the B30.2 intracellular domain.40 The lack of similar sequence features among B7-CD28 family members results in difficulties predicting additional members, potential binding partners, and therapeutic potency.
Structure homology
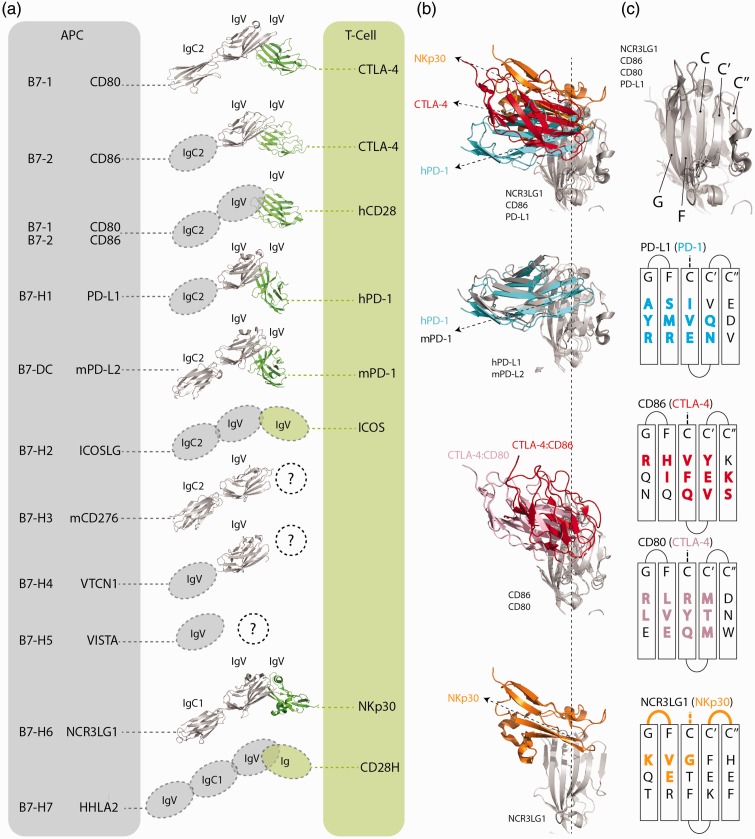
Protein structure is another feature by which a family can be classified. Both B7 and CD28 members utilize the common Ig fold for their extracellular domains (Figure 2(a)), and belong to the IgSF. The Ig fold is widely utilized from antibodies to receptors, and is not associated with any specific biological function. Ig fold can be described as roughly 100 amino acids, consisting of anti-parallel beta strands stabilized through a hydrophobic core and a disulfide bond. The Ig fold is further classified as IgV, IgC1, or IgC2 based on strand number and connectivity,41 which are utilized in B7 and CD28 members, with IgV domains being involved in receptor and counter-receptor binding (Figure 2(a)). It should be noted that strand swapping was observed in some structures of CTLA-4, B7-H4, and murine B7-H3. This strand swap is a potential artifact of the protein refolding process or crystallization conditions and has been observed in other Ig structures, but may also have a physiological role.42,43 With the exception of B7-H5,44 B7 members contain at least two Ig domains, whereas all identified CD28 members consist of a single Ig domain. Overall the B7 members share a consistent structural fold and align well (2.4Å RMSD), with similar number and positioning of beta sheets. We do see variability in loops that connect the beta sheets, and bend angle between the two Ig domains. The variability in loop position is not unexpected as loops are structurally flexible and could vary from structure to structure. The length of these loops can also vary, and this is related to sequence differences. The subtle bend angle differences between the Ig domains can also be attributed to experimental effects of structural determination, and may not reflect actual differences.
Figure 2.
Structural analysis of B7 and CD28 members. (a) Structures of select B7 (gray) and CD28 (green) members. From top to bottom PDB codes: 1I8L (hCD80:hCTLA4), 1I85 (hCD86:hCTLA4), 1YJD (hCD28), 4ZQK (hPD-1:hPD-L1), 3BP5 (mPD-1:mPD-L2), 4I0K (mCD276 has two Ig domains; hCD276 has four), 4GOS (hVTCN1), 3PV6 (hNCR3LG1:NKp30). (b) Comparing IgV domains interaction angle for 4ZQK (hPD-1:hPD-L1), 3BP5 (mPD-1:mPD-L2), 1I8L (hCD80:hCTLA4), 1I85 (hCD86:hCTLA4), and 3PV6 (hNCR3LG1:NKp30). (c) Beta sheets of aligned B7 members at the counter receptor interface, and specific residues that structurally align in 3D space. Residues and loops that are in contact with the CD28 members are colored and in bold.
This Ig structural fold however is not a distinguishing feature for B7 members, as there are other receptors with similar extracellular folds including the butyrophilin family. The butyrophilin family members contain two Ig domains, a membrane distal IgV, and overall look similar to B7 members.40 Members of CD28 consist of a single Ig fold, and the structural layout is shared with many other immune receptors such as the T-cell Ig and mucin domain (TIM) family also having immunological functions.45 Likewise, the two Ig domain CD200 ligand is broadly expressed, while its inhibitory receptor CD200R, also composed of two Ig domains, is expressed on myeloid, NK, B-, and T-cells.46 CD200:CD200R have an interface similar to the B7-CD28 family.47 These examples demonstrate the versatility of the Ig fold and its prevalence in immunology, therefore not unique to B7-CD28 members.
The surfaces utilized by B7-CD28 members to interact, critical for antagonizing therapies blocking the interaction, are comprised of sidechain interactions from beta strands and loops at the interface. This is similar to the classic IgV domain pairing of the variable heavy and light chains of antibodies that can vary by their packing angle,48 which dictates the orientation of the two variable domains. In antibodies, this angle impacts how the binding loops are presented. By aligning and orienting the B7 IgV domains of complex structures (hPD-L1:hPD-1, mPD-L2:mPD1, CD80:CTLA-4, CD86:CTLA-4 and NCR3LG1:NKp30), differences in interface angles are apparent (Figure 2(b)) as previously described.49 The change in interface angle indicates different residues of the IgV domain can be utilized for protein interactions. The angles observed exhibit how CTLA-4 interacts differently with two closely related molecules, CD80 and CD86, whereas PD-1 interacts similarly with both of its ligands. A closer look at the IgV:IgV interface reveals that certain residues on beta strands C, Cʹ, Cʺ, F, and G share spatial positioning, but differ in charge and size of the sidechains, suggesting little is shared at this interface within the family (Figure 2(c)). This positional conservation at the interface is only apparent with structural alignment; IgV domain sequence alignment shows an average percent identity of 22% for B7 and 18% for CD28 members, with different residues aligned at some interface positions due to low conservation. Taken together, the available human complex structures show while they share similar structure, residues at the IgV interface differ in composition and contacts. This further highlights that though members are related through use of the Ig fold, and have beta strand interactions similar to other IgV:IgV complexes, the family has few consistencies in its interactions.
Therapeutic considerations
Since the FDA approval of cancer therapies targeting B7-CD28 family members, anti-CTLA-4 in 2011 and subsequent approvals for anti-PD-1 and PD-L1, there has been tremendous discovery and clinical efforts in reversing immune suppression. B7-CD28 members are amenable targets for antibody therapy, as they rely on extracellular domains to initiate contact and subsequent signaling. However, dissimilarities within B7-CD28 family members have made it difficult to utilize insights gained from research and clinical development targeting the potent PD-1 and CTLA-4 pathways.
Another important aspect to drug discovery is utilizing mouse models. As there is low sequence conservation from human to mouse (Figure 1(a,b)), obtaining cross-species reactive antibodies can be difficult, but more importantly this limits the functional relevance of mouse models. For example there is 50% or less sequence identity between mouse and human for CD80, CD86 and ICOSL, whereas human and rhesus share over 90% sequence identity for these receptors. To further highlight the immunological differences of human and mouse, there are no clear functional mouse orthologues for B7-H6,31 NKp30,50 or B7-H7.51 These differences between mouse and man, and the dynamics of the immune system, make interpreting and translating efficacy difficult for checkpoint inhibitors. An example of this is targeting CTLA-4, where tumor clearance in mouse has been linked to depletion of intratumoral regulatory T-cells,52 but this mechanism may not be reflected in the clinic.53
Discussion
While the core B7-CD28 family can be designated as a trio of related molecules on APC (CD80, CD86, ICOSL) and their counterparts on T-cells (CD28, CTLA-4, ICOS), the broad family criteria of regulating an immune response and containing an Ig fold have led to ever-increasing growth of the family. To illustrate this, NKp30 was initially identified on NK cells, without any notable relationship to the B7-CD28 family.54 A decade later, a previously unannotated gene with a sequence identity “comparable” to other B7 members, therefore named B7-H6 (NCR3LG1), was identified as its receptor.31 Consequently, NKp30 is now considered a CD28 member, and understanding its role in T-cells continues.55 Perhaps a more dramatic example that resulted from lack of similarity among family members is B- and T-lymphocyte attenuator (BTLA), which was initially proposed to be related to CTLA-4 and PD-1 with binding to B7-H4.56 However, it was later revealed that it did not bind B7-H4 and subsequently BTLA is no longer considered part of the B7-CD28 family.57 As two B7 members have not been de-orphanized, there may yet be additional CD28 members. An example of this is the developing story of B7-H5:VSIG-3, which raises the question of VSIG-3 being “CD28-like”. With these lax criteria, there may be more inclusions in the family. This fluidity of B7-CD28 members, with contractions and expansions, limits biological understanding and therapeutic development.
The overwhelming success of targeting the initial B7-CD28 members with therapeutic antibodies altered how cancer is treated. These advances have thrust the complex and dynamic interactions of immune cells into the spotlight, and discovery and classification of these diverse receptors and ligands have created a non-traditional family. Since members are only related in a broad sense, additional members have been included based on Ig domain similarities with little specific functionality in common. Ultimately, family classifications provide value in organizing proteins phylogenetically and evaluating function, but when a family expands without consistency, this value is diminished.
ACKNOWLEDGMENTS
We thank our colleagues Arvind Rajpal, Pavel Strop, John Engelhardt, and Cathy Bolger for their thoughtful comments and insights during the prepartion of this manuscript.
Authors’ contributions
SMW and XAD contributed equally to writing the review and have read and approved the final manuscript.
DECLARATION OF CONFLICTING INTERESTS
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
FUNDING
The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: SMW and XAD are employed by Bristol-Myers Squibb.
References
- 1.Rizvi NA, Hellmann MD, Snyder A, Kvistborg P, Makarov V, Havel JJ, Lee W, Yuan J, Wong P, Ho TS, Miller ML, Rekhtman N, Moreira AL, Ibrahim F, Bruggeman C, Gasmi B, Zappasodi R, Maeda Y, Sander C, Garon EB, Merghoub T, Wolchok JD, Schumacher TN, Chan TA. Cancer immunology. Mutational landscape determines sensitivity to PD-1 blockade in non-small cell lung cancer. Science 2015; 348:124–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hoos A. Development of immuno-oncology drugs – from CTLA4 to PD1 to the next generations. Nat Rev Drug Discov 2016; 15:235. [DOI] [PubMed] [Google Scholar]
- 3.Ni L, Dong C. New B7 family checkpoints in human cancers. Mol Cancer Ther 2017; 16:1203–11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Esensten JH, Helou YA, Chopra G, Weiss A, Bluestone JA. CD28 costimulation: from mechanism to therapy. Immunity 2016; 44:973–88 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Schildberg FA, Klein SR, Freeman GJ, Sharpe AH. Coinhibitory pathways in the B7-CD28 ligand-receptor family. Immunity 2016; 44:955–72 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Stashenko P, Nadler LM, Hardy R, Schlossman SF. Characterization of a human B lymphocyte-specific antigen. J Immunol 1980; 125:1678–85 [PubMed] [Google Scholar]
- 7.Nadler LM, Stashenko P, Hardy R, van Agthoven A, Terhorst C, Schlossman SF. Characterization of a human B cell-specific antigen (B2) distinct from B1. J Immunol 1981; 126:1941–7 [PubMed] [Google Scholar]
- 8.Dörken B, Moldenhauer G, Pezzutto A, Schwartz R, Feller A, Kiesel S, Nadler LM. HD39 (B3), a B lineage-restricted antigen whose cell surface expression is limited to resting and activated human B lymphocytes. J Immunol 1986; 136:4470–79 [PubMed] [Google Scholar]
- 9.Nadler LM, Anderson KC, Marti G, Bates M, Park E, Daley JF, Schlossman SF. B4, a human B lymphocyte-associated antigen expressed on normal, mitogen-activated, and malignant B lymphocytes. J Immunol 1983; 131:244–50 [PubMed] [Google Scholar]
- 10.Freedman AS, Boyd AW, Anderson KC, Fisher DC, Schlossman SF, Nadler LM. B5, a new B cell-restricted activation antigen. J Immunol 1985; 134:2228–35 [PubMed] [Google Scholar]
- 11.Freedman AS, Freeman G, Horowitz JC, Daley J, Nadler LM. B7, a B-cell-restricted antigen that identifies preactivated B cells. J Immunol 1987; 139:3260–67 [PubMed] [Google Scholar]
- 12.Hara T, Fu SM, Hansen JA. Human T cell activation. II. A new activation pathway used by a major T cell population via a disulfide-bonded dimer of a 44 kilodalton polypeptide (9.3 antigen). J Exp Med 1985; 161:1513–24 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Linsley PS, Clark EA, Ledbetter JA. T-cell antigen CD28 mediates adhesion with B cells by interacting with activation antigen B7/BB-1. Proc Natl Acad Sci 1990; 87:5031–5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Linsley PS, Brady W, Urnes M, Grosmaire LS, Damle NK, Ledbetter JA. CTLA-4 is a second receptor for the B cell activation antigen B7. J Exp Med 1991; 174:561–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Harper K, Balzano C, Rouvier E, Mattei MG, Luciani MF, Golstein P. CTLA-4 and CD28 activated lymphocyte molecules are closely related in both mouse and human as to sequence, message expression, gene structure, and chromosomal location. J Immunol 1991; 147:1037–44 [PubMed] [Google Scholar]
- 16.Cohen J. New protein steals the show as 'costimulator' of T cells. Science 1993; 262:844–5 [DOI] [PubMed] [Google Scholar]
- 17.Fernandez-Ruiz E, Somoza C, Sanchez-Madrid F, Lanier LL. CD28/CTLA-4 ligands: the gene encoding CD86 (B70/B7.2) maps to the same region as CD80 (B7/B7.1) gene in human chromosome 3q13-q23. Eur J Immunol 1995; 25:1453–6 [DOI] [PubMed] [Google Scholar]
- 18.Hathcock KS, Laszlo G, Pucillo C, Linsley P, Hodes RJ. Comparative analysis of B7-1 and B7-2 costimulatory ligands: expression and function. J Exp Med 1994; 180:631–40 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Linsley PS, Greene JL, Tan P, Bradshaw J, Ledbetter JA, Anasetti C, Damle NK. Coexpression and functional cooperation of CTLA-4 and CD28 on activated T lymphocytes. J Exp Med 1992; 176:1595–604 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gribben JG, Freeman GJ, Boussiotis VA, Rennert P, Jellis CL, Greenfield E, Barber M, Restivo VA, Jr, Ke X, Gray GS. CTLA4 mediates antigen-specific apoptosis of human T cells. Proc Natl Acad Sci USA 1995; 92:811–5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Krummel MF, Allison JP. CD28 and CTLA-4 have opposing effects on the response of T cells to stimulation. J Exp Med 1995; 182:459–65 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Walunas TL, Lenschow DJ, Bakker CY, Linsley PS, Freeman GJ, Green JM, Thompson CB, Bluestone JA. CTLA-4 can function as a negative regulator of T cell activation. Immunity 1994; 1:405–13 [DOI] [PubMed] [Google Scholar]
- 23.Hutloff A, Dittrich AM, Beier KC, Eljaschewitsch B, Kraft R, Anagnostopoulos I, Kroczek RA. ICOS is an inducible T-cell co-stimulator structurally and functionally related to CD28. Nature 1999; 397:263–6 [DOI] [PubMed] [Google Scholar]
- 24.Dong H, Zhu G, Tamada K, Chen L. B7-H1, a third member of the B7 family, co-stimulates T-cell proliferation and interleukin-10 secretion. Nat Med 1999; 5:1365–9 [DOI] [PubMed] [Google Scholar]
- 25.Freeman GJ, Long AJ, Iwai Y, Bourque K, Chernova T, Nishimura H, Fitz LJ, Malenkovich N, Okazaki T, Byrne MC, Horton HF, Fouser L, Carter L, Ling V, Bowman MR, Carreno BM, Collins M, Wood CR, Honjo T. Engagement of the PD-1 immunoinhibitory receptor by a novel B7 family member leads to negative regulation of lymphocyte activation. J Exp Med 2000; 192:1027–34 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wang S, Zhu G, Chapoval AI, Dong H, Tamada K, Ni J, Chen L. Costimulation of T cells by B7-H2, a B7-like molecule that binds ICOS. Blood 2000; 96:2808–13 [PubMed] [Google Scholar]
- 27.Chapoval AI, Ni J, Lau JS, Wilcox RA, Flies DB, Liu D, Dong H, Sica GL, Zhu G, Tamada K, Chen L. B7-H3: a costimulatory molecule for T cell activation and IFN-gamma production. Nat Immunol 2001; 2:269–74 [DOI] [PubMed] [Google Scholar]
- 28.Tseng SY, Otsuji M, Gorski K, Huang X, Slansky JE, Pai SI, Shalabi A, Shin T, Pardoll DM, Tsuchiya H. B7-DC, a new dendritic cell molecule with potent costimulatory properties for T cells. J Exp Med 2001; 193:839–46 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sica GL, Choi IH, Zhu G, Tamada K, Wang SD, Tamura H, Chapoval AI, Flies DB, Bajorath J, Chen L. B7-H4, a molecule of the B7 family, negatively regulates T cell immunity. Immunity 2003; 18:849–61 [DOI] [PubMed] [Google Scholar]
- 30.Zhu Y, Yao S, Iliopoulou BP, Han X, Augustine MM, Xu H, Phennicie RT, Flies SJ, Broadwater M, Ruff W, Taube JM, Zheng L, Luo L, Zhu G, Chen J, Chen L. B7-H5 costimulates human T cells via CD28H. Nat Commun 2013; 4:2043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Brandt CS, Baratin M, Yi EC, Kennedy J, Gao Z, Fox B, Haldeman B, Ostrander CD, Kaifu T, Chabannon C, Moretta A, West R, Xu W, Vivier E, Levin SD. The B7 family member B7-H6 is a tumor cell ligand for the activating natural killer cell receptor NKp30 in humans. J Exp Med 2009; 206:1495–503 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zhao R, Chinai JM, Buhl S, Scandiuzzi L, Ray A, Jeon H, Ohaegbulam KC, Ghosh K, Zhao A, Scharff MD, Zang X. HHLA2 is a member of the B7 family and inhibits human CD4 and CD8 T-cell function. Proc Natl Acad Sci USA 2013; 110:9879–84 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wang J, Wu G, Manick B, Hernandez V, Renelt M, Erickson C, Guan J, Singh R, Rollins S, Solorz A, Bi M, Li J, Grabowski D, Dirkx J, Tracy C, Stuart T, Ellinghuysen C, Desmond D, Foster C, Kalabokis V. VSIG-3 as a ligand of VISTA inhibits human T-cell function. Immunology 2019; 156:74–85 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Dong H, Strome SE, Salomao DR, Tamura H, Hirano F, Flies DB, Roche PC, Lu J, Zhu G, Tamada K, Lennon VA, Celis E, Chen L. Tumor-associated B7-H1 promotes T-cell apoptosis: a potential mechanism of immune evasion. Nat Med 2002; 8:793–800 [DOI] [PubMed] [Google Scholar]
- 35.Chattopadhyay K, Bhatia S, Fiser A, Almo SC, Nathenson SG. Structural basis of inducible costimulator ligand costimulatory function: determination of the cell surface oligomeric state and functional mapping of the receptor binding site of the protein. J Immunol 2006; 177:3920–9 [DOI] [PubMed] [Google Scholar]
- 36.Rost B. Twilight zone of protein sequence alignments. Protein Eng Des Selection 1999; 12:85–94 [DOI] [PubMed] [Google Scholar]
- 37.El-Gebali S, Mistry J, Bateman A, Eddy SR, Luciani A, Potter SC, Qureshi M, Richardson LJ, Salazar GA, Smart A, Sonnhammer ELL, Hirsh L, Paladin L, Piovesan D, Tosatto SCE, Finn RD. The Pfam protein families database in 2019. Nucleic Acids Res 2019; 47:D427–32 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Escors D, Gato-Canas M, Zuazo M, Arasanz H, Garcia-Granda MJ, Vera R, Kochan G. The intracellular signalosome of PD-L1 in cancer cells. Signal Transduct Target Ther 2018; 3:26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Rhodes DA, Reith W, Trowsdale J. Regulation of immunity by butyrophilins. Annu Rev Immunol 2016; 34:151–72 [DOI] [PubMed] [Google Scholar]
- 40.Arnett HA, Viney JL. Immune modulation by butyrophilins. Nat Rev Immunol 2014; 14:559–69 [DOI] [PubMed] [Google Scholar]
- 41.Bork P, Holm L, Sander C. The immunoglobulin fold. Structural classification, sequence patterns and common core. J Mol Biol 1994; 242:309–20 [DOI] [PubMed] [Google Scholar]
- 42.Sonnen AF, Yu C, Evans EJ, Stuart DI, Davis SJ, Gilbert RJ. Domain metastability: a molecular basis for immunoglobulin deposition? J Mol Biol 2010; 399:207–13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Vigdorovich V, Ramagopal UA, Lazar-Molnar E, Sylvestre E, Lee JS, Hofmeyer KA, Zang X, Nathenson SG, Almo SC. Structure and T cell inhibition properties of B7 family member, B7-H3. Structure 2013; 21:707–17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Mehta N, Maddineni S, Mathews II, Sperberg AP, Huang P-S, Cochran JR. Structure and functional binding epitope of V-domain Ig suppressor of T-cell activation (VISTA). bioRxiv 2019;597716. [DOI] [PubMed] [Google Scholar]
- 45.Freeman GJ, Casasnovas JM, Umetsu DT, DeKruyff RH. TIM genes: a family of cell surface phosphatidylserine receptors that regulate innate and adaptive immunity. Immunol Rev 2010; 235:172–89 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Rygiel TP, Meyaard L. CD200R signaling in tumor tolerance and inflammation: a tricky balance. Curr Opin Immunol 2012; 24:233–8 [DOI] [PubMed] [Google Scholar]
- 47.Hatherley D, Lea SM, Johnson S, Barclay AN. Structures of CD200/CD200 receptor family and implications for topology, regulation, and evolution. Structure 2013; 21:820–32 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Dunbar J, Fuchs A, Shi J, Deane CM. ABangle: characterising the VH–VL orientation in antibodies. Protein Eng Des Selection 2013; 26:611–20 [DOI] [PubMed] [Google Scholar]
- 49.Li Y, Wang Q, Mariuzza RA. Structure of the human activating natural cytotoxicity receptor NKp30 bound to its tumor cell ligand B7-H6. J Exp Med 2011; 208:703–14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Hollyoake M, Campbell RD, Aguado B. NKp30 (NCR3) is a pseudogene in 12 inbred and wild mouse strains, but an expressed gene in Mus caroli. Mol Biol Evol 2005; 22:1661–72 [DOI] [PubMed] [Google Scholar]
- 51.Janakiram M, Chinai JM, Zhao A, Sparano JA, Zang X. HHLA2 and TMIGD2: new immunotherapeutic targets of the B7 and CD28 families. Oncoimmunology 2015; 4:e1026534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Selby MJ, Engelhardt JJ, Quigley M, Henning KA, Chen T, Srinivasan M, Korman AJ. Anti-CTLA-4 antibodies of IgG2a isotype enhance antitumor activity through reduction of intratumoral regulatory T cells. Cancer Immunol Res 2013; 1:32–42 [DOI] [PubMed] [Google Scholar]
- 53.Sharma A, Subudhi SK, Blando J, Scutti J, Vence L, Wargo JA, Allison JP, Ribas A, Sharma P. Anti-CTLA-4 immunotherapy does not deplete FOXP3+ regulatory T cells (Tregs) in human cancers. Clin Cancer Res 2018; 0762 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Pende D, Parolini S, Pessino A, Sivori S, Augugliaro R, Morelli L, Marcenaro E, Accame L, Malaspina A, Biassoni R, Bottino C, Moretta L, Moretta A. Identification and molecular characterization of NKp30, a novel triggering receptor involved in natural cytotoxicity mediated by human natural killer cells. J Exp Med 1999; 190:1505–16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Correia MP, Stojanovic A, Bauer K, Juraeva D, Tykocinski LO, Lorenz HM, Brors B, Cerwenka A. Distinct human circulating NKp30(+)FcepsilonRIgamma(+)CD8(+) T cell population exhibiting high natural killer-like antitumor potential. Proc Natl Acad Sci USA 2018; 115:E5980–E9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Watanabe N, Gavrieli M, Sedy JR, Yang J, Fallarino F, Loftin SK, Hurchla MA, Zimmerman N, Sim J, Zang X, Murphy TL, Russell JH, Allison JP, Murphy KM. BTLA is a lymphocyte inhibitory receptor with similarities to CTLA-4 and PD-1. Nat Immunol 2003; 4:670–9 [DOI] [PubMed] [Google Scholar]
- 57.Sedy JR, Gavrieli M, Potter KG, Hurchla MA, Lindsley RC, Hildner K, Scheu S, Pfeffer K, Ware CF, Murphy TL, Murphy KM. B and T lymphocyte attenuator regulates T cell activation through interaction with herpesvirus entry mediator. Nat Immunol 2005; 6:90–8 [DOI] [PubMed] [Google Scholar]