ABSTRACT
Background
Mesenchymal stem cells (MSCs) are becoming an increasingly attractive option for regenerative therapies due to their availability, self‐renewal capacity, multilineage potential, and anti‐inflammatory properties. Clinical trials are underway to test the efficacy of stem cell‐based therapies for the repair and regeneration of the degenerate intervertebral disc (IVD), a major cause of back pain. Recently, both bone marrow‐derived MSCs and adipose‐derived stem cells (ASCs) have been assessed for IVD therapy but there is a lack of knowledge surrounding the optimal cell source and the response of transplanted cells to the low oxygen, pro‐inflammatory niche of the degenerate disc. Here, we investigated several neurovascular factors from donor‐matched MSCs and ASCs that may potentiate the survival and persistence of sensory nerve fibers and blood vessels present within painful degenerate discs and their regulation by oxygen tensions and inflammatory cytokines.
Methods
Donor‐matched ASCs and MSCs were conditioned with either IL‐1β or TNFα under normoxic (21% O2) or hypoxic (5% O2) conditions. Expression and secretion of several potent neurovascular factors were assessed using qRT‐PCR and human magnetic Luminex assay.
Results
ASCs and MSCs expressed constitutive levels of key neurotrophic factors; and stimulation of ASCs with hypoxia triggered increased secretion of both angiogenic factors (Ang‐2 and VEGF‐A) and neurotrophic (NGF and NT‐3) compared to MSCs. We also report increased transcriptional regulation of pain‐associated neuropeptides in hypoxia stimulated ASCs compared to those in normoxic conditions. We demonstrate transcriptional and translational upregulation of NGF, NT‐3, Ang‐1, and FGF‐2 in response to cytokines in ASCs in 21% and 5% O2.
Conclusions
This work highlights fundamental differences between the neurovascular secretome of donor‐matched ASCs and MSCs, demonstrating the importance of cell‐selection for tissue specific regeneration to reduce ectopic sensory nerve and blood vessel survival and improve patient outcomes.
Keywords: disc degeneration, intervertebral disc, mesenchymal stem cells, regenerative therapy, stem cells
The objectives of this study were to establish whether there is a difference in expression of angiogenic and neurotrophic factors between undifferentiated donor‐matched ASCs and MSCs and whether the expression of these factors is affected by culture in a hypoxic or inflammatory environment alone, or in combination. The data presented here highlights increased responsiveness of ASCs to cytokine stimulation under low oxygen by enhancing secretion of factors involved in neurogenesis (NGF and NT‐3) and neoangiogenesis (VEGF‐A and Ang‐1)within the IVD; a process which could lead to persistent discogenic pain.
Abbreviations
- AF
annulus fibrosus
- Ang‐1
angiopoietin‐1
- Ang‐2
angiopoietin‐2
- ASCs
adipose‐derived mesenchymal stem cells
- BDNF
brain‐derived neurotrophic factor
- CGRP
calcitonin gene related peptide
- ELISA
enzyme‐linked immunosorbent assay
- FGF‐2
fibroblastic growth factor
- IVD
intervertebral disc
- IDD
intervertebral disc degeneration
- IL‐1β
interleukin‐1 beta
- LBP
low back pain
- MSCs
mesenchymal stem cells
- NP
nucleus pulposus
- NGF
nerve growth factor
- NT‐3
neurotrophin‐3
- qRT‐PCR
quantitative real‐time polymerase chain reaction
- TNFα
tumor necrosis factor alpha
- VEGF‐A
vascular endothelial growth factor‐A
1. BACKGROUND
In recent years, mesenchymal stem cell (MSC)‐based therapies have been tested in a wide array of degenerative conditions, including musculoskeletal diseases, Crohn's disease, complications of diabetes, and others. There is a growing clinical database and available results support the preliminary conclusion that these strategies can provide an effective regenerative or disease‐modifying outcome. A definitive conclusion will become available when late stage clinical trials are completed. Pain is a key feature of many musculoskeletal conditions, including, for example, chronic severe lower back pain associated with intervertebral disc (IVD) disease (IDD) or chronic joint pain in osteoarthritis. The impact on patients is immense and leads to reduced quality of life and sometimes serious addictions to opioid medications.
Low back pain (LBP) is an increasingly common health problem and a major cause of disability worldwide, resulting in its inclusion in the World Health Organization's list of 12 priority diseases. Total annual costs associated with back pain are estimated in excess of €12 billion across Europe. Both the UK and the US report LBP as being the most common cause of disability amongst young adults, leading to a loss of more than 100 and 149 million workdays per year, respectively.1 It is starkly clear that as the world population ages, the disease burden of LBP will increase substantially. The causes of LBP remain multifactorial, although approximately 40% of all cases are attributed to the degeneration of one or more IVDs.2
Current treatments include conservative symptomatic pain relief or end‐stage surgical treatments that are often ineffective as they are symptom‐, rather than disease‐modifying.3, 4, 5 Thus, regenerative therapies targeting the degenerate IVD have emerged at the forefront of research within the spinal field; with considerable attention focusing on the development of cell‐based therapeutics that address the underlying pathogenesis of degenerative disc disease.6, 7, 8 Many groups have studied the potential use of MSCs from bone‐marrow or adipose (ASCs) to repair and regenerate the damaged IVD, either alone or in combination with native NP cells, sometimes delivered using biomaterial scaffolds and carriers.9, 10 Stem cells are an attractive option for regenerative therapies due to their availability, self‐renewal capacity, multilineage potential, and immunosuppressive properties. One particular question is the source of stem cells, and various studies have investigated the differences between MSCs and ASCs for their regenerative potential.
Clinical trials are underway to elucidate a successful stem cell‐based therapy for the repair and regeneration of IVD tissue, with preclinical and phase I data showing promising results for the safety and efficacy of allogeneic and autologous ASCs and MSCs.11, 12, 13, 14, 15 Although cell‐therapies have been reported to increase hydration and flexibility of the affected disc, reduce pain, and in some instances, increase disc height, there is a subset of patients who do not show improvement in pain score, thus failing to meet primary endpoints. The causes of patient nonresponse are inconclusive, yet it could be suggested that the delivered MSCs respond negatively to the degenerate microenvironment resulting in persistent pain following delivery.11, 13 While several in vivo studies have demonstrated MSC engraftment and long‐term survival in the harsh environment of normal disc tissue,16, 17, 18, 19, 20 the capacity of MSCs and ASCs to stimulate the survival and persistence of nerve and blood vessels within painful discs has not been addressed.
This study aimed to test the hypothesis that hypoxic and pro‐inflammatory conditioning mimicking that of the degenerate IVD, would modify the expression and secretory profile of donor‐matched ASCs and MSCs by enhancing the expression of potent angiogenic and neurotrophic factors, thus exacerbating pain in patients with innervated discs.
Our objectives were to establish whether there is a difference in expression of angiogenic and neurotrophic factors between undifferentiated ASCs and MSCs and whether the expression of these factors is affected by culture in a hypoxic or inflammatory environment alone, or in combination.
To the authors' knowledge, this is the first study to investigate and report fundamental differences in the neurovascular expression and secretory profile of ASCs and MSCs, identifying an optimal cell source for regenerative therapies of the IVD to reduce the occurrence of neural and vascular growth and improve clinical outcomes.
2. METHODS
2.1. Isolation and culture of MSCs and ASCs
All procedures and experiments were performed with relevant National Research Ethics Service and University of Manchester approvals. Bone marrow from proximal femur and subcutaneous adipose tissue samples were obtained from three donors (one male, two females, mean age 50 years, range 40‐62 years) undergoing hip replacement surgery who provided written informed consent.
Cells were isolated from bone marrow and adipose tissue as previously described.21 Briefly, bone marrow aspirates were washed with PBS and then centrifuged to obtain a cell pellet, and the pellet was resuspended in Minimum Essential Medium α‐modification (αMEM; Sigma‐Aldrich) which included nonessential amino acids, 110 mg/L sodium pyruvate, 1000 mg/L glucose supplemented with final concentrations of 100 U/mL penicillin, 100 μg/mL streptomycin and 0.25 μg/mL amphotericin, 2 mM GlutaMAX (Life Technologies), and 20% (vol/vol) fetal bovine serum (FBS). MSCs were isolated using density gradient centrifugation as previously described.21, 22 Adipose tissue was finely minced with a scalpel and then ASCs were isolated enzymatically as previously described.23 Both cell types were maintained at 37°C in a humidified atmosphere containing 5% (vol/vol) CO2; the culture medium was changed after 5 days and cells cultured to ∼80% confluence in complete αMEM as above supplemented with 10% FBS. MSC and ASC phenotype was confirmed as previously described (data not shown).21, 23, 24
2.2. Cytokine regulation of angiogenic factors, neurotrophic factors, and pain peptides in donor‐matched adipose and bone‐marrow‐derived MSCs
Following expansion to passage three, ASCs and MSCs were plated into wells of a 12‐well culture plate at a density of 50 000 cells per well. The following day cells were washed and exposed to low serum medium (complete medium as above supplemented with 2% FCS) either alone or with the addition of IL‐1β (10 ng/mL) or TNFα (10 ng/mL) for 48 hours in either 21% or 5% O2. All conditions were performed in technical triplicate. Following 48 hours conditioned medium was collected and stored at −80°C until required for further analysis.
2.3. Metabolic activity of MSCs under different oxygen conditions and cytokine stimulations
The metabolic cellular activity of ASCs and MSCs cultured at either 21% or 5% O2 was assessed using Alamar Blue assay (Thermo) in low serum media (2% FBS) at day 0 and day 2 as per the manufacturers protocol. The absorbance was recorded using a fluorescence microplate reader (TECAN) fluorescence excitation and emission wavelengths of 590/540 nm. Fluorescence was recorded for cellular constructs and normalized to the fluorescence of day 0 samples as an indication of cytotoxicity/proliferation.
2.4. Quantitative real‐time PCR
After 48 hours in normoxic or hypoxic culture with or without cytokine stimulation RNA was extracted using PureLink RNeasy Spin Columns (Invitrogen) as per manufacturer's instructions and quantification performed using the NanoDrop (Thermo). cDNA was synthesized using the High‐Capacity cDNA Reverse Transcription Kit (Applied Biosystems) as per manufacturer's instructions and diluted to a final concentration of 5 ng/μL for use in quantitative real‐time polymerase chain reaction (qRT‐PCR).
qRT‐PCR was performed on a StepOnePlus Real‐Time PCR System (Applied Biosystems) in order to investigate gene expression levels of angiogenic factors (Angiopoietin‐1 [Ang‐1], Ang‐2, basic fibroblastic growth factor [FGF‐2], and vascular endothelial growth factor‐A [VEGF‐A]); neurotrophic factors (nerve growth factor [NGF], brain‐derived neurotrophic factor [BDNF], and neurotrophin‐3 [NT‐3]), and pain‐related peptides (substance P and calcitonin gene related peptide [CGRP]).
Reactions were prepared in duplicate using Fast SYBR Green Master Mix (Applied Biosystems, UK) and predesigned KiCqStart Primers (Sigma‐Aldrich; Table 1) to a total volume of 10 μL, containing 10 ng cDNA and 600 nM each primer. Data were analyzed according to the 2−ΔCt method, with expression normalized to 18S reference gene.
Table 1.
Primer sequences used in qRT‐PCR reactions
| Gene | Forward primer (5′➔3′) | Reverse Primer (5′➔3′) | Accession number |
|---|---|---|---|
| 18S | ATCGGGGATTGCAATTATTC | CTCACTAAACCATCCAATCG | NM_003286 |
| NGF | GGTGCATAGCGTAATGTC | TGAAGTTTAGTCCAGTGGG | NM_002506 |
| BDNF | CAAAAGTGGAGAACATTTGC | AACTCCAGTCAATAGGTCAG | NM_001143811 |
| NT‐3 | CGATCTTACAGGTGAACAAG | AATTTTCCTTAACGTCCACC | NM_001102654 |
| Ang‐1 | ATGTTAACAGGAGGATGGTG | GAAGTAGTGCCACTTTATCC | NM_001199859 |
| Ang‐2 | AAGAGAAAGATCAGCTACAGG | CCTTAGAGTTTGATGTGGAC | NM_001118887 |
| FGF‐2 | TGGCTTCTAAATGTGTTACG | GTTTATACTGCCCAGTTCG | NM_002006 |
| VEGF‐A | AATGTGAATGCAGACCAAAG | GACTTATACCGGGATTTCTTG | NM_001204384 |
| Substance P | ATTCTGTGGCTTATGAAAGG | CATTGACACAAATGAAGCTG | NM_003182 |
| CGRP | CAGCTGAATGACTCAAGAAG | GATGCACAATAGGTAACTGC | NM_001033953 |
2.5. Magnetic Luminex Assay analysis of conditioned medium of cytokine stimulated donor‐matched MSCs under normoxic and hypoxic conditions
Levels of Ang‐1, Ang‐2, FGF‐2, and VEGF‐A, NGF, BDNF, and NT‐3, were measured in cell culture supernatant from three patients in technical duplicates using quantitative Luminex assays (R&D Systems) according the manufacturer's instructions, and analyzed using the Biorad Bioplex 200 multiplex ELISA system.
2.6. Enzyme‐linked immunosorbent assay
Substance P levels were measured in cell culture supernatant from three patients in technical duplicate using an ELISA for detection of human substance P (Abcam) as per manufacturer's instructions and the absorbance read at 450 nm and 570 nm using a Victor X3 plate reader (PerkinElmer).
2.7. Statistical analysis
Data were assessed for normality using the Shapiro‐Wilks test and found to be nonparametric. Statistical significance determined using two‐way ANOVA with Tukey's multiple comparisons test, * = P ≤ .05.
3. ETHICAL APPROVAL AND CONSENT TO PARTICIPATE
All procedures and experiments were performed with relevant National Research Ethics Service and University of Manchester approvals. Bone marrow and subcutaneous adipose tissue samples were obtained from three donors undergoing hip replacement surgery who provided written informed consent.
4. RESULTS
4.1. Metabolic activity of ASCs and MSCs was not affected by hypoxic conditions or cytokine stimulation
As a method of confirming cell viability and proliferation, we examined the metabolic activity of MSCs under different treatment conditions. Metabolic activity of ASCs (Figure 1A) and MSCs (Figure 1B) was significantly increased after 48 hours under 21% and 5% O2 irrespective of cytokine stimulation compared to day 0. There were no significant differences between unstimulated controls and cytokine stimulated groups at the same time points and there were no significant differences between cells cultured under 21% or 5% O2 conditions at the same time points. Thus, no changes in metabolic activity of cells were observed due to oxygen levels or cytokine concentration after 48 hours.
Figure 1.
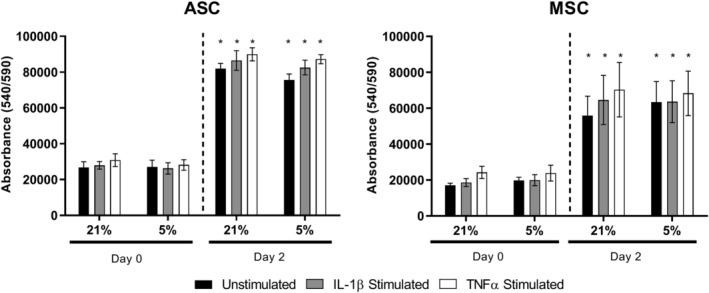
Metabolic activity of ASCs and MSCs exposed to cytokines IL‐1β or TNFα under 21% or 5% oxygen. AlamarBlue assessment of metabolic activity of human donor‐matched ASCs (A) and MSCs (B) under either 21% or 5% O2 and stimulated with either 10 ng/ml of IL‐1β or TNFα for 48 hours. n = 3. Values represent the mean ± SEM. Statistical significance determined using two‐way ANOVA with Tukey's multiple comparisons test, * = P ≤ .05 relative to corresponding group on day 0
4.2. Angiogenic factor expression in ASCs and MSCs in response to low oxygen and inflammatory cytokines
The expression and secretion of several angiogenic factors from donor‐matched MSCs were assessed following stimulation with cytokines under different oxygen concentrations. We report that Ang‐1 expression from ASCs was significantly upregulated at gene and protein level by IL‐1β under 5% O2 (Figure 2A,B) and at protein level only under 21% O2 (Figure 2B). Ang‐1 was significantly upregulated by TNFα in MSCs under 5% O2 compared to unstimulated controls at gene level, with similar insignificant increases seen at secreted protein level (Figure 2A,B).
Figure 2.
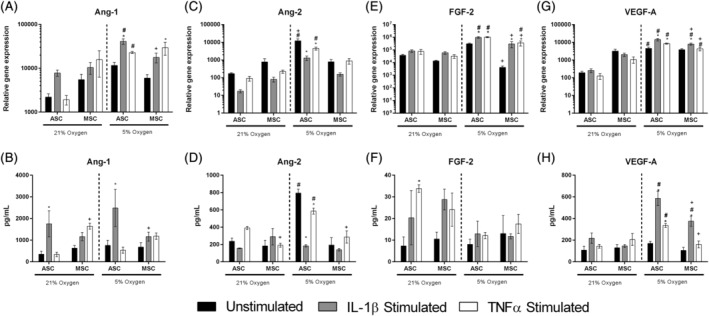
Gene and protein regulation of pro‐angiogenic factors in human donor‐matched ASCs and MSCs exposed to cytokines IL‐1β or TNFα under 21% or 5% oxygen. Pro‐angiogenic factors Ang‐1 (A,B), Ang‐2 (C, D), FGF‐2 (E, F) and VEGF‐A (G, H) mRNA expression (A, C, E, G) and levels of secreted protein (B, D, F, H) in human ASCs and MSCs stimulated with either IL‐1β or TNFα under different oxygen concentrations for 48 hours. Gene expression was normalized to that of reference gene 18S. Values represent the mean ± SEM. Statistical significance determined using two‐way ANOVA with Tukey's multiple comparisons test, * = P ≤ .05 (* = significant compared to unstimulated control within the same group; # = significant compared to corresponding sample in 21% oxygen; + = significance between MSCs and ASCs of the same treatment; n = 3)
Ang‐2 expression was not regulated by cytokines from ASCs or MSCs under 21% oxygen at gene or protein level (Figure 2C,D), yet levels of Ang‐2 were significantly higher in ASCs under 5% O2 compared to 21% O2, and cytokines IL‐1β and TNFα significantly reduced expression of Ang‐2 at gene and protein level in ASCs under 5% O2 compared to unstimulated controls (Figure 2C,D).
FGF‐2 levels were not transcriptionally regulated by cytokines in ASCs or MSCs under 21% O2 (Figure 2E); however, secreted levels of FGF‐2 were significantly increased by TNFα in ASCs under 21% O2 compared to unstimulated controls (Figure 2F). Significant upregulation of FGF‐2 at gene level was evident in ASCs following cytokine stimulation (Figure 2E); however, secreted levels of FGF‐2 remained unaffected by low oxygen and cytokines (Figure 2F).
VEGF‐A was not regulated by cytokines under 21% O2 at gene (Figure 2G) or protein level (Figure 2H). Under 5% O2, VEGF‐A levels were significantly upregulated by IL‐1β and TNFα in ASCs at gene and protein level (Figure 2G‐H). Additionally, MSCs significantly upregulated gene and protein levels of VEGF‐A in response to IL‐1β but not TNFα (Figure 2G‐H).
4.3. Neurotrophic factor expression in ASCs and MSCs in response to low oxygen and inflammatory cytokines
Here, we aimed to assess the effect of inflammatory factors and different oxygen levels on the expression and secretion of several neurotrophic factors from donor‐matched MSCs. While NGF gene expression was not regulated by cytokines under 21% O2 in ASCs or MSCs, ASCs significantly increased its expression when cultured in 5% O2 (Figure 3A). Under hypoxia both ASCs and MSCs responded to TNFα stimulation with an increase in NGF, but a smaller increase was observed following IL‐1β stimulation, yet this change failed to reach significance (Figure 3A). At protein level, NGF secretion into the culture media was significantly increased following TNFα stimulation in both 21% and 5% O2 from ASCs; however, levels were significantly higher from ASCs under 5% O2 compared to 21% O2 (Figure 3B).
Figure 3.
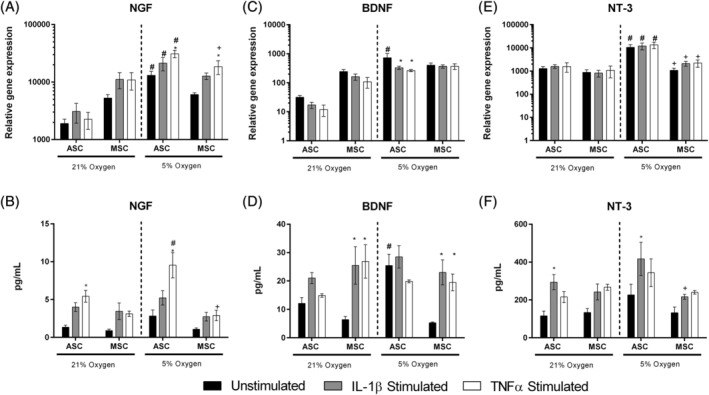
Gene and protein regulation of neurotrophic factors in human donor‐matched ASCs and MSCs exposed to cytokines IL‐1β or TNFα under 21% or 5% oxygen. Neurotrophic factors NGF (A,B), BDNF (C, D) and NT‐3 (E, F) mRNA expression (A,C,E) and secreted protein levels (B,D,F) in human ASCs and MSCs stimulated with either IL‐1β or TNFα under different oxygen concentrations for 48 hours. Gene expression was normalized to that of reference gene 18S. Values represent the mean ± SEM. Statistical significance determined using two‐way ANOVA with Tukey's multiple comparisons test, significance reported if P ≤ .05 (* = significant compared to unstimulated control within the same group; # = significant compared to corresponding sample in 21% oxygen; + = significance between MSCs and ASCs of the same treatment; n = 3)
BDNF gene expression levels were significantly higher in ASCs under 5% O2 compared to ASCs in 21% O2 (Figure 3C). Gene expression was significantly downregulated by IL‐1β and TNFα stimulation in 5% O2 compared to the corresponding unstimulated control (Figure 3C). BDNF expression from MSCs was not altered by cytokine stimulation or O2 levels at gene level (Figure 3C), yet secreted levels of BDNF were significantly upregulated by IL‐1β and TNFα in MSCs at both 21% and 5% O2 compared to unstimulated controls (Figure 3D). BDNF secretion from ASCs was not altered by cytokine stimulation, yet unstimulated ASCs under 5% O2 secreted significantly higher levels of BDNF than unstimulated ASCs under 21% O2 (Figure 3D).
Gene expression of NT‐3 was not altered by cytokine stimulation in either cell type (Figure 3E), yet levels of secreted NT‐3 were significantly increased upon IL‐1β stimulation in ASCs at 21% and 5% O2 (Figure 3F).
4.4. Neuropeptides were transcriptionally regulated by low oxygen in ASCs and MSCs
Here, we aimed to investigate the potential of MSCs to produce pain related peptides in response to low oxygen and inflammatory cytokines, which could subsequently lead to sensitization of ectopic sensory nerve fibers, or increase inflammatory cytokine production from NP cells as previously shown. Substance P gene expression was significantly higher in unstimulated ASCs and MSCs cultured under 5% O2 compared to 21% O2 (Figure 4A). Expression of substance P from ASCs under 5% O2 was downregulated by TNFα stimulation (Figure 4A). Substance P expression was not altered in MSCs in response to cytokine stimulation (Figure 4A). Substance P secretion from both ASCs and MSCs were below the limit of detection (< 9.76 pg/mL) regardless of oxygen levels or cytokine stimulation (data not shown).
Figure 4.
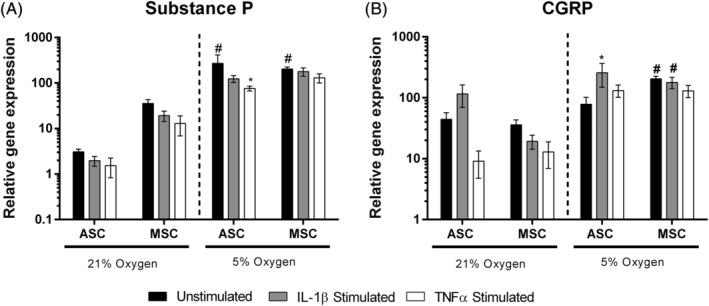
Regulation of neuropeptides in human donor‐matched ASCs and MSCs exposed to cytokines IL‐1β or TNFα under 21% or 5% oxygen. Substance P (A) and CGRP (B) mRNA expression in human ASCs and MSCs stimulated with either IL‐1β or TNFα under different oxygen concentrations for 48 hours. Gene expression was normalized to that of reference gene 18S. Values represent the mean ± SEM. Statistical significance determined using two‐way ANOVA with Tukey's multiple comparisons test, * = P ≤ .05 (* = significant compared to unstimulated control within the same group; # = significant compared to corresponding sample in 21% oxygen; + = significance between MSCs and ASCs of the same treatment; n = 3)
CGRP expression remained unaffected by cytokines in either ASCs or MSCs under 21% O2 (Figure 4B). Gene expression levels of CGRP were significantly upregulated by IL‐1β in ASCs under 5% O2 compared to unstimulated controls (Figure 4B). CGRP levels were significantly higher in MSCs under 5% O2 compared to MSCs under 21% O2 (Figure 4B).
5. DISCUSSION
Cell‐based therapies aim to intercept disease progression through cellular repopulation and exploitation of the immunomodulatory capacity of ASCs and MSCs,25 yet it remains unclear which source of stem cell provides superior functionality for regeneration of the IVD. Interestingly, all cellular and biological therapeutics to date fail to address the presence of ectopic sensory nerve fibers within a subset of painful degenerate IVDs. Of the published clinical trials thus far investigating cell therapies for regeneration of the IVD, several report a cohort of non‐responders; yet it remains unclear as to why these patients do not show any improvements in pain and disability scores. With several reports highlighting the regenerative capacity of ASCs and MSCs in neural and vascular pathologies,26 a likely cause of persistent pain in patients with innervated IVDs is the stem cell‐mediated survival and migration of neural and vascular networks following delivery.
To the best of our knowledge, this is the first report addressing the neurovascular response of human donor‐matched ASCs and MSCs to two characteristic features of degenerate discs; low oxygen and pro‐inflammatory cytokines. It is widely acknowledged that vascularization is accompanied by neural ingrowth within the IVD and so it is important to demonstrate how stem cell‐therapies may promote the persistence of these structures following implantation.27, 28, 29 Therefore, it is important to demonstrate how stem cell‐therapies may promote the persistence of these structures following implantation. Here, we report constitutive expression of several potent angiogenic and neurotrophic factors from ASCs and MSCs and their regulation by low oxygen and cytokines IL‐1β and TNFα.
Vascularization of the degenerate IVD is thought to occur due to alterations in the matrix composition and resultant changes in intradiscal pressure, permitting the survival and inward migration of NGF expressing microvascular blood vessels, and concomitant sensory nerve fibers. Comparison studies investigating the therapeutic potential of ASCs and MSCs following hindlimb ischemia highlight inconsistencies in their ability to re‐vascularize damaged tissue. Studies by Bortolotti et al report effective perfusion and functional recovery following ischemia by MSCs, reporting increased levels of factors involved in vessel remodeling and stabilization, although no differences in Ang‐1 or VEGF‐A were reported.30 In contrast, reports by Kim et al demonstrated superior recovery of blood flow in an ASC treated group, with conditioned medium from ASCs also demonstrating heightened tube formation in vitro compared to MSCs.31 Other studies largely conclude that the therapeutic potential of stem cells is attributed to their differentiation into vascular endothelial cells but also to their ability to produce various angiogenic factors, including VEGF and FGF‐2.31, 32, 33 In the current study, we report increased transcriptional and translational expression of pro‐angiogenic VEGF‐A in response to reduced oxygen in ASCs and MSCs with higher levels evident in ASCs. We also report increased secretion of VEGF‐A from ASCs stimulated with IL‐1β under 5% oxygen. This study demonstrated significantly higher levels of other pro‐angiogenic factors including Ang‐1, Ang‐2, and FGF‐2 at gene level from ASCs when exposed to 5% oxygen compared to 21% oxygen. The presence of Ang‐1 was documented in 2012 by Sakai et al who determined Ang‐1 mRNA expression from disc progenitor cells as well as secreted Ang‐1 within the disc cell culture medium.34 The proposed role of angiogenic factors within a region that is largely avascular surrounds the maintenance and survival of native NP cells that have constitutive expression of HIF1α. Similarly, reports of FGF‐2 localization within the cells of IVD suggest roles for these factors in regulation and survival of NP cells under such hostile conditions.35 The increased levels of angiogenic factors from ASCs and MSCs in response to an environment mimicking that of the target tissue could result in neo‐angiogenesis and stimulation of preexisting blood vessels within the damaged tissue; in turn stimulating concomitant nerve fiber ingrowth.
Neurotrophic factors are associated with the survival, proliferation and migration of neurons, and increased levels of NGF, BDNF, and NT‐3 were evident in the present study from both ASCs and MSCs. NGF and NT‐3 were transcriptionally and translationally upregulated in hypoxic cultured ASCs compared with MSCs, with significantly increased secretion into the culture medium following TNFα conditioning. Previous studies by Redondo‐Castro et al reported native levels of trophic factors from MSCs under 21% oxygen.36 The authors assessed the response of MSCs to IL‐1β and TNFα stimulation in vitro; and in agreement with this study failed to demonstrate cytokine‐mediated NGF secretion. Interestingly however, we report significant TNFα‐mediated upregulation of NGF and IL‐1β‐mediated increases in NT‐3 from ASCs. MSCs but not ASCs increased secretion of BDNF following IL‐1β and TNFα stimulation. It has been hypothesized that ASC differentiation into a neuronal phenotype is enhanced when expression levels of BDNF and NT‐3 are increased simultaneously.37 This was demonstrated by transfection studies, showing increased ASC‐neuronal differentiation in BDNF and NT‐3 overexpressing ASCs alone and in combination. The authors hypothesized this may be due to concurrent increases in their tyrosine kinase receptors.37 Together with the data presented in this study, it could be further hypothesized that ASCs may alter their receptor profiles under different oxygen tensions and therefore become more responsive to cytokines, eliciting increased neurotrophic capabilities compared to MSCs.
Neuropeptide substance P is recognized as a pleiotropic molecule able act on sensory nerve fibers as a classical nociceptor, but more recently reported for its ability to enhance the stem cell phenotype and homing of MSCs to the site of injury.38 Neuropeptides are produced by degenerate NP cells39, 40, 41, 42, 43 and their regulation by pro‐inflammatory cytokines IL‐1β and TNFα is well documented.40, 41 Here, we report significant transcriptional regulation of substance P by low oxygen in ASCs yet this was not evident in the conditioned medium. Induction of substance P gene expression and release has been documented previously in transdifferentiated neural MSCs stimulated with IL‐1α,44 suggesting the induction of a neural phenotype is required for the production and release of substance P into the environment.
The data presented here highlights increased responsiveness of ASCs to cytokine stimulation under low oxygen by enhancing secretion of factors involved in neurogenesis and neo‐angiogenesis within the IVD; a process that could lead to persistent discogenic pain. Although we report that naïve ASCs have a greater potential to increase their expression and secretion of factors involved in nerve survival and vascularization under hypoxic and inflammatory conditions, the minimally invasive availability of these stem cells is still an attractive source for therapy. ASCs retrieved from adipose tissue following liposuction plastic surgeries have been shown to be up to 500 times more prevalent than MSCs when comparing an equivalent volume of tissue.45 ASCs may benefit from being predifferentiated or preconditioned prior to delivery to reduce their responsiveness to low oxygen levels and the inflammatory milieu (Figure 5). Clarke et al were the first to report the use of growth differentiation factor‐6 (GDF‐6) as a differentiation factor promoting NP‐like differentiation of ASCs in replicating matrix synthesis and gene expression of classical NP markers.23 It could therefore be hypothesized that GDF‐6 pre‐differentiated ASCs could enhance NP cell repair as well as attenuating the inflammatory and neurovascular environment as recently reported by Miyazacki et al.46
Figure 5.
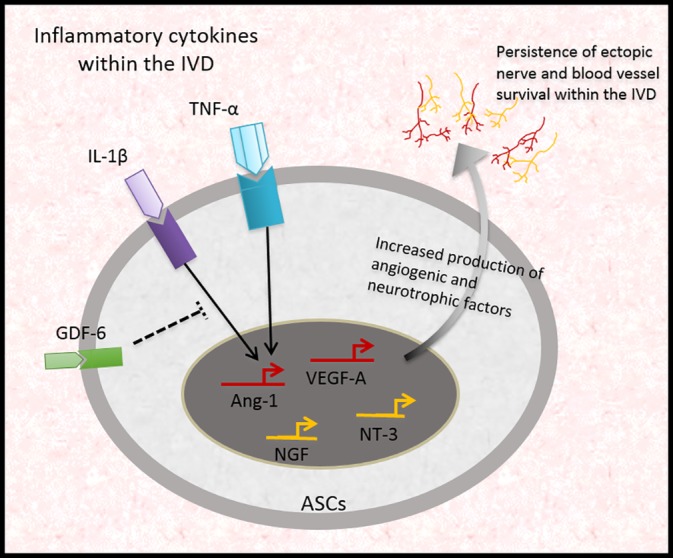
The role of inflammatory cytokines on ASCs under low oxygen environment and their implications in regulating neurovascular persistence and survival within the degenerate IVD. The highly inflammatory nature of the degenerate IVD will expose delivered stem cells to multiple pro‐inflammatory cytokines. TNFα and IL‐1β increased expression of neurotrophic and angiogenic factor expression and secretion from ASCs under low oxygen compared to MSCs. The production of these neurovascular factors may lead to the sensitization of ectopic sensory nerve fibers within the degenerate IVD resulting in sustained pain in these patients. Therefore, we hypothesize that pre‐stimulation or pretreatment of ASCs with GDF‐6 may induce an NP‐like phenotype and reduce their responsiveness to the degenerate microenvironment upon delivery
While this study utilized monolayer culture of human MSCs, it forms a basis for future studies into the mechanistic actions of MSCs when exposed to inflammatory and oxygen depleted environments such as the IVD; and future studies should include 3D cultures of MSCs and NP cells to assess the interaction between different cell types following delivery and their effect on preexisting neural and vascular structures. The IVD is an extremely complex tissue undergoing progressive changes and so it is important to consider all aspects of the target tissue to ensure correct cell types and delivery systems are in place to ensure optimal success rates, including but not limited to a soft, hydrated 3D environment subjected to mechanical load. Therefore, further research should be invested into elucidating the mechanisms by which MSC‐based therapies aid regeneration of the IVD tissue, and avoid the promotion of ectopic structures that may hinder clinical outcomes.
6. CONCLUSION
To conclude, this is the first report to highlight fundamental differences in neurovascular profiles of donor‐matched ASCs and MSCs exposed to inflammatory conditioning under low oxygen tensions in order to provide insights into the importance of stem cell selection for regenerative therapies. This includes other tissues, which have a hypoxic and inflammatory environment in response to injury or disease and which conversely to IVD require angiogenesis and innervation for healing, for example, bone lesions. When considering therapies for regeneration of the IVD, selecting the optimal cell source to repair the tissue as well as preventing neo‐angiogenesis and ectopic sensory nerve fiber ingrowth is crucial to reducing discogenic pain and improving patient outcomes. These observations have allowed us to propose a neurovascular response network associated with cell delivery to the degenerating disc. It appears that NGF, NT‐3, VEGF‐A, and Ang‐1 are key players in this network, and future work should investigate the effect of the ASC/MSC secretome on native NP cells to ensure clinical efficacy of cell‐based regenerative therapies.
CONFLICT OF INTERESTS
F.B. is a shareholder and director of Orbsen Therapeutics Ltd. F.B. is a shareholder of Osiris Therapeutics Inc. A.L.A.B., S.M.R., and J.A.H. declare that they have no competing interests.
AUTHOR CONTRIBUTIONS
A.L.A.B. participated in the study design, data acquisition, analysis and interpretation of data, statistical analysis, and manuscript preparation. S.M.R. interpreted the data, reviewed, and edited draft versions including final manuscript draft. J.A.H. and F.P.B. participated in the study design, interpreted the data, reviewed, and edited draft versions including final manuscript draft. All authors have read and approved the final submitted article.
ACKNOWLEDGMENTS
The authors would like to thank Pauline Baird for her technical assistance at The University of Manchester. We would like to thank Dr Shirley Hanley and the flow cytometry core facility at NUI Galway for the use of the Biorad Bioplex 200 multiplex ELISA system, and Dr Enda O'Connell of the genetics facility at NUI Galway for the use of the qRT‐PCR system. The views expressed in this publication are those of the authors and not necessarily those of the NHS, the National Institute for Health Research, or the Department of Health. We are very grateful for the assistance of research nurses and clinical colleagues at Wrightington Hospital, particularly Mr Tim Board, for the provision of samples.
Binch ALA, Richardson SM, Hoyland JA, Barry FP. Combinatorial conditioning of adipose derived‐mesenchymal stem cells enhances their neurovascular potential: Implications for intervertebral disc degeneration. JOR Spine. 2019;2:e1072 10.1002/jsp2.1072
Funding information European Commission, Grant/Award Number: RESPINE/732163
REFERENCES
- 1. Katz JN. Lumbar disc disorders and low‐back pain: socioeconomic factors and consequences. J Bone Jt Surg. 2006;88(suppl 2):21‐24. [DOI] [PubMed] [Google Scholar]
- 2. Hoy DG, Smith E, Cross M, et al. Reflecting on the global burden of musculoskeletal conditions: lessons learnt from the global burden of disease 2010 study and the next steps forward. Ann Rheum Dis. 2015;74(1):4‐7. [DOI] [PubMed] [Google Scholar]
- 3. Mirza SK, Deyo RA. Systematic review of randomized trials comparing lumbar fusion surgery to nonoperative care for treatment of chronic back pain. Spine (Phila Pa 1976). 2007;32(7):816‐823. [DOI] [PubMed] [Google Scholar]
- 4. Chou R, Loeser JD, Owens DK, et al. Interventional therapies, surgery, and interdisciplinary rehabilitation for low back pain: an evidence‐based clinical practice guideline from the American pain society. Spine (Phila Pa 1976). 2009;34(10):1066‐1077. [DOI] [PubMed] [Google Scholar]
- 5. Chou R, Huffman LH. Medications for acute and chronic low back pain: a review of the evidence for an American pain society/American College of Physicians clinical practice guideline. Ann Intern Med. 2007;147(7):505‐514. [DOI] [PubMed] [Google Scholar]
- 6. Fernandez‐Moure J, Moore CA, Kim K, et al. Novel therapeutic strategies for degenerative disc disease: review of cell biology and intervertebral disc cell therapy. SAGE Open Med. 2018;6:1‐11. 10.1177/2050312118761674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Le Maitre CL, Binch AL, Thorpe AA, Hughes SP. Degeneration of the intervertebral disc with new approaches for treating low back pain. J Neurosurg Sci. 2015;59(1):47‐61. [PubMed] [Google Scholar]
- 8. Sakai D, Schol J. Cell therapy for intervertebral disc repair: clinical perspective. J Orthopaed Transl. 2017;9:8‐18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Henry N, Clouet J, Le Bideau J, Le Visage C, Guicheux J. Innovative strategies for intervertebral disc regenerative medicine: from cell therapies to multiscale delivery systems. Biotechnol Adv. 2017;36:281‐294. [DOI] [PubMed] [Google Scholar]
- 10. Alini M, Mauck R, Sakai D. With or without cells, that is the question! JOR Spine. 2018;1(1):e1010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Noriega DC, Ardura F, Hernandez‐Ramajo R, et al. Intervertebral disc repair by allogeneic mesenchymal bone marrow cells: a randomized controlled trial. Transplantation. 2017;101(8):1945‐1951. [DOI] [PubMed] [Google Scholar]
- 12. Elabd C, Centeno CJ, Schultz JR, Lutz G, Ichim T, Silva FJ. Intra‐discal injection of autologous, hypoxic cultured bone marrow‐derived mesenchymal stem cells in five patients with chronic lower back pain: a long‐term safety and feasibility study. J Transl Med. 2016;14(1):253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Orozco L, Soler R, Morera C, Alberca M, Sanchez A, Garcia‐Sancho J. Intervertebral disc repair by autologous mesenchymal bone marrow cells: a pilot study. Transplantation. 2011;92(7):822‐828. [DOI] [PubMed] [Google Scholar]
- 14. Yoshikawa T, Ueda Y, Miyazaki K, Koizumi M, Takakura Y. Disc regeneration therapy using marrow mesenchymal cell transplantation: a report of two case studies. Spine. 2010;35(11):E475‐E480. [DOI] [PubMed] [Google Scholar]
- 15. Kumar H, Ha D, Lee E, et al. Safety and tolerability of intradiscal implantation of combined autologous adipose‐derived mesenchymal stem cells and hyaluronic acid in patients with chronic discogenic low back pain: 1‐year follow‐up of a phase I study. Stem Cell Res Ther. 2017;8(1):262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Sakai D, Mochida J, Iwashina T, et al. Differentiation of mesenchymal stem cells transplanted to a rabbit degenerative disc model: potential and limitations for stem cell therapy in disc regeneration. Spine. 2005;30(21):2379‐2387. [DOI] [PubMed] [Google Scholar]
- 17. Sakai D, Mochida J, Iwashina T, et al. Regenerative effects of transplanting mesenchymal stem cells embedded in atelocollagen to the degenerated intervertebral disc. Biomaterials. 2006;27(3):335‐345. [DOI] [PubMed] [Google Scholar]
- 18. Jeong JH, Lee JH, Jin ES, Min JK, Jeon SR, Choi KH. Regeneration of intervertebral discs in a rat disc degeneration model by implanted adipose‐tissue‐derived stromal cells. Acta Neurochir. 2010;152(10):1771‐1777. [DOI] [PubMed] [Google Scholar]
- 19. Zhang Y, Guo X, Xu P, Kang L, Li J. Bone mesenchymal stem cells transplanted into rabbit intervertebral discs can increase proteoglycans. Clin Orthop. 2005;430:219‐226. [DOI] [PubMed] [Google Scholar]
- 20. Sobajima S, Vadala G, Shimer A, Kim JS, Gilbertson LG, Kang JD. Feasibility of a stem cell therapy for intervertebral disc degeneration. Spine J. 2008;8(6):888‐896. [DOI] [PubMed] [Google Scholar]
- 21. Burrow KL, Hoyland JA, Richardson SM. Human adipose‐derived stem cells exhibit enhanced proliferative capacity and retain multipotency longer than donor‐matched bone marrow mesenchymal stem cells during expansion in vitro. Stem Cells Int. 2017;2017:1‐15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Strassburg S, Richardson SM, Freemont AJ, Hoyland JA. Co‐culture induces mesenchymal stem cell differentiation and modulation of the degenerate human nucleus pulposus cell phenotype. Regen Med. 2010;5(5):701‐711. [DOI] [PubMed] [Google Scholar]
- 23. Clarke LE, McConnell JC, Sherratt MJ, Derby B, Richardson SM, Hoyland JA. Growth differentiation factor 6 and transforming growth factor‐beta differentially mediate mesenchymal stem cell differentiation, composition, and micromechanical properties of nucleus pulposus constructs. Arthritis Res Ther. 2014;16(2):R67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Strassburg S, Hodson NW, Hill PI, Richardson SM, Hoyland JA. Bi‐directional exchange of membrane components occurs during co‐culture of mesenchymal stem cells and nucleus pulposus cells. PLoS One. 2012;7(3):e33739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Madrigal M, Rao KS. Riordan NH. A review of therapeutic effects of mesenchymal stem cell secretions and induction of secretory modification by different culture methods. J Transl Med. 2014;12(1):260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Cunningham CJ, Redondo‐Castro E, Allan SM. The therapeutic potential of the mesenchymal stem cell secretome in ischaemic stroke. J Cerebral Blood Flow Metabol. 2018;38:1276‐1292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Binch AL, Cole AA, Breakwell LM, et al. Nerves are more abundant than blood vessels in the degenerate human intervertebral disc. Arthritis Res Ther. 2015;17:370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Stefanakis M, Al‐Abbasi M, Harding I, et al. Annulus fissures are mechanically and chemically conducive to the ingrowth of nerves and blood vessels. Spine (Phila Pa 1976). 2012;37(22):1883‐1891. [DOI] [PubMed] [Google Scholar]
- 29. Freemont AJ, Watkins A, Le Maitre C, et al. Nerve growth factor expression and innervation of the painful intervertebral disc. J Pathol. 2002;197(3):286‐292. [DOI] [PubMed] [Google Scholar]
- 30. Bortolotti F, Ukovich L, Razban V, et al. In vivo therapeutic potential of mesenchymal stromal cells depends on the source and the isolation procedure. Stem Cell Reports. 2015;4(3):332‐339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Kim Y, Kim H, Cho H, Bae Y, Suh K, Jung J. Direct comparison of human mesenchymal stem cells derived from adipose tissues and bone marrow in mediating neovascularization in response to vascular ischemia. Cell Physiol Biochem. 2007;20(6):867‐876. [DOI] [PubMed] [Google Scholar]
- 32. Al‐Khaldi A, Al‐Sabti H, Galipeau J, Lachapelle K. Therapeutic angiogenesis using autologous bone marrow stromal cells: improved blood flow in a chronic limb ischemia model. Ann Thorac Surg. 2003;75(1):204‐209. [DOI] [PubMed] [Google Scholar]
- 33. Moon MH, Kim SY, Kim YJ, et al. Human adipose tissue‐derived mesenchymal stem cells improve postnatal neovascularization in a mouse model of hindlimb ischemia. Cell Physiol Biochem. 2006;17(5–6):279‐290. [DOI] [PubMed] [Google Scholar]
- 34. Sakai D, Nakamura Y, Nakai T, et al. Exhaustion of nucleus pulposus progenitor cells with ageing and degeneration of the intervertebral disc. Nat Commun. 2012;3:1264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Lee S, Moon CS, Sul D, et al. Comparison of growth factor and cytokine expression in patients with degenerated disc disease and herniated nucleus pulposus. Clin Biochem. 2009;42(15):1504‐1511. [DOI] [PubMed] [Google Scholar]
- 36. Redondo‐Castro E, Cunningham C, Miller J, et al. Interleukin‐1 primes human mesenchymal stem cells towards an anti‐inflammatory and pro‐trophic phenotype in vitro. Stem Cell Res Ther. 2017;8(1):79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Ji W, Zhang X, Ji L, Wang K, Qiu Y. Effects of brain‐derived neurotrophic factor and neurotrophin‐3 on the neuronal differentiation of rat adipose‐derived stem cells. Mol Med Rep. 2015;12(4):4981‐4988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Hong HS, Lee J, Lee E, et al. A new role of substance P as an injury‐inducible messenger for mobilization of CD29 stromal‐like cells. Nat Med. 2009;15(4):425‐435. [DOI] [PubMed] [Google Scholar]
- 39. Kepler CK, Markova DZ, Koerner JD, et al. Substance P receptor antagonist suppresses inflammatory cytokine expression in human disc cells. Spine (Phila Pa 1976). 2015;40:29. [DOI] [PubMed] [Google Scholar]
- 40. Purmessur D, Freemont AJ, Hoyland JA. Expression and regulation of neurotrophins in the nondegenerate and degenerate human intervertebral disc. Arthritis Res Ther. 2008;10(4):R99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Binch A, Cross A, Le Maitre C. The regulation of nerve and blood vessel ingrowth in aneural and avascular intervertebral disc and articular cartilage. Arthritis Res Ther. 2014;16:416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Kepler CK, Markova DZ, Hilibrand AS, et al. Substance P stimulates production of inflammatory cytokines in human disc cells. Spine (Phila Pa 1976). 2013;38(21):E1291‐E1299. [DOI] [PubMed] [Google Scholar]
- 43. Krock E, Rosenzweig DH, Chabot‐Dore AJ, et al. Painful, degenerating intervertebral discs up‐regulate neurite sprouting and CGRP through nociceptive factors. J Cell Mol Med. 2014;18:1213‐1225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Cho KJ, Trzaska KA, Greco SJ, et al. Neurons derived from human mesenchymal stem cells show synaptic transmission and can be induced to produce the neurotransmitter substance P by interleukin‐1α. Stem Cells. 2005;23(3):383‐391. [DOI] [PubMed] [Google Scholar]
- 45. Strioga M, Viswanathan S, Darinskas A, Slaby O, Michalek J. Same or not the same? Comparison of adipose tissue‐derived versus bone marrow‐derived mesenchymal stem and stromal cells. Stem Cells Dev. 2012;21(14):2724‐2752. [DOI] [PubMed] [Google Scholar]
- 46. Miyazaki S, Diwan AD, Kato K, et al. ISSLS PRIZE IN BASIC SCIENCE 2018: growth differentiation factor‐6 attenuated pro‐inflammatory molecular changes in the rabbit anular‐puncture model and degenerated disc‐induced pain generation in the rat xenograft radiculopathy model. Eur Spine J. 2018;27:1‐13. [DOI] [PMC free article] [PubMed] [Google Scholar]


