Abstract
Genetic tools such as the Cre‐Lox reporter system are powerful aids for tissue‐specific cell tracking. For example, it would be useful in examining intervertebral disc (IVD) cell populations in normal and diseased states. A Cre recombinase and its recognition site, loxP have been adapted from the bacteriophage for use in genetic manipulation. The reporter mice used here express the red fluorescent protein, tdTomato with flanking LoxP sites (Rosa26 TdTomato mice). We compared two different Collagen type II (Col2) promoter constructs that drive Cre‐recombinase expression in mice: (a) Col2‐Cre, which allows constitutive Cre‐recombinase expression under the control of the Col2 promoter/enhancer and (b) Col2‐CreER, which contains a shorter promoter/enhancer region than Col2‐Cre, but has human estrogen binding elements that bind tamoxifen, resulting in Cre‐recombinase expression. The goal of the study is to characterize Cre‐recombinase distribution pattern in Col2‐Cre and Col2‐CreER mice using tdTomato as reporter in the spine. The expression patterns of these two mice were further compared with Col2 gene expression in the native mouse NP and AF tissues by real‐time PCR. We crossed Col2‐Cre mice or Col2‐CreER mice with the tdTomato reporter mice, and compared the tdTomato expression patterns. Col2‐CreER/tdTomato mice were injected with tamoxifen at postnatal day 7 to activate the Cre‐recombinase. TdTomato in the constitutively active Col2‐Cre mice was detected in the nucleus pulposus (NP), the entire annulus fibrosus (AF), and in cartilaginous endplate and growth plate cells in the lower lumbar and coccygeal spine. In contrast, when Col2‐CreER activity was induced by tamoxifen at P7, tdTomato was limited to the inner AF, and was absent from the NP. We have described the differences in Col2 reporter gene expression, in Col2‐Cre/tdTomato and Col2‐Cre‐ER/tdTomato mouse IVD. The information provided here will help to guide future investigations of IVD biology.
Keywords: Cre‐Lox reporter system, gene regulation, intervertebral disc, mouse model, type II collagen (Col2)
1. INTRODUCTION
Genetic tools such as the Cre‐Lox reporter system are powerful aids for tissue‐specific cell tracking. This genetic manipulation allows promoter activities to be reported using a Cre recombinase and its recognition site, loxP adapted from the bacteriophage P1. For example, it would be useful in examining intervertebral disc (IVD) cell populations in normal and diseased states. Examining the mouse IVD would help in understanding the cellular and molecular mechanisms of IVD development and degeneration, and may help in designing new treatment strategies for the human disease.
Healthy IVDs are composed of a central gelatinous nucleus pulposus (NP), contained by concentric rings of annulus fibrosus (AF), sandwiched between cartilaginous endplates. In humans, more than 85% of the collagen in the NP of the lumbar discs is type II collagen (COL2), while the human AF contains about 50% to 65% COL2.1 Furthermore, the distributions of each type of collagen in a radial section of the AF of a 5‐year‐old spine show that their relative proportions varied inversely from being almost all type I collagen (COL1) at the outer edge of the annulus to only COL2 on reaching the NP.1 This distribution gradient of COL1 and COL2 is similar to that of pig IVD.2 In adult humans, with aging and degeneration, notochordal cells are replaced with NP cells, along with collagen fragmentation.3
Because of the importance of type II collagen in the IVD tissues, expression level of Col2 gene4 or presence of COL2 protein5 have been frequently used as desirable phenotype markers in cell therapy or tissue engineering efforts. Increased COL2 post injury has been interpreted as an attempt at repair.6 Although the col2 protein content has been shown to be higher in NP than in AF, we have reported previously that col2 gene expression levels are similar in the bovine NP and AF.4 In this study, the tissues were directly isolated from the bovine tail, and cells used were primary cell cultures passaged only once.4 Thus, a more detailed understanding of temporal‐spatial distribution of collagen gene expression in the normal disc will build a foundation for future studies of pathophysiology, and answer the question whether using Col2 gene expression as a marker for success in disc repair is appropriate.
Animal models of disc degeneration are useful in understanding the events that initiate degeneration/repair, and in identifying critical steps that may be amenable to intervention, despite key differences between most animals and humans (eg, quadruped vs biped, persistence of notochordal cells, etc.).7 The main advantages of the mouse model compared with other animals are the capability to perform genetic manipulations during IVD degeneration, and the large number of genetically modified mouse lines available. Our group have established and refined the mouse tail IVD injury model, to facilitate further mechanistic studies.8, 9, 10 Lumbar spine IVD is prone to degeneration in animals and humans. Therefore, we have included lumbar and tail IVDs in this study.
The reporter mice used here express the red fluorescent protein, tdTomato with flanking LoxP sites (Rosa26 TdTomato mice, also known as Ai9). The reporter mice homozygous for the Rosa‐tdTomato conditional allele contain a loxP‐flanked STOP cassette, preventing transcription of the red fluorescent protein variant tdTomato. Following Cre‐mediated recombination, the STOP cassette would be permanently removed from the genome, resulting in constitutive tdTomato fluorescence expression under control of the synthetic CAG promoter.11
We compared two different Collagen type II (Col2) promoter constructs that drive Cre‐recombinase expression in mice: (a) Col2‐Cre, which allows constitutive Cre‐recombinase expression under the control of the Col2 promoter/enhancer12 and (b) Col2‐CreER, which contains a shorter promoter/enhancer region than Col2‐Cre, but has human estrogen binding elements that bind tamoxifen, resulting in Cre‐recombinase gene transcription, followed by translation into Cre recombinase.13 Specifically, in the Col2‐Cre line, the transgene expresses Cre‐recombinase under the control of a longer version of the Col2a1 (procollagen type II, alpha 1) promoter than that of the Col‐CreER line.12, 13 The Col2‐Cre gene construct consisted of 3 kb of the Col2a1 promoter region, and the first exon and a 3.02 kb fragment of intron 1 enhancer.12 In contrast, the Col2‐CreER transgene contains 1 kb of the Col2a1 promoter, and a 650 bp fragment of the Col2a1 first intron enhancer, along with a mutated ligand‐binding domain of the human estrogen receptor (allowing tamoxifen binding to enhance promoter activity; Figure 1).13 The Col2‐Cre and Col2‐CreER gene expression patterns were compared with real‐time PCR data in native IVD tissues, which evidently contain the full length Col2a1 promoter with all its regulatory elements intact. We have also stained filamentous actin (F‐actin) with fluorescently‐tagged phalloidin, allowing visualization of actin cytoskeleton organization in relation to collagen gene expression.
Figure 1.
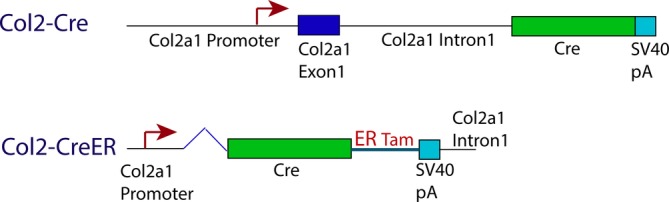
Col2‐Cre and Col2‐CreER gene construct. Col2‐Cre gene includes a 3 kb Col2a1 promoter fragment, Col2a1 exon 1, and a 3.02 kb Col2a1 intron 1. Col2‐CreER gene includes 1 kb Col2a1 promoter, estrogen receptor that binds to tamoxifen (ERTam), and 650 bp Col2a1 intron 1. Both constructs included the Cre Recombinase gene (Cre) and a Simian Virus 40 polyadenylation signal (SV40 pA). Diagram adapted from Ovchinnikov et al and Nakamura et al
2. METHODS
2.1. Mice
All animal use in this study was reviewed and approved by the Institutional Animal Care and Use Committee (IACUC) at the University of Pennsylvania, Philadelphia, PA. Young adult Rosa26 TdTomato (B6.Cg‐Gt(ROSA)26Sortm9(CAG‐tdTomato)Hze/J‐ JAX stock #007909),11 Col2‐Cre (JAX stock #003554),12 Col2‐CreER (JAX stock #006774),13 and C57BL/6j mice were purchased from the Jackson Laboratory (Bar Harbor, ME). Mice were maintained in accordance with the standards for animal housing (group housed with no more than five mice per cage, at 23°C to 25°C with a 12‐hour light/dark cycle, and allowed free access to water and standard laboratory pellets).
To generate mice expressing tdTomato in Col2a1‐expressing cells (Col2‐Cre/tdTomato), Rosa26‐TdTomato mice were bred with Col2‐Cre mice.12 The resulting offspring, both male and female, were sacrificed at 6 weeks of age and lumbar and coccygeal spines were isolated for imaging.
Similarly, tdTomato‐expressing mice under the control of the inducible promoter Col2‐CreER (Col2CreER/Tomato) were generated by breeding Col2‐CreER mice13 with Rosa26‐TdTomato mice. Cre‐recombinase expression in the resulting offspring was induced with tamoxifen. Specifically, tamoxifen (T5684, Sigma‐Aldrich) was dissolved in corn oil at a concentration of 20 mg/mL by shaking overnight at 37°C. Animals received 75 mg tamoxifen/kg body weight intraperitoneally once, at postnatal day 7. The tamoxifen‐oil mixture was stored at 4°C until used.
For real‐time PCR, eight young adult female C57BL/6j mice aged 8 weeks were used to isolate NP and AF, as described previously.14
2.2. Tissue preparation and filamentous actin staining
Lumbar and tail IVDs and portions of the adjacent bony vertebral bodies were isolated immediately after euthanasia. The disc with its surrounding vertebral bodies was lightly fixed with 2% paraformaldehyde for 24 hours. The bone‐disc‐bone segments were partially decalcified with 12.5% EDTA solution. Actin filaments were stained with Alexafluor488 phalloidin (Thermo Fisher Scientific, Waltham, MA), and covered with mounting medium containing DAPI (Vector Laboratories, Burlingame, CA).
2.3. Confocal microscopy
The tissues were embedded in gelatin, as described by Kusumbe et al.15 Briefly, samples were incubated overnight at 4°C in 20% sucrose and 2% polyvinylpyrrolidine. Then, samples were embedded in the above solution with the addition of 8% gelatin. The spine tissues were sectioned to 70 μm thickness in their entirety, and mid‐sagittal sections were imaged in 3D using a confocal fluorescence microscope (Nikon Eclipse Ti, Minato, Tokyo, Japan), using a step size of 5 μm. Maximum intensity projections of the resulting images are shown.
2.4. Quantification of tdTomato positive areas in Col2‐CreER/tdTomato mice
Total and tdTomato‐positive thickness was measured on the section with the largest cross sectional IVD area, in both anterior and posterior AF. The measurement and analysis were achieved using Fiji software, an open‐source platform for biological‐image analysis.16
2.5. NP and AF dissection and RNA isolation
Eight mice were used for NP and AF RNA extraction. The lumbar and coccygeal vertebrae were isolated with a scalpel under a dissecting microscope (VistaVision, VWR International, Radnor, PA). First, we identified the NP and AF, as described previously.14 The gelatinous NP was scraped off with a scalpel, and the AF tissues, identified by their concentric rings, were shaved off the cartilaginous endplate with a scalpel. Lumbar and coccygeal tissues from each mouse NP or AF were dissected and stored, and examined separately. Total cellular RNAs were extracted by the Trizol method, and further purified using an RNeasy Micro Kit (Qiagen, Gaithersburg, MD), as described previously.14 The RNAs from the NP or AF of each animal were reverse transcribed using the SuperScript VILO cDNA synthesis kit (Life Technologies, Carlsbad, CA) containing random hexamers, with added polyDT primers (Invitrogen, Carlsbad, CA).
2.6. Quantitative real‐time PCR
Primer sequences for type I collagen α1 chain (Col1a1) and for Col2a1 were designed based on published sequences, and synthesized by Invitrogen as described previously.14 Five genes were included as endogenous controls: gapdh (glyceraldehyde 3‐phosphate dehydrogenase), gusb (β‐glucuronidase), b2m (β‐2‐microglobulin), actb (β‐actin), and Hsp90ab1 (β‐heat shock 90kD protein 1). The data were analyzed with the GeneGlobe program (Qiagen). This method automatically selects an optimal set of internal control/housekeeping/normalization genes for analysis, from the above housekeeping gene panel. The software measures and identifies the genes with the most stable expression via a non‐normalized calculation. B2m was selected as an optimal internal control with this program. Primers for b2m (β‐2‐microglobulin) were purchased (QuantiTect Primer, Qiagen). The cDNAs were mixed with SYBR Select master mix (Life Technologies) and primers. MicroAmp Optical 96‐well reaction plates (Applied Biosystems, Foster City, CA) were used for PCR reactions (20 μL of reaction mixtures/well), with an annealing temperature of 58°C. Single products were confirmed by determining melting curves at the conclusion of the reaction, and by separating the PCR product in a 2% agarose gel. Relative expression was calculated using the 2‐ΔΔCt method,17, 18 normalized to b2m, which served as an endogenous control. Recently, we reported gene expression profiles in the native mouse NP and AF tissues.14 In this study, we used four internal controls, and b2m was most comparable between NP and AF when RNA concentration had been taken into consideration.14 This is the rationale for using b2m as internal control in intact mouse tissues.
2.7. Statistics
To assess differences in gene expression between NP and AF tissues for each NP/AF pair, ΔCT values were analyzed by paired Student's t test. A P‐value of ≤.05 was considered statistically significant.
3. RESULTS
3.1. Key differences in red fluorescent reporter protein (tdTomato) expression between the Col2‐Cre/tdTomato mice and Col2‐CreER/tdTomato mice
We bred Rosa26TdTomato with Col2‐Cre mice,12 or with tamoxifen‐inducible Col2‐CreER mice.13 In the Col2‐Cre mice, the Cre‐recombinase is expressed under the control of the Col2a1 promoter, which is active in differentiating chondrocytes, notochord and submandibular glands.12 In contrast, in the Col2‐CreER mice the transgene utilizes a form of Col2a1 promoter that is only active with tamoxifen induction.13 In this study, tamoxifen was injected at postnatal day 7 (P7) to induce Col2 promoter activity.
In the lumbar spine of the Col2‐Cre/tdTomato mice, tdTomato was detected in the NP, along with the entire AF, endplates, and growth plates (Figure 2A). However, tdTomato was not detected in the NP of Col2‐CreER/tdTomato mice (Figure 2B). Fluorescent proteins were only detected in the inner AF (along with endplates and growth plates) in the Col2‐CreER/tdTomato mice. Interestingly, tdTomato was detected in 39.9% of the posterior AF cell layers, more than that in the anterior AF (11.6%; Figure 2, right panel; n = 4/group; P = .0135).
Figure 2.
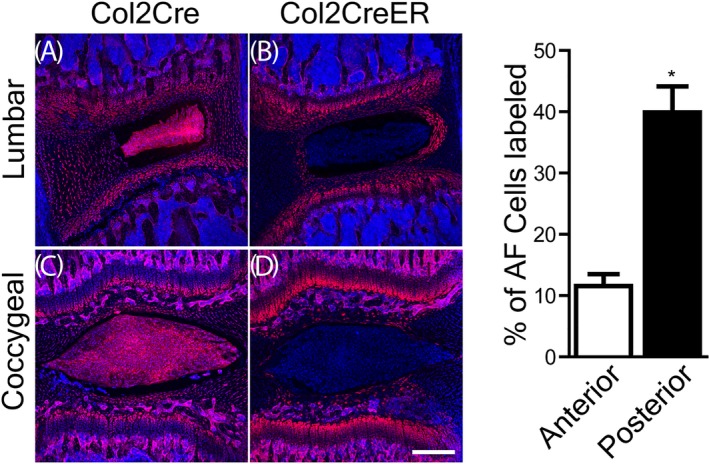
Sagittal section of Col2‐Cre/tdTomato or Col2‐CreER/tdTomato 6‐week old mouse lower lumbar or coccygeal intervertebral disc (IVD). Left panel, confocal images of mouse lumbar, and coccygeal IVDs, with the red fluorescent protein (Tomato) under the control of Col2‐Cre or Col2‐CreER promoters. Red: type II collagen expressing cells; blue: cell nuclei stained with DAPI. A,B, Lumbar spine IVD. C,D, Coccygeal IVD. AF, annulus fibrosus. Scale bar: 250 μm. Right panel, percent (%) of AF cells positive for Tomato in lumbar IVDs of Col2‐CreER mice. Error bar: SE of the mean. *P < .05
In the coccygeal IVDs of Col2‐Cre/tdTomato mice, the distribution of tdTomato is similar to that in lumbar spine: NP, along with AF, endplates, and growth plates were all positive for Tomato (Figure 2C). In the tail IVDs of Col2‐CreER/tdTomato mice, however, only a few layers of the inner AF cells were positive for Tomato, fewer compared with that in the lumbar spine IVDs (n = 4/group; Figure 2D).
3.2. TdTomato was widespread in cartilaginous cells of Col2‐Cre/tdTomato mice
TdTomato was detected in the NP, all layers of anterior and posterior AF, cartilaginous endplate and growth plate cells. As expected, the anterior AF is thicker, with 17 to 23 rings, to accommodate the lumbar lordosis. The posterior AF is thinner, consisting of 10 to 12 rings (Figure 3A,A″). TdTomato is present in the entire NP of both lumbar and coccygeal IVDs (Figure 3A′,D). There is no obvious asymmetry in tdTomato distribution in the coccygeal IVDs: NP, AF, cartilaginous endplates and growth plates, are all positive for tdTomato (Figure 3D).
Figure 3.
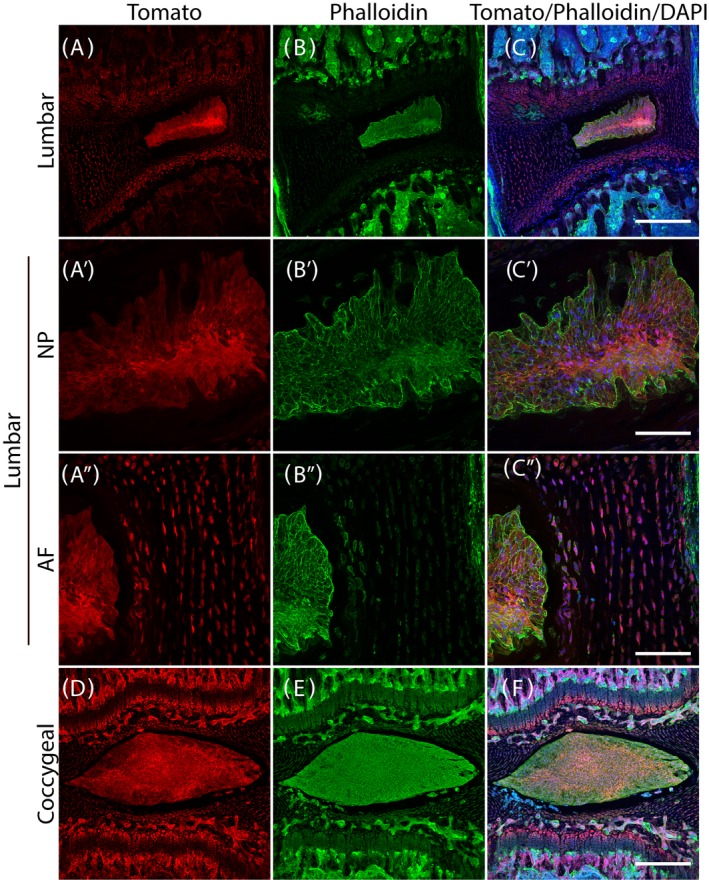
Col2‐Cre/tdTomato 6‐week old mouse lower lumbar and coccygeal spine intervertebral disc (IVD). A‐C, Lumbar spine IVD. A′‐C′, Nucleus pulposus (NP) of the lumbar spine IVD. A″‐C″, Posterior annulus fibrosus (AF) of the lumbar spine. D‐F, Coccygeal IVD. Scale bars: 250 μm for the low magnification images and 25 μm for the high magnification images
To visualize the cytoskeleton, filamentous actin (F‐actin) was stained with phalloidin (Figure 3B,B′,B″,E). Finally, an overview of the Col2 reporter gene expression, F‐actin, and cell nuclei was generated by merging all three images to allow visualization of relative positions of collagen, F‐actin and nuclei (Figure 3C,C′,C″,F).
3.3. Tomato expression is absent from the NP of Col2‐CreER/tdTomato mice
In contrast to the Col2‐Cre/tdTomato mice, in Col2‐CreER/tdTomato mice Col2 reporter gene activity is induced by tamoxifen postnatally. In Col2‐CreER/tdTomato mice, tdTomato expression is limited to the inner AF, cartilaginous endplate, and growth plates. TdTomato is essentially absent from the NP in the lower lumbar spine (n = 4/group; Figure 4A,A′,A″).
Figure 4.
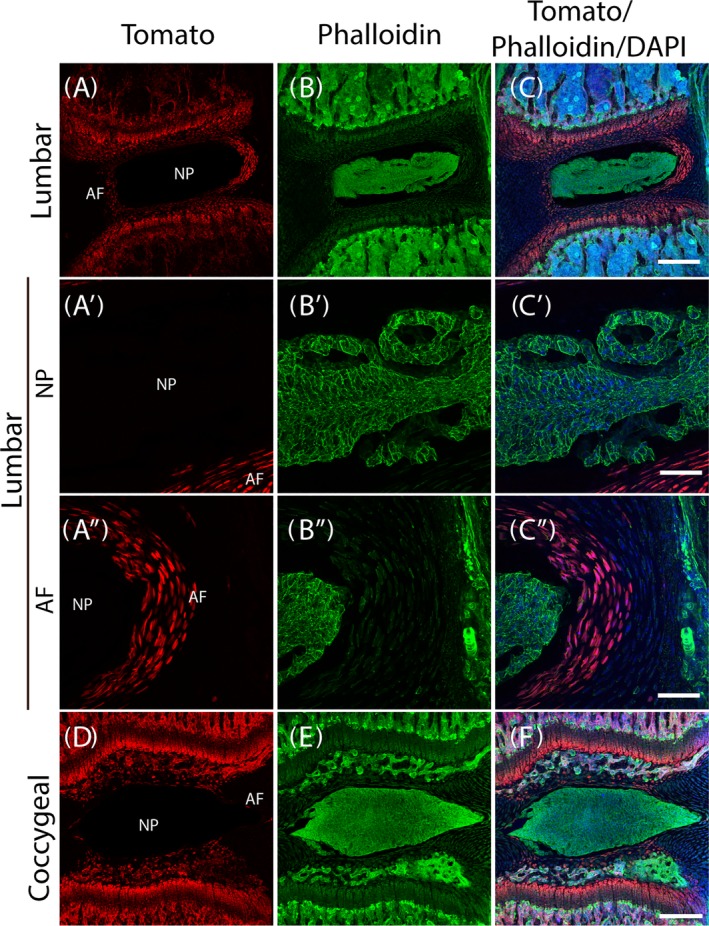
Col2‐CreER/tdTomato 6‐week old mouse lower lumbar and coocygeal spine intervertebral disc (IVD). A‐C, Lumbar spine IVD; A′‐C′, Nucleus pulposus (NP) of the lumbar spine IVD. A″‐C″, posterior annulus fibrosus (AF) of the lumbar spine IVD. D‐F, Coccygeal IVD. Scale bars: 250 μm for the low magnification images and 25 μm for the high magnification images
In the coccygeal IVDs, similar to the lower lumbar IVDs, there is no detectable tdTomato expression in the NP. Only one layer of the anterior and posterior inner AF cells expressed tdTomato. Interestingly, there were several tdTomato‐expressing layers in the rostral and caudal aspects of the AF, adjacent to the cartilaginous endplate (Figure 4D).
Not surprisingly, the distribution pattern of cytoskeleton F‐actin, visualized by phalloidin staining, is similar in Col2‐CreER mice (Figure 4B,B′,B″,E) to that in the Col2‐Cre mice. The relative positions of tdTomato, F‐actin and nuclei are shown with tdTomato and phalloidin staining overlay with DAPI staining (Figure 4C,C′,C″,F).
3.4. Filamentous (F)‐actin aligns along the cell membrane in NP
To reveal cytoskeleton organization in the IVD cells, F‐actin was stained with phalloidin and nuclei were stained blue with DAPI (Figure 5; n = 4 animals). F‐actin, stained green, clearly outlines the NP cell boundaries, suggesting that they align with the cytoplasmic membranes (Figure 5C,D). The F‐actin distribution pattern is less clear in the AF compared with that of the NP (Figure 5A,B). It is surprising that normal NP cells are epithelial cell‐like, because the NP cells are considered “chondrocyte‐like,” and one would expect round cell shape.
Figure 5.
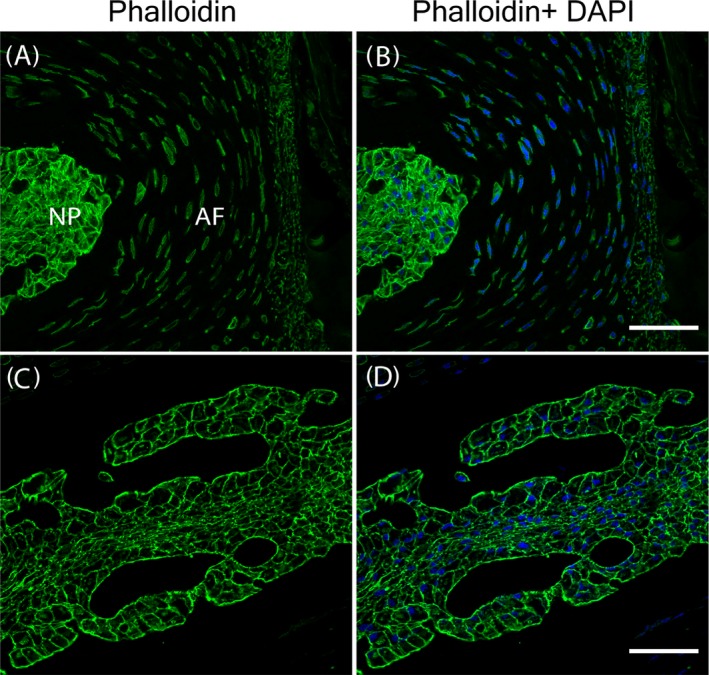
F‐actin aligns along the cell membrane in the mouse lumbar spine intervertebral disc (IVD). A,B, Low magnification images of lumbar spine IVD. C,D, High magnification images of the nucleus pulposus (NP). Scale bars: 250 μm for the low magnification images and 25 μm for the high magnification images
3.5. Type II collagen (col2a1) and Type I (col1a1) gene expression in the adult mouse IVD by real‐time PCR
Although type II collagen is a major component of the IVDs, Col2 gene is expressed at a very low level in both the NP and AF of adult IVD, compared with that of Col1a1 (Figure 6). The average ratio of Col2 to b2m (β‐2‐microglobulin) was 0.0124 in the NP and 0.0138 in the AF. There is no statistically significant difference in col2 gene expression between AF and NP (n = 8, P = .4318; Figure 6A). Col1 gene expression in AF is higher than in NP (col1/b2m ratio in AF: 6.1569; col1/b2m ratio in NP: 0.1388; n = 8, P = .0022; Figure 6B).
Figure 6.
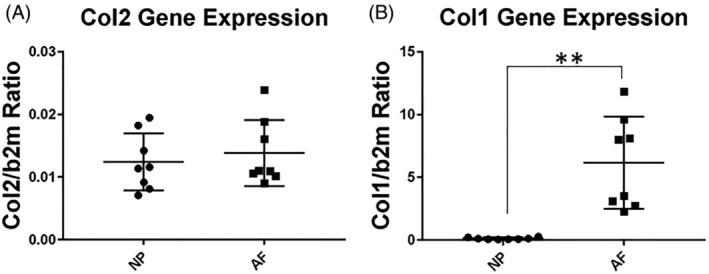
Type II collagen (Col2a1) and type I collagen (Col1a1) gene expression in the 8‐week old murine nucleus pulposus (NP) and annulus fibrosus (AF). A, Col2a1 to b2m gene expression ratio. B, Col1a1 to b2m gene expression ratio in mice. **P ≤ .01
4. DISCUSSION
The major collagen in the NP has been described as type II collagen, and the primary collagen in AF is type I. A previous study performed with Col1‐Cre mice demonstrated that the Col1 promoter is active only in the outer AF,19 consistent with the fact that COL1 is the major collagen in the AF. We were puzzled, however, since we found that Col2 gene expression levels are similar in the bovine NP and AF tissues.4 In this report, we have used a tdTomato reporter gene to visualize Col2 expression pattern in Col2‐Cre and Col2‐CreER mice.
We have shown here that tdTomato expression in the spine of Col2‐Cre/tdTomato mice is widespread, in all cartilaginous cells (NP, AF, cartilaginous endplates and the growth plates). In the Col2‐CreER/tdTomato mice, however, tdTomato is absent in the NP and outer AF when tamoxifen is injected as early as P7. The differences in tdTomato distribution between Col2‐Cre and Col2‐CreER mice are consistent with findings in Col2‐CreER; R26‐lacZ mice20 in that NP did not show significant Cre‐recombinase activity. In comparison to work by Zheng et al,21 our images are of higher quality, due to improved methods as described by Kusumbe et al15 Using wholemount sections promotes the maintenance of tissue integrity with minimal sectioning artifacts, displayed by our clear NP cytoskeletal structure. Additionally, imaging of this delicate structure in 3D allows us to show maximum intensity projection images which more accurately reflect the presence of rare cell populations and determine their spatial arrangement.
Our findings are reminiscent of the articular cartilage in that Col2 reporter gene expression decreased after birth,22 and type II collagen has a long half‐life in cartilage tissue.23 Cartilage is similar to NP, in that it has only low regenerative potential likely due to failure to repair the tissues after trauma or with aging. Col2‐CreER mouse reporter gene expression in the knee joint varies depending on when tamoxifen is administered.22 A similar phenomenon may exist in the IVD; thus, a more detailed study of Col2 gene expression during embryogenesis and at different postnatal ages, especially in skeletally mature mice (10‐12 weeks of age),24 would shed more light on the temporal‐spatial regulation of this gene.
It is worth noting that in the Rosa‐tdTomato reporter mice, robust tdTomato expression is permanent once the Cre‐recombinase removes the STOP cassette, because the tdTomato gene expression in the reporter mouse is under the control of a constitutive CAG promoter.11 Therefore, tdTomato fluorescence pattern only partially recapitulates endogenous native Col2a1 gene expression. Both the Col2‐Cre and Col2‐CreER promoter used are truncated promoters randomly inserted into the genome.12, 13 The Col2‐Cre promoter included a longer promoter region and intron 1 enhancer12 and appears to be active in both NP and AF cells. In comparison, the Col2‐CreER contains a shorter promoter and intron 1 enhancer, and is largely inactive without an additional enhancer that binds to tamoxifen (Figure 1).13 Indeed, although we have observed aberrant tdTomato expression in the absence of tamoxifen in tissues of the long bones, our data suggest no aberrant tdTomato expression within the IVD (data not shown). The data presented here could be helpful in guiding future experiments. For example, it would be more appropriate to use the Col2‐Cre‐ER/tdTomato mice to trace the lineage of AF cells in response to IVD injury, since the inner AF cells are red with the tdTomato protein but NP cells are not. It would be difficult to distinguish lineage of cells in the Col2‐Cre/tdTomato mice since all cells are red.
The differences in tdTomato distribution in the IVD tissues reflect a combination of factors including the timing of tamoxifen binding to the estrogen enhancer, regulatory elements in the transgene, and the position of the constructs inserted in the mouse genome (Figure 1). These factors affect the timing and strength of the promoter activity, as well as the specificity of the promoter. It is interesting that in the Col2‐CreER construct, only the inner AF (not the outer AF) showed tdTomato red signal. This is indeed a fortuitous finding, in that this particular Col2‐CreER mouse strain could be useful to turn on a target gene in the inner AF, or to trace the lineage of the inner AF cells. The study by Zheng et al used different time‐points for their analysis of AF cell lineage tracing with the Col2CreER mouse,21 and the fluorescent protein distribution patterns are consistent with our findings.
Recently, efforts to trace the NP cells or notochord‐lineage cells with genetic methods have been made. For example, with the Krt19 CreERT,25 Noto‐Cre,26 the Sonic Hedgehog (shh)‐Cre, and the Shh‐creERT227 strains. Among these mouse strains, the Krt19 CreERT strain targets the NP cells, thus would be very useful in future studies.25 It is worth noting that tamoxifen‐mediated recombination of Krt19CreERT also occurs in multiple endodermal organs and hair follicle stem cells.28, 29 Noto is expressed in primitive node and hence NotoCre targets all cells from that lineage.26 An inducible ShhCreERT2 allele is available,30 but very few NP cells were targeted following Tamoxifen administration to ShhCreERT2 allele in the postnatal mouse IVDs.21
Real‐time PCR, on the other hand, reflects the activity of the natural, full length promoter at the time of tissue collection. In our analysis with real‐time PCR, Col2 gene expression level did not differ between NP and AF in the wild‐type mice. Mwale et al have shown Col2 gene expression post saline or Link N injection in the rabbit NP or AF.31 However, they did not show gene expression in the intact rabbit disc tissue. In this report, in the NP and AF tissues post saline injection, the Col2a1 gene expression to GAPDH ratio is indeed similar.31 As a future direction, Col2 in situ hybridization analysis will allow direct visualization of RNA to indicate spatial distribution.
Phalloidin staining of the NP reveals that F‐actin aligns along the cell surface, likely playing a major role in supporting the NP cells under the high pressure that the spine endures. In contrast, Col2 is distributed throughout the cytoplasm of the NP cells.
An interesting similarity between the mouse AF and those of larger animals is that the anterior AF is thicker than the posterior AF.32 Clinically, disc herniation is more likely to occur posterolaterally, where the AF is thinner and lacks the structural support from the anterior or posterior longitudinal ligaments. Thus, modeling the human IVD pathology with the mouse model is appropriate, at least from this aspect.
We have described the differences in Col2 gene expression, shown by high resolution images of Col2‐Cre/tdTomato and Col2‐Cre‐ER/tdTomato in the IVD. Specifically, Col2‐Cre/tdTomato exhibited fluorescence in both the NP and AF. However, Col2‐Cre‐ER/tdTomato, with a shorter regulatory region of the Col2 promoter, showed fluorescence only in AF, but not NP. Therefore, care should be taken when considering which mouse to use for understanding IVD development and homeostasis. The information provided here will help to guide future investigations of IVD biology, especially those who use genetic tools to trace IVD cell lineage.
CONFLICT OF INTEREST
The authors declare no competing conflict of interest.
AUTHOR CONTRIBUTIONS
Y.W. and R.J.T. contributed equally to the manuscript. Data acquisition, Y.W., R.J.T., Z.T., and B.M.; Research design and/or data interpretation, Y.W., R.J.T.; R.L.M., L.Q., and Y.Z. Drafting and revising manuscript: Y.Z.; Y.W., R.J.T. Storage and access of all primary data: R.J.T., Y.W., Y.Z., and L.Q. All authors have read and approved the final submitted manuscript. The authors thank Martin F. Heyworth, MD, for critically editing the manuscript, and Dr. Di Chen for valuable insights and discussions.
ACKNOWLEDGMENTS
Y.Z. has been supported by the Eunice Kennedy Shriver National Institute of Child Health and Human Development (NICHD, K08 HD049598). This work is supported, in part, by grants to Y.Z. from the Department of Veterans Affairs Healthcare Network, and the National Institute of Arthritis and Musculoskeletal and Skin Diseases (NIAMS, R21 AR071623, and P30‐AR050950‐10 Pilot). This work is also supported, in part, by research grant to L.Q. from the NIH (NIAMS R01AR066098, R01DK095803, and P30AR069619 to Penn Center for Musculoskeletal Disorders).
Wei Y, Tower RJ, Tian Z, et al. Spatial distribution of type II collagen gene expression in the mouse intervertebral disc. JOR Spine. 2019;2:e1070 10.1002/jsp2.1070
Yulong Wei and Robert J. Tower contributed equally to this study.
Funding information NIH, Grant/Award Numbers: P30AR069619, R01DK095803, R01AR066098; NIH, Grant/Award Numbers: P30‐AR050950‐10, R21 AR071623; Department of Veterans Affairs Healthcare Network; NIH, Grant/Award Number: K08 HD049598
REFERENCES
- 1. Eyre DR, Muir H. Quantitative analysis of types I and II collagens in human intervertebral discs at various ages. Biochim Biophys Acta. 1977;492:29‐42. [DOI] [PubMed] [Google Scholar]
- 2. Eyre DR, Muir H. Types I and II collagens in intervertebral disc. Interchanging radial distributions in annulus fibrosus. Biochem J. 1976;157:267‐270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Urban JP, Roberts S. Degeneration of the intervertebral disc. Arthritis Res Ther. 2003;5:120‐130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Zhang Y, Markova D, Im HJ, et al. Primary bovine intervertebral disc cells transduced with adenovirus overexpressing 12 BMPs and Sox9 maintain appropriate phenotype. Am J Phys Med Rehabil. 2009;88:455‐463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Acosta FL Jr, Metz L, Adkisson HD, et al. Porcine intervertebral disc repair using allogeneic juvenile articular chondrocytes or mesenchymal stem cells. Tissue Eng Part A. 2011;17:3045‐3055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Keorochana G, Johnson JS, Taghavi CE, et al. The effect of needle size inducing degeneration in the rat caudal disc: evaluation using radiograph, magnetic resonance imaging, histology, and immunohistochemistry. Spine J. 2010;10:1014‐1023. [DOI] [PubMed] [Google Scholar]
- 7. Alini M, Eisenstein SM, Ito K, et al. Are animal models useful for studying human disc disorders/degeneration? Eur Spine J. 2008;17:2‐19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Martin JT, Gorth DJ, Beattie EE, Harfe BD, Smith LJ, Elliott DM. Needle puncture injury causes acute and long‐term mechanical deficiency in a mouse model of intervertebral disc degeneration. J Orthop Res. 2013;31:1276‐1282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Tian Z, Ma X, Yasen M, et al. Intervertebral disc degeneration in a percutaneous mouse tail injury model. Am J Phys Med Rehabil. 2018;97:170‐177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Piazza M, Peck SH, Gullbrand SE, et al. Quantitative MRI correlates with histological grade in a percutaneous needle injury mouse model of disc degeneration. J Orthop Res. 2018;36:2771‐2779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Madisen L, Zwingman TA, Sunkin SM, et al. A robust and high‐throughput cre reporting and characterization system for the whole mouse brain. Nat Neurosci. 2010;13:133‐140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Ovchinnikov DA, Deng JM, Ogunrinu G, Behringer RR. Col2a1‐directed expression of cre recombinase in differentiating chondrocytes in transgenic mice. Genesis. 2000;26:145‐146. [PubMed] [Google Scholar]
- 13. Nakamura E, Nguyen MT, Mackem S. Kinetics of tamoxifen‐regulated cre activity in mice using a cartilage‐specific CreER(T) to assay temporal activity windows along the proximodistal limb skeleton. Dev Dyn. 2006;235:2603‐2612. [DOI] [PubMed] [Google Scholar]
- 14. Zhang Y, Tian Z, Ashley JW, et al. Extracellular matrix and adhesion molecule gene expression in the normal and injured murine intervertebral disc. Am J Phys Med Rehabil. 2019;98(1):35‐42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Kusumbe AP, Ramasamy SK, Starsichova A, Adams RH. Sample preparation for high‐resolution 3D confocal imaging of mouse skeletal tissue. Nat Protoc. 2015;10:1904‐1914. [DOI] [PubMed] [Google Scholar]
- 16. Schindelin J, Arganda‐Carreras I, Frise E, et al. Fiji: An open‐source platform for biological‐image analysis. Nat Methods. 2012;9:676‐682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Schmittgen TD, Livak KJ. Analyzing real‐time PCR data by the comparative C(T) method. Nat Protoc. 2008;3:1101‐1108. [DOI] [PubMed] [Google Scholar]
- 18. Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real‐time quantitative PCR and the 2(−delta delta C(T)) method. Methods. 2001;25:402‐408. [DOI] [PubMed] [Google Scholar]
- 19. Bedore J, Quesnel K, Quinonez D, Seguin CA, Leask A. Targeting the annulus fibrosus of the intervertebral disc: Col1a2‐cre(ER)T mice show specific activity of cre recombinase in the outer annulus fibrosus. J Cell Commun Signal. 2016;10:137‐142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Chen M, Li S, Xie W, Wang B, Chen D. Col2CreER(T2), a mouse model for a chondrocyte‐specific and inducible gene deletion. Eur Cell Mater. 2014;28:236‐245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Zheng Y, Fu X, Liu Q, et al. Characterization of cre recombinase mouse lines enabling cell type‐specific targeting of postnatal intervertebral discs. J Cell Physiol. 2019. [A head of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Nagao M, Cheong CW, Olsen BR. Col2‐cre and tamoxifen‐inducible Col2‐CreER target different cell populations in the knee joint. Osteoarthr Cartil. 2016;24:188‐191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Verzijl N, DeGroot J, Thorpe SR, et al. Effect of collagen turnover on the accumulation of advanced glycation end products. J Biol Chem. 2000;275:39027‐39031. [DOI] [PubMed] [Google Scholar]
- 24. Somerville JM, Aspden RM, Armour KE, Armour KJ, Reid DM. Growth of C57BL/6 mice and the material and mechanical properties of cortical bone from the tibia. Calcif Tissue Int. 2004;74:469‐475. [DOI] [PubMed] [Google Scholar]
- 25. Mohanty S, Pinelli R, Dahia CL. Characterization of Krt19 (CreERT) allele for targeting the nucleus pulposus cells in the postnatal mouse intervertebral disc. J Cell Physiol. 2019. [A head of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. McCann MR, Tamplin OJ, Rossant J, Seguin CA. Tracing notochord‐derived cells using a noto‐cre mouse: implications for intervertebral disc development. Dis Model Mech. 2012;5:73‐82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Choi KS, Lee C, Harfe BD. Sonic hedgehog in the notochord is sufficient for patterning of the intervertebral discs. Mech Dev. 2012;129:255‐262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Means AL, Xu Y, Zhao A, Ray KC, Gu G. A CK19(CreERT) knockin mouse line allows for conditional DNA recombination in epithelial cells in multiple endodermal organs. Genesis. 2008;46:318‐323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Zito G, Saotome I, Liu Z, et al. Spontaneous tumour regression in keratoacanthomas is driven by wnt/retinoic acid signalling cross‐talk. Nat Commun. 2014;5:3543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Harfe BD, Scherz PJ, Nissim S, Tian H, McMahon AP, Tabin CJ. Evidence for an expansion‐based temporal shh gradient in specifying vertebrate digit identities. Cell. 2004;118:517‐528. [DOI] [PubMed] [Google Scholar]
- 31. Mwale F, Masuda K, Pichika R, et al. The efficacy of link N as a mediator of repair in a rabbit model of intervertebral disc degeneration. Arthritis Res Ther. 2011;13:R120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Zhang Y, Lenart BA, Lee JK, et al. Histological features of endplates of the mammalian spine: from mice to men. Spine (Phila pa 1976). 2014;39:E312‐E317. [DOI] [PMC free article] [PubMed] [Google Scholar]


