Abstract
Colorectal cancer (CRC) is a worldwide health concern which requires efficient therapeutic strategies. The mechanisms underlying CRC remain an essential subject of investigations in the cancer biology field. The evaluation of human microbiota can be critical in this regard, since the disruption of the normal community of gut bacteria is an important issue in the development of CRC. However, several studies have already evaluated the different aspects of the association between microbiota and CRC. The current study aimed at reviewing and summarizing most of the studies on the modifications of gut bacteria detected in stool and tissue samples of CRC cases. In addition, the importance of metabolites derived from gut bacteria, their relationship with the microbiota, and epigenetic modifications have been evaluated.
Keywords: colorectal cancer, gut bacteria, dysbiosis, epigenetics
1. Introduction
Colorectal cancer (CRC) remains a significant troublesome health issue worldwide [1]. This multifactorial and widespread cancer counts as one of the most common causes of cancer-related death [2]. CRC has a close association with lifestyle, clinically affecting the large intestine and rectum [3,4]. Recent evidence suggests that dysregulation of microbiota-host interactions is associated with various diseases, including diabetes, bowel disease, and cancer [5,6]. In addition to genetic and environmental factors, such as inflammatory processes, diet, alcohol consumption, and smoking, dysbiosis of gut bacteria and epigenetic modifications are a critical link to an increased risk of CRC [7,8]. The term “dysbiosis” refers to an imbalance in the community of healthy human microbiota [9], known as the microbial community inhabiting the skin, oral cavity, lower respiratory tract, vagina, urinary tract and gut [10]. The highest and most varied bacterial density are inhabitants in the large human bowel and interact with the host in a symbiotic relationship [11]. Recent evidence links the response to anticancer immune checkpoint inhibitor therapy to the presence of specific species in the microbiota of patients [12,13,14]. In addition, microbial-derived metabolites also play a fundamental role in host metabolism and CRC progression [15,16]. Therefore, a growing interest in the determination of a possible link between gut bacteria and CRC has been aroused in the last few decades [17,18]. However, causative genera in CRC evolution remains poorly defined. Nevertheless, there is a gap in knowledge on the role of various gut bacteria and their metabolites in CRC, also considering that epigenetic modifications play a significant role in CRC development. Thus, the complete mechanism at the base of CRC pathogenesis is not fully understood and the different aspects of bacterial effects are also entirely unclear. Currently, a major challenge is to define how to integrate microbiota data into medicine approaches in order to introduce an effectiveness prevention, diagnosis, and treatment strategies. The current study furthermore provides a detailed overview of the most critical gut bacteria DNA detected in the standard sample types of CRC. Finally, the most important mechanisms, microbial-derived metabolites, and epigenetic modifications that influence progression to CRC are discussed briefly.
2. The Intestinal Bacteria in Times of CRC:
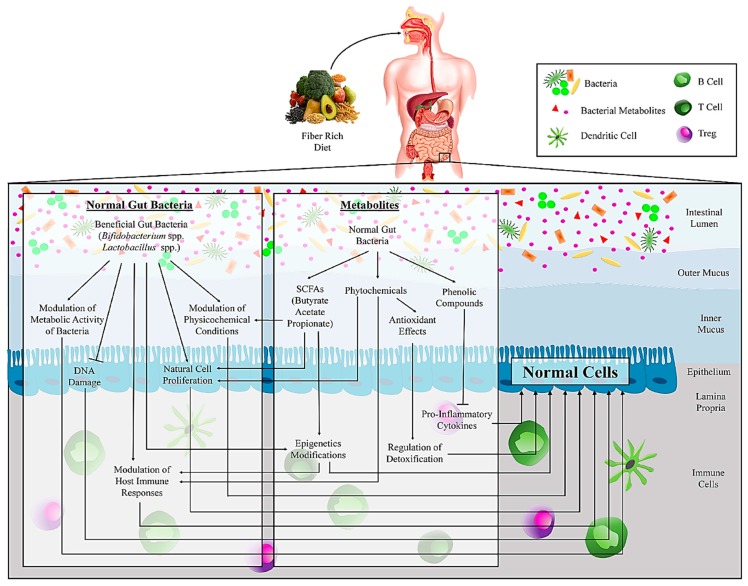
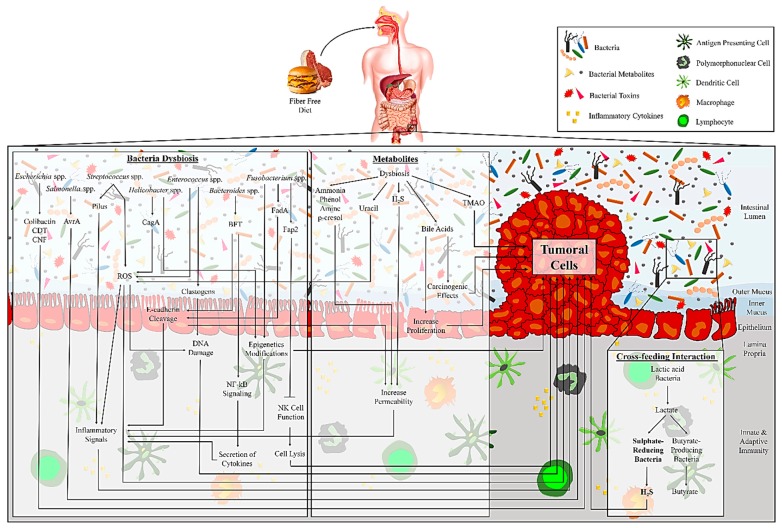
Humans are known as “superorganisms” because of their inherent ability to organize the microbial communities in addition to their cells [19]. The gut bacterial population consists of different phyla species [20]. These organisms have a significant effect on several essential aspects of human health, including nutrient absorption, physiology, metabolism, immune function, and protection against pathogens. Valuable insights have been recently gained into the dysbiosis of the gut bacteria in the development of CRC [19,21]. Some of the most important insights of gut bacteria affects this development, which is discussed in the following sections and is summarized in Figure 1 and Figure 2. There is a long history of association between gut bacteria and CRC progression, which was first introduced by Reddy et al. in 1975 [22], suggesting that a bacteria-dependent dysregulation in the immune system can alter the host metabolism. However, how microbiota can influence CRC development has been a topic of great discussion. A recent study proposed a model that highlights the role of some bacteria as drivers or passengers [23], indicating that pathogenic bacteria (driver) at first rapidly colonize the intestinal epithelium, while opportunistic microorganisms (passengers) then enrich the cancer condition. Accordingly, bacteria with pro-carcinogenic capabilities, especially opportunistic pathogens and polymicrobial anaerobic bacteria, are often detected in early CRC stages [24]. Indeed, a high proportion of bacteria belonging to the Shigella, Salmonella, and Citrobacter genera have been found in early CRC stages compared to healthy controls, while they vanished in a more advanced stage of CRC development [21]. In contrast, the presence of Fusobacterium ssp. and Streptococcaceae families, as passenger bacteria, has not been found in early CRC stages. While, at first the passenger bacteria may use the benefits of changes in the tumor microenvironments to thrive better and expand [25,26], their high proportion in the first stages of CRC might have a role in cancer development [21]. Nakatsu et al. examined bacterial changes across the CRC stages [27]. The enrichment of Fusobacterium, Gemella, Leptotrichia, and Parvimonas and the losses of Alistipes, Bacterioides, Blautia, Collinsella have been reported in early stages (Stage I-II) CRC. Neither of these variations was detected significantly in late stages (Stage III-IV) CRC. Zeller et al. reported a strong enrichment of Fusobacterium and Peptostreptococcus and losses of Eubacterium and Streptococcus in the early stages of CRC [28].
Figure 1.
The schematic association of the gut bacteria and their metabolites in maintaining cell homeostasis.
Figure 2.
The schematic association of gut bacteria and their metabolites, which affects the development of tumorigenesis.
3. The Importance of Gut Bacteria Detected in the Stool and Tissue in CRC:
Several studies compare the evaluation of microbiota derived from tissue and stool samples of CRC patients and healthy controls. As an example of guidance, the data on significant relative abundance of gut bacterial genera in CRC cases are presented in Figure 1, Figure 2, and Table 1. In addition, several studies (not shown in Table 1) evaluated the variation of gut bacteria between tumor tissue and its healthy adjacent tissue in CRC patients [24,26,29,30,31,32,33,34,35]. The incidence risk of CRC is higher in developed countries than in developing ones, which is highly related to dietary differences. However, most of the studies evaluated gut bacteria and CRC in developed countries except for some studies from Malaysia, Indonesia, India, and Morocco [36,37,38,39]. Evidence based on global epidemiological studies suggests an increased risk of CRC by high caloric intake and consumption of some diets like protein (red meat) and animal fat and low consumption of multivitamins and fibers, which affects gut microbial metabolism [40,41]. In the case of local CRC, the range of cure effectiveness is from 70%–90%, while a high mortality rate is reported in advanced CRC cases [42]. Overall, the worldwide incidence of CRC is approximate 4%–5%, and personal traits and lifestyle are considered the most significant risk factors [43]. Moreover, a significant role for CRC development has been ascertained for the dominant gut bacteria [43], although, it is currently unclear how dysbiosis could progress CRC.
Table 1.
Evidence of relative abundance of gut bacterial genera isolated from stool and tissue samples of CRC patients.
| Gut bacteria | Author | Published Time | Enrolment Time | Country | Sample Type (S/Ta) | Cancer Type | Method |
|---|---|---|---|---|---|---|---|
| Increased Gut Bacteria | |||||||
| Acidaminobacter | Weir [85] | 2013 | - | USA | S | CRC | 16S rDNA Sequencing |
| Acidovorax | Sanapareddy [86] | 2012 | - | USA | T | CRA | 16S rDNA Sequencing |
| Actinomyces | Peters [87] | 2016 | 2012–2014 | USA | S | CRC/CRA | Pyrosequencing |
| Kasai [88] | 2015 | 2012–2013 | Japan | S | CRC/CRA | T-RFLP/NGS | |
| Akkermansia | Weir [85] | 2013 | - | USA | S | CRC | 16S rDNA Sequencing |
| Mira-Pascual [89] | 2015 | - | Spain | T | CRC/CRA | qPCR | |
| Alistipes | Feng [90] | 2015 | 2010–2012 | Austria | S | A-CRAb/CRC | Metagenomic Shotgun Sequencing |
| Wu [91] | 2013 | - | China | S | CRC | 16S rRNA Sequencing | |
| Atopobium | Vogtmann [92] | 2016 | 1985–1987 | USA | S | CRC | Whole-genome Shotgun Sequencing |
| Kasai [88] | 2015 | 2012–2013 | Japan | S | CRC/CRA | T-RFLP/NGS | |
| Ahn [93] | 2013 | 1985–1989 | USA | S | CRC | 16S rDNA Sequencing | |
| Anaerococcus | Chen [94] | 2012 | - | China | T | CRC | 16S rDNA Sequencing |
| Anaerotruncus | Chen [94] | 2012 | - | China | T | CRC | 16S rDNA Sequencing |
| Aquabacterium | Sanapareddy [86] | 2012 | - | USA | T | CRA | 16S rDNA Sequencing |
| Bacteriodes | Feng [90] | 2015 | 2010–2012 | Austria | S | A-CRA/CRC | Metagenomic Shotgun Sequencing |
| Sobhani [95] | 2011 | 2004–2006 | France | S | CRC | Pyrosequencing/qPCR | |
| Kasai [88] | 2015 | 2012–2013 | Japan | S | CRC/CRA | T-RFLP/NGS | |
| Xu [96] | 2017 | - | China | T | CRC/CRA | NGS | |
| Brim [97] | 2013 | - | USA | S | Colon polyps | 16S rRNA Sequencing/HITChip/Pyrosequencing | |
| Flemer [98] | 2015 | - | Ireland | S/T | CRC/Polyps | 16S rDNA Sequencing | |
| Wang [99] | 2012 | - | China | S | CRC | 16S rDNA Sequencing | |
| Wu [91] | 2013 | - | China | S | CRC | 16S rDNA Sequencing | |
| Chen [94] | 2012 | - | China | T | CRC | 16S rDNA Sequencing | |
| Drewes [37] | 2017 | - | Malaysia | T | CRC | 16S rDNA Sequencing | |
| Gao [100] | 2015 | - | China | T | CRC | 16S rDNA Sequencing | |
| Liang [101] | 2016 | - | China | S | CRC | duplex qPCR | |
| Nakatsu [27] | 2015 | 2011–2014 | China | T | CRC/CRA | qPCR | |
| Huipeng [102] | 2014 | - | China | T | Colon cancer | PCR/DGGE | |
| Bifidobacterium | Nugent [103] | 2014 | - | USA | T | CRA | qPCR |
| Bilophila | Feng [90] | 2015 | 2010–2012 | Austria | S | A-CRA/CRC | Metagenomic Shotgun Sequencing |
| Hale [104] | 2017 | 2001–2005 | USA | S | CRA | 16S rDNA Sequencing | |
| Hibberd [105] | 2017 | - | USA | T | Colon cancer | 16S rDNA Sequencing | |
| Yazici [106] | 2017 | 2011–2012 | USA | T | CRC | 16S rDNA Sequencing | |
| Blautia | Ai [107] | 2017 | 2012 | China | S | CRC/CRA | 16S rRNA Sequencing |
| Wu [91] | 2013 | - | China | S | CRC | 16S rRNA Sequencing | |
| Mira-Pascual [89] | 2015 | - | Spain | T | CRC/CRA | 16S rRNA Sequencing/qPCR | |
| Butyrivibrio | Dejea [108] | 2014 | - | USA | T | CRC/CRA | Pyrosequencing |
| Campylobacter | Xu [96] | 2017 | - | China | T | CRC/CRA | NGS |
| Wu [91] | 2013 | - | China | S | CRC | 16S rRNA Sequencing | |
| Zhang [109] | 2018 | 2014–2015 | China | S | CRC/A-CRA/BP c | 16S rRNA Sequencing | |
| Citrobacter | Weir [85] | 2013 | - | USA | S | CRC | 16S rDNA Sequencing |
| Cloacibacterium | Sanapareddy [86] | 2012 | - | USA | T | CRA | 16S rDNA Sequencing |
| Clostridium | Dejea [108] | 2014 | - | USA | T | CRC/CRA | Pyrosequencing |
| Hibberd [105] | 2017 | - | USA | T | Colon cancer | 16S rDNA Sequencing | |
| Zhang [109] | 2018 | 2014–2015 | China | S | CRC/A-CRA/BP | 16S rDNA Sequencing | |
| Scanlan [110] | 2008 | - | Ireland | S | Colon cancer | 16S rRNA Sequencing | |
| Allali [36] | 2018 | - | Morocco | S | CRC | 16S rDNA Sequencing | |
| Kasai [88] | 2015 | 2012–2013 | Japan | S | CRC/CRA | T-RFLP/NGS | |
| Fukugaiti [111] | 2015 | - | Brazil | S | CRC | qPCR | |
| Ohigashi [112] | 2013 | 2009–2010 | Japan | S | CRC | qPCR | |
| Xie [113] | 2017 | 2016 | China | S | CRA/CRC/A-CRC | PCR | |
| Liang [101] | 2016 | - | China | S | CRC | duplex qPCR | |
| Collinsella | Chen [94] | 2012 | - | China | T | CRC | 16S rDNA Sequencing |
| Desulfovibrio | Hale [104] | 2017 | 2001–2005 | USA | S | CRA | 16S rRNA Sequencing |
| Chen [94] | 2012 | - | China | T | CRC | 16S rDNA Sequencing | |
| Dialister | Xu [96] | 2017 | - | China | T | CRC/CRA | NGS |
| Zhang [109] | 2018 | 2014–2015 | China | S | CRC/A-CRA/BP | 16S rDNA Sequencing | |
| Hibberd [105] | 2017 | - | USA | T | Colon cancer | 16S rDNA Sequencing | |
| Dorea | Peters [87] | 2016 | 2012–2014 | USA | S | CRC/CRA | Pyrosequencing |
| Wu [91] | 2013 | - | China | S | CRC | 16S rRNA Sequencing | |
| Hibberd [105] | 2017 | - | USA | S | Colon cancer | 16S rRNA Sequencing | |
| Shen [114] | 2010 | - | USA | T | CRA | 16S rDNA Sequencing | |
| Eggerthella | Zhang [109] | 2018 | 2014–2015 | China | S | CRC/A-CRA/BP | 16S rDNA Sequencing |
| Enterococcus | Chen [115] | 2013 | 2010–2011 | China | S | A-CRA | Pyrosequencing |
| Wang [99] | 2012 | - | China | S | CRC | 16S rDNA Sequencing | |
| Balamurugan [39] | 2008 | - | India | S | CRC | Real-time PCR | |
| Escherichia | Feng [90] | 2015 | 2010–2012 | Austria | S | A-CRA/CRC | Metagenomic Shotgun Sequencing |
| Xu [96] | 2017 | - | China | T | CRC/CRA | NGS | |
| Mori [116] | 2018 | 2013–2015 | Italy | S | CRC/CRA | 16S rRNA Sequencing | |
| Wang [99] | 2012 | - | China | S | CRC | 16S rRNA Sequencing | |
| Wu [91] | 2013 | - | China | S | CRC | 16S rRNA Sequencing | |
| Goedert [117] | 2015 | - | USA | S | CRA | 16S rRNA Sequencing | |
| Yoon [118] | 2017 | - | Korea | T | CRC/CRA | 16S rDNA Sequencing | |
| Gao [100] | 2015 | - | China | T | CRC | 16S rDNA Sequencing | |
| Mira-Pascual [89] | 2015 | - | Spain | T | CRC/CRA | qPCR | |
| Kohoutova [44] | 2014 | - | UK | T | CRC/CRA | PCR | |
| Bonnet [119] | 2013 | 2007–2010 | France | T | Colon cancer | PCR | |
| Swidsinski [120] | 1998 | - | Austria | T | CRC/CRA | PCR | |
| Eubacterium | Chen [94] | 2012 | - | China | T | CRC | 16S rDNA Sequencing |
| Faecalibacterium | Sze [121] | 2017 | - | USA | S | CRC/A-CRA/CRA | 16S rDNA Sequencing |
| Shen [114] | 2010 | - | USA | T | CRA | 16S rRNA Sequencing | |
| Fastidiosipila | Wu [91] | 2013 | - | China | S | CRC | 16S rRNA Sequencing |
| Fastidiosipila | Wu [91] | 2013 | - | China | S | CRC | 16S rRNA Sequencing |
| Fusobacterium | Vogtmann [92] | 2016 | 1985–1987 | USA | S | CRC | Whole-genome Shotgun Sequencing |
| Feng [90] | 2015 | 2010–2012 | Austria | S | A-CRA/CRC | Metagenomic Shotgun Sequencing | |
| Yu [122] | 2015 | - | China | S | CRC | Metagenomic Sequencing | |
| Dejea [108] | 2014 | - | USA | T | CRC/CRA | Pyrosequencing | |
| Xu [96] | 2017 | - | China | T | CRC/CRA | NGS | |
| Deng [123] | 2018 | - | China | S | CRC | NGS | |
| Kasai [88] | 2015 | 2012–2013 | Japan | S | CRC/CRA | T-RFLP/ NGS | |
| Kostic [124] | 2012 | - | Spain | T | CRC | WGS/16S rDNA Sequencing/qPCR/FISH | |
| Allali [36] | 2018 | - | Morocco | S | CRC | 16S rDNA Sequencing | |
| Zackular [125] | 2014 | - | Michigan | S | CRC/CRA | 16S rDNA Sequencing | |
| Ahn [93] | 2013 | 1985–1989 | Washington | S | CRC | 16S rDNA Sequencing | |
| Sinha [16] | 2016 | 1985–1987 | USA | S | CRC | 16S rDNA Sequencing | |
| Flemer [98] | 2015 | - | Ireland | S/T | CRC/Polyps | 16S rDNA Sequencing | |
| Flemer [126] | 2017 | - | Ireland | S/T | CRC/Polyps | 16S rDNA Sequencing | |
| Zhang [109] | 2018 | 2014–2015 | China | S | CRC/A-CRA/BP | 16S rDNA Sequencing | |
| Zeller [28] | 2014 | 2004–2006 | France/Germany | S | CRC/CRA | 16S rDNA Sequencing | |
| Baxter [127] | 2016 | - | USA | S | CRC/CRA | 16S rDNA Sequencing | |
| Gao [100] | 2015 | - | China | T | CRC | 16S rDNA Sequencing | |
| Hibberd [105] | 2017 | - | USA | T | Colon cancer | 16S rDNA Sequencing | |
| Chen [94] | 2012 | - | China | T | CRC | 16S rDNA Sequencing | |
| Drewes [37] | 2017 | - | Malaysia | T | CRC | 16S rDNA Sequencing | |
| Yoon [118] | 2017 | - | Korea | T | CRC/CRA | 16S rDNA Sequencing | |
| Amitay [128] | 2017 | 2005–2013 | Germany | S | CRC/A-CRA/CRA | 16S rDNA Sequencing/multiplex PCR | |
| Nakatsu [27] | 2015 | 2011–2014 | China | T | CRC/CRA | 16S rRNA Sequencing/qPCR | |
| Wu [91] | 2013 | - | China | S | CRC | 16S rDNA Sequencing/qPCR | |
| Russo [129] | 2018 | 2015–2016 | Italy | S | CRC | qPCR/16S rDNA Sequencing | |
| Liang [101] | 2016 | - | China | S | CRC | duplex qPCR | |
| Kostic [60] | 2013 | - | USA | S | CRC/CRA | qPCR | |
| Wong [130] | 2016 | - | China | S | CRC/A-CRA | qPCR | |
| Fukugaiti [111] | 2015 | - | Brazil | S | CRC | qPCR | |
| Eklof [131] | 2017 | 2008–2013 | Sweden | S | CRC | qPCR | |
| Mira-Pascual [89] | 2015 | - | Spain | S | CRC/CRA | qPCR | |
| Yu [122] | 2015 | - | China | S | CRC | qPCR | |
| Flanagan [132] | 2014 | 2008–2010 | Ireland | S | CRC | qPCR | |
| Repass [133] | 2016 | - | USA | T | CRC | qPCR | |
| Castellarin [134] | 2012 | - | Canada | T | CRC | qPCR | |
| Tahara [135] | 2014 | - | Japan | T | CRC | qPCR | |
| Ito [136] | 2015 | 2001–2013 | Japan | T | CRC | qPCR | |
| McCoy [137] | 2013 | - | USA | T | CRA | qPCR | |
| Suehiro [138] | 2016 | - | Japan | S | CRC/CRA/A-CRA | PCR | |
| Gemella | Zhang [109] | 2018 | 2014 - 2015 | China | S | CRC/A-CRA/BP | 16S rDNA Sequencing |
| Baxter [127] | 2016 | - | USA | S | CRC/CRA | 16S rDNA Sequencing | |
| Chen [94] | 2012 | - | China | T | CRC | 16S rDNA Sequencing | |
| Nakatsu [27] | 2015 | 2011–2014 | China | T | CRC/CRA | 16S rRNA Sequencing/qPCR | |
| Granulicatella | Nakatsu [27] | 2015 | 2011–2014 | China | T | CRC/CRA | 16S rRNA Sequencing/qPCR |
| Heamophilus | Kasai [88] | 2015 | 2012–2013 | Japan | S | CRC/CRA | T-RFLP/NGS |
| Helicobacter | Goedert [117] | 2015 | - | USA | S | CRA | 16S rRNA Sequencing |
| Sanapareddy [86] | 2012 | - | USA | T | CRA | 16S rDNA Sequencing | |
| Klebsiella | Wang [99] | 2012 | - | China | S | CRC | 16S rDNA Sequencing |
| Goedert [117] | 2015 | - | USA | S | CRA | 16S rRNA Sequencing | |
| Chen [94] | 2012 | - | China | T | CRC | 16S rDNA Sequencing | |
| Lactobacillus | Xu [96] | 2017 | - | China | T | CRC/CRA | NGS |
| Sanapareddy [86] | 2012 | - | USA | T | CRA | 16S rDNA Sequencing | |
| Lactococcus | Lu [139] | 2016 | 2014 | China | T | CRA | Pyrosequencing |
| Sanapareddy [86] | 2012 | - | USA | T | CRA | 16S rDNA Sequencing | |
| Gao [100] | 2015 | - | China | T | CRC | 16S rDNA Sequencing | |
| Methanobrevibacter | Hibberd [105] Mira-Pascual [89] | 2017 | - | USA | T | Colon cancer CRC/CRA | 16S rDNA Sequencing |
| 2015 | - | Spain | S | qPCR | |||
| Methanosphaera | Ai [107] | 2017 | 2012 | China | S | CRC/CRA | 16S rRNA Sequencing |
| Mogibacterium | Xu [96] | 2017 | - | China | T | CRC/CRA | NGS |
| Hale [104] | 2017 | 2001–2005 | USA | S | CRA | 16S rRNA Sequencing | |
| Chen [94] | 2012 | - | China | T | CRC | 16S rDNA Sequencing | |
| Morganella | Goedert [117] | 2015 | - | USA | S | CRA | 16S rRNA Sequencing |
| Odoribacter | Wu [91] | 2013 | - | China | S | CRC | 16S rRNA Sequencing |
| Oscillibacter | Wu [91] | 2013 | - | China | S | CRC | 16S rRNA Sequencing |
| Flemer [98] | 2015 | - | Ireland | S/T | CRC/Polyps | 16S rRNA Sequencing | |
| Oscillospira | Deng [123] | 2018 | - | China | S | CRC | NGS |
| Pantoea | Goedert [117] | 2015 | - | USA | S | CRA | 16S rRNA Sequencing |
| Parabacteroides | Feng [90] | 2015 | 2010–2012 | Austria | S | A-CRA/CRC | Metagenomic Shotgun Sequencing |
| Parvimonas | Feng [90] | 2015 | 2010–2012 | Austria | S | A-CRA/CRC | Metagenomic Shotgun Sequencing |
| Yu [122] | 2015 | - | China | S | CRC | Metagenomic Sequencing | |
| Xu [96] | 2017 | - | China | T | CRC/CRA | NGS | |
| Zhang [109] | 2018 | 2014–2015 | China | S | CRC/A-CRA/BP | 16S rDNA Sequencing | |
| Baxter [127] | 2016 | - | USA | S | CRC/CRA | 16S rRNA Sequencing | |
| Flemer [98] | 2015 | - | Ireland | S/T | CRC/Polyps | 16S rDNA Sequencing | |
| Flemer [126] | 2017 | - | Ireland | S/T | CRC/Polyps | 16S rDNA Sequencing | |
| Sze [121] | 2017 | - | USA | S | CRC/A-CRA/CRA | 16S rDNA Sequencing | |
| Drewes [37] | 2017 | - | Malaysia | T | CRC | 16S rDNA Sequencing | |
| Nakatsu [27] | 2015 | 2011–2014 | China | T | CRC/CRA | 16S rRNA Sequencing /qPCR | |
| Wong [130] | 2016 | - | China | S | CRC/A-CRA | qPCR | |
| Peptostreptococcus | Yu [122] | 2015 | - | China | S | CRC | Metagenomic Sequencing |
| Xu [96] | 2017 | - | China | T | CRC/CRA | NGS | |
| Zhang [109] | 2018 | 2014–2015 | China | S | CRC/A-CRA/BP | 16S rDNA Sequencing | |
| Baxter [127] | 2016 | - | USA | S | CRC/CRA | 16S rRNA Sequencing | |
| Zeller [28] | 2014 | 2004–2006 | France/Germany | S | CRC/CRA | 16S rRNA Sequencing | |
| Flemer [98] | 2015 | - | Ireland | S/T | CRC/Polyps | 16S rRNA Sequencing | |
| Flemer [126] | 2017 | - | Ireland | S/T | CRC/Polyps | 16S rRNA Sequencing | |
| Hibberd [105] | 2017 | - | USA | S/T | Colon cancer | 16S rRNA Sequencing | |
| Chen [94] | 2012 | - | China | T | CRC | 16S rDNA Sequencing | |
| Drewes [37] | 2017 | - | Malaysia | T | CRC | 16S rDNA Sequencing | |
| Gao [100] | 2015 | - | China | T | CRC | 16S rDNA Sequencing | |
| Wang [99] | 2012 | - | China | S | CRC | 16S rDNA Sequencing/qPCR | |
| Nakatsu [27] | 2015 | 2011–2014 | China | T | CRC/CRA | 16S rRNA Sequencing/qPCR | |
| Phascolarctobacterium | Weir [85] | 2013 | - | USA | S | CRC | 16S rDNA Sequencing |
| Wu [91] | 2013 | - | China | S | CRC | 16S rDNA Sequencing | |
| Porphyromonas | Vogtmann [92] | 2016 | 1985–1987 | USA | S | CRC | Whole-genome Shotgun Sequencing |
| Sobhani [95] | 2011 | 2004–2006 | France | S | CRC | Pyrosequencing/qPCR | |
| Baxter [127] | 2016 | - | USA | S | CRC/CRA | 16S rRNA Sequencing | |
| Allali [36] | 2018 | - | Morocco | S | CRC | 16S rRNA Sequencing | |
| Zackular [125] | 2014 | - | Michigan | S | CRC/CRA | 16S rRNA Sequencing | |
| Sze [121] | 2017 | - | USA | S | CRC/A-CRA/CRA | 16S rRNA Sequencing | |
| Ahn [93] | 2013 | 1985–1989 | Washington | S | CRC | 16S rRNA Sequencing | |
| Wang [99] | 2012 | - | China | S | CRC | 16S rRNA Sequencing | |
| Sinha [16] | 2016 | 1985–1987 | USA | S | CRC | 16S rRNA Sequencing | |
| Zhang [109] | 2018 | 2014–2015 | China | S | CRC/A-CRA/BP | 16S rRNA Sequencing | |
| Zeller [28] | 2014 | 2004–2006 | France/Germany | S | CRC/CRA | 16S rRNA Sequencing | |
| Flemer [98] | 2015 | - | Ireland | S/T | CRC/Polyps | 16S rRNA Sequencing | |
| Chen [94] | 2012 | - | China | T | CRC | 16S rDNA Sequencing | |
| Geng [140] | 2014 | - | China | T | CRC/CRA | 16S rRNA Sequencing | |
| Prevotella | Deng [123] | 2018 | - | China | S | CRC | NGS |
| Kasai [88] | 2015 | 2012–2013 | Japan | S | CRC/CRA | T-RFLP/NGS | |
| Baxter [127] | 2016 | - | USA | S | CRC/CRA | 16S rRNA Sequencing | |
| Flemer [126] | 2017 | - | Ireland | S/T | CRC/Polyps | 16S rRNA Sequencing | |
| Gao [100] | 2015 | - | China | T | CRC | 16S rDNA Sequencing | |
| Chen [94] | 2012 | - | China | T | CRC | 16S rDNA Sequencing | |
| Mira-Pascual [89] | 2015 | - | Spain | T | CRC/CRA | 16S rRNA Sequencing/qPCR | |
| Paraprevotella | Chen [94] | 2012 | - | China | T | CRC | 16S rDNA Sequencing |
| Pseudomonas | Lu [139] | 2016 | 2014 | China | T | CRA | Pyrosequencing |
| Zackular [125] | 2014 | - | Michigan | S | CRC/CRA | 16S rDNA Sequencing | |
| Goedert [117] | 2015 | - | USA | S | CRA | 16S rRNA Sequencing | |
| Sanapareddy [86] | 2012 | - | USA | T | CRA | 16S rDNA Sequencing | |
| Yoon [118] | 2017 | - | Korea | T | CRC/CRA | 16S rDNA Sequencing | |
| Ohigashi [112] | 2013 | 2009–2010 | Japan | S | CRC | qPCR | |
| Pyramidobacter | Yazici [106] | 2017 | 2011–2012 | USA | T | CRC | 16S rRNA Sequencing |
| Rhizobium | Yoon [118] | 2017 | - | Korea | T | CRC/CRA | 16S rDNA Sequencing |
| Roseburia | Flemer [98] | 2015 | - | Ireland | S/T | CRC/Polyps | 16S rDNA Sequencing |
| Liang [101] | 2016 | - | China | S | CRC | duplex qPCR | |
| Ruminococcus | Dejea [108] | 2014 | - | USA | T | CRC/CRA | Pyrosequencing |
| Allali [36] | 2018 | - | Morocco | S | CRC | 16S rDNA Sequencing | |
| Zhang [109] | 2018 | 2014–2015 | China | S | CRC/A-CRA/BP CRC/Polyps | 16S rDNA Sequencing | |
| Flemer [98] | 2015 | - | Ireland | S/T | CRA | 16S rDNA Sequencing | |
| Shen [114] | 2010 | - | USA | T | 16S rRNA Sequencing | ||
| Salmonella | Goedert [117] | 2015 | - | USA | S | CRA | 16S rRNA Sequencing |
| Selenomonas | Allali [36] | 2018 | - | Morocco | S | CRC | 16S rDNA Sequencing |
| Hibberd [105] | 2017 | - | USA | T | Colon cancer | 16S rDNA Sequencing | |
| Serratia | Goedert [117] | 2015 | - | USA | S | CRA | 16S rDNA Sequencing |
| Slackia | Chen [94] | 2012 | - | China | T | CRC | 16S rDNA Sequencing |
| Sphingomonas | Richard [141] | 2018 | - | France | T | CACd/CRC | qPCR/16S rRNA Sequencing |
| Shigella | Goedert [117] | 2015 | - | USA | S | CRA | 16S rDNA Sequencing |
| Mori [116] | 2018 | 2013–2015 | Italy | S | CRC/CRA | 16S rRNA Sequencing | |
| Wang [99] | 2012 | - | China | S | CRC | 16S rRNA Sequencing | |
| Wu [91] | 2013 | - | China | S | CRC | 16S rRNA Sequencing | |
| Shen [114] | 2010 | - | USA | T | CRA | 16S rRNA Sequencing | |
| Gao [100] | 2015 | - | China | T | CRC | 16S rDNA Sequencing | |
| Mira-Pascual [89] | 2015 | - | Spain | T | CRC/CRA | qPCR | |
| Solobacterium | Yu [122] | 2015 | - | China | S | CRC | Metagenomic Sequencing |
| Zhang [109] | 2018 | 2014–2015 | China | S | CRC/A-CRA/BP | 16S rDNA Sequencing | |
| Streptococcus | Chen [115] | 2013 | 2010–2011 | China | S | A-CRA | Pyrosequencing |
| Peters [87] | 2016 | 2012–2014 | USA | S | CRC/CRA | Pyrosequencing | |
| Kasai [88] | 2015 | 2012–2013 | Japan | S | CRC/CRA | T-RFLP/NGS | |
| Flemer [126] | 2017 | - | Ireland | S/T | CRC/Polyps | 16S rDNA Sequencing | |
| Wang [99] | 2012 | - | China | S | CRC | 16S rDNA Sequencing | |
| Gao [100] | 2015 | - | China | T | CRC | 16S rDNA Sequencing | |
| Chen [94] | 2012 | - | China | T | CRC | 16S rDNA Sequencing | |
| Geng [140] | 2014 | - | China | T | CRC/CRA | 16S rRNA Sequencing | |
| Richard [141] | 2018 | - | France | T | CAC/CRC | qPCR/16S rRNA Sequencing | |
| Klein [82] | 1977 | - | Chicago | S | CRC | Culture | |
| Subdoligranulum | Wu [91] | 2013 | - | China | S | CRC | 16S rRNA Sequencing |
| Sutterella | Mori [116] | 2018 | 2013–2015 | Italy | S | CRC/CRA | 16S rRNA Sequencing |
| Hale [104] | 2017 | 2001–2005 | USA | S | CRA | 16S rRNA Sequencing | |
| Trabulsiella | Goedert [117] | 2015 | - | USA | S | CRA | 16S rRNA Sequencing |
| Veillonella | Kasai [88] | 2015 | 2012–2013 | Japan | S | CRC/CRA | T-RFLP/NGS |
| Geng [140] | 2014 | - | China | T | CRC/CRA | 16S rRNA Sequencing | |
| Decreased Gut Bacteria | |||||||
| Acidovorax | Gao [100] | 2015 | - | China | T | CRC | 16S rDNA Sequencing |
| Acinetobacter | Gao [100] | 2015 | - | China | T | CRC | 16S rDNA Sequencing |
| Alistipes | Wang [99] | 2012 | - | China | S | CRC | 16S rDNA Sequencing |
| Nakatsu [27] | 2015 | 2011–2014 | China | T | CRC/CRA | 16S rRNA Sequencing | |
| Anaerostipes | Chen [94] | 2012 | - | China | T | CRC | 16S rDNA Sequencing |
| Atopobium | Ohigashi [112] | 2013 | 2009–2010 | Japan | S | CRC | qPCR |
| Bacteriodes | Kostic [124] | 2012 | - | Spain | T | CRC | WGS |
| Zackular [125] | 2014 | - | Michigan | S | CRC/CRA | 16S rDNA Sequencing | |
| Wang [99] | 2012 | - | China | S | CRC | 16S rDNA Sequencing | |
| Weir [85] | 2013 | - | USA | S | CRC | 16S rDNA Sequencing | |
| Allali [36] | 2018 | - | Morocco | S | CRC | 16S rDNA Sequencing | |
| Shen [114] | 2010 | - | USA | T | CRA | 16S rDNA Sequencing | |
| Nakatsu [27] | 2015 | 2011–2014 | China | T | CRC/CRA | 16S rDNA Sequencing | |
| Ohigashi [112] | 2013 | 2009–2010 | Japan | S | CRC | qPCR | |
| Bacillus | Lu [139] | 2016 | 2014 | China | T | CRA | Pyrosequencing |
| Mira-Pascual [89] | 2015 | - | Spain | T | CRC/CRA | qPCR | |
| Bifidobacterium | Feng [90] | 2015 | 2010–2012 | Austria | S | A-CRA/CRC | Metagenomic Shotgun Sequencing |
| Chen [94] | 2012 | - | China | T | CRC | 16S rDNA Sequencing | |
| Mira-Pascual [89] | 2015 | - | Spain | S | CRC/CRA | qPCR | |
| Ohigashi [112] | 2013 | 2009–2010 | Japan | S | CRC | qPCR | |
| Yusuf [38] | 2016 | - | Indonesia | S | CRC | DGGE | |
| Blautia | Xu [96] | 2017 | - | China | T | CRC/CRA | NGS |
| Chen [94] | 2012 | 2011–2014 | China | T | CRC | 16S rDNA Sequencing | |
| Nakatsu [27] | 2015 | - | China | T | CRC/CRA | 16S rDNA Sequencing | |
| Gao [100] | 2015 | - | China | T | CRC | 16S rDNA Sequencing | |
| Buttiauxella | Gao [100] | 2015 | - | China | T | CRC | 16S rDNA Sequencing |
| Caulobacter | Gao [100] | 2015 | - | China | T | CRC | 16S rRNA Sequencing |
| Collinsella | Nakatsu [27] | 2015 | 2011–2014 | China | T | CRC/CRA | 16S rRNA Sequencing |
| Clostridium | Chen [115] | 2013 | 2010–2011 | China | S | A-CRA | Pyrosequencing |
| Zackular [125] | 2014 | - | Michigan | S | CRC/CRA | 16S rDNA Sequencing | |
| Ohigashi [112] | 2013 | 2009–2010 | Japan | S | CRC | qPCR | |
| Coprococcus | Vogtmann [92] | 2016 | 1985–1987 | USA | S | CRC | Whole-genome Shotgun Sequencing |
| Ahn [93] | 2013 | 1985–1989 | USA | S | CRC | 16S rDNA Sequencing | |
| Zhang [109] | 2018 | 2014–2015 | China | S | CRC/A-CRA/BP | 16S rDNA Sequencing | |
| Flemer [98] | 2015 | - | Ireland | S/T | CRC/Polyps | 16S rDNA Sequencing | |
| Shen [114] | 2010 | - | USA | T | CRA | 16S rRNA Sequencing | |
| Desulfovibrio | Scanlan [142] | 2009 | - | UK | S | CRC | qPCR |
| Dialister | Weir [85] | 2013 | - | USA | S | CRC | 16S rDNA Sequencing |
| Dorea | Weir [85] | 2013 | - | USA | S | CRC | 16S rDNA Sequencing |
| Enterococcus | Lu [139] | 2016 | 2014 | China | T | CRA | Pyrosequencing |
| Epilithonimonas | Gao [100] | 2015 | - | China | T | CRC | 16S rDNA Sequencing |
| Eubacterium | Yu [122] | 2015 | - | China | S | CRC | Metagenomic Sequencing |
| Chen [115] | 2013 | 2010–2011 | China | S | A-CRA | Pyrosequencing | |
| Kasai [88] | 2015 | 2012–2013 | Japan | S | CRC/CRA | T-RFLP/NGS | |
| Wang [99] | 2012 | - | China | S | CRC | 16S rDNA Sequencing | |
| Zhang [109] | 2018 | 2014–2015 | China | S | CRC/A-CRA/BP | 16S rDNA Sequencing | |
| Balamurugan [39] | 2008 | - | India | S | CRC | Real-time PCR | |
| Vargo [83] | 1980 | - | USA | S | Colon cancer | Culture | |
| Faecalibacterium | Xu [96] | 2017 | - | China | T | CRC/CRA | NGS |
| Wu [91] | 2013 | - | China | S | CRC | 16S rRNA Sequencing | |
| Chen [94] | 2012 | - | China | T | CRC | 16S rDNA Sequencing | |
| Nakatsu [27] | 2015 | 2011–2014 | China | T | CRC/CRA | 16S rRNA Sequencing | |
| Balamurugan [39] | 2008 | - | India | S | CRC | Real-time PCR | |
| Mira-Pascual [89] | 2015 | - | Spain | T | CRC/CRA | qPCR | |
| Lopez-Siles [143] | 2016 | - | Spain | T | CRC | qPCR | |
| Fusicatenibacter | Zhang [109] | 2018 | 2014–2015 | China | S | CRC/A-CRA/BP | 16S rDNA Sequencing |
| Flavobacterium | Gao [100] | 2015 | - | China | T | CRC | 16S rDNA Sequencing |
| Fusobacterium | Shen [114] | 2010 | - | USA | T | CRA | 16S rRNA Sequencing |
| Richard [141] | 2018 | - | France | T | CAC/CRC | qPCR/16S rRNA Sequencing | |
| Vargo [83] | 1980 | - | USA | S | Colon cancer | Culture | |
| Haemophilus | Hale [104] | 2017 | 2001–2005 | USA | S | CRA | 16S rRNA Sequencing |
| Janthinobacterium | Gao [100] | 2015 | - | China | T | CRC | 16S rDNA Sequencing |
| Lachnobacterium | Weir [85] | 2013 | - | USA | S | CRC | 16S rDNA Sequencing |
| Lachnospira | Weir [85] | 2013 | - | USA | S | CRC | 16S rDNA Sequencing |
| Chen [94] | 2012 | - | China | T | CRC | 16S rDNA Sequencing | |
| Lactobacillus | Feng [90] | 2015 | 2010–2012 | Austria | S | A-CRA/CRC | Metagenomic Shotgun Sequencing |
| Chen [94] | 2012 | - | China | T | CRC | 16S rDNA Sequencing | |
| Megamonas | Weir [85] | 2013 | - | USA | S | CRC | 16S rDNA Sequencing |
| Megasphaera | Ahn [93] | 2013 | 1985–1989 | Washington | S | CRC | 16S rDNA Sequencing |
| Parasutterella | Wang [99] | 2012 | - | China | S | CRC | 16S rDNA Sequencing |
| Pedobacter | Gao [100] | 2015 | - | China | T | CRC | 16S rDNA Sequencing |
| Propionibacterium | Gao [100] | 2015 | - | China | T | CRC | 16S rDNA Sequencing |
| Peptostreptococcus | Ahn [93] | 2013 | 1985–1989 | Washington | S | CRC | 16S rDNA Sequencing |
| Prevotella | Weir [85] | 2013 | - | USA | S | CRC | 16S rDNA Sequencing |
| Pseudobutyrivibrio | Weir [85] | 2013 | - | USA | S | CRC | 16S rDNA Sequencing |
| Chen [94] | 2012 | - | China | T | CRC | 16S rDNA Sequencing | |
| Pseudomonas | Gao [100] | 2015 | - | China | T | CRC | 16S rDNA Sequencing |
| Psychrobacter | Gao [100] | 2015 | - | China | T | CRC | 16S rDNA Sequencing |
| Rahnella | Gao [100] | 2015 | - | China | T | CRC | 16S rDNA Sequencing |
| Roseburia | Chen [115] | 2013 | 2010–2011 | China | S | A-CRA | Pyrosequencing |
| Wang [99] | 2012 | - | China | S | CRC | 16S rDNA Sequencing | |
| Wu [91] | 2013 | - | China | S | CRC | 16S rRNA Sequencing | |
| Hibberd [105] | 2017 | - | USA | S | Colon cancer | 16S rRNA Sequencing | |
| Zhang [109] | 2018 | 2014–2015 | China | S | CRC/A-CRA/BP | 16S rRNA Sequencing | |
| Chen [94] | 2012 | - | China | T | CRC | 16S rDNA Sequencing | |
| Ruminococcus | Feng [90] | 2015 | 2010–2012 | Austria | S | A-CRA/CRC | Metagenomic Shotgun Sequencing |
| Ahn [93] | 2013 | 1985–1989 | Washington | S | CRC | 16S rDNA Sequencing | |
| Weir [85] | 2013 | - | USA | S | CRC | 16S rDNA Sequencing | |
| Zhang [109] | 2018 | 2014–2015 | China | S | CRC/A-CRA/BP | 16S rDNA Sequencing | |
| Richard [141] | 2018 | - | France | T | CAC/CRC | qPCR/16S rRNA Sequencing | |
| Selenomonas | Ahn [93] | 2013 | 1985–1989 | Washington | S | CRC | 16S rDNA Sequencing |
| Slackia | Kasai [88] | 2015 | 2012–2013 | Japan | S | CRC/CRA | T-RFLP/NGS |
| Solibacillus | Lu [139] | 2016 | 2014 | China | T | CRA | Pyrosequencing |
| Sphingobacterium | Gao [100] | 2015 | - | China | T | CRC | 16S rDNA Sequencing |
| Sphingomonas | Gao [100] | 2015 | - | China | T | CRC | 16S rDNA Sequencing |
| Staphylococcus | Ohigashi [112] | 2013 | 2009–2010 | Japan | S | CRC | qPCR |
| Mira-Pascual [89] | 2015 | - | Spain | T | CRC/CRA | qPCR | |
| Streptococcus | Feng [90] | 2015 | 2010–2012 | Austria | S | A-CRA/CRC | Metagenomic Shotgun Sequencing |
| Hale [104] | 2017 | 2001–2005 | USA | S | CRA | 16S rRNA Sequencing | |
| Zhang [109] | 2018 | 2014–2015 | China | S | CRC/A-CRA/BP | 16S rDNA Sequencing | |
| Hibberd [105] | 2017 | - | USA | T | Colon cancer | 16S rDNA Sequencing | |
| Sanapareddy [86] | 2012 | - | USA | T | CRA | 16S rDNA Sequencing | |
| Stenotrophomonas | Gao [100] | 2015 | - | China | T | CRC | 16S rDNA Sequencing |
| Sutterella | Nakatsu [27] | 2015 | 2011–2014 | China | T | CRC/CRA | 16S rRNA Sequencing/qPCR |
| Veillonella | Hale [104] | 2017 | 2001–2005 | USA | S | CRA | 16S rRNA Sequencing |
a Stool/Tissue. b Advanced colorectal adenoma. c Benign polyp. d Colitis-associated cancer. Gut bacteria isolated from stool or tissue samples with both increased and decreased evidence are presented in bold.
Herein, some crucial gut bacterial mechanisms involved in CRC are discussed in detail. Based on molecular methods, one of the first studies identified a secure link between the genus of Escherichia and CRC [42]. Indeed, Escherichia, a commensal gut microbiota, increased in the colon of CRC patients compared with healthy individuals, and some strains, like phylogroups B2 and D, are frequently linked to CRC [44]. The genotoxin colibactin, produced by the polyketide synthase genomic island, pks, presents in E. coli strains of the phylogenetic group B2 and can contribute to the development of CRC [45]. Other E. coli strains that are closely related to CRC can produce a cytotoxic necrotizing factor (CNF) or cytolethal distending toxin (CDT) [46]. CRC and Streptococcus bacteremia have also shown a close association since 1951, when a case of enterococcal endocarditis from S. bovis in association with CRC was reported [47]. Approximately 25%–80% of cases with S. bovis bacteremia progress to CRC, but the primary mechanisms are not identified [47,48]. However, S. bovis and its antigen can stimulate the production of IL-8 in the colon [49] that in turn might contribute to colon carcinogenesis by the induction of NO and ROS [47]. Also, S. gallolyticus subspecies gallolyticus, as biotype 1 S. bovis, has shown a strong association with CRC [50,51]. This organism has been detected in 20%–50% of CRC and colorectal adenoma (CRA) cases [52], the latter being known as a noncancerous colon tumor, which may progress into CRC. S. gallolyticus encodes a pilus with a collagen-binding domain that is more advantageous for CRC development [52,53], through inflammatory signals produced by its pilus [53,54].
In the genera of Bacteroides, B. fragilis strains comprise approximately 0.1% of healthy gut microbiota. The B. fragilis toxin (BFT) of enterotoxigenic B. fragilis (ETBF) has been linked to CRC [55,56], since it was found in 38% of isolates from CRC cases compared with 12% of healthy controls [57]. BFT induces the cleavage of E-cadherin and enhances CRC proliferation and expression of Myc as a proto-oncogene. In addition, BFT initiates NF-kB signaling and induces secretion of cytokines that finally lead to the contribution of mucosal inflammation [42,58]. Another suspected bacterial genus among CRC subjects is Enterococcus. Some E. faecalis strains can stimulate the production of ROS and superoxide anions and to induce genomic instability by DNA damaging [42]. E. faecalis can induce the production of diffusible clastogens, a chromosomal-breaking factor, that causes DNA damages [59]. Therefore, these strains have been proposed as motivators and boosters of CRC. Moreover, the genus Fusobacterium appears as a dominant phylotype influencing CRC. This conclusion is supported by the association between the abundance of Fusobacterium and NF-kB-driven inflammatory genes in human CRCs [42]. Specifically, the abundance of F. nucleatum in CRC is correlated with high production of pro-inflammatory cytokines, leading to upregulation of NF-kB [60]. The carcinogenic properties of F. nucleatum strains are mediated by the unique adhesin, FadA (FadAc) [61], through binding to E-cadherin, with consequent activation of cell growth-related signaling pathways [42]. Moreover, F. nucleatum can inhibit tumor cell lysis by an interaction between its Fap2 protein and NK cells receptors, thus inhibiting the cytotoxic potential of NK cells [62]. Furthermore, Salmonella might enhance CRC-risk through activation of signaling pathways by its pathogenic product, AvrA [63]. On the other hand, the role of Helicobacter pylori in CRC remains controversial, although some new research has introduced the role of H. pylori cytotoxin-associated gene A (CagA), as well as the production of ROS and NOS, in the induction of inflammatory pathways and CRC progression [64,65]. Some meta-analysis studies have also reported a high risk of CRC in H. pylori positive patients, especially in the early stage of CRC [66,67]. Finally, Clostridium septicum infection has been clinically linked to CRC [68], but the related underlying mechanism remains indefinite, and no direct association has been identified. It has been only suggested that C. septicum spores can easily germinate in the hypoxic and acidic tumor condition [69].
Although the above examples indicated the adverse effects of gut bacteria on CRC progression, some positive impacts on CRC prevention have been similarly detected. Frequently, the mechanisms of potentially probiotic gut bacteria are investigated in animal models [70,71]. Nevertheless, several human clinical trials have taken into consideration the protective effect of different probiotics in CRC patients [72,73]. The term “probiotic” refers to the prescription of some live bacteria which provide health benefits [74]. For instance, Bifidobacterium longum and Lactobacillus acidophilus have been introduced as inhibitors of CRC progression [75,76]. L. acidophilus seems to influence the 1,2-dimethylhydrazine-induced CRC, used as a carcinogen agent in the gut lumen, and to reduce the risk of CRC progression in rats [75]. B. adolescentis and B. infantis also suppress 3-methylcholanthrene-induced CRC in the mice model [77]. Also, the protective effect of L. acidophilus in CRC patients seems to derive from its binding to carcinogens in the human gut lumen, thus decreasing intestinal cell proliferation [78]. A clinical trial has indicated the effect of L. casei on the reduction of CRC recurrence [79], while other studies point to the protective effect of L. rhamnosus GG and B. lactis Bb12 in CRC patients [70,80]. Generally, the protective effects of the beneficial gut bacteria in CRC cases is mostly due to the reduction of DNA damage, intestinal cell proliferation, and secretion of interleukin-2, and to the enhancement of host immune responses, interferon-γ production, and modification of physicochemical conditions and metabolic activity of bacteria in the gut [70,80,81]. The increases and decreases of gut bacteria reported in the different studies analyzed are highlighted in Table 1. In addition, Table 1 depicts the use of different techniques of analysis as one of the most important reasons for the vast variations observed. Until the end of the last century, the association between gut bacteria and CRC were identified by culture methods [82,83]. Therefore, the vast majority of gut bacteria that have been recently associated with CRC remain uncharacterized due to their unfeasibility of culturing. The development of molecular techniques, mainly based on the analysis of hypervariable region of 16S ribosomal RNA (rRNA) gene, has provided a large amount of data and lead to better characterization of various bacterial communities [19,47]. Indeed, high throughput sequencing techniques have vastly expanded our knowledge of the significant role of gut bacteria in CRC development [84].
4. Microbial-derived Metabolites and CRC:
New aspects are quickly coming to the fore as possible players of gut bacteria in CRC progression. Different types of diet potentially control the production of microbial-derived metabolites, which have an essential influence on host metabolism and CRC development (Figure 1 and Figure 2) [21]. The data on significant microbial-derived metabolites in stool samples of CRC cases are presented in Table 2. Data are based on The Human Metabolome Database (http://www.hmdb.ca/). The status of all of the reported microbial-derived metabolites in stool samples of CRC cases was “detected but not quantified”. In general, consumption of dietary fiber, which is neither digested nor absorbed, is known as one of the effective strategies to modulate the gut bacteria composition, even for the ones introduced as being potentially prebiotic [144]. The term “prebiotic” refers to selectively food products that induce specific beneficial changes in the gut bacterial community of the host [145]. The association between fiber consumption and gut bacterial pattern is highly under the influence of type of consumed fiber. Different classifications, including origin, physicochemical characteristics, chemical composition, and other subclassifications based on carbohydrate chain length are introduced to describe dietary fibers, because of their heterogeneous nature. The Codex Alimentarius Commission is classified dietary fibers as edible naturally carbohydrates in consumed foods, edible manipulated carbohydrates by enzymatic, chemical or physical modifications in food row materials and edible synthetic carbohydrates [144]. All of them established beneficial physiological effects which approved by scientific evidence and can impact fermentation of different types of gut bacteria and therefore, therapeutic effects of consumers. With regard to physicochemical characteristics, dietary fibers can be separated based on solubility, fermentability, and viscosity. Solubility is indicated to highly impact on the fermentation caused by gut bacteria [144]. Soluble fiber, e.g., pectin and gums, easily digest in the proximal colon and mostly as part of the body metabolism caused by the reduction of carbohydrate absorption, blood pressure, insulin, and LDL level [146]. While insoluble fiber, e.g., cellulose and lignin, is partially fermented in the distal colon, the bacterial density is higher and is commonly involved in intestinal health. In general, fiber from vegetables and fruit is mainly soluble, and cereal fiber is mostly insoluble [147]. The gut bacteria begins fermentation of undigested dietary fibers in the large intestine and produces a huge variety of metabolites [148]. The most original products of gut bacteria in the colon during the fermentation process are short-chain fatty acids (SCFAs), like butyrate, acetate, and propionate, which are modulated by a fiber-rich diet [149]. Butyrate and propionate influence on the regulation of gut physiology and immune system, while acetate is a substrate in gluconeogenesis and lipogenesis process [145]. The members of the phylum Firmicutes frequently produce butyrate, which induces several controversial actions in the colon [150]. There are plenty of data describing the role of butyrate in cancer prevention, but its role in CRC remains inconclusive. Butyrate stimulates the natural proliferation of epithelial cells in the colon [151]. In addition, the phenolic compounds, by inhibiting several pro-inflammatory mediators, can lead to alterations of the gut bacterial community [152]. Nevertheless, its capability to interact dependently on the genetic backgrounds has increased concerns about its role in CRC development [21]. Consequently, considering the type of microbial-derived metabolites is essential, but their interaction with genetic and epigenetic backgrounds are challenging tasks that also need to be considered.
Table 2.
Evidence of the microbial-derived metabolites in CRC.
| Metabolite | Chemical Class | Bacterial Source | Bacterial Level in CRC a | Reference |
|---|---|---|---|---|
| Benzoic Acid | Benzenoid (Benzene) |
Serratia | + | [15,16,171] |
| Hippuric Acid(Benzamidoacetic Acid) | Benzenoid (Benzene) |
Clostridium
Eubacterium Ruminococcus Faecalibacterium |
± ± ± ± |
[171] |
| Hydroxybenzoic Acid | Benzenoid (Benzene) |
Arthrobacter
Bifidobacterium Microbulbifer Escherichia Eubacterium Corynebacterium Clostridium |
* ± * ± ± * ± |
[16,171] |
| Syringic Acid | Benzenoid (Benzene) |
Bifidobacterium | ± | [171] |
| 3-Hydroxyphenylacetic Acid | Benzenoid (Phenol) |
Klebsiella
Clostridium |
+ ± |
[15,171] |
| 4-Hydroxyphenylacetic Acid | Benzenoid (Phenol) |
Pseudomonas
Klebsiella Acinetobacter Clostridium |
± + - ± |
[15,16,171] |
| p-Cresol | Benzenoid (Phenol) |
Bacteriodes
Bifidobacterium Enterobacter Lactobacillus Clostridium |
± ± * ± ± |
[15] |
| Allantoin | Organoheterocyclic Compound (Azole) |
Bacillus
Streptomyces |
- * |
[171] |
| N-Acetylputrescine | Organic Acid (Organic Carboximidic Acid) |
Corynebacterium | * | [15,16,171] |
| 5-Aminopentanoic Acid | Organic Acid (Organic Carboximidic Acid) |
Corynebacterium | * | [15,16,171] |
| Acetic Acid | Organic Acid (Organic Carboximidic Acid) |
Acinetobacter
Bacteriodes Bifidobacterium Enterobacter Prevotella Ruminococcus Streptococcus Staphylococcus Pseudomonas Proteus Klebsiella Escherichia Enterococcus Citrobacter Akkermansia |
- ± ± * ± ± ± - ± * + ± ± + + |
[85,172,173,174,175,176] |
| Gamma-Aminobutyric Acid (GABA) | Organic Acid (Organic Carboximidic Acid) |
Bifidobacterium
Lactobacillus |
± ± |
[15,16,171] |
| Glutaric Acid | Organic Acid (Organic Carboximidic Acid) |
Escherichia | ± | [15,16,171] |
| Succinic Acid | Organic Acid (Organic Carboximidic Acid) |
Acinetobacter
Enterobacter Corynebacterium Basfia Pseudomonas Proteus Mannheimia Klebsiella Escherichia Enterococcus Citrobacter Anaerobiospirillum Actinobacillus |
- * * * ± * * + ± ± + * * |
[15,16,171,174] |
| 5-Keto-D-gluconate | Organic Acid (Organic Hydroxy Acid) |
Gluconobacter | * | [15,171] |
| Hydroxypropionic Acid | Organic Acid (Organic Hydroxy Acid) |
Escherichia
Klebsiella |
± + |
[15,16,171] |
| Lactic Acid | Organic Acid (Organic Hydroxy Acid) |
Acinetobacter
Enterobacter Corynebacterium Bacillus Streptococcus Staphylococcus Pseudomonas Proteus Klebsiella Escherichia Enterococcus Citrobacter |
- * * - ± - ± * + ± ± + |
[15,16,171,174,176] |
| Hydroxyacetic Acid(Glycolic Acid) | Organic Acid (Organic Hydroxy Acid) |
Alcaligenes
Acetobacter Rhodococcus Pseudomonas Leptospirillum Gluconobacter Escherichia Acidithiobacillus Corynebacterium |
* * * ± * * ± * * |
[15,16,171] |
| Pyruvic Acid | Organic Acid (Organic Keto Acid) |
Corynebacterium
Escherichia |
* ± |
[16,171] |
| Oxoglutaric Acid(Ketoglutaric Acid) | Organic Acid (Organic Keto Acid) |
Corynebacterium | * | [15] |
| p-Cresol sulfate | Organic Acid (Organic Sulfuric Acid) |
Clostridium
Lactobacillus Enterobacter Bifidobacterium |
± ± * ± |
[15,16,171] |
| Cadaverine | Organonitrogen Compound (Amine) |
Corynebacterium | * | [15,16,171] |
| Putrescine | Organonitrogen Compound (Amine) |
Enterobacter
Cronobacter Citrobacter Corynebacterium |
* * + * |
[15,16,171] |
| 2,3-Butanediol | Organooxygen Compound (Alcohol) |
Serratia
Klebsiella Bacillus Enterobacter |
+ + - * |
[15] |
| D-Arabinose | Organooxygen Compound (Carbohydrate) |
Streptococcus
Pediococcus Lactococcus Lactobacillus Geobacillus Escherichia Enterococcus Enterobacter Clostridium Alicyclobacillus Bifidobacterium |
± * + ± * ± ± * ± * ± |
[15] |
| Mannitol | Organooxygen Compound (Carbohydrate) |
Clostridium
Streptococcus Leuconostoc Zymomonas Torulaspora Rhodobacter Pseudomonas Lactococcus Gluconobacter Lactobacillus |
± ± * * * * ± + * ± |
[171] |
| Ribulose | Organooxygen Compound (Carbohydrate) |
Acetobacter
Gluconobacter |
* * |
[15] |
| Tartaric Acid | Organooxygen Compound (Carbohydrate) |
Agrobacterium
Nocardia Rhizobium |
* * + |
[171] |
| Indoleacetic Acid | Organoheterocyclic Compound (Indole) |
Bradyrhizobium
Rhizobium Pseudomonas Pantoea Enterobacter Clostridium Bacillus Agrobacterium Azospirillum |
* + ± + * ± - * * |
[15,16,171] |
| 5-Hydroxytryptamine(Serotonin) | Indole |
Enterococcus
Streptococcus Escherichia |
± ± ± |
[15] |
| Tryptamine | Indole |
Ruminococcus
Clostridium |
± ± |
[15,171] |
| Ferulic Acid | Phenylpropanoid Polyketide (Phenylpropanoic Acid) |
Pseudomonas | ± | [15,16,171] |
| Desaminotyrosine (4-Hydroxyphenylpropionic Acid) | Phenylpropanoid Polyketide (Phenylpropanoic Acid) |
Klebsiella
Staphylococcus Pseudomonas Lactobacillus Eubacterium Enterococcus Clostridium Bifidobacterium Acinetobacter Bacteriodes |
+ - ± ± ± ± ± ± - ± |
[15,16,171] |
| Hydrocinnamic Acid | Phenylpropanoid Polyketide (Phenylpropanoic Acid) |
Clostridium
Eubacterium |
± ± |
[15,16,171] |
| Hydroxyphenyllactic Acid | Phenylpropanoid Polyketide (Phenylpropanoic Acid) |
Clostridium
Bifidobacterium Staphylococcus Pseudomonas Lactobacillus Klebsiella Eubacterium Escherichia Enterococcus Acinetobacter Bacteriodes |
± ± - ± ± + ± ± ± - ± |
[15,171] |
| Phenyllactic Acid | Phenylpropanoid Polyketide (Phenylpropanoic Acid) |
Clostridium Klebsiella
Staphylococcus Pseudomonas Lactobacillus Eubacterium Escherichia Enterococcus Bifidobacterium Acinetobacter Bacteriodes |
± + - ± ± ± ± ± ± - ± |
[15] |
| 6-Hydroxynicotinic Acid | Organoheterocyclic Compound (Pyridine) |
Serratia
Achromobacter Pseudomonas |
+ * ± |
[15,16,171] |
| Butyric Acid | Lipid (Fatty Acyl) |
Anaerostipes
Eubacterium Roseburia Faecalibacterium Coprococcus |
- ± ± ± - |
[85,174,176] |
| Coprosterol | Steroid (Cholesterol) |
Lactobacillus | ± | [15] |
| Glycocholic Acid | Steroid (Bile Acid) |
Bacteriodes
Bifidobacterium Clostridium Lactobacillus |
± ± ± ± |
[15,16,171] |
a The bacterial relative abundance in CRC based on reported data in Table 1. Increase (+), Decrease (-), both increase and decrease (±), Not available (*).
Despite the beneficial SCFAs fermentation, amino acids can produce potentially harmful compounds during fermentation. Some of these, like ammonia, p-cresol, hydrogen sulfide, and some amines, may be important in CRC and in other gut disorders, which is controlled by a fiber-free diet [115,153]. These compounds may increase the risk of DNA damage, leaky gut, inflammation, and CRC development [153]. For instance, a secure connection has been established between gut bacteria and the metabolism of sulfate to produce cysteine, methionine, and hydrogen sulfide (H2S) that, in turn are toxic in high concentrations and contribute to the proliferation of colon cells and CRC progression [154]. Production of H2S in the gut is mostly done by members of Desulfovibrio spp., as specialist sulfate-reducing bacteria. They can utilize lactate to improve their growth, and sulfide formation [155] to stimulate CRC progression by the inhibition of butyrate oxidation and by inducing the breakdown of the gut barrier. The level of hydrogen sulfide is mainly influenced by bacterial activity, rather than by their abundance [156,157]. Butyrate-producing bacteria can also utilize lactate in competition with sulfate-reducing bacteria, especially Desulfovibrio spp. Lactate is one of the beneficial products of colonized lactic acid gut bacteria, including Lactobacillus, Streptococcus, Bifidobacterium, Enterococcus, and Eubacterium, which usually utilized by other gut bacterial genera in a cross-feeding interaction [158]. An evaluation is compared produced butyrate of Eubacterium hallii and Anaerostipes caccae, as two main butyrate-producing bacteria, from lactate in coculture with Desulfovibrio piger [155]. The results confirmed the high reduction of produced butyrate from lactate in this condition. In addition, the results of the triculture experiment involving Bifidobacterium adolescentis, as a lactic acid gut bacteria, have been strongly established inhibition of butyrate formation and induction of sulfide formation in the presence of Eubacterium hallii, Anaerostipes caccae and Desulfovibrio piger. Similarly, a high level of amines, especially polyamines, are toxic and are associated with CRC [157]. Several gut bacteria like Salmonella enterica subsp. enterica serovar Typhimurium, S. flexneri, H. pylori, and S. pneumonia, increase their virulence by abuse of polyamines [159]. Phytochemicals are also crucial because of their antioxidant effects and their potency in the regulation of detoxification, cell proliferation, apoptosis, and inflammation [160]. The reactive oxygen species (ROS), can damage DNA and increase the risk of CRC through neutralizing the antioxidants [157]. The nitrogen metabolites, like N‑nitroso compounds (NOCs), potentially promote CRC by the induction of DNA damage [157].
It has been postulated that an imbalance in the gut bacterial community can enhance the proliferation of damaging bacteria and their carcinogenic products [161]. However, additional investigations are required to establish this hypothesis. Bile acids can induce cytotoxic effects and increase the proliferation of malignant cells [162]. Overall, bile acids, like deoxycholic acid and lithocholic acid, have been potentially introduced as carcinogenic agents having a negative correlation with the level of anti-carcinogenic products in the colon [163]. Uracil, another microbial-derived metabolite, is also associated with ROS production in the intestine [164]. Gut bacteria metabolism can also induce trimethylamine N-oxide (TMAO), which is intensely associated with CRC [165]. Furthermore, many gut bacteria, via ethanol induction, produce highly carcinogenic acetaldehyde [166]. Generally, the fermentation is not the only metabolism process of gut bacteria; indeed they can also induce anaerobic metabolism. For example, sulfate, nitrate, and different organic compounds can function as electron receptors in the respiratory process [167]. Also, oxygen may count as an electron receptor of the facultative anaerobes Bacteroides spp. and Faecalibacterium prausnitzii [168,169].
In addition to the direct effect of gut bacteria and their metabolites on the development of homeostasis and tumorigenesis, they can be indirectly involved. For instance, bacteria commonly exchange primary metabolites with other organisms, known as cross-feeding interaction [170]. Dietary fiber extensively increases metabolic interaction in the gut bacterial community [144]. Competition of sulfate-reducing bacteria and butyrate-producing bacteria on exchanging of produced lactate by lactic acid bacteria in order to produce H2S or butyrate in different conditions is one of the most identified cross-feeding examples [155]. In addition, some gut bacteria utilize hydrogen and formate, and they mainly participate in anaerobic metabolism through a cross-feeding interaction [156]. These interactions play a vital role in the formation of gut microbial communities [170]. In brief, it can be concluded which a complex bidirectional network involved in the regulation of gut bacterial community by metabolites and metabolites by the gut bacterial community.
5. The Role of Bacterial Metabolites in Epigenetic Modifications of CRC
It is well known that epigenetic modifications influence many cellular processes by regulating gene expression, notably without direct modification of DNA sequence in the genome. Several types of epigenetic modifications, including histone modifications, DNA methylation, chromatin remodeling, and RNA-based regulation, are identified [172]. However, the value of epigenetic modifications in the development of different disorders in comparison with genetic mutations had been mostly ignored. With the increasing knowledge of the potential association between epigenetics and gene expression, evaluation of epigenetic modifications in different disorders has become a popular area of research [7]. Bacteria and their metabolites have a profound effect on the transcriptional profile of the host cells by the induction of epigenetic modifications [177]. These metabolites are crucial messengers in the crosstalk between microbiota and host cells, and microbiota can cooperate in the development of several major disorders by induction of epigenetic modifications [7]. A growing area of interest is the association between different epigenetic modifications in CRC progression and gut bacteria. Epigenetic regulation of many common genes (like GATA4, MLH1, p16INK4a, LKB1, and APC) and genetic pathways in CRC are well documented [178]. As mentioned, SCFAs are known as the major products of gut bacteria, which induce histone modification [179]. Butyrate and acetate act as histone deacetylase inhibitors, which affect the epigenetic modifications governing CRC development [180]. Propionate is known as a less effective histone deacetylase inhibitor, with respect to butyrate, because of its higher bioavailability and lower accumulation in colonocytes [178]. In particular, Faecalibacterium, Eubacterium, and Roseburia were identified as the most important butyrate producer in the gut microbiota. However, other butyrate-producers also have been found, such as Fusobacterium, Peptoniphilus, Coprococcus, Porphyromonas, Clostridium, Megasphaera, and others [181]. Evidence indicates that Fusobacterium increases methylation of the hMLH1 gene and microsatellite instability [182]. The loss of histone H4 lysine monoacetylation and H4K16 and H4K20 trimethylation has been identified as a hallmark in CRC [183]. A detailed evaluation indicated acetylation of H3K27 along with methylation of H3K4 as the possible cause of activation of variant enhancer loci in tissue samples of CRC cases [184]. Besides, trimethylation of H3K4, H3K9, and H4K20 has been also evaluated in CRC [185]. Also, gut bacteria produce methionine during the metabolism of sulfate. Methionine modulates bacterial metabolism to increase S-adenosyl methionine (SAM) synthesis, which is a methyl donor for DNA methyltransferase [186]. F. nucleatum was also found concerning DNA methylation by targeting innate immune signaling [187]. Several investigations of CRC epigenome have introduced numerous aberrant methylated genes in CRC cases, such as RAAS F2A, WIF1, ALX4, MGM2, APC, RUNX3, p14, p16, SOX2, and NDRG4 [188,189,190]. It is noteworthy that aberrant methylation of cMyc gene, encoding the c-myc oncoprotein, has been detected in CRC cases [191]. Moreover, H. pylori induces methylation of some genes related to cell growth, cell adherence, and DNA repair [192]. Besides, trimethylamine, mainly produced by Escherichia coli, induces DNA methylation [179]. Also, dysregulation of miRNAs, potential cancer biomarkers, is frequently reported in many studies [193,194]. For instance, overexpression of miR-21 and miR-106 has been detected in stool samples of CRC cases [195] and F. nucleatum has been shown to decrease the miR-18a level and to modulate some innate immune signaling in CRC [196]. In addition, an array of candidate miRNAs, which are involved in different process like signaling, proliferation, apoptosis, differentiation, migration, and invasion (i.e., let-7 family, miR-17–92, miR-34a, miR-34b/c, miR-92a, miR-135a/b, miR-139, miR-145, miR-126, miR-133b, miR-141, miR-143, miR-144, miR-192, miR-195, miR-200c, miR-215, and miR-675) have been suggested in association with CRC [195,196,197,198]. Overall, various links have been found between different miRNAs and gut bacteria to impact on CRC developments [199]. In summary, several studies have explained in more detail the crosstalk between the microbiota and epigenetic modifications in CRC [7,178,198]. It is suggested that the prescription of L. acidophilus, L. casei, and B. breve in CRC cases can enhance expression of some tumor suppressor genes, which were typically suppressed by methylation process [180]. To date, the existing data about the epigenome strongly validate the fact that epigenetic factors rather than genetics could account as more precise disease pathogenetic biomarkers. In this context, further studies are required to deeply explore the correlation between epigenetic modifications and microbiota in CRC subjects.
6. Conclusions
Emerging scientific advances of the role of gut bacteria community in the pathogenesis of CRC continue to be elucidated and refined. Given existing evidence of dysbiosis in CRC, the link between gut bacteria and CRC development has become an urgent topic for future biomedical research. We tried to review the effect of the gut bacteria community and their metabolites in CRC cases and the salient epigenetic mechanisms. Ultimately, the combined use of epigenetic, microbiota, and metabolites analyses can be very significant for reaching a targeted therapeutics and innovative precision strategy for CRC. Therefore, introducing a personalized modulation of the pattern of gut bacteria and their metabolites activity or epigenetic modifications may be a new and useful approach to reduce the risk of CRC progression.
Acknowledgments
The authors are grateful to the personnel of the Gastrointestinal Research Institute of Shahid Beheshti University of Medical Sciences, and Mycobacteriology and Pulmonary Research Department, Pasteur Institute of Iran, for their assistance in Project entitled “Microbiota and Colorectal Cancer”. We tender our apologies to those authors whose deserving research was not cited in this manuscript.
Author Contributions
Investigation and evaluation of published studies, S.T., S.D.S., S.A.B., and A.M.; Contributing in writing the manuscript, R.B. and M.Z.; Major contributors in writing and editing, S.T., S.D.S., A.M and M.P.; Designing of figures, S.T.; Supervision of the study, M.P. and A.M.
Conflicts of Interest
The authors declare that they have no competing interests.
References
- 1.Azadeh S., Moghimi-Dehkordi B., Fatem S.R., Pourhoseingholi M.A., Ghiasi S., Zali M.R. Colorectal cancer in Iran: An epidemiological study. Asian Pac. J. Cancer Prev. 2008;9:123–126. [PubMed] [Google Scholar]
- 2.Stewart B., Wild C.P. World Cancer Report 2014. International Agency for Research on Cancer, WHO; Geneva, Switzerland: 2014. World Cancer Report Publisher. [Google Scholar]
- 3.Van T.R., Allen-Vercoe E. Microbial Interactions and Interventions in Colorectal Cancer. Microbiology. 2017;47:777–780. doi: 10.1128/microbiolspec.bad-0004-2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Pourhoseingholi M.A., Zali M.R. Colorectal cancer screening: Time for action in Iran. World J. Gastrointest. Oncol. 2012;4:82–83. doi: 10.4251/wjgo.v4.i4.82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.McQuade J.L., Daniel C.R., Helmink B.A., Wargo J.A. Modulating the microbiome to improve therapeutic response in cancer. Lancet Oncol. 2019;20:e77–e91. doi: 10.1016/S1470-2045(18)30952-5. [DOI] [PubMed] [Google Scholar]
- 6.Jobin C.J.S. Precision medicine using microbiota. Science. 2018;359:32–34. doi: 10.1126/science.aar2946. [DOI] [PubMed] [Google Scholar]
- 7.Yang T., Owen J.L., Lightfoot Y.L., Kladde M.P., Mohamadzadeh M., Lightfooot Y.L. Microbiota impact on the epigenetic regulation of colorectal cancer. Trends Mol. Med. 2013;19:714–725. doi: 10.1016/j.molmed.2013.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Moghimi-Dehkordi B., Safaee A., Zali M.R. Prognostic factors in 1,138 Iranian colorectal cancer patients. Int. J. Color. Dis. 2008;23:683–688. doi: 10.1007/s00384-008-0463-7. [DOI] [PubMed] [Google Scholar]
- 9.Petersen C., Round J.L. Defining dysbiosis and its influence on host immunity and disease. Cell. Microbiol. 2014;16:1024–1033. doi: 10.1111/cmi.12308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tarashi S., Badi S.A., Moshiri A., Nasehi M., Fateh A., Vaziri F., Siadat S.D. The human microbiota in pulmonary tuberculosis: Not so innocent bystanders. Tuberculosis. 2018;113:215–221. doi: 10.1016/j.tube.2018.10.010. [DOI] [PubMed] [Google Scholar]
- 11.Candela M., Guidotti M., Fabbri A., Brigidi P., Franceschi C., Fiorentini C. Human intestinal microbiota: Cross-talk with the host and its potential role in colorectal cancer. Crit. Rev. Microbiol. 2011;37:1–14. doi: 10.3109/1040841X.2010.501760. [DOI] [PubMed] [Google Scholar]
- 12.Vétizou M., Pitt J.M., Daillère R., Lepage P., Waldschmitt N., Flament C., Rusakiewicz S., Routy B., Roberti M.P., Duong C.P.M., et al. Anticancer immunotherapy by CTLA-4 blockade relies on the gut microbiota. Science. 2015;350:1079–1084. doi: 10.1126/science.aad1329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Adachi K., Tamada K. Microbial biomarkers for immune checkpoint blockade therapy against cancer. J. Gastroenterol. 2018;53:999–1005. doi: 10.1007/s00535-018-1492-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Garrett W.S. The gut microbiota and colon cancer. Science. 2019;364:1133–1135. doi: 10.1126/science.aaw2367. [DOI] [PubMed] [Google Scholar]
- 15.Brown D.G., Rao S., Weir T.L., O’Malia J., Bazan M., Brown R.J., Ryan E.P. Metabolomics and metabolic pathway networks from human colorectal cancers, adjacent mucosa, and stool. Cancer Metab. 2016;4:11. doi: 10.1186/s40170-016-0151-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sinha R., Ahn J., Sampson J.N., Shi J., Yu G., Xiong X., Hayes R.B., Goedert J.J. Fecal Microbiota, Fecal Metabolome, and Colorectal Cancer Interrelations. PLoS ONE. 2016;11:e0152126. doi: 10.1371/journal.pone.0152126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Papastergiou V., Karatapanis S., Georgopoulos S.D. Helicobacter pylori and colorectal neoplasia: Is there a causal link? World J. Gastroenterol. 2016;22:649–658. doi: 10.3748/wjg.v22.i2.649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tilg H., Adolph T.E., Gerner R.R., Moschen A.R. The Intestinal Microbiota in Colorectal Cancer. Cancer Cell. 2018;33:954–964. doi: 10.1016/j.ccell.2018.03.004. [DOI] [PubMed] [Google Scholar]
- 19.Walsh C.J., Guinane C.M., O’Toole P.W., Cotter P.D., O’Toole P.W. Beneficial modulation of the gut microbiota. FEBS Lett. 2014;588:4120–4130. doi: 10.1016/j.febslet.2014.03.035. [DOI] [PubMed] [Google Scholar]
- 20.Qin J., Li R., Raes J., Arumugam M., Burgdorf K.S., Manichanh C., Nielsen T., Pons N., Levenez F., Yamada T. A human gut microbial gene catalogue established by metagenomic sequencing. Nature. 2010;464:59. doi: 10.1038/nature08821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Oke S., Martin A. Insights into the role of the intestinal microbiota in colon cancer. Ther. Adv. Gastroenterol. 2017;10:417–428. doi: 10.1177/1756283X17694832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Reddy B.S., Narisawa T., Wright P., Vukusich D., Weisburger J.H., Wynder E.L. Colon carcinogenesis with azoxymethane and dimethylhydrazine in germ-free rats. Cancer Res. 1975;35:287–290. [PubMed] [Google Scholar]
- 23.Tjalsma H., Boleij A., Marchesi J.R., Dutilh B.E. A bacterial driver–passenger model for colorectal cancer: Beyond the usual suspects. Nat. Rev. Genet. 2012;10:575–582. doi: 10.1038/nrmicro2819. [DOI] [PubMed] [Google Scholar]
- 24.Warren R.L., Freeman D.J., Pleasance S., Watson P., Moore R.A., Cochrane K., Allen-Vercoe E., Holt R.A. Co-occurrence of anaerobic bacteria in colorectal carcinomas. Microbiome. 2013;1:16. doi: 10.1186/2049-2618-1-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lazarovitch T., Shango M., Levine M., Brusovansky R., Akins R., Hayakawa K., Lephart P., Sobel J., Kaye K., Marchaim D. The relationship between the new taxonomy of Streptococcus bovis and its clonality to colon cancer, endocarditis, and biliary disease. Infection. 2013;41:329–337. doi: 10.1007/s15010-012-0314-x. [DOI] [PubMed] [Google Scholar]
- 26.Marchesi J.R., Dutilh B.E., Hall N., Peters W.H.M., Roelofs R., Boleij A., Tjalsma H. Towards the Human Colorectal Cancer Microbiome. PLoS ONE. 2011;6:e20447. doi: 10.1371/journal.pone.0020447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Nakatsu G., Li X., Zhou H., Sheng J., Wong S.H., Wu W.K.K., Ng S.C., Tsoi H., Dong Y., Zhang N., et al. Gut mucosal microbiome across stages of colorectal carcinogenesis. Nat. Commun. 2015;6:8727. doi: 10.1038/ncomms9727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zeller G., Tap J., Voigt A.Y., Sunagawa S., Kultima J.R., Costea P.I., Amiot A., Böhm J., Brunetti F., Habermann N., et al. Potential of fecal microbiota for early-stage detection of colorectal cancer. Mol. Syst. Biol. 2014;10:766. doi: 10.15252/msb.20145645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Li Y.Y., Ge Q.X., Cao J., Zhou Y.J., Du Y.L., Shen B., Wan Y.J.Y., Nie Y.Q. Association of Fusobacterium nucleatum infection with colorectal cancer in Chinese patients. World J. Gastroenterol. 2016;22:3227–3233. doi: 10.3748/wjg.v22.i11.3227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mangifesta M., Mancabelli L., Milani C., Gaiani F., De’Angelis N., De’Angelis G.L., Van Sinderen D., Ventura M., Turroni F. Mucosal microbiota of intestinal polyps reveals putative biomarkers of colorectal cancer. Sci. Rep. 2018;8:13974. doi: 10.1038/s41598-018-32413-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Geng J., Fan H., Tang X., Zhai H., Zhang Z. Diversified pattern of the human colorectal cancer microbiome. Gut Pathog. 2013;5:2. doi: 10.1186/1757-4749-5-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zhou Y., He H., Xu H., Li Y., Li Z., Du Y., He J., Zhou Y., Wang H., Nie Y. Association of oncogenic bacteria with colorectal cancer in South China. Oncotarget. 2016;7:80794–80802. doi: 10.18632/oncotarget.13094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Gao R., Kong C., Huang L., Li H., Qu X., Liu Z., Lan P., Wang J., Qin H. Mucosa-associated microbiota signature in colorectal cancer. Eur. J. Clin. Microbiol. Infect. Dis. 2017;36:2073–2083. doi: 10.1007/s10096-017-3026-4. [DOI] [PubMed] [Google Scholar]
- 34.Burns M.B., Lynch J., Starr T.K., Knights D., Blekhman R. Virulence genes are a signature of the microbiome in the colorectal tumor microenvironment. Genome Med. 2015;7:55. doi: 10.1186/s13073-015-0177-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wei Z., Cao S., Liu S., Yao Z., Sun T., Li Y., Li J., Zhang D., Zhou Y. Could gut microbiota serve as prognostic biomarker associated with colorectal cancer patients’ survival? A pilot study on relevant mechanism. Oncotarget. 2016;7:46158–46172. doi: 10.18632/oncotarget.10064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Allali I., Boukhatem N., Bouguenouch L., Hardi H., Boudouaya H.A., Cadenas M.B., Ouldim K., Amzazi S., Azcarate-Peril M.A., Ghazal H. Gut microbiome of Moroccan colorectal cancer patients. Med. Microbiol. Immunol. 2018;207:211–225. doi: 10.1007/s00430-018-0542-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Drewes J.L., White J.R., Dejea C.M., Fathi P., Iyadorai T., Vadivelu J., Roslani A.C., Wick E.C., Mongodin E.F., Loke M.F. High-resolution bacterial 16S rRNA gene profile meta-analysis and biofilm status reveal common colorectal cancer consortia. NPJ Biofilms Microbiomes. 2017;3:34. doi: 10.1038/s41522-017-0040-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Yusuf F., Ilyas S., Damanik H.A., Fatchiyah F. Microbiota Composition, HSP70 and Caspase-3 Expression as Marker for Colorectal Cancer Patients in Aceh, Indonesia. Acta Med. Indones. 2016;48:289–299. [PubMed] [Google Scholar]
- 39.Balamurugan R., Rajendiran E., George S., Samuel G.V., Ramakrishna B.S. Real-time polymerase chain reaction quantification of specific butyrate-producing bacteria, Desulfovibrio and Enterococcus faecalis in the feces of patients with colorectal cancer. J. Gastroenterol. Hepatol. 2008;23:1298–1303. doi: 10.1111/j.1440-1746.2008.05490.x. [DOI] [PubMed] [Google Scholar]
- 40.O’keefe S.J. Diet, microorganisms and their metabolites, and colon cancer. Gastroenterol. Hepatol. 2016;13:691. doi: 10.1038/nrgastro.2016.165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Giovannucci E., Rimm E.B., Stampfer M.J., Colditz G.A., Ascherio A., Willett W.C. Intake of fat, meat, and fiber in relation to risk of colon cancer in men. Cancer Res. 1994;54:2390–2397. [PubMed] [Google Scholar]
- 42.Sears C.L., Garrett W.S. Microbes, Microbiota, and Colon Cancer. Cell Host Microbe. 2014;15:317–328. doi: 10.1016/j.chom.2014.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Mármol I., Sánchez-De-Diego C., Dieste A.P., Cerrada E., Yoldi M.J.R. Colorectal Carcinoma: A General Overview and Future Perspectives in Colorectal Cancer. Int. J. Mol. Sci. 2017;18:197. doi: 10.3390/ijms18010197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kohoutova D., Smajs D., Moravkova P., Cyrany J., Moravkova M., Forstlova M., Cihak M., Rejchrt S., Bures J. Escherichia coli strains of phylogenetic group B2 and D and bacteriocin production are associated with advanced colorectal neoplasia. BMC Infect. Dis. 2014;14:733. doi: 10.1186/s12879-014-0733-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Cuevas-Ramos G., Petit C.R., Marcq I., Boury M., Oswald E., Nougayrède J.P. Escherichia coli induces DNA damage in vivo and triggers genomic instability in mammalian cells. Proc. Natl. Acad. Sci. USA. 2010;107:11537–11542. doi: 10.1073/pnas.1001261107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Buc E., Dubois D., Sauvanet P., Raisch J., Delmas J., Darfeuille-Michaud A., Pezet D., Bonnet R. High Prevalence of Mucosa-Associated E. coli Producing Cyclomodulin and Genotoxin in Colon Cancer. PLoS ONE. 2013;8:e56964. doi: 10.1371/journal.pone.0056964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Compare D., Nardone G. The bacteria-hypothesis of colorectal cancer: Pathogenetic and therapeutic implications. Transl. Gastrointest. Cancer. 2013;3:44–53. [Google Scholar]
- 48.Tsai C.E., Chiu C.T., Rayner C.K., Wu K.L., Chiu Y.C., Hu M.L., Chuah S.K., Tai W.C., Liang C.M., Wang H.M. Associated factors in Streptococcus bovis bacteremia and colorectal cancer. Kaohsiung J. Med. Sci. 2016;32:196–200. doi: 10.1016/j.kjms.2016.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Ellmerich S., Duranton B., Gosse F., Galluser M., Klein J.P., Raul F., Scholler M. Promotion of intestinal carcinogenesis by Streptococcus bovis. Carcinogenesis. 2000;21:753–756. doi: 10.1093/carcin/21.4.753. [DOI] [PubMed] [Google Scholar]
- 50.Boleij A., Tjalsma H. The itinerary of Streptococcus gallolyticus infection in patients with colonic malignant disease. Lancet Infect. Dis. 2013;13:719–724. doi: 10.1016/S1473-3099(13)70107-5. [DOI] [PubMed] [Google Scholar]
- 51.Boleij A., Van Gelder M.M.H.J., Swinkels D.W., Tjalsma H. Clinical Importance of Streptococcus gallolyticus Infection Among Colorectal Cancer Patients: Systematic Review and Meta-analysis. Clin. Infect. Dis. 2011;53:870–878. doi: 10.1093/cid/cir609. [DOI] [PubMed] [Google Scholar]
- 52.Abdulamir A.S., Hafidh R.R., Abu Bakar F. Molecular detection, quantification, and isolation of Streptococcus gallolyticus bacteria colonizing colorectal tumors: Inflammation-driven potential of carcinogenesis via IL-1, COX-2, and IL-8. Mol. Cancer. 2010;9:249. doi: 10.1186/1476-4598-9-249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Boleij A., Dutilh B.E., Kortman G.A.M., Roelofs R., Laarakkers C.M., Engelke U.F., Tjalsma H. Bacterial Responses to a Simulated Colon Tumor Microenvironment. Mol. Cell. Proteom. 2012;11:851–862. doi: 10.1074/mcp.M112.019315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Boleij A., Muytjens C.M.J., Bukhari S.I., Cayet N., Glaser P., Hermans P.W.M., Swinkels D.W., Bolhuis A., Tjalsma H. Novel Clues on the Specific Association of Streptococcus gallolyticus subsp gallolyticus With Colorectal Cancer. J. Infect. Dis. 2011;203:1101–1109. doi: 10.1093/infdis/jiq169. [DOI] [PubMed] [Google Scholar]
- 55.Sears C.L., Geis A.L., Housseau F. Bacteroides fragilis subverts mucosal biology: From symbiont to colon carcinogenesis. J. Clin. Investig. 2014;124:4166–4172. doi: 10.1172/JCI72334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Zamani S., Shariati S.H., Zali M.R., Aghdaei H.A., Asiabar A.S., Bokaie S., Nomanpour B., Sechi L.A., Feizabadi M.M. Detection of enterotoxigenic Bacteroides fragilis in patients with ulcerative colitis. Gut Pathog. 2017;9:53. doi: 10.1186/s13099-017-0202-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Toprak N.U., Yagci A., Güllüoglu B.M., Akin M., Demirkalem P., Celenk T., Soyletir G. A possible role of Bacteroides fragilis enterotoxin in the aetiology of colorectal cancer. Clin. Microbiol. Infect. 2006;12:782–786. doi: 10.1111/j.1469-0691.2006.01494.x. [DOI] [PubMed] [Google Scholar]
- 58.Soler A.P., Miller R., Laughlin K.V., Carp N.Z., Klurfeld D.M., Mullin J.M. Increased tight junctional permeability is associated with the development of colon cancer. Carcinogenesis. 1999;20:1425–1432. doi: 10.1093/carcin/20.8.1425. [DOI] [PubMed] [Google Scholar]
- 59.Yang Y., Wang X., Huycke T., Moore D.R., Lightfoot S.A., Huycke M.M. Colon Macrophages Polarized by Commensal Bacteria Cause Colitis and Cancer through the Bystander Effect. Transl. Oncol. 2013;6:596–606. doi: 10.1593/tlo.13412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Kostic A.D., Chun E., Robertson L., Glickman J.N., Gallini C.A., Michaud M., Clancy T.E., Chung D.C., Lochhead P., Hold G.L., et al. Fusobacterium nucleatum potentiates intestinal tumorigenesis and modulates the tumor immune microenvironment. Cell Host Microbe. 2013;14:207–215. doi: 10.1016/j.chom.2013.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Rubinstein M.R., Wang X., Liu W., Hao Y., Cai G., Han Y.W. Fusobacterium nucleatum promotes colorectal carcinogenesis by modulating E-cadherin/β-catenin signaling via its FadA adhesin. Cell Host Microbe. 2013;14:195–206. doi: 10.1016/j.chom.2013.07.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Bashir A., Miskeen A.Y., Hazari Y.M., Asrafuzzaman S., Fazili K.M. Fusobacterium nucleatum, inflammation, and immunity: The fire within human gut. Tumor Biol. 2016;37:2805–2810. doi: 10.1007/s13277-015-4724-0. [DOI] [PubMed] [Google Scholar]
- 63.Lu R., Wu S., Zhang Y.G., Xia Y., Liu X., Zheng Y., Chen H., Schaefer K.L., Zhou Z., Bissonnette M., et al. Enteric bacterial protein AvrA promotes colonic tumorigenesis and activates colonic beta-catenin signaling pathway. Oncogenesis. 2014;3:e105. doi: 10.1038/oncsis.2014.20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Shmuely H., Passaro D., Figer A., Niv Y., Pitlik S., Samra Z., Koren R., Yahav J. Relationship between Helicobacter pylori CagA status and colorectal cancer. Am. J. Gastroenterol. 2001;96:3406–3410. doi: 10.1111/j.1572-0241.2001.05342.x. [DOI] [PubMed] [Google Scholar]
- 65.Handa O., Naito Y., Yoshikawa T. Helicobacter pylori: A ROS-inducing bacterial species in the stomach. Inflamm. Res. 2010;59:997–1003. doi: 10.1007/s00011-010-0245-x. [DOI] [PubMed] [Google Scholar]
- 66.Zumkeller N., Brenner H., Zwahlen M., Rothenbacher D. Helicobacter pylori Infection and Colorectal Cancer Risk: A Meta-Analysis. Helicobacter. 2006;11:75–80. doi: 10.1111/j.1523-5378.2006.00381.x. [DOI] [PubMed] [Google Scholar]
- 67.Guo Y., Li H.Y. Association between Helicobacter pylori infection and colorectal neoplasm risk: A meta-analysis Based on East Asian population. J. Cancer Res. Ther. 2014;10:263. doi: 10.4103/0973-1482.151482. [DOI] [PubMed] [Google Scholar]
- 68.Mirza N.N., McCloud J.M., Cheetham M.J. Clostridium septicum sepsis and colorectal cancer—A reminder. World J. Surg. Oncol. 2009;7:73. doi: 10.1186/1477-7819-7-73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Dylewski J., Luterman L. Septic arthritis and Clostridium septicum: A clue to colon cancer. Can. Med. Assoc. J. 2010;182:1446–1447. doi: 10.1503/cmaj.091946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Davis C.D., Milner J.A. Gastrointestinal microflora, food components and colon cancer prevention. J. Nutr. Biochem. 2009;20:743–752. doi: 10.1016/j.jnutbio.2009.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Mendes M.C.S., Paulino D.S., Brambilla S.R., Camargo J.A., Persinoti G.F., Carvalheira J.B.C. Microbiota modification by probiotic supplementation reduces colitis associated colon cancer in mice. World J. Gastroenterol. 2018;24:1995–2008. doi: 10.3748/wjg.v24.i18.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Ali R.A.R., Zaharuddin L., Chan S.-N., Wong Z., Ngiu C.S., Mokhtar N.M. Sa1838—The Clinical and Circulating Inflammatory Cytokines Effects of Probiotic Containing Lactobacillus and Bifidobacterium Strains in Patients with Colorectal Cancer: A Randomized Double Blind Controlled Trial. Gastroenterology. 2018;154:414. doi: 10.1016/S0016-5085(18)31661-5. [DOI] [Google Scholar]
- 73.Drago L.J.M. Probiotics and Colon Cancer. Microorganisms. 2019;7:66. doi: 10.3390/microorganisms7030066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Rafter J. Probiotics and colon cancer. Best Pract. Res. Clin. Gastroenterol. 2003;17:849–859. doi: 10.1016/S1521-6918(03)00056-8. [DOI] [PubMed] [Google Scholar]
- 75.McIntosh G.H., Royle P.J., Playne M.J. A Probiotic Strain of L. Acidophilus Reduces DMH-Induced Large Intestinal Tumors in Male Sprague-Dawley Rats. Nutr. Cancer. 1999;35:153–159. doi: 10.1207/S15327914NC352_9. [DOI] [PubMed] [Google Scholar]
- 76.Rowland I.R., Bearne C.A., Fischer R., Pool-Zobel B.L. The effect of lactulose on DNA damage induced by DMH in the colon of human flora-associated rats. Nutr. Cancer. 1996;26:37–47. doi: 10.1080/01635589609514461. [DOI] [PubMed] [Google Scholar]
- 77.Kohwi Y., Imai K., Tamura Z., Hashimoto Y. Antitumor effect of Bifidobacterium infantis in mice. Gan. 1978;69:613–618. [PubMed] [Google Scholar]
- 78.Lidbeck A., Övervik E., Rafter J., Nord C.E., Gustafsson J.Å. Effect of Lactobacillus acidophilus Supplements on Mutagen Excretion in Faeces and Urine in Humans. Microb. Ecol. Health Dis. 1992;5:59–67. doi: 10.3109/08910609209141305. [DOI] [Google Scholar]
- 79.Ishikawa H., Akedo I., Otani T., Suzuki T., Nakamura T., Takeyama I., Ishiguro S., Miyaoka E., Sobue T., Kakizoe T. Randomized trial of dietary fiber andLactobacillus casei administration for prevention of colorectal tumors. Int. J. Cancer. 2005;116:762–767. doi: 10.1002/ijc.21115. [DOI] [PubMed] [Google Scholar]
- 80.Rafter J., Bennett M., Caderni G., Clune Y., Hughes R., Karlsson P.C., Klinder A., O’Riordan M., O’Sullivan G.C., Pool-Zobel B., et al. Dietary synbiotics reduce cancer risk factors in polypectomized and colon cancer patients. Am. J. Clin. Nutr. 2007;85:488–496. doi: 10.1093/ajcn/85.2.488. [DOI] [PubMed] [Google Scholar]
- 81.Hirayama K., Rafter J. The role of probiotic bacteria in cancer prevention. Microbes Infect. 2000;2:681–686. doi: 10.1016/S1286-4579(00)00357-9. [DOI] [PubMed] [Google Scholar]
- 82.Klein R.S., Recco R.A., Catalano M.T., Edberg S.C., Casey J.I., Steigbigel N.H. Association ofStreptococcus boviswith Carcinoma of the Colon. N. Engl. J. Med. 1977;297:800–802. doi: 10.1056/NEJM197710132971503. [DOI] [PubMed] [Google Scholar]
- 83.Vargo D., Moskovitz M., Floch M.H. Faecal bacterial flora in cancer of the colon. Gut. 1980;21:701–705. doi: 10.1136/gut.21.8.701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Del Vecchio F., Mastroiaco V., Di Marco A., Compagnoni C., Capece D., Zazzeroni F., Capalbo C., Alesse E., Tessitore A. Next-generation sequencing: Recent applications to the analysis of colorectal cancer. J. Transl. Med. 2017;15:246. doi: 10.1186/s12967-017-1353-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Weir T.L., Manter D.K., Sheflin A.M., Barnett B.A., Heuberger A.L., Ryan E.P. Stool Microbiome and Metabolome Differences between Colorectal Cancer Patients and Healthy Adults. PLoS ONE. 2013;8:e70803. doi: 10.1371/journal.pone.0070803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Sanapareddy N., Legge R.M., Jovov B., McCoy A., Burcal L., Araujo-Perez F., Randall T.A., Galanko J., Benson A., Sandler R.S., et al. Increased rectal microbial richness is associated with the presence of colorectal adenomas in humans. ISME J. 2012;6:1858–1868. doi: 10.1038/ismej.2012.43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Peters B.A., Dominianni C., Shapiro J.A., Church T.R., Wu J., Miller G., Yuen E., Freiman H., Lustbader I., Salik J., et al. The gut microbiota in conventional and serrated precursors of colorectal cancer. Microbiome. 2016;4:69. doi: 10.1186/s40168-016-0218-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Kasai C., Sugimoto K., Moritani I., Tanaka J., Oya Y., Inoue H., Tameda M., Shiraki K., Ito M., Takei Y., et al. Comparison of human gut microbiota in control subjects and patients with colorectal carcinoma in adenoma: Terminal restriction fragment length polymorphism and next-generation sequencing analyses. Oncol. Rep. 2016;35:325–333. doi: 10.3892/or.2015.4398. [DOI] [PubMed] [Google Scholar]
- 89.Mira-Pascual L., Cabrera-Rubio R., Ocon S., Costales P., Parra A., Suarez A., Moris F., Rodrigo L., Mira A., Collado M.C. Microbial mucosal colonic shifts associated with the development of colorectal cancer reveal the presence of different bacterial and archaeal biomarkers. J. Gastroenterol. 2015;50:167–179. doi: 10.1007/s00535-014-0963-x. [DOI] [PubMed] [Google Scholar]
- 90.Feng Q., Liang S., Jia H., Stadlmayr A., Tang L., Lan Z., Zhang D., Xia H., Xu X., Jie Z., et al. Gut microbiome development along the colorectal adenoma–carcinoma sequence. Nat. Commun. 2015;6:6528. doi: 10.1038/ncomms7528. [DOI] [PubMed] [Google Scholar]
- 91.Wu N., Yang X., Zhang R., Li J., Xiao X., Hu Y., Chen Y., Yang F., Lu N., Wang Z., et al. Dysbiosis Signature of Fecal Microbiota in Colorectal Cancer Patients. Microb. Ecol. 2013;66:462–470. doi: 10.1007/s00248-013-0245-9. [DOI] [PubMed] [Google Scholar]
- 92.Vogtmann E., Hua X., Zeller G., Sunagawa S., Voigt A.Y., Hercog R., Goedert J.J., Shi J., Bork P., Sinha R. Colorectal Cancer and the Human Gut Microbiome: Reproducibility with Whole-Genome Shotgun Sequencing. PLoS ONE. 2016;11:e0155362. doi: 10.1371/journal.pone.0155362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Ahn J., Sinha R., Pei Z., Dominianni C., Wu J., Shi J., Goedert J.J., Hayes R.B., Yang L. Human Gut Microbiome and Risk for Colorectal Cancer. J. Natl. Cancer Inst. 2013;105:1907–1911. doi: 10.1093/jnci/djt300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Chen W., Liu F., Ling Z., Tong X., Xiang C. Human Intestinal Lumen and Mucosa-Associated Microbiota in Patients with Colorectal Cancer. PLoS ONE. 2012;7:e39743. doi: 10.1371/journal.pone.0039743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Sobhani I., Tap J., Roudot-Thoraval F., Roperch J.P., Letulle S., Langella P., Corthier G., Van Nhieu J.T., Furet J.P. Microbial Dysbiosis in Colorectal Cancer (CRC) Patients. PLoS ONE. 2011;6:e16393. doi: 10.1371/journal.pone.0016393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Xu K., Jiang B. Analysis of Mucosa-Associated Microbiota in Colorectal Cancer. Med. Sci. Monit. 2017;23:4422–4430. doi: 10.12659/MSM.904220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Brim H., Yooseph S., Zoetendal E.G., Lee E., Torralbo M., Laiyemo A.O., Shokrani B., Nelson K., Ashktorab H. Microbiome Analysis of Stool Samples from African Americans with Colon Polyps. PLoS ONE. 2013;8:e81352. doi: 10.1371/journal.pone.0081352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Flemer B., Lynch D.B., Brown J.M., Jeffery I.B., Ryan F.J., Claesson M.J., O’riordain M., Shanahan F., O’toole P.W. Tumour-associated and non-tumour-associated microbiota in colorectal cancer. Gut. 2017;66:633–643. doi: 10.1136/gutjnl-2015-309595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Wang T., Cai G., Qiu Y., Fei N., Zhang M., Pang X., Jia W., Cai S., Zhao L. Structural segregation of gut microbiota between colorectal cancer patients and healthy volunteers. ISME J. 2012;6:320–329. doi: 10.1038/ismej.2011.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Gao Z., Guo B., Gao R., Zhu Q., Qin H. Microbiota disbiosis is associated with colorectal cancer. Front. Microbiol. 2015;6:20. doi: 10.3389/fmicb.2015.00020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Liang Q., Chiu J., Chen Y., Huang Y., Higashimori A., Fang J., Brim H., Ashktorab H., Ng S.C., Ng S.S.M. Fecal bacteria act as novel biomarkers for noninvasive diagnosis of colorectal cancer. Clin. Cancer Res. 2017;23:2061–2070. doi: 10.1158/1078-0432.CCR-16-1599. [DOI] [PubMed] [Google Scholar]
- 102.Huipeng W., Lifeng G., Chuang G., Jiaying Z., Yuankun C. The Differences in Colonic Mucosal Microbiota between Normal Individual and Colon Cancer Patients by Polymerase Chain Reaction-denaturing Gradient Gel Electrophoresis. J. Clin. Gastroenterol. 2014;48:138–144. doi: 10.1097/MCG.0b013e3182a26719. [DOI] [PubMed] [Google Scholar]
- 103.Nugent J.L., McCoy A.N., Addamo C.J., Jia W., Sandler R.S., Keku T.O. Altered Tissue Metabolites Correlate with Microbial Dysbiosis in Colorectal Adenomas. J. Proteome Res. 2014;13:1921–1929. doi: 10.1021/pr4009783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Hale V.L., Chen J., Johnson S., Harrington S.C., Yab T.C., Smyrk T.C., Nelson H., Boardman L.A., Druliner B.R., Levin T.R., et al. Shifts in the Fecal Microbiota Associated with Adenomatous Polyps. Cancer Epidemiol. Biomark. Prev. 2017;26:85–94. doi: 10.1158/1055-9965.EPI-16-0337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Hibberd A.A., Lyra A., Ouwehand A.C., Rolny P., Lindegren H., Cedgård L., Wettergren Y. Intestinal microbiota is altered in patients with colon cancer and modified by probiotic intervention. BMJ Open Gastroenterol. 2017;4:e000145. doi: 10.1136/bmjgast-2017-000145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Yazici C., Wolf P.G., Kim H., Cross T.W.L., Vermillion K., Carroll T., Augustus G.J., Mutlu E., Tussing-Humphreys L., Braunschweig C., et al. Race-dependent association of sulfidogenic bacteria with colorectal cancer. Gut. 2017;66:1983–1994. doi: 10.1136/gutjnl-2016-313321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Ai L., Tian H., Chen Z., Chen H., Xu J., Fang J.Y. Systematic evaluation of supervised classifiers for fecal microbiota-based prediction of colorectal cancer. Oncotarget. 2017;8:9546–9556. doi: 10.18632/oncotarget.14488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Dejea C.M., Wick E.C., Hechenbleikner E.M., White J.R., Welch J.L.M., Rossetti B.J., Peterson S.N., Snesrud E.C., Borisy G.G., Lazarev M. Microbiota organization is a distinct feature of proximal colorectal cancers. Proc. Natl. Acad. Sci. USA. 2014;111:18321–18326. doi: 10.1073/pnas.1406199111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Zhang Y., Yu X., Yu E., Wang N., Cai Q., Shuai Q., Yan F., Jiang L., Wang H., Liu J., et al. Changes in gut microbiota and plasma inflammatory factors across the stages of colorectal tumorigenesis: A case-control study. BMC Microbiol. 2018;18:92. doi: 10.1186/s12866-018-1232-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Scanlan P.D., Shanahan F., Clune Y., Collins J.K., O’Sullivan G.C., O’Riordan M., Holmes E., Wang Y., Marchesi J.R. Culture-independent analysis of the gut microbiota in colorectal cancer and polyposis. Environ. Microbiol. 2008;10:789–798. doi: 10.1111/j.1462-2920.2007.01503.x. [DOI] [PubMed] [Google Scholar]
- 111.Fukugaiti M.H., Ignacio A., Fernandes M.R., Ribeiro U., Nakano V., Avila-Campos M.J. High occurrence of Fusobacterium nucleatum and Clostridium difficile in the intestinal microbiota of colorectal carcinoma patients. Braz. J. Microbiol. 2015;46:1135–1140. doi: 10.1590/S1517-838246420140665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Ohigashi S., Sudo K., Kobayashi D., Takahashi O., Takahashi T., Asahara T., Nomoto K., Onodera H. Changes of the Intestinal Microbiota, Short Chain Fatty Acids, and Fecal pH in Patients with Colorectal Cancer. Dig. Dis. Sci. 2013;58:1717–1726. doi: 10.1007/s10620-012-2526-4. [DOI] [PubMed] [Google Scholar]
- 113.Xie Y.H., Gao Q.Y., Cai G.X., Sun X.M., Zou T.H., Chen H.M., Yu S.Y., Qiu Y.W., Gu W.Q., Chen X.Y., et al. Fecal Clostridium symbiosum for Noninvasive Detection of Early and Advanced Colorectal Cancer: Test and Validation Studies. EBioMedicine. 2017;25:32–40. doi: 10.1016/j.ebiom.2017.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Shen X.J., Rawls J.F., Randall T.A., Burcal L., Mpande C.N., Jenkins N., Jovov B., Abdo Z., Sandler R.S., Keku T.O. Molecular characterization of mucosal adherent bacteria and associations with colorectal adenomas. Gut Microbes. 2010;1:138–147. doi: 10.4161/gmic.1.3.12360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Chen H.M., Yu Y.N., Wang J.L., Lin Y.W., Kong X., Yang C.Q., Yang L., Liu Z.J., Yuan Y.Z., Liu F., et al. Decreased dietary fiber intake and structural alteration of gut microbiota in patients with advanced colorectal adenoma. Am. J. Clin. Nutr. 2013;97:1044–1052. doi: 10.3945/ajcn.112.046607. [DOI] [PubMed] [Google Scholar]
- 116.Mori G., Rampelli S., Orena B.S., Rengucci C., De Maio G., Barbieri G., Passardi A., Gardini A.C., Frassineti G.L., Gaiarsa S., et al. Shifts of Faecal Microbiota During Sporadic Colorectal Carcinogenesis. Sci. Rep. 2018;8:10329. doi: 10.1038/s41598-018-28671-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Goedert J.J., Gong Y., Hua X., Zhong H., He Y., Peng P., Yu G., Wang W., Ravel J., Shi J., et al. Fecal Microbiota Characteristics of Patients with Colorectal Adenoma Detected by Screening: A Population-based Study. EBioMedicine. 2015;2:597–603. doi: 10.1016/j.ebiom.2015.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Yoon H., Kim N., Park J.H., Kim Y.S., Lee J., Kim H.W., Choi Y.J., Shin C.M., Park Y.S., Lee D.H., et al. Comparisons of Gut Microbiota Among Healthy Control, Patients with Conventional Adenoma, Sessile Serrated Adenoma, and Colorectal Cancer. J. Cancer Prev. 2017;22:108–114. doi: 10.15430/JCP.2017.22.2.108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Bonnet M., Buc E., Sauvanet P., Darcha C., Dubois D., Pereira B., Déchelotte P., Bonnet R., Pezet D., Darfeuille-Michaud A. Colonization of the human gut by E. coli and colorectal cancer risk. Clin. Cancer Res. 2014;20:859–867. doi: 10.1158/1078-0432.CCR-13-1343. [DOI] [PubMed] [Google Scholar]
- 120.Swidsinski A., Khilkin M., Kerjaschki D., Schreiber S., Ortner M., Weber J., Lochs H. Association between intraepithelial Escherichia coli and colorectal cancer. Gastroenterology. 1998;115:281–286. doi: 10.1016/S0016-5085(98)70194-5. [DOI] [PubMed] [Google Scholar]
- 121.Sze M.A., Baxter N.T., Ruffin M.T., Rogers M.A.M., Schloss P.D. Normalization of the microbiota in patients after treatment for colonic lesions. Microbiome. 2017;5:150. doi: 10.1186/s40168-017-0366-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Yu J., Feng Q., Wong S.H., Zhang D., Yi Liang Q., Qin Y., Tang L., Zhao H., Stenvang J., Li Y. Metagenomic analysis of faecal microbiome as a tool towards targeted non-invasive biomarkers for colorectal cancer. Gut. 2017;66:70–78. doi: 10.1136/gutjnl-2015-309800. [DOI] [PubMed] [Google Scholar]
- 123.Deng X., Li Z., Li G., Li B., Jin X., Lv G. Comparison of microbiota in patients treated by surgery or chemotherapy by 16S rRNA sequencing reveals potential biomarkers for colorectal cancer therapy. Front. Microbiol. 2018;9:1607. doi: 10.3389/fmicb.2018.01607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Kostic A.D., Gevers D., Pedamallu C.S., Michaud M., Duke F., Earl A.M., Ojesina A.I., Jung J., Bass A.J., Tabernero J. Genomic analysis identifies association of Fusobacterium with colorectal carcinoma. Genome Res. 2012;22:292–298. doi: 10.1101/gr.126573.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Zackular J.P., Rogers M.A.M., Ruffin M.T., Schloss P.D. The Human Gut Microbiome as a Screening Tool for Colorectal Cancer. Cancer Prev. Res. 2014;7:1112–1121. doi: 10.1158/1940-6207.CAPR-14-0129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Flemer B., Warren R.D., Barrett M.P., Cisek K., Das A., Jeffery I.B., Hurley E., Micheal O.R., Shanahan F., Paul W.T. The oral microbiota in colorectal cancer is distinctive and predictive. Gut. 2018;67:1454–1463. doi: 10.1136/gutjnl-2017-314814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Baxter N.T., Ruffin M.T., Rogers M.A.M., Schloss P.D. Microbiota-based model improves the sensitivity of fecal immunochemical test for detecting colonic lesions. Genome Med. 2016;8:37. doi: 10.1186/s13073-016-0290-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Amitay E.L., Werner S., Vital M., Pieper D.H., Höfler D., Gierse I.-J., Butt J., Balavarca Y., Cuk K., Brenner H. Fusobacterium and colorectal cancer: Causal factor or passenger? Results from a large colorectal cancer screening study. Carcinogenesis. 2017;38:781–788. doi: 10.1093/carcin/bgx053. [DOI] [PubMed] [Google Scholar]
- 129.Russo E., Bacci G., Chiellini C., Fagorzi C., Niccolai E., Taddei A., Ricci F., Ringressi M.N., Borrelli R., Melli F. Preliminary Comparison of Oral and Intestinal Human Microbiota in Patients with Colorectal Cancer: A Pilot Study. Front. Microbiol. 2018;8:2699. doi: 10.3389/fmicb.2017.02699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Wong S.H., Kwong T.N., Chow T.-C., Luk A.K., Dai R.Z., Nakatsu G., Lam T.Y., Zhang L., Wu J.C., Chan F.K. Quantitation of faecal Fusobacterium improves faecal immunochemical test in detecting advanced colorectal neoplasia. Gut. 2017;66:1441–1448. doi: 10.1136/gutjnl-2016-312766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Eklöf V., Löfgren-Burström A., Zingmark C., Edin S., Larsson P., Karling P., Alexeyev O., Rutegård J., Wikberg M.L., Palmqvist R. Cancer-associated fecal microbial markers in colorectal cancer detection. Int. J. Cancer. 2017;141:2528–2536. doi: 10.1002/ijc.31011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Flanagan L., Schmid J., Ebert M., Soucek P., Kunicka T., Liška V., Bruha J., Neary P., DeZeeuw N., Tommasino M., et al. Fusobacterium nucleatum associates with stages of colorectal neoplasia development, colorectal cancer and disease outcome. Eur. J. Clin. Microbiol. Infect. Dis. 2014;33:1381–1390. doi: 10.1007/s10096-014-2081-3. [DOI] [PubMed] [Google Scholar]
- 133.Repass J., Maherali N., Owen K., Reproducibility Project: Cancer B., Reproducibility Project Cancer B. Registered report: Fusobacterium nucleatum infection is prevalent in human colorectal carcinoma. eLife. 2016;5:e10012. doi: 10.7554/eLife.10012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Castellarin M., Warren R.L., Freeman J.D., Dreolini L., Krzywinski M., Strauss J., Barnes R., Watson P., Allen-Vercoe E., Moore R.A. Fusobacterium nucleatum infection is prevalent in human colorectal carcinoma. Genome Res. 2012;22:299–306. doi: 10.1101/gr.126516.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Tahara T., Yamamoto E., Suzuki H., Maruyama R., Chung W., Garriga J., Jelinek J., Yamano H.-O., Sugai T., An B., et al. Fusobacterium in colonic flora and molecular features of colorectal carcinoma. Cancer Res. 2014;74:1311–1318. doi: 10.1158/0008-5472.CAN-13-1865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Ito M., Kanno S., Nosho K., Sukawa Y., Mitsuhashi K., Kurihara H., Igarashi H., Takahashi T., Tachibana M., Takahashi H. Association of Fusobacterium nucleatum with clinical and molecular features in colorectal serrated pathway. Int. J. Cancer. 2015;137:1258–1268. doi: 10.1002/ijc.29488. [DOI] [PubMed] [Google Scholar]
- 137.McCoy A.N., Araujo-Perez F., Azcárate-Peril A., Yeh J.J., Sandler R.S., Keku T.O. Fusobacterium Is Associated with Colorectal Adenomas. PLoS ONE. 2013;8:e53653. doi: 10.1371/journal.pone.0053653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Suehiro Y., Sakai K., Nishioka M., Hashimoto S., Takami T., Higaki S., Shindo Y., Hazama S., Oka M., Nagano H., et al. Highly sensitive stool DNA testing of Fusobacterium nucleatum as a marker for detection of colorectal tumours in a Japanese population. Ann. Clin. Biochem. Int. J. Lab. Med. 2017;54:86–91. doi: 10.1177/0004563216643970. [DOI] [PubMed] [Google Scholar]
- 139.Lu Y., Chen J., Zheng J., Hu G., Wang J., Huang C., Lou L., Wang X., Zeng Y. Mucosal adherent bacterial dysbiosis in patients with colorectal adenomas. Sci. Rep. 2016;6:26337. doi: 10.1038/srep26337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Geng J., Song Q., Tang X., Liang X., Fan H., Peng H., Guo Q., Zhang Z. Co-occurrence of driver and passenger bacteria in human colorectal cancer. Gut Pathog. 2014;6:26. doi: 10.1186/1757-4749-6-26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Richard M.L., Liguori G., Lamas B., Brandi G., da Costa G., Hoffmann T.W., Pierluigi Di Simone M., Calabrese C., Poggioli G., Langella P., et al. Mucosa-associated microbiota dysbiosis in colitis associated cancer. Gut Microbes. 2018;9:131–142. doi: 10.1080/19490976.2017.1379637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Scanlan P.D., Shanahan F., Marchesi J.R. Culture-independent analysis of desulfovibrios in the human distal colon of healthy, colorectal cancer and polypectomized individuals. FEMS Microbiol. Ecol. 2009;69:213–221. doi: 10.1111/j.1574-6941.2009.00709.x. [DOI] [PubMed] [Google Scholar]
- 143.Lopez-Siles M., Martinez-Medina M., Surís-Valls R., Aldeguer X., Sabat-Mir M., Duncan S.H., Flint H.J., Garcia-Gil L.J. Changes in the Abundance of Faecalibacterium prausnitzii Phylogroups I and II in the Intestinal Mucosa of Inflammatory Bowel Disease and Patients with Colorectal Cancer. Inflamm. Bowel Dis. 2016;22:28–41. doi: 10.1097/MIB.0000000000000590. [DOI] [PubMed] [Google Scholar]
- 144.Holscher H.D. Dietary fiber and prebiotics and the gastrointestinal microbiota. Gut Microbes. 2017;8:172–184. doi: 10.1080/19490976.2017.1290756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Macfarlane G.T., Macfarlane S. Fermentation in the human large intestine: Its physiologic consequences and the potential contribution of prebiotics. J. Clin. Gastroenterol. 2011;45:S120–S127. doi: 10.1097/MCG.0b013e31822fecfe. [DOI] [PubMed] [Google Scholar]
- 146.Lattimer J.M., Haub M.D. Effects of Dietary Fiber and Its Components on Metabolic Health. Nutrients. 2010;2:1266–1289. doi: 10.3390/nu2121266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Terry P., Giovannucci E., Michels K.B., Bergkvist L., Hansen H., Holmberg L., Wolk A. Fruit, Vegetables, Dietary Fiber, and Risk of Colorectal Cancer. J. Natl. Cancer Inst. 2001;93:525–533. doi: 10.1093/jnci/93.7.525. [DOI] [PubMed] [Google Scholar]
- 148.Flint H.J., Scott K.P., Louis P., Duncan S.H. The role of the gut microbiota in nutrition and health. Nat. Rev. Gastroenterol. Hepatol. 2012;9:577–589. doi: 10.1038/nrgastro.2012.156. [DOI] [PubMed] [Google Scholar]
- 149.Bishehsari F., Engen P.A., Preite N.Z., Tuncil Y.E., Naqib A., Shaikh M., Rossi M., Wilber S., Green S.J., Hamaker B.R., et al. Dietary Fiber Treatment Corrects the Composition of Gut Microbiota, Promotes SCFA Production, and Suppresses Colon Carcinogenesis. Genes. 2018;9:102. doi: 10.3390/genes9020102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Belcheva A., Irrazabal T., Martin A. Gut microbial metabolism and colon cancer: Can manipulations of the microbiota be useful in the management of gastrointestinal health? BioEssays. 2015;37:403–412. doi: 10.1002/bies.201400204. [DOI] [PubMed] [Google Scholar]
- 151.Donohoe D.R., Collins L.B., Wali A., Bigler R., Sun W., Bultman S.J. The Warburg Effect Dictates the Mechanism of Butyrate Mediated Histone Acetylation and Cell Proliferation. Mol. Cell. 2012;48:612–626. doi: 10.1016/j.molcel.2012.08.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Cardona F., Andres-Lacueva C., Tulipani S., Tinahones F.J., Queipo-Ortuño M.I. Benefits of polyphenols on gut microbiota and implications in human health. J. Nutr. Biochem. 2013;24:1415–1422. doi: 10.1016/j.jnutbio.2013.05.001. [DOI] [PubMed] [Google Scholar]
- 153.Windey K., De Preter V., Verbeke K. Relevance of protein fermentation to gut health. Mol. Nutr. Food Res. 2012;56:184–196. doi: 10.1002/mnfr.201100542. [DOI] [PubMed] [Google Scholar]
- 154.Marchesi J.R., Adams D.H., Fava F., Hermes G.D., Hirschfield G.M., Hold G., Quraishi M.N., Kinross J., Smidt H., Tuohy K.M. The gut microbiota and host health: A new clinical frontier. Gut. 2016;65:330–339. doi: 10.1136/gutjnl-2015-309990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Marquet P., Duncan S.H., Chassard C., Bernalier-Donadille A., Flint H.J. Lactate has the potential to promote hydrogen sulphide formation in the human colon. FEMS Microbiol. Lett. 2009;299:128–134. doi: 10.1111/j.1574-6968.2009.01750.x. [DOI] [PubMed] [Google Scholar]
- 156.Carbonero F., Benefiel A.C., Gaskins H.R. Contributions of the microbial hydrogen economy to colonic homeostasis. Nat. Rev. Gastroenterol. Hepatol. 2012;9:504–518. doi: 10.1038/nrgastro.2012.85. [DOI] [PubMed] [Google Scholar]
- 157.Louis P., Hold G.L., Flint H.J. The gut microbiota, bacterial metabolites and colorectal cancer. Nat. Rev. Genet. 2014;12:661–672. doi: 10.1038/nrmicro3344. [DOI] [PubMed] [Google Scholar]
- 158.Duncan S.H., Louis P., Flint H.J. Lactate-Utilizing Bacteria, Isolated from Human Feces, That Produce Butyrate as a Major Fermentation Product. Appl. Environ. Microbiol. 2004;70:5810–5817. doi: 10.1128/AEM.70.10.5810-5817.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Di Martino M.L., Campilongo R., Casalino M., Micheli G., Colonna B., Prosseda G. Polyamines: Emerging players in bacteria–host interactions. Int. J. Med. Microbiol. 2013;303:484–491. doi: 10.1016/j.ijmm.2013.06.008. [DOI] [PubMed] [Google Scholar]
- 160.Ramos S. Cancer chemoprevention and chemotherapy: Dietary polyphenols and signalling pathways. Mol. Nutr. Food Res. 2008;52:507–526. doi: 10.1002/mnfr.200700326. [DOI] [PubMed] [Google Scholar]
- 161.Arthur J.C., Jobin C. The struggle within: Microbial influences on colorectal cancer. Inflamm. Bowel Dis. 2010;17:396–409. doi: 10.1002/ibd.21354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Kahouli I., Tomaro-Duchesneau C., Prakash S. Probiotics in colorectal cancer (CRC) with emphasis on mechanisms of action and current perspectives. J. Med. Microbiol. 2013;62:1107–1123. doi: 10.1099/jmm.0.048975-0. [DOI] [PubMed] [Google Scholar]
- 163.Ou J., Delany J.P., Zhang M., Sharma S., O’Keefe S.J.D. Association Between Low Colonic Short-Chain Fatty Acids and High Bile Acids in High Colon Cancer Risk Populations. Nutr. Cancer. 2012;64:34–40. doi: 10.1080/01635581.2012.630164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Lee K.A., Kim B., Bhin J., Kim D.H., You H., Kim E.K., Kim S.H., Ryu J.H., Hwang D., Lee W.J. Bacterial Uracil Modulates Drosophila DUOX-Dependent Gut Immunity via Hedgehog-Induced Signaling Endosomes. Cell Host Microbe. 2015;17:191–204. doi: 10.1016/j.chom.2014.12.012. [DOI] [PubMed] [Google Scholar]
- 165.Xu R., Wang Q., Li L. A genome-wide systems analysis reveals strong link between colorectal cancer and trimethylamine N-oxide (TMAO), a gut microbial metabolite of dietary meat and fat. BMC Genom. 2015;16:S4. doi: 10.1186/1471-2164-16-S7-S4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Homann N. Alcohol and upper gastrointestinal tract cancer: The role of local acetaldehyde production. Addict. Biol. 2001;6:309–323. doi: 10.1080/13556210020077028. [DOI] [PubMed] [Google Scholar]
- 167.Sieber J.R., McInerney M.J., Gunsalus R.P. Genomic Insights into Syntrophy: The Paradigm for Anaerobic Metabolic Cooperation. Annu. Rev. Microbiol. 2012;66:429–452. doi: 10.1146/annurev-micro-090110-102844. [DOI] [PubMed] [Google Scholar]
- 168.Baughn A.D., Malamy M.H. The strict anaerobe Bacteroides fragilis grows in and benefits from nanomolar concentrations of oxygen. Nature. 2004;427:441–444. doi: 10.1038/nature02285. [DOI] [PubMed] [Google Scholar]
- 169.Khan M.T., Duncan S.H., Stams A.J.M., Van Dijl J.M., Flint H.J., Harmsen H.J.M. The gut anaerobe Faecalibacterium prausnitzii uses an extracellular electron shuttle to grow at oxic–anoxic interphases. ISME J. 2012;6:1578–1585. doi: 10.1038/ismej.2012.5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.D’Souza G., Shitut S., Preussger D., Yousif G., Waschina S., Kost C. Ecology and evolution of metabolic cross-feeding interactions in bacteria. Nat. Prod. Rep. 2018;35:455–488. doi: 10.1039/C8NP00009C. [DOI] [PubMed] [Google Scholar]
- 171.Goedert J.J., Sampson J.N., Moore S.C., Xiao Q., Xiong X., Hayes R.B., Ahn J., Shi J., Sinha R. Fecal metabolomics: Assay performance and association with colorectal cancer. Carcinogenesis. 2014;35:2089–2096. doi: 10.1093/carcin/bgu131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Wang X., Wang J., Rao B., Deng L. Gut flora profiling and fecal metabolite composition of colorectal cancer patients and healthy individuals. Exp. Ther. Med. 2017;13:2848–2854. doi: 10.3892/etm.2017.4367. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 173.Lin Y., Ma C., Liu C., Wang Z., Yang J., Liu X., Shen Z., Wu R. NMR-based fecal metabolomics fingerprinting as predictors of earlier diagnosis in patients with colorectal cancer. Oncotarget. 2016;7:29454–29464. doi: 10.18632/oncotarget.8762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Ni Y., Xie G., Jia W. Metabonomics of Human Colorectal Cancer: New Approaches for Early Diagnosis and Biomarker Discovery. J. Proteome Res. 2014;13:3857–3870. doi: 10.1021/pr500443c. [DOI] [PubMed] [Google Scholar]
- 175.Monleon D., Morales J.M., Barrasa A., López J.A., Vázquez C., Celda B. Metabolite profiling of fecal water extracts from human colorectal cancer. NMR BioMed. 2009;22:342–348. doi: 10.1002/nbm.1345. [DOI] [PubMed] [Google Scholar]
- 176.De Monerri N.C.S., Kim K.J.T.A. Pathogens hijack the epigenome: A new twist on host-pathogen interactions. Am. J. Pathol. 2014;184:897–911. doi: 10.1016/j.ajpath.2013.12.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Hullar M.A.J., Fu B.C. Diet, the Gut Microbiome, and Epigenetics. Cancer J. 2014;20:170–175. doi: 10.1097/PPO.0000000000000053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Bultman S.J. Interplay between diet, gut microbiota, epigenetic events, and colorectal cancer. Mol. Nutr. Food Res. 2017;61:1500902. doi: 10.1002/mnfr.201500902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Tetro J., Allen-Vercoe E. The Human Microbiome Handbook. DEStech Publications, Inc.; Lancaster, PA, USA: 2016. [Google Scholar]
- 180.Lightfoot Y.L., Yang T., Sahay B., Mohamadzadeh M. Targeting aberrant colon cancer-specific DNA methylation with lipoteichoic acid-deficient Lactobacillus acidophilus. Gut Microbes. 2013;4:84–88. doi: 10.4161/gmic.22822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Demehri F.R., Frykman P.K., Cheng Z., Ruan C., Wester T., Nordenskjöld A., Kawaguchi A., Hui T.T., Granström A.L., Funari V.J.J. Altered fecal short chain fatty acid composition in children with a history of Hirschsprung-associated enterocolitis. J. Pediatr. Surg. 2016;51:81–86. doi: 10.1016/j.jpedsurg.2015.10.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Mima K., Nishihara R., Qian Z.R., Cao Y., Sukawa Y., Nowak J.A., Yang J., Dou R., Masugi Y., Song M.J.G. Fusobacterium nucleatum in colorectal carcinoma tissue and patient prognosis. Gut. 2016;65:1973–1980. doi: 10.1136/gutjnl-2015-310101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Fraga M.F., Ballestar E., Villar-Garea A., Boix-Chornet M., Espada J., Schotta G., Bonaldi T., Haydon C., Ropero S., Petrie K., et al. Loss of acetylation at Lys16 and trimethylation at Lys20 of histone H4 is a common hallmark of human cancer. Nat. Genet. 2005;37:391–400. doi: 10.1038/ng1531. [DOI] [PubMed] [Google Scholar]
- 184.Akhtar-Zaidi B., Cowper-Sal·lari R., Corradin O., Saiakhova A., Bartels C.F., Balasubramanian D., Myeroff L., Lutterbaugh J., Jarrar A., Kalady M.F., et al. Epigenomic enhancer profiling defines a signature of colon cancer. Science. 2012;336:736–739. doi: 10.1126/science.1217277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Benard A., Goossens-Beumer I.J., Van Hoesel A.Q., De Graaf W., Horati H., Putter H., Zeestraten E.C., Van De Velde C.J., Kuppen P.J. Histone trimethylation at H3K4, H3K9 and H4K20 correlates with patient survival and tumor recurrence in early-stage colon cancer. BMC Cancer. 2014;14:531. doi: 10.1186/1471-2407-14-531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Ye X., Wang R., Bhattacharya R., Boulbes D.R., Fan F., Xia L., Adoni H., Ajami N.J., Wong M.C., Smith D.P., et al. Fusobacterium Nucleatum Subspecies Animalis Influences Proinflammatory Cytokine Expression and Monocyte Activation in Human Colorectal Tumors. Cancer Prev. Res. 2017;10:398–409. doi: 10.1158/1940-6207.CAPR-16-0178. [DOI] [PubMed] [Google Scholar]
- 187.Yu T., Guo F., Yu Y., Sun T., Ma D., Han J., Qian Y., Kryczek I., Sun D., Nagarsheth N., et al. Fusobacterium nucleatum Promotes Chemoresistance to Colorectal Cancer by Modulating Autophagy. Cell. 2017;170:548–563.e16. doi: 10.1016/j.cell.2017.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Mirchev M., Kahl P., Friedrichs N., Kotzev I., Buettner R. DNA Methylation in Patients with Colorectal Cancer—Correlation with Some Clinical and Morphological Features and with Local Tumour Invasion. Folia Med. 2010;52:22–30. doi: 10.2478/v10153-010-0043-9. [DOI] [PubMed] [Google Scholar]
- 189.Fan X.Y., Hu X.L., Han T.M., Wang N.N., Zhu Y.M., Hu W., Ma Z.H., Zhang C.J., Xu X., Ye Z.Y., et al. Association between RUNX3 promoter methylation and gastric cancer: A meta-analysis. BMC Gastroenterol. 2011;11:92. doi: 10.1186/1471-230X-11-92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Farhana L., Banerjee H.N., Verma M., Majumdar A.P.N. Advanced Structural Safety Studies. Volume 1856. Springer; Berlin/Heidelberg, Germany: 2018. Role of Microbiome in Carcinogenesis Process and Epigenetic Regulation of Colorectal Cancer; pp. 35–55. [DOI] [PubMed] [Google Scholar]
- 191.Sharma S., Kelly T.K., Jones P.A. Epigenetics in cancer. Carcinogenesis. 2010;31:27–36. doi: 10.1093/carcin/bgp220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Wen X.Z., Akiyama Y., Pan K.F., Liu Z.J., Lu Z.M., Zhou J., Gu L.K., Dong C.X., Zhu B.D., Ji J.F., et al. Methylation of GATA-4 and GATA-5 and development of sporadic gastric carcinomas. World J. Gastroenterol. 2010;16:1201–1208. doi: 10.3748/wjg.v16.i10.1201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Fabbri M.J. TLRs as miRNA receptors. Cancer Res. 2012;72:6333–6337. doi: 10.1158/0008-5472.CAN-12-3229. [DOI] [PubMed] [Google Scholar]
- 194.Tanaka T., Tanaka M., Tanaka T., Ishigamori R.J.I. Biomarkers for colorectal cancer. Int. J. Mol. Sci. 2010;11:3209–3225. doi: 10.3390/ijms11093209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Kong Y.W., Ferland-McCollough D., Jackson T.J., Bushell M.J. microRNAs in cancer management. Lancet Oncol. 2012;13:e249–e258. doi: 10.1016/S1470-2045(12)70073-6. [DOI] [PubMed] [Google Scholar]
- 196.Wu C.W., Dong Y.J., Liang Q.Y., He X.Q., Ng S.S.M., Chan F.K.L., Sung J.J.Y., Yu J. MicroRNA-18a Attenuates DNA Damage Repair through Suppressing the Expression of Ataxia Telangiectasia Mutated in Colorectal Cancer. PLoS ONE. 2013;8:e57036. doi: 10.1371/journal.pone.0057036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Hu S., Liu L., Chang E.B., Wang J.-Y., Raufman J.-P. Butyrate inhibits pro-proliferative miR-92a by diminishing c-Myc-induced miR-17-92a cluster transcription in human colon cancer cells. Mol. Cancer. 2015;14:1221. doi: 10.1186/s12943-015-0450-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Bardhan K., Liu K. Epigenetics and Colorectal Cancer Pathogenesis. Cancers. 2013;5:676–713. doi: 10.3390/cancers5020676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Yuan C., Burns M.B., Subramanian S., Blekhman R. Interaction between host MicroRNAs and the gut microbiota in colorectal cancer. MSystems. 2018;3:e00205-17. doi: 10.1128/mSystems.00205-17. [DOI] [PMC free article] [PubMed] [Google Scholar]