Abstract
Broccoli is a popular brassica vegetable and its consumption may decrease the occurrence of cancer in certain populations. To gain insight into the metabolites that may induce physiological responses to broccoli intake, a non-targeted metabolomic approach and a targeted approach for analysis of glucosinolate metabolites were developed using high resolution accurate mass spectrometry. A human study was conducted in which 6 subjects consumed a single meal of 200 g of uncooked broccoli florets. The metabolomic analysis revealed changes in endogenous metabolites and a decrease in hippuric acid after broccoli consumption. Targeted analysis using high-resolution, accurate mass-mass spectrometry (HRAM-MS) enabled detection of low concentrations (nM) of glucosinolate metabolites in human urine and plasma. Glucosinolate metabolites were found in human urine (13) and plasma (8), respectively. Metabolites from methoxyl-indole glucosinolates, arising from broccoli consumption, are reported for the first time. Most glucosinolate metabolites reached their peak concentration in urine 2-4 h after consumption while, in plasma, peak maxima were achieved 2 h after intake. The results suggest that glucoraphanin metabolites (sulforaphane, sulforaphane cysteine, sulforaphane N-acetyl cysteine) and indole metabolites (ascorbigen and methoxyl ascorbigen from indole glucosinolates) may serve as marker compounds for the intake of broccoli.
Keywords: Glucosinolate; Broccoli; Human study; High resolution mass spectrometry; Metabolomics, targeted analysis; Glucoraphanin; Glucobrassicin; Methoxyl glucobrassicin
1. Introduction
Epidemiological studies suggest an inverse association between intakes of brassica vegetables and cancer occurrence (Verhoeven, Goldbohm, van Poppel, Verhagen, & van den Brandt, 1996; Verkerk, Schreiner, Krumbein, Ciska, Holst, Rowland, et al., 2009; L. I. Wang, Giovannucci, Hunter, Neuberg, Su, & Christiani, 2004). Among all brassica vegetables, broccoli is one of the most studied and its consumption is associated with decreased occurrence of prostate and lung cancer in some populations (Kalpana Deepa Priya, Gayathri, Gunassekaran, Murugan, & Sakthisekaran, 2013; Traka, Gasper, Melchini, Bacon, Needs, Frost, et al., 2008; van Poppel, Verhoeven, Verhagen, & Goldbohm, 1999).
Glucosinolates in broccoli are considered to be the most important secondary metabolites and are the parent form of isothiocyanates. Isothiocyanates are reported to be potent inducers of phase II detoxification enzymes (Mithen, Faulkner, Magrath, Rose, Williamson, & Marquez, 2003; Munday & Munday, 2002; Ye & Zhang, 2001) and can induce cell cycle arrest and apoptosis (Arumugam & Abdull Razis, 2018; Kalpana Deepa Priya, Gayathri, Gunassekaran, Murugan, & Sakthisekaran, 2013). Dietary glucosinolates are relatively inactive and are not absorbed by humans or mammals directly. Instead, they are converted to isothiocyanates in the presence of plant myrosinase or by the activity of enteric microflora. Isothiocyanates can be absorbed and further metabolized in humans, as parent and conjugated forms (Holst & Williamson, 2004).
Sulforaphane (1-isothiocyanato-4-methylsulfinyl butane) is the isothiocyanate derived from glucoraphanin (4-methylsulfinylbutyl glucosinolates) and has been studied widely. Following absorption, it can be conjugated with glutathione and metabolized via the mercapturic acid pathway. The major metabolites of sulforaphane are N-acetylcysteine and cysteine conjugates (Egner, Kensler, Chen, Gange, Groopman, & Friesen, 2008; Fuentes, Paredes-Gonzalez, & Kong, 2015; Platz, Piberger, Budnowski, Herz, Schreiner, Blaut, et al., 2015; H. Wang, Lin, Shen, Khor, Nomeir, & Kong, 2011). In addition to glucoraphanin, indole glucosinolates, such as glucobrassicin, 1-methoxyl-glucobrassicin (neoglucobrassicin), and 4-methoxyl-glucobrassicin are other major glucosinolates reported in broccoli (Ares, Nozal, Bernal, & Bernal, 2014; Lippmann, Lehmann, Florian, Barknowitz, Haack, Mewis, et al., 2014; Tian, Rosselot, & Schwartz, 2005; Troyer, Stephenson, & Fahey, 2001). However, isothiocyanates produced through hydrolysis of indole glucosinolates are not stable and are further degraded into indole derivatives, depending on pH and the co-existence of epithiospecifier proteins (Barba, Nikmaram, Roohinejad, Khelfa, Zhu, & Koubaa, 2016). Indole-3-carbinol (I3C), a degradation product of glucobrassicin, has been reported as having chemo-preventative activity (Weng, Tsai, Kulp, & Chen, 2008). The detection of I3C metabolites in human urine and plasma, after consumption of selenium-fortified broccoli, has been reported and the major metabolites were indole-3-carboxaldehyde, indole-3-carboxylic acid, and ascorbigen (Hauder, Winkler, Bub, Rufer, Pignitter, & Somoza, 2011). However, other indole metabolites from methoxyl glucobrassicin have not been considered.
For the quantification of isothiocyanates, such as sulforaphane, a cyclocondensation reaction of isothiocyanates with 1,2-benzenedithiol in combination with HPLC-UV detection at 365 nm, was proposed by Zhang et al. (Zhang, Cho, Posner, & Talalay, 1992). This method can be employed for the quantification of isothiocyanates and their metabolites in brassicaceous extracts or biological samples (Tang, Paonessa, Zhang, Arnbrosone, & McCann, 2013; Zhang, Cho, Posner, & Talalay, 1992). However, compared to the HPLC-MS/MS-based methodology, cyclo-condensation methods have low sensitivity and are not able to differentiate among individual isothiocyanates or their conjugates (Egner, Kensler, Chen, Gange, Groopman, & Friesen, 2008; Hauder, Winkler, Bub, Rufer, Pignitter, & Somoza, 2011; Platz, et al., 2015; H. Wang, Lin, Shen, Khor, Nomeir, & Kong, 2011). Generally, quantification of indole derivatives from indole glucosinolates is performed using HPLC, with UV or fluorescence detection, coupled with mass spectrometry. Targeted analysis using multi-reaction monitoring (MRM) with mass spectrometry has also been developed for quantitation of indole metabolites (Hauder, Winkler, Bub, Rufer, Pignitter, & Somoza, 2011).
Metabolomic approaches may be used to gain insight into the metabolites that might induce physiological responses to diet as well as changes in metabolite concentrations in biological matrices, such as plasma, urine, and tissues. Numerous studies have used metabolomics to characterize different dietary intakes and metabolite changes (Andersen, Rinnan, Manach, Poulsen, Pujos-Guillot, Larsen, et al., 2014; Brennan, 2013; Guasch-Ferre, Bhupathiraju, & Hu, 2018; Guertin, Moore, Sampson, Huang, Xiao, Stolzenberg-Solomon, et al., 2014; Koulman & Volmer, 2008; Primrose, Draper, Elsom, Kirkpatrick, Mathers, Seal, et al., 2011; Zheng, Clausen, Dalsgaard, & Bertram, 2015). However, since analytical methods for non-targeted metabolomics are usually developed to detect as many metabolites as possible, signals associated with biologically meaningful metabolites, which present at low levels, may be lost in noise or much higher concentrations of endogenous metabolites (Dettmer, Aronov, & Hammock, 2007; Koal & Deigner, 2010). Hence, monitoring changes in low-concentration metabolites requires a combination of both non-targeted metabolomic and targeted analysis approaches.
In this study, we employed these two approaches to pinpoint changes in glucosinolate metabolite concentrations in human urine and plasma after broccoli consumption. We developed an efficient procedure to discover metabolites in urine and plasma, using high-resolution, accurate mass-mass spectrometry (HRAM MS) detection with selected ion monitoring (SIM). The results expand evidence of changes in endogenous metabolites and glucosinolate metabolites in human urine and plasma following consumption of raw broccoli, changes that suggest or explained their proposed or demonstrated roles in human health.
2. Experimental methods
2.1. Chemicals
Sulfatase (from Helix pomatia), (DL)-sulforaphane (90%), indole-3-carbinol, indole-3-carboxaldehyde, indole-3-carboxylic acid, indole-3-acetonitrile (I3-ACN), 3,3’-diindolylmethane and (−)-sinigrin hydrate were purchased from Sigma-Aldrich (St. Louis, MO, USA). Standards were dissolved in methanol to obtain solutions of 1 mg/mL stock solutions. Formic acid and HPLC grade methanol and acetonitrile were purchased from Fisher Scientific (Waltham, MA, USA). HPLC-grade water was prepared from distilled water using a Milli-Q system (Millipore Lab., Bedford, MA, USA).
2.2. Quantitation of Glucosinolates in Broccoli
Quantitation of total and individual glucosinolates was based on IS09167-1 (https://www.iso.org/standard/16763.html) with slight modifications (Sun, Kou, Geng, Huang, Yang, Luo, et al., 2015). The results are reported as μmol/g dry weight.
The PDA signal at 229 nm was used to quantify desulfoglucosinolates (desulfo-GLs). Peak areas were integrated using Xcalibur 4.1. and identities were confirmed using high accuracy mass measurement. The quantities of glucosinolates in broccoli were calculated using sinigrin as an internal standard and recommended relative response factors. HPLC analyses were performed using a Vanquish UHPLC (Thermo Fisher Scientific, San Jose, CA, USA) equipped with a diode array detector. A Vanquish Accucore C18+ UHPLC column (100 mm × 2.1 mm, 1.5-μm) column was used for separation. Elution was performed using mobile phase A (0.1% formic acid aqueous solution) and mobile phase B (0.1% formic Acid in acetonitrile). The elution program was as follows: 2–50% B, 0–10 min; 50–95% B, 10–15 min; and kept at 95% B for 15–25 min; The re-equilibration time is 5 min. The flow rate was 0.2 mL/min, and detection was at 229 nm. A Q-Exactive MS (Thermo Fisher Scientific, San Jose, CA, USA) was used to confirm each desulfo-glucosinolate. The optimized conditions for MS were set as follows: sheath gas at 40 (arbitrary unit), aux gas at 10 (arbitrary unit), and sweep gas at 5 (arbitrary unit), spray voltage at −4.0 kV, capillary temp at 320 °C, S-Lens RF level at 50 V. The mass range was from m/z 100 to 1000 with a resolution of 35,000, FTMS AGC target at 1e6 and FT-MS/MS AGC target at 1e5. Source CID was set at 20%. Quantification was conducted using desulfated glucosinolates, with desulfo-sinigrin as the internal standard and response factors were calculated against internal standard.
2.3. Experimental Design of the Human Study
This study was conducted at the Beltsville Human Nutrition Research Center (BHNRC) in Beltsville, MD, USA. Subjects were recruited from the Washington, D.C. area and were screened for general health. Eligibility was determined by routine clinical blood and urine screening and completion of a self-report on a health history questionnaire. Potential subjects were excluded if they met any of the following criteria: 1) pregnant, lactating, or intending to become pregnant during the study period, 2) known allergy or intolerance to Brassica vegetables, 3) colonoscopy during the three weeks prior to start of study, 4) use of probiotics during three weeks prior to start of study, 5) history of bariatric surgery or nutrient malabsorption disease, 6) use of tobacco products, 7) Crohn’s disease or diverticulitis, 8) suspected or known strictures, fistulas or physiological/mechanical gastrointestinal obstruction, 9) type 2 diabetes requiring the use of medication including insulin or 10) use of blood-thinning medication.
Six subjects were selected (characteristics are listed in Table S1) using random number generation in Microsoft Excel. There were 3 males and 3 females, aged 28-67 y (mean 46.2 ± 17.3) with BMIs in the range 25.3 – 28.1 kg/m2 (mean 25.5 ± 1.7, i.e. all over-weight). This study was conducted according to the guidelines laid down in the Declaration of Helsinki. All procedures involving human subjects were approved by the Chesapeake Institutional Review Board (Columbia, MD, USA) and written informed consent was obtained from all subjects. This trial was registered at clinicaltrials.gov ().
The study design was a controlled feeding intervention for one 12-day period. For seven days, the subjects consumed a diet of their choice excluding brassica vegetables. For the next three days, subjects consumed brassica-free meals prepared by the BHNRC. On day 11, fasting blood and urine samples were collected. Then, the subjects consumed a breakfast consisting of 1) 200 g of fresh, uncooked broccoli florets with 50 g of a mayonnaise-type dressing containing soybean oil, egg yolk, white vinegar, salt, pepper, and sugar, 2) a 100 g of white flour roll, and 3) 10 g of margarine. Subjects consumed breakfast within 15 minutes, and hour 0 was the point at which they began eating. This meal was the only consumption of brassica vegetables during the study.
Blood samples were collected hourly at 0, 1, 2, 3 ,4, 5, 6 and a final sample was collected at the 24 h. Blood was collected into EDTA-coated vacutainers and, following centrifugation (2000 g, 10 min.), and plasma aliquots were snap-frozen in liquid nitrogen and stored at −80 °C until analysis. Urine samples were collected in a container at hour 0 and, then, in separate containers, over the periods 0-2, 2-4, 4-6, and 6-24 h. Urines collected from 0-2, 2-4, and 4-6 hours were at BHNRC and were acidified immediately (0.25 mL of 0.7% ascorbic acid to 1.5 mL of urine) before being stored at −80 °C. Urine from 6-24 h was collected at the homes of the subjects using jugs containing 2 g of ascorbic acid and aliquots were stored at −80 °C. Urinary creatinine was measured using a clinical chemistry analyzer (Vitros 5,1, Ortho Clinical Diagnostics, Raritan, NJ, USA) according to the manufacturer’s instructions (product code 680 2584).
2.4. Preparation of Urine and Plasma Samples for UHPLC-HRAM MS analysis
Urine samples were centrifuged at 5,000 g and 4 °C for 10 min, and the supernatant was passed through a 0.22 μm PTFE syringe filter. For plasma samples, 1 mL of methanol was added to 0.5 mL plasma to precipitate the proteins. After homogenization for 1 min, samples were further centrifuged at 5,000 g and 4 °C for 15 min. The upper layer was collected and passed through a 0.22 μm PTFE syringe filter. The injection volume was 2 μL for both urine and plasma samples.
2.5. Non-targeted Metabolomics Analysis
The UHPLC-HRAM MS system consisted of a hybrid quadrupole-orbitrap mass spectrometer with a Vanquish UHPLC system (Q-Exactive, Thermo Fisher Scientific, San Jose, CA, USA). For non-targeted metabolites profiling, the separation was carried out on a Vanquish Accucore C18+ UHPLC column (100 mm × 2.1 mm, 1.5-μm) (Thermo Fisher Scientific, San Jose, CA, USA) at a flow rate of 0.2 mL/min. The mobile phase consisted of 0.1 % formic acid in water (A) and 0.1 % formic acid in acetonitrile (B). The re-equilibration time was 10 min. Sample temperature was set at 4 °C and the column temperature was set at 40 °C. The injection volume was 2 μL. The gradient started with 10% B, then ramped to 95% B in 20 min and was held for 10 min. The mass scan range was set at m/z 100-1500 under electro-spray with positive ionization. The MS source parameters were as follows: sheath gas, 30 arbitrary unit; auxiliary gas and sweep gas, 5 arbitrary unit; spray voltage, 3.2 kv; capillary temperature, 320 °C.
UHPLC-HRAM MS raw files were processed using Nonlinear Progenesis QI (Durham, NC, USA) for peak detection, noise filtering, and peak alignment. A 2D data matrix was generated from Progenesis Qi, including variable index (paired m/z-retention time), sample names (observations), and peak intensities. Multivariate analysis was performed on the output table using SIMCA 13.0 (Sartorius Stedim Biotech, Umeå, Sweden). To obtain reliable, high-quality metabolomic data, a pooled urine sample (containing equal volumes from six 0 h urine samples from each volunteer) was used as a quality control (QC) sample. The QC sample was processed in the same way as other samples and was placed randomly in the sample queue to monitor stability of the system. All samples were run randomly and repeated in 4 different batches to minimize the batch effects and instrument variation. Structures were annotated by searching HMDB (http://www.hmdb.ca/) and Scripps Metlin (http://metlin.scripps.edu/), using exact mass and MS/MS spectra and structures.
2.6. Targeted metabolites analysis
For targeted metabolites analysis, the separation was carried out using the same column under the same HPLC conditions. A high-resolution selected ion monitoring (HR-SIM) method was used for targeted metabolites analysis. Resolution was set at 70,000 with max IT time of 50 ms. The scan width was set at 0.4 Da. Data were acquired under electrospray ionization with positive ionization mode. The m/z of targeted metabolites and the abbreviations used are listed in Table 1.
Table 1.
Targeted metabolites by selected ion monitoring of HRAM-MS
| Theoretical Mass [M+H]+ | Formula [M] | Metabolites Name | Time Range (min) | Abbreviation |
|---|---|---|---|---|
| 130.06513 | C9H7N* | indole-3-carbinol | 4-8 | I3C |
| 146.06004 | C9H7NO | indole-3-carboxaldehyde | 1.6-10 | I3CAL |
| 160.07569 | C10H9NO* | methoxyl-indole-3-carbinol | 2-8 | MI3C |
| 162.05496 | C9H7NO2 | indole-3-carboxylic acid | 3-8 | I3CA |
| 176.07061 | C10H9NO2 | methoxyl-indolealdehyde | 5-9 | MI3CAL |
| 178.03548 | C6H11NOS2 | sulforaphane | 1.2-6.5 | SFN |
| 192.06552 | C10H9NO3 | methoxyl-indole-3 carboxylic acid | 4.5-7.6 | MI3CA |
| 299.05523 | C9H18N2O3S3 | sulforaphane-cysteine | 1.2-6.5 | SFN-CYS |
| 306.09721 | C15H15NO6 | ascorbigen | 5.1-5.7 | ABG |
| 322.09213 | C15H15NO7 | hydroxy-ascorbigen | 3.9-5.5 | HABG |
| 336.10778 | C16H17NO7 | methoxyl-ascorbigen | 7-8.5 | MABG |
| 341.06580 | C11H20N2O4S3 | sulforaphane-N-acetyl-cysteine | 3-9 | SFN-N-CYS |
| 485.11929 | C16H28N4O7S3 | sulforaphane-glutathione | 7.4-7.9 | SFN-GSH |
[M-H2O]+
2.7. Method Validation
Matrix effects were examined by comparing peak areas obtained for blank urine or plasma samples spiked with sulforaphane(SFN), indole-3-carbinol(I3C), indole-3-carboxaldehyde(I3CAL) and indole-3-carboxylic acid(I3CA) to those of pure standard solutions containing the same amounts of the analytes. LOD and LOQ were calculated as 3.3 and 10 times the signal-to-noise ratio (S/N). A calibration plot with five standards was prepared and analyzed with each run.
2.8. Statistical Analysis.
Data are expressed as the mean value with the standard deviation (SD) of measurements (mean ± SD) from six subjects unless otherwise stated. Principal component analysis was performed using SIMCA 13.0.
3. Results and Discussion
3.1. Quantitative Analysis Individual and Total Glucosinolates in Broccoli
As shown in Fig.S1., a total of 10 glucosinolates were detected in broccoli and the total glucosinolate concentration was 14.67 μmol/g dry weight using the modified ISO-9167-1 method. Instead of using rapeseed oil as a control sample, High resolution accurate mass measurement was used to confirm desulfo-glucosinolates. The product ion at m/z 195.03 (C6H11O5S), representing the common thioglucose group, was used to confirm desulfo-forms of glucosinolates. In a previous broccoli-feeding studies (Bricker, Riedl, Ralston, Tober, Oberyszyn, & Schwartz, 2014; Charron, Vinyard, Ross, Seifried, Jeffery, & Novotny, 2018; Hauder, Winkler, Bub, Rufer, Pignitter, & Somoza, 2011) , glucoraphanin (4-methylsulfinylbutyl glucosinolate) metabolites, such as SFN and related conjugates, were investigated. However, indole glucosinolate-related metabolites are not widely covered, apart from perhaps glucobrassicin (Indol-3-ylmethyl glucosinolate, 3.15 μmol/g dry weight). The other major indole glucosinolates are neoglucobrassicin (1-methoxyindol-3-ylmethyl glucosinolate, 4.98 μmol/g dry weight) and 4-methoxyl-glucobrassicin (0.44 μmol/g dry weight). These two methoxyl glucobrassicin comprised 37% of total glucosinolates, so it is necessary to study these metabolites from methoxyl glucobrassicin broccoli after consumption.
3.2. Non-Targeted Metabolomics Analysis of Urine Samples
For the non-targeted metabolomic study, samples were submitted randomly for analysis to minimize variance from instrument performance. Intensity variances for the major ion features (ion feature was defined as the combination of retention time and mass to charge ratio or neutral molecular weight of a metabolite) in QC samples are shown in Table S2. Variation in these ion features across the QC samples was less than 15%. The reproducibility of chromatographs was determined based on retention time variation, which were generated by Progenesis QI; retention times deviated by less than 0.2 min overall. Based on this, differences between test samples from different subjects are more likely to reflect varied metabolite profiles than analytical variation. The multivariate analysis of QC samples showed that deviation of the analytical system was acceptable and differences in metabolite profiles between the groups were due to designed experimental factors.
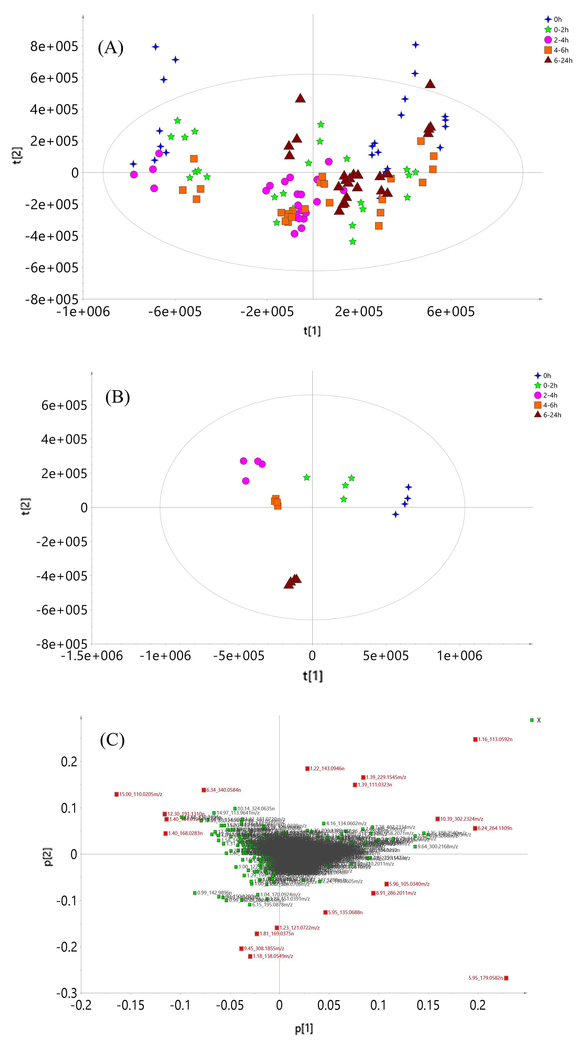
Principal component analysis (PCA) was employed to visualize sample clustering, trends and outliers among the observations. After preprocessing using Progenesis QI, the data matrix contained 3761 metabolite ion features in positive mode. The abundance of each ion feature was normalized to the total ion abundance and scaled using pareto scaling method (van den Berg, Hoefsloot, Westerhuis, Smilde, & van der Werf, 2006). Using this approach, there was no clear discrimination between samples collected at different time intervals. Further PCA was carried out on four analytical replicates and no clear separation was found, as shown in Fig. 1(A). These results suggest that variance came mostly from differences observed within each subject in response to the broccoli meal. Thus, the dataset was separated into 6 sub-datasets corresponding to each individual and re-examined.
Fig. 1.
The PCA score plot of all urine samples from six healthy subjects (A), from subject 3 (B), and the loading plot from subject 3, (C)
Using PCA, distinct clusters of samples formed reflecting individuals’ responses at different times, as shown in Fig. 1(B) and Fig.S2. It was also found that individuals had different metabolic trajectories in response to broccoli consumption (i.e. between individual differences as well as within-individual responses), as clustering trends were different. The inter-individual differences in the urinary metabolite profiles were considerable and suggested that comparing metabolite changes within each subject would be more informative.
A loading plot was used to select important ion features responsible for group separation. Nineteen ion features, marked as red squares in Fig. 1C, were determined to be important, based on an absolute loading value greater than 0.1 on either the first or second component. Among these, seven ion features were identified tentatively following searching of the open-source databases Metlin, HMDB and PubChem; tentative identifications are listed in Table 2. From the loading plot, it was worth noting that hippuric acid (5.95_179.0582n) had the highest loading and a similar changing trend among subjects after broccoli consumption. The control sample (hour 0) had the highest concentrations of hippuric acid, which decreased after broccoli consumption reaching the lowest point 2 to 4 hours after consumption, exception one subject (minimum occurred at 4-6 hours) and then increased up to 24 h (Fig.S3). Hippuric acid is thought to be related to consumption of fruits and vegetables in health children and adolescents in epidemiologic studies. The elevated level of hippuric acid were observed with the increase of fruits and vegetable juice consumption (Krupp, Doberstein, Shi, & Remer, 2012). In a following study by Penczynski and Krupp (Penczynski, Krupp, Bring, Bolzenius, Remer, & Buyken, 2017), the dietary flavonoids were believed to have the association with urinary hippuric acid excretion. However, these studies used 3-day weighed dietary records instead of controlled feeding, and no detailed information of fruits and vegetable were provided. In our study, we did not observe the increase of hippuric acid after broccoli meal. Besides hippuric acid, endogenous urinary metabolites, such as creatinine, uric acid and carnitines, were also detected.
Table 2.
The tentative identification of the important ion features in urine
| Ion Feature | Formula | Mass error (ppm) | Name | Database used for identification |
|---|---|---|---|---|
| 1.16_113.0592n* | C4H7N3O | 2.5 | Creatinine | Metlin, HMDB |
| 5.95_179.0582n | C9H9NO3 | −0.2 | Hippuric acid | Metlin, HMDB |
| 1.40_168.0283n | C5H4N4O3 | 0.0 | Uric acid | Metlin |
| 1.31_176.0320n | C6H8O6 | −0.1 | Ascorbic acid | Metlin, HMDB |
| 1.70_232.1543m/z | C11H21NO4 | −0.1 | Butyryl-L-carnitine or Isobutyryl carnitine | Metlin, HMDB |
| 6.24_264.1109n | C13H16N2O4 | −0.4 | Phenylacetylglutamine | Metlin, Pubchem |
| 10.39_302.2324m/z | C16H31NO4 | −0.6 | Dimethylheptanoyl carnitine | Metlin, HMDB |
n -- Neutral mass, means the ion features were deconvoluted into a neutral mass by Progenesis QI Metlin metlin.scripps.edu/ HMDB www.hmdb.ca/ Pubchem pubchem.ncbi.nlm.nih.gov
Changes in glucosinolate metabolites were not found using non-targeted metabolomic study, probably because variations were small compared to higher intensity endogenous metabolites and, thus, indistinguishable from noise.
3.3. Targeted Analysis of Metabolites of Glucoraphanin and Indole Glucosinolates
The non-targeted metabolomic approach, using a full scan MS method, lacked sensitivity. Thus, a more sensitive, specific, and precise method, based on selected ion monitoring with high resolution accurate mass spectrometry (HRSIM), was developed. Previous studies reported only metabolites from glucoraphanin and glucobrassicin metabolites, and did not include metabolites of 4-methoxyl glucobrassicin and 1-methoxyl glucobrassicin in their method (Hauder, Winkler, Bub, Rufer, Pignitter, & Somoza, 2011). As methoxyl glucobrassicins are also significant components in broccoli (37% of the total glucosinolates), the metabolites from these two indole glucosinolates were also taken into consideration in this study.
A time aligned HRSIM was optimized according to the retention behavior of metabolites on the C18 column. A ±0.005 Da window was used for reconstruction of selected ion monitoring chromatograms. The mass error for metabolites was less than 1 ppm.
Under optimum experimental conditions, SFN, I3C, I3CAL, and I3CA exhibited good linearities, sensitivities, and reproducibilities in urine and plasma. The correlation coefficient (r2) were all > 0.99. Limits of detection (LOD) and quantification (LOQ), and matrix effects are presented in Table S3. Due to the unavailability of commercial reference standards, sulforaphane mercapturic pathway metabolites were quantified as sulforaphane. Methoxyl indole carboxylic acid, methoxyl indole carbinol, and methoxyl indole carboxaldehyde were quantified using indole-3-carboxylic acid, indole-3-carbinol and indole-3-carboxaldehyde as reference standards, respectively. Ascorbigen and methoxyl-ascorbigen were quantified using indole-3-carbinol as reference standard. Methoxyl indole carboxylic acid, methoxyl indole carbinol, and methoxyl indole carboxaldhyde have several isomers and were quantified using the sum of isomer peak areas.
3.4. Glucosinolate Metabolites in Urine Samples
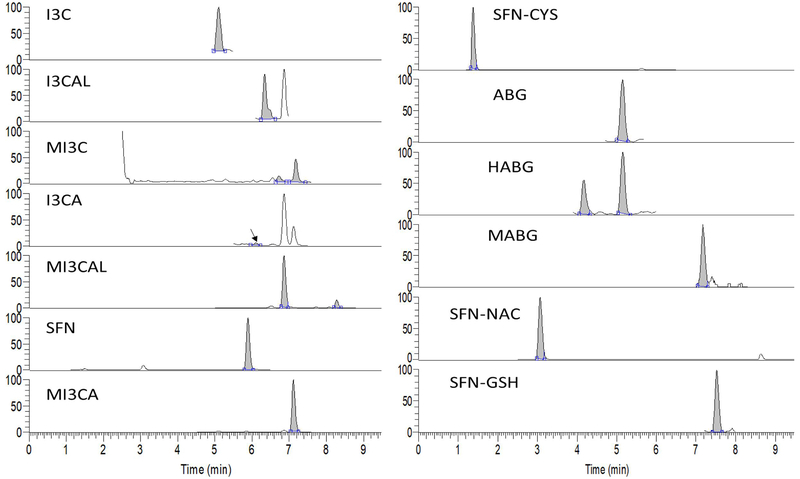
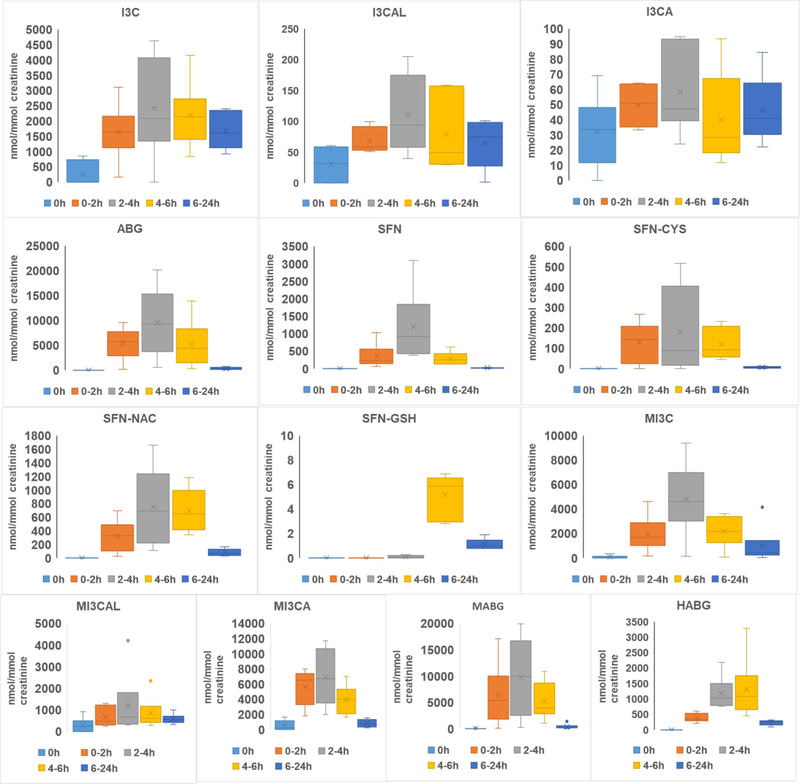
Fig. 2 shows typical SIM chromatograms for metabolites of glucoraphanin, glucobrassicin, and methoxyl-glucobrassicin. A total of 13 metabolites were detected. Sulforaphane (SFN), sulforaphane-cysteine (SFN-CYS), sulforaphane-N-acetyl-cysteine (SFN-NAC), and sulforaphane-glutathione (SFN-GSH) from glucoraphanin were detected in all but the fasted urine samples (t= 0). This is consistent with a previous report (Hauder, Winkler, Bub, Rufer, Pignitter, & Somoza, 2011), except no sulforaphane-cysteine-glycine was found in our study. Concentrations of metabolites were normalized against creatinine concentrations. Average concentrations of SFN and SFN metabolites reached their peak at 2-4 h, except for SFN-GSH at 4-6 h. SFN had a mean maximum concentration of 1197.80 ± 942.43 nmol/mmol at 2-4 h, while SFN-GSH had a very low concentration of 5.15 ± 1.62 nmol/mmol at its maximum (4-6 h). Indole-3-carbinol (I3C), indole-3-carboxaldehyde (I3CAL), and indole-3-carboxylic acid (I3CA) from glucobrassicin were also detected in the urine samples. It is worth noting that for I3C, the protonated [M+H]+ form was not observed, while the [M+H-H2O]+ form was observed as the major peak. This is consistent with a previous report (Kokotou, Revelou, Pappas, & Constantinou-Kokotou, 2017) and might explain why I3C was not detected by Hauder et al. (Hauder, Winkler, Bub, Rufer, Pignitter, & Somoza, 2011) in a broccoli feeding study where the [M+H]+ ion was used for selected ion monitoring in their targeted analysis.
Fig. 2.
HRSIM chromtograms of glucosinolate metabolites in human urine
Indole-3-acetonitrile (I3-ACN) and 3,3’-diindolylmethane were not detected in any urine samples. Notably, in the fasting urine samples, collected prior to broccoli intake, I3C, I3CAL, and I3CA were observed even though the subjects had been asked to avoid glucosinolates before the study. However, given I3-CAL has been identified as an endogenous metabolite in rat and human urine (van Haard, 1988), this might not indicate lack of compliance so much as natural variation in a small group of individuals related to a metabolic pathway where I3C is converted to I3CAL and I3CA via oxidative reaction. Consequently, glucobrassicin metabolites, specifically I3-CA and I3-CAL, may not be suitable dietary marker compounds for cruciferous vegetables. However, concentrations of these metabolites increased significantly after broccoli consumption. Ascorbigen was also detected in blank urine samples from two subjects but at very low concentrations, whereas it was the most abundant metabolite at 2-4 h (9631.13 ± 6285.15 nmol/mmol).
Based on the chemical structure of indole metabolites from glucobrassicin, it is reasonable to deduce that metabolites from methoxyl glucosinolates were methoxyl indole-3-carbinol (MI3C), methoxyl-indole-3-carboxaldehyde(MI3CAL), methoxyl-indole-3-carboxylic acid (MI3CA), and methoxyl-ascorbigen (MABG). Similar to glucobrassicin metabolites, these metabolites reached peak concentrations at 4-6 h, and MABG achieved the highest concentration 9824.68 ± 7145.73 nmol/mmol.
Hydroxyl-ABGs are reported for the first time and these metabolites may be derived from hydroxy glucosinolates in broccoli or produced via oxidation of ABG. However, due to the absence of reference standards for these compounds, we were not able to differentiate metabolites arising from isomeric 1-methoxyl-glucobrassicin and 4-methoxyl-glucobrassicin, as they share the same elemental compositions. The concentrations of metabolites in urine are shown in Fig. 3 and possible metabolic pathways were proposed for indole glucosinolates are shown in Fig.S4.
Fig. 3.
The concentration of glucosinolates metabolites in human urine (Normalized with creatinine level, nmol/mmol)
3.5. Glucosinolate Metabolites in Plasma samples
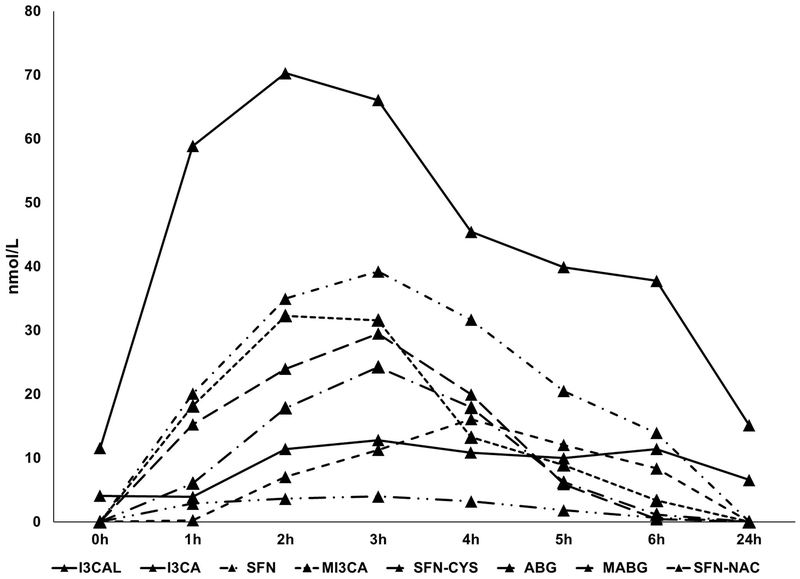
SFN, SFN-CYS, and SFN-NAC were the three major glucoraphanin metabolites detected in plasma samples. Concentrations reached maximum levels at 2-4 h, but were below the LOD at 24 h. The major glucobrassicin metabolites identified in plasma were I3-CAL, I3-CA, and ABG. I3CA was the predominant metabolite and reached its maximum at 2 h with a concentration of 70.30 ± 9.20 nmol/L. Methoxyl glucobrassicin metabolites detected in plasma were MI3CA and MABG, which had peak concentrations of 32.31 ± 27.98 and 29.49 ± 8.4 nmol/L, respectively. In comparison to metabolite concentrations in urine, plasma metabolites concentrations were much lower. Also, differences between subjects were significant for metabolites such as methoxyl-indole carboxylic acid and SFN-CYS. ABG or MABG were not detected at 0 h and reached their maxima at 3 h.
Our results suggest that glucosinolate metabolites, SFN, SFN-CYS, and SFN-N-CYS, can serve reliably as markers for intake of brassica vegetables containing glucoraphanin or sulforaphane. They did not occur in control samples (t= 0) but were the predominant metabolites in plasma after broccoli consumption. Indole metabolites, such as I3CAL, I3CA, or MI3CA from glucobrassicin or methoxyl glucobrassicin, were detected in the control samples (t= 0), but their concentrations were elevated 3 to 5-times after broccoli consumption. Derivatives of ABG or MABG, including 4-hydroxyascorbigen, 4-methoxyl ascorbigen, and (the N-methoxyl derivative) neoascorbigen, have also been reported previously in brassica vegetables such as broccoli and kale (Buskov, Hansen, Olsen, Sorensen, Sorensen, & Sorensen, 2000; Buskov, Olsen, Sorensen, & Sorensen, 2000; Ku, Becker, & Juvik, 2016; Ku, Jeffery, & Juvik, 2013, 2014). Therefore, ABG or MABG in plasma might originate not only from indole derivatives (degraded from indole glucosinolates via the mediation of myrosinase) that react with ascorbate (Wagner & Rimbach, 2009), but also from direct absorption. The time-concentration curves of metabolites in plasma are shown in Fig. 4.
Fig. 4.
The time and mean concentration curves of glucosinolate metabolites in human plasma following consumption of 200 g of uncooked broccoli florets (n=6 for hours 0-6; n=5 for 24-h samples due to one subject was unable to complete the blood draw)
3.6. Conclusion
In summary, metabolite profiles determined using non-targeted metabolomic studies can characterize responses to broccoli intake in humans. These profiles changed with time, after broccoli consumption, despite differential responses among subjects. The decrease observed in urinary hippuric acid might be associated with broccoli consumption. The very sensitive, high-resolution, accurate mass-mass spectrometry (HRAM-MS) needed for targeted analysis of glucosinolate metabolites was established and, for the first time, plasma metabolites from methoxyl glucosinolates are reported. The method detected multiple glucosinolate metabolites at the nM level in human urine and plasma after a single meal of uncooked broccoli.
Supplementary Material
Highlights.
Metabolomic analysis revealed changes in endogenous metabolites after broccoli consumption.
Metabolites from methoxyl-indole glucosinolates consumption are reported for the first time.
Targeted analysis HRAM-MS enabled detection of low concentrations of glucosinolate metabolites at nM level.
Acknowledgements
This research was supported by the Agricultural Research Service of the U.S. Department of Agriculture Project 8040-51000-056-00D and the Agricultural Research Service of the U.S. Department of Agriculture, an Interagency Agreement with the Office of Dietary Supplements at the National Institutes of Health.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Andersen MB, Rinnan A, Manach C, Poulsen SK, Pujos-Guillot E, Larsen TM, Astrup A, & Dragsted LO (2014). Untargeted metabolomics as a screening tool for estimating compliance to a dietary pattern. J Proteome Res, 13(3), 1405–1418. [DOI] [PubMed] [Google Scholar]
- Ares AM, Nozal MJ, Bernal JL, & Bernal J (2014). Optimized extraction, separation and quantification of twelve intact glucosinolates in broccoli leaves. Food Chemistry, 152, 66–74. [DOI] [PubMed] [Google Scholar]
- Arumugam A, & Abdull Razis AF (2018). Apoptosis as a Mechanism of the Cancer Chemopreventive Activity of Glucosinolates: a Review. Asian Pac J Cancer Prev, 19(6), 1439–1448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barba FJ, Nikmaram N, Roohinejad S, Khelfa A, Zhu ZZ, & Koubaa M (2016). Bioavailability of Glucosinolates and Their Breakdown Products: impact of Processing. Frontiers in Nutrition, 3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brennan L (2013). Metabolomics in nutrition research: current status and perspectives. Biochem Soc Trans, 41(2), 670–673. [DOI] [PubMed] [Google Scholar]
- Bricker GV, Riedl KM, Ralston RA, Tober KL, Oberyszyn TM, & Schwartz SJ (2014). Isothiocyanate metabolism, distribution, and interconversion in mice following consumption of thermally processed broccoli sprouts or purified sulforaphane. Mol Nutr Food Res, 55(10), 1991–2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buskov S, Hansen LB, Olsen CE, Sorensen JC, Sorensen H, & Sorensen S (2000). Determination of ascorbigens in autolysates of various Brassica species using supercritical fluid chromatography. J Agric Food Chem, 45(7), 2693–2701. [DOI] [PubMed] [Google Scholar]
- Buskov S, Olsen CE, Sorensen H, & Sorensen S (2000). Supercritical fluid chromatography as basis for identification and quantitative determination of indol-3-ylmethyl oligomers and ascorbigens. J Biochem Biophys Methods, 43(1-3), 175–195. [DOI] [PubMed] [Google Scholar]
- Charron CS, Vinyard BT, Ross SA, Seifried HE, Jeffery EH, & Novotny JA (2018). Absorption and metabolism of isothiocyanates formed from broccoli glucosinolates: effects of BMI and daily consumption in a randomised clinical trial. Br J Nutr, 120(12), 1370–1379. [DOI] [PubMed] [Google Scholar]
- Dettmer K, Aronov PA, & Hammock BD (2007). Mass spectrometry-based metabolomics. Mass Spectrom Rev, 26(1), 51–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Egner PA, Kensler TW, Chen JG, Gange SJ, Groopman JD, & Friesen MD (2008). Quantification of sulforaphane mercapturic acid pathway conjugates in human urine by high-performance liquid chromatography and isotope-dilution tandem mass spectrometry. Chem Res Toxicol, 21(10), 1991–1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuentes F, Paredes-Gonzalez X, & Kong AN (2015). Dietary Glucosinolates Sulforaphane, Phenethyl Isothiocyanate, Indole-3-Carbinol/3,3’-Diindolylmethane: Anti-Oxidative Stress/Inflammation, Nrf2, Epigenetics/Epigenomics and In Vivo Cancer Chemopreventive Efficacy. Curr Pharmacol Rep, 1(3), 179–196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guasch-Ferre M, Bhupathiraju SN, & Hu FB (2018). Use of Metabolomics in Improving Assessment of Dietary Intake. Clin Chem, 64(1), 82–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guertin KA, Moore SC, Sampson JN, Huang WY, Xiao Q, Stolzenberg-Solomon RZ, Sinha R, & Cross AJ (2014). Metabolomics in nutritional epidemiology: identifying metabolites associated with diet and quantifying their potential to uncover diet-disease relations in populations. Am J Clin Nutr, 100(1), 208–217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hauder J, Winkler S, Bub A, Rufer CE, Pignitter M, & Somoza V (2011). LC-MS/MS quantification of sulforaphane and indole-3-carbinol metabolites in human plasma and urine after dietary intake of selenium-fortified broccoli. J Agric Food Chem, 59(15), 8047–8057. [DOI] [PubMed] [Google Scholar]
- Holst B, & Williamson G (2004). A critical review of the bioavailability of glucosinolates and related compounds. Nat Prod Rep, 21(3), 425–447. [DOI] [PubMed] [Google Scholar]
- Kalpana Deepa Priya D, Gayathri R, Gunassekaran GR, Murugan S, & Sakthisekaran D (2013). Apoptotic role of natural isothiocyanate from broccoli (Brassica oleracea italica) in experimental chemical lung carcinogenesis. Pharmaceutical Biology, 51(5), 621–628. [DOI] [PubMed] [Google Scholar]
- Koal T, & Deigner HP (2010). Challenges in mass spectrometry based targeted metabolomics. Current Molecular Medicine, 10(2), 216–226. [DOI] [PubMed] [Google Scholar]
- Kokotou MG, Revelou PK, Pappas C, & Constantinou-Kokotou V (2017). High resolution mass spectrometry studies of sulforaphane and indole-3-carbinol in broccoli. Food Chemistry, 237, 566–573. [DOI] [PubMed] [Google Scholar]
- Koulman A, & Volmer DA (2008). Perspectives for Metabolomics in Human Nutrition: An Overview. Nutr Bull, 33(4), 324–330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krupp D, Doberstein N, Shi L, & Remer T (2012). Hippuric acid in 24-hour urine collections is a potential biomarker for fruit and vegetable consumption in healthy children and adolescents. J Nutr, 142(7), 1314–1320. [DOI] [PubMed] [Google Scholar]
- Ku KM, Becker TM, & Juvik JA (2016). Transcriptome and Metabolome Analyses of Glucosinolates in Two Broccoli Cultivars Following Jasmonate Treatment for the Induction of Glucosinolate Defense to Trichoplusia ni (Hubner). Int J Mol Sci, 17(7). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ku KM, Jeffery EH, & Juvik JA (2013). Influence of seasonal variation and methyl jasmonate mediated induction of glucosinolate biosynthesis on quinone reductase activity in broccoli florets. J Agric Food Chem, 61(40), 9623–9631. [DOI] [PubMed] [Google Scholar]
- Ku KM, Jeffery EH, & Juvik JA (2014). Exogenous methyl jasmonate treatment increases glucosinolate biosynthesis and quinone reductase activity in kale leaf tissue. PLoS One, 9(8), e103407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lippmann D, Lehmann C, Florian S, Barknowitz G, Haack M, Mewis I, Wiesner M, Schreiner M, Glatt H, Brigelius-Flohe R, & Kipp AP (2014). Glucosinolates from pak choi and broccoli induce enzymes and inhibit inflammation and colon cancer differently. Food Funct, 5(6), 1073–1081. [DOI] [PubMed] [Google Scholar]
- Mithen R, Faulkner K, Magrath R, Rose P, Williamson G, & Marquez J (2003). Development of isothiocyanate-enriched broccoli, and its enhanced ability to induce phase 2 detoxification enzymes in mammalian cells. Theor Appl Genet, 106(4), 727–734. [DOI] [PubMed] [Google Scholar]
- Munday R, & Munday CM (2002). Selective induction of phase II enzymes in the urinary bladder of rats by allyl isothiocyanate, a compound derived from Brassica vegetables. Nutr Cancer, 44(1), 52–59. [DOI] [PubMed] [Google Scholar]
- Penczynski KJ, Krupp D, Bring A, Bolzenius K, Remer T, & Buyken AE (2017). Relative validation of 24-h urinary hippuric acid excretion as a biomarker for dietary flavonoid intake from fruit and vegetables in healthy adolescents. Eur J Nutr, 56(2), 757–766. [DOI] [PubMed] [Google Scholar]
- Platz S, Piberger AL, Budnowski J, Herz C, Schreiner M, Blaut M, Hartwig A, Lamy E, Hanske L, & Rohn S (2015). Bioavailability and biotransformation of sulforaphane and erucin metabolites in different biological matrices determined by LC-MS-MS. Anal Bioanal Chem, 407(7), 1819–1829. [DOI] [PubMed] [Google Scholar]
- Primrose S, Draper J, Elsom R, Kirkpatrick V, Mathers JC, Seal C, Beckmann M, Haldar S, Beattie JH, Lodge JK, Jenab M, Keun H, & Scalbert A (2011). Metabolomics and human nutrition. Br J Nutr, 105(8), 1277–1283. [DOI] [PubMed] [Google Scholar]
- Sun J, Kou L, Geng P, Huang H, Yang T, Luo Y, & Chen P (2015). Metabolomic vassessment reveals an elevated level of glucosinolate content in CaCl(2) treated broccoli microgreens. J Agric Food Chem, 63(6), 1863–1868. [DOI] [PubMed] [Google Scholar]
- Tang L, Paonessa JD, Zhang YS, Arnbrosone CB, & McCann SE (2013). Total isothiocyanate yield from raw cruciferous vegetables commonly consumed in the United States. Journal of Functional Foods, 5(4), 1996–2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tian Q, Rosselot RA, & Schwartz SJ (2005). Quantitative determination of intact glucosinolates in broccoli, broccoli sprouts, Brussels sprouts, and cauliflower by high-performance liquid chromatography-electrospray ionization-tandem mass spectrometry. Anal Biochem, 343(1), 93–99. [DOI] [PubMed] [Google Scholar]
- Traka M, Gasper AV, Melchini A, Bacon JR, Needs PW, Frost V, Chantry A, Jones AM, Ortori CA, Barrett DA, Ball RY, Mills RD, & Mithen RF (2008). Broccoli consumption interacts with GSTM1 to perturb oncogenic signalling pathways in the prostate. PLoS One, 3(7), e2568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Troyer JK, Stephenson KK, & Fahey JW (2001). Analysis of glucosinolates from broccoli and other cruciferous vegetables by hydrophilic interaction liquid chromatography. J Chromatogr A, 919(2), 299–304. [DOI] [PubMed] [Google Scholar]
- van den Berg RA, Hoefsloot HC, Westerhuis JA, Smilde AK, & van der Werf MJ (2006). Centering, scaling, and transformations: improving the biological information content of metabolomics data. BMC Genomics, 7, 142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Haard PM (1988). Chromatography of urinary indole derivatives. J Chromatogr, 429, 59–94. [DOI] [PubMed] [Google Scholar]
- van Poppel G, Verhoeven DT, Verhagen H, & Goldbohm RA (1999). Brassica vegetables and cancer prevention. Epidemiology and mechanisms. Adv Exp Med Biol, 472, 159–168. [DOI] [PubMed] [Google Scholar]
- Verhoeven DT, Goldbohm RA, van Poppel G, Verhagen H, & van den Brandt PA (1996). Epidemiological studies on brassica vegetables and cancer risk. Cancer Epidemiol Biomarkers Prev, 5(9), 733–748. [PubMed] [Google Scholar]
- Verkerk R, Schreiner M, Krumbein A, Ciska E, Holst B, Rowland I, De Schrijver R, Hansen M, Gerhauser C, Mithen R, & Dekker M (2009). Glucosinolates in Brassica vegetables: the influence of the food supply chain on intake, bioavailability and human health. Mol Nutr Food Res, 53 Suppl 2, S219. [DOI] [PubMed] [Google Scholar]
- Wagner AE, & Rimbach G (2009). Ascorbigen: chemistry, occurrence, and biologic properties. Clin Dermatol, 27(2), 217–224. [DOI] [PubMed] [Google Scholar]
- Wang H, Lin W, Shen G, Khor TO, Nomeir AA, & Kong AN (2011). Development and validation of an LC-MS-MS method for the simultaneous determination of sulforaphane and its metabolites in rat plasma and its application in pharmacokinetic studies. J Chromatogr Sci, 49(10), 801–806. [DOI] [PubMed] [Google Scholar]
- Wang LI, Giovannucci EL, Hunter D, Neuberg D, Su L, & Christiani DC (2004). Dietary intake of Cruciferous vegetables, Glutathione S-transferase (GST) polymorphisms and lung cancer risk in a Caucasian population. Cancer Causes Control, 15(10), 977–985. [DOI] [PubMed] [Google Scholar]
- Weng JR, Tsai CH, Kulp SK, & Chen CS (2008). Indole-3-carbinol as a chemopreventive and anti-cancer agent. Cancer Letters, 262(2), 153–163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ye L, & Zhang Y (2001). Total intracellular accumulation levels of dietary isothiocyanates determine their activity in elevation of cellular glutathione and induction of Phase 2 detoxification enzymes. Carcinogenesis, 22(12), 1987–1992. [DOI] [PubMed] [Google Scholar]
- Zhang Y, Cho CG, Posner GH, & Talalay P (1992). Spectroscopic quantitation of organic isothiocyanates by cyclocondensation with vicinal dithiols. Anal Biochem, 205(1), 100–107. [DOI] [PubMed] [Google Scholar]
- Zheng H, Clausen MR, Dalsgaard TK, & Bertram HC (2015). Metabolomics to Explore Impact of Dairy Intake. Nutrients, 7(6), 4875–4896. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.