Abstract
The effect of high-pressure processing (HPP) on Listeria monocytogenes, the indigenous microbiota and the shelf-life of chicken fillets was evaluated. Chicken fillets were inoculated with different inocula (2, 4, and 6 log CFU/g) of a 4-strain cocktail of L. monocytogenes, vacuum-packed, processed or not with HPP (500 MPa/10 min) and stored at 4 °C and 12 °C. Total viable counts (TVC), L. monocytogenes, Pseudomonas spp., Brochothrix thermosphacta, lactic acid bacteria (LAB), Enterobacteriaceae and yeasts/molds were determined along with the pH and sensory analysis. Pulsed-field gel electrophoresis (PFGE) was used to monitor the succession of indigenous Brochothrix isolates and inoculated Listeria strains. The main spoilage microorganism of HPP-treated samples was B. thermosphacta detected after 3 days of storage. HPP decreased the inoculated Listeria population. For the low and medium inoculum case it was detected throughout the shelf-life at both temperatures in populations near to the detection limit or after enrichment. In the high inoculum case, the pathogen decreased ≥5-log cycles after HPP, while increased subsequently to 1.6 and 4.5 log CFU/g at 4 °C and 12 °C, respectively, by the end of the shelf-life. PFGE showed that Brochothrix isolates exhibited a significant diversity among control samples, whereas this was limited for the HPP-treated samples. The survival and distribution of different Listeria strains depended on the initial inoculum and storage temperature. In conclusion, HPP increased the shelf-life (for 5 and 4 days, at 4 °C and 12 °C, respectively) and enhanced the safety of chicken meat.
Keywords: high-pressure processing, Listeria monocytogenes, Brochothrix thermosphacta, PFGE, poultry, safety, quality
1. Introduction
It is well established that poultry meat is a highly perishable food with a short shelf-life limited from 4 to 15 days under refrigeration, depending mostly on the packaging type used [1]. Microbial contamination on fresh chicken meat is diversified and mostly depends on the poultry carcass quality, the slaughtering process and the environmental microbiota [2]. Spoilage of poultry is caused only by a specific microbial association the “ephemeral/specific spoilage micro-organisms E(S)SO,” which can be introduced to the meat during processing, transportation, and storage in the market [3,4]. The metabolic activity of the specific microbiota leads to changes dependent on the availability of energy substrates i.e., low molecular weight compounds (glucose, lactate, amino acids, etc.) of meat and spoilage prevails because of accumulation of metabolic by-products [4,5]. Brochothrix thermosphacta can be recognized as a common spoilage microorganism often found in poultry meat [6]. B. thermosphacta is capable of growing at a wide range of pH (5–9), temperature (0–30 °C), or NaCl (up to 10%) and is thought to be a ubiquitous microorganism throughout the meat production chain [7]. It is responsible for spoilage of meat stored under aerobic, modified atmosphere packaging (MAP) or/and vacuum packaged conditions and can become the dominant microbiota leading to souring of the meat. During spoilage of meat, Brochothrix can utilize glucose and produce unpleasant off-flavors, like cheesy odors responsible for a distinct type of meat spoilage [4,8].
Apart from the E(S)SO microbiota, poultry meat might contain endogenous pathogens like Listeria monocytogenes, Campylobacter spp., Staphylococcus aureus, or Salmonella enterica and raw poultry products have been responsible for a significant number of cases of food poisoning to humans, especially when contaminated with Campylobacter spp., or Salmonella enterica [9,10,11]. However, since the incidence of L. monocytogenes in chicken meat is not required to be reported to EFSA, little information is available about the prevalence in chicken meat [11]. A recent meta-analysis study reported that L. monocytogenes was frequently found in chicken meat (prevalence of about 21%) at the industry or retail level and for both packed or unpacked products [11]. L. monocytogenes has the capacity to form biofilms and the ability to withstand acids and detergents, is resistant to chemical biocides, which result in the extended persistence of this pathogen in the industrial equipment and environment [12,13,14]. Therefore, it is of importance to perform risk assessment studies to help reveal what may be done to reduce or eliminate the pathogen prevalence in chicken meat.
High-pressure processing (HPP) is an effective nonthermal preservation technology, which is being used as an alternative food preservation method to improve food safety, to control food spoilage, and to extend the shelf-life with minimal changes in the nutritional, functional, or sensorial characteristics of the original food material [15,16,17]. Up to date, a variety of HPP products, such as ready to eat (RTE) meats, fruit juices, packaged vegetables, sea food, dairy products, sliced ham and meat are commercially available [13,15]. Depending on the product microbiota, the pressure levels that are usually used in the meat industry are between 400–600 MPa for a relatively short processing time [18,19]. Although many studies have been conducted for measuring the effect of HPP on the spoilage microbiota of red meat and meat products [20,21,22], limited information is available for raw chicken meat. In addition, the complexity of the reactions taking place in a biological system such as meat, makes it difficult to predict the effect of HPP treatments on meat microbiota, since the resistance of the different microorganisms present in meat is variable and depends on many factors (type of bacteria, food matrix, etc.,) [23]. Liu et al. [24] have investigated the pressure resistance of inoculated Campylobacter jejuni and Escherichia coli strains with or without the presence of the specific spoilage microbiota of poultry meat on aseptically prepared minced poultry meat using pressure of 400 MPa for 30 min holding time at 40 °C. The researchers reported that at this pressure level the cell counts of the inoculated (7–8 log CFU/g initial inoculum level) microorganisms (B. thermosphacta, Carnobacterium divergens, Campylobacter jejuni, and Pseudomonas fluorescens) were found below the detection limit after pressure, except the E. coli strain which was reduced by 3.5 log CFU/g. In spite of that, in a previous study by Argyri et al. [25] it was shown that the main spoilage microorganism after HPP (500 MPa/10 min) on chicken fillets was B. thermosphacta. Furthermore, many research articles have focused on the behavior of L. monocytogenes in meat products after HPP [26,27,28], but only a few studies have focused on chicken meat [6,29]. Therefore, it is of great importance to monitor the behavior of the pathogen after HPP on fresh chicken fillets and during shelf-life, because any cells that might survive HPP, could potentially recover and grow during storage of the product because of the psychrotrophic nature of L. monocytogenes.
In this context, the current study was designed to monitor the survival and growth of the indigenous Brochothrix thermosphacta and the inoculated L. monocytogenes strains after the application of HPP (500 MPa/10 min/18–20 °C) and storage of vacuum-packaged chicken fillets stored at 4 °C and 12 °C. Moreover, the shelf-life of the product was determined by sensory analysis of non-inoculated samples. Finally, the strain distribution of Brochothrix isolates and the inoculated L. monocytogenes were evaluated by using molecular tools.
2. Materials and Methods
2.1. Experimental Design and Preparation of Chicken Fillet Samples
Chicken breast fillets of two different batches were obtained from the local meat industry and transported under refrigeration to the laboratory with minimal delay, where they were held at 1 °C for 1–2 h. The meat was then aseptically cut and weighted into portions of 30 g and subsequently inoculated with L. monocytogenes (cocktail culture of four strains) as is described at Section 2.2. A portion of samples was not inoculated with the pathogen and served as samples for sensory analysis. All samples were packed under vacuum into plastic pouches (100 mm wide–100 mm long, 90 mm thickness), of O2 permeability of ca. 75 mL/m2/24 h/1 atm at 23 °C and 75% relative humidity of ca. 75 cc/m2/24 h/1 atm (Flexo–Pack S.A., Athens, Greece), using a HenkoVac 1700Machine (Howden Food Equipment B.V., The Netherlands). Half of the prepared samples were treated with HPP of 500 MPa for 10 min. All samples were stored at 4 °C and 12 °C in high precision (±0.5 °C) incubation chambers (VELP Scientifica Srl, Usmate Velate MB, Milan, Italy), for up to 14 days, until spoilage was pronounced (discoloration and presence of off–odors) and non-inoculated samples were characterized from sensory analysis as spoiled. Microbiological analysis was assessed in parallel with molecular analysis and sensory evaluation of non-inoculated samples, as is already described by Argyri et al. [25].
2.2. Inoculation of the Chicken Fillet Samples
Four strains of L. monocytogenes (FMCC–B–128, FMCC–B–129, FMCC–B–131, FMCC–B–133) were used as a cocktail to inoculate the chicken fillets at three different inoculum levels (2, 4, and 6 log CFU/g). The strains were provided by the Food Microbiology Culture Collection (FMCC), Laboratory of Microbiology and Biotechnology of Foods, Agricultural University of Athens and all strains originated from Greek food industries (FMCC–B–128 and FMCC–B–133 were isolated from soft cheese, FMCC–B–129 was isolated from RTE minced meat based frozen meal and FMCC–B–131 was isolated from conveyor belt of RTE frozen food). The strains were activated from a stock culture stored at −80 °C and were grown overnight at 37 °C in 10 mL of brain heart infusion broth (BHI, LAB049, LabM, Lancashire, UK). Each strain was harvested by centrifugation at 10,000× g for 5 min, washed twice with ¼ strength Ringers solution (Ringers solution tablets, 96724–100TAB, Merck, Darmstadt, Germany) and resuspended in 10 mL Ringers solution in a final concentration of approximately 9 log CFU/mL. To prepare the cocktail culture of the four strains, each strain was serially diluted on the decimal scale with ¼ strength Ringers solution and then all strains were mixed in equal volumes to give final concentrations of 2, 4 and 6 log CFU/g. Chicken fillets were then inoculated with the three inoculation levels and packed under vacuum. Half of the prepared samples were treated with HPP. To verify the counts of the inocula, the same dilutions were spread-plated on Palcam agar (Palcam agar, BK145HA, Biokar Diagnostics, Allonne, France).
2.3. High-Pressure Processing (HPP)
HPP experiments were conducted by applying 500 MPa for 10 min at room temperature (18–20 °C). Further details of the HPP treatment and the selection of 500 MPa/10 min, can be found at the study by Argyri et al. [25]. After the HPP treatment, the samples were divided according to their origin from the two different meat batches/different level of inoculum/HPP-treated samples/control samples (without HPP treatment) and then stored at 4 °C and 12 °C in vacuum packaging.
2.4. Microbiological and pH Analyses
Microbiological and pH analyses were carried out until the end of storage at 4 °C and 12 °C. From each sampling case, three packages of inoculated samples were subjected to microbiological and pH analyses and three additional packages were used for the enrichment method. Simultaneously, three samples from the non-inoculated samples were implemented in sensory evaluation. To estimate the number of viable cells, chicken fillet samples (25 g) were weighed aseptically, added to sterile ¼ Ringers solution (50 mL), and homogenized in a stomacher (Stomacher 400 Circulator, Seward Limited, Norfolk, UK) for 60 s at room temperature according to ISO 7218:2013. Serial decimal dilutions were prepared with the Ringers solution and duplicate 1 or 0.1 mL samples of the appropriate dilutions were mixed or spread on non–selective and selective media. To reduce the detection limit of the enumeration method (for spread plating) to 0.48 log CFU/g, 1 mL of the first decimal dilution was spread equally on three agar plates of each substrate. The studied agar media were the following: plate count agar (CM0325, Oxoid, Basingstoke, UK) for the enumeration of total viable counts (TVC), incubated at 30 °C for 48–72 h; de Man–Rogosa–Sharp (MRS) medium (CM 0361, Oxoid, Basingstoke, UK) adjusted to pH 5.7, overlaid with the same medium for the enumeration of LAB incubated at 30 °C for 48–72 h; streptomycin thallous acetate-actidione agar (STAA, 4020792, Biolife, Milano, Italy) with STAA selective supplement (4240052, Biolife, Milano, Italy) for the enumeration of Brochothrix thermosphacta, incubated at 25 °C for 72 h; rose bengal chloramphenicol agar base (LAB036, LAB M, Lancashire, UK) with selective supplement (X009, LAB M, Lancashire, UK) for the enumeration of yeasts and molds incubated at 25 °C for 48–72 h; violet red bile glucose agar (CM 0485, Oxoid, Basingstoke, UK) overlaid with the same medium, for the enumeration of Enterobacteriaceae incubated at 37 °C for 24 h; Pseudomonas agar base (CM559, Oxoid, Basingstoke, UK) supplemented with CFC selective supplement (SR0103, Oxoid, Basingstoke, UK), for the enumeration of Pseudomonas spp. incubated at 25 °C for 48 h; Palcam agar (BK145HA, Biokar Diagnostics, Allonne, France) supplemented with Palcam selective supplement (BS00408, Biokar Diagnostics, Allonne, France) for the enumeration of L. monocytogenes incubated at 37 °C for 24 and 48 h. For the HPP-treated samples, incubation time was extended by 1–2 days in all growth media to allow recovery of lethally/sublethally injured or stressed by HPP treatment cells. For the samples inoculated with L. monocytogenes, enrichment was performed according to ISO 11290–1:1996/Amnd1:2004 for L. monocytogenes. To ensure the absence of the three major pathogens at the non-inoculated samples, the samples were analyzed periodically throughout storage using the enrichment methods for Salmonella spp. (ISO 6579: 2002/Cor.1:2004), E. coli O157:H7 (ISO 16654:2001) and L. monocytogenes (ISO 11290–1:1996/Amd 1:2004).
The pH values were measured throughout storage with a digital pH meter (HI 2211 pH–ORP Meter, HANNA Instruments, Smithfield, RI, USA), by immersing the electrode in the meat homogenate (stomacher homogenate) after the microbiological analysis.
2.5. Isolation and Growth of Listeria sp. and Brochothrix sp.
Chicken meat was sampled at specific time intervals depending on the storage temperature. Listeria colonies were randomly isolated from plates that corresponded to the beginning (day 0) and final storage time for the two storage temperatures. From each of the aforementioned samplings, 20% of the colonies (i.e., 10–20 colonies) were randomly collected from the appropriate countable dilution on Palcam agar plates. When the pathogen was not detected using the enumeration method, random colonies (i.e., 6–8, depending on the purity) were isolated from the Palcam petri dishes from the enrichment method. Brochothrix colonies were randomly isolated from STAA plates that corresponded to the beginning (day 0) and to the end of the storage period for control cases of 4 °C and 12 °C, while for HPP-treated samples Brochothrix isolates were picked from the third day of storage and at the end of the storage period for both temperatures. Day 3 was the first day that colonies of Brochothrix were detected. From each of the aforementioned samplings, 20% of the colonies (i.e., 10–20 colonies) were randomly collected from the appropriate countable dilution of STAA plates. The purified cultures were stored at −80 °C in BHI broth supplemented with 20% (v/v) glycerol (Serva, Heidelberg, Germany). Before experimental use, each isolate was grown twice in BHI at 37 °C for 16 h and 25 °C for 24 h for Listeria and Brochothrix isolates respectively, while the purity of the cultures was always checked in BHI agar plates before use. The isolates (294 isolates for Listeria and 110 isolates for Brochothrix) were subsequently screened with pulsed-field gel electrophoresis (PFGE) as described in Section 2.6.
2.6. Pulsed-Field Gel Electrophoresis
The PFGE was applied to monitor the survival and distribution of each inoculated L. monocytogenes strain and also to monitor the succession of Brochothrix sp. isolates in the control and HPP-treated samples during storage at 4 °C and 12 °C. PFGE was performed according to Papadopoulou et al. [30]. The restriction enzyme ApaI (10 U) (New England Biolabs, Ipswich, MA, USA) was used for both Listeria and Brochothrix isolates, following manufacturer’s recommendation for 16 h. The restriction fragments were separated by pulsed–field gel electrophoresis on CHEF–DRII equipment (BIO–RAD, Hercules, CA, USA) in 1% (w/v) PFGE grade agarose gel (BIO–RAD, Hercules, CA, USA) in 0.5 mM tris–borate buffer and the running parameters were the following: 6 V/cm, 4 s initial switching time, 40 s final switching time and a 18 h of total run at 14 °C. Gel was stained with ethidium bromide (0.5 μg/mL) (Sigma, Missouri, USA) and bands were visualized using GelDoc system (BIO–RAD, Hercules, CA, USA). The PFGE fingerprints of L. monocytogenes FMCC–B128, FMCC–B129, FMCC–B131, FMCC–B133 strains were used as reference strains to compare the obtained restriction profiles of the Listeria isolates. For Brochothrix isolates, representative isolates per distinct PFGE cluster were subjected to species identification by sequencing the V1–V3 variable region of the 16S rRNA gene according to Doulgeraki et al. [31]. PCR products were purified using the QIAquick® PCR Purification Kit (Qiagen, Hilden, Germany) according to the manufacturer’s instructions and sent for sequencing to CeMIA, (Department of Immunology and Histocompatibility, Faculty of Medicine, University of Thessaly, Volos, Greece). Obtained results of the 16S rRNA gene sequence were aligned with those in GenBank using the program BLAST to determine their closest known relatives to the partial 16S rRNA gene (Table S1).
2.7. Sensory Analysis
The sensory evaluation of the chicken fillets was performed throughout storage as previously reported [25], by a five-membered sensory panel (trained staff from the laboratory of Institute of Technology of Agricultural Products, HAO–DEMETER) at the same time intervals as for microbiological analyses. The same sensory panel was used in all evaluations and was unaware of the tested sample. The sensory assessment was conducted in triplicate under artificial light in individual booths in a special sensory analysis room allocated in the Institute of Technology of Agricultural Products and meat samples were left to reach ambient temperature prior to analysis. In brief, the sensory panel evaluated the color, smell, and taste (after cooking) of the meat in a five-point hedonic scale. Scores ranged from 1 (fresh) to 3 (unacceptable). Scores above 2 were given to characterize the meat as spoiled and indicated the end of shelf-life. No score was given by the panelists when differentiation in the sensory characteristics of the product were not linked to spoilage attributes (e.g., in the basic characteristics of the product), but to HPP treatment.
2.8. Statistical Analysis
All experiments were carried out in triplicate with two independent batches of chicken fillets each. Significance was established at p < 0.05. Results were analyzed for statistical significance with analysis of variance (ANOVA). Duncan’s multiple range test was used to determine the significant differences among results [coefficients, ANOVA tables and significance (p < 0.05) were computed using Statistica v.5.0 (Statsoft Inc., Tulsa, OK, USA)].
3. Results
3.1. Population Dynamics/Inactivation of the Indigenous Microbiota
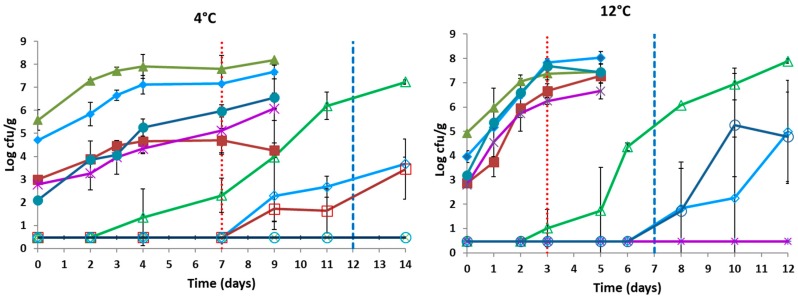
The changes of the indigenous microbiota in raw chicken fillets untreated or treated with HPP (500 MPa/10 min) during storage under vacuum package at 4 °C and 12 °C is presented in Figure 1. For the untreated samples (control), the microbiological analysis showed that the initial population of the indigenous microbiota on chicken samples stored at 4 °C and 12 °C consisted of B. thermosphacta (5.25 ± 0.41 log CFU/g), Pseudomonas spp. (4.26 ± 0.44 log CFU/g), yeasts/molds (2.92 ± 0.14 log CFU/g), LAB (2.79 ± 0.23 log CFU/g), and Enterobacteriaceae (2.65 ± 0.64 log CFU/g). The population of all the microbial groups on the untreated samples (without HPP) increased during storage at both temperatures, with B. thermosphacta (increase of 2.61 and 2.52 log CFU/g, at 4 °C and 12 °C, respectively) being the dominant microbiota followed by Pseudomonas spp. (increase of 2.96 and 4.07 log CFU/g, at 4 °C and 12 °C, respectively), Enterobacteriaceae (increase of 4.45 and 4.23 log CFU/g, at 4 °C and 12 °C, respectively), yeasts/molds (increase of 1.27 and 4.41 log CFU/g, at 4 °C and 12 °C, respectively), and LAB (increase of 3.30 and 3.86 log CFU/g at 4 °C and 12 °C, respectively) (Figure 1). Only after the end of the product’s shelf-life at 12 °C (third day of storage), Pseudomonas spp. and Enterobacteriaceae exhibited population numbers similar or higher than those of B. thermosphacta (Figure 1).
Figure 1.
Growth curves of B. thermosphacta (▲) or (△), Pseudomonas spp. (♦) or (◊), LAB (*) or (x), Enterobacteriaceae (●) or (○), yeasts/molds (■) or (□), on untreated (closed symbols), or HPP-treated (500 MPa/10 min) (open symbols) chicken fillets, respectively, during storage under vacuum package at 4 °C (left) and at 12 °C (right). Vertical red dotted line: end of the control samples shelf-life according to sensory analysis. Vertical blue dashed line: end of the HPP-treated samples shelf-life according to sensory analysis. Error bars represent the mean values ± standard deviation.
The population dynamics of the aforementioned microbial groups and their contribution to the final microbiota were greatly affected by the application of HPP (500 MPa/10 min), as exemplified by the evolution of all microbial counts. In brief, the application of HPP on chicken meat resulted in the reduction of all the microbial counts below the detection limit of the enumeration method, reaching a total reduction of ≥ 5 log cycles (Figure 1). During storage of the HPP-treated samples at both temperatures, the main spoilage microorganism was B. thermosphacta, whereas the population of the rest microbial groups was found below the detection limit of the enumeration method. However, toward and after the end of the products shelf-life a minor increase of Pseudomonas and yeasts/molds at 4 °C and Pseudomonas and Enterobacteriaceae at 12 °C was observed (Figure 1). B. thermosphacta was detected after the third day of storage at both temperatures and increased until the end of the storage period (Figure 1). Nevertheless, the final counts of Brochothrix population were found lower compared to those of all of the microbial groups in the control samples. Finally, the estimated shelf-life of HPP samples was 12 days at 4 °C and 7 days at 12 °C, where the counts of B. thermosphacta were estimated at 6 log CFU/g and 5 log CFU/g at 4 °C and 12 °C, respectively. In contrary, the end of shelf-life in control samples was after 7 days at 4 °C and 3 days at 12 °C, where Brochothrix population reached over 7 log CFU/g at both temperatures.
The initial pH values of chicken fillets stored at 4 °C were 5.82 ± 0.03 and 6.05 ± 0.05 for the control and the HPP–treated samples, respectively, while the corresponding values for 12 °C were 5.96 ± 0.05 and 6.14 ± 0.06, demonstrating a small increase of ca. 0.2 units (p < 0.05) after the application of HPP. During storage, pH values remained similar to the initial pH within the same treatment (p > 0.05). Nevertheless, the changes in the pH values between the different treatments were found to vary significantly during storage (p < 0.05), ranging between 0.1–0.23 (Figure S1).
3.2. Population Dynamics/Inactivation of Listeria monocytogenes
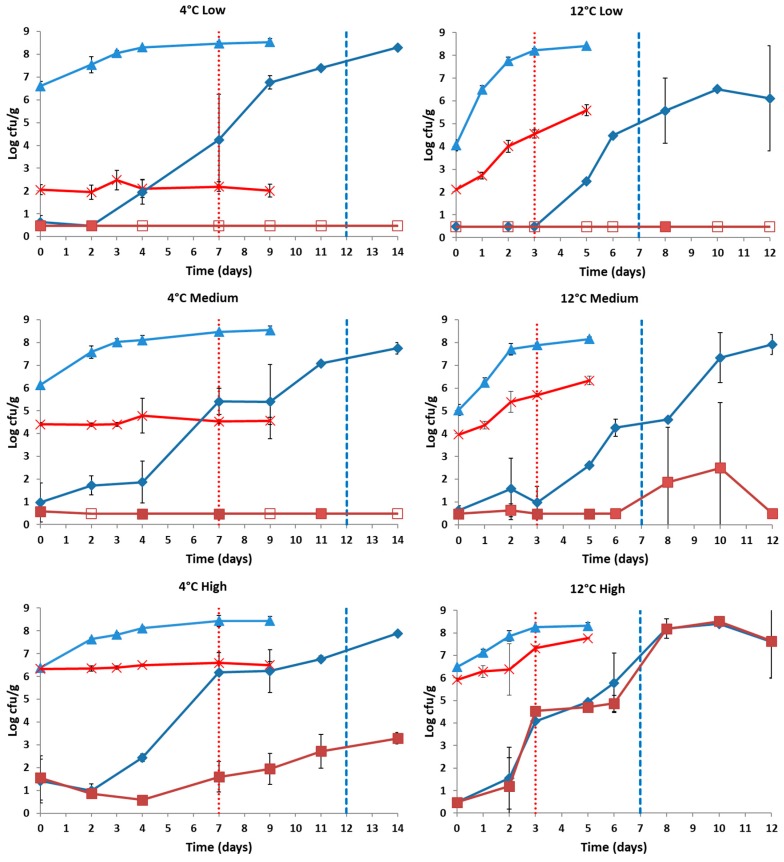
The survival of the pathogen L. monocytogenes (cocktail culture of four strains) inoculated in three different initial levels in both control and HPP-treated samples followed by storage in vacuum packaging at 4 °C and 12 °C is presented in Figure 2. Regarding the samples without HPP treatment (control), the population of the pathogen at all inocula (2, 4, and 6 log CFU/g) remained similar to the initial inoculum level in all cases during storage at 4 °C. For the samples stored at 12 °C, results showed that the population of the pathogen for the three different inocula cases showed an increase of 1.85–3.48 log CFU/g, depending on the case (Figure 2).
Figure 2.
Growth curves of TVC on untreated (▲) or HPP–treated (500 MPa/10 min) (♦) chicken fillets and of L. monocytogenes on untreated (x) or HPP–treated (500 MPa/10 min) (■) chicken fillets inoculated with low, medium, and high inoculum level of a 4-strain cocktail of Listeria monocytogenes and stored under vacuum package at 4 °C (left) and at 12 °C (right). Open symbols (□) indicate absence of the pathogen after applying the enrichment method. Vertical red dotted line: end of the control samples shelf-life according to sensory analysis. Vertical blue dashed line: end of the HPP-treated samples shelf-life according to sensory analysis. Error bars represent the mean values ± standard deviation.
On the other hand, the samples that were treated with HPP and stored at 4 °C showed a decrease of the pathogen population which was depended on the different inoculum. More specifically, for the low and medium inoculum no growth of the pathogen was observed, while for the high inoculum (6 log CFU/g) a 5-log cycle reduction was recorded during cold storage. In detail, in the samples with the low initial inoculum L. monocytogenes was detectable after enrichment at the first days of cold storage until the fourth day that the pathogen was absent. In the samples with medium inoculum L. monocytogenes was found periodically present after enrichment during storage at 4 °C (Figure 2). At the high inoculum the pathogen increased during cold storage at a population level of 3 log CFU/g. At 12 °C the application of HPP resulted in the reduction of the pathogen in all the inoculation cases on day 0 to the detection limit of the enumeration method. More specifically, during storage at 12 °C pathogen population remained below the detection limit of the enumeration method for the low and medium inoculum. In the low inoculum case, L. monocytogenes was absent during storage at 12 °C, except from the eighth day of storage that it was detected after enrichment in one out of the six samples analyzed. Though, in the medium inoculum case L. monocytogenes was found always present after enrichment, while a slight increase was observed after the end of the shelf-life (seventh day) of the product. Likewise, in the high inoculum case the population of the pathogen increased reaching the level of 7 log CFU/g by the end of storage at 12 °C, as it is evident from Figure 2. Lastly, Figure 2 displays the TVC population, which represented the dominant microbiota in each case. At control samples the dominant microbiota consisted of B. thermosphacta and Pseudomonas sp., while for the HPP-treated samples TVC most likely corresponded to B. thermosphacta, which was found to be the main spoilage microorganism during cold storage. Similarly, at 12 °C the dominant microbiota of the control samples was B. thermosphacta and Pseudomonas sp., whereas for the HPP-treated samples TVC corresponded to B. thermosphacta (low and medium inoculum) and B. thermosphacta along with L. monocytogenes in the case of high inoculum.
3.3. Effect of HPP on the Brochothrix Isolates According to PFGE Analysis
In this study the diversity as well as the succession of B. thermosphacta at strain level was examined during storage after different treatments (HPP or control) and storage temperatures (4 °C and 12 °C) for chicken meat stored under vacuum packaging. Brochothrix isolates (52 isolates) were recovered from fresh chicken fillets (day 0) and at the end of storage for control samples at 4 °C and 12 °C, while for HPP-treated samples Brochothrix isolates (58 isolates) were recovered after the third day of storage and at the end of storage for both temperatures.
According to the PFGE profiles for Brochothrix isolates, different profiles between treatments (HPP or control) and storage temperatures (4 °C and 12 °C) were obtained. In detail, a significant diversity among isolates recovered from control samples was observed, whereas for the isolates recovered after HPP their diversity was lower. Moreover, storage conditions also affected the strain diversity resulting in a lower diversity of the isolates when samples were stored at 12 °C.
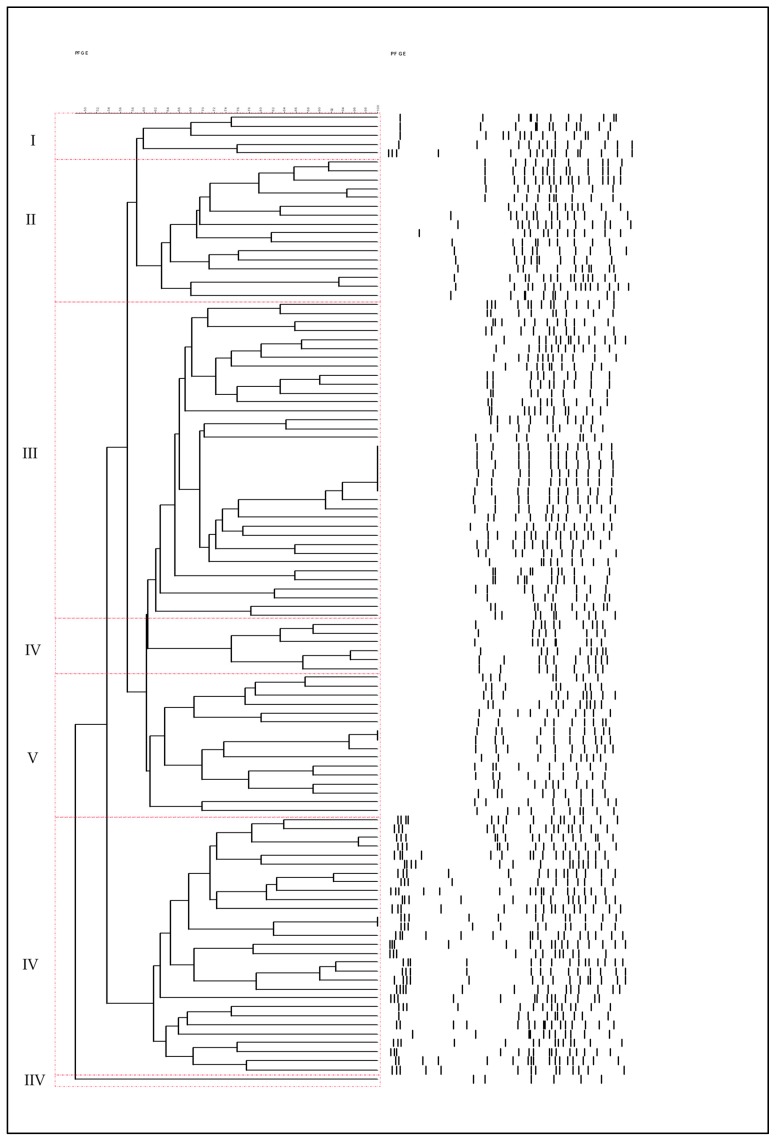
The dendrogram obtained after cumulative image analysis of PFGE patterns using a coefficient of similarity of 60%, resulted in seven different groups (Figure 3). In Table 1, the prevalence of the different groups related to the different conditions (untreated and treated with HPP and storage temperatures) is summarized. In detail, Group I had five isolates all recovered from the end of cold storage of HPP-treated samples. Group II encompassed 16 isolates and the diversity of the recovered PFGE profiles was observed mostly at control samples stored at both temperatures. Additionally, no isolates were recovered from control samples at 12 °C. Group III was the most abundant in terms of isolates (37). In detail, two PFGE profiles were found in HPP samples (day 3 and end of storage), while nine different profiles were recovered from the control samples at 4 °C. At 12 °C no isolates were recovered from control samples, whereas 26 isolates were recovered from HPP samples. Group IV consisted of six isolates recovered mainly from control samples for both temperatures at 0 h and at the end of storage. Only one isolate in this group was recovered from an HPP sample on the third day of storage at 4 °C. Group V contained mainly isolates (16) from control and HPP-treated samples from 12 °C. Group VI consisted of 29 isolates, where no isolates were recovered from HPP samples from 12 °C. The last group (VII) consisted of a single isolate recovered from the end of 12 °C of control samples. A representative number of isolates from the above seven groups were subjected to 16S rRNA gene sequencing, revealing that all groups were assigned to B. thermosphacta at a percentage over 99% (Table S1).
Figure 3.
Cluster analysis of pulsed-field gel electrophoresis (PFGE) ApaI digestion fragments of the Brochothrix thermosphacta isolates calculated by the unweighted pair group method.
Table 1.
Distribution of B. thermosphacta isolates at the beginning and the end of storage under vacuum package of chicken fillets at 4 °C and 12 °C untreated (control) or treated with HPP (500 MPa/10 min).
| T (°C) | Treatment | Storage Time | Group | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| I | II | III | IV | V | VI | VII | Total Isolates | |||
| 4 °C | Control | Beginning | 0 | 2 a | 2 | 1 | 3 | 3 | 0 | 11 |
| End | 0 | 4 | 7 | 1 | 0 | 3 | 0 | 15 | ||
| HPP | Beginning b | 0 | 4 | 0 | 1 | 0 | 7 | 0 | 12 | |
| End | 5 | 1 | 2 | 0 | 0 | 6 | 0 | 14 | ||
| 12 °C | Control | Beginning | 0 | 0 | 0 | 2 | 6 | 6 | 0 | 14 |
| End | 0 | 0 | 0 | 1 | 6 | 4 | 1 | 12 | ||
| HPP | Beginning b | 0 | 2 | 7 | 0 | 1 | 0 | 0 | 10 | |
| End | 0 | 3 | 19 | 0 | 0 | 0 | 0 | 22 | ||
| Total isolates | 5 | 16 | 37 | 6 | 16 | 29 | 1 | 110 | ||
a: number of isolates. b: colonies isolated on day 3 (first appearance of colonies on STAA– no colonies were grown at day 0).
3.4. Effect of HPP on the Different Listeria Strains According to PFGE Analysis
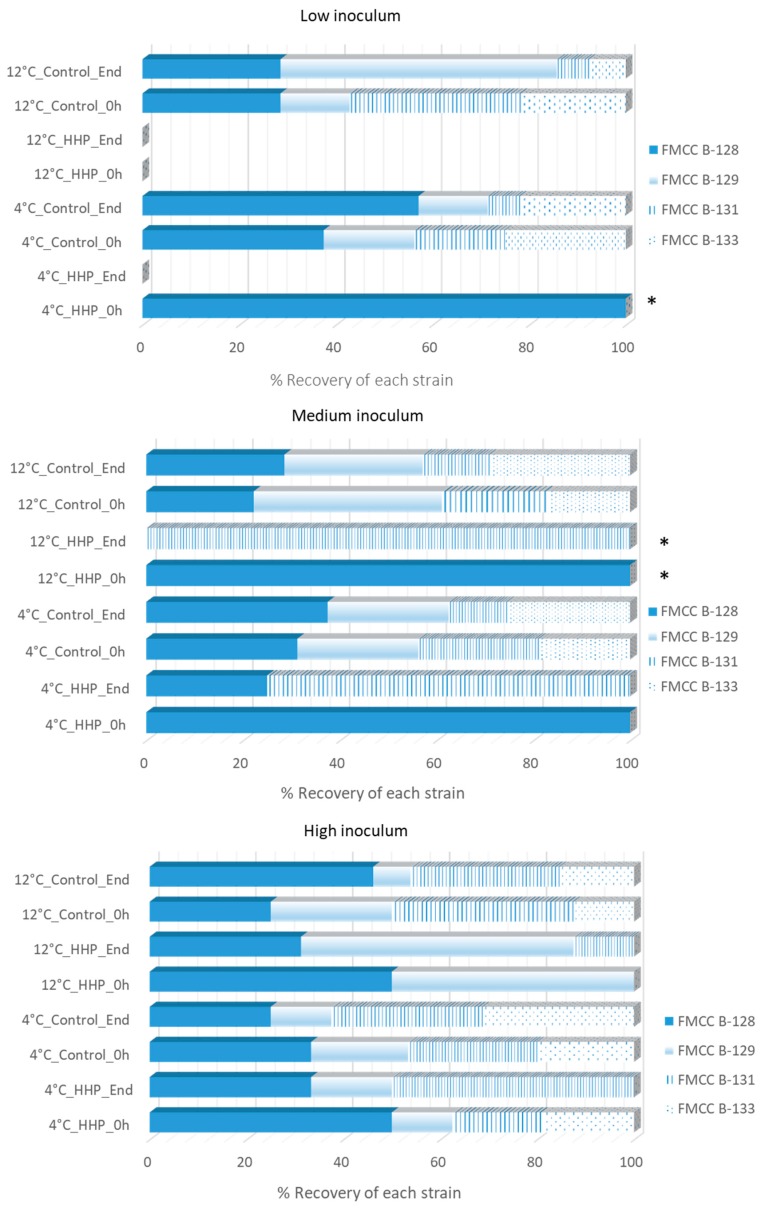
A total of 294 isolates were recovered from Palcam agar plates and the isolates were screened with PFGE to monitor the survival and the distribution of the L. monocytogenes strains on chicken samples untreated or treated with HPP, stored under vacuum packaging for both temperatures. The distribution of the above recovered isolates based on their PFGE profiles is presented in Figure 4. According to the PFGE analysis, the survival and distribution of the different Listeria strains depend on the initial inoculum level, the application of HPP along with the storage temperature (Figure 4). At 0 h for the three inoculum levels on control samples, the distribution of the strains was FMCC–B–128 (ca. 32–37%)> FMCC–B–131 (ca. 19–27%) > FMCC–B–133 (ca. 19–25%), and FMCC–B–129 (ca. 19–25%). At the end of cold storage for control samples the strain FMCC–B–128 was the dominant strain in low and medium inoculum case, while FMCC–B–131 displayed the lowest recovery percentage. Regarding the low inoculum, it has to be noted that the pathogen was detected only in samples analyzed with the enrichment method. For samples inoculated with the high inoculum strains FMCC–B–131 and FMCC–B–133 exhibited the highest recovery percentage at the end of cold storage of control samples. Subsequently, after the application of HPP the strain distribution varied between the different inoculum levels. For low (after enrichment) and medium inoculum FMCC–B–128 was the only strain recovered, whereas for the high inoculum the distribution of the strains was FMCC–B–128 (ca. 50%), FMCC–B–131 (ca. 19%), FMCC–B–133 (ca. 19%), and FMCC–B–129 (ca. 12%). At the end of storage at 4 °C of HPP-treated samples no strains were recovered from the low inoculum case (after enrichment), the strain FMCC–B–131 dominated in medium inoculum case and lastly, in the high inoculum case all strains but FMCC–B–133 were recovered.
Figure 4.
Survival of the different L. monocytogenes strains (FMCC–B–128, FMCC–B–129, FMCC–B–131, FMCC–B–133) on chicken fillets untreated (control) or HPP–treated (500 MPa/10 min) with low, medium, and high levels of L. monocytogenes initial inoculum, at the beginning (0 h) and the end of storage under vacuum package at 4 °C and 12 °C. * Pathogen detected after applying the enrichment method.
For the samples stored at 12 °C the initial distribution of the strains for the three inocula was FMCC–B–131 (ca. 22–38%) > FMCC–B–129 (ca. 14–39%)> FMCC–B–128 (ca. 22–29%)> and FMCC–B–133 (ca. 12–22%), while at the end of storage differences were observed between the three inoculum levels in terms of recovery percentages of each strain. However, in contrast to the cold storage, no specific recovery pattern in strain distribution was observed for control samples (Figure 4). After HPP, no colonies were recovered after enrichment in the low inoculum case, FMCC–B–128 was the only recovered strain (after enrichment) in the medium inoculum case, while at high inoculum the recovered strains were FMCC–B–128 and FMCC–B–129 in a ratio of 1:1. Finally, at the end of storage at 12 °C no strains were recovered after enrichment in the low inoculum case, in the medium inoculum case the only recovered strain (after enrichment) was FMCC–B–131, while at high inoculum all strains but FMCC–B–133 were recovered. The latter finding is of interest, since the same strains were absent or present in the high inoculum case after cold storage of HPP–treated samples. For comparison reasons, it has to be noted that in the cases that the isolates were recovered after enrichment the presence of each strain has to be characterized as random and the appearance of the strains in the figures should be interpreted as qualitative information rather than % contribution in the samples [25].
3.5. Sensory Analysis
The organoleptic changes of the control and HPP-treated (non–inoculated) samples were similar with our previous published work for chicken fillets stored at 4 °C and 12 °C under vacuum packaging [25]. In brief, the time required by the sensory panel to consider a sample as spoiled, differed for each temperature and for HPP-treated or untreated (control) samples. Concerning cold storage, the shelf-life of the HPP-treated chicken fillets was increased to 12 days of storage, while for control samples the end of shelf-life was estimated after 7 days of storage (Figure 1). In addition, at the time of the sensory rejection the TVC values were ca. 7.5 log CFU/g and 6.0 log CFU/g for the control and HPP-treated samples, respectively (Figure 1). At 12 °C meat was characterized as spoiled by the sensory panel at the third day of storage for control samples and at the seventh day of storage for HPP-treated samples (Figure 2). The mean values of TVC at the time of sensory rejection (meat characterized as spoiled) were ca. 8.0 log CFU/g and 5.2 log CFU/g for control and HPP-treated samples, respectively (Figure 2). The organoleptic attribute that was mostly affected by the HPP treatment was the color of the product. In more details, panelists mentioned that in the raw chicken the color was found to be whiter with increased lightness, but after cooking the appearance of the product was similar to the control. However, the juiciness and coherence were improved in the case of HPP-treated samples, characteristics that were pointed out by all the panelists.
4. Discussion
The microbiological results obtained in this study demonstrated similar or higher reductions in the population of indigenous microbiota after the application of HPP, as compared to literature findings of poultry products or other meat products treated with HPP. In more details, in the current work B. thermosphacta was found to be the dominant microorganism after HPP, a result that was also observed by Argyri et al. [25], while a small increase of Pseudomonas spp. and yeasts/molds at 4 °C and Pseudomonas and Enterobacteriaceae at 12 °C was observed after the end of the shelf-life of the HPP-treated chicken fillets. Previous studies have reported that HPP caused a reduction in the population of the aerobic and anaerobic mesophiles, LAB, Listeria spp., Staphylococcus spp., B. thermosphacta, coliforms, yeasts, and molds to below the detection limit of the enumeration method on a variety of RTE meat products at 600 MPa/180 sec [20], and to levels below the detection limit of enumeration method for B. thermosphacta, Carnobacterium divergens, Campylobacter jejuni and Ps. fluorescens after 400 MPa/30 min on sterile minced poultry meat [24]. A reduction of 3.7 log CFU/g in the aerobic mesophiles of the mechanically recovered poultry meat at 450 MPa/15 min was observed by Yuste et al. [32]. Also, in the recent work by Al–Nehlawi et al. [6] poultry sausages were inoculated with 7–9 log CFU/g of various monocultures of the common meat microbiota, HPP was applied (350 MPa/10 min) and then products were packed without or with CO2 and stored at 2 °C. From the results it was evident that the application of HPP has resulted in a significant reduction of 4 log CFU/g of Leuconostoc carnosum, while in samples inoculated with B. thermosphacta the population of this bacterium suffered a reduction of 5 log CFU/g and no recovery was observed throughout the tested poultry products packed with CO2. Additionally, in another study B. thermosphacta was inactivated after HPP (400 MPa/10 min) when initial population of the samples was ca. 4.5 log CFU/g on a sliced packed cooked ham stored in vacuum packages under refrigeration temperatures [33]. Finally, Rodríguez–Calleja et al. [34] studied the multiple effect of HPP, MAP, and edible coatings on the microbiota of chicken fillets stored at 4 °C and the results demonstrated that B. thermosphacta was inactivated when all hurdles were applied, although this bacterium consisted of 89% of the primary microbiota. When less hurdles were applied (MAP and HPP treatment) B. thermosphacta was capable of growing and becoming the dominant microbiota throughout storage [34]. In the current study, HPP increased the shelf-life of chicken fillets for 5 and 4 days, at 4 °C and 12 °C, respectively, which was similar to Argyri et al. [25] previous findings for cold storage (6 days) but different for 12 °C (2 days). It has to be noted that besides the fact that the trained sensory panel consisted of a limited number of persons, they all agreed for the end of the product’s shelf-life (sensory scores ≥ 2).
As mentioned above, B. thermosphacta was found to be the dominant population after the application of HPP on chicken meat. Accordingly, PFGE was used to study the diversity, as well as, the succession of B. thermosphacta isolates under different treatments (untreated and treated with HPP) and temperatures (4 °C and 12 °C) during storage of chicken meat. Results showed that Brochothrix isolates exhibited a significant diversity among control samples, whereas this was limited for the HPP-treated samples. These results were in line with previous studies in meat and meat products [33,35], where HPP treatment was found to decrease the strain diversity. Moreover, storage at abuse temperatures (12 °C) affected the strain diversity resulting in a lower diversity. Similar results were also observed in other studies dealing with meat spoilage at various temperatures [30,31]. B. thermosphacta has gained much attention because of its ubiquitous nature and its potential to dominate as a food spoiler on meat and seafood products stored under MAP. For instance, B. thermosphacta was found to be one of the dominant species in French chicken cuts stored under various MAP concentrations after a 16S rRNA gene pyrosequencing approach in the study of Rouger et al. [1]. At the aforenoted study, the importance of the slaughterhouse environment in the diversity of the microbiota was also discussed. This result was also evident in the work by Samapundo et al. [36], where it was shown that B. thermosphacta was isolated from cutting blades and leg hooks used for the preparation of chicken cuts. Recent studies dealt with the typing of Brochothrix sp. isolates in an effort to evaluate genotypic and phenotypic diversity of this species and finally correlate spoilage potential of the isolates [37,38]. Illikoud et al. [37] evaluated different molecular methods for the typing of 161 Brochothrix isolates from different foods and results showed that Brochothrix isolates that were derived from different ecological and geographical origins were widely distributed in all the groups. In addition, the formed groups encompassed isolates from different animals, or/and for fresh and spoiled food, or/and from raw and processed food and suggested that the strains did not share any ecotype. Finally, from both studies it was shown that the spoilage potential of Brochothrix isolates is mostly strain dependent [37,38].
In the current study, a cocktail culture of four strains of Listeria monocytogenes in three inoculum levels was used to inoculate chicken fillets to acquire information regarding microbial risk assessment. After the application of HPP the pathogen population decreased depending on the inoculum case. For the low and medium inoculum case the pathogen was detected throughout the shelf-life at both temperatures in populations near to the detection limit or after enrichment. However, the pathogen decreased ≥ 5-log cycles after HPP and subsequently increased to 1.6 and 4.5 log CFU/g at 4 °C and 12 °C, respectively, by the end of shelf-life in the high inoculum case. Many studies have confirmed that HPP treatments sub-lethally injure microorganisms, but they are capable of partial recovery and growth after some days of storage, as it was evident in this study too. So far, several studies have focused on the effect of HPP on L. monocytogenes in a variety of meats and meat products. In a study by Jofre et al. [39] cooked ham was inoculated with three strains of L. monocytogenes (4 log CFU/g initial inoculum level) among other pathogens and pressurized at 600 MPa/5 min at 10 °C. From the results it was evident that the pathogen population was reduced more than 3.5 log CFU/g and remained at levels below 10 CFU/g during refrigerated storage [39]. Similar results were observed in the study of Hayman et al. [20] in a variety of RTE meats inoculated with 4 log CFU/g of L. monocytogenes, where after HPP treatment (600 MPa/180 s/20 °C) L. monocytogenes was found sporadically present after enrichment for a storage period of 91 days at 4 °C. Reduction of 7 log units of a 3-strain cocktail of L. monocytogenes (7 log CFU/g initial inoculum level) was observed at 600 MPa/3 min on cooked ham in the study of Bover-Cid et al. [40]. Yet, the cells that survived were able to initiate growth just after HPP treatment [40]. Schneinberg et al. [41] reported that inoculated beef jerky which was treated with two cycles of HPP (550 MPa/60 sec/22 °C) resulted in a reduction of approximately 1 log of L. monocytogenes (7 log CFU/g initial inoculum level) and subsequently it increased during storage under vacuum packaging, in contrast to the other examined inoculated pathogens that exhibited a significant reduction in the population levels. Marcos et al. [42] studied the effect of HPP in tandem with antimicrobial packaging films on cooked ham and reported that HPP (400 MPa/10 min) reduced the initial level of L. monocytogenes population from 4 log CFU/g to 0.6 log CFU/g and the latter population remained stable throughout storage at 6 °C. However, in the aforementioned study the application of HPP without the use of antimicrobial packaging was found inefficient to prevent pathogen growth during storage at 6 °C [42]. In other studies, pressures less than 400 MPa and holding times of more than 10 min did not result in a meaningful reduction of L. monocytogenes in a variety of meat products [43,44]. Finally, Kruk et al. [45] inoculated sterilized chicken breast fillets with 7 log CFU/g of L. monocytogenes, S. Typhimurium, and E. coli and applied three different pressure treatments (300, 450, and 600 MPa/5 min/15 °C). More than a 7 log cycle reduction was achieved at 450 and 600 MPa, while at 300 MPa cell counts were reduced by 4 log CFU/g. L. monocytogenes was not detected in samples pressurized with 450 and 600 MPa during storage at 4 °C, but its population remained close to 4 log CFU/g in samples treated with 300 MPa [45]. According to the above, it was evident that inactivation of L. monocytogenes varies and depends on the product type, the different HPP treatments, and most likely the different strains tested.
Several studies have monitored the survival of L. monocytogenes strains, however, to our knowledge, no work until now has implemented in typing with molecular methods the survival of Listeria strains after HPP treatment, although, the survival of different strains of the pathogen has been studied when pathogen was inoculated either as a monoculture [46,47] or as mixed culture [40] in a variety of products after different HPP treatments. Thus, PFGE typing revealed the distribution of the inoculated pathogenic strains and it was evident that pathogen survival was strain dependent. Each strain behaved different in relation to the different inoculum level, storage temperature, and HPP treatment (untreated or HPP–treated samples). For instance, the strain FMCC–B–128 was found to be the main recovered strain after HPP, however, at the end of storage for both temperatures, the aforementioned strain exhibited the lowest recovery percentages, while the control showed no specific recovery pattern for the aforenoted strain. Moreover, in the high inoculum case the same strains were absent or present at both storage temperatures in HPP–treated samples. According to literature findings, in a variety of products inoculated with different strains of L. monocytogenes the survival of L. monocytogenes was found to be strain-dependent and was affected by a variety of temperatures used for storage [48,49,50].
5. Conclusions
The aim of this study was to evaluate the synergistic effect of combining vacuum packaging and HPP treatment in reducing or inactivating spoilage and pathogenic bacteria (Listeria monocytogenes), as well as, monitoring the shelf-life of chicken fillets. Results demonstrated that HPP increased the shelf-life and enhanced the safety of chicken meat. Additionally, the microbiota of HPP-treated samples consisted only of a single species, i.e., B. thermosphacta. Strain diversity of Brochothrix isolates was affected by HPP treatment leading to a lower diversity of the pressed samples, while strain diversity of the pathogen was mostly depended on the inoculum level and storage temperature conditions. However, further research is needed to better understand the inhibitory effects of HPP on target strains of chicken microbiota.
Acknowledgments
This work has been co–financed by the European Union (European Social Fund–ESF) and Greek national funds through the Operational Program “Education and Lifelong Learning” of the National Strategic Reference Framework (NSRF)–Research Funding Program THALES: Reinforcement of the interdisciplinary and/or inter–institutional research and innovation.
Supplementary Materials
The following are available online at https://www.mdpi.com/2076-2607/7/11/520/s1.
Author Contributions
Conceptualization, A.A.A., N.C.; methodology, A.A.A., N.C.; validation, A.A.A., N.C.; formal analysis, A.A.A., O.S.P.; investigation, A.A.A., O.S.P. and P.S.; resources, C.C.T.; data curation, A.A.A., O.S.P.; writing—original draft preparation, A.A., N.C., and O.S.P.; writing—review and editing, C.C.T.; visualization, A.A.A., O.S.P. and N.C.; supervision, C.C.T.; project administration, N.C., C.C.T.
Funding
This research received no external funding.
Conflicts of Interest
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
References
- 1.Rouger A., Moriceau N., Prevost H., Remenant B., Zagorec M. Diversity of bacterial communities in French chicken cuts stored under modified atmosphere packaging. Food Microbiol. 2018;70:7–16. doi: 10.1016/j.fm.2017.08.013. [DOI] [PubMed] [Google Scholar]
- 2.Chaillou S., Chaulot-Talmon A., Caekebeke H., Cardinal M., Christieans S., Denis C., Desmonts M.H., Dousset X., Feurer C., Hamon E., et al. Origin and ecological selection of core and food-specific bacterial communities associated with meat and seafood spoilage. ISME J. 2015;9:1105–1118. doi: 10.1038/ismej.2014.202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Argyri A.A., Panagou E.Z., Nychas G.J.E. Advances in traditional, vacuum and modified atmosphere packaging MAP of fresh and processed poultry products. In: Kerry J.P., editor. Advances in Meat, Poultry and Seafood Packaging. 1st ed. Elsevier; London, UK: 2012. pp. 205–247. [Google Scholar]
- 4.Nychas G.J.E., Skandamis P.N., Tassou C.C., Koutsoumanis K.P. Meat spoilage during distribution. Meat Sci. 2008;78:77–89. doi: 10.1016/j.meatsci.2007.06.020. [DOI] [PubMed] [Google Scholar]
- 5.Nychas G.J.E., Skandamis P.N. Fresh meat spoilage and modified atmosphere packaging MAP. Improving the Safety of Fresh Meat. Woodhead Publ. 2005:461–502. [Google Scholar]
- 6.Al-Nehlawi A., Guri S., Guamis B., Saldo J. Synergistic effect of carbon dioxide atmospheres and high hydrostatic pressure to reduce spoilage bacteria on poultry sausages. LWT Food Sci. Technol. 2014;58:404–411. doi: 10.1016/j.lwt.2014.03.041. [DOI] [Google Scholar]
- 7.Stanborough T., Fegan N., Powell S.M., Tamplin M., Chandry P.S. Insight into the.genome of Brochothrix thermosphacta, a problematic meat spoilage bacterium. Appl. Environ. Microbiol. 2017;83:e02786-16. doi: 10.1128/AEM.02786-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Pennacchia C., Villani F., Ercolini D. Development of a Real-Time PCR assay for the specific detection of Brochothrix thermosphacta in fresh and spoiled meat. Int. J. Food Microbiol. 2009;134:230–236. doi: 10.1016/j.ijfoodmicro.2009.07.005. [DOI] [PubMed] [Google Scholar]
- 9.Del Olmo A., Calzada J., Nunez M. Effect of lactoferrin and its derivatives, high hydrostatic pressure, and their combinations, on Escherichia coli O157:H7 and Pseudomonas fluorescens in chicken filets Innovative. Innov. Food Sci. Emerg. Technol. 2012;13:51–56. doi: 10.1016/j.ifset.2011.07.016. [DOI] [Google Scholar]
- 10.EFSA European Food Safety Authority. [(accessed on 12 December 2017)]; Available online: https://www.efsa.europa.eu/en/efsajournal/pub/5077.
- 11.Gonçalves-Tenório A., Nunes Silva B., Rodrigues V., Cadavez V., Gonzales-Barron U. Prevalence of Pathogens in Poultry Meat: A Meta-Analysis of European Published Surveys. Foods. 2018;7:69. doi: 10.3390/foods7050069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Allen K.J., Walecka-Zacharska E., Chen J.C., Kosek-Paszkowska K., Devlieghere F., Van Meervenne E., Osek J., Wieczorek K., Bania J. Listeria Monocytogenes: An Examination of Food Chain Factors Potentially Contributing to Antimicrobial Resistance. Food Microbiol. 2018;54:178–189. doi: 10.1016/j.fm.2014.08.006. [DOI] [Google Scholar]
- 13.Ferreira M., Almeida A., Delgadillo I., Saraiva J., Cunha A. Susceptibility of Listeria monocytogenes to high pressure processing: A review. Food Rev. Int. 2016;32:377–399. doi: 10.1080/87559129.2015.1094816. [DOI] [Google Scholar]
- 14.Zhang Q.Q., Han Y.Q., Cao J.X., Xu X.L., Zhou G.H., Zhang W.Y. The spoilage of air-packaged broiler meat during storage at normal and fluctuating storage temperatures. Poultry Sci. 2012;91:208–214. doi: 10.3382/ps.2011-01519. [DOI] [PubMed] [Google Scholar]
- 15.Huang H.W., Wu S.Z., Lu J.K., Shyu Y.T., Wang C.Y. Current status and future trends of high-pressure processing in food industry. Food Control. 2017;72:1–8. doi: 10.1016/j.foodcont.2016.07.019. [DOI] [Google Scholar]
- 16.Bravo D., de Alba M., Medina M. Combined treatments of high-pressure with the lactoperoxidase system or lactoferrin on the inactivation of Listeria monocytogenes, Salmonella Enteritidis and Escherichia coli O157:H7 in beef carpaccio. Food Microbiol. 2014;41:27–32. doi: 10.1016/j.fm.2014.01.010. [DOI] [PubMed] [Google Scholar]
- 17.Hugas M., Garriga M., Monfort J.M. New mild technologies in meat processing: High pressure as a model technology. Meat Sci. 2002;62:359–371. doi: 10.1016/S0309-1740(02)00122-5. [DOI] [PubMed] [Google Scholar]
- 18.Chien S.Y., Sheen S., Sommers C.H., Sheen L.Y. Modeling the Inactivation of Intestinal Pathogenic Escherichia coli O157:H7 and Uropathogenic E. coli in Ground Chicken by High Pressure Processing and Thymol. Front. Microbiol. 2016;7:920. doi: 10.3389/fmicb.2016.00920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Simonin H., Duranton F., de Lamballerie M. New Insights into the High-Pressure Processing of Meat and Meat Products. Comp. Rev. Food Sci. Food Saf. 2012;11:285–306. doi: 10.1111/j.1541-4337.2012.00184.x. [DOI] [Google Scholar]
- 20.Hayman M.M., Baxter I., O’Riordan P.J., Stewart C.M. Effects of High-Pressure Processing on the Safety, Quality, and Shelf Life of Ready-to-Eat Meats. J. Food Prot. 2004;67:1709–1718. doi: 10.4315/0362-028X-67.8.1709. [DOI] [PubMed] [Google Scholar]
- 21.Jofré A., Aymerich T., Grebol N., Garriga M. Efficiency of high hydrostatic pressure at 600 MPa against food-borne microorganisms by challenge tests on convenience meat products. LWT Food Sci. Technol. 2009;42:924–928. doi: 10.1016/j.lwt.2008.12.001. [DOI] [Google Scholar]
- 22.Lerasle M., Guillou S., Simonin H., Anthoine V., Chéret R., Federighi M., Membré J.-M. Assessment of Salmonella and Listeria monocytogenes level in ready-to-cook poultry meat: Effect of various high pressure treatments and potassium lactate concentrations. Int. J. Food Microbiol. 2014;186:74–83. doi: 10.1016/j.ijfoodmicro.2014.06.019. [DOI] [PubMed] [Google Scholar]
- 23.Rendueles E., Omer M.K., Alvseike O., Alonso-Calleja C., Capita R., Prieto M. Microbiological food safety assessment of high hydrostatic pressure processing: A review. LWT Food Sci. Technol. 2011;44:1251–1260. doi: 10.1016/j.lwt.2010.11.001. [DOI] [Google Scholar]
- 24.Liu Y., Betti M., Ganzle M.G. High Pressure Inactivation of Escherichia coli, Campylobacter jejuni, and Spoilage Microbiota on Poultry Meat. J. Food Prot. 2012;75:497–503. doi: 10.4315/0362-028X.JFP-11-316. [DOI] [PubMed] [Google Scholar]
- 25.Argyri A.A., Papadopoulou O.S., Nisiotou A., Tassou C.C., Chorianopoulos N. Effect of high pressure processing on the survival of Salmonella Enteritidis and shelf-life of chicken fillets. Food Microbiol. 2018;70:55–64. doi: 10.1016/j.fm.2017.08.019. [DOI] [PubMed] [Google Scholar]
- 26.Hereu A., Dalgaard P., Garriga M., Aymerich T., Bover-Cid S. Analyzing and modelling the growth behavior of Listeria monocytogenes on RTE cooked meat products after a high pressure treatment at 400 MPa. Int. J. Food Microbiol. 2014;186:84–94. doi: 10.1016/j.ijfoodmicro.2014.06.020. [DOI] [PubMed] [Google Scholar]
- 27.Rubio B., Possas A., Rincon F., García-Gímeno R.M., Martínez B. Model for Listeria monocytogenes inactivation by high hydrostatic pressure processing in Spanish chorizo sausage. Food Microbiol. 2018;69:18–24. doi: 10.1016/j.fm.2017.07.012. [DOI] [PubMed] [Google Scholar]
- 28.Valdramidis V.P., Patterson M.F., Linton M. Modelling the recovery of Listeria monocytogenes in high pressure processed simulated cured meat. Food Control. 2015;47:353–358. doi: 10.1016/j.foodcont.2014.07.022. [DOI] [Google Scholar]
- 29.Stratakos A.C.H., Linton M., Patterson M.F., Koidis A. Effect of high-pressure processing on the shelf life, safety and organoleptic characteristics of lasagne ready meals during storage at refrigeration and abuse temperature. Innov. Food Sci. Emerg. Technol. 2015;30:1–7. doi: 10.1016/j.ifset.2015.05.010. [DOI] [Google Scholar]
- 30.Papadopoulou O.S., Doulgeraki A.I., Botta C., Cocolin L., Nychas G.J.-E. Genotypic characterization of Brochothrix thermosphacta isolated during storage of minced pork under aerobic or modified atmosphere packaging conditions. Meat Sci. 2012;92:735–738. doi: 10.1016/j.meatsci.2012.06.030. [DOI] [PubMed] [Google Scholar]
- 31.Doulgeraki A.I., Paramithiotis S., Nychas G.-J.E. Characterization of the Enterobacteriaceae community that developed during storage of minced beef under aerobic or modified atmosphere packaging conditions. Int. J. Food Microbiol. 2011;145:77–83. doi: 10.1016/j.ijfoodmicro.2010.11.030. [DOI] [PubMed] [Google Scholar]
- 32.Yuste J., Capellas M., Fung D.Y.C., Mor-Mur M. Inactivation and sublethal injury of foodborne pathogens by high pressure processing: Evaluation with conventional media and thin agar layer method. Food Res. Int. 2001;37:861–866. doi: 10.1016/j.foodres.2004.05.002. [DOI] [Google Scholar]
- 33.Han Y., Jiang Y., Xu Y., Sun X., Xu B., Zhou G. Effect of high pressure treatment on microbial populations of sliced vacuum-packed cooked ham. Meat Sci. 2011;88:682–688. doi: 10.1016/j.meatsci.2011.02.029. [DOI] [PubMed] [Google Scholar]
- 34.Rodríguez-Calleja M., Cruz-Romero M.C., O’Sullivan M.G., García-López M.L., Kerry J.P. High-pressure-based hurdle strategy to extend the shelf-life of fresh chicken breast fillets. Food Control. 2012;25:516–524. doi: 10.1016/j.foodcont.2011.11.014. [DOI] [Google Scholar]
- 35.Han Y., Xu Y., Jiang Y., Zhou G., Sun X., Xu B. Inactivation of food spoilage bacteria by high pressure processing: Evaluation with conventional media and PCR–DGGE analysis. Food Res. Int. 2010;43:1719–1724. doi: 10.1016/j.foodres.2010.05.012. [DOI] [Google Scholar]
- 36.Samapundo S., de Baenst I., Aerts M., Cnockaert M., Devlieghere F., Van Damme P. Tracking the sources of psychrotrophic bacteria contaminating chicken cuts during processing. Food Microbiol. 2019;81:40–50. doi: 10.1016/j.fm.2018.06.003. [DOI] [PubMed] [Google Scholar]
- 37.Illikoud N., Rossero A., Chauvet R., Courcoux P., Pilet M.-F., Charrier T., Jaffres E., Zagorec M. Genotypic and phenotypic characterization of the food spoilage bacterium Brochothrix thermosphacta. Food Microbiol. 2019;81:22–31. doi: 10.1016/j.fm.2018.01.015. [DOI] [PubMed] [Google Scholar]
- 38.Casaburi A., De Filippis F., Villani F., Ercolini D. Activities of strains of Brochothrix thermosphacta in vitro and in meat. Food Res. Int. 2014;62:366–374. doi: 10.1016/j.foodres.2014.03.019. [DOI] [Google Scholar]
- 39.Jofré A., Garriga M., Aymerich T. Inhibition of Salmonella sp. Listeria monocytogenes and Staphylococcus aureus in cooked ham by combining antimicrobials, high hydrostatic pressure and refrigeration. Meat Sci. 2008;78:53–59. doi: 10.1016/j.meatsci.2007.06.015. [DOI] [PubMed] [Google Scholar]
- 40.Bover-Cid S., Serra-Castellóa C., Dalgaard P., Garriga M., Jofré A. New insights on Listeria monocytogenes growth in pressurised cooked ham: A piezo-stimulation effect enhanced by organic acids during storage. Int. J. Food Microbiol. 2019;290:150–158. doi: 10.1016/j.ijfoodmicro.2018.10.008. [DOI] [PubMed] [Google Scholar]
- 41.Scheinberg J.A., Svoboda A.L., Cutter C.N. High-pressure processing and boiling water treatments for reducing Listeria monocytogenes, Escherichia coli O157:H7, Salmonella spp.; and Staphylococcus aureus during beef jerky processing. Food Control. 2014;39:105–110. doi: 10.1016/j.foodcont.2013.11.002. [DOI] [Google Scholar]
- 42.Marcos B., Jofre A., Aymerich T., Monfort J.P., Garriga M. Combined effect of natural antimicrobials and high pressure processing to prevent Listeria monocytogenes growth after a cold chain break during storage of cooked ham. Food Control. 2008;19:76–81. doi: 10.1016/j.foodcont.2007.02.005. [DOI] [Google Scholar]
- 43.Balamurugan S., Ahmed R., Chibeu A., Gao A., Koutchma T., Strange P. Effect of salt types and concentrations on the high-pressure inactivation of Listeria monocytogenes in ground chicken. Int. J. Food Microbiol. 2016;218:51–56. doi: 10.1016/j.ijfoodmicro.2015.11.010. [DOI] [PubMed] [Google Scholar]
- 44.Bover-Cid S., Belletti N., Garriga M., Aymerich T. Model for Listeria monocytogenes inactivation on dry-cured ham by high hydrostatic pressure processing. Food Microbiol. 2011;28:804–809. doi: 10.1016/j.fm.2010.05.005. [DOI] [PubMed] [Google Scholar]
- 45.Kruk Z.A., Yun H., Rutley D.L., Lee E.J., Kim Y.J., Jo C. The effect of high pressure on microbial population, meat quality and sensory characteristics of chicken breast fillet. Food Control. 2011;22:6–12. doi: 10.1016/j.foodcont.2010.06.003. [DOI] [Google Scholar]
- 46.Evert-Arriagada K., Trujilloa A.G., Amador-Espejob G.G., Hernández-Herreroa M.M. High pressure processing effect on different Listeria spp. in a commercial starter-free fresh cheese. Food Microbiol. 2018;76:481–486. doi: 10.1016/j.fm.2018.07.012. [DOI] [PubMed] [Google Scholar]
- 47.Jofré A., Aymerich T., Bover-Cid S., Garriga M. Inactivation and recovery of Listeria monocytogenes, Salmonella enterica and Staphylococcus aureus after high hydrostatic pressure treatments up to 900 MPa. Int. Microbiol. 2010;13:105–112. doi: 10.2436/20.1501.01.115. [DOI] [PubMed] [Google Scholar]
- 48.Kagli D.M., Iliopoulos V., Stergiou V., Lazaridou A., Nychas G.J. Differential Listeria monocytogenes Strain Survival and Growth in Katiki, a Traditional Greek Soft Cheese, at Different Storage Temperatures. Appl. Environ. Microbiol. 2009;75:3621–3626. doi: 10.1128/AEM.01571-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Papadopoulou O., Chorianopoulos N. Production of a functional fresh cheese enriched with the probiotic strain Lb. plantarum T571 isolated from traditional Greek product. Curr. Res. Nutr. Food Sci. 2016;4:169–181. [Google Scholar]
- 50.Papadopoulou O.S., Argyri A.A., Varzakis E.E., Tassou C.C., Chorianopoulos N.G. Greek functional Feta cheese: Enhancing quality and safety using a Lactobacillus plantarum strain with probiotic potential. Food Microbiol. 2018;74:21–33. doi: 10.1016/j.fm.2018.02.005. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.