Abstract
Background/purpose
Mini-implant screws are now routinely used as anchorage devices in orthodontic treatments. This study used synthetic bone models to investigate how the primary stability of an orthodontic mini-implant (OMI) as measured by resonance frequency (RF) is affected by varying cortical bone thickness and trabecular bone density.
Materials and methods
Three synthetic cortical shells (thicknesses of 1, 2, and 3 mm) and three polyurethane foam blocks (densities of 40, 20, and 10 pound/cubic foot) were used to represent jawbones of varying cortical bone thicknesses and varying trabecular bone densities. Twenty-five stainless steel OMIs (2 × 10 mm) were sequentially inserted into artificial bone blocks to depths of 2, 4, and 6 mm. Five experimental groups of bone blocks with OMIs were examined by Implomates® RF analyzer. Statistical and correlation analyses were performed by Kruskal-Wallis test, Wilcoxon rank-sum test, and simple linear regression.
Results
As trabecular bone density decreased, RF decreased; as cortical bone thickness decreased, RF also decreased. Simple linear regression analysis showed highly linear correlations between trabecular bone density and RF (R2 > 0.99; P < 0.0001) and between cortical bone thickness and RF (R2 > 0.98; P < 0.0001).
Conclusion
The stability of an OMI at the time of placement is influenced by both cortical bone thickness and trabecular bone density. Both cortical bone thickness and trabecular bone density have strong linear correlations with RF.
Keywords: Mini-implants, Primary stability, Cortical bone thickness, Trabecular bone density, Resonance frequency
Introduction
The need for orthodontic treatment modalities that provide maximal anchorage control but with minimal patient compliance requirements has led to the development of mini-implant anchorage devices for orthodontics.1 Mini-implants allow orthodontists to achieve treatment goals that were previously considered extremely difficult, if not impossible. The clinical success rate of mini-implants in orthodontics now exceeds 85%,2,3 which is a considerable improvement from the past. However, this success rate is still unsatisfactory, especially in comparison with the success rate for dental implants (>95%).4 Anchorage stability is a key factor in the success of orthodontic treatment assisted with mini-implants. Orthodontic mini-implants (OMIs) are used mainly for primary loading. Primary stability is an important factor in the success rate of OMIs. If primary stability is not achieved upon insertion, the OMI may loosen during orthodontic treatment.5 Primary stability is affected by bone quantity (bone volume), bone quality (bone density), surgical technique, and implant geometry (length, diameter, and surface characteristics).6, 7, 8 The primary stability of an OMI mainly depends on mechanical retention between the OMI and bone.9 Cortical bone thickness is important in the success of an OMI because insufficient cortical bone thickness often causes inadequate primary stability. Low bone density in the posterior maxilla is another major cause of implant loss.10
The stability of an OMI is difficult to evaluate and is often measured in terms of mobility. Resonance frequency analysis (RFA) is a noninvasive diagnostic method of assessing the stability of OMIs and dental implants. Two RFA devices that are currently in clinical use are the Osstell® (Integration Diagnostics AB, Göteborg, Sweden) and Implomates® (BioTech One, Inc., Taipei, Taiwan) devices. For clinical use in detecting resonance frequency (RF) values of an unmodified OMI, Implomates® is more convenient than Osstell®. Although the Osstell® system has been used to assess the stability of dental implants, the system is less than ideal for this purpose. For RF measurement, for example, the head designs of OMIs are not threaded to enable coupling with SmartPeg for RF measurement.11
The objective of this study was to determine precisely how bone quality and quantity (i.e., cortical bone thickness and trabecular bone density) correlate with primary OMI stability by using the Implomates® device to measure RF in synthetic bone samples.
Materials and methods
Orthodontic mini-implants
Twenty-five stainless steel OMIs (2.0 mm in diameter and 10 mm in length; Bio-Ray Biotech Instruments Co., Ltd., New Taipei City, Taiwan) were used for the experiments in this study (five mini-implants per experimental group). The OMIs were placed without pre-drilling. Each OMI was inserted manually with a hand driver. Measurements of RF were performed in OMIs sequentially inserted to depths of 2, 4, and 6 mm.
Bone specimens
Mechanical test blocks of artificial bone (Sawbones®; Pacific Research Laboratories Inc., Vashon Island, WA, USA) were selected as a jaw bone equivalent (Table 1). The mean bone mineral density was 0.55 g/cm3 for the anterior maxilla and 0.31 g/cm3 for the posterior maxilla.12 Polyurethane foam blocks of artificial bone with densities of 40 pcf (pound/cubic foot) (0.64 g/cm3), 20 pcf (0.32 g/cm3), and 10 pcf (0.16 g/cm3) were selected as the trabecular bone equivalent in the experimental groups. For trabecular bone samples, the moduli of elasticity were 759, 210, and 58 MPa. The average cortical bone thickness ranged from 1.09 to 2.12 mm in the maxilla and from 1.59 to 3.03 mm in the mandible.13 Sheets of artificial bone used in the experiments had cortical layer thicknesses of 1, 2, and 3 mm and an elastic modulus of 16.7 GPa.
Table 1.
Mechanical properties of artificial bone (Sawbones®) used in this study.
| Density | Compressive |
Tensile |
|||
|---|---|---|---|---|---|
| Strength | Modulus | Strength | Modulus | ||
| Cortical bone | 1.64 g/cm3 | 157 MPa | 16.7 GPa | 157 MPa | 16 GPa |
| Trabecular bone | 0.64 g/cm3 | 31 MPa | 759 MPa | 19 MPa | 1000 MPa |
| Trabecular bone | 0.32 g/cm3 | 8.4 MPa | 210 MPa | 5.6 MPa | 284 MPa |
| Trabecular bone | 0.16 g/cm3 | 2.2 MPa | 58 MPa | 2.1 MPa | 86 MPa |
Resonance frequency analysis
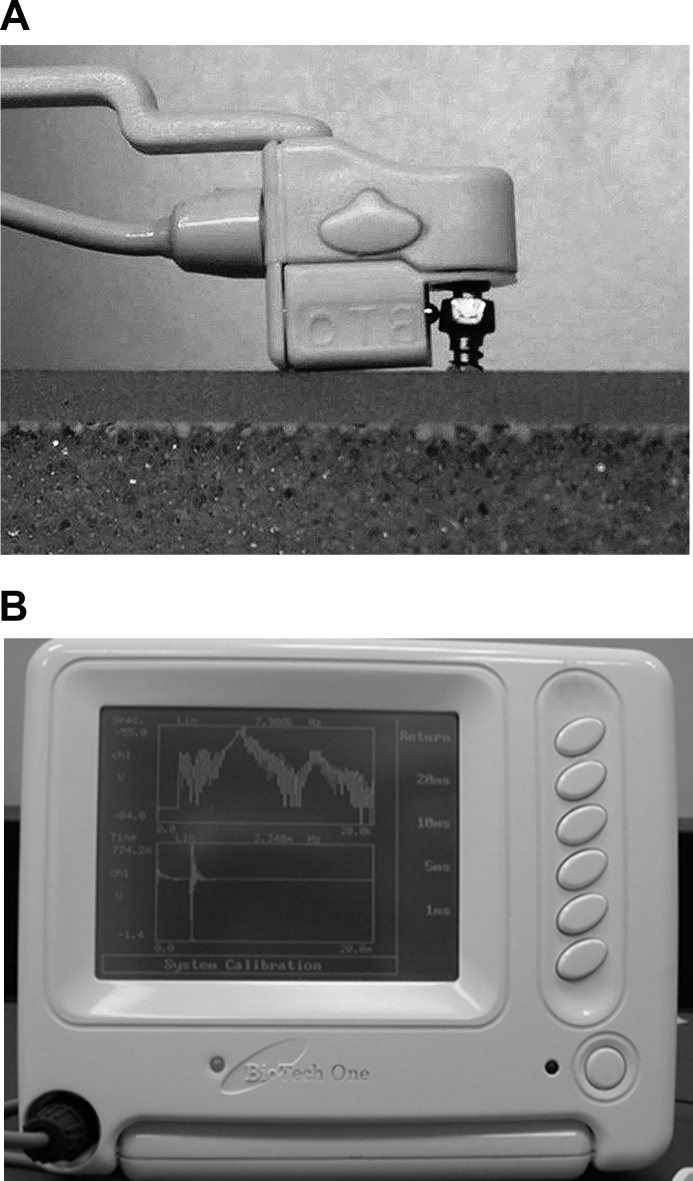
The RF of OMIs inserted into artificial bone was measured at three different insertion depths (2, 4, and 6 mm). All measurements were performed with an RF analyzer (Implomates®) (Fig. 1). For each OMI, RF was measured three times at each insertion depth, and the mean RF was recorded. The mean values for the five OMIs were used as the measured variables.
Figure 1.
The Implomates® resonance frequency analyzer used in this study. The device uses an impact force to excite resonance in the mini-implant screw. (A) The impact force is provided by a small electrically driven impact rod inside the transducer. (B) The received response signal is then transferred to a computer for frequency spectrum analysis.
Statistical analysis
The Kruskal-Wallis test was used to compare RF values for OMIs implanted under varying trabecular bone densities, varying cortical bone thicknesses, and varying insertion depths. Post-hoc pairwise comparisons were also performed by Wilcoxon rank-sum test. Simple linear regression analysis was performed to determine correlations between RF and trabecular bone density and between RF and cortical bone thickness in OMIs placed at each of the three insertion depths. A P value of <0.05 was considered statistically significant. All statistical analyses were performed using JMP 8 (SAS Institute Inc., Cary, NC, USA).
Results
Trabecular bone density versus resonance frequency
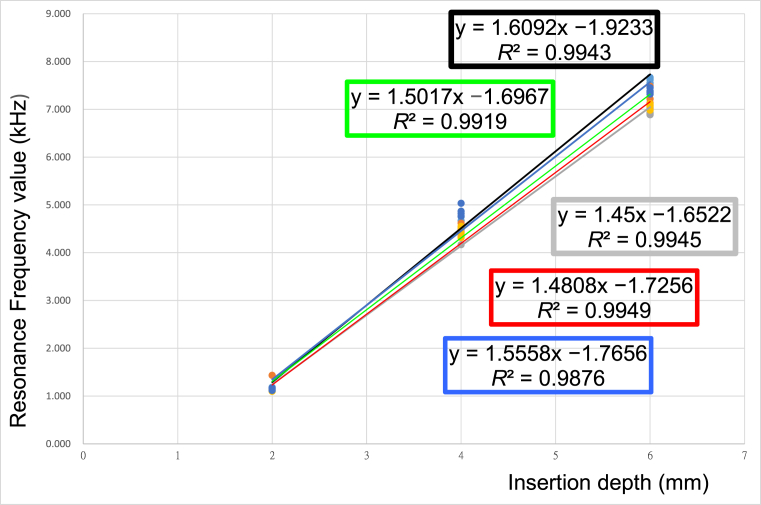
Table 2 presents the mean RFs and standard deviations for the OMIs. The OMIs were sequentially implanted into artificial bone with a cortical bone thickness of 2 mm and trabecular bone densities of 40, 20 and 10 pcf at depths of 2, 4, and 6 mm. The RF values were progressively increased with insertion depth. The Kruskal-Wallis test revealed that OMIs inserted to a depth of 2 mm did not significantly differ in RF (P = 0.1067). However, OMIs inserted to depths of 4 and 6 mm significantly differed in RF (P = 0.0111 and P = 0.0024, respectively). Post-hoc pairwise comparisons demonstrated that, at an insertion depth of 4 mm, RF values were significantly lower in Group 3 (10 pcf) than in Group 1 (40 pcf); at an insertion depth of 6 mm, RF values were significantly lower in Group 2 (20 pcf) and in Group 3 (10 pcf) than in Group 1 (40 pcf). For OMIs inserted to depths of 2, 4, and 6 mm, RF had a significant linear correlation with trabecular bone density. All R2 values exceeded 0.99 (P < 0.0001) with decreasing slopes (Group 1: β = 1.609; Group 2: β = 1.502; Group 3: β = 1.45) (Table 4 and Fig. 2). That is, as trabecular bone density decreased, RF values also decreased.
Table 2.
Mean ± standard deviation of the resonance frequency values (kHz) for mini-implants inserted with different depths in artificial bone with various trabecular bone densities.
| Group | n | 2 mm | 4 mm | 6 mm |
|---|---|---|---|---|
| 1 (40 pcf) | 5 | 1.160 ± 0.015 | 4.783 ± 0.077 | 7.597 ± 0.070 |
| 2 (20 pcf) | 5 | 1.190 ± 0.138 | 4.543 ± 0.180 | 7.197 ± 0.175 |
| 3 (10 pcf) | 5 | 1.133 ± 0.012 | 4.377 ± 0.121 | 6.933 ± 0.059 |
| P-valuea | 0.1067 | 0.0111 | 0.0024 | |
| Significant differenceb | Group 3 < Group 1 | Group 2 < Group 1 Group 3 < Group 1 |
||
Kruskal-Wallis test.
Post hoc pairwise comparisons were conducted by Wilcoxon rank-sum test.
Table 4.
A simple linear regression was used to analyze the relationship between the experimental groups of orthodontic mini-implants placed at 2, 4, and 6-mm depths and the measured resonance frequencies.
| Group | y = α + β x | β | P-value | R2 |
|---|---|---|---|---|
| 1 | y = −1.923333 + 1.6091667 x | 1.6091667 | <0.0001 | 0.994325 |
| 2 | y = −1.696667 + 1.5016667 x | 1.5016667 | <0.0001 | 0.991895 |
| 3 | y = −1.652222 + 1.45 x | 1.45 | <0.0001 | 0.994496 |
| 4 | y = −1.725556 + 1.4808333 x | 1.4808333 | <0.0001 | 0.99495 |
| 5 | y = −1.765556 + 1.5558333 x | 1.5558333 | <0.0001 | 0.987616 |
x, insertion depth of orthodontic mini-implant (2, 4, and 6 mm).
y, measured resonance frequency (kHz).
Figure 2.
Relationships between cortical bone thickness, trabecular bone density and measured resonance frequency values of the mini-implants.
Cortical bone thickness versus resonance frequency
Table 3 presents the mean RFs and standard deviations for OMIs sequentially inserted to depths of 2, 4, and 6 mm into artificial bone with a trabecular bone density of 20 pcf and cortical bone thicknesses of 1, 2 and 3 mm. The table shows that RF values increased as insertion depth increased. The RF values for OMIs inserted to a depth of 2 mm did not significantly differ (P = 0.2622). However, RF values significantly differed for OMIs with insertion depths of 4 mm (P = 0.0122) or 6 mm (P = 0.0116). Post-hoc pairwise comparisons revealed that RF values in Group 4 (1 mm) were significantly lower than those in Group 5 (3 mm) at an insertion depth of 4 mm and were significantly lower in Group 4 (1 mm) than in Group 5 (3 mm) at an insertion depth of 6 mm. Table 4 and Fig. 2 show that RF values had significant linear correlations with cortical bone thicknesses in OMIs inserted to depths of 2, 4, and 6 mm; all R2 values were higher than 0.98 (P < 0.0001) and had decreasing slopes (Group 5: β = 1.556; Group 2: β = 1.502; Group 4: β = 1.480). As cortical bone thickness decreased, RF also decreased.
Table 3.
Mean ± standard deviation of the resonance frequency values (kHz) for mini-implants inserted with different depths in artificial bone with various cortical bone thicknesses.
| Group | n | 2 mm | 4 mm | 6 mm |
|---|---|---|---|---|
| 5 (3 mm) | 5 | 1.150 ± 0.020 | 4.850 ± 0.111 | 7.373 ± 0.049 |
| 2 (2 mm) | 5 | 1.190 ± 0.138 | 4.543 ± 0.180 | 7.197 ± 0.175 |
| 4 (1 mm) | 5 | 1.123 ± 0.019 | 4.423 ± 0.109 | 7.047 ± 0.061 |
| P-valuea | 0.2622 | 0.0122 | 0.0116 | |
| Significant differenceb | Group 4 < Group 5 | Group 4 < Group 5 | ||
Kruskal-Wallis test.
Post hoc pairwise comparisons were conducted by Wilcoxon rank-sum test.
Discussion
The RF analysis is currently considered the gold standard for clinical assessment of implant stability.14 The Osstell® RFA device is routinely used to monitor the stability of a dental implant during treatment. A dental implant study showed that RF values measured with the Implomates® have a strong positive linear correlation with implant stability quotient (ISQ) values derived with the Ossstell® device (r = 0.991, P < 0.001).15 The Implomates® RFA device developed by Huang et al.15,16 utilizes an impact force for resonance excitation of an OMI. Impact force is provided by a small electrically driven rod inside the transducer. The design of the Implomates® transducer minimizes contact and no torque force is required during its application. The received response signal is then transferred to a computer for frequency spectrum analysis. High and low RF values are interpreted as high and low stability, respectively.
Titanium alloy OMIs and stainless steel OMIs are currently used in orthodontic practice. Compared to titanium alloy OMIs, stainless steel OMIs have better penetration and do not require a pilot hole. Another advantage of stainless steel OMIs is their ease of placement. Our study revealed that OMI stability is significantly associated with insertion depth, which is consistent with the results of a pig study in which OMIs placed in the pelvic bone blocks showed linear associations between insertion depth and stability.17 However, the primary stability of an OMI mainly depends on insertion depth rather than on the implant materials (e.g., titanium alloy or stainless steel).18
Artificial bone was used to simulate OMIs implanted in human jaw bones. However, the cortical layer density specification of the artificial bone is only 102 pcf (1.64 g/cm3). Therefore, this study compared varying trabecular bone density to determine whether changes in bone density affect RF. According to Nackaerts et al.,19 the bone mineral density (BMD) of the mandible ranges from 0.528 to 0.820 g/cm3 and averages 0.661 g/cm3. Based on this data, artificial bone with a density of 40 pcf (0.64 g/cm3) was used to simulate the trabecular BMD of the mandible. Artificial bone with a density of 20 pcf (0.32 g/cm3) was used to simulate the posterior maxilla,12 and artificial bone with a density of 10 pcf (0.16 g/cm3) was used to simulate a jawbone with low BMD. As mentioned above, the average cortical bone thickness ranges from 1.09 to 2.12 mm in the maxilla and from 1.59 to 3.03 mm in the mandible.13 Therefore, artificial bone with cortical layer thicknesses of 1, 2, and 3 mm were selected.
Primary stability had a strong correlation with cortical bone thickness, which is in agreement with a systematic review and meta-analysis of clinical studies.20 The primary stability of an OMI was positively associated with cortical bone thickness at the receptor site. However, the likelihood of OMI failure is higher in cortical bone with a thickness less than 1 mm compared to that with a thickness of 1 mm or more.21,22 Numerical analyses using finite element models have shown that deflection of OMIs decreases as cortical bone thickness increases23 and that cortical bone with thickness less than 1 mm is vulnerable to stresses that can cause bone resorption in this region.24 Motoyoshi et al.22,25 reported that a cortical bone thickness greater than 1 mm was needed for adequate primary stability and acceptable success rates in OMI placements.
Deguchi et al.26 used three-dimensional computed tomography to evaluate cortical bone thickness in various locations in the maxilla and the mandible and to compare cortical bone thickness between implant angulation measured by 3 angulations (30°, 45°, and 90°) from the long axis of each tooth mesial and distal to the first molar and distal to the second molar. In the maxilla, cortical bone thickness in the buccal region distal to the second molar (1.3 mm) was significantly lower than that mesial and distal to the first molar (1.8 mm and 1.5 mm). Cortical bone thickness was significantly higher on the lingual side of the second molar (1.7 mm) compared with the buccal side. In the mandible, cortical bone thickness was significantly higher mesial and distal to the second molar (1.8–2.0 mm) compared with the maxilla. Additionally, cortical bone thickness at 30° to the long axis of the tooth was as much as 1.5 times higher than cortical bone thickness at 90° (perpendicular) to the long axis of the tooth. Therefore, in most clinical cases, altering the OMI insertion angle can compensate for insufficient cortical bone thickness and can increase insertion depth.
A systematic review of clinical studies indicate that implant primary stability is positively associated with bone mineral density at the receptor site: as the bone density increases, the primary stability of dental implants also increases.27 In the present study, measured RF decreased as trabecular bone density decreased. When OMIs were implanted into artificial bone at a depth of 4 mm, RF in Group 3 (10 pcf) was significantly lower than Group 1 (40 pcf). At a depth of 6 mm, RF in Group 2 (20 pcf) and Group 3 (10 pcf) were significantly lower than Group 1. These experimental results indicate that a change in the density of the trabecular bone can be detected by a change in RF value.
Skeletal anchorage with mini-implant screws is widely used in orthodontic practice because it has no patient compliance requirements. Two key determinants of primary stability are bone quality and quantity.28 Cortical bone quantity and quality affect the long-term stability of an OMI. Stationary anchorage failure often results from low bone density due to inadequate cortical thickness.29 The primary implant stability of an OMI can be estimated by computed tomography measurements of cortical bone thickness and trabecular bone density before treatment.30
In conclusion, the primary stability of an OMI depends on both cortical bone thickness and trabecular bone density. Furthermore, these factors have strong linear correlations with RF value. Further clinical research is still needed to confirm the findings of this in vitro study performed using synthetic bone models. Measuring RF with an Implomates® device is a practical, noninvasive, and nondestructive approach to evaluating OMI stability.
Conflicts of interest
The authors have no conflicts of interest relevant to this article.
Acknowledgments
This study was supported by a grant from the Kaohsiung Medical University Hospital, Taiwan (KMUH96-6R05). The authors thank Dr. Yi-Hsin Yang for her valuable assistant with statistical analysis.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.jds.2019.06.002.
Appendix A. Supplementary data
The following is the Supplementary data to this article:
References
- 1.Favero L., Brollo P., Bressan E. Orthodontic anchorage with specific fixtures: related study analysis. Am J Orthod Dentofacial Orthop. 2002;122:84–94. doi: 10.1067/mod.2002.124870. [DOI] [PubMed] [Google Scholar]
- 2.Papadopoulos M.A., Papageorgiou S.N., Zogakis I.P. Clinical effectiveness of orthodontic miniscrew implants: a meta-analysis. J Dent Res. 2011;90:969–976. doi: 10.1177/0022034511409236. [DOI] [PubMed] [Google Scholar]
- 3.Papageorgiou S.N., Zogakis I.P., Papadopoulos M.A. Failure rates and associated risk factors of orthodontic miniscrew implants: a meta-analysis. Am J Orthod Dentofacial Orthop. 2012;142:577–595. doi: 10.1016/j.ajodo.2012.05.016. e7. [DOI] [PubMed] [Google Scholar]
- 4.Jang H.W., Kang J.K., Lee K., Lee Y.S., Park P.K. A retrospective study on related factors affecting the survival rate of dental implants. J Adv Prosthodont. 2011;3:204–215. doi: 10.4047/jap.2011.3.4.204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Schätzle M., Golland D., Roos M., Stawarczyk B. Accuracy of mechanical torque-limiting gauges for mini-screw placement. Clin Oral Implants Res. 2010;21:781–788. doi: 10.1111/j.1600-0501.2010.01927.x. [DOI] [PubMed] [Google Scholar]
- 6.Wilmes B., Rademacher C., Olthoff G., Drescher D. Parameters affecting primary stability of orthodontic mini-implants. J Orofac Orthop. 2006;67:162–174. doi: 10.1007/s00056-006-0611-z. [DOI] [PubMed] [Google Scholar]
- 7.Cheng S.J., Tseng I.Y., Lee J.J., Kok S.H. A prospective study of the risk factors associated with failure of mini-implants used for orthodontic anchorage. Int J Oral Maxillofac Implant. 2004;19:100–106. [PubMed] [Google Scholar]
- 8.Trisi P., Rao W., Rebaudi A. A histometric comparison of smooth and rough titanium implants in human low-density jawbone. Int J Oral Maxillofac Implant. 1999;14:689–698. [PubMed] [Google Scholar]
- 9.Maino B.G., Bednar J., Pagin P., Mura P. The spider screw for skeletal anchorage. J Clin Orthod. 2003;37:90–97. [PubMed] [Google Scholar]
- 10.Tolstunov L. Implant zones of the jaws: implant location and related success rate. J Oral Implantol. 2007;33:211–220. doi: 10.1563/1548-1336(2007)33[211:IZOTJI]2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- 11.Su Y.Y., Wilmes B., Hönscheid R., Drescher D. Application of a wireless resonance frequency transducer to assess primary stability of orthodontic mini-implants: an in vitro study in pig ilia. Int J Oral Maxillofac Implant. 2009;24:647–654. [PubMed] [Google Scholar]
- 12.Devlin H., Horner K., Ledgerton D. A comparison of maxillary and mandibular bone mineral densities. J Prosthet Dent. 1998;79:323–327. doi: 10.1016/s0022-3913(98)70245-8. [DOI] [PubMed] [Google Scholar]
- 13.Ono A., Motoyoshi M., Shimizu N. Cortical bone thickness in the buccal posterior region for orthodontic mini-implants. Int J Oral Maxillofac Surg. 2008;37:334–340. doi: 10.1016/j.ijom.2008.01.005. [DOI] [PubMed] [Google Scholar]
- 14.Lachmann S., Laval J.Y., Jäger B. Resonance frequency analysis and damping capacity assessment. Part 2: peri-implant bone loss follow-up. An in vitro study with the Periotest and Osstell instruments. Clin Oral Implants Res. 2006;17:80–84. doi: 10.1111/j.1600-0501.2005.01174.x. [DOI] [PubMed] [Google Scholar]
- 15.Chang W.J., Lee S.Y., Wu C.C. A newly designed resonance frequency analysis device for dental implant stability detection. Dent Mater J. 2007;26:665–671. doi: 10.4012/dmj.26.665. [DOI] [PubMed] [Google Scholar]
- 16.Huang H.M., Cheng K.Y., Chen C.F., Ou K.L., Li C.T., Lee S.Y. Design of a stability-detecting device for dental implants. Proc Inst Mech Eng H. 2005;219:203–211. doi: 10.1243/095441105X9336. [DOI] [PubMed] [Google Scholar]
- 17.Nienkemper M., Santel N., Hönscheid R., Drescher D. Orthodontic mini-implant stability at different insertion depths: sensitivity of three stability measurement methods. J Orofac Orthop. 2016;77:296–303. doi: 10.1007/s00056-016-0036-2. [DOI] [PubMed] [Google Scholar]
- 18.Brown R.N., Sexton B.E., Gabriel Chu T.M. Comparison of stainless steel and titanium alloy orthodontic miniscrew implants: a mechanical and histologic analysis. Am J Orthod Dentofacial Orthop. 2014;145:496–504. doi: 10.1016/j.ajodo.2013.12.022. [DOI] [PubMed] [Google Scholar]
- 19.Nackaerts O., Jacobs R., Horner K. Bone density measurements in intra-oral radiographs. Clin Oral Investig. 2007;11:225–229. doi: 10.1007/s00784-007-0107-2. [DOI] [PubMed] [Google Scholar]
- 20.Marquezan M., Mattos C.T., Sant'Anna E.F., de Souza M.M., Maia L.C. Does cortical thickness influence the primary stability of miniscrews? : a systematic review and meta-analysis. Angle Orthod. 2014;84:1093–1103. doi: 10.2319/093013-716.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Motoyoshi M., Yoshida T., Ono A., Shimizu N. Effect of cortical bone thickness and implant placement torque on stability of orthodontic mini-implants. Int J Oral Maxillofac Implant. 2007;22:779–784. [PubMed] [Google Scholar]
- 22.Motoyoshi M., Inaba M., Ono A., Ueno S., Shimizu N. The effect of cortical bone thickness on the stability of orthodontic mini-implants and on the stress distribution in surrounding bone. Int J Oral Maxillofac Surg. 2009;38:13–18. doi: 10.1016/j.ijom.2008.09.006. [DOI] [PubMed] [Google Scholar]
- 23.Stahl E., Keilig L., Abdelgader I., Jäger A., Bourauel C. Numerical analyses of biomechanical behavior of various orthodontic anchorage implants. J Orofac Orthop. 2009;70:115–127. doi: 10.1007/s00056-009-0817-y. [DOI] [PubMed] [Google Scholar]
- 24.Motoyoshi M., Ueno S., Okazaki K., Shimizu N. Bone stress for a mini-implant close to the roots of adjacent teeth–3D finite element analysis. Int J Oral Maxillofac Surg. 2009;38:363–368. doi: 10.1016/j.ijom.2009.02.011. [DOI] [PubMed] [Google Scholar]
- 25.Motoyoshi M., Matsuoka M., Shimizu N. Application of orthodontic mini-implants in adolescents. Int J Oral Maxillofac Surg. 2007;36:695–699. doi: 10.1016/j.ijom.2007.03.009. [DOI] [PubMed] [Google Scholar]
- 26.Deguchi T., Nasu M., Murakami K., Yabuuchi T., Kamioka H., Takano-Yamamoto T. Quantitative evaluation of cortical bone thickness with computed tomographic scanning for orthodontic implants. Am J Orthod Dentofacial Orthop. 2006;129:721. doi: 10.1016/j.ajodo.2006.02.026. e7–12. [DOI] [PubMed] [Google Scholar]
- 27.Marquezan M., Osório A., Sant'Anna E., Souza M.M., Maia L. Does bone mineral density influence the primary stability of dental implants? A systematic review. Clin Oral Implants Res. 2012;23:767–774. doi: 10.1111/j.1600-0501.2011.02228.x. [DOI] [PubMed] [Google Scholar]
- 28.Kravitz N.D., Kusnoto B. Risks and complications of orthodontic miniscrews. Am J Orthod Dentofacial Orthop. 2007;131:S43–S51. doi: 10.1016/j.ajodo.2006.04.027. [DOI] [PubMed] [Google Scholar]
- 29.Melsen B., Verna C. Miniscrew implants: the Aarhus anchorage system. Semin Orthod. 2005;11:24–31. [Google Scholar]
- 30.Rozé J., Babu S., Saffazadeh A., Gayet-Delacroix M., Hoomaert A., Layrolle P. Correlating implant stability to bone structure. Clin Oral Implants Res. 2009;20:1140–1145. doi: 10.1111/j.1600-0501.2009.01745.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.