Abstract
Background/Purpose
Sialolithiasis, the so-called salivary gland stone, is a condition forming salivary calculi within a salivary gland or ducts. Little is known about the epidemiological survey of sialolithiasis in Taiwanese population. In this study, we conducted an age-period-cohort (APC) analysis evaluating the prevalence of sialolithiasis.
Materials and methods
A retrospective study was conducted to analyze the registered database compiled by the Taiwanese National Health Insurance Research Database from 1996 to 2013. The APC analysis was performed to investigate the effects of age, diagnosis period, and birth cohort with sialolithiasis.
Results
We found that the prevalence of sialolithiasis varied from 1.4 (105) to 2.3 (105). The mean age ±standard deviation with sialolithiasis from 1996 to 2013 was 37.7 ± 18.5 and 46.2 ± 18.6 years old, respectively. The prevalence was higher among male than female (RR: 1.10; 95% CI: 1.05–1.15, p < 0.001). The age >65 group had higher risk compared to age <40 group (RR: 2.27; 95% CI: 2.13–2.43, p < 0.001). The relative risk for sialolithiasis demonstrated significant age effect (p < 0.001). The relative risk for sialolithiasis did not show the significant period effect (p = 0.742). The relative risk for sialolithiasis demonstrated significant cohort effect (p = 0.01). The relative risk for sialolithiasis demonstrated significant APC effect (p = 0.002).
Conclusion
Form this nationwide population-based database, the prevalence of sialolithiasis occurs more frequently in male than in female. In addition, the relative risk for sialolithiasis demonstrated the significant APC effects.
Keywords: Sialolithiasis, Taiwan, Prevalence, Nationwide population-based study, Age-period-cohort
Introduction
Sialolithiasis is characterized by a calcified mass within a salivary gland or duct mainly in major salivary gland and very rare in minor salivary glands.1 This condition usually leads to the obstruction of salivary duct and subsequently results in increased risk of bacterial infections. The symptoms are swelling, inflammation, and pain caused by the obstruction of physiological salivary flow and commonly meal related.2,3
The prevalence of sialolithiasis was between 1 and 2%.4 From the literature review, the incidence rate of sialolithiasis was found about 2.9 and 5.5 per 100,000 person-years based on selected hospital data.5, 6, 7 Recently, the incidence rate of symptomatic sialolithiasis in Denmark was between 7.3 and 14.1 per 100,000 person-years.8
Little is known about the status of sialolithiasis in Taiwan. In this study, the prevalence of sialolithiasis was investigated by using National Health Insurance Research Database (NHIRD) from 1996 to 2013. The age-period-cohort (APC) analysis was performed to investigate the effects of age, diagnosis period, and birth cohort on sialolithiasis.
Materials and methods
Data source and ethical consideration
This study was approved by the Ethics Review Board at the Chung Shan Medical University Hospital. Dental dataset (DN),9 dental original claim data for ambulatory care expenditures by dental visit, was used for this retrospective study. Due to this dataset was analyzed anonymously, no informed consent from participants was required. This DN has been used for the epidemiology survey for dental diseases10,11 and even the conditions of emergency dental visits.12
Patient identification and measurements
The diagnosis of sialolithiasis was identified according to the International Classification of Disease, Ninth revision (ICD-9), code of 527.5. In this study, we identified dental visit patients with diagnosed sialolithiasis during the period between January 1, 1996 and December 31, 2013 for annual prevalence rate of sialolithiasis from DN.
APC analysis was performed to investigate the effects of age, diagnosis period, and birth cohort with sialolithiasis as described by Yu et al.13 Cases of sialolithiasis were categorized into 17 age groups (0–4 to 80–84), 3 period groups (1999–2004, 2005–2009, and 2010–2013), and 19 birth cohort groups (1918–2008) with a corresponding 5-year interval. Several models such as age alone, period alone, cohort alone, and age-period-cohort were generated. The goodness of fit for the specified model was evaluated by the deviance/degree of freedom (DF).
Statistical analysis
The relative risk of sialolithiasis from 1996 to 2013 after adjusting for year, age, and gender was evaluated by multivariate Poisson regression. Relative risk (RR), percent per year (PPY), and 95% confidence interval (CI) of sialolithiasis by APC analysis were calculated. All statistical analyses were performed with the SPSS version 18 (SPSS, Chicago, IL, USA).
Results
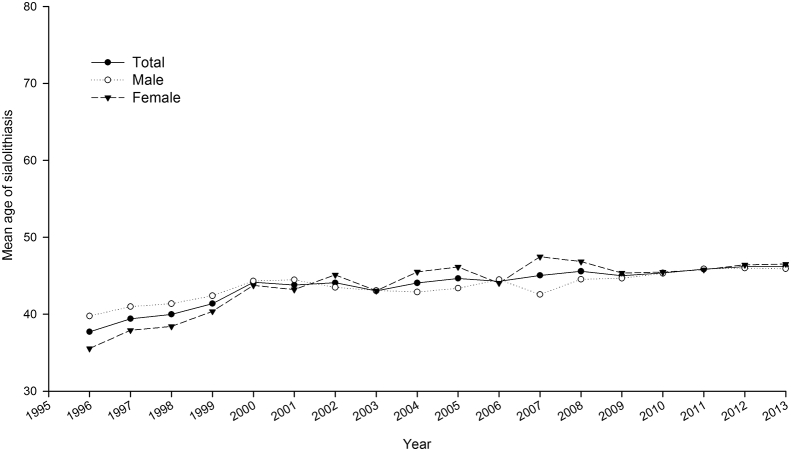
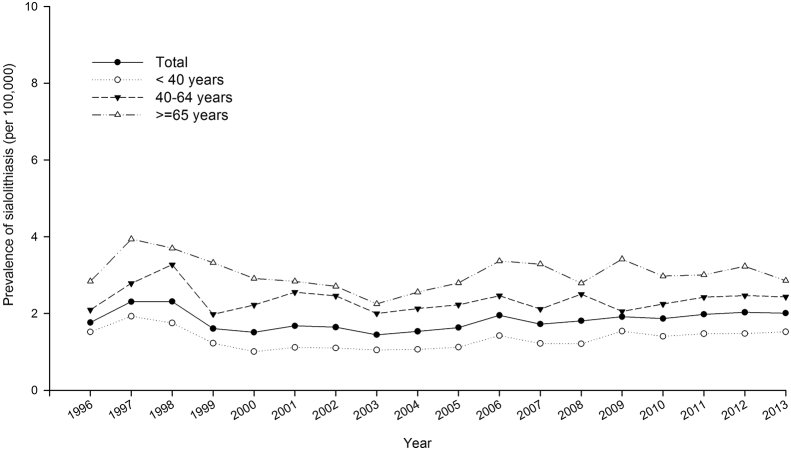
The sex-specific annual prevalence of sialolithiasis from 1996 to 2013 is presented in Table 1. The prevalence of sialolithiasis was ranged from 1.4 (per 105) to 2.3 (per 105). The average annual prevalence was 1.918 (per 105). As shown in Fig. 1, the mean age of patients with sialolithiasis was 46.24 years old. The prevalence of sialolithiasis stratified by age was shown in Fig. 2. The relative risk of sialolithiasis by multivariate Poisson regression demonstrated in Table 2. The risk was no significant change in annual increments (RR: 1.00; 95% confidence interval (CI), 0.99–1.00, p = 0.281). Compared to age <40 years old, the elder group (≧65 years old) had higher risk of sialolithiasis (RR: 2.27; 95% CI: 2.13–2.43, p < 0.001). The male had a significantly higher sialolithiasis risk than females (RR: 1.10; 95% CI: 1.05–1.15, p < 0.001).
Table 1.
Prevalence of sialolithiasis by gender.
|
Year |
Total |
Male |
Female |
|||||||
|---|---|---|---|---|---|---|---|---|---|---|
| N | No. of Sialolithiasis | P | N | No. of Sialolithiasis | P | N | No. of Sialolithiasis | P | Sex ratio | |
| 1996 | 21,525,433 | 380 | 1.8 | 11,065,798 | 198 | 1.8 | 10,459,635 | 181 | 1.7 | 1.1 |
| 1997 | 21,742,815 | 502 | 2.3 | 11,163,764 | 248 | 2.2 | 10,579,051 | 253 | 2.4 | 1.0 |
| 1998 | 21,928,591 | 507 | 2.3 | 11,243,408 | 270 | 2.4 | 10,685,183 | 237 | 2.2 | 1.1 |
| 1999 | 22,092,387 | 355 | 1.6 | 11,312,728 | 179 | 1.6 | 10,779,659 | 176 | 1.6 | 1.0 |
| 2000 | 22,276,672 | 336 | 1.5 | 11,392,050 | 200 | 1.8 | 10,884,622 | 135 | 1.2 | 1.5 |
| 2001 | 22,405,568 | 376 | 1.7 | 11,441,651 | 215 | 1.9 | 10,963,917 | 158 | 1.4 | 1.4 |
| 2002 | 22,520,776 | 370 | 1.6 | 11,485,409 | 221 | 1.9 | 11,035,367 | 148 | 1.3 | 1.5 |
| 2003 | 22,604,550 | 327 | 1.4 | 11,515,062 | 160 | 1.4 | 11,089,488 | 166 | 1.5 | 1.0 |
| 2004 | 22,689,122 | 348 | 1.5 | 11,541,585 | 192 | 1.7 | 11,147,537 | 156 | 1.4 | 1.2 |
| 2005 | 22,770,383 | 372 | 1.6 | 11,562,440 | 199 | 1.7 | 11,207,943 | 173 | 1.5 | 1.2 |
| 2006 | 22,876,527 | 447 | 2.0 | 11,591,707 | 236 | 2.0 | 11,284,820 | 211 | 1.9 | 1.1 |
| 2007 | 22,958,360 | 396 | 1.7 | 11,608,767 | 196 | 1.7 | 11,349,593 | 200 | 1.8 | 1.0 |
| 2008 | 23,037,031 | 417 | 1.8 | 11,626,351 | 229 | 2.0 | 11,410,680 | 188 | 1.6 | 1.2 |
| 2009 | 23,119,772 | 443 | 1.9 | 11,636,734 | 221 | 1.9 | 11,483,038 | 222 | 1.9 | 1.0 |
| 2010 | 23,162,123 | 433 | 1.9 | 11,635,225 | 220 | 1.9 | 11,526,898 | 213 | 1.8 | 1.0 |
| 2011 | 23,224,912 | 460 | 2.0 | 11,645,674 | 225 | 1.9 | 11,579,238 | 235 | 2.0 | 1.0 |
| 2012 | 23,315,822 | 474 | 2.0 | 11,673,319 | 261 | 2.2 | 11,642,503 | 213 | 1.8 | 1.2 |
| 2013 | 23,373,517 | 470 | 2.0 | 11,684,674 | 245 | 2.1 | 11,688,843 | 225 | 1.9 | 1.1 |
P: prevalence rate per 100,000 population.
Figure 1.
Time trends for the mean age of patients with sialolithiasis from 1996 to 2013.
Figure 2.
Age-specific group in the prevalence of sialolithiasis in Taiwan from 1996 to 2013.
Table 2.
Relative risk (RR) of sialolithiasis by multivariate Poisson regression.
| 95% C.I. |
p value | |||
|---|---|---|---|---|
| RRa | Lower | Upper | ||
| Year (per 1 year) | 1.00 | 0.99 | 1.00 | 0.281 |
| Age (ref: <40) | ||||
| 40-64 | 1.76 | 1.68 | 1.85 | <0.001 |
| ≧65 | 2.27 | 2.13 | 2.43 | <0.001 |
| Gender (ref: female) | ||||
| Male | 1.10 | 1.05 | 1.15 | <0.001 |
Adjusted for year, age and gender.
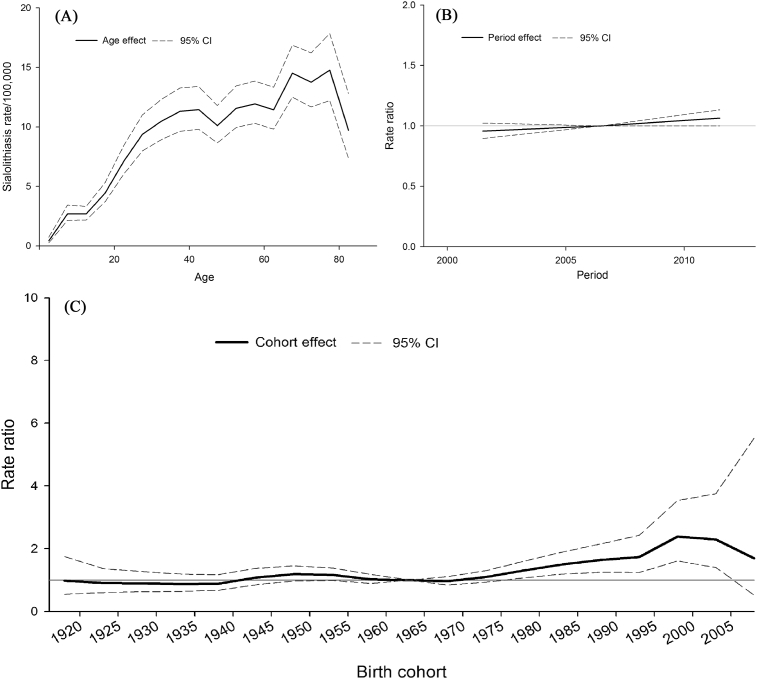
The effects of age, diagnosis period, and birth cohort of sialolithiasis were revealed in Table 3. In terms of age effect, the prevalence of sialolithiasis increased with age. The slope of trend was steeper before the age of 40 years old and the peak were around 65 and 75 years old, respectively (Fig. 3A). The relative risk for sialolithiasis demonstrated significant age effect (p < 0.001).
Table 3.
The results of age-period-cohort model for sialolithiasis.
| Model | DF | Deviance | Deviance/DF | P-Value |
|---|---|---|---|---|
| Age | 15 | 118.49 | 7.90 | <0.001 |
| Period | 1 | 0.11 | 0.11 | 0.742 |
| Cohort | 17 | 33.53 | 1.97 | 0.01 |
| Age-Period-Cohort | 1 | 9.57 | 9.57 | 0.002 |
DF: degree of freedom.
Figure 3.
Cross–sectional curve from age–period–cohort model (a) age effect (b) period effect (c) birth effect in the rate of sialolithiasis in Taiwan.
In the period effect, there was not significant difference in the relative risk of sialolithiasis in different eras (Fig. 3B). As shown in Table 3, the relative risk for sialolithiasis did not show the significant period effect (p = 0.742).
In the cohort effect, the results demonstrated a significant higher sialolithiasis risk than the generation from 1978 to 2003 as compared with 1963 as a reference generation (Fig. 3C). As shown in Table 3, the relative risk for sialolithiasis demonstrated significant cohort effect (p = 0.01).
In summary, the age and cohort effect were associated with the risk for sialolithiasis. As shown in Table 3, the relative risk for sialolithiasis demonstrated significant APC effect (p = 0.002).
Discussion
In this study, the average annual prevalence of sialolithiasis was 1.918 (per 105) in Taiwan. The nationwide incidence of hospital-admitted sialolithiasis have been evaluated in Denmark based on ICD10 diagnosis.7,8 However, this is the first nationwide population based study to investigate the prevalence of sialolithiasis.
In this study, male had higher risk suffering from sialolithiasis than female. Similar results were found by Sigismund et al.3 in Germany, and Stelmach et al.14 in Poland. There was no significant gender difference in a University Hospital, San Francisco, USA. However, Schrøder SA et al.8 who reported female had higher risk suffering from sialolithiasis than male in Denmark. The reason is not quite clear. There may be due to different race, geography region, and the contents of drinking water.
APC model is as a parametric statistical model widely used in epidemiology research to estimate the independent effect of age, time period and birth cohort rates of a particular event.15,16 Age effects are associated with the outcome of time. Period effects can affect all ages simultaneously over time. Birth cohort effects involve changes across groups with the same birth year who presented the same outcome during the same period. APC provides important clues for social, historical, and environmental factors that impacts disease morbidity or mortality. To the best of our knowledge, this is the first APC analysis of sialolithiasis in the world. The relative risk for sialolithiasis demonstrated significant APC effect.
The strength of this study was the use of same methodology to investigate the prevalence of sialolithiasis in data available from cross-sectional analysis conducted from 1996 to 2013. The use of a nationwide population-based database can provide sufficient sample size, generalizability, and statistical power to assess the status of sialolithiasis in Taiwan.
With APC analysis may be beneficial to provide the age effects, period effects, and cohort effects for sialolithiasis. The age effects mainly reflect the physiological and social changes in different ages. In this study, there are at least three peaks of the sialolithiasis around age 40, 65, and 80 years old. This suggests that the age might impact the occurrence of sialolithiasis.
Birth cohort effects involve the changes across groups with the same birth year who presented the same outcome during the same period. The birth cohorts showed the highest ratio of sialolithiasis from 1998 to 2003. The reason may explain as following. In Taiwan, the National Health Insurance (NHI) program launched in 1995. Therefore, it is speculated that the NHI system has successfully improved the early diagnosis and the treatment demand of this disease. Taken together, this result can be a reference for developing the relevant oral health policies or promoting the prevention of sialolithiasis in specific age groups. It may assist in treatment strategies in this national dental care system for sialolithiasis.
However, there are still some limitations in this study. First, the prevalence of sialolithiasis might be underestimated by using DN database. It would miss to collect the diagnosis of sialolithiasis from medical doctors, especially the specialty on ear, nose, and throat. However, DN is the records of all dental visit per year from 1996 to 2013. This could provide the long-term monitor of diagnosed sialolithiasis from dental treatment. Second, the diagnosis of sialolithiasis was based on ICD-9 code. It could not obtain the actual locations of sialolithiasis form which salivary glands, either major or minor. Third, sialolithiasis can be difficult to diagnose because the sialolith is not always possible to visualize and the variation in the severity of symptoms. Therefore, further studies are required to identify the histopathological, examination with X-ray or sialography to improve the accurate diagnosis of sialolithiasis. Finally, one of the proposed factors is tobacco smoking.17 The DN dataset without the status of smoking should be noted.
Although the above limitations raise that may need future investigations, this study still remains valuable because it provides the first nationwide population-based survey in Taiwan currently. Our study revealed the prevalence of sialolithiasis occurs more frequently in male than in female. The relative risk for sialolithiasis demonstrated significant APC effects.
Conflicts of interest
The authors declare no conflict of interest.
References
- 1.Wang W.C., Chen C.Y., Hsu H.J., Kuo J.H., Lin L.M., Chen Y.K. Sialolithiasis of minor salivary glands: a review of 17 cases. J Dent Sci. 2016;11:152–155. doi: 10.1016/j.jds.2015.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lustmann J., Regev E., Melamed Y. Sialolithiasis: a survey on 245 patients and a review of the literature. Int J Oral Maxillofac Surg. 1990;19:135–138. doi: 10.1016/s0901-5027(05)80127-4. [DOI] [PubMed] [Google Scholar]
- 3.Sigismund P.E., Zenk J., Koch M., Schapher M., Rudes M., Iro H. Nearly 3000 salivary stones: some clinical and epidemiologic aspects. The Laryngoscope. 2015;125:1879–1882. doi: 10.1002/lary.25377. [DOI] [PubMed] [Google Scholar]
- 4.Rauch S., Gorlin R.J. Disease of the salivary glands. In: Gorlin R.J., Goldmann H.M., editors. Oral pathology. Mosby; St Louis, MO: 1970. pp. 997–1003. [Google Scholar]
- 5.Sherman J.A., McGurk M. Lack of correlation between water hardness and salivary calculi in England. Br J Oral Maxillofac Surg. 2000;38:50–53. doi: 10.1054/bjom.1999.0074. [DOI] [PubMed] [Google Scholar]
- 6.Escudier M.P., McGurk M. Symptomatic sialoadenitis and sialolithiasis in the English population, an estimate of the cost of hospital treatment. Br Dent J. 1999;186:463–466. doi: 10.1038/sj.bdj.4800141. [DOI] [PubMed] [Google Scholar]
- 7.Schroder S., Homoe P., Wagner N., Vataire A.L., Lundager Madsen H.E., Bardow A. Does drinking water influence hospital admitted sialolithiasis on an epidemiological level in Denmark? BMJ Open. 2015;5 doi: 10.1136/bmjopen-2014-007385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Schrøder S.A., Andersson M., Wohlfahrt J., Wagner N., Bardow A., Homøe P. Incidence of sialolithiasis in Denmark: a nationwide population-based register study. Eur Arch Oto-Rhino-Laryngol. 2017;274:1975–1981. doi: 10.1007/s00405-016-4437-z. [DOI] [PubMed] [Google Scholar]
- 9.National Health Insurance Administration, Ministry of health and welfare, Taiwan, R.O.C. National health insurance research database. Data subsets. Assessed from nhird.nhri.org.tw/en.
- 10.Yang S.F., Wang Y.H., Su N.Y. Changes in prevalence of pre-cancerous oral submucous fibrosis from 1996-2013 in Taiwan: a nationwide population-based retrospective study. J Formos Med Assoc. 2018;117:147–152. doi: 10.1016/j.jfma.2017.01.012. [DOI] [PubMed] [Google Scholar]
- 11.Chen Y.T., Wang Y.H., Yu H.C., Yu C.H., Chang Y.C. Time trend in the prevalence of oral lichen planus based on Taiwanese National Health Insurance Research Database 1996-2013. J Dent Sci. 2018;13:274–280. doi: 10.1016/j.jds.2018.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Huang S.M., Huang J.Y., Yu H.C., Su N.Y., Chang Y.C. Trends, demographics, and conditions of emergency dental visits in Taiwan 1997-2013: a nationwide population-based retrospective study. J Formos Med Assoc. 2019;118:582–587. doi: 10.1016/j.jfma.2018.11.012. [DOI] [PubMed] [Google Scholar]
- 13.Yu H.C., Su N.Y., Huang J.Y., Lee S.S., Chang Y.C. Trends in the prevalence of periodontitis in Taiwan from 1997 to 2013: a nationwide population-based retrospective study. Medicine. 2017;96 doi: 10.1097/MD.0000000000008585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Stelmach R., Pawłowski M., Klimek L., Janas A. Biochemical structure, symptoms, location and treatment of sialoliths. J Dent Sci. 2016;11:299–303. doi: 10.1016/j.jds.2016.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Holford T.R. Understanding the effects of age, period and cohort on incidence and mortality rates. Annu Rev Public Health. 1991;12:425–457. doi: 10.1146/annurev.pu.12.050191.002233. [DOI] [PubMed] [Google Scholar]
- 16.Keyes K.M., Li G. A multiphase method for estimating cohort effects in age-period contingency table data. Ann Epidemiol. 2010;20:779–785. doi: 10.1016/j.annepidem.2010.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Williams M.F. Sialolithiasis. Otolaryngol Clin. 1999;32:819–834. doi: 10.1016/s0030-6665(05)70175-4. [DOI] [PubMed] [Google Scholar]