Abstract
This meta-analysis was conducted to assess mortality, clinical and microbiological response following antibiotic therapy among patients with carbapenem-resistant Klebsiella pneumoniae (CRKP) infections. Fifty-four observational studies involving 3195 CRKP-infected patients who received antibiotic treatment were included. We found combination therapy to be associated with lower mortality than monotherapy, but no differences in clinical and microbiological response. Among the various combination therapies, no significant differences in mortality, clinical and microbiological response were found. Moreover, clinical outcomes did not differ significantly among various monotherapies. This report describes the data related to the article entitled: “A systematic review and meta-analysis of treatment outcomes following antibiotic therapy among patients with carbapenem-resistant Klebsiella pneumoniae infections”.
Keywords: Antibiotic resistance, Carbapenem-resistant, Infectious diseases, Pharmacotherapy, Klebsiella pneumoniae
Specifications Table
| Subject | Infectious diseases |
| Specific subject area | Antibiotic efficacy against carbapenem-resistant Klebsiella pneumoniae (CRKP) infections. |
| Type of data | Table Chart Figure |
| How data were acquired | Systematic review and meta-analysis |
| Data format | Raw data and analyzed data |
| Parameters for data collection | Outcomes (mortality, clinical and microbiological response) among antibiotic-treated patients with carbapenem-resistant Klebsiella pneumonia (CRKP) infections. |
| Description of data collection | The data presented is based on fifty-five articles (54 studies) selected based on a systematic literature review that involved searches performed in Medline, Embase, Cochrane Central, and the International Pharmaceutical Abstracts databases from their inception to December 2018. |
| Data source location | Monash University, Melbourne, Australia |
| Data accessibility | Data are with this article |
| Related research article | Agyeman AA, Bergen PJ, Rao GG, Nation RL, Landersdorfer CB A systematic review and meta-analysis of treatment outcomes following antibiotic therapy among patients with carbapenem-resistant Klebsiella pneumoniae infections International Journal of Antimicrobial Agents. |
Value of the Data
|
1. Data
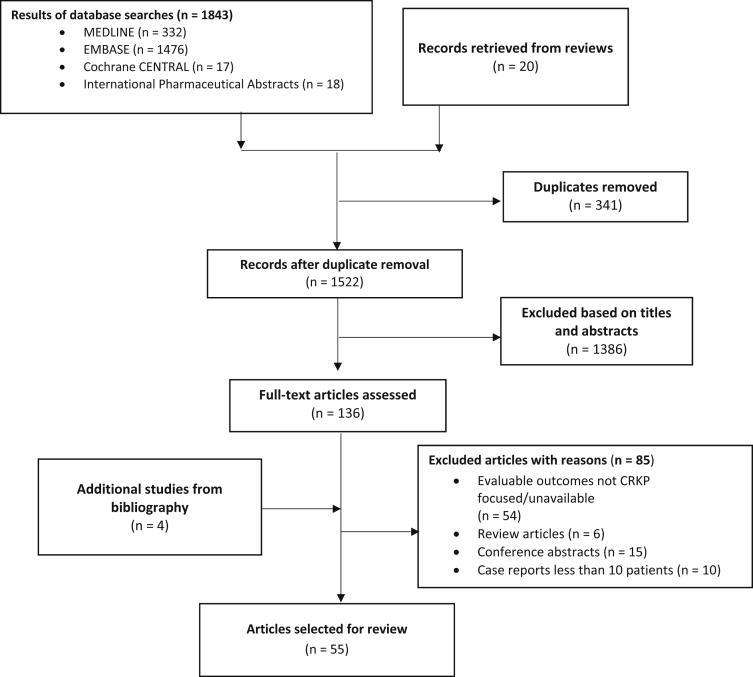
Based on the inclusion criteria, out of 1863 articles initially screened, fifty-five articles (54 studies) reporting treatment outcomes among antibiotic-treated CRKP-infected patients were included in the meta-analysis (Fig. 1). The included studies were of good quality as per their quality appraisal scores (Table 1) which were evaluated using the Newcastle-Ottawa scale (NOS) for nonrandomized trials included in meta-analyses [1].
Fig. 1.
Flow diagram of the systematic review process.
Table 1.
Newcastle-Ottawa Scale for quality assessment of included studies.
| Article No. | First author, year | Criteria for quality assessment |
Total quality score | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Selection |
Comparability |
Outcome/Exposure |
||||||||
| 1 | 2 | 3 | 4 | 1 | 2 | 3 | ||||
| 1 | Alexander, 2012 [2] | * | n.a | * | * | n.a | * | * | * | 6 |
| 2 | Bergamasco, 2012 [3] | * | n.a | * | * | n.a | * | * | * | 7 |
| 3 | Brizendine, 2015 [4] | * | * | * | * | * * | * | * | * | 9 |
| 4 | Capone, 2013 [5] | * | n.a | * | * | n.a | * | * | - | 5 |
| 5 | Cprek, 2016 [6] | * | n.a | * | * | n.a | * | * | * | 6 |
| 6 | Daikos, 2009 [7] | * | * | * | * | ** | * | * | * | 9 |
| 7 | Daikos, 2014 [8] | * | n.a | * | * | n.a | * | * | * | 6 |
| 8 | Dubrovskaya, 2013 [9] | * | n.a | * | * | n.a | * | * | * | 6 |
| 9 | Gomez-Simmonds, 2016 [10] | * | n.a | * | * | n.a | * | * | * | 6 |
| 10 | Ji, 2015 [11] | * | n.a | * | * | n.a | * | * | * | 6 |
| 11 | Machuca, 2017 [12] | * | n.a | * | * | n.a | * | * | * | 6 |
| 12 | Michalopoulos, 2010 [13] | * | n.a | * | * | n.a | * | * | * | 6 |
| 13 | Mouloudi, 2010 [14] | * | * | - | - | ** | * | * | n.a | 6 |
| 14 | Nguyen, 2010 [15] | * | n.a | * | * | n.a | * | * | * | 6 |
| 15 | Qureshi, 2012 [16] | * | n.a | * | * | n.a | * | * | * | 6 |
| 16 | Qureshi, 2014 [17] | * | * | * | * | ** | * | * | * | 9 |
| 17 | Sanchez-Romero, 2012 [18] | * | * | * | * | ** | * | - | * | 8 |
| 18 | Satlin, 2011 [19] | * | n.a | * | * | n.a | * | * | * | 6 |
| 19 | Souli, 2008 [20] | * | n.a | * | * | n.a | * | * | * | 6 |
| 20 | Souli, 2010 [21] | * | n.a | * | * | n.a | * | * | * | 6 |
| 21 | Souli, 2017 [22] | * | n.a | * | * | n.a | * | * | * | 6 |
| 22 | Shields, 2016a [23] | * | n.a | * | * | n.a | * | * | * | 6 |
| 23 | Trecarichi, 2016 [24] | * | * | * | * | ** | * | * | * | 9 |
| 24 | Tumbarello, 2012 [25] | * | n.a | * | * | n.a | * | * | * | 6 |
| 25 | Tumbarello, 2015 [26] | * | n.a | * | * | n.a | * | * | * | 6 |
| 26 | Vardakas, 2015 [27] | * | n.a | * | * | n.a | * | * | * | 6 |
| 27 | Venugopalan, 2017 [28] | * | * | * | * | ** | * | * | n.a | 8 |
| 28 | Weisenberg, 2009 [29] | * | n.a | * | * | n.a | * | - | * | 5 |
| 29 | Daikos, 2007 [30] | * | * | - | - | ** | * | * | n.a | 6 |
| 30 | Maltezou, 2009 [31] | * | n.a | * | * | n.a | * | * | * | 6 |
| 31 | Navarro-San, 2013 [32] | * | n.a | * | * | n.a | * | * | * | 6 |
| 32 | Di Carlo, 2013 [33] | * | n.a | * | * | n.a | * | * | * | 6 |
| 33 | Balandin, 2014 [34] | * | n.a | * | * | n.a | * | * | * | 6 |
| 34 | Kontopidou, 2014 [35] | * | n.a | * | * | n.a | * | * | * | 6 |
| 35 | McLaughlin, 2014 [36] | * | * | - | * | ** | * | * | n.a | 7 |
| 36 | Pontikis, 2014 [37] | * | n.a | * | * | n.a | * | * | * | 6 |
| 37 | Mammina, 2010 [38] | * | n.a | * | * | n.a | * | * | * | 6 |
| 38 | Oliva, 2017 [39] | * | n.a | * | * | n.a | * | * | * | 6 |
| 39 | Gonzalez-Padilla, 2015 [40] | * | n.a | * | * | n.a | * | * | * | 6 |
| 40 | Neuner, 2011 [41] | * | n.a | * | * | n.a | * | * | * | 6 |
| 41 | Falagas, 2007 [42] | * | * | - | * | ** | * | * | n.a | 7 |
| 42 | Sbrana, 2013 [43] | * | n.a | * | * | n.a | * | * | * | 6 |
| 43 | Papadimitriou-Olivgeris, 2017 [44] | * | * | - | * | ** | * | * | n.a | 7 |
| 44 | Falcone, 2016 [45] | * | n.a | * | * | n.a | * | * | * | 6 |
| 45 | Liao, 2017 [46] | * | n.a | * | * | n.a | * | - | * | 5 |
| 46 | De Pascale, 2017 [47] | * | * | - | - | ** | * | * | n.a | 6 |
| 47 | Freire, 2015 [48] | * | * | * | * | ** | * | * | * | 9 |
| 48 | Hussein, 2013 [49] | * | * | - | * | ** | * | * | n.a | 7 |
| 49 | Simkins, 2014 [50] | * | * | - | * | ** | * | * | n.a | 7 |
| 50 | Shields, 2016b [51] | * | n.a | * | * | n.a | * | * | * | 6 |
| 51 | Russo, 2018 [52] | * | * | - | * | ** | * | * | n.a | 7 |
| 52 | Su, 2018 [53] | * | n.a | * | * | n.a | * | * | * | 6 |
| 53 | Varotti, 2017 [54] | * | * | - | * | ** | * | * | n.a | 7 |
| 54 | Pouch, 2015 [55] | * | * | - | * | ** | * | * | n.a | 7 |
| 55 | Duani, 2018 [56] | * | * | - | * | ** | * | * | n.a | 7 |
*Fulfillment of items within a section; n.a, not applicable.
1.1. Mortality
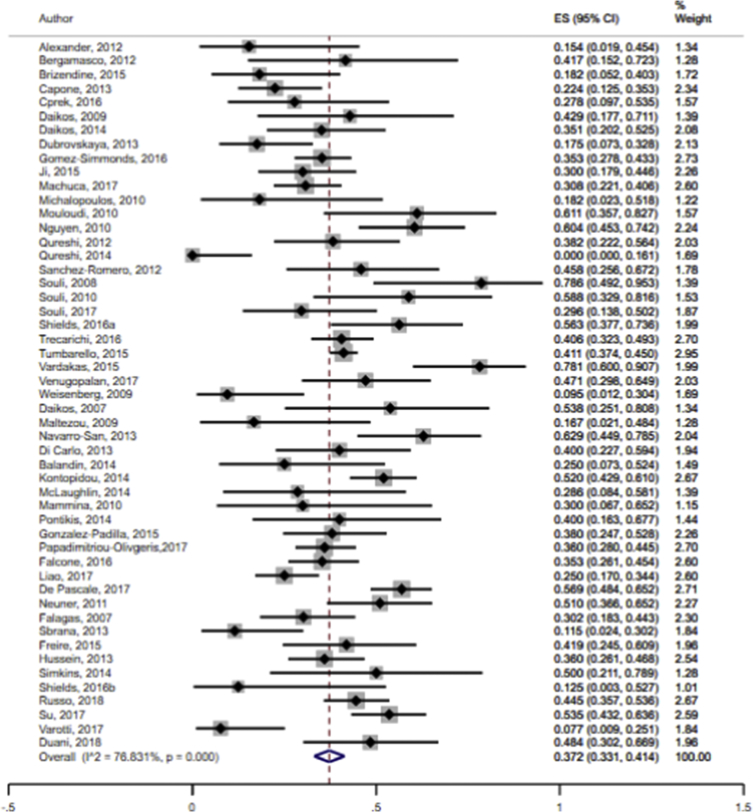
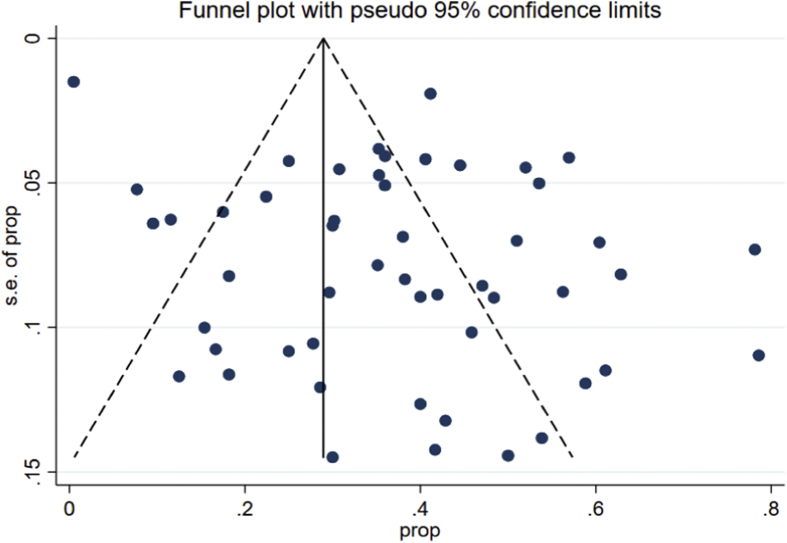
The data showed that the overall pooled mortality rate among the CRKP-infected patients treated with antibiotics was 37.2% (95% CI 33.1–41.4%; I2 = 76.8%) (Fig. 2). Sub-group analyses based on geographic region (North America: 30.4%, 95% CI 20.9–40.8%, I2 = 80.4%; other: 39.5%, 95% CI 35.1–44.1%, I2 = 74.7%), publication years (≤2012: 40.8%, 95% CI 31.4–50.6%; I2 = 67.2%; 2013–2018: 36.1%, 95% CI 31.5–40.8%, I2 = 79.8%) and study design (retrospective: 37.5%, 95% CI 32.6–42.5%, I2 = 79.1%: prospective: 35.4%, 95% CI 28.2–42.9%; I2 = 56.6%) did not result in significant reduction in heterogeneity levels for the pooled mortality rates. Moreover, funnel plot visualization showed no evidence of publication bias (Fig. 3).
Fig. 2.
Pooled mortality rates following antibiotic treatment among CRKP-infected patients in the included studies.
Fig. 3.
Funnel plot depicting reported mortality rates following antibiotic therapy across included studies.
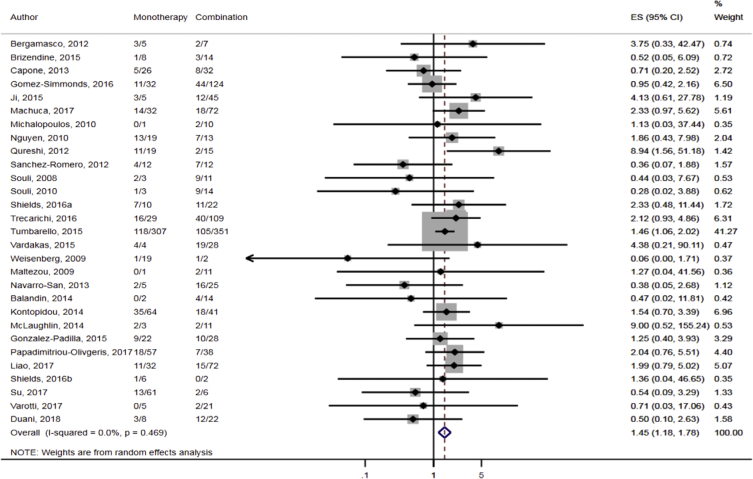
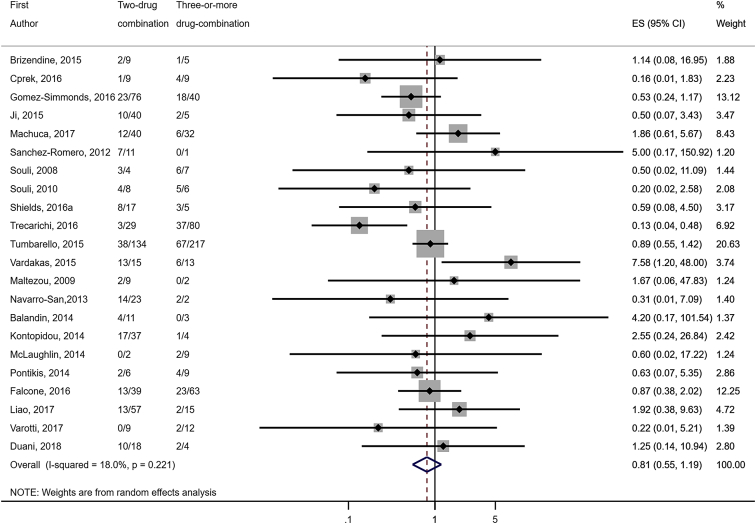
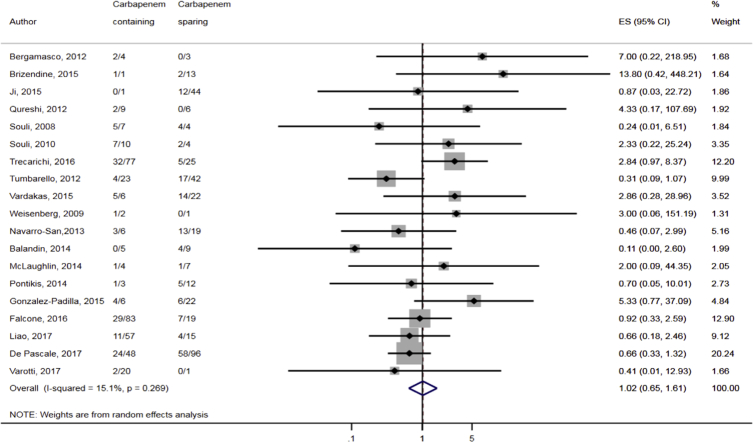
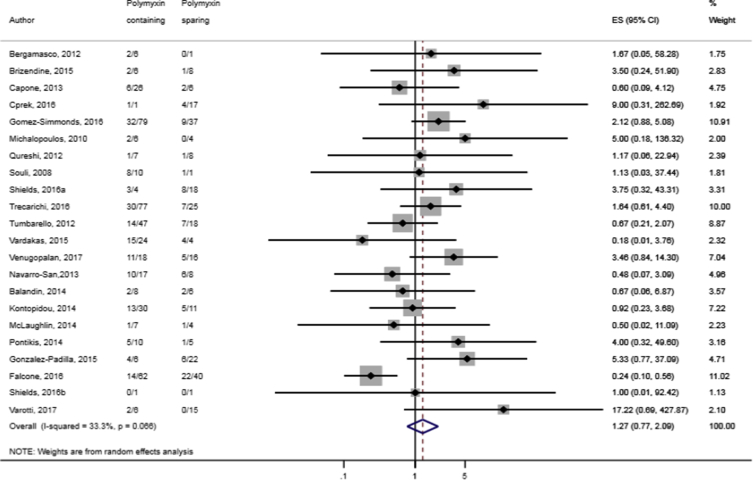
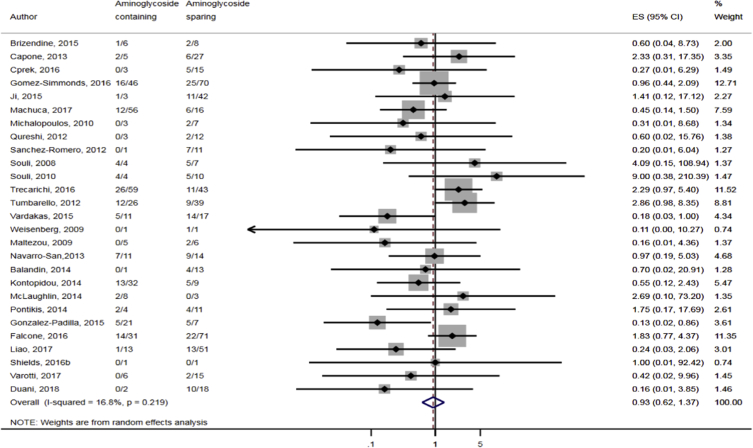
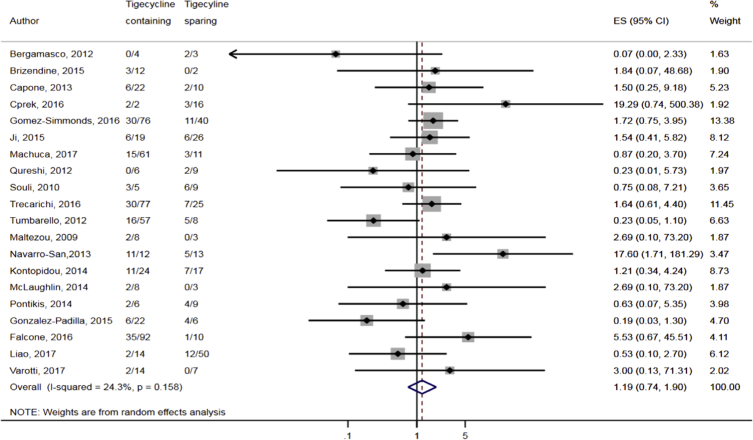
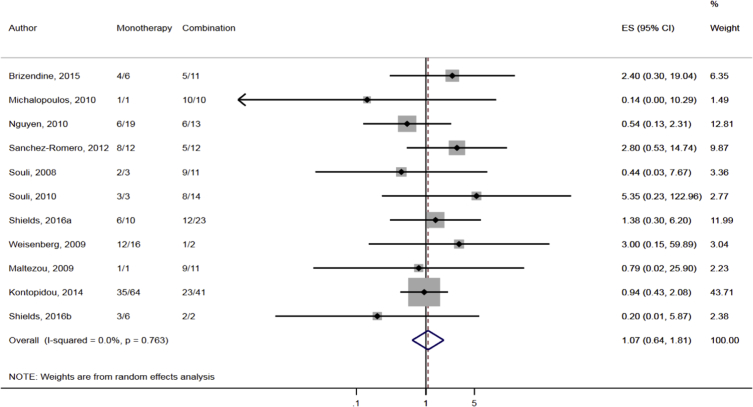
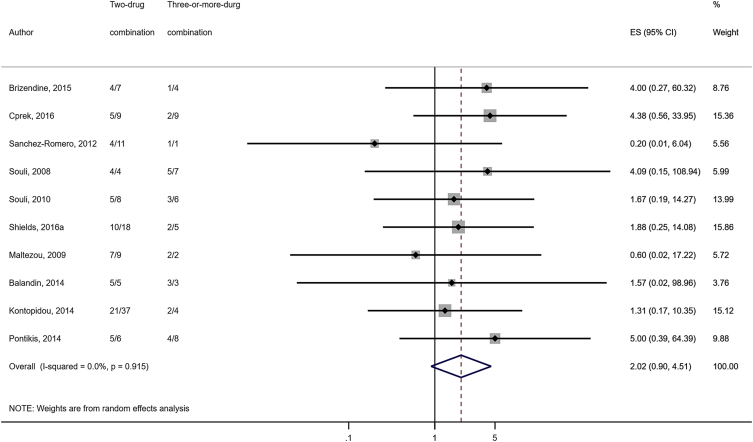
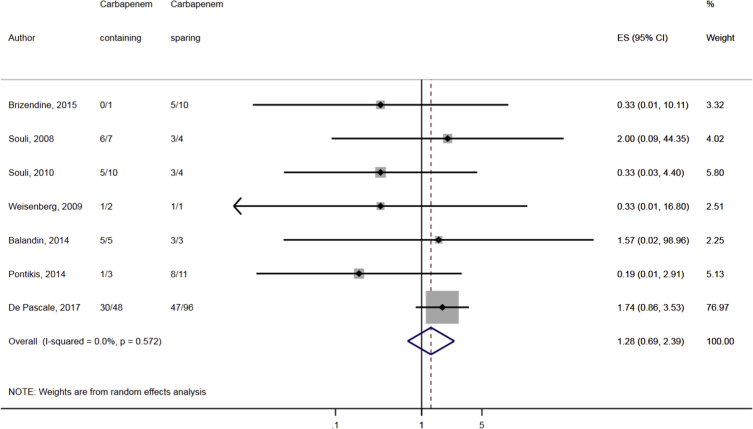
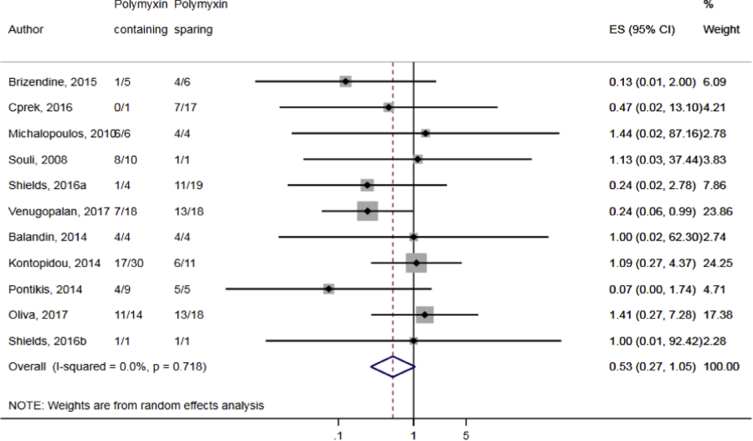
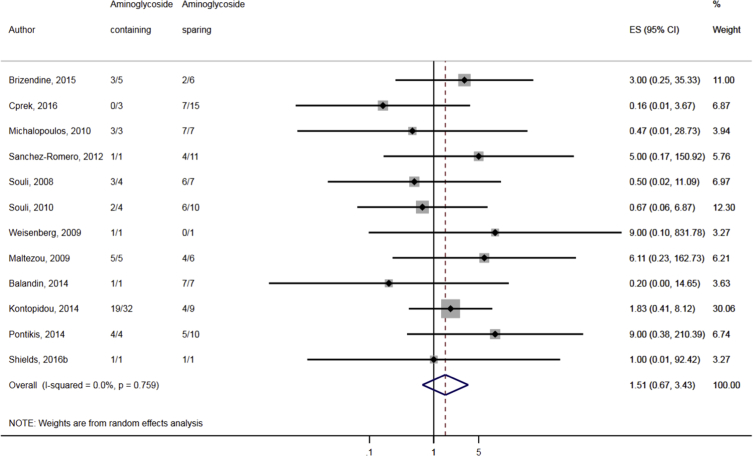
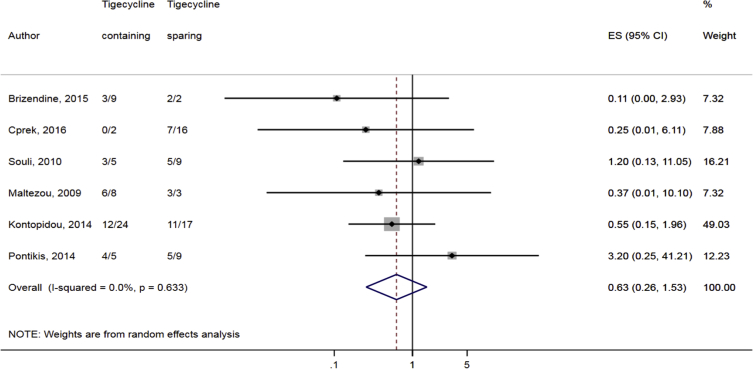
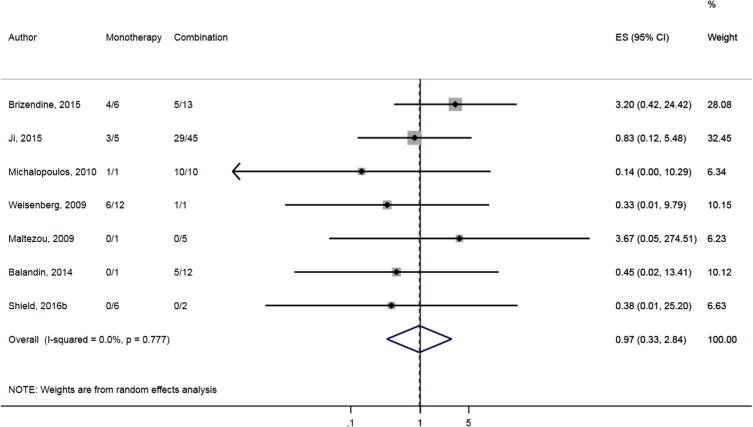
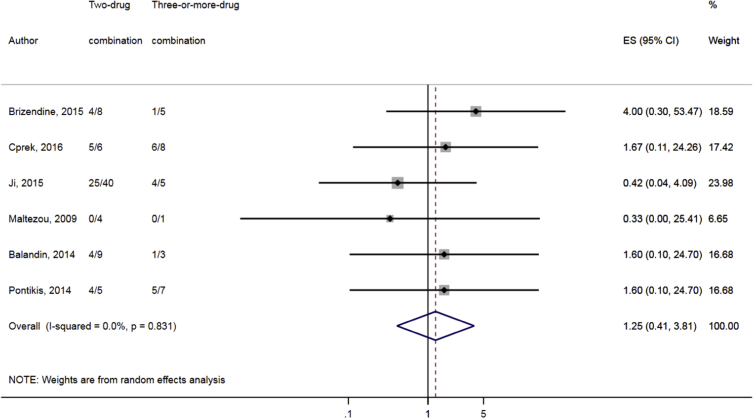
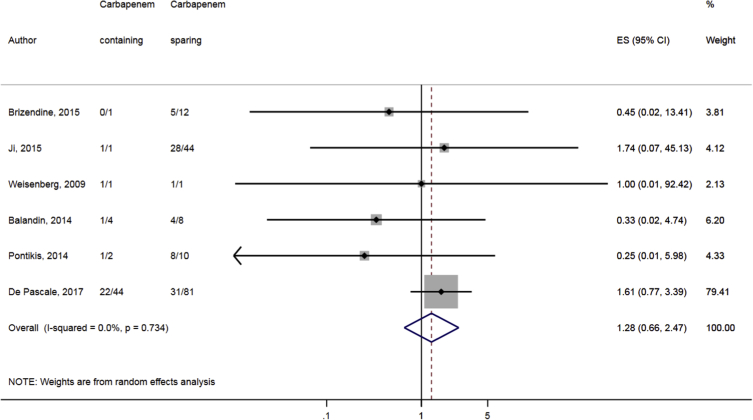
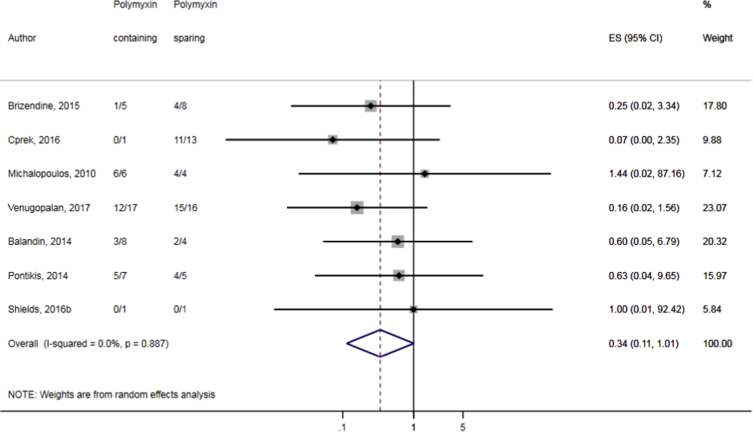
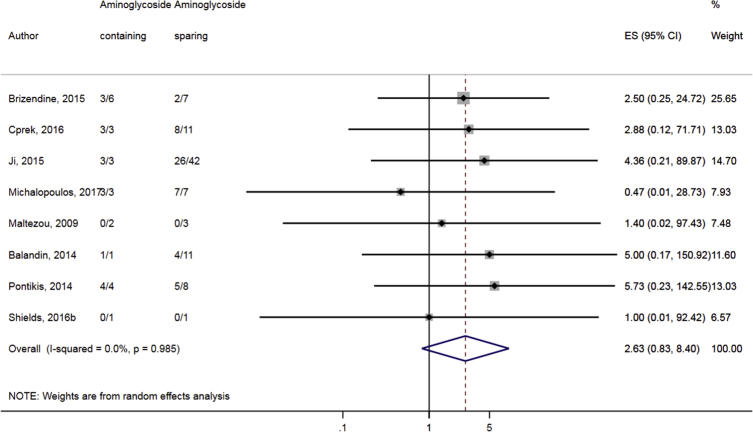
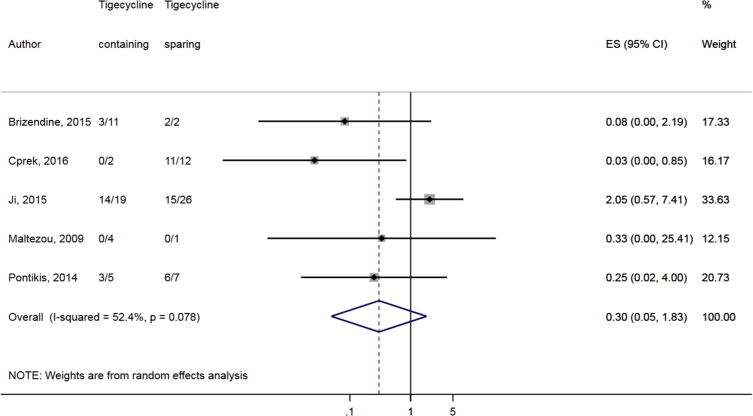
Compared to combination therapy, monotherapy was associated with a higher likelihood of mortality (odds ratio [OR] 1.45, 95% CI 1.18–1.78; I2 = 0.0%) (Fig. 4). However, there were no significant differences in the likelihood of mortality between CRKP-infected patients treated with 2-drug and ≥3-drug combination regimens (Fig. 5) or between the combination containing and sparing regimens of carbapenems (Fig. 6), polymyxins (Fig. 7), aminoglycosides (Fig. 8) and tigecycline (Fig. 9). Moreover, there were no significant differences in the likelihood of mortality between the various monotherapies (Table 2). The comparison of the mortality outcomes across the various antibiotic combination regimens did not change when the analysis was restricted to 14- and 30-day mortality (Table 3).
Fig. 4.
Comparison of mortality between CRKP-infected patients treated with monotherapy and combination therapies.
Fig. 5.
Comparison of mortality between CRKP-infected patients treated with ≥3-drug and those on 2-drug combination therapies.
Fig. 6.
Comparison of mortality between CRKP-infected patients treated with carbapenem-containing and carbapenem-sparing combination therapies.
Fig. 7.
Comparison of mortality between CRKP-infected patients treated with polymyxin-containing and polymyxin-sparing combination therapies.
Fig. 8.
Comparison of mortality between CRKP-infected patients treated with aminoglycoside-containing and aminoglycoside-sparing combination therapies.
Fig. 9.
Comparison of mortality between CRKP-infected patients treated with tigecycline-containing and tigecycline-sparing combination therapies.
Table 2.
Comparison of mortality, microbiological and clinical response rate according to various antibiotic monotherapies.
| Outcome | No. of studies pooled | No. of patients | Odds Ratio (OR) (95% CI) | Heterogeneity of included studies |
|---|---|---|---|---|
| Mortality | ||||
| Carbapenem vs polymyxin | 7 | 98 | 0.83 (0.29–2.40) | 18.3%, p = 0.290 |
| Carbapenem vs aminoglycoside | 10 | 110 | 1.83 (0.67–4.97) | 11.3%, p = 0.339 |
| Carbapenem vs tigecycline | 8 | 103 | 1.43 (0.56–3.69) | 0.0%, 0.540 |
| Polymyxin vs aminoglycoside | 11 | 377 | 1.10 (0.70–1.71) | 0.0%, p = 0.557 |
| Polymyxin vs tigecycline | 11 | 422 | 0.84 (0.56–1.25) | 0.0%, p = 0.788 |
| Aminoglycoside vs tigecycline | 11 | 188 | 0.53 (0.27–1.04) | 0.0%, p = 0.980 |
| Clinical response | ||||
| Carbapenem vs polymyxin | No pooled data (1 datapoint) | – | – | – |
| Carbapenem vs aminoglycoside | 3 | 19 | 0.75 (0.08–7.08) | 0.0%, p = 0.592 |
| Carbapenem vs tigecycline | 3 | 27 | 0.92 (0.14–5.91) | 0.0%, p-0.554 |
| Polymyxin vs aminoglycoside | 2 | 50 | 1.10 (0.13–9.61) | 28.7%, p = 0.236 |
| Polymyxin vs tigecycline | 4 | 69 | 2.27 (0.46–11.27) | 0.0%, p = 0.564 |
| Aminoglycoside vs tigecycline | 3 | 56 | 2.58 (0.79–8.41) | 0.0%, p = 0.997 |
| Microbiological response | ||||
| Carbapenem vs polymyxin | No pooled data (0 datapoint) | – | – | – |
| Carbapenem vs aminoglycoside | No pooled data (1 datapoint) | – | – | – |
| Carbapenem vs tigecycline | No pooled data (1 datapoint) | – | – | – |
| Polymyxin vs aminoglycoside | No pooled data (1 datapoint) | – | – | – |
| Polymyxin vs tigecycline | 2 | 50 | 2.76 (0.87–8.68) | 0.0%, p = 0.344 |
| Aminoglycoside vs tigecycline | 2 | 65 | 3.00 (0.60–15.1) | 0.0%, p = 0.580 |
Table 3.
Sub-group analyses of 14-day and 30-day mortality following specific antibiotic therapies among CRKP-infected patients.
| Mortality | No. of studies pooled | No. of patients | Odds Ratio (OR) (95% CI) | Heterogeneity of included studies |
|---|---|---|---|---|
| By 14-day | ||||
| Monotherapy vs combination | 8 | 935 | 1.42 (1.06–1.90); p = 0.020 | I2 = 0.0%; p = 0.907 |
| 2-drug vs ≥ 3-drug combination | 8 | 490 | 0.91 (0.59–1.41); p = 0.674 | I2 = 0.0%; p = 0.844 |
| Carbapenem-containing vs carbapenem-sparing | 4 | 65 | 1.39 (0.31–6.16); p = 0.664 | I2 = 0.0%; p = 0.700 |
| Polymyxin-containing vs polymyxin-sparing | 5 | 114 | 1.45 (0.50–4.16); p = 0.491 | I2 = 0.0%; p = 0.531 |
| Aminoglycoside-containing vs aminoglycoside-sparing | 6 | 117 | 0.46 (0.17–1.24); p = 0.125 | I2 = 0.0%; p = 0.504 |
| Tigecycline-containing vs tigecycline-sparing | 5 | 106 | 1.70 (0.60–4.78); p = 0.315 | I2 = 0.0%; p = 0.890 |
| By 28-day or 30-day | ||||
| Monotherapy vs combination | 14 | 763 | 1.54 (1.09–2.17); p = 0.015 | I2 = 1.2%; p = 0.436 |
| 2-drug vs ≥ 3-drug combination | 13 | 555 | 0.91 (0.59–1.42); p = 0.684 | I2 = 9.2%; p = 0.353 |
| Carbapenem-containing vs carbapenem-sparing | 11 | 470 | 0.65 (0.37–1.12); p = 0.123 | I2 = 14.2%; p = 0.309 |
| Polymyxin-containing vs polymyxin-sparing | 12 | 457 | 1.33 (0.64–2.75); p = 0.446 | I2 = 53.8%; p = 0.014 |
| Aminoglycoside-containing vs aminoglycoside-sparing | 13 | 545 | 0.99 (0.58–1.67); p = 0.956 | I2 = 27.0%; p = 0.172 |
| Tigecycline-containing vs tigecycline-sparing | 11 | 507 | 1.09 (0.48–2.45); p = 0.844 | I2 = 56.1%; p = 0.012 |
The bold values represent the significant results.
1.2. Clinical response
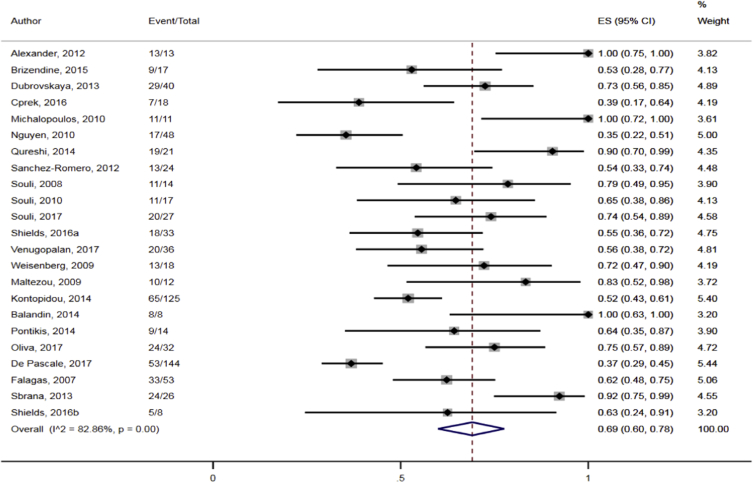
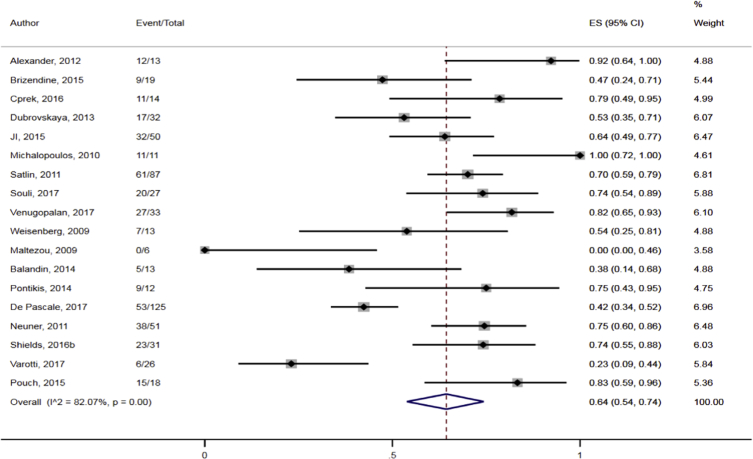
The data showed that the overall pooled clinical response rate among the CRKP-infected patients treated with antibiotics was 69.0% (95% CI 60.1–78.2%; I2 = 82.8%) (Fig. 10). Sub-group analyses based on geographic region (North America: 64.9%, 95% CI 50.1–78.5%, I2 = 80.4; other: 72.4%, 95% CI 60.0–83.4%; I2 = 85.4%), publication years (≤2012: 73.9%, 95% CI 57.1–88.0%; I2 = 82.5%; 2013–2018: 66.3%, 95% CI 54.9–76.9%, I2 = 83.6%) and study design (retrospective: 67.1%, 95% CI 55.5–77.7%, I2 = 84.4%: prospective: 79.7%, 95% CI 63.2–92.6%; I2 = 59.4%) did not result in significant reduction in heterogeneity levels for the pooled clinical response rate. Moreover, direct observation of the funnel plot did not show any obvious evidence of publication bias (Fig. 11).
Fig. 10.
Pooled clinical response rates following antibiotic treatment among CRKP-infected patients in the included studies.
Fig. 11.
Funnel plot depicting reported clinical response rates following antibiotic therapy across included studies.
There was no significant difference in the clinical response rate between monotherapy and combination regimens (Fig. 12), nor between 2-drug and ≥3-drug combination regimens (Fig. 13). Furthermore, no significant differences were noted in the pooled clinical response between combination containing and sparing regimens of carbapenems (Fig. 14), polymyxins (Fig. 15), aminoglycosides (Fig. 16) and tigecycline (Fig. 17). Moreover, there were no significant differences in the likelihood of clinical response between the various monotherapies (Table 2).
Fig. 12.
Comparison of clinical response between CRKP-infected patients treated with monotherapy and combination therapies.
Fig. 13.
Comparison of clinical response between CRKP-infected patients treated with ≥3-drug and those on 2-drug combination therapies.
Fig. 14.
Comparison of clinical response between CRKP-infected patients treated with carbapenem-containing and carbapenem-sparing combination therapies.
Fig. 15.
Comparison of clinical response between CRKP-infected patients treated with polymyxin-containing and polymyxin-sparing combination therapies.
Fig. 16.
Comparison of clinical response between CRKP-infected patients treated with aminoglycoside-containing and aminoglycoside-sparing combination therapies.
Fig. 17.
Comparison of clinical response between CRKP-infected patients treated with tigecycline-containing and tigecycline-sparing combination therapies.
1.3. Microbiological response
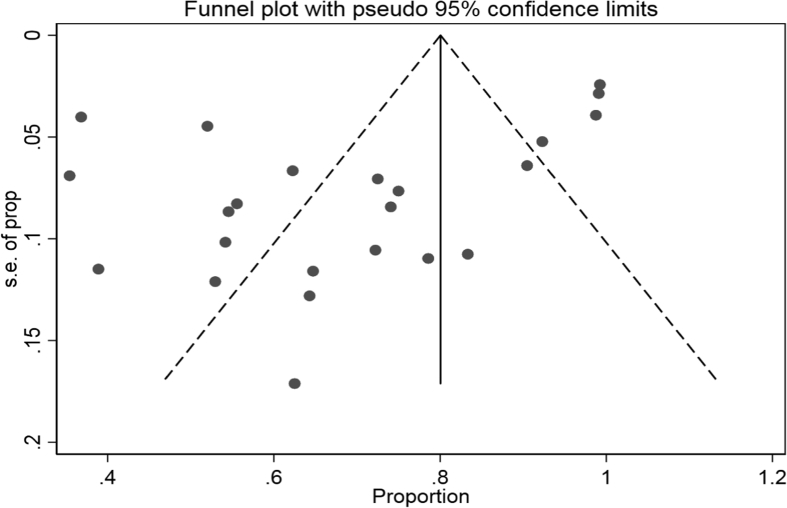
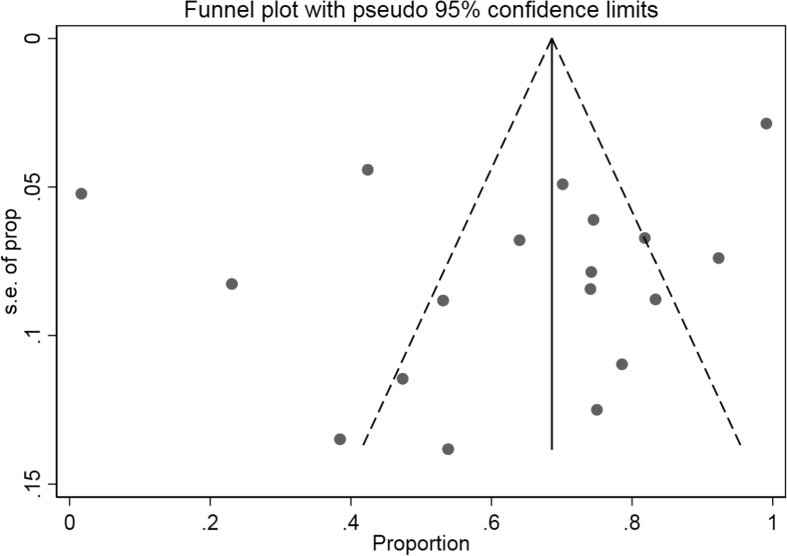
The data showed that the overall pooled microbiological response rate among the CRKP-infected patients treated with antibiotics was 63.7% (95% CI 53.7–74.1%; I2 = 82.1%) (Fig. 18). Sub-group analyses based on geographic region (North America: 71.6%, 95% CI 63.6–79.1%, I2 = 48.7%; other: 53.9%, 95% CI 34.5–72.7%, I2 = 86.7%), publication years (≤2012: 67.8%, 95% CI 49.1–84.2%; I2 = 82.6%; 2013–2018: 62.2%, 95% CI 49.3–74.4%, I2 = 80.9%) and study design (retrospective: 63.2%, 95% CI 51.6–74.1%, I2 = 81.9%; prospective: 78.8%, 95% CI 60.6–92.9%; I2 = 68.0%) did not result in significant reduction in heterogeneity levels for the pooled microbiological response rate. Moreover, as per the funnel plot visualization, there was no obvious presence of publication bias (Fig. 19).
Fig. 18.
Pooled microbiological response rates following antibiotic treatment among CRKP-infected patients in the included studies.
Fig. 19.
Funnel plot depicting reported microbiological response rates following antibiotic therapy across included studies.
There was no significant difference in the microbiological response rate between monotherapy and combination regimens (Fig. 20), nor between 2-drug and ≥3-drug combination regimens (Fig. 21). Furthermore, no significant differences were noted in the pooled microbiological response between combination containing and sparing regimens of carbapenems (Fig. 22), polymyxins (Fig. 23), aminoglycosides (Fig. 24) and tigecycline (Fig. 25). Moreover, there were no significant differences in the likelihood of clinical response between the various monotherapies (Table 2).
Fig. 20.
Comparison of microbiological response between CRKP-infected patients treated with monotherapy and combination therapies.
Fig. 21.
Comparison of microbiological response between CRKP-infected patients treated with ≥3-drug and those on 2-drug combination therapies.
Fig. 22.
Comparison of microbiological response between CRKP-infected patients treated with carbapenem-containing and carbapenem-sparing combination therapies.
Fig. 23.
Comparison of microbiological response between CRKP-infected patients treated with polymyxin-containing and polymyxin-sparing combination therapies.
Fig. 24.
Comparison of microbiological response between CRKP-infected patients treated with aminoglycoside-containing and aminoglycoside-sparing combination therapies.
Fig. 25.
Comparison of microbiological response between CRKP-infected patients treated with tigecycline-containing and tigecycline-sparing combination therapies.
2. Experimental design, materials and methods
2.1. Search strategy
In this article, treatment outcomes (mortality, clinical and microbiological response) among antibiotic-treated CRKP-infected patients were reviewed based on published literature. More specifically, a thorough systematic literature search was conducted in Medline, EMBASE, the Cochrane Central Register of Controlled Trials, and the International Pharmaceutical Abstracts databases from their inception to December 26 2018 using the using search terms such as Klebsiella pneumoniae, antibiotic therapy and carbapenem resistance. The full search strategy is presented in Table 4. The database searches were also supplemented by manual reference screening of the included articles.
Table 4.
Search strategy for Ovid Medline which was adapted for other databases.
|
3. Methodological quality assessment
All studies that met the selection criteria were assessed for quality via the Newcastle-Ottawa scale (NOS) for nonrandomized trials included in meta-analyses [1]. Studies achieving a NOS score of ≥5 were deemed to be of sufficient quality for inclusion in the review.
3.1. Inclusion and exclusion criteria
All studies addressing treatment outcomes for patients with infections caused by CRKP who received antibiotic therapy were eligible for inclusion. Studies involving both infected and colonized patients were included if the treatment outcomes of the infected patients could be separately extracted. Studies were excluded if they were based upon case reports or case series of <10 patients, focused on children or were in vitro or animal studies. Conference abstracts and meeting reports were also excluded.
3.2. Data extraction
A pre-designed data extraction form was used to collect relevant data. The extracted information included study details (first author, publication year, sample size, period, design, and country), population characteristics (gender distribution, mean age, site of infection etc.), antibiotic susceptibility testing (AST), details of antibiotic regimen, treatment outcomes (mortality, clinical response, and microbiological response) and any reported adverse events. All-cause mortality evaluated at end of patient follow-up was the primary outcome measurement. We additionally extracted data specifically for 14-day and 30-day mortality. The secondary outcomes were clinical response, microbiological response and adverse events. Due to the lack of standard and uniform criteria for the assessment and reporting of clinical response and microbiological response we adopted the definitions as employed in individual studies. The articles’ screening and selection process was conducted according to the PRISMA Guidelines [57].
3.3. Data analysis
The overall all-cause mortality, clinical response and microbiological response rates were determined via meta-analysis proportion. The meta-analysis was performed using the Freeman-Tukey double arcsine transformed proportions to stabilize the variance [58]. A random-effects (DerSimonian and Laird) model was used in the meta-analysis due to the anticipated heterogeneity across studies. For the comparative assessment of treatment outcomes following specific antibiotic therapies, the effect measure was expressed as odds ratios (ORs). Cochran's Q test and the Ι2 statistic were used to quantify the presence of statistical heterogeneity [59]. I2 values of 25%, 50%, and 75% were considered to be low, moderate, and high degrees of heterogeneity, respectively. To examine the potential sources of heterogeneity in the pooled mortality, clinical and microbiological response rates, we performed subgroup analyses based on the following characteristics: geographic region (North America vs. other), publication years (≤2012 vs. 2013–2018) and study design (prospective vs. retrospective). The presence of publication bias was assessed by direct observation of funnel plots and quantified with Egger's regression test [60]. To examine the robustness of our pooled estimates, leave-one-out sensitivity analyses were performed. A study was considered influential if the pooled estimate without it was outside the 95% CIs of the overall pooled estimate. All analyses were performed using Stata 15/IC (StataCorp LP, College Station, Texas, USA). P-value <0.05 was considered as statistically significant.
Acknowledgments
The authors are grateful to Dr. Richard Ofori-Asenso of the Department of Epidemiology and Preventive Medicine, Monash University for cross-checking the extracted data and to Dr. Jenni Ilomaki of the Centre for Medicine Use and Safety, Monash University for constructive review of the manuscript. AAA is supported by a Monash Graduate Scholarship and Monash International Postgraduate Research Scholarship for her doctoral studies. CBL was supported by an Australian National Health and Medical Research Council (NHMRC) Career Development Fellowship (APP1062509). The funders had no role in the research design, data collection, analysis and interpretation, or the decision to submit the work for publication.
Conflict of Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- 1.The Newcastle-Ottawa Scale (NOS) for assessing the quality of nonrandomised studies in meta-analyses. 2011. http://www.ohri.ca/programs/clinical_epidemiology/oxford.asp
- 2.Alexander B.T., Marschall J., Tibbetts R.J., Neuner E.A., Ritchie D.J. Treatment and Clinical Outcomes of Urinary Tract Infections Caused by KPC-Producing Enterobacteriaceae in a Retrospective Cohort. Clin. Ther. 2012:1314–1323. doi: 10.1016/j.clinthera.2012.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bergamasco M.D., Barroso Barbosa M., de Oliveira Garcia D., Cipullo R., Moreira J.C.M., Baia C. Infection with Klebsiella pneumoniae carbapenemase (KPC)-producing K. pneumoniae in solid organ transplantation. Transpl. Infect. Dis. 2012;14:198–205. doi: 10.1111/j.1399-3062.2011.00688.x. [DOI] [PubMed] [Google Scholar]
- 4.Brizendine K.D., Richter S.S., Cober E.D., Van Duin D. Carbapenem-resistant Klebsiella pneumoniae urinary tract infection following solid organ transplantation. Antimicrob. Agents Chemother. 2015;59:553–557. doi: 10.1128/AAC.04284-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Capone A., Giannella M., Fortini D., Giordano A., Meledandri M., Ballardini M. High rate of colistin resistance among patients with carbapenem-resistant Klebsiella pneumoniae infection accounts for an excess of mortality. Clin. Microbiol. Infect. : Off. Pub. Eur. Soc. Clin. Microbiol. Infect. Dis. 2013;19:E23–E30. doi: 10.1111/1469-0691.12070. [DOI] [PubMed] [Google Scholar]
- 6.Cprek J.B., Gallagher J.C. Ertapenem-containing double-carbapenem therapy for treatment of infections caused by carbapenem-resistant Klebsiella pneumoniae. Antimicrob. Agents Chemother. 2016;60:669–673. doi: 10.1128/AAC.01569-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Daikos G.L., Petrikkos P., Psichogiou M., Kosmidis C., Petrikkos G. 2009. Prospective Observational Study of the Impact of VIM-1 Metallo-Beta-Lactamase on the Outcome of Patients with Klebsiella pneumoniae Bloodstream Infections; pp. 1868–1873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Daikos G.L., Tsaousi S., Tzouvelekis L.S., Anyfantis I., Psichogiou M., Argyropoulou A. Carbapenemase-producing Klebsiella pneumoniae bloodstream infections: lowering mortality by antibiotic combination schemes and the role of carbapenems. Antimicrob. Agents Chemother. 2014;58:2322–2328. doi: 10.1128/AAC.02166-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dubrovskaya Y., Chen T.Y., Scipione M.R., Esaian D., Phillips M.S., Papadopoulos J. Risk factors for treatment failure of polymyxin B monotherapy for carbapenem-resistant Klebsiella pneumoniae infections. Antimicrob. Agents Chemother. 2013;57:5394–5397. doi: 10.1128/AAC.00510-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gomez-Simmonds A., Nelson B., Eiras D.P., Loo A., Jenkins S.G., Whittier S. Combination regimens for treatment of carbapenem-resistant Klebsiella pneumoniae bloodstream infections. Antimicrob. Agents Chemother. 2016;60:3601–3607. doi: 10.1128/AAC.03007-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ji S., Lv F., Du X., Wei Z., Fu Y., Mu X., Jiang Y., Yu Y. Cefepime combined with amoxicillin/clavulanic acid: a new choice for the KPC-producing K. pneumoniae infection. Int. J. Infect. Dis. : Int. J. Infect. Dis. : Off. Pub. Int. Soc. Infect. Dis. 2015;38:108. doi: 10.1016/j.ijid.2015.07.024. [DOI] [PubMed] [Google Scholar]
- 12.Machuca I., Gutierrez-Gutierrez B., Gracia-Ahufinger I., Rivera Espinar F., Cano A., Guzman-Puche J. Mortality associated with bacteremia due to colistin-resistant Klebsiella pneumoniae with high-level meropenem resistance: importance of combination therapy without colistin and carbapenems. Antimicrob. Agents Chemother. 2017;61 doi: 10.1128/AAC.00406-17. e00406-e00417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Michalopoulos A., Virtzili S., Rafailidis P., Chalevelakis G., Damala M., Falagas M.E. Intravenous fosfomycin for the treatment of nosocomial infections caused by carbapenem-resistant Klebsiella pneumoniae in critically ill patients: a prospective evaluation. Clin. Microbiol. Infect. 2010;16:184–186. doi: 10.1111/j.1469-0691.2009.02921.x. [DOI] [PubMed] [Google Scholar]
- 14.Mouloudi E., Protonotariou E., Zagorianou A., Iosifidis E., Karapanagiotou A., Giasnetsova T., Tsioka A., Roilides E., Sofianou D., Gritsi Bloodstream infections caused by metallo-β-lactamase/Klebsiella pneumoniae carbapenemase–producing K. pneumoniae among intensive care unit patients in Greece: risk factors for infection and impact of type of resistance on outcomes. Infect. Control Hosp. Epidemiol. 2010;31(12):1250–1256. doi: 10.1086/657135. [DOI] [PubMed] [Google Scholar]
- 15.Nguyen M., Eschenauer G.A., Bryan M., O'Neil K., Furuya E.Y., Della-Latta P. Carbapenem-resistant Klebsiella pneumoniae bacteremia: factors correlated with clinical and microbiologic outcomes. Diagn. Microbiol. Infect. Dis. 2010;67:180–184. doi: 10.1016/j.diagmicrobio.2010.02.001. [DOI] [PubMed] [Google Scholar]
- 16.Qureshi Z.A., Paterson D.L., Potoski B.A., Kilayko M.C., Sandovsky G., Sordillo E. Treatment outcome of bacteremia due to KPC-producing Klebsiella pneumoniae: superiority of combination antimicrobial regimens. Antimicrob. Agents Chemother. 2012;56:2108–2113. doi: 10.1128/AAC.06268-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Qureshi Z.A., Syed A., Clarke L.G., Doi Y., Shields R.K. Epidemiology and clinical outcomes of patients with carbapenem- resistant Klebsiella pneumoniae bacteriuria. Antimicrob. Agents Chemother. 2014;58:3100–3104. doi: 10.1128/AAC.02445-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sanchez-Romero I., Asensio A., Oteo J., Munoz-Algarra M., Isidoro B., Vindel A. Nosocomial outbreak of VIM-1-producing Klebsiella pneumoniae isolates of multilocus sequence type 15: molecular basis, clinical risk factors, and outcome. Antimicrob. Agents Chemother. 2012;56:420–427. doi: 10.1128/AAC.05036-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Satlin M.J., Kubin C.J., Blumenthal J.S., Cohen A.B., Furuya E.Y., Wilson S.J. Comparative effectiveness of aminoglycosides, polymyxin B, and tigecycline for clearance of carbapenem-resistant Klebsiella pneumoniae from urine. Antimicrob. Agents Chemother. 2011;55:5893–5899. doi: 10.1128/AAC.00387-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Souli M., Kontopidou F.V., Papadomichelakis E., Galani I., Armaganidis A., Giamarellou H. Clinical experience of serious infections caused by Enterobacteriaceae producing VIM-1 metallo-beta-lactamase in a Greek university hospital. Clin. Infect. Dis.: Off. Pub. Infect. Dis. Soc. Am. 2008;46:847–854. doi: 10.1086/528719. [DOI] [PubMed] [Google Scholar]
- 21.Souli M., Galani I., Antoniadou A., Papadomichelakis E., Poulakou G., Panagea T. An outbreak of infection due to beta-Lactamase Klebsiella pneumoniae Carbapenemase 2-producing K. pneumoniae in a Greek University Hospital: molecular characterization, epidemiology, and outcomes. Clin. Infect. Dis. : Off. Pub. Infect. Dis. Soc. Am. 2010;50:364–373. doi: 10.1086/649865. [DOI] [PubMed] [Google Scholar]
- 22.Souli M., Karaiskos I., Masgala A., Galani L., Barmpouti E., Giamarellou H. Double-carbapenem combination as salvage therapy for untreatable infections by KPC-2-producing Klebsiella pneumoniae. Eur. J. Clin. Microbiol. Infect. Dis. 2017;36:1305–1315. doi: 10.1007/s10096-017-2936-5. [DOI] [PubMed] [Google Scholar]
- 23.Shields R.K., Clancy C.J., Press E.G., Nguyen M.H. Aminoglycosides for treatment of bacteremia due to carbapenem-resistant Klebsiella pneumoniae. Antimicrob. Agents Chemother. 2016;60:3187–3192. doi: 10.1128/AAC.02638-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Trecarichi E.M., Pagano L., Martino B., Candoni A., Di Blasi R., Nadali G. Bloodstream infections caused by Klebsiella pneumoniae in onco-hematological patients: clinical impact of carbapenem resistance in a multicentre prospective survey. Am. J. Hematol. 2016;91:1076–1081. doi: 10.1002/ajh.24489. [DOI] [PubMed] [Google Scholar]
- 25.Tumbarello M., Viale P., Viscoli C., Trecarichi E.M., Tumietto F., Marchese A. Predictors of mortality in bloodstream infections caused by Klebsiella pneumoniae carbapenemase-producing K. pneumoniae: importance of combination therapy. Clin. Infect. Dis. : Off. Pub. Infect. Dis. Soc. Am. 2012;55:943–950. doi: 10.1093/cid/cis588. [DOI] [PubMed] [Google Scholar]
- 26.Tumbarello M., Trecarichi E.M., De Rosa F.G., Giannella M., Giacobbe D.R., Bassetti M. Infections caused by KPC-producing Klebsiella pneumoniae: differences in therapy and mortality in a multicentre study. J. Antimicrob. Chemother. 2015;70:2133–2143. doi: 10.1093/jac/dkv086. [DOI] [PubMed] [Google Scholar]
- 27.Vardakas K.Z., Matthaiou D.K., Falagas M.E., Antypa E., Koteli A., Antoniadou E. Tigecycline for carbapenem-resistant Klebsiella pneumoniae infections in the intensive care unit. Infect. Dis. 2015;47:751–753. doi: 10.3109/23744235.2015.1049659. [DOI] [PubMed] [Google Scholar]
- 28.Venugopalan V., Nogid B., Le T.N., Rahman S.M., Bias T.E. Double carbapenem therapy (DCT) for bacteremia due to carbapenem-resistant Klebsiella pneumoniae (CRKP): from test tube to clinical practice. Infect. Dis. 2017;49:867–870. doi: 10.1080/23744235.2017.1350880. [DOI] [PubMed] [Google Scholar]
- 29.Weisenberg S.A., Morgan D.J., Espinal-Witter R., Larone D.H. Clinical outcomes of patients with Klebsiella pneumoniae carbapenemase-producing K. pneumoniae after treatment with imipenem or meropenem. Diagn. Microbiol. Infect. Dis. 2009;64:233–235. doi: 10.1016/j.diagmicrobio.2009.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Daikos G.L., Karabinis A., Paramythiotou E., Syriopoulou V.P., Kosmidis C., Avlami A. VIM-1-producing Klebsiella pneumoniae bloodstream infections: analysis of 28 cases. Int. J. Antimicrob. Agents. 2007;29:471–473. doi: 10.1016/j.ijantimicag.2006.11.006. [DOI] [PubMed] [Google Scholar]
- 31.Maltezou H.C., Giakkoupi P., Maragos A., Bolikas M., Raftopoulos V., Papahatzaki H. Outbreak of infections due to KPC-2-producing Klebsiella pneumoniae in a hospital in Crete (Greece) J. Infect. 2009;58:213–219. doi: 10.1016/j.jinf.2009.01.010. [DOI] [PubMed] [Google Scholar]
- 32.Navarro-San Francisco C., Mora-Rillo M., Romero-Gomez M.P., Moreno-Ramos F., Rico-Nieto A., Ruiz-Carrascoso G. Bacteraemia due to OXA-48-carbapenemase-producing Enterobacteriaceae: a major clinical challenge. Clin. Microbiol. Infect. : Off. Pub. Eur. Soc. Clin. Microbiol. Infect. Dis. 2013;19:E72–E79. doi: 10.1111/1469-0691.12091. [DOI] [PubMed] [Google Scholar]
- 33.Di Carlo P., Gulotta G., Casuccio A., Pantuso G., Raineri M., Farulla C.A. KPC - 3 Klebsiella pneumoniae ST258 clone infection in postoperative abdominal surgery patients in an intensive care setting: analysis of a case series of 30 patients. BMC Anesthesiol. 2013;13:13. doi: 10.1186/1471-2253-13-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Balandin Moreno B., Fernandez Simon I., Pintado Garcia V., Sanchez Romero I., Isidoro Fernandez B., Romera Ortega M.A. Tigecycline therapy for infections due to carbapenemase-producing Klebsiella pneumoniae in critically ill patients. Scand. J. Infect. Dis. 2014;46:175–180. doi: 10.3109/00365548.2013.861608. [DOI] [PubMed] [Google Scholar]
- 35.Kontopidou F., Giamarellou H., Katerelos P., Maragos A., Kioumis I., Trikka-Graphakos E. Infections caused by carbapenem-resistant Klebsiella pneumoniae among patients in intensive care units in Greece: a multi-centre study on clinical outcome and therapeutic options. Clin. Microbiol. Infect. : Off. Pub. Eur. Soc. Clin. Microbiol. Infect. Dis. 2014;20:O117–O123. doi: 10.1111/1469-0691.12341. [DOI] [PubMed] [Google Scholar]
- 36.McLaughlin M.M., Advincula M.R., Malczynski M., Barajas G., Qi C., Scheetz M.H. Quantifying the clinical virulence of Klebsiella pneumoniae producing carbapenemase Klebsiella pneumoniae with a Galleria mellonella model and a pilot study to translate to patient outcomes. BMC Infect. Dis. 2014;14:31–37. doi: 10.1186/1471-2334-14-31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Pontikis K., Karaiskos I., Bastani S., Dimopoulos G., Kalogirou M., Katsiari M. Outcomes of critically ill intensive care unit patients treated with fosfomycin for infections due to pandrug-resistant and extensively drug-resistant carbapenemase-producing Gram-negative bacteria. Int. J. Antimicrob. Agents. 2014;43:52–59. doi: 10.1016/j.ijantimicag.2013.09.010. [DOI] [PubMed] [Google Scholar]
- 38.Mammina C., Palma D.M., Bonura C., Anna Plano M.R., Monastero R., Sodano C. Outbreak of infection with Klebsiella pneumoniae sequence type 258 producing Klebsiella pneumoniae carbapenemase 3 in an intensive care unit in Italy. J. Clin. Microbiol. 2010;48:1506–1507. doi: 10.1128/JCM.00315-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Oliva A., Scorzolini L., Castaldi D., Gizzi F., De Angelis M., Storto M. Double-carbapenem regimen, alone or in combination with colistin, in the treatment of infections caused by carbapenem-resistant Klebsiella pneumoniae (CR-Kp) J. Infect. 2017;74:103–106. doi: 10.1016/j.jinf.2016.10.002. [DOI] [PubMed] [Google Scholar]
- 40.Gonzalez-Padilla M., Torre-Cisneros J., Rivera-Espinar F., Pontes-Moreno A., Lopez-Cerero L., Pascual A. Gentamicin therapy for sepsis due to carbapenem-resistant and colistin-resistant Klebsiella pneumoniae. J Antimicrob. Chemother. 2015;70:905–913. doi: 10.1093/jac/dku432. [DOI] [PubMed] [Google Scholar]
- 41.Neuner E.A., Yeh J.Y., Hall G.S., Sekeres J., Endimiani A., Bonomo R.A. Treatment and outcomes in carbapenem-resistant Klebsiella pneumoniae bloodstream infections. Diagn. Microbiol. Infect. Dis. 2011;69:357–362. doi: 10.1016/j.diagmicrobio.2010.10.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Falagas M.E., Rafailidis P.I., Kofteridis D., Virtzili S., Chelvatzoglou F.C., Papaioannou V. Risk factors of carbapenem-resistant Klebsiella pneumoniae infections: a matched case control study. J Antimicrob. Chemother. 2007;60:1124–1130. doi: 10.1093/jac/dkm356. [DOI] [PubMed] [Google Scholar]
- 43.Sbrana F., Malacarne P., Viaggi B., Costanzo S., Leonetti P., Leonildi A. Carbapenem-sparing antibiotic regimens for infections caused by Klebsiella pneumoniae carbapenemase-producing K. pneumoniae in intensive care unit. Clin. Infect. Dis. : Off. Pub. Infect. Dis. Soc. Am. 2013;56:697–700. doi: 10.1093/cid/cis969. [DOI] [PubMed] [Google Scholar]
- 44.Papadimitriou-Olivgeris M., Fligou F., Bartzavali C., Zotou A., Spyropoulou A., Koutsileou K. Carbapenemase-producing Klebsiella pneumoniae bloodstream infection in critically ill patients: risk factors and predictors of mortality. Eur. J. Clin. Microbiol. Infect. Dis. : Off. Pub. Eur. Soc. Clin. Microbiol. 2017;36:1125–1131. doi: 10.1007/s10096-017-2899-6. [DOI] [PubMed] [Google Scholar]
- 45.Falcone M., Russo A., Iacovelli A., Restuccia G., Ceccarelli G., Giordano A. Predictors of outcome in ICU patients with septic shock caused by Klebsiella pneumoniae carbapenemase-producing K. pneumoniae. Clin. Microbiol. Infect. : Off. Pub. Eur. Soc. Clin. Microbiol Infect. Dis. 2016;22:444–450. doi: 10.1016/j.cmi.2016.01.016. [DOI] [PubMed] [Google Scholar]
- 46.Liao Y., Hu G.-H., Xu Y.-F., Che J.-P., Luo M., Zhang H.-M. Retrospective analysis of fosfomycin combinational therapy for sepsis caused by carbapenem-resistant Klebsiella pneumoniae. Exp. Ther. Med. 2017;13:1003–1010. doi: 10.3892/etm.2017.4046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.De Pascale G., Martucci G., Montini L., Panarello G., Cutuli S.L., Di Carlo D. Double carbapenem as a rescue strategy for the treatment of severe carbapenemase-producing Klebsiella pneumoniae infections: a two-center, matched case-control study. Crit. Care. 2017;21:173. doi: 10.1186/s13054-017-1769-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Freire M.P., Abdala E., Moura M.L., de Paula F.J., Spadao F., Caiaffa-Filho H.H. Risk factors and outcome of infections with Klebsiella pneumoniae carbapenemase-producing K. pneumoniae in kidney transplant recipients. Infection. 2015;43:315–323. doi: 10.1007/s15010-015-0743-4. [DOI] [PubMed] [Google Scholar]
- 49.Hussein K., Raz-Pasteur A., Finkelstein R., Neuberger A., Shachor-Meyouhas Y., Oren I. Impact of carbapenem resistance on the outcome of patients' hospital-acquired bacteraemia caused by Klebsiella pneumoniae. J. Hosp. Infect. 2013;83:307–313. doi: 10.1016/j.jhin.2012.10.012. [DOI] [PubMed] [Google Scholar]
- 50.Simkins J., Muggia V., Cohen H.W., Minamoto G.Y. Carbapenem-resistant Klebsiella pneumoniae infections in kidney transplant recipients: a case-control study. Transpl. Infect. Dis. : Off. J. Transplant. Soc. 2014;16:775–782. doi: 10.1111/tid.12276. [DOI] [PubMed] [Google Scholar]
- 51.Shields R.K., Press E.G., Haidar G., Doi Y., Potoski B.A., Nguyen M.H. Clinical outcomes, drug toxicity, and emergence of ceftazidime-avibactam resistance among patients treated for carbapenem-resistant enterobacteriaceae infections. Clin. Infect. Dis. 2016;63:1615–1618. doi: 10.1093/cid/ciw636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Russo A., Giuliano S., Ceccarelli G., Alessandri F., Giordano A., Brunetti G. Comparison of septic shock due to multidrug-resistant acinetobacter baumannii or Klebsiella pneumoniae carbapenemase-producing K. pneumoniae in intensive care unit patients. Antimicrob. Agents Chemother. 2018;62 doi: 10.1128/AAC.02562-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Su C.F., Chuang C., Lin Y.T., Chan Y.J., Lin J.C., Lu P.L. Treatment outcome of non-carbapenemase-producing carbapenem-resistant Klebsiella pneumoniae infections: a multicenter study in Taiwan. Eur. J. Clin. Microbiol. Infect. Dis. : Off. Pub. Eur. Soc. Clin. Microbiol. 2018;37:651–659. doi: 10.1007/s10096-017-3156-8. [DOI] [PubMed] [Google Scholar]
- 54.Varotti G., Dodi F., Terulla A., Santori G., Mariottini G., Bertocchi M. Impact of carbapenem-resistant Klebsiella pneumoniae (CR-KP) infections in kidney transplantation. Transpl. Infect. Dis. : Off. J. Transplant. Soc. 2017;19 doi: 10.1111/tid.12757. [DOI] [PubMed] [Google Scholar]
- 55.Pouch S.M., Kubin C.J., Satlin M.J., Tsapepas D.S., Lee J.R., Dube G. Epidemiology and outcomes of carbapenem-resistant Klebsiella pneumoniae bacteriuria in kidney transplant recipients. Transpl. Infect. Dis. : Off. J. Transplant. Soc. 2015;17:800–809. doi: 10.1111/tid.12450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Duani H., Moreira C.M., da Silva L.C., Clemente W.T., Romanelli R.M.C. A shorter period of therapy is associated with higher mortality in bloodstream infections caused by carbapenemase-producing Klebsiella pneumoniae in a Brazilian centre. Infect. Dis (London, England) 2018;50(2):156–161. doi: 10.1080/23744235.2017.1370129. [DOI] [PubMed] [Google Scholar]
- 57.Moher D., Liberati A., Tetzlaff J., Altman D.G., Group P. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. BMJ. 2009;339:b2535. doi: 10.1136/bmj.b2535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Barendregt J.J., Doi S.A., Lee Y.Y., Norman R.E., Vos T. Meta-analysis of prevalence. J. Epidemiol. Community Health. 2013;67(11):974–978. doi: 10.1136/jech-2013-203104. [DOI] [PubMed] [Google Scholar]
- 59.Higgins J.P., Thompson S.G., Deeks J.J., Altman D.G. Measuring inconsistency in meta-analyses. BMJ. 2003;327(7414):557–560. doi: 10.1136/bmj.327.7414.557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Higgins J., Green S., Higgins J., Green S. 2011. Cochrane Handbook for Systematic Reviews of Interventions the Cochrane Collaboration. [Google Scholar]