Highlights
-
•
This is one of the first studies on cervical cancer survival in a low-income country.
-
•
In Uganda, cervical cancer is often incompletely treated and survival remains poor.
-
•
HIV infection in this cohort was not associated with stage at diagnosis.
-
•
HIV was weakly associated with shorter survival.
Keywords: HIV/AIDS, Cervical cancer, Uganda, Global health, Survival, Sub-Saharan Africa
Abstract
Our objective was to determine how HIV infection impacts cervical cancer stage at presentation and overall survival (OS) among Ugandan women. This was a prospective study of 149 women diagnosed with cervical cancer from 2013 to 2015 at the Uganda Cancer Institute. Poisson regression models were fit to calculate prevalence ratios (PR) for the association between HIV infection and late stage at cancer diagnosis. The association between HIV infection and OS after cervical cancer diagnosis was evaluated using Cox proportional hazards models. The cohort included 53 HIV-positive and 96 HIV-negative participants. Median age at diagnosis was 44 years for HIV-positive and 54 years for HIV-negative participants. Seventy-seven percent of HIV-positive participants received antiretroviral therapy. Median baseline CD4 count was 373 cells/mm3 for HIV-positive participants versus 926 cells/mm3 for HIV-negative participants. Thirty-two percent of HIV-positive participants were diagnosed with late stage cervical cancer (III-IV) versus 39% of HIV-negative participants. No association was found between late stage at cancer diagnosis and HIV infection (PR adjusted for age, parity and transport cost 1.0, 95%CI 0.6–1.8). Most women presenting for care received cancer treatment, though almost half who received radiotherapy did not complete treatment. The median OS was 13.7 months for HIV-positive participants and 24.3 months for HIV-negative participants. After adjusting for age and stage, HIV infection was weakly associated with OS (HR 1.3, 95%CI 0.8–2.2). In Uganda, cervical cancer is often incompletely treated and survival remains poor. HIV infection was not associated with cervical cancer stage at diagnosis, but may be weakly associated with shorter survival.
1. Introduction
Cervical cancer is the most common cancer in women in East Africa (Ferlay et al., 2012), and was recognized as an “AIDS-defining cancer” early in the HIV epidemic (Maiman et al., 1997). Even in the era of antiretroviral therapy (ART), the incidence of cervical cancer is at least four times higher among HIV-positive than HIV-negative women (Chen et al., 2015). Almost all cases of cervical cancer are caused by oncogenic strains of the human papillomavirus (HPV) (Walboomers et al., 1999). Women with HIV however are more likely to experience persistent HPV infection, development and persistence of cervical dysplasia, and more rapid progression to cancer (Lomalisa et al., 2000). Recent studies from upper-middle-income economies, as defined by the World Bank, (World Bank Data, 2019) have begun to explore the impact of HIV infection on survival in cervical cancer patients and shown differing results (Grover et al., 2018, Ferreira et al., 2017, Dryden-Peterson et al., 2016, Simonds et al., 2018).
Uganda is a low-income economy (World Bank Data, 2019) with an estimated adult HIV prevalence of 7.1% (UNAIDS AIDSinfo, 2017). It has a well-established national cancer center that has had the ability to provide surgery, radiotherapy, and chemotherapy. However, the healthcare system and its patients often lack the resources to initiate or complete treatment after a cancer diagnosis. Understanding the presentation and outcomes of cervical cancer in patients who seek care in these environments can better inform care delivery. The impact of HIV on cervical cancer survival has not yet been reported in low-income economies. Therefore the aims of this study are to compare cervical cancer stage at diagnosis between HIV-positive and HIV-negative individuals and to characterize the relationship between HIV disease and cervical cancer survival in Uganda.
2. Materials and methods
2.1. Study design and participants
We conducted a prospective cohort study of cervical cancer patients diagnosed between August 2013 and August 2015 at the Uganda Cancer Institute (UCI) in Kampala, Uganda, which is the country’s only national cancer referral hospital. Adult women undergoing diagnostic workup for suspected cervical cancer were referred to the study for assessment. Patients were eligible for inclusion if they (1) had pathologically confirmed cervical cancer, (2) underwent their initial evaluation at the UCI, and (3) committed to attending all study visits during the three-year observation period. Written informed consent was obtained from all subjects.
For a complete description of materials and methods, see Supplemental Content 1.
2.2. Data collection
On enrollment, a standard set of assessments was made for each participant, including medical and demographic history, physical exam, blood draw, and tumor biopsy. Participants returned for follow-up visits to review treatment history and health status 6, 12, 24, and 36 months after enrollment. Patients who received treatment were considered to have received adequate radiation therapy if their combined external-beam and brachytherapy equivalent dose (EQD2) was at least 73.75 Gy. This is the EQD2 dose for 45 Gy of external-beam radiation delivered over 15 fractions followed by 25 Gy of low-dose rate brachytherapy delivered in one fraction, which was the standard definitive treatment regimens at the UCI
2.3. Statistical analysis
The primary exposure of interest was HIV serostatus, as determined by HIV antibody testing, assessed using the enrollment blood draw.
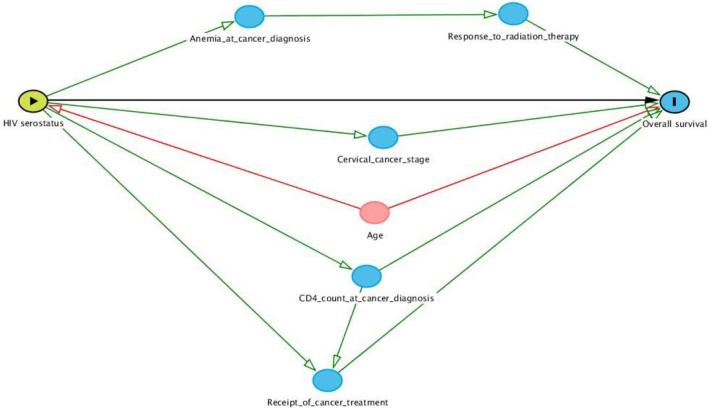
The primary outcome of interest was overall survival (OS), which was measured from enrollment to the date of death or last known contact. Patients were censored at the time of their last known contact or the conclusion of study follow-up (September 10, 2016). The Kaplan-Meier method was used to compare OS in HIV-positive and HIV-negative cervical cancer patients using the log-rank test. A Cox proportional hazards model was used to determine the adjusted association between HIV serostatus and OS. A directed acyclic graph (DAG) illustrating causal assumptions between key clinicopathologic characteristics was developed to determine the role of each variable in this analysis and reasons for inclusion in the multivariable Cox model (see Supplemental Content 2). As seen in the DAG, only age at diagnosis met criteria as a potential confounder. While FIGO stage does not meet the criteria as a potential confounder, it was included in our model to assess its role as a mediator. Because almost all cervical cancer tumors in Uganda are squamous histology and poorly or undifferentiated grade, these factors were not included in the final model. An exploratory analysis was performed to examine the potential role of hemoglobin as a mediator (see Supplemental Content 3).
The secondary outcome of interest was FIGO stage at diagnosis. Stages III and IV were considered late. Poisson regression using robust standard errors was used to calculate prevalence ratios (PR) as measures of the association between HIV serostatus and late stage of cancer at diagnosis, adjusted for age, number of live births, and cost of transportation to the UCI.
3. Results
From August 2013 to August 2015, 149 women enrolled in this study. There were 53 (35.6%) HIV-positive and 96 (64.4%) HIV-negative participants. The demographic, clinical, and treatment characteristics of the study population are shown in Table 1. The HIV-positive group was younger than the HIV-negative group (median age 44 and 54 respectively) and reported fewer live births. FIGO stage distribution was similar. HIV-positive individuals had lower median CD4 count at enrollment (373 versus 926 cells/mm3) compared to HIV-negative individuals. The majority of HIV-positive individuals were enrolled in care for HIV, using ART, and with undetectable viral loads.
Table 1.
Selected cervical cancer patient demographic, clinical and treatment characteristics, cervical cancer prognosis study, Kampala, Uganda, 2013–2015.
| Characteristic | HIV-negative, # (%) (n = 96) |
HIV-positive, # (%) (n = 53) |
|---|---|---|
| Median age (IQR), years | 54 (47–62) | 44 (39–48) |
| Highest education completed | ||
| - None | 25 (34) | 8 (19) |
| - Primary | 42 (57) | 22 (52) |
| - Secondary or greater | 7 (9) | 12 (29) |
| - Unknown* | 22 | 11 |
| Tobacco use | ||
| - Lifelong non-smoker | 90 (94) | 51 (98) |
| - Current smoker | 1 (1) | 0 (0) |
| - Former smoker | 5 (5) | 1 (2) |
| - Unknown* | 0 | 1 |
| Live births | ||
| - 0 | 1 (1) | 0 (0) |
| - 1–3 | 12 (13) | 20 (38) |
| - 4–7 | 33 (34) | 24 (45) |
| - 8+ | 50 (52) | 9 (17) |
| Median BMI (IQR) | 23 (20–27) | 23 (19–25) |
| ECOG performance status | ||
| - 0 | 2 (2) | 0 (0) |
| - 1 | 80 (84) | 48 (91) |
| - 2 | 8 (8) | 4 (7) |
| - 3 | 6 (6) | 1 (2) |
| Transport cost to UCI (USh) | ||
| - ≤20,000 | 50 (52) | 42 (79) |
| - 21,000–40,000 | 26 (27) | 8 (15) |
| - 41,000–60,000 | 16 (17) | 3 (6) |
| - >60,000 | 4 (4) | 0 (0) |
| FIGO stage | ||
| - I | 12 (12) | 10 (19) |
| - II | 47 (49) | 26 (49) |
| - III | 33 (35) | 15 (28) |
| - IV | 4 (4) | 2 (4) |
| Tumor histology | ||
| - Squamous | 95 (95) | 48 (90) |
| - Adenocarcinoma | 4 (4) | 4 (8) |
| - Other | 1 (1) | 1 (2) |
| Tumor grade | ||
| - Well differentiated | 1 (1) | 5 (10) |
| - Moderately differentiated | 22 (23) | 13 (25) |
| - Poorly/undifferentiated | 71 (76) | 33 (65) |
| - Unknown | 2 | 2 |
| Type of initial treatment | ||
| - Chemoradiation | 21 (25) | 10 (21) |
| - Chemotherapy only | 1 (1) | 1 (2) |
| - Radiation therapy only | 40 (49) | 27 (56) |
| - Surgery only | 0 (0) | 1 (2) |
| - Surgery + adjuvant treatmenta | 0 (0) | 2 (4) |
| - No treatment | 21 (25) | 7 (15) |
| - Unknown* | 13 | 5 |
| Total radiation therapy received | ||
| - <73.75 Gy | 25 (42) | 15 (43) |
| - ≥73.75 Gy | 34 (58) | 20 (57) |
| - Unknown* | 2 | 2 |
| - Did not receive upfront radiotherapy | 35 | 16 |
| Median baseline hemoglobinb (IQR), g/dL | 12.0 (9.9–12.9) | 10.5 (8.6–12.5) |
| Median CD4 at enrollment, (IQR), cells/mm3 | 926 (639–1045) | 373 (300–502) |
| Enrolled in HIV care services at enrollment | – | |
| - Yes | – | 45 (88) |
| - No | – | 6 (12) |
| - Unknown* | 2 | |
| Duration of HIV infection | ||
| - <1 year | – | 8 (16) |
| − 1–5 years | – | 17 (33) |
| - >5 years | – | 26 (51) |
| - Unknown* | – | 2 |
| HIV plasma RNA level at enrollment | ||
| - <500 copies/mL | – | 41 (77) |
| - ≥500 copies/mL | – | 12 (23) |
| HAART use at enrollment | ||
| - No | – | 12 (23) |
| - Yes | – | 41 (77) |
Abbreviations: IQR = interquartile range; UCI = Uganda Cancer Institute; USh = Ugandan shillings; ECOG = Eastern Cooperative Oncology Group.
Missing data are shown as separate category for categorical variables, but percentages were calculated among non-missing data only.
1 patient received radiation therapy; 1 patient received concurrent chemoradiation.
Baseline hemoglobin was not available for 34 (35%) of HIV-negative and 16 (30%) of HIV-positive patients.
3.1. HIV associations with stage at diagnosis
Seventeen (32.1%) HIV-positive and 37 (38.5%) HIV-negative patients presented with late stage cervical cancer (FIGO stage III-IV). Table 2 shows the unadjusted and adjusted PRs for late stage at cancer diagnosis for HIV serostatus, age, number of live births, and cost of transportation to the UCI. The unadjusted PR for late stage at cancer diagnosis comparing HIV-positive with HIV-negative participants was 0.8 (95%CI 0.5–1.3); the adjusted PR was 1.0 (95%CI 0.6–1.8).
Table 2.
Univariable and multivariable associations of selected characteristics with late stage at cervical cancer diagnosis, Kampala, Uganda, 2013–2015.*
| Characteristic | Univariable analysis PR (95%CI) (n = 149) |
Multivariable analysis**PR (95%CI) (n = 149) |
|---|---|---|
| HIV serostatus | ||
| - HIV-negative | 1.0 (reference) | 1.0 (reference) |
| - HIV-positive | 0.8 (0.5–1.3) | 1.0 (0.6–1.8) |
| Age | ||
| (Per 10-year age increase) | 1.2 (1.0–1.4) | 1.1 (0.9–1.3) |
| Live births | ||
| (Per each additional 1 live birth) | 1.2 (1.0–1.1) | 1.0 (0.9–1.1) |
| Transport cost to UCI (USh) | ||
| - ≤20,000 | 1.0 (reference) | 1.0 (reference) |
| - 21,000–40,000 | 0.7 (0.3–1.3) | 0.7 (0.3–1.3) |
| - 41,000–60,000 | 1.5 (0.9–2.4) | 1.4 (0.8–2.4) |
| - >60,000 | 2.1 (1.1–3.9) | 1.9 (0.9–4.0) |
Abbreviations: PR = prevalence ratio; USh = Ugandan Shillings.
In the model, 54 patients were late stage at diagnosis, and 95 were earlier stage at diagnosis.
Multivariable analysis adjusted for HIV serostatus, age, number of live births, transport cost.
Table 3 shows HIV-specific treatment characteristics by FIGO stage at diagnosis among the HIV-positive patients. Persons presenting with late-stage disease tended to have lower CD4 cell counts, but similar HIV plasma concentrations.
Table 3.
HIV-specific treatment characteristics by cervical cancer stage at diagnosis among HIV-positive patients, Kampala, Uganda, 2013–2015.
| Characteristic | Early stage, % (#) (n = 36) |
Late stage, % (#) (n = 17) |
|---|---|---|
| Duration of HIV infection | ||
| - <1 year | 6 (17) | 2 (12) |
| − 1–5 years | 10 (29) | 7 (44) |
| - >5 years | 19 (54) | 7 (44) |
| - Unknown* | 1 | 1 |
| CD4 T-cell count at enrollment | ||
| - >500 cells/mm3 | 9 (25) | 5 (29) |
| − 201–500 cells/mm3 | 24 (67) | 8 (47) |
| − 0–200 cells/mm3 | 3 (8) | 4 (24) |
| HIV plasma RNA level at enrollment | ||
| - <500 copies/mL | 27 (75) | 14 (82) |
| - ≥500 copies/mL | 9 (25) | 3 (18) |
| HAART use at enrollment | ||
| - No | 8 (22) | 4 (24) |
| - Yes | 28 (78) | 13 (76) |
| Enrolled in HIV care services at enrollment | ||
| - No | 3 (9) | 3 (18) |
| - Yes | 31 (91) | 14 (82) |
| - Unknown* | 2 | 0 |
Missing data are shown as separate category for categorical variables, but percentages were calculated among non-missing data only.
3.2. Treatment characteristics
Among patients for whom treatment status was known, a greater proportion of HIV-positive individuals received cancer treatment compared to HIV-negative individuals (85% and 75% respectively), the majority of which included radiation. Among those who received radiation as part of their upfront therapy, a similar proportion of HIV-positive and HIV-negative patients received adequate radiation therapy (57% and 58% respectively) and a smaller proportion of HIV-positive individuals received concurrent chemotherapy (27% vs 34%).
3.3. HIV associations with overall survival
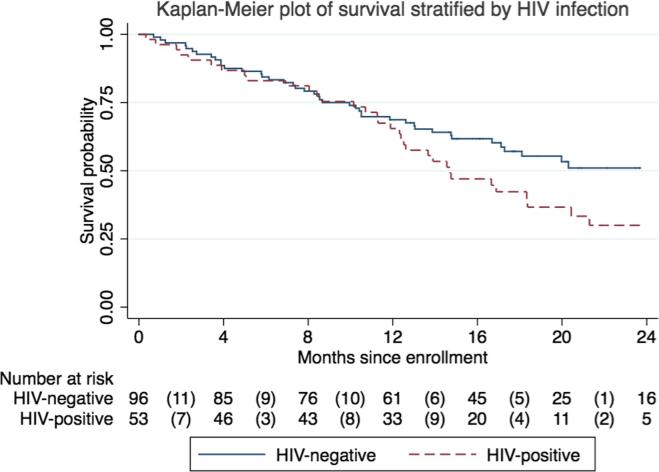
The median follow-up was 14.8 months among the entire cohort and 18.9 months among those alive and not lost to follow-up. One HIV-positive and one HIV-negative participant were lost to follow-up. There were 35 deaths among HIV-positive and 45 deaths among HIV-negative individuals. One-year survival was 65% (95%CI 51–77%) among HIV-positive and 69% (95%CI 58–77%) among HIV-negative participants. Two-year survival was 30% (95%CI 17–44%) among HIV-positive and 51% (95%CI 39–62%) among HIV-negative patients. The median survival was 18.3 months—14.7 months among HIV-positive individuals and 24.3 months among HIV-negative individuals. As seen in Fig. 1, HIV-positive patients had shorter unadjusted OS compared to HIV-negative patients, with a divergence in survival curves starting at 12 months.
Fig. 1.
Kaplan-Meier plot of overall cervical cancer survival, stratified by HIV serostatus, Kampala, Uganda, 2013–2015. Risk table below plot shows number at risk at each labeled time point and number of deaths (in parentheses) during each labeled time interval.
Table 4 shows the univariable and multivariable Cox regression analyses of characteristics in relation to OS. In univariable analysis, HIV infection (HR 1.6, 95%CI 1.0–2.4) and late FIGO stage (HR 2.3, 95%CI 1.5–3.6) were associated with an increased hazard of death. After adjusting for age and FIGO stage, there was a weaker association between HIV infection and OS (HR 1.3, 95%CI 0.8–2.2).
Table 4.
Univariable and multivariable associations of selected characteristics with overall survival among cervical cancer patients, Kampala, Uganda, 2013–2015.
| Characteristic | Univariable analysis HR (95%CI) | Multivariable analysis*HR (95%CI) |
|---|---|---|
| HIVSeroStatus | ||
| - HIV-negative | 1.0 (reference) | 1.0 (reference) |
| - HIV-positive | 1.6 (1.0–2.4) | 1.3 (0.8–2.2) |
| FIGO stage | ||
| - Early (stage I-II) | 1.0 (reference) | 1.0 (reference) |
| - Late (stage III-IV) | 2.3 (1.5–3.6) | 2.8 (1.7–4.4) |
Abbreviations: HR = hazard ratio; CI = confidence interval; FIGO = International Federation of Gynecology and Obstetrics.
Adjusted for HIV serostatus, age, and FIGO stage.
4. Discussion
In this prospective study, we found that among newly diagnosed Ugandan cervical cancer patients, mortality was high and optimal treatment rates were low, irrespective of HIV status. While the stage distribution was similar between HIV-positive and HIV-negative women, there was an approximately 60% poorer survival in HIV-positive patients, with a divergence in survival curves starting at 12 months. A multivariable model that included age and FIGO stage showed a weaker association between HIV serostatus and hazard of death, most likely reflecting a confounding effect of age. In our analyses, neither stage nor hemoglobin were found to be mediating factors.
This is one of the first studies to examine the relationship between HIV status and cervical cancer survival in a low-income economy. Findings from our study are consistent with those reported from upper-middle-income countries, such as Botswana and Brazil, where most HIV-positive individuals studied were also on ART. In an early Botswana study, investigators found that HIV infection nearly doubled the adjusted hazard of death after a cervical cancer diagnosis (Dryden-Peterson et al., 2016). In Brazil, no differences in survival were noted at one year, but after one year, HIV infection doubled the stage-adjusted hazards of death. Survival curves from the Brazilian cohort showed a similar divergence at one year as observed in our study (Ferreira et al., 2017). In South Africa, two- and five-year unadjusted survival was significantly worse in HIV-positive individuals even when excluding patients with low CD4 counts (Simonds et al., 2018). Possible explanations include earlier cancer recurrence (seen in the Brazilian cohort and not measured in our cohort), more rapid progression after a partial response, and more frequent late toxicities (not measured in any of the cohorts). In many lower resource settings, post-treatment assessments are often not performed, making it difficult to distinguish between those who have recurred after a complete response versus those who have progressed after a partial response—scenarios in which HIV infection may have differing impact.
Interestingly, a subsequent publication from the group in Botswana reported on cervical cancer patients who received curative intent concurrent chemotherapy and radiation therapy (Grover et al., 2018). This was a more highly selected population compared to other studies: median ART treatment duration was longer (7 years), median CD4 count was higher (481 cells/mm3), and a higher proportion of individuals completed treatment. In this study, HIV infection was not associated with 2-year OS.
The majority of people who received radiation therapy in our study did not receive adequate radiotherapy nor concurrent chemotherapy, which has been shown to improve survival (Vale et al., 2008). This was a similar problem in other lower-resourced settings, such as the earlier Botswana study where only 48% of women in Botswana treated with curative intent completed the recommended radiation therapy (Dryden-Peterson et al., 2016). Even in the later Botswana study, only 76% of HIV-positive patients completed brachytherapy (Grover et al., 2018).
In addition to cancer treatment, a mediator of poorer survival associated with HIV infection could include subtle differences in immune status. While the median CD4 count at enrollment (373 cells/mm3) among HIV-positive individuals in our study was above the threshold at which most severe opportunistic infections are considered a risk, this was still far below the median CD4 count among HIV-negative individuals (926 cells/mm3). The authors from the second Botswana study suggest that outcomes by HIV status were similar in part because HIV infections were well-controlled in their population. However, the CD4 counts and duration of ART treatment were only slightly better than in our study and in the earlier Botswana study (Grover et al., 2018, Dryden-Peterson et al., 2016). Perhaps these small differences in immune status are sufficient to drive variation in outcome. Increasing evidence supports the adaptive immune system’s role in ongoing clinical benefits from cancer therapy (Coulie et al., 2014). Immune dysfunction and T-cell depletion characterize HIV infection (Okoye and Picker, 2013), and subtle functional immune deficits may potentially persist in women with optimally-treated HIV infection (Nkwanyana et al., 2009, Trimble et al., 2010). Furthermore, T-cell responses in the peripheral blood are not necessarily reflective of mucosal immunity, such as in the cervix (Trimble et al., 2010), and mucosal CD4 cell populations in HIV-positive women may not recover after initiating ART (Nkwanyana et al., 2009).
Our study is limited by a small sample size. While our results may be applicable to patients who present to the UCI for care, they may not reflect the general population in Uganda where ART coverage is 57%, compared to 77% in our study (UNAIDS AIDSinfo, 2017). Nevertheless, our study population represents a group of patients among whom effort could be made to substantially improve survival.
This is one of the first studies on cervical cancer survival in a low-income economy, where it is important to better define optimal interventions. With the majority of new cervical cancer cases occurring in low- and middle-income economies, it is imperative to continue detailed studies of the natural history of cervical cancer and explore novel treatment and retention strategies in these populations.
Author contribution section
Study conception and design: all authors.
Data collection: ESW.
Data analysis: ESW, RRU, EMK, SMS, CC.
Drafting and editing of manuscript: all authors.
Declaration of Competing Interest
RU has received royalties from UpToDate, Inc. The authors otherwise have no conflicts of interest to report
Footnotes
Research reported in this publication was supported by the National Institutes of Health under award numbers T32 CA009515, U54 CA190146, R01 CA206466, and P30 AI02775.
Supplementary data to this article can be found online at https://doi.org/10.1016/j.gore.2019.100516.
Appendix A. Supplementary material
The following are the Supplementary data to this article:
Supplementary Fig. 1.
Directed Acyclic Graph illustrating causal assumptions applied to potential confounding and mediating variables in a cohort of patients with cervical cancer where the exposure of interest is HIV serostatus and the outcome of interest is overall survival. Arrows represent direction of causal effects between two variables. The black pathway represents the hypothesized association between HIV and survival; the red pathway represents a potential confounding relationship; green pathways represent potential mediating relationships.
Mediation analysis showing relationship between potential mediator and HIV serostatus; and whether the association between HIV and cervical cancer is diminished when mediator is added into the regression model.
References
- Ferlay, J.B.F., Soerjomataram, I., Ervik, M., Dikshit, R., Eser, S., Mathers, C., Rebelo, M., Parkin, D.M., Forman, D., n.d. GLOBOCAN 2012 v1.0, Cancer Incidence and Mortality Worldwide: IARC CancerBase No. 11. http://globocan.iarc.fr/Pages/fact_sheets_cancer.aspx (accessed August 25, 2015). [DOI] [PubMed]
- Maiman M., Fruchter R.G., Clark M., Arrastia C.D., Matthews R., Gates E.J. Cervical cancer as an AIDS-defining illness. Obstet. Gynecol. 1997;89:76–80. doi: 10.1016/s0029-7844(96)00378-x. [DOI] [PubMed] [Google Scholar]
- Chen C.-H., Chung C.-Y., Wang L.-H., Lin C., Lin H.-L.H.-C., Lin H.-L.H.-C. Risk of cancer among HIV-infected patients from a population-based nested case-control study: implications for cancer prevention. BMC Cancer. 2015;15:133. doi: 10.1186/s12885-015-1099-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walboomers J.M., Jacobs M.V., Manos M.M., Bosch F.X., Kummer J.A., Shah K.V., Snijders P.J., Peto J., Meijer C.J., Muñoz N. Human papillomavirus is a necessary cause of invasive cervical cancer worldwide. J. Pathol. 1999;189:12–19. doi: 10.1002/(SICI)1096-9896(199909)189:1<12::AID-PATH431>3.0.CO;2-F. [DOI] [PubMed] [Google Scholar]
- Lomalisa P., Smith T., Guidozzi F. Human immunodeficiency virus infection and invasive cervical cancer in South Africa. Gynecol. Oncol. 2000;77:460–463. doi: 10.1006/gyno.2000.5775. [DOI] [PubMed] [Google Scholar]
- World Bank Data Help Desk, World Bank Country and Lending Groups, (n.d.). https://datahelpdesk.worldbank.org/knowledgebase/articles/906519-world-bank-country-and-lending-groups (accessed April 16, 2019).
- Grover S., Bvochora-Nsingo M., Yeager A., Chiyapo S., Bhatia R., MacDuffie E., Puri P., Balang D., Ratcliffe S., Narasimhamurthy M., Gwangwava E., Tsietso S., Kayembe M.K.A., Ramogola-Masire D., Dryden-Peterson S., Mahantshetty U., Viswanathan A.N., Zetola N.M., Lin L.L. Impact of human immunodeficiency virus infection on survival and acute toxicities from chemoradiation therapy for cervical cancer patients in a limited-resource setting. Int. J. Radiat. Oncol. Biol. Phys. 2018;101:201–210. doi: 10.1016/j.ijrobp.2018.01.067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferreira M.P., Coghill A.E., Chaves C.B., Bergmann A., Thuler L.C., Soares E.A., Pfeiffer R.M., Engels E.A., Soares M.A. Outcomes of cervical cancer among HIV-infected and uninfected women treated at the Brazilian National Institute of Cancer (2001–2013) AIDS. 2017;31:523–531. doi: 10.1097/QAD.0000000000001367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dryden-Peterson S., Bvochora-Nsingo M., Suneja G., Efstathiou J.A., Grover S., Chiyapo S., Ramogola-Masire D., Kebabonye-Pusoentsi M., Clayman R., Mapes A.C., Tapela N., Asmelash A., Medhin H., Viswanathan A.N., Russell A.H., Lin L.L., Kayembe M.K.A., Mmalane M., Randall T.C., Chabner B., Lockman S. HIV Infection and survival among women with cervical cancer. J. Clin. Oncol. 2016 doi: 10.1200/JCO.2016.67.9613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simonds H.M., Botha M.H., Neugut A.I., Van Der Merwe F.H., Jacobson J.S. Five-year overall survival following chemoradiation among HIV-positive and HIV-negative patients with locally advanced cervical carcinoma in a South African cohort. Gynecol. Oncol. 2018;151:215–220. doi: 10.1016/j.ygyno.2018.08.038. [DOI] [PubMed] [Google Scholar]
- UNAIDS AIDSinfo, HIV Prevalence, (n.d.). http://aidsinfo.unaids.org/ (accessed April 3, 2017).
- Vale W.C.C., Tierney J.F., Stewart L.A., Brady M., Dinshaw K., Jakobsen A., Parmar M.K., Thomas G., Trimble T., Alberts D.S., Chen H., Cikaric S., Eifel P.J., Garipagaoglu M., Keys H., Kantardzic N., Lal P., Lanciano R., Leborgne F., Lorvidhaya V., Onishi H., Pearcey R.G., Pras E., Rober Reducing uncertainties about the effects of chemoradiotherapy for cervical cancer: a systematic review and meta-analysis of individual patient data from 18 randomized trials. J. Clin. Oncol. 2008;26:5802–5812. doi: 10.1200/JCO.2008.16.4368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coulie P.G., Van den Eynde B.J., van der Bruggen P., Boon T. Tumour antigens recognized by T lymphocytes: at the core of cancer immunotherapy. Nat. Rev. Cancer. 2014;14:135–146. doi: 10.1038/nrc3670. [DOI] [PubMed] [Google Scholar]
- Okoye A.A., Picker L.J. CD4(+) T-cell depletion in Hiv infection: mechanisms of immunological failure. Immunol. Rev. 2013;254:54–64. doi: 10.1111/imr.12066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nkwanyana N.N., Gumbi P.P., Roberts L., Denny L., Hanekom W., Soares A., Allan B., Williamson A.L., Coetzee D., Olivier A.J., Burgers W.A., Passmore J.A. Impact of human immunodeficiency virus 1 infection and inflammation on the composition and yield of cervical mononuclear cells in the female genital tract. Immunology. 2009;128:e746–e757. doi: 10.1111/j.1365-2567.2009.03077.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trimble C.L., Peng S., Thoburn C., Kos F., Wu T.C. Naturally occurring systemic immune responses to HPV antigens do not predict regression of CIN2/3. Cancer Immunol. Immunother. 2010;59:799–803. doi: 10.1007/s00262-009-0806-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Mediation analysis showing relationship between potential mediator and HIV serostatus; and whether the association between HIV and cervical cancer is diminished when mediator is added into the regression model.