Abstract
The remote Dallol Hot Springs, an active hydrothermal system in the volcanic region of Danakil (Ethiopia), is an interesting yet poorly studied polyextreme environment for investigating the limits of life. Here, we explored the presence of signs of life in five samples of sinter deposits at Dallol, by means of lipid biomarkers and stable isotope composition. The results reveal the existence of biological material with predominance of (presently or recently active) microbial sources, according to the relative abundance of low-over-high molecular weight moieties (n-alkanes, n-carboxylic acids, or n-alkanols), and the detection of diverse microbial-diagnostic compounds (i.e., monomethyl alkanes; C16:1 ω7, C18:1 ω9, C18:1 ω10, C18:2 ω6,9 and iso/anteiso C15 and C17 carboxylic acids; or short-chained dicarboxylic acids). The molecular lipid patterns at Dallol suggest a microbial community largely composed of thermophilic members of the Aquificae, Thermotogae, Chroroflexi, or Proteobacteria phyla, as well as microbial consortia of phototrophs (e.g., Cyanobacteria-Chloroflexi) in lower-temperature and higher-pH niches. Autotrophic sources most likely using the Calvin cycle, together with the acetyl coenzyme A (CoA) pathway, were inferred from the depleted bulk δ13C ratios (−25.9/−22.6‰), while sulfate-reducing bacteria were considered according to enriched sulfate (7.3/11.7‰) and total sulfur (20.5/8.2‰) δ34S ratios. The abundance of functionalized hydrocarbons (i.e., n-carboxylic acids and n-alkanols) and the distinct even-over-odd predominance/preference on the typically odd n-alkanes (CPIalkanes ≤ 1) pointed to active or recent microbial metabolisms. This study documents the detection of biosignatures in the polyextreme environment of Dallol and raises the possibility of finding life or its remnants in other remote locations on Earth, where the harsh environmental conditions would lead to expect otherwise. These findings are relevant for understanding the limits of life and have implications for searching for hypothetical life vestiges in extreme environments beyond Earth.
Key Words: Lipid biomarkers, Bulk stable isotopes, Polyextreme environments, Limits of life, Dallol hydrothermal system
1. Introduction
Hydrothermal systems have great significance in the early evolution of the biosphere. They are thriving ecosystems containing thermophilic microorganisms similar to those that existed early in Earth's history (Ward et al., 1989). While deep-sea hydrothermal vents have been traditionally postulated to be the environment where life started out, recent geological, chemical, and computational findings rather point to a land-based alternative scenario (Damer, 2016, and references therein). According to this theory, a system of volcanic pools and hot springs on land provides, apart from the basic ingredients for life (i.e., energy and nutrients), a way to create complex molecules and bring them together to promote prebiotic reactions (Van Kranendonk et al., 2017). The alternation of drying and wetting spells combined with the continuous heat supply results in the formation of complex molecules (i.e., polymers) from simpler units such as amino acids or fatty acids (Deamer and Georgiou, 2015). These systems have been operating on Earth for very long periods, as documented in the oldest known subaerial hydrothermal deposits in Australia (Djokic et al., 2017). This land-based perspective is relevant from an astrobiological point of view because it guides scientists to different places in the Solar System to search for life beyond Earth. The examination of geothermal areas and their microbial community may contribute to decipher the origin and expansion of life on Earth and beyond, for instance in analogous hydrothermal systems on Mars (e.g., Gusev Crater) or on Jupiter's and Saturn's icy moons (e.g., Europa and Enceladus, respectively).
Subaerial thermal springs are important ecosystems not only as hosts of life but as long-term preservers of biosignatures (Djokic et al., 2017). Microorganisms representative of early-evolved lineages of chemosynthetic life inhabit modern hot springs (Ward et al., 1989), occurring as planktonic cells in fluids and as biofilms on the surface on interior fractures of mineral deposits (Pancost et al., 2005). These microbial communities are largely composed of thermophiles, majorly inhabiting vent areas or occupying lower-temperature niches, such as hot-spring discharge channels and aprons (Campbell et al., 2015a). The mineral entombment of biofilms and microbial mats living on the hydrothermal deposits facilitates the preservation of numerous microbial biosignatures (Cady et al., 2003; Ruff and Farmer, 2016). In ancient thermal springs, the preservation of biological signatures provides great paleobiological and paleoenvironmental information for understanding early life (Knoll and Walter, 1996).
The search for molecular evidence of life is crucial for understanding the emergence and evolution of life on Earth and other Solar System bodies. Learning about habitability in other planetary bodies requires a deep knowledge of adaptability and life boundaries on Earth. Remote and inhospitable environments on Earth provide excellent settings for assessing the capability of the most resistant forms of life (extremophiles) to endure and thrive in the harshest conditions. The presence of life or its remnants has been investigated in diverse extreme environments on Earth (geothermal regions, hypersaline desert, acidic rivers, hyperarid frozen soils, deep caves, etc.), where life adapts to thrive in a variety of hostile conditions such as hypersalinity (e.g., Cheng et al., 2017; Sánchez-García et al., 2018), aridity (e.g., Wilhelm et al., 2017), acidity (Fernández-Remolar et al., 2005; Fernández-Remolar and Knoll, 2008), thermal systems (Farmer and Des Marais, 1999; Cady et al., 2003; Sánchez-García et al., 2019), or subzero temperatures (Rivkina et al., 2007; Steven et al., 2008). However, although it is well known that life can tolerate or even thrive under extreme conditions (Rothschild and Mancinelli, 2001), the impact of multiple physicochemical factors on the development of life is poorly understood (Harrison et al., 2013). What are the limits of life and to what extent is life able to thrive in environments holding several extreme conditions at the same time are issues that need to be investigated.
Biological studies on polyextreme environments are scarce (Ngugi et al., 2016; Pérez et al., 2018; Wierzchos et al., 2018), with only a few sites, such as the Chilean Altiplano or Red Sea brines, investigated from that perspective. In the Danakil Depression, in northeast Ethiopia (Fig. 1), the volcanic features of the Erta Ale range at the Afar Triangle have created a polyextreme hydrothermal system. Categorized as hot desert climate, Danakil is considered one of the driest (annual precipitation between 50 and 100 mm; Garland, 1980) and hottest (mean annual temperature of 35°C; Fazzini and Bisci, 2015) places on the planet. Located in one of the most remote, inhospitable, and poorly studied regions in the world (i.e., Danakil), Dallol is a complex and active hydrothermal system (Fig. 1c) composed of diverse hot springs that open into an arid desert. In Dallol, seawater and hydrothermal fluids mix, resulting in a hypersaline environment, where the springs discharge extremely hot (temperature from 90°C to 108°C; Franzson et al., 2015; Kotopoulou et al., 2019), oxygen-free, hyperacidic (pH ranging from −1.7 to 4; Gebresilassie et al., 2011; Kotopoulou et al., 2019), Fe-rich hydrothermal brines, which are halite supersaturated as soon as they are in contact with the atmosphere (Kotopoulou et al., 2019). The heat and aridity in Dallol give rise to the development of large evaporitic deposits of about 1000 m depth that are rich in K, Mn, Fe, Mg, or Zn (Tadesse et al., 2003). The abundance of metals makes Dallol Hot Springs an important area for mining exploitation (e.g., rock salt, potassium salts, or manganese deposits) and trading (e.g., Gebresilassie et al., 2011; Darrah et al., 2013; Franzson et al., 2015). In addition, geotourism based on geothermal spring and volcano visiting is becoming more popular in the area (Erfurt-Cooper and Cooper, 2010), where the Dallol Springs are some of the major attractions because of their stunning colored waters, mineral salts, and landforms (Edelman and Roscoe, 2010). The scarce studies existing on Dallol are mostly focused on geological (Nobile et al., 2012; Darrah et al., 2013) and geophysical (Hovland et al., 2006; Carniel et al., 2010) interests related to the recent seismicity and volcanic activity, with a few works reporting on the hydrochemistry operating in this geothermal system (Gonfiantini et al., 1973; Kotopoulou et al., 2019). Little is known about the ecology and biochemistry in the polyextreme environment, with the only biological studies focused on studying the diversity or genome sequencing of halophilic microorganisms on industrially processed, and thus likely human and environmentally contaminated, samples (commercial salts) from Dallol (Gibtan et al., 2016, 2017). There is no study that we know exploring the autochthonous distribution of microbial populations at Dallol. If microorganisms are present in the polyextreme hydrothermal brines and evaporitic fields, their existence would expand the limits of life supporting habitability on Earth and analogous extraterrestrial sites, thus rendering Dallol a site of unique astrobiological significance.
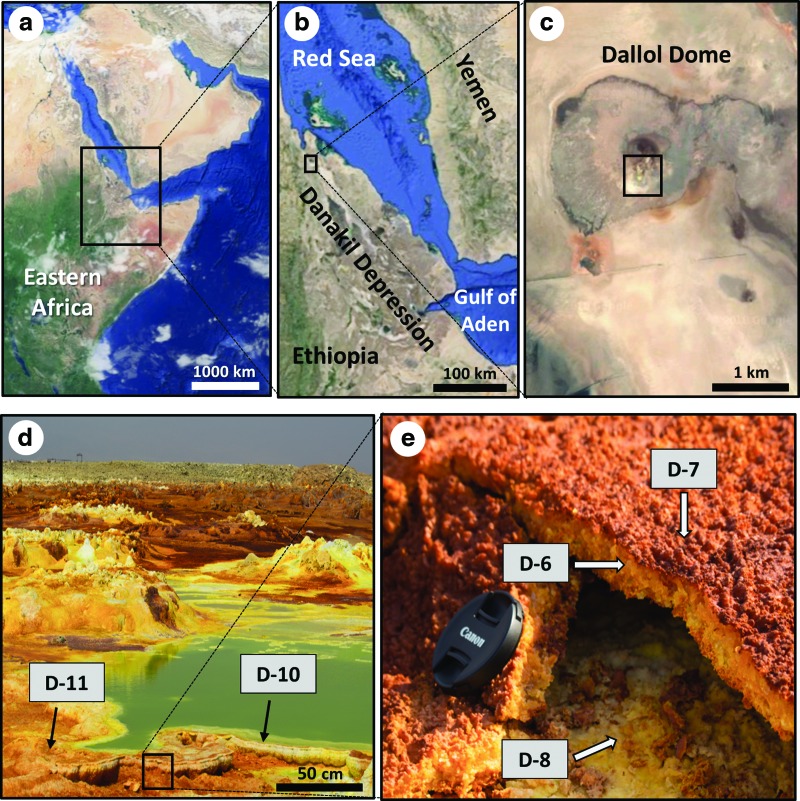
FIG. 1.
Map of eastern Africa (a), showing the Danakil Depression in Ethiopia (b), where the Dallol Hot Springs are located (c). An overview of the survey area with the three sampling sites (an active fumarole chimney, a terrace of evaporitic precipitates, and a hydrothermal pool) is shown (d). A close-up of the three evaporite samples from the small fumarole chimney is also included (e). The maps are shown as satellite images from Google Maps.
This work aimed to investigate the presence of biomarkers in evaporitic deposits of the Dallol Hot Springs to assess the habitability and/or preservation of biomolecules in the polyextreme environment. A geochemical approach based on the combination of lipid and bulk isotopic (δ13C and δ32S) analysis was proposed to assess the presence of life or its remnants in the inhospitable Dallol region. The distribution of lipid molecules with source-diagnosis value is analyzed to infer biological sources and assess activity level. The presence of lipids and isotopic biosignatures is interpreted in a mineralogical context. This study is framed within the UE-funded Europlanet Project (H2020), which, among other activities, investigates extreme environments for detecting biosignatures in very harsh conditions for life to exist, and seeks to validate those extreme environments as terrestrial analogs of other planets (e.g., Mars, Jupiter's icy moon Europa).
1.1. Field settings
The present study is located in the Danakil Depression (Fig. 1), one of the hottest and most tectonically active regions in the world (Darrah et al., 2013). The Danakil Depression is located near the triple junction of the Red Sea, Gulf of Aden, and the Ethiopian Rift, and is part of the East African Rift System, an active continental drifting between Africa and Arabia (Ebinger et al., 2008), which causes the floor of the Danakil Depression to be located about 120 m below sea level (Holwerda and Hutchinson, 1968). From a geological point of view, the Danakil Depression is composed of three formations alternating as follows, from west to east: (a) Neoproterozoic metavolcanic and metasedimentary rocks, (b) Quaternary alluvial deposits intercalated with red beds, and (c) evaporites mainly consisting of halite and potash deposits, as well as sulfur (Gebresilassie et al., 2011). The Neoproterozoic setting includes the mount Dallol and the surrounding hot-spring area (Fig. 1c), where the present study was conducted. The area around Dallol is occupied by an ephemeral salt lake sitting atop a 2 km thick evaporite sequence (Behle et al., 1975) formed when an arm of the Red Sea was isolated by an uplifted horst block, causing almost complete evaporation of the water (Beyene and Abdelsalam, 2005). The evaporite sequence is dominated by halite and sylvite, and subordinate layers rich in carnallite and kainite (Holwerda and Hutchinson, 1968).
The volcanic heat causes the ascent of hot water through the layers of halite and anhydrite that dissolve and deposit over the basaltic lava flows, originating fumaroles, subaerial and subaqueous hydrothermal springs, and acidic brines that produce salt chimneys, pillars, hornitos, terraces, and pools of ephemeral colors (Carniel et al., 2010; Kotopoulou et al., 2019). The broad color palette of the landscape is one of the most striking features of Dallol, as colors range from pale green to dark brown and reds, due to the combined action of the continuous discharge of oxygen-free Fe(II)-rich spring brines, the low solubility of oxygen in high temperature, hyperacidic brines, and hypersaline brines, and therefore the slow oxidation of the Fe(II) species (Kotopoulou et al., 2019). Three principal evaporitic formations in the Dallol hydrothermal system are (a) pillars, or circular columns composed of salt formed from outflows of water supersaturated with sodium chloride; (b) circular manifestations formed by deposition episodes, where mineral-supersaturated water begins precipitating out upon cooling, exsoluting and disintegrating material (i.e., halides becoming richer in anhydrite and sulfur over time); and (c) acid pools (pH ∼0.7 to 4) derived from the oxidation of geothermal hydrogen sulfide to sulfuric acid upon mixing with groundwater (Franzson et al., 2015). Our study focused on the acidic pools of the main Dallol hydrothermal area (14°14′19″N, 40°17′38″E) and their associated evaporites (Fig. 1c, 1d). The active subsidence in Dallol causes the delivery of salts coming from the subsurface hot water, which precipitate and form evaporitic structures, such as small chimneys surrounded by pools of hot water (Fig. 1d), enriched in metals and colorful precipitates of halite (NaCl), pyrolusite (MnO2), chlorargyrite (AgCl), wurtzite (ZnS), and iron-rich salts (Master, 2013).
2. Materials and Methods
2.1. Sample collection
In January 2016, geological samples were collected from three evaporitic sites in the Dallol Hot Springs (ca. 146 m below sea level), as part of a Europlanet sampling campaign (Grant agreement N° 654208). Three sampling locations were chosen aiming to cover different hydrothermal environments: an active fumarole chimney, an inactive terrace of evaporitic precipitates, and a hydrothermal pool. Five samples were collected in total (Fig. 1d, 1e). Salt precipitates were sampled from three different parts of the fumarole chimney: yellow (D6) and brownish (D7) precipitates from the outer and inner part of the chimney top layer, and yellow precipitates from the base of the fumarole structure (D8). One sample was taken from the yellow precipitates lying in the terrace nearby the fumarole chimney (D11), and another was taken of yellow-greenish precipitate from the edge of the green thermal pool (D10). Temperature and pH in the water pool were measured at 90°C and 2, respectively. The pH of the sampled material was measured to be around 4 in the five sites, whereas the in situ temperature was not measured but assumed to be in between atmospheric (diurnal range from 25°C to 45°C; Garland, 1980) and that measured in the water (90°C). The five evaporite samples were collected with a solvent-clean (dichloromethane and methanol) stainless-steel spatula, stored in polypropylene containers at −20°C, and, once in the laboratory, freeze-dried and ground in a pestle prior to lipid analysis.
2.2. Geochemical and mineralogical analyses
The mineralogical composition of the evaporites was measured with a Bruker X-ray diffractometer (AXS D8-Focus, XRD). The freeze-dried samples were ground and scanned in the 2·θ-diffraction angle from 5° to 70°, with a scanning step size of 0.01°, at 40 kV and 40 mA with a Cu X-ray source (Cu Kα1,2, λ = 1.54056 Å).
Anions and low-molecular-weight organic acids were measured by ion chromatography (IC) in the water-extractable phase of the samples. For this analysis, 2 g of sample was sonicated (3 × 1 min cycles) and diluted in 10 mL of deionized water, then filtered through a 22 μm GF/F. The filtrates were collected and loaded into a Metrohm 861 Advanced compact ion chromatograph (Metrohm AG, Herisau, Switzerland) undiluted or at dilution values, depending on ion concentrations. For all the anions, the column Metrosep A supp 7−250 was used with 3.6 mM sodium carbonate (NaCO3) as eluent. The pH of the water solutions was measured with a pH meter (WTW, GmbH & Co. KG, Weilheim, Germany) after 24 h of solution stabilization.
2.3. Lipid extraction, fractionation, and analysis
The evaporitic samples were Soxhlet extracted (∼30 g) with a mixture of dichloromethane/methanol (DCM:MeOH, 3:1, v/v) for 24 h, after addition of three internal standards (tetracosane-D50, myristic acid-D27, 2-hexadecanol). The total lipid extract (TLE) was concentrated to ca. 2 mL by using rotary evaporation, and activated copper was added to remove elemental sulfur. TLE was separated into polarity fractions by using Bond-Elut (bond phase NH2, 500 mg, 40 μm particle size) and Al2O3 (activated, neutral, 0.05–0.15 mm particle size) columns (Supplementary Fig. S1, https://www.liebertpub.com/suppl/doi/10.1089/ast.2018.1963). First, the neutral and acidic lipid fractions were obtained by eluting the TLE through a Bond-Elut column with 15 mL of DCM:2-propanol (2:1, v/v) and 15 mL of acetic acid (2%) in diethyl ether, respectively. Then, a further separation of the neutral lipid fraction into nonpolar and polar subfractions was done using 0.5 g of Al2O3 in a Pasteur pipette (ca. 2.5 cm high). The nonpolar fraction was obtained by eluting 4.5 mL of hexane/DCM (9:1, v/v), and the polar fraction by subsequently eluting 3 mL of DCM/methanol (1:1, v/v). The acidic and polar fractions were trans-esterified (BF3 in methanol) and tri-methylsilylated (N,O-bis(trimethylsilyl)trifluoroacetamide, BSTFA), respectively, and then analyzed as fatty acid methyl esters (i.e., FAMEs) and trimethylsilyl (i.e., TMS) alkanols by gas chromatography–mass spectrometry (GC-MS).
The three lipid fractions (i.e., nonpolar, polar, and acidic) were analyzed by GC-MS using a 6850 GC system coupled to a 5975 VL MSD with a triple axis detector (Agilent Technologies) operating with electron ionization at 70 eV and scanning from m/z 50 to 650. The analytes were injected (1 μL) and separated on a HP-5MS column (30 m × 0.25 mm i.d. × 0.25 μm film thickness) with He as a carrier gas at 1.1 mL min−1. For the nonpolar fraction, the oven temperature was programmed from 50°C to 130°C at 20°C min−1, then to 300°C at 6°C min−1 (held 20 min). For the acidic fraction, the oven temperature was programmed from 70°C to 130°C at 20°C min−1 and to 300°C at 10°C min−1 (held 10 min). For the polar fraction, the oven temperature program was the same as for the acidic fraction, but the oven was held for 15 min at 300°C. The injector temperature was 290°C, the transfer line 300°C, and the MS source 240°C. Compound identification was based on the comparison of mass spectra and/or reference materials and quantification on the use of external calibration curves of n-alkanes (C10 to C40), n-FAMEs (i.e., C8 to C24), n-alkanols (C10, C14, C18, and C20), and branched isoprenoids (2,6,10-trimethyl-docosane, crocetane, pristane, phytane, squalane, and squalene), all from Sigma-Aldrich. The recoveries of the internal standards averaged 72 ± 23 %.
2.4. Stable isotopic analysis of organic carbon, sulfate, and total sulfur
The stable isotopic composition of the bulk organic carbon, sulfate, and total sulfur was determined by isotope ratio mass spectrometry (IRMS), with a MAT 253 (Thermo Fisher Scientific). The isotopic analyses were conducted according to the respective USGS methods for carbon (Révész et al., 2012a), sulfur in sulfate, (Kester et al., 2011), and total sulfur (Révész et al., 2012b). For organic carbon and total sulfur, 2 g of sample was ground and homogenized with a corundum mortar and pestle. The samples were decarbonated with concentrated HCl (37%) and, given the abundance of NaCl reported by IC (>98%), washed with deionized water to eliminate the dissolved salts through GF/F filtering (0.7 μm pore size, Whatman). Precombusted filters (8 h at 450°C) were dried in an oven (50°C) and analyzed by IRMS. For sulfate analysis, approximately 1 g of the sample was extracted with deionized water (20:1), by shaking for several hours. The supernatant was then extracted again with a solution of preheated HCl (1 M, 70°C) to ensure a complete extraction of the sulfate from the sample. The sulfate concentration is measured by IC. The pH of the extracted solution was adjusted to less than 2, and sulfate was then precipitated by using a saturated solution of barium chloride (BaCl2). The solution was allowed to stand overnight and then filtrated to collect the formed BaSO4. After drying in an oven at 50°C, the BaSO4 was analyzed for the isotopic composition of the sulfate by using the method by Kester and coauthors (2011).
The carbon and sulfates/sulfur results were reported as δ13C (abbreviation from δ[13C/12C]) and δ34S (abbreviation from δ[34S/32S]) values relative to the Pee Dee Belemnite limestone standard (PDB) and Vienna-Canyon Diablo Troilite (VCDT), respectively, in the usual parts per mill (‰) notation. An analytical precision of 0.1‰ was determined by using three certified standards for carbon (USGS41, IAEA-600, and USGS40), three for sulfur on sulfate (IAEA-SO5, IAEA-SO6, and NBS 127), and three for sulfur (IAEA-S1, IAEA S-2, and IAEA-S3). The total organic carbon content (TOC %) was measured with an elemental analyzer (Flash HT, Thermo Fisher Scientific), during the stable isotope measurements.
3. Results
3.1. Mineralogy and bulk geochemistry
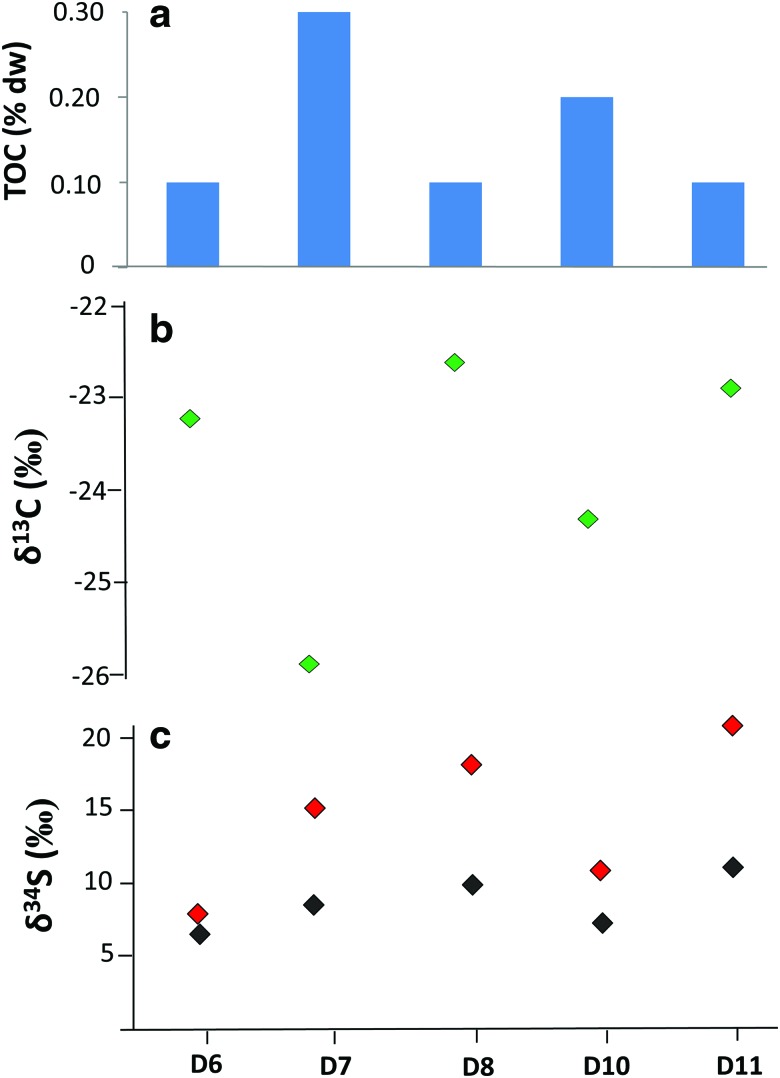
The XRD analysis indicated that the sample mineralogy majorly consisted of halite (NaCl, >90%), with lower presence of hydrothermal minerals such as bromian chlorargyrite (Ag(Cl,Br)) and wurtzite (ZnS). The only anions measured by IC were chloride (2215 ± 115 mg/g), fluoride (54 ± 3.4 μg/g), and sulfate (14 ± 0.83 mg/g), whereas no organic anions were detected. The TOC content was measured to vary from 0.12% to 0.35% of dry weight (dw) (Fig. 2a). The sample isotopic composition ranged from −22.6‰ to −25.9‰ for δ13C (Fig. 2b), from 6.5‰ to 11.7‰ for δ34S from sulfate (Fig. 2c), and from 8.2‰ to 20.5‰ for δ34S for total sulfur (Fig. 2c).
FIG. 2.
Geochemical composition of the bulk organic fraction in the Dallol samples; (a) concentration of total organic carbon (% of the sample dry weight), (b) stable carbon isotopic composition of organic carbon (‰ PDB), and (c) stable sulfur isotopic composition of total sulfur (red) and sulfur from sulfate (black) (‰ VCDT).
3.2. Lipid biomarkers
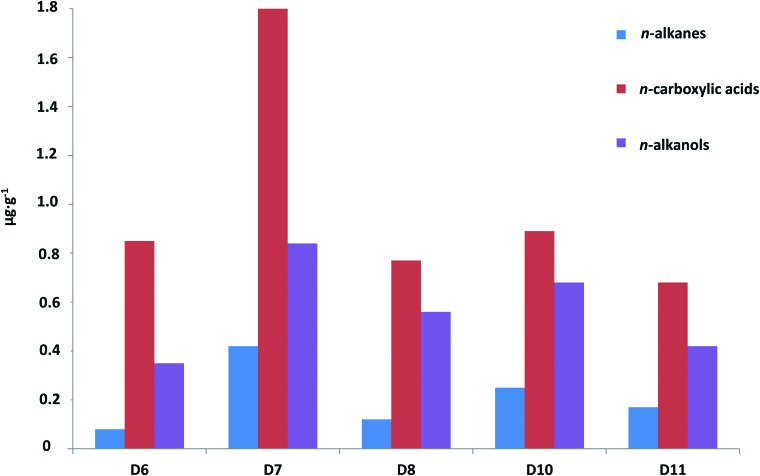
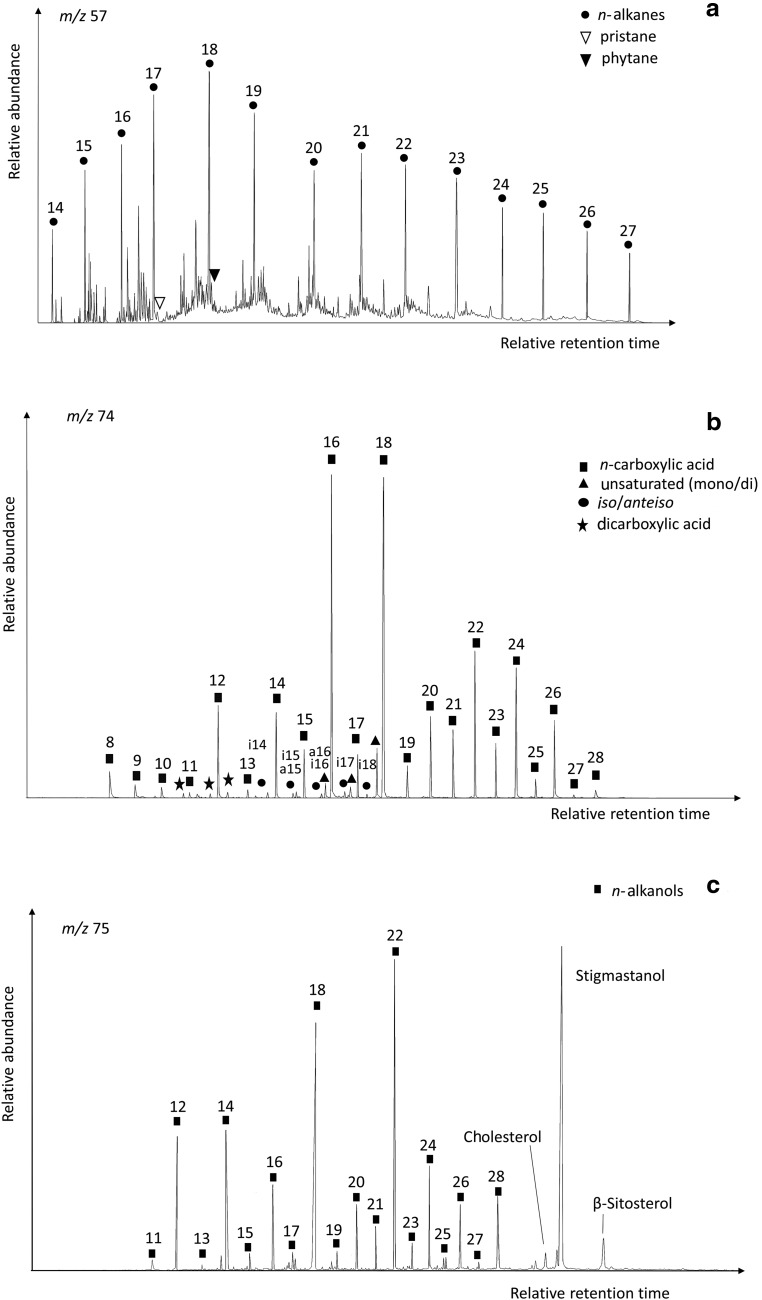
The GC-MS analysis of the lipid extracts detected the presence of diverse families (Table 1) using mass-to-charge ratios (m/z) of 57 (n-alkanes and isoprenoids), m/z = 74 (n-carboxylic acids, including saturated, unsaturated, and branched moieties), and m/z = 75 (n-alkanols and sterols) (Tables 2–4). Among all families, the n-carboxylic acids were the most abundant lipid compounds, followed by the n-alkanols, and n-alkanes (Fig. 3). The three major lipid families showed a clear even-over-odd predominance/preference. Straight-chain alkanes (aka normal, or n-alkanes) ranging from 14 to 27 carbons (C14 to C27) were measured at concentrations from 0.08 to 0.42 μg·g−1 (Tables 1 and 2). The n-alkane molecular distribution exhibited an average chain length (ACL) of 19–20 (Table 1) and a bimodal pattern with a maximum peak at C18 and a secondary peak at C21 (Fig. 4a). The relative abundance of low-molecular-weight (LMW) over high-molecular-weight (HMW) n-alkanes resulted in LMW/HMW ratios always larger than one (1.6–3.1; Table 1). Branched alkanes (mono-, di-, tri-, and tetramethyl congeners) were also found in the m/z = 57 ratio, with the monomethyl alkanes being the most abundant (Table 2). Among the branched alkanes, pristane (Pr) and phytane (Ph) were found (Fig. 4a) at concentrations from 0.05 to 0.10 μg·g−1 and from 0.02 to 0.07 μg·g−1, respectively (Table 2). Other isoprenoids such as squalane or crocetane were not detected.
Table 1.
Concentration (μg·g−1) and Compositional Distribution of Lipid Biomarkers and Bulk Stable Isotopes in the Dallol Samples
| D6 | D7 | D8 | D10 | D11 | |
|---|---|---|---|---|---|
| TOC (% dw) | 0.12 | 0.35 | 0.15 | 0.22 | 0.14 |
| δ13COC (‰) | −23.2 | −25.9 | −22.6 | −24.3 | −22.9 |
| δ34S total (‰) | 8.2 | 15.3 | 18.4 | 10.9 | 20.5 |
| δ34S SO4 (‰) | 6.5 | 8.5 | 9.5 | 7.3 | 11.7 |
| n-alkanes | 0.08 | 0.42 | 0.12 | 0.25 | 0.17 |
| branched n-alkanesa | 0.05 | 0.25 | 0.09 | 0.16 | 0.12 |
| ACL n-alkanesb | 19 | 20 | 19 | 20 | 20 |
| LMWn-alkanes/HMWn-alkanesc | 3.10 | 1.62 | 2.46 | 1.82 | 1.53 |
| CPI n-alkanes (c14–C27)d | 0.86 | 1.05 | 0.98 | 0.98 | 1.01 |
| pristanee | 0.05 | 0.10 | 0.06 | 0.07 | n.d. |
| phytanef | n.d. | 0.07 | 0.03 | 0.02 | n.d. |
| n-carboxylic acids | 0.85 | 1.77 | 0.78 | 0.88 | 0.68 |
| unsaturated carboxylic acidsg | 0.12 | 0.15 | 0.12 | 0.13 | 0.10 |
| dicarboxylic acids | 0.02 | 0.02 | 0.01 | 0.01 | 0.01 |
| iso/anteiso-carboxylic acidsh | 0.01 | 0.05 | 0.02 | 0.02 | 0.01 |
| MM-carboxylic acidsi | n.d. | 0.02 | 0.01 | 0.03 | 0.01 |
| Σ carboxylic acidsj | 1.01 | 2.01 | 0.93 | 1.07 | 0.82 |
| ACL n-carboxylic acidsb | 18 | 19 | 17 | 17 | 18 |
| LMWn-acids/HMWn-acidsc | 3.12 | 2.36 | 63.67 | 6.40 | 3.07 |
| CPI n-carboxylic acids (c8–C28)d | 7.40 | 4.34 | 28.85 | 3.68 | 5.74 |
| n-alkanols | 0.35 | 0.83 | 0.56 | 0.68 | 0.42 |
| ACL n-alkanolsb | 21 | 20 | 20 | 20 | 20 |
| LMWn-alkanols/HMWn-alkanolsc | 0.70 | 0.98 | 0.92 | 0.98 | 0.98 |
| CPI n-alkanols (c11–C28)d | 6.71 | 4.38 | 3.45 | 4.29 | 8.25 |
| cholesterolk | 0.01 | 0.02 | 0.01 | 0.03 | 0.01 |
| stigmastanoll | 0.22 | 0.35 | 0.13 | 0.15 | 0.11 |
| β-sitosterolm | 0.02 | 0.03 | 0.02 | 0.01 | 0.01 |
| Σ c32+C34 wax ester | n.d. | 0.04 | 0.01 | 0.03 | n.d. |
Sum of mono-, di-, trimethyl n-alkanes.
Average chain length of C14–C27 n-alkanes; C8–C28 n-carboxylic acids and C11–C28 n-alkanols. ACLi-n = Σ(i·Xi + … + n·Xn)/ΣXi + … + Xn), where X is concentration (van Dongen et al., 2008).
Low molecular weight (LMW) over high molecular weight (HMW). LMW is the sum of C14–C20 (n-alkanes), C8–C20 (n-carboxylic acids), and C11–C20 (n-alkanols). HMW is the sum of C21–C27 (n-alkanes), C21–C28 (n-carboxylic acids), and C21–C28 (n-alkanols).
Carbon preference index, CPIi-n = ½Σ(Xi + Xi+2 + … + Xn)/Σ(Xi−1 + Xi+1 + … + Xn−1) + ½Σ(Xi + Xi+2 + … + Xn)/Σ(Xi+1 + Xi+3 + … + Xn+1), where X is concentration.
2,6,10,14-tetramethyl-pentadecane.
2,6,10,14-tetramethyl-hexadecane.
Sum of monounsaturated n-carboxylic acids (C16 and C18).
Sum of iso- and anteiso- n-carboxylic acids (C14–C18).
Sum of monomethyl C22 and C24 n-carboxylic acids.
Sum of all carboxylic acids (saturated, unsaturated, dicarboxylic, iso/anteiso- and monomethyl-).
Cholest-5-en-3b-ol.
24-ethyl-5a-cholest-22-en-3b-ol.
4-Ethylcholest-5-en-3β-ol.
n.d. = not detected.
Table 2.
Concentration (μg·g−1) of Normal (i.e., Straight-Chain) and Branched Alkanes in the Dallol Samples
| Alkanes | Abbreviationa | D6 | D7 | D8 | D10 | D11 |
|---|---|---|---|---|---|---|
| Tetradecane | C14 | 0.004 | 0.022 | 0.004 | 0.01 | 0.006 |
| Dodecane. 5.8-dimethyl- | DiM-C12 | 0.002 | 0.005 | 0.005 | 0.005 | 0.004 |
| Dodecane. 2.6.11-trimethyl | TriM-C12 | 0.015 | 0.071 | 0.022 | 0.055 | 0.019 |
| Tridecane. 3-methyl- | MM-C13 | 0.002 | 0.016 | 0.012 | 0.016 | 0.004 |
| Pentadecane | C15 | 0.006 | 0.027 | 0.009 | 0.017 | 0.009 |
| Tetradecane. 4-methyl- | MM-C14 | 0.018 | 0.090 | 0.025 | 0.055 | 0.041 |
| Hexadecane | C16 | 0.009 | 0.039 | 0.011 | 0.021 | 0.011 |
| Pentadecane. 2.6.10.14-tetramethylb | TetraM-C15 | 0.052 | 0.102 | 0.062 | 0.073 | n.d. |
| Hexadecane. 6-methyl | MM-C16 | 0.002 | 0.040 | n.d. | n.d. | 0.033 |
| Heptadecane | C17 | 0.012 | 0.045 | 0.014 | 0.028 | 0.015 |
| Hexadecane. 2.6.10.14-tetramethylc | TetraM-C16 | n.d. | 0.071 | 0.032 | 0.021 | n.d. |
| Pentadecane. 3.6.11-trimethyl- | TriM-C15 | 0.004 | 0.004 | 0.003 | 0.006 | 0.005 |
| Octadecane | C18 | 0.015 | 0.059 | 0.019 | 0.042 | 0.027 |
| Nonadecane | C19 | 0.008 | 0.041 | 0.016 | 0.024 | 0.019 |
| Eicosane | C20 | 0.009 | 0.028 | 0.013 | 0.022 | 0.017 |
| Eicosane. 2-methyl | MM-C20 | 0.010 | 0.029 | 0.026 | 0.024 | 0.016 |
| Heneicosane | C21 | 0.006 | 0.027 | 0.008 | 0.020 | 0.013 |
| Docosane | C22 | 0.006 | 0.026 | 0.008 | 0.018 | 0.011 |
| Tricosane | C23 | 0.005 | 0.024 | 0.006 | 0.015 | 0.010 |
| Tetracosane | C24 | 0.003 | 0.023 | 0.007 | 0.012 | 0.009 |
| Pentacosane | C25 | n.d. | 0.022 | 0.006 | 0.009 | 0.009 |
| Hexacosane | C26 | n.d. | 0.021 | n.d. | 0.008 | 0.008 |
| Heptacosane | C27 | n.d. | 0.018 | n.d. | 0.008 | 0.008 |
| Σ br-alkanesd | 0.105 | 0.428 | 0.187 | 0.255 | 0.122 | |
| Σ n-alkanes | 0.083 | 0.422 | 0.121 | 0.254 | 0.172 |
Cx is used to denominate the linear and saturated (normal) alkanes; MM, DiM, TriM, and TetraM stand for mono-, di-, tri- and tetramethyl alkanes.
Pristane.
Phytane.
Sum of branched alkanes.
n.d. = not detected.
Table 4.
Concentration (μg·g−1) of Normal Alkanols in the Dallol Samples
| Alkanols | Acronym | D6 | D7 | D8 | D10 | D11 |
|---|---|---|---|---|---|---|
| Undecanol | C11 | 0.002 | 0.005 | 0.005 | 0.005 | 0.002 |
| Dodecanol | C12 | 0.010 | 0.065 | 0.033 | 0.061 | 0.018 |
| Tridecanol | C13 | 0.002 | 0.008 | 0.016 | 0.016 | 0.002 |
| Tetradecanol | C14 | 0.018 | 0.060 | 0.040 | 0.070 | 0.038 |
| Pentadecanol | C15 | 0.002 | 0.004 | 0.004 | 0.004 | 0.004 |
| Hexadecanol | C16 | 0.028 | 0.070 | 0.035 | 0.030 | 0.027 |
| Heptadecanol | C17 | 0.010 | 0.031 | 0.013 | 0.021 | 0.010 |
| Octadecanol | C18 | 0.049 | 0.119 | 0.085 | 0.119 | 0.072 |
| Nonadecanol | C19 | 0.002 | 0.002 | 0.005 | 0.002 | 0.002 |
| Eicosanol | C20 | 0.022 | 0.070 | 0.050 | 0.030 | 0.039 |
| Heneicosanol | C21 | 0.003 | 0.038 | 0.018 | 0.022 | 0.006 |
| Docosanol | C22 | 0.071 | 0.140 | 0.101 | 0.111 | 0.082 |
| Tricasanol | C23 | 0.009 | 0.012 | 0.012 | 0.012 | 0.009 |
| Tetracosanol | C24 | 0.052 | 0.070 | 0.050 | 0.070 | 0.038 |
| Pentacosanol | C25 | 0.006 | 0.033 | 0.023 | 0.023 | 0.006 |
| Hexacosanol | C26 | 0.025 | 0.051 | 0.029 | 0.031 | 0.025 |
| Heptacosanol | C27 | 0.012 | 0.016 | 0.006 | 0.016 | 0.012 |
| Octacosanol | C28 | 0.025 | 0.039 | 0.031 | 0.039 | 0.025 |
| Σ n-Alkanols | 0.348 | 0.833 | 0.556 | 0.682 | 0.417 |
FIG. 3.
Relative concentration (μg·g−1) of the three major lipid families in the Dallol evaporites, the straight chain n-alkanes, n-carboxylic acids, and n-alkanols.
FIG. 4.
Mass chromatograms of the three major lipid families in the Dallol sample D7; n-alkanes (m/z 57) (a), carboxylic acids as methyl esters (m/z 74) (b), and n-alkanols as trimethyl-silyl esters (m/z 75) (c).
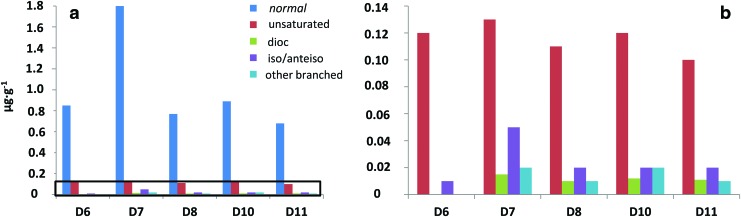
The n-carboxylic acids were measured at concentrations between 0.68 and 1.77 μg·g−1 (Tables 1 and 3), with chain lengths ranging from C8 to C28 (ACL ≤19). The straight-chained were the most abundant carboxylic acids, whereas unsaturated, branched, or dicarboxylic acids were scarcer (Table 1 and Fig. 4b). The molecular distribution of the majority n-carboxylic acids showed a clear dominance of the C16 and C18 peaks, with secondary peaks at C12, C14, and C22 (Fig. 4b). The resulting ratio of LMW over HMW carboxylic acids ranged from 2.3 to 63 (Table 1). Carboxylic moieties with different unsaturations (mono- and di-) were observed at C16, C17, and C18 at concentrations from 0.10 to 0.15 μg·g−1 (Tables 1 and 3). Acids with two carboxyl groups (i.e., dioic or dicarboxylic acids) were also found from C8 to C10 at small concentrations ranging from 0.01 to 0.02 g·g−1 (Table 1). Branched n-carboxylic acids with iso and anteiso configuration (i/a) were detected between C14 and C18 at concentrations from 0.01 to 0.05 μg·g−1 (Table 1), with predominance of the i/a-C15 and i/a-C17 (Fig. 4b). Other branched n-carboxylic acids included monomethyl C22 and C24 acids (Table 1).
Table 3.
Concentration (μg·g−1) of Carboxylic Acids (Saturated, Unsaturated, Branched, and Dicarboxylic) in the Dallol Samples
| Carboxylic acids | Abbreviationa | D6 | D7 | D8 | D10 | D11 |
|---|---|---|---|---|---|---|
| Octanoic acid | C8 | 0.011 | 0.014 | 0.001 | 0.001 | 0.011 |
| Nonanoic acid | C9 | 0.003 | 0.028 | 0.002 | 0.002 | 0.003 |
| Decanoic acid | C10 | 0.013 | 0.018 | 0.001 | 0.001 | 0.013 |
| Undecanoic acid | C11 | 0.002 | 0.001 | 0.001 | n.d. | 0.002 |
| Octanedioic acid | di-C8 | 0.004 | 0.003 | 0.004 | 0.005 | 0.004 |
| Dodecanoic acid | C12 | 0.024 | 0.070 | 0.010 | 0.008 | 0.02 |
| Nonanedioic acid | di-C9 | 0.014 | 0.011 | 0.005 | 0.003 | 0.005 |
| Tridecanoic acid | C13 | 0.002 | 0.002 | 0.010 | 0.019 | 0.002 |
| Decanedioic acid | di-C10 | 0.002 | 0.001 | 0.002 | 0.003 | 0.002 |
| Tridecanoic acid, 12-methyl- | isoC14 | n.d. | 0.002 | 0.002 | n.d. | n.d. |
| cis-9-Tetradecenoic acid | anteisoC14 | n.d. | 0.004 | n.d. | n.d. | n.d. |
| Tetradecanoic acid | C14 | 0.019 | 0.050 | 0.012 | 0.071 | 0.019 |
| Methyl 13-methyltetradecanoate | isoC15 | 0.003 | 0.003 | n.d. | n.d. | 0.003 |
| Tetradecanoic acid, 12-methyl | anteisoC15 | n.d. | 0.005 | n.d. | 0.001 | 0.001 |
| Pentadecanoic acid | C15 | 0.01 | 0.058 | 0.013 | 0.041 | 0.010 |
| Pentadecanoic acid, 13-methyl | isoC16 | 0.001 | 0.002 | n.d. | 0.001 | n.d. |
| Pentadecanoic acid, 14-methyl | anteisoC16 | n.d. | 0.002 | n.d. | 0.002 | 0.002 |
| 9-Hexadecenoic acid (Z) | C16:1 (ω7) | 0.017 | 0.022 | n.d. | 0.001 | 0.020 |
| Hexadecanoic acid | C16 | 0.27 | 0.413 | 0.374 | 0.280 | 0.174 |
| Hexadecanoic acid, 15-methyl | isoC17 | 0.006 | 0.011 | 0.004 | n.d. | 0.004 |
| Methyl 8-heptadecenoate | anteisoC17 | 0.003 | 0.009 | n.d. | n.d. | 0.003 |
| Heptadecanoic acid | C17 | 0.017 | 0.021 | n.d. | 0.046 | 0.017 |
| Heptadecanoic acid, 16-methyl | isoC18 | n.d. | 0.012 | 0.011 | 0.018 | n.d. |
| 9,12-Octadecadienoic acid (Z,Z) | C18:2 (ω6,9) | 0.021 | 0.023 | 0.015 | 0.011 | 0.021 |
| 9-Octadecenoic acid (Z)- | C18:1 (ω9) | 0.056 | 0.062 | 0.065 | 0.093 | 0.056 |
| 8-Octadecenoic acid (E)- | C18:1 (ω10) | 0.024 | 0.042 | 0.035 | 0.024 | 0.004 |
| Octadecanoic acid | C18 | 0.244 | 0.463 | 0.322 | 0.237 | 0.214 |
| Nonadecanoic acid | C19 | 0.006 | 0.030 | n.d. | 0.037 | 0.006 |
| Octadecanoic acid, 10-oxo- | Oxo-C19 | 0.002 | 0.002 | n.d. | n.d. | 0.002 |
| Eicosanoic acid | C20 | 0.025 | 0.076 | 0.018 | 0.019 | 0.025 |
| Heneicosanoic acid | C21 | 0.023 | 0.057 | n.d. | 0.015 | 0.023 |
| Methyl 11-docosenoate | MM-C22 | n.d. | 0.004 | 0.004 | 0.010 | n.d. |
| Docosanoic acid | C22 | 0.052 | 0.017 | 0.012 | 0.037 | 0.042 |
| Tricosanoic acid | C23 | 0.020 | 0.074 | n.d. | 0.011 | 0.02 |
| Tetracosanoic acid | C24 | 0.053 | 0.146 | n.d. | 0.030 | 0.034 |
| Methyl,22-methyl-tetracosanoate | MM-C24 | n.d. | 0.016 | 0.007 | 0.015 | 0.010 |
| Pentacosanoic acid | C25 | 0.013 | 0.029 | n.d. | 0.004 | 0.013 |
| Hexacosanoic acid | C26 | 0.036 | 0.115 | n.d. | 0.021 | 0.026 |
| Heptacosanoic acid | C27 | 0.003 | 0.020 | n.d. | n.d. | 0.003 |
| Octacosanoic acid | C28 | 0.007 | 0.070 | n.d. | 0.001 | 0.007 |
| Σ n-carboxylic acids | 0.853 | 1.772 | 0.776 | 0.881 | 0.684 | |
| Σ unsaturated carboxylic acids | 0.118 | 0.149 | 0.115 | 0.129 | 0.101 | |
| Σ dicarboxylic acids | 0.020 | 0.015 | 0.011 | 0.011 | 0.011 | |
| Σ iso/anteiso carboxylic acids | 0.013 | 0.050 | 0.017 | 0.022 | 0.013 | |
| Σ MM carboxylic acids | n.d. | 0.020 | 0.011 | 0.025 | 0.010 |
Cx is used to denominate the linear and saturated (normal) carboxylic acids, where x is the number of carbons; Cx:y is used to denominate the unsaturated carboxylic acids, where x is the number of carbons and y the number of unsaturations; MM stands for mono-methyl chains and Oxo for the ketoacids.
Straight-chained alkanols (i.e., n-alkanols) were the second most abundant lipids (0.35–0.83 μg·g−1) in the Dallol samples (Fig. 3 and Table 4). They showed even distributions (carbon preference index, CPI = 3.4–8.2) from C11 to C28 (ACL ≤21), with dominance of the C22 and C18 congeners and minority but important presence of the C12-C14 and C24-C26-C28 peaks (Fig. 4c). In the same ion (i.e., m/z = 75), three sterols belonging to the cholesterol and phytosterol (stigmastanol and β-sitosterol) classes were also detected (Fig. 4c), with stigmastanol being the most abundant sterol (0.11–0.35 μg g−1; Table 1).
Finally, a few wax esters (i.e., hexadecyl hexadecanoate, C32; and octadecyl hexadecanoate, C34) were measured in the polar fraction (ions m/z 257, 480; and m/z 257, 508, respectively) at low concentrations (≤0.04 μg g−1) in the D7, D8, and D10 samples (Table 1).
4. Discussion
4.1. Bulk geochemistry in the Dallol hot springs
The mineralogical composition of the Dallol samples explained the color range observed in the sampled precipitates. The light yellow and whitish tones (Fig. 1d, 1e) reflected the majority content of halite and the presence of freshly exposed bromian chlorargyrite, whereas the brown and orangish tones revealed the presence of wurtzite and oxidized chlorargyrite. The high halite content is typical of evaporitic deposits and common in hypersaline environments such as the Atacama Desert (e.g., Fernández-Remolar et al., 2013; Sánchez-García et al., 2018). The abundance of halite is known to play a role in the preservation of organics including microbial biosignatures, a process that has been described in different hypersaline (Fernández-Remolar et al., 2013; Schinteie and Brocks, 2016; Cheng et al., 2017) and hydrothermal (Pancost et al., 2005; Zhang et al., 2007) systems. In hypersaline and hyperarid environments similar to Dallol, a wide variety of preservation strategies have been reported such as entrapment in salt crystals (e.g., de los Ríos et al., 2010; Cheng et al., 2017), xeropreservation (Wilhelm et al., 2017; Sánchez-García et al., 2018), or cellular modifications as survival mechanisms upon stress (Morgan et al., 2006). Hydrothermal minerals such as bromian chlorargyrite and wurtzite are known to form in acidic and saline hydrothermal solutions that undergo surface deposition (Nickel, 2002).
The low values of TOC in the five Dallol samples (Fig. 2a) illustrated the limited content of organic matter in a system where the polyextreme conditions hamper the development of life. Dallol is an unvegetated region, where the intense solar radiation, elevated temperatures, hyperacidity, and high salinity make the hydrothermal substrate fairly inhospitable. In fact, no lichens, higher plants, or native animals were observed in the place during the sampling period. The only form of life capable of adapting and thriving in such a hostile environment is expected to be majorly prokaryotic (i.e., bacteria, archaea), with potential presence of acidophilic fungi, and to occupy specific niches. The diversity of geochemical conditions existing in pools and fumaroles, where hydrothermal fluids and surrounding evaporites are characterized by abrupt gradients of temperature, pH, redox potential, oxygen concentration, mineral content, and metal availability, provides a variety of niches for the microbial communities, in a similar way as in deep-sea hydrothermal vents (McCollom and Shock, 1997).
The δ13C ratios (−22.6‰ to −25.9‰) measured in the Dallol samples (Fig. 2b) are in the range of those reported in similar geothermal environments such as the Obsidian Pool in the Yellowstone National Park (Schuler et al., 2017), El Tatio geyser field in Chile (Sánchez-García et al., 2019), Uzon Caldera in Kamchatka (Russia; Burgess et al., 2011), or California (Eagleville) and Nevada (Paradise Valley and Crescent Valley) hot springs (Zhang et al., 2007), and suggest a dominant autotrophic fingerprint. According to kinetic and thermodynamic methods, microorganisms using the acetyl coenzyme A (CoA) pathway express the largest fractionation of 13C during fixation of CO2 (Δδ13C from 15‰ to 36‰), whereas those using the Calvin cycle display somewhat less discrimination (Δδ13C from 11‰ to 26‰) (Hayes, 2001). Fractionations even lower occur with CO2 incorporations using the reductive citric acid (Δδ13C from 3‰ to 13‰) or hydroxypropionate (Δδ13C from 2‰ to 13‰) cycles (van der Meer et al., 2000; Hayes, 2001). In the Dallol Hot Springs, three potential carbon substrates were considered for the autotrophs to grow. Together with atmospheric CO2 and dissolved inorganic carbon (DIC), dissolved CO2 is an important source of carbon in the hyperacidic springs generated from mixing of CO2-rich ascending magmatic fluids, boiling meteoric water, and seawater trapped in the evaporitic sequence (Kotopoulou et al., 2019). Assuming a mean δ13C value for atmospheric CO2 of −8‰ (Graven et al., 2017), a δ13C value ranging from −2.8‰ to −6.2‰ for dissolved CO2 as measured by others on CO2-emitting fumaroles from the Dallol region (Darrah et al., 2013), and a DIC δ13C value of −2.5‰ as that measured on DIC from similar hot springs (Obsidian Pool) in the Yellowstone National Park (Schuler et al., 2017), we considered that fractionation upon biological incorporation of carbon in the Dallol hydrothermal system ranged from 15‰ to 23‰ relative to the three potential carbon substrates. These fractionation values are in the range of those involved in the Calvin cycle (e.g., Cyanobacteria or α-, β-, and γ-Proteobacteria; Hayes et al., 1983; Bar-Even et al., 2011) and in the lower edge of those using the CoA pathway for CO2 incorporation (e.g., some Firmicutes, methanogenic Euryarchaeota, or chemolithotrophic Planctomycetes; Bar-Even et al., 2011; Havig et al., 2011). Interestingly, in microbial mats constructed by Chloroflexus in conjunction with Cyanobacteria, where the former grows photoheterotrophically by consuming cyanobacterial photosynthate, organic matter may show isotopic signatures more typical of the Calvin cycle (e.g., −23.5‰) than of the hydroxypropionate cycle characteristically used by Chloroflexi (van der Meer et al., 2001). Members of Chloroflexi, Proteobacteria, Firmicutes, Euryarchaeota, Bacteroidetes, Actinobacteria, or Acidobacteria have been previously described in commercial salt from the Dallol hydrothermal system (Gibtan et al., 2016, 2017).
As for the sulfur isotopic composition, the enriched δ34S signatures measured both on sulfate (6.5‰ to 11.7‰) and total sulfur (8.2‰ to 20.5‰) suggested the participation of sulfate-reducing bacteria in the microbial community of Dallol. Bacterial sulfate reduction produces isotopic fractionation during the microbial sulfur cycle, which results in enriched δ34S values in sulfate and total sulfur in contrast to the lighter δ34S values of sulfide (Canfield, 2001). In Dallol, the presence of sulfate as one of the few inorganic ions detected in the evaporitic samples (14 ± 0.83 mg/g) would ensure the substrate required by sulfate-reducing bacteria to grow.
4.2. Lipid biosignatures in the Dallol evaporites
The lipid analysis of the Dallol samples revealed a dominant microbial signature. The three major lipid families (i.e., n-alkanes, n-carboxylic acids, and n-alkanols) showed a characteristic even-over-odd distribution (Fig. 4). In the nonpolar fraction (Fig. 4a), the relative abundance of LMW n-alkanes (≤C20) with maximum at C18 (ACL ≤20) and the prevailing even character (CPI ≤1) (Table 1) reflected microbial signatures (Grimalt and Albaigés, 1987; Meyers and Ishiwatari, 1993; Volkman et al., 1998). This was supported by the relative abundance of monomethyl alkanes among the detected branched alkanes (Table 2), components that have been described in bacterial communities (including Cyanobacteria) from hydrothermal (Cady and Farmer, 1996; Campbell et al., 2015b) and hypersaline (Dembitsky et al., 2001) environments. Other microbial biomarkers found in the Dallol samples were the isoprenoids pristane and phytane (Fig. 4a). These compounds are mainly originated from phytol, the esterifying alcohol of phototrophic chlorophylls (Didyk et al., 1978), which degradation gives rise to pristane or phytane in the presence or absence of oxygen, respectively (Peters et al., 2005). In addition, phytane may have alternative sources such as archaeols (Brocks and Summons, 2003) or tocopherols (E vitamins; Goossens et al., 1984). In Dallol, the lack of autochthonous vegetation led us to consider different phototrophic sources of pristane and phytane such as cyanobacteria or bacteria containing bacteriochlorophylls a and b (Peters and Moldowan, 1993). Although the growth of cyanobacteria and other phototrophic bacteria is seriously hampered at temperatures higher than 73°C (Ward et al., 1989; Miller and Castenholz, 2000) and pH values below ∼4 in the case of cyanobacteria (Cirés et al., 2017), in hydrothermal systems such as Dallol these parameters typically change with abrupt gradients in short distance (McCollom and Shock, 1997) and temporal ranges, with active spring sites going inactive and new springs emerging in new locations in the range of days (Kotopoulou et al., 2019). This dynamicity and variability in the Dallol hydrothermal system make it possible to find niches of lower temperature and higher pH, where phototrophic microorganisms are able to thrive in a polyextreme environment otherwise unsuitable for photosynthetic microorganisms. To a lesser extent, pristane and phytane could also come from the degradation of phytol present in vegetal masses extending in neighboring areas, where wind may have played a role in transporting the allochthonous material. Woods and biomass extensions spreading about 7 km away from the hydrothermal zone could provide the vegetal material to be aerially introduced into the hydrothermal system. In either case, the relative abundance of pristane relative to phytane would reflect the predominance of oxic conditions in the degradation of phytol, which is consistent with the prevailing oxicity existing in evaporitic environments (Chong-Díaz et al., 1999) such as Dallol. On the other hand, the lower concentration of phytane relative to pristane could be due to a different origin than phytol for this compound (i.e., archaeols or tocopherols).
The microbial signature was also reflected in the distribution of carboxylic acids. First, the majoritarian straight-chain carboxylic acids showed a distinct even character (CPI = 3.7–28) and a predominance of short chains (ACL <19) with maximum peaks at C16 and C18 (Fig. 4b). These even and short distributions are typically associated with microbial sources (Cranwell, 1974), as the majority of bacteria have a simple carboxylic acid composition with significant proportion of myristic (C14), palmitic (C16), and stearic (C18) acids (Kaneda, 1991). Second, unsaturations were ubiquitously detected on the C16 (C16:1 ω7) and C18 (C18:1 ω9, C18:1 ω10, and C18:2 ω6,9) carboxylic acids (Fig. 4b and Table 3). The detection of these and other polyunsaturated acids in Octopus Spring (temperature of 87°C and pH of 8.3) in Yellowstone (USA) was related to the presence of thermophilic bacteria from the orders Aquificales and Thermotogales (Jahnke et al., 2001). In particular, the C18:1 (ω9) acid was recognized in thermophilic microorganisms within the Chloroflexi phylum (i.e., Thermomicrobium) (Jahnke et al., 2001; Kaur et al., 2015). Third, the Dallol acidic fraction contained low concentrations of dicarboxylic acids ranging from C8 to C10 (Fig. 5b). Comparable distributions of short dicarboxylic acids (C6–C10) in the Octopus Spring were described as core lipids of Thermotogales members (Carballeira et al., 1997; Jahnke et al., 2001). Finally, the ubiquitous detection of short chains (C14–C18) of iso/anteiso carboxylic acids in the Dallol samples (Fig. 4b) reinforced the microbial hypothesis. The branched carboxylic acids, in particular the iso/anteiso C15 and C17 pairs, are typically associated with bacterial sources (Kaneda, 1991), being particularly abundant in sulfate-reducing bacteria (Langworthy et al., 1983). They were found in thermophilic bacteria from the Thermus and Meiothermus genera in cultured strains (Nobre et al., 1996; Yang et al., 2006) and in thermophilic bacteria from the Octopus Springs in Yellowstone (Jahnke et al., 2001). The iso/anteiso C15 and C17 pairs have also been described in other hot springs, for example, in New Zealand (Kaur et al., 2015), as well as in microbial mats from the Shark Bay in Australia (Pagés et al., 2015), or in ooids (small and spheroidal, concentric layered sedimentary grains of calcium carbonate) from Bahamas and Australia (Summons et al., 2013). Other branched carboxylic acids such as the iso-C18 were described in certain Chloroflexi members in hydrothermal systems in New Zealand and in Octopus Spring (Jahnke et al., 2001; Kaur et al., 2015), as well as in the Desulfobacter spp. (Dowling et al., 1988; Summons et al., 2013). The detection of wax esters of 32 and 34 carbons in three of the five Dallol samples (i.e., D7, D8, and D10), although at small concentrations (Table 1), also supported the presence of anoxygenic phototroph Chloroflexus, as it was described in hydrothermal systems of New Zealand (Campbell et al., 2015b). Thermophiles such as Chloroflexus and Roseiflexus typically dwell in microbial mats together with Cyanobacteria, growing photo-heterotrophically by consuming the cyanobacterial photosynthate (van der Meer et al., 2000). In Dallol, the combined detection of monomethyl alkanes, C18:1 and C18:2 carboxylic acids, pristine and phytane, and wax esters suggests the presence of Cyanobacteria in the Dallol evaporites, likely forming Cyanobacteria-Chloroflexus mats based on the reported presence of Chloroflexus in commercial salts from the area (Gibtan et al., 2017).
FIG. 5.
Qualitative composition of the acidic fraction isolated from the Dallol evaporite samples (a), with a zoom view on the area within the black inlet (b). In the legend, “normal” stands for straight-chain acids, “unsaturated” for mono- and diunsaturated acids, “dioic” for dicarboxylic acids, “iso/anteiso” for branched acids with methyl groups in iso and anteiso positions, and “other branched” for other methylated non iso/anteiso carboxylic acids.
In addition to the microbial character dominating the Dallol signatures, some contribution of vegetal sources was inferred from the presence of secondary groups of HMW n-carboxylic acids (Fig. 4b) and n-alkanols (Fig. 4c) of even character and maximum peaks at C22–C28. These types of distributions are typically observed in organic matter from macrophytes or higher plants (Eglinton and Hamilton, 1967; Feng and Simpson, 2007). Their identification in Dallol, together with certain sterols (i.e., stigmastanol or β-sitosterol), was consistent with the presence of vegetal vestiges. Sterols are ubiquitous constituents in all eukaryotic organisms, where stigmastanol or β-sitosterol are almost exclusively produced by higher plants (Patterson and Nes, 1991; Goad and Akihisa, 1997), whereas cholesterol is majorly produced from animals (Volkman, 1986). The vegetal and animal signatures in Dallol were explained by external inputs from nearby areas of woods or human settlements. In the nearby Gaet'ale spring, 3.8 km southeast of Dallol, Master (2016) described the presence of vegetal and human debris, washed in by rains from the woods and sparse villages located in the highlands. These runoff episodes are common in this area given the low altitude of the floor in the Danakil Depression. In addition, the presence of animals (at least birds and insects) in the region (Master, 2016) may also explain the observation of animal biosignatures. Altogether, the molecular distribution of alkanes, carboxylic acids, alkanols, sterols, and wax esters revealed the presence of biological vestiges in the Dallol evaporite samples. In particular, microbial sources appeared to be dominant according to the relative abundance of the short over the long n-alkanes, n-carboxylic acids, or n-alkanols (ACL ≤21; Table 1). Despite the inhospitability of the Dallol Hot Springs, at least some extremophilic microorganisms appear to be resistant enough to endure the extremely low pH and high temperatures and live (past or present) in the polyextreme environment.
4.3. Molecular indications of active or recent metabolisms
The molecular distribution of the diverse lipid families manifested a generalized presence of microbial vestiges in the Dallol evaporites. While a definitive distinction between presently active metabolisms or fossilized biological fingerprints cannot be accomplished, we argue here a couple of observations that led us to consider that active or recent metabolisms dominate in Dallol. The relative abundance of functionalized (i.e., n-carboxylic acids and n-alkanols) versus saturated (i.e., n-alkanes) hydrocarbons (Fig. 3) was indicative of extant communities or recent biogenesis (Simoneit et al., 1998). n-Carboxylic acids and n-alkanols are labile lipids that tend to be rapidly destroyed during diagenesis (Brocks and Summons, 2004) by cleavage of alkyl chains that produces n-alkanes without odd-over-even predominance (Killops and Killops, 2005). Without active metabolisms, the presence of functionalized groups tends to be minority relative to the saturated moieties. In the Dallol samples, n-carboxylic acids and n-alkanols were not only considerably more abundant than n-alkanes (i.e., two to four times), but the latest showed beside an atypical even distribution pattern with maximum at C18 that coincided with the microbial-diagnostic C18 maximum peaks in the carboxylic (Fig. 5b) and hydroxyl (Fig. 5c) fractions. Given the short residence time of the labile n-carboxylic acids and n-alkanols in most environments, their detection in Dallol at such proportions suggested that they derive from either extant biomass or exceptionally well-preserved fossil lipids.
On the other hand, the relative enrichment of even compounds in the three majority families, even the n-alkanes, supports the hypothesis of active metabolisms or very good preservation. n-Alkanes derived from biological sources typically show preference for odd (plants; Eglinton and Hamilton, 1967) or even (microorganisms; Meyers and Ishiwatari, 1993) carbons, whereas the absence of even/odd patterns may reveal an advanced diagenesis (Killops and Killops, 2005) or abiotic origin (McKay, 2004) of those lipids. In Dallol, CPI values lower than or equal to the unit in the n-alkanes (Table 1) denote organic matter decay by either active or past microbial metabolisms.
In sum, the lipid analysis enabled us to identify molecular evidence of life (mostly microbial) in the polyextreme environment of Dallol Hot Springs. The abundance of functionalized relative to saturated hydrocarbons pointed to present or recently active metabolisms producing typical microbial signatures. The preservation of functionalized-fossil lipids by encasing inside salt precipitation is another plausible way (Conner and Benison, 2013) to explain the “fresh” biosignatures detected in the halite-rich hydrothermal system. This is the first study reporting the detection of (present or recent) life in the Dallol evaporitic system. These findings are relevant for constraining the limits of life and have implications for the search for extraterrestrial life. The existence of viable life in the polyextreme environment of Dallol expands our understanding of the limits of life and supports the habitability on analogous environments in other planetary bodies (e.g., martian geological sites; Klingelhofer et al., 2004; Bishop et al., 2015). Whereas further investigation is needed to more comprehensively identify the microbial communities associated to the hydrothermal and evaporitic substrates, the present study constitutes the first biogeochemical approach to describe the Dallol polyextreme environment. In the future, additional analyses such as that of phospholipid fatty acids will be conducted to assess the presence of presently operative metabolisms.
5. Conclusion
This study is the first to show lipid molecular evidence of life in hydrothermal deposits of the Dallol Hot Springs, a geothermal extreme environment in eastern Ethiopia combining hypersalinity, acidity, and high water temperatures. A lipid biomarker approach was used to elucidate preserved biosignatures in a polyextreme environment with interest for understanding the limits of life. The abundance of low- over high-molecular-weight chains (n-alkanes, n-carboxylic acids, and n-alkanols) together with the detection of a number of bacterial-diagnostic compounds documented the predominance of microbial lipids in the Dallol samples. We hypothesize that the microbial signatures most likely correspond to present or recently active metabolisms, according to the large proportion of functionalized hydrocarbons and the distinct even-over-odd pattern of all lipid families, including the n-alkanes. The observed molecular distributions suggest that, despite the inhospitability of the Dallol Hot Springs, there are at least some microorganisms capable of living in such extreme pH, temperature, and salinity conditions. These findings contribute to elucidate where the limits of life may be and where it may be interesting to search for life in analogous extraterrestrial environments. Whereas further investigation is needed to identify comprehensively the microbial communities associated to the hydrothermal and evaporitic substrates, the present study constitutes the first biogeochemical approach to identify (present or recent) life vestiges in the Dallol polyextreme environment. Future work is planned to combine proteomics and compound specific-isotopic analysis for achieving a metabolic and phylogenetic characterization of the microbial community inhabiting Dallol.
Supplementary Material
Acknowledgments
D. Carrizo and L. Sánchez acknowledge the Spanish Ministry of Economy and Competitiveness (MINECO/FEDER) for funding their respective projects RYC-2014-19446 and CGL2015-74254-JIN. D. Carrizo is co-I of NASA Astrobiology Institute (NAI) CAN7 team Changing Planetary Environment and the Fingerprints of Life. The present study was carried out in the context of the Europlanet Project (H2020 RI), which received funding from the European Union's Horizon 2020 research and innovation program under grant agreement No 654208.
Abbreviations Used
- ACL
average chain length
- CPI
carbon preference index
- DIC
dissolved inorganic carbon
- FAMEs
fatty acid methyl esters
- GC-MS
gas chromatography–mass spectrometry
- HMW
high-molecular-weight
- IC
ion chromatography
- IRMS
isotope ratio mass spectrometry
- LMW
low-molecular-weight
- m/z
mass-to-charge ratios
- PDB
Pee Dee Belemnite limestone standard
- TLE
total lipid extract
- TOC
total organic carbon
- VCDT
Vienna-Canyon Diablo Troilite
- XRD
X-ray diffractometer
Author Disclosure Statement
No competing financial interests exist.
Associate Editor: Jack Mustard
Supplementary Information: The graphical abstract and Supplementary Fig. S1 may be found in the online version of this article at www.liebertonline.com/ast
References
- Bar-Even A., Noor E., and Milo R. (2011) A survey of carbon fixation pathways through a quantitative lens. J Exp Bot 63:2325–2342 [DOI] [PubMed] [Google Scholar]
- Behle A., Makris J., Baier B., and Delibasis N. (1975) Salt thickness near Dallol (Ethiopia) from seismic reflection measurement and gravity data. In Afar Depression of Ethiopia, Proceedings of an International Symposium on the Afar Region and Related Rift Problems, held in Bad Bergzabern, FRG, 1974, edited by A. Pilzger. and Rösler A., Schweizerbart'sche Verlagbuchhandlung E., Stuttgart, Germany, pp 379–390 [Google Scholar]
- Beyene A. and Abdelsalam M.G. (2005) Tectonics of the Afar Depression—a review and synthesis. J Afr Earth Sci 41:41–59 [Google Scholar]
- Bishop J.L., Murad E., and Dyar M.D. (2015) Akaganeite and schwertmannite: spectral properties and geochemical implications of their possible presence on Mars. Am Mineral 100:738–746 [Google Scholar]
- Brocks J.J. and Summons R.E. (2003) Sedimentary hydrocarbons: biomarkers for early life. In Biogeochemistry, edited by Holland H.D. and Turekian K.K., Elsevier, Amsterdam, pp 63–115 [Google Scholar]
- Brocks J.J. and Summons R.E. (2004) Sedimentary hydrocarbons, biomarkers for early life. In Biogeochemistry, Treatise on Geochemistry Vol. 8, edited by W.H. Schlesinger, Elsevier Pergamon, Oxford, pp 63–115 [Google Scholar]
- Burgess E.A., Unrine J.M., Mills G.L., Romanek C.S., and Wiegel J. (2012) Comparative geochemical and microbiological characterization of two thermal pools in the Uzon Caldera, Kamchatka, Russia. Microb Ecol 63:471–489 [DOI] [PubMed] [Google Scholar]
- Cady S.L. and Farmer J.D. (1996) Fossilization processes in siliceous thermal springs: trends in preservation along thermal gradients. Ciba Found Symp 202:150–170 [DOI] [PubMed] [Google Scholar]
- Cady S.L., Farmer J.D., Grotzinger J.P., Schopf J.W., and Steele A. (2003) Morphological biosignatures and the search for life on Mars. Astrobiology 3:351–368 [DOI] [PubMed] [Google Scholar]
- Campbell K.A., Guido D.M., Gautret P., Foucher F., Ramboz C., and Westall F. (2015a) Geyserite in hot-spring siliceous sinter: window on Earth's hottest terrestrial (paleo) environment and its extreme life. Earth-Science Rev 148:44–64 [Google Scholar]
- Campbell K.A., Lynne B.Y., Handley K.M., Jordan S., Farmer F.D., Guido D.M., Fourcher F., Turner S., and Perry R.S. (2015b) Tracing biosignature preservation of geothermally silicified microbial textures into the geological record. Astrobiology 15:858–882 [DOI] [PubMed] [Google Scholar]
- Canfield D.E. (2001) Isotope fractionation by natural populations of sulfate-reducing bacteria. Geochim Cosmochim Acta 65:1117–1124 [Google Scholar]
- Carballeira N.M., Reyes M., Sostre A., Huang H., Verhagen M.F.J.M., and Adams M.W.W. (1997) Unusual fatty acid compositions of the hyperthermophilic archaeon Pyrococcus furiosus and the bacterium Thermotoga maritima. J Bacteriol 179:2766–2768 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carniel R., Munoz J.E., and Jones J. (2010) A geophysical multi-parametric analysis of hydrothermal activity at Dallol, Ethiopia. J Afr Earth Sci 58:812–819 [Google Scholar]
- Cheng Z., Xiao L., Wang H., Yang H., Li J., Huang T., Xu Y., and Ma N. (2017) Bacterial and archaeal lipids recovered from subsurface evaporites of Dalangtan Playa on the Tibetan Plateau and their astrobiological implications. Astrobiology 17:1112–1122 [DOI] [PubMed] [Google Scholar]
- Chong-Díaz G.M., Mendoza M., García-Veigas J., Pueyo J.J., and Turner P. (1999) Evolution and geochemical signatures in a Neogene forearc evaporitic basin: the Salar Grande (Central Andes of Chile). Palaeogeogr Palaeoclimatol Palaeoecol 151:39–54 [Google Scholar]
- Cirés S., Casero M.C., and Quesada A. (2017) Toxicity at the edge of life: a review of cyanobacterial toxins from extreme environments. Mar Drugs 15, doi: 10.3390/md15070233 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conner A.J. and Benison K.C. (2013) Acidophilic halophilic microorganisms in fluid inclusions in halite from Lake Magic, Western Australia. Astrobiology 13:850–860 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cranwell P.A. (1974) Monocarboxylic acids in lake sediments: indicators derived from terrestrial and aquatic biota, of paleo-environmental trophic levels. Chem Geol 14:1–14 [Google Scholar]
- Damer B. (2016) A field trip to the Archaean in search of Darwin's warm little pond. Life 6:21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Darrah T.H., Tedesco D., Tassi F., Vaselli O., Cuoco E., and Poreda R.J. (2013) Gas chemistry of the Dallol region of the Danakil Depression in the Afar region of the northern most East African Rift. Chem Geol 339:16–29 [Google Scholar]
- Deamer D.W. and Georgiou C.D. (2015) Hydrothermal conditions and the origin of cellular life. Astrobiology 15:1091–1095 [DOI] [PubMed] [Google Scholar]
- de los Ríos A., Valea S., Ascaso C., Davila A., Kastovsky J., McKay C.P., Gómez-Silva B., and Wierzchos J. (2010) Comparative analysis of the microbial communities inhabiting halite evaporites of Atacama Desert. Int Microbiol 13:79–89 [DOI] [PubMed] [Google Scholar]
- Dembitsky V.M., Dor I., Shkrob I., and Aki M. (2001) Branched alkanes and other apolar compounds produced by the cyanobacterium Microcoleus vaginatus from the Negev Desert. Russ J Bioorgan Chem 27:110–119 [PubMed] [Google Scholar]
- Didyk B.M., Simmoneit B.R.T., Brassell S.C., and Eginton G. (1978) Organic geochemical indicators of palaeoenvironmental conditions of sedimentation. Nature 272:216–222 [Google Scholar]
- Djokic T., Van Kranendonk M.J., Campbell K.A., Walter M.R., and Ward C.R. (2017) Earliest signs of life on land preserved in ca. 3.5 Ga hot spring deposits. Nat Commun 8, doi: 10.1038/ncomms15263 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dowling N.J.E., Nichols P.D., and White D.C. (1988) Phospholipid fatty acid and infra-red spectroscopic analysis of a sulphate-reducing consortium. FEMS Microbiol Lett 53:325–333 [Google Scholar]
- Ebinger C.J., Keir D., Ayele A., Calais E., Wright T.J., Belachew B., Hammond J.O.S., Campbell E., and Buck W.R. (2008) Capturing magma intrusion and faulting processes during continental rupture: seismicity of the Dabbahu (Afar) rift. Geophys J Int 174:1138–1152 [Google Scholar]
- Edelman J. and Roscoe R. (2010) Volcano tourism in Ethiopia and the Danakil rift zone. In Volcano and Geothermal Geotourism, Sustainable Geo-Resources for Leisure and Recreation, edited by Erfurt-Cooper P. and Cooper M., Earthscan and Taylor & Francis; Abingdon, UK, and New York, USA [Google Scholar]
- Eglinton G. and Hamilton R.J. (1967) Leaf epicuticular waxes. Science 156:1322–1335 [DOI] [PubMed] [Google Scholar]
- Erfurt-Cooper P. and Cooper M., editors. (2010) Volcano and Geothermal Geotourism, Sustainable Geo-Resources for Leisure and Recreation, Earthscan and Taylor & Francis; Abingdon, UK, and New York, USA [Google Scholar]
- Farmer J.D. and Des Marais D.J. (1999) Exploring for a record of ancient martian life. J Geophys Res Planets 104:26977–26995 [DOI] [PubMed] [Google Scholar]
- Fazzini M. and Bisci C. (2015) The climate of Ethiopia. In Landscapes and Landforms of Ethiopia, edited by Billi P., World Geomorphological Landscapes, Springer [Google Scholar]
- Feng X. and Simpson M.J. (2007) The distribution and degradation of biomarkers in Alberta grassland soil profiles. Org Geochem 38:1558–1570 [Google Scholar]
- Fernández-Remolar D. and Knoll A.H. (2008) Fossilization potential of iron-bearing minerals in acidic environments of Río Tinto, Spain: implications for Mars exploration. Icarus 194:72–85 [Google Scholar]
- Fernández-Remolar D., Morris R.V., Gruener J.E., Amils R., and Knoll A.H. (2005) The Río Tinto basin, Spain: mineralogy, sedimentary geobiology, and implications for interpretation of outcrop rocks at Meridiani Planum, Mars. Earth Planet Sci Lett 240:149–167 [Google Scholar]
- Fernández-Remolar D., Chong-Díaz G., Ruíz-Bermejo M., Harir M., Schitt-Kopplin P., Tziotis D., Gómez-Ortiz D., García-Villadangos M., Martín-Redondo M.P., Gómez F., Rodríguez-Manfredi J.A., Moreno-Paz M., De Diego-Castilla D., Echeverría A., Urtuvia V.N., Blanco Y., Rivas L., Izawa M.R.M., Banerjee N.R., Demergasso C., and Parro V. (2013) Molecular preservation in halite- and perchlorate-rich hypersaline subsurface deposits in the Salar Grande basin (Atacama Desert, Chile): implications for the search for molecular biomarkers on Mars. J Geophys Res Biogeosciences 118:1–18 [Google Scholar]
- Franzson H., Helgadóttir H.M., and Óskarsson F. (2015) Surface exploration and first conceptual model of the Dallol geothermal area, northern Afar, Ethiopia [#11043]. In Proceedings World Geothermal Congress 2015, Melbourne, Australia, pp 19–25 [Google Scholar]
- Garland C.R. (1980) Geology of the Adigrat Area. Ministry of Mines, Addis Ababa Memoir 1:1–51 [Google Scholar]
- Gebresilassie S., Tsegab H., Kabeto K., Gebreyohannes T., Sewale A., Amare K., Mabrahtu A., Zarabruk S., Mebrahtu G., Bebrehiwot K., and Haile M. (2011) Preliminary study on geology, mineral potential, and characteristics of hot springs from Dallol area, Afar rift, northeastern Ethiopia: implications for natural resource exploration. Momona Ethiopian Journal of Science 3:17–30 [Google Scholar]
- Gibtan A., Woo M., Oh D., Park K., Lee H.-S., Sohn J.H., Lee D.-W., Sohn J.-K., and Lee S-J. (2016) Draft genome sequence of the extremely halophilic Halorubrum sp. SAH-A6 isolated from rock salts of the Danakil depression, Ethiopia. Genom Data 10:30–32 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibtan A., Park K., Woo M., Shin J.-K., Lee D.-W., Sohn J.H., Song M., Roh S.W., Lee S.-J., and Lee H.-S. (2017) Diversity of extremely halophilic archaeal and bacterial communities from commercial salts. Front Microbiol 8:799–810 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goad L.J. and Akihisa T. (1997) Analysis of Sterols, Blackie Academic & Professional, London [Google Scholar]
- Gonfiantini R., Borsi S., Ferrara G., and Panichi C. (1973) Isotopic composition of waters from the Danakil depression (Ethiopia). Earth Planet Sci Lett 18:13–21 [Google Scholar]
- Goossens H., de Leeuw J.W., Schenck P.A., and Brassell S.C. (1984) Tocopherols as likely precursors of pristane in ancient sediments and crude oils. Nature 312:440–442 [Google Scholar]
- Graven H., Allison C.E., Etheridge D.M., Hammer S., Keeling R.F., Levin I., Meljer H.A.J., Rubino M., Tans P.P., Trudinger C.M., Vaughn B.H., and White J.W.C. (2017) Compiled records of carbon isotopes in atmospheric CO2 for historical simulations in CMIP6. Geoscientific Model Development 10:4405–4417 [Google Scholar]
- Grimalt J.O. and Albaigés J. (1987) Sources and occurrence of C12–C22 n-alkane distributions with even carbon-number preference in sedimentary environments. Geochim Cosmochim Acta 51:1379–1384 [Google Scholar]
- Harrison J.P., Gheeraert N., Tsigelnitskiy D., and Cockell C.S. (2013) The limits for life under multiple extremes. Trends Microbiol 21:204–212 [DOI] [PubMed] [Google Scholar]
- Havig J.F., Raymond J., Meyer-Dombard D.R., Zolotova N., and Shock E.L. (2011) Merging isotopes and community genomics in a siliceous sinter-depositing hot spring. J Geophys Res 116, doi: 10.1029/2010JG001415 [DOI] [Google Scholar]
- Hayes J.M. (2001) Fractionation of the Isotopes of Carbon and Hydrogen in Biosynthetic Processes, National Meeting of the Geological Society of America, Boston, MA
- Hayes J.M., Kaplan I.R., and Wedeking K.W. (1983) Precambrian organic geochemistry, preservation of the record. In The Earth's Earliest Biosphere: Its Origin and Evolution, edited by Schopf J.W., Princeton University Press, Princeton, NJ, pp 93–134 [Google Scholar]
- Holwerda U.G. and Hutchinson R.W. (1968) Potash-bearing evaporites in the Danakil Region, Ethiopia. Econ Geol 63:124–150 [Google Scholar]
- Hovland M., Rueslaåttenb H.G., Johnsenc H.K., Kvammed B., and Kuznetsova T. (2006) Salt formation associated with sub-surface boiling and supercritical water. Mar Pet Geol 23:855–869 [Google Scholar]
- Jahnke L.L., Eder W., Huber R., Hope J.M., Hinrichs K.U., Hayes J.M., Des Marais D.J., Cady S.L., and Summons R.E. (2001) Signature lipids and stable carbon isotope analyses of Octopus Spring hyperthermophilic communities compared with those of Aquificales representatives. Appl Environ Microbiol 67:5179–5189 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaneda T. (1991) Iso- and anteiso-fatty acids in bacteria: biosynthesis, function, and taxonomic significance. Microbiol Rev 55:288–302 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaur G., Mountain B.W., Stott M.B., Hopmans E.C., and Pancost R.D. (2015) Temperature and pH control on lipid composition of silica sinters from diverse hot springs in the Taupo Volcanic Zone, New Zealand. Extremophiles 19:327–344 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kester C.L., Rye R.O., Johnson C.A., Schwartz C., and Holmes C. (2011) On-line sulfur isotope analysis of organic material by direct combustion—preliminary results and potential applications. Isotopes Environ Health Stud 37:53–65 [DOI] [PubMed] [Google Scholar]
- Killops S. and Killops V. (2005) Introduction to Organic Geochemistry, Blackwell Publishing, Oxford, UK
- Klingelhofer G., Morris R.V., Bernhardt B., Schroder C., Rodionov D.S., de Souza P.A., Jr, Yen A., Gellert R., Evlanov E.N., Zubkov B., Foh J., Bonnes U., Kankeleit E., Gutlich P., Ming D.W., Renz F., Wdowiak T., Squyres S.W., and Arvidson R.E. (2004) Jarosite and hematite at Meridiani Planum from Opportunity's Mössbauer spectrometer. Science 306:1740–1745 [DOI] [PubMed] [Google Scholar]
- Knoll A. and Walter M.R. (1996) The limits of Palaeontological knowledge: finding the gold among the dross. Ciba Found Symp 202:198–209 [DOI] [PubMed] [Google Scholar]
- Kotopoulou E., Delgado Huertas A., Garcia-Ruiz J.M., Dominguez-Vera J.M., Lopez-Garcia J.M., Guerra-Tschuschke I., and Rull F. (2019) A polyextreme hydrothermal system controlled by iron: the case of Dallol at the Afar Triangle. ACS Earth Space Chem 3:90–99 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langworthy T.A., Holzer G., Zeikus J.G., and Tornabene T.G. (1983) Iso- and anteiso-branched glycerol diethers of the thermophilic anaerobe Thermodesulfotobacterium commune. Syst Appl Microbiol 4:1–17 [DOI] [PubMed] [Google Scholar]
- Master S. (2013) The unique hydrothermal spring deposits at Dallol (Danakil Depression, Afar, Ethiopia), products of the interaction between mafic magma and marine evaporites. Johannesburg, South Africa: Turbine Hall [abstract]. Geocongress, Biennial Congress of the Geological Society of South Africa, Geological Society of South Africa, Johannesburg, South Africa [Google Scholar]
- Master S. (2016) Gaet'ale—a reactivated thermal spring and potential tourist hazard in the Asale salt flats, Danakil Depression, Ethiopia. Journal of Applied Volcanology 5, doi: 10.1186/s13617-015-0042-x [DOI] [Google Scholar]
- McCollom T.M. and Shock E.L. (1997) Geochemical constraints on chemolithoautotrophic metabolism by microorganisms in seafloor hydrothermal systems. Geochim Cosmochim Acta 61:4375–4391 [DOI] [PubMed] [Google Scholar]
- McKay C.P. (2004) What is life and how do we search for it in others worlds? PLoS Biol 2, doi: 10.1371/journal.pbio.0020302 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyers P.A. and Ishiwatari R. (1993) Lacustrine organic geochemistry: an overview of indicators of organic matter sources and diagenesis in lake sediments. Org Geochem 20:867–900 [Google Scholar]
- Miller S.R. and Castenholz R.W. (2000) Evolution of thermotolerance in hot spring cyanobacteria of the genus Synechococcus. Appl Environ Microbiol 66: 4222–4229 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morgan C.A., Herman N., White P.A., and Vewey G. (2006) Preservation of micro-organisms by drying: a review. J Microbiol Methods 66:183–193 [DOI] [PubMed] [Google Scholar]
- Ngugi D.K., Blom J., Stepanauskas R., and Stingl U. (2016) Diversification and niche adaptations of Nitrospina-like bacteria in the polyextreme interfaces of Red Sea brines. ISME J 10:1383–1399 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nickel E.H. (2002) An unusual occurrence of Pd, Pt, Au, Ag and Hg minerals in the Pilbara region of Western Australia. Can Mineral 40:419–433 [Google Scholar]
- Nobile A., Pagli C., Keir D., Wright T.J., Ayele A., Ruch J., and Acocella V. (2012) Dike-fault interaction during the 2004 Dallol intrusion at the northern edge of the Erta Ale Ridge (Afar, Ethiopia). Geophys Res Lett 39:L19305 [Google Scholar]
- Nobre M.F., Carreto L., Wait R., Tenreiro S., Fernandes O., Sharp R.J., and De Costa M.S. (1996) Fatty composition of the species of the genera Thermus acid Meiothermus. Syst Appl Microbiol 19:303–311 [Google Scholar]
- Pagés A., Grice K., Welsh D.T., Teasdale P.T., Van Kranendonk M.J., and Greenwood P. (2015) Lipid biomarker and isotopic study of community distribution and biomarker preservation in a laminated microbial mat from Shark Bay, Western Australia. Microb Ecol 70:459–472 [DOI] [PubMed] [Google Scholar]
- Pancost R.D., Pressley S., Coleman J.M., Benning L.G., and Mountain B.W. (2005) Lipid biomolecules in silica sinters: indicators of microbial diversity. Environ Microbiol 7:66–77 [DOI] [PubMed] [Google Scholar]
- Patterson G.W. and Nes W.D. (1991) Physiology and Biochemistry of Sterols, American Oil Chemists' Society, Champaign, IL
- Pérez V., Dorador C., Molina V., Yáñez C., and Hengst M. (2018) Rhodobacter sp. Rb3, an aerobic anoxygenic phototroph which thrives in the polyextreme ecosystem of the Salar de Huasco, in the Chilean Altiplano. Antonie van Leeuwenhoek Journal of Microbiology 111:1449–1465 [DOI] [PubMed] [Google Scholar]
- Peters K.E. and Moldowan J.M. (1993) The Biomarker Guide, Prentice Hall, Engelwood Cliffs, NJ
- Peters K.E., Walters C.C., and Moldowan J.M. (2005) The Biomarker Guide—Part II—Biomarkers and Isotopes in Petroleum Exploration and Earth History, Cambridge University Press, New York [Google Scholar]
- Révész K., Qi H., and Coplen T.B. (2012a) Determination of the δ15N and δ13C of total nitrogen and carbon in solids; RSIL lab code 1832. In Chapter 5 of Stable Isotope-Ratio Methods, Section C of Methods of the Reston Stable Isotope Laboratory (slightly revised from version 1.1 released in 2007), U.S. Geological Survey Techniques and Methods, book 10, edited by Révész K. and Coplen T.B., U.S. Geological Survey, Reston, VA: Available online at https://pubs.usgs.gov/tm/2006/tm10c5 [Google Scholar]
- Révész K., Qi H., and Coplen T.B. (2012b). Determination of the δ34S of total sulfur in solids; RSIL lab code 1800. In Chapter 4 of Stable Isotope-Ratio Methods, Section C of Methods of the Reston Stable Isotope Laboratory (slightly revised from version 1.1 released in 2007), U.S. Geological Survey Techniques and Methods, book 10, edited by Révész K. and Coplen T.B., U.S. Geological Survey, Reston, VA: Available online at https://pubs.usgs.gov/tm/2006/tm10c4 [Google Scholar]
- Rivkina E., Shcherbakova V., Laurinavichius K., Petrovskaya L., Krivushin K., Kraev G., Pecheritsina S., and Gilichinsky D. (2007) Biogeochemistry of methane and methanogenic archaea in permafrost. FEMS Microbiol Ecol 61:1–15 [DOI] [PubMed] [Google Scholar]
- Rothschild L.J. and Mancinelli R.L. (2001) Life in extreme environments. Nature 409:1092–1101 [DOI] [PubMed] [Google Scholar]
- Ruff S.W. and Farmer J.D. (2016) Silica deposits on Mars with features resembling hot spring biosignatures at El Tatio in Chile. Nat Commun 7, doi: 10.1038/ncomms13554 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sánchez-García L., Aeppli C., Parro V., Fernandez-Remolar D., García-Villadangos M., Chong-Díaz G., Blanco Y., and Carrizo D. (2018) Molecular biomarkers in the subsurface of the Salar Grande (Atacama, Chile) evaporitic deposits. Biogeochemistry 140:31–52 [Google Scholar]
- Sánchez-García L., Fernandez-Martinez M.A., García-Villadangos M., Blanco Y., Cady S.L., Hinman N., Bowden M.E., Pointing S.B., Lee K.C., Warren-Rhodes K., Lacap-Bugler D., Cabrol N.A., Parro V., and Carrizo D. (2019) Microbial biomarker transition in El Tatio (Chile) sinter mounds through different stages of hydrothermal activity. Front Microbiol 9, doi: 10.3389/fmicb.2018.03350 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schinteie R. and Brocks J.J. (2016) Paleoecology of Neoproterozoic hypersaline environments: biomarker evidence for haloarchaea, methanogens, and cyanobacteria. Geobiology 15:641–663 [DOI] [PubMed] [Google Scholar]
- Schuler C.G., Havig J.R., and Hamilton T.L. (2017) Hot spring microbial community composition, morphology, and carbon fixation: implications for interpreting the ancient rock record. Front Earth Sci 5, doi: 10.3389/feart.2017.00097 [DOI] [Google Scholar]
- Simoneit B.R., Summons R.E., and Jahnke L.L. (1998) Biomarkers as tracers for life on early Earth and Mars. Orig Life Evol Biosph 28:475–483 [DOI] [PubMed] [Google Scholar]
- Steven B., Pollard W.H., Greer C.W., and Whyte L.G. (2008) Microbial diversity and activity through a permafrost/ground ice core profile from the Canadian High Arctic. Environ Microbiol 10:3388–3403 [DOI] [PubMed] [Google Scholar]
- Summons R.E., Bird L.R., Gillespie A.L., Pruss S.B., Roberts M., and Sessions A.L. (2013) Lipid biomarkers in ooids from different locations and ages: evidence for a common bacterial flora. Geobiology 11:420–436 [DOI] [PubMed] [Google Scholar]
- Tadesse S., Milesi J.P., and Deschamps Y. (2003) Geology and mineral potential of Ethiopia: a note on geology and mineral map of Ethiopia. J Afr Earth Sci 36:273–313 [Google Scholar]
- van der Meer M.T.J., Schouten S., de Leeuw J.W., and Ward D.M. (2000) Autotrophy of green non-sulphur bacteria in hot spring microbial mats: biological explanations for isotopically heavy organic carbon in the geological record. Environ Microbiol 2:428–435 [DOI] [PubMed] [Google Scholar]
- van der Meer M.T.J., Schouten S., van Dongen B.E., Rijpstra W.I.C., Fuchs G., Sinninghe Damsté J.S., de Leeuw J.W., and Ward D.M. (2001) Biosynthetic controls on the 13C contents of organic components in the photoautotrophic bacterium Chloroflexus aurantiacus. J Biol Chem 276:10971–10976 [PubMed] [Google Scholar]
- van Dongen B.E., Semiletov I.P., Weijers J.W.H., and Gustafsson Ö. (2008) Contrasting lipid biomarker composition of terrestrial organic matter exported from across the Eurasian Arctic by the five Great Russian Arctic Rivers. Global Biogeochemical Cycles vol 22, GB1011. 10.1029/2007GB002974 [DOI]
- Van Kranendonk M.J., Deamer D.W., and Djokic T. (2017) Life springs. Sci Am 317:28–35 [DOI] [PubMed] [Google Scholar]
- Volkman J.K. (1986) A review of sterol markers for marine and terrigenous organic matter. Org Geochem 9:83–99 [Google Scholar]
- Volkman J.K., Barrett S.M., Blackburn S.I., Manssur M.P., Sises E.L., and Gelin F. (1998) Microalgal biomarkers: a review of recent research developments. Org Geochem 29:1163–1179 [Google Scholar]
- Ward D.M., Weller R., Shiea J., Castenholtz R.W., and Cohen Y. (1989) Hot spring microbial mats: anoxygenic and oxygenic mats of possible evolutionary significance. In Microbial Mats, Physiological Ecology of Benthic Microbial Communities, edited by Cohen Y. and Rosenberg E., American Society for Microbiology, Washington, DC [Google Scholar]
- Wierzchos J., Casero M.C., Artieda O., and Ascaso C. (2018) Endolithic microbial habitats as refuges for life in polyextreme environment of the Atacama Desert. Curr Opin Microbiol 43:124–131 [DOI] [PubMed] [Google Scholar]
- Wilhelm M.B., Davila A.F., Eigenbrode J.L., Parenteau M.N., Jahnke L.L., Liu X.-L., Summons R.E., Wray J.J., O'Reilly S.S., and Williams A. (2017) Xeropreservation of functionalized lipid biomarkers in hyperarid soils in the Atacama Desert. Org Geochem 103:97–104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Y.-L., Yang F.-L., Jao S.-C., Chen M.-Y., Tsay S.-S., Zou W., and Wu S.-H. (2006) Structural elucidation of phosphoglycolipids from strains of bacterial thermophiles, Thermus and Meiothermus. J Lipid Res 47:1823–1832 [DOI] [PubMed] [Google Scholar]
- Zhang C.L., Huang Z., Li Y.-L., Romanek C.S., and Mills G.L. (2007) Lipid biomarkers, carbon isotopes, and phylogenetic characterization of bacteria in California and Nevada hot springs. Geomicrobiol J 24:519–534 [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.