Highlights
-
•
PAS improved hand function in 5 patients with incomplete non-traumatic tetraplegia.
-
•
The effect persisted for at least 6 months after the end of the 6-week stimulation.
-
•
There were no adverse effects.
Keywords: TMS, Rehabilitation, Plasticity, Spinal Cord, Paired Associative Stimulation, Spinal cord injury
Abstract
Objectives
Long-term paired associative stimulation (PAS) is a non-invasive combination of transcranial magnetic stimulation and peripheral nerve stimulation and leads to improved hand motor function in individuals with incomplete traumatic tetraplegia. Spinal cord injuries (SCIs) can also be induced by neurological diseases. We tested a similar long-term PAS approach in patients with non-traumatic neurological SCI.
Methods
In this case series, five patients with non-traumatic tetraplegia received PAS to the weaker upper limb 3 to 5 times per week for 6 weeks. Patients were evaluated by manual muscle testing (MMT) before and immediately after the therapy and at the 1- and 6-month follow-ups. Patients were also evaluated for spasticity, hand mechanical and digital dynamometry, pinch test and Box and Block test.
Results
MMT values of all patients improved at all post-PAS evaluations. The mean ± standard error MMT increase was 1.44 ± 0.37 points (p = 0.043) immediately after PAS, 1.57 ± 0.4 points (p = 0.043) at the 1-month follow-up and 1.71 ± 0.47 points (p = 0.043) at the 6-month follow-up. The pinch test, digital dynamometry and Box and Block test results also improved in all patients.
Conclusions
Long-term PAS may be a safe and effective treatment for improving hand function in patients with non-traumatic tetraplegia.
Significance
This is the first report demonstrating the therapeutic potential of PAS for neurological SCI.
1. Introduction
Spinal cord injury (SCI) can be caused by trauma or disease and leads to disruption of corticospinal connectivity. Although most SCI research is focused on traumatic SCI, the percentage of disease-related SCI is estimated to be between 30% and 80% of all cases (Scivoletto et al., 2014).
For tetraplegic individuals, regaining hand control remains the highest priority regardless of aetiology (Anderson, 2004). Development of activity-based therapies is acknowledged as the top priority in SCI rehabilitation (van Middendorp et al., 2014). We have shown that long-term paired associative stimulation (PAS) (Suppa et al., 2017, Stefan et al., 2000), a non-invasive technique involving multiple repetitions of peripheral nerve stimulation (PNS) combined with transcranial magnetic stimulation (TMS), improves hand motor function in patients with traumatic tetraplegic SCI (Shulga et al., 2016a, Tolmacheva et al., 2017, Rodionov et al., 2019). Spinal PAS aims at coincidence of orthodromic and antidromic neuronal impulse volleys induced by TMS and PNS, respectively, at the corticomotoneuronal synapses (Shulga et al., 2016a, Shulga et al., 2016b, Shulga et al., 2015). This is expected to lead to a beneficial neuroplasticity at spared corticospinal connections. PAS induces a more profound and persistent improvement than PNS only (Tolmacheva et al., 2017).
Neurological SCIs can profoundly impair motor function and quality of life. Nevertheless, present treatments may spare a considerable amount of neuronal connectivity. Therefore, many patients with non-traumatic SCI might be responsive to neuroplasticity-enhancing protocols. On the other hand, the cellular and molecular mechanisms underlying neurological SCI can differ dramatically from those of traumatic SCI, and it is not clear whether treatments effective for traumatic SCI would also be effective in disease-induced injuries. In this case series, we investigated whether our promising PAS technique could also be beneficial for patients with neurological SCI. For this purpose, we applied PAS for 6 weeks on 5 patients with chronic non-traumatic tetraplegia.
2. Methods
2.1. Study design
The study was approved by the Ethics Committee of Medicine of the Helsinki University Hospital (study identifier: NCT03104803). All patients provided written informed consent. Five patients with disease-related chronic tetraplegia (American Spinal Injury Association Impairment Scale grade D, right-handed) participated in the study (Fig. 1B). Patients’ hands were evaluated with the Daniels and Worthingham’s manual muscle test (MMT); the weaker hand was selected for PAS. The contralateral hand was not stimulated. Each patient underwent 22 PAS sessions (5 days per week for 2 weeks and 3 days per week for 4 weeks). The physiotherapist evaluated motor performance (MMT of muscles innervated by the stimulated nerves on a 0–5 scale, Fig. 1A). The muscles with a score <5 at initial evaluation were followed up (Fig. 1A). Spasticity (Modified Ashworth Scale from the elbow and wrist and digital dynamometry [PABLO, Tyromotion]) was assessed before, immediately after and at 1 month and 6 months after the stimulation. The physiotherapist was not informed about the rule of hand selection and did not know which hand was stimulated. Another researcher familiar with the PAS protocol performed measurements for pinch (Baseline® Mechanical Pinch Gauge), manual dynamometry (Exacta™ Hydraulic Hand Dynamometer) and Box and Block tests at the same time points. As we did not have a sham control group, this study needs to be considered as a case series.
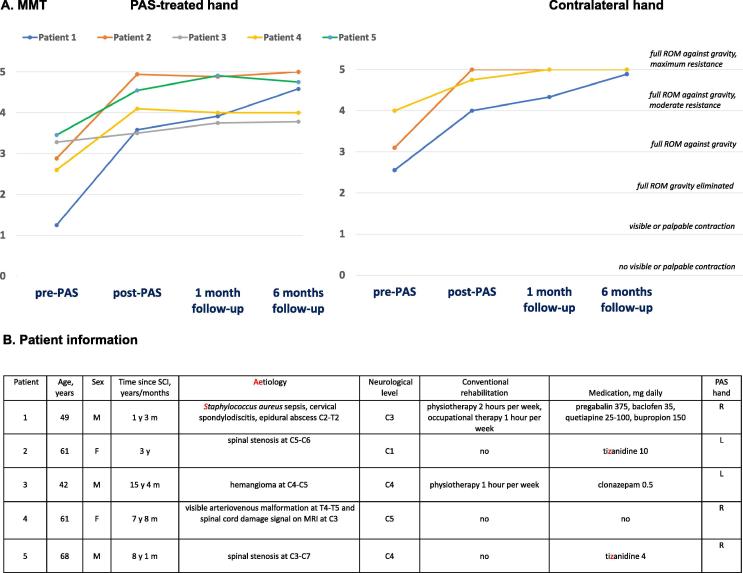
Fig. 1.
Individual results of manual motor test (MMT) and patient-specific information. A) The average score of all evaluated muscles having a score of <5 at initial evaluation is shown for each patient at all time points. In PAS-treated hand, MMT score improved in all five patients immediately after 6 weeks of stimulation and kept improving or remained stable during the 1-month and 6-month follow-up periods. Contralateral hand MMT score improved as well (patients 3 and 5 with normal contralateral hand MMT score before intervention are not shown). ROM – range of motion B) Individual information of each patient. M - male, F – female, C/T – cervical/thoracic spinal level, PNS – peripheral nerve stimulation, ISI – interstimulus interval, R – right, L – left.
2.2. Rehabilitation, medication and nutrition
Conventional rehabilitation and medication (Fig. 1B) were not modified and continued during the study. As in our previous studies (Shulga et al., 2016a, Tolmacheva et al., 2017), all patients were instructed to take a standard dose of multivitamin during the stimulation period and up to 1-month follow-up to ensure that any lack of vitamins or minerals would not prevent the therapeutic effect. No other changes to medication or nutrition were made.
2.3. Patient evaluation
2.3.1. Manual motor test and analysis
The physiotherapist assessed the patient’s motor score of all hand muscles innervated by the stimulated nerves of both hands by Daniels and Worthingham’s muscle test (Hislop et al., 2014) on a 0 to 5 scale (Fig. 1A, see Supplementary Table for raw data). The muscles that had a motor score <5 at initial evaluation were evaluated immediately after the treatment and at 1 month and 6 months after the last stimulation session. We calculated the differences between initial evaluation and each subsequent evaluation for each muscle and obtained one average of all muscle change values at each time point for each patient (Supplementary Table). These averages were used for statistical analysis.
2.3.2. Spasticity
Spasticity was assessed with the Modified Ashworth Scale from the elbow (extensors, flexors) and wrist (extensors, flexors) in both hands.
2.3.3. Mechanical pinch and grip dynamometry
Pinch dynamometry was performed with a Baseline® Mechanical Pinch Gauge (Fabrication Enterprises Inc., USA). Grip-force evaluation was performed with the Exacta™ Hydraulic Hand Dynamometer (North Coast Medical, Inc., USA). The patients were seated in a chair with their back straight, shoulder adducted, and elbow flexed at 90°. The pinch gauge was placed between the proximal interphalangeal joint of the index finger and the tip of the thumb for key pinch, between the tip of the thumb and the tip of the index finger for tip pinch and between the tip of the thumb and tips of the index and middle fingers for palmar pinch. For grip strength dynamometry, the handle of the hand dynamometer was adjusted to a comfortable grasp according to the patient’s hand size. Both hands were tested. For each test, the best result out of three attempts was recorded (in kilograms).
2.3.4. Digital grip strength dynamometry
We utilised digital dynamometry in addition to mechanical dynamometry. Digital dynamometry better reflects functionality, as the patient can use the most functionally advantageous position that maximizes the use of all available muscles. The compressive force of the hand was performed with the assessment tool of the PABLO (Tyromotion GmbH, Austria) rehabilitation device. Any comfortable position of the shoulder and forearm was allowed for this measurement. The best result out of three attempts was recorded (in kilograms).
2.3.5. Box and block test
The patients received instructions before the test and practiced for 15 s before the actual test. The patient grasped one block at a time and transported the blocks from one compartment of the box to the other for 1 min. The total number of the transferred blocks was recorded.
2.4. Paired associative stimulation
2.4.1. Transcranial magnetic stimulation
We administered TMS with an eXimia magnetic stimulator (Nexstim Ltd., Helsinki, Finland) with a cooled figure-of-eight coil (outer loop diameter 70 mm). A navigation system based on individual brain magnetic resonance imaging (MRI) was utilised to guarantee accuracy of the site of the delivered stimuli during the repeated stimulations. We defined hotspots in the primary motor cortex (M1) for abductor digiti minimi (ADM), abductor pollicis brevis (APB) and brachioradialis (BR) muscles as the sites where TMS induced movement in the corresponding muscles and elicited the largest and the most consistent MEPs. When no movement could be elicited, only MEP size and consistency were used for the selection. The resting motor threshold (RMT) of the hotspots was identified as the minimum TMS intensity inducing an MEP size ≥50 µA (peak-to-peak amplitude) in 5 attempts out of 10. We recorded 15 MEPs from ADM, APB and BR with a 3.3-second interpulse interval at a TMS intensity of 120% RMT. We visually analysed EMG epochs on a 200-ms time window before a given stimuli to detect pre-activation (any increase in spontaneous EMG activity amplitude exceeding baseline) and excluded such recordings from the analysis.
2.4.2. Peripheral nerve stimulation and F-response measurement
We stimulated peripheral nerves with a Dantec Keypoint device (Natus Medical Inc., Pleasanton, CA). Two surface electrodes (Neuroline 720, AMBU A/S, Ballerup, Denmark) were placed at the wrist for median or ulnar nerve stimulation and on the arm proximal to the elbow for radial nerve stimulation. During the radial nerve stimulation, the electrodes were pressed against the skin. For the F-response recording, two surface electrodes were used with the active electrode over the bulk of APB, ADM, and BR during median, ulnar and radial nerve stimulation, respectively. We recorded 10 F-responses to a single 0.2-millisecond electrical stimulus at suprathreshold intensity to detect the minimum F latency. In addition, we determined the minimum intensity eliciting F-responses with a 1-millisecond single pulse.
2.4.3. Paired associative stimulation (PAS)
Each patient had 22 PAS sessions (5 days per week for 2 weeks and 3 days per week for 4 weeks). The weaker hand was selected for PAS. Stimulations of the median, ulnar and radial nerves were paired with the corresponding TMS sites that were determined as described above (transcranial magnetic stimulation) and performed one nerve/TMS site at a time. The stimulation of one nerve/cortex pair took 20 min (240 pairs of TMS and PNS trains), and the stimulation of all three pairs took 60 min, correspondingly. The contralateral hand did not receive stimulation. We calculated the interstimulus interval (ISI) between TMS and PNS with the formula (minimal F latency minus MEP latency) as described previously (positive ISI means that PNS precedes and negative ISI that PNS follows the TMS) (Shulga et al., 2015). This calculation aims at timing simultaneous arrival of a TMS-induced volley with the first volley of the PNS train at the spinal-cord level (Shulga et al., 2015); however, possible minor calculation errors due to technical challenges in measurements do not abolish the PAS effect on MEPs (Shulga et al., 2016b). MEP latency was defined from the average response of 15 MEPs in each patient. TMS was delivered as single pulses at 100% of stimulator output (SO) (Tolmacheva et al 2019). PNS was delivered as a 100-Hz train6 (Tolmacheva et al., 2019) consisting of six biphasic square-wave 1-millisecond pulses at the minimum intensity individually defined to induce F responses (with 1-millisecond pulses). The TMS pulse and PNS train were delivered at 0.2 Hz. Patients were instructed to imagine the movements of the muscles innervated by the stimulated nerve. The individual settings used for each patient (defined or calculated as described above) are presented in Table 1.
Table 1.
Stimulation parameters.
| Patient | Stimulated hand |
PNS intensity, mA (med, uln, rad) |
ISI, ms (med, uln, rad) |
|---|---|---|---|
| Patient 1 | Right | 20, 62, 20 | +10, +10, +2 |
| Patient 2 | Left | 8, 6, 65 | −1, +1, +6 |
| Patient 3 | Left | 3, 10, 17 | +5, +3, +1 |
| Patient 4 | Right | 4, 4, 9 | +1, +1, −9 |
| Patient 5 | Right | 2, 11, 33 | −2, −3, −4 |
2.4.4. Statistical analysis
Wilcoxon signed-rank test was performed with IBM SPSS statistics 25 software. The test was selected based on the number of patients. The test inherently produces p = 0.043 when all “post” values are larger than “pre” values in five compared pairs. The data are presented as a mean ± standard error.
3. Results
3.1. Manual motor testing (MMT)
After treatment, the MMT score of the PAS-treated hand was higher than the pre-PAS score in all 5 patients at all evaluations; the average increase across patients was 1.4 ± 0.4 points (p = 0.043) immediately after PAS, 1.6 ± 0.4 (p = 0.043) at 1-month follow-up and 1.7 ± 0.5 (p = 0.043) at 6-month follow-up (Fig. 1A). MMT scores also improved in the contralateral, non-stimulated hand (Fig. 1A). In each patient with abnormal pre-PAS motor scores in both hands (patients 1, 2 and 4), MMT score improvement was higher in the stimulated than in the non-stimulated hand at all evaluations. The improvement in the stimulated hand normalised to the non-stimulated hand averaged across the 3 comparable patients was 157 ± 27% immediately after PAS and 129 ± 12% at the 1-month and 130 ± 9% at the 6-month evaluations. Joint spasticity as assessed using the Modified Ashworth Scale did not change significantly.
3.2. Hand strength and functional tests
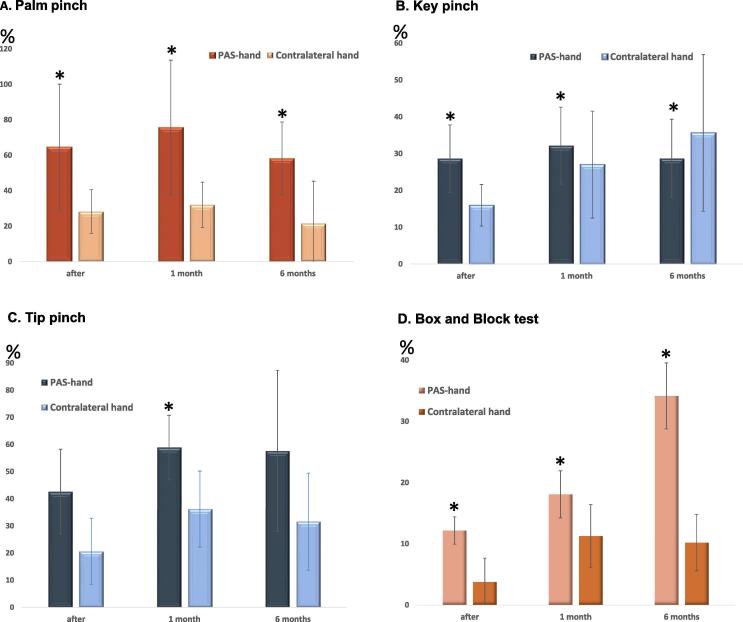
All patients had abnormal palm, key and tip pinch test results in the stimulated hand before PAS. In the contralateral hand, all patients had abnormal values for key pinch and 3/5 patients had abnormal values for tip and palm pinches. Palm and key pinch of the stimulated hand improved in all patients at all evaluations (p = 0.043 for each evaluation; Fig. 2A-B). Tip pinch (Fig. 2C) of the stimulated hand improved in all patients (p = 0.043) at the 1-month follow-up. The stimulated hand improved in all patients (p = 0.043 for each evaluation; Fig. 2D) also in the box and block test. Mechanical hand dynamometry did not reveal significant improvement at any evaluation in either hand. Digital hand dynamometry, however, revealed a significant improvement at the 1-month and 6-month follow-ups (p = 0.043 for each evaluation; Fig. 3). Patients reported post-therapy functional gains (such as improved use of the stimulated hand for hair washing, food slicing, dressing, handling a steering wheel) and more confident use of hands in all tasks of daily life. One patient resumed practicing guitar playing, which was not possible before therapy. No adverse effects of PAS were reported.
Fig. 2.
Pinch test and Box and Block test results. The results are shown as average per cent increase from the pre-PAS evaluation level (post-PAS normalised to pre-PAS minus 100%).
Fig. 3.
Dynamometry. A) Digital hand dynamometry was performed with the PABLO hand rehabilitation device. B) The values obtained by digital dynamometry improved in all patients at 1-month and 6-month follow-up. Average per cent increase from the pre-PAS level is shown (post-PAS normalised to pre-PAS minus 100%).
4. Discussion
The present case series support our previous data showing that long-term PAS can be effectively utilised for the rehabilitation of a tetraplegic hand at the chronic stage after SCI (Tolmacheva et al., 2017, Shulga et al., 2016a, Shulga et al., 2016b, Rodionov et al., 2019) and suggest that these findings might be applicable also to patients with neurological SCI. PAS was effective in patients up to 68 years of age and with time after disease onset of up to 15 years. Patients with more recent SCI derived greater benefit (Fig. 1). We used PAS with 100 Hz PNS for the first time in patients. PNS 100 Hz is more effective than PNS 50 Hz in PAS of healthy subjects (Tolmacheva et al., 2019).
We also showed for the first time that the beneficial effect of PAS can persist for up to 6 months and even improve during the follow-up. This post-PAS improvement is most probably due to the more versatile use of hands in everyday life enabled by PAS. Therefore, for the patients with milder SCI represented by our patients, even a short treatment time of 6 weeks may be sufficient to induce long-lasting positive meaningful hand function changes. The increased use of hands in daily life possibly contributes to the observed effects also during the 6-week stimulation period, and its effect cannot be separated from the effects of PAS. All patients, however, had attempted to use their hands as much as possible also before treatment; it was clear that PAS therapy enabled increased use. As more versatile use of hands is the actual goal of treatment, it would have been impossible to restrict the use of the hands to the pre-PAS level to separate the effect of PAS alone.
MMT results (Fig. 1) indicated improvement not only in the stimulated but also in the non-stimulated (contralateral), less severely affected hand in all three patients (two out of five patients had normal MMT scores in the contralateral hand from the beginning). This might be explained by several factors. First, improved use of the more severely affected hand might encourage the patient to engage in bilateral tasks in a more versatile way, promoting the rehabilitation of both hands. Second, severe impairment in one limb might worsen the impairment of the contralateral limb (which is less affected by the primary injury) through unfavourable interhemispheric cortico-cortical or intraspinal neuronal interactions. For example, patients with chronic post-stroke motor impairment recruit larger portions of secondary motor areas than patients with no residual impairment (Ward and Cohen, 2004). Larger activation of supplementary motor areas was also detected in patients with incomplete spinal cord injury (Sharp et al., 2017). This increased activation might impair the function of the less affected limb by interhemispheric inhibition (Boddington and Reynolds, 2017). Achieving a more normal state in the sensorimotor network governing the more severely affected limb might alleviate this impairment. We have previously shown that when one hand of the patient receives PAS and the other only PNS, the hand receiving PAS recovers better than the contralateral limb receiving PNS only (Tolmacheva et al., 2017). The present data indicate that the improved function in the hand treated with PNS only (Tolmacheva et al., 2017) might be due at least in part to such factors and not to the application of PNS. In all other tests apart from MMT, however, significant improvement was observed for the stimulated hand only (Fig. 2, Fig. 3), and in MMT, the stimulated hand improved more than the non-stimulated one.
Patient 4 responded to the therapy already at 10 days after the stimulation onset (Supplementary Video). Mechanisms of injury are variable in patients with neurological SCI. The main cause of paresis in patients responding quickly might not be disruption of corticospinal connectivity but, rather, a disturbed balance of excitatory and inhibitory drives between the corticospinal tract and spinal sensorimotor networks (Wagner et al., 2018), which might be driven towards a more normal state by PAS.
We present a case series of a small sample size. Importantly, all 5 patients benefited from the treatment. All patients were enrolled at the chronic stage where no spontaneous recovery was expected to occur. Patients received conventional physical therapy and medication for years before the 6-week PAS period, and these were not changed or increased during the treatment or the 6-month follow-up period; physical therapy and medication thus cannot explain the observed effects. PAS was combined with motor imagery. All patients had undergone extensive rehabilitation and had attempted to use the hands in their daily life for years before this intervention; thus, it is highly improbable that 6 weeks of motor imagery alone would have had a greater effect than that from the previous years of conventional training.
The possible therapeutic effects of TMS or PNS alone were not evaluated in this study. In our previous studies, however, we have shown that neither TMS nor PNS components of the PAS protocol utilised in this study potentiate MEPs in healthy subjects (Shulga et al., 2016b, Tolmacheva et al., 2019). We have also shown that PAS is more effective than PNS alone (Tolmacheva et al., 2017). Low-frequency TMS applied in this study is known to have an inhibitory effect (Hallett and Berrardelli, 2008) and thus is not expected to produce the observed results.
Patient spasticity was variable and fluctuated depending on the time of day, medication and overall health condition. As spasticity strongly affects the electromyographic baseline activity and hence MEP amplitudes, it was not possible to reliably assess the effect of PAS with MEPs.
PAS applied as a long-term treatment to neurological patients might alter conduction of neural fibres with time. However, measurements of motoneuron conductance may be challenging in these patients. In addition, cortical mapping may be difficult due to spasticity. We have previously demonstrated that our novel variant of PAS induces robust potentiation of MEPs at a wide range of intervals between TMS and PNS (Shulga et al., 2016b), whereas conventional PAS protocols require a very narrow, precisely defined TMS-PNS interval. This protocol is also not sensitive to small errors in motor cortex mapping (Tolmacheva et al., 2019). The efficacy of this protocol at a wide range of TMS-PNS intervals could be the reason why it is better suitable for clinical use than conventional PAS protocols, and thus in part explaining the mechanism of its therapeutic action. A single high-intensity TMS pulse results in a high-frequency repetitive discharge of corticospinal neurons (Di Lazzaro et al., 2008). The combination of high-intensity TMS pulses with high-frequency trains for the peripheral component of PAS plausibly enables LTP-like effects at a wider range of ISIs. Spike timing is not the only requirement for plasticity induction, which depends also on firing rate, postsynaptic voltage and synaptic cooperativity (Feldman, 2012). The 10- to 20-ms pulse interval in a stimulus train may increase the probability of some of the orthodromic and antidromic volleys arriving at the corticomotoneuronal synapses within the LTP-inducing time window. When LTP-inducing and LTD-inducing interactions occur at the same time, LTP can override LTD (Sjostrom et al., 2001). Consistent with this hypothesis, we have shown that an additional increase in PNS frequency enhances the effectiveness of the TMS-PNS protocol in healthy subjects (Tolmacheva et al., 2019).
The present and previous data (Suppa et al., 2017, Tolmacheva et al., 2017, Shulga et al., 2016a, Shulga et al., 2016b, Rodionov et al., 2019) indicate that PAS is a safe, well-tolerated and feasible method to promote functionally meaningful hand rehabilitation in some patients with tetraplegia with incomplete SCI. Further trials with more patients and at a subacute stage after SCI are justified.
Acknowledgments
Acknowledgements
This work was supported by the Academy of Finland (AS, 307951), governmental subsidiary funds (AS), the Maire Taponen Foundation (AS) and the University of Helsinki (AT). The study sponsors had no role in the collection, analysis and interpretation of data or in the writing of the manuscript. We thank David Monter-Danger for excellent technical assistance (MRI).
Declaration of Competing Interest
JPM has received travel and accommodation expenses for international lectures from Nexstim Plc, outside the submitted work. Other authors have nothing to disclose.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.cnp.2019.07.002.
Appendix A. Supplementary data
The following are the Supplementary data to this article:
Hand movements (spreading and extending the fingers) of patient 4 before and 10 days after the beginning of stimulation.
References
- Anderson K.D. Targeting recovery: priorities of the spinal cord-injured population. J. Neurotrauma. 2004;21:1371–1383. doi: 10.1089/neu.2004.21.1371. [DOI] [PubMed] [Google Scholar]
- Boddington L.J., Reynolds J.N.J. Targeting interhemispheric inhibition with neuromodulation to enhance stroke rehabilitation. Brain Stimul. 2017;10:214–222. doi: 10.1016/j.brs.2017.01.006. [DOI] [PubMed] [Google Scholar]
- Di Lazzaro V., Ziemann U., Lemon R.N. State of the art: Physiology of transcranial motor cortex stimulation. Brain Stimul. 2008;1:345–362. doi: 10.1016/j.brs.2008.07.004. [DOI] [PubMed] [Google Scholar]
- Feldman D.E. The spike-timing dependence of plasticity. Neuron. 2012;75:556–571. doi: 10.1016/j.neuron.2012.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hallett M., Berrardelli A. Oxford Handbook of Transcranial Stimulation. Oxford University Press; New York: 2008. Movement disorders. [Google Scholar]
- Hislop H.J., Avers D., Brown M. nineth ed. Elsevier; 2014. Daniels and Worthingham's Muscle Testing: Techniques of Manual Examination and Performance Testing. [Google Scholar]
- Rodionov A., Savolainen S., Kirveskari E., Mäkelä J.P., Shulga A. Restoration of hand function with long-term paired associative stimulation after chronic incomplete tetraplegia: a case study. Spinal Cord Ser. Cases. 2019 doi: 10.1038/s41394-019-0225-5. (accepted manuscript) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scivoletto G., Tamburella F., Laurenza L., Torre M., Molinari M. Who is going to walk? A review of the factors influencing walking recovery after spinal cord injury. Front. Hum. Neurosci. 2014;8:141. doi: 10.3389/fnhum.2014.00141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharp K.G., Gramer R., Page S.J., Cramer S.C. Increased brain sensorimotor network activation after incomplete spinal cord injury. J. Neurotrauma. 2017;34:623–631. doi: 10.1089/neu.2016.4503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shulga A., Lioumis P., Kirveskari E., Savolainen S., Makela J.P., Ylinen A. The use of F-response in defining interstimulus intervals appropriate for LTP-like plasticity induction in lower limb spinal paired associative stimulation. J. Neurosci Methods. 2015;242C:112–117. doi: 10.1016/j.jneumeth.2015.01.012. [DOI] [PubMed] [Google Scholar]
- Shulga A., Zubareva A., Lioumis P., Makela J.P. Paired associative stimulation with high-frequency peripheral component leads to enhancement of corticospinal transmission at wide range of interstimulus intervals. Front. Hum. Neurosci. 2016;10:470. doi: 10.3389/fnhum.2016.00470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shulga A., Lioumis P., Zubareva A., Brandstack N., Kuusela L., Kirveskari E. Long-term paired associative stimulation can restore voluntary control over paralyzed muscles in incomplete chronic spinal cord injury patients. Spinal. Cord. Ser. Cases. 2016;2:16016. doi: 10.1038/scsandc.2016.16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sjostrom P.J., Turrigiano G.G., Nelson S.B. Rate, timing, and cooperativity jointly determine cortical synaptic plasticity. Neuron. 2001;32:1149–1164. doi: 10.1016/s0896-6273(01)00542-6. [DOI] [PubMed] [Google Scholar]
- Stefan K., Kunesch E., Cohen L.G., Benecke R., Classen J. Induction of plasticity in the human motor cortex by paired associative stimulation. Brain. 2000;123:572–584. doi: 10.1093/brain/123.3.572. [DOI] [PubMed] [Google Scholar]
- Suppa A., Quartarone A., Siebner H., Chen R., Di Lazzaro V., Del Giudice P. The associative brain at work: Evidence from paired associative stimulation studies in humans. Clin. Neurophysiol. 2017;128:2140–2164. doi: 10.1016/j.clinph.2017.08.003. [DOI] [PubMed] [Google Scholar]
- Tolmacheva A., Savolainen S., Kirveskari E., Lioumis P., Kuusela L., Brandstack N.M. Long-term paired associative stimulation enhances motor output of the tetraplegic hand. J Neurotrauma. 2017;34:2668–2674. doi: 10.1089/neu.2017.4996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tolmacheva A., Mäkelä J.P., Shulga A. Increasing the frequency of peripheral component in paired associative stimulation strengthens its efficacy. Sci. Rep. 2019;9:3849. doi: 10.1038/s41598-019-40474-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Middendorp J.J., Allison H., Cowan K. Spinal cord injury priority setting partnership. Top ten research priorities for spinal cord injury. Lancet Neurol. 2014;13:1167. doi: 10.1016/S1474-4422(14)70253-4. [DOI] [PubMed] [Google Scholar]
- Wagner F.B., Mignardot J.B., Le Goff-Mignardot C.G., Demesmaeker R., Komi S., Capogrosso M. Targeted neurotechnology restores walking in humans with spinal cord injury. Nature. 2018;563:65–71. doi: 10.1038/s41586-018-0649-2. [DOI] [PubMed] [Google Scholar]
- Ward N.S., Cohen L.G. Mechanisms underlying recovery of motor function after stroke. Arch. Neurol. 2004;61:1844–1848. doi: 10.1001/archneur.61.12.1844. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Hand movements (spreading and extending the fingers) of patient 4 before and 10 days after the beginning of stimulation.