Graphical abstract
Keywords: Argyreia speciosa, Antioxidant, Polyphenol, Fractionation
Abstract
Traditional pertinence
Argyreia speciosa Sweet (Linn.), belongs to the family convolvulaceae, a traditional Indian medicinal herb, has been used to treat acute/chronic ulcers, gonorrhea, rheumatoid arthritis and several nervous disorders having a long history.
Aim of the study
A broad spectrum approach of this work was to find out the antioxidant activity of Argyreia speciosa seeds, in vitro and in vivo antioxidant assay were performed.
Material and methods
Total phenolic content (TPC), reducing power (RP), antioxidant activity (AOA), (superoxide anion), DPPḢ (1,1-diphenyl-2-picrylhydrazyl) and ˙OH (hydroxyl) radicals scavenging activities, GSH (glutathione), CAT (catalase), SOD (superoxide dismutase) and LPO (lipid peroxidase) are the major parameters which were studied for determining in vitro and in vivo antioxidant property of seed extract & their six fractions obtained from A. speciosa. Carbon tetrachloride (CCl4) induced rat model was used to determine in vivo antioxidant assay of extract and its fractions.
Results
Butanol fraction (AS-BF) showed strong antioxidant property and protected oxidative DNA damage. AS-BF was found best as compared to all other fraction for determining antioxidant property of seeds with the reduction in lipid peroxide formation and increment in GSH, CAT and SOD. AS-BF showed the presence of phenolic compounds viz. gallic acid, chlorogenic acid, and ellagic acid.
Conclusion
From these results, it was proved that A. speciosa seeds prevent tissue damage due to oxidative stress with strong antioxidant activity.
1. Introduction
In the case of human body, oxidative free radicals possess a very important role for various biological activities, such as the intercellular killing of microbes by phagocytic cells like macrophages and granulocytes (Bhagat et al., 2016). They are involved in a certain cell signaling process known as redox signaling (Pacher et al., 2007). From the latest researches, it is found that oxygen-centered ROS which is abbreviated as reactive oxygen species e.g. superoxide and hydroxyl radical play a very important role in the case of cell signaling (Singh et al., 2017b). However, because of their reactive nature, they are involved in unwanted side reactions causes cell damage. Excess of these ROS resulting in cell damage and cell death. Due to these reasons they pony up maximum diseases e.g. diabetes, cancer, cardiac damage and immunological disease. The body has strong mechanisms to minimize cellular damage by ROS (Vlasova, 2018). In addition, antioxidants obtained from different sources play a very important role in the defense mechanisms of ROS (Sarma et al., 2010). Plants are a rich source of bioactive molecules, having a broad spectrum of pharmacological and antioxidant activity. Ethnopharmacological studies showed that secondary metabolites from plant sources like carotenoids, polyphenols, ascorbates and natural phenolic compounds having a strong chemical defense mechanism against ROS (Benzie, 2003). The literature survey reveals that in 2014, global demand of antioxidant products was near about 2.25 billion $ and its expected consumption level will increase up to 3.25 billion $ in 2020 (5.5% between 2015 and 2020) (Market Research Store, 2017).
Argyreia speciosa Sweet (Linn.) is one of the most popular Indian traditional plant commonly known as Ghav-patta belongs to Convolvulaceae family (Singh et al., 2017a). The seeds are eaten in some parts of Bihar due to the rich sources of fatty acid, proteins and amino acids. Roots have been reported for antispasmodic (Goel et al., 2002), nootropic (Hanumanthachar et al., 2007), aphrodisiac activity and immunomodulatory activities (Gokhale et al., 2003). Flowers showed significant antidiarrhoeal and antiulcer activity (Rao et al., 2004) whereas leaves were found to possessed nematicidal and antimicrobial activity (Shukla et al., 1999). Alcoholic extract of A. speciosa flowers has been reported for antiviral activity (Sahu Alakh et al., 2013). Traditionally seeds of A. speciosa is used as a tonic. There is no scientific evidence for the antioxidant activity of A. speciosa seeds. Due to its valuable use, antioxidant activity (in vitro and in vivo assay) and oxidative DNA damage protecting potential of all fractions obtained from A. speciosa seeds were performed.
2. Experimental procedure
2.1. Chemicals
Chlorogenic acid, kaempferol, ellagic acid, ferrozine, ferulic acid, caffeic acid and DPPH for determining antioxidant activity were obtained from Merck, Mumbai, India; β-carotene, linolic acid and gallic acid from Merck, Mumbai, India. Standard bioactive compounds like quercetin & rutin were obtained from HI media, Delhi, India and solvents of HPLC grade were brought from Merck, Mumbai, India.
2.2. Authentication and collection
A. speciosa seeds were collected from nearby villages of Lucknow. After collection, seeds were authenticated as specimen no: A. speciosa-NBR-894563 from Department of Herbarium of National Botanical Research Institute, Lucknow, India (CSIR-NBRI).
2.3. 50% hydroalcoholic extraction and fractionation
Sun-dried and finely powdered seeds (150 g) were first defatted with the help of petroleum ether. After that seeds were extracted with 50% methanol for the next 50 h. Filter paper (No. 4) was used for the filtration of extract. The procedure was repeated 3 times to get the extract and solvents were removed from extract with the help of Rotavapor (150 psi). Dried extract of seeds was obtained with the help of lyophilization. The freshly dried seed extract was carried out for partition separation in distilled water and ethyl ether. To remove the impurities from the phenolic part, the aqueous seed extract was treated with 20% NaHCO3 (to get pH range between 8 and 9), and non phenolics were treated with chloroform. Polyphenolic fraction (pH 3–4) was obtained from the aqueous extract by adding HCl into it. Six different fractions of A. speciosa seeds such as crude dried extract (AS-CE), the fraction of diethyl ether (AS-DF), the fraction of CCL4 (AS-CF), the fraction of ethyl acetate (AS-EF), the butanol fraction (AS-BF) and the aqueous fraction (AS-AF) were obtained. Solvents from these fractions were evaporated and dried completely. The dried extracts were used for the preliminary screening, in vitro and in vitro antioxidant activity.
2.4. Polyphenolic content determination (TPC)
Phenolic contents of A. speciosa seeds extract were calculated with slight modification (Ragazzi and Veronese, 1973). 10 mg of seeds extract was mixed with 1.0 ml of Folin’s reagent (1 N) and 2.0 ml of Na2CO3 (20%) was added subsequently. The spectrum of samples was measured at 727 nm and phenolic contents were expressed as mg/g equivalence of Gallic acid (GAE).
2.5. Flavonoid content determination (TFC)
The colorimetric assay method was used to calculate total flavonoid content with some modification (Oyaizu, 1986). UV/visible absorbance of seeds extract was determined at 510 nm. Quercetin (0 to 500 mg/L, Merck, Mumbai, India) was taken as a standard and flavonoid content was determined as (Quercetin)/g of the seeds extract.
2.6. In vitro antioxidant activity
Beta-carotene and linoleic acid test was used for determination of antioxidant activity with some modification in the method explained by Emmons and Peterson (1999) and it was represented as percentage inhibition (PI%). Ferric reduction antioxidant assay method was used to measure the reducing capacity (RP) (Apati et al., 2003), 1,1-diphenyl-2-picrylhydrazyl solution (DPPḢ–6 × 10–5 M in methanol) was used to determine free radicals by some modification in method given by Yen and Pin-Der (1994), efficiency concentration (EC50), inhibitory concentration (IC50), anti-radical power (ARP) and superoxide ion free radical scavenging activity assay (FRSA) (Kroyer, 2004, Nishikimi et al., 1972). Hydroxyl radical scavenging activity was determined by Halliwell and Gutteridge (2015). Chelation capacity of ferrous ion was determined by Decker and Welch (1990). Lipid peroxide formation from lipid-rich rat liver homogenates and its inhibition were determined by TBARS abbreviated as Thiobarbituric acid-reactive species assay (Ohkawa et al., 1979).
2.7. Chromatographical analysis
For purification and determination of HPLC analysis silica gel which is pre-coated-60 F254 plate (Merck, Mumbai, India) having a thickness of 0.2 mm was used. Linomat IV (CAMAG) was used for sample application and plates were scanned under the scanner (CAMAG-3).
2.8. Nicking protective effect of DNA induced by OH free radical
Supercoiled pBR322 DNA was used for determining the protective effect of seeds extract by DNA nicking. Lee method with some modification was used for determining DNA nicking (Lee et al., 2002), quercetin (50 µM) and catalase (5 units) were used as reference one.
2.9. In vivo antioxidant activity
Wister rats were used to determine in vivo antioxidant activity. Rats were divided into 5 respective groups (n = 6 in each group). First group: Normal control group (1% CMC, p.o.); Second group: Negative control group (CCl4 induced group, CCl4: liquid paraffin (1:1; 2 ml/kg body weight/day, s.c.); Third group: Treatment group (CCl4 + AS-BE 50 mg/kg body weight/day, p.o.); Fourth group: (CCl4 + AS-BE 100 mg/kg body weight/day, p.o.); Fifth group: Reference group (CCl4 + Vitamin E, 50 mg/kg body weight/day, p.o.). Rats were sacrificed after 24 h of the last dose of CCl4. Supernatants was used for determination of biochemical parameters. Malondialdehyde (MDA) level were estimated by the method of Ohkawa et al. (1979). CAT, SOD, and GSH most important parameters of in vivo antioxidant activity were estimated by the method explained by Aebi, 1974, Kakkar et al., 1984, and Ellman (1959).
2.10. Statistical determination
One-way ANOVA was used for statistical determination and it was completely assayed by Newman Keuls Multiple Comparison followed by Scheffe’s test with software Graph pad Prism 7. Studies were explained in the form of mean ± SEM to measure the difference in each group and (P < 0.05).
3. Results
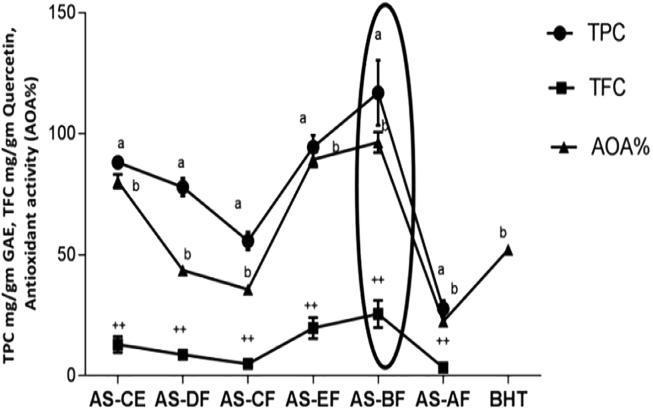
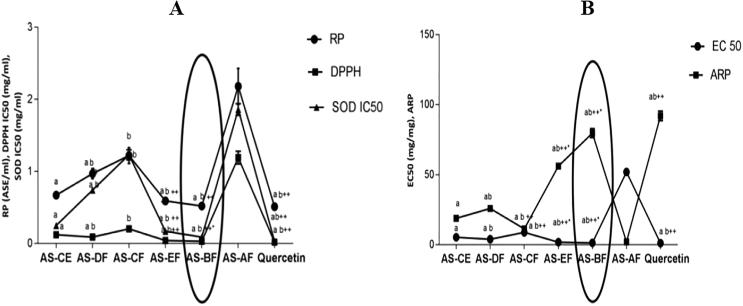
TPC ranged from 27.7–117.2 mg/g in all six fractions of A. speciosa seeds extract. AS-BF showed maximum phenolic content and it has decreased respectively in this order AS-BF > AS-EF > AS-CE > AS-DF > AS-CF > AS-AF (Fig. 1.). The TFC in various fractions were varied from 3.367 to 25.54 mg/g (quercetin maximum). The antioxidant activity of all fractions of seed extract ranged from 21.43%–96.78%. Maximum antioxidant activity was found in AS-BF and range went down in respective pattern AS-BF > AS-EF > AS-CE > AS-DF > AS-CF > AS-AF, in AS-BF antioxidant activity percentage was found to be 52.13 ± 2.01% (Fig. 1.). Reducing power (RP) level ranged from 0.56 to 2.21 (Fig. 2A.). Maximum reducing power was shown in AS-BF, it was determined by the low value of ASE/ml (0.56) and other fractions having RP value respectively: AS-BF > AS-EF > AS-CE > AS-DF > AS-CF > AS-AF.
Fig. 1.
TPC (mg/g GAE dry extract), TFC (mg/gm Quercetin) and antioxidant activity (%) of different fractions of A. speciosa seeds. Values were expressed as mean ± SEM. a Significantly different (p < 0.001) from one another in the same columns. b Significantly different (p < 0.01) from one another in the same columns. ++Significantly different (p < 0.05) from one another in the same columns.
Fig. 2.
A) Reducing power (RP), DPPH and superoxide anion radical scavenging activity of different fractions of A. speciosa seeds. Values were expressed as mean ± SEM. Values showing the same letter are not significantly different (p > 0.05) from one other in the same columns. B) EC50 and ARP of different fractions of A. speciosa seeds. Values were expressed as mean ± SEM. Values showing the same letter are not significantly different (p > 0.05) from one other in the same columns.
Inhibitory concentration ranged from 0.032 to 1.20 mg/ml in all six fractions of A. speciosa seeds and effective concentration was 1.24–52.21 mg/mg DPPḢ and level of ARP was found as 1.87–79.68. Butanol fraction showed maximum DPPH scavenging activity and it was proved by the lower level of IC50 and EC50 and higher ARP (Fig. 2A.). Scavenging activity of all six fractions was expressed in the given order: AS-BF > AS-EF > AS-CE > AS-DF > AS-CF > AS-AF. Superoxide anion radical activity in AS-EF (0.17 mg/ml) and AS-BF (0.09 mg/ml) was determined very potent and in case of AS-DF, AS-CF, AS-AF and quercetin values were ranges as 0.72, 1.21, 1.83 and 0.03 mg/ml (Fig. 2B).
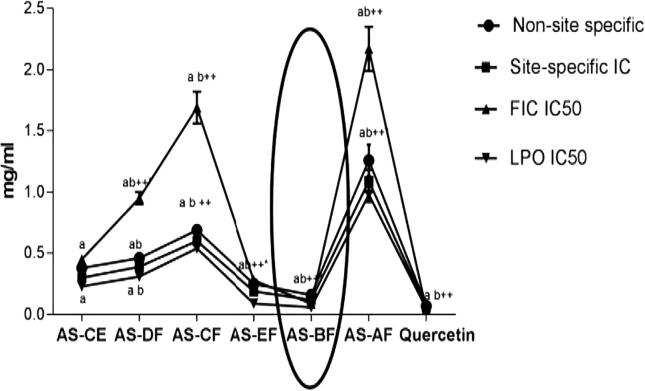
AS-BF showed the maximum value of non-site specific inhibition of hydroxyl radicals. The result was proved by the low value of IC50 (0.16 mg/ml) and data were analyzed for rest fractions as follows: AS-BF > AS-EF > AS-CE > AS-DF > AS-CF > AS-AF. These conditions proved that AS-BF work as metal chelates. Alcoholic fraction has minimum antioxidant property due to least IC50 1.21 mg/ml. Metal chelation ranged for all fraction as: AS-BF > AS-EF > AS-CE > AS-DF > AS-CF > AS-AF (Fig. 3). Chelating capacity was expressed as the value of IC50 and it was varied from 0.09 to 2.13 mg/ml (Fig. 3). MDA generation was inhibited by AS-BF in its concentration-dependent way and its IC50 value were decreased in this way: AS-BF (0.061 mg/ml) > AS-EF (0.09) > AS-CE (0.21 mg/ml) > AS-DF (0.29 mg/ml) > AS-CF (0.52 mg/ml) > AS-AF (0.98 mg/ml) (Fig. 3). From these data it was showed that butanol fraction has the best antioxidant activity in in vitro study as compared with other fractions. Henceforth, AS-BF was further used to determine protective oxidative damage and in vivo antioxidant property determination.
Fig. 3.
Effect of different fractions of A. speciosa seeds on hydroxyl radical scavenging activity, ferrous ion chelating (FIC) and ferrous sulphate induced lipid peroxidation in rat liver homogenate. Values were expressed as mean ± SEM. Values showing the same letter is not significantly different (p > 0.05) from one other in the same columns.
HPLC analysis was used for determining the presence of polyphenols: Gallic acid (556.7 μg/gm), kaempferol (497.6 μg/g), ferulic acid (128.2 μg/g), ellagic acid (199.1 μg/g), rutin (190 μg/g), quercetin (804 μg/g) and chlorogenic acid (482.5 μg/g) in AS-BF. Presence of these bioactive compounds was further proved by IR and MS analysis of seed extracts.
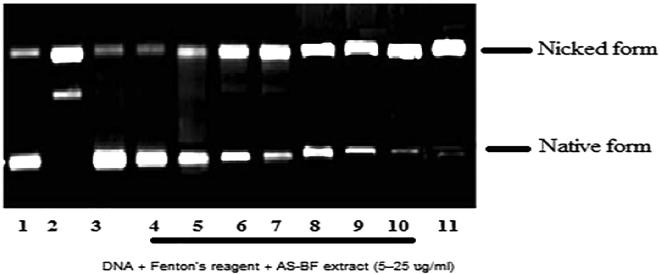
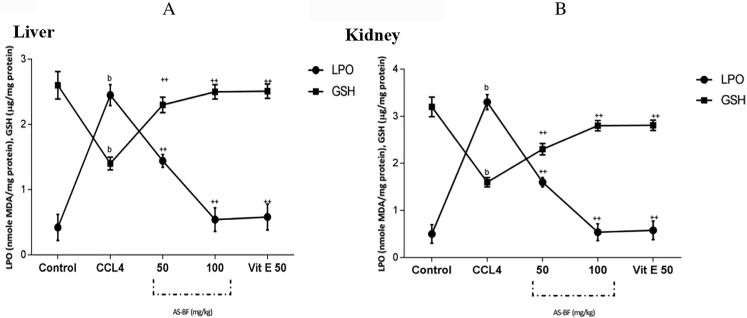
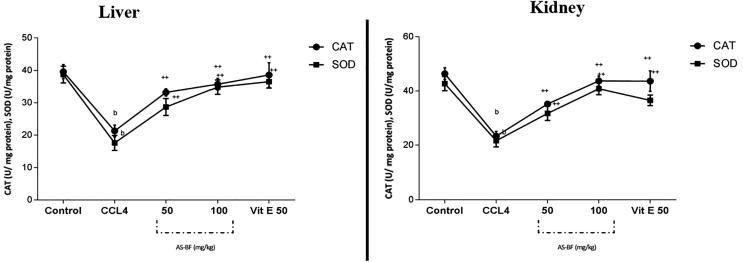
AS-BF (Lane 4 to 10) showed concentration-dependent DNA nicking protection it was found in the range 5–25 μg/ml. Lowest concentration and protection was at Lane 4 and the highest level of protection of DNA was at 10 μg/ml. Results were compared with 5U of Catalase at Lane 3 and standard quercetin 50 µM - Lane 11 (Fig. 4.). CCl4 intoxication leads to an increase in the concentration of LPO. Its significant coefficient value is<0.001, there is quite a reduction in GSH value (p < 0.001), CAT level (p < 0.001) and SOD content (p value is < 0.001) in different organs especially in kidney and liver as compared to healthy one (Fig. 5, Fig. 6). AS-BF treatment at dose 50 and 100 mg/kg showed strong effect against CCl4 intoxication and also inhibited increased content of LPO, increased SOD, GSH and CAT levels in CCl4 intoxicated rats with a significant coefficient (p < 0.001) (Fig. 5, Fig. 6).
Fig. 4.
Protective effect of AS-BF on super coiled DNA nicking caused by hydroxyl radicals. Lane (1) native pBR322 DNA; Lane (2) DNA + Fenton’s reagent; Lane (3) DNA + Fenton’s reagent + Catalase (5 units); Lane (4) – (10) DNA + Fenton’s reagent + AS-BF extract (5–25 µg/ml) and Lane (11) DNA + Fenton’s reagent + Quercetin (50 µM).
Fig. 5.
Effects of AS-BF on level of LPO (nmole MDA/mg protein) and glutathione (µg /mg protein) in liver (A) and kidney (B) of CCl4 intoxicated rats. Values were expressed as mean ± SEM; p value: b < 0.01 and ++ < 0.001 compared to respective control group; p value: b < 0.05, ++ < 0.01 and c < 0.001 compared to respective CCl4 treated group.
Fig. 6.
Effects of AS-BF on level of antioxidant enzymes CAT (U/mg protein) and SOD (U/mg protein) in liver (A) and kidney (B) of CCl4 intoxicated rats. Values were expressed as mean ± SEM; p value: b < 0.01 and ++ < 0.001 compared to respective control group; p value: b < 0.05, ++ < 0.01 and c < 0.001compared to respective CCl4 treated group.
4. Discussion
A. speciosa seeds and its six fractions were studied for their in vitro and in vivo antioxidant activity, at the same time their oxidative DNA nicking study was also performed. As compared to all five fractions of seed extract, butanol fraction showed the highest level of phenolic and flavonoid content. This was the one of the most important reasons for its strength in in vitro and in vivo antioxidant activity. Antioxidant phenolic acids were present in seed extracts and their results were proved by HPLC analysis.
Linoleic acid and beta-carotene were used to determine oxidative free radicals scavenging activity of A. speciosa. As β-carotene bleaching capacity was hindered by free radical neutralization showed the presence of different antioxidants (Juntachote and Berghofer, 2005). AS-BF represents strong antioxidant activity due to a decrease in β-carotene bleaching. Reducing power of A. speciosa seeds was determinant to its ability of electron transfer which is the strong determination of antioxidant activity (Chung et al., 2002). DPPH assay was determined at 517 nm and their scavenging property was determined by discoloration of DPPH. The highest level of RP and DPPH were shown in the butanol fraction of A. speciosa seeds.
From all findings, it was observed that AS-BF showed the highest scavenging activity as compared to other fractions. Some researches has proven that polyphenols are superoxide scavengers and have strong antioxidant activity (Benavente-García and Castillo, 2008). Hydroxyl free radicals initiate a chain type of reaction for the promotion of irreversible oxidation of bioactive molecules and propagating free radical regeneration. That is why deoxyribose assay was used for the confirmation of the antioxidant activity of AS-BF fraction. Both site-specific and non-site specific degeneration of free radicals were studied.
Antioxidant activity of A. speciosa seeds was further proved with the help of chelation capacity of ferrous ion and they are in vitro lipid peroxide formation from rat liver homogenate. Transition metals were well stabilized by chelating agents and follow the catalytic type of reaction (Moore and Yu, 2008). Lipid peroxidation assay was determined by MDA formation. This study represented that butanol fraction inhibited the level of MDA which was due to the absence of ferryl/perferryl complex (Govindarajan et al., 2003). From results, it was observed that AS-BF showed free radical scavenging activity at different concentration were observed at DNA damage of plasmid. A most important reason for DNA nicking may be due to phenolic contents (Sestili et al., 1998).
Free radical scavenging enzymes have the capacity to augment SOD and CAT activity to determine antioxidant activity of natural products (Cheeseman and Slater, 1993). In case of CCl4 induced toxicity, the antioxidant activity was altered, Cyt-P450 type of metabolic system was used for metabolism of CCl4 and it releases some radical like trichloromethyl (CCl3•). These radicals further undergo oxidation and they form trichloroperoxy (CCl3O2•) radical; these products cause lipid peroxidation which affect the defense mechanism of antioxidants (Ahr et al., 1982). AS-BF protected antioxidant degradation and decreased MDA level due to an increase in antioxidant activity in the liver and kidney. AS-BF leads to increased CAT and SOD levels in CCl4 induced rats and decreases in GSH levels. At a dose of 100 mg/kg, AS-BF showed the best finding as compared to vitamin E at dose 50 mg/kg. Form these results it was proven that AS-BF has strong antioxidant activity.
5. Conclusion
A. speciosa seeds having huge amounts of polyphenols that are responsible for free radicals scavenging property. Butanol fraction of A. speciosa seeds represented the strong chelating capacity for metal ions and they protected DNA damage. From an in vivo study, it was also proven that AS-BF prevents tissue damage which was due to oxidative stress. These findings promote the preventive effect of A. speciosa seeds and explain the traditional use of it in every day for healthy life.
Declaration of Competing Interest
None.
Acknowledgement
One of the authors Lubna Azmi is thankful to DST-INSPIRE (Department of Science & Technology) New Delhi for their support. All authors are thankful to the Director of CSIR-NBRI for providing necessary facilities.
Footnotes
Peer review under responsibility of King Saud University.
References
- Aebi H. Methods of Enzym Analysis. second ed. Academic press; New York: 1974. Catalase; pp. 673–684. [Google Scholar]
- Ahr H.J., King L.J., Nastainczyk W., Ullrich V. The mechanism of reductive dehalogenation of halothane by liver cytochrome P450. Biochem. Pharmacol. 1982;31:383–390. doi: 10.1016/0006-2952(82)90186-1. [DOI] [PubMed] [Google Scholar]
- Apati P., Szentmihalyi K., Kristo S.T., Papp I., Vinkler P., Szoke E., Kery A. Herbal remedies of Solidago Correlation of phytochemical characteristics and antioxidative properties. J. Pharma. Biomed. Anal. 2003;32:1045–1053. doi: 10.1016/s0731-7085(03)00207-3. [DOI] [PubMed] [Google Scholar]
- Benavente-García O., Castillo J. Update on uses and properties of citrus flavonoids: New findings in anticancer, cardiovascular, and anti-inflammatory activity. J. Agric. Food Chem. 2008;56:6185–6205. doi: 10.1021/jf8006568. [DOI] [PubMed] [Google Scholar]
- Bhagat J., Ingole B., Singh N. Glutathione S-transferase, catalase, superoxide dismutase, glutathione peroxidase, and lipid peroxidation as biomarkers of oxidative stress in snails: a review. Invertebr. Surviv. J. 2016 [Google Scholar]
- Benzie I.F.F. Evolution of dietary antioxidants. Comp Biochem. Physiol. Part A Mol. Integr. Physiol. 2003;136:113–126. doi: 10.1016/s1095-6433(02)00368-9. [DOI] [PubMed] [Google Scholar]
- Cheeseman K.H., Slater T.F. An introduction to free radical biochemistry. Br. Med. Bull. 1993;49:481–493. doi: 10.1093/oxfordjournals.bmb.a072625. [DOI] [PubMed] [Google Scholar]
- Chung Y.C., Chang C.T., Chao W.W., Lin C.F., Chou S.T. Antioxidative activity and safety of the 50% ethanolic extract from red bean fermented by Bacillus subtilis IMR-NK1. J. Agric. Food Chem. 2002;50:2454–2458. doi: 10.1021/jf011369q. [DOI] [PubMed] [Google Scholar]
- Decker E.A., Welch B. Role of ferritin as a lipid oxidation catalyst in muscle food†. J. Agric. Food Chem. 1990;38:674–677. [Google Scholar]
- Ellman G.L. Tissue sulfhydryl groups. Arch. Biochem. Biophys. 1959;82:70–77. doi: 10.1016/0003-9861(59)90090-6. [DOI] [PubMed] [Google Scholar]
- Emmons C.L., Peterson D.M. Antioxidant activity and phenolic contents of oat groats and hulls. Cereal Chem. 1999;76:902–906. [Google Scholar]
- Goel A.K., Kulshreshtha D.K., Dubey M.P., Rajendran S.M. Screening of Indian plants for biological activity: Part XVI. Indian J. Exp. Biol. 2002;40:812–827. [PubMed] [Google Scholar]
- Gokhale A.B., Damre A.S., Saraf M.N. Investigations into the immunomodulatory activity of Argyreia speciosa. J. Ethnopharmacol. 2003;84:109–114. doi: 10.1016/s0378-8741(02)00168-x. [DOI] [PubMed] [Google Scholar]
- Govindarajan R., Rastogi S., Vijayakumar M., Shirwaikar A., Rawat A.K.S., Mehrotra S., Pushpangadan P. Studies on the antioxidant activities of Desmodium gangeticum. Biol. Pharm. Bull. 2003;26:1424–1427. doi: 10.1248/bpb.26.1424. [DOI] [PubMed] [Google Scholar]
- Halliwell B., Gutteridge J.M.C. fifth ed. Oxford University Press; Oxford: 2015. Free radicals in biology and medecine. [Google Scholar]
- Hanumanthachar J., Navneet K., Jyotibala C. Evaluation of nootropic effect of argyreia speciosa in mice. J. Heal. Sci. 2007;53:382–388. [Google Scholar]
- Juntachote T., Berghofer E. Antioxidative properties and stability of ethanolic extracts of Holy basil and Galangal. Food Chem. 2005;92:193–202. [Google Scholar]
- Kakkar P., Das B., Viswanathan P.N. A modified spectrophotometric assay of superoxide dismutase. Indian J. Biochem. Biophys. 1984;21:130–132. [PubMed] [Google Scholar]
- Kroyer G.T. Red clover extract as antioxidant active and functional food ingredient. Innov. Food Sci. Emerg. Technol. 2004;5:101–105. [Google Scholar]
- Lee J.C., Kim H.R., Kim J., Jang Y.S. Antioxidant property of an ethanol extract of the stem of Opuntia ficus-indica var. saboten. J. Agric. Food Chem. 2002;50:6490–6496. doi: 10.1021/jf020388c. [DOI] [PubMed] [Google Scholar]
- Market Research Store, 2017. Global Antioxidants (Natural and Synthetic) Market Poised to Surge From USD 2.25 Billion in 2014 to USD 3.25 Billion by 2020, Growing at 5.5% CAGR. GlobalNewswire, El Segundo, CA. 19 January 2016. Retrieved 30 January 2017.
- Moore J., Yu L.L. Methods for antioxidant capacity estimation of wheat and wheat-based food products. Wheat Antioxidants. 2008:118–172. [Google Scholar]
- Nishikimi M., Appaji Rao N., Yagi K. The occurrence of superoxide anion in the reaction of reduced phenazine methosulfate and molecular oxygen. Biochem. Biophys. Res. Commun. 1972;46:849–854. doi: 10.1016/s0006-291x(72)80218-3. [DOI] [PubMed] [Google Scholar]
- Ohkawa H., Ohishi N., Yagi K. Assay for lipid peroxides in animal tissues by thiobarbituric acid reaction. Anal. Biochem. 1979;95:351–358. doi: 10.1016/0003-2697(79)90738-3. [DOI] [PubMed] [Google Scholar]
- Oyaizu M. Studies on products of browning reaction. Antioxidative activities of products of browning reaction prepared from glucosamine. Japanese J. Nutr. Diet. 1986;44:307–315. [Google Scholar]
- Pacher P., Beckman J.S., Liaudet L. Nitric oxide and peroxynitrite in health and disease. Physiol. Rev. 2007;87:315–424. doi: 10.1152/physrev.00029.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ragazzi E., Veronese G. Quantitative analysis of phenolic compounds after thin-layer chromatographic separation. J. Chromatogr. A. 1973;77:369–375. doi: 10.1016/s0021-9673(00)92204-0. [DOI] [PubMed] [Google Scholar]
- Rao C.V., Ojha S.K., Reddy G.D., Rawat A.K.S., Rao G.M.M., Pushpangadan P. Antidiarrhoeal activity of Argyreia speciosa flower: An ethnopharmacological study. Acta Pharm. Turc. 2004;46:149–159. [Google Scholar]
- Sarma A.D., Mallick A.R., Ghosh A. Free radicals and their role in different clinical conditions: An overview. Int. J. Pharma Sci. Res. 2010;1:185–192. [Google Scholar]
- Sahu Alakh N., Hemalatha S., Sairam K. HPTLC fingerprinting and in vitro antioxidant studies of argyreia speciosa sweet leaves and mesua ferrea linn. flowers. Int. J. Res. Ayurveda Pharm. 2013 [Google Scholar]
- Sestili P., Guidarelli A., Dacha M., Cantoni O. Quercetin prevents DNA single strand breakage and cytotoxicity caused by tert-butylhydroperoxide: Free radical scavenging versus iron chelating mechanism. Free Radic. Biol. Med. 1998;25:196–200. doi: 10.1016/s0891-5849(98)00040-9. [DOI] [PubMed] [Google Scholar]
- Shukla Y.N., Srivastava A., Kumar S., Kumar S. Phytotoxic and antimicrobial constituents of Argyreia speciosa and Oenothera biennis. J. Ethnopharmacol. 1999;67:241–245. doi: 10.1016/s0378-8741(99)00017-3. [DOI] [PubMed] [Google Scholar]
- Singh A., Dayal R., Ojha R., Mishra K. Phytocomponents of argyreia speciosa (Linn. f.) confer radioprotection. J. Radiat Cancer Res. 2017 [Google Scholar]
- Singh P., Kesharwani R.K., Keservani R.K. Sustained Energy for Enhanced Human Functions and Activity. 2017. Antioxidants and vitamins: roles in cellular function and metabolism. roles in cellular function and metabolism. [Google Scholar]
- Vlasova I.I. Peroxidase activity of human hemoproteins: keeping the fire under control. Molecules. 2018 doi: 10.3390/molecules23102561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yen G.C., Pin-Der D. Scavenging effect of methanolic extracts of peanut hulls on free-radical and active-oxygen species. J. Agric. Food Chem. 1994;42:629–632. [Google Scholar]