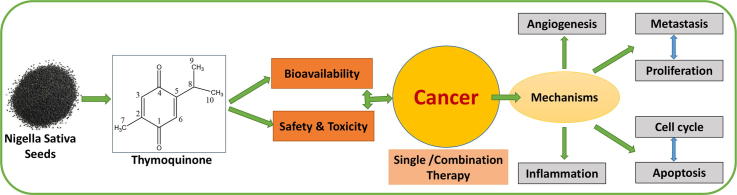
Graphical abstract
Keywords: Thymoquinone, Phytopharmaceuticals, Natural compounds, Anti-cancer therapeutics, Plant products
Abbreviations: TQ, thymoquinone; WHO, world health organization; THQ, thymohydroquinone; USD, United States Dollar; ROS, reactive oxygen species; RNS, reactive nitrogen species; PXRD, powder x-ray diffraction; FTIR, fourier-transform infrared spectroscopy; IUPAC, international union of pure and applied chemistry; NMR, nuclear magnetic resonance; SLNs, solid lipid nanoparticles; NLCs, nanostructured lipid carriers; NSAIDs, non-steroidal anti-inflammatory drugs; LPS, lipopolysaccharide; FGFs, fibroblast growth factors; TNFα, tumor necrosis factor alpha; VEGF, vascular endothelial growth factor; CDKs, cyclin-dependent kinases; TNBC, triple negative breast cancer; eEF-2K, elongation factor 2 kinase; EMT, epithelial to mesenchymal transition; TMZ, temozolomide; LKB1, liver kinase B1; AMPK, AMP-activated protein kinase; MC-A, myrtucommulone-A; RES, resveratrol; CDDP, cisplatin; SCLC, small cell lung carcinoma; OEC, oral epithelial cells; APC, adenomatous polyposis coli; XIAP, X-linked inhibitor of apoptosis protein; GBM, glioblastoma multiforme; HPDE, human pancreatic ductal epithelial cells; UMSCC, university of Michigan squamous cell carcinoma; PCNA, proliferating cell nuclear antigen
Abstract
Cancer remains the topmost disorders of the mankind and number of cases is unceasingly growing at unprecedented rates. Although the synthetic anti-cancer compounds still hold the largest market in the modern treatment of cancer, natural agents have always been tried and tested for potential anti-cancer properties. Thymoquinone (TQ), a monoterpene and main ingredient in the essential oil of Nigella sativa L. has got very eminent rankings in the traditional systems of medicine for its anti-cancer pharmacological properties. In this review we summarized the diverse aspects of TQ including its chemistry, biosynthesis, sources and pharmacological properties with a major concern being attributed to its anti-cancer efficacies. The role of TQ in different aspects involved in the pathogenesis of cancer like inflammation, angiogenesis, apoptosis, cell cycle regulation, proliferation, invasion and migration have been described. The mechanism of action of TQ in different cancer types has been briefly accounted. Other safety and toxicological aspects and some combination therapies involving TQ have also been touched. A detailed literature search was carried out using various online search engines like google scholar and pubmed regarding the available research and review accounts on thymoquinone upto may 2019. All the articles reporting significant addition to the activities of thymoquinone were selected. Additional information was acquired from ethno botanical literature focusing on thymoquinone. The compound has been the centre of attention for a long time period and researched regularly in quite considerable numbers for its various physicochemical, medicinal, biological and pharmacological perspectives. Thymoquinone is studied for various chemical and pharmacological activities and demonstrated promising anti-cancer potential. The reviewed reports confirmed the strong anti-cancer efficacy of thymoquinone. Further in-vitro and in-vivo research is strongly warranted regarding the complete exploration of thymoquinone in ethnopharmacological context.
1. Introduction
A wide variety of vegetables, fruits, spices and herbs have been consumed since distant past for a number of applications, including disease preventive and therapeutic, and are known to contain various bioactive compounds. Mother nature has bestowed mankind with a huge sum of contemporary therapeutic resources since the distant past. Plants are the natural source of many of the phytoconstituents as contemporary drugs which have found their place in modern therapeutics (Kaur et al., 2018). Of all the medical prescriptions today in developed countries like the US, almost 25–30% compounds are from the plant origin, which are employed for the treatment of various ailments in the contemporary practice of medicine. While in developing countries like India and China this data may reach to as much as 80%. Present estimation of world market of drugs obtained from plant sources may lie somewhere near 30,000 million USD (Roy et al., 2018). (See Table 1)
Table 1.
13C and 1H NMR spectra of thymoquinone in CDCl3.
| Designation | Type of carbon atom | 1H NMR signal δ [ppm], J [Hz] | 13C NMR signal δ [ppm] |
|---|---|---|---|
| C-1 | Cq | – | 188.6 |
| C-2 | Cq | – | 145.2 |
| C-3 | CH | 6.59 J = 1.58 |
133.8 |
| C-4 | Cq | – | 187.4 |
| C-5 | Cq | – | 155.0 |
| C-6 | CH | 6.52 J = 1.18 |
130.4 |
| C-7 | CH3 | 2.05 J = 1.58 |
15.4 |
| C-8 | CH | 3.03 J = 6.94, 1.18 |
26.5 |
| C-9/10 | CH3 | 1.13 J = 6.94 |
21.4 |
The plants which possess medicinal properties have always proven their worth and vitality for the benefits of mankind. Plants possess a vast assortment of exuberant and eternal resources of bioactive constituents which are applied for the treatment of numerous diseases. Some of the vital and eminent bioactive constituents of plants include alkaloids, glycosides, tannins, flavonoids, phenolic compounds, resins, lectins, etoposides, tanniposides, fatty acids, waxes, terpenoids, phenolic acids and polypropanoids, etc., (Sut et al., 2018, Ahmad et al., 2018a, Ahmad et al., 2018b). These bioactive constituents exhibit a wide array of medicinal and pharmacological activities like anti-tubercular, anti-cancer, anticoagulant, immunomodulatory, aldehyde oxidase inhibitors, anti-oxidant, antidiabetic, anti-inflammatory, influenza virus neuraminidase inhibitors, antibacterial, antifungal, antiplatelet, anti-degranulating, anti-trypanosomal activities (Rehman et al., 2019, Bhat et al., 2019).
Among these, Nigella sativa L. is one of the promising plant sources to a range of bioactive constituents like Thymoquinone, α-pinene, p-cymene, monoterpenes etc. Nigella sativa L. and its seeds are known by a number of other names like Nigella sativa L., black cumin, black caraway, nutmeg flower, fennel flower, Roman coriander and kalonji (Singh et al., 2017).
Thymoquinone (2-Isopropyl-5-methyl-1, 4-benzoquinone) is the bioactive constituent of the volatile oil of black cumin (Nigella sativa L.) seeds that has been extensively utilised traditionally in Middle East and Southeast Asian countries owing to its various health promoting capacities (Majdalawieh et al., 2017). It has been widely analyzed for its broad range of medicinal and pharmacological activities including but not limited to anti-inflammatory (Taka et al., 2018), anti-oxidant (Armutcu et al., 2018), antihistaminic (Kanter et al., 2006), antitumor (Singh et al., 2018), analgesic (Amin and Hosseinzadeh, 2016), anti-Alzheimer’s (Cascella et al., 2018), hepatoprotective, neuroprotective (Noorbakhsh et al., 2018), renoprotective, histone protein modulator (Rasheed et al., 2018), insecticidal (Scott et al., 2017), anti-ischemic (Bouhlel et al., 2017), Leishmanicidal (Mahmoudvand et al., 2015), radioprotective effects (Akyuz et al., 2017). TQ has also been clinically tested for a diverse kind of ailments like arthritis, diabetes, hypercholesterolemia etc. and is known for its eminent activity against a range of human carcinomas as it has negligible toxicity against normal cells. A number of studies have revealed the exploration of different molecular pathways for different cellular mechanisms for the therapeutic potentiality of TQ in the management of different types of metastatic tumors (Barkat et al., 2018). Multiple molecular targets are acted upon by TQ through different pathways, and also it shows its actions via many cellular mechanisms like proliferation inhibition, induction of apoptosis, cell cycle interruption, reactive oxygen species (ROS) production, and prevention of angiogenesis and cellular metastasis.
2. Chemistry
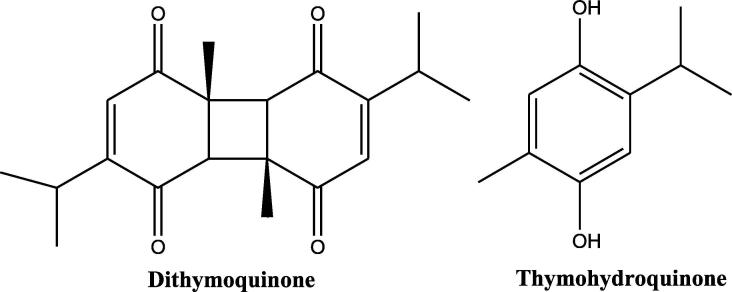
TQ is a non-toxic major bioactive compound obtained from the oil of black seeds of Nigella sativa L., has a chemical structure as shown in Fig. 2, and bears the chemical formula C10H12O2 and molecular weight of 164.204 g/mol. The concentration of TQ in the seed oil has been reported to be between 18 and 25 µg/mL. TQ exhibits important properties such as antimicrobial, analgesic and anti-inflammatory, protecting organs against oxidative damage, induced by an array of free radical producing agents, neuromodulatory, inhibition of eicosanoid generation and membrane lipid peroxidation, providing it the sufficient attraction for modern pharmacology (Glamočlija et al., 2018). In TGA analysis the thermal decomposition of TQ starts at 65 °C and finishes at ∼213 °C. Alkharfy et al.; report that TQ belongs to the monoterpenes class of natural compounds (Fig. 1) and has been reported to be showing keto-enol tautomerism. Specifically, TQ is a tautomeric compound where keto-form is the major configuration which exhibits pharmacological actions (Ahmad et al., 2018a, Ahmad et al., 2018b). TQ is a 10 carbon compound having a basic quinone ring moiety of 6 carbons while the 7th methyl group carbon at position C2 and 8th, 9th and 10th propyl group at position C5. Two of the most frequently synthesized close derivatives of TQ are dithymoquinone and thymohydroquinone (Fig. 3). Thymoquinone closely resembles basic monoterpene skeletal moiety with 10 carbon structure having the basic molecular formula C6H16 and composed of two isoprene units containing a ring (Fig. 1) having significant relevance to pharmaceutical, cosmetic, agricultural and food industries. High resolution powder x-ray diffraction (PXRD) and simulated annealing based molecular location method shows a triclinic unit cell with a = 6.73728(8) Å, b = 6.91560(8) Å, c = 10.4988(2) Å, α = 88.864(2)°, β = 82.449(1)°, γ = 77.0299(9)°; cell volume = 472.52(1) Å3, Z = 2. The UV–Visible absorption spectra of TQ shows the absorption maxima at λmax of 257 nm in physiological phosphate buffered saline pH 7.4, (Khalife and Lupidi, 2007) while its spectra in ethanol exhibits a π → π* transition spectra with a maxima at 252 nm with an extinction coefficient of 25000 cm2 × mmol−1 (Sicker et al., 2019). The FTIR spectrum of TQ establishes the presence of a carbonyl within a conjugate system by two specific bands at 1669 and 1621 cm−1 (because of Fermi resonance) and C-H strong stretching of aliphatic groups which are specific features in such p-benzoquinones, the weaker bands at higher wavenumber 3040 cm−1 represent the stretching observed in vinyl C-H in the C = C-H groups (Alwadei et al., 2019).
Fig. 2.
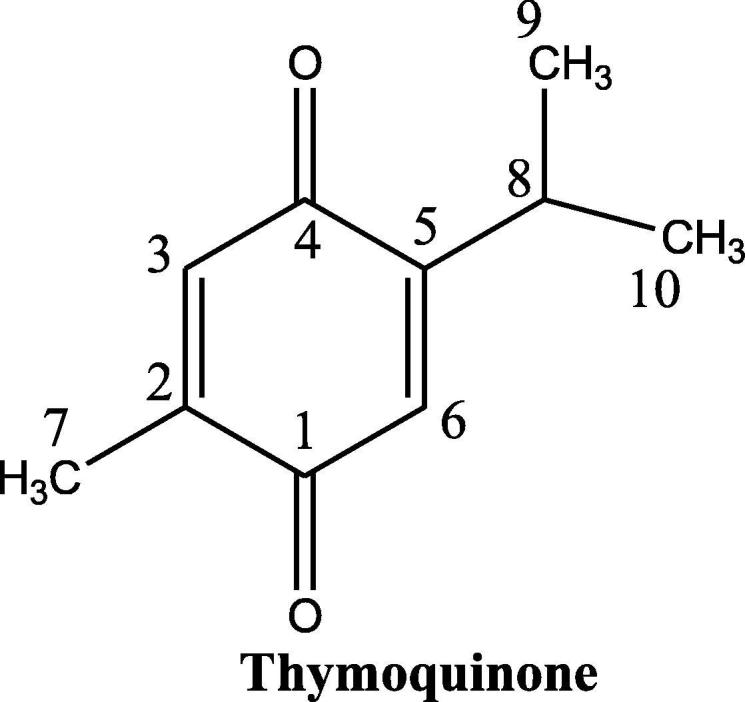
The chemical structure and atom numbering for Thymoquinone.
Fig. 1.
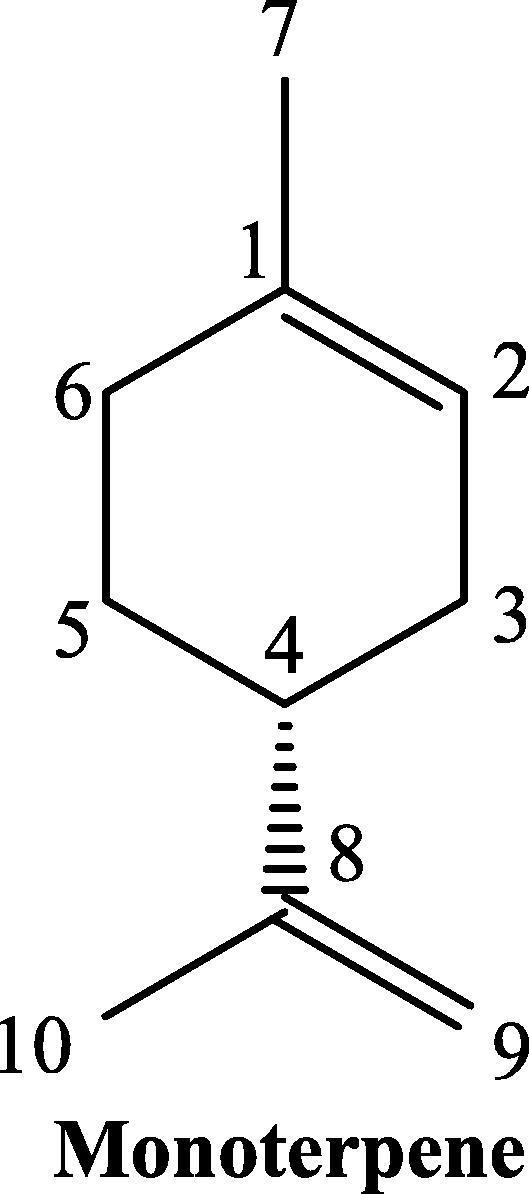
Basic chemical structure and atom numbering of monoterpene skeleton.
Fig. 3.
The chemical structure of close derivatives of thymoquinone.
2.1. Structural chemistry of thymoquinone
TQ is a 10 carbon compound having the IUPAC name 2-isopropyl-5methyl-1,4-benzoquinone. It is a solid bright yellow compound having scaly crystals with a melting point of 49–50 °C and gives a characteristic intense smell of pepper. The solubility of TQ varies in the range of 549–669 µg/mL in all aqueous solutions (Ahmad et al., 2018a, Ahmad et al., 2018b). TQ is unstable in aqueous solutions particularly at alkaline pH and also possess severe light sensitivity. It contains two carbon–carbon double bonds (C C) and two carbon-oxygen double bonds (C O). It possesses 0 hydrogen bond donor count, while hydrogen bond acceptor count is 2 and a rotatable bond count of 1 with the logP value of 2. The topological polar surface area of TQ is 34.1 A2 (Alwadei et al., 2019).
The NMR spectrum of TQ elucidates as, at δH = 1.13 gives the doublet for methyl groups 9 and 10 along with J = 6.94 Hz. Consequently, a singlet of methyl group for proton number 7 at δH = 2.04. The signal from the methine proton at eighth position of the isopropyl group comes as a septet. It exhibits an extra pairing with 6th proton of 1.18 Hz. The second one repeats in the olefinic doublet at δH = 6.52 that has to be attributed to 6th proton. 3rd proton at δH = 6.59 is shown as a quadruplet because of the pairing with the CH3 group 7th proton. The concept, that 6th proton is more protected than 3rd proton is because of the plus inductive effect (+I-effect) of the isopropyl functionality (Sicker et al., 2019).
The electro-ionization stimulated atomization of TQ is controlled by decarbonylation step and CH3-elimination, which in separate episodes tends to the most vivid ion having formula C7H9+ (m/z 93). The range of unsubstituted 1,4-benzoquinone is controlled by the voiding of ethyne from the molecular ionic part. If this was being employed to TQ, it would further extends to the exit of propyne group and 3-methylbutyne to impart C7H6O2+. m/z 124 and C5H4O2+. m/z 96, but, this does not happen. Any ionic part with m/z 124 cannot be detected and the ionic part with m/z 96 as per the determination of mass at high resolution has the constitution C6H8O+…. It is shaped after the first decarbonylation maneuver by the disintegration of the ionic part with mass by charge ratio of 136. However, if rather than that propyne, 3-methylbutyne is annihilated as a neutral part, then the similar C4H4O+. (m/z 68) is received. Successive decarbonylation results from m/z 68 or respectively 96 to the ionized part of alkynes C3H4+. (m/z 40) or respectively C5H8+. (M/z 68). The contribution of C5H8+. To the total intensity at m/z 68, which majorly comes from C4H4O+, is small (Sicker et al., 2019).
2.2. Biosynthesis of thymoquinone
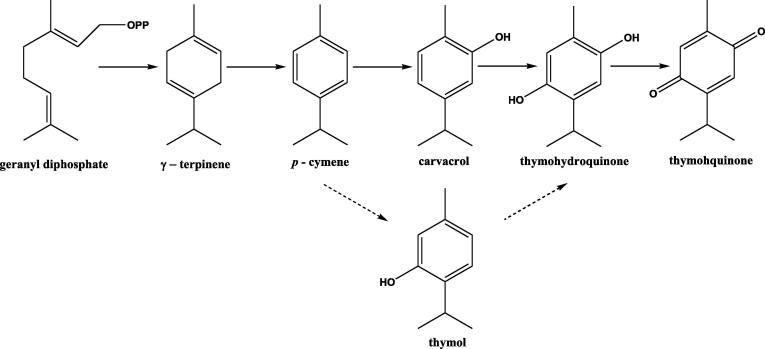
TQ obtained from Nigella sativa L. (Black Cumin), an annual herb frequently used in Middle Eastern countries and India has been gaining a worldwide acceptance presently. An extensive range of historical traditional and medicinal uses have been documented in the text. TQ is a secondary metabolite biosynthesized by plants mainly by terpene biosynthetic pathway. A striking similarity exists in the structures of different monoterpene components of Nigella sativa L. volatile oil and their presence in the species that accumulate similar compounds.
TQ belongs to the class of monoterpene compounds which are formally descended by condensation of two isoprene units. The secondary metabolism in plants predominantly leads to the formation of these, which can be isolated by steam distillation or solvent extraction of plant parts. The universally accepted “biogenic isoprene rule” generalizes that naturally occurring terpenoids are derived from either directly or by predictable stereospecific cyclization, rearrangement and dimerization from acyclic C-10 precursors like geraniol.
Geranyl diphosphate undergoes cyclization and forms γ-terpinene, which in turn is aromatized into p-cymene and then it is followed by hydroxylation to carvacrol. Further hydroxylation of carvacrol takes place and leads to the formation of thymohydroquinone. Thymohydroquinone undergoes oxidation and finally leads to the formation of TQ (Fig. 4). In some species and chemotypes which accumulate thymol in place of carvacrol, an alternate biosynthetic pathway occurs, where hydroxylation of p-cymene to thymol takes place. Monoterpene composition generally alters during the maturation of seeds. In immature seeds the major monoterpenes are γ-Terpinene and α-thujene and the former is slowly substituted by p-cymene, carvacrol, thymohydroquinone, and TQ as the seed development occurs. Zawirska Wojtasiak et al.; isolated a number of compounds from Nigella sativa L. essential oil and separated six pairs of enantiomers. They reported that the main odorant of Nigella sativa L. –thymoquinone and p-cymene – are not chiral (Zawirska-Wojtasiak et al., 2010).
Fig. 4.
A schematic diagram for the biosynthesis pathway of thymoquinone.
3. Source and occurrence of thymoquinone
TQ is a naturally occurring quinone derivative, commonly called black caraway seed or black cumin obtained from Nigella sativa L. of family Ranunculaceae. Nigella sativa L. is a flowering plant which is found in countries adjoining Mediterranean sea, India, Pakistan, and Iran. The plant has a varying composition of many constituents like fixed oils, volatile oils, alkaloids, saponins, coumarins, minerals, fibers etc (Mashayekhi-Sardoo et al., 2018). Nigella sativa L. contains the bioactive compound TQ which constitutes like 54% of the volatile oil in the annual herbal flowering plant. Traditionally it has been used as a spice, but also as a medicine. Certain essential fatty acids are also present in the triglycerides of oil but TQ is the main constituent (Ong et al., 2016). Thymoquinone is obtained as a result of the oxidation of thymol, which is also a natural product, in the presence of manganese oxide in acidic medium to thymol. The quantity of essential oil in the oil of black seed varies between 0.5 and 1% and approximately one-third of it is TQ which gives yellow color to the black seed oil (Karandrea et al., 2017). TQ is also reported in other plants such as Nepeta Leucophylla, Tetraclinis articulata, Juniperus Cedrus, Callitris quadrivalvis, Monarda fistulosa etc. It has also been reported in genus Tetraclinis, Cupressus, and Juniperus of the Cupressaceae family (Hou et al., 2018).
Besides Ranunculaceae, where only minute quantities of TQ are found, its presence has also been confirmed in other genera of Laminaceae family like Agastache, Coridothymus, Monarda, Mosla, Origanum, Satureja, Thymbra, and Thymus (Hou et al., 2018). In plants, TQ is found along with its dimeric and reduced forms dithymoquinone and thymohydroquinone, the latter being a compound with significant biological activities (Imran et al., 2018). Similar to TQ, THQ is found in a number of genera of the family like Coridothymus, Origanum, Monarda, Mosla, Satureja, and Thymus (Taborsky et al., 2012).
4. Bioavailability, safety and toxicity
The rate and extent of a drug which reaches the systemic circulation from the site of administration is known as bioavailability of that particular drug. It is the major factor to be considered for good therapeutic efficacy of the drug (Beg et al., 2011). Among various physicochemical parameters which affect the bioavailability of drug or its dosage form, solubility is one of the major parameter that plays a crucial role to determine the bioavailability of any drug moiety. To enhance the bioavailability and efficacy of TQ people are trying various nanoformulations, i.e., liposomes, solid lipid nanoparticles (SLNs), niosomes, nanostructured lipid carriers (NLCs), nanoemulsions etc. When self nanoemulsifying drug delivery system of TQ was prepared, its bioavailability has been increased 3.87 fold in comparison to TQ suspension (Tubesha et al., 2013, Surekha and Sumathi, 2016, Goyal et al., 2017, Kalam et al., 2017). The bioavailability of TQ has been reported to get enhanced 5 times by SLN preparation in comparison to conventional formulations when tested on albino wistar rats (Singh et al., 2013). In an in vivo study performed on albino Wistar rats TQ shows enhanced bioavailability in NLC formulation when compared to suspension by 3.97 fold with increased plasma half-life (Elmowafy et al., 2016).
Toxicity studies have immense importance in the safety assessment of new compound before these get tested in human trials. In this regard, the toxic effects of the drug on laboratory animals have been tested and then the similar effect has been assessed on human individuals. The LD50 (lethal dose) of TQ is dependent on route of administration and vehicle used because of its insoluble nature in water. It also varies based on the type of rodent, i.e., mice and rats mainly. The LD50 of TQ on oral administration in mice was 870.9 mg/kg and after i.p. (intraperitoneal) injection, it became 104.7 mg/kg. The same has been reported in rats as 794.3 mg/kg and 57.5 mg/kg after oral and i.p. administration respectively (Tubesha et al., 2013, Al-Ali et al., 2008, Abukhader, 2012). The toxicity signs after oral administration of TQ reported are difficulty in respiration (dyspnoea) and peritonitis after i.p. injection in rats and mice (Abdelwahab et al., 2013). However, it is also reported that on long term administration of TQ alone or TQ-NLC causes liver toxicity but at the tolerable dose, it does not affects the organ function. They authors also concluded that the NLC formation of TQ increased the tolerability of TQ alone in their experiment upto 100 mg/kg (Abdelwahab et al., 2013, Ong et al., 2016).
5. Thymoquinone and inflammation
Inflammation is the first line of body defense mechanism from noxious external stimuli. The ultimate purpose of this reaction is to protect the organism from the initial injury and then the result of it. When these inflammatory reactions are not controlled by natural physiological processes then it may cause inflammatory diseases which are commonly spread over worldwide. For the treatment of these diseases, there are two categories of drugs are used, i.e., steroidal anti-inflammatory drugs and non-steroidal anti-inflammatory drugs (NSAIDs). Steroidal drugs are proven potent for inflammatory diseases but prolonged use may impact serious adverse effects. On the other hand prolonged use of NSAIDs also leads to side effects (Ansari et al., 2019).
Due to the above mentioned troubles associated with these synthetic drugs, there is a strong need for research on natural products which are justified as safer. TQ is one of the principle constituent of Nigella sativa L., a herb which is used in the traditional system of Indian medicines for the treatment of various systems of inflammatory conditions. TQ is reported to inhibit eicosanoids productions mainly thromboxane B and leukotrienes B4 by affecting the level of COX and LOX respectively. These enzymes are the main factor in the inflammatory pathway and act by expressing different inflammatory cytokines which cause oxidative stress and infiltration of neutrophils and macrophages and finally lead to tissue damage. TQ has a role in inhibition of nitric oxide production by macrophages which proves its anti-inflammatory action (Paarakh, 2010, Pise and Padwal, 2017). Reports also suggested that it has an activity to preserve the action of antioxidant enzymes such as catalase, glutathione peroxidase, and glutathione-S-transferase and acts as free radical as well as superoxide radical scavenger (Jain et al., 2017). There are numerous cytokines such as TNF-α, IL-1, IL-2, IL-6, and IL-10 which have a considerable role in inflammation. Among these TNF-α and IL-1 is important for the overexpression of other pro-inflammatory cytokines (IL-6 and IL-8), reactive oxygen/nitrogen species (ROS/RNS) and lipid mediators which may cause organ failure while sepsis. The level of these cytokines is modulated in sepsis when treated with TQ. Apart from this NF-κB also play an important role in sepsis by upsurging the level of proinflammatory cytokines. TQ also has an inhibitory effect on NF-κB during sepsis such that it inhibits the production of proinflammatory cytokines thereby decreasing the infiltration of inflammatory cells and showed protective action against tissue and organ damage (Alkharfy et al., 2018).
Oxidative stress in systemic inflammation has also been reported with a critical role in memory and learning impairment. Studies also suggested that neuro-inflammation induced by lipopolysaccharide (LPS) causes impairment of learning and memory through release of pro-inflammatory cytokines and over production of ROS. In a mouse model, learning and memory impairment induced by LPS was associated with an increased level of TNF-α and IL-6 in hippocampal and IL-6 in cortical tissue. Administration of TQ is associated with a reduction of hippocampal TNF-α and IL-6 levels and also TQ exhibited its anti-inflammatory activity via suppressing NF-κB and inhibition of cytokine production. In this study authors explained that the neuroprotective effects of TQ are expressed in terms of the improvement in learning and memory in the LPS induced model has occurred (Bargi et al., 2017).
6. Thymoquinone and angiogenesis
The formation of new blood vessels or the development of blood vessels is known as angiogenesis. More strictly it is also known as the development of blood vessels from pre-existing vessels (Potente et al., 2011). It is controlled by some factors such as fibroblast growth factors (FGFa and FGFb), transforming growth factor (TGF-α and TGF-β), hepatocyte growth factor (HGF), tumor necrosis factor (TNF-α), angiogenin, interleukin-8, and angiopoietins. Among all these, one of the most important factors that contributes to angiogenesis is vascular endothelial growth factor (VEGF). In in-vitro condition VEGF stimulates the endothelial cell growth which is mainly originated from veins, arteries and lymph drainage vessels. It is an endurance mechanism for the endothelial origin cells in vitro as well as in vivo circumstances (Karamysheva, 2008).
TQ treatment significantly decreases angiogenesis via regulating the signal of VEGF through Akt and extracellular receptor kinases pathway (Mercan et al., 2018) (Peng et al., 2013). TQ reduces the tumor blood vessels in human prostate cancer (PC3)53 and osteosarcoma (SaOS-2) cells xenograft model in nude mice. In osteosarcoma it suppresses the angiogenesis markers VEGF and CD34. In the same research it is also demonstrated that it affects the angiogenesis by acting on NF-κB. Some reports are also available for antiangiogenic activity of TQ with the same VEGF mechanistic activity in different cell lines (Peng et al., 2013, Paramasivam et al., 2012, Kundu et al., 2014, ElKhoely et al., 2015, Ozturk et al., 2018). Inhibition of VEGF not only directly inhibits the angiogenesis but also it causes the necrosis in that particular area of tumor development and in this way it may hamper the blood vessel formation (Ahmad et al., 2019). This effect was observed in the combination treatment of TQ with resveratrol (Alobaedi et al., 2017).
In 2008 Tingfang Yi et al. Reported that thymoquinone hinders with the angiogenesis which is a vital manoeuvre in tumor growth and metastasis. They explained that TQ downregulated the AKT activation and extracellular signal-regulated kinase and inhibits angiogenesis in-vitro and in-vivo. It also stops the tumor angiogenesis in xenograft human prostate cancer (PC3) mice model (Yi et al., 2008). Another report by Woo et al. reports that TQ exerts its anti-tumor effects via diverse mechanisms including the inhibition of angiogenesis, and through a number of molecular targets like p53, p73, STAT3 and PPAR-ϒ etc. Co-administration of TQ with Tamoxifen was assessed for its anti-angiogenic potential via regulation of a number of molecular signalling targets viz. Akt, XIAP, PI3-K-AKT, PARP etc. and was reported to cause tumor necrosis and tumor growth arrest through angiogenesis inhibition in MCF-7 breast tumor xenograft (Woo et al., 2012).
In 2010, Banerjee et al., reviewed the molecular and therapeutic potential of thymoquinone in cancer and explained the anti-inflammatory and anti-tumor potential of thymoquinone. They suggested that TQ inhibits tumor cell proliferation via regulation of apoptosis signalling, angiogenesis inhibition and arresting of cell cycle (Banerjee et al., 2010). Another report suggests that TQ activates the tumor suppressor genes and also inhibits angiogenesis process by activation of some significant motifs of angiogenesis (Kensara et al., 2016).
TQ also inhibits endothelial cell migration and as seen in the HUVEC cell migration inhibition in a dose dependent manner. The authors also report that suppression of VEGF production and thus prevention of tumor growth through anti-tumor angiogenesis by TQ is justified by the significantly decreased serum concentration of VEGF in TQ treated group compared to normal control. TQ also exerts its anti-angiogenesis effects via NF-κB pathway and its downstream molecules, by inhibition of HUVEC differentiation into tube like structure, decreasing the tube formation capacity by endothelial cells. TQ exerts its effects on COX2 expression and prostaglandins production in mouse model, and overexpression of COX2 has a significant part in the angiogenesis upregulation (Rahmani et al., 2014). The effect of TQ on angiogenesis was studied by CD31 immunostaining in lung cancer xenograft in nude mice, where the authors report that the antitumor effects of thymoquinone are due to the anti-apototic effects and not because of the anti-angiogenesis effects as no significant neoangiogenesis was observed between the TQ treated group and control LNM35 tumor xenograft (Attoub et al., 2013).
7. Thymoquinone and apoptosis
Apoptosis is programmed cell death in response to pathological or physiological changes which are directed to eliminate the dead, mutated or aged cell. In other words, it can be said that it is the cleaning pathway of a biological system from dead cells that may cause a potential health threat to the body if not removed. There are two main pathways of apoptosis - first, intrinsic pathway/mitochondrial pathway which is regulated by Bcl protein family. In this pathway initially, various stimuli trigger the increment of mitochondrial membrane permeability and finally releases apoptogenic factors that cause the membrane disruption and mitochondrial dysfunction. This dysfunction activates various apoptogenic proteases, e.g., caspases. Second, these caspases are also activated by the formation of a death receptor at the cellular surface (Baig et al., 2016). Drugs acting on either pathway of apoptosis are currently focused on anticancer therapy. For example activities of caspases like caspase-3, caspase-9 or cleaved caspase -3 are studied as hall marks of apoptosis in cancer cells (Gupta et al., 2018a, Gupta et al., 2018b). Treatment of TQ in spinal cord injury reduces the activity of caspase-3 and caspase-9 and inhibited apoptosis in an animal model (Chen et al., 2018) and the same mechanism has also been reported in gastric carcinoma (Gali-Muhtasib et al., 2004).
Also cell may undergo DNA damage which may be repairable or irrepairable which implies that either DNA damage is repaired correctly or may be rendered as irrepairable (Gupta et al., 2018a, Gupta et al., 2018b). When cells undergo DNA damage and if it is unrepairable then apoptosis may occur which is attributed through the treatment of TQ on glioblastoma cell. In the same study author conclude that TQ induced apoptosis via the elevated level of Bax and cytochrome c proteins. They have also shown that TQ induces apoptosis through p53 independent pathway (Gurung et al., 2010). In contrast to that, the authors also reported that p53 dependent pathway may also get involved in apoptosis. Although in this study they reported that the apoptosis induced by TQ was concentration dependent which involves p53 elevation as well as down regulation of Bcl-2. Multiple pathways are reported for the induction of apoptosis by TQ treatment which are Jak-STAT, Akt, Notch, ERK-JNK, and NF-κB. In bladder cancer cell lines TQ induces apoptosis through endoplasmic reticulum mediated mitochondrial pathway in which increased ratio of Bax/Bcl-2 as well as cytochrome-c was found (Zhang et al., 2018). In another study, performed in the prostate cancer cell line (DU-145) the apoptotic activity of TQ has been reported and was due to ROS mediation. In the same study, TQ also showed synergistic activity in combination with zoledronic acid (Dirican et al., 2014). It showed the apoptotic effect in breast cancer cell line (T47D) in combination with gemcitabine as well as alone (Bashmail et al., 2018).
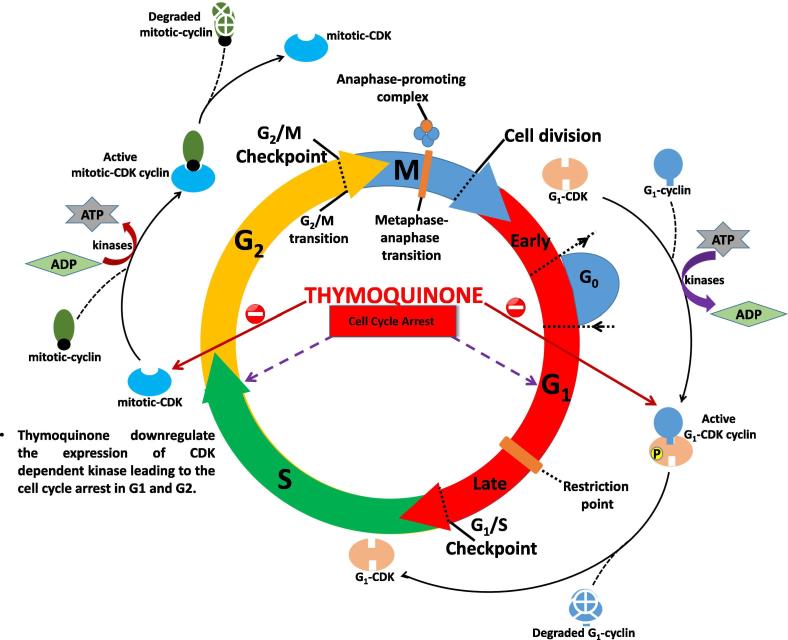
8. Thymoquinone and cell cycle regulation
The cell cycle of eukaryotes is divided into two major event DNA replication (S-phase) and mitosis (M-phase) with G1 and G2 gap events. All events are correlated in such a way to ensure the sequential regeneration of cell cycle events, and all these events are conserved throughout eukaryotes. In all eukaryotes, cyclin-dependent kinase (CDK) plays a crucial role and is highly conserved molecules responsible in the cell cycle (Sexton et al., 2018). CDK controls both cell cycle transition event as G1/S transition and G2/M transition. In yeast CDK encoded by CDC28 and mutation blocked the cell cycle progression at G1-phase by which cells enters in the sexual cycle instead of the mitotic cycle (Fig. 5). CDKs require positive regulatory partners for activity, termed as cyclins and identified as proteins that fluctuate in bulk via cell cycle in progressive cleaving of early embryonic cells.
Fig. 5.
Role of thymoquinone in cell cycle regulation.
Cyclin-dependent kinase resides a serine/threonine-specific catalytic core with regulatory subunits as cyclins, controls kinase activity, and substrate specificity. The first evidence of the role of Cdk/cyclin complex in cell cycle control was noted in yeast. Further evidence was found by researchers that Cdk responsible for cell cycle progression and cyclins manages the transition between cell cycle phases (Lim and Kaldis, 2013). In every tumor cells the machinery that controls the process of cells to forward from resting to cell cycle are found altered (Li et al., 2010).
Thymoquinone downregulates interleukin-6 downstream inducible STAT3 stimulation in multiple melanoma cells in humans along with the supressing the c-Src and JAK2 stimulation including appearance of STAT3 regulated genetic consequences, like-cyclin D1, Bcl-2, Bcl-xL, surviving, Mcl-1, and VEGF. It has also been reported to inhibit the proliferation, accumulation of cancer cells in the sub-G1 phase and apoptotic phase (Li et al., 2010).
TQ potentiates natural killer cells activity and showed potent toxicity in WEHI-3 cell line, seen as the increment of early apoptosis, upregulation of anti-apoptotic protein, Bcl2, and downregulates the apoptotic protein, Bax. In the experiment, where both treated and untreated cells were analyzed for cell cycle distribution, it confirmed that TQ promotes decrement in the S-phase cells. The significant increment in the apoptosis is seen at 24 and 48 hr of treatment, confirms S-phase arrest in a time-dependent manner (Salim et al., 2014).
Antineoplastic activity of TQ was observed against multifarious tumors. DNA concentrations in thymoquinone treated T47D and MDA-MB-468 cells were evaluated for cell cycle distribution. Cell cycle arrest at the G1 phase was observed within 12 and 24 hr incubation. Whereas 25% increment in the first G1 cell cycle phase was found in all types of breast cancer cells within 24 hr treatment with TQ followed by changing of G1 cell phase halt to sub G1 cell cycle phase towards apoptotic cell death within 48 hr treatment (Rajput et al., 2013a, Rajput et al., 2013b).
TQ effect on cell proliferation, apoptosis and signalling pathways in HCT116 and HCT116p53-/- colon cancer and HepG2 hepatoma cells in vitro was observed by Wirries et al. and they found that HCT116 cells were most sensitive towards TQ treatment (10 uM) induces shifting of cell cycle distribution in S/G2 phase after 72 and 96 hr treatment. The increment of 67.8 to 89.1% and from 62.4 to 88.6% after 72 or 96 hr incubation with TQ (Wirries et al., 2010).
9. Thymoquinone and proliferation/invasion/ migration
The effect of TQ on the expression of eukaryotic elongation factor-2 kinase (eEF-2K) was explored in search for the new molecular target in Triple negative breast cancer (TNBC). The study revealed that TQ inhibits cell proliferation, migration/invasion and tumor growth in TNBC via NF-kB/miR-603/eFF-2K signalling axis, eEF-2K promotes tumor growth and progression by modulating the activity of Src, FAK, PI3K/Akt, c-Myc and cyclinD1 (Kabil et al., 2018).
TQ potential was evaluated on SiHa and CaSki cervical cancer cell lines in the modulation of epithelial to mesenchymal transition (EMT)-regulated proteins and cancer metastasis. TQ showed time-dependent and dose-dependent cytotoxic effect and restricts migration and invasion of cervical cancer cells. TQ restricts the expression of Twist1, Zeb1 expression and upregulates E-Cadherin expression and this inhibition strongly suggests that TQ directly targets the Twist1 and Zeb1. The decrement in the activity of Twist1 and Zeb1 promotes assertions that TQ might be the main target of TQ. So, TQ inhibitory effects in metastasis of cervical cancer cells are directly caused by Twist1/E-Cadherin/EMT or/and Zeb1/E-Cadherin/EMT signalling pathway (Li et al., 2017).
TQ effect was evaluated on cell proliferation, migration, invasion and anti-metastatic effect in lung cancer cell line A549 cells. Wound healing assay suggests dose-dependent increase in the inhibition of cell migration after 24, 48 or 72 hr in A549 cells. At 40 µM/L for 24, 48 or 72 hr as compared with negative control, cell migration inhibition follows time-dependent increase in A549 cells. Transwell invasion assay suggests dose-dependent increase in cellular invasion inhibition rate as compared to negative control after 24, 48 or 72 hr in A549 cells. Similarly at 20 µM/L dose of TQ shows time-dependent increment in inhibition of invasion after 24, 48 or 72 hr in A549 cells (Yang et al., 2015).
Synergistic effect of TQ and temozolomide (TMZ) was observed in human glioblastoma multiforme cell line (U87MG). The TMZ is an alkylating agent and develops resistance in the treatment of cancer. Combination of TMZ and TQ showed the synergistic effect and inhibit 77% cell migration restricts the invasion of cancer cells by 43% leading to restrict the proliferation of cells in a time and dose-dependent fashion and inhibit the expression of MMP-2 and MMP-9 required for glioma malignancy (Pazhouhi et al., 2018).
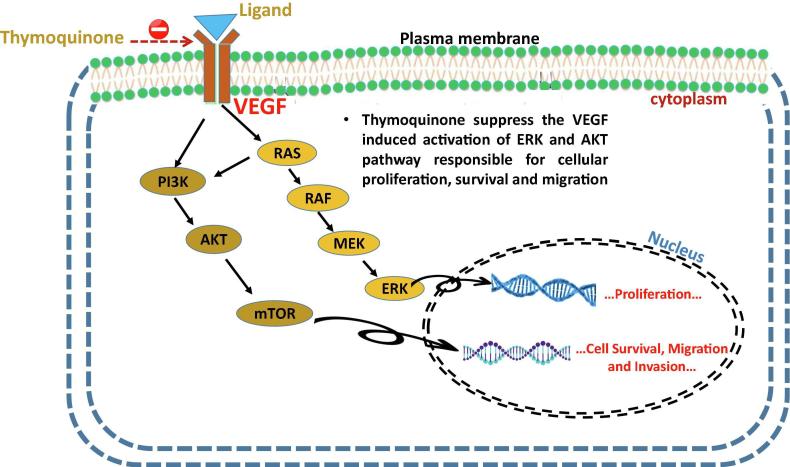
TQ restricts the expression of AKT and extracellular signal-regulated kinase signalling pathway leading to the inhibition of angiogenesis and development of the tumor. The study was performed in human umbilical vein endothelial cell for justifying the role of TQ in migration, invasion and tube formation properties of these endothelial cells. The results indicated that, these endothelial cells exhibited inhibition of proliferation and restrict the activation of AKT and extracellular signal-regulated kinase. In a wound-healing assay performed in HUVEC cells, it was found that TQ inhibits HUVEC migration in a concentration-dependent manner and in transwell invasion assay TQ significantly restricts invasion of cells at 80–100 nM/L. TQ efficiently suppresses the activation of VEGF-induced ERK and AKT excluding inhibition of VEGFR2 (Fig. 6). Both AKT and ERK play a critical role in mediating cellular proliferation, survival and migration and TQ efficiently inhibits the activation of both AKT and ERK in endothelial cells (Yi et al., 2008).
Fig. 6.
Inhibition of VEGF receptors by thymoquinone leading to the downregulation of tumor metastasis.
Another study performed in renal cell carcinoma for determining the efficacy of thymoquinone on the migration and invasion of cancer cell, confirmed the inhibition of migrating and invasive ability of human RCC 769-P and 786-O cell lines. Results showed that TQ potentiates E-cadherin expression and downregulates the mRNA and protein level expression of Snail, ZEB1 and vimentin in a dose-dependent fashion. It also upregulates the phosphorylation levels of liver kinase B1 (LKB1) and AMP-activated protein kinase (AMPK). TQ showed nonsignificant changes in normal renal tubular epithelial HK2 cell line upon 24 hr; however slight decrement in the growth observed after 48 and 72 hr suggests low cytotoxicity over normal epithelial cells but concentration-dependent effect of TQ was observed over renal cell carcinoma cells in proliferation and migration of cancer cells. The migration of 769-P and 786-O cells significantly diminished and treatment of TQ with 10 uM for 24 hr significantly reduced the invasiveness of 769-P and 786-O cells. LKB1/AMPK signalling is required for the cancer metastasis and a concentration-dependent TQ treatment increases the phosphorylation level of both LKB1 and AMPK upon in both the cancer cell lines (Kou et al., 2018).
10. Thymoquinone and metastasis
Although the anti-metastatic effects of thymoquinone have been reported in numerous studies, the exact mechanism of action is not well understood. TQ showed remarkable suppression of metastatic phenotype and inverse transition from epithelial to mesenchymal portion in carcinoma of renal cells by modulating LKB1/AMPK signalling pathway. Enhanced expression of LKB1 and AMPK was observed in western blot results upon TQ treatment, also, upregulation in the phosphorylation of LKB1 and AMPK was shown in the 769-p cell line. A concentration-dependent enhancement in phosphorylation of LKB1 and AMPK was shown over 24 hr TQ treatment in 786-O cells. Upregulation in the expression of LKB1 enhanced the E-cadherin expression and reduces the Snail expression upon thymoquinone treatment in 786-O and 769-P cells (Chen et al., 2017, Kou et al., 2018).
TQ efficiently reduced phosphorylation of IKKα/β and NF-κB and reduced metastasis of CPT-11-R cells. Irinotecan resistant cells (CPT-11-R) LoVo colon cancer cell line upon TQ treatment showed the diminished activity of ERK1/2 and PI3K and increased activity of JNK and p38. NF-κB activation induces metastasis in CPT-11-R cells. Gelatin zymography, invasion assay, migration assay, and western blot showed dose-dependent reduction in the protein level of EMT marker, MMP-2 and MMP-9 upon TQ treatment. TQ treatment increases phosphorylation of JNK and p38, MAPKs, and reduction in the activity of ERK1/2 and PI3K (Chen et al., 2017, Mu et al., 2015).
Ahmad et al., studied the anti-, metastasis effects of thymoquinone on human A375 and mouse B16F10 melanoma cell lines and tried to evaluate whether TQ inhibits metastasis of melanoma cells by targeting NLRP3 subunits of inflammasomes. Their results suggested that TQ may prove to be vital immunotherapeutic agent both alone and in combination therapy for melanoma in the control and prevention of metastasis (Ahmad et al., 2013). In this study, 10 and 20 mg/kg body weight of thymoquinone significantly inhibited the metastatic tumor nodule formation in lungs, as compared to the vehicle control treated group.
Khan et al., in 2015 studied the effects of TQ on cancer metastasis inhibition by downregulation of TWIST1 expression and suggested that reduction of epithelial to mesenchymal transition is the key mechanism involved in that. The study was carried out in cancer cell line on EMT regulatory proteins and TQ was used in dose dependent manner to reduce the transcriptional activity of the TWIST1 promoter and its mRNA expression. The authors showed that TQ treatment reduced the expression of TWIST1 gene and inhibited cancer cell metastasis in cancer cells in xenograft tumors in mice (Khan et al., 2015). The authors later reported that TQ leads to NF-κB inhibition, reduced expression of p38 MPAK through ROS generation and reduces the cancer cell metastasis by downregulation of EMT and inhibition of serine/threonine kinases Plk1 (Khan et al., 2017).
Another study was performed to assess the anti-metastatic efficacy of thymoquinone on the pancreatic cancer in vitro and in vivo. Human pancreatic cancer simulating metastatic model was established where histologically intact pancreatic tumor tissue was orthotopically implanted into the pancreatic wall of nude mice. TQ treatment significantly decreased the tumor metastasis in comparison to normal control. It also led to the reduction in NF-κB and MMP9 expression in tumor tissues and established the anti-metastatic potential of TQ on pancreatic cancer (Wu et al., 2011).
Mostofa et al., recently reviewed the potential of TQ as an adjuvant therapy in the treatment of cancer and provided evidences from preclinical studies and suggested that in addition to the cell death and tumor growth inhibitory potential, TQ also hinders in the many other diverse tumorigenic processes like angiogenesis, invasion and metastasis via metastatic signalling pathways. TQ treatment downregulates a number of signalling molecules like TGF-β, VEGF, FCF, EGF, metastatic factors, Nrf-2, STAT-3, that are responsible for and lead to transcriptional activation of genes involved in metastasis (Mostofa et al., 2017).
11. Combinational therapy using thymoquinone
The insensitivity of gemcitabine in pancreatic cancer was overcome by pre-treatment of pancreatic cancer cell lines PANC-1, AsPC-1 and BxPC-3 cells with TQ. The synergistic effect shows potent antitumor effect via Notch1/PI3K/Akt/mTOR regulated signalling. Pre-treatment with TQ downregulates the anti-apoptotic Bcl-2, Bcl-xL, XIAP and upregulates pro-apoptotic molecules like caspase-3, caspase-9, Bax and enhances the release of cytochrome-c (Mu et al., 2015, Iskender et al., 2016).
Epithelial to mesenchymal transition (EMT) pertains to the progression and metastasis in cancer. The role of TQ and myrtucommulone-A (MC-A) in EMT showed negative regulation of EMT process via cancer cell signalling pathways. The effect of TQ and MC-A were compared with inhibitors, LY294002, SB431542 and U0126 in HTB-9 cells. Partial expression of phosphorylation of ERK ½ was showed by LY294002 while increase in AKT and β-catenin phosphorylation is a result of inhibition of ERK1/2. Activation of p38 MAPK, AKT and ERK1/2 shown by SB431542 and no stable pattern was seen in U0126 treatment. On the other hand, no inhibitors were able to match the pattern of effects of MC-A or TQ in HTB-9 or in MDB-MB-231 cells (Jafri et al., 2010, Iskender et al., 2016).
The combined effect of TQ and resveratrol (RES) were shown on mice transplanted with breast cancer xenograft, and it caused a significant reduction of tumor size, induced apoptosis, geographic necrosis and reduced VEGF expression. The dose-dependent increment in response was seen upon TQ and RES in different cell lines. 15 uM RES with increasing TQ concentration showed a significant decrement in cell viability in EMT6/P cell line. The combination therapy of TQ and RES showed strongest antitumor activity as compared to TQ or RES treatment alone (Alaufi et al., 2017, Alobaedi et al., 2017).
TQ in combination with most active chemotherapeutic agent, cisplatin (CDDP) showed a remarkable effect in lung cancer. Nearly 90% inhibition of cell proliferation was recorded in NCI-H146 cell line of small cell lung carcinoma (SCLC) at the dose of 100 uM of TQ and 5 uM of CDDP upon 48 and 72 hr treatment. 20 mg/kg TQ and 2.5 mg/kg CDDP were combined to shrink the tumor volume by 79% (Khader and Eckl, 2014, Jafri et al., 2010).
The cytotoxicity of TQ and CDDP were recorded in oral squamous cell carcinoma in UMSCC-14C and normal oral epithelial cells (OEC). Improvement in the cytotoxicity and reduction in IC50 was seen in UMSCC-14C cells upon 24, 48 and 72 hr incubation with a combination of TQ and CDDP. Combination of TQ and CDDP with 5 uM each, produces apoptosis in 99.1% of cells upon 24 hr incubation. TQ and CDDP combined treatment enhances P53 expression in UMSCC-14C and OEC cells by 3 and 5 times as compared to control. 5 and 6 times increment in the expression of caspase-9 was observed upon TQ and CDDP combined treatment as compared to control in UMSCC-14C and OEC cells (Subramanian and Govindan, 2007, Alaufi et al., 2017).
12. Chemopreventive and anticancer effects of thymoquinone
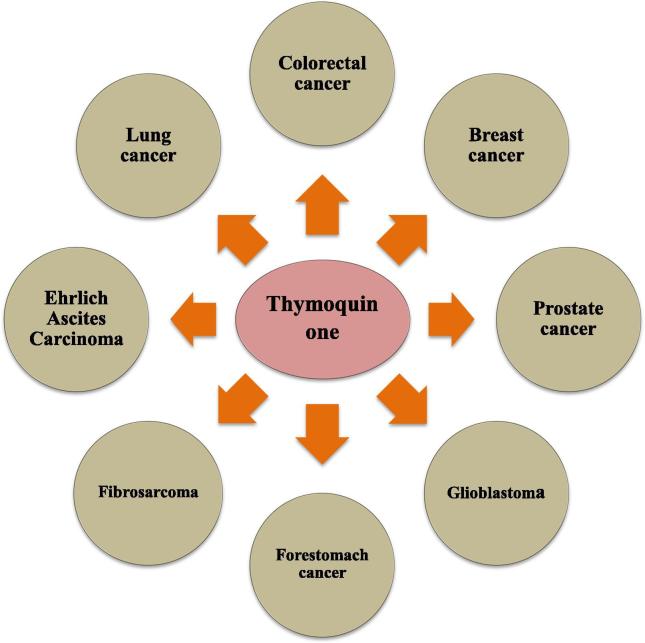
Cancer is a malignant growth or tumor resulting from an uncontrolled division of cells. It is estimated that around 9.6 million world population died due to cancer in 2018 (WHO 2018). It not only affects certain parts of the body but also spreads throughout the body and damages various vital organs. To treat cancer, already existing therapies like chemotherapy and radiation therapy have various side effects that are limiting its clinical use, so it becomes necessary to find new molecules without these limitations; hence, nowadays scientists are more focusing on natural products, which are safer as compare to other existing therapies. One such molecule is TQ which is obtained from N. sativa, however, very few authors have reviewed the chemopreventive and anticancer property of TQ in past (Khader and Eckl, 2014). Recently a lot more studies are going on the active constituents of N. sativa on various cancers such as lung cancer, colorectal cancer, prostate cancer, breast cancer, glioblastoma, and various other types of cancer (Fig. 7). Acute and chronic toxicity studies have recently been performed to assess the safety of N. sativa oil and its primary active component, TQ, particularly when given via oral route. The above mentioned topic is aimed at summarizing some of the recent and significant work done by various scientists on the effects of TQ against various types of cancer (Kundu et al., 2014).
Fig. 7.
Thymoquinone possess the potential in the prevention and treatment of different kinds of cancer.
12.1. Thymoquinone and lung cancer
Lung cancer is the most common cancer worldwide, accounting for 1.3 million deaths annually. It has accounted for 1.76 million deaths worldwide in 2018 (WHO, 2018). Smoking is considered as the major cause of lung cancer; however, there are many other causes like passive smoking, lung disease, radon gas, familial predisposition, asbestos fibres and air pollution. Many researchers have studied the antitumor activity of TQ in vitro and in vivo. Recently scientists have shown cytotoxic effect of TQ on lung cancer cell line (Subramanian and Govindan, 2007). However, the detailed anticancer effects and its molecular mechanisms of TQ on lung cancer are yet to be elucidated. Scientists studied TQ effect on A549 lung cancer cell line to elucidate the cell proliferation, migration, and invasion as well as its underlying mechanisms of antimetastasis. The study showed that TQ is a potent anticancer drug in A549 cells, and showed selective cytotoxicity towards A549 cells. It also inhibits the proliferation rate of A549 cells in a dose/time-dependent manner. TQ downregulated the expression of proliferation marker PCNA and cyclin D1 in A549 cells after treatment with TQ. Hence, it was confirmed that TQ has a clear antiproliferative effect in vitro (Nithya et al., 2014).
It is also established that thymoquinone causes p53-independent apoptotic cell deaths by the stimulation of caspase-8 and caspase-9, followed by the downregulation of caspase-3 in the caspase ladder. Also caspase-8 activation increases the cytochrome-c discharge taking place from mitochondria to cytoplasm. It also controls the ratio of Bax/Bcl2 by upregulating the apoptosis favouring the Bax and down-regulating the apoptotic opposing Bcl2 proteins in p53-null HL-60 cell lines simultaneously with the process of apoptosis. Evaluating the anti-tumor effects of thymoquinone on A549 NSCLC cells treated with benzo (α) pyrene, Ulasli et al showed that thymoquinone exposure enhanced the Bax and reduced Bcl2 proteins, and subsequently enhanced the ratio of Bax/Bcl2. It also downregulated the cyclin D expression and upregulated the p21 expression, also it has also increased the expression of TRAIL receptor 1 and 2. The molecular outcomes finally result in a modulatory p53 level which affects the initiation cell cycle arrest in of G2/M phase and finally apoptosis (Khader and Eckl, 2014).
In the study of Benzo (α) pyrene induced animal model where animals showed considerably altered levels of heme indices with concomitantly decreased levels of membrane bound ATPases in the lung tissue and erythrocyte membrane, the TQ treated animals showed significant increase in the activities of membrane integrity enzymes and established the protective role of TQ in maintaining membrane-bound ATPases (Nithya et al., 2014).
12.2. Thymoquinone and colorectal cancer
Colorectal cancer mostly affects the colon and rectum which are the final parts of the gastrointestinal system. Colorectal cancer is one of the most universally diagnosed with gastrointestinal cancers and among the main causes of cancer-related death in western developed countries. Colorectal cancer is the third most common diagnosed cancer in both men and women. One in 22 men and one in 24 women will be diagnosed with colorectal cancer in their lifetime (Smith et al., 2018). Scientists investigated the effect of TQ on colorectal cancer in vitro and in vivo. One of the researchers found that TQ significantly decreased the human LoVo colon cancer cell proliferation and also decreased the levels of p-PI3K, p-Akt, p-GSK3β, and β-catenin and thereby downregulated the downstream COX-2 expression which resulted in a reduction in PGE2 levels and the suppression of EP2 and EP4 activation. Further analysis showed that TQ treatment inhibited the nuclear translocation of β-catenin in LoVo cancer cells. TQ reduced the COX-2 expression at the transcriptional level and the reduction of COX-2 expression efficiently reduced LoVo cell migration. The results were further verified in an animal tumor xenograft model (Hsu et al., 2017). TQ, when given intraperitoneally reduced the formation of aberrant crypt foci and colon adenoma burden in Balb/c mice and also elicited a reduction in the incidence and multiplicity of colon tumors in Wistar rats both before and after treatment with 1,2-dimethylhydrazine. A decrease in the number of large polyps in the intestine of adenomatous polyposis coli (APC) mice was reported after oral feeding of TQ (Lang et al., 2011).
12.3. Thymoquinone and prostate cancer
Prostate cancer is the cancer of prostate gland which is mainly present in men. It starts when cells in the prostate gland starts to grow uncontrollably. It makes fluid that is part of the semen. About 1 man in 9 is prone to prostate cancer during his lifetime. Among them, the older men aged above 65 are more prone to prostate cancer, and it is rare before the age of 40. The average age at the time of diagnosis is about 66 (Smith et al., 2018). Several scientists have investigated the anticancer effect of TQ in prostate cancer both in vitro and in vivo. The authors have studied the TQ-induced growth inhibition in PC-3 and C4-2B prostate cancer cells, due to the generation of ROS. Results suggested that up-regulation of GADD45a and AIF and down-regulation of Bcl-2-related proteins might have a role in cell death by TQ in PC-3 cells. They also investigated that TQ-induced cell death in C4-2B cells is ROS dependent and not AR-dependent. TQ specifically inhibits proliferation and viability of cancerous cells but not the normal cells. Scientists observed that TQ stunted the growth of C4–2B derived tumors in nude mice in a xenograft prostate tumor model. This was associated with drastic downregulation of androgen receptor, transcription factor E2F-1, and cyclin A which was determined by Western blot analysis. Their findings clearly suggest that TQ may prove to be an effective agent in treating the hormone-sensitive, as well as hormone-refractory, prostate cancers with a reasonable degree of selectivity (Banerjee et al., 2010).
12.4. Thymoquinone and breast cancer
Breast cancer is the leading cause of death among different kinds of cancer. Antitumor activity of TQ in breast cancer was shown to be mediated through different modes of mechanisms, which includes induction of apoptosis, stimulation of immune system and inhibition of VEGF expression. RES showed the ability to enhance the anticancer activity of TQ through converging with the same pathways followed by TQ.
TQ reduced the proliferation rate of breast cancer cells and found to be significantly effective against tumor cell growth, invasion, and migration. It was shown that the TQ exhibits its anticancer effects through PPAR-γ pathway via binding to the receptors, and affecting their regulated gene products. It was also revealed that thymoquinone enhances the production of ROS, which ultimately leads to the phosphorylation of p38, resulting in the antiproliferative and pro-apoptotic efficacy of thymoquinone in breast cancer (Woo et al., 2011).
In the xenograft mouse model, scientists explained the capability of thymoquinone to inhibit the growth of breast cancer, and the simultaneous treatment with doxorubicin showed significantly enhanced tumor inhibition. Moreover, thymoquinone also showed the increased expression of p-p38 protein in tumors, and a decrease in the XIAP, survivin, Bcl-xL, and Bcl-2 anti-apoptotic proteins (Woo et al., 2013).
12.5. Thymoquinone and glioblastoma
Glioblastoma multiforme (GBM) is among the most devastating brain tumors with an average survival of not more than one year and comes with unique challenges to therapy because of its aggressive behaviour. It accounts for more than 40% of all malignant brain tumors and approximately 54.4% among all malignant gliomas with an average age at diagnosis being 64 years and more common in men rather than women (Weil, 2005).
Thymoquinone also possess the capacity to induce concentration and exposure time-dependent apoptosis. The antiapoptotic efficacy of thymoquinone was established by the formation of DNA ladder, mitochondrial membrane potential loss in the cells undergoing apoptosis, however, this thymoquinone induced apoptosis largely depends upon activation of caspases since apoptosis was majorly curbed by the caspase inhibitor z-VAD-FMK in general. Therefore, these effects distinctly established that thymoquinone induced apoptosis could happen independently of p53- arbitrated cellular consequences. It is also established that around 50% of cancers in human entertain p53 mutations, the effects have significant entailments for establishing thymoquinone as a chemopreventive or chemotherapeutic agent (Chowdhury et al., 2018).
It has also been reported that TQ has the ability to interfere in normal cell cycle progression and thereby inhibits GBM growth. Several studies have reported that TQ can cause cell cycle arrest at different phases. TQ treatment can alter the expression of multiple cell cycle regulatory proteins, such as cyclin D1, cyclin E, and the CDK inhibitor p27, and induces apoptosis (accumulation of sub-G1 population) through caspase activation and PARP cleavage (Chowdhury et al., 2018).
12.6. Thymoquinone and other types of cancer
The antitumor efficacy of thymoquinone appears to be significant both for tumor prevention and in treating various types of cancer. TQ exhibits selectivity to cancer cells, since normal cells, human pancreatic ductal epithelial cells (HPDE) and mouse keratinocytes are resistant to the apoptotic effects of TQ (Banerjee et al., 2010). We briefly summarize below, the antitumor effects of TQ various other types of cancer.
12.6.1. Forestomach cancer
In the mice model, animals were protected against benzo (α) pyrene [B(α)P] induced forestomach cancer and chromosomal abnormalities in bone marrow cells. It was also established that daily consumption of the thymoquinone either before, during, or after the exposure to B(α)P decreased the occurrence of CAs damaged cells significantly as against the extremely mutagenic B(α)P solely, along with that tumor and heterogeneity was proved to be curbed in as much as 70 and 67% cases (Badary et al., 1997).
12.6.2. Fibrosarcoma
The growth inhibitory and antitumor effects of TQ studied by Badary et al., in fibrosarcoma male Swiss albino mice induced by 20-methylcholanthrene. TQ was found to be significantly supressing the tumor incidences and tumor load (34% compared to 100% in normal control animals), as well as it also checked the onslaught of MC-induced fibrosarcoma cancers which is an indication of chemopreventive action against MC-induced fibrosarcomas (Badary et al., 1997).
12.6.3. Ehrlich ascites carcinoma
Badary et al. found that thymoquinone enhanced the activity of cisplatin in Ehrlich ascites carcinoma-bearing animal model and concluded that thymoquinone (50 mg/l in ad libitum) when administered 5 days before and 5 days after single dose of cisplatin, reduced nephrotoxicity and potentiated the antitumor activity of cisplatin. Another study done by Badary in mice bearing EAC xenograft, revealed that TQ (10 mg/kg/day) in drinking water significantly potentiated the antitumor effect of Ifosfamide (Badary et al., 1997).
13. Future perspective and conclusion
The present day research has been focusing on novel therapeutic strategies which are preferably based upon natural resources and can provide some potent anti-cancer compounds eligible for application in the clinical settings. A great variety of natural compounds have been studies that possess a wide spectrum of pharmacological studies. A large number of studies have been undertaken on TQ and growing evidence of its application in an array of in-vitro and in-vivo designs. In the current manuscript, we documented from available literature that TQ possess great potential in acting as both a chemopreventive agent in cancer studies as well as, can also be applied in anti-tumor therapeutic paradigms. In addition, we have compiled a number of studies briefly describing chemistry and biosynthetic pathway of TQ synthesis, its occurrence in natural resources and a number of its pharmacological actions. Different toxicological and safety studies of TQ have also been taken into consideration in the present manuscript. Till date description of TQ has been quite broad in the available literature, in its capacity to be acting at a number of different steps in various disease processes including but not limited to inflammation, angiogenesis, apoptosis, cell cycle regulation, tumor metastasis etc. Additionally, the detailed role of TQ in its ability in targeting different kinds of cancer has been explained. Looking at the number of studies about TQ and the pace at which these studies have taken place and still growing it can be predicted that this compound will be explored and will exhibit a broad array of other further pharmacological actions as well. The authors came across diverse studies about the pharmacokinetics and pharmacodynamics of TQ, therefore a strong suggestion regarding the further exploration and compilation of pharmacokinetics and pharmacodynamics of TQ can be placed here. Also, a number of comprehensive dose-dependent toxicological studies of TQ need to be undertaken before it can be thoroughly applied in the clinical settings.
14. Authors’ contribution
AA, RKM, AV and AK contributed to the sections from introduction part upto thymoquinone and metastasis. Rest part from combination therapy upto conclusion was contributed by MUR, AQK, WQ and RK.
Declaration of Competing Interest
The authors declared that there is no conflict of interest.
Acknowledgement
This work is supported by Department of Science and Technology SERB, Grant nos. YSS/2015/001851 and DST-Nanomission with grant no. SR/NM/NB-1044/2016(G). AA and RKM and AV are thankful to INST for providing fellowship.
Footnotes
Peer review under responsibility of King Saud University.
References
- Abdelwahab S.I., Sheikh B.Y., Taha M.M.E., How C.W., Abdullah R., Yagoub U., El-Sunousi R., Eid E.E. Thymoquinone-loaded nanostructured lipid carriers: preparation, gastroprotection, in vitro toxicity, and pharmacokinetic properties after extravascular administration. Int. J. Nanomed. 2013;8:2163–2172. doi: 10.2147/IJN.S44108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abukhader M.M. The effect of route of administration in thymoquinone toxicity in male and female rats. Ind. J. Pharmaceut. Sci. 2012;74(3):195–200. doi: 10.4103/0250-474X.106060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahmad A., Fauzia E., Kumar M., Mishra R.K., Kumar A., Khan M.A., Raza S.S., Khan R. Gelatin-coated polycaprolactone nanoparticle-mediated naringenin delivery rescue human mesenchymal stem cells from oxygen glucose deprivation-induced inflammatory stress. ACS Biomater. Sci. Eng. 2018;5(2):683–695. doi: 10.1021/acsbiomaterials.8b01081. [DOI] [PubMed] [Google Scholar]
- Ahmad A., Raish M., Alkharfy K.M., Alsarra I.A., Khan A., Ahad A., Jan B.L., Shakeel F. Solubility, solubility parameters and solution thermodynamics of thymoquinone in different mono solvents. J. Mol. Liq. 2018;272:912–918. [Google Scholar]
- Ahmad A., Khan F., Mishra R.K., Khan R. Precision cancer nanotherapy: evolving role of multifunctional nanoparticles for cancer active targeting. J. Med. Chem. 2019 doi: 10.1021/acs.jmedchem.9b00511. [DOI] [PubMed] [Google Scholar]
- Ahmad I., Muneer K.M., Tamimi I.A., Chang M.E., Ata M.O., Yusuf N. Thymoquinone suppresses metastasis of melanoma cells by inhibition of NLRP3 inflammasome. Toxicol. Appl. Pharmacol. 2013;270(1):70–76. doi: 10.1016/j.taap.2013.03.027. [DOI] [PubMed] [Google Scholar]
- Akyuz M., Taysi S., Baysal E., Demir E., Alkis H., Akan M., Binici H., Karatas Z.A. Radioprotective effect of thymoquinone on salivary gland of rats exposed to total cranial irradiation. Head Neck. 2017;39(10):2027–2035. doi: 10.1002/hed.24861. [DOI] [PubMed] [Google Scholar]
- Al-Ali A., Alkhawajah A.A., Randhawa M.A., Shaikh N.A. Oral and intraperitoneal LD50 of thymoquinone, an active principle of Nigella sativa, in mice and rats. J Ayub Med Coll Abbottabad. 2008;20(2):25–27. [PubMed] [Google Scholar]
- Alaufi O.M., Noorwali A., Zahran F., Al-Abd A.M., Al-Attas S. Cytotoxicity of thymoquinone alone or in combination with cisplatin (CDDP) against oral squamous cell carcinoma in vitro. Sci. Rep. 2017;7(1):1–12. doi: 10.1038/s41598-017-13357-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alkharfy K.M., Ahmad A., Jan B.L., Raish M. Thymoquinone reduces mortality and suppresses early acute inflammatory markers of sepsis in a mouse model. Biomed. Pharmacother. 2018;98:801–805. doi: 10.1016/j.biopha.2018.01.028. [DOI] [PubMed] [Google Scholar]
- Alobaedi O.H., Talib W.H., Basheti I.A. Antitumor effect of thymoquinone combined with resveratrol on mice transplanted with breast cancer. Asian Pac. J. Trop. Med. 2017;10(4):400–408. doi: 10.1016/j.apjtm.2017.03.026. [DOI] [PubMed] [Google Scholar]
- Alwadei M., Kazi M., Alanazi F.K. Novel oral dosage regimen based on self-nanoemulsifying drug delivery systems for codelivery of phytochemicals-curcumin and thymoquinone. Saudi Pharmaceut. J. 2019:1–11. doi: 10.1016/j.jsps.2019.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amin B., Hosseinzadeh H. Black cumin (Nigella sativa) and its active constituent, thymoquinone: an overview on the analgesic and anti-inflammatory effects. Planta Med. 2016;82(01/02):8–16. doi: 10.1055/s-0035-1557838. [DOI] [PubMed] [Google Scholar]
- Ansari M.M., Ahmad A., Mishra R.K., Raza S.S., Khan R. Zinc gluconate-loaded chitosan nanoparticles reduce severity of collagen-induced arthritis in Wistar rats. ACS Biomater. Sci. Eng. 2019;5(7):3380–3397. doi: 10.1021/acsbiomaterials.9b00427. [DOI] [PubMed] [Google Scholar]
- Armutcu F., Akyol S., Akyol O. The interaction of glutathione and thymoquinone and their antioxidant properties. Electron. J. General Med. 2018;15(4):1–8. [Google Scholar]
- Attoub S., Sperandio O., Raza H., Arafat K., Al-Salam S., Al Sultan M.A., Al Safi M., Takahashi T., Adem A. Thymoquinone as an anticancer agent: evidence from inhibition of cancer cells viability and invasion in vitro and tumor growth in vivo. Fundam. Clin. Pharmacol. 2013;27(5):557–569. doi: 10.1111/j.1472-8206.2012.01056.x. [DOI] [PubMed] [Google Scholar]
- Badary O.A., Nagi M.N., Al-Shabanah O.A., Al-Sawaf H.A., Al-Sohaibani M.O., Al-Bekairi A.M. Thymoquinone ameliorates the nephrotoxicity induced by cisplatin in rodents and potentiates its antitumor activity. Can. J. Phys. Pharmacol. 1997;75(12):1356–1361. [PubMed] [Google Scholar]
- Baig S., Seevasant I., Mohamad J., Mukheem A., Huri H.Z., Kamarul T. Potential of apoptotic pathway-targeted cancer therapeutic research: where do we stand? Cell Death Dis. 2016;7(1):1–11. doi: 10.1038/cddis.2015.275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banerjee S., Padhye S., Azmi A., Wang Z., Philip P.A., Kucuk O., Sarkar F.H., Mohammad R.M. Review on molecular and therapeutic potential of thymoquinone in cancer. Nutr. Cancer. 2010;62(7):938–946. doi: 10.1080/01635581.2010.509832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bargi R., Asgharzadeh F., Beheshti F., Hosseini M., Sadeghnia H.R., Khazaei M. The effects of thymoquinone on hippocampal cytokine level, brain oxidative stress status and memory deficits induced by lipopolysaccharide in rats. Cytokine. 2017;96:173–184. doi: 10.1016/j.cyto.2017.04.015. [DOI] [PubMed] [Google Scholar]
- Barkat M.A., Ahmad J., Khan M.A., Beg S., Ahmad F.J. Insights into the targeting potential of thymoquinone for therapeutic intervention against triple-negative breast cancer. Curr. Drug Targets. 2018;19(1):70–80. doi: 10.2174/1389450118666170612095959. [DOI] [PubMed] [Google Scholar]
- Bashmail H.A., et al. Thymoquinone synergizes gemcitabine anti-breast cancer activity via modulating its apoptotic and autophagic activities. Sci. Rep. 2018;8(1):1–11. doi: 10.1038/s41598-018-30046-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beg S., Swain S., Rizwan M., Irfanuddin M., Shobha Malini D. Bioavailability enhancement strategies: basics, formulation approaches and regulatory considerations. Curr. Drug Deliv. 2011;8(6):691–702. doi: 10.2174/156720111797635504. [DOI] [PubMed] [Google Scholar]
- Bhat R.R., Rehman M.U., Shabir A., Mir M.U.R., Ahmad A., Khan R., Masoodi M.H., Madkhali H., Ganaie M.A. Plant and Human Health. Springer; 2019. Chemical Composition and Biological Uses of Artemisia absinthium (Wormwood) pp. 37–63. [Google Scholar]
- Bouhlel A., Mosbah I.B., Abdallah N.H., Ribault C., Viel R., Mannaï S., Corlu A., Abdennebi H.B. Thymoquinone prevents endoplasmic reticulum stress and mitochondria-induced apoptosis in a rat model of partial hepatic warm ischemia reperfusion. Biomed. Pharmacother. 2017;94:964–973. doi: 10.1016/j.biopha.2017.08.018. [DOI] [PubMed] [Google Scholar]
- Cascella M., Bimonte S., Barbieri A., Del Vecchio V., Muzio M.R., Vitale A., Benincasa G., Ferriello A.B., Azzariti A., Arra C. Dissecting the potential roles of nigella sativa and its constituent thymoquinone on the prevention and on the progression of alzheimer’s disease. Front. Aging Neurosci. 2018;10(16):1–10. doi: 10.3389/fnagi.2018.00016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen M.-C., Lee N.-H., Hsu H.-H., Ho T.-J., Tu C.-C., Chen R.-J., Lin Y.-M., Viswanadha V.P., Kuo W.-W., Huang C.-Y. Inhibition of NF-κB and metastasis in irinotecan (CPT-11)-resistant LoVo colon cancer cells by thymoquinone via JNK and p38. Environ. Toxicol. 2017;32(2):669–678. doi: 10.1002/tox.22268. [DOI] [PubMed] [Google Scholar]
- Chen Y., Wang B., Zhao H. Thymoquinone reduces spinal cord injury by inhibiting inflammatory response, oxidative stress and apoptosis via PPAR-γ and PI3K/Akt pathways. Exp. Therap. Med. 2018;15(6):4987–4994. doi: 10.3892/etm.2018.6072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chowdhury F.A., Hossain M.K., Mostofa A.G.M., Akbor M.M., Sayeed B., Shahdaat M. Therapeutic potential of thymoquinone in glioblastoma treatment: Targeting major gliomagenesis signaling pathways. Biomed. Res. Int. 2018;2018:1–15. doi: 10.1155/2018/4010629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dirican A., Erten C., Atmaca H., Bozkurt E., Kucukzeybek Y., Varol U., Oktay Tarhan M., Karaca B., Uslu R. Enhanced cytotoxicity and apoptosis by thymoquinone in combination with zoledronic acid in hormone-and drug-resistant prostate cancer cell lines. J. Buon. 2014;19(4):1055–1061. [PubMed] [Google Scholar]
- ElKhoely A., Hafez H.F., Ashmawy A.M., Badary O., Abdelaziz A., Mostafa A., Shouman S.A. Chemopreventive and therapeutic potentials of thymoquinone in HepG2 cells: mechanistic perspectives. J. Nat. Med. 2015;69(3):313–323. doi: 10.1007/s11418-015-0895-7. [DOI] [PubMed] [Google Scholar]
- Elmowafy M., Samy A., Raslan M.A., Salama A., Said R.A., Abdelaziz A.E., El-Eraky W., El Awdan S., Viitala T. Enhancement of bioavailability and pharmacodynamic effects of thymoquinone via nanostructured lipid carrier (NLC) formulation. AAPS Pharm. Sci. Tech. 2016;17(3):663–672. doi: 10.1208/s12249-015-0391-0. [DOI] [PubMed] [Google Scholar]
- Gali-Muhtasib H., Diab-Assaf M., Boltze C., Al-Hmaira J., Hartig R., Roessner A., Schneider-Stock R. Thymoquinone extracted from black seed triggers apoptotic cell death in human colorectal cancer cells via a p53-dependent mechanism. Int. J. Oncol. 2004;25(4):857–866. [PubMed] [Google Scholar]
- Glamočlija U., Padhye S., Špirtović-Halilović S., Osmanović A., Veljović E., Roca S., Novaković I., Mandić B., Turel I., Kljun J. Synthesis, biological evaluation and docking studies of benzoxazoles derived from thymoquinone. Molecules. 2018;23(12):1–17. doi: 10.3390/molecules23123297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goyal S.N., Prajapati C.P., Gore P.R., Patil C.R., Mahajan U.B., Sharma C., Talla S.P., Ojha S.K. Therapeutic potential and pharmaceutical development of thymoquinone: a multitargeted molecule of natural origin. Front. Pharmacol. 2017;8(656):1–19. doi: 10.3389/fphar.2017.00656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gupta A., Ahmad A., Singh H., Kaur S., Ansari M.M., Jayamurugan G., Khan R. Nanocarrier composed of magnetite core coated with three polymeric shells mediates LCS-1 delivery for synthetic lethal therapy of BLM-defective colorectal cancer cells. Biomacromolecules. 2018;19(3):803–815. doi: 10.1021/acs.biomac.7b01607. [DOI] [PubMed] [Google Scholar]
- Gupta A., Ahmad A., Dar A.I., Khan R. Synthetic lethality: from research to precision Cancer Nanomedicine. Curr. Cancer Drug Targets. 2018;18(4):337–346. doi: 10.2174/1568009617666170630141931. [DOI] [PubMed] [Google Scholar]
- Gurung R.L., Lim S.N., Khaw A.K., Soon J.F.F., Shenoy K., Ali S.M., Jayapal M., Sethu S., Baskar R., Hande M.P. Thymoquinone induces telomere shortening, DNA damage and apoptosis in human glioblastoma cells. PloS one. 2010;(8):1–11. doi: 10.1371/journal.pone.0012124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hou Q., Liu L., Dong Y., Wu J., Du L., Dong H., Li D. Effects of Thymoquinone on radiation enteritis in mice. Sci. Rep. 2018;8(1):15122. doi: 10.1038/s41598-018-33214-3. 1-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsu H.-H., Chen M.-C., Day C.H., Lin Y.-M., Li S.-Y., Tu C.-C., Padma V.V., Shih H.-N., Kuo W.-W., Huang C.-Y. Thymoquinone suppresses migration of LoVo human colon cancer cells by reducing prostaglandin E2 induced COX-2 activation. World J. Gastroenterol. 2017;23(7):1171–1179. doi: 10.3748/wjg.v23.i7.1171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Imran M., Rauf A., Khan I.A., Shahbaz M., Qaisrani T.B., Fatmawati S., Abu-Izneid T., Imran A., Rahman K.U., Gondal T.A. Thymoquinone: A novel strategy to combat cancer: a review. Biomed. Pharmacother. 2018;106:390–402. doi: 10.1016/j.biopha.2018.06.159. [DOI] [PubMed] [Google Scholar]
- Iskender B., Izgi K., Canatan H. Novel anti-cancer agent myrtucommulone-A and thymoquinone abrogate epithelial–mesenchymal transition in cancer cells mainly through the inhibition of PI3K/AKT signalling axis. Mol. Cell. Biochem. 2016;416(1–2):71–84. doi: 10.1007/s11010-016-2697-y. [DOI] [PubMed] [Google Scholar]
- Jafri S.H., Glass J., Shi R., Zhang S., Prince M., Kleiner-Hancock H. Thymoquinone and cisplatin as a therapeutic combination in lung cancer: in vitro and in vivo. J. Experimen. Clin. Cancer Res. 2010;29(1):87. doi: 10.1186/1756-9966-29-87. 1-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jain A., Pooladanda V., Bulbake U., Doppalapudi S., Rafeeqi T.A., Godugu C., Khan W. Liposphere mediated topical delivery of thymoquinone in the treatment of psoriasis. Nanomed. Nanotechnol. Biol. Med. 2017;13(7):2251–2262. doi: 10.1016/j.nano.2017.06.009. [DOI] [PubMed] [Google Scholar]
- Kabil N., Bayraktar R., Kahraman N., Mokhlis H.A., Calin G.A., Lopez-Berestein G., Ozpolat B. Thymoquinone inhibits cell proliferation, migration, and invasion by regulating the elongation factor 2 kinase (eEF-2K) signaling axis in triple-negative breast cancer. Breast Cancer Res. Treat. 2018;171(3):593–605. doi: 10.1007/s10549-018-4847-2. [DOI] [PubMed] [Google Scholar]
- Kalam M.A., Raish M., Ahmed A., Alkharfy K.M., Mohsin K., Alshamsan A., Al-Jenoobi F.I., Al-Mohizea A.M., Shakeel F. Oral bioavailability enhancement and hepatoprotective effects of thymoquinone by self-nanoemulsifying drug delivery system. Mater. Sci. Eng., C. 2017;76:319–329. doi: 10.1016/j.msec.2017.03.088. [DOI] [PubMed] [Google Scholar]
- Kanter M., Coskun O., Uysal H. The antioxidative and antihistaminic effect of Nigella sativa and its major constituent, thymoquinone on ethanol-induced gastric mucosal damage. Arch. Toxicol. 2006;80(4):217–224. doi: 10.1007/s00204-005-0037-1. [DOI] [PubMed] [Google Scholar]
- Karamysheva A.F. Mechanisms of angiogenesis. Biochemistry (Moscow) 2008;73(7):751. doi: 10.1134/s0006297908070031. [DOI] [PubMed] [Google Scholar]
- Karandrea S., Yin H., Liang X., Slitt A.L., Heart E.A. Thymoquinone ameliorates diabetic phenotype in Diet-Induced Obesity mice via activation of SIRT-1-dependent pathways. PLoS ONE. 2017;12(9):1–19. doi: 10.1371/journal.pone.0185374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaur G., Kataria H., Mishra R. New Age Herbals. Springer; 2018. Medicinal plants as novel promising therapeutics for neuroprotection and neuroregeneration; pp. 437–453. [Google Scholar]
- Kensara O.A., El-Shemi A.G., Mohamed A.M., Refaat B., Idris S., Ahmad J. Thymoquinone subdues tumor growth and potentiates the chemopreventive effect of 5-fluorouracil on the early stages of colorectal carcinogenesis in rats. Drug Des. Develop. Therapy. 2016;10:2239–2253. doi: 10.2147/DDDT.S109721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khader M., Eckl P.M. Thymoquinone: an emerging natural drug with a wide range of medical applications. Iranian J. Basic Med. Sci. 2014;17(12):950–957. [PMC free article] [PubMed] [Google Scholar]
- Khalife K.H., Lupidi G. Nonenzymatic reduction of thymoquinone in physiological conditions. Free Radical Res. 2007;41(2):153–161. doi: 10.1080/10715760600978815. [DOI] [PubMed] [Google Scholar]
- Khan M.A., Tania M., Wei C., Mei Z., Fu S., Cheng J., Xu J., Fu J. Thymoquinone inhibits cancer metastasis by downregulating TWIST1 expression to reduce epithelial to mesenchymal transition. Oncotarget. 2015;6(23):19580–19591. doi: 10.18632/oncotarget.3973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khan M.A., Tania M., Fu S., Fu J. Thymoquinone, as an anticancer molecule: from basic research to clinical investigation. Oncotarget. 2017;8(31):51907–51919. doi: 10.18632/oncotarget.17206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kou B., Kou Q., Ma B., Zhang J., Sun B., Yang Y., Li J., Zhou J., Liu W. Thymoquinone inhibits metastatic phenotype and epithelial-mesenchymal transition in renal cell carcinoma by regulating the LKB1/AMPK signaling pathway. Oncol. Rep. 2018;40(3):1443–1450. doi: 10.3892/or.2018.6519. [DOI] [PubMed] [Google Scholar]
- Kundu J., Chun K.-S., Aruoma O.I., Kundu J.K. Mechanistic perspectives on cancer chemoprevention/chemotherapeutic effects of thymoquinone. Mutation Res./Fundamen. Mol. Mech. Mutagen. 2014;768:22–34. doi: 10.1016/j.mrfmmm.2014.05.003. [DOI] [PubMed] [Google Scholar]
- Lang M., Borgmann M., Oberhuber G., Campregher C., Evstatiev R., Gasche C. Thymoquinone attenuates tumor growth in ApcMin/+ mice by induction of apoptosis. Gastroenterology. 2011;140(5) doi: 10.1186/1476-4598-12-41. S–401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li F., Rajendran P., Sethi G. Thymoquinone inhibits proliferation, induces apoptosis and chemosensitizes human multiple myeloma cells through suppression of signal transducer and activator of transcription 3 activation pathway. Br. J. Pharmacol. 2010;161(3):541–554. doi: 10.1111/j.1476-5381.2010.00874.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li J., Khan M., Wei C., Cheng J., Chen H., Yang L., Ijaz I., Fu J. Thymoquinone inhibits the migration and invasive characteristics of cervical cancer cells SiHa and CaSki in vitro by targeting epithelial to mesenchymal transition associated transcription factors Twist1 and Zeb1. Molecules. 2017;22(12):2105. doi: 10.3390/molecules22122105. 1-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lim S., Kaldis P. Cdks, cyclins and CKIs: roles beyond cell cycle regulation. Development. 2013;140(15):3079–3093. doi: 10.1242/dev.091744. [DOI] [PubMed] [Google Scholar]
- Mahmoudvand H., Tavakoli R., Sharififar F., Minaie K., Ezatpour B., Jahanbakhsh S., Sharifi I. Leishmanicidal and cytotoxic activities of Nigella sativa and its active principle, thymoquinone. Pharm. Biol. 2015;53(7):1052–1057. doi: 10.3109/13880209.2014.957784. [DOI] [PubMed] [Google Scholar]
- Majdalawieh A.F., Fayyad M.W., Nasrallah G.K. Anti-cancer properties and mechanisms of action of thymoquinone, the major active ingredient of Nigella sativa. Crit. Rev. Food Sci. Nutr. 2017;57(18):3911–3928. doi: 10.1080/10408398.2016.1277971. [DOI] [PubMed] [Google Scholar]
- Mashayekhi-Sardoo H., Rezaee R., Karimi G. An overview of in vivo toxicological profile of thymoquinone. Toxin Rev. 2018:1–8. [Google Scholar]
- Mercan T., Yamasan B.E., Erkan O., Özdemir S. Thymoquinone alters ionic currents and decreases β adrenergic response in rat ventricle myocytes. J. Mol. Cell. Cardiol. 2018;120:22. [Google Scholar]
- Mostofa A.G.M., Hossain M.K., Basak D., Sayeed B., Shahdaat M. Thymoquinone as a potential adjuvant therapy for cancer treatment: Evidence from preclinical studies. Front. Pharmacol. 2017;8(295):1–12. doi: 10.3389/fphar.2017.00295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mu G., Zhang L., Li H., Liao Y., Yu H. Thymoquinone pretreatment overcomes the insensitivity and potentiates the antitumor effect of gemcitabine through abrogation of Notch1, PI3K/Akt/mTOR regulated signaling pathways in pancreatic cancer. Dig. Dis. Sci. 2015;60(4):1067–1080. doi: 10.1007/s10620-014-3394-x. [DOI] [PubMed] [Google Scholar]
- Nithya G., Mani R., Sakthisekaran D. Oral administration of thymoquinone attenuates benzo (a) pyrene induced lung carcinogenesis in male Swiss albino mice. Int. J. Pharm. Pharm. Sci. 2014;6:260–263. [Google Scholar]
- Noorbakhsh M.-F., Hayati F., Samarghandian S., Shaterzadeh-Yazdi H., Farkhondeh T. An overview of hepatoprotective effects of thymoquinone. Recent Pat. Food, Nut. Agri. 2018;9(1):14–22. doi: 10.2174/2212798410666180221105503. [DOI] [PubMed] [Google Scholar]
- Ong Y.S., Yazan L.S., Ng W.K., Noordin M.M., Sapuan S., Foo J.B., Tor Y.S. Acute and subacute toxicity profiles of thymoquinone-loaded nanostructured lipid carrier in BALB/c mice. Int. J. Nanomed. 2016;11:5905–5915. doi: 10.2147/IJN.S114205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ozturk S.A., Alp E., Saglam A.S.Y., Konac E., Menevse E.S. The effects of thymoquinone and genistein treatment on telomerase activity, apoptosis, angiogenesis, and survival in thyroid cancer cell lines. J. Cancer Res. Ther. 2018;14(2):328–334. doi: 10.4103/0973-1482.202886. [DOI] [PubMed] [Google Scholar]
- Paarakh P.M. Nigella sativa Linn.–A comprehensive review. 2010;1:409–429. [Google Scholar]
- Paramasivam A., Kalaimangai M., Sambantham S., Anandan B., Jayaraman G. Anti-angiogenic activity of thymoquinone by the down-regulation of VEGF using zebrafish (Danio rerio) model. Biomed. Prevent. Nutr. 2012;2(3):169–173. [Google Scholar]
- Pazhouhi M., Sariri R., Khazaei M.R., Moradi M.T., Khazaei M. Synergistic effect of temozolomide and thymoquinone on human glioblastoma multiforme cell line (U87MG) J. Cancer Res. Ther. 2018;14(5):1023–1028. doi: 10.4103/0973-1482.187241. [DOI] [PubMed] [Google Scholar]
- Peng L., Liu A., Shen Y., Xu H.-Z., Yang S.-Z., Ying X.-Z., Liao W., Liu H.-X., Lin Z.-Q., Chen Q.-Y. Antitumor and anti-angiogenesis effects of thymoquinone on osteosarcoma through the NF-κB pathway. Oncol. Rep. 2013;29(2):571–578. doi: 10.3892/or.2012.2165. [DOI] [PubMed] [Google Scholar]
- Pise H.N., Padwal S.L. Evaluation of anti-inflammatory activity of Nigella sativa: An experimental study. Nat. J. Physiol. Pharm. Pharmacol. 2017;7(7):707–711. [Google Scholar]
- Potente M., Gerhardt H., Carmeliet P. Basic and therapeutic aspects of angiogenesis. Cell. 2011;146(6):873–887. doi: 10.1016/j.cell.2011.08.039. [DOI] [PubMed] [Google Scholar]
- Rajput S., Kumar B.P., Dey K.K., Pal I., Parekh A., Mandal M. Molecular targeting of Akt by thymoquinone promotes G1 arrest through translation inhibition of cyclin D1 and induces apoptosis in breast cancer cells. Life Sci. 2013;93(21):783–790. doi: 10.1016/j.lfs.2013.09.009. [DOI] [PubMed] [Google Scholar]
- Rajput S., Kumar B.P., Sarkar S., Das S., Azab B., Santhekadur P.K., Das S.K., Emdad L., Sarkar D., Fisher P.B., Mandal M. Targeted apoptotic effects of thymoquinone and tamoxifen on XIAP mediated Akt regulation in breast cancer. PLoS ONE. 2013;8(4):e61342. doi: 10.1371/journal.pone.0061342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rasheed Z., Altorbag A.A., Al-Bossier A.S., Alnasser N.A., Alkharraz O.S., Altuwayjiri K.M., Alobaid A.S., Alsaif A.K., Alanazi Y.H., Alghidani B.A. Protective potential of thymoquinone against peroxynitrite induced modifications in histone H2A: in vitro studies. Int. J. Biol. Macromol. 2018;112:169–174. doi: 10.1016/j.ijbiomac.2018.01.157. [DOI] [PubMed] [Google Scholar]
- Rehman M.U., Wali A.F., Ahmad A., Shakeel S., Rasool S., Ali R., Rashid S.M., Madkhali H., Ganaie M.A., Khan R. Neuroprotective strategies for neurological disorders by natural products: an update. Curr. Neuropharmacol. 2019;17(3):247–267. doi: 10.2174/1570159X16666180911124605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rahmani Arshad H., Alzohairy Mohammad A., Khan Masood A., Aly Salah M. Therapeutic implications of black seed and its constituent thymoquinone in the prevention of cancer through inactivation and activation of molecular pathways. Evidence-Based Complemen. Alternat. Med. 2014;14:1–13. doi: 10.1155/2014/724658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roy A., Jauhari N., Bharadvaja N. Anticancer Plants: Natural Products and Biotechnological Implements. Springer; 2018. Medicinal Plants as a Potential Source of Chemopreventive Agents; pp. 109–139. [Google Scholar]
- Salim L.Z.A., Othman R., Abdulla M.A., Al-Jashamy K., Ali H.M., Hassandarvish P., Dehghan F., Ibrahim M.Y., Omer F.A.E.A., Mohan S. Thymoquinone inhibits murine leukemia WEHI-3 cells in vivo and in vitro. PLoS One. 2014;9(12):e115340. doi: 10.1371/journal.pone.0115340. 1-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scott M.L., Adams M.F., Karchesy J.J., McAllister J.C. Insecticidal Activity of Thymoquinone and Related Compounds Against Culex quinquefasciatus (Diptera: Culicidae) J. Med. Entomol. 2017;54(3):785–787. doi: 10.1093/jme/tjw181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sexton P. Advancing translational understanding for cancer and obesity therapy. ACS Pharmacol. Transl. Sci. 2018;1 doi: 10.1021/acsptsci.8b00014. 2 2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sicker D., Zeller K.-P., Siehl H.-U., Berger S. Wiley-VCH; 2019. Natural Products: Isolation, Structure Elucidation, History. [Google Scholar]
- Singh A., Ahmad I., Akhter S., Jain G.K., Iqbal Z., Talegaonkar S., Ahmad F.J. Nanocarrier based formulation of Thymoquinone improves oral delivery: stability assessment, in vitro and in vivo studies. Colloids Surf., B. 2013;102:822–832. doi: 10.1016/j.colsurfb.2012.08.038. [DOI] [PubMed] [Google Scholar]
- Singh R.P., Gangadharappa H.V., Mruthunjaya K. Cuminum cyminum–A popular spice: An updated review. Pharmaco. J. 2017;9(3):292–301. [Google Scholar]
- Singh S.K., Mishra M.K., Lillard J.W., Singh R. Thymoquinone enhanced the tumoricidal activity of NK Cells against Lung Cancer. Am. Assoc. Immunol. 2018;200:124–125. [Google Scholar]
- Smith R.A., Andrews K.S., Brooks D., Fedewa S.A., Manassaram-Baptiste D., Saslow D., Brawley O.W., Wender R.C. Cancer screening in the United States, 2018: a review of current American Cancer Society guidelines and current issues in cancer screening. CA: A Cancer J. Clin. 2018;68(4):297–316. doi: 10.3322/caac.21446. [DOI] [PubMed] [Google Scholar]
- Subramanian J., Govindan R. Lung cancer in never smokers: a review. J. Clin. Oncol. 2007;25(5):561–570. doi: 10.1200/JCO.2006.06.8015. [DOI] [PubMed] [Google Scholar]
- Surekha R., Sumathi T. An efficient encapsulation of thymoquinone using solid lipid nanoparticle for brain targeted drug delivery: physicochemical characterization, pharmacokinetics and bio-distribution studies. Int. J. Pharmac. Clin. Res. 2016;8(12):1616–1624. [Google Scholar]
- Sut S., et al. New drugs from old natural compounds: scarcely investigated sesquiterpenes as new possible therapeutic agents. Current Med. Chem. 2018;25(10):1241–1258. doi: 10.2174/0929867324666170404150351. [DOI] [PubMed] [Google Scholar]
- Taborsky J., Kunt M., Kloucek P., Lachman J., Zeleny V., Kokoska L. Identification of potential sources of thymoquinone and related compounds in asteraceae, cupressaceae, lamiaceae, and ranunculaceae families. Open Chem. 2012;10(6):1899–1906. [Google Scholar]
- Taka E., Mendonca P., Mazzio E.A., Reed S.D., Reams R., Soliman K.F. Molecular targets underlying the anti-inflammatory effects of thymoquinone in LPS activated BV-2 Cells. FASEB J. 2018;32(1S):40–42. [Google Scholar]
- Tubesha Z., Imam M., Mahmud R., Ismail M. Study on the potential toxicity of a thymoquinone-rich fraction nanoemulsion in Sprague Dawley rats. Molecules. 2013;18(7):7460–7472. doi: 10.3390/molecules18077460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weil R.J. Glioblastoma multiforme—Treating a deadly tumor with both strands of RNA. PLoS Med. 2005;3(1):21–23. doi: 10.1371/journal.pmed.0030031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wirries A., Breyer S., Quint K., Schobert R., Ocker M. Thymoquinone hydrazone derivatives cause cell cycle arrest in p53-competent colorectal cancer cells. Experimen. Therap. Med. 2010;1(2):369–375. doi: 10.3892/etm_00000058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woo C.C., Hsu A., Kumar A.P., Sethi G., Tan K.H.B. Thymoquinone inhibits tumor growth and induces apoptosis in a breast cancer xenograft mouse model: the role of p38 MAPK and ROS. PloS one. 2013;8(10):e75356. doi: 10.1371/journal.pone.0075356. 1-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woo C.C., Loo S.Y., Gee V., Yap C.W., Sethi G., Kumar A.P., Tan K.H.B. Anticancer activity of thymoquinone in breast cancer cells: possible involvement of PPAR-γ pathway. Biochem. Pharmacol. 2011;82(5):464–475. doi: 10.1016/j.bcp.2011.05.030. [DOI] [PubMed] [Google Scholar]
- Woo C.C., Hsu A., Kumar A.P., Sethi G., Tan K.H.B. Thymoquinone: potential cure for inflammatory disorders and cancer. Biochem. Pharmacol. 2012;83(4):443–451. doi: 10.1016/j.bcp.2011.09.029. [DOI] [PubMed] [Google Scholar]
- Wu Z.H., Chen Z., Shen Y., Huang L.L., Jiang P. Anti-metastasis effect of thymoquinone on human pancreatic cancer. Acta Pharmac. Sinica. 2011;46(8):910–914. [PubMed] [Google Scholar]
- Yang J., Kuang X., Lv P., Yan X. Thymoquinone inhibits proliferation and invasion of human nonsmall-cell lung cancer cells via ERK pathway. Tumor Biol. 2015;36(1):259–269. doi: 10.1007/s13277-014-2628-z. [DOI] [PubMed] [Google Scholar]
- Yi T., Cho S.-G., Yi Z., Pang X., Rodriguez M., Wang Y., Sethi G., Aggarwal B.B., Liu M. Thymoquinone inhibits tumor angiogenesis and tumor growth through suppressing AKT and extracellular signal-regulated kinase signaling pathways. Mol. Cancer Ther. 2008;7(7):1789–1796. doi: 10.1158/1535-7163.MCT-08-0124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zawirska-Wojtasiak R., Mildner-Szkudlarz S., Wasowicz E., Pacynski M. Gas chromatography, sensory analysis and electronic nose in the evaluation of black cumin (Nigella sativa L.) artoma quality. Herba Polonica. 2010;56(4):20–30. [Google Scholar]
- Zhang M., Du H., Huang Z., Zhang P., Yue Y., Wang W., Liu W., Zeng J., Ma J., Chen G. Thymoquinone induces apoptosis in bladder cancer cell via endoplasmic reticulum stress-dependent mitochondrial pathway. Chem. Biol. Interact. 2018;292:65–75. doi: 10.1016/j.cbi.2018.06.013. [DOI] [PubMed] [Google Scholar]